Abstract
Hospital-onset bacteremia and fungemia (HOB), a potential measure of healthcare-associated infections, was evaluated in a pilot study among 60 patients across 3 hospitals. Two-thirds of all HOB events and half of nonskin commensal HOB events were judged as potentially preventable. Follow-up studies are needed to further develop this measure.
Rates of central-line–associated bloodstream infections (CLABSIs) decreased 50% between 2008 and 2014 in the United States.1 CLABSI reporting to the Centers for Disease Control and Prevention’s National Healthcare Safety Network (NHSN) and use of the CLABSI data in Centers for Medicare and Medicaid Services (CMS) public reporting and pay for performance programs likely prompted enhanced infection prevention efforts to reduce CLABSI rates, though reductions since 2014 have diminished.2
CLABSIs are a subset of all hospital-onset bacteremia and fungemia (HOB). Prior studies have speculated whether HOB could replace CLABSI as a performance measure that better measures patient safety and quality because it assesses all patients, not just those with central lines. HOB could theoretically drive further improvements in patient care and could be used for public reporting. In prior studies, HOB rates decreased with CLABSI rates during implementation of CLABSI prevention bundles and may better differentiate performance across intensive care units (ICUs) compared to CLABSI.3,4
The clinical relevance and preventability of CLABSIs, when using evidence-based insertion and maintenance practices, led to its broad acceptance as a quality measure.5 In contrast, HOB has many more potential causes, encompassing infections at multiple anatomic sites and associated with many medical devices and procedures. The overall preventability of HOB is unknown; thus, determining the degree of preventability is critical to the potential use of HOB as a quality measure.
The aim of this study was to develop methods for determining the infectious causes and preventability of HOB, with the goal of informing the design for a larger follow-up study.
Methods
The HOB has been defined as microorganism growth from a blood culture obtained at least 3 calendar days after hospital admission, when admission date is day 1.
We included 20 HOB events each from 3 academic medical centers. These events were randomly selected from HOBs among all hospitalized adults (Emory University Hospital and the University of Maryland Medical Center) and critically ill children (Johns Hopkins Hospital) between October 1, 2014, and September 30, 2015.
Physicians reviewed medical records to identify potential risk factors and sources of bacteremia and fungemia from clinical documentation. When medical record documentation was ambiguous, the physician reviewer was instructed to use clinical judgement to determine the most likely source. Two physician reviewers with infection prevention experience at each hospital used underlying patient factors, causative microorganism(s), source of infection, and other clinical data to rate the preventability of each HOB event on a 6-point Likert scale in an “ideal hospital” that practices “flawless infection control and patient care.” To support adjudication of preventability, a rating grid was created that listed the comparative risk of bacteremia due to underlying conditions on one axis and the likelihood of preventing the infection type under ideal conditions on the other axis (Fig. 1). For example, bacteremia resulting from mucosal-barrier injuries (low preventability) among immunosuppressed patients (high susceptibility) were suggested to be classified as “definitely not-preventable,” as previously described.6 In contrast, bacteremia resulting from CLABSIs (high preventability) in an otherwise healthy patient (low susceptibility) were suggested to be classified as “definitely preventable.” Reviewers could either review cases independently or together but were asked to reach a consensus on the preventability rating for each HOB event.
Fig. 1.
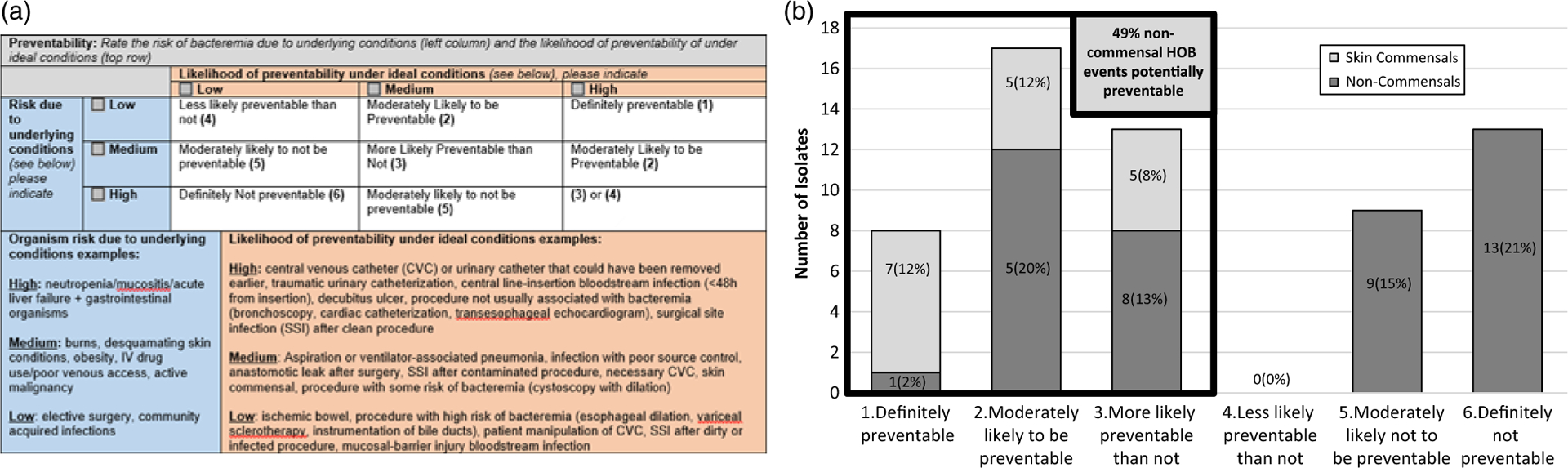
Tool for adjudicating preventability of hospital onset bacteremia and fungemia (HOB) events (panel A), and HOB preventability across 3 academic medical centers, (panel B) (n = 60).
Results
The median hospitalization duration until the HOB event was 13 days (interquartile range [IQR] 7–24 days) among patients in primarily adult hospitals and 24 days (IQR, 9–60 days) among pediatric ICU patients. Half (50%) of adult HOB events originated from ICUs (Table 1).
Table 1.
Clinical Characteristics of 60 Patients with Hospital-Onset Bacteremia and Fungemia (HOB) across 3 Academic Medical Centers (n = 60)
| All Patients | UMMC | JHH | EUH | |||||
|---|---|---|---|---|---|---|---|---|
| No. | (%) | No. | (%) | No. | (%) | No. | % | |
| Total HOB events | 60 | (100) | 20 | (100) | 20 | (100) | 20 | (100) |
| Time from admission to HOB event, median d (IQR) | 16 (7–30) | 18 (7–30) | 24 (9–60) | 11 (8–15) | ||||
| Patient location during blood culture | ||||||||
| Intensive care unit (ICU) | 40 | (67) | 12 | (60) | 20 | (100) | 8 | (40) |
| All adult ICUs | 18 | (30) | 10 | (50) | 0 | (0) | 8 | (40) |
| Neonatal ICU | 2 | (3) | 2 | (10) | 0 | (0) | 0 | (0) |
| Pediatric ICU | 20 | (33) | 0 | (0) | 20 | (100) | 0 | (0) |
| Intermediate acuity unit | 3 | (5) | 3 | (15) | 0 | (0) | 0 | (0) |
| Ward | 17 | (28) | 5 | (25) | 0 | (0) | 12 | (60) |
| Indwelling Devices and Procedures | ||||||||
| Central venous catheter, anyb | 44 | (73) | 9 | (45) | 17 | (85) | 18 | (90) |
| Temporary central line | 14 | (23) | 3 | (15) | 3 | (15) | 8 | (40) |
| Peripherally inserted central catheter | 13 | (22) | 3 | (15) | 4 | (20) | 6 | (30) |
| Port or tunneled catheter | 12 | (20) | 0 | (0) | 8 | (40) | 4 | (20) |
| Dialysis catheter | 8 | (13) | 3 | (15) | 0 | (0) | 5 | (25) |
| Midline | 1 | (2) | 0 | (0) | 0 | (0) | 1 | (5) |
| No central line | 12 | (20) | 7 | (35) | 4 | (20) | 1 | (5) |
| Urinary catheterb | 12 | (20) | 4 | (20) | 1 | (5) | 7 | (35) |
| Mechanical ventilatory supportb | 15 | (25) | 3 | (15) | 6 | (30) | 6 | (30) |
| Other indwelling deviceb,c | 24 | (40) | 7 | (35) | 12 | (60) | 5 | (25) |
| No indwelling deviceb | 6 | (10) | 5 | (25) | 0 | (0) | 1 | (5) |
| Surgery or other invasive procedured prior to HOB | 7 | (12) | 2 | (10) | 3 | (15) | 2 | (10) |
| Microbiology | ||||||||
| Gram positive | 33 | (55) | 10 | (50) | 10 | (50) | 13 | (65) |
| Coagulase-negative Staphylococcus | 17 | (28) | 4 | (20) | 6 | (30) | 7 | (35) |
| Enterococcus spp. | 6 | (10) | 4 | (20) | 1 | (5) | 1 | (5) |
| Methicillin-susceptible S. aureus | 6 | (10) | 2 | (10) | 3 | (15) | 1 | (5) |
| Methicillin-resistant S. aureus | 2 | (3) | 1 | (5) | 0 | (0) | 1 | (5) |
| Streptococci spp. | 3 | (5) | 0 | (0) | 0 | (0) | 3 | (15) |
| Gram negative | 21 | (35) | 7 | (35) | 7 | (35) | 7 | (35) |
| Eschericia coli | 5 | (8) | 2 | (10) | 1 | (5) | 2 | (10) |
| Klebsiella spp. | 4 | (7) | 2 | (10) | 1 | (5) | 1 | (5) |
| Serratia spp. | 4 | (7) | 0 | (0) | 2 | (10) | 2 | (10) |
| Pseudomonas aeruginosa | 3 | (5) | 2 | (10) | 1 | (5) | 0 | (0) |
| Other | 6 | (10) | 2 | (10) | 1 | (5) | 3 | (15) |
| Fungi | 8 | (13) | 2 | (10) | 3 | (15) | 2 | (10) |
| Candida spp | 7 | (12) | 2 | (10) | 2 | (10) | 2 | (10) |
| Other fungi | 1 | (2) | 0 | (0) | 1 | (5) | 0 | (0) |
| Source of bacteremia or fungemia | ||||||||
| Skin contamination | 11 | (18) | 3 | (15) | 4 | (20) | 4 | (20) |
| CLABSI nonmucosal barrier injury | 17 | (28) | 4 | (20) | 6 | (30) | 7 | (35) |
| CLABSI with mucosal barrier injury | 5 | (8) | 0 | (0) | 0 | (0) | 5 | (25) |
| Respiratory tract | 6 | (10) | 2 | (10) | 4 | (20) | 0 | (0) |
| Peripheral IV related | 3 | (5) | 3 | (15) | 0 | (0) | 0 | (0) |
| Pyelonephritis | 3 | (5) | 3 | (15) | 0 | (0) | 0 | (0) |
| CAUTI | 3 | (5) | 1 | (5) | 0 | (0) | 2 | (10) |
| Intra-abdominal abscess or peritonitis | 3 | (5) | 2 | (10) | 1 | (5) | 0 | (0) |
| Ischemic bowel | 2 | (3) | 0 | (0) | 1 | (5) | 1 | (5) |
| Other | 5 | (8) | 2 | (10) | 2 | (10) | 1 | (5) |
| Unknown | 2 | (3) | 0 | (0) | 2 | (10) | 0 | (0) |
| NHSN-reported CLABSI | 12 | (20) | 3 | (15) | 7 | (35) | 2 | (10) |
| Secondary bloodstream infection | 2 | (3) | 0 | (0) | 2 | (10) | 0 | (0) |
Note. EUH, Emory University Hospital; HOB, hospital-onset bacteremia and fungemia; JHH, Johns Hopkins Hospital; NHSN, National Healthcare Safety Network; UMMC, University of Maryland Medical Center; CLABSI, central line-associated bloodstream infection; CAUTI, catheter-associated urinary tract infection; IQR, interquartile range; ICU, intensive care unit.
Pediatric ICU patients in JHH were selected a priori.
Indicates presence within 2 days before or after the date of the blood culture.
Other indwelling devices included arterial line, biliary drain, chest tube, external ventricular drain, extracorporeal membrane oxygenation, fistula drain, gastrostomy tube, intra-aortic balloon pump, intraosseous line, intermittent urinary catheterization, jejunostomy tube, noninvasive high flow respiratory support system, nasogastric tube, surgical drain, ventricular assist device, and ureteral stent.
Invasive procedures included bronchoscopy, cardiac catheterization, feeding tube placement, insertion of a tunneled hemodialysis central line, interventional radiology percutaneous procedure, and transesophageal echocardiogram.
Central venous catheters were frequently present in the time period 2 days before and after blood cultures were obtained from patients (44 of 60, 73%). Presence of urinary catheters (20%) and invasive mechanical ventilation were less common (25%). Few HOB events (6 of 60, 10%) occurred among patients without any indwelling medical device, catheters or invasive mechanical ventilation (Table 1).
Coagulase-negative staphylococci were the single most common organism identified among HOB events (17 of 60, 28%), followed by Candida spp (7 of 60, 12%), methicillin-susceptible S. aureus (6 of 60, 10%), and Enterococcus spp (6 of 60, 10%) (Table 1).
Clinical sources of HOB varied, and reviewers identified 14 separate categories of HOB sources during this study; the most common sources were nonmucosal barrier injury CLABSI (11 of 60, 28%), followed by skin contamination (11 of 60, 18%), and the respiratory tract (6 of 60, 10%) (Table 1).
Overall, 38 of 60 HOB events (63%) were adjudicated as potentially preventable; 17 were due to skin commensal organisms and attributed to contaminated blood cultures. Among HOB events not due to skin commensal organisms, 21 of 43 of these HOB events (49%) were determined to be potentially preventable (Fig. 1).
A minority (12 of 60, 20%) of all HOB events and potentially preventable HOB events (5 of 38, 13%) were reported to NHSN as CLABSI based on 2014 CDC definitions.
Discussion
In a pilot study, a variety of microorganisms and clinical sources were implicated in HOB events that were systematically evaluated by expert medical record reviewers. Approximately two-thirds of all HOB events and half of non-skin commensal HOB events were judged to be potentially preventable with current recommendations in an ideal healthcare setting.
For HOB to serve as a meaningful and actionable quality measure, a substantial and quantifiable proportion of these events should be preventable with good clinical care and infection prevention practices. In this study, to judge HOB preventability, physician adjudicators weighed the underlying patient susceptibility and the preventability of the microorganism and source of infection. To simplify this process, we created a grid with suggested preventability ratings based on these 2 dimensions of susceptibility and infection type (Fig. 1). We acknowledge that our list of clinical scenarios was not exhaustive, and an important qualitative finding was the need to develop expert consensus using a systematic framework to determine the preventability of a wider range of potential HOB clinical scenarios. Standardized provider training and calculation of interrater reliability was not assessed in this pilot study but should be performed in a larger study.
The role of skin commensal organisms in an HOB measure must also be considered; nearly one-third of bacteremia events in this study and 38% of bacteremias among already hospitalized patients in another study were due to skin commensal organisms.7 Because skin commensal bacteremia events most often do not represent true infection, arguably these may not “count” the same as noncommensal bacteremias in a quality measure. However, blood cultures with skin commensals are often initially interpreted as true infections, and they frequently result in unnecessary antibiotic use and prolonged hospitalization.8,9 Furthermore, skin commensal contamination is preventable with proper blood culture collection techniques, and reduction of blood culture contamination is a relevant goal for quality improvement.10
An important finding of this study is that only 20% of HOB events resulted in an NHSN-reported CLABSI, suggesting that HOB events beyond CLABSIs are preventable and should be evaluated as targets for prevention. Although broad generalizations about microorganisms, clinical sources, and preventability cannot be drawn from this limited study, we demonstrated an approach for assessing preventability of HOB events. Larger studies across a variety of hospital settings are needed to assess the generalizability of these results, understand current risk factors for HOB, and develop prevention strategies.
Acknowledgments.
The findings and conclusions are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Financial support.
C.R., A.M.M., J.T.J., A.D.H., and S.L. are supported by the Centers for Disease Control Prevention Epicenters Program. A.D.H. is also supported by the National Institutes of Health (grant no. 5K24AI079040-05).
Footnotes
Conflicts of interest. The authors report no financial conflicts of interest related to this article.
References
- 1.Centers for Disease Control and Prevention. National and State Healthcare Associated Infections Progress Report. In: Division of Healthcare Quality Promotion NCEZID. Atlanta, GA: Centers for Disease Control and Prevention; 2016. [Google Scholar]
- 2.Healthcare-associated infections in the United States, 2006–2016: a story of progress. Centers for Disease Control and Prevention website. https://www.cdc.gov/hai/surveillance/data-reports/data-summary-assessing-progress.html. Published 2018. Accessed July 17, 2018.
- 3.Leekha S, Li S, Thom KA, et al. Comparison of total hospital-acquired bloodstream infections to central line-associated bloodstream infections and implications for outcome measures in infection control. Infect Control Hosp Epidemiol 2013;34:984–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rock C, Thom KA, Harris AD, et al. A multicenter longitudinal study of hospital-onset bacteremia: time for a new quality outcome measure? Infect Control Hosp Epidemiol 2016;37:143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pronovost P, Needham D, Berenholtz S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med 2006;355:2725–2732. [DOI] [PubMed] [Google Scholar]
- 6.Blijlevens NM, Donnelly JP, De Pauw BE.Mucosal barrier injury: biology, pathology, clinical counterparts and consequences of intensive treatment for haematological malignancy: an overview. Bone Marrow Transpl 2000; 25:1269–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Linsenmeyer K, Gupta K, Strymish JM, Dhanani M, Brecher SM, Breu AC. Culture if spikes? Indications and yield of blood cultures in hospitalized medical patients. J Hosp Med 2016;11:336–340. [DOI] [PubMed] [Google Scholar]
- 8.Alahmadi YM, Aldeyab MA, McElnay JC, et al. Clinical and economic impact of contaminated blood cultures within the hospital setting. J Hosp Infect 2011;77:233–236. [DOI] [PubMed] [Google Scholar]
- 9.Waltzman ML, Harper M. Financial and clinical impact of false-positive blood culture results. Clin Infect Dis 2001;33:296–299. [DOI] [PubMed] [Google Scholar]
- 10.Towns ML, Jarvis WR, Hsueh PR. Guidelines on blood cultures. J Microbiol Immunol Infect 2010;43:347–349. [DOI] [PubMed] [Google Scholar]


