Abstract
Background
Glaucoma is the term for a group of eye disorders that causes progressive damage to the optic nerve, which can lead to visual impairment and, potentially, irreversible blindness. Minimally invasive bleb surgery (MIBS) reduces eye pressure through the implantation of a device that creates a new subconjunctival outflow pathway for eye fluid drainage. MIBS is a less invasive alternative to conventional/incisional glaucoma surgery (e.g., trabeculectomy). We conducted a health technology assessment of MIBS for people with glaucoma, which included an evaluation of effectiveness, safety, the budget impact of publicly funding MIBS, and patient preferences and values.
Methods
We performed a systematic literature search of the clinical evidence. We assessed the risk of bias of each included study using the Cochrane Risk of Bias 1.0 tool for randomized controlled trials (RCTs) and the Risk of Bias Assessment tool for Nonrandomized Studies (RoBANS) for comparative observational studies, and the quality of the body of evidence according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Working Group criteria. We conducted an economic literature search and we estimated the budget impact of publicly funding MIBS in Ontario. We did not conduct a primary economic evaluation due to the limited long-term effectiveness data. We summarized the preferences and values evidence from previous health technology assessments to understand the perspectives and experiences of patients with glaucoma.
Results
We included 41 studies (2 RCTs and 39 comparative observational studies) in the clinical evidence review. MIBS may reduce intraocular pressure and the number of medications used, but we are uncertain if MIBS results in outcomes similar to trabeculectomy (GRADE: Moderate to Very low). Compared with trabeculectomy, MIBS may result in fewer follow-up visits and interventions, and adverse events (GRADE: Moderate to Very Low). MIBS may also reduce intraocular pressure and the number of antiglaucoma medications used, compared with other glaucoma treatments, but the evidence is uncertain (GRADE: Very low). Our economic evidence review identified two directly applicable studies. The results of these studies indicate that the cost-effectiveness of MIBS is highly uncertain, and the cost of glaucoma interventions are likely to vary across provinces. The annual budget impact of publicly funding MIBS in Ontario ranged from $0.11 million in year 1 to $0.67 million in year 5, for a total 5-year budget impact estimate of $1.93 million. Preferences and values evidence suggests that fear of ultimate blindness and difficulty managing medication for glaucoma led patients to explore other treatment options such as MIBS. Glaucoma patients found minimally invasive glaucoma surgery (MIGS) procedure beneficial, with minimal side effects and recovery time.
Conclusions
Minimally invasive bleb surgery reduces intraocular eye pressure and the number of antiglaucoma medications needed, but we are uncertain if the outcomes are similar to trabeculectomy (GRADE: Moderate to Very low). However, MIBS may be safer than trabeculectomy (GRADE: Moderate to Very low) and result in fewer follow-ups (GRADE: Moderate to Very low). MIBS may also improve glaucoma symptoms compared with other glaucoma treatments, but the evidence is very uncertain (GRADE: Very low).
We estimate that publicly funding MIBS would result in an additional cost of $1.93 million over 5 years. Patients who underwent MIGS procedures found them to be generally successful and beneficial, with minimal side effects and recovery time. We could not draw conclusions about specific MIBS procedures or long-term outcomes.
Objective
This health technology assessment evaluates the effectiveness, safety, and cost-effectiveness of minimally invasive bleb surgery (MIBS) for people with glaucoma. It also evaluates the budget impact of publicly funding MIBS and the experiences, preferences, and values of people with glaucoma.
Background
Health Condition
Glaucoma is a group of eye disorders that cause progressive damage to the optic nerve, which can lead to visual impairment and, potentially, irreversible blindness. Most cases of glaucoma involve the accumulation of aqueous humour (fluid in the eye) in the anterior chamber (front part of the eye) due to poor drainage, which builds pressure in the eye (intraocular pressure, IOP), gradually damaging the optic nerve. About 90% of the time, glaucoma starts by affecting the loss of peripheral (side) vision. Other symptoms include decreased contrast sensitivity, seeing halos around lights, blurred or hazy vision, eye redness, and severe headache or eye pain. The symptoms of glaucoma are often not apparent until irreversible damage to the optic nerve has occurred. As such, glaucoma is sometimes referred to as the “sneak” or “silent thief of sight.”1 Risk factors for glaucoma include elevated IOP, increasing age, family history, race or ethnicity (e.g., African, Hispanic, Southeast Asian), thinner cornea (which may delay diagnosis by causing IOP-measuring devices to produce a false low estimation of the IOP), and myopia (nearsightedness).2
Aqueous humour is a transparent fluid inside the eye that is produced by the ciliary body (part of the middle layer of the wall of the eye). The main functions of the aqueous humour are to maintain IOP, provide nutrients and oxygen to the eye, remove metabolic byproducts from the eye, and facilitate the passage of light to the retina. Aqueous humour leaves the eye by passive flow via two pathways: the trabecular (conventional) pathway and the uveoscleral pathway. About 85% of fluid drainage occurs through the trabecular meshwork (spongy tissue located in the anterior chamber angle of the eye), which is drained through a structure known as Schlemm's canal.3 The fluid then joins the venous blood system and returns to the heart.
Glaucoma may be categorized as primary or secondary, and open- or closed-angle. Primary glaucoma is due to unknown causes (idiopathic) and may also be referred to as chronic glaucoma. Glaucoma due to identifiable underlying causes (e.g., injury to the eye, inflammation in the eye, certain medications, advanced cases of cataracts or diabetes, etc.) is categorized as secondary glaucoma. Open-angle (or wide-angle) glaucoma occurs when the trabecular meshwork and Schemm's canal are anatomically open but do not allow for optimal drainage. Closed-angle (or angle-closure, narrow-angle) glaucoma occurs when the coloured part of the eye (the iris) is positioned against the trabecular meshwork and blocks the flow of fluid out of the eye. The most common type of glaucoma is primary open-angle glaucoma, which accounts for about 90% of glaucoma cases in Canada.4
Glaucoma is diagnosed through a comprehensive eye exam, which must include measuring the IOP (tonometry), a dilated eye exam to examine the shape and colour of the optic nerve (ophthalmoscopy), a field of vision test (perimetry), measuring corneal thickness (pachymetry), and inspecting the drainage angle (gonioscopy). The Ontario Health Insurance Plan (OHIP) diagnostic code for any type of glaucoma is 365. There are no separate codes for specific types of glaucoma. In addition, in Canada, there is no diagnostic code to reflect glaucoma severity or progression.
The most common staging system used by published researchers for glaucoma is the Hoddap-Parrish-Anderson (HPA) criteria, which is described in Table 1. The HPA classification system considers two criteria: the overall extent of damage (using both the mean deviation value and the number of defective points in the Humphrey Statpac-2 pattern deviation probability map) and the proximity of defect(s) to fixation (fixation target). The mean deviation (also referred to as the mean defect, or MD) gives an overall value of the total amount of visual field loss, with normal values typically within 0 to -2 decibels (dB). A dB is the logarithmic representation of the intensity of the light stimulus and has a direct correlation to the sensitivity of the retina. Zero dB represents the brightest light stimulus; higher dB values correspond to dimmer stimuli. However, there are some disadvantages to the HPA classification system: the visual field defect is characterized into four relatively coarse stages, accurate and time-consuming analysis of every visual field test result is required (reducing its day-to-day clinical usefulness), and there is no information about the location and depth of the defect(s).
Table 1:
Hoddap-Parrish-Anderson Criteria for Glaucoma
| Classification of defect | Criteria |
|---|---|
| Early | Mean deviation < -6 dB |
| On the pattern deviation plot, < 25% of the points depressed below the 5% level and < 15% points depressed below the 1% level | |
| No point within central 5° with sensitivity < 15 dB | |
| Moderate | Mean deviation < -12 dB |
| On pattern deviation plot, < 50% points depressed below the 5% level and < 25% points depressed below the 1% level | |
| No point within central 5° with sensitivity < 0 dB | |
| Only 1 hemifield containing a point sensitivity < 15 dB within 5° of fixation | |
| Severe (e.g., advanced, end-stage) | Mean deviation > -12 dB |
| On pattern deviation plot, > 50% points depressed below the 5% level or > 25% points depressed below the 1% level | |
| Any point within central 5° with sensitivity < 0 dB | |
| Both hemifields containing point(s) with sensitivity < 15 dB within 5° of fixation |
Abbreviation: dB, decibel.
The International Classification of Diseases (ICD) staging may be used when discussing the suitability of glaucoma treatment devices. The ICD is used in the United States and Europe based on government and private insurance systems.
Clinical Need and Population of Interest
Glaucoma is the leading cause of irreversible blindness in the world.5 Glaucoma affects more than 400,000 Canadians. More than 250,000 Canadians have primary open-angle glaucoma, which is the most common form of glaucoma.4 It was estimated that 290,000 people in Ontario had glaucoma in 2019, with the number increasing to 323,000 in 2023.6
Current Treatment Options
Glaucoma treatments aim to reduce the IOP, which is the only modifiable risk factor. Table 2 describes the different types of treatment through the glaucoma continuum of care (approximately from least to most invasive).
Table 2:
Glaucoma Treatments
| Treatment name | Mechanism of action | Examples |
|---|---|---|
| Eye drops | Increase trabecular or uveoscleral outflow | Prostaglandin analogues, rho kinase inhibitors, nitric oxides, miotic or cholinergic agents |
| Decrease aqueous humour production | Alpha-adrenergic agonists, beta blocks, carbonic anhydrase inhibitors | |
| Oral medications | Decrease aqueous production | Carbonic anhydrase inhibitor |
| Laser treatment | Increase trabecular outflow | Selective laser trabeculoplasty, argon laser trabeculoplasty (has been replaced by selective laser trabeculoplasty due to risk/safety) |
| Decrease aqueous production | Cyclophotocoagulation, cycloablation, transscleral cyclodiode therapy, micropulse cyclo therapy | |
| Alleviate pupil block | Peripheral iridotomy | |
| Minimally (micro) invasive glaucoma surgery | Increase trabecular outflow | Tissue ablation/removal: Trabectome, Kahook Dual Blade Device: iStent, iStent Inject, Hydrus Microstent 360° suture: gonioscopy-assisted transluminal trabeculotomy, ab interno canaloplasty |
| Increase uveoscleral outflow | CyPass Micro-Stent (withdrawn from the global market in 20187) | |
| Minimally invasive bleb surgery | Create new subconjunctival outflow pathway | XEN Gel Stent, PreserFlo MicroShunt |
| Conventional or incisional glaucoma surgery | Penetrating glaucoma surgery (filtration surgery) | Trabeculectomy, device-modified trabeculectomy (Ex-PRESS Filtration Device) |
| Non-penetrating glaucoma surgery | Deep sclerectomy, canaloplasty, viscocanalostomy | |
| Drainage implant (also called an aqueous shunt, tube shunt, seton, or glaucoma drainage device) surgery | Ahmed Valve, Baerveldt Implant, Ahmed ClearPath, Molteno3 Glaucoma Drainage Device |
Glaucoma treatment is a compromise between reducing the risk of symptomatic vision loss and the consequences of therapy to maintain a person's quality of life. The treatment goal is typically a specific IOP level or a percentage reduction in IOP. There is no single target IOP level that is appropriate for every person and the target IOP needs to be estimated for each eye. The factors considered when setting the target IOP may include glaucoma stage, pre-existing glaucoma damage, the person's age and life expectancy, untreated IOP level, the rate of progression during follow-up, the adverse consequences of intervention, patient preference, family history, and the status of the other eye.
Glaucoma treatment often starts with prescription eye drops, which decreases IOP either by increasing aqueous humour outflow or by decreasing aqueous humour production. People may be prescribed multiple eye drops or they may need to use artificial tears (eye drops used to lubricate dry eyes and help maintain moisture on the outer surface of the eye). Oral medications may be prescribed if eye drops alone cannot control IOP, but they are often used as a last resort due to systemic side effects such as hypokalemia (low blood potassium levels).
Other treatments, such as laser therapy and minimally invasive glaucoma surgery (MIGS) may be tried for early to moderate glaucoma. Laser therapy involves the use of a very focused light beam to improve fluid drainage or reduce aqueous production. Selective laser trabeculoplasty is now used earlier and more often before exhausting all possible combinations of eye drops due to its improved compliance, fewer side effects, and improved preservation of the ocular surface. Minimally invasive glaucoma surgery describes a range of implants, devices, and techniques that use tiny incisions designed to provide a safer and less invasive approach compared with conventional glaucoma surgery.
Surgery may be indicated for people who continue to show progressive vision loss despite maximal medical therapy, are intolerant of glaucoma medications, or have difficulty adhering to medical treatment plans. For moderate to severe glaucoma, minimally invasive bleb surgery (MIBS) or conventional or incisional glaucoma surgery may be performed. Conventional glaucoma surgery can be further classified as either penetrating or non-penetrating (i.e., whether the surgery involves penetration of the anterior chamber of the eye) or if a (conventional) drainage implant is involved.
Glaucoma treatments that are currently in development include injectable, dissolvable pellets of medications that sit in the anterior chamber of the eye, and intraocular implantable medications.
Health Technology Under Review
Minimally invasive bleb surgery aims to reduce IOP through the creation of a new subconjunctival outflow pathway. Devices are implanted in the eye and create a small channel for aqueous humour drainage via the subconjunctival space. The procedure forms a blister-like fluid collection (known as a bleb) at the surface of the eye that allows drainage.
These devices are used primarily to treat moderate to severe glaucoma and refractory glaucoma, unlike MIGS, which is primarily for early to moderate glaucoma. They provide an alternative option for people who are higher risk or are poor candidates for or do not wish to undergo more invasive conventional glaucoma surgery (in particular, trabeculectomy, and possibly also glaucoma drainage implant surgery). They also have the potential to delay or replace conventional glaucoma surgery and may reduce the use of eye drops.
Depending on the device, they may be inserted using an ab interno (inside the eye) or ab externo (outside the eye) technique. The devices are often combined with mitomycin C (MMC), an antimetabolite used to prevent postoperative bleb fibrosis (scarring). Bleb fibrosis is the most common cause of surgical failure and may require postoperative bleb needling (using a fine needle to break down scar tissue) to restore drainage.
Compared with conventional glaucoma surgery, the minimally invasive nature of these devices allows for shorter procedure time, fewer complications, faster recovery, and fewer follow-up visits, but at a higher device cost.8,9 Minimally invasive bleb surgeries may be performed as an outpatient procedure or in small operating rooms or private clinics. The implantation procedure takes about 30-60 minutes and is performed by a glaucoma specialist using local neuroleptanalgesia (combination of an analgesic and sedative or tranquilizer). In addition, these devices may be implanted as a standalone procedure or in combination with cataract surgery. Conventional glaucoma surgery is also an outpatient surgery, but may be performed under local or general anesthesia and typically takes about 45-60 minutes. Conventional glaucoma surgery may also be performed in combination with cataract surgery.
Possible adverse events and complications of MIBS include choroidal effusion (abnormal accumulation of fluid in suprachoroidal space), hypotony (low IOP), hyphema (bleeding in the eye), implant migration or exposure, wound leak, endophthalmitis (infection of the interior cavity of the eye), the need for secondary surgical intervention, and intraocular surgery complications.8,9 Most procedure complications are usually transient and self-resolving, but may be managed with medical attention.
Currently, only two MIBS devices are available worldwide: the XEN Gel Stent (AbbVie Corp., previously from AqueSys and Allergan) and the PreserFlo MicroShunt (Santen Pharmaceutical Co., previously from InnFocus, Inc.). Each device is described in more detail below.
XEN Gel Stent
The XEN Gel Stent was developed with the aim of improving the predictability and safety profile of conventional bleb-forming glaucoma surgeries.10 It is a flexible and permanent collagen implant (a hydrophilic tube composed of a porcine gel cross-linked with glutaraldehyde) that drains aqueous fluid from the anterior chamber of the eye to the subconjunctival space through a scleral channel. The stent is designed to create resistance to outflow of around 6-8 mmHg under conditions of physiologically normal aqueous production (2-2.5 mL/min) without the need for a valve.10 Implantation is performed using a sterilized, single-hand inserter containing a needle that is preloaded with one gel stent.
Three designs were created—the XEN 45, 63, and 140. They are all 6 mm in length with an external diameter of 150 µm, but they differ in the inner diameter of their lumen (45, 63, and 140 µm, respectively). However, the XEN 140 is no longer available. The XEN 45 Gel Stent is the primary commercially available version, but there has been recent commercialization and use of the XEN 63. The XEN 45 and XEN 63 Gel Stents have the same population of interest and now use the same stent injector (previously the XEN 63 used a different injector). The larger lumen size of the XEN 63 Gel Stent allows for increased flow and a potential for greater IOP reductions, compared with the XEN 45.11 With the XEN 63, there is also an increased incidence of postoperative hypotony for a prolonged period of time, during which choroidal effusions and choroidal hemorrhages may occur.
The XEN Gel Stent was the first device to create a subconjunctival space for aqueous outflow drainage. It was originally developed to be implanted through an ab interno technique; however, new surgical techniques have been developed so that now it may also be implanted ab externo and opening the conjunctiva.12,13 The ab externo technique is more invasive but eliminates the need for corneal incisions and allows for complete control over the final positioning of the stent (and removal of the stent for a second placement if necessary), compared with the original ab interno technique. In addition, postoperative bleb needling is also less common with the ab externo technique because MMC can be applied directly to the sclera.
Contraindications include closed-angle glaucoma, where angle has not been surgically opened, previous glaucoma shunt/valve or conjunctival scarring/pathologies in the target quadrant, active inflammation, active iris neovascularization (formation of new blood vessels), anterior chamber intraocular lens, intraocular silicone oil, and vitreous (clear gel-like fluid in the eye) in the anterior chamber.8
PreserFlo MicroShunt
The PreserFlo MicroShunt (formerly known as the InnFocus MicroShunt, DE-128 MicroShunt, MIDI Tube/Ray/Arrow) is 8.5 mm in length and divided by a 1-mm “fin” into distal (3 mm) and proximal (4.5 mm) segments, to prevent migration of the device into the anterior chamber.9 The external lumen of the PreserFlo MicroShunt is 350 μm and the internal lumen is 70 µm with a bevelled tip at the proximal end.9 It is composed of poly(styrene-block-isobutylene-block-styrene; SIBS), which is biocompatible and bioinert. The PreserFlo MicroShunt is implanted ab externo with opening the conjunctiva and a bleb is produced under the conjunctiva and Tenon's capsule. The lumen size and possible decrease in IOP of the PreserFlo MicroShunt is more comparable to XEN 63 than XEN 45.
Contraindications include bacterial conjunctivitis, bacterial corneal ulcers, endophthalmitis (infection of the tissues or fluids inside the eye), orbital cellulitis (infection of the soft tissues of the eye socket), bacteremia (presence of bacteria in the bloodstream) or septicemia (serious bloodstream infection), active scleritis (inflammation in the episcleral and scleral tissue), uveitis (inflammation inside the eye), severe dry eye, severe blepharitis (inflammation of the eyelids), pre-existing ocular or systemic pathology that is likely to cause postoperative complications (e.g., severe myopia and thin conjunctiva), closed-angle glaucoma, shallow anterior chamber, inability of the patient to adhere to postoperative visits and/or medications, and/or intolerance or allergy to MMC.9
Regulatory Information
Both the XEN Gel Stent and PreserFlo MicroShunt have been approved by Health Canada as class III medical devices. Table 3 describes their regulatory status in Canada, the United States, Europe, and Australia. All three types of the XEN Gel Stent (45, 63, and 140) have Health Canada approval, however the XEN 63 Gel Stent has not yet been approved in some other countries. The PreserFlo MicroShunt was granted Health Canada approval in March 2021 and launched commercially in November 2021, but was used in Canada as early as 2015 at some specific sites under investigative and special access (Iqbal Ahmed, MD, email communication, February 11, 2023).
Table 3:
Regulatory Status Information for the XEN Gel Stent and PreserFlo MicroShunt
| Device name | Manufacturer | HC license No. & date issued | HC approved indication | US, European, and Australian regulatory status |
|---|---|---|---|---|
| XEN Gel Stent | AbbVie Corp. (previously Allergan and AqueSys) | 94691 Feb 17, 2015 |
Reduce IOP in people with open angle glaucoma where previous medical treatments have failed (XEN 45, 63, and 140) | FDA approval, 2016 (XEN 45) CE mark, 2013 (XEN 45) ARTG, 2017(XEN 45) |
| PreserFlo MicroShunt | Santen (previously InnFocus) Glaukos holds exclusive commercialization rights in Canada |
105971 Mar 29, 2021 |
Reduce IOP in people with primary open-angle glaucoma with uncontrollable IOP despite maximum tolerated medical therapy, or where surgery is warranted | Pending FDA approval (submitted Jun 2020) CE mark, 2012 ARTG,2021 |
Abbreviations: ARTG, Australian Register of Therapeutic Goods; CE, Conformité Européenne; FDA, US Food and Drug Administration; HC, Health Canada; IOP, intraocular pressure.
Ontario, Canadian, and International Context
In 2019, Ontario Health, in collaboration with the Canada's Drug and Health Technology Agency (CADTH), completed a health technology assessment evaluating MIGS,14 and re-evaluated iStent (a type of MIGS) for glaucoma in 2021.15 Minimally invasive bleb surgery was included as a type of MIGS in the 2019 health technology assessment (HTA) and limited evidence was found at the time. Ontario Health and CADTH found uncertainty about the comparative effectiveness of MIGS versus pharmacotherapy, laser, or filtration surgery, as well as MIGS combined with cataract surgery versus conventional glaucoma surgery combined with cataract surgery.14 As a result, Ontario Health recommended against publicly funding MIGS.6
In 2021, Ontario Health found that the iStent, used in combination with cataract surgery, may improve IOP and reduce the number of eye drop medications needed.15 However, they also found uncertainty about the effectiveness of iStent when used alone compared with treatments such eye drops or filtration surgery. As a result, Ontario Health recommended publicly funding iStent in combination with cataract surgery for adults with mild to moderate glaucoma that cannot be well controlled with pressure-lowering medications.16
Minimally invasive bleb surgery is publicly funded in Ontario; device and associated costs are covered by hospital global budgets. However, not all hospitals fund MIBS as part of their global budget, resulting in considerable variability of access and inequitable treatment across the province. A review of the evidence to support a public funding recommendation for MIBS may improve allocation of funding and reduce the current inequity.
Despite the approval and use of MIGS for over a decade (and later also MIBS), there is currently no specific physician fee code for MIGS or MIBS in Ontario. There is also no official guidance on which code to use for these procedures. Glaucoma specialists who perform MIBS use a surrogate billing code for MIGS (E132: glaucoma-filtering procedures) along with the code for implantation of a drainage device (E136: with intraocular implant of seton) (I. Ahmed, MD, email communication, February 11, 2023; D. Jinapriya, MD, email communication, March 2, 2023).17
About 892 XEN Gel Stents were implanted in Ontario in 2022 and almost all were within large urban hospitals (AbbVie Corp., email communications, December 6, 2022). XEN 45 is currently used in Ontario, and more recently also XEN 63 (XEN 140 is not available). The cost of the XEN 45 and 63 stents is about $1,200 CAD.
The recent commercialization of XEN 63 may lead to its increased use in Ontario. XEN 63 may provide greater reductions in IOP with similar safety compared with the XEN 45 for the same device cost (David Yan, MD, email communication, March 31, 2023). The majority of XEN Gel Stents in Canada are used in Ontario and Quebec (about 44% and 22%, respectively), followed by British Columbia and Saskatchewan (about 12% and 8%, respectively; AbbVie Corp., email communication December 6, 2022).
About 450 PreserFlo MicroShunts were implanted in Ontario in 2022, and all were within large urban hospitals. In Canadian provinces outside Ontario, the majority of PreserFlo MicroShunts are used in British Columbia and Quebec (about 500 and 450, respectively, in 2022). In total, about 1,800 PreserFlo MicroShunts were implanted in Canada in 2022. The cost of the PreserFlo MicroShunt in Canada is about $1,400 CAD. Device number and cost estimates were provided by Glaukos Canada (email communication, February 2, 2023). In addition to Canada, Glaukos holds the exclusive commercialization rights to the PreserFlo MicroShunt in jurisdictions such as the United States, Australia, New Zealand, and Latin America.
Access to MIBS devices is limited by funding and procedure availability, despite clinical need. Use of MIBS is anticipated to increase over time and, under ideal use conditions, MIBS devices may replace 50% to 90% of trabeculectomies in Ontario (D. Jinapriya, MD, email communication, March 2, 2023; D. Yan, MD, email communication, March 31, 2023). The Canadian Ophthalmological Society's clinical practice guideline on the management of glaucoma in the adult eye was published in 2009 and does not include any mention of MIBS (currently, no update is anticipated).18
Within Canada, there is inconsistency in MIGS reimbursement and inclusion in the physician schedule of benefits.14 For example, fee codes for MIGS exist in Alberta and Quebec, but not in Ontario or Manitoba.14 The National Institute of Excellence in Health and Social Services (Institut national d'excellence en santé et en services sociaux, or INESSS) in Quebec evaluated XEN 45 in 2020 and found that public coverage of XEN 45 may represent a fair and reasonable option if measures are taken to mitigate the economic burden.19 It was also noted that, given the uncertainties in the effectiveness of XEN 45 over the long term, it should be reassessed in light of new available data (INESSS recommended a 3-year time frame). However, an update is not planned at this time.
In 2021, the Centre for Clinical Epidemiology and Evaluation in British Columbia published an HTA on MIGS that included XEN.20 At the time of publication, the PreserFlo MicroShunt was not yet available in the province. The HTA included and summarized previously published Canadian evaluations on MIGS (Ontario Health and CADTH MIGS HTA,14 Ontario Health MIGS budget impact analysis and patient preferences evaluation6, and INESSS iStent HTA19,21). They found limited evidence for the clinical and cost effectiveness of MIGS for open-angle glaucoma20 and, based on their results, the Health Technology Assessment Committee did not recommend expanding the use of MIGS in British Columbia.22 Their recommendation also noted that ongoing use of MIGS in British Columbia should be monitored to inform future analyses, and recommended reviewing MIGS again when compelling new evidence of its clinical benefits becomes available.
Minimally invasive bleb surgery devices are available and used internationally, including the United States, Europe, and Australia. The XEN Gel Stent is covered in the United States by Medicare and some commercial payers.23 The PreserFlo MicroShunt currently does not have FDA approval and is not available in the United States. According to the American Academy of Ophthalmology guidelines on primary open-angle glaucoma (2020),24 selection of the XEN Gel Stent should be left to the discretion of the treating ophthalmologist in consultation with the individual patient (discretionary recommendation, insufficient quality evidence). The guidelines did not mention the PreserFlo MicroShunt.
The National Institute for Health and Care Excellence (NICE) in the United Kingdom reviewed the XEN Gel Stent in 2018 and found that evidence on the safety and efficacy of the XEN Gel Stent for primary open-angle glaucoma was limited in quantity and quality.25 They recommended that the procedure be used only with special arrangements for clinical governance, consent, and audit or research. Further research, including randomized controlled trials (RCTs), was encouraged, as well as details on patient selection and long-term outcomes.
The European Glaucoma Society's 2020 glaucoma guidelines noted that there is insufficient evidence for superiority or equivalence in efficacy between any MIGS procedures versus trabeculectomy (there was no specific mention of the XEN Gel Stent or PreserFlo MicroShunt).26 Similarly, the Asia-Pacific Glaucoma Society's glaucoma guidelines from 2016 noted that further studies are required to establish the long-term effectiveness of MIGS devices (which included the XEN Gel Stent).27 Appendix 1 summarizes clinical guideline recommendations for MIBS.
Equity Context
Studies have found that Black patients are more affected by glaucoma and suffer from more advanced disease.28 Diagnostic challenges include lower rates of diagnostic testing and thinner average central corneal thickness, which affects IOP measurement.28 Treatment challenges described in the literature include poor follow-up, medication adherence, and trust in health care providers.28 Black people undergoing trabeculectomy have also been found to have higher rates of failure compared with white people.28
There is currently varying geographic access to MIBS within Ontario, with access primarily available at large, urban, academic hospitals. In addition, there is a limited number of glaucoma specialists in the province of Ontario who perform glaucoma surgeries. Increased public funding of these devices would improve patient access and reduce glaucoma surgery wait times.
Systematic Reviews
Multiple systematic reviews have been conducted on MIBS in recent years (see Appendix 2). These systematic reviews differed slightly in their interventions (e.g., only included XEN Gel Stent or PreserFlo MicroShunt), study design (e.g., only RCTs, inclusion of noncomparative studies), comparators, outcomes of interest (e.g., focus on specific outcomes), method of analysis, and study eligibility criteria compared with our research question. We used these reviews as a reference source for relevant studies that may meet our inclusion criteria.
Expert Consultation
We engaged with experts in the specialty areas of ophthalmology and glaucoma to help inform our understanding of aspects of the health technology and our methodologies and to contextualize the evidence.
PROSPERO Registration
This health technology assessment has been registered in PROSPERO, the international prospective register of systematic reviews (CRD42023409090), available at crd.york.ac.uk/PROSPERO.
Clinical Evidence
Research Question
What are the effectiveness and safety of minimally invasive bleb surgery (MIBS) compared with other treatment alternatives for people with glaucoma?
Methods
Clinical Literature Search
We performed a clinical literature search on March 6, 2023, to retrieve studies published from database inception until the search date. We used the Ovid interface in the following databases: MEDLINE, Embase, the Cochrane Central Register of Controlled Trials, the Cochrane Database of Systematic Reviews, and the National Health Service Economic Evaluation Database (NHS EED).
A medical librarian developed the search strategies using controlled vocabulary (e.g., Medical Subject Headings) and relevant keywords. The final search strategy was peer-reviewed using the PRESS Checklist.29
We created database auto-alerts in MEDLINE and Embase and monitored them until June 12, 2023. We also performed a targeted grey literature search of the International HTA Database, the websites of health technology assessment organizations and regulatory agencies, and clinical trial and systematic review registries, following a standard list of sites developed internally. See Appendix 3 for our literature search strategies, including all search terms.
Eligibility Criteria
Studies
Inclusion Criteria
English-language full-text publications
Studies published from database inception until March 6, 2023
Randomized controlled trials (RCTs), comparative observational studies, systematic reviews, and meta-analyses
Exclusion Criteria
Animal and in vitro studies
Nonsystematic reviews, narrative reviews, abstracts, editorials, letters, case reports, and commentaries
Studies where outcomes of interest cannot be extracted
Participants
Inclusion Criteria
Adults (≥ 18 years old) with any type of glaucoma and of any cataract status
Exclusion Criteria
People with only increased IOP, or who otherwise have not been diagnosed with glaucoma
Interventions
Inclusion Criteria
XEN Gel Stent (XEN 45, 63, or 140) or PreserFlo MicroShunt, with or without concomitant cataract surgery
Exclusion Criteria
Minimally invasive glaucoma surgery (MIGS) that does not create a subconjunctival outflow pathway
Conventional or incisional glaucoma surgery: penetrating and non-penetrating glaucoma surgeries, including device-modified trabeculectomy (e.g., Ex-PRESS Glaucoma Filtration Device); glaucoma drainage implant surgery using conventional glaucoma drainage devices (e.g., Ahmed Valve, Baerveldt Implant)
Comparator
Inclusion Criteria
-
Conventional or incisional glaucoma surgery
-
—
Penetrating and non-penetrating glaucoma surgeries, including device-modified trabeculectomy
-
—
Glaucoma drainage implant surgery using conventional glaucoma drainage devices
-
—
Different MIBS device (i.e., head-to-head comparisons of different devices)
-
—
Any type of MIGS
-
—
Exclusion Criteria
Different version of the same device
Different surgical technique using the same device
Different patient population using the same device
Outcome Measures
Changes in IOP (mmHg)
Success rate (as defined by study authors; e.g., ≥ 20% reduction in IOP, IOP ≤ 21 mmHg)
Quality of life (e.g., Glaucoma Quality of Life-15, Glaucoma Symptom Scale)
Visual impairment (visual field, visual acuity)
Number of medications required
Number of reinterventions (e.g., a second procedure)
Number of follow-up visits
Adverse events and complications (e.g., choroidal effusion, hypotony, hyphemia, wound leak)
Literature Screening
Two reviewers screened title and abstracts to assess the eligibility of a sample of 100 citations to validate the inclusion and exclusion criteria. A single reviewer then screened all remaining ciations using Covidence30 and then obtained the full texts of studies that appeared eligible for review, according to the inclusion criteria. A single reviewer then examined the full-text articles and selected studies eligible for inclusion. A single reviewer also examined reference lists.
Data Extraction
We extracted relevant data on study characteristics and risk-of-bias items using a data form to collect information on the following:
Source (e.g., citation information, study type)
Methods (e.g., study design, study duration and years, participant allocation, allocation sequence concealment, blinding, reporting of missing data, reporting of outcomes, whether the study compared two or more groups)
Outcomes (e.g., outcomes measured, number of participants for each outcome, number of participants missing for each outcome, outcome definition and source of information, unit of measurement, upper and lower limits [for scales], time points at which the outcomes were assessed)
Equity Considerations
We used PROGRESS-Plus, a health equity framework recommended by the Campbell and Cochrane Equity Methods Group,31 to explore potential inequities for this health technology assessment. Factors that may lead to disadvantage or inequities in the framework include place of residence; race or ethnicity, culture or language; gender or sex; disability; occupation; religion; education; socioeconomic status; social capital; and other key characteristics that stratify health opportunities and outcomes. Relevant equity considerations in the effect of race or ethnicity are reported to the extent that information was available in the included studies.
Statistical Analysis
We did not conduct a meta-analysis due to the clinical and statistical heterogeneity of the studies. We summarized the results narratively and in tabular form. We were unable to undertake an equity subgroup analysis because information on the impact of race was not available in most studies (but see above for equity considerations).
Critical Appraisal of Evidence
We assessed the risk of bias using the Cochrane Risk of Bias 1.0 Tool32 for RCTs and the Risk of Bias Assessment Tool for Nonrandomized Studies (RoBANS) tool33 for comparative observational studies (Appendix 4).
We evaluated the quality of the body of evidence for each outcome according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Handbook.34 The body of evidence was assessed based on the following considerations: risk of bias, inconsistency, indirectness, imprecision, and publication bias. The overall rating reflects our certainty in the evidence.
Results
Clinical Literature Search
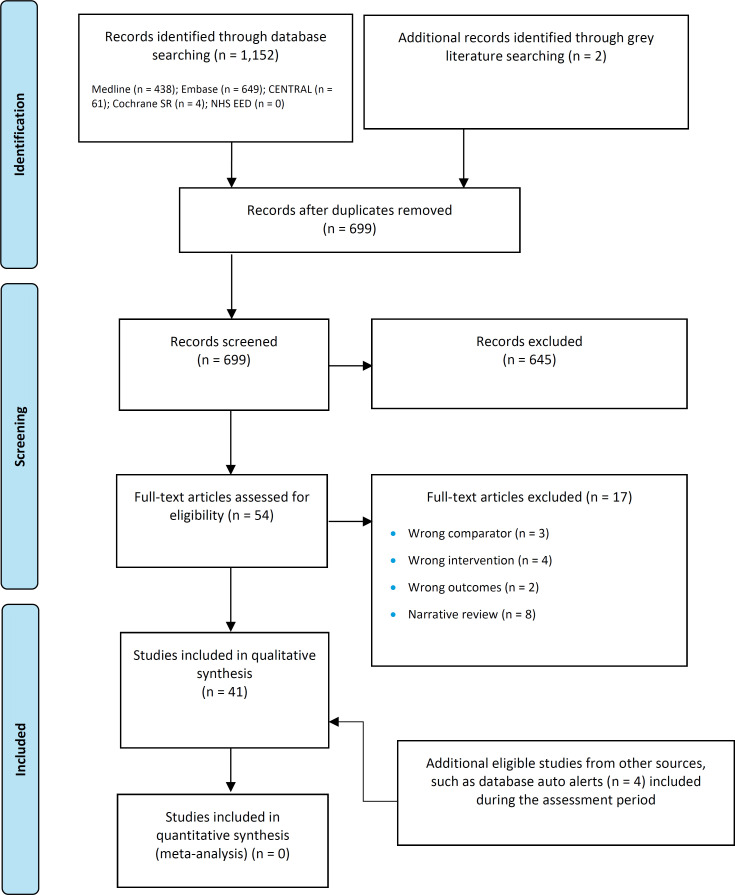
The database search of the clinical literature yielded 699 citations published between database inception and March 6, 2023, including grey literature searches and after duplicates were removed. We identified 4 additional eligible studies from other sources, including database alerts (monitored until June 12, 2023). In total, we identified 41 studies (2 RCTs and 39 comparative observational studies) that met our inclusion criteria. Figure 1 presents the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow diagram for the clinical literature search.
Figure 1: PRISMA Flow Diagram - Clinical Search Strategy.
Abbreviation: PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analyses.
Source: Adapted from Page et al.35
PRISMA flow diagram showing the clinical search strategy. The database search of the clinical literature yielded 699 citations published between database inception and March 6, 2023. We identified four additional eligible studies from other sources. After removing duplicates, we screened the abstracts of 699 studies and excluded 645. We assessed the full text of 54 articles and excluded a further 17. In the end, we included 41 articles in the qualitative synthesis.
Characteristics of Included Studies
We found 41 studies from Australia, Austria, Belgium, Canada, France, Germany, Italy, Netherlands, Portugal, Singapore, Spain, Switzerland, Thailand, Turkey, the United States, and the United Kingdom. There were two RCTs on MIBS (one each comparing XEN36 and PreserFlo37 to trabeculectomy) and 39 comparative observational studies (which were primarily retrospective, often using data from chart reviews). We found 27 studies on XEN, 8 on PreserFlo, and 4 that included both XEN and PreserFlo. Most studies included trabeculectomy as a comparator; other comparators included gonioscopy-assisted transluminal trabeculotomy,38 filtering canaloplasty,39 non-penetrating deep sclerotomy,40-42 EX-PRESS,43 iStent with phacoemulsification and endocyclophotocoagulation (ICE2),44 Kahook Dual Blade goniotomy,45 and Ahmed valve.46 All included studies evaluated only XEN 45 (we did not find any comparative evidence for XEN 63).
The comparative studies varied in their population inclusion criteria and reporting detail (e.g., severity and duration of glaucoma, previous glaucoma treatments, clinical criteria, race or ethnicity), surgical technique (e.g., ab interno vs. externo approach, other surgical modifications, amount of mitomycin C used, bilateral MIBS, inclusion of concomitant cataract surgery), reporting of outcomes (e.g., different definitions of clinical success or failure, grouping and reporting of adverse events). In addition, the number of surgeons and their level of experience was often not reported. Long-term comparative evidence was limited, with most studies having a follow-up duration of 1 year or less. The longest follow-up duration of the included studies was 3 years.47,48
We excluded studies comparing MIBS within different populations (e.g., different glaucoma types49,50 or population,51,52 with or without cataract surgery53-57), different types of the same MIBS (e.g., XEN 45 vs. 6358), different surgical techniques (e.g., ab interno vs. ab externo),12,59-61 and cataract surgery with or without MIBS,62 since the focus of our review was on the effectiveness and safety of MIBS compared with other glaucoma treatments.
Additional details of the included studies can be found in Appendix 2.
Risk of Bias in the Included Studies
The two RCTs36,37 were noninferiority in design, with possible reporting bias (e.g., intention-to-treat analysis, unclear if participants who discontinued the study were similar to those who completed the study, industry sponsorship and involvement in study development and analysis). There was likely a low risk of bias for the other domains. One RCT also reported interim 1-year results from a 2-year study.37
There was generally a moderate to high risk of bias for the comparative observational studies, which were primarily retrospective studies using chart reviews. Baseline characteristics of groups were different in some studies (e.g., glaucoma type or severity, previous glaucoma treatments), which were often not accounted for. There was also selective reporting of study outcomes and confounding concerns among certain studies.
Details of the risk of bias of the included studies can be found in Appendix 5.
Changes in Intraocular Pressure
Tables 4 and 5 present the changes in IOP for MIBS compared with trabeculectomy or other glaucoma treatments. Significant reductions in IOP were found within all groups (i.e., MIBS and the study comparators), but studies did not consistently report on whether there were significant differences between groups, which makes direct comparability unclear. In general, changes in IOP were often similar or smaller compared with trabeculectomy.
Table 4:
Changes in Intraocular Pressure for Minimally Invasive Bleb Surgery Versus Trabeculectomy
| Author, year | N | Changes in IOP (mmHg) for MIBS vs. Trab | P value |
|---|---|---|---|
| Randomized controlled studies | |||
| Baker et al, 202137 | PF: 395 | Mean IOP ± SD, 1 y | Significantly reduced in both groups at 1 y (P < .01) |
| Trab: 132 | PF: 21.1 ± 4.9 to 14.3 ± 4.3 (mean % change: 29.1%) | ||
| Trab: 21.1 ± 5.0 to 11.1 ± 4.3 (mean % change: 45.4%) | |||
|
Between-group least-squares mean reduction difference
|
< .01, but noninferiority criterion was not met (noninferiority P = .94) | ||
| 3.2 (95% CI: 2.3-4.1) | |||
| Sheybani et al, 202336 | XEN: 95 | Mean ± IOP, 1 y | |
| Trab: 44 | XEN: 23.1 ± 5.8 to 14.4 ± 4.1 | < .001 | |
| Trab: 22.6 ± 5.7 to 11.8 ± 3.5 | < .001 | ||
| Between group difference: 2.8 (95% CI: 0.4-5.2) | = .024 | ||
| Comparative observational studies | |||
| Aghayeva et al, 202139 | PF: 23 Trab: 187 |
Median IOP (range) 1 d: -10 (-14.5 to-7) vs.-8 (-14 to 0) 1 wk: -9.5 (-14.8 to -6.3) vs. -12 (-18 to -7) |
Significant correlation in IOP change between both eyes for PF (P < .0001) and trab (P = .001) |
| Bormann et al, 202264 | XEN: 69 Trab: 50 |
Mean IOP ± SD, 6 mo 15.3 ± 0.4 vs. 13.6 ± 0.7 |
= .01 |
|
Mean IOP ± SD, 12 mo 15.2 ± 0.4 vs. 13.5 ± 0.6 |
< .01 | ||
|
Mean IOP ± SD, 24 mo 15.0 ± 0.4 vs. 13.3 ± 0.6 (37% and 36% reduction, respectively) |
< 0.1 | ||
| Cappelli et al, 202247 | XEN: 34 Trab: 34 |
IOP in trab group had lower IOP values and a better IOP profile vs. XEN | NR |
| Fili et al, 202265 | PF: 98 Trab: 92 |
Mean IOP ± SD, 1 mo PF: 23.5 ± 8.4 to 10.6 ± 4.9 Trab: 22.03 ± 5.2 to 9.6 ± 3.5 Mean IOP ± SD, 6 mo PF: 23.5 ± 8.4 to 12.4 ± 3.6 Trab: 22.03 ± 5.2 to 10.5 ± 4.4 Mean IOP ± SD, 12 mo PF: 23.5 ± 8.4 to 12.9 ± 3.4 Trab: 22.03 ± 5.2 to 11.4 ± 4.5 |
Significantly reduced compared with baseline for PF at 6 mo (P = .0009), trab at 6 mo (P = .00082), PF at 12 mo (P = .00053), and trab at 12 mo (P = .0006) Significant difference between groups at 12 mo (P = .00151) |
| Fu et al, 202266 | PF: 101 Trab: 101 |
Median IOP, 3 mo PF: 22 (17-29) to 11 (9-15) Trab: 20 (16-28) to 10 (7-13) |
= .006 |
|
Median IOP, 6 mo PF: 22 (17-29) to 12 (10-16) Trab: 20 (16-28) to 11 (8-14) |
= .048 | ||
|
Median IOP, 18 mo PF: 22 (17-29) to 15 (10-17) Trab: 20 (16-28) to 11 (10-13) |
= .183 | ||
| Jamke et al, 202367 | PF: 29 Trab: 30 |
Median IOP reduction (IQR), 12 mo -6.3 (-11.3 to -4.2) vs. -7.5 (-14.2 to -4.0) |
= .596 |
| Kee et al, 202168 | XEN + phaco: 46 | Between-group difference in mean reduction (95% CI) | |
| Trab + phaco: 91 | 1 mo: -0.7 (95% CI: -3.5 to 2.0) 3 mo: -3.6 (95% CI: -6.0 to -1.2) 6 mo: -3.8 (95% CI: -6.3 to -1.3) 12 mo: -2.9 (95% CI: -5.2 to -0.7) |
= .599 = .004 = .003 = .012 |
|
| Marcos-Parra et al, 201969 | XEN: 17 XEN + phaco: 48 Trab: 30 Trab + phaco: 26 |
Mean IOP (95% CI), 12 mo XEN: -6.7 (95% CI: -10.4 to -3.0) XEN + phaco: -3.5 (95% CI: -5.0 to -2.0) Trab: -8.1 (95% CI: -10.4 to -5.9) Trab + phaco: -7.3 (95% CI: -9.3 to -5.3) XEN and XEN + phaco vs. trab and trab + phaco: 18.5 (27.0%) vs. 33.8 (22.9%) |
= .0013 < .0001 < .0001 < .0001 = .001 |
| Median IOP reduction (IQR), 12 mo | |||
| XEN vs. trab: 36.4% (18.1% to 44.3%) vs. 36.2% (14.3% to 52.2%) | = .4063 | ||
| XEN + phaco vs. trab + phaco: 19.6% (0.0% to 35.1%) vs. 37.1% (20.0% to 45.0%) | = .048 | ||
| No significant difference between XEN and XEN + phaco, except for mean IOP at 6 mo (lower for XEN + phaco) No significant difference between trab and trab + phaco |
|||
| Ozcelik Kose et al, 202170 | XEN: 18 Trab: 30 |
Mean difference between IOP in DLDP and sitting positions ± SE 2.4 ± 0.3 vs. 2.5 ± 0.2 Mean difference between IOP in supine and sitting positions ± SE 0.85 ± 0.19 vs. 1.00 ± 0.11 |
NR specifically for XEN vs. trab |
| Schargus et al, 202171 | XEN: 38 XEN + phaco: 42 |
Mean IOP ± SD, XEN vs. XEN + phaco vs. trab 6 mo: 15.6 ± 3.41 vs. 15.0 ± 3.4 vs. 4.1 ± 4.8 12 mo: 15.2 ± 2.9 vs. 15.3 ± 2.9 vs. 1.3 ± 4.3 |
= .19 = .12 |
| Trab: 52 | 24 mo: 15.7 ± 3.0 vs. 14.7 ± 3.2 vs. 13.9 ± 4.2 | = .04 | |
| Marcos-Parra et al, 202272 | XEN: 17 XEN + phaco: 46 Trab: 41 Trab + phaco: 30 |
Mean IOP reduction (95% CI), 36 mo XEN: -6.3 (95% CI: -11.0 to -1.6) XEN + phaco: -2.5 (95% CI: -4.5 to -0.4) Trab: -2.5 (95% CI: -4.5 to -0.4) Trab + phaco: -5.6 (95% CI: -7.7 to -3.4) Mean difference (95% CI), 36 mo XEN vs. trab: -2.6 (95% CI: -7.6 to 2.4) |
= .025 < .001 = .019 < .001 = .170 |
| Sharpe et al, 202073 | XEN: 90 Trab: 89 |
Mean IOP ± SD XEN: 17.8 ± 6.0 to 13.5 ± 5.9 (24.1% reduction) Trab: 20.4 ± 9.0 to 10.8 ± 4.8 (47% reduction) At 6 mo, trab had lower mean IOP vs. XEN |
= .03 < .001 < .003 |
| Teus et al, 201974 | XEN: 10 Trab: 15 |
Mean IOP change ± SD -8.5 ± 5.3 vs. -8.8 ± 5.2 |
= .9 |
| Sacchi et al, 202375 | XEN: 7 Trab: 7 |
Mean IOP ± SD 1 wk: 8.43 ± 2.44 vs. 5.43 ± 2.44 1 mo: 10.71 ± 1.25 vs. 5.43 ± 2.513 3 mo: 13.00 ± 3.61 vs. 8.00 ± 2.58 6 mo: 14.50 ± 2.35 vs. 8.50 ± 2.26 |
Significant difference within groups (P < .0001) |
| 12 mo: 12.00 ± 1.90 vs. 10.00 ± 2.83 24 mo: 14.83 ± 3.97 vs. 12.83 ± 4.62 |
|||
| Nobl et al, 202376 | PF: 31 Trab: 29 |
Mean IOP reduction ± SD, 12 mo PF: 20.8 ± 5.9 to 12.4 ± 2.8 (40.4% reduction) Trab: 22.3 ± 6.5 to 11.1 ± 3.7 (50.2% reduction) |
Significantly reduced within both groups (P < .0001 for PF and trab) No difference between groups (P = .07) |
| Nuzzi et al, 202148 | XEN: 23 Trab: 39 |
Mean IOP ± SD 1 mo: 11.6 ± 4.2 vs. 10.8 ± 1.7 3 mo: 13.4 ± 3.9 vs. 12.2 ± 3.9 6 mo: 20.8 ± 6.1 vs. 15.1 ± 3.3 12 mo: 18.5 ± 2.4 vs. 15.8 ± 3.4 24 mo: 19.3 ± 2.3 vs. 15.0 ± 3.0 36 mo: 19.6 ± 2.1 vs. 15.7 ± 3.8 |
NR specifically for XEN vs. trab comparison |
| Olgun et al, 202177 | XEN: 49 Trab: 31 |
Mean decrease ± SD 2.5± 1.2 vs. 2.3 ± 1.1 Mean % difference ± SD 73.5± 35.5 vs. 61.1 ± 28.4 |
= .303 = .015 |
| Ponnusamy et al, 202178 | XEN: 17 Trab: 14 |
Mean IOP ± SE 1 wk: 16.2 ± 2.5 vs. 14.7 ± 2.2 1 mo: 16.9 ± 1.4 vs. 17.8 ± 2.5 2 mo: 16.3 ± 1.5 vs. 15.1 ± 1.8 6 mo: 15.1 ± 1.2 vs. 13.4 ± 0.9 |
NR specifically for XEN vs. trab comparison |
| Pillunat et al, 202279 | PF: 26 Trab: 26 |
Median IOP reduction (range), 6 mo PF: 5.3 (3.0-12.5) Trab: 7.1 (3.9-10.0) Median IOP reduction %, 6 mo PF: 33.9 (19.4-52.1) Trab: 40.5 (29.6-52.5) |
No significant difference in median IOP reduction or reduction % between groups at 6 mo (P = .458 and .337, respectively) |
| Van Lancker et al, 202280 | PF: 70 Trab: 64 |
Mean IOP ± SD 1 d: 8 ± 4.8 vs. 11.5 ± 7.4 1 wk: 10.0 ± 4.4 vs. 11.1 ± 7.7 1 mo: 13.0 ± 7.3 vs. 12.6 ± 6.8 3 mo: 13.8 ± 5.3 vs. 12.0 ± 6.6 6 mo: 13.0 ± 4.1 vs. 12.6 ± 5.6 12 mo: 14.0 ± 5.9 vs. 12.5 ± 6.3 18 mo: 12.9 ± 3.9 vs. 11.7 ± 5.3 24 mo: 13.8 ± 5.4 vs. 12.2 ± 5.9 |
No difference between groups at any time point, except at 1 d (P = .004) |
| Wagner et al, 202081 | XEN: 82 Trab: 89 |
IOP reduction ± SD 6 mo: 5.5 ± 7.6 vs. 11.9 ± 9.0 12 mo: 7.2 ± 8.2 vs. 10.5 ± 9.2 |
< .001 = .003 |
Abbreviations: CI, confidence interval; DLDP, dependent lateral decubitus position; IOP, intraocular pressure; IQR, interquartile range; MIBS, minimally invasive bleb surgery; NR, not reported; PF, PreserFlo; phaco, phacoemulsification; SD, standard deviation; SE, standard error; trab, trabeculectomy.
Table 5:
Changes in Intraocular Pressure for Minimally Invasive Bleb Surgery Versus Other Glaucoma Treatments
| Author, year | N | Change in IOP (mmHg) for MIBS vs. other glaucoma tx | P value |
|---|---|---|---|
| Aghayeva et al, 202139 | PF: 23 Filtering canaloplasty: 25 |
Median IOP (range), PF vs. filtering canaloplasty 1 d: -10 (-14.5 to -7) vs. -10 (-14 to -4) 1 wk: -9.5 (-14.8 to -6.3) vs. -7.5 (-11.3 to -5) |
No correlation in IOP change between both eyes |
| Almendral-Gomez et al, 202340 | XEN: 63 NPDS: 65 |
Mean IOP reduction ± SE, XEN vs. NPDS 1 mo: -4.0 ± 0.6 vs. -5.8 ± 0.6 Mean difference: 1.8 ± 0.9 (95% CI: 0.02 to 3.6) 3 mo: -3.6 ± 0.5 vs. -5.7 ± 0.5 Mean difference: 2.1 ± 0.7 (95% CI: 0.7 to 3.5) 6 mo: -3.9 ± 0.4 vs. -4.8 ± 0.4 Mean difference: 0.9 ± 0.6 (95% CI: -0.3 to 2.1) 12 mo: -4.9 ± 0.4 vs. -3.9 ± 0.4 Mean difference: -1.0 ± 0.5 (95% CI: -2.1 to -0.04) Mean IOP significantly greater at 1 d and 12 mo in NPDS group vs. XEN, but significantly lower at 1 and 3 mo vs. XEN |
= .0474 = .0046 = .1414 = .0385 |
|
Adjusting for age, preop IOP, No. of preop AGMs, cataract surgery Mean IOP lowering significantly greater at 1 d and 12 mo for XEN, but significantly greater in the NPDS group at 1 and 3 mo |
|||
| Duong et al, 202245 | XEN: 57 KDB: 18 |
Mean IOP ± SD at 24 mo, XEN vs. KDB 14.7 ± 3.2 (32.7% reduction, P = .018) vs. 16.7± 3.2 (40.4% reduction, P = .049) Between groups: P= .416 |
Significantly greater IOP reduction in XEN group from 1 d until 1 mo, but no differences from 3 to 24 mo |
| Gambini et al, 202263 | PF: 29 XEN: 29 |
Mean IOP ± SD, XEN vs. PF 1 d: 10.6 ± 2.7 vs. 8.1 ± 2.8 1 wk: 12.5 ± 2.6 vs. 8.7 ± 2.0 1 mo: 14.2 ± 2.1 vs. 11.3 ± 2.1 |
Differences within group was significant at all time points |
| 3 mo: 13.8 ± 2.0 vs. 12.1 ± 2.2 6 mo: 14.2 ± 2.0 vs. 12.9 ± 2.1 |
Lower IOP at all time points for PF, but difference was not significant at day 1 (P = .0087), wk 1 (P = .0001), or 1 mo (P = .0005) | ||
| Nuzzi et al, 202148 | XEN: 23 Cypass: 18 Baerveldt: 15 |
Mean IOP ± SD, XEN vs. Cypass vs. Baerveldt 1 mo: 11.6 ± 4.2 vs. 10.6 ± 1.8 vs. 10.9 ± 0.8 3 mo: 13.4 ± 3.9 vs. 18.1 ± 11.3 vs. 12.3 ± 1.2 6 mo: 20.8 ± 6.1 vs. 16.3 ± 2.6 vs. 17.5 ± 7.1 12 mo: 18.5 ± 2.4 vs. 17.4 ± 3.2 vs. 16.3 ± 2.9 24 mo: 19.3 ± 2.3 vs. 17.7 ± 3.0 vs. 15.6 ± 3.1 36 mo: 19.6 ± 2.1 vs. 18.2 ± 3.1 vs. 15.7 ± 3.8 |
= .832 = .047 = .012 = .142 = .005 = .034 Above P values for comparison of XEN, Cypass, Baerveldt, and trab |
| Olgun et al, 202038 | XEN: 114 GATT: 107 |
Mean IOP ± SD, XEN vs. GATT 3 mo: 12.7 ± 2.6 vs. 18.2 ± 7.1 6 mo: 13.4 ± 3.2 vs. 16.2 ± 5.1 12 mo: 13.5 ± 2.3 vs. 15.0 ± 4.1 18 mo: 13.9 ± 2.5 vs. 15.5 ± 4.2 24 mo: 13.8 vs. 2.1 vs. 15.3 ± 3.8 |
< .001 < .001 < .001 < .001 < .001 |
| Ozcelik Kose et al, 202170 | XEN: 18 Medical tx: 30 |
Mean difference between DLDP and sitting positions ± SE 2.4 ± 0.3 vs. 3.5 ± 0.2 Mean difference between supine and sitting positions ± SE 0.83 ± 0.19 vs. 1.64 ± 0.13 |
< .001 < .001 Above values for XEN vs. trab vs. medical tx |
| Qidwai et al, 202244 | XEN: 37 PF: 48 ICE2: 162 |
Mean IOP ± SD, XEN vs. PF vs. ICE2 7 d: 13.1 ± 3.3 vs. 10.1 ± 6.2 vs. 10.0 ± 4.2 1 mo: 13.7 ± 4.5 vs. 11.9 ± 5.5 vs. 13.1 ± 5.3 3 mo: 13.5 ± 2.7 vs. 10.0 ± 3.9 vs. 16.3 ± 5.0 6 mo: 13.9 ± 2.9 vs. 12.6 ± 1.5 vs. 14.4 ± 3.7 12 mo: 13.9 ± 3.1 vs. 13.5 ± 4.4 vs. 14.3 ± 1.8 18 mo: 14.3 ± 3.3 vs. 13.6 ± 3.0 vs. 16.0 ± 5.2 24 mo: 13.4 ± 3.0 vs. 12.3 ± 4.0 vs. 13.1 ± 4.3 Mean IOP reduction from baseline, XEN vs. PF vs. ICE2 |
Significant difference in reduction from baseline for all groups and all time points |
| 7 d: 5.4 vs. 10.4 vs. 9.9 6 mo: 4.6 vs. 7.9 vs. 5.5 12 mo: 4.6 vs. 7.0 vs. 5.6 24 mo: 5.1 vs. 8.2 vs. 6.8 |
|||
| Ponnusamy et al, 202178 | XEN: 17 EX-PRESS: 16 |
Mean IOP ± SE, XEN vs. EX-PRESS 1 wk: 16.2 ± 2.5 vs. 15.0 ± 1.8 1 mo: 16.9 ± 1.4 vs. 15.4 ± 2.1 2 mo: 16.3 ± 1.5 vs. 16.2 ± 2.3 6 mo: 15.1 ± 1.2 vs. 14.5 ± 1.4 |
= .871 = .711 = .883 = .688 Values above for XEN vs. trab vs. EX-PRESS |
| Stoner et al, 202143 | XEN: 52 EX-PRESS: 48 |
Mean IOP ± SD, XEN vs. EX-PRESS at 1 y XEN: 21.4 ± 1.2 to 13.0 ± 0.6 EX-PRESS: 18.9 ± 1.1 to 11.5 ± 0.8 No significant difference between groups at 1 y, but mean IOP was significantly higher for XEN at 1, 3, and 6 mo |
< .0001 < .0001 |
| Saletta et al, 202282 | PF: 30 XEN: 30 |
Mean IOP ± SD, XEN vs. PF 1 d: 8.8 ± 6.0 vs. 8.9 ± 4.4 1 wk: 10.6 ± 6.4 vs. 11.4 ± 7.0 1 mo: 18.3 ± 9.4 vs. 13.0 ± 6.8 3 mo: 33.4 ± 5.8 vs. 16.6 ± 6.7 6 mo: 14.9 ± 5.4 vs. 15.9 ± 6.4 12 mo: 14.5 ± 4.8 vs. 15.4 ± 6.7 |
Significant decrease within XEN and PF groups (P < 0.01) No significance difference between XEN and PF groups (P > .5) |
|
% IOP change from baseline, XEN vs. PF 1 d: 62.5 ± 4.4 vs. 59.0 ± 4.4 1 wk: 53.2 ± 5.9 vs. 47.9 ± 5.9 1 mo: 19.4 ± 7.2 vs. 42.6 ± 7.2 3 mo: 33.4 ± 5.8 vs. 22.9 ± 5.8 6 mo: 35.5 ± 5.4 vs. 27.0 ± 5.4 12 mo: 34.9 ± 5.3 vs. 31.9 ± 5.3 |
|||
| Scheres et al, 202183 | XEN: 41 PF: 41 |
Mean IOP ± SD, XEN vs. PF 1 mo: 13.1 ± 6.4 vs. 10.3 ± 3.2 3 mo: 13.8 ± 4.6 vs. 10.9 ± 2.8 6 mo: 14.5 ± 4.8 vs. 12.5 ± 4.2 12 mo: 13.3 ± 2.9 (31%) vs. 12.1 ± 3.5 (40%) 24 mo: 13.8 ± 3.8 (28%) vs. 12.1 ± 3.5 (39%) |
= .019 = .002 = .07 = .17 = .19 Lower IOP values for PF at all time points, but difference only significant at 1 and 3 mo |
| Wagner et al, 202284 | XEN: 35 PF: 35 Trab: 35 |
IOP reduction ± SD, XEN vs. PF vs. trab Trab 12.1 ± 7.9 was 5.8 (95% CI: 2.2-9.6) higher than XEN (P < .001) and 4.8 (95% CI: 0.9-8.7) higher than PF (P = .01) |
IOP reduction at 6 mo was significantly different between the 3 groups IOP reduction not significantly different between XEN and PF (P = .81) |
Abbreviations: AGM, antiglaucoma medication; CI, confidence interval; GATT, gonioscopy-assisted transluminal trabeculotomy; ICE2, iStent with endoscopic cyclophotocoagulation; IOP, intraocular pressure; KDB, Kahook Dual Blade; MIBS, minimally invasive bleb surgery; NPDS, nonpenetrating deep sclerectomy; PF, PreserFlo; preop, preoperative; SD, standard deviation; SE, standard error; trab, trabeculectomy; tx, treatment.
The reasons for differences among study groups included differences in baseline population demographics and risk factors, glaucoma type (commonly primary open angle glaucoma, but most studies also included pseudoexfoliative glaucoma, pigmentary glaucoma, secondary glaucoma, or other type), and previous glaucoma treatments. Gambini et al63 found no significant differences in IOP by age, sex, and standalone vs. combined surgery for XEN and PreserFlo.
The GRADE certainty for MIBS versus trabeculectomy was Moderate for the RCTs (downgrading for risk of bias) and Very low for the observational studies (downgrading for risk of bias; Table A6). The GRADE certainty for MIBS versus other glaucoma treatments was Very low, downgrading for risk of bias (Table A6).
Success Rate
Tables 6 and 7 present the success rate of MIBS compared with trabeculectomy and other glaucoma treatments. Success rate (and failure) was variably defined within studies, but typically included a combination of a specific range of change in IOP and a percentage reduction from baseline, with or without the need for antiglaucoma medications. Studies often subcategorized clinical success as absolute success (typically, no use of antiglaucoma medications and no subsequent glaucoma treatments) or qualified success (typically allows for the use of antiglaucoma medications). Definitions of success varied based on the upper or lower limit of IOP changes.
Table 6:
Success Rate for Minimally Invasive Bleb Surgery Versus Trabeculectomy
| Author, year | N | Definition of success | MIBS vs. trab success rate |
|---|---|---|---|
| Randomized controlled trials | |||
| Baker et al, 202137 | PF: 295 Trab: 132 |
Surgical success IOP ≥ 20% reduction without AGM increase, clinical hypotony, vision loss to counting fingers, or secondary surgical intervention at a noninferiority test with 24% margins Overall success IOP > 6 to < 21 mmHg and 20% reduction in IOP on 2 consecutive follow-up visits after 3 mo, with or without AGMs Complete success Overall success without AGMs |
Probability of surgical success 53.9% vs. 72.7%, P < .01 Overall success IOP < 21: 77.0% vs. 80.3%, difference -3.3 (95% CI: -11.5 to 4.8) IOP < 17: 66.1% vs. 78.0%, difference -12.0 (95% CI: -21.1 to -2.8) IOP < 14: 39.7% vs. 63.6%, difference -23.9 (95% CI: -33.7 to -14.1) Complete success IOP < 21: 60.8% vs. 68.2, difference -7.4 (95% CI: -17.0 to -2.1) IOP < 17: 53.9% vs. 66.7%, difference -12.7 (95% CI: -22.5 to -3.0) IOP < 14: 35.2% vs. 56.1%, difference -20.9 (95% CI: -30.5 to -11.2) Cumulative failure rate (95% CI) at 1 y 41.6% (95% CI: 36.4-46.3) vs. 29.9% (21.5-37.5), P = .02 |
| Sheybani et al, 202336 | XEN: 95 Trab: 44 |
Surgical success IOP > 20% reduction without increase in AGMs, clinical hypotony, vision loss to counting fingers, secondary surgical intervention Complete success IOP ≤ 18 mmHg (excluding eyes with hypotony) with ≥ 20% IOP reduction without AGMs Qualified success IOP ≤ 18 mmHg (excluding eyes with hypotony) with ≥ 20% IOP reduction with AGMs |
Complete success at 12 mo 44.2% vs. 59.1%, P > .144 Qualified success at 12 mo 62.1% vs. 72.7%, P > .144 Overall success at 12 mo 62.1% vs. 68.2% Between group difference: -6.1% (95% CI: -22.9% to 10.8%), P = .487; XEN statistically noninferior to trab |
| Comparative observational studies | |||
| Bormann et al, 202264 | XEN: 69 Trab: 50 |
Complete success |
< 21 mmHg, XEN vs. trab Complete: 71.0% vs. 80.0%, P = .89 |
| lOP reduction ≥ 20%, lOP < 21 mmHg, no AGM use, no additional glaucoma surgery during 12 mo follow-up except laser suture lysis or needling Qualified success IOP reduction > 20%, with additional use of AGMs if the preop no. of AGMs was not exceeded |
Qualified: 91.3% vs. 92.0%, P = .27 < 18 mmHg, XEN vs. trab Complete: 65.2% vs. 80.0%, P = .14 Qualified: 82.6% vs. 92.0%, P = .08 < 15 mmHg, XEN vs. trab Complete: 43.5% vs. 72.0%, P = .01 Qualified: 52.2% vs. 76.0%, P = .01 < 12 mmHg, XEN vs. trab Complete: 23.2% vs. 44.0%, P = .03 Qualified: 24.6% vs. 44.0%, P = .02 |
||
| Fili et al, 202265 | PF: 98 Trab: 92 |
Absolute success lOP 6-15 mmHg, ≥ 20% lOP reduction, no use of AGMS, no subsequent glaucoma procedures Qualified success: lOP 6-18 mmHg, ≥ 20% lOP reduction, use of fewer AGMs than before surgery, no subsequent glaucoma procedures |
Absolute success 1 mo: 93.3% vs. 99.3% 3 mo: 92% vs. 99.3% 6 mo: 90.7% vs. 98% 1 y: 81.3% vs. 94% Between groups: P = .042 Qualified success 1 mo: 95.3% vs. 99.3% 3 mo: 94% vs. 99.3% 6 mo: 94% vs. 98% 1 y: 93.3% vs. 96% Between groups: P = .082 |
| Kee et al, 202168 | XEN + phaco: 46 Trab + phaco: 91 |
Complete success No surgical failures, no use of AGMs Qualified success Complete success, additional use of AGMs Surgical failure Sustained IOP > 18 mmHg or < 20% reduction for at least 2 consecutive follow-up visits despite additional AGMs from (and inclusive of) 1 mo and onwards Sustained lOP ≤ 5 mmHg on 2 consecutive follow-up visits, from (and inclusive of) 1 mo and onwards No perception of light after surgery or presence of vision-threatening severe complications |
Complete success, 12 mo 52.5% vs. 83.5%, P < .001 21.7% vs. 11.0% needed eyedrops, P = .0924 Significant difference between groups for complete and qualified success, P = .00087 |
| Reoperations for glaucoma, but not including slit lamp-based interventions Revision or removal of XEN implant |
|||
| Fu et al, 202266 | PF: 101 Trab: 101 |
Complete success IOP < 21 mmHg, no further surgical reintervention, no loss of light perception vision, no chronic hypotony (IOP ≤ 5 mmHg at 2 consecutive followups from mo 3, no use of AGMs Qualified success Complete success, but allows use of AGMs Strict success Complete success, but with ≥ 20% IOP reduction |
Complete success 6 mo: 65.3% vs. 63.9% 12 mo: 55.1% vs. 58.1% 18 mo: 42.8% vs. 53.9% Qualified success 6 mo: 72.0% vs. 68.1% 12 mo: 68.2% vs. 62.1% 18 mo: 68.2% vs. 62.1% Strict success 6 mo: 52.9% vs. 46.4% 12 mo: 44.5% vs. 40.1% 18 mo: 35.6% vs. 35.6% No significant differences between groups for all success criteria |
| Jamke et al, 202367 | PF: 30 Trab: 30 |
Complete success
Both groups without hypotony (IOP ≤ 5 mmHg) and without need for any AGMs Qualified success Complete success but allows AGMs Overall success Included both complete and qualified success |
Complete success
Qualified success 5% vs. 0% Overall success 87% vs. 87% |
| Nuzzi et al, 202148 | XEN: 23 Trab: 39 |
Complete success IOP ≤ 21 mmHg without any AGM or surgery Qualified success IOP ≤ 21 mmHg with AGMs |
Complete success 42.9% vs. 94.9%, P = NR Qualified success 52.4% vs. 93.8%, P = NR |
| Nobl et al, 202376 | PF: 31 Trab: 29 |
Complete success Absence of all failure criteria (IOP > 17 or < 5 mmHg at 12 mo postop, surgical revision, secondary glaucoma surgery, loss of light perception) Qualified success Complete success but allows AGMs |
Complete success 5-15 mmHg: 71.0% vs. 75.9%, P = .77 5-17 mmHg: 83.9% vs. 82.8%, P > .9999 5-19 mmHg: 83.9% vs. 82.8%, P > .9999 Qualified success 5-15 mmHg: 77.4% vs. 82.8%, P = .75 5-17 mmHg: 90.3% vs. 93.1%, P > .9999 5-19 mmHg: 90.3% vs. 96.6%, P = .61 |
| Pillunat et al, 202279 | PF: 26 Trab: 26 |
Complete success Median IOP and peak diurnal IOP
Qualified success Complete success but allows AGMs |
Complete success
Qualified success PF: 5% vs. 0%, P = 1.0 |
| Schargus et al, 202171 | XEN: 38 XEN + phaco: 42 Trab: 52 |
Complete success IOP reduction ≥ 20% without additional use of AGMs, no further surgical procedures for 24 mo (except laser suture lysis for trab and needling for both XEN and trab) Qualified success Complete success with additional use of AGMs, no further surgical procedures for 24 mo (except laser suture lysis for trab and needling for both XEN and trab) |
Complete success, XEN vs. XEN + phaco vs. trab IOP < 21 at 12 mo: 61% vs. 74% vs. 69% IOP < 21 at 24 mo: 58% vs. 74% vs. 67% IOP < 18 at 12 mo: 53% vs. 67% vs. 69% IOP < 18 at 24 mo: 53% vs. 67% vs. 67% IOP < 15 at 12 mo: 47% vs. 52% vs. 67% IOP < 15 at 24 mo: 29% vs. 57% vs. 64% Qualified success, XEN vs. XEN + phaco vs. trab IOP < 21 at 12 mo: 29% vs. 10% vs. 14% IOP < 21 at 24 mo: 24% vs. 14% vs. 14% IOP < 18 at 12 mo: 26% vs. 10% vs. 10% IOP < 18 at 24 mo: 24% vs. 10% vs. 14% IOP < 15 at 12 mo: 21% vs. 0% vs. 2% IOP < 15 at 24 mo: 13% vs. 2% vs. 8% Between-group comparisons were comparable for each level of success |
| Schlenker et al, 201886 | XEN: 185 Trab: 169 |
Complete success No failure, > 17 mmHg without any AGMs at least 1 mo after surgery despite in-clinic interventions (including needling), undergoing reoperation, or loss of light perception vision Qualified success Complete success allowing for use of AGMs Failure 2 consecutive IOP readings of < 6 mmHg with > 2 lines of vision loss |
HR (95% CI) for PF vs. trab Complete success IOP 6-14: crude HR 1.00 (95% CI: 0.71-1.41); adjusted HR 1.15 (95% CI: 0.73-1.81) IOP 6-17: crude HR 1.00 (95% CI: 0.68-1.45); adjusted HR 1.20 (95% CI: 0.73-1.96) IOP 6-21: crude HR 0.94 (95% CI: 0.64-1.38); adjusted HR 1.14 (95% CI: 0.70-1.85) Qualified success IOP 6-14: crude HR 1.28 (95% CI: 0.81-2.01); adjusted HR 1.59 (95% CI: 0.86-2.91) IOP 6-17: crude HR 1.13 (95% CI: 0.61-2.09); adjusted HR 1.34 (95% CI: 0.64-2.81) IOP 6-21: crude HR 1.30 (95% CI: 0.63-2.69); adjusted HR 1.43 (95% CI: 0.61-3.33) |
| Theilig et al, 202087 | XEN: 100 Trab: 100 |
Complete success IOP reduction ≥ 20%, without additional use of AGMs, no further surgical procedure (except for laser suture lysis for the trab group, or needling in both XEN and trab) Qualified success Complete success with the additional use of AGMs when pre-surgical number of AGMs was not exceeded |
Complete success 9 mo: 37% vs. 47% 12 mo: 33% vs. 39% Qualified success 9 mo: 63% vs. 77% 12 mo: 67% vs. 74% No significant difference between groups |
| Van Lancker et al, 202280 | PF: 70 Trab: 64 |
Complete success Absence of failure criteria (IOP > 21 mmHg or < 20% from baseline on 2 consecutive visits after 3 mo, IOP ≤ 5 mmHg on 2 consecutive visits after 3 mo, reoperation for glaucoma, or loss of light vision), without AGMs Qualified success Complete success, but allows AGMs |
Complete success 12 mo: 60% vs. 53% 18 mo: 55% vs. 47% Qualified success 12 mo: 19% vs. 14% 18 mo: 20% vs. 16% |
| Wagner et al, 202081 | XEN: 82 Trab: 89 |
Complete success No failure and did not need AGMs Qualified success Complete success but needed AGM and no surgery Failure IOP > 18 mmHg, or IOP reduction < 20%, or hypotony (IOP ≤ 5 mmHg), or revision surgery, or loss of light perception |
Complete success 6 mo: 59.8% (95% CI: 49.3%-69.3%) vs. 72.4% (95% CI: 62.8%-81.3%); crude OR 0.50 (95% CI: 0.25%-1.002%), P = .051; adjusted OR 0.48 (95% CI: 0.22%-1.07%), P = .07 1 y: 58.5% (95% CI: 47.6%-69.4%) vs. 65.5% (95% CI: 55.6%-75.9%); crude OR 0.61 (95% CI: 0.31%-1.22%), P = .16; adjusted OR 0.66 (95% CI: 0.32%-1.37%), P = .26 Qualified success 6 mo: 70.7% (95% CI: 61.0%-80.0%) vs. 81.6% (95% CI: 72.9%-89.2%); crude OR 0.51 (95% CI: 0.24%-1.10%), P = .08; adjusted OR 0.44 (95% CI: 0.18%-1.09%), P = .08 1 y: 72.0% (95% CI: 61.7%-81.0%) vs. 72.4% (95% CI: 62.7%-81.8%); crude OR 0.81 (95% CI: 0.39%-1.69%), P = .57; adjusted OR 0.72 (95% CI: 0.32%-1.62%) |
| Wanichwecharungruang et al, 202188 | N = 57 Trab = 57 |
Complete success IOP reduction ≥ 20%, without AGMs Overall success IOP reduction ≥ 20%, with or without AGMs Failure Not fulfilling overall success criteria, or loss of light perception |
Complete success 3 mo: 69.6% vs. 70.2%, P = .951 6 mo: 71.7% vs. 70.2%, P = .861 12 mo: 66.7% vs. 65.5%, P = .897 18 mo: 65.9% vs. 64.2%, P = .857 24 mo: 62.9% vs. 62.2%, P = .954 Overall success 3 mo: 80.4% vs. 78.9%, P = .852 6 mo: 79.2% vs. 78.9%, P = .969 12 mo: 77.1% vs. 74.5%, P = .764 18 mo: 72.7% vs. 73.6%, P = .924 24 mo: 71.4% vs. 73.3%, P = .850 |
Abbreviations: AGM, antiglaucoma medication; CI, confidence interval; HR, hazard ratio; IOP, intraocular pressure; MD, mean deviation; MIBS, minimally invasive bleb surgery NR, not reported; OR, odds ratio; PF, PreserFlo; phaco, phacoemulsification; trab, trabeculectomy.
Table 7:
Success Rate for Minimally Invasive Bleb Surgery Versus Other Glaucoma Treatments
| Author, year | N | Definition of success | Success rate of MIBS vs. other glaucoma tx |
|---|---|---|---|
| Gambini et al, 202263 | XEN: 29 PF: 29 |
Complete success IOP ≤ 18 mmHg at 2 consecutive follow-ups after 3 mo, no further medical treatment Qualified success Eyes that had not failed but needed medical treatment to manage IOP |
Complete success, XEN vs. PF 3 mo: 51% vs. 62% 6 mo: 44% vs. 56% Qualified success, XEN vs. PF 3 mo: 78% vs. 82% 6 mo: 73% vs. 79% |
| Olgun et al, 202038 | XEN: 114 GATT: 107 |
Complete success IOP ≤ 21 mmHg and ≥ 20% reduction, without any further AGMs or IOP-lowering surgery Qualified success Complete success with or without AGMs, without any further IOP-lowering surgery |
Complete success, XEN vs. GATT 34.2% vs. 50.5% Qualified success, XEN vs. GATT 97.4% vs. 89.7% |
| Saletta et al, 202282 | XEN: 30 PF: 30 |
Complete success IOP 6-16 mmHg without AGMs Qualified success IOP 6-16 mmHg with ≥ 1 AGM |
Probability of complete success at 12 mo XEN vs. PF: 62.2% vs. 55.2% (P = .96) Probability of qualified success at 12 mo XEN vs. PF: 69.3% vs. 70.4% (P = .64) If needling as a standalone intervention was considered a censored observation: difference between groups for complete (P = .035) and qualified (P = .02) success showing survival superiority of PF |
| Scheres et al, 202183 | XEN: 41 PF: 41 |
Complete success IOP ≤ 18 mmHg at 2 consecutive follow-up visits after 3 mo, no AGMs or additional glaucoma surgery Qualified success Complete success ± AGM use and no additional glaucoma interventions |
Probability of complete success at: 12 mo: XEN vs. PF: 46% vs. 58% (NS) 24 mo: XEN vs. PF: 34% vs. 49% (NS) Probability of qualified success at: 12 mo: XEN vs. PF: 78% vs. 79% (NS) 24 mo: XEN vs. PF: 73% vs. 79% (NS) |
| Almendral-Gomez et al, 202340 | XEN: 63 NPDS: 65 |
Complete success An IOP reduction ≥ 20% at 12 mo with IOP absolute value ≤ 18 mmHg, without AGMs Qualified success Complete success with AGMs |
Total success, XEN vs. NPDS 57.1% (36/63 eyes) vs. 52.3% (34/65 eyes) Mean difference 4.8%; 95% CI: -30.5% to 20.8%; P = .7115) |
| Duong et al, 202245 | XEN: 57 KDB: 18 |
Success at 2 IOP thresholds IOP < 21 mmHg and IOP < 18 mmHg, both with or without AGMs, without glaucoma surgery |
IOP < 21 mmHg, XEN vs. KDB Both at 1 y and 2 y: 72% vs. 61%, P = .06 IOP < 18 mm Hg, XEN vs. KDB Both at 1 y and 2 y: 67% vs. 33%, P = .001 |
| Stoner et al, 201243 | XEN: 52 EX-PRESS: 48 |
Complete success IOP ≥ 6 and ≤ 18 mmHg, without reoperation for uncontrolled glaucoma, loss of light perception, or use of AGMs Qualified success Complete success, but allows for AGMs |
Complete success, HR (95% CI) IOP 6-12: crude HR 3.52 (95% CI: 1.84-6.73); adjusted HR 4.73 (95% CI: 1.97-11.3) IOP 6-15: crude HR 3.37 (95% CI: 1.75-6.46); adjusted HR 4.08 (95% CI: 1.78-9.40): IOP 6-18: crude HR 3.15 (95% CI: 1.65-6.00); adjusted HR 3.94 (95% CI: 1.73-9.00) IOP 6-21: crude 3.15 (95% CI: 1.65-6.00); adjusted HR 3.94 (95% CI 1.73-9.00) Qualified success IOP 6-12: crude HR 2.40 (95% CI: 1.25-4.62); adjusted HR 2.94 (95% CI: 1.21-7.14) IOP 6-15: crude HR 1.23 (95% CI: 0.52-2.90); adjusted HR 1.49 (95% CI: 0.48-4.58) IOP 6-18: crude HR 1.14 (95% CI: 0.43-3.00); adjusted HR 1.61 (95% CI: 0.40-6.38) IOP 6-21: crude HR 1.13 (95% CI: 0.42-3.00); adjusted HR 1.87 (95% CI: 0.41-8.44) |
| Teixeira et al, 202046 | XEN-Baerveldt: 12 Ahmed: 12 |
Complete success IOP ≤ 21 and > 5, and reduction of ≥ 20%, without AGMs Qualified success Complete success with AGMs |
Complete success, XEN-Baerveldt vs. Ahmed 3 (25.0%) vs. 7 (58.3%), P = NR Qualified success, XEN-Baerveldt vs. Ahmed 6 (50.0%) vs. 4 (33.3%), P = NR Total success, XEN-Baerveldt vs. Ahmed 9 (75%) vs. 11 (91.7%), P = .72 |
| Theillac et al, 202041 | XEN: 46 NPDS: 58 |
Complete success IOP ≤ 18, 15, or 12 mmHg in the absence of antiglaucoma tx Qualified success IOP ≤ 18, 15, or 12 mmHg, ≤ 1 AGMs Failure IOP > 18 mmHg, with or without treatment |
Complete success, XEN vs. NPDS IOP ≤ 18 mmHg: 69.6% vs. 63.8%, P = .54 IOP ≤ 15 mmHg: 54.4% vs. 50.0%, P = .67 IOP ≤ 12 mmHg: 28.3% vs. 29.3%, P = .91 Qualified success, XEN vs. NPDS IOP ≤ 18 mmHg: 89.1% vs. 89.7%, P = .93 IOP ≤ 15 mmHg: 69.6% vs. 65.5%, P = .66 IOP ≤ 12 mmHg: 39.1% vs. 36.2%, P = .76 |
| Touboul et al, 202242 | XEN: 70 NPDS: 103 |
Complete success IOP reduction ≥ 20% and IOP ≤ 18 mmHg at 12 mo, without AGMs Qualified success Complete success with use of AGMs Failure Absence of surgical success, reoperation, or loss of light perception attributable to glaucoma |
Complete success, XEN vs. NPDS 28.6% vs. 42.7% Qualified success, XEN vs. NPDS 20.0% vs. 16.5% Between-group difference (95% CI) 10.65 (95% CI: -4.42 to 25.72), P = .17 |
| Nuzzi et al, 202148 | XEN: 23 Cypass: 18 Baerveldt: 15 |
Complete success IOP < 21 mmHg without any AGM or surgery Qualified success IOP ≤ 21 mmHg with AGMs |
Complete success, XEN vs. Cypass vs. Baerveldt 42.9% vs. 50.0% vs. 92.9%, P = NR Qualified success 52.4% vs. 55.6% vs. 93.3%, P = NR |
| Wagner et al, 202284 | XEN: 35 PF: 35 Trab: 35 |
Complete success Did not fail (IOP > 18 mmHg, or hypotony [IOP ≤ 5 mmHg], or revision surgery, or loss of light perception) and no AGMs Qualified success Complete success with AGMs Strict success IOP reduction ≥ 20% |
Complete success, XEN vs. PF vs. trab 51.4% (95% CI 34.0%-68.8%) vs. 74.2% (95% CI: 57.9%-90.5%) vs. 73.5% (95% CI: 57.9%-89.2%) No difference between groups (P = .08) Qualified success, XEN vs. PF vs. trab 77.1% (95% CI: 62.5%-91.58%) vs. 90.6% (95% CI: 79.9%-100%) vs. 94.1% (95%-CI: 85.8%-100%) No difference between groups (P = .08) Strict success, XEN vs. PF vs. trab 64.7% (95% CI: 47.8%-81.6%) vs. 31.4% (95% CI: 15.2%-47.6%) vs. 54.8% (95% CI: 36.3%-73.4%) |
| Significant difference between groups (P = .02) | |||
| • Trab had higher strict success rate vs. XEN (P = .006) | |||
| • No difference between trab and PF (P = .42) | |||
| • No difference between XEN and PF (P = .06) |
Abbreviations: AGM, antiglaucoma medication; GATT, gonioscopy-assisted transluminal trabeculotomy; KDB, Kahook Dual Blade; HR, hazard ratio; IOP, intraocular pressure; MIBS, minimally invasive bleb surgery; NPDS, nonpenetrating deep sclerotomy; NR, not reported; NS, not significant; PF, PreserFlo; trab, trabeculectomy; tx, treatment.
In general, studies found that the success rate of MIBS may be lower or possibly similar to trabeculectomy or other glaucoma treatments, both at initial time points and also at later time points. Time to failure was also not significant between XEN and PreserFlo.83 Similar to the reporting of changes in IOP, not all studies provided information on the differences between groups, limiting direct comparability.
Among studies, the most common reasons for treatment failure reported were inadequate IOP reduction, the need for reoperation or reintervention, persistent hypotony, and loss of light perception. Kee et al68 found possible factors affecting outcomes included differences in risk of failure, and there was a higher likelihood of surgical failure in eyes with a higher preoperative number of antiglaucoma medications (odds ratio [OR] 3.403; P = .096) versus lower likelihood of surgical failure in people with underlying diabetes (OR 0.282; P = .074). Schlenker et al85 found that both on a crude and adjusted basis, white ethnicity was associated with a decreased rate of failure (crude hazard radio [HR]: 0.49 [95% CI: 0.30-0.81]; adjusted HR: 0.49 [95% CI: 0.25-0.96]), and diabetes was associated with an increased rate of failure (crude HR: 3.28 [95% CI: 2.03-5.32]; adjusted HR: 4.21 [95% CI: 2.10-8.45)]. Duong et al45 found that surgical success was not significantly associated with baseline glaucoma severity or diagnosis during the study period for XEN versus Kahook Dual Blade goniotomy.
The GRADE certainty for MIBS versus trabeculectomy was Moderate for the RCTs (downgrading for risk of bias) and Very low for the observational studies (downgrading for risk of bias; see Table A6). The GRADE certainty for MIBS versus other glaucoma treatments was Very low for observational studies due to downgrading for risk of bias and inconsistency (Table A6).
Changes in Vision
Changes in vision were most often reported as best-corrected visual acuity (BCVA, which measures the possible ability to distinguish shapes and details of objects at a given distance with corrected lenses) and visual field (measures peripheral vision; Tables 8 and 9). A higher BCVA value indicates better vision.
Table 8:
Changes in Vision for Minimally Invasive Bleb Surgery Versus Trabeculectomy
| Author, year | N | Vision changes for MIBS vs. trab |
|---|---|---|
| Randomized controlled trials | ||
| Sheybani et al, 202336 | XEN: 95 Trab: 44 |
Mean BCVA (logMAR) 1 mo: 0.20 vs. 0.19 3 mo: 0.18 vs. 0.14 6 mo: 0.17 vs. 0.13 9 mo: 0.16 vs. 0.18 12 mo: 0.12 vs. 0.16 Between group difference at 12 mo: P = .021 |
| Comparative observational studies | ||
| Bormann et al, 202264 | XEN: 69 Trab: 50 |
Mean VA ± SD (logMAR) 6 mo: 0.23 ± 0.03 vs. 0.16 ± 0.03, P = .15 12 mo: 0.22 ± 0.03 vs. 0.17 ± 0.03, P = .67 |
| 24 mo: 0.22 ± 0.03 vs. 0.22 ± 0.06, P = .75 | ||
|
Mean MD ± SD (dB) 6 mo: 11.6 ± 0.5 vs. 7.9 ± 0.8, P = .15 12 mo: 11.3 ± 0.5 vs. 8.0 ± 0.7, P < .01 24 mo: 11.3 ± 0.5 vs. 8.1 ± 0.8, P < .01 |
||
|
Mean RNFL thickness ± SD (μm) 6 mo: 60.5 ± 1.9 vs. 64.3 ± 2.6, P < .01 12 mo: 59.3 ± 1.8 vs. 62.9 ± 2.6, P = .01 24 mo: 58.9 ± 1.8 vs. 63.2 ± 2.6, P = .04 |
||
| Fili et al, 202265 | PF: 98 Trab: 92 |
Mean VF ± SD at 12 mo PF: -11.8 ± 9.27 to -10.51 ± 8.63 (P = .17) Trab: -12.64 ± 8.32 to -11.4 ± 9.27 (P = .4) Mean RNFL thickness ± SD at 12 mo PF: 66.06 ± 14.44 to 65.6 ± 12.93 (P = .21) Trab: 65.43 ± 15.72 to 64.52 ± 12.82 (P = .43) |
| Fu et al, 202266 | PF: 101 Trab: 101 |
Mean VF ± SD 6 mo: -13.80 ± 7.53 vs. -14.47 ± 8.56 (P = .74) 12 mo: -14.52 ± 8.18 vs. -15.73 ± 7.39 (P = .638) 18 mo: -15.97 ± 8.26 vs. -16.45 ± 8.08 (P = .882) No difference between groups at any time point |
| Jamke et al, 202367 | PF: 29 Trab: 30 |
BCVA (logMAR) at 12 mo PF: 0.05 (0.00-0.10) to 0.07 (0.00-0.14), P = .311 Trab: 0.15 (0.05-0.30) to 0.18 (0.10-0.30), P = .259 VF MD (dB) at 12 mo PF: -5.7 (-11.9 to -3.0) to -4.2 (-8.3 to -1.9), P = .002 Trab: -9.1 [-19.2 to -6.5) to -11.3 (-16.6 to -7.7), P = .604 |
| Olgun et al, 202177 | XEN: 49 Trab: 31 |
Mean BCVA (logMAR) No significant difference between groups |
| Schargus et al, 202171 | XEN: 38 XEN + phaco: 42 Trab: 52 |
Mean BCVA (logMAR), XEN vs. XEN + phaco vs. trab 6 mo: 0.28 ± 0.30 vs. 0.18 ± 0.22 vs. 0.16 ± 0.19, P =.17 12 mo: 0.27 ± 0.29 vs. 0.18 ± 0.23 vs. 0.17 ± 0.17, P =.33 24 mo: 0.28 ± 0.29 vs. 0.16 ± 0.22 vs. 0.16 ± 0.17, P =.11 |
| Schlenker et al, 201785 | XEN: 185 Trab: 169 |
Median (IQR) BCVA (logMAR) at last follow-up or before reoperation 0.2 (IQR 0.1-0.5) vs. 0.3 (IQR 0.1-0.5), P = .24 |
| Schlenker et al, 201886 | XEN: 185 Trab: 169 |
OR of trab relative to XEN, losing vision Crude OR: 1.53 (95% CI: 0.87-2.71), P = .1379 Adjusted OR (for poor preop vision only): 1.66 (95% CI: 0.93-2.95), P = .0846) |
| Adjusted OR (full model): 1.99 (95% CI: 1.04-3.81), P = .0383 HR of XEN relative to trab, recovering to baseline vision Crude HR 1.20 (95% CI: 0.94-1.54), P = .1455 Adjusted HR (for poor preop vision only): 1.39 (95% CI: 2.07-3.96), P = .0247 Adjusted HR (full model): 1.46 (95% CI: 1.10-2.00), P = .0173 |
||
| Sharpe et al, 202073 | XEN: 90 Trab: 89 |
VA similar between groups both preop and postop, no significant changes at 6 mo (P > .3) |
| Pillunat et al, 202279 | PF: 26 Trab: 26 |
BCVA (logMAR) at 12 mo PF: 0.1 (0.0-0.24) to 0.1 (0.0-0.22), P = .484 Trab: 0.19 (0.05-0.33) to 0.15 (0.10-0.30), P = .715 VF MD (logMAR) at 12 mo, dB PF: -8.7 (-19.0 to -3.1) to -8.9 (-5.3 to-3.7), P = .737 Trab: -12.9 (-18.5 to -7.4) to -10.4 (-15.1 to -7.2), P = .058 |
| Van Lancker et al, 202280 | PF: 70 Trab: 64 |
Mean change in Snellen VA ± SD, pinhole (logMAR) -0.09 ± 0.37 vs. -0.17 ± 0.48, P = .28 Loss of ≥ 2 Snellen lines 19% vs. 27%, P = .27 |
Abbreviations: BCVA, best-corrected visual acuity; CI, confidence interval; dB, decibel; HR, hazard ratio; IQR, interquartile range; logMAR, logarithm of the minimum angle of resolution; MD, mean deviation; MIBS, minimally invasive bleb surgery; PF, PreserFlo; phaco, phacoemulsification; SD, standard deviation; trab, trabeculectomy; VA, visual acuity; VF, visual field.
Table 9:
Changes in Vision of Minimally Invasive Bleb Surgery Versus Other Glaucoma Treatments
| Author, year | N | Vision changes for MIBS vs. other glaucoma tx |
|---|---|---|
| Almendral-Gomez et al, 202340 | XEN: 63 NPDS: 65 |
Mean RNFL thickness ± SD (µm), XEN vs NPDS XEN: 77.8 ± 17.4 to 77.2 ± 17.0 Mean difference: -0.6 (95% CI: -2.0 to 0.8); P = .3881 NPDS: 70.6 ± 17.5 to 72.1 ± 16.6 Mean difference: 1.5 (95% CI: -0.0 to 3.0); P = .0501 |
|
Mean VF ± SD (µm), XEN vs. NPDS XEN: -5.4 ± 4.9 to -5.1 ± 5.5 Mean difference: 0.3 (95% CI: -0.2 to 0.7); P = .5467 NPDS: -10.5 ± 6.7 to -10.8 ± 7.4 Mean difference: -0.3 (95% CI: -1.1 to 0.6); P = .5467 No significant changes in RNFL thickness or VF at any time point, except an improvement in RNFL thickness in the nasal quadrant of the NPDS group and a significant reduction in pattern SD in the XEN group |
||
| Olgun et al, 202038 | XEN: 114 GATT: 107 |
Mean VA ± SD (logMAR), XEN vs. GATT 0.11 ± 0.26 vs. 0.42 ± 0.34, P < .001 |
| Qidwai et al, 202244 | XEN: 37 PF: 48 ICE2: 162 |
Mean BCVA ± SD (logMAR), XEN vs. PF vs. ICE2 7 d: 0.20 ± 0.2 vs. 0.45 ± 0.4 vs. 0.14 ± 0.1, P = NR 1 mo: 0.17 ± 0.2 vs. 0.36 ± 0.3 vs. 0.16 ± 0.2, P = NR 3 mo: 0.17 ± 0.2 vs. 0.39 ± 0.2 vs. 0.13 ± 0.1, P = NR 6 mo: 0.21 ± 0.25 vs. 0.34 ± 0.3 vs. 0.13 ± 0.2, P = NR 12 mo: 0.18 ± 0.2 vs. 0.40 ± 0.4 vs. 0.12 ± 0.1, P = NR 18 mo: 0.16 ± 0.1 vs. 0.33 ± 0.14 vs. 0.10 ± 0.1, P = NR 24 mo: 0.20 ± 0.2 vs. 0.33 ± 0.3 vs. 0.12 ± 0.1, P = NR Mean change from baseline, XEN vs. PF vs. ICE2 7 d: 0.07 (P = .34) vs. -0.09 (P < .001) vs. 0.14 (P < .001) 12 mo: 0.09 (P < .006) vs. -0.04 (P = .23) vs. 0.16 (P < .001) 24 mo: 0.07 (P < .04) vs. 0.03 (P = .44) vs. 0.16 (P = .001) |
| Stoner et al, 202143 | XEN: 52 EX-PRESS: 48 |
VA not significantly different between groups at any study time |
| Teixeira et al, 202046 | XEN-Baerveldt: 12 Ahmed: 12 |
Mean VA ± SD (logMAR), XEN-Baerveldt vs. Ahmed 1 mo: 1.3 ± 1.1 vs. 1.3 ± 1.1, P = .30 6 mo: 1.4 ± 1.2 vs. 1.0 ± 1.0, P = .64 12 mo: 1.3 ± 1.2 vs. 1.0 ± 1.0, P = .61 |
| Wagner et al, 202284 | XEN: 35 PF: 35 Trab: 35 |
BCVA ± SD (logMAR), XEN vs. PF vs. trab 1 wk: 0.32 ± 0.33 vs. 0.51 ± 0.37 vs. 0.48 ± 0.31, P = .06 1 mo: 0.34 ± 0.49 vs. 0.28 ± 0.32 vs. 0.45 ± 0.31, P = .41 6 mo: 0.23 ± 0.40 vs. 0.23 ± 0.25 vs. 0.22 ± 0.24, P = .93 |
Abbreviations: BCVA, best-corrected visual acuity; GATT, gonioscopy-assisted transluminal trabeculotomy; logMAR, logarithm of the minimum angle of resolution; MIBS, minimally invasive bleb surgery; NPDS, nonpenetrating deep sclerectomy; NR, not reported; PF, PreserFlo; RNFL, retinal nerve fiber layer; SD, standard deviation; trab, trabeculectomy; tx, treatment; VA, visual acuity; VF, visual field.
The mean deviation for the visual field test becomes negative as the overall field of vision becomes worse, with normal values typically within 0 and -2 dB. Most studies found no significant differences within groups or between MIBS and other glaucoma treatments; however, a few studies showed possible improvement in vision within the MIBS group.
The GRADE for MIBS versus trabeculectomy was Moderate for the RCTs (downgrading for risk of bias) and Very low for the observational studies (downgrading for risk of bias and inconsistency). The GRADE for MIBS versus other glaucoma treatment was Very low for the observational studies due to downgrading for risk of bias and inconsistency (Table A6).
Changes in Antiglaucoma Medications
Tables 10 and 11 present the changes in antiglaucoma medications for MIBS compared with trabeculectomy and other glaucoma treatments. Studies consistently found significant reductions in medication use within groups and possibly no difference between groups. Baseline medication use generally ranged from three to four medications, and reduced to about zero (medication-free) and one medication. Most studies did not provide information on how medication use was determined (e.g., patient-reported or verified chart review), which may impact the accuracy of changes in medication use.
Table 10:
Changes in Antiglaucoma Medications for Minimally Invasive Bleb Surgery Versus Trabeculectomy
| Author, year | N | No. of AGMs, MIBS vs trab | P value |
|---|---|---|---|
| Randomized controlled studies | |||
| Baker et al, 202137 | PF: 384 Trab: 125 |
Mean ± SD 0.6 ± 1.1 vs. 0.3 ± 0.9 % AGM-free 71.6% vs. 84.8% |
No. of AGMs per patient significantly reduced from baseline in both groups at 1 y |
| Sheybani et al, 202336 | XEN: 95 Trab: 44 |
Mean ± SD at 12 mo XEN: 2.8 ± 1.2 to 0.6 ± 1.1 Trab: 2.5 ± 1.3 to 0.3 ± 0.6 Between-group mean reduction: 0.3 (95% CI: 0.0 to 0.7) % AGM-free at 12 mo 62.1% vs. 70.5% Between group difference: 0.0 (95% CI: -0.23 to 0.20), P = .904 |
< .001 < .001 = .068 |
| Comparative observational studies | |||
| Kee et al, 202168 | XEN + phaco: 46 Trab + phaco: 91 |
Between-group differences in mean reduction (95% CI) 1 mo: 0.0 (95% CI: -0.3 to 0.4) 3 mo: -0.2 (95% CI: -0.4 to 0.5) 6 mo: -0.4 (95% CI: -0.9 to 0.0) 12 mo: -0.5 (95% CI: -1.0 to 0.0) |
= .439 = .463 = .153 = .092 |
| Bormann et al, 202264 | XEN: 69 Trab: 50 |
Mean ± SD 6 mo: 0.7 ± 0.1 vs. 0.3 ± 0.1 12 mo: 0.6 ± 0.1 vs. 0.5 ± 0.1 12 mo: 0.6 ± 0.1 vs. 0.4 ± 0.1 |
= .08 = .29 = .27 |
| Fili et al, 202265 | PF: 98 Trab: 92 |
Mean ± SD at 6 mo PF: 2.5 ± 1.2 to 0.1 ± 0.5 Trab: 2.7 ± 0.9 to 0.3 ± 0.5 Mean ± SD at 12 mo PF 0.4 ± 0.8 (84% change) Trab: 0 (100% change) |
Significantly reduced No. of AGMs for PF (P = .00091) and trab (P = .00072) at 12 mo |
| Fu et al, 202266 | PF: 101 Trab: 101 |
Median (range) at 12 mo PF: 0 (0-1) Trab: 0 (0-0) Median (range) at 18 mo PF: 0 (0-2) Trab: 0 (0-0) |
Both groups had significantly lower need for AGMs than baseline for all time points (P < .001) Significantly lower in trab group at 12 mo (P = .024) and 18 mo (P = .019) |
| Jamke et al, 202367 | PF: 29 Trab: 30 |
Median (range) at 12 mo PF: 4 (3-4) to 0 (0-0) Trab: 4 (4-4) to 0 (0-0) |
< .001 < .001 |
| Marcos-Parra et al, 201969 | XEN: 17 XEN + phaco: 48 Trab: 30 Trab + phaco: 26 |
Mean ± SD at 12 mo XEN: 2.5 ± 0.8 to 0.2 ± 0.6 XEN + phaco: 2.1 ± 0.9 to 0.1 ± 0.3 Trab: 2.5 ± 0.7 to 0.2 ± 0.5 Trab + phaco: 2.4 ± 0.8 to 0.1 ± 0.2 XEN and XEN + phaco: 2.2 ± 0.9 to 0.1 ± 0.4 |
< .0001 < .0001 < .0001 < .0001 < .0001 |
| Trab and trab + phaco: 2.4 ± 0.7 to 0.2 ± 0.5 | < .0001 No significant differences between XEN vs. trab and XEN + phaco vs. trab + phaco |
||
| Schargus et al, 202171 | XEN: 38 XEN + phaco: 42 Trab: 52 |
Mean ± SD, XEN vs. XEN + phaco vs. trab 6 mo: 0.9 ± 1.2 vs. 0.5 ± 1.1 vs. 0.4 ± 0.9 12 mo: 0.8 ± 1.2 vs. 0.5 ± 1.1 vs. 0.6 ± 1.2 24 mo: 0.8 ± 1.2 vs. 0.4 ± 1.0 vs. 0.5 ± 1.1 |
= .08 = .37 = .19 |
| Schlenker et al, 201785 | XEN: 185 Trab: 169 |
Median (IQR) at last follow-up 0.0 (IQR 0.0-1.0) vs. 0.0 (IQR 0.0-0.0) |
= .98 |
| Marcos-Parra et al, 202372 | XEN: 17 XEN + phaco: 46 Trab: 41 Trab + phaco: 30 |
Mean difference (95% CI), 36 mo XEN: -1.7 (95% CI: -2.5 to -0.9) XEN + phaco: -1.8 (95% CI: -2.2 to -1.5) Trab: -2.1 (95% CI: -2.5 to -1.8) Trab + phaco: -2.1 (95% CI: -2.6 to -1.7) XEN vs. trab: -0.5 (95% CI: -1.3 to 0.4) XEN + phaco vs. trab + phaco: |
< .001 < .001 < .001 < .001 <. 001 = .256 = .753 |
| Nobl et al, 202349 | PF: 31 Trab: 29 |
Mean ± SD at 12 mo PF: 2.7 ± 1.2 to 0.2 ± 0.7 Trab: 2.9 ± 1.2 to 0.3 ± 0.9 No difference between groups |
< .0001 < .0001 = .44 |
| Sharpe et al, 202273 | XEN: 90 Trab: 89 |
Mean ± SD XEN: 2.9 ± 1.1 to 1.1 ± 2.3 Trab: 3.1 ± 0.9 to 0.8 ± 1.4 |
< .0001 < .0001 = .39 |
| At 6 mo, No. of AGMs was similar between groups | |||
| Ponnusamy et al, 202178 | XEN: 17 Trab: 14 |
Mean ± SE 1 wk: 1.2 ± 0.3 vs. 0.3 ± 0.3 1 mo: 1.7 ± 0.3 vs. 0.3 ± 0.3 2 mo: 1.6 ± 0.4 vs. 1 ± 0.5 6 mo: 1.9 ± 0.5 vs. 1.1 ± 0.5 |
NR specifically for XEN vs. trab |
| Van Lancker et al, 202280 | PF: 70 Trab: 64 |
Mean ± SD 1 wk: 0.0 ± 0.3 vs. 0.0 ± 0.2 1 mo: 0.2 ± 0.5 vs. 0.0 ± 0.1 3 mo: 0.4 ± 1.0 vs. 0.3 ± 0.9 6 mo: 0.3 ± 06 vs. 0.4 ± 0.9 12 mo: 0.5 ± 1.1 vs. 0.6 ± 1.3 18 mo: 0.7 ± 1.2 vs. 0.5 ± 1.2 24 mo: 0.8 ± 1.4 vs. 0.5 ± 1.1 |
NS = .03 NS NS NS NS NS |
| Wagner et al, 202081 | XEN: 82 Trab: 89 |
Mean ± SD No. classes of AGMs 0.3 ± 0.5 vs. 0.2 ± 0.4 |
= .17 |
Abbreviations: AGM, antiglaucoma medication; CI, confidence interval; phaco, phacoemulsification; MIBS, minimally invasive bleb surgery; NR, not reported; NS, not significant; PF, PreserFlo; SD, standard deviation; SE, standard error; trab, trabeculectomy.
Table 11:
Changes in Number of Antiglaucoma Medications for Minimally Invasive Bleb Surgery Versus Other Glaucoma Treatments
| Author, year | N | No. of AGMs for MIBS vs. other glaucoma tx |
|---|---|---|
| Gambini et al, 202263 | XEN: 29 PF: 29 |
Mean ± SD at 6 mo, XEN vs. PF XEN: 2.5 ± 1.0 to 0.7 ± 1.1 PF: 2.7 ± 0.8 to 0.4 ± 1.2 % AGM-free, XEN vs. PF 44% vs. 56% (P = .14) |
| Duong et al, 202245 | XEN: 57 KDB: 18 |
Mean ± SD at 24 mo, XEN vs. KDB 1.5 ± 1.5 (54% reduction, P = .008) vs. 1.7 ± 0.6 (50% reduction, P = .038 Between-group differences in reduction: P = .710 Significantly greater reduction in AGM after XEN for 1 wk until 9 mo, but no significant differences from 12 mo to remainder of follow-up |
| Nuzzi et al, 202148 | XEN: 23 Cypass: 18 |
Mean ± SD, XEN vs. Cypass 3 mo: 0.13 ± 0.5 vs. 18.06 ± 1.60 6 mo: 0.45 ± 0.82 vs. 0.33 ± 0.65 12 mo: 0.45 ± 0.82 vs. 0.5 ± 0.85 24 mo: 0.45 ± 0.82 vs. 0.5 ± 0.85 36 mo: 0.55 ± 0.82 vs. 0.5 ± 0.85 |
| No significant difference (P = .44) | ||
| Olgun et al, 202038 | XEN: 114 GATT: 107 |
Mean ± SD, XEN vs. GATT 3 mo: 0.4 ± 0.8 vs. 1.4 ± 0.7, P < .001 6 mo: 0.8 ± 1.2 vs. 1.2 ± 0.6, P = .013 12 mo: 1.1 ± 1.4 vs. 1.3 ± 0.4, P = .084 18 mo: 1.7 ± 1.9 vs. 1.2 ± 0.4, P = .022 24 mo: 1.8 ± 1.8 vs. 1.2 ± 0.4, P = .009 |
| Ponnusamy et al, 202178 | XEN: 17 EX-PRESS: 16 |
Mean ± SE, XEN vs. EX-PRESS 1 wk: 1.2 ± 0.3 vs. 1.1 ± 0.5, P = .244 1 mo: 1.7 ± 0.3 vs. 1 ± 0.5, P = .060 2 mo: 1.6 ± 0.4 vs. 1.6 ± 0.4, P = .549 6 mo: 1.9 ± 0.5 vs. 1.4 ± 0.4, P = .475 |
| Stoner et al, 202143 | XEN: 52 EX-PRESS: 48 |
Mean ± SD, XEN vs. EX-PRESS at 1 y XEN: 2.8 ± 0.2 to 1.5 ± 0.2 (P = .002) EX-PRESS: 3.1 ± 0.2 to 0.5 ± 0.2 (P < .0001) No. of medications significantly higher for XEN at 1 mo, 3 mo, 6 mo, and 1 y |
| Saletta et al, 202282 | XEN: 30 PF: 30 |
Mean, XEN vs. PF 1 mo: 0.4 vs. 0.2 3 mo: 0.7 vs. 0.5 6 mo: 0.7 vs. 0.4 12 mo: 0.8 vs. 1.2 P = NR |
| Teixeira et al, 202046 | XEN-Baerveldt:12 Ahmed: 12 |
Mean, XEN-Baerveldt vs. Ahmed 1 mo: 0.9 ± 0.9 vs. 1.1 ± 0.8, P = .64 3 mo: 0.9 ± 1.1 vs. 0.7 ± 0.7, P = .47 6 mo: 1.5 ± 1.1 vs. 0.8 ± 1.2, P = .52 12 mo: 1.3 ± 1.0 vs. 0.75 ± 1.2, P = .29 Mean reduction, XEN-Baerveldt vs. Ahmed -1.8 ± -2.0 vs. -2.17 ± 1.59, P = .59 |
| Wagner et al, 202284 | XEN: 35 PF: 35 Trab: 35 |
Mean ± SD, XEN vs. PF vs. trab 0.7 ± 1.0 vs. 0.4 ± 0.8 vs. 0.5 ± 1.0 No difference between groups (P = .50) |
| Almendral-Gomez et al, 202340 | XEN: 63 NPDS: 65 |
Mean ± SE, XEN vs. NPDS XEN: 2.1 ± 0.7 to 0.2 ± 0.5 (P < .0001) NPDS: 2.0 ± 0.8 to 0.3 ± 0.6 (P < .0001) No difference between groups: mean difference: 0.2 (95% CI: -0.1 to 0.5); P = .2629 |
Abbreviations: AGM, antiglaucoma medication; CI, confidence interval; GATT, gonioscopy-assisted transluminal trabeculotomy; KDB, Kahook Dual Blade; MIBS, minimally invasive bleb surgery; NPDS, nonpenetrating deep sclerectomy; NR, not reported; PF, PreserFlo; SD, standard deviation; SE, standard error; trab, trabeculectomy; tx, treatment.
The GRADE certainty for MIBS versus trabeculectomy was Moderate for the RCTs (downgrading for risk of bias) and Very low for the observational studies (downgrading for risk of bias and imprecision). The GRADE certainty for MIBS versus other glaucoma treatments was Very low for the observational studies, also downgrading for risk of bias and imprecision (Table A6).
Health-Related Quality of Life
Sheybani et al36 evaluated work impairment using the Work Productivity and Activity Impairment: General Health questionnaire and glaucoma symptom burden using a shortened, 18-item checklist based on the Collaborative Initial Glaucoma Treatment Study Symptom and Health Problem Checklist among participants. The authors found that XEN had a consistently higher proportion of participants reporting complete resumption of their activities and daily routine compared with trabeculectomy. They also found that XEN had lower frequency and bothersome scores for both domains at all postoperative visits and a quicker reduction in frequency of eye symptoms and visual problems, compared with trabeculectomy.
Basilio et al89 evaluated the health-related quality of life for XEN using the Glaucoma Symptom Scale (GSS), which consists of 10 items and two domains: six visual items (SYMP-6: blurry/dim vision, hard to see in daylight, hard to see in darkness, and halos around lights) and four functional or symptom-related items (FUNC-4: feeling something in the eye, burning/smarting/stinging, tearing, itching, soreness/tiredness, dryness). The GSS is a 5-level scale rating how troublesome the symptom is (0 for very troublesome and 4 if the complaint was absent). A higher score indicates higher quality of life. The authors found no significant difference in GSS scores between XEN and trabeculectomy when symptoms were compared alone (P = .34) or as visual (P = 0.16) and non-visual (P = .43) symptoms. Analysis of the relationship between GSS and other study variables found a strong negative correlation between the administration of antiglaucoma medications and GSS results in both groups (P < .01 and = .01, respectively). There was also a moderate negative correlation between GSS and IOP for the XEN group (P = .03). No significant correlations were established for age, postoperative follow-up time, or BCVA. See Table 12 for details. The GRADE certainty for MIBS versus trabeculectomy was Moderate for the RCT (downgrading for imprecision) and Very low the observational study (downgrading for risk of bias) (Table A6).
Table 12:
Health-Related Quality of Life for Minimally Invasive Bleb Surgery Versus Trabeculectomy
| Author, year | N | Health-related quality of life for MIBS vs. trab |
|---|---|---|
| Randomized controlled trials | ||
| Sheybani et al, 202336 | XEN: 95 | Mean WPAI-GH, XEN vs. trab |
| Trab: 44 | Inability to work was max at 1 wk in both groups Between group difference at 1 wk: -32.3% (95% CI: -62.7 to -1.9), P = .038 Between group difference at 3 mo: -14.3% ± 6.7%, P = .033 WPAI-GH change from baseline, mean ± SD XEN: 18.0% ± 38.3%, P < .001 Trab: 22.1% ± 35.0%, P < .002 % of people with resumption of activities and daily routine, XEN vs. trab 2 wk: 26.3% vs. 13.6%, P = .125 1 mo: 41.4% vs. 31.8%, P = .350 SHPC-18 score, XEN vs. trab 1 d: 18.9 vs. 21.8 1 wk: 20.5 vs. 26.0 2 wk: 16.4 vs. 20.3 1 mo: 14.0 vs. 23.0 3 mo: 12.1 vs. 18.7 6 mo: 11.0 vs. 23.3 Between group differences suggest faster reduction for XEN vs. trab |
|
| Comparative observational studies | ||
| Basilio et al, 201889 | XEN: 17 | Mean GSS score ± SD, XEN vs. trab |
| Trab: 17 | 42.6 ± 6.8 vs. 41.6 ± 7.0, P = .34 Mean GSS non-visual symptoms, XEN vs. trab 19.8 vs. 19.5, P = .43 Mean GSS visual symptoms, XEN vs. trab 13.5 vs. 12.1, P = .16 |
|
Abbreviations: MIBS, minimally invasive bleb surgery; GSS, Glaucoma Symptom Scale; SD, standard deviation; SHPC-18, Symptom and Health Problem Checklist; trab, trabeculectomy; WPAI-GH, Work Productivity and Activity Impairment Questionnaire: General Health.
Follow-Up Visits
Studies generally found that the number of follow-up visits (e.g., postoperative visit, clinic visit, or follow-up visit) was significantly reduced for MIBS compared with trabeculectomy (Table 13). We did not find any studies evaluating follow-up visits for MIBS and other glaucoma treatments. Cappelli et al47 also evaluated mean hospitalization days, which was significantly reduced for MIBS compared with trabeculectomy. The GRADE certainty for MIBS versus trabeculectomy was Moderate for the RCT (downgrading for risk of bias) and Very low for the observational studies (downgrading for risk of bias and imprecision).
Table 13:
Follow-Up Visits for Minimally Invasive Bleb Surgery Versus Trabeculectomy
| Author, year | N | Follow-up visits for MIBS vs. trab |
|---|---|---|
| Randomized controlled trials | ||
| Sheybani et al, 202336 | XEN: 95 | % participants/eyes requiring postop intervention |
| Trab: 44 | Office-based: 34.7% vs. 63.6%, P < .0001 Office-based excluding laser suture lysis: 33 (34.7%) vs. 18 (40.9%), P = .024 OR-based: 13 (13.7%) vs. 8 (18.2%), P = .163 |
|
| Comparative observational studies | ||
| Cappelli et al, 202247 | XEN: 34 | Visit |
| Trab: 34 | 12 mo: 12.76 (4.41) vs. 10.88 (4.87), P = .15 24 mo: 22.04 (8.87) vs. 17.20 (8.87), P = .055 Mean (SD) hospitalization days 1 (0) vs. 1.9 (0.9), P < .001 |
|
| Fu et al, 202266 | PF: 101 Trab: 101 |
Median postop visits (range) 8 (6-10) vs. 10 (7-13), P = .001 |
| Kee et al, 202168 | XEN + phaco: 46 | Mean No. of postop visits within 1 y |
| Trab + phaco: 91 | 10.0 vs. 8.3, P = .002 | |
| Schlenker et al, 20 1890 | XEN: 185 | Mean No. clinic visits ± SD at 1 mo |
| Trab: 169 | 3.94 ± 1.59 vs. 4.62 ± 2.12 | |
| Crude: PF had 0.68 ± 2.56 fewer visits vs. trab | ||
| Adjusted for baseline differences: PF had 1.00 ± 2.32 fewer visits vs. trab (P < .001) | ||
|
Mean No. clinic visits ± SD at 3 mo 5.94 ± 3.00 vs. 6.70 ± 3.65 Crude: PF had 0.76 ± 4.91 fewer visits vs. trab Adjusted for baseline differences: 1.21 ± 4.06 (P < 1.001). > 6 visits within 1 mo 12 6.5%) vs. 26 (15.4%) > 14 visits within 3 mo 3 (1.6%) vs. 11 (6.5%) |
||
| Van Lancker et al, 202280 | PF: 70 | Mean No. of follow-up visits ± SD |
| Trab: 64 | < 12 mo: 8.2 ± 3.5 vs. 14.6 ± 6.7, P < .05 > 12 mo: 3.6 ± 4.4 vs. 4.0 ± 4.0, P = NS |
|
Abbreviations: MIBS, minimally invasive bleb surgery; NS, not significant; OR, operating room; phaco, phacoemulsification; PF, PreserFlo; SD, standard deviation; trab, trabeculectomy.
Adverse Events
In general, the total number of adverse events was lower for MIBS compared with trabeculectomy, as well as the number of severe adverse events (e.g., blebitis, endophthalmitis, retinal detachment) (Tables 14 and 15). The most commonly reported adverse events for both groups were hypotony (self-resolving), hyphema, choroidal effusion or detachment, and bleb-related complications.
Table 14:
Adverse Events of Minimally Invasive Bleb Surgery Versus Trabeculectomy
| Adverse event | Author, year | MIBS, N (%) | Trab, N (%) | P value |
|---|---|---|---|---|
| Increased IOP | Baker et al, 202137 | 100/395 (25.3%) | 65/131 (49.6%) | < .01 |
| Sheybani et al, 202336 | 20/95 (23.2%) | 22/44 (50.0%) | NR | |
| IOP decompensation | Fili et al, 202265 | 19/98 (19.4%) | 11/92 (12.0%) | NR |
| Hypotony | Baker et al, 202137 | 104/395 (26.3%) | 63/131 (48.1%) | < .01 |
| Fili et al, 202265 | 24/98 (24.5%) | 11/92 (12.0%) | NR | |
| Fu et al, 202266 | 46/101 (45.5%) | 51/101 (50.5%) | = .573 | |
| Kee et al, 202168 | 0/46 (0%) | 7/91 (7.7%) | = .095 | |
| Jamke et al, 202367 | 16/30 (53%) | 9/30 (30%) | = .115 | |
| Nobl et al, 202349 | 14/31 (45%) | 10/29 (35%) | = .44 | |
| Sacchi et al, 202375 | 2/7 (28.6%) | 6/7 (85.7%) | NR | |
| Ponnusamy et al, 202178 | 0/17 (0%) | 0/14 (0%) | NR | |
| Pillunat et al, 202179 | 18/26 (69%) | 7/26 (27%) | = .005 | |
| Pillunat et al, 202279 | 7/26 (27%) | 6/26 (23%) | = .76 | |
| Hyphema | Nobl et al, 202349 | 5/31 (16%) | 2/29 (7%) | = .43 |
| Cappelli et al, 202247 | 2/34 (5.9%) | 0/34 (0%) | NR | |
| Pillunat et al, 202279 | 0/26 (0%) | 2/26 (8%) | = .49 | |
| Marcos-Parra et al, 201969 | 5/65 (7.7%) | 17 (30.4%) | = .0013 | |
| Marcos-Parra et al, 202372 | 5/63 (7.9%) | 26/71 (36.6%) | < .001 | |
| Sacchi et al, 202375 | 1/7 (14.3%) | 0/7 (0%) | NR | |
| Olgun et al, 202177 | 6/49 (12.2%) | 2/28 (7.1%) | NR | |
| Kee et al, 202168 | 1/46 (2.2%) | 0/91 (0%) | = .336 | |
| Sheybani et al, 202336 | 6/95 (6.3%) | 3/44 (6.8%) | NR | |
| Van Lancker et al, 202280 | 5/70 (7%) | 0/65 (0%) | NS | |
| Conjunctival buttonhole | Sheybani et al, 202336 | 0/95 (0%) | 1/44 (2.3%) | NR |
| Subconjunctival bleeding | Baker et al, 202137 | 66/395 (16.7%) | 22/131 (16.8%) | = .98 |
| Ponnusamy et al, 202178 | 0/17 (0%) | 0/14 (0%) | NR | |
| Suprachoroidal hemorrhage | Ponnusamy et al, 202178 | 0/17 (0%) | 1/14 (7.1%) | NR |
| AC bleeding | Sheybani et al, 202336 | 1/95 (1.1%) | 2/44 (4.5%) | NR |
| Shallow AC | Baker et al, 202137 | 18/395 (4.6%) | 11/131 (8.4%) | = .98 |
| Kee et al, 202168 | 3/46 (6.5%) | 2/91 (2.2%) | = .334 | |
| Ponnusamy et al, 202178 | 0/17 (0%) | 0/14 (0%) | NR | |
| Seidel + | Fili et al, 202265 | 1/98 (1.0%) | 3/92 (3.3%) | NR |
| Cappelli et al, 202247 | 1/34 (2.9%) | 3/34 (8.8%) | = .30 | |
| Jamke et al, 202367 | 0/30 (0%) | 6/30 (20%) | = .023 | |
| Marcos-Parra et al, 201969 | 2/65 (4.1%) | 1/56 (1.8%) | = .6501 | |
| Marcos-Parra et al, 202372 | 2/63 (3.2%) | 2/71 (2.8%) | = 1.000 | |
| Nobl et al, 202349 | 3/31 (10%) | 0/29 (0%) | = .24 | |
| Flat AC | Cappelli et al, 202247 | 0/34 (0%) | 5/34 (14.7%) | NR |
| Marcos-Parra et al, 201969 | 1/65 (1.5%) | 11/56 (19.6%) | = .0009 | |
| Marcos-Parra et al, 202372 | 1/63 (1.6%) | 2/71 (2.8%) | = .498 | |
| Sacchi et al, 202375 | 0/7 (0%) | 2/7 (28.6%) | NR | |
| Ponnusamy et al, 202178 | 0/17 (0%) | 0/14 (0%) | NR | |
| Nobl et al, 202349 | 5/31 (16%) | 2/29 (7%) | = .43 | |
| Corneal complications | Nobl et al, 202349 | 5/31 (16%) | 6/29 (21%) | = .75 |
| Choroidal effusion/detachment | Baker et al, 202137 | 18/395 (4.6%) | 8/131 (6.1%) | = .98 |
| Sheybani et al, 202336 | 2/95 (2.1%) | 4/44 (9.1%) | NR | |
| Ponnusamy et al, 202178 | 0/17 (0%) | 0/14 (0%) | NR | |
| Cappelli et al, 202247 | 6/34 (17.6%) | 2/34 (5.9%) | = .13 | |
| Fili et al, 202265 | 23/98 (23.4%) | 27/92 (29.3%) | NR | |
| Fu et al, 202266 | 13/101 (12.9%) | 15/101 (14.9%) | = .839 | |
| Fu et al, 202266 | 0/101 (0%) | 2/101 (2.0%) | = .498 | |
| Jamke et al, 202367 | 8/30 (27%) | 7/30 (23%) | = .776 | |
| Olgun et al, 202138 | 0/49 (0%) | 0/31 (0%) | NR | |
| Sacchi et al, 202375 | 1/7 (14.3%) | 4/7 (57.1%) | NR | |
| Nobl et al, 202349 | 11/31 (36%) | 5/29 (17%) | = .15 | |
| Pillunat et al, 202279 | 4/26 (15%) | 5/26 (20%) | = 1.0 | |
| Van Lancker et al, 202280 | 2/70 (3%) | 8/64 (13%) | NS | |
| Bleb leak | Baker et al, 202137 | 26/395 (6.6%) | 17/131 (13.0%) | = .74 |
| Sacchi et al, 202375 | 0/7 (0%) | 1/7 (14.3%) | NR | |
| Van Lancker et al, 202280 | 3/70 (4%) | 5/64 (8%) | NR | |
| Sheybani et al, 202336 | 0/95 (0%) | 7/44 (15.9%) | NR | |
| Bleb-related complications | Baker et al, 202137 | 15/395 (3.8%) | 8 (6.1%) | = .98 |
| Bleb fibrosis | Marcos-Parra et al, 201969 | 8/65 (12.3%) | 1/56 (1.8%) | = .0285 |
| Marcos-Parra et al, 202372 | 8/63 (12.7%) | 2/71 (2.8%) | = .045 | |
| Sheybani et al, 202336 | 4/95 (4.2%) | 0/44 (0%) | NR | |
| Blebitis or endophthalmitis | Nobl et al, 202349 | 0/31 (0%) | 0/29 (0%) | = .24 |
| Ponnusamy et al, 202178 | 0/17 (0%) | 0/14 (0%) | NR | |
| Sacchi et al, 202375 | 0/7 (0%) | 0/7 (0%) | NR | |
| Van Lancker et al, 202280 | 0/70 (0%) | 0/64 (0%) | NS | |
| Pillunat et al, 202279 | 0/26 (0%) | 0/26 (0%) | = 1.0 | |
| Tenon's cyst | Marcos-Parra et al, 201969 | 7/65 (10.8%) | 5/56 (8.9%) | = .7366 |
| Marcos-Parra et al, 202372 | 7 (11.1%) | 6 (8.5%) | = .771 | |
| Iris damage | Sheybani et al, 202336 | 0/95 (0%) | 1/44 (2.3%) | NR |
| Iris adhesions | Sheybani et al, 202336 | 3/95 (3.2%) | 2/44 (4.5%) | NR |
| Retinal detachment | Jamke et al, 202367 | 0/30 (0%) | 0/30 (0%) | = 1.0 |
| Pillunat et al, 202279 | 0/26 (0%) | 0/26 (0%) | = 1.0 | |
| Van Lancker et al, 202280 | 0/70 (0%) | 0/64 (0%) | = 1.0 |
Abbreviations: AC, anterior chamber; IOP, intraocular pressure; MIBS, minimally invasive bleb surgery; NR, not reported; NS, not significant; trab, trabeculectomy.
Table 15:
Adverse Events of Minimally Invasive Bleb Surgery Versus Other Glaucoma Treatments
| Adverse event | Author, year | MIBS, N (%) | Other glaucoma tx, N (%) | P value |
|---|---|---|---|---|
| Hypotony | Scheres et al, 202183 | XEN: 10/41 (24%) | PF: 16/41 (39%) | NR |
| Stoner et al, 202143 | XEN: 15/52 (28.9%) | EX-PRESS: 25/48 (52.1%) | = .023 | |
| Olgun et al, 202038 | XEN: 0/114 (0%) | GATT: 0/107 (0%) | NR | |
| Elevated IOP | Almendral-Gomez et al, 202340 | XEN: 1/63 (1.6%) | NPDS: 0/65 (0%) | = .3097 |
| (Micro)hyphema | Scheres et al, 202183 | XEN: 9/41 (22%) | PF: 8/41 (20%) | NR |
| Stoner et al, 202143 | XEN: 11/52 (21.2%) | EX-PRESS: 6/48 (12.5%) | = .248 | |
| Olgun et al, 202038 | XEN: 30/114 (26.3%) | 30/107 (28.0%) | NR | |
| Seidel | Almendral-Gomez et al, 202340 | XEN: 0/63 (0%) | NPDS: 2/65 (3%) | = .1638 |
| Choroidal effusion | Stoner et al, 202143 | XEN: 1/52 (1.9%) | EX-PRESS: 9/48 (18.8%) | = .022 |
| Teixeira et al, 202046 | XEN-Baerveldt: 0/12 (0%) | Ahmed: 2/12 (16.7%) | NR | |
| Choroidal detachment | Saletta et al, 202282 | XEN: 4/30 (13%) | PF: 3/30 (10%) | NR |
| Scheres et al, 202183 | XEN: 1/41 (2%) | PF: 1/41 (2%) | NR | |
| Theillac et al, 202041 | XEN: 0/47 (0%) | NPDS: 1/58 (1.7%) | = .91 | |
| Almendral-Gomez et al, 202340 | XEN: 0/63 (0%) | NPDS: 0/65 (0%) | = .1638 | |
| Endophthalmitis | Saletta et al, 202282 | XEN: 0/30 (0%) | PF: 0/30 (0%) | NR |
| Olgun et al, 202038 | XEN: 1/114 (0.9%) | GATT: 0/107 (0%) | NR | |
| XEN obstruction | Teixeira et al, 202046 | XEN-Baerveldt: 3/12 (25.0%) | Ahmed: NA/12 | NR |
| Poorly functioning bleb | Teixeira et al, 202046 | XEN-Baerveldt: 0/12 (0%) | Ahmed: 3/12 (25.0%) | NR |
| Choroidal hemorrhage | Saletta et al, 202282 | XEN: 0/30 (0%) | PF: 0/30 (0%) | NR |
| Suprachoroidal hemorrhage | Olgun et al, 202038 | XEN: 0/114 (0%) | GATT: 1/107 (0.9%) | NR |
| Vitreous hemorrhage | Almendral-Gomez et al, 202340 | XEN: 1/63 (1.6%) | NPDS: 0/65 (0%) | = .3097 |
| Device migration | Scheres et al, 202183 | XEN: 1/41 (2%) | PF: 0/41 (0%) | NR |
| Ptosis | Scheres et al, 202183 | XEN: 0/41 (0%) | PF: 1/41 (2%) | NR |
Abbreviations: GATT, gonioscopy-assisted transluminal trabeculotomy; IOP, intraocular pressure; MIBS, minimally invasive bleb surgery; NPDS, nonpenetrating deep sclerotomy; NR, not reported; PF, PreserFlo; tx, treatment.
Follow-Up Interventions
Studies found that MIBS generally resulted in similar or fewer follow-up interventions compared with trabeculectomy and other glaucoma treatments, with the possible exception of bleb needling (Tables 16 and 17). Bleb needling and revision may be increased in the MIBS groups compared with other glaucoma treatments, indicating that, while MIBS is a minimally invasive procedure, people may still need followup for bleb fibrosis revision and needling.
Table 16:
Follow-Up Interventions for Minimally Invasive Bleb Surgery Versus Trabeculectomy
| Author, year | N | MIBS vs. trab | P value |
|---|---|---|---|
| Randomized controlled trials | |||
| Baker et al, 202137 | PF: 395 Trab: 132 |
40.8% vs. 67.4% had ≥ 1 postoperative intervention Between-group difference: -26.7% (95% CI: -36.0 to -17.3) |
< .01 |
| Comparative observational studies | |||
| Cutolo et al, 202391 | XEN: 284 Trab: 173 |
Unplanned reoperations within 90 d 4/284 (1.4%) vs. 9/226 (4.0%) Causes included bleb scarring, bleb leak, aqueous misdirection, suprachoroidal hemorrhage, overfiltration and hypotony maculopathy, corneal decompensation |
= .07 |
| Fili et al, 202265 | PF: 98 Trab: 92 |
AC reformation: 4 (4.1%) vs. 8 (8.7%) AC washout: 3 (3.1%) vs. 0 (0%) Bleb revision: 19 (19.4%) vs. 4 (4.3%) Needling: 4 (4.1%) vs. 0 (0%) |
NR |
| Fu et al, 202266 | PF: 101 Trab: 101 |
Bleb revision in clinic: 3 (3.0%) vs. 1 (1.0%) Bleb revision in theatre: 11 (10.9%) vs. 25 (24.8%) AC reformation: 0 (0%) vs. 4 (4.0%) AC washout: 3 (3.0%) vs. 0 (0%) Laser suture lysis: 0% vs. 4 (4.0%) |
= .621 = .016 = .121 = .498 = .121 |
| Jamke et al, 202367 | PF: 30 Trab: 30 |
Laser suture lysis: 0 (0%) vs. 13 (43%) Encapsulation and bleb needling: 1 (3%) vs. 4 (13%) AC reformation: 8 (27%) vs. 7 (23%) |
< .001 = .353 = .776 |
| Kee et al, 202168 | XEN + phaco: 46 Trab + phaco: 91 |
Bleb massage: 9/91 (19.6%) vs. 50/91(54.9%) Bleb interventions: 38/46 (82.6%) vs. 11/46 (12.1%) Non-bleb interventions: 5/46 (10.9%) vs. 55/91 (60.4%) |
< .001 < .001 < .001 |
| Nobl et al, 202376 | PF: 31 Trab: 29 |
Further surgical intervention needed: 3 (9.7%) vs. 1 (3.4%) Needling: 7 (0.23/eye) vs. 8 (0.28/eye) Postoperative subconjunctival 5-FU injections: 32 (1.03/eye) vs. 28 (0.97/eye) |
NR = .055 = .42 |
| Schlenker et al, 201785 | XEN: 185 Trab: 169 |
Reoperation rate: 19 (10.3%) vs. 9 (5.3%) Needling: 80 (43.2%) vs. 52 (30.8%) Laser suture lysis: 0 (0%) vs. 84 (49.7%) AC reformation: 22 (11.2%) vs. 13 (7.7%) Bleb repair: 2 vs. 10 (1.1%) vs. 10 (5.9%) Iris sweep/synecialysis: 3 (1.6%) vs. 4 (2.4%) |
= .11 |
| YAG to implant/ostomy: 3 (1.6%) vs. 2 (1.2%) Iridoplasty: 2 (1.1%) vs. 0 (0%) Bleb cautery: 1 (0.5%) vs. 0 (0%) |
|||
| Pillunat et al, 202279 | PF: 26 Trab: 26 |
Laser suture lysis: 0% vs. 12% Encapsulation and bleb needling: 0% vs. 15% Secondary surgery: 0% vs. 0% |
= .24 = .11 = 1.0 |
| Van Lancker et al, 202280 | PF: 70 Trab: 64 |
AC reformation: 3 vs. 10 AC washout: 2 vs. 0 Laser suture lysis: 0 vs. 1 Laser iridoplasty: 2 vs. 0 Laser peripheral iridotomy: 1 vs. 0 Laser capsulotomy: 1 vs. 1 Bleb revision: 0 vs. 2 |
NR NR NR NR NR NR NR |
| Schlenker et al, 201886 | XEN: 185 Trab: 169 |
Postoperative intervention: 95 (51.4%) vs. 105 (62.1%) Laser suture lysis: 0 (0%) vs. 124 (73.4%) AC reformation: 33 (17.8%) vs. 16 (9.5%) Bleb repair/conjunctival suturing: 3 (1.6%) vs. 14 (8.3%) Iris sweep/synecialysis: 3 (1.6%) vs. 4 (2.4%) YAG to implant/ostomy: 7 (3.8%) vs. 2 (1.2%) Iridoplasty: 2 (1.1%) vs. 0 (0%) Laser ostomy: NA vs. 0 (0%) Bleb cautery: 1 (0.5%) vs. 0 (0%) |
= .0004 NA = .21 = .015 = .71 = 1.0 = .50 NA = 1.0 |
| Marcos-Parra et al, 201969 | XEN: 17 XEN + phaco: 48 Trab: 30 Trab + phaco: 26 |
Needling: 13/65 (20.0%) vs. 3/56 (5.4%) Surgical revision: 6/65 (9.2%) vs. 3/56 (5.4%) |
= .2749 = .182 |
| Marcos-Parra et al, 202372 | XEN: 63 Trab: 71 |
Needling: 12 (19.0%) vs. 4 (5.6%) Surgical revision: 10 (15.9%) vs. 7 (9.9%) |
= .313 = .771 |
| Ponnusamy et al, 202178 | XEN: 17 Trab: 14 |
Re-needling rate: 18% vs. 22% | NR specifically for XEN vs. trab |
| Olgun et al, 202177 | XEN: 31 Trab: 49 |
Bleb needling: 12 (24.%) vs. 7 (22.6%) | NS |
| Sacchi et al, 202375 | XEN: 7 Trab: 7 | Bleb needling: 2 (28.6%) vs. 2 (28.6%) Blood injection: 0 (0%) vs. 3 (42.3%) Laser suture lysis: 0 (0%) vs. 1 (14.3%) AC visoelastic injection: 0 (0%) vs. 2 (28.6%) |
NR NR NR NR |
| Surgical bleb revision: 1 (14.3%) vs. 1 (14.3%) | NR | ||
Abbreviations: AC, anterior chamber; FU, fluorouracil; MIBS, minimally invasive bleb surgery; YAG, yttrium aluminum garnet; NA, not applicable; NR, not reported; NS, not significant; PF, PreserFlo; phaco, phacoemulsification; trab, trabeculectomy.
Table 17:
Follow-Up Interventions for Minimally Invasive Bleb Surgery Versus Other Glaucoma Treatments
| Author, year | N | Follow-up interventions for MIBS vs. other glaucoma tx | P value |
|---|---|---|---|
| Gambini et al, 202263 | XEN: 29 PF: 29 |
AC reformation: 2 (6.9%) vs. 1 (3.4%) Needling: 21% vs. 7% Bleb revision: 7% vs. 3% |
NR = .14 NR |
| Saletta et al, 202282 | XEN: 30 PF: 30 |
Needling: 10/30 (33%) vs. 3/30 (10%) Bleb revision: 0/30 (0%) vs. 4/30 (13%) |
NR NR |
| Scheres et al, 202183 | XEN: 41 PF: 41 |
Needling: 8/41 (20%) vs. 2/41 (5%) Bleb revision: 2/41 (5%) vs. 2/41 (5%) Glaucoma filtration surgery: 3/41 (7%) vs. 6/41 (15%) |
= .09 = 1.00 = .48 |
| Phacoemulsification: 6/41 (38%) vs. 4/41 (18%) MP-TSCPC: 8/41 (20%) vs. 1/41 (2%) Trabecular microbypass stent: 1/41 (2%) vs. 1/41 (2%) |
= .27 = .029 = 1.00 |
||
| Almendral-Gomez et al, 202340 | XEN: 63 NPDS: 65 |
Needling failure: 1/63 (1.6%) vs. 0/65 (0%) | = .3097 |
| Stoner et al, 202143 | XEN: 52 EX-PRESS: 48 |
Needling: 15 (28.9%) vs. 9 (18.8%) Bleb revision: 11 (21.2%) vs. 13 (27.1%) Laser suture lysis: 3 (5.8%) vs. 0% |
= .262 = .558 NA |
| Ponnusamy et al, 202178 | XEN: 17 EX-PRESS: 16 |
Re-needling rate: 18% vs. 13% | = .92 (for XEN vs. trab vs. EX-PRESS |
| Duong et al, 202245 | XEN: 57 KDB: 18 |
8/57 (24%) vs. 8/18 (44%) needed additional glaucoma surgery Needling: 21/57 (37%) vs. NR Nd:YAG goniopuncture: 0/57 (0%) vs. 1 (5.6%) |
= .006 NR NR |
| Olgun et al, 202038 | XEN: 114 GATT: 107 |
Further glaucoma surgery: 5/114 (4.4%) vs. 7/107 (6.5%) | = .480 |
| Theillac et al, 202041 | XEN: 47 NPDS: 58 |
Second surgery: 2/47 (4.3%) vs. 0/58 (0%) Bleb revision surgery: 1/47 (2.1%) vs. 1/58 (1.7%) |
= .82 = .88 |
| Touboul et al, 202242 | XEN: 70 NPDS: 103 |
Reoperation: 17.14% vs. 8.74% | = .14 |
| Mean difference for reoperation: -8.40 (95% CI: -18.78 to 1.97) | = .10 | ||
| Median (IQR) before reoperation: 9.00 mo (2.75-16.25) vs. 11.00 mo (6.00-15.00) 1 needling procedure: 11.4% vs. 7.8% 2 needling procedures: 12.9% vs. 10.7% ≥ 3 needling procedures: 15.7% vs. 18.5% |
= .57 |
Abbreviations: AC, anterior chamber; GATT, gonioscopy-assisted transluminal trabeculotomy; IQR, interquartile range; KDB, Kahook Dual Blade; MIBS, minimally invasive bleb surgery; MIBS, minimally invasive bleb surgery; Nd:YAG: neodymium-doped yttrium aluminum garnet; NPDS, nonpenetrating deep sclerectomy; NR, not reported; PF, PreserFlo; phaco, phacoemulsification; trab, trabeculectomy; tx, treatment.
The included studies differed in their categorization (e.g., short-term vs. long-term, mild vs. severe, overall) and nomenclature of follow-up interventions. We have reported the events as listed within the included studies. The GRADE certainty for MIBS versus trabeculectomy was Moderate for the RCT (downgrading for imprecision) and Very low for the observational studies (downgrading for risk of bias and imprecision). The GRADE certainty for MIBS versus other glaucoma treatments was Very low for the observational studies, also downgrading for risk of bias and imprecision (Table A6).
Discussion
Our results align with previous related systematic reviews (Appendix 2). In general, we found significant improvements in outcomes for both MIBS and the included study comparator compared with baseline. Minimally invasive bleb surgery may result in improvements less than or similar to other glaucoma treatments; however, there is uncertainty in the evidence.
We found limited high-quality comparative evidence for MIBS. There has been only one noninferiority RCT conducted on each of the XEN Gel Stent36 and the PreserFlo MicroShunt37; however, the results were inconsistent for some outcomes (e.g., noninferior or inferior compared with trabeculectomy). While the RCTs did evaluate different MIBS devices, study methods and analyses differed (e.g., noninferiority analysis margins and power, primary outcomes). Due to their noninferiority design, the RCTs can only demonstrate that MIBS is no worse than trabeculectomy and cannot comment on whether MIBS is superior to trabeculectomy.
The majority of the studies compared MIBS with trabeculectomy, with some studies also evaluating MIBS compared with gonioscopy-assisted transluminal trabeculotomy,38 filtering canaloplasty,39 nonpenetrating deep sclerotomy,40-42 EX-PRESS,43 Kahook Dual Blade,45 iStent with phacoemulsification and endocyclophotocoagulation (ICE2).44 This reflects clinical practice, where, according to experts, trabeculectomy is the most relevant comparator for MIBS.
There were risk-of-bias concerns among the included studies. Differences in baseline characteristics in studies were not always adjusted for, which affected study results. Some observational studies were too underpowered to detect differences among study groups. The majority of the included comparative observational studies had high risk of bias, primarily due to their retrospective design. For these reasons, the overall generalizability of these included studies may be limited.
We found comparative studies that included both standalone XEN and combined XEN cataract surgery in their population, but the impact of concurrent cataract surgery was not consistently evaluated or reported. Included studies that examined combined cataract surgery found no significant changes in IOP between groups, except for the early postoperative period (lower for XEN with concurrent cataract surgery). However, a systematic review of standalone XEN versus XEN and cataract surgery found that standalone XEN may be better in lowering IOP in the early postoperative period.92
Some studies included both naïve glaucoma participants (with no previous glaucoma procedures) and those who had previously failed a glaucoma procedure. Other studies found that MIBS is effective for people who had previously failed glaucoma procedures due to its minimally invasive nature, but that this population may require more antiglaucoma medications compared with naïve glaucoma participants.51
While we did not specifically evaluate the impact of surgical techniques on outcomes, studies have found that deeper implant positions in the conjunctiva for XEN may achieve better outcomes and lower the need for follow-up needling.93 The ab interno versus ab externo surgical technique may also affect clinical outcomes, although other studies have found they may be similar in outcomes such as changes in IOP and medication use.13,61
Clinical outcomes were assessed using standardized or validated tools, which reduced measurement variability. However, clinical success or failure was variably defined within the studies and did not always reflect standard clinical definitions. Surgeon experience has also been shown to potentially impact clinical outcomes, but this information was often not provided or accounted for among the studies. Studies have found that the learning curve for MIBS may be significant and that device position is crucial for optimal results.93 94
In general, the included studies found that there were fewer overall adverse events (and serious adverse events) and fewer follow-ups for MIBS compared with conventional or incisional glaucoma surgery. This aligns with the less invasive nature of MIBS. However, an exception may be the outcome of bleb needling. Some studies found that MIBS had higher rates of bleb needling, which can result in additional follow-ups, than did trabeculectomy. We also found that the reporting of adverse events differed between studies (e.g., category or severity of adverse events, nomenclature and description of adverse events), which makes direct comparisons between studies difficult. A systematic review on the method and quality of reporting complications in MIGS studies also found that complications were not reported uniformly well or in the same manner.95
Long-term follow-up results were also limited, with the majority of studies evaluating outcomes at 1 year or earlier. The longest follow-up duration was 3 years.47,48 The longer term direct comparative effectiveness of MIBS compared with glaucoma treatment is therefore unclear. However, noncomparative studies with up to 5-year follow-up have shown the continued effectiveness of MIBS (reduction in IOP and use of medications).96,97
In addition, there was a lack of comparative studies on XEN 63, which may provide higher reductions in IOP compared with XEN 45.58 XEN 63 has only recently been commercialized in Canada, but may be beginning to replace XEN 45 in clinical use.
While there is uncertainty within the evidence, MIBS provides an alternative for people with moderate to advanced/severe glaucoma who may have failed maximum medical therapy and who cannot or choose not to undergo conventional or incisional glaucoma surgery. Minimally invasive bleb is currently the only minimally invasive option that exists for this patient population. The procedure time may also be shorter than that of trabeculectomy. But prospective studies with low risk of bias are required, in particular to evaluate the comparative effectiveness of XEN 63 due to its increasing clinical use. Future longer-term studies are also needed to compare MIBS with other glaucoma treatments.
Strengths and Limitations
We have included the most recent evidence on the effectiveness and safety of MIBS compared with other treatments for people with glaucoma. While multiple other systematic reviews (Appendix 2) have been published on MIBS, they differed in either their population, comparator, outcomes of interest, or methods, and the study results were not consistently analyzed within the reviews. The majority of the studies conducted on MIBS are noncomparative studies. We chose not to conduct a meta-analysis due to these differences in population and study methodology.
The evaluation of MIBS is also rapidly evolving, with most studies having been published within the last 2 years. Most systematic reviews were conducted earlier and did not capture these recent studies (e.g., Sheybani et al,36 a pivotal new randomized controlled trial comparing XEN with trabeculectomy, was published in 2023).
Conclusions
Minimally invasive bleb surgery reduces IOP (clinically meaningful) and the number of medications used, but we are uncertain if MIBS results in similar outcomes as trabeculectomy (GRADE: Moderate to Very low). Compared with trabeculectomy, MIBS may result in improved health-related quality of life, fewer follow-up visits, adverse events, and follow-up interventions (GRADE: Moderate to Very low). MIBS may also reduce IOP and the number of medications used, compared with other glaucoma treatments, but the evidence is very uncertain (GRADE: Very low). We found limited long-term data on MIBS.
Economic Evidence
Research Question
What is the cost-effectiveness of minimally invasive bleb surgery (MIBS) compared with other treatment alternatives for people with glaucoma?
Methods
Economic Literature Search
We performed an economic literature search on March 8, 2023, to retrieve studies published from database inception until the search date. To retrieve relevant studies, we developed a search using the clinical search strategy with an economic and costing filter applied.
We created database auto-alerts in MEDLINE and Embase and monitored them until June 2, 2023. We also performed a targeted grey literature search following a standard list of websites developed internally, which includes the International HTA Database and the Tufts Cost-Effectiveness Analysis Registry. See Clinical Literature Search, above, for further details on methods used. See Appendix 3 for our literature search strategies, including all search terms.
Eligibility Criteria
Studies
Inclusion Criteria
English-language full-text publications
Cost-benefit analyses, cost-effectiveness analyses, cost-consequence analyses, cost-minimization analyses, cost-utility analyses, budget impact analyses, or systematic reviews of economic analyses
Exclusion Criteria
Studies in which the outcomes of interest are not reported or cannot be extracted
Nonsystematic reviews, editorials, case reports, commentaries, conference abstracts, letters, and unpublished studies
Noncomparative costing studies or feasibility analyses
POPULATION
Inclusion Criteria
Adults (≥ 18 years old) with any type of glaucoma and any cataract status
Exclusion Criteria
People with only increased intraocular pressure (IOP), or who otherwise have not been diagnosed with glaucoma
Interventions
Inclusion Criteria
MIBS with or without concomitant cataract surgery
Received XEN Gel Stent (XEN 45, 63, or 140)
Received PreserFlo MicroShunt
Exclusion Criteria
Minimally invasive glaucoma surgeries (MIGS) that do not create a subconjunctival outflow pathway
-
Conventional or incisional glaucoma surgery
-
—
Penetrating and non-penetrating glaucoma surgeries, including device-modified trabeculectomy (e.g., Ex-PRESS Glaucoma Filtration Device)
-
—
Glaucoma drainage implant surgery using conventional glaucoma drainage devices (e.g., Ahmed Valve, Baerveldt Implant)
Comparators
Inclusion Criteria
-
Conventional or incisional glaucoma surgery
-
—
Penetrating and non-penetrating glaucoma surgeries, including device-modified trabeculectomy
-
—
Glaucoma drainage implant surgery using conventional glaucoma drainage devices
-
—
Different MIBS device (i.e., head-to-head comparisons of different devices)
Any type of MIGS
Exclusion Criteria
Different version of the same device
Different surgical technique using the same device
Different patient population using the same device
Outcome Measures
Costs
Health outcomes (e.g., quality-adjusted life-years)
Incremental costs
Incremental effectiveness
Incremental cost-effectiveness ratios
Literature Screening
A single reviewer conducted an initial screening of titles and abstracts using Covidence30 and then obtained the full texts of studies that appeared eligible for review according to the inclusion criteria. The same reviewer then examined the full-text articles and selected studies eligible for inclusion. The reviewer also examined reference lists and consulted content experts for any additional relevant studies not identified through the search.
Data Extraction
We extracted relevant data on study characteristics and outcomes to collect information about the following:
Source (e.g., citation information, study type)
Methods (e.g., study design, analytic technique, perspective, time horizon, population, intervention[s], comparator[s])
Outcomes (e.g., health outcomes, costs, incremental cost-effectiveness ratios)
We contacted study authors to provide clarification as needed.
Study Applicability and Limitations
We determined the usefulness of each identified study for decision-making by applying a modified quality appraisal checklist for economic evaluations originally developed by the National Institute for Health and Care Excellence (NICE) in the United Kingdom to inform the development of NICE's clinical guidelines.98 We modified the wording of the questions to remove references to guidelines and to make it specific to Ontario. Next, we separated the checklist into two sections. In the first section, we assessed the applicability of each study to the research question (directly, partially, or not applicable). In the second section, we assessed the limitations (minor, potentially serious, or very serious) of the studies that we found to be directly applicable.
Results
Economic Literature Search
The database search of the economic literature yielded 47 citations published from database inception until March 8, 2023, including grey literature searches and after duplicates were removed. We did not identify any eligible studies from other sources, including database alerts (monitored until June 2, 2023). In total, we identified six studies that met our inclusion criteria. Appendix 6 describes the single study that was excluded after full-text review.99 Figure 2 presents the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow diagram for the economic literature search.
Figure 2: PRISMA Flow Diagram - Economic Search Strategy.
Abbreviation: PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analyses.
Source: Adapted from Page et al.35
PRISMA flow diagram showing the clinical search strategy. The database search of the clinical literature yielded 73 citations published from database inception to March 8, 2023. After removing duplicates, we screened the abstracts of 47 studies and excluded 40. We assessed the full text of seven articles and excluded a single study. In the end, we included six articles in the qualitative synthesis.
Overview of Included Economic Studies
We identified six 14,19,80,100-102 relevant studies published from database inception to March 8, 2023. Table 18 describes the study design, population, interventions, comparators, and results of the included studies. We identified two Canadian studies14,19 and four international studies.80,100-102 Four economic studies examined the XEN 45 or the XEN 63 Gel Stent,14,19,101,102 and two studies examined PreserFlo MicroShunt.80,100
Table 18:
Results of Economic Literature Review - Summary
| Author, year, country | Analytic technique, study design, perspective, time horizon | Population | Intervention(s) and comparator(s) | Results | ||
|---|---|---|---|---|---|---|
| Health outcomes | Costs | Cost-effectiveness | ||||
| CADTH, 2019,14 Canada | Cost-utility analysis Markov model Canadian public payer Lifetime horizon |
Adults with moderate glaucoma Age: 65 Baseline VF: -6.54 |
Interventions: XEN 45 Comparator: Trabeculectomy |
QALYs (vs. trabeculectomy) 12.49 (-0.0003) 12.49 |
2018 CAD (vs. trabeculectomy) $12,322 (-$1,053) $13,375 |
ICUR of trabeculectomy vs. ($/QALY) $3,050,721 |
| INESSS, 2020,19 Canada | Cost-minimization analysis Model based Quebec public payer 1-year horizon |
Adults with moderate or advanced primary open angle glaucoma | Interventions: XEN 45 with phacoemulsification XEN 45 without phacoemulsification Comparator: Trabeculectomy with phacoemulsification Trabeculectomy without phacoemulsification |
NA NA NA NA |
2020 CAD Confidential Confidential $3,624 $3,281 |
The XEN 45 is more costly than trabeculectomy, primarily due to device costs |
| Atik et al, 2022,100 United States | Cost-utility analysis Markov model US societal 1-year horizon |
Adults with primary open angle glaucoma | Intervention: PreserFlo MicroShunt Comparator: Trabeculectomy |
QALYs 0.85 0.86 |
2021 USD $6,318 $4,260 |
The PreserFlo MicroShunt is more costly and less effective than trabeculectomy Scenario analyses indicated that the model was most sensitive to the price of the PreserFlo MicroShunt and the |
| frequency of adverse events. The model was not sensitive to the time horizon duration | ||||||
| Van Lancker et al,80 2022, United Kingdom | Cost-consequence Retrospective UK public payer 19 months |
Adults with uncontrolled glaucoma | Intervention: PreserFlo MicroShunt Comparator: Trabeculectomy |
Surgical failure at 18 months: 25% 35% |
2021 GBP £3,972 £4,261 |
The PreserFlo MicroShunt was associated with lower costs and a lower probability of surgical failure when compared with trabeculectomy |
| Martínez-de-la-Casa et al, 2019,101 Spain | Budget impact Model-based Spanish National Health System 3-year horizon |
Adults with mild, moderate, or uncontrolled glaucoma | Intervention: Standard care includes the XEN 45 Comparator: Standard care does not include the XEN 45 |
NA NA |
2016 EUR €4,665.41 €4,097.78 |
Introducing the XEN 45 to the Spanish standard of care is estimated to reduce the total expenditures |
| Vila Arteaga et al, 2022,102 Spain | Budget impact Model based Spanish National Health System 1-year horizon |
Adults with primary open angle glaucoma | Intervention: Standard care includes the XEN 63 Comparator: Standard care does not include the XEN 63 |
NA NA |
1-year budget impact, 2021 EUR €85.9 milliona €83.3 million |
Introducing the XEN 65 into the Spanish standard of care is estimated to reduce the budget impact by €2.56 million EUR |
Abbreviations: CADTH, Canadian Agency for Drugs and Technologies in Health; ICUR, incremental cost-utility ratio; INESSS, Institut national d'excellence en santé et en services sociaux; NA, not applicable; QALY, quality-adjusted life-years.
Population size of 25,718 individuals in Spain eligible for the XEN 63 Gel Stent.
Canadian Evidence
The Canadian Agency for Drugs and Technologies in Health (CADTH) in collaboration with Ontario Health conducted a cost-utility analysis comparing various minimally invasive glaucoma surgery (MIGS) devices as a class to alternative treatments (pharmacotherapy, laser therapy, and filtration surgery) for adults with glaucoma in 2019.14 As part of this analysis, a MIBS device (the XEN 45) was compared with trabeculectomy (a type of filtration surgery often performed in individuals with moderate to severe glaucoma). A probabilistic Markov model was developed from the perspective of a Canadian public payer with a lifetime horizon. In the model, reductions in IOP measured in mmHg resulted in reduced rates of vision loss. Effectiveness inputs for the XEN 45 and trabeculectomy were sourced from Schlenker et al,85 which estimated that both treatments were associated with an 11-mmHg reduction in IOP.14,85 Owing to the lack of long-term effectiveness estimates of glaucoma interventions, the analysis assumed that IOP would gradually return to pre-treatment levels after 10 years. Costs associated with surgery, medications, adverse events, and ophthalmologist consultations were considered. The cost of the XEN 45 device ($1,087) was assumed to be double the cost of the iStent MIGS device ($543.50). Costs were sourced to resemble those incurred in Alberta and were reported in 2018 CAD. Health and cost outcomes were both discounted at a rate of 1.5%. Cost inputs from the cost-utility analysis were used to inform a budget impact analysis conducted by Ontario Health.104
CADTH and Ontario Health were unable to make conclusions on the cost-effectiveness of MIGS as a class because of the uncertainty related to 1) the relative effectiveness of glaucoma interventions, 2) the relationship between IOP reduction and changes in health-related quality of life, and 3) the lack of long-term effectiveness data. In a scenario analysis, the authors found that the XEN 45 was associated with similar QALYs (-0.0003) and fewer costs (-$1,053) when compared with trabeculectomy over a lifetime horizon.
The Institut national d'excellence en santé et en services sociaux (INESSS) in Quebec conducted a cost-minimization analysis comparing the XEN 45 (with and without phacoemulsification) with trabeculectomy (with and without phacoemulsification).19 Phacoemulsification is a form of cataract surgery often done alongside MIBS. This analysis was conducted by modifying a sponsor (industry)-submitted probabilistic model that used a 1-year time horizon and took the perspective of a public health care payer in Quebec. Costs related to device acquisition, medication (including wastage and dispensing fees), postoperative follow-up, and complications were considered. The cost of the XEN 45 was provided by the manufacturer and was not published in the report. Costs were reported in 2020 CAD.
INESSS estimated that the 1-year costs of trabeculectomy with and without phacoemulsification were $3,624 and $3,281, respectively. The XEN 45 was associated with higher costs than trabeculectomy. Total cost estimates for individuals who received the XEN 45 with and without phacoemulsification were confidential and not reported. Higher costs were owing to the increased costs of the XEN 45 device despite reductions related to the costs associated with medication, postoperative follow-up, and complications. The results of the cost-minimization analysis were used to inform a 3-year budget impact analysis from the perspective of a Quebec provincial public payer. The budget impact analysis used costs sourced from the economic analysis and volume of procedures sourced from Quebec administrative billing data. The budget impact results were confidential and not reported.
International Evidence
Atik et al100 conducted a cost-utility analysis comparing the PreserFlo MicroShunt with trabeculectomy for adults with open-angle glaucoma. The authors used a probabilistic Markov model with a 1-year time horizon and a US societal perspective. Effectiveness data for the PreserFlo MicroShunt and trabeculectomy were sourced from a randomized clinical trial.105 Costs were sourced to resemble those incurred in the Alabama Medicare system and included physician fees, equipment, supplies, medications, and post-operative visits. Costs were reported in 2021 USD and were not discounted in the reference case given the short time horizon. Scenario analyses with longer time horizons were discounted at a rate of 3%. Atik et al100 found that the PreserFlo MicroShunt was associated with fewer quality-adjusted life years (QALYs; -0.01) and higher costs ($2,058). Scenario analyses found that when extending the time horizon from 1 to 20 years, the PreserFlo MicroShunt was still associated with fewer QALYs (-0.29) and higher costs ($3,679). Scenario analyses found that even if the device was free, trabeculectomy would still be associated with lower costs.
Van Lancker et al80 conducted a retrospective cost-consequence analysis comparing the PreserFlo MicroShunt with trabeculectomy. The authors evaluated surgical outcomes for 129 individuals with glaucoma in the United Kingdom. The authors matched observed resource use with cost inputs sourced from published studies, schedule of benefits, and national formularies. The authors considered costs related to surgical procedures, follow-up visits, and post-operative procedures. The authors included costs related to non-glaucoma medications, but excluded those related to glaucoma medications because they found no difference in glaucoma medication usage between the treatment arms. The cost of the PreserFlo MicroShunt device was £1,070. Costs were reported in 2021 GBP and were not discounted. The principal effectiveness measure in the study was surgical failure, which was defined as IOP > 21 mmHg or < 20% reduction from baseline IOP. The authors found that PreserFlo MicroShunt was associated with a reduction in costs (-£245) and a lower probability of surgical failure than with trabeculectomy.80 The reduction in costs were owing to PreserFlo MicroShunt having fewer post operative visits and lower costs related to non-glaucoma medications.
Martínez-de-la-Casa et al101 conducted a model-based analysis of the budget impact of adding the XEN 45 (with and without cataract surgery) to the Spanish standard of care for individuals with open angle glaucoma. The authors modelled a cohort of 405 individuals with mild, moderate, or uncontrolled open-angle glaucoma. The authors estimated that if the XEN 45 was publicly available, 35.5% of the individuals in the modelled cohort would elect to receive it. The budget impact model used a 3-year time horizon and took the perspective of the Spanish National Health System. The Spanish standard of care depended on the glaucoma stage, but included non-perforating deep sclerectomy (NPDS), EXPRESS, iStent Inject, and trabeculectomy, all with and without cataract surgery. Costs related to device acquisition, surgical interventions, follow-up visits, complications, and additional procedures were considered. The model did not consider costs that occurred after the first year of the initial treatment. The authors based their determination of market share of current glaucoma interventions and the uptake of the XEN 45 on expert opinion. The cost of the XEN 45 device was €790.00 and was sourced from the manufacturer. Costs were reported in 2016 EUR and no discount rate was applied.
Martínez-de-la-Casa et al101 found the average treatment cost per individual with glaucoma with standard care (no XEN 45) was €4,665.41, and the average treatment cost per individual with glaucoma when the XEN 45 was available was €4,097.78. The introduction of the XEN 45 was associated with increased device acquisition costs and decreased costs related to surgical interventions, follow-up visits, complications, and additional procedures. The authors did not provide a breakdown of cost estimates stratified by the glaucoma intervention.
Vila-Arteaga et al102 conducted a model-based analysis that assessed the budget impact of including the XEN 63 to the current standard of care (trabeculectomy, NPDS, PreserFlo MicroShunt, iStent inject, the XEN 45, and Ahmed valve) for adults with primary open-angle glaucoma. The authors used a 1-year time horizon and took the perspective of the Spanish National Health System. Costs related to device acquisition, surgical interventions, post-operative complications, follow-up visits, and additional procedures were considered. Costs were reported in 2021 EUR and no discount rate was applied. The authors sourced market share of primary open-angle glaucoma treatments from expert opinion and manufacturer consultation. The size of the study population was estimated from previously published prevalence data. The authors estimated that, over a 1-year time horizon, 25,718 individuals would be eligible to receive the XEN 63 if introduced into the Spanish National Health System and that 6,173 of those individuals would receive the XEN 63. They estimated that the XEN 63 would completely replace the XEN 45 and reduce the market share of trabeculectomy, Ahmed valve, and non-perforating deep sclerectomy.
Vila-Arteaga et al102 estimated that adding the XEN 63 to the standard of care in Spain would result in a €2.56 million reduction in health care costs. The average treatment cost per individual with glaucoma under standard care (no XEN 63) was €3,395.35, and the average treatment cost per individual with glaucoma when the XEN 63 was available was €3,239.43. The reduction in budget impact was primarily owing to lower costs associated with additional procedures, follow-up visits, and post-surgery complications.
Applicability and Limitations of the Included Studies
Appendix 7 provides the results of the quality appraisal checklist for economic evaluations applied to the included studies. The population and comparators of all six studies matched our inclusion criteria.14,19,80,100-102 The studies by Atik et al,100 Van Lancker et al,80 Martínez-de-la-Casa et al,101 and Vila-Arteaga et al102 were set outside of Canada. It is unclear if the estimated costs, resource utilization, and clinical management of glaucoma would be comparable between these jurisdictions and Ontario. We deemed the cost-utility analysis conducted by CADTH and Ontario Health14 as well as the cost-minimization analysis conducted by INESSS19 to be directly applicable, and we assessed them to have minor limitations.14,19 Both studies are relevant to the Ontario setting.
Discussion
We identified six relevant studies that compared either the XEN Gel Stent or PreserFlo MicroShunt to trabeculectomy. Two Canadian studies were deemed directly applicable. The cost-utility analysis conducted by CADTH and Ontario Health was unable to make strong conclusions on the cost-effectiveness of MIGS as a class owning to 1) uncertainty in the relative clinical efficacy, 2) the relationship between IOP reduction and changes in health-related quality of life, and 3) the lack of long-term effectiveness data.14 The analysis comparing the XEN 45 with trabeculectomy found that the XEN 45 was associated with similar QALYs and lower costs. The model is further limited because 1) the effectiveness parameters were not varied in the probabilistic analysis, 2) the assumption that the XEN 45 device would have double the device cost of iStent Inject, 3) the assumption that the XEN 45 would have similar procedure duration time as less invasive MIGS, and 4) the exclusion of drug wastage costs. The analysis also used cost inputs to resemble those that were incurred in Alberta. Therefore, it is unclear if the XEN 45 would remain less costly than trabeculectomy when using Ontario cost inputs (Alberta's trabeculectomy costs ranged from $3,837 to $5,113, while Ontario's estimates ranged from $2,500 to $3,717). It is also unclear how the uncertainty surrounding the XEN 45 device costs, the exclusion of drug wastage, and the assumption of procedure duration would impact cost-effectiveness. Cost inputs from the CADTH and Ontario Health cost-utility analysis were used to inform an Ontario Health budget impact analysis. The analysis did not consider costs for MIBS devices.
The cost-minimization analysis conducted by INESSS did not consider differences in health-related quality of life.19 The analysis found that the XEN 45 was more costly than trabeculectomy based on device acquisition cost. INESSS estimated lower physician fees, lower post-operative complication rates, lower medication costs, and fewer ophthalmological visits when compared to trabeculectomy. The 1-year time horizon also meant that the study did not consider any long-term changes in costs associated with the XEN 45 or trabeculectomy. Due to the confidential nature of the manufacturer-provided device costs, it is unclear if the device cost inputs resemble those used in the CADTH and Ontario Health cost-utility analysis.
The cost-utility analysis conducted by Atik et al100 found that the PreserFlo MicroShunt was more costly and was associated with fewer QALYs when compared to trabeculectomy. Their scenario analyses indicated that the cost-effectiveness of trabeculectomy when compared to the PreserFlo MicroShunt increased as the time horizon was extended. This is due to trabeculectomy being associated with a larger IOP reduction and reductions in IOP pressure impacting the long-term trajectory of individuals with glaucoma. Unlike the study by Atik et al, Van Lancker et al80 found that PreserFlo MicroShunt was associated with fewer costs (£289, 2021 GBP), primarily due to lower non-glaucoma medication costs and reduced reoperations. The budget impact analyses from the Spanish system perspective by Martínez-de-la-Casa et al101 and Vila-Arteaga et al102 found that introducing the XEN 45 and the XEN 63 would reduce health care costs.101,102 The more recent analysis by Vila-Arteaga et al assumed that the XEN 63 would replace the XEN 45 completely. The authors of both analyses declared conflicts of interests related to employment or financial support from manufacturers of MIBS devices.
Strengths and Limitations
We conducted a review of the economic literature comparing MIBS to alternative treatments for individuals with glaucoma. The primary strength of this review is its comprehensive nature, providing a summary of the latest economic evidence for all currently available MIBS devices. We also identify evidence for MIBS devices from a variety of jurisdictions and perspectives. This review is limited in that we were unable to identify Canadian evidence for the PreserFlo MicroShunt. Additionally, most of the identified studies only compared MIBS to trabeculectomy. We were unable to identify any cost-effectiveness analysis comparing MIBS to another MIBS.
Conclusions
The cost-utility analysis conducted by CADTH and Ontario Health and the cost-minimization analysis conducted by INESSS were deemed to have minor limitations and were relevant to the Ontario context. The results of the two studies indicate that the cost-effectiveness of MIBS in Canada is highly uncertain. While most studies found or assumed similar effectiveness between MIBS and trabeculectomy, the incremental cost remains uncertain because the costs associated with MIBS and trabeculectomy vary across jurisdictions.
Primary Economic Evaluation
The economic evidence review identified two studies comparing MIBS with trabeculectomy that were deemed directly applicable to the Ontario context.14,19 The two studies either found or assumed no differences in the effectiveness between MIBS and trabeculectomy. The long-term cost-effectiveness of MIBS when compared to trabeculectomy is unknown due to the uncertainty in the relative effectiveness of MIBS and lack of long-term data. Modelling inputs from Ontario, Alberta, and Quebec analyses indicate that the cost of glaucoma interventions is likely to be different between the provinces.
While new clinical evidence comparing the XEN Gel Stents and the PreserFlo MicroShunt has been published since the CADTH and Ontario Health cost-utility analysis,73,79,84 the limitations related to the long-term trajectory of disease in individuals with glaucoma remain and are unlikely to be addressed with a new cost-effectiveness analysis. Therefore, we did not conduct a primary economic evaluation and instead we estimated Ontario costs for MIBS by conducting a budget impact analysis.
Budget Impact Analysis
Research Question
What is the potential 5-year budget impact for the Ontario Ministry of Health of publicly funding minimally invasive bleb surgery (MIBS) compared with other treatment alternatives for people with glaucoma?
Methods
Analytic Framework
We estimated the budget impact of publicly funding MIBS using the cost difference between two scenarios: (1) the current clinical practice (the current scenario), in which MIBS are funded through global budgets at a few hospitals in Ontario; and (2) anticipated change in funding with increased uptake at more Ontario hospitals (the new scenario). The current scenario includes the costs of MIBS procedures currently being done in the province. Figure 3 presents the model schematic.
Figure 3: Schematic Model of Budget Impact.
Flow chart describing the model for the budget impact analysis. The current scenario explores resource use under current clinical practice, in which MIBS is funded under some hospital's global budgets. The new scenario explores resource use and total costs with a public funding recommendation for MIBS. The budget impact is the difference in cost between the two scenarios.
Key Assumptions
-
We assumed that the percentage of individuals who receive MIBS or trabeculectomy in both eyes approximates estimates sourced from the literature
-
—
We conducted scenario analyses assuming a lower and higher number of individuals receiving treatment in both eyes
-
—
We assumed an average cost and same relative effectiveness for MIBS devices (the XEN 45 and XEN 63, and the PreserFlo MicroShunt), although the costs and relative effectiveness of each device may vary slightly
-
We assumed that the population of interest corresponds to individuals who would otherwise have received trabeculectomy
-
—
We conducted scenario analyses varying the size of the population of interest
-
—
-
We assumed that trabeculectomy would be the most relevant comparator to MIBS
-
—
This assumption was based on previously published economic evaluations by CADTH and Ontario Health, as well as by INESSS
-
—
-
We assumed that MIBS has a surgical time requirement of 30 minutes
-
—
We conducted scenario analyses varying the time to conduct MIBS
-
—
-
We assume that individuals who receive MIBS will not experience a re-operation within 1-year after surgery
-
—
Similar to the INESSS cost-minimization analysis, we conducted a scenario analysis where 3.8% of individuals who received MIBS would require another MIBS surgery
-
—
Population of Interest
The population of interest for this analysis is adults with glaucoma. We assumed, similar to the INESSS cost-minimization analysis, that the population of interest corresponds to individuals who in the absence of MIBS would otherwise have received trabeculecomy.19 The number of trabeculectomy procedures was sourced from the Medical Services Database (accessed through IntelliHealth Ontario106), published studies, and expert opinion.
The Medical Services Database contains claims for Ontario Health Insurance Plan (OHIP) Schedule of Benefits fee codes. The OHIP Schedule of Benefits does not have a trabeculectomy-specific billing code. Instead, these procedures, along with other procedures, such as tube shunt implantation, are grouped under the filtration surgery-related billing codes107:
E132: Glaucoma filtering procedure
E214: Glaucoma filtering procedure and cataract extraction
Additionally, billing code ‘E136: with intraocular implant of seton’ can be claimed alongside E132 or E214. We sourced all patient visits with claims for any of the billing codes E132, E214, and E136 between January 1, 2010, and December 31, 2021. We excluded patient visits where E136 was claimed alongside E132 or E214 as these are unlikely to correspond to trabeculectomy procedures.107
The INESSS cost-minimization analysis reported the number of trabeculectomy procedures occurring in Quebec.19 We divided the number of trabeculectomy procedures in Quebec by the Quebec provincial glaucoma prevalence estimates from Kansal et al,107 to estimate the rate of trabeculectomy per 100,000 individuals with glaucoma. Applying this rate to Ontario glaucoma prevalence estimates, also sourced from Kansal et al,107 indicate that 60% of visits for billing codes E132 or E214 correspond to trabeculectomy procedures. This estimate was validated by clinical experts (P. Hooper, MD, email communication, March 21, 2023). We describe this calculation in Appendix 8.
We observed an increase in the number of procedure claims for E132 or E214 between 2010 (N = 1,946) and 2021 (N = 2,836). To account for changing provincial demographics, as well as changes in clinical practices, we used a linear regression model and estimated age-stratified rates for billing claims (codes E132 or E214) over the 5-year budget impact time horizon.108 We than matched estimated rates with Statistics Canada population projections for Ontario.109 A detailed description of the methods used to predict the number of claims are provided in Appendix 8. We used the upper and lower 95% confidence interval estimates of our predictions on the number of trabeculectomy procedures to conduct scenario analyses. All analyses of the IntelliHealth Ontario data were conducted using R.110
As mentioned above, expert consultation indicated that MIBS is currently funded through some hospitals’ global budgets. We sourced an estimate of 1,320 MIBS procedures currently being conducted in the province from manufacturers (Glaukos, email communication, April 13, 2023; AbbVie, email communication, April 21, 2023). We conducted scenario analyses with ± 25% changes in the number of MIBS procedures. Table 19 provides estimates of our population of interest.
Table 19:
Estimation of the Size of the Population of interest
| Year 1 (2024) | Year 2 (2025) | Year 3 (2026) | Year 4 (2027) | Year 5 (2028) | |
|---|---|---|---|---|---|
| Estimated number of trabeculectomy procedures | 1,936 | 2,016 | 2,099 | 2,184 | 2,274 |
| Estimated number of MIBS procedures | 1,320 | 1,320 | 1,320 | 1,320 | 1,320 |
| Estimated size of population of interest | 3,256 | 3,336 | 3,419 | 3,504 | 3,594 |
Current Intervention Mix
The current intervention mix was estimated based on administrative data and manufacturer responses (Table 19). We assumed that the number of MIBS procedures in the current scenario will remain constant during the 5-year budget impact time horizon.
Uptake of the New Intervention and New Intervention Mix
The future uptake was estimated based on clinical expert and manufacturer opinion (P. Hooper, MD, and D. Jinapriya, MD, email communication, April-May 2023; Glaukos Corp., email communication, April 13, 2023; Abbvie Corp., email communication, April 21, 2023). We estimated an average uptake of 65%, with a range of 40% to 95%. Table 20 provides estimates for the uptake of MIBS assuming an increase to 65% in year 5. We conducted scenario analyses varying the uptake rate of MIBS.
Table 20:
Uptake of MIBS devices and Trabeculectomy in Ontario
| Year 1 (2024) | Year 2 (2025) | Year 3 (2026) | Year 4 (2027) | Year 5 (2028) | |
|---|---|---|---|---|---|
| Current Scenario: Standard care | |||||
| Trabeculectomy | 59% | 60% | 61% | 62% | 63% |
| MIBS | 41% | 40% | 39% | 38% | 37% |
| New Scenario: Increased funding for MIBS | |||||
| Trabeculectomy | 54% | 49% | 45% | 40% | 35% |
| MIBS | 46% | 51% | 55% | 60% | 65% |
Abbreviation: MIBS, minimally invasive bleb surgery.
Resources and Costs
We considered costs and resource use incurred by the Ontario Ministry of Health and did not include out-of-pocket expenditures. We used cost inputs sourced from the CADTH and Ontario Health cost-utility analysis,14 the INESSS cost-minimization analysis,19 previously published studies,80,85,87,111 the OHIP Schedule of benefits,112 and the Ontario Drug Benefit Formulary.113 Similar to the INESSS cost-minimization analysis for the XEN 45, we considered only costs occurring within the first year after a MIBS or trabeculectomy procedure.
We sourced from the clinical literature that 10% of individuals would receive a MIBS or trabeculectomy procedure in both eyes (see clinical review, above). For individuals receiving MIBS in both eyes, we included the cost of an additional MIBS device. The frequency of cataract surgery alongside MIBS was sourced from the clinical review (3%-24%), and we used the midpoint of the range (13.5%) as the frequency that MIBS or trabeculectomy is done alongside cataract surgery. This value was verified by expert opinion (P. Hooper, MD, email communication, June 26, 2023). We conducted scenario analyses varying the frequency of cataract surgery by +/- 50%. We also conducted a scenario analysis with a frequency of cataract surgery sourced from the Medical Service Database (accessed through IntelliHealth Ontario).106
In 2021, the last year we had data available, cataract surgery was done 52.9% of the time alongside a glaucoma filtering procedure. The rate of cataract surgery alongside MIBS or trabeculectomy estimated from administrative data was higher than that observed in the clinical review.
Appendix 8 provides a detailed breakdown of how we estimated cost and resource use. All costs were expressed in 2023 CAD. Where necessary, we used the all-items component of the Canadian Consumer Price Index (CPI) to adjust costs to present values.114
Device Costs
The MIBS device was estimated to cost $1,314.40—the average of device cost of $1,228.80 for the XEN device (Abbvie Corp., email communication, April 21, 2023) and $1,400.00 for the PreserFlo MicroShunt (Glaukos Corp., email communication, April 13, 2023). We conducted scenario analyses with ± 25% changes in the cost of MIBS devices.
Surgery Costs
Similar to the CADTH and Ontario Health cost-utility analysis,14 we sourced the cost of trabeculectomy from the Ontario Case Costing Initiative (OCCI).115 We sourced estimates of inpatient and day surgery costs for visits using the Canadian Classification of Intervention (CCI) codes related to filtration surgery.116 We also sourced cataract surgery inpatient and day surgery direct and indirect costs by filtering the OCCI with the associated CCI codes.117
We followed the CADTH and Ontario Health cost-utility analysis in assuming that the cost of MIBS would have similar indirect costs as cataract surgery.14 We assumed that the operating room-specific direct costs would vary based on the time to conduct cataract surgery compared with the time to conduct MIBS.
We asked clinical experts the duration to conduct a MIBS procedure and selected the most frequent answer of 30 min (P. Hooper, MD, and D. Jinapriya, MD, email communications, April-May 2023). We conducted scenario analyses varying the time to conduct a MIBS procedure. The time to conduct cataract surgery was assumed to be 20 minutes. This estimate was sourced from the CADTH and Ontario Health cost-utility analysis. The three studies in the clinical review reported a wider range of procedure duration for MIBS (15-58 minutes). The three studies all reported that MIBS had a longer surgical duration than trabeculectomy. We used expert opinion to inform this model parameter as we are unclear if the reported values in the clinical studies would resemble those observed in Ontario.
Physician fees for both trabeculectomy and MIBS were sourced from the OHIP Schedule of benefits.113 Similar to the CADTH and Ontario Health cost-utility analysis, we assumed that trabeculectomy would require anesthesia-related physician fee codes, but MIBS would not.14
For trabeculectomy or MIBS procedures done in combination with cataract surgery, we followed the CADTH and Ontario Health cost-utility analysis in assuming that the costs consisted of the cost of cataract surgery sourced from the OCCI plus the direct costs associated with either procedure.
Medication Costs
To estimate medication costs, we first sourced the number of medications used postoperation from the clinical review (MIBS: 0-1.4; trabeculectomy: 0-0.7) and selected the midpoint of both ranges (MIBS: 0.7; trabeculectomy: 0.35). We then estimated the average yearly cost of a medication received after MIBS or trabeculectomy using the medication listed in the INESSS cost-minimization analysis19; dosing and wastage information were sourced from Iordanous et al,111 the frequency that an individual would receive each medication was sourced from INESSS cost-minimization analysis. Drug unit costs were sourced from the Ontario Drug Benefit Formulary.112 We estimated that the yearly cost for a medication received after MIBS or trabeculectomy would be $128.39.
From the OHIP Schedule of Benefits database (accessed through IntelliHealth Ontario106), we estimated that 77.2% of individuals receiving glaucoma filtration surgery would be over the age of 65, and thus eligible for the Ontario Drug Benefit Program. We multiplied yearly medication costs by 77.2% to determine the estimated cost to the Ontario Drug Benefit Program would be $99.12 per glaucoma medication regimen.
We then multiplied the yearly medication cost by the number of medications used postoperation sourced from the clinical review (MIBS: $99.12 × 0.7 = $69.38; trabeculectomy $99.12 × 0.35 = $34.69). Appendix 8, Table A10, provides a detailed description of the medication cost calculation. Due to the overlapping ranges on the number of medications used postoperation, we conducted scenario analyses varying the number of medications for both MIBS and trabeculectomy.
Adverse Events
Similar to the INESSS cost-minimization analysis, we sourced the frequency of adverse events for trabeculectomy and MIBS from Schlenker et al.85 Additionally, we sourced the frequency of retinal detachment from two studies identified in the clinical review.80,87
We assumed that all adverse events except retinal detachment would incur only physician fees and so we excluded facility costs. We included facility costs for retinal detachment due to the expected operating room requirements and substantive facility costs (P. Hooper, MD, email communication, July 4, 2023). Inpatient and ambulatory facility costs were sourced from the Cost Analysis Tool (accessed via IntelliHealth Ontario).118
Detailed adverse events calculations are available in Appendix 9. We conducted scenario analyses varying the relative frequency of each adverse event for both MIBS and trabeculectomy, as well as alternative fee codes associated with each adverse event (Table 21).
Table 21:
Budget Impact Analysis Cost Inputs, Reference Case
| Variable | Unit costa | Duration, quantity, frequency | Total costa | Reference |
|---|---|---|---|---|
| Device costs | ||||
| MIBS device | $1,314.40 | 1.10b | $1,445.84 | MIBS manufacturers |
| Inpatient and day surgery costs | ||||
| Trabeculectomy | $1,951.33 | 1 | $1,951.33 | OCCI,115 CADTH & OH14 |
| and cataract surgery | $2,354.09 | 1 | $2,354.09 | OCCI,115 CADTH & OH14 |
| MIBS | $1,149.76 | 1 | $1,149.76 | OCCI,115 CADTH & OH14 |
| and cataract surgery | $1,804.39 | 1 | $1,804.39 | OCCI,115 CADTH & OH14 |
| Surgery physician fees | ||||
| Trabeculectomy | $642.94 | 1 | $642.94 | OHIP SoB,113 CADTH & OH14 |
| with cataract surgery | $914.44 | 1 | $914.44 | OHIP SoB,113 CADTH & OH14 |
| MIBS | $840.00 | 1 | $840.00 | OHIP SoB,113 CADTH & OH14 |
| with cataract surgery | $1,111.50 | 1 | $1,111.50 | OHIP SoB,113 CADTH & OH14 |
| Yearly medication costs | ||||
| Yearly medication costc | $99.12 | Ontario Drug Formulary,112 INESSS,19 Iordanous et al111 |
||
| Trabeculectomy MIBS | 0.35 | $34.69 | ||
| MIBS | 0.70 | $69.38 | ||
| Ophthalmologist visits | ||||
| Trabeculectomy | $276.69 | 1 | $276.69 | OHIP SoB,113 INESSS,19 CADTH & OH14 |
| MIBS | $234.90 | 1 | $234.90 | OHIP SoB,113 INESSS,19 CADTH & OH14 |
| Ophthalmologists’ tests | ||||
| Trabeculectomy | $590.72 | 1 | $590.72 | OHIP SoB,113 INESSS,19 CADTH & OH14 |
| MIBS | $481.50 | 1 | $481.50 | OHIP SoB,113 INESSS,19 CADTH & OH14 |
| Adverse events physician fees | ||||
| Needling | $161.75 | OHIP SoB113 | ||
| Trabeculectomy | 30.8% | $49.82 | Code E137 | |
| MIBS | 43.2% | $69.88 | Schlenker et al85 | |
| Laser suture lysis | $182.75 | OHIP SoB113 | ||
| Trabeculectomy | 49.7% | $90.83 | Code E133 | |
| MIBS | 0% | $0.00 | Schlenker et al85 | |
| Anterior chamber reformation | $182.75 | OHIP SoB113 | ||
| Trabeculectomy | 7.7% | $14.07 | Code E133 | |
| MIBS | 11.9% | $21.75 | Schlenker et al85 | |
| Bleb repair/conjunctival suturing | $210.00 | OHIP SoB113 | ||
| Trabeculectomy | 5.9% | $12.39 | Code E212 | |
| MIBS | 1.1% | $2.31 | Schlenker et al85 | |
| Iris sweep/syneochiolysis | $182.75 | OHIP SoB113 | ||
| Trabeculectomy | 2.4% | $4.39 | Code E133 | |
| MIBS | 1.6% | $2.92 | Schlenker et al85 | |
| YAG to implant/ostomy | $161.75 | OHIP SoB113 | ||
| Trabeculectomy | 1.2% | $1.94 | Code E139 | |
| MIBS | 1.6% | $2.59 | Schlenker et al85 | |
| Iridoplasty | $350.00 | OHIP SoB113 | ||
| Trabeculectomy | 0.0% | $0.00 | Code E156 | |
| MIBS | 1.1% | $3.85 | Schlenker et al85 | |
| Retinal detachment | $282.65 | OHIP SoB113 | ||
| Trabeculectomy | 1.2% | $13.33 | Code E151, E148, and E936 | |
| MIBS | 0.6% | $6.43 | Theilig et al,87 Van Lancker80 | |
| Adverse events facility costs | ||||
| Retinal detachment | $3,444.88 | CAT118 | ||
| Trabeculectomy | 1.2% | $42.01 | Theilig et al,87 Van Lancker80 | |
| MIBS | 0.6% | $20.26 | ||
Abbreviations: CADTH, Canadian Agency for Drugs and Technologies in Health; CAT, cost-analysis tool; INESSS, Institut national d'excellence en santé et en services sociaux; MIBS: minimally invasive bleb surgery; OCCI: Ontario case costing Initiative; OH, Ontario Health; OHIP SoB: Ontario Health Insurance Plan Schedule of Benefits; YAG, yttrium-aluminum-garnet.
2023 CAD. Appendix 8 provides a detailed description of all costing items and unit costs prior to CPI adjustment.
Device costs multiplied by 1.10 to account for 10% of individuals receiving MIBS or trabeculectomy in both eyes.
Yearly medication costs are calculated by multiplying each drug's unit cost by the frequency and summing across all medications. See Appendix 8, Table A10 for detailed calculation.
Internal Validation
The secondary health economist conducted formal internal validation. This process included checking for errors and ensuring the accuracy of parameter inputs and equations in the budget impact analysis.
Analysis
We conducted a reference case analysis and sensitivity analyses. Our reference case analysis represents the analysis with the most likely set of input parameters and model assumptions. Our scenario analyses explored how the results are affected by varying input parameters and model assumptions.
-
1.
Higher number of trabeculectomy procedures in population estimation
-
We estimated a higher number of trabeculectomy procedures from the upper 95% CI of the linear models used to estimate trabeculectomy volume
-
-
Reference case: year 1 = 1,936, year 2 = 2,016, year 3 = 2,099, year 4 = 2,184, year 5 = 2,274
-
-
Scenario 1: year 1 = 2,844, year 2 = 3,017, year 3 = 3,200, year 4 = 3,389, year 5 = 3,589)
-
-
-
-
2.
Lower number of trabeculectomy procedures in population estimation
-
We estimated a lower number of trabeculectomy procedures from the lower 95% CI of the linear model used to estimate trabeculectomy volume
-
-
Reference case: year 1 = 1,936, year 2 = 2,016, year 3 = 2,099, year 4 = 2,184, year 5 = 2,274
-
-
Scenario 2: year 1 = 1,027, year 2 = 1,013, year 3 = 998, year 4 = 979, year 5 = 957)
-
-
-
-
3.
Increased number of MIBS procedures already funded out of global hospital budgets in population estimation
We estimated a 25% increase in the yearly number of MIBS procedures currently being conducted (reference case: 1,320; scenario 3: 1,650)
-
4.
Decreased number of MIBS procedures already funded out of global hospital budgets in population estimation
We estimated a 25% decrease in the yearly number of MIBS procedures currently being conducted (reference case: 1,320; scenario 4: 990)
-
5.
Increased rate of adoption of MIBS
We increased the rate of adoption for MIBS in the New Scenario so that adoption in year 5 was 75% (compared to 65% in the reference case)
-
6.
MIBS replaces trabeculectomy
We increased the rate of adoption for MIBS in the New Scenario so that adoption in year 5 was 95% (compared to 65% in the reference case)
-
7.
Lower rate of adoption for MIBS
We reduced the rate of adoption for MIBS in the New Scenario so that adoption in year 5 was 50% (compared to 65% in the reference case)
-
8.
Higher proportion of MIBS and trabeculectomy procedures occurring in both eyes
We used a higher percentage of MIBS or trabeculectomy procedures occurring in both eyes (reference case = 10%; scenario 8 = 15%)
-
9.
Lower proportion of MIBS and trabeculectomy procedures occurring in both eyes
We used a lower percentage of MIBS or trabeculectomy procedures occurring in both eyes (reference case = 10%; scenario 9 = 5%)
-
10.
Higher proportion of MIBS and trabeculectomy procedures occurring alongside cataract surgery
We used a higher percentage of procedures occurring alongside cataract surgery (reference = 13.5%; scenario 10 = 20%)
-
11.
Lower proportion of MIBS and trabeculectomy procedures occurring alongside cataract surgery
We used a lower percentage of procedures occurring alongside cataract surgery (reference = 13.5%; scenario 11 = 9%)
-
12.
Proportion of MIBS and trabeculectomy procedures occurring alongside cataract surgery resembles studies identified from administrative data
We used the frequency of cataract surgery sourced from administrative data (reference = 13.5%; scenario 12 = 52.9%)
-
13.
Increased MIBS device acquisition costs
MIBS device acquisition costs increased by 20% (reference case device cost = $1,314.40; scenario 13 device cost = $1,577.28)
-
14.
Decreased MIBS device acquisition costs
MIBS device acquisition costs decreased by 20% (reference case device cost = $1,314.40; scenario 14 device cost = $1,051.52)
-
15.
45 min MIBS surgery duration
Increased MIBS surgery time from 30 to 45 minutes (reference case MIBS surgery cost = $974.20; scenario 15 surgery cost = $1,331.96)
-
16.
60 min MIBS surgery duration
Increased MIBS surgery time from 30 to 60 minutes (reference case MIBS surgery cost = $974.20; scenario 16 surgery cost = $1,689.72)
-
17.
Evidence related to the number of medications required postoperation favors MIBS (reference case, proportion of medications after surgery MIBS = 0.7 and trabeculectomy = 0.35; scenario 17, MIBS = 0.35 and trabeculectomy = 0.52)
-
18.
Evidence related to the number of required postoperation favors trabeculectomy (reference case, proportion of medications after surgery MIBS = 0.7 and trabeculectomy = 0.35; scenario 18, MIBS = 1.05 and trabeculectomy = 0.18)
-
19.
Evidence related to adverse events favors MIBS (reference case, probability of having an adverse event was sourced from clinical evidence80,85,87; scenario 19, probability of having an adverse event decreased by 10% for MIBS and increased by 10% for trabeculectomy)
-
20.
Evidence related to adverse events favors trabeculectomy (reference case, probability of having an adverse event was sourced from clinical evidence80,85,87; scenario 20, probability of having an adverse event increased by 10% for MIBS and decreased by 10% for trabeculectomy)
-
21.
Alternative fee codes for adverse events (reference case, see Table 21; scenario 21, bleb repair/conjunctival suturing associated with OHIP fee code E132, YAG to implant/ostomy and Iridoplasty both associated with OHIP fee code E134)
-
22.
Reoperation at 1-year (reference case, no reoperation within the 1 year, we consider costs; scenario 22, similar to the INESSS cost-minimization analysis, 3.8% of individuals who receive MIBS would incur MIBS surgery and MIBS device acquisition fee costs due to reoperation)
-
23.
Less expensive MIBS procedure physician codes:
Reduced MIBS physician fee codes (reference case, MIBS procedure physician's cost include OHIP fee code E132 and E136; scenario 23, MIBS procedure physician's costs consist of only E132)
Results
Reference Case
Table 22 provides the results of the reference case analysis (see Appendix 9 for detailed results). We estimate that public funding for MIBS would lead to an additional cost of 0.11 million in year 1 to $0.67 million in year 5, for a total of $1.93 million over 5 years. We estimated that in the Current Scenario, 10,509 trabeculectomy and 6,600 MIBS procedures are likely to occur in the province in the next 5 years. In the New Scenario, with a positive public funding recommendation for MIBS, we estimated that the number of MIBS procedures performed over the next 5 years would increase to 9,519. This corresponds to additional costs of MIBS device acquisition ranging from $0.25 million to $1.47 million over the next 5 years (Table A14, Appendix 9).
Table 22:
Budget Impact Analysis Results—Reference Case
| Scenario | Budget impact, $ milliona | |||||
|---|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | Totalb | |
| Current Scenario | ||||||
| Trabeculectomy | 7.39 | 7.69 | 8.01 | 8.33 | 8.68 | 40.10 |
| MIBS | 5.91 | 5.91 | 5.91 | 5.91 | 5.91 | 29.54 |
| Total | 13.30 | 13.60 | 13.92 | 14.24 | 14.59 | 69.65 |
| New Scenario | ||||||
| Trabeculectomy | 6.73 | 6.29 | 5.82 | 5.32 | 4.80 | 28.97 |
| MIBS | 6.68 | 7.56 | 8.48 | 9.44 | 10.46 | 42.61 |
| Total | 13.41 | 13.85 | 14.30 | 14.76 | 15.26 | 71.58 |
| Budget impact | ||||||
| Cost difference | 0.11 | 0.24 | 0.38 | 0.52 | 0.67 | 1.93 |
Abbreviation: MIBS, minimally invasive bleb surgery.
2023 CAD.
Results may appear inexact due to rounding.
We estimated that the per individual cost to conduct MIBS was $4,351.37 (including device acquisition: $1,445.84; surgery: $1,989.76; medications: $69.38; ophthalmologist visits and tests: $716.40; adverse events: $129.99). The cost to conduct MIBS with cataract surgery was estimated to be $5,227.50. The increase in costs was due to higher costs of surgery ($2,915.89). The cost to conduct trabeculectomy was estimated to be $3,725.14 (including surgery: $2,594.27; medications: $34.69; ophthalmologist visits and tests: $867.41; adverse events:$228.77). The cost of trabeculectomy with cataract surgery was estimated to be $4,339.40, and this was also higher than the cost of trabeculectomy alone owing to higher costs of surgery ($3,268.53).
Scenario Analysis
Table 23 provides the results of the scenario analyses. The estimates of the total budget impact over 5 years ranged from $0.89 million in the scenario with a lower rate of MIBS adoption to $4.39 million in the scenario with a 60-minute MIBS surgery duration. The model results were most sensitive to changes in the assumptions related to the rate of adoption of MIBS (including almost full replacement of trabeculectomy), the duration of MIBS surgery, the cost of the MIBS device, and the size of the population of interest.
Table 23:
Budget Impact Analysis Results—Scenario Analyses
| Scenario | Total budget impact, millionsa,b | Changec |
|---|---|---|
| Reference case | 1.93 | 0% |
| Scenario 1: Higher number of trabeculectomy procedures | 2.58 | 34% |
| Scenario 2: Lower number of trabeculectomy procedures | 1.28 | -34% |
| Scenario 3: Increased number of MIBS procedures (current scenario) | 2.11 | 9% |
| Scenario 4: Decreased number of MIBS procedures (current scenario) | 1.74 | -9% |
| Scenario 5: Increased rate of adoption of MIBS (new scenario) | 2.62 | 36% |
| Scenario 6: MIBS replaces trabeculectomy (new scenario) | 3.99 | 107% |
| Scenario 7: Lower rate of adoption for MIBS (new scenario) | 0.89 | -54% |
| Scenario 8: Higher proportion of MIBS and trabeculectomy procedures occurring in both eyes | 3.00 | 55% |
| Scenario 9: Lower proportion of MIBS and trabeculectomy procedures occurring in both eyes | 2.54 | 32% |
| Scenario 10: Higher proportion of MIBS and trabeculectomy procedures occurring alongside cataract surgery | 1.98 | 3% |
| Scenario 11: Lower proportion of MIBS and trabeculectomy procedures occurring alongside cataract surgery | 1.89 | -2% |
| Scenario 12: MIBS and trabeculectomy cataract surgery rate sourced from administrative data | 2.21 | 15% |
| Scenario 13: Increased MIBS device acquisition costs | 3.78 | 96% |
| Scenario 14: Decreased MIBS device acquisition costs | 1.75 | -9% |
| Scenario 15: 45 min MIBS surgery duration | 3.16 | 64% |
| Scenario 16: 60 min MIBS surgery duration | 4.39 | 128% |
| Scenario 17: Medication evidence favors MIBS | 1.78 | -8% |
| Scenario 18: Medication evidence favors trabeculectomy | 2.08 | 8% |
| Scenario 19: AE evidence favors MIBS | 1.92 | 0% |
| Scenario 20: AE evidence favors trabeculectomy | 2.09 | 8% |
| Scenario 21: Alternative fee codes for adverse events | 1.95 | 1% |
| Scenario 22: Reoperation at 1-year | 2.31 | 20% |
| Scenario 23: Lower MIBS procedure physician costs | 1.08 | -44% |
Abbreviations: AE, adverse events; MIBS, minimally invasive bleb surgery.
2023 CAD.
Results may appear inexact due to rounding.
Percent change calculated as the total budget impact of the scenario analysis divided by the total budget impact of the reference case.
Discussion
We conducted a budget impact analysis estimating the 5-year budget impact of a public funding recommendation for MIBS devices for the management of glaucoma. We found that a public funding recommendation for these devices in Ontario would increase the budget over the next 5 years by about $1.93 million. The increase is primarily due to device acquisition costs, as we estimated that individuals who receive MIBS would have lower medication costs, lower surgery costs, and fewer ophthalmological visits and tests compared to those treated with trabeculectomy. We estimated that currently 1,320 MIBS procedures are occurring yearly in Ontario, funded via some hospital's global budgets. Scenario analyses indicated that our budget impact estimates were most sensitive to the uptake rate of MIBS. This is significant because the future uptake of MIBS in Ontario is highly uncertain. In the reference case, we estimated that the number of MIBS procedures conducted over 5 years would increase from 6,600 to 9,519. The scenario with the largest increase in MIBS uptake (scenario 6) estimated that the number of procedures would increase from 6,600 to 12,649. The scenario with the largest increase in budget impact used a 60-minute surgery duration (scenario 16). In this scenario, we no longer observe savings related to surgery costs; however, this scenario assumes extreme and unlikely values because MIBS is less invasive when compared to trabeculectomy and is unlikely to incur similar surgical costs. A detailed micro-costing exercise may provide more accurate cost estimates of MIBS surgery.
Similar to the cost-minimization analysis conducted by INESSS, we found that MIBS was more costly than trabeculectomy. We estimated lower drug costs compared to the INESSS analysis, primarily due to the lower unit cost of Bimatoprost (sourced from the Ontario Drug Formulary). We also estimated lower costs of adverse events and follow-up visits due to lower physician fees compared to the ones sourced in the INESSS analysis.
The differences in the relative cost estimates between this analysis and the previous CADTH and Ontario Health cost-utility analysis14 are likely due to increased surgery costs for MIBS and lower surgery costs for trabeculectomy. We used a longer duration of MIBS procedure compared to the estimate for MIGS used in the CADTH and Ontario Health analysis as we expect MIBS to be more invasive when compared to other MIGS devices. Additionally, the reference case inputs for surgery with trabeculectomy used in the CADTH and Ontario Health cost-utility analysis were reflective of Alberta's health care system costs, while our scenario analysis inputs estimated lower trabeculectomy costs under Ontario's health care system.
Clinical experts indicated that a subset of individuals with glaucoma would not be good candidates to receive a trabeculectomy procedure due to the required follow-up appointments, but would be able to receive a MIBS procedure (D. Jinapriya, MD, email communication, July 11, 2023). We were unable to include these individuals in the estimate of our population of interest and were not able to quantify any health-related benefits that these individuals would receive. Increased access to MIBS throughout the province would likely provide treatment pathways for these individuals. Current surgical wait times for glaucoma surgeries may create a barrier to accessing MIBS and may require expanding the pool of ophthalmologists who can perform this procedure.119 (Ophthalmologists who have gone through a glaucoma fellowship may perform MIBS procedures; thus some expansion may be possible without additional system changes.) This is especially true for individuals who have to travel to receive surgery, as many of the centres conducting glaucoma surgery are located in Southern Ontario.120
Strengths and Limitations
Our analysis has several strengths. We were able to source the cost of a MIBS device from manufacturers. We were also able to leverage pre-existing analyses of MIBS in Canada to more accurately estimate the costs and resource use associated with the MIBS procedure. Our projections for the number of trabeculectomy procedures taking place in Ontario considered both changing practices and changing demographics in Ontario.
Our analysis has several limitations. First, the uptake of MIBS in the next 5 years is highly uncertain and we estimated the potential uptake based on expert opinion. The responses from clinical experts and manufacturers indicated that there is a wide range of plausible uptake scenarios for MIBS; consequently, we conducted comprehensive scenario analyses to address this uncertainty. Second, we were unable to estimate the frequency of trabeculectomy from administrative data alone; instead we relied on expert opinion and data from previously published studies.107 Additionally, we heard from experts that certain individuals would not be candidates for trabeculectomy, but would be for MIBS. We addressed these two limitations by conducting scenario analyses varying the size of the population of interest.
Conclusions
We estimated that public funding for MIBS would increase expenditures by $0.11 million in year 1 to $0.67 million in year 5, for a total 5-year budget impact of $1.93 million. We expect that the number of MIBS procedures would increase from 6,600 in the current funding scenario to about 9,519 over the next 5 years. However, the true future uptake of MIBS is highly uncertain.
Preferences and Values Evidence
Objective
The objective of this analysis was to explore the underlying values, needs, and priorities of those who have lived experience with treatment for glaucoma, as well as the preferences and perceptions of patients who have received minimally invasive bleb surgery (MIBS).
Background
Exploring patient preferences and values provides a unique source of information about people's experiences of a health condition and the health technologies or interventions used to manage or treat that health condition. It includes the impact of the condition and its treatment on the person with the health condition, their family and other caregivers, and the person's personal environment. Engagement also provides insights into how a health condition is managed by the province's health system.
Information shared from lived experience can also identify gaps or limitations in published research (e.g., outcomes important to those with lived experience that are not reflected in the literature).121-123 Additionally, lived experience can provide information and perspectives on the ethical and social values implications of health technologies or interventions.
Because the needs, preferences, priorities, and values of those with lived experience in Ontario are important to consider to understand the impact of the technology in people's lives, we may speak directly with people who live with a given health condition, including those with experience of the technology or intervention we are exploring.
From direct engagement with patients, the previous health technology assessments (HTAs), as well as consultation with clinical experts, we learned that patients are not always familiar with the specific type of glaucoma surgery they underwent. In response, we made the decision to leverage two previous HTAs on the topic of MIGS that explored the perspectives and experiences of patients with glaucoma and those of their caregivers. The 2019 HTA by Ontario Health104 included direct patient engagement as well as qualitative evidence from a systematic review. The 2020 HTA by the Institut national d'excellence en santé et en services sociaux (INESSS)19 highlights challenges faced by glaucoma patients and references previous work by CADTH.
Qualitative Evidence
Research Question
What are the perspectives and experiences of patients with glaucoma regarding glaucoma and their treatment, and of their clinical and non-clinical caregivers?
Methods
As part of the 2019 HTA,14 CADTH conducted a systematic review and thematic synthesis of primary qualitative research describing the perspectives and experiences of patients with glaucoma, and those of their caregivers. The results of included studies were synthesized using thematic synthesis, an approach that draws on methods for analysis from grounded theory and meta-ethnography. Patient engagement occurred throughout the project and involved conversations with three female patients with glaucoma, two of whom had undergone MIGS (the specific procedure was not identified).
Results
The systematic review of qualitative evidence included 15 studies that reported on patients’ and caregivers’ perspectives and experiences of glaucoma.14 These studies included a total of 329 participants with glaucoma and 31 family members and were assessed to be of low quality. The CADTH report found that:
The results of the thematic synthesis centred around patients’ experiences and perceptions of glaucoma. A diagnosis of glaucoma was unexpected, typically patients explained vision changes as part of normal aging, not as a prompt to seek vision care. This means that those without routine vison care may be more at risk for being diagnosed with more advanced glaucoma and therefore be ineligible for MIGS. Pharmacotherapy in the form of eye drops was disruptive to patients’ lives. Despite a range of creative and committed responses, patients with comorbidities and busy lives with travel or lack of routine made adherence difficult. Reducing the number and frequency of medications was valued by patients. Patients expressed a range of views on glaucoma surgeries, from being a last resort to freedom from eye drops. Some may be conservative in assuming the risks of a surgery where blindness is a possibility. Patients experienced glaucoma as an illness, not as a disease…. [“Illness” can comprise the full range of symptoms that make up a feeling of unwellness and can include more than just the clinically outlined symptoms]. While surgical treatments can offer patients improved clinical outcomes, patients still worried about the need to use additional medications or future surgery and the need for vigilance about the return of elevated IOP, pointing to the lingering impact of glaucoma.
Direct Patient Engagement
Research Question
What are the underlying values, needs, impacts, and preferences of people with lived experience with glaucoma and its treatment options, including MIGS?
Methods
Ontario Health conducted direct engagement through qualitative interviews with 10 people. Four participants had received a MIGS procedure (unknown if procedures were MIBS), three had received trabeculectomy (filtration surgery), and three were treated with drops and laser therapy. Interview questions sought to examine the lived experience of people with glaucoma and its impact on their daily activities and quality of life. Participants were asked about their decision-making and values related to glaucoma treatment, their experiences with treatment, the impact of their treatments, and their impressions of MIGS procedures, if applicable. Participants didn't know the specific type of MIGS procedure they had received.
Results
Glaucoma Diagnosis
Participants described glaucoma as a fairly unfamiliar condition. Diagnosis was often surprising and unexpected, with asymptomatic patients typically diagnosed through routine eye examinations. Participants reported that prior to their diagnosis, their symptoms were mild and attributed to other causes such as aging or natural vision changes. The results are consistent with CADTH's qualitative review, which found that patients “experienced vision changes as a symptom of normal aging [and] did not interpret or perceive their vision changes as pathological.” A glaucoma diagnosis could be emotionally distressing because of the absence of symptoms, but the potential for serious adverse consequences, including blindness. Participants reported that the largest impact of glaucoma was the emotional distress and fear of potentially losing their eyesight:
It's sort of like, “You don't have to worry; your pressure's a little bit higher.” But then you realize, “Oh no, your pressure's going higher, [and] you might go blind. Let's do something about this.” The other [end of the spectrum] is very severe.
I needed to do everything I could to save my sight.
Glaucoma Treatment
Treatment with medication, such as eye drops, a common first-line treatment for glaucoma, was a challenge for some people. Generally, participants reported that their eye-drop protocol was simple and initially effective. However, participants admitted that compliance could be an issue and that the eyedrop regimen tended to dictate their daily schedule:
The drops did rule my scheduling, and now I'm free of that, so it's really nice to go out and not have to worry, “Oh, I have to hurry up or I'll miss a drop.”
In addition, some participants spoke of the challenges eye drops could have for those who were not physically capable of administering the drops due to lack of dexterity or mobility:
My aunt had glaucoma…and she just had a horrible time because … she had to put the eye drops in. It was difficult for her; she was an older woman at the time.
The majority of those interviewed eventually found that eye drops were not sufficient to treat their glaucoma, and were eventually required to attempt other treatment options, such as laser surgery, MIGS, or more invasive surgeries:
So I did [drops] for a couple of weeks, and I went and saw [the physician], and the pressure had decreased but not enough. So that's when he started talking about surgery.
The majority of those interviewed had received surgical intervention for glaucoma, meaning eye-drop treatment had been ineffective. This participation bias likely skewed the perceived effectiveness of eye drops as a treatment for glaucoma and therefore do not reflect a wider medical consensus.
Surgical Decision-Making
Following unsuccessful eye-drop treatment for glaucoma, participants reported that laser surgery was often the next option presented by their physicians. If this was not successful, more invasive surgeries, such as a MIGS procedure or trabeculectomy, could be offered. The key factor in patients’ decisionmaking about their treatment for glaucoma was the patient—physician relationship. Patients felt that a trusting relationship was essential for them to be comfortable making a decision about surgery as a treatment option, perhaps because, as the literature suggests, they were unfamiliar with the nature of glaucoma and its symptoms.
It comes down to how much you trust your doctor, the surgeon, and what they're telling you. I wouldn't hesitate to go forward. I guess there's always a risk, but it's a risk—are you going to lose your sight totally if you don't do it?… You put your trust in these people that they know what they're doing. That's the whole thing; you put your trust in their hands.
Contemplating eye surgery caused anxiety and fear in participants:
Well, I'll tell you the truth: when I was going to have the trabeculotomy done, I was very anxious because I had never had surgery in my life before.
Especially the eyes—no one wants to have surgery on [their eyes].
The potential to avoid using eye drops was also expressed as a factor in the decision to undergo surgery:
It's quality of life. It's a lifestyle thing. If you can be released from putting eye drops in and then doing all this other stuff and your eyes really don't…[the drops] stabilize your eyes, but you really have to do that your whole life.
Impact of Surgery
Participants who had undergone a MIGS procedure generally found it to be successful and beneficial, with minimal side effects and recovery time. Specific information about people's experiences with MIBS was not obtained because participants were generally unaware of the type of MIGS procedure they had received.
[The doctor] did that for my left eye and the pressure did go down…. There was no problem after the surgery. Then he did the right eye and put the drain in and I had no problems following the surgery on that [eye] either.
I think what I'm saying is, I can [be] more productive [in] the community and [in] society in general, because I've had this surgery and I don't have the fear,… At the beginning, I had a fear of blindness and depending on others and also the anxiety of being a burden on others.
A few participants noted the costs associated with ongoing glaucoma treatment as a barrier to care. Others noted that wait times for some surgical procedures could impact their quality of life as they waited for a procedure to treat their glaucoma.
Health Technology Assessment by INESSS
Methods
As part of its 2020 health technology assessment on iStent, INESSS consulted with patient associations. Representatives were contacted by email to inform them of the ongoing evaluation and to ensure that the interests, needs, and perspectives of people with glaucoma would be considered in this assessment.19 Additionally, INESSS conducted a secondary analysis of results from a survey of Canadians with glaucoma, capturing information on the physical, psychological, and financial burdens, as well as other burdens associated with the condition.19 The survey was conducted by the Foundation Fighting Blindness, and results were used in the health technology assessment done by CADTH and Ontario Health.14
Results
INESSS highlighted the challenges faced by people with glaucoma, including adherence to medications, the impact on activities of daily living, and the psychological dimension of dealing with glaucoma. The report also noted that some people were not inclined to seek professional help for challenges with their eyes due to the belief that the effects of glaucoma were a normal part of aging.
Preferences and Values Evidence Conclusions
Glaucoma poses significant challenges to patients. Fear of ultimate blindness and difficulty managing eye-drop medication led patients to explore other treatment options, such as MIGS. Patients relied on the trust they had in their physician to determine if surgery was necessary. Those who underwent MIGS procedures found them to be generally successful and beneficial, with minimal side effects and recovery time needed. We could not draw conclusions about specific MIBS procedures.
Conclusions of the Health Technology Assessment
Minimally invasive bleb surgery reduces IOP and the number of medications used, but we are uncertain if MIBS results in similar outcomes as trabeculectomy (GRADE: Moderate to Very low). Compared with trabeculectomy, MIBS may result in improved health-related quality of life and fewer follow-up visits, adverse events, and follow-up interventions (GRADE: Moderate to Very low). MIBS may also reduce IOP and the number of medications used compared with other glaucoma treatments, but the evidence is very uncertain (GRADE: Very low). We estimate that publicly funding MIBS would results in an additional cost of $1.93 million over 5 years. Patients who underwent MIGS procedures found them to be generally successful and beneficial, with minimal side effects and recovery time needed. We could not draw conclusions about specific MIBS procedures.
Acknowledgments
This report was developed by a multidisciplinary team from Ontario Health. The primary clinical epidemiologist was Myra Wang, the secondary clinical epidemiologist was Sonia Thomas, the primary medical librarian was Corinne Holubowich, the secondary medical librarian was Caroline Higgins, the primary health economist was David Rios, the secondary health economist was Olga Gajic-Veljanoski, and the primary patient engagement analyst was Samrawit Lemma.
The medical editor was Tim Maguire. Others involved in the development and production of this report were Emma Tolmie, Claude Soulodre, Susan Harrison, Sarah McDowell, Chunmei Li, Jigna Mistry, Andrée Mitchell, Charles de Mestral, and Nancy Sikich.
We would like to thank the following people and organizations for lending their expertise to the development of this report:
Iqbal Ahmed, Prism Eye Institute
Philip Hooper, St. Joseph's Health Care London
Cindy Hutnik, Western University
Delan Jinapriya, Galen Eye Centre
David Yan, Sinai Health
AbbVie Corporation
Glaukos Corporation
The statements, conclusions, and views expressed in this report do not necessarily represent the views of those we consulted.
Citation
Ontario Health. Minimally invasive bleb surgery for glaucoma: a health technology assessment. Ont Health Technol Assess Ser [Internet]. 2024 Jan;24(1):1-151. Available from: https://hqontario.ca/evidence-to-improve-care/health-technology-assessment/reviews-and-recommendations/minimally-invasive-bleb-surgery-for-glaucoma
Abbreviations
- CI:
confidence interval
- GRADE:
Grading of Recommendations Assessment, Development, and Evaluation
- BCVA:
best-corrected visual acuity
- CADTH:
Canadian Agency for Drugs and Technologies in Health
- CCI:
Canadian Classification of Intervention
- dB:
decibel
- FDA:
US Food & Drug Administration
- GSS:
Glaucoma Symptom Scale
- HR:
hazard ratio
- HTA:
health technology assessment
- INESSS:
Institut national d'excellence en santé et en services sociaux
- IOP:
intraocular pressure
- MD:
mean deviation (or mean defect), for visual field
- MIBS:
minimally invasive bleb surgery
- MIGS:
minimally invasive glaucoma surgery
- OCCI:
Ontario Case Costing Initiative
- OHIP:
Ontario Health Insurance Plan
- OR:
odds ratio
Glossary
- Adverse event:
An adverse event is an unexpected medical problem that happens during treatment for a health condition. Adverse events may be caused by something other than the treatment.
- Base case:
In economic evaluations, the base case is the “best guess” scenario, including any assumptions, considered most likely to be accurate. In health technology assessments conducted by Ontario Health, the reference case is used as the base case.
- Budget impact analysis:
A budget impact analysis estimates the financial impact of adopting a new health care intervention on the current budget (i.e., the affordability of the new intervention). It is based on predictions of how changes in the intervention mix will impact the level of health care spending for a specific population. Budget impact analyses are typically conducted for a short-term period (e.g., 5 years). The budget impact, sometimes referred to as the net budget impact, is the estimated cost difference between the current scenario (i.e., the anticipated amount of spending for a specific population without using the new intervention) and the new scenario (i.e., the anticipated amount of spending for a specific population following the introduction of the new intervention).
- Cost-benefit analysis:
A cost-benefit analysis is a type of economic evaluation that expresses the effects of a health care intervention in terms of a monetary value so that these effects can be compared with costs. Results can be reported either as a ratio of costs to benefits or as a simple sum that represents the net benefit (or net loss) of one intervention over another. The monetary valuation of the different intervention effects is based on either prices that are revealed by markets or an individual or societal willingness-to-pay value.
- Cost-consequence analysis:
A cost-consequence analysis is a type of economic evaluation that estimates the costs and consequences (i.e., the health outcomes) of two or more health care interventions. In this type of analysis, the costs are presented separately from the consequences.
- Cost-effective:
A health care intervention is considered cost-effective when it provides additional benefits, compared with relevant alternatives, at an additional cost that is acceptable to a decision-maker based on the maximum willingness-to-pay value.
- Cost-effectiveness analysis:
Used broadly, “cost-effectiveness analysis” may refer to an economic evaluation used to compare the benefits of two or more health care interventions with their costs. It may encompass several types of analysis (e.g., cost-effectiveness analysis, cost-utility analysis). Used more specifically, “cost-effectiveness analysis” may refer to a type of economic evaluation in which the main outcome measure is the incremental cost per natural unit of health (e.g., life-year, symptom-free day) gained.
- Cost-minimization analysis:
In economic evaluations, a cost-minimization analysis compares the costs of two or more health care interventions. It is used when the intervention of interest and its relevant alternative(s) are determined to be equally effective.
- Cost-utility analysis:
A cost-utility analysis is a type of economic evaluation used to compare the benefits of two or more health care interventions with their costs. The benefits are measured using quality-adjusted life-years, which capture both the quality and quantity of life. In a cost-utility analysis, the main outcome measure is the incremental cost per quality-adjusted life-year gained.
- Equity:
Unlike the notion of equality, equity is not about treating everyone the same way.124 It denotes fairness and justice in process and in results. Equitable outcomes often require differential treatment and resource redistribution to achieve a level playing field among all individuals and communities. This requires recognizing and addressing barriers to opportunities for all to thrive in our society.
- Equity-deserving groups:
- Those who exhibit the socially stratifying characteristics identified in the PROGRESS-Plus framework.31 These characteristics involve:
- Place of residence (e.g., rural and remote populations)
- Race/ethnicity/culture (e.g., First Nations, Métis, and Inuit populations, immigrant populations, and linguistic minority populations)
- Occupation or labour-market experiences more generally (e.g., those in “precarious work” arrangements like minimum-wage, seasonal, or part-time work)
- Gender
- Religion
- Educational level (e.g., health literacy)
- Socio-economic status (e.g., economically disadvantaged populations)
- Social capital/social exclusion (e.g., citizenship/residence)
- Personal characteristics associated with discrimination (e.g., age, disability, sexual orientation)
- Time-dependent relationships (e.g., leaving the hospital, in respite care)
- Generic preference-based measures:
Generic preference-based measures are generic (i.e., not disease specific) instruments used to obtain the quality-adjusted weight (i.e., the utility value) of being in a given health state. Generic preference-based measures typically consist of a self-completed questionnaire, a health-state classification system, and a scoring formula that calculates the utility value. Examples include the Health Utilities Index Mark 3 (HUI3), the EQ-5D, and the Short Form-Six Dimensions (SF-6D). The quality-adjusted weights are obtained from the public or from patients, who are provided with a descriptive profile of each predefined health state and asked to fill out a questionnaire. The benefit of using a generic instrument is the ability to obtain utility values that are comparable across different health care interventions and diseases.
- Health inequity:
Health inequities are avoidable inequalities in health between groups of people within countries and between countries.125 These inequities arise from inequalities within and between societies. Social and economic conditions and their effects on people's lives determine their risk of illness and the actions taken to prevent them becoming ill or treat illness when it occurs.
- Health-related quality of life:
Health-related quality of life is a measure of the impact of a health care intervention on a person's health. It includes the dimensions of physiology, function, social life, cognition, emotions, sleep and rest, energy and vitality, health perception, and general life satisfaction.
- Health state:
A health state is a particular status of health (e.g., sick, well, dead). A health state is associated with some amount of benefit and may be associated with specific costs. Benefit is captured through individual or societal preferences for the time spent in each health state and is expressed in quality-adjusted weights called utility values. In a Markov model, a finite number of mutually exclusive health states are used to represent discrete states of health.
- Incremental cost:
The incremental cost is the additional cost, typically per person, of a health care intervention versus a comparator.
- Incremental cost-effectiveness ratio (ICER):
The incremental cost-effectiveness ratio (ICER) is a summary measure that indicates, for a given health care intervention, how much more a health care consumer must pay to get an additional unit of benefit relative to an alternative intervention. It is obtained by dividing the incremental cost by the incremental effectiveness. Incremental cost-effectiveness ratios are typically presented as the cost per life-year gained or the cost per quality-adjusted life-year gained.
- Market distribution:
When evaluating more than two technologies, the market distribution is the proportion of the population that uses each technology.
- Markov model:
A Markov model is a type of decision-analytic model used in economic evaluations to estimate the costs and health outcomes (e.g., quality-adjusted life-years gained) associated with using a particular health care intervention. Markov models are useful for clinical problems that involve events of interest that may recur over time (e.g., stroke). A Markov model consists of mutually exclusive, exhaustive health states. Patients remain in a given health state for a certain period of time before moving to another health state based on transition probabilities. The health states and events modelled may be associated with specific costs and health outcomes.
- Microsimulation model:
In economic evaluations, a microsimulation model (e.g., an individual-level or patient-level model) is used to simulate the health outcomes for a heterogeneous group of patients (e.g., patients of different ages or with different sets of risk factors) after receiving a particular health care intervention. The health outcomes and health events of each patient are modelled, and the outcomes of several patients are combined to estimate the average costs and benefits accrued by a group of patients. In contrast, a cohort model follows a homogeneous cohort of patients (e.g., patients of the same age or with the same set of risk factors) through the model and estimates the proportion of the cohort who will experience specific health events.
- Ministry of Health perspective:
The perspective adopted in economic evaluations determines the types of costs and health benefits to include. Ontario Health develops health technology assessment reports from the perspective of the Ontario Ministry of Health. This perspective includes all costs and health benefits attributable to the Ministry of Health, such as treatment costs (e.g., drugs, administration, monitoring, hospital stays) and costs associated with managing adverse events caused by treatments. This perspective does not include out-of-pocket costs incurred by patients related to obtaining care (e.g., transportation) or loss of productivity (e.g., absenteeism).
- Probabilistic analysis:
A probabilistic analysis (also known as a probabilistic sensitivity analysis) is used in economic models to explore uncertainty in several parameters simultaneously and is done using Monte Carlo simulation. Model inputs are defined as a distribution of possible values. In each iteration, model inputs are obtained by randomly sampling from each distribution, and a single estimate of cost and effectiveness is generated. This process is repeated many times (e.g., 10,000 times) to estimate the number of times (i.e., the probability) that the health care intervention of interest is cost-effective.
- Quality-adjusted life-year (QALY):
The quality-adjusted life-year (QALY) is a generic health outcome measure commonly used in cost-utility analyses to reflect the quantity and quality of life-years lived. The life-years lived are adjusted for quality of life using individual or societal preferences (i.e., utility values) for being in a particular health state. One year of perfect health is represented by one quality-adjusted life-year.
- Reference case:
The reference case is a preferred set of methods and principles that provide the guidelines for economic evaluations. Its purpose is to standardize the approach of conducting and reporting economic evaluations, so that results can be compared across studies.
- Risk difference:
Risk difference is the difference in the risk of an outcome occurring between one health care intervention and an alternative intervention.
- Scenario analysis:
A scenario analysis is used to explore uncertainty in the results of an economic evaluation. It is done by observing the potential impact of different scenarios on the cost-effectiveness of a health care intervention. Scenario analyses include varying structural assumptions from the reference case.
- Sensitivity analysis:
Every economic evaluation contains some degree of uncertainty, and results can vary depending on the values taken by key parameters and the assumptions made. Sensitivity analysis allows these factors to be varied and shows the impact of these variations on the results of the evaluation. There are various types of sensitivity analysis, including deterministic, probabilistic, and scenario.
- Thematic synthesis:
Thematic synthesis is a system of organizing and analyzing data to generate descriptive and analytical themes. Thematic synthesis is a three stage process: the analyst (1) creates a database in which the data to be analyzed is entered word for word and then assigned a code according to its meaning and content; (2) identifies descriptive themes so that the concepts from different studies can be compared and grouped based on similarities and differences; and, finally, (3) identifies analytical themes that go beyond the content of the original articles and can be used to determine the key messages.
- Uptake rate:
In instances where two technologies are being compared, the uptake rate is the rate at which a new technology is adopted. When a new technology is adopted, it may be used in addition to an existing technology, or it may replace an existing technology.
- Willingness-to-pay value:
A willingness-to-pay value is the monetary value a health care consumer is willing to pay for added health benefits. When conducting a cost-utility analysis, the willingness-to-pay value represents the cost a consumer is willing to pay for an additional quality-adjusted life-year. If the incremental cost-effectiveness ratio is less than the willingness-to-pay value, the health care intervention of interest is considered cost-effective. If the incremental cost-effectiveness ratio is more than the willingness-to-pay value, the intervention is considered not to be cost-effective.
Appendices
Appendix 1: Guideline Recommendations for Minimally Invasive Bleb Surgery
Table A1:
Guideline Recommendations for Minimally Invasive Bleb Surgery
| Author, year | Recommendation |
|---|---|
| Canadian guidelines | |
| Canadian Ophthalmological Society, 200918 | No mention of MIBS |
| International guidelines | |
| Asia-Pacific Glaucoma Society, 201627 |
Mention of XEN Gel Stent as a type of MIGS Further studies are required to establish the long-term effectiveness of MIGS devices. |
| National Institute for Health and Care Excellence, 201825 |
Mention of XEN Gel Stent only Procedure should be performed only with special arrangements for clinical governance, consent, and audit or research. Further research, including RCTs, was encouraged, as well as details on patient selection and long-term outcomes. |
| American Academy of Ophthalmology, 202024 |
Mention of XEN Gel Stent only Selection of XEN should be left to the discretion of the treating ophthalmologist, in consultation with the individual patient (insufficient quality, discretionary recommendation). |
| European Glaucoma Society, 202026 |
No specific mention of MIBS Currently there is not sufficient evidence to support the superiority or equivalence in efficacy between any MIGS nor versus trabeculectomy. |
Abbreviations: MIBS, minimally invasive bleb surgery; MIGS, minimally invasive glaucoma surgery.
Appendix 2: Summary of Published Systematic Reviews on Minimally Invasive Bleb Surgery
Table A2:
Summary of Published Systematic Reviews on Minimally Invasive Bleb Surgery
| Author, year | Search period | Databases searched | Inclusion criteria | Critical appraisal | No. included studies | Main conclusions |
|---|---|---|---|---|---|---|
| King et al, 2018126 | Up to July 2018 | MEDLINE, Embase, ISRCTN registry; ClinicalTrials.gov, WHO ICTRP |
MIBS ± cataract surgery compared with other glaucoma treatment MIBS ± cataract surgery |
Cochrane Risk of Bias tool | 0 | There is currently no high-quality evidence on MIBS for medically uncontrolled OAG |
| National Institute for Health and Care Excellence, 201825 | Up to December 2017 | MEDLINE, Embase, Cochrane library | People with primary OAG Clinical studies in English language on XEN Excluded: abstracts with no clinical outcomes, review, editorial, lab or animal study, conference abstracts, unless reported specific adverse events not available in published literature |
NR | 11 (1 retrospective comparative case series, 7 case series, 3 case reports) |
Reduces IOP and medication use Adverse events include device exposure, removal and reposition, implantation failure, increased IOP, loss of BCVA, bleb complications and needling, internal ostium obstruction, choroidal detachment, hypotony, wound problems, bleeding, secondary surgical interventions |
| Buffault et al, 2019127 | 2016-2018 | PubMed | People with primary OAG, PXG or PG XEN ± cataract surgery Cohort studies with ≥ 1 y follow-up |
NR | 8 case series (6 prospective, 2 retrospective) | XEN appears to be effective in reducing IOP and the No. of medications in OAG patients within 1 y with an acceptable safety profile |
| INESSS, 202019 | 2000-2020 | PubMed, Embase, grey literature, studies identified through expert consultation | Adults ≥ 18 y with primary OAG XEN ± cataract surgery vs. trab RCTs, observational studies, economic studies, HTAs, data submitted by manufacturer |
Downs and Black 3/3 studies were good quality | 3 comparative Additional 8 noncomparative | XEN 45 is effective in reducing IOP and amount of AGM required Extent of benefit appears to be similar to trab, but low level of evidence for the comparison XEN 45 has a safety profile similar to trab |
| Chen et al, 2022128 | September 2015 to December 2021 | MEDLINE, Embase, Cochrane library | People with glaucoma with no restriction for age, sex, ethnicity, or use of AGM XEN ± cataract surgery Case series or cohort studies; studies with the longest follow-up for included studies with overlapping populations Exclusion: case reports, reviews with < 1 y follow-up |
NR | 56 | XEN was effective and safe for primary and secondary OAG |
| Betzler et al, 2023129 | NR | MEDLINE, Embase, Current Contents, CENTRAL | ≥ 10 patients in study XEN ± cataract surgery Pilot, cohort, observational studies, and RCTs | ROBINS-I 8/33 studies had moderate to high risk of bias |
33 | Low incidence of complications and secondary glaucoma procedures after XEN 45 Relatively high incidence of postoperative bleb needling Lack of high-quality, longer-term evidence on the safety of XEN 45 for OAG |
Abbreviations: BCVA, best-corrected visual acuity; CENTRAL, Cochrane Central Register of Controlled Trials; HTA, health technology assessment; ICTRP, International Clinical Trials Registry Platform; IOP, intraocular pressure; ISRCTN, International Standard Randomized Controlled Trial Number; MIBS, minimally invasive bleb surgery; NR, not reported; OAG, open angle glaucoma; PG, pigmentary glaucoma; PXG, pseudoexfoliative glaucoma; RCT, randomized controlled trial; ROBINS-I, Risk Of Bias in Non-randomized Studies - of Interventions; trab, trabeculectomy; WHO, World Health Organization.
Appendix 3: Literature Search Strategies
Clinical Evidence Search
Search Date: March 6, 2023
Databases searched: Ovid MEDLINE, Embase, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, and NHS Economic Evaluation Database
Database segments: EBM Reviews - Cochrane Central Register of Controlled Trials <February 2023>, EBM Reviews - Cochrane Database of Systematic Reviews <2005 to February 28, 2023>, EBM Reviews - NHS Economic Evaluation Database <1st Quarter 2016>, Embase <1980 to 2023 Week 09>, Ovid MEDLINE(R) ALL <1946 to March 03, 2023>
Search Strategy:
-
1
exp Glaucoma/ (157405)
-
2
Glaucoma Drainage Implants/ (5169)
-
3
(glaucoma* or anti-glaucoma* or antiglaucoma* or OAG or COAG or POAG).ti,ab,kf. (165316)
-
4
((open or wide or closed or narrow) adj3 angle* adj5 (eye or eyes or ocular*)).ti,ab,kf. (10147)
-
5
(glaucoma* or opthalmol*).jw. (24187)
-
6
Intraocular Pressure/ (112520)
-
7
(intraocular pressure* or intraocular tension* or IOP).ti,ab,kf. (115454)
-
8
Ocular Hypertension/ (18072)
-
9
(intraocular hypertension* or ocular hypertension* or OHT).ti,ab,kf. (20405)
-
10
or/1-9 (285672)
-
11
((gel* adj2 (implant* or stent*)) or gelstent*).ti,ab,kf. (3681)
-
12
(micro fistula* or microfistula* or micro stent* or microstent* or micro shunt* or microshunt*).ti,ab,kf. (1339)
-
13
(XEN*1 or XEN 45* or XEN45* or XEN 63* or XEN63* or XEN 140* or XEN140* or Xen R* or (XEN* adj4 (ab* externo* or ab* interno* or bleb* or gel* or implant* or lumen* or shunt* or stent*))).ti,ab,kf. (12256)
-
14
(((minimal* invasiv* or micro incision* or microincision* or micro invas* or microinvas* or micro surg* or microsurg* or less invasiv*) adj3 (bleb* or drainage device* or drainage implant* or subconjunct* or sub-conjunct*)) or (bleb* form* adj3 surg*) or MIBS).ti,ab,kf. (526)
-
15
((abbvie* or allergan* or aquesys*) adj5 (gel* or shunt* or stent* or xen*)).ti,ab,kf. (224)
-
16
(preserflo* or midi arrow* or midiarrow* or midi ray* or midiray* or midi tube* or miditube*).ti,ab,kf. (170)
-
17
((glaukos* or innfocus* or santen*) adj5 (gel* or shunt*)).ti,ab,kf. (16)
-
18
or/11-17 (17042)
-
19
10 and 18 (1581)
-
20
Case Reports/ or Comment.pt. or Conference Proceeding.pt. or Congress.pt. or Editorial.pt. or (Letter not (Letter and Randomized Controlled Trial)).pt. (6545510)
-
21
19 not 20 (1369)
-
22
exp Animals/ not Humans/ (16270943)
-
23
21 not 22 (1249)
-
24
limit 23 to english language [Limit not valid in CDSR; records were retained] (1174)
-
25
24 use medall,cctr,coch,cleed (503)
-
26
exp glaucoma/ (157405)
-
27
glaucoma drainage implant/ (5169)
-
28
(glaucoma* or anti-glaucoma* or antiglaucoma* or OAG or COAG or POAG).tw,kw,kf. (165758)
-
29
((open or wide or closed or narrow) adj3 angle* adj5 (eye or eyes or ocular*)).tw,kw,kf. (10200)
-
30
(glaucoma* or opthalmol*).jw. (24187)
-
31
intraocular pressure/ (112520)
-
32
(intraocular pressure* or intraocular tension* or IOP).tw,kw,kf. (115949)
-
33
intraocular hypertension/ (13437)
-
34
(intraocular hypertension* or ocular hypertension* or OHT).tw,kw,kf. (20613)
-
35
or/26-34 (286005)
-
36
nonvalved ophthalmic drainage device/ (693)
-
37
((gel* adj2 (implant* or stent*)) or gelstent*).tw,kw,kf,dv. (3782)
-
38
(micro fistula* or microfistula* or micro stent* or microstent* or micro shunt* or microshunt*).tw,kw,kf,dv. (1462)
-
39
(XEN*1 or XEN 45* or XEN45* or XEN 63* or XEN63* or XEN 140* or XEN140* or Xen R* or (XEN* adj4 (ab* externo* or ab* interno* or bleb* or gel* or implant* or lumen* or shunt* or stent*))).tw,kw,kf,dv. (12611)
-
40
(((minimal* invasiv* or micro incision* or microincision* or micro invas* or microinvas* or micro surg* or microsurg* or less invasiv*) adj3 (bleb* or drainage device* or drainage implant* or subconjunct* or sub-conjunct*)) or (bleb* form* adj3 surg*) or MIBS).tw,kw,kf,dv. (550)
-
41
((abbvie* or allergan* or aquesys*) adj5 (gel* or shunt* or stent* or xen*)).tw,kw,kf,dv. (364)
-
42
(preserflo* or midi arrow* or midiarrow* or midi ray* or midiray* or midi tube* or miditube*).tw,kw,kf,dv. (189)
-
43
((glaukos* or innfocus* or santen*) adj5 (gel* or shunt*)).tw,kw,kf,dv. (17)
-
44
or/36-43 (17778)
-
45
35 and 44 (1931)
-
46
Case Report/ or Comment/ or Editorial/ or (letter.pt. not (letter.pt. and randomized controlled trial/)) or conference abstract.pt. or conference review.pt. (11053386)
-
47
45 not 46 (1447)
-
48
(exp animal/ or nonhuman/) not exp human/ (11803208)
-
49
47 not 48 (1407)
-
50
limit 49 to english language [Limit not valid in CDSR; records were retained] (1299)
-
51
50 use emez (649)
-
52
25 or 51 (1152)
-
53
52 use medall (438)
-
54
52 use emez (649)
-
55
52 use cctr (61)
-
56
52 use coch (4)
-
57
52 use cleed (0)
-
58
remove duplicates from 52 (721)
Economic Evidence Search
Search Date: March 8, 2023
Databases searched: Ovid MEDLINE, Embase, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, and NHS Economic Evaluation Database
Database segments: EBM Reviews - Cochrane Central Register of Controlled Trials <February 2023>, EBM Reviews - Cochrane Database of Systematic Reviews <2005 to March 7, 2023>, EBM Reviews - NHS Economic Evaluation Database <1st Quarter 2016>, Embase <1980 to 2023 Week 09>, Ovid MEDLINE(R) ALL <1946 to March 07, 2023>
Search Strategy:
-
1
exp Glaucoma/ (157430)
-
2
Glaucoma Drainage Implants/ (5169)
-
3
(glaucoma* or anti-glaucoma* or antiglaucoma* or OAG or COAG or POAG).ti,ab,kf. (165374)
-
4
((open or wide or closed or narrow) adj3 angle* adj5 (eye or eyes or ocular*)).ti,ab,kf. (10148)
-
5
(glaucoma* or opthalmol*).jw. (24192)
-
6
Intraocular Pressure/ (112528)
-
7
(intraocular pressure* or intraocular tension* or IOP).ti,ab,kf. (115486)
-
8
Ocular Hypertension/ (18072)
-
9
(intraocular hypertension* or ocular hypertension* or OHT).ti,ab,kf. (20408)
-
10
or/1-9 (285748)
-
11
((gel* adj2 (implant* or stent*)) or gelstent*).ti,ab,kf. (3682)
-
12
(micro fistula* or microfistula* or micro stent* or microstent* or micro shunt* or microshunt*).ti,ab,kf. (1339)
-
13
(XEN*1 or XEN 45* or XEN45* or XEN 63* or XEN63* or XEN 140* or XEN140* or Xen R* or (XEN* adj4 (ab* externo* or ab* interno* or bleb* or gel* or implant* or lumen* or shunt* or stent*))).ti,ab,kf. (12260)
-
14
(((minimal* invasiv* or micro incision* or microincision* or micro invas* or microinvas* or micro surg* or microsurg* or less invasiv*) adj3 (bleb* or drainage device* or drainage implant* or subconjunct* or sub-conjunct*)) or (bleb* form* adj3 surg*) or MIBS).ti,ab,kf. (526)
-
15
((abbvie* or allergan* or aquesys*) adj5 (gel* or shunt* or stent* or xen*)).ti,ab,kf. (224)
-
16
(preserflo* or midi arrow* or midiarrow* or midi ray* or midiray* or midi tube* or miditube*).ti,ab,kf. (170)
-
17
((glaukos* or innfocus* or santen*) adj5 (gel* or shunt*)).ti,ab,kf. (16)
-
18
or/11-17 (17047)
-
19
10 and 18 (1581)
-
20
Case Reports/ or Comment.pt. or Conference Proceeding.pt. or Congress.pt. or Editorial.pt. or (Letter not (Letter and Randomized Controlled Trial)).pt. (6547525)
-
21
19 not 20 (1369)
-
22
exp Animals/ not Humans/ (16272568)
-
23
21 not 22 (1249)
-
24
23 use coch,cleed (4)
-
25
economics/ (264352)
-
26
economics, medical/ or economics, pharmaceutical/ or exp economics, hospital/ or economics, nursing/ or economics, dental/ (1037268)
-
27
economics.fs. (469930)
-
28
(econom* or price or prices or pricing or priced or discount* or expenditure* or budget* or pharmacoeconomic* or pharmaco-economic*).ti,ab,kf. (1248288)
-
29
exp “costs and cost analysis”/ (679905)
-
30
(cost or costs or costing or costly).ti. (328875)
-
31
cost effective*.ti,ab,kf. (442266)
-
32
(cost* adj2 (util* or efficacy* or benefit* or minimi* or analy* or saving* or estimate* or allocation or control or sharing or instrument* or technolog* or increment*)).ab,kf. (305991)
-
33
models, economic/ (15899)
-
34
markov chains/ or monte carlo method/ (105932)
-
35
(decision adj1 (tree* or analy* or model*)).ti,ab,kf. (65441)
-
36
(markov or markow or monte carlo).ti,ab,kf. (176189)
-
37
quality-adjusted life years/ (55213)
-
38
(QOLY or QOLYs or HRQOL or HRQOLs or QALY or QALYs or QALE or QALEs).ti,ab,kf. (109813)
-
39
((adjusted adj1 (quality or life)) or (willing* adj2 pay) or sensitivity analys*s).ti,ab,kf. (188877)
-
40
or/25-39 (3318026)
-
41
23 and 40 (69)
-
42
41 use medall,cctr (31)
-
43
24 or 42 (35)
-
44
exp glaucoma/ (157430)
-
45
glaucoma drainage implant/ (5169)
-
46
(glaucoma* or anti-glaucoma* or antiglaucoma* or OAG or COAG or POAG).tw,kw,kf. (165816)
-
47
((open or wide or closed or narrow) adj3 angle* adj5 (eye or eyes or ocular*)).tw,kw,kf. (10201)
-
48
(glaucoma* or opthalmol*).jw. (24192)
-
49
intraocular pressure/ (112528)
-
50
(intraocular pressure* or intraocular tension* or IOP).tw,kw,kf. (115981)
-
51
intraocular hypertension/ (13437)
-
52
(intraocular hypertension* or ocular hypertension* or OHT).tw,kw,kf. (20617)
-
53
or/44-52 (286082)
-
54
nonvalved ophthalmic drainage device/ (693)
-
55
((gel* adj2 (implant* or stent*)) or gelstent*).tw,kw,kf,dv. (3783)
-
56
(micro fistula* or microfistula* or micro stent* or microstent* or micro shunt* or microshunt*).tw,kw,kf,dv. (1462)
-
57
(XEN*1 or XEN 45* or XEN45* or XEN 63* or XEN63* or XEN 140* or XEN140* or Xen R* or (XEN* adj4 (ab* externo* or ab* interno* or bleb* or gel* or implant* or lumen* or shunt* or stent*))).tw,kw,kf,dv. (12615)
-
58
(((minimal* invasiv* or micro incision* or microincision* or micro invas* or microinvas* or micro surg* or microsurg* or less invasiv*) adj3 (bleb* or drainage device* or drainage implant* or subconjunct* or sub-conjunct*)) or (bleb* form* adj3 surg*) or MIBS).tw,kw,kf,dv. (550)
-
59
((abbvie* or allergan* or aquesys*) adj5 (gel* or shunt* or stent* or xen*)).tw,kw,kf,dv. (364)
-
60
(preserflo* or midi arrow* or midiarrow* or midi ray* or midiray* or midi tube* or miditube*).tw,kw,kf,dv. (189)
-
61
((glaukos* or innfocus* or santen*) adj5 (gel* or shunt*)).tw,kw,kf,dv. (17)
-
62
or/54-61 (17783)
-
63
53 and 62 (1931)
-
64
Case Report/ or Comment/ or Editorial/ or (letter.pt. not (letter.pt. and randomized controlled trial/)) or conference abstract.pt. or conference review.pt. (11054442)
-
65
63 not 64 (1447)
-
66
(exp animal/ or nonhuman/) not exp human/ (11804833)
-
67
65 not 66 (1407)
-
68
Economics/ (264352)
-
69
Health Economics/ or Pharmacoeconomics/ or Drug Cost/ or Drug Formulary/ (146368)
-
70
Economic Aspect/ or exp Economic Evaluation/ (547380)
-
71
(econom* or price or prices or pricing or priced or discount* or expenditure* or budget* or pharmacoeconomic* or pharmaco-economic*).tw,kw,kf. (1268543)
-
72
exp “Cost”/ (679905)
-
73
(cost or costs or costing or costly).ti. (328875)
-
74
cost effective*.tw,kw,kf. (451050)
-
75
(cost* adj2 (util* or efficac* or benefit* or minimi* or analy* or saving* or estimate* or allocation or control or sharing or instrument* or technolog* or increment*)).ab,kw,kf. (315454)
-
76
Monte Carlo Method/ (82185)
-
77
(decision adj1 (tree* or analy* or model*)).tw,kw,kf. (68828)
-
78
(markov or markow or monte carlo).tw,kw,kf. (179654)
-
79
Quality-Adjusted Life Years/ (55213)
-
80
(QOLY or QOLYs or HRQOL or HRQOLs or QALY or QALYs or QALE or QALEs).tw,kw,kf. (113143)
-
81
((adjusted adj1 (quality or life)) or (willing* adj2 pay) or sensitivity analys*s).tw,kw,kf. (209515)
-
82
or/68-81 (2845148)
-
83
67 and 82 (82)
-
84
83 use emez (40)
-
85
43 or 84 (75)
-
86
limit 85 to english language [Limit not valid in CDSR; records were retained] (73)
-
87
86 use medall (23)
-
88
86 use emez (38)
-
89
86 use cctr (8)
-
90
86 use coch (4)
-
91
86 use cleed (0)
-
92
remove duplicates from 86 (50)
Grey Literature Search
Performed on: Mar 9 - 16, 2023
Websites searched: Alberta Health Evidence Reviews, BC Health Technology Assessments, Canadian Agency for Drugs and Technologies in Health (CADTH), Institut national d'excellence en santé et en services sociaux (INESSS), Institute of Health Economics (IHE), University Of Calgary Health Technology Assessment Unit, Ontario Health Technology Assessment Committee (OHTAC), McGill University Health Centre Health Technology Assessment Unit, Centre Hospitalier de l'Universite de Quebec-Universite Laval, Contextualized Health Research Synthesis Program of Newfoundland (CHRSP), Health Canada Medical Device Database, International HTA Database (INAHTA), Agency for Healthcare Research and Quality (AHRQ) Evidence-based Practice Centers, Centers for Medicare & Medicaid Services Technology Assessments, Veterans Affairs Health Services Research and Development, Institute for Clinical and Economic Review, Oregon Health Authority Health Evidence Review Commission, Washington State Health Care Authority Health Technology Reviews, National Institute for Health and Care Excellence (NICE), National Health Service England (NHS), Healthcare Improvement Scotland, Health Technology Wales, Ireland Health Information and Quality Authority Health Technology Assessments, Australian Government Medical Services Advisory Committee, Australian Safety and Efficacy Register of New Interventional Procedures - Surgical (ASERNIP-S), Health Council of Australian Governments Health Technologies, Italian National Agency for Regional Health Services, Belgian Health Care Knowledge Centre, Ludwig Boltzmann Institute for Health Technology Assessment, Swedish Agency for Health Technology Assessment and Assessment of Social Services, Ministry of Health Malaysia Health Technology Assessment Section, Tuft's Cost-Effectiveness Analysis Registry, PROSPERO, EUnetHTA, clinicaltrials.gov
Keywords used: xen, preserflo, MIBS, glaucoma, gel, gel stent, gelstent, gelatin, microstent, microstunt, minimally invasive, bleb, subconjunctival, drainage, intraocular, glaucome, sous-conjonctival, intraoculaire
Clinical results (included in PRISMA): 2
Economic results (included in PRISMA): 1
Ongoing HTAs (PROSPERO/EUnetHTA/NICE/MSAC): 20
Ongoing clinical trials: 40
Appendix 4: Characteristics of Included Comparative Studies on Minimally Invasive Bleb Surgery
Table A3:
Characteristics of Included Comparative Studies on Minimally Invasive Bleb Surgery
| Author, year Country | Study design | MIBS vs. Comparator | No. of eyes and patients | Participants | Glaucoma type | Age, y | Mean no. meds used ± SD | Followup time |
|---|---|---|---|---|---|---|---|---|
| Baker et al, 202137 United States | Noninferiority RCT | PF vs. trab | 527 eyes 527 pts PF: 395 pts Trab: 132 pts |
40-85 y, mild-to-severe POAG inadequately controlled on max tolerated medical therapy, IOP > 15 and < 40 mmHg, VF MD -3.00 dB or worse | POAG | PF: 66.4 ± 9.3 Trab male: 67.8 ± 9.3 P = .14 |
PF: 3.1 ± 1.0 Trab: 3.0 ± 0.9 P = .31 |
2 y (results available for 1 y) |
| Sheybani et al, 202336 United States | Noninferiority RCT | XEN vs. trab | 158 eyes 139 pts XEN: 95 pts Trab: 44 pts |
≥ 18 y and had OAG with IOP ≥ 15 and ≤ 44 mmHg, uncontrolled on current medical therapy, BCVA 20/100 Snellen or better, VF MD no worse than -18.0 dB, Shaffer grade ≥ 2 in the target area or open angle in eyes with prior failed angle surgery | POAG, PEXG, PG, other | PF: 69.5 ± 9.6 Trab: 69.4 ± 9.7 |
XEN: 2.8 ± 1.2 Trab: 2.5 ± 1.3 P = .29 |
1 y |
| Aghayeva et al, 202139 Germany | Retrospective observational | PF vs. trab, FCP | 235 eyes 235 pts PF: 23 pts Trab: 187 pts FCP: 25 pts |
MMC-augmented PF, trab, or FCP as initial unilateral glaucoma surgery from January 2019 to January 2020 Surgery indications: deterioration of optic nerve head, retinal nerve fiber layer thickness, or VF |
POAG, PEXG, PG, NTG, secondary uveal, other | PF: 68.6 ± 13.3 (range 25-86) Trab: 67.1 ± 10.4 (range 40-88) FCP: 60.2 ± 12.6 (range 35-84) |
PF: 7.6 ± 6.6 Trab: 8.1 ± 8.5 FCP: 9.4 ± 6.5 Presence of topical therapy in fellow eye: PF: 19 (83%) Trab: 170 (91%) FCP: 24 (96%) |
1 wk |
| Fili et al, 202265 Germany | Prospective observational | PF vs. trab | 300 eyes 300 pts PF: 150 eyes and pts Trab: 150 eyes and pts |
Moderate or advanced OAG and IOP > 18 mmHg VF MD worse than than -6 db Secondary: cup-to-disc ratio 0.7 to 1.0, IOP higher than target IOP with medical therapy |
POAG, PEXG, PG | PF: 73.3 ± 11.2 (range 38-91) Trab: 69.0 ± 9.2 (range 48-87) |
PF: 2.6 ± 1.17 Trab: 2.7 ± 0.7 P = .1 |
1 y |
| Fu et al, 202266 United Kingdom | Retrospective observational | PF vs. trab | 202 eyes 202 pts PF: 101 eyes and pts Trab: 101 eyes and pts |
Primary incisional glaucoma surgery, minimum 3-mo follow-up | POAG, PACG, SOAG, NTG tension | PF: 69 (57-78) Trab: 66 (57-76) P = .25 |
PF: 4 (3-4) Trab: 4 (3-4) P = .27 |
18 mo |
| Jamke et al, 202367 Germany | Prospective observational | PF vs. trab | 60 eyes 60 pts PF: 30 eyes and pts Trab: 30 eyes and pts |
Insufficient IOP-control on maximum tolerated medical therapy, VF progression, poor adherence/intolerance to topical medication with topical and/or systemic side effects, minimum 1-y followup | HPG, NPG | PF: 68.0 (62.8—79.0) Trab: 68.5 (61.0—77.0) P = .441 |
PF: 4.0 (3.0-4.0) Trab: 4.0 (3.8-4.0) |
1 y |
| Nobl et al, 202376 Germany | Retrospective observational | PF vs. trab | 60 eyes 54 pts PF: 31 eyes, 28 pts Trab: 29 eyes, 26 pts |
IOP above the target pressure or progression glaucoma disease under maximum tolerable medical therapy, PEXG, at least 18 y at time of surgery | PEXG | PF: 77.3 ± 7.7 Trab: 71.3 ± 8.3 P = .001 |
PF: 2.7 ± 1.2 Trab: 2.9 ± 1.2 P = .68 |
1 y |
| Pillunat et al, 202279 Germany | Prospective observational | PF vs. trab | 52 eyes 52 patients PF: 26 eyes and pts Trab: 26 eyes and pts |
Inadequately controlled on maximum tolerated medical therapy, VF progression, poor adherence/ intolerance to topical medications with topical and/or systemic side effects, minimum 6-mo follow-up | HPG, NPG | PF: 75 (65-79) Trab: 75 (67.8-77) P = .76 |
PF: 4 (3-5) Trab: 4 (3-4) P = .79 |
1 y |
| Van Lancker et al, 202280 United Kingdom | Retrospective Observational | PF vs. trab | 134 eyes 129 pts PF: 70 eyes Trab: 64 eyes |
Uncontrolled glaucoma on maximally tolerated medical therapy, any age, with any prior glaucoma procedure and any level of IOP Exclusion: neovascular glaucoma, concurrent cataract surgery |
POAG, PACG, uveitic SOAG, other secondary OAG, NTG, PEXG, PG, angle recession, juvenile OAG P = .5 | PF: 69.0 ± 13 Trab: 65.7 ± 14 P = .17 |
PF: 3.4 ± 1.2 Trab: 3.6 ± 1.0 |
18 mo |
| Almendral-Gomez et al, 202340 Spain | Retrospective observational | XEN vs. NPDS | 129 eyes XEN: 53 eyes NPDS: 65 eyes |
≥ 18 y with insufficient medically controlled early to moderate OAG, uncontrolled ocular hypertension despite medical therapy | POAG, PEX, PG, HTO, other | XEN: 71.0 (7.2) NPDS: 69.1 (9.3) P = .32 |
XEN: 2.1 (0.7) NPDS: 2.0 (0.8) |
1 y |
| Basilio et al, 201889 Portugal | Cross sectional observational | XEN vs. trab | 34 eyes 34 pts XEN: 17 eyes and pts Trab: 17 eyes and pts |
Advanced OAG, uncontrolled with medical therapy, satisfactorily preserved conjunctiva Excluded: combined surgery, prior ipsilateral eye surgery, ocular surface disease, cognitive or auditory impairment |
OAG | XEN: 69.9 ± 12.4 Trab: 67.3 ± 15.4 |
XEN: 0: 9 (52.9%) 1: 2 (11.8%) 2: 4 (23.5%) 3: 2 (11.8%) 4: 0 (0%) Trab: 0: 7 (41.2%) 1: 3 (17.6%) 2: 1 (5.9%) 3: 5 (29.5%) 4: 1 (5.9%) |
NA |
| Bormann et al, 202264 Germany | Retrospective observational | XEN vs. trab | 119 pts XEN: 69 pts Trab: 50 pts |
POAG, pseudophakia, at least 40 y, IOP repeatedly documented above target pressure and not sufficiently controllable by medical therapy, no prior incisional glaucoma surgery | POAG | XEN: 75.5 ± 0.8 Trab: 73.9 ± 1.3 |
XEN: 3.0 ± 0.1 Trab: 3.2 ± 0.2 P = .25 |
2 y |
| Bormann et al, 202264 Germany | Retrospective observational | XEN vs. trab | 149 pts XEN: 56 pts Trab: 93 pts |
POAG or PEXG with typical optical disc changes, pseudophakia, no prior incisional glaucoma surgery, IOP repeatedly measured above individual target pressure by applanation tonometry despite maximum tolerable medical therapy, increasing scotomas or increase in MD (2 dB/y) in static-automated VF exam (during last 12 mo before surgery) Exclusion: < 35 y, glaucoma other than POAG or PEXG, entity other than POAG or PEX, concurrent cataract surgery |
POAG,PEXG | XEN: 70.4 ± 8.8 Trab: 70.3 ± 10.7 P = .58 |
XEN: 3.2 ± 1.1 Trab: 3.3 ± 1.3 P = .89 |
2 y |
| Cappelli et al, 202247 Italy | Retrospective observational | XEN vs. trab | 78 pts XEN: 34 pts Trab: 34 pts |
> 45 y with uncontrolled IOP, POAG or PEXG, at least 36- mo follow-up, previous ocular surgeries were excluded except for laser trabeculoplasty or phacoemulsification with IOL implantation > 6 mo before | POAG, PEXG | XEN: 72.7 (9.5) Trab: 74.2 (7.7) P = .46 |
XEN: 2.91 (0.82) Trab: 2.72 (1.16) P = .51 |
3 y |
| Cutolo et al, 202391 Italy | Retrospective observational | XEN vs. trab | 510 eyes 392 pts XEN: 284 eyes, 219 pts, |
XEN or trab procedure that had additional surgery in same 90-d period, standalone or concurrent cataract surgery | POAG, PACG, PEXG, juvenile OAG | XEN: 70.4 ± 13.3 Trab: 69.0 ± 11.2 P = .25 |
NR | 90 d |
| Trab: 226 eyes, 173 pts | ||||||||
| Duong et al, 202245 United States | Retrospective observational | XEN vs. Kahook | 75 eyes XEN: 57 eyes and pts Kahook: eyes and pts |
OAG (all types), > 18 y, at least 24-mo follow-up, with or without previous laser or surgical treatment Exclusion: < 18 y, concurrent cataract surgery |
POAG, mixed, PEXG, PG, juvenile onset open angle, chronic angle closure, NTG, traumatic glaucoma, uveitic glaucoma | XEN: 70.3 ± 16.4 Trab: 70.7 ± 14.7 P = .925 |
XEN 0-1: 2 (3.5%) 2-3: 31 (54.4%) 4+: 24 (42.1%) Kahook: 0-1: 2 (11.1%) 2-3: 6 (33.3%) 4+: 10 (55.6%) |
2 y |
| Kee et al, 202168 Singapore | Retrospective observational | Phaco-XEN vs. phaco-trab | 137 pts Phaco-XEN: 91 pts Phaco-trab: 46 pts |
> 40 y; POAG, PEXG, PG, NTG, or primary angle closure; ≥ 1 antiglaucoma medication; presence of cataract Exclusion: other secondary glaucoma, previous intraocular surgery/procedures (except laser procedures), < 6-mo follow-up |
POAG, primary angle closure, NTG, PEXG, PG | Phaco-XEN: 71.8 (7.1) Phaco-trab: 71.2 (7.9) P = .66 |
Phaco-XEN: 2.9 (SD 0.8; 95% CI 2.6-3.1) Phaco-trab: 2.7 (SD 0.8; 95% CI 2.5-2.8) P = .15 |
1 y |
| Marcos-Parra et al, 201969 Spain | Retrospective observational | XEN vs. trab | 121 eyes 91 pts XEN: 17 eyes Phaco-XEN: 48 eyes Trab: 30 eyes Phaco-trab: 26 eyes |
Uncontrolled OAG, with or without cataract, ≥ 40 y, minimum 12-mo follow-up Exclusion: other type of glaucoma, previous intraocular surgery except cataract surgery, progressive retinal or optic nerve disease |
POAG, PEXG, other | XEN: 71.2 (11.7) Phaco-XEN: 72.7 (6.2) P = .50 Trab: 67.0 (9.8) |
XEN: 2.5 (0.8) Phaco-XEN: 2.1 (0.9) P = .11 Trab: 2.5 (0.7) Phaco-trab: 2.4 (0.8) |
1 y |
| Phaco-trab: 72.3 (10.2) P = .05 |
P = .68 | |||||||
| Marcos-Parra et al, 202372 Spain | Retrospective observational | XEN vs. trab | 121 eyes 91 pts XEN: 17 eyes Phaco-XEN: 48 eyes Trab: 30 eyes Phaco-trab: 26 eyes |
> 40 y, uncontrolled OAG, with or without cataract, minimum 36-mo follow-up Exclusion: other types of glaucoma, previous intraocular surgery except cataract surgery, progressive retinal or optic nerve disease |
NR | XEN: 71.2 (11.7) Phaco-XEN: 73 (6.2) P = .89 Trab: 66.8 (9.7) Phaco-trab: 72.0 (10.4) P = .02 |
XEN: 2.5 (0.8) Phaco-XEN: 2.1 (0.9) P = .09 Trab: 2.4 (0.8) Phaco-trab: 2.4 (0.9) P = .73 |
3 y |
| Nuzzi et al, 202148 Italy | Retrospective observational | XEN vs. Cypass vs. trab vs. Baerveldt | 95 pts XEN: 23 pts Cypass: 18 pts Trab: 39 pts Baerveldt: 15 pts |
50-85 y; POAG, PEXG, or PG; treated IOP > 19 mmHg in ≥ 2 measurements; elevated IOP regardless of full medical therapy, intolerance, or low compliance | POAG, PEXG | XEN: 2.26 ± 1.0 Cypass: 2.5 ± 0.98 Trab: 2.15 ± 0.96 Baerveldt: 2.53 ± 0.99 P = .47 |
XEN: 73.6 ± 6.4 Cypass: 68.1 ± 7.5 Trab: 74.9 ± 8.5 Baerveldt: ± 7.6 P = .015 |
3 y |
| Olgun et al, 202038 Turkey | Retrospective observational | XEN vs. GATT | 221 eyes XEN: 114 eyes GATT: 107 eyes |
OAG, IOP > 21 mmHg Exclusion: normal IOP despite medication intolerance, previous glaucoma surgery history |
POAG, PEXG, PG, uveitic, steroid | XEN: 65.8 ± 10.6 GATT: 59.1 ± 14.3 P = .001 |
XEN: 3.4 ± 0.5 GATT: 3.3 ± 0.6 P = .97 |
2 y |
| Olgun et al, 202177 Turkey | Retrospective observational | XEN vs. trab | 80 eyes 64 pts XEN: 31 eyes, 28 pts |
Perimetric OAG unresponsive to max medical therapy Exclusion: previous ocular surgery except for cataract |
POAG, PEXG, PG | XEN: 61.6 ± 16.2 Trab: 61.7 ± 12.1 P = .91 |
XEN: 3.8 ± 0.4 Trab: 3.4 ± 0.5 |
3 mo |
| Trab: 49 eyes, 36 pts | surgery ≥ 6 mo before enrollment, any corneal disease, wearing of contact lenses, presence of intraocular lens in anterior chamber, aphakia, postop complications requiring surgical intervention, history of previous ocular inflammation, significant comorbid disease that could interfere with follow-up | |||||||
| Ozcelik Kose et al, 202170 Turkey | Prospective | XEN vs. trab vs. medical tx | 48 pts XEN: 18 pts Trab: 30 pts |
Open angle in gonioscopy, VF defect consistent with glaucomatous optic nerve damage in at least 1 eye, absence of optic neuropathy other than glaucoma Excluded: other types of glaucoma, chronic eye diseases (uveitis, neovascular glaucoma, endophthalmitis), congenital and closed-angle glaucoma, history of ocular trauma |
POAG, PEXG, PG | XEN: 70 (62-77) Trab: 68 (60-72) P = .20 |
XEN: 0 Trab: 0 Medical tx: 3.5 (2.75-4) P < .01 |
Up to 3 y |
| Ponnusamy et al, 202178 United States | Retrospective observational | XEN vs. trab vs. ExPRESS | 47 eyes 41 pts XEN: 17 eyes Trab: 14 eyes ExPRESS: 16 eyes |
Poorly controlled IOP ± medications, evidence of bleb fibrosis in patients with prior XEN or trab or ExPress, minimum 6-mo follow-up after bleb Exclusion: received any other procedure (Kahook Dual Blade goniotomy, MicroPulse cyclophotocoagulation Ahmed valve) at time of bleb needling |
NR | Overall: 71.6 ± 8.6 | XEN: 1.1 ± 1.4 Trab: 2.5 ± 1.6 ExPRESS: 2.3 ± 1.6 P = .025 |
Minimum 6 mo |
| Sacchi et al, 202375 Italy | Retrospective observational | XEN vs. trab | 14 eyes and pts XEN: 7 eyes and pts Trab: 7 eyes and pts |
Unmet target IOP despite maximum medical therapy and laser, or significant perimetric progression on 3 consecutive VFs, or intolerance to medical medical therapy | POAG | XEN: 52.96 ± 10.15 Trab: 63.9 ± 13.06 P = .13 |
XEN: 3.14 ± 0.38 Trab: 3.57 ± 0.53 P = .13 |
2 y |
| Myopia > 6 D, ≥ 18 y, ≥ 2-y follow-up, with or without cataract surgery; if laser trab, then ≥ 6 mo prior Exclusion: previous surgery except for cataract surgery if ≥ 6 mo prior, anterior chamber intraocular lens, other types of glaucoma (neovascular, uveitic, angle closure, syndromic) |
||||||||
| Schargus et al, 202171 Germany | Retrospective observational | XEN vs. trab | 132 eyes 132 pts XEN: 38 eyes and pts Trab: 42 eyes and pts |
POAG, ≥ 40 y, no prior incisional glaucoma surgery, with or without cataract surgery, typical glaucomatous optic disc changes with untreated IOP ≥ 21 mmHg, evidence of disease progression under max tolerable medical therapy (3 consecutive VF results during last 12 mo showing increase ≥ 2.0 dB Exclusion: < 40 y, other types of glaucoma, history of previous glaucoma surgery |
POAG | XEN: 73.3 ± 5.9 Phaco-XEN: 73.4 ± 6.2 Trab: 69.9 ± 9.2 P = .24 |
N (%) of taken preservative-free eye drops XEN: 12 (32%) Phaco-XEN: 15 (36%) Trab: 19 (37%) P = .72 |
2 y |
| Schlenker et al, 2017,85 201886Canada, Germany, Austria, Belgium | Retrospective observational | XEN VS. trab | 354 eyes 293 pts XEN: 185 eyes, 159 pts Trab: 169 eyes, 139 pts |
30-90 Y; POAG, PEXG, PG, NTG, angle-recession, mixed glaucoma, history of angle-closure, or juvenile glaucoma; above target IOP on max medical therapy Exclusion: prior incisional filtering glaucoma surgery, other types of glaucoma (neovascular, uveitic, iridocorneal endothelial syndrome, and Axenfeld-Rieger syndrome), fibrous or epithelial downgrowth, previous corneal graft surgery, previous retinal surgery, < 1-mo follow-up |
POAG, PEXG, PG, PACG, mixed, NTG, juvenile open angle, other | XEN: median 65.0 (IQR: 53.7-73.6) Trab: median 67.2 (IQR: 59.2-74.8) p = .038 |
Med classes XEN: median 3.0 (IQR: 3.0-4.0) Trab: median 3.0 (IQR: 3.0-4.0) P = .43 |
≥ 1 mo |
| Sharpe et al, 202073 United States | Retrospective observational | XEN vs. trab | 179 eyes XEN: 90 eyes Trab: 89 eyes |
XEN or trab with up to 6-mo follow-up Exclusion: prior incisional glaucoma surgery, combined cataract surgery, < 1-mo follow-up |
POAG, low-tension, PEXG, PG, secondary | XEN: 74.5 ± 7.6 TRAB: 68.1 ± 8.2 P = < .001 |
XEN: 2.9 ± 1.1 Trab: 3.1 ± 0.9 P = .24 |
6 mo |
| Stoner et al, 202143 United States | Retrospective observational | XEN vs. ExPRESS | 188 eyes XEN: 52 eyes ExPRESS: 48 eyes |
Uncontrolled IOP despite maximum tolerated medical therapy or confirmed progression of glaucoma, with or without cataract surgery Exclusion: < 1-mo follow-up, other additional concurrent surgeries |
POAG, PEXG, PG, chronic angle closure, NTG, juvenile, second-ary, steroid | XEN: 77.8 ± 9.5 ExPRESS: 67.5 ± 12.7 P < .001 |
XEN: 2.8 ± 1.2 (range 0-6) ExPRESS: 3.1 ± 1.4 (range 0-6) P = . 37 |
3 mo |
| Teixeira et al, 202046 Portugal | Prospective observational | XEN-augmented Baerveldt vs. Ahmed | 24 pts XEN-augmented: 12 eyes and pts Ahmed: 12 eyes and pts |
Adult, inadequately controlled IOP > 21 mmHg on maximum tolerated medical therapy, had aqueous shunt as planned procedure, primary or secondary glaucoma, previous failed trab or other intraocular surgery, minimum 12-mo follow-up If no previous intraocular surgery, then secondary glaucoma known to have a high failure rate with trab (e.g., uveitic, iridocorneal endothelial syndrome, aniridia, secondary glaucoma following vitreoretinal surgery |
POAG, SOAG, primary and secondary closed angle | XEN-augmented: 59 ± 19 (range 14-83) Ahmed: 60 ± 19 (range 14-78) |
N (%) oral acetazolamide XEN-augmented: 7 (58.3%) Ahmed: 5 (41.7%) P = .54 Topical XEN-augmented: 3.7 ± 0.9 (range 2-5) AHMED: 2.9 ± 0.9 P = .69 |
12 mo |
| Teus et al, 201974 Spain | Retrospective observational | XEN vs. trab | 38 eyes XEN: 10 eyes Trab: 15 eyes Control: 23 eyes |
POAG, procedure performed ≥ 1 y before study Exclusion: neck problems, collaborated poorly, difficulty maintaining eye in appropriate position |
POAG | XEN: 72.7 ± 12.51 Trab: 70.19 ± 9.61 |
Eye drops XEN: 0.3 ± 0.4 Trab: 0.33 ± 0.6 P = .8 |
≥ 1 y |
| Theilig et al, 202087 Germany | Retrospective observational | XEN vs. trab | 200 eyes 200 patients XEN: 100 eyes and pts Trab: 100 eyes and pts |
POAG based on presence of typical glaucomatous optic disc changes, ≥ 40 y, untreated IOP ≥ 21 mmHg, diseases progression under maximum tolerable medical therapy (≥ 3 consecutive VF results during last 12 mo showing increase ≥ 2 dB), with or without cataract surgery Also included > 1 y since prior glaucoma surgery and > 1 y since prior trab for XEN Exclusion: < 40 y, other type of glaucoma |
POAG | XEN: 70.9 (95% CI:68.6-73.2) Trab: 70.3 (95% CI:68.6-72.1) P = .12 |
XEN: 2.96 ± 1.13 (95% CI: 2.74-3.18) Trab: 3.26 ± 1.23 (95% CI: 3.01-3.50) |
1 y |
| Theillac et al, 202041 France | Retrospective observational | XEN vs. NPDS | 105 eyes 75 patients XEN: 47 eyes, 36 pts NPDS: 58 eyes, 39 pts |
OAG patients with no previous glaucoma surgery | POAG, PEXG, PG, NTG, high myopia, cortisone-induced | XEN: 72.1 ± 8.7 NPDS: 69.3 ± 8.2 P = .10 |
XEN: 2.66 ± 1.07 NPDS: 2.93 ± 0.88 |
1 y |
| To et al, 2023130United States | Retrospective observational | XEN vs. trab, GDD, or CPC | 54 eyes XEN: 18 eyes Comparator: 36 eyes |
XEN: Surgically refractory glaucoma (uncontrolled IOP after a trabeculectomy or GDD implantation) Comparator: surgically refractory glaucoma, had additional filtering procedure (trab or GDD) or continuous wave CPC Exclusion: CPC prior to procedure, < 3-mo follow-up |
POAG, primary angle closure, PEXG, PG, uveitic | XEN: 65.4 ± 15.6 Comparator: 63.1 ± 12.5 P = .55 |
XEN: 2.3 ± 1.2 (range 0-4) Comparator: 2.8 ± 1.1 (range 0-5) P = .093 |
1 y |
| Touboul et al, 202242France | Retrospective observational | XEN vs. NPDS | 173 eyes and pts XEN: 70 eyes and pts NPDS: 103 eyes and pts |
> 18 y, glaucoma Exclusion: < 6-mo follow-up, lack of data, did not have the scheduled procedure, previous angle closure glaucoma, previous glaucoma surgery |
POAG, SOAG, PEXG, PG, steroid-induced, uveitic, aphakic, traumatic, NTG | XEN: 67.2 ± 10.4 NPDS: 64.6 ± 14.4 P = .16 |
Medication classes XEN: 2.59 ± 0.97 NPDS: 2.79 ± 0.85 P = .31 |
1 y |
| Wagner et al, 202081 Germany | Retrospective observational | XEN vs. trab | 171 eyes, 144 pts XEN: 82 eyes, 58 pts Trab: 89 eyes, 86 pts |
> 18 y, refractory OAG (POAG, PEXG, PG, NTG) Exclusion: prior filtering glaucoma surgery |
POAG, PEXG, PG, NTG, uveitic | XEN: median 73.0 (IQR: 65.8-80.0) Trab: median 67.2 (IQR: 59.2-74.8) P = .002 |
Medication classes XEN: median 2.0 (range 1.0-3.0) Trab: median 3.0 (range 2.0-4.0) P = .004 |
1 y |
| Wanichwecharung-ruang et al, 202188 Thailand | Retrospective observational | XEN vs. trab | 114 eyes XEN: 57 eyes Trab: 57 eyes |
≥ 18 y, primary glaucoma (POAG or PACG) Also included: phakic or pseudophakic pts with history of uneventful phacoemulsification ≥ 6 mo prior, previous laser iridotomy, iridoplasty, trabeculoplasty, both eyes if surgery for each eye ≥ 30 d apart |
POAG, PACG | XEN: 70.4 ± 8.4 Trab: 68.9 ± 8.6 P = .33 |
XEN: 2.2 ± 1.4 Trab: 2.4 ± 0.7 |
2 y |
| Gambini et al, 202263 Italy | Retrospective observational | XEN vs. PF | 58 eyes XEN: 29 PF: 29 |
POAG, BCVA 20/200 or better, uncontrolled glaucoma on maximum tolerated medical therapy, IOP of 12-45 mmHg, phakic or pseudophakic patients treated with intracapsular lens implantation, rapid and significant loss of visual function Both eyes may be included if required, with ≥ 1 mo between procedures |
POAG | XEN: 73.2 ± 4.8 PF: 72.2 ± 5.7 P = .68 |
XEN: 2.5 ± 1.0 Trab: 2.7 ± 0.8 P = .53 |
6 mo |
| Qidwai et al, 202244 United Kingdom | Retrospective observational | XEN vs. PF vs. ICE2 | 247 eyes and pts XEN: 37 eyes and pts PF: 48 eyes and pts ICE2: 162 eyes and pts |
Treated (on topical medication or previous selective laser trabeculoplasty) ocular hypertension or mild-to-moderate glaucoma | POAG, SOAG, NTG, ocular hypertension, PACG | XEN: 76.3 ± 8.3 PF: 74.6 ± 11.9 iStent and ICE2: 77.4 ± 6.9 P = .16 |
Number of drops XEN: 2.9 ± 0.7 PF: 2.9 ± 0.8 iStent and ICE2: 2.0 ± 1.0 P < .001 |
2 y |
| Saletta et al, 202282Switzerland | Retrospective observational | XEN vs. PF | 60 eyes and pts XEN: 30 eyes and pts PF: 30 eyes and pts |
Glaucoma surgery performed as standalone procedure, no concurrent cataract surgery or any other surgery including intravitreal injections, complete 12 mo follow-up data Exclusion: narrow chamber angle/history of angle closure, neovascular glaucoma, any previous glaucoma surgery other than laser trabeculoplasty |
POAG, PEXG, PG | XEN: 70 for women, 63 for men PF: 71 for women, 62 for men |
XEN: 2.9 PF: 3.4 |
1 y |
| Scheres et al, 202183 Netherlands | Retrospective observational | XEN vs. PF | 82 eyes 64 pts XEN: 41 eyes, 31 pts PF: 41 eyes, 33 pts |
POAG, inadequately controlled glaucoma despite maximum tolerated medical therapy, progression of VF loss Exclusion: < 6-mo follow-up |
POAG | XEN: 69±8 PF: 66 ± 9 P = .13 |
XEN: 2.5 ± 1.4 PF: 2.3 ± 1.5 P = .65 |
2 y |
| Wagner et al, 202284 Germany | Retrospective observational | XEN vs. PF vs. trab | 105 eyes 105 pts XEN: 35 PF: 35 Trab: 35 |
> 18 y, refractory OAG (POAG, secondary OAG, NTG) Exclusion: prior filtering glaucoma surgery |
POAG, PEXG, PG, NTG | XEN: median 70.0 (IQR: 63.0-75.5) PF: median 73.0 (IQR: 66.0- 78.5) Trab: median 68.0 (IQR: 61.5-76.0) P = .31 |
Medication classes XEN: median 2.0 (IQR: 1.0-3.5) PF: median 2.0 (IQR: 2.0-3.0) Trab: median 3.0 (IQR: 2.0-3.0) P = .02 |
6 mo |
Appendix 5: Critical Appraisal of Clinical Evidence
Table A4:
Risk of Biasa Among Randomized Controlled Trials (Cochrane Risk-of-Bias Tool 1.0)
| Author, year | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Incomplete outcome data | Selective reporting | Other bias |
|---|---|---|---|---|---|---|
| Baker et al, 202137 | Low | Low | Lowb | Unclearc | Highd | Manufacturer sponsored the study and supported development of manuscript |
| Sheybani et al, 202336 | Low | Low | Lowb | Low | Highd | Manufacturer funded and participated in trial design, analysis, data interpretation, review, and approval of publication |
Risk of bias assessed with Cochrane Risk of Bias tool 1.0 for randomized controlled trials.32 Possible risk-of-bias levels: low, high, and unclear.
Blinding of participants and personnel not possible due to the type of interventions studied. No mention of blinding for data analysis.
Interim 1-year results from a 2-year study.
Intention-to-treat analysis. Unclear if participants who discontinued the study were similar to those who completed the study. Possible reporting bias due to industry involvement.
Table A5:
Risk of Biasa Among Comparative Observational Studies (RoBANS)
| Author, year | Selection of participants | Confounding variables | Measurement of the intervention | Blinding of the outcome assessment | Incomplete outcome data | Selective outcome reporting |
|---|---|---|---|---|---|---|
| Aghayeva et al, 202139 | Highb | Unclear | Low | Low | Low | Low |
| Almendral-Gomez et al, 202340 | Highb | Unclear | Low | Low | Highc | High |
| Basilio et al, 201889 | Highb | Unclear | Low | Low | Uncleard | Unclear |
| Bormann et al, 202264 | Low | Unclear | Low | Low | Low | High |
| Cappelli et al, 202247 | Low | Unclear | Low | Low | Highc | High |
| Cutolo et al, 202391 | Highb | Highe | Low | Low | Low | Low |
| Duong et al, 202345 | Highb | Highe | Low | Low | Highc | Low |
| Kee et al, 202168 | Highb | Highe | Low | Low | Uncleard | Low |
| Marcos-Parras et al, 202372 | Highb | Highe | Low | Low | Low | Low |
| Nuzzi et al, 202148 | Highb | Highe | Low | Low | Low | Low |
| Olgun et al, 202038 | Low | Highe | Low | Low | Uncleard | Low |
| Olgun et al, 202177 | Low | Highe | Low | Low | Low | Low |
| Ozcelik Kose et al, 202170 | Low | Highe | Low | Low | Low | Low |
| Ponnusamy et al, 202178 | Highb | Low | Low | Low | Low | Low |
| Sacchi et al, 202375 | Highb | Highe | Low | Low | Low | Low |
| Schargus et al, 202171 | Highb | Highe | Low | Low | Low | Low |
| Schlenker et al, 201785 | Highb | Unclear | Low | Low | Low | Low |
| Schlenker et al, 20 1886 | Highb | Highe | Low | Low | Low | Low |
| Sharpe et al, 202273 | Highb | Highe | Low | Low | Low | Low |
| Stoner et al, 202143 | Low | Highe | Low | Low | Uncleard | Low |
| Teixeira et al, 202046 | Highb | Highe | Low | Low | Low | High |
| Teus et al, 201974 | Highb | Low | Low | Low | Low | Unclear |
| Theilig et al, 202087 | Highb | Highe | Low | Low | Low | Low |
| Theillac et al, 202041 | Highb | Highe | Low | Low | Low | Low |
| To et al, 2022130 | Highb | Highe | Low | Low | Low | Low |
| Touboul et al, 202242 | Highb | Highe | Low | Low | Uncleard | Low |
| Wagner et al, 202081 | Highb | Highe | Low | Low | Low | Low |
| Wanichwecharungruang et al, 202188 | Highb | Low | Low | Low | Low | High |
| Fili et al, 202265 | Low | Unclear | Low | Low | Low | Low |
| Fu et al, 202266 | Low | Highe | Low | Low | Highc | Low |
| Jamke et al, 202367 | Higha | Unclear | Low | Low | Low | Low |
| Nobl et al, 202149 | Higha | Highe | Low | Low | Low | Low |
| Pillunat et al, 202279 | Highb | Highe | Low | Low | Low | Low |
| Van Lancker et al, 202280 | Highb | Highe | Low | Low | Highc | Low |
| Gambini et al, 202263 | Low | Unclear | Low | Low | Highc | High |
| Qidwai et al, 202244 | Highb | Highe | Low | Low | Low | Low |
| Saletta et al, 202282 | Highb | Highe | Low | Low | Low | Low |
| Scheres et al, 202183 | Highb | Highe | Low | Low | Low | High |
| Wagner et al, 202284 | Highb | Low | Low | Low | Low | Low |
Risk of bias assessed using RoBANS, Risk of Bias Assessment Tool for Nonrandomized Studies33. Possible risk of bias levels: low, high, unclear.
Retrospective study design.
Selective/incomplete reporting of outcomes.
No information/details about participants lost to follow-up.
Unadjusted baseline differences between groups.
Table A6:
GRADE Evidence Profile for Minimally Invasive Bleb Surgery Versus Trabeculectomy
| Number of studies (design) | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | Upgrade considerations | Quality |
|---|---|---|---|---|---|---|---|
| Changes in intraocular pressure | |||||||
| 2 (RCTs) | Serious limitations (-1)a | No serious limitations | No serious limitations | No serious limitations | No serious limitations | None | ⊕⊕⊕ Moderate |
| 20 (observational studies) | Serious limitations (-1)b | No serious limitations | No serious limitations | No serious limitations | No serious limitations | None | ⊕ Very low |
| Success rate | |||||||
| 2 (RCTs) | Serious limitations (-1)a | Serious limitations (-1)c | No serious limitations | No serious limitations | No serious limitations | None | ⊕⊕⊕ Moderate |
| 19 (observational studies) | Serious limitations (-1)b | Serious limitations (-1)c | No serious limitations | No serious limitations | No serious limitations | None | ⊕ Very low |
| Change in vision | |||||||
| 1 (RCT) | Serious limitations (-1)a | No serious limitations | No serious limitations | No serious limitations | No serious limitations | None | ⊕⊕⊕ Moderate |
| 13 (observational studies) | Serious limitations (-1)b | Serious limitations (-1)c | No serious limitations | No serious limitations | No serious limitations | None | ⊕ Very low |
| Antiglaucoma medications needed | |||||||
| 2 (RCTs) | Serious limitations (-1)a | No serious limitations | No serious limitations | No serious limitations | No serious limitations | None | ⊕⊕⊕ Moderate |
| 15 (observational studies) | Serious limitations (-1)b | No serious limitations | No serious limitations | Serious limitations (-1)d | No serious limitations | None | ⊕ Very low |
| Health-related quality of life | |||||||
| 1 (RCT) | No serious limitations | No serious limitations | No serious limitations | Serious limitations (-1)d | No serious limitations | None | ⊕⊕⊕ Moderate |
| 1 (observational study) | Serious limitationsb | No serious limitations | No serious limitations | No serious limitations | No serious limitations | None | ⊕ Very low |
| Number of follow-up visits | |||||||
| 1 (RCT) | Serious limitations (-1)a | No serious limitations | No serious limitations | No serious limitations | No serious limitations | None | ⊕⊕⊕ Moderate |
| 5 (observational studies) | Serious limitations (-1)b | No serious limitations | No serious limitations | Serious limitations (-1)d | No serious limitations | None | ⊕ Very low |
| Number of follow-up interventions | |||||||
| 1 (RCT) | No serious limitations | No serious limitations | No serious limitations | Serious limitations (-1)d | No serious limitations | None | ⊕⊕⊕ Moderate |
| 7 (observational studies | Serious limitations (-1)b | No serious limitations | No serious limitations | Serious limitations (-1)d | No serious limitations | None | ⊕ Very low |
Abbreviations: GRADE, Grading of Recommendations Assessment, Development, and Evaluation; RCT, randomized controlled trial.
Possible reporting bias.
Primarily retrospective observational studies. Sometimes studies had unadjusted baseline differences between groups and unclear/incomplete patient/follow-up data.
Inconsistent results between studies.
Differences in outcome measurement between studies.
Table A7:
GRADE Evidence Profile for Minimally Invasive Bleb Surgery Versus Other Glaucoma Treatments
| Number of studies (design) | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | Upgrade considerations | Quality |
|---|---|---|---|---|---|---|---|
| Changes in intraocular pressure | |||||||
| 10 (observational studies) | Serious limitations (-1)a | No serious limitations | No serious limitations | No serious limitations | No serious limitations | None | ⊕ Very low |
| Success rate | |||||||
| 12 (observational studies) | Serious limitations (-1)a | Serious limitations (-1)b | No serious limitations | No serious limitations | No serious limitations | None | ⊕ Very low |
| Changes in vision | |||||||
| 12 (observational studies) | Serious limitations (-1)a | No serious limitations | No serious limitations | No serious limitations | No serious limitations | None | ⊕ Very low |
| Antiglaucoma medications needed | |||||||
| 10 (observational studies) | Serious limitations (-1)a | No serious limitations | No serious limitations | Serious limitations (-1)c | No serious limitations | None | ⊕ Very low |
| Number of follow-up interventions | |||||||
| 7 (observational studies | Serious limitations (-1)a | No serious limitations | No serious limitations | Serious limitations (-1)c | No serious limitations | None | ⊕ Very low |
Abbreviation: GRADE, Grading of Recommendations Assessment, Development, and Evaluation.
Primarily retrospective observational studies. Sometimes studies had unadjusted baseline differences between groups and unclear/incomplete patient/follow-up data.
Inconsistent results between studies.
Differences in outcome measurement between studies.
Appendix 6: Selected Excluded Studies - Economic Evidence
For transparency, we provide a list of studies that readers might have expected to see but that did not meet the inclusion criteria, along with the primary reason for exclusion.
| Citation | Primary reason for exclusion |
|---|---|
| Mendez-Hernandez C, Palomino-Bautista C, Torres-Imaz R, Pena-Urbina P, Perucho-Gonzalez L, Garcia-Feijoo J. Glaucoma medical treatment as a predictor of XEN45 subconjunctival gel implant hypotensive efficacy. Graefes Arch Clin Exp Ophthalmol. 2023;261(2): 521-33. | Non-comparative study |
Appendix 7: Results of Applicability and Limitation Checklists for Studies Included in the Economic Literature Review
Table A8:
Assessment of the Applicability of Studies Evaluating the Cost-Effectiveness of Minimally Invasive Bleb Surgery
| Author, year, country | Is the study population similar to the question? | Are the interventions similar to the question? | Is the health care system studied sufficiently similar to Ontario? | Were the perspectives clearly stated? If yes, what were they? | Are all direct effects included? Are all other effects included where they are material? | Are all future costs and outcomes discounted? If yes, at what rate? | Is the value of health effects expressed in terms of quality-adjusted life-years? | Are costs and outcomes from other sectors fully and appropriately measured and valued? | Overall judgmenta |
|---|---|---|---|---|---|---|---|---|---|
| CADTH & OH, 2019,14 Canada | Yes | Yes | Yes | Yes, Canadian public health payer | Yes | Yes, 1.5% | Yes | Yes | Directly applicable |
| INESSS 2020,19 Canada | Yes | Yes | Yes | Yes, Quebec public health payer | Yes | NA, 1-y time horizon | NA, cost minimization analysis | Yes | Directly applicable |
| Atik et al, 2022,100 United States | Yes | Yes | Partially | Yes, US Societal perspective | Yes | NA, 1-y time horizon | Yes | Yes | Partially applicable |
| Van Lancker et al, 2022,80 United Kingdom | Yes | Yes | Partially | Yes, UK public payer | Yes | NA, 19-mo time horizon | NA, cost-minimization analysis | Partially, exclusion of glaucoma medication costs | Partially applicable |
| Martínez-de-la-Casa, 2019,101 Spain | Yes | Yes | Partially | Yes, Spanish National Health System | Yes | No, 3-y time horizon, no discounting | NA, budget impact analysis | Yes | Partially applicable |
| Vila-Arteaga et al, 2022,102 Spain | Yes | Yes | Partially | Yes, Spanish National Health System | Yes | NA, 1-y time horizon | NA, budget impact analysis | Yes | Partially applicable |
Note: Response options for all items were “yes,” “partially,” “no,” “unclear,” and “NA” (not applicable).
Overall judgment may be “directly applicable,” “partially applicable,” or “not applicable.”
Table A9:
Assessment of the Limitations of Studies Evaluating the Cost-Effectiveness of Minimally Invasive Bleb Surgery
| Author, year, country | Does the model structure adequately reflect the nature of the health condition under evaluation? | Is the time horizon sufficiently long to reflect all important differences in costs and outcomes? | Are all important and relevant health outcomes included? | Are the clinical inputsa obtained from the best available sources? | Do the clinical inputsa match the estimates contained in the clinical sources? | Are all important and relevant (direct) costs included in the analysis? | Are the estimates of resource use obtained from the best available sources? | Are the unit costs of resources obtained from the best available sources? | Is an appropriate incremental analysis presented, or can it be calculated from the reported data? | Are all important and uncertain parameters subjected to appropriate sensitivity analysis? | Is there a potential conflict of interest? | Overall judgmentb |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CADTH & Ontario Health, 2019,14 Canada | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Minor limitations |
| INESSS 2020,19 Canada | Yes | No | Partially, no health-related quality of life outcomes | Yes | Yes | Yes | Yes | Yes | NA, cost-minimization analysis | Yes | No | Minor limitations |
Note: Response options for all items were “yes,” “partially,” “no,” “unclear,” and “NA” (not applicable).
Clinical inputs include relative treatment effects, natural history, and utilities.
Overall judgment may be “minor limitations,” “potentially serious limitations,” or “very serious limitations.”
Appendix 8: Detailed Budget Impact Analysis Model Inputs
Reference Case
All costs in 2023 CAD unless otherwise marked.
Device Costs
MIBS device: $1,314.40
Taken as the average of the cost of the XEN Gel Stent device ($1,228.80) and the PreserFlo MicroShunt device ($1,400.00) (Glaukos Corp., email communication, April 13 2023; Abbvie Corp., email communication, April 21 2023). The cost is the same for the XEN 45 and the XEN 63.
Surgery Costs (Procedure Costs, Excluding Physician Fees)
Trabeculectomy: $1,951.33
We sourced the cost of trabeculectomy by querying the OCCI for cost estimates of visits with CCI codes related to filtration surgery occurring during the 2017/2018 fiscal year. The CCI codes associated with filtration surgery were sourced from Armstrong et al (1CJ52LA, 1CJ52LASJ, 1CJ52LAQB, and 1CJ52WJ).116 Inpatient and day surgery costs were estimated to be $1,653.37 (direct cost $1,258.94, indirect cost $393.43). Adjusting for inflation (CPI 2023/CPI 2017 = 153.9/130.4 = 118%), we arrived at a cost of $1,951.33.
MIBS: $1,149.76
We used a similar approach to the CADTH and Ontario Health for costing MIBS.14 First, we sourced the cost of cataract surgery from the OCCI by querying for CCI codes associated with cataract surgery occurring during the 2017/2018 fiscal year (CCI Codes: 1CL53LAFE, 1CL53LALM, 1CL53LALN, 1CL53LALO, 1CL53LALP, 1CL53LALR, 1CL89NVLR, 1CL89VOLM, 1CL89VOLN, 1CL89VOLP, 1CL89VRLR, 1CL89NPLM, 1CL89NPLN, 1CL89NPLP, 1CL89NVLP, 1CL89VRLM, 1CL89VRLN, and 1CL89VRLP).117 This resulted in an estimated cataract surgery cost of $735.69 in 2017 CAD (direct cost $554.67, indirect cost $181.02). From OCCI data, we also estimated that the operating room functional center related costs accounted for 86% of all direct costs associated with cataract surgery.
We assumed that MIBS has a time requirement of 30 minutes, while cataract surgery has a time requirement of 20 minutes (CADTH and Ontario Health, p. 8514). We assume that the direct operation room functional center costs will increase by 150% (30 min/20 min) when conducting MIBS. This results in an estimated cost for MIBS of $974.20 (2017 CAD; direct cost $793.18 = $554.67 x (1 - 86%) + $554.67 x (86% x 1.5); indirect cost = $181.02). Adjusting for inflation (CPI 2023/CPI 2017 = 153.9/130.4 = 118%), we arrived at a cost of $1,149.76.
Trabeculectomy and Cataract Surgery: $2,354.09
We follow the CADTH and Ontario Health analysis in assuming that the cost of trabeculectomy and cataract surgery consists of the cost of cataract surgery ($735.69, 2017 CAD; see MIBS cost calculation, plus the direct costs associated with trabeculectomy, $1,258.94) for a cost of $1,994.63 in 2017 CAD. Adjusting for inflation (CPI 2023/ CPI 2017 = 153.9/130.4 = 118%), we arrive at a cost of $2,354.09.
MIBS and Cataract Surgery: $1,804.39
Similar to trabeculectomy and cataract surgery, we assume that the cost of MIBS with cataract surgery consists of the cost of cataract surgery ($735.69, 2017 CAD) plus the direct costs associated with MIBS ($793.18, 2017 CAD), for a cost of $1,528.87, 2017 CAD. Adjusting for inflation(CPI 2023/CPI 2017 = 153.9/130.4 = 118%, we arrive at a cost of $1,804.39.
Physician Fees
MIBS Physician Fees: $840.00
We note that OHIP Schedule of Benefits does not have a specific MIBS or MIGS billing code. We follow the CADTH and Ontario Health cost-utility analyses14 in assuming that physicians billing for MIBS will claim OHIP billing codes E132 (Glaucoma filtering procedures, $550.00) and E136 (with intraocular implant of seton, $290.00). This results in a total physician fee cost of $840.00.
Trabeculectomy Physician Fees: $642.94
We follow the CADTH and Ontario Health analysis14 in assuming that physician billing for trabeculectomy will be using the code E132 (Glaucoma filtering procedures, $550.00). We also included fees for six units of anesthetics ($15.49 × 6), for a cost of $642.94.
MIBS With Cataract Surgery Physician Fees: $1,111.50
We follow the CADTH and Ontario Health analysis14 in assuming that for MIBS with cataract surgery, physicians will claim E214 (Glaucoma filtering procedure and cataract extraction, $729.00), E136 (with intraocular implant of seton, $290.00), and E950 (insertion of intraocular lens, $92.50). This results in a physician fee cost of $1,111.50.
Trabeculectomy and Cataract Surgery Physician Fees: $914.44
We follow the CADTH and Ontario Health analysis14 in assuming that physicians billing for trabeculectomy and cataract surgery will claim E214 (Glaucoma filtering procedure and cataract extraction, $729.00) and E950 (insertion of intraocular lens, $92.50). We also include fees for 6 units of anesthetics ($15.49 × 6) for a total physician fee cost of $914.44.
Medications
Yearly Medication Costs: $99.12
We used similar methods as the INESSS cost-minimization analysis to estimate the cost of medications received after MIBS or trabeculectomy.19
We then sourced the medications that an individual would receive after either MIBS or trabeculectomy from the INESSS analysis (Table A9).19 We also sourced the frequency that a specific medication would be used (Table A9).19 We matched medications with unit costs sourced from the Ontario Drug Benefit Formulary.112 We sourced dosing information and 21% drug wastage from Iordanous et al.111 The weighted yearly drug cost of a medication received after trabeculectomy or MIBS was calculated by multiplying the cost per year, including drug wastage, by the usage rates and summing for all seven medications: ($146.84 × 13.5%) + ($111.96 × 13.5%) + ($206.84 × 13.5%) + ($100.91 × 40.5%) + ($125.86 × 4.1%) + ($148.33 × 9.5%) + ($100.61 × 5.5%) = $128.39.
We estimated from administrative data that 77.2% of individuals receiving MIBS or trabeculectomy would be over the age of 65 and eligible for the Ontario Drug Benefit program.131 To estimate the cost to the program, we multiplied the yearly medication cost by the percentage of individuals eligible ($128.39 × 77.2%) for a cost of $99.12.
Table A10:
Per-Person Medication Cost Calculation
| Medication, DIN | Unit price | Unit price with markupa | Drops/mL | Doses/Day | Bottles/Year | Cost per year | Cost per year with wastageb | Frequency |
|---|---|---|---|---|---|---|---|---|
| Travoprost 0.004% 5 mL, 2413167 | $43.14 | $56.52 | 34 | 1 | 2.15 | $121.35 | $146.84 | 13.5% |
| Latanoprost 0.005% 2.5 mL, 2436256 | $9.58 | $20.28 | 32 | 1 | 4.56 | $92.53 | $111.96 | 13.5% |
| Bimatoprost 0.01% 5 mL, 9857368 | $60.19 | $74.93 | 32 | 1 | 2.28 | $170.94 | $206.84 | 13.5% |
| Timolol 0.5% 5 mL, 2171899 | $8.05 | $18.62 | 32.6 | 2 | 4.48 | $83.39 | $100.91 | 40.5% |
| Dorzolamide HCL 2% 5 mL, 2453347 | $2.11 | $12.21 | 25.7 | 3 | 8.52 | $104.02 | $125.86 | 4.1% |
| Brinzolamide 1% 5 mL, 2238873 | $3.56 | $13.77 | 24.6 | 3 | 8.90 | $122.59 | $148.33 | 9.5% |
| Brimonidine 0.15% 5 mL, 2248151 | $1.93 | $12.02 | 21.1 | 2 | 6.92 | $83.15 | $100.61 | 5.5% |
| Yearly medication costc | $128.39 | |||||||
| Yearly medication cost for individuals eligible for the Ontario Drug Benefit Program | $99.12 | |||||||
Abbreviation: DIN: drug identification number.
Note: Bottles per year was calculated by multiplying the drops per milliliter by the medication volume to get an estimated drops per bottle.
Drops per bottle was divided by doses per day to get an estimate of days per bottle. Bottles per year rescales days per bottle to a yearly scale.
Values may not match due to rounding.
Assuming an 8% markup and $9.93 dispensing fee.132
Including 21% drug wastage.111
Calculated by multiplying cost per year with wastage with frequency and summing for all drugs.
Ophthalmologist Visits and Vision Tests
-
Ophthalmologist visits
-
—
MIBS: $234.90
-
—
Trabeculectomy: $276.69
-
—
-
Vision tests
-
—
MIBS: $481.50
-
—
Trabeculectomy: $590.72
-
—
We used similar methods as the CADTH and Ontario Health cost-utility analysis14 for estimating costs of ophthalmologist and vision tests. We assume that physicians would claim OHIP fee code A235 ($82.40) for an initial visit and A234 ($30.50) for subsequent visits. Similar to the CADTH and Ontario Health analysis, we sourced the number of visits required from the INESSS cost-minimization analysis.19 MIBS was associated with 6 visits and trabeculectomy with 7.37 visits. This results in a cost of $234.90 ($82.40 + 5 x $30.50) for MIBS, and $276.69 ($82.40 + 6.37 x $30.50) for trabeculectomy.
We followed the CADTH and Ontario Health analysis14 in assuming that each ophthalmologist visit includes a visual field test (G435: $5.10, except for first visits), IOP test (G858: $14.05 + G432: $26.95), and optic disc test (G820: $35.00). Using the number of visit inputs listed above, the vision test cost was $481.50 for MIBS (5 x $5.10 + [$14.05+ $26.95 + $35] x 6), and $590.72 for trabeculectomy (6.37 x $5.10 + [$13.75+ $26.95 + $35] x 7.37).
Adverse Events
-
Physician fees, adverse events
-
—
Needling: $161.75
-
—
Laser suture lysis: $182.75
-
—
Anterior chamber reformation: $182.75
-
—
Bleb repair: $210.00
-
—
Iris sweep/syneochiolysis: $182.75
-
—
Yttrium-aluminume-garnet (YAG) to implant/ostomy: $161.75
-
—
Iridoplasty: $350.00
-
—
Retinal detachment: $1,092.65
-
—
-
Facility fees
-
—
Retinal detachment: $3,444.88
-
—
We sourced physician fees for adverse events from the OHIP Schedule of Benefits. Codes were verified through expert consultation. Similar to the INESSS cost-minimization analysis, the frequency of adverse events was sourced from Table 3 of Schlenker et al.85 Additionally, we sourced the frequency of retinal detachment from two studies identified in the clinical review (Table 3 from Schlenker et al,85 and Table 5 from Van Lancker et al80).80,87 We assumed that an individual may have multiple adverse events, but each adverse event can only occur once.
We considered facility costs for retinal detachment due to the expected operating room requirements and substantive facility costs. Inpatient and ambulatory facility costs were sourced from the Cost Analysis Tool (IntelliHealth Ontario).118 We queried the cost analysis tool for inpatient and ambulatory visits occurring during the 2021 fiscal year and associated with the International Classification of Diseases (ICD)-10 code ‘H330 Retinal Detachment with Retinal Break.’ From this, we estimate costs of $3,169.56 in 2021 CAD and adjusted for inflation (CPI 2023/CPI 2021 = 153.9/141.6 = 108.7%), for a cost estimate of $3,444.88.
Table A11:
Cost and Frequency of Adverse Events
| Adverse event | Cost | Fee code | Frequency MIBS | Frequency trabeculectomy |
|---|---|---|---|---|
| Needling | $161.75 | E137 Needling (discission) - primary or subsequent | 43.2% | 30.8% |
| Laser suture lysis | $182.75 | E133 Extraocular glaucoma procedures | 0.0% | 49.7% |
| Anterior chamber reformation | $182.75 | E133 Extraocular glaucoma procedures | 11.9% | 7.7% |
| Bleb repair/conjunctival suturing | $210.00 | E212 Bleb repair with conjunctival pull-down | 1.1% | 5.9% |
| Iris sweep | $182.75 | E133 Extraocular glaucoma procedures | 1.6% | 2.4% |
| YAG to implant/ostomy | $161.75 | E139 Capsulotomy | 1.6% | 1.2% |
| Iridoplasty | $350.00 | E156 Intraocular suturing of iris/ pupillary defect | 1.1% | 0.0% |
| Retinal detachment | $282.65 | E151 Re-attachment of retina and choroid by diathermy, photocoagulation, or cryopexy as an initial procedure | 0.6% | 1.2% |
| $720.00 | E148 Vitrectomy by infusion suction cutter technique | |||
| $90.00 | E936 Vitreous exchange (air, gas, or artificial vitreous substance) | |||
| $3,444.88 | Retinal detachment facility costs |
Abbreviations: MIBS, minimally invasive bleb surgery; YAG: yttrium-aluminum-garnet.
Percentage of Ontario Filtration Surgery Billing Codes Associated With Trabeculectomy
Table A12:
Calculation of the Percentage of OHIP Filtration Surgery Billing Codes Associated With Trabeculectomy
| Variable | Row | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 |
|---|---|---|---|---|---|---|---|
| Quebec Trabeculectomy estimatesa | 1 | 724 | 760 | 798 | 838 | 880 | 924 |
| Quebec glaucoma prevalenceb | 2 | 57444 | 59003 | 60650 | 62215 | 63969 | 65744 |
| Calculation | 3 = 1/2 | 0.013 | 0.013 | 0.013 | 0.013 | 0.014 | 0.014 |
| Ontario glaucoma prevalenceb | 4 | 89000 | 91856 | 94599 | 97235 | 99937 | 102801 |
| Calculation | 5 = 3/4 | 1122 | 1183 | 1245 | 1310 | 1375 | 1445 |
| Ontario claims for filtration surgery codesc | 6 | 2106 | 2121 | 2018 | 2059 | 2126 | 2346 |
| Percent of filtration surgery codes associated with trabeculectomy | 7= 5/6 | 53% | 56% | 62% | 64% | 65% | 62% |
| Mean percentage of filtration surgery codes associated with trabeculectomy (2012-2017) | 60% (mean of row 7) | ||||||
Definitions: INESSS, Institut national d'excellence en santé et en services sociaux; MIBS, minimally invasive bleb surgery.
Calculated from INESSS MIBS budget impact analysis, which reported claims for 2021-2023 and a 5% growth rate.19
Sourced from Appendix B of Kansal et al.107
Sourced from IntelliHealth analysis.106
Number of Trabeculectomy Procedures Conducted in Ontario
To estimate the number of trabeculectomy procedures in the province, we multiplied the number claims for filtration surgery related billing codes (E132 and E214, sourced from Intellihealth) by 60%.
We matched the historical Ontario population estimates (2015-2021, sourced from Statistics Canada Table: 17-10-0005-01109) to estimate the rate of trabeculectomy procedures per 100,000 individuals for various age groups. We used linear models to predict future rates of trabeculectomy procedures. We used data from 2015 to 2021 to inform our linear models as we observed a decrease in the rate of claims for glaucoma filtering procedures prior to 2015 for the 80+ age group and then matched these predicted rates with the Statistics Canada populations projections. See Table A12 for detailed results of the rate of trabeculectomy procedures, as well as trabeculectomy estimates.
Table A13:
Detailed Population Estimates
| Age | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | 2023 | 2024 | 2025 | 2026 | 2027 | 2028 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ontario population (in 100,000's) | ||||||||||||||
| Total | 137.1 | 138.8 | 140.7 | 143.1 | 145.4 | 147.3 | 148.1 | 151.2 | 153.7 | 156.1 | 158.4 | 160.6 | 162.7 | 164.8 |
| < 50 | 86.2 | 86.7 | 87.6 | 89 | 90.3 | 91.2 | 91.2 | 93.3 | 94.9 | 96.4 | 97.8 | 99.1 | 100.4 | 101.6 |
| 50-65 | 29 | 29.4 | 29.7 | 29.8 | 30 | 30 | 30 | 30.1 | 30 | 29.9 | 29.7 | 29.5 | 29.3 | 29 |
| 65-80 | 19 | 19.6 | 20.3 | 21 | 21.8 | 22.6 | 23.4 | 24.3 | 25.2 | 26.1 | 27 | 28 | 28.9 | 29.9 |
| 80+ | 2.9 | 3 | 3.1 | 3.2 | 3.3 | 3.4 | 3.5 | 3.5 | 3.6 | 3.7 | 3.8 | 4 | 4.1 | 4.3 |
| Rate of trabeculectomy procedures (per 100,000 individuals) | ||||||||||||||
| < 50 | 0.8 | 1.1 | 0.7 | 0.8 | 1 | 1 | 0.9 | 1 | 1 | 1 | 1 | 1 | 1 | 1.1 |
| 50-65 | 8.2 | 8.3 | 8.8 | 8.9 | 10.8 | 7.5 | 9.9 | 10.5 | 10.7 | 10.9 | 11.1 | 11.3 | 11.5 | 11.7 |
| 65-80 | 34.6 | 33.6 | 37 | 34.9 | 39.7 | 32.3 | 40.9 | 41.6 | 42.3 | 43 | 43.7 | 44.4 | 45 | 45.7 |
| 80+ | 93 | 93.1 | 107 | 99.6 | 104.5 | 84 | 106.1 | 103.5 | 104.2 | 104.8 | 105.5 | 106.2 | 106.8 | 107.5 |
| Trabeculectomy estimates | ||||||||||||||
| Total | 1237 | 1277 | 1410 | 1389 | 1623 | 1334 | 1704 | 1784 | 1858 | 1936 | 2016 | 2099 | 2184 | 2274 |
| < 50 | 72 | 93 | 63 | 67 | 87 | 93 | 82 | 92 | 95 | 98 | 100 | 103 | 105 | 108 |
| 50-65 | 239 | 243 | 261 | 267 | 323 | 225 | 298 | 315 | 320 | 325 | 329 | 332 | 336 | 338 |
| 65-80 | 656 | 659 | 751 | 734 | 867 | 731 | 957 | 1011 | 1065 | 1121 | 1181 | 1242 | 1303 | 1366 |
| 80+ | 270 | 282 | 335 | 321 | 346 | 285 | 367 | 366 | 378 | 392 | 406 | 422 | 440 | 462 |
Appendix 9: Detailed Budget Impact Results
Table A14:
Detailed Budget Impact Results
| Budget impact, $ milliona | Year 1 | Year 2 | Year 3 | Year 4 | Year 5 |
|---|---|---|---|---|---|
| Current scenario | |||||
| Trabeculectomy total | 7.39 | 7.69 | 8.01 | 8.33 | 8.68 |
| Surgery | 5.20 | 5.41 | 5.64 | 5.86 | 6.11 |
| Medications | 0.07 | 0.07 | 0.07 | 0.08 | 0.08 |
| Ophthalmologist visits and tests | 1.68 | 1.75 | 1.82 | 1.89 | 1.97 |
| Adverse events | 0.44 | 0.46 | 0.48 | 0.50 | 0.52 |
| MIBS total | 5.91 | 5.91 | 5.91 | 5.91 | 5.91 |
| Device acquisition | 1.91 | 1.91 | 1.91 | 1.91 | 1.91 |
| Surgery | 2.79 | 2.79 | 2.79 | 2.79 | 2.79 |
| Medications | 0.09 | 0.09 | 0.09 | 0.09 | 0.09 |
| Ophthalmologist visits and tests | 0.95 | 0.95 | 0.95 | 0.95 | 0.95 |
| Adverse events | 0.17 | 0.17 | 0.17 | 0.17 | 0.17 |
| Total current scenario | 13.30 | 13.60 | 13.92 | 14.24 | 14.59 |
| New scenario | |||||
| Trabeculectomy total | 6.73 | 6.29 | 5.82 | 5.32 | 4.80 |
| Surgery | 4.74 | 4.43 | 4.09 | 3.74 | 3.38 |
| Medications | 0.06 | 0.06 | 0.05 | 0.05 | 0.04 |
| Ophthalmologist visits and tests | 1.53 | 1.43 | 1.32 | 1.21 | 1.09 |
| Adverse events | 0.40 | 0.38 | 0.35 | 0.32 | 0.29 |
| MIBS total | 6.68 | 7.56 | 8.48 | 9.44 | 10.46 |
| Device acquisition | 2.16 | 2.44 | 2.74 | 3.05 | 3.38 |
| Surgery | 3.15 | 3.57 | 4.01 | 4.46 | 4.94 |
| Medications | 0.10 | 0.12 | 0.13 | 0.15 | 0.16 |
| Ophthalmologist visits and tests | 1.07 | 1.21 | 1.36 | 1.51 | 1.67 |
| Adverse events | 0.19 | 0.22 | 0.25 | 0.27 | 0.30 |
| Total new scenario | 13.41 | 13.85 | 14.30 | 14.76 | 15.26 |
| Budget impact | |||||
| Device acquisition | 0.25 | 0.53 | 0.83 | 1.14 | 1.47 |
| Surgery | -0.10 | -0.21 | -0.33 | -0.45 | -0.58 |
| Medications | 0.01 | 0.01 | 0.02 | 0.03 | 0.04 |
| Ophthalmologist visits and tests | -0.03 | -0.06 | -0.09 | -0.12 | -0.15 |
| Adverse events | -0.02 | -0.04 | -0.06 | -0.08 | -0.10 |
| Total Budget impact | 0.11 | 0.24 | 0.38 | 0.52 | 0.67 |
Abbreviation: MIBS, minimally invasive bleb surgery.
All totals in 2023 CAD; results may appear inexact due to rounding. Negative values indicate cost savings.
KEY MESSAGES
What Is This Health Technology Assessment About?
Glaucoma refers to any of a group of eye disorders that cause progressive damage to the optic nerve, which can lead to visual impairment and potentially irreversible blindness. Most cases of glaucoma involve the accumulation of fluid in the eye due to poor drainage, which builds pressure in the eye (known as intraocular pressure, or IOP), gradually damaging the optic nerve.
Glaucoma treatment often starts with prescription eye drops and may progress to oral medications, laser therapy, or minimally invasive glaucoma surgery, for early to moderate glaucoma. For moderate to severe (advanced) glaucoma, a procedure, such as minimally invasive bleb surgery (MIBS) or conventional/incisional glaucoma surgery (e.g., trabeculectomy), may be performed. In a MIBS procedure, eye pressure is reduced through the implantation of a device that creates a new pathway for eye fluid drainage (known as subconjunctival outflow).
This health technology assessment looked at how safe, effective, and cost-effective MIBS is for people with glaucoma. It also looked at the budget impact of publicly funding MIBS and at the experiences, preferences, and values of people with glaucoma.
What Did This Health Technology Assessment Find?
Minimally invasive bleb surgery reduces intraocular pressure and also the number of medications people with glaucoma use to manage their condition, but we are uncertain if MIBS results in similar outcomes to trabeculectomy (GRADE: Moderate to Very low). Compared with trabeculectomy, MIBS may result in improved health-related quality of life and fewer follow-up doctor visits, adverse events, and follow-up interventions (GRADE: Moderate to Very low). The procedure may also reduce intraocular pressure and the number of medications used compared with other glaucoma treatments, but the evidence is very uncertain (GRADE: Very low).
We were unable to determine the cost-effectiveness of MIBS. We estimated that publicly funding MIBS would result in a total cost increase of $1.93 million over the next 5 years.
People who underwent minimally invasive glaucoma surgery found it to be generally successful and beneficial, with minimal side effects and recovery time needed. However, we could not draw conclusions about specific bleb surgery procedures.
Contributor Information
Ontario Health:
Myra Wang, Sonia Thomas, Corinne Holubowich, Caroline Higgins, David Rios, Olga Gajic-Veljanoski, and Samrawit Lemma
About Us
We are an agency created by the Government of Ontario to connect, coordinate and modernize our province's health care system. We work with partners, providers and patients to make the health system more efficient so everyone in Ontario has an opportunity for better health and wellbeing. We work to enhance patient experience, improve population health, enhance provider experiences, improve value and advance health equity.
For more information about Ontario Health, visit OntarioHealth.ca.
Equity, Inclusion, Diversity and Anti-Racism
Ontario Health is committed to advancing equity, inclusion and diversity and addressing racism in the health care system. As part of this work, Ontario Health has developed an Equity, Inclusion, Diversity and Anti-Racism Framework, which builds on existing legislated commitments and relationships and recognizes the need for an intersectional approach.
Unlike the notion of equality, equity is not about sameness of treatment. It denotes fairness and justice in process and in results. Equitable outcomes often require differential treatment and resource redistribution to achieve a level playing field among all individuals and communities. This requires recognizing and addressing barriers to opportunities for all to thrive in our society.
References
- (1).Glaucoma: the sneak thief of sight. 2013. [cited 2023 Aug 28]. Available from: https://www.aao.org/eye-health/news/glaucoma-sneak-thief-of-sight.
- (2).McMonnies CW. Glaucoma history and risk factors. J Optom. 2017; 10(2):71–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Abu-Hassan DW, Acott TS, Kelley MJ. The trabecular meshwork: a basic review of form and function. J Ocul Biol. 2014; 2(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).CNIB Foundation. Glaucoma [Internet]. Toronto: CNIB; 2023. [cited 2023 Feb 3]. Available from: https://www.cnib.ca/en/sight-loss-infovour-eveseye-diseases/glaucoma. [Google Scholar]
- (5).Sun Y, Chen A, Zou M, Zhang Y, Jin L, Li Y, et al. Time trends, associations and prevalence of blindness and vision loss due to glaucoma: an analysis of observational data from the Global Burden of Disease Study 2017. BMJ Open. 2022; 12(1):e053805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Ontario Health (Quality). Minimally invasive glaucoma surgery: a budget impact analysis and evaluation of patients' experiences, preferences, and values. 2019 Dec; 19(9):1–57. [PMC free article] [PubMed] [Google Scholar]
- (7).Alcon. Media release: Alcon announces voluntary global market withdrawal of CyPass Micro-Stent for surgical glaucoma [Internet]. Fort Worth (TX): Alcon; 2018. [cited 2023 Mar 1]. Available from: https://www.alcon.com/media-release/alcon-announces-voluntary-global-market-withdrawal-cypass-micro-stent-surgical. [Google Scholar]
- (8).AbbVie Corp. What is the XEN gel stent? [Internet]. North Chicago (IL): AbbVie Corp.; 2021. [cited 2023 Feb 3]. Available from: https://www.xengelstent.com/XENGelStent. [Google Scholar]
- (9).Glaukos Corp. Preserflo MicroShunt: resources for healthcare professionals [Internet]. San Clemente (CA): Glaukos Corp.; 2023. [cited 2023 Feb 3]. Available from: https://preserflo.glaukos.com/healthcare-professionals. [Google Scholar]
- (10).Fea AM, Durr GM, Marolo P, Malinverni L, Economou MA, Ahmed I. XEN gel stent: a comprehensive review on its use as a treatment option for refractory glaucoma. Clin Ophthalmol. 2020; 14:1805–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Fea AM, Menchini M, Rossi A, Posarelli C, Malinverni L, Figus M. Early experience with the new XEN63 implant in primary open-angle glaucoma patients: clinical outcomes. J Clin Med. 2021; 10(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Ruda RC, Yuan L, Lai GM, Raiciulescu S, Kim WI. Clinical outcomes of ab interno placement versus ab externo placement of XEN45 gel stents. Ophthalmol Glaucoma. 2023; 6(1):4–10. [DOI] [PubMed] [Google Scholar]
- (13).Gallardo MJ, Vincent LR, Porter M. Comparison of clinical outcomes following gel stent implantation via ab-externo and ab-interno approaches in patients with refractory glaucoma. Clin Ophthalmol. 2022; 16:2187–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Canadian Agency for Drugs and Technologies in Health, Ontario Health. Optimal use of minimally invasive glaucoma surgery: a health technology assessment [Internet]. Ottawa (ON): CADTH; 2019. [cited 2023 Feb 3]. Available from: https://www.cadth.ca/sites/default/files/pdf/op0532-migs-science-report.pdf. [PubMed] [Google Scholar]
- (15).Ontario Health. iStent for adults with glaucoma: a health technology assessment. 2021 Jul; 21(10):1–42. [PMC free article] [PubMed] [Google Scholar]
- (16).Ontario Health. iStent for adults with glaucoma: recommendation [Internet]. Toronto: Queen's Printer for Ontario; 2021. Jul [cited 2023 Feb 3]. Available from: https://www.hqontario.ca/evidence-to-improve-care/health-technology-assessment/reviews-and-recommendations/istent-for-adults-with-glaucoma. [Google Scholar]
- (17).Ontario Ministry of Health. Schedule of benefits: physician services under the Health Insurance Act [Internet]. Toronto: Queen's Printer for Ontario; 2022. [cited 2023 Feb 3]. Available from: https://www.health.gov.on.ca/en/pro/programs/ohip/sob/physserv/sob_master_20221201.pdf. [Google Scholar]
- (18).Canadian Ophthalmological Society. Canadian Ophthalmological Society evidence-based clinical practice guidelines for the management of glaucoma in the adult eye [Internet]. Ottawa: Canadian Ophthalmological Society; 2009. [cited 2023 Feb 3]. Available from: http://www.ophtalmo.net/bv/Doc/2009-426-guide-glaucome.pdf. [DOI] [PubMed] [Google Scholar]
- (19).Institut national d'excellence en santé et en services sociaux (INESSS). XEN45 gel implant for microinvasive glaucoma surgery (MIGS): English summary [Internet]. Quebec City: INESSS; 2020. [cited 2023 Feb 3]. Available from: https://www.inesss.qc.ca/fileadmin/doc/INESSS/Rapports/Technologies/INESSS_Summary_XEN45_Gel.pdf. [Google Scholar]
- (20).Vancouver Coastal Health Research Institute. Minimally invasive glaucoma surgery [Internet]. Vancouver (BC): Vancouver Coastal Health Research Institute; 2020. [cited 2023 Feb 3]. Available from: https://www2.gov.bc.ca/assets/gov/health/about-bc-s-health-care-system/heath-care-partners/health-authorities/bc-health-technology-assessments/minimally-invasive-glaucoma-surgery-hta.pdf. [Google Scholar]
- (21).Cloutier L, Désy F, Nshimyumukiza L. iStent® and iStent inject® trabecular bypass for micro-invasive glaucoma surgery (MIGS) [Internet]. Quebec 2020. [cited 2023 Oct 31]. Available from: https://www.inesss.qc.ca/en/publications/publications/publication/istentr-and-istent-iniectr-trabecular-bypass-for-micro-invasive-glaucoma-surgery-migs.html.
- (22).Government of British Columbia. Minimally invasive glaucoma surgery [Internet]. Vancouver (BC): Government of British Columbia; 2023. [cited 2023 Feb 3]. Available from: https://www2.gov.bc.ca/gov/content/health/about-bc-s-health-care-system/partners/health-authorities/bc-health-technology-assessment/health-technology-assessments/glaucoma-surgery. [Google Scholar]
- (23).AbbVie Corp. The XEN gel stent procedure: comprehensive billing and coding guide [Internet]. North Chicago (IL): Abbvie Corp.; 2021. [cited 2023 Feb 3]. Available from: https://hcp.xengelstent.com/-/media/proiect/xenhcp2021/page/reimbursement/xen104454v6xen-comprehensive-billing–coding-guide–digital-version.ashx?la=en&hash=79B89164B53AA4693CD819C920723CEF6214730D. [Google Scholar]
- (24).American Academy of Ophthalmology. Primary open-angle glaucoma preferred practice pattern [Internet]. San Francisco (CA): American Academy of Ophthalmology; 2020. [cited 2023 Feb 3]. Available from: https://www.aao.org/preferred-practice-pattern/primary-open-angle-glaucoma-ppp. [Google Scholar]
- (25).National Institute for Health and Care Excellence. Microinvasive subconjunctival insertion of a transscleral gelatin stent for primary open-angle glaucoma [Internet]. London: National Institute for Health and Care Excellence; 2018. [cited 2023 Feb 3]. Available from: https://www.nice.org.uk/guidance/ipg612/resources/microinvasive-subconiunctival-insertion-of-a-trans-scleral-gelatin-stent-for-primary-openangle-glaucoma-pdf-1899873918817477. [Google Scholar]
- (26).European Glaucoma Society. European Glaucoma Society terminology and guidelines for glaucoma, 5th edition. Br J Ophthalmol. 2021;105(Suppl 1):1–169. [DOI] [PubMed] [Google Scholar]
- (27).Asian Pacific Glaucoma Society. Asia Pacific glaucoma guidelines [Internet]. Amsterdam: Kugler Publications; 2016. [cited 2023 Feb 3]. Available from: https://www.apglaucomasociety.org/sites/default/files/content-files/APGG3%20-%20For%20public.pdf. [Google Scholar]
- (28).Wu AM, Shen LQ. Racial disparities affecting black patients in glaucoma diagnosis and management. Semin Ophthalmol. 2023:1–11. [DOI] [PubMed]
- (29).McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. 2016; 75:40–6. [DOI] [PubMed] [Google Scholar]
- (30).Covidence (systematic review software) [Computer program]. Veritas Health Innovation. Melbourne (Australia). Available at: https://www.covidence.org/home.
- (31).Cochrane Equity Methods Group. Evidence for equity: PROGRESS-Plus [Internet]. London, United Kingdom: The Cochrane Collaboration; c2023. [cited 2023 Feb 3]. Available from: https://methods.cochrane.org/equity/proiects/evidence-equity/progress-plus. [Google Scholar]
- (32).Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ [Internet]. 2011. [cited 2023 Feb 3]; 343:d5928. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3196245. [DOI] [PMC free article] [PubMed]
- (33).Kim SY, Park JE, Lee YJ, Seo HJ, Sheen SS, Hahn S, et al. Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J Clin Epidemiol. 2013; 66(4):408–14. [DOI] [PubMed] [Google Scholar]
- (34).Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GRADE handbook [Internet]. Hamilton (ON): Grade Working Group; 2013. [cited 2017 Dec]. Available from http://gdt.guidelinedevelopment.org/app/handbook/handbook.html. [Google Scholar]
- (35).Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. J Clin Epidemiol [Internet]. 2021. [cited 2021 May 27]; 134:178–89. Available from: https://www.sciencedirect.com/science/article/pii/S0895435621000731. [DOI] [PubMed] [Google Scholar]
- (36).Sheybani A, Vera V, Grover DS, Vold SD, Cotter F, Bedrood S, et al. Gel stent versus trabeculectomy: the randomized, multicenter, gold-standard pathway study (GPS) of effectiveness and safety at 12 months. Am J Ophthalmol. 2023; 252:306–25. [DOI] [PubMed] [Google Scholar]
- (37).Baker ND, Barnebey HS, Moster MR, Stiles MC, Vold SD, Khatana AK, et al. Ab-externo microshunt versus trabeculectomy in primary open-angle glaucoma: one-year results from a 2-year randomized, multicenter study. Ophthalmology. 2021; 128(12):1710–21. [DOI] [PubMed] [Google Scholar]
- (38).Olgun A, Aktas Z, Ucgul AY. XEN gel implant versus gonioscopy-assisted transluminal trabeculotomy for the treatment of open-angle glaucoma. Int Ophthalmol. 2020; 40(5):1085–93. [DOI] [PubMed] [Google Scholar]
- (39).Aghayeva FA, Chronopoulos P, Schuster AK, Pfeiffer N, Hoffmann EM. Inter-eye relationship of intraocular pressure change after unilateral trabeculectomy, filtering canaloplasty, or PreserFlo microshunt implantation. Graefes Arch Clin Exp Ophthalmol. 2021; 259(10):3045–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Almendral-Gomez J, Perucho-Martinez S, Martin-Giral E, Fernandez-Escamez C, Buenasmananas-Maeso M, Monja-Alarcon N, et al. XEN gel stent versus nonpenetrating deep sclerectomy in ocular hypertension and open angle glaucoma patients. J Glaucoma. 2023; 32(6):511–9. [DOI] [PubMed] [Google Scholar]
- (41).Theillac V, Blumen-Ohana E, Akesbi J, Hamard P, Sellam A, Brasnu E, et al. Cataract and glaucoma combined surgery: XEN® gel stent versus nonpenetrating deep sclerectomy, a pilot study. BMC Ophthalmol. 2020; 20(1):231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Touboul A, Fels A, Morin A, Bensmail D, Lachkar Y. One-year outcomes of standalone XEN gel stent versus nonpenetrating deep sclerectomy. J Glaucoma. 2022; 31(12):955–65. [DOI] [PubMed] [Google Scholar]
- (43).Stoner AM, Capitena Young CE, SooHoo JR, Pantcheva MB, Patnaik JL, Kahook MY, et al. A comparison of clinical outcomes after XEN gel stent and EX-PRESS glaucoma drainage device implantation. J Glaucoma. 2021; 30(6):481–8. [DOI] [PubMed] [Google Scholar]
- (44).Qidwai U, Jones L, Ratnarajan G. A comparison of iStent combined with phacoemulsification and endocyclophotocoagulation (ICE2) with the PreserFlo MicroShunt and XEN-45 implants. Ther Adv Ophthalmol. 2022; 14:25158414221125697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Duong RT, Pittner AC, Roa TM, Dirghangi AJ, Netland PA. Stand-alone XEN gel microstent implantation compared with Kahook Dual Blade goniotomy. J Glaucoma. 2022; 31(11):898–902. [DOI] [PubMed] [Google Scholar]
- (46).Teixeira FJ, Sousa DC, Machado NM, Caiado F, Barao R, Sens P, et al. XEN-augmented Baerveldt surgical success rate and comparison with the Ahmed valve. Acta Ophthalmol. 2020; 98(7):e870–e5. [DOI] [PubMed] [Google Scholar]
- (47).Cappelli F, Cutolo CA, Olivari S, Testa V, Sindaco D, Pizzorno C, et al. Trabeculectomy versus XEN gel implant for the treatment of open-angle glaucoma: a 3-year retrospective analysis. BMJ Open Ophthalmol. 2022; 7(1):e000830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Nuzzi R, Gremmo G, Toja F, Marolo P. A retrospective comparison of trabeculectomy, Baerveldt glaucoma implant, and microinvasive glaucoma surgeries in a three-year follow-up. Semin Ophthalmol. 2021; 36(8):839–49. [DOI] [PubMed] [Google Scholar]
- (49).Nobl M, Freissinger S, Kassumeh S, Priglinger S, Mackert MJ. One-year outcomes of microshunt implantation in pseudoexfoliation glaucoma. PLoS One. 2021; 16(8):e0256670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Gillmann K, Bravetti GE, Mermoud A, Rao HL, Mansouri K. XEN gel stent in pseudoexfoliative glaucoma: 2-year results of a prospective evaluation. J Glaucoma. 2019; 28(8):676–84. [DOI] [PubMed] [Google Scholar]
- (51).Lewczuk K, Konopinska J, Jablonska J, Rudowicz J, Laszewicz P, Dmuchowska DA, et al. XEN glaucoma implant for the management of glaucoma in naive patients versus patients with previous glaucoma surgery. J Clin Med. 2021; 10(19). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Hengerer FH, Auffarth G, Conrad-Hengerer I. Comparison of minimally invasive XEN45 gel stent implantation in glaucoma patients without and with prior interventional therapies. Ophthalmol Ther. 2019; 8(3):447–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Martinez-de-la-Casa JM, Saenz-Frances F, Morales-Fernandez L, Perucho L, Mendez C, Fernandez-Vidal A, et al. Clinical outcomes of combined Preserflo Microshunt implantation and cataract surgery in open-angle glaucoma patients. Sci Rep. 2021; 11(1):15600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Mansouri K, Guidotti J, Rao HL, Ouabas A, D'Alessandro E, Roy S, et al. Prospective evaluation of standalone XEN gel implant and combined phacoemulsification-XEN gel implant surgery: 1-year results. J Glaucoma. 2018; 27(2):140–7. [DOI] [PubMed] [Google Scholar]
- (55).Lenzhofer M, Strohmaier C, Hohensinn M, Hitzl W, Steiner V, Baca B, et al. Change in visual acuity 12 and 24 months after transscleral ab interno glaucoma gel stent implantation with adjunctive Mitomycin C. Graefes Arch Clin Exp Ophthalmol. 2019; 257(12):2707–15. [DOI] [PubMed] [Google Scholar]
- (56).Karimi A, Lindfield D, Turnbull A, Dimitriou C, Bhatia B, Radwan M, et al. A multi-centre interventional case series of 259 ab-interno Xen gel implants for glaucoma, with and without combined cataract surgery. Eye (Lond). 2019; 33(3):469–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Ibarz Barbera M, Martinez-Galdon F, Caballero-Magro E, Rodriguez-Pinero M, Tana-Rivero P. Efficacy and safety of the PreserFlo microshunt with mitomycin C for the treatment of open angle glaucoma. J Glaucoma. 2022; 31(7):557–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Hussien IM, De Francesco T, Ahmed IIK. Intermediate outcomes of the novel 63μm gelatin microstent versus the conventional 45μm gelatin microstent. Ophthalmol Glaucoma. 2023. [DOI] [PubMed]
- (59).Tan NE, Tracer N, Terraciano A, Parikh HA, Panarelli JF, Radcliffe NM. Comparison of safety and efficacy between ab interno and ab externo approaches to XEN gel stent placement. Clin Ophthalmol. 2021; 15:299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Ucar F, Cetinkaya S. XEN implantation in patients with primary open-angle glaucoma: comparison of two different techniques. Int Ophthalmol. 2020; 40(10):2487–94. [DOI] [PubMed] [Google Scholar]
- (61).Yuan L, Rana HS, Lee I, Lai G, Raiciulescu S, Kim W. Short-term outcomes of XEN 45 gel stent ab interno versus ab externo transconjunctival approaches. J Glaucoma. 2023. [DOI] [PubMed]
- (62).Gillmann K, Bravetti GE, Rao HL, Mermoud A, Mansouri K. Impact of phacoemulsification combined with XEN gel stent implantation on corneal endothelial cell density: 2-year results. J Glaucoma. 2020; 29(3):155–60. [DOI] [PubMed] [Google Scholar]
- (63).Gambini G, Carla MM, Giannuzzi F, Boselli F, Grieco G, Caporossi T, et al. Anterior segment-optical coherence tomography bleb morphology comparison in minimally invasive glaucoma surgery: XEN Gel Stent vs. PreserFlo MicroShunt. Diagnostics (Basel). 2022; 12(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Bormann C, Schmidt M, Busch C, Rehak M, Scharenberg CT, Unterlauft JD. Implantation of XEN after failed trabeculectomy: an efficient therapy? Klin Monbl Augenheilkd. 2022; 239(1):86–93. [DOI] [PubMed] [Google Scholar]
- (65).Fili S, Kontopoulou K, Vastardis I, Perdikakis G, Kohlhaas M. PreserFlo MicroShunt versus trabeculectomy in patients with moderate to advanced open-angle glaucoma: 12-month follow-up of a single-center prospective study. Cureus. 2022; 14(8):e28288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Fu MX, Normando EM, Luk SMH, Deshmukh M, Ahmed F, Crawley L, et al. Microshunt versus trabeculectomy for surgical management of glaucoma: a retrospective analysis. J Clin Med. 2022; 11(18). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Jamke M, Herber R, Haase MA, Jasper CS, Pillunat LE, Pillunat KR. PRESERFLO MicroShunt versus trabeculectomy: 1-year results on efficacy and safety. Graefes Arch Clin Exp Ophthalmol. 2023:1–15. [DOI] [PMC free article] [PubMed]
- (68).Kee AR, Vivien Yip CH, Chua CH, Bryan Ang CH, Jeremy Hu Y, Guo X, et al. Comparison of efficacy and safety of XEN45 implant versus trabeculectomy in Asian eyes. J Glaucoma. 2021; 30(12):1056–64. [DOI] [PubMed] [Google Scholar]
- (69).Marcos Parra MT, Salinas Lopez JA, Lopez Grau NS, Ceausescu AM, Perez Santonja JJ. XEN implant device versus trabeculectomy, either alone or in combination with phacoemulsification, in open-angle glaucoma patients. Graefes Arch Clin Exp Ophthalmol. 2019; 257(8):1741–50. [DOI] [PubMed] [Google Scholar]
- (70).Ozcelik Kose A, Esen F, Imamoglu S, Ercalik NY, Tekcan H, Kugu S. Effects of ab interno XEN gel implantation on postural intraocular pressure elevations. Semin Ophthalmol. 2021; 36(3):82–7. [DOI] [PubMed] [Google Scholar]
- (71).Schargus M, Busch C, Rehak M, Meng J, Schmidt M, Bormann C, et al. Functional monitoring after trabeculectomy or XEN microstent implantation using spectral domain optical coherence tomography and visual field indices-a retrospective comparative cohort study. Biology (Basel). 2021; 10(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Marcos-Parra MT, Salinas-Lopez JA, Mateos-Marcos C, Moreno-Castro L, Mendoza-Moreira AL, Perez-Santonja JJ. Long-term effectiveness of XEN 45 gel-stent in open-angle glaucoma patients. Clin Ophthalmol. 2023; 17:1223–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (73).Sharpe R, Pham G, Chang P. Comparison of ab interno XEN gelatin stent vs trabeculectomy with mitomycin C: a retrospective study. J Curr Glaucoma Pract. 2020; 14(3):87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (74).Teus MA, Paz Moreno-Arrones J, Castano B, Castejon MA, Bolivar G. Optical coherence tomography analysis of filtering blebs after long-term, functioning trabeculectomy and XEN(R) stent implant. Graefes Arch Clin Exp Ophthalmol. 2019; 257(5):1005–11. [DOI] [PubMed] [Google Scholar]
- (75).Sacchi M, Fea AM, Monsellato G, Tagliabue E, Villani E, Ranno S, et al. Safety and efficacy of ab interno XEN 45 gel stent in patients with glaucoma and high myopia. J Clin Med. 2023; 12(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Nobl M, Grun C, Kassumeh S, Priglinger S, Mackert MJ. One-year outcomes of PreserFlo(TM) microshunt implantation versus trabeculectomy for pseudoexfoliation glaucoma. J Clin Med. 2023; 12(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Olgun A, Duzgun E, Yildiz AM, Atmaca F, Yildiz AA, Sendul SY. XEN gel stent versus trabeculectomy: short-term effects on corneal endothelial cells. Eur J Ophthalmol. 2021; 31(2):346–53. [DOI] [PubMed] [Google Scholar]
- (78).Ponnusamy V, Nguyen V, An JA. Comparative outcome analysis of bleb needling of fibrotic blebs in the clinic versus the operating room: a retrospective case series. BMC Ophthalmol. 2021; 21(1):115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (79).Pillunat KR, Herber R, Haase MA, Jamke M, Jasper CS, Pillunat LE. PRESERFLO MicroShunt versus trabeculectomy: first results on efficacy and safety. Acta Ophthalmol. 2022; 100(3):e779–e90. [DOI] [PubMed] [Google Scholar]
- (80).Van Lancker L, Saravanan A, Abu-Bakra M, Reid K, Quijano C, Goyal S, et al. Clinical outcomes and cost analysis of PreserFlo versus trabeculectomy for glaucoma management in the United Kingdom. Ophthalmol Glaucoma. 2022. [DOI] [PubMed]
- (81).Wagner FM, Schuster AK, Emmerich J, Chronopoulos P, Hoffmann EM. Efficacy and safety of XEN®-implantation vs. trabeculectomy: data of a “real-world” setting. PLoS One. 2020; 15(4):e0231614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (82).Saletta G, Alexoudis A, Gatzioufas Z, Grieshaber M, Papazoglou A, Tschopp M, et al. Retrospective analysis of 12 months glaucoma implant efficacy: XEN45 and PreserFlo microshunt. Klin Monbl Augenheilkd. 2022; 239(4):429–34. [DOI] [PubMed] [Google Scholar]
- (83).Scheres LMJ, Kujovic-Aleksov S, Ramdas WD, de Crom R, Roelofs LCG, Berendschot T, et al. XEN® gel stent compared to PRESERFLO MicroShunt implantation for primary open-angle glaucoma: two-year results. Acta Ophthalmol. 2021; 99(3):e433–e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (84).Wagner FM, Schuster AK, Munder A, Muehl M, Chronopoulos P, Pfeiffer N, et al. Comparison of subconjunctival microinvasive glaucoma surgery and trabeculectomy. Acta Ophthalmol. 2022; 100(5):e1120–e6. [DOI] [PubMed] [Google Scholar]
- (85).Schlenker MB, Gulamhusein H, Conrad-Hengerer I, Somers A, Lenzhofer M, Stalmans I, et al. Efficacy, safety, and risk factors for failure of standalone ab interno gelatin microstent implantation versus standalone trabeculectomy. Ophthalmology. 2017; 124(11):1579–88. [DOI] [PubMed] [Google Scholar]
- (86).Schlenker MB, Gulamhusein H, Conrad-Hengerer I, Somers A, Lenzhofer M, Stalmans I, et al. Standalone ab interno gelatin stent versus trabeculectomy: postoperative interventions, visual outcomes, and visits. Ophthalmol Glaucoma. 2018; 1(3):189–96. [DOI] [PubMed] [Google Scholar]
- (87).Theilig T, Rehak M, Busch C, Bormann C, Schargus M, Unterlauft JD. Comparing the efficacy of trabeculectomy and XEN gel microstent implantation for the treatment of primary open-angle glaucoma: a retrospective monocentric comparative cohort study. Sci Rep. 2020; 10(1):19337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (88).Wanichwecharungruang B, Ratprasatporn N. 24-month outcomes of XEN45 gel implant versus trabeculectomy in primary glaucoma. PLoS One. 2021; 16(8):e0256362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (89).Basilio AL, Moura-Coelho N, Passos I, Cardoso MS, Domingues I, Reina M, et al. XEN(R) implant and trabeculectomy medium-term quality of life assessment and comparison of results. Int J Ophthalmol. 2018; 11(12):1941–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (90).Schlenker MB, Gulamhusein H, Conrad-Hengerer I, Somers A, Lenzhofer M, Stalmans I, et al. Standalone Ab Interno Gelatin Stent versus Trabeculectomy: Postoperative Interventions, Visual Outcomes, and Visits. Ophthalmol Glaucoma. 2018; 1(3):189–96. [DOI] [PubMed] [Google Scholar]
- (91).Cutolo CA, Bonzano C, Catti C, Pizzorno C, Bagnis A, Traverso CE, et al. Reoperations for complications within 90 days after gel stent implantation or trabeculectomy. Int Ophthalmol. 2023; 43(5):1745–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (92).Lim SY, Betzler BK, Yip LWL, Dorairaj S, Ang BCH. Standalone XEN45 gel stent implantation versus combined XEN45-phacoemulsification in the treatment of open angle glaucoma-a systematic review and meta-analysis. Graefes Arch Clin Exp Ophthalmol. 2021; 259(11):3209–19. [DOI] [PubMed] [Google Scholar]
- (93).Lenzhofer M, Strohmaier C, Sperl P, Hohensinn M, Hitzl W, Steiner V, et al. Effect of the outer stent position on efficacy after minimally invasive transscleral glaucoma gel stent implantation. Acta Ophthalmol. 2019; 97(8):e1105–e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (94).Busch T, Skiljic D, Rudolph T, Bergstrom A, Zetterberg M. Learning curve and one-year outcome of XEN 45 gel stent implantation in a Swedish population. Clin Ophthalmol. 2020; 14:3719–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (95).Bonnar J, Azuara-Blanco A. Systematic review of the method and quality of reporting of complications from studies evaluating innovative glaucoma surgical procedures. Eye (Lond). 2023 Jun; 37(9):1774–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (96).Lavin-Dapena C, Cordero-Ros R, D'Anna O, Mogollon I. XEN 63 gel stent device in glaucoma surgery: A 5-years follow-up prospective study. Eur J Ophthalmol. 2021; 31(4):1829–35. [DOI] [PubMed] [Google Scholar]
- (97).Lenzhofer M, Kersten-Gomez I, Sheybani A, Gulamhusein H, Strohmaier C, Hohensinn M, et al. Four-year results of a minimally invasive transscleral glaucoma gel stent implantation in a prospective multi-centre study. Clin Exp Ophthalmol. 2019; 47(5):581–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (98).National Institute for Health and Care Excellence. Process and methods guides. Appendix I: quality appraisal checklist-economic evaluations [Internet]. London: The Institute; 2012. [cited 2016 Jan]. Available from: https://www.nice.org.uk/process/pmg4/chapter/appendix-i-quality-appraisal-checklist-economic-evaluations. [Google Scholar]
- (99).Mendez-Hernandez C, Palomino-Bautista C, Torres-Imaz R, Pena-Urbina P, Perucho-Gonzalez L, Garcia-Feijoo J. Glaucoma medical treatment as a predictor of XEN45 subconjunctival gel implant hypotensive efficacy. Graefes Arch Clin Exp Ophthalmol. 2023; 261(2):521–33. [DOI] [PubMed] [Google Scholar]
- (100).Atik A, Fahy ET, Rhodes LA, Samuels BC, Mennemeyer ST, Girkin CA. Comparative cost-effectiveness of trabeculectomy versus MicroShunt in the US medicare system. Ophthalmology. 2022; 129(10):1142–51. [DOI] [PubMed] [Google Scholar]
- (101).Martínez-de-la-Casa JM, Amaro-Barra A, Teus MA, Vila-Arteaga J, Oyagüez I, Martínez C. Budget impact analysis of the XEN® gel stent implant for glaucoma treatment. Expert Rev Ophthalmol. 2019; 14(1):5–13. [Google Scholar]
- (102).Vila Arteaga J, Gutierrez Díaz E, Martínez de la Casa JM, Millá Griñó E, Asorey García A, Salvador Alepuz J, et al. Budget impact analysis of the XEN®63 for the treatment of primary openangle glaucoma in Spain. Arch Soc Esp Oftalmol (Engl Ed). 2023; 98(1):2–10. [DOI] [PubMed] [Google Scholar]
- (103).Canadian Agency for Drugs Technologies in Health. Optimal use of minimally invasive glaucoma surgery: a health technology assessment. Ottawa: The Agency; 2018. [PubMed] [Google Scholar]
- (104).Ontario Health (Quality). Minimally invasive glaucoma surgery: a budget impact analysis and evaluation of patients' experiences, preferences, and values. Ont Health Technol Assess Ser [Internet]. 2019; 19(9):1–57. [PMC free article] [PubMed] [Google Scholar]
- (105).Baker ND, Barnebey HS, Moster MR, Stiles MC, Vold SD, Khatana AK, et al. Ab-Externo MicroShunt versus Trabeculectomy in Primary Open-Angle Glaucoma: One-Year Results from a 2-Year Randomized, Multicenter Study. Ophthalmology. 2021; 128(12):1710–21. [DOI] [PubMed] [Google Scholar]
- (106).Medical Services Database [Internet]. Toronto (ON): IntelliHealth Ontario. c2023. [cited 2023 Feb 02]. Available from: https://intellihealth.moh.gov.on.ca/. [Google Scholar]
- (107).Kansal V, Armstrong JJ, Hutnik CM. Trends in glaucoma filtration procedures: a retrospective administrative health records analysis over a 13-year period in Canada. Clin Ophthalmol. 2020; 14:501–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (108).Statistics Canada. Population estimates on July 1st, by age and sex. Table: 17-10-0005-01 [Internet]. Ottawa (ON): Statistics Canada. c2023. [cited 2023 Mar 01]. Available from: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1710000501. [Google Scholar]
- (109).Statistics Canada. Projected population, by projection scenario, age and sex, as of July 1. Table: 17-10-0057-01 [Internet]. Ottawa (ON): Statistics Canada. c2023. [cited 2023 Mar 01]. Available from: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1710000501. [Google Scholar]
- (110).R: A language and environment for statistical computing [Computer program]. Vienna: R Foundation for Statistical Computing; 2013. [Google Scholar]
- (111).Iordanous Y, Kent JS, Hutnik CM, Malvankar-Mehta MS. Projected cost comparison of trabectome, iStent, and endoscopic cyclophotocoagulation versus glaucoma medication in the Ontario Health Insurance Plan. J Glaucoma. 2014; 23(2):e112–8. [DOI] [PubMed] [Google Scholar]
- (112).Ontario Ministry of Health. Ontario drug benefit formulary [Internet]. Toronto (ON): King's Printer for Ontario. c2023. Available from: https://www.formulary.health.gov.on.ca/formulary. [Google Scholar]
- (113).Ministry of Health. Schedule of benefits: physician services under the Health Insurance Act [Internet]. Toronto (ON): Queen's Printer for Ontario; 2023. [cited 2023 Feb 02]. Available from: https://www.health.gov.on.ca/en/pro/programs/ohip/sob/physserv/sob_master.pdf. [Google Scholar]
- (114).Statistics Canada. Consumer Price Index, annual average, not seasonally adjusted: Table: 18-10-0005-01 [Internet]. Ottawa (ON): Statistics Canada. c2023. [cited 2023 Mar 06]. Available from: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1810000501. [Google Scholar]
- (115).Ministry of Health, Ministry of Long-Term Care. Health data branch web portal: Ontario case costing initiative [Internet]. Toronto (ON): Queen's Printer for Ontario. c2023. [cited 2023 Mar 01]. Available from: https://hsim.health.gov.on.ca/HDBPortal. [Google Scholar]
- (116).Armstrong JJ, Welk BK, Reid JNS, Kansal V, Hutnik CML. Secondary surgical intervention after primary glaucoma filtration surgery: an Ontario population-based study. Can J Ophthalmol. 2019; 54(2):212–22. [DOI] [PubMed] [Google Scholar]
- (117).Canadian Institute for Health Information. Implantable medical devices in Canada: insights into high-volume procedures and associated costs. Ottawa (ON): CIHI; 2020. [Google Scholar]
- (118).IntelliHealth. Cost analysis tool [Internet]. Toronto: King's Printer for Ontario. c2023. Available from: https://intellihealth.moh.gov.on.ca. [Google Scholar]
- (119).Felfeli T, Balas M, Austria G, Menalo R, Vasiliu D, Zanchetta C. Regional trends in ophthalmic surgical wait times in Ontario, Canada. Can J Ophthalmol. 2023; 58(3):e104–e6. [DOI] [PubMed] [Google Scholar]
- (120).Ontario Health. Public reporting wait times: glaucoma (eye pressure lowering) [Internet]. Toronto (ON): King's Printer for Ontario. c2023. Available from: https://www.ontariohealth.ca/public-reporting/wait-times-results. [Google Scholar]
- (121).Barham L. Public and patient involvement at the UK National Institute for Health and Clinical Excellence. Patient. 2011; 4(1):1–10. [DOI] [PubMed] [Google Scholar]
- (122).Messina J, Grainger DL. A pilot study to identify areas for further improvements in patient and public involvement in health technology assessments for medicines. Patient. 2012; 5(3):199–211. [DOI] [PubMed] [Google Scholar]
- (123).Ontario Health Technology Advisory Committee Public Engagement Subcommittee. Public engagement for health technology assessment at Health Quality Ontario—final report from the Ontario Health Technology Advisory Committee Public Engagement Subcommittee [Internet]. Toronto (ON): Queen's Printer for Ontario; 2015. Apr [cited 2018 Apr 30]. Available from: http://www.hqontario.ca/Portals/0/documents/evidence/special-reports/report-subcommittee-20150407-en.pdf. [Google Scholar]
- (124).Ontario Health. Ontario Health's equity, inclusion, diversity and anti-racism framework [Internet]. Toronto: King's Printer for Ontario; 2022. [cited 2023 Mar 22]. Available from: https://www.ontariohealth.ca/sites/ontariohealth/files/2020-12/Equity%20Framework.pdf. [Google Scholar]
- (125).World Health Organization. Social determinants of health: key concepts [Internet]. Geneva, Switzerland: WHO; 2013. [cited 2022 Mar 22]. Available from: https://www.who.int/news-room/questions-and-answers/item/social-determinants-of-health-key-concepts. [Google Scholar]
- (126).King AJ, Shah A, Nikita E, Hu K, Mulvaney CA, Stead R, et al. Subconjunctival draining minimally-invasive glaucoma devices for medically uncontrolled glaucoma. Cochrane Database Syst Rev. 2018; 12(12):CD012742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (127).Buffault J, Baudouin C, Labbe A. XEN(R) gel stent for management of chronic open angle glaucoma: a review of the literature. J Fr Ophtalmol. 2019; 42(2):e37–e46. [DOI] [PubMed] [Google Scholar]
- (128).Chen XZ, Liang ZQ, Yang KY, Lv K, Ma Y, Li MY, et al. The outcomes of XEN gel stent implantation: a systematic review and meta-analysis. Front Med (Lausanne). 2022; 9:804847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (129).Betzler BK, Lim SY, Lim BA, Yip VCH, Ang BCH. Complications and post-operative interventions in XEN45 gel stent implantation in the treatment of open angle glaucoma-a systematic review and meta-analysis. Eye (Lond). 2023; 37(6):1047–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (130).To LK, Dhoot RK, Chuang AZ, Karimaghaei S, Guevara-Abadia F, Shah RD, et al. Defining the role of ab externo XEN gel stent in glaucomatous eyes with prior failed surgical intervention. Graefes Arch Clin Exp Ophthalmol. 2023; 261(3):779–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (131).Ontario Ministry of Health. Get coverage for prescription drugs [Internet]. Toronto: King's Printer for Ontario; c2023. [cited 2023 May 30]. Available from: https://www.ontario.ca/page/get-coverage-prescription-drugs. [Google Scholar]
- (132).Ontario Ministry of Health. Ontario drug benefit program: dispensing fees [Internet]. Toronto: King's Printer for Ontario; c2023. [cited 2023 May 30]. Available from: https://www.health.gov.on.ca/en/public/programs/drugs/programs/odb/opdp_dispensing_fees.aspx. [Google Scholar]