Abstract
Background
Studies have reported correlations between various oral behaviors and painful temporomandibular disorders (TMD), yet comprehensive research on the independent effects of each oral behavior within the general population remains sparse.
Objective
This cross-sectional study aimed to investigate the association between painful TMD (PT) and various oral behaviors in general population.
Methods
A questionnaire survey was conducted with participants to collect data encompassing demographic characteristics, eight specific oral behaviors, and the 5 major TMD symptoms(5Ts) checklist. Participants were categorized into PT and non-PT (NPT) groups based on their responses to the 5Ts checklist. Those reporting TMJ/facial pain or headaches were assigned to the PT group, while all other participants constituted the NPT group. Both univariate and multivariate logistic regression analyses were employed to evaluate the association between individual oral behavior and the presence of PT, controlling for demographic confounders including age, sex, systemic diseases, and dental treatments history.
Results
A total of 441 valid questionnaires were received, including 156 males and 285 females. The prevalence of PT was identified to be 33.33%, with 61.00% of participants engaging in one or more types of oral behaviors. Each oral behavior was more frequently reported in the PT group compared to the NPT group. The univariate logistic regression analysis identified positive correlations between all eight oral behaviors and PT. In the multivariate logistic regression analysis, these associations persisted after adjustment for demographic confounders including age, sex, history of systemic diseases and dental treatments (P<0.01). The behaviors most strongly associated with PT were “Hold or jut jaw forward/to the side” (OR:4.478), “Hold, tighten or tense muscles without clench” (OR:3.343) and “Hold jaw in rigid or tense position” (OR:3.209).
Conclusion
The presence of oral behaviors has significant association with PT. Individuals exhibiting multiple oral behaviors are more likely to experience PT. Additional studies are needed to clarify the effects of reducing oral behaviors on pain-related symptoms.
Keywords: oral behaviors, painful temporomandibular disorders, Temporomandibular Joint, general population, cross-sectional study
Introduction
Temporomandibular disorders (TMDs) encompass a group of musculoskeletal conditions involving pain or dysfunction of the masticatory muscles, temporomandibular joint (TMJ) and related structures.1,2 According to Diagnostic Criteria for Temporomandibular Disorders (DC/TMD), a TMD with pain symptoms is defined as painful TMDs (PT). The prevalence of TMDs is significantly high globally.3 Studies targeting Chinese college students and medical students showed the prevalence of TMD were 29.1% and 31.7%, respectively.4 A Canadian survey indicated that up to 50% of adults may experience PT-related symptoms, including pain and functional disturbances in joint and muscle activity.5 In addition to the chronic pain itself and noise from the joints, patients with PT also tend to suffer from dysphonia and eating difficulties due to pain when opening the mouth, which can lead to impairment in quality of life.6 Moreover, the economic burden of TMDs is substantial; patients in the United States alone may incur approximately $100 billion annually in treatment costs.7
The etiology of PT is multifactorial, including biological, psychological and social factors. Biological factors can roughly be attributed to joint and muscle lesions. Moreover, studies have shown that the elevation of inflammatory markers such as tumor necrosis factor (TNF-α), interleukin-1(IL-1) and prostaglandin (PG) in blood and saliva may be related to PT.8 Psychosocial factors have long been a research hotspot in TMDs. There are evidence-based conclusions about the significant association between psychological disorders and orofacial musculoskeletal pain.9 Cross-sectional studies have demonstrated that PT patients exhibit higher levels of anxiety, depression, and psychological stress compared to those with non-painful TMD or without TMD, with stress scores positively correlated to pain intensity and duration.10 A few prospective studies have also suggested that psychological variables might be potential risk factors for onset and chronicity of PT.11,12
Oral behaviors, particularly parafunctional behaviors, refer to the activities of the masticatory muscles occurring outside of the usual functions of speaking and chewing, such as clenching teeth, holding jaw in rigid position consciously or unconsciously.13 These behaviors have been implicated as potential risk factors for TMDs.14 The temporomandibular region features neural and biomechanical connections that form a complex functional unit, with studies suggesting that neural pathways associated with PT can be influenced by posture.15 Interestingly, PT appears to be associated with Parkinson’s disease, possibly due to the clenching or grinding of teeth caused by muscle rigidity, spasm and jaw tremors caused by uncoordinated muscle contractions.16 Bruxism is recognized as a prevalent oral behavior that may exacerbate PT through joint damage from the bite force exerted during such episodes. Additionally, patients with bruxism have been reported to possess a heightened pain sensitivity, leading to more frequent PT reports.17 However, other researchers believe that bruxism in the case of obstructive sleep apnea (OSA) reduces the incidence of the pain.18 Therefore, the independent role of bruxism is not clear. The difference between the effects of awake and sleep bruxism on PT has also not been elucidated. Previous studies have largely focused on dental clinic or hospital patients,19,20 with research on the general population remaining scarce.
The objective of this study is to ascertain the relationship between the prevalence of PT and oral behaviors in the general population. The null hypothesis was proposed that there was no correlation between oral behaviors and PT.
Materials and Methods
Study Design
This cross-sectional study was carried out among general population using data from convenient questionnaires We have attached the questionnaire in the Supplementary Material. The questionnaires were available from June 14th, 2023 to August 23rd, 2023, and the analysis was completed in August 29th, 2023.
Based on a 95% confidence level, 5% margin of error for confidence interval and 34.9% prevalence of TMDs, a minimum sample size of 350 subjects was determined with a sample size calculator (https://www.calculator.net/sample-size-calculator.html).
A total of 441 voluntary participants from different communities took part in this study. The purpose of this study was fully explained at the beginning of the questionnaire, so as to ensure the informed consent of the participants.
The inclusion criteria were as follows: (a) ability to read and understand the questionnaire; (b) ability to read on electrical devices. The exclusion criteria included: (a) history of medication that could obscure the symptoms of TMDs; (b) history of drug abuse; (c) incomplete or improperly filled out questionnaires.
Data Collection
The questionnaires consisted of three distinct sections.
Demographic information including sex, age, education level, dental treatments history and systemic diseases history were collected in the first part.
The second part was an oral behavior checklist. Eight items were selected to assess oral behaviors according to Cláudia Barbosa,21 including six waking behaviors and two sleep behaviors to identify and quantify the overuse of the joints and muscles, including: (1) Bruxism (sleep); (2) Sleep position pressure jaw; (3) Bruxism (waking); (4) Clench teeth (waking); (5) Press, touch or hold teeth together; (6) Hold, tighten or tense muscles without clench; (7) Hold or jut jaw forward/to the side; (8) Hold jaw in rigid or tense position. A five-point scale ranging from 1(not at all) to 5(always) was used in each item.
In the third part, the 5 major TMD symptoms(5Ts) were chosen as a TMD screening tool, which has been demonstrated high sensitivity (96.1% at least) and high specificity (100%) for identifying PT in a previous study.22 Two of the 5 symptoms involved TMD-related facial pain and headaches were used to identify PTs, and each question requires a yes or no answer.
Do you have any pain in your jaw, temple, in the ear or front of the ear?
Do you have any headaches (including the temple areas)?
Any positive answer indicated possible PT. Participants with TMJ/facial pain or headache were divided into PT group and the others were divided into the NPT group.
Statistical Analysis
Statistical analysis was performed with the R package (https://www.R-project.org, the R Foundation) and Empowerstats (http://www.empowerstats.com, X&Y Solutions, Inc., Boston, USA). Quantitative data were presented in the form of mean±standard deviation and evaluated by the Mann–Whitney U-test. Categorical data, including dental treatments history, systemic diseases history and oral behaviors, as well as the occurrence of PTs symptoms were presented by frequency and evaluated by the R×C chi-square test. A univariate logistic regression was performed to evaluate the influence of each oral behavior on the presence of PT. Demographic confounders including age (as a continuous variable), sex (male or female), history of systemic diseases (with or without) and history of dental treatments (with or without) were adjusted in the multivariate regression model. The correlations between the oral behaviors were assessed with Spearman’s rank correlation test. In general, an ɑ level of 0.05 was used to determine statistical significance.
Results
A total of 519 questionnaires were received. After excluding incomplete or duplicate questionnaires, 441 valid questionnaires were included (Table 1), including 156 males and 285 females, with a mean age of 33.37 ± 12.47 years. The participants mostly graduated from college (74.60%), received dental treatments (52.61%) and did not have systematic diseases (71.88%). Among all respondents in this study, there were 66.67% (N=294) in NPT group and 33.33% (N=147) in PT group. No significant difference in sex and education was observed between two groups, while the situation of age, systemic diseases and experience of dental treatments were significantly different (P<0.05).
Table 1.
Demographic Characteristics of the Participants
| Variable | NPT group(N=294) | PT group(N=147) | p value |
|---|---|---|---|
| Age | 32.39 ± 12.30 | 35.31 ± 12.63 | 0.021* |
| Sex | 0.833 | ||
| Male | 105 (35.71%) | 51 (34.69%) | |
| Female | 189 (64.29%) | 96 (65.31%) | |
| Education level | 0.460 | ||
| High school or below | 66 (22.45%) | 26 (17.69%) | |
| College | 214 (72.79%) | 115 (78.23%) | |
| Above college | 14 (4.76%) | 6 (4.08%) | |
| Systemic diseases | <0.001** | ||
| No | 242 (82.31%) | 75 (51.02%) | |
| Yes | 52 (17.69%) | 72 (48.98%) | |
| Dental treatments | <0.001** | ||
| No | 157 (53.40%) | 52 (35.37%) | |
| Yes | 137 (46.60%) | 95 (64.63%) |
Notes: Quantitative data presented by mean±SD; categorical data presented by frequency (constituent ratio); *P<0.05 and **P<0.01.
Abbreviation: PT, Painful Temporomandibular Disorder.
In Table 2, the univariate logistic regression analysis for all 8 oral behaviors revealed positive correlations with PT. In the multivariate logistic regression analysis, such influence remained after adjustment for demographic confounders, including “Bruxism (sleep)” (OR:1.958; 95% CI 1.212–3.163), “Sleep position pressure jaw” (OR:2.511; 95% CI 1.608–3.919), “Bruxism (waking)” (OR:2.138; 95% CI 1.181–3.872), “Clench teeth” (waking) (OR:2.587; 95% CI 1.591–4.206), “Press, touch or hold teeth together” (OR:2.673; 95% CI 1.710–4.181), “Hold, tighten or tense muscles without clench” (OR:3.343; 95% CI 2.120–5.272), “Hold or jut jaw forward/to the side” (OR:4.478; 95% CI 2.737–7.327), and “Hold jaw in rigid or tense position” (OR:3.209; 95% CI 2.024–5.090)(Table 3).
Table 2.
Univariate Logistic Regression Analysis for PT and NPT Groups
| Variable | OR | 95% CI | p value |
|---|---|---|---|
| Bruxism (sleep) | 2.468 | (1.583, 3.847) | <0.001** |
| Sleep position pressure jaw | 3.100 | (2.053, 4.680) | <0.001** |
| Bruxism (waking) | 2.749 | (1.599, 4.728) | <0.001** |
| Clench teeth (waking) | 2.989 | (1.899, 4.704) | <0.001** |
| Press, touch or hold teeth together | 3.394 | (2.246, 5.129) | <0.001** |
| Hold, tighten or tense muscles without clench | 3.894 | (2.553, 5.938) | <0.001** |
| Hold or jut jaw forward/to the side | 5.760 | (3.637, 9.121) | <0.001** |
| Hold jaw in rigid or tense position | 3.617 | (2.375, 5.509) | <0.001** |
Notes: OR, odds ratio; 95% CI, 95% confidence interval. **P<0.01.
Table 3.
Multivariate Logistic Regression Analysis for PT and NPT Groups
| Variable | Adjusted I | Adjusted II | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p value | OR | 95% CI | p value | |
| Bruxism (sleep) | 2.415 | (1.536, 3.797) | <0.001** | 1.958 | (1.212, 3.163) | 0.0060** |
| Sleep position pressure jaw | 3.163 | (2.075, 4.821) | <0.001** | 2.511 | (1.608, 3.919) | <0.001** |
| Bruxism (waking) | 2.793 | (1.605, 4.859) | <0.001** | 2.138 | (1.181, 3.872) | 0.0121* |
| Clench teeth (waking) | 3.021 | (1.905, 4.791) | <0.001** | 2.587 | (1.591, 4.206) | <0.001** |
| Press, touch or hold teeth together | 3.464 | (2.274, 5.278) | <0.001** | 2.673 | (1.710, 4.181) | <0.001** |
| Hold, tighten or tense muscles without clench | 4.024 | (2.614, 6.195) | <0.001** | 3.343 | (2.120, 5.272) | <0.001** |
| Hold or jut jaw forward/to the side | 5.783 | (3.620, 9.238) | <0.001** | 4.478 | (2.737, 7.327) | <0.001** |
| Hold jaw in rigid or tense position | 3.858 | (2.498, 5.959) | <0.001** | 3.209 | (2.024, 5.090) | <0.001** |
Notes: Adjust I adjust for: Sex (1, 2); Age (As a continuous variable). Adjust II adjust for: Sex (1, 2); Age (As a continuous variable); Systemic diseases (with a history of systemic diseases or without); Dental treatments (with a history of dental treatments or without). *P<0.05 and **P<0.01.
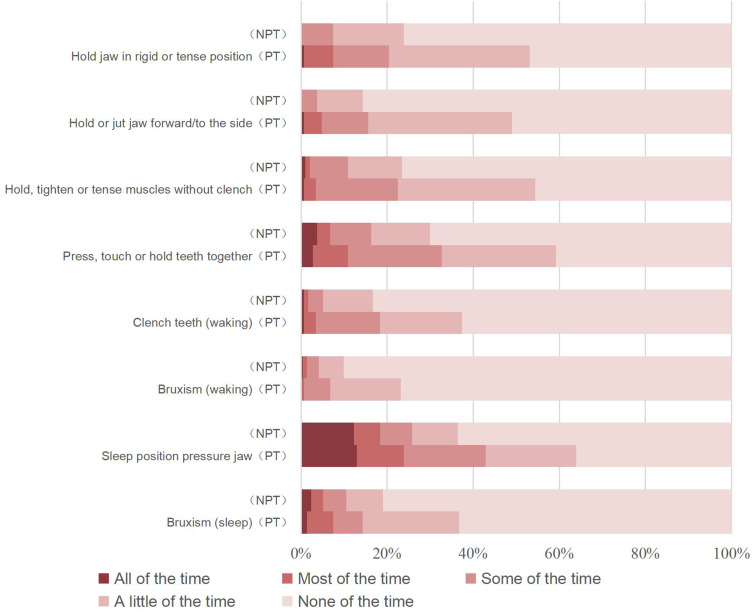
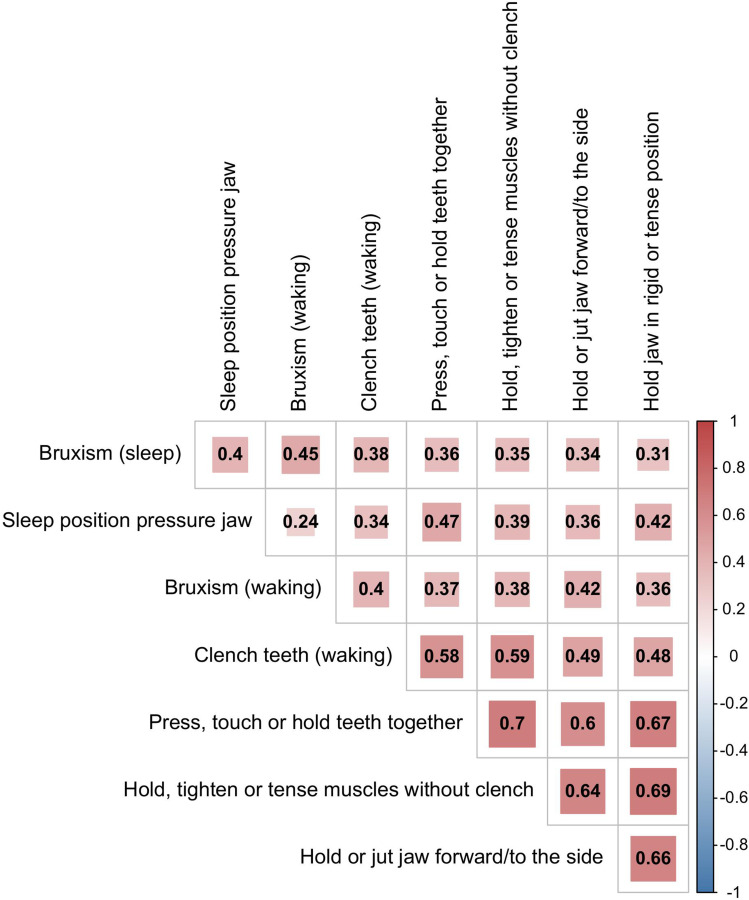
Figure 1 indicates that every individual oral behavior was more frequent in the PT group than in the NPT group. Notably, 61.00% of the participants had one or more oral behaviors. The PT prevalence in participants having from 0 to 8 oral behaviors were 16.28%, 30.77%, 26.47%, 37.84%, 39.02%, 50.00%, 60.00%, 82.35% and 60.00%, successively. And the behaviors “Hold jaw in rigid or tense position”, “Hold, tighten or tense muscles without clench”, “Press, touch or hold teeth together” and “Hold or jut jaw forward/to the side” often appeared together (Figure 2).
Figure 1.
Frequency of oral behaviors in PT group and NPT group.
Abbreviation: PT, Painful Temporomandibular Disorder.
Figure 2.
The heatmap of correlations among the oral behaviors.
Discussion
This general-population-based cross-sectional study examined the link between PT and oral behaviors. PT participants displayed a higher total score of oral behaviors after adjusting for age, sex, history of systemic diseases and dental treatments confounders.
The most common oral behaviors in this study were “Sleep position pressure jaw” (45.58%) and “Press, touch or hold teeth together” (39.68%), which were associated with the presence of PTs. In this study, there were 28.80% of the participants reported they had a habit of grinding their teeth (while waking or sleeping), which was similar to Atsü SS’s study but slightly lower than that in Gavish A’s researches.23,24 This difference may be explained by the fact that Gavish A’s study was based on teenagers or female high school students. Such adolescence often had a habit of biting their nails and holding objects in their mouths. As they grow older, these behaviors are gradually abandoned, and the accompanying teeth grinding behavior is less and less.25 In this study, the behaviors “Hold jaw in rigid or tense position”, “Hold, tighten or tense muscles without clench”, “Press, touch or hold teeth together” and “Hold or jut jaw forward/to the side” had correlation with each other, which implied the majority of these oral behaviors were not isolated.
Studies based on Chinese college students showed the prevalence of PT was 9.9% using clinical signs instead of 5Ts.4 However, in this study, the prevalence of PT was as high as 33.33% among respondents. The age distribution of the sample in this study was 33.37±12.47, and the high incidence age of TMDs is 20 to 40 years old.26 The sample of this study overlapped with the age of high incidence of TMD, so the prevalence in this study was relatively high. Moreover, diagnosis based on clinical signs is more stringent, whereas diagnosis based on questionnaires relies mostly on self-recall and self-report, tending to overestimate the prevalence of PT.
Sex differences in PT have been a subject of debate. Many studies have shown a higher prevalence of PT in females than in males.27 However, a prospective study showed that the incidence of acute TMDs was not significantly different between male and female, and only chronic TMDs showed a higher prevalence in females than in males.28 Therefore, the situation of higher prevalence of females in cross-sectional surveys may be due to a longer course of TMDs in females. In this cross-sectional study, the difference in PT between males and females was not significant, which might be related to the larger sample size of females due to their higher response to the questionnaire. Males and females showed similar constituent of oral behaviors, while other studies have demonstrated that there is no difference in PT between boys and girls during childhood, but from late puberty, females begin to show more TMDs symptoms.29 This difference may be caused by neuropsychological or physiological differences, such as more estrogenic hormone, lower pain thresholds and greater psychological stress in females than those in males.30
Existing studies have shown that oral behaviors occurring in non-physiological functional situations may be an etiology or at least a risk factor for PT,31,32 suggesting that even low-grade but persistent oral habits may lead to joint degeneration over time.1,2 In addition, several studies have shown that there is a positive correlation between oral behaviors and PT,33,34 which is consistent with our result. In the univariate analysis, all the 8 oral behaviors listed showed obvious correlations with PT. This difference remained after multivariate logistic regression analysis adjusting the confounders. However, Emodi-Perlman A. questioned this association.35 This difference may be due to the fact that their study was mainly based on Israeli children between the ages of 5 and 12, younger children have lower self-awareness of oral behaviors, and many behaviors without loud noise were often ignored by their parents.
Bruxism, also referred to as habitual clenching or grinding teeth, is thought to result in microtrauma to the TMJ and surrounding musculature, potentially leading to inflammation and pain.36 This study contrasted sleep and awake bruxism. In the multivariate regression analysis, the association of awake bruxism on PT appeared to be greater than that in sleep bruxism. Some researchers believed that the incidence of TMDs was associated only with sleep bruxism,37,38 while in other studies the TMDs incidence was associated only with awake or mixed bruxism.39,40 This difference may be due to the fact that compared with awake bruxism, sleep bruxism can also indirectly affect PT by affecting sleep quality and psychological stress.41
Previous studies had shown different mechanisms between sleep and awake bruxism.42 Sleep bruxism is mainly related to neurological diseases, while awake bruxism is more associated with psychological factors. The mechanical overload or microtrauma caused by oral behaviors such as bruxism may be responsible to TMD pathway. Overload joint activity cannot provide sufficient rest time of the orofacial muscles and cause consequent pain.43 This pain may lead to local muscle spasm, further aggravating the pain and creating a vicious cycle. In addition, the joint disc displacement caused by increased joint load is also an important factor causing PT.36
Despite its insights, several limitations should be considered when interpreting our findings. This study was a cross-sectional study based on the general population. The causal relationship between PT and oral behaviors was not completely determined. And more prospective trials are needed to clarify it. Secondly, the oral behaviors were self-reported, which may underestimate or overestimate their true prevalence due to lack of awareness.44 More objective measures like electromyography could improve accuracy. Third, our community sample lacked representation across all ages, education levels and localities. In addition, this study focused on pain in the oral facial region. The different location, intensity and nature of pain can be further studied in detail.
Conclusions
This cross-sectional study showed a higher frequency of oral behaviors in PT patients compared to those without PT. The simultaneous presence of multiple oral behaviors in individuals with PT suggests that non-functional oral activities may be an important associated factor for the presence of PT. Clinicians are advised to recommend patients reduce teeth grinding, clenching, and related behaviors. Public health promotion of these behavioral changes could be valuable for primary prevention. Further prospective researches are necessary to clarify causal mechanisms and effects of reducing oral behaviors on PT outcomes.
Funding Statement
This work was supported by China Postdoctoral Science Foundation (Grant number 2023M732464), Technology Innovation Project of Science and Technology Bureau of Chengdu (grant number 2022-YF05 −01691-SN) and Clinical Research Project of West China Hospital of Stomatology, Sichuan University (grant number LCYJ-2023-YY-2).
Abbreviations
TMD, temporomandibular disorders; PT, painful TMD; 5Ts, the 5 major TMD symptoms; TMJ, temporomandibular joint; TNF, tumor necrosis factor; IL-1, interleukin-1; PG, prostaglandin; OSA, obstructive sleep apnea.
Ethics Approval and Informed Consent
This cross-sectional study was approved by the Institutional Review Board of West China Hospital of Stomatology (Approval no. WCHSIRB-D-2022-118) and was conducted in conformity to the Declaration of Helsinki. Consent was obtained from all study subjects.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Beaumont S, Garg K, Gokhale A, Heaphy N. Temporomandibular Disorder: a practical guide for dental practitioners in diagnosis and management. Aust Dent J. 2020;65(3):172–180. doi: 10.1111/adj.12785 [DOI] [PubMed] [Google Scholar]
- 2.Magalhães BG, Freitas JLM, Barbosa A, et al. Temporomandibular disorder: otologic implications and its relationship to sleep bruxism. Braz J Otorhinolaryngol. 2018;84(5):614–619. doi: 10.1016/j.bjorl.2017.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alrizqi AH, Aleissa BM. Prevalence of temporomandibular disorders between 2015–2021: a literature review. Cureus. 2023;15(4):e37028. doi: 10.7759/cureus.37028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xie C, Lin M, Yang H, Ren A. Prevalence of temporomandibular disorders and its clinical signs in Chinese students, 1979–2017: a systematic review and meta-analysis. Oral Dis. 2019;25(7):1697–1706. doi: 10.1111/odi.13016 [DOI] [PubMed] [Google Scholar]
- 5.Li DTS, Leung YY. Temporomandibular disorders: current concepts and controversies in diagnosis and management. Diagnostics. 2021;11(3):3. doi: 10.3390/diagnostics11030459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Osiewicz M, Manfredini D, Biesiada G, et al. Prevalence of function-dependent temporomandibular joint and masticatory muscle pain, and predictors of temporomandibular disorders among patients with lyme disease. J Clin Med. 2019;8(7):929. doi: 10.3390/jcm8070929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.List T, Jensen RH. Temporomandibular disorders: old ideas and new concepts. Cephalalgia. 2017;37(7):692–704. doi: 10.1177/0333102416686302 [DOI] [PubMed] [Google Scholar]
- 8.Farré-Guasch E, Aliberas JT, Spada NF, de Vries R, Schulten E, Lobbezoo F. The role of inflammatory markers in Temporomandibular Myalgia: a systematic review. Jpn Dent Sci Rev. 2023;59:281–288. doi: 10.1016/j.jdsr.2023.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karkazi F, Özdemir F. Temporomandibular disorders: fundamental questions and answers. Turk J Orthod. 2020;33(4):246–252. doi: 10.5152/TurkJOrthod.2020.20031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fillingim RB, Ohrbach R, Greenspan JD, et al. Potential psychosocial risk factors for chronic TMD: descriptive data and empirically identified domains from the OPPERA case-control study. J Pain. 2011;12(11 Suppl):T46–T60. doi: 10.1016/j.jpain.2011.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fillingim RB, Ohrbach R, Greenspan JD, et al. Psychological factors associated with development of TMD: the OPPERA prospective cohort study. J Pain. 2013;14(12 Suppl):T75–T90. doi: 10.1016/j.jpain.2013.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kindler S, Samietz S, Houshmand M, et al. Depressive and anxiety symptoms as risk factors for temporomandibular joint pain: a prospective cohort study in the general population. J Pain. 2012;13(12):1188–1197. doi: 10.1016/j.jpain.2012.09.004 [DOI] [PubMed] [Google Scholar]
- 13.Mehdipour A, Aghaali M, Janatifar Z, Saleh A. Prevalence of oral parafunctional habits in children and related factors: an observational cross-sectional study. Int J Clin Pediatr Dent. 2023;16(2):308–311. doi: 10.5005/jp-journals-10005-2520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abe S, Kawano F, Matsuka Y, Masuda T, Okawa T, Tanaka E. Relationship between oral parafunctional and postural habits and the symptoms of temporomandibular disorders: a survey-based cross-sectional cohort study using propensity score matching analysis. J Clin Med. 2022;11(21):6396. doi: 10.3390/jcm11216396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Minervini G, Franco R, Marrapodi MM, et al. Correlation between Temporomandibular Disorders (TMD) and Posture Evaluated trough the Diagnostic Criteria for Temporomandibular Disorders (DC/TMD): a systematic review with meta-analysis. J Clin Med. 2023;12(7):2652. doi: 10.3390/jcm12072652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Minervini G, Franco R, Marrapodi MM, Ronsivalle V, Shapira I, Cicciù M. Prevalence of temporomandibular disorders in subjects affected by Parkinson disease: a systematic review and metanalysis. J Oral Rehabil. 2023;50(9):877–885. doi: 10.1111/joor.13496 [DOI] [PubMed] [Google Scholar]
- 17.Ommerborn MA, Özbek A, Grunwald M, et al. Effects on general pain perception and dental pulp sensibility in probable sleep bruxism subjects by experimentally induced pain in a pilot study. Sci Rep. 2023;13(1):5836. doi: 10.1038/s41598-023-33019-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raphael KG, Santiago V, Lobbezoo F. Is bruxism a disorder or a behaviour? Rethinking the international consensus on defining and grading of bruxism. J Oral Rehabil. 2016;43(10):791–798. doi: 10.1111/joor.12413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meloto CB, Slade GD, Lichtenwalter RN, et al. Clinical predictors of persistent temporomandibular disorder in people with first-onset temporomandibular disorder: a prospective case-control study. J Am Dental Assoc. 2019;150(7):572–581.e510. doi: 10.1016/j.adaj.2019.03.023 [DOI] [PubMed] [Google Scholar]
- 20.Sharma S, Wactawski-Wende J, LaMonte MJ, et al. Incident injury is strongly associated with subsequent incident temporomandibular disorder: results from the OPPERA study. Pain. 2019;160(7):1551–1561. doi: 10.1097/j.pain.0000000000001554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barbosa C, Manso MC, Reis T, Soares T, Gavinha S, Ohrbach R. Are oral overuse behaviours associated with painful temporomandibular disorders? A cross-sectional study in Portuguese university students. J Oral Rehabil. 2021;48(10):1099–1108. doi: 10.1111/joor.13226 [DOI] [PubMed] [Google Scholar]
- 22.Yap AU, Zhang MJ, Zhang XH, Cao Y, Fu KY. Viability of the quintessential 5 temporomandibular disorder symptoms as a TMD screener. Oral Surg Oral Med Oral Radiol. 2022;133(6):643–649. doi: 10.1016/j.oooo.2021.11.009 [DOI] [PubMed] [Google Scholar]
- 23.Atsü SS, Güner S, Palulu N, Bulut AC, Kürkçüoğlu I. Oral parafunctions, personality traits, anxiety and their association with signs and symptoms of temporomandibular disorders in the adolescents. Afr Health Sci. 2019;19(1):1801–1810. doi: 10.4314/ahs.v19i1.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gavish A, Halachmi M, Winocur E, Gazit E. Oral habits and their association with signs and symptoms of temporomandibular disorders in adolescent girls. J Oral Rehabil. 2000;27(1):22–32. doi: 10.1046/j.1365-2842.2000.00484.x [DOI] [PubMed] [Google Scholar]
- 25.Vanderas AP, Papagiannoulis L. Multifactorial analysis of the aetiology of craniomandibular dysfunction in children. Int J Paediatr Dent. 2002;12(5):336–346. doi: 10.1046/j.1365-263X.2002.00380.x [DOI] [PubMed] [Google Scholar]
- 26.Maixner W, Diatchenko L, Dubner R, et al. Orofacial pain prospective evaluation and risk assessment study--The OPPERA study. J Pain. 2011;12(11 Suppl):T4–T11.e2. doi: 10.1016/j.jpain.2011.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gauer RL, Semidey MJ. Diagnosis and treatment of temporomandibular disorders. Am Family Phys. 2015;91(6):378–386. [PubMed] [Google Scholar]
- 28.Slade GD, Bair E, Greenspan JD, et al. Signs and symptoms of first-onset TMD and sociodemographic predictors of its development: the OPPERA prospective cohort study. J Pain. 2013;14(12 Suppl):T20–T32.e3. doi: 10.1016/j.jpain.2013.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barbosa Tde S, Miyakoda LS, Pocztaruk Rde L, Rocha CP, Gavião MB. Temporomandibular disorders and bruxism in childhood and adolescence: review of the literature. Int J Pediatr Otorhinolaryngol. 2008;72(3):299–314. doi: 10.1016/j.ijporl.2007.11.006 [DOI] [PubMed] [Google Scholar]
- 30.Sena MF, Mesquita KS, Santos FR, Silva FW, Serrano KV. Prevalence of temporomandibular dysfunction in children and adolescents. Rev Paulista Pediatria. 2013;31(4):538–545. doi: 10.1590/S0103-05822013000400018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bergmann A, Edelhoff D, Schubert O, Erdelt KJ, Pho Duc JM. Effect of treatment with a full-occlusion biofeedback splint on sleep bruxism and TMD pain: a randomized controlled clinical trial. Clin Oral Investig. 2020;24(11):4005–4018. doi: 10.1007/s00784-020-03270-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carra MC, Huynh N, Fleury B, Lavigne G. Overview on sleep bruxism for sleep medicine clinicians. Sleep Med Clin. 2015;10(3):375–384s. doi: 10.1016/j.jsmc.2015.05.005 [DOI] [PubMed] [Google Scholar]
- 33.Karibe H, Shimazu K, Okamoto A, Kawakami T, Kato Y, Warita-Naoi S. Prevalence and association of self-reported anxiety, pain, and oral parafunctional habits with temporomandibular disorders in Japanese children and adolescents: a cross-sectional survey. BMC Oral Health. 2015;15(1):8. doi: 10.1186/1472-6831-15-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Michelotti A, Cioffi I, Festa P, Scala G, Farella M. Oral parafunctions as risk factors for diagnostic TMD subgroups. J Oral Rehabil. 2010;37(3):157–162. doi: 10.1111/j.1365-2842.2009.02033.x [DOI] [PubMed] [Google Scholar]
- 35.Emodi-Perlman A, Eli I, Friedman-Rubin P, Goldsmith C, Reiter S, Winocur E. Bruxism, oral parafunctions, anamnestic and clinical findings of temporomandibular disorders in children. J Oral Rehabil. 2012;39(2):126–135. doi: 10.1111/j.1365-2842.2011.02254.x [DOI] [PubMed] [Google Scholar]
- 36.Chisnoiu AM, Picos AM, Popa S, et al. Factors involved in the etiology of temporomandibular disorders - a literature review. Clujul Med. 2015;88(4):473–478. doi: 10.15386/cjmed-485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fernandes G, Siqueira JT, Godoi Gonçalves DA, Camparis CM. Association between painful temporomandibular disorders, sleep bruxism and tinnitus. Braz Oral Res. 2014;28:28. doi: 10.1590/s1806-83242013005000031 [DOI] [PubMed] [Google Scholar]
- 38.Blanco Aguilera A, Gonzalez Lopez L, Blanco Aguilera E, et al. Relationship between self-reported sleep bruxism and pain in patients with temporomandibular disorders. J Oral Rehabil. 2014;41(8):564–572. doi: 10.1111/joor.12172 [DOI] [PubMed] [Google Scholar]
- 39.Castrillon EE, Exposto FG. Sleep bruxism and pain. Dent Clin N Am. 2018;62(4):657–663. doi: 10.1016/j.cden.2018.06.003 [DOI] [PubMed] [Google Scholar]
- 40.Berger M, Szalewski L, Szkutnik J, Ginszt M, Ginszt A. Different association between specific manifestations of bruxism and temporomandibular disorder pain. Neurol Neurochir Pol. 2017;51(1):7–11. doi: 10.1016/j.pjnns.2016.08.008 [DOI] [PubMed] [Google Scholar]
- 41.Peixoto KO, Resende C, Almeida EO, et al. Association of sleep quality and psychological aspects with reports of bruxism and TMD in Brazilian dentists during the COVID-19 pandemic. J Appl Oral Sci. 2021;29:e20201089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhong J, Gao X, Hu S, Yue Y, Liu Y, Xiong X. A worldwide bibliometric analysis of the research trends and hotspots of bruxism in adults during 1991–2021. J Oral Rehabil. 2023;51(1):5–14. doi: 10.1111/joor.13577 [DOI] [PubMed] [Google Scholar]
- 43.Matre DA, Sinkjaer T, Svensson P, Arendt-Nielsen L. Experimental muscle pain increases the human stretch reflex. Pain. 1998;75(2–3):331–339. doi: 10.1016/S0304-3959(98)00012-8 [DOI] [PubMed] [Google Scholar]
- 44.Vlăduțu D, Popescu SM, Mercuț R, et al. Associations between Bruxism, Stress, and manifestations of temporomandibular disorder in young students. Int J Environ Res Public Health. 2022;19(9):5415. doi: 10.3390/ijerph19095415 [DOI] [PMC free article] [PubMed] [Google Scholar]