ABSTRACT
Data on reinfection in large Asian populations are limited. In this study, we aimed to evaluate the reinfection rate, disease severity, and time interval between the infections in the symptomatic and asymptomatic populations which are firstl infected with BA.2 Omicron Variant. We retrospectively included adult patients with COVID-19 discharged from four designated hospitals between 27 April 2021 and 30 November 2022, who were interviewed via telephone from 29 January to 1 March 2023. Univariable and multivariable analyses were used to explore risk factors associated with reinfection. A total of 16,558 patients were followed up, during the telephone survey of an average of 310.0 days, 1610 (9.72%) participants self-reported reinfection. The mean time range of reinfection was 257.9 days. The risks for reinfection were analysed using multivariable logistic regression. Patients with severe first infection were at higher risk for reinfection (aORs, 2.50; P < 0.001). The male (aORs,0.82; P < 0.001), the elderly (aORs, 0.44; P < 0.001), and patients with full vaccination (aORs, 0.67; P < 0.001) or booster (aORs, 0.63; P < 0.001) had the lower risk of reinfection. Patients over 60 years of age (aORs,9.02; P = 0.006) and those with ≥2 comorbidities (aORs,11.51; P = 0.016). were at higher risk for severe reinfection. The number of clinical manifestations of reinfection increases in people with severe first infection (aORs, 2.82; P = 0.023). The overall reinfection rate was 9.72%, and the reinfection rate of Omicron-to-Omicron subvariants was 9.50% at one year. The severity of Omicron-Omicron reinfection decreased. Data from our clinical study may provide clinical evidence and bolster response preparedness for future COVID-19 reinfection waves.
KEYWORDS: SARS-CoV-2, COVID-19, Omicron variant, reinfection, follow-up study
As of 28 June 2023, there have been 760 million confirmed cases of COVID-19, including about 7 million deaths reported to the World Health Organization (WHO) globally [1]. Current research shows that the immune barrier to COVID-19 can be retained for about 6 months. However, the incubation period of COVID-19 is about 1–5 days, and the formation of memory humoral immunity is difficult to respond to in the short term, leading to the occurrence of reinfections. Currently, there are conflicting reports on the disease severity of reinfection on COVID-19. Some studies held the opinion reinfection increased risks of death, hospitalization, and sequelae in multiple organ systems, while other studies indicated the severity rate decreased in the reinfection phase [2]. Considering the ongoing global circulation of the Omicron variant, understanding the characteristics of Omicron reinfection rates in the entire population remains crucial for future public health policies. A few studies cover a comprehensive assessment of reinfection risk among asymptomatic and symptomatic infected individuals. Due to the early large-scale nucleic acid screening strategy, we can obtain relatively accurate data on secondary infections.
We conducted telephone follow-ups with the patients who were confirmed with or clinician diagnosed with initial COVID-19 from 27 April 2021 to 30 November 2022, after they were discharged from Huashan Hospital affiliated to Fudan University, Nanjing Hospital of Chinese Medicine, Wuxi No.5 People’s Hospital, and the Sixth People’s Hospital of Shenyang, and we have followed-up the cohort through telephone, which started on 17 February 2023 and ended on 6 March 2023. According to the 10th version of the national COVID-19 protocol [3], severe COVID-19 infection was defined by at least one of the following items: (a) respiratory rate ≥30, (b) finger oxygen saturation less than 93 when inhaling air at rest, (c) PaO2/FiO2 ≤ 300mmHg, and (d) progressive clinical symptoms with lung images showing >50% significant progression of the lesion in 24–48 h. Critical COVID-19 infection was defined by at least one of the following items: (a) respiratory failure and the need for mechanical ventilation, (b) shock, and (c) other organ failure requiring ICU monitoring treatment. The study was approved by the Huashan Ethics Committee (2022-721). Mann–Whitney U was used to compare the demographic data for continuous variables. Differences in proportions were evaluated using χ² tests. Logistic regression was used to estimate the odds ratios (ORs) and 95% CIs to explore risk factors associated with reinfection and severe reinfection. Logistic regression was adjusted for the following potential confounders: gender (male/female), age (<60 years/≥60 years), number of comorbidities (0/1/≥2), vaccination status (unvaccinated/partially vaccinated/ fully vaccinated/ booster), and severity of first infection (non-severe/severe).
Most of the patients in the cohort had only experienced one COVID-19 infection with the Omicron Variant before December 2022, and among which, we included asymptomatic, mild-to-moderate, and severe to critically ill patients. The reinfection events under investigation, excluding those related to the Delta variant, occurred between December 2022 and January 2023. During this period, the BA.5 lineage and its related sub-lineages were the predominant variants in China, representing over 99.5% of the infections. Therefore, the data from this cohort can objectively reflect the Omicron-to-Omicron subvariants reinfection situation among the overall population. Data from our clinical study may provide the clinical evidence to support the policy strategy for the recent Omicron reinfection outbreak in China.
A total of 24,884 patients with COVID-19 participatedand were invited to participate in our telephone follow-up, of whom 16,558 (66.54%) responded. The demographic and clinical characteristics of the 16,558 participants are shown in Supplementary Table 1. During the telephone survey of an average of 310.0 days after the first infection of SARS-CoV-2, 1610 (9.72%) participants self-reported positive RT–PCR test or antigen test. The mean time to reinfection from the first positive RT–PCR test or antigen test was 257.9 days, with the shortest reinfection time being 94 days, and the longest reinfection time being 611 days. The reinfection rate of Omicron-to-Omicron subvariants was 9.50% at one year. The time-varying curves of reinfection rates for different subgroups are shown in Supplementary Figure 1.
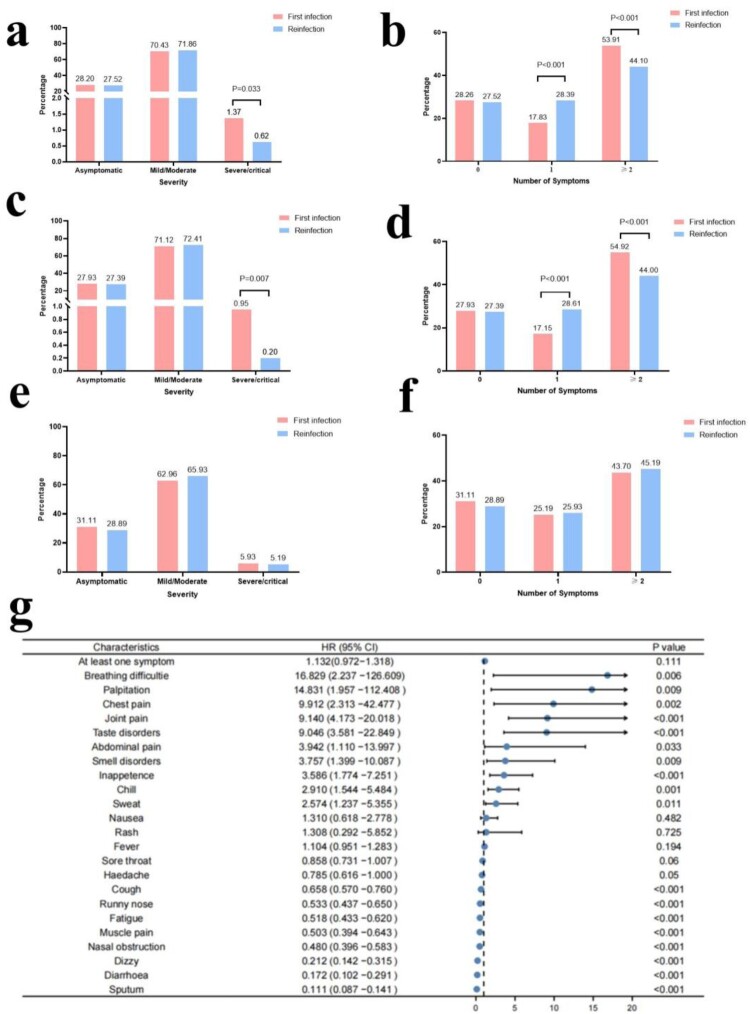
The proportion of severe-critical reinfections was lower than that of the first infection (0.62% vs. 1.37%, P = 0.033), and the proportion of reinfections with two or more symptoms was also lower than that of the first infection (44.10% vs. 53.91%, P < 0.001) (Figure 1(a,b)). The same phenomenon was observed in adults aged <60 years (Figure 1(c,d)), but in the older age group over 60 years, there was no significant difference in the number of symptoms and severity between reinfection and first infection (Figure 1(e,f)). Reinfection was associated with an increased risk of dyspnoea, palpitation, chest pain, joint pain, etc. (Figure 1(g)). The dynamics of the number of symptoms and severity of first infection and reinfection are shown in Supplementary Figure 2.
Figure 1.
Comparison of severity and number of symptoms of the first infection and reinfection. a. Comparison of severity of the first infection and reinfection in all patients. b. Comparison of the number of symptoms of the first infection and reinfection in all patients. c. Comparison of severity of the first infection and reinfection in patients aged less than 60 years. d. Comparison of the number of symptoms of the first infection and reinfection in patients aged less than 60 years. e. Comparison of severity of the first infection and reinfection in patients aged equal to or over 60 years. f. Comparison of the number of symptoms of the first infection and reinfection in patients aged equal to or over 60 years. g. Risk of clinical manifestations in people with SARS-CoV-2 first infection versus reinfection.
In our cohort, 1567 (1567/1610, 97.33%) reinfections occurred during the Omicron wave, and 43 (43/1610, 2.67%) reinfections occurred during the Delta wave (Supplementary Table 4). Although a higher proportion of Delta-infected patients went to the emergency than Omicron patients after reinfection (11.63% vs 2.36%, P < 0.001) (Supplementary Figure 3(a,b)), there was no significant difference in the admission rate of reinfection between the two groups (0% vs. 0.64%, P = 1.000) (Supplementary Figure 3(c,d)).
In multivariable logistic regression, patients with severe first infection were at higher risk for reinfection than those with non-severe first infection (aORs, 2.50; P < 0.001). The male (aORs,0.82; P < 0.001), the elderly (aORs, 0.44; P < 0.001), and patients with full vaccination (aORs, 0.67; P < 0.001) or booster (aORs, 0.63; P < 0.001) (Supplementary Table 2) had the lower risk of reinfection. We also evaluated risk factors for severe reinfections of SARS-CoV-2. We found an increased risk in patients over 60 years of age (aORs,9.02; P = 0.006) and in those with ≥2 comorbidities (aORs,11.51; P = 0.016) (Supplementary Table 3). We found an increased number of clinical manifestations in reinfections in the populations with severe first infection (aORs, 2.82; P = 0.023) (Supplementary Table 5).
Discussion
In our study, the overall reinfection rate was about 9.72%. The reinfection rate was higher than that in Alpha-Delta, but it was similar in the Omicron wave. During the Alpha-Delta wave, the incidence of suspected reinfection remained at 1%–2.7% of cases [4]. In the Omicron wave, a rapid increase was observed in the incidence of reinfection, from 2% during the week of 5–11 December to 11% during the week of 19–25 December [5]. Several factors might contribute to the high reinfection rate, including the emergence of variants capable of immune evasion and waning immunity as the interval from initial infection or vaccination increases for many persons [6,7]. Previous studies showed the effectiveness of previous infections in preventing reinfection varied. The effectiveness was 90.2% against the alpha variant, 85.7% against the beta variant, 92.0% against the delta variant, and 56.0% against the omicron variant [8].
In our study, the average interval between two infections was 257.9 days. In a previous study, the overall average time from first infection to reinfection was 334 ± 146 days [9]; however, Omicron-to-Omicron reinfection events appear in as little as 3 weeks after the initial infection, with a mean of 22 weeks. Advanced age, female sex, and underlying comorbidities appeared to be the first risk factors for reinfection. Females had a higher prevalence of recurrence [10,11]. Previous studies showed Omicron-Omicron reinfections over a shorter time interval than seen after a first infection with non-Omicron VOCs [12]. Previous studies showed protection from re-infection from ancestral, alpha, and delta variants declined over time but remained at 78.6% at 40 weeks [13]. However, protection for the Omicron variant was significantly lower and declined more rapidly over time. When it comes to the XBB variant, previous BA.1 infection and first series vaccination conferred no or minimal protection. Protection, conferred by a previous BA.2 infection against XBB reinfection, was lower within 6 months of first infection (74%,) and waned at a faster rate, decreasing to 49% at 7–8 months and 37% at 8 months [14].
Importantly, our study found the severity of SARS-CoV-2 reinfection decreased. The proportion of severe-critical reinfections was lower than that of the first infection (0.82% vs 1.37%, P = 0.033). The study of veterans showed reinfection contributed to additional risks of death (hazard ratio (HR) = 2.17) and hospitalization (HR = 3.32) compared to no reinfection. However, in our cohort, we had similar conclusions to most of the other studies, the SARS-CoV-2 reinfection risk remained substantially lower compared with the first infection [15,16]. The protection of previous infections against hospitalization or death caused by reinfection was robust [8]. One possible reason for the discrepancy is the inclusion of biased population samples, with some studies including the entire population and others focusing only on hospitalized patients. In most of the regions in the world, widespread nucleic acid testing has not been implemented since 2022, leading to the loss of information regarding asymptomatic and mild reinfections.
Our analysis has some limitations. First, it relied on patients’ self-reported information, including results from RT–PCR or antigen tests, oxygen saturation levels, and treatment records. it might be fewer patients who did not undergo antigen or oxygen saturation tests, leading to a small bias of the occurrence and severity rate of reinfections. Secondly, there might be a bias in the time interval of reinfection because most of the reinfections were recorded in the initial wave of widespread community transmission at the end of 2022, and the re-exposure risk among previously infected individuals remained artificially controlled before adjustments in epidemic prevention policies. Thirdly, there were seasonal differences between the first infection and reinfection. In our study, most first infections occurred in spring, but most reinfections occurred in winter, the seasonal effects cannot be excluded in terms of symptoms and severity of disease.
Our study showed the overall reinfection rate was 9.72%, and the reinfection rate of Omicron-to-Omicron subvariants was 9.50% at one year. The severity of Omicron-Omicron reinfection decreased among the general population. The most predictive factors for the risk of severe reinfection were age and multiple comorbidities. The male, the elderly, and patients being vaccinated had a lower risk for reinfection. The disease severity at the first episode was the risk factor for reinfection. China is recently experiencing a reinfection wave; data from our clinical study may provide clinical evidence and bolster response preparedness for future epidemics or endemics around the world.
Supplementary Material
Funding Statement
This study was funded by a project supported by the National Natural Science Foundation of China (82341033).
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.WHO . WHO Coronavirus (COVID-19) dashboard June 28 2023 [cited 2023 July 5]. Available from: https://covid19.who.int/
- 2.Bowe B, Xie Y, Al-Aly Z.. Acute and postacute sequelae associated with SARS-CoV-2 reinfection. Nat Med. 2022;28(11):2398–2405. doi: 10.1038/s41591-022-02051-3. PubMed PMID: 36357676; PubMed Central PMCID: PMCQ1. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.China NHCotPRo . The 10th version of the national COVID-19 protocol 2023 [cited 2023 October 18]. Available from: http://www.nhc.gov.cn/xcs/zhengcwj/202301/32de5b2ff9bf4eaa88e75bdf7223a65a.shtml
- 4.District SNH . Southern Nevada Health District. B.1.617.2 variant detected in Clark County 2023 [cited 2023 July 5]. Available from: https://www.southernnevadahealthdistrict.org/news-release/b-1-617-2-variant-detected-in-clark-county/
- 5.District SNH . Southern Nevada Health District reports first Omicron case in Clark County resident 2021 [cited 2023 July 5]. Available from: https://www.southernnevadahealthdistrict.org/news-release/southern-nevada-health-district-reports-first-omicron-case-in-clark-county-resident
- 6.Hall V, Foulkes S, Insalata F, et al. Protection against SARS-CoV-2 after Covid-19 vaccination and previous infection. N Engl J Med. 2022;386(13):1207–1220. doi: 10.1056/NEJMoa2118691. PubMed PMID: 35172051; PubMed Central PMCID: PMCQ1. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(US) CfDCaP . Science brief: SARS-CoV-2 infection-induced and vaccine-induced immunity 2021 [cited 2023 July 5]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK575088/
- Altarawneh HN, Chemaitelly H, Hasan MR, et al. Protection against the Omicron variant from previous SARS-CoV-2 infection. N Engl J Med. 2022;386(13):1288–1290. doi: 10.1056/NEJMc2200133. PubMed PMID: 35139269; PubMed Central PMCID: PMCQ1. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nguyen NN, Houhamdi L, Hoang VT, et al. High rate of reinfection with the SARS-CoV-2 Omicron variant. J Infect. 2022;85(2):174–211. doi: 10.1016/j.jinf.2022.04.034. PubMed PMID: 35472367; PubMed Central PMCID: PMCQ1. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mensah AA, Lacy J, Stowe J, et al. Disease severity during SARS-COV-2 reinfection: a nationwide study. J Infect. 2022;84(4):542–550. doi: 10.1016/j.jinf.2022.01.012. PubMed PMID: 35085659; PubMed Central PMCID: PMCQ1. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sotoodeh Ghorbani S, Taherpour N, Bayat S, et al. Epidemiologic characteristics of cases with reinfection, recurrence, and hospital readmission due to COVID-19: a systematic review and meta-analysis. J Med Virol. 2022;94(1):44–53. doi: 10.1002/jmv.27281. PubMed PMID: 34411311; PubMed Central PMCID: PMCQ1. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burkholz S, Rubsamen M, Blankenberg L, et al. Analysis of well-annotated next-generation sequencing data reveals increasing cases of SARS-CoV-2 reinfection with Omicron. Commun Biol. 2023;6(1):288. doi: 10.1038/s42003-023-04687-4. PubMed PMID: 36934204; PubMed Central PMCID: PMCQ1. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.COVID-19 Forecasting Team . Past SARS-CoV-2 infection protection against re-infection: a systematic review and meta-analysis. Lancet. 2023;401(10379):833–842. doi: 10.1016/S0140-6736(22)02465-5. PubMed PMID: 36930674; PubMed Central PMCID: PMCQ1. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan CY, Chiew CJ, Pang D, et al. Protective immunity of SARS-CoV-2 infection and vaccines against medically attended symptomatic omicron BA.4, BA.5, and XBB reinfections in Singapore: a national cohort study. Lancet Infect Dis. 2023;23(7):799–805. doi: 10.1016/S1473-3099(23)00060-9. PubMed PMID: 36924786; PubMed Central PMCID: PMCQ1. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sacco C, Petrone D, Del Manso M, et al. Risk and protective factors for SARS-CoV-2 reinfections, surveillance data, Italy, August 2021 to March 2022. Euro Surveill. 2022;27(20). doi: 10.2807/1560-7917.ES.2022.27.20.2200372. PubMed PMID: 35593164; PubMed Central PMCID: PMCQ1. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Medic S, Anastassopoulou C, Lozanov-Crvenkovic Z, et al. Incidence, risk, and severity of SARS-CoV-2 reinfections in children and adolescents between March 2020 and July 2022 in Serbia. JAMA Netw Open. 2023;6(2):e2255779. doi: 10.1001/jamanetworkopen.2022.55779. PubMed PMID: 36780157; PubMed Central PMCID: PMCQ1. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.