Abstract
In critical care, the specific, structured approach to patient care known as a “time-limited trial” has been promoted in the literature to help patients, surrogate decision makers, and clinicians navigate consequential decisions about life-sustaining therapy in the face of uncertainty. Despite promotion of the time-limited trial approach, a lack of consensus about its definition and essential elements prevents optimal clinical use and rigorous evaluation of its impact. The objectives of this American Thoracic Society Workshop Committee were to establish a consensus definition of a time-limited trial in critical care, identify the essential elements for conducting a time-limited trial, and prioritize directions for future work. We achieved these objectives through a structured search of the literature, a modified Delphi process with 100 interdisciplinary and interprofessional stakeholders, and iterative committee discussions. We conclude that a time-limited trial for patients with critical illness is a collaborative plan among clinicians and a patient and/or their surrogate decision makers to use life-sustaining therapy for a defined duration, after which the patient’s response to therapy informs the decision to continue care directed toward recovery, transition to care focused exclusively on comfort, or extend the trial’s duration. The plan’s 16 essential elements follow four sequential phases: consider, plan, support, and reassess. We acknowledge considerable gaps in evidence about the impact of time-limited trials and highlight a concern that if inadequately implemented, time-limited trials may perpetuate unintended harm. Future work is needed to better implement this defined, specific approach to care in practice through a person-centered equity lens and to evaluate its impact on patients, surrogates, and clinicians.
Keywords: critical care, palliative care, life-sustaining therapy, shared decision making
Contents
- Overview
- Key Conclusions
Introduction
- Methods
- Committee Membership
- Literature Review and Search Strategy
- Committee Meetings and Iterative Discussions
- Modified Delphi Process
- Committee Report
Section 1: An Operational Definition of Time-limited Trials for Patients with Critical Illness
Section 2: Essential Elements for Conducting a Time-limited Trial in Critical Care
Section 3: Collaborative Planning and Clear Communication with Patients and Surrogate Decision Makers During a Time-limited Trial
Section 4: Potential Challenges for Time-limited Trials
Section 5: Priorities for Future Work on Time-limited Trials
Limitations
Conclusions
Overview
A “time-limited trial” is a specific, structured approach to patient care that has been promoted for patients with critical illness, with the intention of helping patients, surrogate decision makers, and clinicians navigate decisions about life-sustaining therapy in the face of uncertainty. Despite the promotion of time-limited trials, this approach to care lacks a clear definition and consensus about its essential elements. To inform future research and implementation efforts, the American Thoracic Society organized a workshop committee to establish a consensus definition of a time-limited trial in critical care, identify the essential elements for conducting a time-limited trial, and prioritize directions for future work. The committee’s key conclusions are as follows.
Key Conclusions
-
•
The time-limited trial in critical care is operationally defined as a collaborative plan among clinicians and a patient and/or their surrogate decision maker(s) to use life-sustaining therapy for a defined duration, after which the patient’s response to therapy informs the decision to continue care directed toward recovery, transition to care focused exclusively on comfort, or extend the trial’s duration.
-
•
Time-limited trials are designed to help patients, surrogate decision makers, and clinicians collaboratively navigate uncertainty in critical illness. This specific approach to patient care aims to uphold a set of goals held by many patients: to extend life when possible and to avoid prolonged life-sustaining therapy if the chance of survival is low or the impact on quality of life is unacceptable.
-
•
A time-limited trial is composed of 16 essential elements that follow four sequential phases: consider, plan, support, and reassess.
-
•
For patients with critical illness, time-limited trials are primarily led by intensive care unit (ICU) clinicians and do not require routine clinical ethics consultation. The support of other healthcare teams (e.g., consultants, specialty palliative care, continuity physicians) can be beneficial in some cases.
-
•
If inadequately or inappropriately implemented in clinical practice, time-limited trials may contribute to conflict with patients and surrogates or exacerbate existing healthcare inequities.
-
•
Future work on time-limited trials should evaluate how best to implement this approach to patient care in practice through a person-centered equity lens and determine its impact on patients, surrogates, clinicians, and health systems.
Introduction
Providing care that aligns with an individual patient’s goals, priorities, and treatment preferences is a cornerstone of high-quality critical care. However, achieving this standard in clinical practice can be challenging because critical illness is often fraught with both diagnostic and prognostic uncertainty. Amid this uncertainty, patients and their surrogate decision makers (herein surrogates) face the challenge of making unfamiliar, complex, and highly consequential medical decisions.
The time-limited trial is an existing, specific approach to patient care that has been promoted in the literature by palliative and critical care experts to help patients, surrogates, and clinicians navigate these challenging decisions (1–3). This approach has been described as an attempt or “trial” of life-sustaining therapy, with specific, agreed-on guidelines designed to help clinicians, patients, and surrogates evaluate whether the therapy is providing benefit to the patient. These guidelines typically include a boundary on how long the trial lasts and clinical criteria that help determine the patient’s response to the therapy. After the trial is over, patients (if able), surrogates, and clinicians use these criteria to consider whether to continue life-sustaining therapies or, instead, focus exclusively on comfort (1).
In critical care, the time-limited trial approach aims to provide greater nuance to a clinical decision that is often oversimplified as a dichotomous choice between either open-ended life-sustaining therapy or an immediate transition to comfort-focused, end-of-life care. Rather, many critically ill patients have more nuanced goals, such as extending life when possible and avoiding prolonged life-sustaining therapy if the chance of survival is low or the impact on quality of life is unacceptable (4, 5). Time-limited trials offer a middle ground may better uphold this set of patient goals within the uncertain context of critical illness and the complex system of the ICU environment.
Since time-limited trials were first described in healthcare literature, clinicians and researchers have increasingly discussed and debated about this specific approach to patient care. Critical care and palliative care professional societies have recognized the potential of time-limited trials (6, 7), and the lay media has reported favorably on their use in the ICU (8). Time-limited trials have also been promoted in the fields of nephrology, neurology, and surgery (9–11).
However, there remains a lack of clarity about the definition of a time-limited trial and a lack of consensus about the essential elements required for its conduct, which has hindered the clinical use of this approach and our understanding of its impact (12–14). For example, emerging observational data suggest clinicians currently lack adequate guidance and tools to optimally and safely operationalize time-limited trials in practice (12, 15). In one study, clinicians perceived surrogates who requested extensions to the time-limited trial as a barrier to successful implementation, despite the original description of this approach as not binding with an option to extend the trial if desired (1, 15). Furthermore, it remains unclear whether and to what extent time-limited trials are distinct from current, usual care for patients with critical illness and from other best practices for palliative care and communication in the ICU. Because of this imprecision in what constitutes a time-limited trial, there is limited understanding about how time-limited trials affect outcomes for patients, surrogates, clinicians, and health systems.
The objectives of this American Thoracic Society (ATS) Workshop Committee were to establish a consensus definition of a time-limited trial in critical care, identify the essential elements for conducting a time-limited trial, and prioritize directions for future work. The intended audience of this report includes interprofessional clinicians who are either considering or already using time-limited trials; researchers and ethicists who are investigating these trials and their impact on patients, surrogates, and clinicians; and policy makers and administrators who influence the organization and delivery of critical care. We also developed a companion ATS Patient Information Series fact sheet (https://www.atsjournals.org/doi/abs/10.1164/rccm.209i3p1) for patients, surrogates, and other friends and family members.
Methods
Committee Membership
The objectives and activities of this committee were reviewed, approved, and funded by the ATS Project Review Committee and Board of Directors. The committee had 27 members, including healthcare professionals (n = 25), a family member of a prior ICU patient, and a person who survived critical illness. The committee cochairs identified potential members with clinical, academic, or personal experience related to critical care and time-limited trials (e.g., evidenced by authorship on relevant articles or recommendation from another committee member) and sought to achieve diverse committee representation. Of those invited, two potential members (a physician and a family member of an ICU patient) declined to participate. The final committee included clinicians, researchers, and administrators representing the clinical fields of cardiology, critical care, neurology, palliative care, pulmonary, and surgery. Members’ professional backgrounds included chaplaincy, clinical ethics, law, nursing, medicine (physicians), physical therapy, respiratory therapy, and healthcare administration. Committee members’ research fields of expertise included bioethics, clinical trials, epidemiology, health services and outcomes, health equity and disparities, ICU organization and operations, implementation science, and palliative care. Potential conflicts of interest were disclosed and managed in accordance with the policies and procedures of the ATS; no significant conflicts were identified.
Literature Review and Search Strategy
In May 2022, the workshop chairs conducted a structured literature search before the first committee meeting to build a comprehensive reference library of time-limited trials literature. We searched the title and abstract fields in MEDLINE via PubMed and the Cumulative Index to Nursing and Allied Health Literature for the exact phrases “time-limited trial” and “time-limited trials” (see the data supplement for search details). We excluded papers that did not refer to serious illness care or life-sustaining interventions. We did not restrict the search results by date of publication, publication format, or study design. We included additional articles recommended by committee members and newly published articles that met our criteria throughout the workshop process. The committee used these references to inform committee objectives, identify items for a modified Delphi process, support committee findings, and put committee findings into context with prior publications.
Committee Meetings and Iterative Discussions
Workshop committee members attended a series of virtual teleconference meetings between June 2022 and November 2022. Before the first committee meeting, patient and family representatives met separately with the cochairs to learn about the concept of a time-limited trial, prepare for committee activities, and provide input on committee objectives and activities. At the first full committee meeting, all members discussed, refined, and accepted the project objectives. The committee was then divided into five subcommittees on the basis of members’ expertise: definition, essential elements, contextual factors (e.g., sociocultural and spiritual), future directions, and patient and family engagement. After the first full committee meeting, each subcommittee held separate virtual teleconference meetings to review and synthesize the existing literature published on its subtopic, use the literature synthesis to develop items for the Delphi process, and develop preliminary findings and statements to be reviewed by the full committee. We held a final full committee meeting in November 2022 to review the Delphi results, clarify and arbitrate areas of consensus and nonconsensus, and finalize contents for this report.
Modified Delphi Process
We used a modified Delphi process to measure stakeholder consensus on key items related to time-limited trials and their essential elements, following the Guidance on Conducting and Reporting Delphi Studies guidelines (16). The ATS Survey Screening Committee approved the survey, and the University of Wisconsin Institutional Review Board approved the Delphi study. The Delphi survey participants included the committee members and other relevant stakeholders with time-limited trial expertise, identified through snowball sampling (17). The snowball sampling process continued until we reached 100 respondents (Table 1), which required sending the survey invitation to 193 identified stakeholders (i.e., 52% initial survey response rate).
Table 1.
Characteristics of the modified Delphi participants (n = 100)
| Characteristic* | n (%) |
|---|---|
| Geographical area† | |
| North America | 81 (87) |
| Europe | 8 (9) |
| Asia | 4 (4) |
| Gender | |
| Female | 48 (51) |
| Male | 46 (49) |
| Role‡ | |
| Physician | 78 (82) |
| Researcher/scientist | 22 (23) |
| Personal experience as family member of an intensive care unit patient | 11 (12) |
| Nurse | 5 (5) |
| Advanced practice provider | 3 (3) |
| Ethicist | 3 (3) |
| Chaplain | 1 (1) |
| Occupational therapist | 1 (1) |
| Physical therapist | 1 (1) |
| Respiratory therapist | 1 (1) |
| Years in practice§ | |
| 0–5 | 13 (13) |
| 6–10 | 21 (22) |
| 11–20 | 36 (37) |
| 21–30 | 15 (15) |
| 31–40 | 7 (7) |
| ⩾41 | 1 (1) |
No response: n = 7 (geographical area), n = 6 (gender), n = 5 (role), and n = 3 (years in practice).
Countries included the United States, Canada, China, the Czech Republic, France, Germany, Greece, Israel, Norway, and the United Kingdom.
Percentages may total greater than 100% because some participants had more than one role.
Among participants who identified as healthcare practitioners.
Given the scope of this workshop committee, we prespecified that the Delphi process would consist of two rounds. In the first round, participants received a 29-item survey about time-limited trial elements and statements based on the subcommittees’ review of the literature. In the second round, we sent a follow-up survey to the 100 first-round participants, 87 of whom responded (i.e., 87% second-round response rate). The 23-item second-round survey included items that did not reach consensus in the first round (modified, if necessary, on the basis of free-text comments) and new items suggested by participants. The surveys are provided in the data supplement. After the second round, as described above, we held a meeting with the full committee to review and arbitrate all items not reaching consensus.
The surveys used a nine-point scale to measure consensus for each item. For general statements, participants rated their agreement with the statement using a scale ranging from 1 (strong disagreement) to 9 (strong agreement). A priori, we defined consensus agreement as ⩾80% of participants rating the item ⩾7 and consensus disagreement as ⩾80% of participants rating the item ⩽3. For potential steps of time-limited trials, respondents were asked to rate each step on a scale ranging from 1 (neither beneficial nor necessary for a time-limited trial) to 9 (beneficial and necessary for a time-limited trial), with scores of 4–6 representing grades of benefit without necessity. A priori, we defined a consensus essential element of a time-limited trial as ⩾80% of participants rating the step ⩾7. We also a priori defined potentially beneficial elements as ⩾80% of participants rating the item ⩾4 without meeting essential element criteria. All other results were classified as nonconsensus. During each round, participants also provided free-text commentary on survey items and suggested new items to include, as necessary.
Committee Report
This workshop report was drafted by the committee cochairs and the leaders of each of the subcommittees. The full committee then reviewed, revised, and approved the report before submission for peer review. The patient and family engagement subcommittee created a companion fact sheet for ICU patients and families that will be published within the ATS Patient Information Series (https://www.atsjournals.org/doi/abs/10.1164/rccm.209i3p1).
Section 1: An Operational Definition of Time-limited Trials for Patients with Critical Illness
This workshop committee proposes the following operational definition for a time-limited trial in critical care:
A collaborative plan among clinicians and a patient and/or their surrogate decision maker(s) to use life-sustaining therapy for a defined duration, after which the patient’s response to therapy informs the decision to continue care directed toward recovery, transition to care focused exclusively on comfort, or extend the trial’s duration.
We developed this definition by synthesizing 1) published definitions in the healthcare literature, 2) stakeholder consensus obtained through the modified Delphi process, and 3) iterative revision and discussion within the workshop committee.
During our review of published definitions in the healthcare literature, we identified two common approaches to defining time-limited trials (Table 2): an operational approach (focused on essential elements and pragmatic steps of conducting a trial) and a conceptual approach (focused on a trial’s objectives and rationales). Through the Delphi process, we measured the degree of consensus around each of these major themes. We identified strong consensus about the operational themes, which led to our formulation of the operational definition above, characterizing the major elements of conducting a time-limited trial.
Table 2.
Major themes in the healthcare literature on the definition of a time-limited trial in critical care
| Operational Approaches (Essential Elements and Pragmatic Steps) |
Conceptual Approaches (Objectives and Rationales) |
|
|---|---|---|
| Theme (subthemes) | Interventions (life-sustaining care in general, specific life-sustaining therapies) | Prognosis (uncertain, poor) |
| Engagement with patients or surrogates (agreement, planning, standardization) | Decision making (uncertainty, readiness, disagreement) |
Early in our process, a fundamental question arose about whether time-limited trials can or should be distinguished from all critical care delivery in general, which implicitly consists of a trial of life-sustaining therapy with assessment of clinical response. Our committee concluded that the time-limited trial approach is distinct from current “usual care” for patients with critical illness, largely because of strong consensus that the trial is explicitly proposed to the patient and/or surrogate(s) who can then endorse the structured plan. This explicit, agreed-on nature of a time-limited trial is reflected in its operational definition.
We also identified strong consensus among Delphi participants that time-limited trials can be considered for an individual, specific life-sustaining therapy (e.g., a trial of mechanical ventilation) or for life-sustaining care in general, which could include all available life-sustaining therapies or could be an agreed-on, limited set of acceptable therapies.
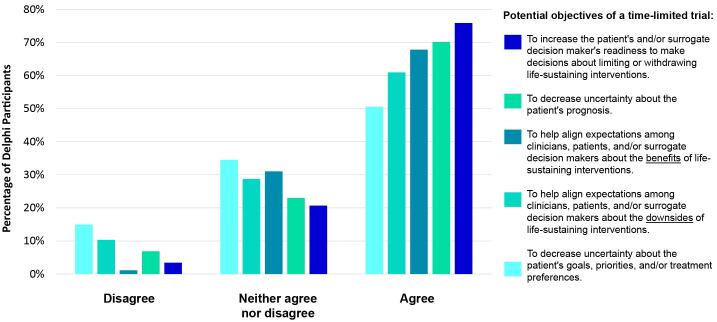
Despite strong consensus on how to operationally define a time-limited trial, we found a range of opinions on the conceptual definition of a time-limited trial, including its main objectives (Figure 1). Although the majority (>50%) of Delphi participants agreed or strongly agreed with each potential objective of a time-limited trial, none met our a priori defined consensus threshold.
Figure 1.
Degree of stakeholder agreement that a statement should be considered a main objective of a time-limited trial.
This committee supports the fundamental, conceptual view that the time-limited trial approach should be primarily framed by and focused on the uncertainty inherent in critical illness, which can be experienced and voiced by clinicians, patients, and/or surrogates. Others, including thought leaders and clinicians, propose that time-limited trials should be used when clinicians believe with near certainty that the patient will not survive, but our committee concludes this is an overly narrow conception. By focusing on the acknowledgment and management of uncertainty instead of on “poor prognosis,” time-limited trials may safeguard against well-described prognostic inaccuracies and biases about which patients are likely to benefit from critical care (18–21). Our conclusion is further supported by the dynamic and fraught nature of prognostication, because of ever-evolving technology and the inability of clinicians’ prognostic estimates to account for individual patients’ priorities and values about acceptable health states.
Section 2: Essential Elements for Conducting a Time-limited Trial in Critical Care
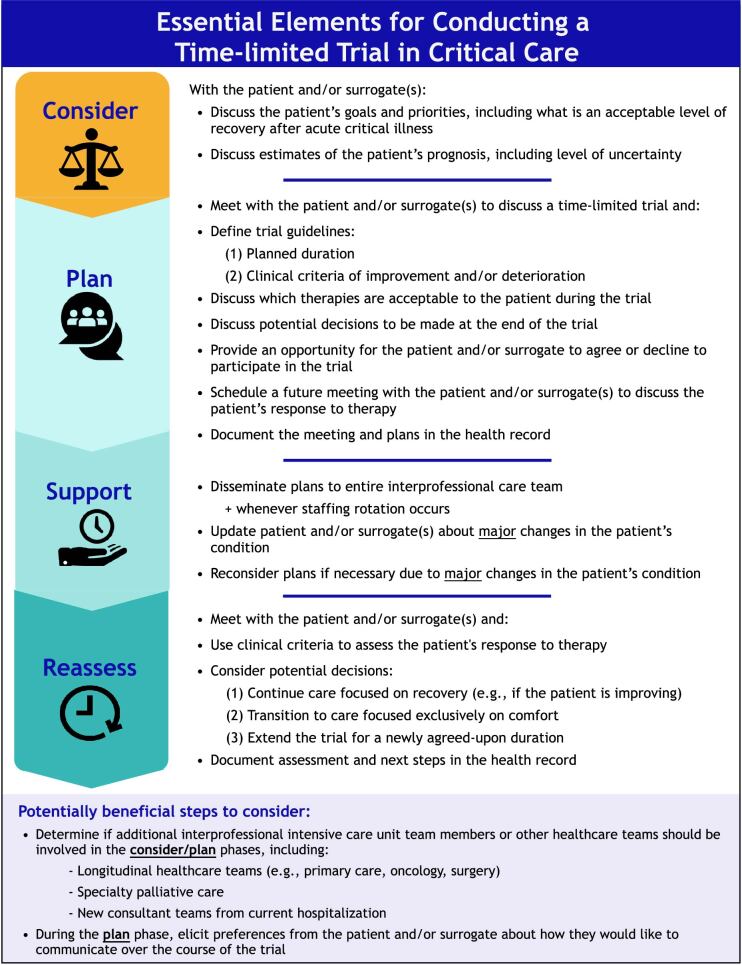
We first identified 18 potential steps of a time-limited trial in the healthcare literature and presented them to the 100 Delphi participants in the first-round survey. During this round, we identified consensus that 11 of these steps are essential elements of a time-limited trial, according to our a priori definition. During the second round, we identified seven additional essential elements on the basis of suggested modifications and newly proposed steps from the first round (see the data supplement for details). The committee then combined 4 related items into two steps (to reach 16 essential elements), made minor language modifications for clarity, and organized steps into four phases of care: consider, plan, support, and reassess (Figure 2).
Figure 2.
The essential elements for conducting a time-limited trial in critical care.
We also identified steps to consider when conducting a time-limited trial that may be helpful in some cases, but not necessary for all trials. For example, Delphi participants noted the need to involve other disciplines and healthcare teams (e.g., primary care providers, primary oncologists, specialty palliative care) is highly dependent on a patient’s specific situation and on the available hospital resources. We also found near consensus (78% agreement among Delphi participants, with unanimous consensus within the committee) that time-limited trials in critical care do not require routine clinical ethics consultation.
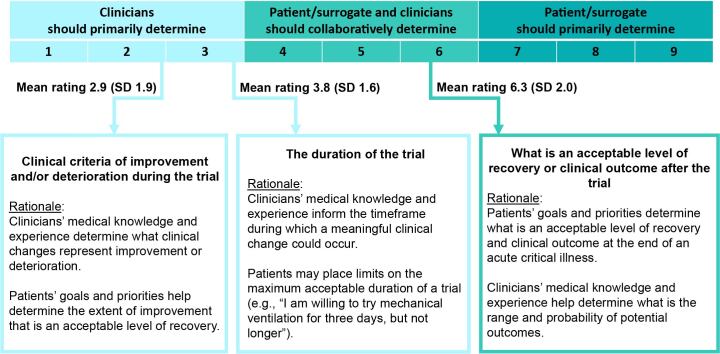
Although the time-limited trial is fundamentally a collaborative process between clinicians, patients, and surrogates, their respective roles may vary across the different elements of a trial. In the Delphi process, we measured participants’ views on these respective roles for three major elements of a time-limited trial: 1) the clinical criteria of improvement and deterioration, 2) the duration, and 3) the acceptable degree of recovery or the clinical outcome after the time-limited trial. We asked participants to rate each element on a scale of 1 through 9, with 1 representing an element that should be exclusively determined by clinicians and 9 representing an element that should be exclusively determined by the patient and/or surrogate(s) (Figure 3).
Figure 3.
The respective roles of patients, surrogates, and clinicians during the collaborative planning process of a time-limited trial. Delphi participants were asked to rate the different elements of a time-limited trial on a scale ranging from 1 (element exclusively determined by clinicians) to 9 (element exclusively determined by patient and/or surrogate[s]). The mean score and SD of scores among participants are labeled. The rationales describe findings from Delphi free-text comments and committee consensus about the nature of the roles. SD = standard deviation.
Conducting a time-limited trial also requires support of the patient and surrogates throughout its duration, after the initial plans are made. An important component of this support is ongoing communication among clinicians, patients, and surrogates about the patient’s condition. However, we identified important considerations about the amount, extent, and frequency of this communication and updating about the patient’s condition. On one hand, a time-limited trial should be flexible and modifiable, to account for unexpected clinical changes and patients and/or surrogates whose preferences or priorities evolve during the trial. On the other hand, time-limited trials may benefit patients and surrogates by minimizing information or decision “overload,” including minor, daily fluctuations in physiologic status that may have minimal clinical significance. Continual updating and revision of trial plans according to these minor changes is likely to undermine this benefit. In the second round of the Delphi process, we found strong consensus on an approach that balances these two considerations: patients and/or surrogates should be updated according to major changes in the patient’s condition, and plans should be revised if necessary due to these major changes.
Section 3: Collaborative Planning and Clear Communication with Patients and Surrogate Decision Makers during a Time-limited Trial
As established in Sections 1 and 2 of this report, the time-limited trial approach is fundamentally a collaborative, structured process among patients, surrogates, and clinicians. Communication is a key aspect of this process, with the primary goal of fostering transparency and collaboration during the time-limited trial (Text Box).
Box 1. The perspective of a surrogate decision maker who participated in a time-limited trial as the surrogate decision maker for her husband.
Surrogate Decision Maker Voice: I think employing the terminology [time-limited trial] is helpful. To me, it would help patients and families feel there is a solid plan with a start and end date. When there is so much unknown, the more clarity the better. Goal setting gives everyone something to target and focus on, rather than feeling adrift with endless ICU days ahead. And to know that if a treatment isn’t working it will be discontinued is also important since so many treatments are uncomfortable for the patient. I want to hear that there is goal setting, with a plan to evaluate and re-evaluate at specific times. Also, if Plan A is not effective, what is Plan B? At the same time, I would want the doctor to express flexibility, understanding that every patient is different and his or her response to treatment might be wildly different than the patient who came before. I would advise patients/families to never be afraid to (respectfully) speak up or ask questions if they don’t understand why a treatment is being implemented.
Communication among clinicians, critically ill patients, and their surrogate decision makers is an integral component of all care delivery in the ICU (22–25), regardless of whether a patient is undergoing a time-limited trial. Communication skills can be taught, learned, and deliberately practiced by clinicians, similar to other medical procedures (26–29). In the specific setting of a time-limited trial, the importance of high-quality communication in building trust and consensus is heightened.
Communication during a time-limited trial should be viewed as a longitudinal process requiring continuous engagement and support throughout the four trial phases (consider, plan, support, and reassess) instead of as a one-time event (e.g., a single planning meeting). These moments for communication and support are also opportunities to appropriately frame the time-limited trial, to prevent or mitigate concerns, misconceptions, and mistrust (Table 3).
Table 3.
Important framing for time-limited trials to promote collaboration among patients, surrogates, and clinicians
| Time-Limited Trials | |
|---|---|
| Should Be | Should Not Be |
| Framed by uncertainty | Framed by notions of “poor prognosis” |
| Transparent | Coercive |
| Individualized and adaptable | Prescriptive or binding |
| Iterative | Time pressured or finite |
| Oriented to patients’ goals and priorities | Oriented to one specific outcome |
| Trials of high-quality, standard-of-care therapies | Lower quality care |
| A collaborative process between patients, surrogates, and clinicians | Determined exclusively by clinicians |
The terminology of time-limited trials, including the name of the approach itself, was somewhat controversial within the committee and among the Delphi participants. Although the term “time-limited trial” is accepted and widely used in the literature, some committee members and Delphi participants expressed concerns about potential misinterpretation when this terminology is used with patients and surrogates. First, the word “limited” could be misinterpreted to mean lower quality care or that restrictions are being imposed on a patient’s care. Second, the word “trial” could be misinterpreted as meaning a research study or experimentation. Through the Delphi process, we did not reach consensus about whether it is important to use consistent terminology across contexts, including clinical care and research. Although consistency in terminology may promote transparency and trust, we acknowledge many examples of technical terms in healthcare that promote communication among clinicians and researchers but that are not typically used with patients and families (e.g., “myocardial infarction,” “cerebrovascular accident”).
The committee ultimately concluded that these questions of potential misinterpretation and optimal terminology should be informed by future empirical work that includes patients and surrogates. Despite these unanswered questions, the committee unanimously agreed that the most important aspects of communication with patients and surrogates are transparency, clear framing, and communication describing what the time-limited trial approach to care is instead of what the care approach is called. As described in Figure 2, this transparency includes providing an explicit opportunity for patients to agree or decline to participate and to revise plans during the trial. To support this communication, the committee has developed a companion patient- and surrogate-facing resource about time-limited trials that is available at (https://www.atsjournals.org/doi/abs/10.1164/rccm.209i3p1).
A time-limited trial is a specific, structured approach to patient care rather than a communication tool, though successful and appropriate use of this approach to care relies on well-established best practices for communication and shared decision making (30). This includes tailoring language and using linguistic strategies that are culturally, ethnically, and racially inclusive and sensitive (31–36). There is little evidence about how best to communicate about time-limited trials with patients and surrogates, but several approaches have been suggested (1, 2, 11, 37, 38). For example, some experts propose using the combined framing of “hope” and “worry” statements to express uncertainty and the range of potential outcomes to patients and surrogates when considering a time-limited trial (37). Communication with patients, surrogates, and families during a time-limited trial also presents an opportunity to introduce complementary palliative care concepts, such as spiritual care, symptom assessment and management, caregiver and family support, and care focused exclusively on comfort (e.g., hospice care models) (31, 39).
Communication with patients and surrogates during a time-limited trial also requires an interprofessional approach. As illustrated in Figure 2, we reached consensus that time-limited trials are an interprofessional process and require dissemination to and engagement from team members with different lenses. This team-based approach can also leverage the unique relationships between patients and families and each member of the team (40), including case managers, chaplains, dietitians, nurses, occupational and physical therapists, pharmacists, physicians, respiratory therapists, and social workers. In some cases, the important roles of cultural brokers and chaplains may help clinicians engage patients and surrogates and accurately describe a time-limited trial plan.
Section 4: Potential Challenges for Time-limited Trials
Although experts have highlighted the potential for time-limited trials to increase the alignment of care with patients’ goals, improve the therapeutic alliance, and reduce decisional conflict, there is a small evidence base for efficacy (3, 12, 38, 41, 42). To our knowledge, the time-limited trial approach has been tested in only one pre-post study of a quality improvement program focused on implementation. In that study, surrogates experienced earlier and more frequent family meetings, though there was no change in their satisfaction with medical care or amount of decision making conflict (38).
It is also important to acknowledge the potential for unintended harm due to time-limited trial use, which may be disproportionately accrued by structurally marginalized patients and surrogates. First, identifying candidates for a time-limited trial is a subjective process that currently depends on individual clinicians and may be prone to unwarranted variation and biases. For example, the largest investigation of time-limited trials to date included patients whom a clinician deemed to be at risk for “potentially nonbeneficial” ICU treatments (38). Although this practical approach resembles usual care, inaccuracies and biases in physicians’ prognostication for mortality and other nonmortal outcomes (e.g., quality of life) are well described (18, 21), and quantitative, prognostic algorithms have not fared much better (19, 43, 44). Furthermore, assessment of “benefit” from medical treatments is highly influenced by patients’ and surrogates’ cultural norms and expectations (45, 46). Indeed, the valuation of treatment preferences has been shown to occur in a racialized and classed manner, such that the values of structurally minoritized patients and surrogates are contested or deemed inappropriate (47–51). Because of these factors, this committee has unanimously agreed that time-limited trials should be framed around the uncertainty inherent in critical illness instead of on perceptions or measures of poor prognosis (see Section 1).
Second, as discussed in Section 3, successful implementation of time-limited trials relies on communication and collaboration with patients and surrogates. Although there is little evidence about the quality of this communication and collaboration specifically during time-limited trials, we know from other studies of the ICU context that communication is less common and of lower quality with people from racially and ethnically minoritized populations (47, 52–63). For example, the Institute of Medicine’s seminal “Unequal Treatment” report from 2002 and more recent data have demonstrated that clinicians are less likely to ask about treatment preferences, share all treatment options, and provide emotional support for decision making when communicating with racially and ethnically minoritized surrogates and families than White surrogates and families (55–62). In the context of time-limited trials, these observations raise the potential for inadequate informed consent, limited agency among patients and surrogates to participate in setting the guidelines of a time-limited trial, and a lack of available remedies if conflicts arise. This finding suggests additional interventions are needed to ensure that patients and surrogates are incorporated as equal partners in time-limited trials.
Section 5: Priorities for Future Work on Time-limited Trials
This committee has formulated key, unanswered questions to help motivate future work on time-limited trials, on the basis of gaps identified in the healthcare literature and on areas of nonconsensus identified during our modified Delphi process (Table 4). The three most pressing objectives for future work are to determine 1) how to optimize the use of time-limited trials in clinical practice; 2) if time-limited trials benefit patients, surrogates, and clinicians compared with usual care; and 3) what are the potential unintended harms caused by inappropriate implementation of time-limited trials and how to safeguard against these. To realize the potential of time-limited trials in critical care, this future work will require a patient- and family-centered equity lens.
Table 4.
Summary of prioritized future questions for time-limited trials in critical care
| Stakeholder | Key Unanswered Questions |
|---|---|
| Patients, surrogates, and families | What are patient, surrogate, and other family member perspectives about the concept of a time-limited trial? |
| What are the best words or phrases to use with patients, surrogates, and families when discussing and planning time-limited trials? | |
| What potential benefits of time-limited trials are important to patients, surrogates, and other family members (e.g., decisional conflict and regret, psychological outcomes, interpersonal experiences)? | |
| How do we best ensure that the time-limited trial process is collaborative and centers the patient’s perspective? | |
| What factors influence whether patients and/or surrogates agree or decline to participate? | |
| What potential harms could patients, surrogates, or other family members experience during time-limited trials? | |
| How do time-limited trials influence patients’, surrogates’, or other family members’ perceptions of trust in clinicians and health systems? | |
| Interprofessional clinicians who care for critically ill patients | Consider phase
|
Plan phase
| |
Support phase
| |
Reassess phase
| |
| Health systems | How can health systems support the optimal implementation of time-limited trials? |
| What, if any, are the impacts of time-limited trials on health systems? | |
| How can health systems ensure that systemic biases do not propagate inequities in the implementation of time-limited trials? | |
| Should time-limited trials be used in the context of or for the purpose of allocation of limited healthcare resources? If yes, how can this be transparently and equitably implemented? |
Limitations
The committee members and Delphi participants who contributed to this report were predominantly from the United States and from academic medical centers, so our consensus findings may not represent those who practice in other geographic regions or nonacademic settings. Given the scope of this committee, we also prespecified that our modified Delphi process consisted of two rounds, which were followed by arbitration of any nonconsensus items within the 27-member workshop committee. Nevertheless, we did reach our a priori threshold for consensus on all the essential element items after two rounds (see the data supplement for details), and this report provides our analysis of the nonconsensus items.
Conclusions
The time-limited trial is a specific, structured approach to patient care designed to help patients, surrogates, and clinicians collaboratively navigate the uncertainty and complexity inherent in critical illness. This approach aims to operationalize simultaneous goals held by many patients: extending life when possible and avoiding prolonged life-sustaining therapy if the chance of survival is low or the impact on quality of life is unacceptable (4, 5). Time-limited trials are composed of 16 essential elements and follow four sequential phases: consider, plan, support, and reassess. For patients with critical illness, time-limited trials are led primarily by ICU clinicians and do not require routine clinical ethics consultation. The support of other healthcare teams (e.g., consultants, specialty palliative care, continuity physicians) can be beneficial in some cases. Future work should use a person-centered equity lens to better operationalize this defined approach to patient care in clinical practice and to evaluate its impact on patients, surrogates, and clinicians.
Acknowledgments
This workshop report was prepared by an ad hoc subcommittee of the ATS Assembly on Behavioral Science and Health Services Research.
Members of the subcommittee are as follows:
Jacqueline M. Kruser, M.D., M.S. (Co-Chair)1
Christopher E. Cox, M.D., M.P.H. (Co-Chair)2
Writing committee members:
Deepshikha C. Ashana, M.D., M.B.A., M.S.2
Katherine R. Courtright, M.D., M.S.H.P.5
Erin K. Kross, M.D.6,8
Thanh H. Neville, M.D., M.S.H.S.9
Eileen Rubin, J.D.10
Yael Schenker, M.D., M.A.S.11
Donald R. Sullivan, M.D., M.A., M.C.R.12,13
J. Daryl Thornton, M.D., M.P.H.14,15
Elizabeth M. Viglianti, M.D., M.P.H., M.Sc.16
Workshop committee members:
Deena Kelly Costa, Ph.D., R.N.17,18
Claire J. Creutzfeldt, M.D.7
Michael E. Detsky, M.D., M.S.H.P.19
Heidi J. Engel, P.T., D.P.T.20
Neera Grover, M.I.M.S.10
Aluko A. Hope, M.D., M.S.C.E.12
Jason N. Katz, M.D., M.H.S.3
Rachel Kohn, M.D., M.S.C.E.5
Andrew G. Miller, M.Sc., R.R.T.-A.C.C.S., R.R.T.-N.P.S.,4,21
Michael J. Nabozny, M.D.22
Judith E. Nelson, M.D., J.D.23,24
Hasan Shanawani, M.D., M.P.H.25
Jennifer P. Stevens, M.D., M.S.26
Alison E. Turnbull, D.V.M., M.P.H., Ph.D.27,28,29
Curtis H. Weiss, M.D., M.S.30
M. Jeanne Wirpsa, M.A., B.C.C., H.E.C.-C.31
1Division of Allergy, Pulmonary and Critical Care, Department of Medicine, School of Medicine and Public Health, University of Wisconsin, Madison, Wisconsin; 2Division of Pulmonary, Allergy, and Critical Care Medicine and 3Division of Cardiology, Department of Medicine, and 4Division of Pediatric Critical Care, Department of Pediatrics, Duke University, Durham, North Carolina; 5Division of Pulmonary, Allergy, and Critical Care, Department of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania; 6Division of Pulmonary, Critical Care, and Sleep Medicine, Department of Medicine, and 7Department of Neurology, University of Washington, Seattle, Washington; 8Cambia Palliative Care Center of Excellence at UW Medicine, Seattle, Washington; 9Division of Pulmonary, Critical Care, and Sleep Medicine, Department of Medicine, David Geffen School of Medicine, University of California, Los Angeles, Los Angeles, California; 10ARDS Foundation, Northbrook, Illinois; 11Division of General Internal Medicine, Department of Medicine, University of Pittsburgh, Pittsburgh, Pennsylvania; 12Division of Pulmonary, Allergy, and Critical Medicine, Oregon Health and Sciences University, Portland, Oregon; 13Center to Improve Veteran Involvement in Care, VA Portland Health Care System, Portland, Oregon; 14Center for Health Equity, Engagement, Education, and Research, MetroHealth Campus, and 15Division of Pulmonary, Critical Care, and Sleep Medicine, Case Western Reserve University, Cleveland, Ohio; 16Department of Internal Medicine, University of Michigan Medical School, Ann Arbor, Michigan; 17Yale School of Nursing, Orange, Connecticut; 18Section of Pulmonary, Critical Care, and Sleep Medicine, Yale School of Medicine, New Haven, Connecticut; 19Department of Medicine, Sinai Health System, Toronto, Ontario, Canada; 20Department of Rehabilitative Services, University of California, San Francisco, San Francisco, California; 21Respiratory Care Services, Duke University Medical Center, Durham, North Carolina; 22Department of Surgery, University of Rochester, Rochester, New York; 23Division of Pulmonary, Critical Care, and Sleep Medicine, Department of Medicine, and 24Hertzberg Palliative Care Institute, Mount Sinai School of Medicine, New York, New York; 25VA National Center for Patient Safety, Ann Arbor, Michigan; 26Division for Pulmonary, Critical Care, and Sleep Medicine, Department of Medicine, Beth Israel Deaconess Medical Center, Boston, Massachusetts; 27Division of Pulmonary and Critical Care Medicine, School of Medicine, 28Department of Epidemiology, Bloomberg School of Public Health, and 29Outcomes After Critical Illness and Surgery Group, Johns Hopkins University, Baltimore, Maryland; 30Division of Pulmonary, Critical Care, Allergy, and Immunology, North Shore University Health System, Evanston, Illinois; and 31Northwestern Memorial Hospital, Chicago, Illinois
Acknowledgment: The authors gratefully acknowledge the 73 additional participants in the modified Delphi process, whose survey responses became the foundation for the committee’s statements. These participants include (with permission to be named) Rebecca A. Aslakson, M.D., Ph.D., F.A.A.H.P.M., F.C.C.M. (Burlington, Vermont), Catherine Auriemma, M.D., M.S. (Philadelphia, Pennsylvania), Jan Bakker, M.D., Ph.D., F.C.C.M., F.C.C.P. (New York, New York), Michael D. Barnett, M.D., M.S. (Birmingham, Alabama), Cameron Baston, M.D., M.S.C.E. (Philadelphia, Pennsylvania), Michael Beil, M.D., Ph.D., Sc.D. (Inverness, UK), Brian L. Block, M.D. (San Francisco, California), Jori Bogetz, M.D. (Seattle, Washington), Dong Chang, M.D., M.S. (Torrance, California), Jen-Ting (Tina) Chen, M.D., M.S. (San Francisco, California), Jonathan C. H. Cheung, M.B.Ch.B., B.Sc., M.Clin.US (Hong Kong SAR, China), Justin Clapp, Ph.D., M.P.H. (Philadelphia, Pennsylvania), Karin Clifton, B.A., M.S.N., A.C.N.P.-B.C. (San Francisco, California), Martha A. Q. Curley, R.N., Ph.D., F.A.A.N. (Philadelphia, Pennsylvania), J. Randall Curtis, M.D., M.P.H. (Seattle, Washington), Justin Fiala, M.D. (Chicago, Illinois), Hans Flaatten, M.D., Ph.D., Prof. (Bergen, Norway), Sean Forsythe, M.D. (Maywood, Illinois), Rob Fowler, M.D., M.D.C.M., M.Sc. (Toronto, Ontario, Canada), Hayley B. Gershengorn, M.D. (Miami, Florida), Michelle Ng Gong, M.D., M.S. (New York, New York), Bertrand Guidet, M.D. (Paris, France), Rachel Hadler, M.D. (Atlanta, Georgia), Joanna Hart, M.D., M.S.H.P. (Philadelphia, Pennsylvania), Breanna Hetland, Ph.D., R.N., C.C.R.N.-K. (Omaha, Nebraska), Dana Holiona, M.S.W., L.C.S.W. (Chicago, Illinois), Robert Holloway, M.D., M.P.H. (Rochester, New York), May Hua, M.D., M.S. (New York, New York), Caroline J. Hurd, M.D., F.A.C.P., F.A.A.H.P.M. (Seattle, Washington), Paul J. Hutchison, M.D., M.A. (Maywood, Illinois), David Y. Hwang, M.D. (Chapel Hill, North Carolina), Theodore J. Iwashyna, M.D., Ph.D. (Baltimore, Maryland), Anand S. Iyer, M.D., M.S.P.H. (Birmingham, Alabama), Gavin M. Joynt, M.B. Ch.B., F.C.I.C.M. (Hong Kong SAR, China), Christian Jung, M.D., Ph.D., Prof. (Duesseldorf, Germany), Jeremy Kahn, M.D., M.Sc. (Pittsburgh, Pennsylvania), Nader Kamangar, M.D., F.A.C.P., F.C.C.P., F.C.C.M., Prof. (Los Angeles, California), Nita Khandelwal, M.D., M.S. (Seattle, Washington), Kristina Kordesch, R.N., A.C.N.P.-A.G. (San Francisco, California), Anna Krupp, Ph.D., M.S.H.P., R.N. (Iowa City, Iowa), Dustin Krutsinger, M.D., M.S. (Omaha, Nebraska), May M. Lee, M.D. (Los Angeles, California), Theresa Lombardo, D.N.P., A.P.R.N. (Chicago, Illinois), Jan Maláska, M.D., Ph.D., E.D.I.C. (Brno, Czech Republic), Spyros D. Mentzelopoulos, M.D., Ph.D., D.E.A.A., E.D.I.C. (Athens, Greece), Andrej Michalsen, M.D., M.P.H. (Konstanz, Germany), Jayson Neagle, M.D. (Chicago, Illinois), Simon Oczkowski, M.D., M.H.Sc., M.Sc. (Hamilton, Ontario, Canada), Tracy Ann Pasek, R.N., D.N.P., C.C.N.S., C.C.R.N.-K. (Pittsburgh, Pennsylvania), Shruti Patel, M.D., M.B.A. (Maywood, Illinois), Christiane Perme, P.T., C.C.S., F.C.C.M. (Houston, Texas), Chiagozie Pickens, M.D. (Chicago, Illinois), Nida Qadir, M.D. (Los Angeles, California), Caroline M. Quill, M.D., M.S.H.P. (Rochester, New York), Katerina Rusinova, M.D., Ph.D. (Prague, Czech Republic), Gregory A. Schmidt, M.D. (Iowa City, Iowa), Margaret L. (Gretchen) Schwarze, M.D., M.P.P., F.A.C.S. (Madison, Wisconsin), Katharine Secunda, M.D. (Philadelphia, Pennsylvania), Melanie M. Smith, M.D. (Chicago, Illinois), Megan Staley, O.T.D., O.T.R./L., C.L.T. (San Francisco, California), Sigal Sviri, M.D., M.H.A. (Jerusalem, Israel), Amy Trowbridge, M.D. (Seattle, Washington), P. Vernon van Heerden, M.D., Ph.D., Prof. (Jerusalem, Israel), Kelly C. Vranas, M.D., M.C.R. (Portland, Oregon), James M. Walter, M.D. (Chicago, Illinois), Gary Weissman, M.D., M.S.H.P. (Philadelphia, Pennsylvania), and Douglas B. White, M.D., M.A.S. (Pittsburgh, Pennsylvania).
Footnotes
This official Workshop Report of the American Thoracic Society was approved September 2023
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures: J.M.K.’s spouse served as consultant for Radialogica; served as co-editor for CHEST; spouse received honoraria from Astra Zeneca; received research support from NIH/NHLBI. D.C.A. received research support from NIH/NHLBI. E.K.K. served on advisory board for NIH and PCORI. E.R. served as president and CEO of ARDS Foundation. Y.S. served as consultant and medical advisor for EMMI Solutions, Wolters Kluwer; received research support from a K24 grant; received royalties from UpToDate. D.S. received honoraria from UpToDate. E.M.V. received honoraria from Brown University and Southeastern Critical Care Summit; received research support from NIH/NHLBI; received travel support from Society of Women in Urology. D.K.C. received research support from Agency for Healthcare Research and Quality and NHLBI; spouse/partner holds stock in Microsoft. C.J.C. received research support from NIH. H.E. served as consultant for Arjo-Huntleigh; served as speaker for American Physical Therapy Association. A.A.H. received research support from NIH/NHLBI. A.G.M. employee of Respiratory Care; served as consultant for S2N Health; received honoraria from Fisher and Paykel and Saxe Communications. J.P.S. received royalties from McGraw Hill and UpToDate. C.E.C. received research support from NIH. K.R.C., T.H.N., J.D.T., M.E.D., N.G., J.N.K., R.K., M.J.N., J.E.N., H.S., A.E.T., C.H.W., M.J.W. reported no commercial or relevant non-commercial interests from ineligible companies.
References
- 1. Quill TE, Holloway R. Time-limited trials near the end of life. JAMA . 2011;306:1483–1484. doi: 10.1001/jama.2011.1413. [DOI] [PubMed] [Google Scholar]
- 2. Siropaides CH, Arnold RM. Time-limited trials for serious illness #401. J Palliat Med . 2020;23:1540–1541. doi: 10.1089/jpm.2020.0490. [DOI] [PubMed] [Google Scholar]
- 3. Vink EE, Azoulay E, Caplan A, Kompanje EJO, Bakker J. Time-limited trial of intensive care treatment: an overview of current literature. Intensive Care Med . 2018;44:1369–1377. doi: 10.1007/s00134-018-5339-x. [DOI] [PubMed] [Google Scholar]
- 4. Cox CE, White DB, Hough CL, Jones DM, Kahn JM, Olsen MK, et al. Effects of a personalized web-based decision aid for surrogate decision makers of patients with prolonged mechanical ventilation: a randomized clinical trial. Ann Intern Med . 2019;170:285–297. doi: 10.7326/M18-2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Auriemma CL, Harhay MO, Haines KJ, Barg FK, Halpern SD, Lyon SM. What matters to patients and their families during and after critical illness: a qualitative study. Am J Crit Care . 2021;30:11–20. doi: 10.4037/ajcc2021398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kon AA, Shepard EK, Sederstrom NO, Swoboda SM, Marshall MF, Birriel B, et al. Defining futile and potentially inappropriate interventions: a policy statement from the Society of Critical Care Medicine Ethics Committee. Crit Care Med . 2016;44:1769–1774. doi: 10.1097/CCM.0000000000001965. [DOI] [PubMed] [Google Scholar]
- 7.American Academy of Hospice and Palliative Medicine. Chicago: American Academy of Hospice and Palliative Medicine; 2011. https://aahpm.org/positions/withholding-nonbeneficial-interventions [Google Scholar]
- 8.Span P. New York: The New York Times; 2021. https://www.nytimes.com/2021/05/10/health/elderly-hospitals-palliative-care.html [PubMed] [Google Scholar]
- 9. Rinehart A. Beyond the futility argument: the fair process approach and time-limited trials for managing dialysis conflict. Clin J Am Soc Nephrol . 2013;8:2000–2006. doi: 10.2215/CJN.12191212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Creutzfeldt CJ, Holloway RG. Treatment decisions after severe stroke: uncertainty and biases. Stroke . 2012;43:3405–3408. doi: 10.1161/STROKEAHA.112.673376. [DOI] [PubMed] [Google Scholar]
- 11. Neuman MD, Allen S, Schwarze ML, Uy J. Using time-limited trials to improve surgical care for frail older adults. Ann Surg . 2015;261:639–641. doi: 10.1097/SLA.0000000000000939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Viglianti EM, Ervin JN, Newton CA, Kruser JM, Iwashyna TJ, Valley TS. Time-limited trials in the ICU: a mixed-methods sequential explanatory study of intensivists at two academic centres. BMJ Open . 2022;12:e059325. doi: 10.1136/bmjopen-2021-059325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Barnato AE, Tate JA, Rodriguez KL, Zickmund SL, Arnold RM. Norms of decision making in the ICU: a case study of two academic medical centers at the extremes of end-of-life treatment intensity. Intensive Care Med . 2012;38:1886–1896. doi: 10.1007/s00134-012-2661-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schenker Y, Tiver GA, Hong SY, White DB. Discussion of treatment trials in intensive care. J Crit Care . 2013;28:862–869. doi: 10.1016/j.jcrc.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bruce CR, Liang C, Blumenthal-Barby JS, Zimmerman J, Downey A, Pham L, et al. Barriers and facilitators to initiating and completing time-limited trials in critical care. Crit Care Med . 2015;43:2535–2543. doi: 10.1097/CCM.0000000000001307. [DOI] [PubMed] [Google Scholar]
- 16. Jünger S, Payne SA, Brine J, Radbruch L, Brearley SG. Guidance on Conducting and Reporting Delphi Studies (CREDES) in palliative care: recommendations based on a methodological systematic review. Palliat Med . 2017;31:684–706. doi: 10.1177/0269216317690685. [DOI] [PubMed] [Google Scholar]
- 17.Tracy SJ. In: Qualitative research methods: collecting evidence, crafting analysis, communicating impact. Tracy SJ, editor. Oxford: Blackwell; 2013. Interview planning and design: sampling, recruiting, and questioning. [Google Scholar]
- 18. Detsky ME, Harhay MO, Bayard DF, Delman AM, Buehler AE, Kent SA, et al. Discriminative accuracy of physician and nurse predictions for survival and functional outcomes 6 months after an ICU admission. JAMA . 2017;317:2187–2195. doi: 10.1001/jama.2017.4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ashana DC, Anesi GL, Liu VX, Escobar GJ, Chesley C, Eneanya ND, et al. Equitably allocating resources during crises: racial differences in mortality prediction models. Am J Respir Crit Care Med . 2021;204:178–186. doi: 10.1164/rccm.202012-4383OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Christakis NA, Iwashyna TJ. Attitude and self-reported practice regarding prognostication in a national sample of internists. Arch Intern Med . 1998;158:2389–2395. doi: 10.1001/archinte.158.21.2389. [DOI] [PubMed] [Google Scholar]
- 21. Christakis NA, Lamont EB. Extent and determinants of error in doctors’ prognoses in terminally ill patients: prospective cohort study. BMJ . 2000;320:469–472. doi: 10.1136/bmj.320.7233.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Azoulay E, Sprung CL. Family-physician interactions in the intensive care unit. Crit Care Med . 2004;32:2323–2328. doi: 10.1097/01.ccm.0000145950.57614.04. [DOI] [PubMed] [Google Scholar]
- 23. Kynoch K, Chang A, Coyer F, McArdle A. The effectiveness of interventions to meet family needs of critically ill patients in an adult intensive care unit: a systematic review update. JBI Database Syst Rev Implement Reports . 2016;14:181–234. doi: 10.11124/JBISRIR-2016-2477. [DOI] [PubMed] [Google Scholar]
- 24. Curtis JR. Communicating about end-of-life care with patients and families in the intensive care unit. Crit Care Clin . 2004;20:363–380. doi: 10.1016/j.ccc.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 25. Curtis JR, Patrick DL, Shannon SE, Treece PD, Engelberg RA, Rubenfeld GD. The family conference as a focus to improve communication about end-of-life care in the intensive care unit: opportunities for improvement. Crit Care Med . 2001;29:N26–N33. doi: 10.1097/00003246-200102001-00006. [DOI] [PubMed] [Google Scholar]
- 26. Lakin JR, Tulsky JA, Bernacki RE. Time out before talking: communication as a medical procedure. Ann Intern Med . 2021;174:96–97. doi: 10.7326/M20-4223. [DOI] [PubMed] [Google Scholar]
- 27. Piazza O, Cersosimo G. Communication as a basic skill in critical care. J Anaesthesiol Clin Pharmacol . 2015;31:382–383. [PMC free article] [PubMed] [Google Scholar]
- 28. Arnold RM, Back AL, Barnato AE, Prendergast TJ, Emlet LL, Karpov I, et al. The Critical Care Communication project: improving fellows’ communication skills. J Crit Care . 2015;30:250–254. doi: 10.1016/j.jcrc.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 29. Back AL, Arnold RM, Baile WF, Fryer-Edwards KA, Alexander SC, Barley GE, et al. Efficacy of communication skills training for giving bad news and discussing transitions to palliative care. Arch Intern Med . 2007;167:453–460. doi: 10.1001/archinte.167.5.453. [DOI] [PubMed] [Google Scholar]
- 30. Kon AA, Davidson JE, Morrison W, Danis M, White DB, American College of Critical Care Medicine American Thoracic Society. Shared decision making in ICUs: an American College of Critical Care Medicine and American Thoracic Society policy statement. Crit Care Med . 2016;44:188–201. doi: 10.1097/CCM.0000000000001396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sullivan DR, Iyer AS, Enguidanos S, Cox CE, Farquhar M, Janssen DJA, et al. Palliative care early in the care continuum among patients with serious respiratory illness: an official ATS/AAHPM/HPNA/SWHPN policy statement. Am J Respir Crit Care Med . 2022;206:e44–e69. doi: 10.1164/rccm.202207-1262ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Butler M, McCreedy E, Schwer N, Burgess D, Call K, Przedworski J, et al. Improving cultural competence to reduce health disparities. Rockville, MD: Agency for Healthcare Research and Quality; 2016. [PubMed] [Google Scholar]
- 33. Andrulis DP, Brach C. Integrating literacy, culture, and language to improve health care quality for diverse populations. Am J Health Behav . 2007;31:S122–S133. doi: 10.5555/ajhb.2007.31.supp.S122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sudore RL, Landefeld CS, Pérez-Stable EJ, Bibbins-Domingo K, Williams BA, Schillinger D. Unraveling the relationship between literacy, language proficiency, and patient-physician communication. Patient Educ Couns . 2009;75:398–402. doi: 10.1016/j.pec.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kelley AS, Wenger NS, Sarkisian CA. Opiniones: end-of-life care preferences and planning of older Latinos. J Am Geriatr Soc . 2010;58:1109–1116. doi: 10.1111/j.1532-5415.2010.02853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schillinger D, Duran ND, McNamara DS, Crossley SA, Balyan R, Karter AJ. Precision communication: physicians’ linguistic adaptation to patients’ health literacy. Sci Adv . 2021;7:eabj2836. doi: 10.1126/sciadv.abj2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Downer K, Gustin J, Lincoln T, Goodman L, Barnett MD. Communicating about time-limited trials. Chest . 2022;161:202–207. doi: 10.1016/j.chest.2021.08.071. [DOI] [PubMed] [Google Scholar]
- 38. Chang DW, Neville TH, Parrish J, Ewing L, Rico C, Jara L, et al. Evaluation of time-limited trials among critically ill patients with advanced medical illnesses and reduction of nonbeneficial ICU treatments. JAMA Intern Med . 2021;181:786–794. doi: 10.1001/jamainternmed.2021.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lanken PN, Terry PB, Delisser HM, Fahy BF, Hansen-Flaschen J, Heffner JE, et al. ATS End-of-Life Care Task Force An official American Thoracic Society clinical policy statement: palliative care for patients with respiratory diseases and critical illnesses. Am J Respir Crit Care Med . 2008;177:912–927. doi: 10.1164/rccm.200605-587ST. [DOI] [PubMed] [Google Scholar]
- 40. Kruser JM, Solomon D, Moy J, Holl JL, Viglianti EM, Detsky ME, et al. Impact of interprofessional teamwork on aligning icu care with patient goals: a qualitative study of transactive memory systems. Ann Am Thorac Soc . 2023;20:548–555. doi: 10.1513/AnnalsATS.202209-820OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vincent J-L. Withdrawing may be preferable to withholding. Crit Care . 2005;9:226–229. doi: 10.1186/cc3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. VanKerkhoff TD, Viglianti EM, Detsky ME, Kruser JM. Time-limited trials in the intensive care unit to promote goal-concordant patient care. Clin Pulm Med . 2019;26:141–145. doi: 10.1097/cpm.0000000000000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Miller WD, Han X, Peek ME, Charan Ashana D, Parker WF. Accuracy of the sequential organ failure assessment score for in-hospital mortality by race and relevance to crisis standards of care. JAMA Netw Open . 2021;4:e2113891. doi: 10.1001/jamanetworkopen.2021.13891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sarkar R, Martin C, Mattie H, Gichoya JW, Stone DJ, Celi LA. Performance of intensive care unit severity scoring systems across different ethnicities in the USA: a retrospective observational study. Lancet Digit Health . 2021;3:e241–e249. doi: 10.1016/S2589-7500(21)00022-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Geros-Willfond KN, Ivy SS, Montz K, Bohan SE, Torke AM. Religion and spirituality in surrogate decision making for hospitalized older adults. J Relig Health . 2016;55:765–777. doi: 10.1007/s10943-015-0111-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Braun UK, Beyth RJ, Ford ME, McCullough LB. Voices of African American, Caucasian, and Hispanic surrogates on the burdens of end-of-life decision making. J Gen Intern Med . 2008;23:267–274. doi: 10.1007/s11606-007-0487-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hauschildt KE.Whose good death? Valuation and standardization as mechanisms of inequality in hospitals J Health Soc Behav 2022. [online ahead of print] 15 Dec 2022; DOI: 10.1177/00221465221143088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ashana DC, D’Arcangelo N, Gazarian PK, Gupta A, Perez S, Reich AJ, et al. “Don’t talk to them about goals of care”: understanding disparities in advance care planning. J Gerontol A Biol Sci Med Sci . 2022;77:339–346. doi: 10.1093/gerona/glab091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ashana DC, Jan A, Parish A, Johnson KS, Steinhauser KE, Olsen MK, et al. Interpersonal perception: family- and physician-reported conflict in the intensive care unit. Ann Am Thorac Soc . 2022;19:1937–1942. doi: 10.1513/AnnalsATS.202202-147RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lane-Fall MB, Miano TA, Aysola J, Augoustides JGT. Diversity in the emerging critical care workforce: analysis of demographic trends in critical care fellows from 2004 to 2014. Crit Care Med . 2017;45:822–827. doi: 10.1097/CCM.0000000000002322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Choi PJ, Curlin FA, Cox CE. Addressing religion and spirituality in the intensive care unit: a survey of clinicians. Palliat Support Care . 2019;17:159–164. doi: 10.1017/S147895151800010X. [DOI] [PubMed] [Google Scholar]
- 52. Scheunemann LP, Ernecoff NC, Buddadhumaruk P, Carson SS, Hough CL, Curtis JR, et al. Clinician-family communication about patients’ values and preferences in intensive care units. JAMA Intern Med . 2019;179:676–684. doi: 10.1001/jamainternmed.2019.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cooper L, Roter D. In: Unequal treatment: confronting racial and ethnic disparities in health care. Smedley BD, Stith AY, Nelson AR, editors. Washington, DC: The National Academies Press; 2003. Patient-provider communication: the effect of race and ethnicity on process and outcomes of healthcare; pp. 552–593. [PubMed] [Google Scholar]
- 54. Muni S, Engelberg RA, Treece PD, Dotolo D, Curtis JR. The influence of race/ethnicity and socioeconomic status on end-of-life care in the ICU. Chest . 2011;139:1025–1033. doi: 10.1378/chest.10-3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Shen MJ, Peterson EB, Costas-Muñiz R, Hernandez MH, Jewell ST, Matsoukas K, et al. The effects of race and racial concordance on patient-physician communication: a systematic review of the literature. J Racial Ethn Health Disparities . 2018;5:117–140. doi: 10.1007/s40615-017-0350-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Martin KD, Roter DL, Beach MC, Carson KA, Cooper LA. Physician communication behaviors and trust among black and white patients with hypertension. Med Care . 2013;51:151–157. doi: 10.1097/MLR.0b013e31827632a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Johnson RL, Roter D, Powe NR, Cooper LA. Patient race/ethnicity and quality of patient-physician communication during medical visits. Am J Public Health . 2004;94:2084–2090. doi: 10.2105/ajph.94.12.2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Reid HW, Lin OM, Fabbro RL, Johnson KS, Svetkey LP, Olsen MK, et al. Racial differences in patient perception of interactions with providers are associated with health outcomes in type II diabetes. Patient Educ Couns . 2021;104:1993–2003. doi: 10.1016/j.pec.2021.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Johnson KS. Racial and ethnic disparities in palliative care. J Palliat Med . 2013;16:1329–1334. doi: 10.1089/jpm.2013.9468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Johnson KS, Kuchibhatla M, Tulsky JA. Racial differences in self-reported exposure to information about hospice care. J Palliat Med . 2009;12:921–927. doi: 10.1089/jpm.2009.0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Park J, Beach MC, Han D, Moore RD, Korthuis PT, Saha S. Racial disparities in clinician responses to patient emotions. Patient Educ Couns . 2020;103:1736–1744. doi: 10.1016/j.pec.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Drwecki BB, Moore CF, Ward SE, Prkachin KM. Reducing racial disparities in pain treatment: the role of empathy and perspective-taking. Pain . 2011;152:1001–1006. doi: 10.1016/j.pain.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 63. Peek ME, Odoms-Young A, Quinn MT, Gorawara-Bhat R, Wilson SC, Chin MH. Racism in healthcare: its relationship to shared decision-making and health disparities: a response to Bradby. Soc Sci Med . 2010;71:13–17. doi: 10.1016/j.socscimed.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]