Abstract
Rationale
Bronchiectasis is a chronic, progressive disease of bronchial dilation, inflammation, and scarring leading to impaired mucociliary clearance and increased susceptibility to infection. Identified causes include previous severe respiratory infections. A small, single-center UK study demonstrated a reduction in bronchiectasis exacerbations during the first year of the coronavirus disease (COVID-19) pandemic. No studies have been conducted in a U.S. (commercially insured) cohort to date.
Objectives
To explore the impact of the COVID-19 pandemic on the frequency of exacerbations in a large cohort of commercially insured U.S. patients with bronchiectasis by testing the hypothesis that U.S. patients with bronchiectasis had fewer exacerbations during the pandemic.
Methods
This retrospective observational cohort study used health insurance claims data from Optum’s deidentified Clinformatics Data Mart database, which included U.S. patients and their covered dependents. Eligible patients were ⩾18 years of age with bronchiectasis; patients with other respiratory conditions were excluded. The main study cohort excluded patients with frequent asthma and/or chronic obstructive pulmonary disease diagnoses. The primary objective was to compare the bronchiectasis exacerbation rates before and during the COVID-19 pandemic.
Results
The median number of exacerbations per patient per year decreased significantly from the year before the COVID-19 pandemic to the first year of the pandemic (1 vs. 0; P < 0.01). More patients had zero exacerbations during the first year of the pandemic than the year prior (57% vs. 24%; McNemar’s chi-square = 122.56; P < 0.01).
Conclusions
In a U.S. population-based study of patients with International Classification of Diseases codes for bronchiectasis, the rate of exacerbations during Year 1 of the COVID-19 pandemic was reduced compared with the 2-year time period preceding the pandemic.
Keywords: bronchiectasis, exacerbation, severe acute respiratory syndrome coronavirus 2, pandemics
Non–cystic fibrosis bronchiectasis, hereafter referred to as bronchiectasis, is a chronic, progressive disease of bronchial dilation, inflammation, and scarring that leads to impaired mucociliary clearance and increased susceptibility to infection (1–3). Numerous causes of bronchiectasis have been identified, including previous severe respiratory infections (e.g., bacterial pneumonia or tuberculosis), genetic abnormalities, immunologic conditions, autoimmune diseases, airway lesions, and chronic aspiration (1). Bronchiectasis is characterized by chronic respiratory symptoms (e.g., cough, phlegm or sputum production, and dyspnea), and exacerbation of bronchiectasis is defined as a deterioration in at least three of six key symptoms (i.e., cough, phlegm and/or sputum volume and consistency, phlegm and/or sputum purulence, breathlessness or exercise intolerance, fatigue or malaise, and hemoptysis) for at least 48 hours and a clinician determination that a change in treatment is required (1, 4).
The etiology of bronchiectasis exacerbations is unclear, but potential triggers include viral and bacterial infections, neutrophilic and eosinophilic inflammation, and environmental exposures (5–8). Lockdowns and social distancing measures enacted during the coronavirus disease (COVID-19) pandemic reduced the number and frequency of interpersonal interactions and by extension the circulation of respiratory viruses such as rhinovirus and influenza (9–11). A small, single-center, prospective study was recently conducted in the United Kingdom in which researchers examined exacerbation rates among patients with computed tomography–confirmed bronchiectasis before and during the first year of the pandemic (12). More than 80% of patients in the study reported using additional protective measures (e.g., minimizing person-to-person contact through social distancing and staying home) during the pandemic, as recommended for high-risk and vulnerable patients. Researchers observed a marked reduction in bronchiectasis exacerbations among patients in the study during the first 12 months of the COVID-19 pandemic, which they associated with social distancing measures adopted during that time period. Another small study conducted at three centers in Spain revealed similar reductions in exacerbations and severe exacerbations among patients with bronchiectasis during the pandemic (13). These observations require confirmation in larger, multicenter studies. No studies have been conducted in a commercially insured U.S. cohort to date.
Therefore, the objective of this analysis was to explore the impact of the COVID-19 pandemic on the frequency of exacerbations in U.S. patients with bronchiectasis by testing the hypothesis that U.S. patients with bronchiectasis had fewer exacerbations during the COVID-19 pandemic.
Methods
Cohort
Our study had a retrospective observational cohort design and used health insurance claims data from Optum’s deidentified Clinformatics Data Mart database, which is derived from a database of administrative health claims for members of large commercial and Medicare Advantage health plans as well as their covered dependents. Eligible patients were ⩾18 years of age by January of the first year of the time window (time window 1: March 1, 2018, to February 29, 2020; time window 2: March 1, 2019, to February 28, 2021), had continuous health plan enrollment during the time window (i.e., 2 yr), had at least one bronchiectasis diagnosis code before March in the year that the time window started, and had at least one bronchiectasis exacerbation within the time window. Patients were excluded if they had tuberculosis, nontuberculous mycobacterial respiratory infection, pulmonary fibrosis, cystic fibrosis, lung cancer, primary ciliary dyskinesia, pulmonary hypertension, or alpha-1 antitrypsin deficiency; excluded diagnoses were identified via diagnosis codes in the claims database. A full list of the International Classification of Diseases, Tenth Revision (ICD-10), codes used to identify patients with bronchiectasis, as well as the codes used in the exclusionary diagnoses, is provided in the data supplement.
The study included a main cohort, which comprised all patients who met the eligibility criteria and who did not have two asthma and/or chronic obstructive pulmonary disease (COPD) diagnoses at least 30 days apart (a proxy for a confirmed asthma and/or COPD diagnosis), and a sensitivity cohort, which had no asthma or COPD exclusion criteria. For the main cohort, the ICD-10 codes for bronchiectasis used to define an exacerbation could be in any diagnostic position for the claim, whereas for the sensitivity cohort, the diagnosis was required to be in the primary diagnostic position. Exacerbations were defined as claims for inpatient visits (defined as an inpatient hospitalization with ICD-10 codes for bronchiectasis) or outpatient visits (defined as an emergency department, urgent care, physician’s office, or outpatient hospital visit with ICD-10 codes for bronchiectasis followed by an antibiotic claim within ±7 days of the visit). It should be noted that although an antibiotic prescription should follow the encounter, in practice there can be subtle inaccuracies with dates in claims data (i.e., it can be difficult to distinguish between an antibiotic claim for an exacerbation vs. a maintenance antibiotic claim, and antibiotic e-prescriptions are precise but do not inform on the exact date of antibiotic use for an exacerbation). Therefore, an antibiotic claim within ±7 days of the visit was chosen to ensure that we did not miss an exacerbation.
Study Time Periods and Impact of the Pandemic
March 1 was selected as the starting date for each time window because lockdowns related to the COVID-19 pandemic in the U.S. started in March 2020. The study consisted of two distinct 2-year time windows: time window 1, which took place before the COVID-19 pandemic from March 1, 2018, to February 29, 2020, and time window 2, which began before and continued into the first year of the COVID-19 pandemic from March 1, 2019, to February 28, 2021. Patients had to meet the inclusion and exclusion criteria for each time window separately.
The key outcome measure was the number of bronchiectasis exacerbations per patient per year. Patients were also categorized according to the number of exacerbations (i.e., zero, one, two, three, and four or more) per year. Subgroup analyses were conducted for patients who were “frequent exacerbators,” who were defined as patients with at least two bronchiectasis exacerbations during the first year of the time window in any diagnostic position.
The primary objective of this study was to compare the rate of bronchiectasis exacerbations before and during the COVID-19 pandemic. Secondary objectives were to determine whether the number of bronchiectasis exacerbations experienced by a patient varied from year to year before (i.e., time window 1) and during (i.e., time window 2) the COVID-19 pandemic and to assess whether pandemic-related restrictions were associated with a greater decrease in the number of exacerbations compared with normal (i.e., prepandemic) conditions.
Statistical Analysis
Analysis was conducted for all patients with bronchiectasis as well as for patients with more than two bronchiectasis exacerbations. Analysis was also performed in the main and sensitivity cohorts. McNemar’s chi-square tests were conducted to test differences in treatments prescribed to patients with bronchiectasis between Year 1 and Year 2 of time windows 1 and 2. Wilcoxon signed rank tests were used to evaluate differences in the median number of exacerbations per patient per year for each time window. Logistic regression was used to assess the association between frequent exacerbation in prior year with frequent exacerbation. The significance threshold was set at 0.05, without correction for multiple testing, as the primary objective (year-to-year differences in the two time windows for the main cohort) was assessed using only two P values.
Results
In the main cohort, 905 patients were included in time window 1, and 954 patients were included in time window 2 (i.e., the patients had bronchiectasis exacerbation diagnoses in any position and did not have two asthma or COPD diagnoses ⩾30 d apart) (Figure 1A). In addition, in the sensitivity cohorts, which had no asthma or COPD exclusion criteria, 2,164 patients were included in time window 1, and 2,141 patients were included in time window 2 (Figure 1B). In all cohorts, nearly all patients (≈99.7%) had at least two bronchiectasis diagnosis codes ⩾30 days apart (see Table E1 in the data supplement). Distribution of patients by state is presented in the data supplement (see Figure E1). A greater number of patients with bronchiectasis in the database were from the Southwestern and Southeastern U.S., whereas fewer patients were from the Northern U.S.; otherwise, patients were located throughout the contiguous U.S. More patients in the main cohort were female (77% in time window 1 and 75% in time window 2), and the patients’ median (interquartile range) age was 75 (69–81) years in time window 1 and 76 (69–82) years in time window 2; patient demographics were similar in the sensitivity cohort (Table 1).
Figure 1.
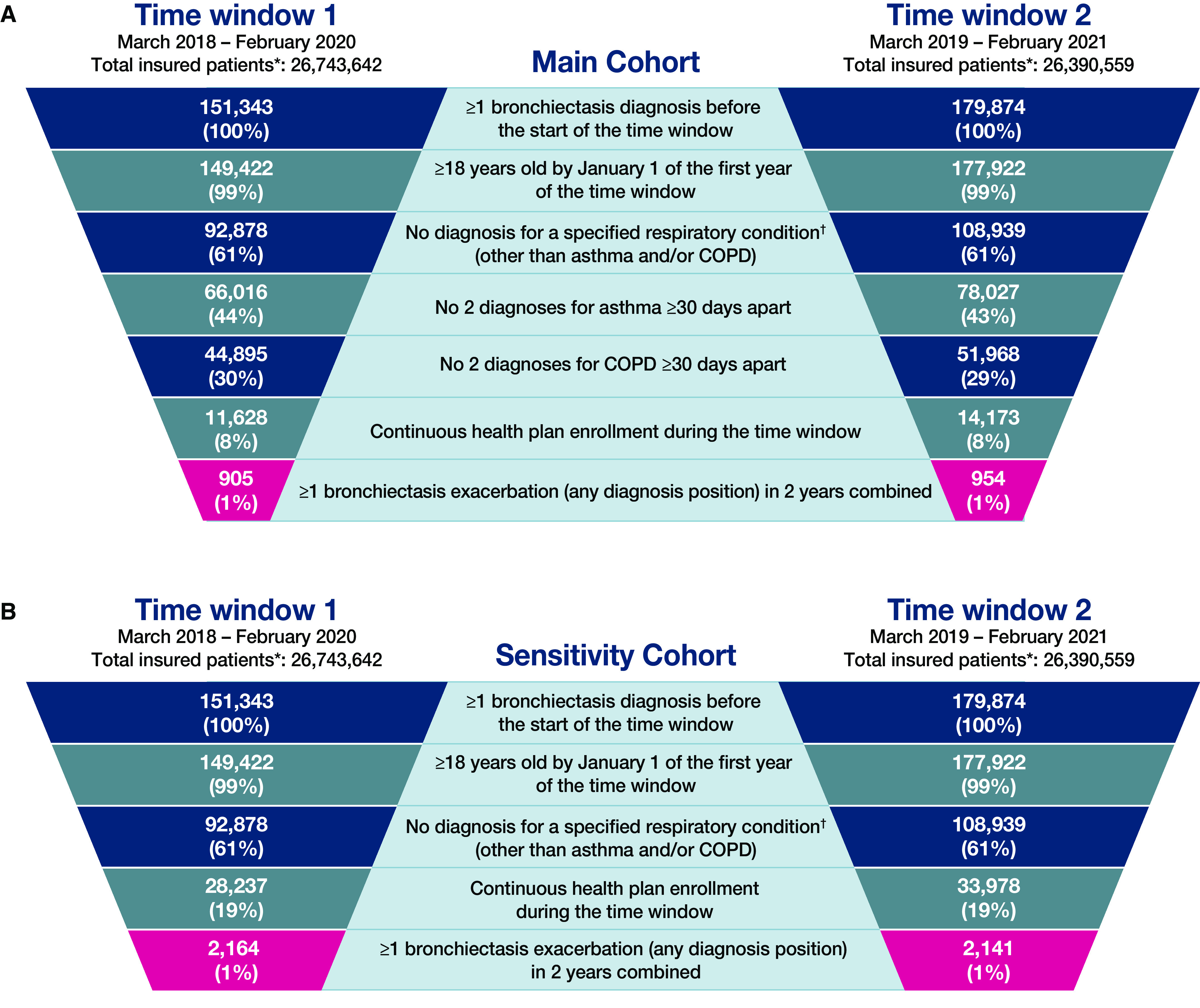
Patient selection. (A) Main cohort (excludes patients with asthma and chronic obstructive pulmonary disease [COPD]). (B) Sensitivity cohort (does not exclude patients with asthma and COPD). *Total number of patients with ⩾1 day of insurance coverage in the Optum dataset during the specified time window. †Other respiratory conditions include tuberculosis, nontuberculous mycobacterial respiratory infection, pulmonary fibrosis, cystic fibrosis, lung cancer, primary ciliary dyskinesia, pulmonary hypertension, and alpha-1 antitrypsin deficiency.
Table 1.
Patient demographics
| Category | All Patients |
Patients with Two or More Exacerbations in the First Year |
||
|---|---|---|---|---|
| Time Window 1: March 2018 to February 2020 | Time Window 2: March 2019 to February 2021 | Time Window 1: March 2018 to February 2020 | Time Window 2: March 2019 to February 2021 | |
| Main cohort, N | 905 | 954 | 159 | 197 |
| Age, yr, median (IQR) | 75 (69–81) | 76 (69–82) | 75 (69–82) | 75 (69–81) |
| Female, n (%) | 695 (77) | 717 (75) | 128 (81) | 151 (77) |
| Medicare, n (%) | 746 (82) | 809 (84) | 133 (84) | 164 (83) |
| Suspected asthma any time, n (%) | 123 (14) | 147 (15) | 26 (16) | 34 (17) |
| Confirmed asthma any time, n (%) | 0 | 0 | 0 | 0 |
| Suspected COPD and/or bronchitis any time, n (%) | 220 (24) | 217 (23) | 41 (26) | 53 (27) |
| Confirmed COPD and/or bronchitis any time, n (%) | 0 | 0 | 0 | 0 |
| Sensitivity cohort, N | 2,164 | 2,141 | 461 | 536 |
| Age, yr, median (IQR) | 75 (69–81) | 75 (69–81) | 75 (68–80) | 75 (69–80) |
| Female, n (%) | 1,579 (73) | 1,547 (72) | 342 (74) | 407 (76) |
| Medicare, n (%) | 1,852 (86) | 1,845 (86) | 392 (85) | 468 (87) |
| Suspected asthma any time, n (%) | 1,217 (56) | 1,241 (58) | 294 (64) | 334 (62) |
| Confirmed asthma any time, n (%) | 987 (46) | 1,007 (47) | 240 (52) | 276 (51) |
| Suspected COPD and/or bronchitis, n (%) | 1,589 (73) | 1,560 (73) | 366 (79) | 410 (76) |
| Confirmed COPD and/or bronchitis any time, n (%) | 1,341 (62) | 1,307 (61) | 326 (71) | 355 (66) |
Definition of abbreviations: COPD = chronic obstructive pulmonary disease; IQR = interquartile ratio.
Suspected” refers to the presence of at least one asthma/COPD diagnosis code any time in the patient’s records. “Confirmed” refers to the presence of at least 2 asthma and/or COPD diagnosis codes ⩾30 d apart any time in the patient’s records.
Patient treatments for bronchiectasis during the study included inhaled bronchodilators; inhaled, oral, and intravenous corticosteroids; and oral and intravenous antibiotics (Table 2). Among all patients in the main cohort, within time window 1, there were no statistically significant changes in prescribed treatment from the first year to the second year. Within time window 1, for all patients in the sensitivity cohort, there were significant reductions in prescribed treatment only for inhaled corticosteroids. Within time window 2, for all patients in the main cohort, there were statistically significant reductions in prescribed treatment with oral macrolides, antibiotics, and corticosteroids; inhaled bronchodilators and corticosteroids; and intravenous corticosteroids. Within time window 2, for all patients in the sensitivity cohort, there were statistically significant reductions in prescribed treatment with all medications.
Table 2.
Bronchiectasis prescription patterns by drug class
| Category | All Patients |
Patients with Two or More Exacerbations in the First Year |
|||
|---|---|---|---|---|---|
| Time Window 1: March 2018 to February 2020 | Time Window 2: March 2019 to February 2021 | Time Window 1: March 2018 to February 2020 | Time Window 2: March 2019 to February 2021 | ||
| Main cohort, N | 905 | 954 | 159 | 197 | |
| Any inhaled bronchodilator, n (%) | Year 1 | 298 (33) | 322 (34) | 87 (55) | 102 (52) |
| Year 2 | 300 (33) | 275 (29) | 72 (45) | 80 (41) | |
| McNemar’s chi-square | 0.02 | 11.2 | 5.23 | 9.68 | |
| P Value | 0.8 | <0.01 | 0.02 | <0.01 | |
| Any oral macrolides, n (%) | Year 1 | 319 (35) | 361 (38) | 85 (53) | 114 (58) |
| Year 2 | 309 (34) | 196 (21) | 53 (33) | 52 (26) | |
| McNemar’s chi-square | 0.30 | 85.9 | 19.0 | 45.8 | |
| P Value | 0.6 | <0.01 | <0.01 | <0.01 | |
| Any oral corticosteroids, n (%) | Year 1 | 266 (29) | 309 (32) | 72 (45) | 95 (48) |
| Year 2 | 274 (30) | 205 (21) | 52 (33) | 48 (24) | |
| McNemar’s chi-square | 0.27 | 39.5 | 8.70 | 29.5 | |
| P Value | 0.6 | <0.01 | <0.01 | <0.01 | |
| Any oral antibiotic, n (%) | Year 1 | 666 (74) | 720 (75) | 147 (92) | 184 (93) |
| Year 2 | 648 (72) | 597 (63) | 124 (78) | 131 (66) | |
| McNemar’s chi-square | 1.09 | 42.4 | 15.1 | 46.0 | |
| P Value | 0.3 | <0.01 | <0.01 | <0.01 | |
| Any inhaled corticosteroid, n (%) | Year 1 | 204 (23) | 204 (21) | 47 (30) | 52 (26) |
| Year 2 | 202 (22) | 157 (16) | 36 (23) | 38 (19) | |
| McNemar’s chi-square | 0.03 | 18.0 | 4.50 | 7.00 | |
| P Value | 0.9 | <0.01 | 0.04 | <0.01 | |
| Any intravenous corticosteroid, n (%) | Year 1 | 231 (26) | 258 (27) | 49 (31) | 66 (34) |
| Year 2 | 231 (26) | 192 (20) | 50 (31) | 48 (24) | |
| McNemar’s chi-square | 0 | 18.6 | 0.03 | 5.23 | |
| P Value | 1.0 | <0.01 | 0.9 | 0.02 | |
| Any intravenous antibiotic, n (%) | Year 1 | 96 (11) | 132 (14) | 28 (18) | 37 (19) |
| Year 2 | 112 (12) | 109 (11) | 18 (11) | 23 (12) | |
| McNemar’s chi-square | 1.60 | 2.92 | 3.13 | 4.45 | |
| P Value | 0.2 | 0.1 | 0.1 | 0.03 | |
| Any inhaled bronchodilator and any inhaled corticosteroid, n (%) | Year 1 | 97 (11) | 106 (11) | 30 (19) | 34 (17) |
| Year 2 | 106 (11) | 81 (9) | 24 (15) | 27 (14) | |
| McNemar’s chi-square | 0.87 | 7.91 | 1.25 | 1.71 | |
| P Value | 0.4 | <0.01 | 0.3 | 0.2 | |
| Sensitivity cohort, N | 2,164 | 2,141 | 461 | 536 | |
| Any inhaled bronchodilator, n (%) | Year 1 | 1,459 (67) | 1,495 (70) | 371 (80) | 423 (79) |
| Year 2 | 1,450 (67) | 1,441 (67) | 343 (74) | 390 (73) | |
| McNemar’s chi-square | 0.19 | 7.25 | 9.56 | 13.4 | |
| P Value | 0.7 | <0.01 | <0.01 | <0.01 | |
| Any oral macrolides, n (%) | Year 1 | 1,036 (48) | 1,117 (52) | 288 (62) | 370 (69) |
| Year 2 | 1,033 (48) | 821 (38) | 239 (52) | 238 (44) | |
| McNemar’s chi-square | 0.01 | 126.6 | 18.1 | 99.0 | |
| P Value | 0.9 | <0.01 | <0.01 | <0.01 | |
| Any oral corticosteroids, n (%) | Year 1 | 1,142 (53) | 1,225 (57) | 326 (71) | 368 (69) |
| Year 2 | 1,149 (53) | 955 (45) | 284 (62) | 262 (49) | |
| McNemar’s chi-square | 0.08 | 111.8 | 16.0 | 68.5 | |
| P Value | 0.8 | <0.01 | <0.01 | <0.01 | |
| Any oral antibiotic, n (%) | Year 1 | 1,758 (81) | 1,804 (84) | 440 (95) | 502 (94) |
| Year 2 | 1,734 (80) | 1,523 (71) | 375 (81) | 395 (74) | |
| McNemar’s chi-square | 1.05 | 124.3 | 52.2 | 88.8 | |
| P Value | 0.3 | <0.01 | <0.01 | <0.01 | |
| Any inhaled corticosteroid, n (%) | Year 1 | 716 (33) | 710 (33) | 184 (40) | 191 (36) |
| Year 2 | 667 (31) | 619 (29) | 159 (34) | 158 (29) | |
| McNemar’s chi-square | 6.40 | 24.4 | 7.91 | 12.5 | |
| P Value | 0.01 | <0.01 | <0.01 | <0.01 | |
| Any intravenous corticosteroid, n (%) | Year 1 | 709 (33) | 747 (35) | 188 (41) | 203 (38) |
| Year 2 | 744 (34) | 570 (27) | 164 (36) | 142 (26) | |
| McNemar’s chi-square | 2.00 | 49.8 | 4.11 | 22.0 | |
| P Value | 0.2 | <0.01 | 0.04 | <0.01 | |
| Any intravenous antibiotic, n (%) | Year 1 | 321 (15) | 387 (18) | 96 (21) | 135 (25) |
| Year 2 | 348 (16) | 266 (12) | 67 (15) | 78 (15) | |
| McNemar’s chi-square | 1.68 | 31.9 | 8.01 | 22.7 | |
| P Value | 0.2 | <0.01 | <0.01 | <0.01 | |
| Any inhaled bronchodilator and any inhaled corticosteroid, n (%) | Year 1 | 559 (26) | 581 (27) | 152 (33) | 161 (30) |
| Year 2 | 534 (25) | 499 (23) | 131 (28) | 130 (24) | |
| McNemar’s chi-square | 1.70 | 20.1 | 5.07 | 11.9 | |
| P Value | 0.2 | <0.01 | 0.02 | <0.01 | |
P values are for the comparison of Year 1 to Year 2 for each time window using McNemar’s chi-square test. For each test pair, df = 1.
Among patients in both cohorts with at least two exacerbations in the first year, significant reductions in all prescribed medications were observed from the first year to the second year in both time windows, with the exception of intravenous antibiotics in time window 1 in the main cohort and inhaled bronchodilators and/or corticosteroids in time windows 1 and 2 in the main cohort (Table 2).
After patients were grouped according to number of exacerbations (i.e., zero, one, two, three, and four or more) (Figure 2 and Table 3), those who had frequent (two or more) exacerbations during the first year of time window 1 (prepandemic) were more likely than patients who had zero or one exacerbation to have frequent exacerbations in the subsequent prepandemic year (main cohort, 31% vs. 14% [logistic regression P < 0.01]; sensitivity cohort, 32% vs. 15% [logistic regression P < 0.01]).
Figure 2.
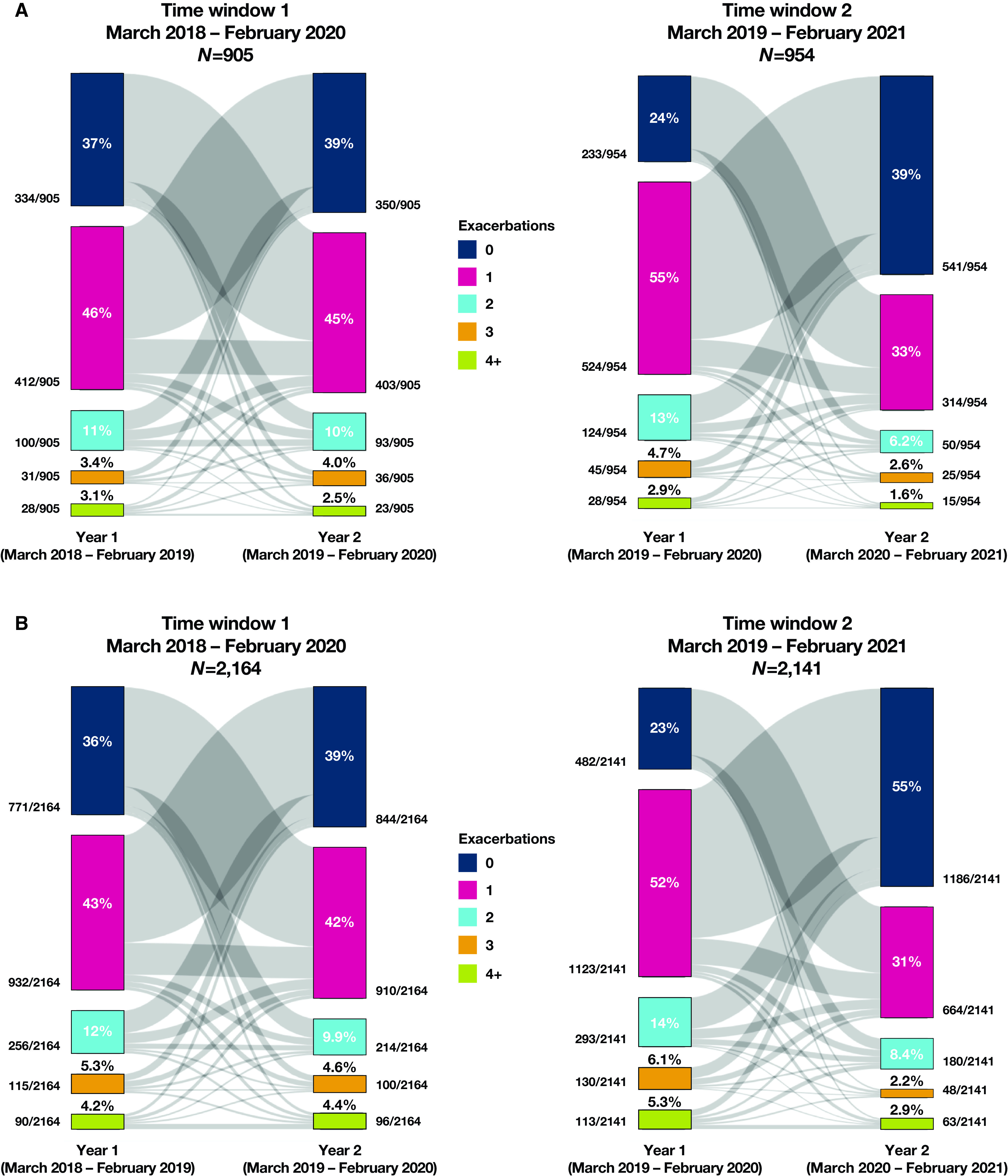
Dynamics of bronchiectasis exacerbations. (A) Main cohort (excludes patients with asthma and chronic obstructive pulmonary disease [COPD]). (B) Sensitivity cohort (does not exclude patients with asthma and COPD). The bands showing year-to-year variation in exacerbation class are scaled to the percentage that each transitioning group represents on each end (i.e., this percentage can and does differ between the respective ends of the gray bands).
Table 3.
Patients with bronchiectasis exacerbations before and during the coronavirus disease pandemic
| Time Window 1 |
Time Window 2 |
|||
|---|---|---|---|---|
| Year 1: March 2018 to February 2019 | Year 2: March 2019 to February 2020 | Year 1: March 2019 to February 2020 | Year 2: March 2020 to February 2021 | |
| Main cohort | ||||
| Total patients, N | 905 | 905 | 954 | 954 |
| 0 exacerbations, n (%) | 334 (37) | 350 (39) | 233 (24) | 541 (57) |
| 1 exacerbation, n (%) | 412 (46) | 403 (45) | 524 (55) | 314 (33) |
| 2 exacerbations, n (%) | 100 (11) | 93 (10) | 124 (13) | 59 (6.2) |
| 3 exacerbations, n (%) | 31 (3.4) | 36 (4.0) | 45 (4.7) | 25 (2.6) |
| ⩾4 exacerbations, n (%) | 28 (3.1) | 23 (2.5) | 28 (2.9) | 15 (1.6) |
| Sensitivity cohort | ||||
| Total patients, N | 2,164 | 2,164 | 2,141 | 2,141 |
| 0 exacerbations, n (%) | 771 (36) | 844 (39) | 482 (23) | 1,186 (55) |
| 1 exacerbation, n (%) | 932 (43) | 910 (42) | 1,123 (52) | 664 (31) |
| 2 exacerbations, n (%) | 256 (12) | 214 (9.9) | 293 (14) | 180 (8.4) |
| 3 exacerbations, n (%) | 115 (5.3) | 100 (4.6) | 130 (6.1) | 48 (2.2) |
| ⩾4 exacerbations, n (%) | 90 (4.2) | 96 (4.4) | 113 (5.3) | 63 (2.9) |
From the first year (the year before the COVID-19 pandemic) to the second year (the first year of the pandemic) of time window 2, more patients had zero exacerbations in the second year of time window 2 (the first year of the pandemic) in both the main cohort (24% during the first year vs. 57% during the second year; McNemar’s chi-square = 122.56; df = 1; P < 0.01) and the sensitivity cohort (23% during the first year vs. 55% during the second year; McNemar’s chi-square = 297.13; df = 1; P < 0.01).
During time window 1, the median number of exacerbations per patient per year did not differ in the main cohort from Year 1 to Year 2 (Wilcoxon signed rank test statistic [W] = 162,924; P = 0.5), whereas a significant difference was observed in the sensitivity cohort (W = 973,007; P = 0.04) (Table 4). In contrast, the median number of exacerbations per patient per year decreased significantly from the year before the COVID-19 pandemic to the first year of the pandemic (time window 2) in the main cohort (W = 263,614; P < 0.01) and the sensitivity cohort (W = 1,337,065; P < 0.01).
Table 4.
Number of bronchiectasis exacerbations per patient per year
| Time Window 1 |
Time Window 2 |
|||
|---|---|---|---|---|
| Year 1: March 2018 to February 2019 | Year 2: March 2019 to February 2020 | Year 1: March 2019 to February 2020 | Year 2: March 2020 to February 2021 | |
| Main cohort | ||||
| Total patients, N | 905 | 954 | ||
| Exacerbations per patient, mean (SD) | 0.93 (1.04) | 0.89 (1.01) | 1.09 (0.99) | 0.61 (0.92) |
| Number of exacerbations, median (IQR) | 1 (0–1) | 1 (0–1) | 1 (1–1) | 0 (0–1) |
| Wilcoxon signed rank test statistic (W) | 162,924 | 263,614 | ||
| P value* | 0.5 | <0.01 | ||
| Sensitivity cohort | ||||
| Total patients, N | 2,164 | 2,141 | ||
| Exacerbations per patient, mean (SD) | 1.05 (1.33) | 0.99 (1.34) | 1.27 (1.40) | 0.72 (1.27) |
| Number of exacerbations, median (IQR) | 1 (0–1) | 1 (0–1) | 1 (1–2) | 0 (0–1) |
| Wilcoxon signed rank test statistic (W) | 973,007 | 1,337,065 | ||
| P value* | 0.04 | <0.01 | ||
Definition of abbreviations: IQR = interquartile ratio; SD = standard deviation.
P values are for the comparison of Year 1 to Year 2 for each cohort and the time window using the two-sided Wilcoxon signed rank test.
Discussion
Under ordinary circumstances (i.e., during the 2-year time window before the COVID-19 pandemic), the median number of exacerbations per patient per year did not differ in the main cohort, despite some normal year-to-year variation in their exacerbation rates (Figure 2). A significant difference in median exacerbation numbers was seen in the main cohort in time window 2, which compares prepandemic and pandemic years. This is evidence that this change is distinct from background year-to-year variability. In contrast, a nominally significant difference was observed during time window 1 in the sensitivity cohort, a difference that may be an artifact of multiple testing, whereas the lower P value for time window 2 in the sensitivity cohort is stronger evidence of year-to-year change in median exacerbation rates, coinciding with the start of the pandemic.
During the COVID-19 pandemic, patients were more likely to have zero exacerbations in the first year of the pandemic than in the year before the pandemic, and the percentage of patients with each number of exacerbations (i.e., one, two, three, and four or more) was smaller. The difference in the median number of exacerbations per patient per year was larger in the time window that included the COVID-19 pandemic than in the time window before the pandemic. It seems the expected “regression to the mean” was mitigated by the study design in including all patients with at least one exacerbation in either of the 2 consecutive years in each window. These data thus support our hypothesis that patients experienced fewer bronchiectasis exacerbations during the COVID-19 pandemic.
In the prepandemic time window, we observed that patients who had frequent exacerbations appeared more likely than those with only zero or one exacerbation to continue having frequent exacerbations in the following year, in line with expectations. These results concur with previous observations in clinical practice about the likelihood of prior exacerbations in bronchiectasis predicting future exacerbation risk (14). A predictive likelihood is also seen in COPD and asthma (15, 16). However, only 31–32% of patients with frequent (at least two) exacerbations remained in that category the following prepandemic year, indicating that the previous exacerbation rate in bronchiectasis will enrich for subsequent exacerbation rates but not as strongly as previously reported in the literature (14). It is possible that our results differ because insurance claims coding practices provide only a limited picture of a patient’s clinical state. Furthermore, our observations may reflect the dynamics of bronchiectasis exacerbations among commercially insured Americans only and, as such, may not be applicable in the broader context of the disease.
Our findings of decreasing exacerbations during the pandemic are consistent with an earlier UK study by Crichton and colleagues that showed a significant reduction in the frequency of exacerbations during the pandemic, from 2.08 exacerbations in 2018–2019 to 2.01 in 2019–2020 to 1.12 in 2020–2021 (12), as well as with a Spanish study by Martínez-Vergara and colleagues that showed a 57% reduction in all exacerbations during the pandemic (13). Thus, the collective data suggest that exacerbations among patients with bronchiectasis were reduced by approximately half during the COVID-19 pandemic. It is notable that the current large U.S. population-based study produced similar findings as those described in countries with universal healthcare systems (12, 13). Studies conducted during the pandemic also revealed significant reductions in exacerbations related to asthma and COPD (17, 18). Multiple reasons have been posited to explain the decrease in exacerbations among patients with bronchiectasis during the pandemic, including reductions in circulating viruses, improvements in air quality, changes in the use of healthcare services, and an increase in general health anxiety leading to improved adherence to background medications (9–11, 19–24). Taken together, these studies offer evidence that public health measures adopted during the COVID-19 pandemic (e.g., social distancing and personal hygiene practices) may be helpful for patients with chronic respiratory conditions. It is unlikely that the results are entirely explained by a lack of access to healthcare services during the pandemic. However, we acknowledge the impact of decreased clinical personnel and resources, as well as changes in the extent to which enrollees sought medical care, particularly during the early part of the pandemic.
Strengths and Limitations
Strengths of our study include the large sample size and the use of a claims database that could provide clinician-confirmed diagnoses rather than having to rely on patient-reported data, which allowed us to expand on existing research related to the impact of the COVID-19 pandemic on exacerbations of bronchiectasis.
Our study also had several limitations. Because we used retrospective claims data, we were unable to provide insight into the patient perspective and experience of bronchiectasis during the pandemic, and we may have underestimated the frequency of exacerbations. Two separate 2-year windows were studied instead of a single 3-year window to maintain sample size, as 3 years of continuous enrollment from March 1, 2018, is not common. This is important to have enough statistical power to study inpatient visits but resulted in a more complex study design that is more difficult to interpret.
Other limitations associated with our study design include residual confounding, a bias toward a White population (which was possibly wealthier given that they were insured), and an inability to determine causality. Furthermore, we observed geographical inhomogeneity in the distribution of the enrollees. The use of a commercial claims database did not allow us to distinguish between a maintenance antibiotic prescription and an antibiotic prescription used for an exacerbation. The use of two diagnostic codes for COPD or asthma within a 30-day period as a proxy for a confirmed diagnosis precludes definite diagnostic confidence. However, this is a standard approach used in epidemiology studies of real-world data in the absence of other, stronger proxies for diagnostic confirmation. In addition, although our bronchiectasis definition was carefully constructed, it has not been validated, and we acknowledge that the study population comprises a heterogeneous mix of bronchiectasis subtypes. Additional studies that combine radiographic, spirometry, clinical, and claims data may improve interpretation of the findings of the current administrative claims-based studies. The fraction of the total bronchiectasis population within the Optum database (∼1% of all patients with a bronchiectasis diagnosis code in the four overlapping study samples) selected by the study inclusion criteria (e.g., the requirement of at least one exacerbation within the time window, the requirement of continuity of insurance, and the exclusion of mycobacterial diseases) combined with the (uninvestigated) demographic biases inherent in an insured U.S. population may not be representative of the overall bronchiectasis population. Therefore, data should be interpreted with caution.
Finally, the use of a U.S. claims database and our study design precluded us from studying the impact of the COVID-19 pandemic on mortality rates. To allow within-individual year-to-year comparisons, we required 2 years of continuous enrollment; however, the trade-off was that this introduced a degree of selection bias and prevented us from studying mortality (which would prematurely end enrollment). Above all, it is important to note that many aspects of health care have been in flux during the pandemic, making it difficult to interpret data collected during this time period.
Conclusions
During the first year of the COVID-19 pandemic, broad public health measures, including social distancing and improved personal hygiene, were enacted worldwide. The results of our study show that patients with bronchiectasis in the U.S. experienced reductions in exacerbation rates during the first year of the pandemic compared with the 12-month period immediately prior. Possible reasons for this reduced exacerbation rate include decreased numbers of circulating viruses combined with voluntary self-isolation of vulnerable individuals, improvements in air quality, and changes in the use of healthcare services. Our findings, when considered together with similar studies of exacerbation rates among patients with bronchiectasis (12, 13), asthma (17), and COPD (18), provide evidence that public health measures adopted during the COVID-19 pandemic may have resulted in some benefit for patients with bronchiectasis.
Acknowledgments
Acknowledgment
Medical writing support was provided by Sara N. Fischer, Ph.D. (Citrus Health Group), and was funded by AstraZeneca in accordance with Good Publication Practice (GPP 2022) guidelines. The authors also thank Hana Mullerova (AstraZeneca) for providing her expertise in real-world datasets.
Footnotes
Supported by AstraZeneca (Cambridge, United Kingdom).
Author Contributions: A.Å., R.S.G.M., C.S., J.D.C., and I.P. conceived the study and contributed to the study design. A.Å., S.J.K., S.A., and R.S.G.M. contributed to the data acquisition and extraction. A.Å., S.J.K., R.S.G.M., S.P., K.C., S.A., C.S., J.D.C., and I.P. contributed to the data analysis and interpretation. All authors helped revise the manuscript, commented on the manuscript, contributed to discussion, and approved the final version for submission.
Availability of data and materials: The data that support the findings of this study are available from Optum, but restrictions apply to the availability of these data, which were used under license for the present study and so are not publicly available. Additional information regarding AstraZeneca’s data-sharing policy can be accessed at https://www.astrazenecaclinicaltrials.com/our-transparency-commitments/.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Lonni S, Chalmers JD, Goeminne PC, McDonnell MJ, Dimakou K, De Soyza A, et al. Etiology of non-cystic fibrosis bronchiectasis in adults and its correlation to disease severity. Ann Am Thorac Soc . 2015;12:1764–1770. doi: 10.1513/AnnalsATS.201507-472OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. O’Donnell AE. Bronchiectasis. Chest . 2008;134:815–823. doi: 10.1378/chest.08-0776. [DOI] [PubMed] [Google Scholar]
- 3. Aksamit TR, O’Donnell AE, Barker A, Olivier KN, Winthrop KL, Daniels MLA, et al. Bronchiectasis Research Registry Consortium Adult patients with bronchiectasis: a first look at the US Bronchiectasis Research Registry. Chest . 2017;151:982–992. doi: 10.1016/j.chest.2016.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hill AT, Haworth CS, Aliberti S, Barker A, Blasi F, Boersma W, et al. EMBARC/BRR Definitions Working Group Pulmonary exacerbation in adults with bronchiectasis: a consensus definition for clinical research. Eur Respir J . 2017;49:1700051. doi: 10.1183/13993003.00051-2017. [DOI] [PubMed] [Google Scholar]
- 5. Gao YH, Guan WJ, Xu G, Lin ZY, Tang Y, Lin ZM, et al. The role of viral infection in pulmonary exacerbations of bronchiectasis in adults: a prospective study. Chest . 2015;147:1635–1643. doi: 10.1378/chest.14-1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Polverino E, Dimakou K, Hurst J, Martinez-Garcia MA, Miravitlles M, Paggiaro P, et al. The overlap between bronchiectasis and chronic airway diseases: state of the art and future directions. Eur Respir J . 2018;52:1800328. doi: 10.1183/13993003.00328-2018. [DOI] [PubMed] [Google Scholar]
- 7. Tsikrika S, Dimakou K, Papaioannou AI, Hillas G, Thanos L, Kostikas K, et al. The role of non-invasive modalities for assessing inflammation in patients with non-cystic fibrosis bronchiectasis. Cytokine . 2017;99:281–286. doi: 10.1016/j.cyto.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 8. Dente FL, Bilotta M, Bartoli ML, Bacci E, Cianchetti S, Latorre M, et al. Neutrophilic bronchial inflammation correlates with clinical and functional findings in patients with noncystic fibrosis bronchiectasis. Mediators Inflamm . 2015;2015:642503. doi: 10.1155/2015/642503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Olsen SJ, Winn AK, Budd AP, Prill MM, Steel J, Midgley CM, et al. Changes in influenza and other respiratory virus activity during the COVID-19 pandemic—United States, 2020–2021. MMWR Morb Mortal Wkly Rep . 2021;70:1013–1019. doi: 10.15585/mmwr.mm7029a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Feng L, Zhang T, Wang Q, Xie Y, Peng Z, Zheng J, et al. Impact of COVID-19 outbreaks and interventions on influenza in China and the United States. Nat Commun . 2021;12:3249. doi: 10.1038/s41467-021-23440-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kıymet E, Böncüoğlu E, Şahinkaya Ş, Cem E, Çelebi MY, Düzgöl M, et al. Distribution of spreading viruses during COVID-19 pandemic: effect of mitigation strategies. Am J Infect Control . 2021;49:1142–1145. doi: 10.1016/j.ajic.2021.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Crichton ML, Shoemark A, Chalmers JD. The impact of the COVID-19 pandemic on exacerbations and symptoms in bronchiectasis: a prospective study. Am J Respir Crit Care Med . 2021;204:857–859. doi: 10.1164/rccm.202105-1137LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Martínez-Vergara A, Girón Moreno RM, Olveira C, Victoria Girón M, Peláez A, Ancochea J, et al. Impact of the SARS-CoV-2 virus pandemic on patients with bronchiectasis: a multicenter study. Antibiotics (Basel) . 2022;11:1096. doi: 10.3390/antibiotics11081096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chalmers JD, Aliberti S, Filonenko A, Shteinberg M, Goeminne PC, Hill AT, et al. Characterization of the “frequent exacerbator phenotype” in bronchiectasis. Am J Respir Crit Care Med . 2018;197:1410–1420. doi: 10.1164/rccm.201711-2202OC. [DOI] [PubMed] [Google Scholar]
- 15. Vogelmeier CF, Diesing J, Kossack N, Pignot M, Friedrich FW. COPD exacerbation history and impact on future exacerbations—8-year retrospective observational database cohort study from Germany. Int J Chron Obstruct Pulmon Dis . 2021;16:2407–2417. doi: 10.2147/COPD.S322036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sears MR. Can we predict exacerbations of asthma? Am J Respir Crit Care Med . 2019;199:399–400. doi: 10.1164/rccm.201811-2122ED. [DOI] [PubMed] [Google Scholar]
- 17. Shah SA, Quint JK, Nwaru BI, Sheikh A. Impact of COVID-19 national lockdown on asthma exacerbations: interrupted time-series analysis of English primary care data. Thorax . 2021;76:860–866. doi: 10.1136/thoraxjnl-2020-216512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alsallakh MA, Sivakumaran S, Kennedy S, Vasileiou E, Lyons RA, Robertson C, et al. EAVE II Collaborators Impact of COVID-19 lockdown on the incidence and mortality of acute exacerbations of chronic obstructive pulmonary disease: national interrupted time series analyses for Scotland and Wales. BMC Med . 2021;19:124. doi: 10.1186/s12916-021-02000-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ropkins K, Tate JE. Early observations on the impact of the COVID-19 lockdown on air quality trends across the UK. Sci Total Environ . 2021;754:142374. doi: 10.1016/j.scitotenv.2020.142374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zangari S, Hill DT, Charette AT, Mirowsky JE. Air quality changes in New York City during the COVID-19 pandemic. Sci Total Environ . 2020;742:140496. doi: 10.1016/j.scitotenv.2020.140496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Roy CM, Bollman EB, Carson LM, Northrop AJ, Jackson EF, Moresky RT. Assessing the indirect effects of COVID-19 on healthcare delivery, utilization and health outcomes: a scoping review. Eur J Public Health . 2021;31:634–640. doi: 10.1093/eurpub/ckab047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moynihan R, Sanders S, Michaleff ZA, Scott AM, Clark J, To EJ, et al. Impact of COVID-19 pandemic on utilisation of healthcare services: a systematic review. BMJ Open . 2021;11:e045343. doi: 10.1136/bmjopen-2020-045343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Whaley CM, Pera MF, Cantor J, Chang J, Velasco J, Hagg HK, et al. Changes in health services use among commercially insured US populations during the COVID-19 pandemic. JAMA Netw Open . 2020;3:e2024984. doi: 10.1001/jamanetworkopen.2020.24984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Losier A, Gupta G, Caldararo M, Dela Cruz CS. The impact of coronavirus disease 2019 on viral, bacterial, and fungal respiratory infections. Clin Chest Med . 2023;44:407–423. doi: 10.1016/j.ccm.2022.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]


