Abstract
Pasteurella haemolytica serotype 1 is the bacterial agent responsible for the pathophysiological events associated with bovine pneumonic pasteurellosis. Our previous studies support a role for the lipopolysaccharide (LPS) from P. haemolytica in the induction of proinflammatory cytokines. One of the pathological hallmarks of bovine pneumonic pasteurellosis is an influx of neutrophils into the alveolar spaces. This pronounced influx suggests the local production of a chemotactic factor(s) such as interleukin-8 (IL-8). In the context of the lung, the alveolar macrophage appears to be the major producer of IL-8, a proinflammatory cytokine with potent neutrophil chemotactic activity. By using Northern blot analysis, we have examined the kinetics of IL-8 mRNA expression in P. haemolytica LPS-stimulated bovine alveolar macrophages and found that 1 ng of LPS per ml induces maximal expression of IL-8 mRNA. The results also indicate a biphasic time course expression pattern in which IL-8 mRNA levels peak between 1 and 2 h in the first phase and between 16 and 24 h in the second phase (P < 0.01). In addition, monospecific polyclonal antibodies were used to demonstrate the role of tumor necrosis factor alpha (TNF-α) in the second phase of IL-8 mRNA expression. Our findings support a role for P. haemolytica LPS and TNF-α in the induction of IL-8 from bovine alveolar macrophages.
Economic losses from bovine pneumonic pasteurellosis, commonly known as shipping fever, cost the cattle industry billions of dollars annually (1). Although shipping fever is a multifactorial disease involving infection by a variety of microorganisms in conjunction with stressful management practices and environmental factors, Pasteurella haemolytica serotype 1 is the primary agent responsible for the clinical disease and pathophysiologic events (17, 32). Bovine pneumonic pasteurellosis is an acute fibrinonecrotizing pleuropneumonia characterized by an influx of neutrophils into the alveoli; accumulation of fibrinous edema fluid within the alveoli, pleural surface, and interlobular septa; hemorrhage; vascular thrombosis; and coagulative parenchymal necrosis of the lung (31). A substantial amount of evidence implicates the neutrophil in the pathogenesis of lung injury in bovine pneumonic pasteurellosis (19, 28, 30). Studies involving a calf model of experimental pneumonic pasteurellosis have shown that marked neutrophil influx into the alveoli occurs within the first few hours after bacterial inoculation and that peracute lung lesions are evident within the first 6 h postinfection (19, 28). In these studies, neutrophil depletion ameliorated the lung injury and the pathophysiologic alterations that occur in the intact animal (19). These findings imply that neutrophils are the primary effector cells of the peracute lung injury associated with the disease. The influx of neutrophils into the alveolar space early in the disease suggests the generation of specific chemotactic factors which promote neutrophil recruitment into the alveolar compartment.
Pasteurella haemolytica possesses several virulence factors, of which the lipopolysaccharide (LPS) and leukotoxin (Lkt) appear to be the most important. P. haemolytica LPS is similar to LPS produced by other gram-negative bacteria and is composed of biologically active lipid A, core oligosaccharide, and an antigenic polysaccharide side chain (O antigen) (4). We have shown that purified LPS from P. haemolytica A1 given intrabronchially causes neutrophil and platelet influx, fibrin exudation, and edema in the alveolar spaces, neutrophil aggregation in the capillaries, and other pathophysiological derangements in the lungs (30). More recently, we reported that purified LPS from P. haemolytica induced tumor necrosis factor alpha (TNF-α) and interleukin-1β (IL-1β) mRNA expression in bovine alveolar macrophages (AMs) and secretion of these biologically active cytokines (33).
TNF-α is a proinflammatory cytokine hypothesized to be involved in the inflammatory cascade caused by P. haemolytica. TNF-α has a profound effect on tissue remodeling, repair, and inflammation by coordinating the activities of many other cells, including endothelial cells, granulocytes, fibroblasts, and lymphoid cells (12). Although not directly chemotactic, TNF-α facilitates leukocyte recruitment by upregulating leukocyte adhesion proteins on endothelial cells (9), as well as through the paracrine induction of leukocyte chemotactic factor synthesis from immune (22) and nonimmune (20, 23–25) cells of the lung.
IL-8 is a CXC chemokine that is produced by many cell types, including monocytes/macrophages, fibroblasts, epithelial cells, endothelial cells, and neutrophils (2). Although IL-8 has been shown to have chemotactic activity for T lymphocytes, eosinophils, and basophils, it is the most potent chemoattractant for neutrophils. In addition, IL-8 is able to induce many neutrophil activities including oxidative burst, exocytosis of specific granules, and release of proteases (16, 27).
We hypothesize that the initial interaction of P. haemolytica LPS with resident AMs leads to the production and release of TNF-α and IL-8, along with other proinflammatory molecules, into the alveolar spaces. This is followed by recruitment of neutrophils, eruption of a cytokine-mediated inflammatory cascade, and neutrophil activation, resulting in the release of toxic oxygen radicals, proteases, and cytokines which participate in direct lung tissue injury. The focus of this study is to characterize the expression of IL-8 mRNA from bovine AMs stimulated with purified LPS from P. haemolytica A1.
MATERIALS AND METHODS
Antibodies and reagents.
Monospecific polyclonal antibodies against recombinant bovine TNF-α produced in rabbit (anti-bovine TNF-α) and preimmune rabbit serum were both generously provided by T. H. Elsasser, U.S. Department of Agriculture, Beltsville, Md. LPS from P. haemolytica 12296 was isolated by the hot-phenol–water extraction technique as described previously (29, 33). The concentration of endotoxin present in the LPS, as determined by the Limulus amebocyte lysate assay (BioWhittaker, Walkersville, Md.), revealed that 1 μg of LPS per ml was equivalent to approximately 150 endotoxin units. Recombinant human TNF-α (rhTNF-α) was purchased from PharMingen (San Diego, Calif.) and contained less than 0.1 ng of LPS/μg of human TNF-α.
Bacterial strains, plasmids, and media.
Escherichia coli DH5α competent cells were obtained from Gibco BRL (Grand Island, N.Y.), pGEM3zf(+) was purchased from Promega (Madison, Wis.), and pET15b was purchased from Novagen (Madison, Wis.). E. coli DH5α transformed with recombinant pGEM3zf(+) was grown in Luria-Bertani medium containing 50 μg of ampicillin per ml.
Recovery and isolation of AMs.
AMs were collected by lung lavage with sterile, endotoxin-free phosphate-buffered saline (pH 7.4) from healthy, 3- to 6-week-old calves sedated with an intravenous injection of xylazine hydrochloride (Miles Inc., Shawnee Mission, Kan.). Approximately 107 cells in Dulbecco’s modified Eagle’s medium (Celox, Oakdale, Minn.) (supplemented with 2% fetal bovine serum, 1 mM l-glutamine, 0.1 mM nonessential amino acids, 14 mM HEPES, 100 U of penicillin per ml, 0.1 mg of streptomycin per ml, and 25 μg of amphotericin B per ml in 0.9% sodium chloride) were plated onto 10-cm tissue culture petri dishes and allowed to adhere for 3 h at 37°C in a humidified atmosphere containing 5% CO2. Nonadherent cells were removed, and adherent AMs were incubated with fresh medium for at least 36 h. Adherent populations were >95% macrophages and >98% viable as determined by nonspecific esterase staining and trypan blue dye exclusion, respectively. Following incubation, inducers or treatments were added directly to the existing media.
Stimulation of AMs.
When stimulating AMs, the cells were cultured in the presence of either purified LPS from P. haemolytica 12296 or rhTNF-α. Concentrations of LPS or rhTNF-α and exposure times varied as described for individual experiments. In the experiments with polymyxin B (Sigma Chemical Co., St. Louis, Mo.), 10 μg of polymyxin B per ml was preincubated with 1 μg of LPS per ml for 30 min before AM stimulation. When the anti-bovine TNF-α antibodies and the preimmune rabbit serum were used, different dilutions were preincubated with 1 μg of LPS per ml for 30 min before AM stimulation. Supernatants were collected by centrifugation at 1,000 × g for 10 min, aliquoted, and frozen at −70°C.
Cloning of bovine IL-8 cDNA.
Total RNA was extracted from AMs by the guanidinium isothiocyanate-phenol-chloroform procedure (3). The concentration of RNA was determined by measuring the absorbance at 260 nm with a spectrophotometer. Contaminating DNA was removed from the total RNA with RNase-free DNase I by incubation at 37°C for 15 min. By using 2 μl of random hexamer primers (Perkin Elmer, Foster City, Calif.), RNA was reverse transcribed at 42°C for 1 h in a final volume of 20 μl. PCR was performed for 30 cycles (93°C for 1 min, 50°C for 30 s, and 72°C for 30 s) with the synthesized single-stranded cDNA and the following primers based on conserved IL-8 sequence from pig, human, and rabbit: 5′CTCT(CG)TGTGA(GA)GCTGCAGTTCTG-3′ and 5′-T(TG)CTCAG(TC)(TC)CTCTTCAA(AG)AA(TC)AT-3′. The amplified product was ligated into the pGEM3zf(+) vector and sequenced by the dideoxy chain termination method (26). The remaining sequence at the 3′ end was acquired through the use of 3′ RACE (rapid amplification of cDNA ends) (Gibco BRL, Gaithersburg, Md.), and the entire mature protein sequence was cloned into the pET15b expression vector.
Northern blot analysis.
Approximately 5 μg of RNA from each sample was electrophoresed on 1.2% formaldehyde–agarose gels and transferred to nitrocellulose membranes. Hybridization was performed at 45°C with either [α-32P]dCTP (Amersham, Arlington Heights, Ill.)-labeled bovine IL-8 cDNA insert or [α-32P]dCTP-labeled bovine TNF-α cDNA insert (33) at 1 × 106 to 2 × 106 cpm/ml for at least 4 h in a solution containing 50% deionized formamide, 30% 20× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), 4% 50X Denhardt’s solution, 5% 1 M NaH2PO4 (pH 7.0), 4 mg of yeast tRNA per ml, and 10% dextran. The membranes were washed and exposed to X-ray film (Kodak) with an intensifying screen for 4 to 48 h at −80°C. All blots were rehybridized with a human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA probe as an internal control to normalize the signal between samples. To quantify relative levels of hybridization signal, membranes were placed in a phosphorimaging cassette and analyzed with phosphorimaging software (Molecular Dynamics, Sunnyvale, Calif.).
The results are expressed as a percentage of the band representing the maximal intensity for each blot. Values are presented as means ± standard errors of the mean (SEM). Data were analyzed by a two-tailed t test, and statistical significance was set at P < 0.01.
TNF-α bioassay.
TNF-α was measured by a bioassay (8) with WEHI-13VAR cells, a variant of WEHI 164 clone 13. The cells were plated on 96-well plates at 3.5 × 104 cells per well in RPMI 1640 medium (BioWhittaker) containing 5% fetal bovine serum. The plates were incubated at 37°C overnight in a humidified atmosphere containing 5% CO2. The medium was removed the following morning, and 50 μl of a 1-μg/ml actinomycin D (Sigma) solution was added to each well. Samples containing either rhTNF-α (positive control), medium plus 5% fetal bovine serum (negative control), or macrophage supernatants were used in duplicate (100 μl/well) and incubated as above. At 24 h later, 25 μl of 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyl tetrazolium bromide (MTT; Sigma) dissolved in phosphate-buffered saline at 5 mg/ml was added to each well. The plates were allowed to incubate for an additional 3 h at 37°C, and then 100 μl of 50% dimethyl formamide–20% sodium dodecyl sulfate (pH 4.7) (10) was added per well. After 1 h, the optical density at 570 nm was measured with a microplate enzyme-linked immunosorbent assay reader (Molecular Device Corp., Menlo Park, Calif.). Concentrations of TNF-α were expressed in picograms per milliliter, which were extrapolated based on a standard curve established with rhTNF-α. The results of the bioassay are expressed as means ± SEM.
RESULTS
Cloning of bovine IL-8 cDNA.
Through the use of primer sequences based on IL-8 cDNA sequence data from human, pig, and rabbit, a 230-bp cDNA fragment encoding the first 77 amino acids of the bovine IL-8 mature protein was amplified by PCR. The amplified fragment was purified, ligated into pGEM3zf(+), and then transformed into E. coli DH5α. The remaining sequence at the 3′ end of the coding region was obtained by 3′ RACE. After subcloning into the pET15b expression vector and sequence analysis, the entire mature protein cDNA sequence of 84 amino acids was found to be identical to bovine IL-8 sequence data published after these studies were initiated (15).
Kinetics of IL-8 mRNA expression in bovine AMs stimulated with P. haemolytica LPS.
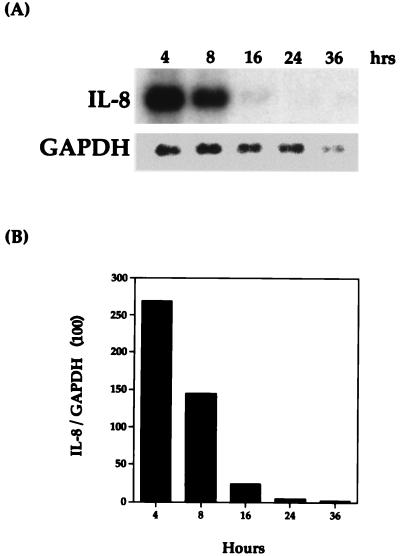
Preliminary experiments revealed that IL-8 mRNA was induced during the isolation of bovine AMs. Therefore, before any induction experiments could be performed, it was important to establish the duration of culture that bovine AMs required to reach undetectable levels of IL-8 mRNA. AMs were plated, allowed to adhere for 3 h, washed, and then harvested at 4, 8, 16, 24, and 36 h. As shown in Fig. 1, IL-8 mRNA levels were undetectable by Northern blot analysis at 24 h. Since the exact culture time needed to reach undetectable levels of IL-8 mRNA was somewhat variable, the cells were allowed to incubate for at least 36 h in all subsequent experiments.
FIG. 1.
Length of time required by bovine AMs to become quiescent for bovine IL-8 mRNA. (A) Northern blot analysis with the IL-8 probe was performed as described in Materials and Methods. (B) Relative levels of hybridization signal were normalized to GAPDH. The data shown are representative of two separate experiments.
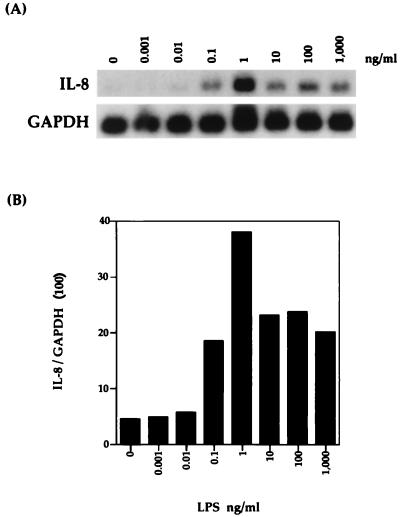
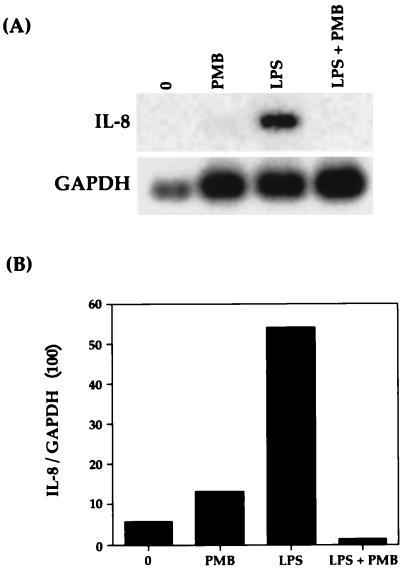
Bovine AMs were stimulated with various concentrations (0.001 to 1,000 ng/ml) of P. haemolytica LPS for 1 h. As shown by Northern blot analysis (Fig. 2), P. haemolytica LPS induced bovine AMs to express IL-8 mRNA. Expression steadily increased to a peak at 1 ng/ml and then decreased slightly to a level that remained constant with increasing concentrations of LPS. This induced expression was entirely due to LPS, since preincubation of LPS with polymyxin B completely abrogated any expression of IL-8 mRNA (Fig. 3).
FIG. 2.
Bovine IL-8 mRNA expression in bovine AMs stimulated with various concentrations of P. haemolytica LPS for 1 h. (A) Northern blot analysis with the IL-8 probe was performed as described in Materials and Methods. (B) Relative levels of IL-8 mRNA were normalized to GAPDH mRNA levels. The data shown are representative of three separate experiments.
FIG. 3.
Effect of polymyxin B (PMB) on bovine IL-8 mRNA expression. Bovine AMs were cultured in the presence of 1 μg of P. haemolytica LPS per ml with or without preincubation of 10 μg of polymyxin B per ml. (A) After 1 h, the cells were harvested and Northern blot analysis was performed as described in Materials and Methods. (B) Relative levels of IL-8 mRNA were normalized to GAPDH. The data shown are representative of three separate experiments.
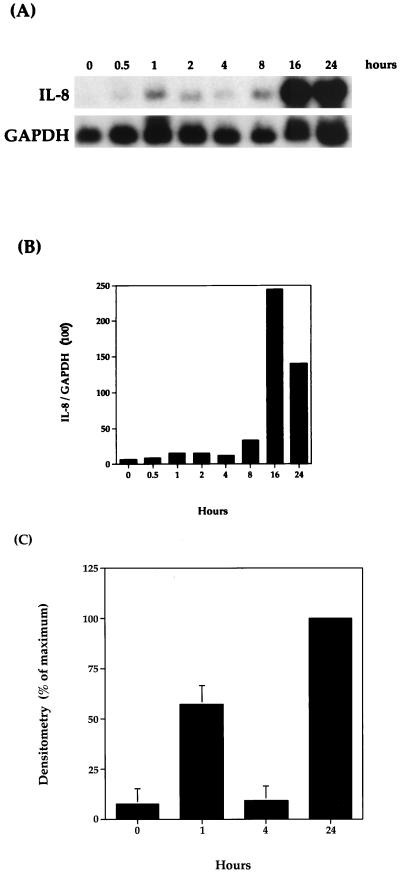
To determine the kinetics of IL-8 mRNA expression, bovine AMs were harvested at various times following stimulation with 1 μg of P. haemolytica LPS per ml. This concentration was used in the following experiments since it had been shown to induce maximal expression of bovine TNF-α mRNA in AMs (33). In related studies, 1 μg of LPS from E. coli per ml was used to stimulate human whole blood, which resulted in a similar IL-8 mRNA expression pattern to the one described below (5, 7). As shown by Northern blot analysis (Fig. 4; also see Fig. 8), LPS-stimulated AMs expressed IL-8 mRNA in a biphasic pattern. Although the relative amount of IL-8 mRNA expressed at specific time points is somewhat variable among experiments, the biphasic pattern of expression is statistically significant (P < 0.01), as demonstrated in Fig. 4C. IL-8 mRNA levels increased to a peak between 1 and 2 h, decreased to baseline levels at 4 h, and peaked again between 16 and 24 h (Fig. 4A). The higher level of IL-8 expression at 1 h in Fig. 8 compared to Fig. 4 is most probably due to the increased amount of IL-8 mRNA in the unstimulated cells. Results also indicated that maximal mRNA expression in the second phase was approximately 10 times greater than that in the first phase. This biphasic pattern suggests that the first phase is mediated by the initial LPS stimulation whereas the second phase results from stimulation by a macrophage-derived molecule produced in response to the initial LPS stimulation.
FIG. 4.
Time course of bovine IL-8 mRNA expression. Bovine AMs were stimulated with 1 μg of P. haemolytica LPS per ml for the indicated times. (A) Northern blot analysis with the IL-8 probe was performed as described in Materials and Methods. (B) Relative levels of hybridization signal were normalized to GAPDH. The data shown are representative of three separate experiments. (C) The results are expressed as a percentage of the band representing the maximal intensity for each blot. The final results represent mean ± SEM (n = 3).
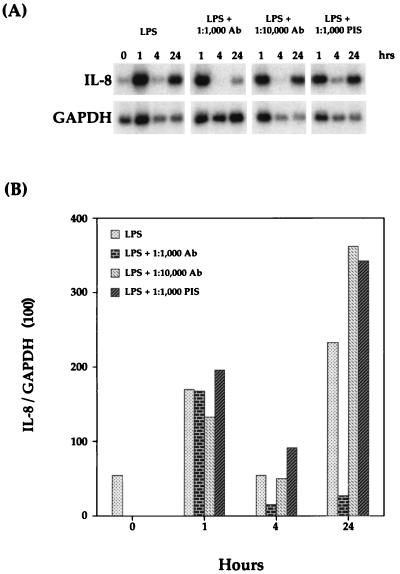
FIG. 8.
Inhibition of bovine IL-8 mRNA expression with monospecific polyclonal antibodies against bovine TNF-α. (A) Northern blot analysis with the IL-8 probe was performed as described in Materials and Methods. (B) Relative levels of IL-8 mRNA were normalized to GAPDH mRNA levels. The data shown are representative of two separate experiments. PIS, preimmune serum.
rhTNF-α induces expression of bovine IL-8 mRNA in bovine AMs.
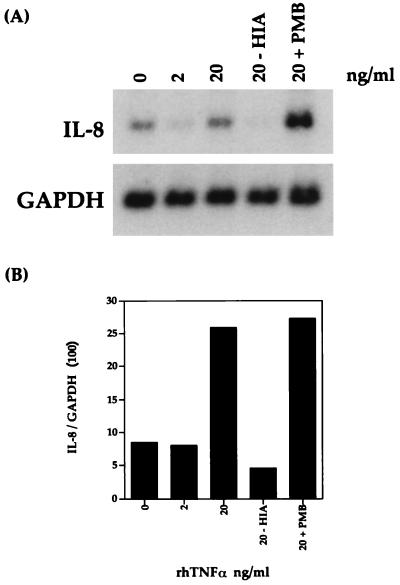
Previous studies with LPS-stimulated human whole blood have also shown a biphasic pattern for IL-8 mRNA and protein (5, 7). It was found that the second phase was in part the result of stimulation by TNF-α (5). To address the role of TNF-α in the second phase of IL-8 mRNA induction, bovine AMs were stimulated with 2 and 20 ng of rhTNF-α per ml for 4 h. As shown by Northern blot analysis (Fig. 5), rhTNF-α induced IL-8 mRNA in a dose-dependent manner. To demonstrate that rhTNF-α-induced IL-8 mRNA expression was not due to the small amount of contaminating LPS found in recombinant proteins produced by E. coli, rhTNF-α was either boiled or preincubated with polymyxin B prior to AM stimulation. IL-8 mRNA was not induced when 20 ng of rhTNF-α per ml was boiled for 30 min (Fig. 5, lane 20-HIA), and preincubation of 20 ng of rhTNF-α per ml with 10 μg of polymyxin B per ml for 30 min had no effect on the level of induction compared to the results obtained with 20 ng of rhTNF-α per ml alone (lane 20-PMB).
FIG. 5.
IL-8 mRNA expression is induced by recombinant human TNF-α. (A) After 4 h, the cells were harvested and Northern blot analysis was performed as described in Materials and Methods. HIA, 20 ng of rhTNF-α per ml was boiled for 30 min before being added to AMs. (B) Relative levels of IL-8 mRNA were normalized to GAPDH. The data shown are representative of two separate experiments.
P. haemolytica LPS-stimulated AMs express bovine TNF-α mRNA and secrete bioactive TNF-α protein.
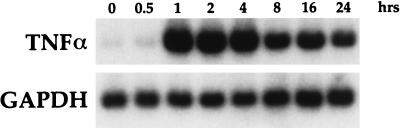
To demonstrate the ability of LPS to induce TNF-α mRNA, blots from LPS-stimulated time course experiments were rehybridized with a bovine TNF-α probe. As shown by Northern blot analysis (Fig. 6), 1 μg of LPS per ml induced TNF-α mRNA expression in bovine AMs. The kinetics of TNF-α mRNA expression were quite different from those of IL-8 expression, since a peak in mRNA at 1 h was followed by a steady decline over time. These results are consistent with those previously published by Yoo et al. (33).
FIG. 6.
Time course of bovine TNF-α mRNA expression. Bovine AMs were stimulated with 1 μg of P. haemolytica LPS per ml for the indicated times. Northern blot analysis with the bovine TNF-α probe was performed as described in Materials and Methods.
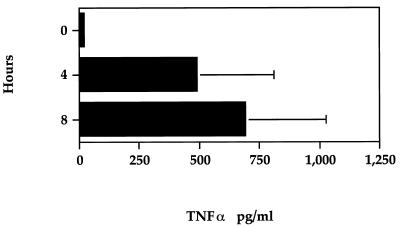
To determine the kinetics of TNF-α protein secretion from AMs, macrophage supernatants which had been aliquoted and frozen from LPS-stimulated time course experiments were assayed for bioactive TNF-α. As demonstrated in Fig. 7, 1 μg of P. haemolytica LPS per ml stimulated bovine AMs to secrete bioactive TNF-α. After 8 h of LPS stimulation, the amount of TNF-α increased to approximately 700 pg/ml.
FIG. 7.
Time-dependent secretion of extracellular TNF-α by bovine AMs stimulated with 1 μg of P. haemolytica LPS per ml for the indicated times, as assessed by a bioassay. The TNF-α concentration was calculated by using the mean absorbance of duplicate wells and is expressed in picograms per milliliter as described in Materials and Methods. The values shown represent mean ± SEM (n = 3).
Antibodies against bovine TNF-α reduced IL-8 mRNA in the second phase of expression.
To further demonstrate the role of TNF-α in the second phase of IL-8 mRNA induction, various dilutions of monospecific polyclonal antibodies against bovine TNF-α were preincubated with 1 μg of P. haemolytica LPS per ml for 30 min before macrophage stimulation. AMs were harvested at 0, 1, 4, and 24 h after stimulation. As shown by Northern blot analysis (Fig. 8), an antibody dilution of 1:1,000 significantly reduced IL-8 mRNA levels at 24 h compared to the results obtained with LPS alone. As a control, the same dilution of preimmune serum was preincubated with 1 μg of LPS per ml, and as expected, the preimmune serum did not reduce IL-8 mRNA levels at any time point.
DISCUSSION
One of the pathological hallmarks of bovine pneumonic pasteurellosis is an influx of neutrophils into the alveolar spaces. Although no definitive evidence has been presented about which factor(s) is responsible for the recruitment of neutrophils into the alveolar milieu, the influx is thought to be mediated by a locally produced chemotactic factor(s). IL-8 is a potent neutrophil chemotactic factor involved in the pathology of several inflammatory diseases including psoriasis (18) and adult respiratory distress syndrome (11). In the context of the lungs, there is compelling evidence which suggests that the AM is the major source of IL-8. Since bovine IL-8 had not been cloned at the start of this project, we designed primers based on IL-8 cDNA sequences from other species and used PCR and 3′ RACE to amplify the entire mature protein cDNA coding region. Sequence analysis revealed that this clone was identical to one used in a recently published experiment (15).
Since the AM seems to be the central cell in orchestrating the inflammatory response against P. haemolytica infection, we investigated the kinetics of IL-8 mRNA expression in P. haemolytica LPS-stimulated AMs. Our preliminary studies revealed that when bovine AMs were incubated only overnight, IL-8 mRNA levels in unstimulated cells were nearly or as high as levels in LPS-stimulated cells. We hypothesize that this level of expression was residual mRNA that had been induced during the isolation and plating of cells (21). To quantify the amount of mRNA induction in stimulated cells with respect to that in unstimulated cells, IL-8 mRNA expression needed to start at a basal level. In unstimulated cells, undetectable levels of IL-8 mRNA were consistently reached only when AMs were allowed to rest for at least 36 h.
Our in vitro experiments have shown that bovine IL-8 is abundantly expressed in AMs stimulated with P. haemolytica LPS and TNF-α. mRNA was elevated above resting levels with as little as 10 pg of LPS per ml, and maximum induction was seen at 1 ng/ml. Northern blot analysis also revealed that AMs stimulated with 1 μg of LPS per ml over a 24-h period expressed IL-8 mRNA in a biphasic expression pattern. This observation is consistent with a role for IL-8 in the initial recruitment of neutrophils into the alveolar space and in the subsequent neutrophil activation which can lead to tissue damage. Although many other laboratories have studied IL-8 mRNA expression in LPS-stimulated AMs, our findings of a biphasic expression pattern have put us in an exclusive group. Similar results have been documented only in studies of IL-8 mRNA and protein in human whole blood stimulated with LPS from E. coli (5, 7). A biphasic expression pattern for IL-8 has not been demonstrated in LPS-stimulated AMs from other species including pigs, sheep, and dogs (13). In support of our results, we have shown IL-8 mRNA levels at 0 and 4 h to be significantly different from those at 1 and 24 h (P < 0.01).
TNF-α has been implicated as a primary mediator of the inflammatory response, with a characteristic rapid burst of production initiating the release of a cascade of other mediators (6). One key function of this early mediator may be the induction of IL-8 release, which can attract neutrophils to tissue sites of inflammation (14). TNF-α has been shown to induce the expression of IL-8 in several cell types, including fibroblasts and epithelial cells, which are nonresponsive to direct LPS stimulation (2, 14). DeForge et al. have shown that a mixture of anti-TNF, anti-IL-1α, and anti-IL-1β antibodies could nearly completely ablate the secondary wave of IL-8 mRNA expression and protein secretion (5). We have demonstrated the induction of TNF-α mRNA and protein secretion from LPS-stimulated AMs and the induction of IL-8 mRNA in rhTNF-α-stimulated AMs. Using polyclonal antibodies against bovine TNF-α, we have also demonstrated a substantial decrease in the IL-8 mRNA level at 24 h. Although the initial phase of IL-8 mRNA expression was not altered by antibodies against bovine TNF-α, the levels in the prolonged, secondary phase were significantly reduced in the presence of antibody. We hypothesize that the remaining mRNA may be due to other inflammatory mediators such as IL-1α and IL-1β (5). These results provide evidence that the first phase of IL-8 mRNA expression from AMs is an LPS-mediated event whereas the second phase is due to the early, LPS-stimulated release of mediators such as TNF-α, which act in an autocrine or paracrine fashion on other AMs.
Taken together, these results suggest that inflammatory cytokines such as IL-8 may play an important role in the pathogenesis of lung injury seen in bovine pneumonic pasteurellosis. Our results also emphasize the complex network of cytokines in an inflammatory response. Because a pronounced influx of neutrophils into the alveolar spaces occurs in many pulmonary diseases including bovine pneumonic pasteurellosis, further experiments are needed to explore the direct role of IL-8 in the recruitment of neutrophils into the lung.
ACKNOWLEDGMENTS
We thank Christie Malazdrewich for her help in collecting the alveolar macrophages, and Elaina Bleifield for her assistance with the TNF-α bioassay, and Shih-Ling Hsuan for providing purified LPS from P. haemolytica.
This study was supported by USDA-NRI competitive grant 95-37204-1963 (to S.K.M.).
REFERENCES
- 1.Babiuk L A, Lawman M J P, Biol M I, Gifford G A. A seminar in bovine immunology. Western Veterinary Conference. Las Vegas, Nev: Veterinary Learning Systems Co., Inc.; 1987. Bovine respiratory disease: pathogenesis and control by interferon; pp. 12–24. [Google Scholar]
- 2.Baggiolini M, Walz A, Kunkel S L. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J Clin Invest. 1989;84:1045–1049. doi: 10.1172/JCI114265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 4.Confer A W, Panciera R J, Clinkenbeard K D, Mosier D A. Molecular aspects of virulence of Pasteurella haemolytica. Can J Vet Res. 1990;54:S48–S52. [PubMed] [Google Scholar]
- 5.DeForge L E, Kenney J S, Jones M L, Warren J S, Remick D G. Biphasic production of IL-8 in lipolysaccharide (LPS)-stimulated human whole blood. J Immunol. 1992;148:2133–2141. [PubMed] [Google Scholar]
- 6.DeForge L E, Nguyen D T, Kunkel S L, Remick D G. Regulation of the pathophysiology of tumor necrosis factor. J Lab Clin Med. 1990;116:429–438. [PubMed] [Google Scholar]
- 7.DeForge L E, Remick D G. Kinetics of TNF, IL-6, and IL-8 gene expression in LPS-stimulated human whole blood. Biochem Biophys Res Commun. 1991;174:18–24. doi: 10.1016/0006-291x(91)90478-p. [DOI] [PubMed] [Google Scholar]
- 8.Ellis J A, Godson D, Campos M, Sileghem M, Babiuk L A. Capture immunoassay for ruminant tumor necrosis factor-α: comparison with bioassay. Vet Immunol Immunopathol. 1993;35:289–300. doi: 10.1016/0165-2427(93)90040-b. [DOI] [PubMed] [Google Scholar]
- 9.Gamble J R, Harlan J M, Klebanoff S J, Vadas M A. Stimulation of the adherence of neutrophils to umbilical vein endothelium by human recombinant tumor necrosis factor. Proc Natl Acad Sci USA. 1985;82:8667–8671. doi: 10.1073/pnas.82.24.8667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hansen M B, Nielsen S E, Berg K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J Immunol Methods. 1989;119:203–210. doi: 10.1016/0022-1759(89)90397-9. [DOI] [PubMed] [Google Scholar]
- 11.Jorens P G, Van Damme J, DeBacker W, Bossaert L, DeJongh R F, Herman A G, Rampart M. Interleukin 8 (IL-8) in the bronchoalveolar lavage fluid from patients with the adult respiratory distress syndrome (ARDS) and patients at risk for ARDS. Cytokine. 1992;4:592–597. doi: 10.1016/1043-4666(92)90025-m. [DOI] [PubMed] [Google Scholar]
- 12.Larrick J W, Kunkel S L. The role of tumor necrosis factor and interleukin 1 in the immunoinflammatory response. Pharm Res. 1988;5:129–138. doi: 10.1023/a:1015904721223. [DOI] [PubMed] [Google Scholar]
- 13.Lin G, Pearson A E, Scamurra R W, Zhou Y, Baarsch M J, Weiss D J, Murtaugh M P. Regulation of interleukin-8 expression in porcine alveolar macrophages by bacterial lipopolysaccharide. J Biol Chem. 1994;269:77–85. [PubMed] [Google Scholar]
- 14.Matsushima K, Oppenheim J J. Interleukin 8 and MCAF: novel inflammatory cytokines inducible by IL-1 and TNF. Cytokine. 1989;1:2–13. doi: 10.1016/1043-4666(89)91043-0. [DOI] [PubMed] [Google Scholar]
- 15.Morsey M A, Popowych Y, Kowalski J, Gerlach G, Godson D, Campos M, Babiuk L A. Molecular cloning and expression of bovine interleukin-8. Microb Pathog. 1996;20:203–212. doi: 10.1006/mpat.1996.0019. [DOI] [PubMed] [Google Scholar]
- 16.Peveri P, Walz A, Dewald B, Baggiolini M. A novel neutrophil-activating factor produced by human mononuclear phagocytes. J Exp Med. 1988;167:1547–1559. doi: 10.1084/jem.167.5.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rehmtulla A J, Thomson R G. A review of the lesions of shipping fever of cattle. Can Vet J. 1981;22:1–8. [PMC free article] [PubMed] [Google Scholar]
- 18.Schroeder J M, Christophers E. Identification of C5ades arg and an anionic neutrophil-activating peptide (ANAP) in psoriatic scales. J Invest Dermatol. 1986;87:53–58. doi: 10.1111/1523-1747.ep12523566. [DOI] [PubMed] [Google Scholar]
- 19.Slocombe R F, Malark J, Ingersoll R, Derksen F J, Robinson N E. Importance of neutrophils in the pathogenesis of acute pneumonic pasteurellosis in calves. Am J Vet Res. 1985;46:2253–2258. [PubMed] [Google Scholar]
- 20.Standiford T J, Kunkel S L, Basha M A, Chensue S W, Lynch III J P, Toews G B, Westwick J, Strieter R M. Interleukin-8 gene expression by a pulmonary epithelial cell line: a model for cytokine networks in the lung. J Clin Invest. 1990;86:1945–1953. doi: 10.1172/JCI114928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Standiford T J, Kunkel S L, Kasahara K, Milia M J, Rolfe M W, Strieter R M. Interleukin-8 gene expression from human alveolar macrophages: the role of adherence. Am J Respir Cell Mol Biol. 1991;5:579–585. doi: 10.1165/ajrcmb/5.6.579. [DOI] [PubMed] [Google Scholar]
- 22.Strieter R M, Chensue S W, Basha M A, Standiford T J, Lynch J P, Baggiolini M, Kunkel S L. Human alveolar macrophage gene expression of interleukin-8 by tumor necrosis factor-alpha, lipopolysaccharide, and interleukin-1 beta. Am J Respir Cell Mol Biol. 1990;2:3221–3226. doi: 10.1165/ajrcmb/2.4.321. [DOI] [PubMed] [Google Scholar]
- 23.Strieter R M, Kunkel S L, Showell H J, Remick D G, Phan S H, Ward P A, Marks R M. Endothelial cell gene expression of a neutrophil chemotactic factor by TNF-α, LPS, and IL-1β. Science. 1989;243:1467–1469. doi: 10.1126/science.2648570. [DOI] [PubMed] [Google Scholar]
- 24.Strieter R M, Phan S H, Showell H J, Remick D G, Lynch J P, Genord M, Raiford C, Eskandari M, Marks R M, Kunkel S L. Monokine-induced neutrophil chemotactic factor gene expression in human fibroblasts. J Biol Chem. 1989;264:10621–10626. [PubMed] [Google Scholar]
- 25.Strieter R M, Wiggins R, Phan S H, Wharram B L, Showell H J, Remick D G, Chensue S W, Kunkel S L. Monocyte chemotactic protein gene expression by cytokine-treated human fibroblasts and endothelial cells. Biochem Biophys Res Commun. 1989;162:694–700. doi: 10.1016/0006-291x(89)92366-8. [DOI] [PubMed] [Google Scholar]
- 26.Tabor S, Richardson C C. DNA sequence analysis with a modified bacteriophage T7 polymerase. Proc Natl Acad Sci USA. 1987;84:4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thelen M, Peveri P, Keren P, Von Tscharner V, Walz A, Baggiolini M. Mechanism of neutrophil activation by NAF, a novel monocyte-derived peptide agonist. FASEB J. 1988;2:2702–2706. [PubMed] [Google Scholar]
- 28.Walker R D, Hopkins F M, Schultz T W, McCracken M D, Moore R N. Changes in leukocyte populations in pulmonary lavage fluids of calves after inhalation of Pasteurella haemolytica. Am J Vet Res. 1985;46:2429–2433. [PubMed] [Google Scholar]
- 29.Westphal O, Jann K. Bacterial lipopolysaccharide: extraction with phenol-water and further applications of the procedure. Methods Carbohydr Chem. 1965;5:83–91. [Google Scholar]
- 30.Whiteley L O, Maheswaran S K, Weiss D J, Ames T R. Alterations in pulmonary morphology and peripheral coagulation profiles caused by intratracheal inoculation of live and ultraviolet light-killed Pasteurella haemolytica A1 in calves. Vet Pathol. 1991;28:275–285. doi: 10.1177/030098589102800403. [DOI] [PubMed] [Google Scholar]
- 31.Whiteley L O, Maheswaran S K, Weiss D J, Ames T R, Kannan M S. Pasteurella haemolytica A1 and bovine respiratory disease: pathogenesis. J Vet Intern Med. 1992;6:11–22. doi: 10.1111/j.1939-1676.1992.tb00980.x. [DOI] [PubMed] [Google Scholar]
- 32.Wilke B N, Shewen P E. Defining the role that Pasteurella haemolytica plays in shipping fever. Vet Med. 1988;83:1053–1058. [Google Scholar]
- 33.Yoo H S, Maheswaran S K, Lin G, Townsend E L, Ames T R. Induction of inflammatory cytokines in bovine alveolar macrophages following stimulation with Pasteurella haemolytica lipopolysaccharide. Infect Immun. 1995;63:381–388. doi: 10.1128/iai.63.2.381-388.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]