Abstract
Infection with Plasmodium berghei ANKA is usually lethal. The parasite causes in some mouse strains a neurovascular syndrome, experimental cerebral malaria (ECM), involving immunopathological reactions. The effects on the development of ECM of the mouse genetic background have been clearly demonstrated, but nothing is known about the effects of the clonal diversity of the parasite. We showed that various cloned lines derived from a polyclonal line of P. berghei ANKA caused ECM but that the extent of ECM induction was dependent on the amount of inoculum. Subtle differences in ECM characteristics (survival time and hypothermia) were also observed. We also confirmed, using the 1.49L cloned line, that the mouse genetic background strongly affects ECM.
The infection of some mouse strains with the ANKA or K173 strain of Plasmodium berghei leads to the development of a neurovascular disease, experimental cerebral malaria (ECM), resulting in death 6 to 14 days after inoculation with the parasite. These two parasite strains have been extensively used in experimental models for cerebral malaria (13, 20, 38). However, in recent years the relevance of this model system has often been called into question. It has been suggested that the major difference between human and murine cerebral malarias is that there is no major cerebral sequestration of parasitized erythrocytes (PE) in the vascular endothelium of the mouse brain (4, 45). Lymphocytes and monocytes are the major cell populations sequestered in the venules of rodents, but recent studies have suggested that PE can also be sequestered (8, 15, 29, 36). Margination and sequestration of mononuclear cells have also been reported in the brains of patients who died of cerebral malaria (35, 37).
The idea that erythrocyte sequestration is the key factor in the pathogenesis of the human syndrome has been challenged (4, 6, 23, 33), and it has been suggested that this process is actually one of the terminal events of cerebral malaria (33). Cytokine induction and nitric oxide generation have also been implicated in the pathogenesis of cerebral malaria in both humans and rodents (5, 6). Recent studies have shown an association between tumor necrosis factor alpha (TNF-α) concentrations, interleukin 1 (IL-1) concentrations, and disease severity (19, 30, 31). Grau et al. used a polyclonal line of P. berghei ANKA in CBA/Ca mice and monoclonal or polyclonal antibodies to cytokines to demonstrate the involvement of gamma interferon (IFN-γ), TNF-α, IL-3, and granulocyte-macrophage colony-stimulating factor (16, 17, 20, 21). Different results were obtained when the K173 strain of P. berghei was used in C57BL/6 mice; antibodies to IFN-γ and TNF-α were unable to prevent ECM (10, 13). In a recent study, Hermsen et al. reported that circulating TNF-α was not involved in the development of ECM in C57BL/6 mice infected with P. berghei K173 (27). This finding demonstrated the importance of defining precisely the host-parasite combination.
The genetic backgrounds in mice (12, 21, 32, 38) and in humans (28) affect the development of cerebral malaria, whereas there is no firm evidence as yet that clonal variation of the parasite has any effect. In this study, we investigated the capacity of various cloned lines of P. berghei ANKA to cause ECM.
MATERIALS AND METHODS
Mice.
Six- to 8-week-old pathogen-free female C57BL/6, BALB/c, C3H/HeN, DBA/2, and Swiss mice were purchased from Charles River Breeding Laboratories (Saint Aubin Les Elbeuf, France). CBA/Ca (6- to 8-week-old) mice were obtained from Harlan Olac (Leicester, United Kingdom). C3H/HeJ and 129Sv/eV (6- to 8-week-old) mice were kindly donated by P. A. Cazenave (Institut Pasteur).
Body temperature.
Body temperature was measured with a digital thermometer (Quick-check; Polylabo, Strasbourg, France) inserted into the rectum and read when the readout stabilized (20 s).
Parasites.
Lines 1.49L, 1.97L, 4, and 5 of P. berghei ANKA were cloned by one of us (D.W.) from an initially polyclonal line of P. berghei ANKA obtained from M. Wéry (Institute of Tropical Medicine, Antwerp, Belgium) as previously described (44). Cloned lines 1.49L and 1.97L were derived from cloned line 1 and differ in the numbers of passages in mice. The polyclonal line used in this study was obtained from G. Grau (University of Geneva, Geneva, Switzerland). This line was derived from the same polyclonal line of P. berghei ANKA as that used to derive the various cloned lines and was maintained as a stabilate in liquid nitrogen. It was passaged sequentially in OF1, BALB/c, and CBA/Ca mice in Geneva (7, 15) and then in C57BL/6 mice in our laboratory. Blood stages of the cloned lines were stored as stabilates (107 or 108 PE/ml in Alsever’s solution containing 10% glycerol) in liquid nitrogen. Mice were infected intraperitoneally (i.p.) with various doses of PE. Parasitemia was monitored daily by counting the number of infected cells per 2,000 erythrocytes in Giemsa-stained slides.
Disease assessment.
Mice were considered to have ECM if they had neurological signs (ataxia, paralysis, deviation of the head, and convulsions). Some mice were killed when they displayed clinical signs. Brains were removed and fixed in 10% paraformaldehyde for histological examination by standard techniques to detect neurological lesions (hemorrhage, edema, and endothelial damage). The cumulative incidence of ECM was defined as the appearance of neurological signs followed by death after P. berghei ANKA infection.
RESULTS
Induction of cerebral malaria by a polyclonal line of P. berghei ANKA.
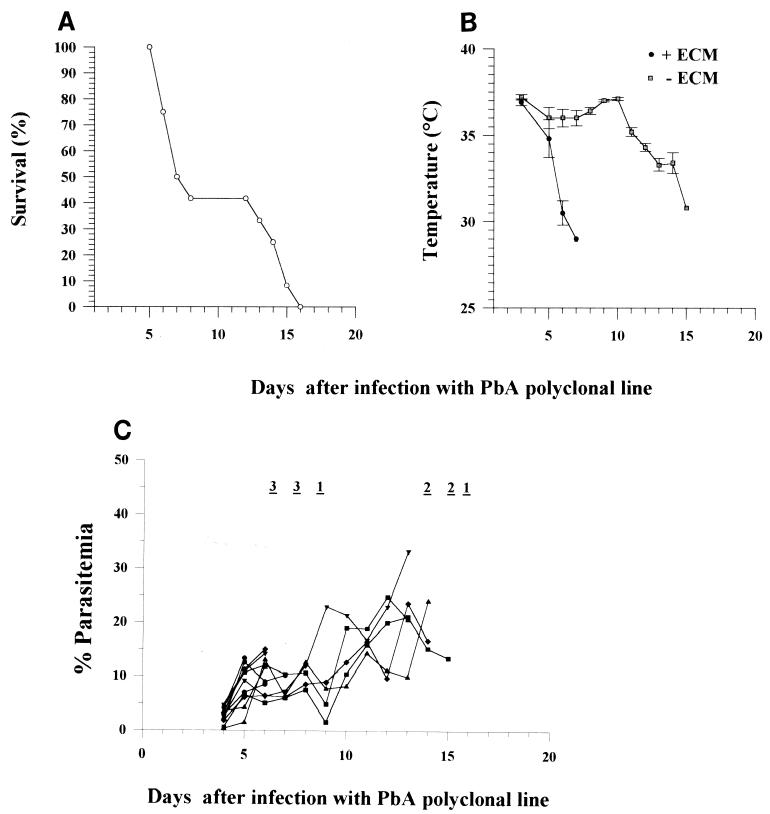
We found that different aliquots of 106 PE obtained from frozen stabilates of blood from a C57BL/6 mouse infected with a polyclonal line of P. berghei ANKA induced ECM in replicated experiments with an efficiency of 50 to 100% (Table 1). In each experiment, the mice that developed ECM showed neurological signs and early hypothermia (Fig. 1B) between days 6 and 12 and had about 5 to 15% parasitemia (Fig. 1C). Histological studies showed that mice with ECM had widespread damage to the microvasculature in the brain, with edema and hemorrhage (data not shown).
TABLE 1.
Cumulative incidence of ECM in C57BL/6 mice infected with the P. berghei ANKA polyclonal linea
| Expt | Cumulative incidence of ECM (%)b |
|---|---|
| 1 | 66.6 |
| 2 | 100 |
| 3 | 90 |
| 4 | 58.3 |
PE of the P. berghei ANKA polyclonal line were obtained from infected C57BL/6 mice, and 106 PE from various batches were injected i.p. into mice (7 to 12 mice per group).
Between days 6 and 12 after infection with the P. berghei ANKA polyclonal line.
FIG. 1.
Survival (A), body temperature (B), and parasitemia (C) after infection of 12 C57BL/6 mice with 106 PE of the P. berghei ANKA polyclonal line. This experiment is experiment 4 reported in Table 1. + ECM, mice with neurological signs of ECM; − ECM, mice with no neurological signs of ECM. Mortality is indicated at the top of panel C as the number of mice that died on that day, and each curve represents the progression of a single mouse.
We also found that the mouse strains from which the PE originated affected the incidence of ECM. PE taken from C57BL/6 or CBA/Ca mice consistently induced ECM in a high proportion of C57BL/6 or CBA/Ca mice but not in BALB/c mice (Table 2). PE taken from Swiss mice induced ECM in few C57BL/6 or CBA/Ca mice. PE taken from BALB/c mice did not induce ECM in C57BL/6 or BALB/c mice.
TABLE 2.
Cumulative incidence of ECM in mouse strains infected with the P. berghei ANKA polyclonal line passaged in various mouse strains
| Expt | Mouse straina
|
Cumulative incidence of ECM (%)b | |
|---|---|---|---|
| PE donor | Recipient | ||
| 1 | Swiss | C57BL/6 | 33 |
| CBA/Ca | 20 | ||
| 2 | C57BL/6 | C57BL/6 | 62.5 |
| BALB/c | 20 | ||
| CBA/Ca | 70 | ||
| 3 | BALB/c | C57BL/6 | 0 |
| BALB/c | 0 | ||
| 4 | CBA/Ca | C57BL/6 | 70 |
| CBA/Ca | 80 | ||
PE of the P. berghei ANKA polyclonal line were obtained from an infected C57BL/6 mouse, and 106 PE were injected i.p. into recipient mice (five to eight mice per group).
Between days 6 and 12 after infection with the P. berghei ANKA polyclonal line.
Induction of cerebral malaria by various cloned lines of P. berghei ANKA.
The results described above suggested that the line of P. berghei ANKA used in these experiments contained a mixture of clones with various degrees of virulence. They also suggested that the proportion of these clones could be altered by the genetic background of the mouse or could differ between mice depending on the proportions of the various clones in the infectious inoculum or the growth capacity of each clone.
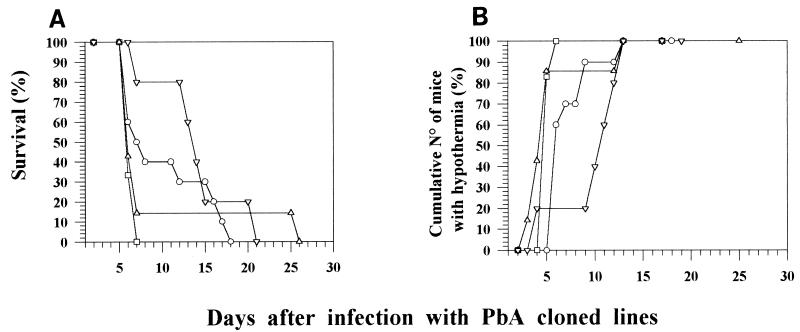
We used cloned lines derived from a polyclonal line of P. berghei ANKA (44) from which our own polyclonal line was derived (7, 15). PE of four cloned lines were obtained from C57BL/6 mice, and their capacity to cause ECM was compared with that of the polyclonal line of P. berghei ANKA. We observed that all of the cloned lines induced ECM in C57BL/6 mice infected with 2 × 106 PE. However, there were differences between the cloned lines in survival time and the time taken to develop hypothermia and neurological manifestations. In a typical experiment (Table 3 and Fig. 2), cloned lines 1.97L and 4 induced ECM in 6 to 7 days, whereas cloned lines 1.49L and 5 induced neurological signs over a longer period (6 to 14 days) (Table 3). Mice died 2 days after the onset of neurological signs; therefore, extension of the time taken to develop ECM manifestations led to a delay in mortality (Fig. 2A) and in the development of hypothermia (Fig. 2B). The time taken to develop ECM in a single mouse strain could not be used as a feature to characterize a parasite cloned line, since the values obtained were highly variable in different experimental inoculations with parasite stabilates of the same or different batches (data not shown). Nonetheless, the proportion of mice which developed ECM was constant for the different clones.
TABLE 3.
Cumulative incidence of ECM for various cloned lines of P. berghei ANKA in C57BL/6 micea
| Cloned line | Cumulative incidence of ECM (%) | Time taken to neurological signs (days)b |
|---|---|---|
| 1.49L | 70 | 6–12 |
| 1.97L | 100 | 6–7 |
| 4 | 86 | 6–7 |
| 5 | 80 | 6–14 |
PE of different P. berghei ANKA cloned lines were obtained from C57BL/6 mice previously infected with the respective cloned lines. An aliquot of 2 × 106 PE of each cloned line was injected i.p. into recipient mice (5 to 12 mice per group).
Time between inoculation with PE and appearance of the neurological signs.
FIG. 2.
Survival (A) and cumulative percentage of C57BL/6 mice displaying a body temperature below 35°C (B) after infection with 2 × 106 PE of four P. berghei ANKA cloned lines. Symbols: ○, line 1.49L; □, line 1.97L; ▵, line 4; ▿, line 5.
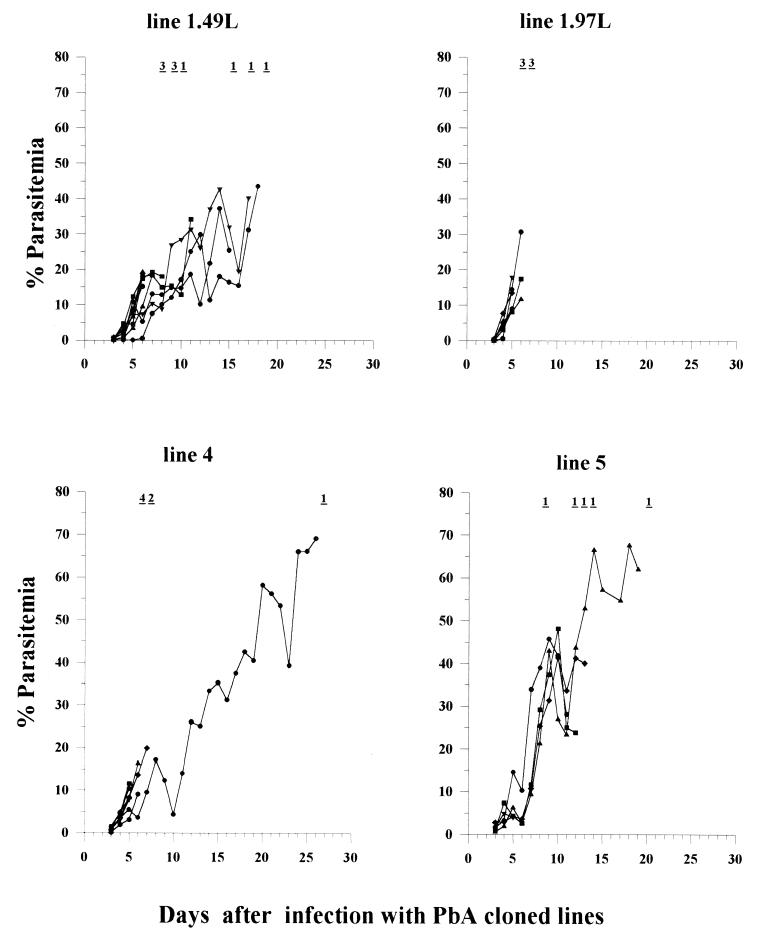
All of the cloned lines produced similar parasitemia curves (Fig. 3). However, cloned lines 1.49L, 1.97L, and 4 resulted in lower parasitemia levels (10 to 20%) when they induced ECM than did cloned line 5 (20 to 40%).
FIG. 3.
Parasitemia of C57BL/6 mice infected with four P. berghei ANKA cloned lines. Mortality is indicated at the top of each panel as the number of mice that died on that day. Each curve represents the progression of a single mouse.
Effects of the amount of infectious inoculum of three cloned lines on the incidence of ECM.
We investigated the effects of the amount of infectious inoculum of three cloned lines because it was previously shown that this factor could affect the incidence of ECM (9). For cloned lines 1.49L and 5, the injection of a large or small quantity of PE resulted in a smaller proportion of mice having ECM than for the other cloned line. For cloned line 1.97L, only a very large amount of inoculum (107 PE) affected the incidence of ECM (Table 4).
TABLE 4.
Effect of the amount of inoculum of the various cloned lines of P. berghei ANKA on the cumulative incidence of ECM in C57BL/6 micea
| Cloned line | Dose of PE | Cumulative incidence of ECM (%)b |
|---|---|---|
| 1.49L | 1 × 105 | 40 |
| 1 × 106 | 60 | |
| 2 × 106 | 80 | |
| 1 × 107 | 20 | |
| 1.97L | 1 × 104 | 70 |
| 1 × 105 | 100 | |
| 1 × 106 | 75 | |
| 2 × 106 | 100 | |
| 1 × 107 | 0 | |
| 5 | 1 × 104 | 40 |
| 1 × 105 | 10 | |
| 1 × 106 | 20 | |
| 2 × 106 | 80 | |
| 1 × 107 | 40 |
PE of various P. berghei ANKA cloned lines were obtained from C57BL/6 mice previously infected with the respective cloned lines. Aliquots of various quantities of PE from the same batch of each cloned line were injected i.p. into recipient mice (five mice per group).
Between days 6 and 12 (cloned lines 1.49L and 1.97L) and between days 6 and 14 (cloned line 5).
Infection of several mouse strains with cloned line 1.49L.
We (Table 2) and others (7, 12, 18, 21, 24–26, 32, 34, 38, 41) have shown that the mouse genetic background affects the incidence of ECM caused by polyclonal P. berghei, so cloned line 1.49L (106 PE from C57BL/6 mice) was injected into various mouse strains. Some strains were very susceptible to ECM, some were partially susceptible, and others were not susceptible at all (Table 5). Mice developed ECM between days 7 and 12, had moderate parasitemia at that time, and suffered early hypothermia, which varied from a low (BALB/c) to a high (C57BL/6) degree. Infection with cloned line 1.49L was always lethal in all of the mouse strains and resulted in similar parasitemia curves in all of the strains (data not shown). Mice which did not die of ECM died of anemia and hyperparasitemia.
TABLE 5.
Incidence of ECM in various mouse strains infected with P. berghei ANKA cloned line 1.49La
| Mouse strain | Haplotype | No. of experiments | Total no. of mice | Cumulative incidence of ECM (%)b | Parasitemia (%)c | Temperature (°C)c | Time taken to neurological signs (days)d |
|---|---|---|---|---|---|---|---|
| DBA/2 | d | 4 | 25 | 0 | |||
| BALB/c | d | 5 | 35 | 35 | 24.73 ± 5.4 | 35.7 ± 0.56 | 7–11 |
| C3H/HeN | k | 2 | 10 | 30 | 28.37 ± 2.67 | 29.52 ± 2.67 | 8–11 |
| 129Sv/eV | k | 2 | 12 | 66 | 19.13 ± 2.25 | 28.55 ± 0.64 | 7–10 |
| CBA/Ca | k | 2 | 21 | 57.1 | 16.9 ± 0.52 | 33.12 ± 0.75 | 7–10 |
| C57BL/6 | b | 3 | 19 | 68.4 | 16.11 ± 0.52 | 21.27 ± 2.44 | 7–12 |
PE of P. berghei ANKA cloned line 1.49L were obtained from an infected C57BL/6 mouse, and 106 PE were injected i.p. into recipient mice (7 to 12 mice per group).
Between days 6 and 12.
Values are for mice with ECM on the day of death.
Time between inoculation with PE and appearance of the neurological signs.
Effect of the mouse strain from which PE were taken on ECM induced by P. berghei ANKA cloned line 1.49L.
The origin of the PE had an effect on the development of ECM in recipient mice (Table 2). Three strains of mice were infected with PE taken from a C57BL/6 mouse infected with cloned line 1.49L. Infected blood was obtained from these different mouse strains, and 2 × 106 PE were injected into C57BL/6 mice. Compared with PE taken from C57BL/6 mice, PE taken from BALB/c mice caused less ECM and PE taken from C3H/HeJ mice caused more ECM (Table 6).
TABLE 6.
Cumulative incidence of ECM in C57BL/6 mice infected with P. berghei ANKA cloned line 1.49L passaged in various mouse strains
| Mouse strain in which PE werea:
|
Cumulative incidence of ECM (%)b | |
|---|---|---|
| Passaged | Injected | |
| C57BL/6 | C57BL/6 | 71.4 |
| BALB/c | C57BL/6 | 42.8 |
| C3H/HeJ | C57BL/6 | 100 |
PE of P. berghei ANKA cloned line 1.49L were obtained from C57BL/6, BALB/c, and C3H/HeJ donor mice infected with PE obtained from C57BL/6 mice. A total of 106 PE were injected i.p. into C57BL/6 mice. There were six to seven mice in each recipient group.
Between days 6 and 12.
DISCUSSION
In this study, we have shown that cloned lines of P. berghei ANKA can differ in their ability to induce ECM in the same host. Unlike the polyclonal line, aliquots from the same stock of cloned lines induced consistently similar levels of ECM in C57BL/6 mice. All of the cloned lines caused ECM, but the degree of ECM induction was dependent on the amount of inoculum and thus differed for the cloned lines (Table 4). This finding is consistent with a previous report showing that the amount of infectious P. berghei K173 inoculum used affects the development of ECM in C57BL/6 mice (9). ECM is thought to develop via a complex pathway. Grau et al. have suggested that P. berghei ANKA antigens induce the release of IL-3, granulocyte-macrophage colony-stimulating factor, and IFN-γ by CD4 Th1 cells (20). These lymphokines activate macrophages, causing them to release TNF-α. TNF-α then induces adhesion molecules (e.g., intracellular adhesion molecule 1) in the brain endothelium, leading to leukocyte adhesion (14, 22, 40). Changes in the amount of the inoculum could lead to the release of a different array of lymphokines (e.g., IL-4 and IL-10) by activation of CD4 Th2 cells. Consistent with this notion, we previously reported that IL-10 prevents the induction of ECM (11).
We used four cloned lines in this study. Two of these lines (1.49L and 1.97L) were derived from the same clone and differed only in the number of passages in mice. If differences between clones can be accounted for by antigenic differences, the differences between lines 1.49L and 1.97L showed that a clonal phenotype may not be stable. A clone may also generate new clones after propagation. Such clonal variation has been demonstrated for P. falciparum, in which it occurred at a rate of 2% per generation in vitro (39). Clonal variation is linked to the adhesion capacity of clones and has been shown to be due to antigenic variation of a parasite protein, PfEMP-1 (3, 42, 43). No homologous molecules have been described for P. berghei, but there is evidence that antigenic clonal variation exists in this parasite species. Antigenic variation has been demonstrated by homologous and heterologous challenges of white mice with various clones of P. berghei ANKA (1, 2, 44).
It has been shown that the genetic background of a mouse affects the course of parasitemia and disease (7, 12, 18, 21, 24–26, 32, 34, 38, 41). We assessed the development of ECM in six inbred mouse strains infected with 106 PE of P. berghei ANKA cloned line 1.49L. There were marked differences in susceptibilities to ECM between the strains, but the levels of parasitemia were similar in all of the strains (Table 5). Mice were either susceptible or resistant to the neurological complications. Genes in the mouse major histocompatibility complex do not seem to control ECM susceptibility, because some mouse strains with the same H-2d haplotype were susceptible (BALB/c) whereas others were resistant (DBA/2). Genetic studies are required to define precisely the role of the mouse genetic background and to identify the genes involved in ECM resistance.
Studies with various host-parasite combinations revealed another level of complexity. The mouse strain from which the PE were taken affected the incidence of ECM. When the polyclonal line or cloned line 1.49L was used, the mouse strain in which the line was passaged affected the incidence of ECM (Tables 2 and 6). Similar findings have been reported with another polyclonal line of P. berghei ANKA (41). It is possible that passage of a P. berghei ANKA line in one mouse strain results in the selection of a specific group of clones which may differ from that selected in a different mouse strain. Our data may also explain the conflicting reports on the susceptibility or resistance to ECM of BALB/c mice infected with P. berghei ANKA polyclonal lines (15, 21, 32, 34). As these lines were passaged in different mouse strains, they may differ in parasite clonal content. There were also differences in the incidence of ECM in BALB/c mice obtained from different suppliers (unpublished results). This finding suggests that there may be differences in the genetic backgrounds of BALB/c mice from different suppliers. Thus, different clones could induce ECM in different mouse strains in specific combinations.
We used blood-stage parasites in this study. These parasite forms multiply by mitosis, with mutations occurring at a very low rate. In contrast, passage through mosquitoes may result in a large number of new clones being created by recombination, which may occur during the sexual stage when the parasite multiplies by meiosis. Consistent with this idea, we were unable to induce consistently high levels of ECM in mice through P. berghei ANKA-infected-mosquito bites or sporozoite injection (unpublished results).
In summary, the clonal composition of the parasite inoculum may affect the incidence of ECM, and the selection of various virulent clones is regulated by the mouse genetic background and the dynamic interplay between the clones and their hosts. Further work is needed to identify, at the molecular level, which P. berghei ANKA antigens are involved in the induction of ECM. Our results also emphasize the importance of taking this phenomenon into account in studies of pathophysiology, virulence, or protection in rodent or human malaria.
ACKNOWLEDGMENTS
This work was partly supported by a grant from Institut Electricité et Santé to Laurent Rénia.
We thank Ana Margarida Vigario and Georges Snounou for critical review of the manuscript. The paper was edited by Owen Parkes.
REFERENCES
- 1.Alger N A, Branton M, Harant J, Silverman P H. Plasmodium berghei NK65 in the inbred A/J mouse. Variations in virulence of P. berghei demes. J Protozool. 1971;18:598–601. doi: 10.1111/j.1550-7408.1971.tb03382.x. [DOI] [PubMed] [Google Scholar]
- 2.Alger N A, Branton M, Silverman P H. Plasmodium berghei NK65 in the inbred A/J mouse: immunity in the A/J mouse naturally recovered from NK65C and challenged with NK65E. J Protozool. 1977;19:516–518. doi: 10.1111/j.1550-7408.1972.tb03517.x. [DOI] [PubMed] [Google Scholar]
- 3.Baruch D J, Pasloke B L, Singh H B, Bi X, Ma X C, Feldman M, Tarashi T F, Howard R J. Cloning the P. falciparum gene encoding PfEMP1, a malaria variant antigen and adherence receptor on the surface of parasitized human erythrocytes. Cell. 1995;82:77–88. doi: 10.1016/0092-8674(95)90054-3. [DOI] [PubMed] [Google Scholar]
- 4.Berendt A R, Turner G D H, Newbold C I. Cerebral malaria: the sequestration hypothesis. Parasitol Today. 1994;10:412–414. doi: 10.1016/0169-4758(94)90238-0. [DOI] [PubMed] [Google Scholar]
- 5.Clark I A, Rockett K A, Cowden W B. Possible central role of nitric oxide in conditions clinically similar to cerebral malaria. Lancet. 1992;340:894–896. doi: 10.1016/0140-6736(92)93295-x. [DOI] [PubMed] [Google Scholar]
- 6.Clark I A, Rockett K A. The cytokine theory of human cerebral malaria. Parasitol Today. 1994;10:410–412. doi: 10.1016/0169-4758(94)90237-2. [DOI] [PubMed] [Google Scholar]
- 7.Contreras C E, June C H, Perrin L H, Lambert P-H. Immunopathological aspects of Plasmodium berghei infection in five strains of mice. I. Immune complexes and other serological features during the infection. Clin Exp Immunol. 1980;42:403–411. [PMC free article] [PubMed] [Google Scholar]
- 8.Curfs J H A J, Schetters T P M, Hermsen C C, Jerusalem C R, Van Zon A, Eling W M C. Immunological aspects of cerebral lesions in murine malaria. Clin Exp Immunol. 1988;74:136–140. [PMC free article] [PubMed] [Google Scholar]
- 9.Curfs J H A J, Hermsen C C, Meuwissen J H E T, Eling W M C. Immunization against cerebral pathology in Plasmodium berghei-infected mice. Parasitology. 1992;105:7–14. doi: 10.1017/s0031182000073625. [DOI] [PubMed] [Google Scholar]
- 10.Curfs J H A J, Van Der Meide P H, Billiau A, Meuwissen J H E T, Eling W M C. Plasmodium berghei: recombinant interferon-γ and the development of parasitemia and cerebral lesions in malaria-infected mice. Exp Parasitol. 1993;77:212–223. doi: 10.1006/expr.1993.1078. [DOI] [PubMed] [Google Scholar]
- 11.Eckwalanga M, Marussig M, Dias Tavares M, Bouanga J C, Hulier E, Pavlovitch J, Minoprio P, Portnoï D, Rénia L, Mazier D. Murine AIDS protects against experimental cerebral malaria: down-regulation by interleukin 10 of a T-helper type 1 CD4+ cell-mediated pathology. Proc Natl Acad Sci USA. 1994;91:8097–8101. doi: 10.1073/pnas.91.17.8097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eling W, Van Zon A, Jerusalem C. The course of a Plasmodium berghei infection in six different mouse strains. Z Parasitenkd. 1977;54:29–45. doi: 10.1007/BF00380634. [DOI] [PubMed] [Google Scholar]
- 13.Eling W M C, Kremsner P G. Cytokines in malaria, pathology and infection. Biotherapy. 1994;7:211–221. doi: 10.1007/BF01878487. [DOI] [PubMed] [Google Scholar]
- 14.Falanga P B, Butcher E C. Late treatment with anti-LFA1 (CD11a) antibody prevents cerebral malaria in a mouse model. Eur J Immunol. 1991;21:2259–2260. doi: 10.1002/eji.1830210938. [DOI] [PubMed] [Google Scholar]
- 15.Finley R W, Mackey J, Lambert P-H. Virulent P. berghei malaria: prolonged survival and decreased cerebral pathology in T cell deficient nude mice. J Immunol. 1982;129:2213–2217. [PubMed] [Google Scholar]
- 16.Grau G E, Fajardo L F, Piguet P F, Allet B, Lambert P-H, Vassalli P. Tumor necrosis factor (cachectin) as an essential mediator in murine cerebral malaria. Science. 1987;237:1210–1212. doi: 10.1126/science.3306918. [DOI] [PubMed] [Google Scholar]
- 17.Grau G E, Kindler V, Piguet P F, Lambert P-H, Vassalli P. Prevention of experimental cerebral malaria by anticytokine antibodies. Interleukin 3 and granulocyte macrophage colony-stimulating factor are intermediates in increased tumor necrosis factor production and macrophage accumulation. J Exp Med. 1988;168:1499–1504. doi: 10.1084/jem.168.4.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grau G E, Heremans H, Piguet P F, Pointaire P, Lambert P-H, Billiau A, Vassalli P. Monoclonal antibody against interferon-gamma can prevent experimental cerebral malaria and its associated overproduction of tumor necrosis factor. Proc Natl Acad Sci USA. 1989;86:5572–5574. doi: 10.1073/pnas.86.14.5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grau G E, Taylor T E, Molyneux M E, Wirima J J, Vassalli P, Hommel M, Lambert P-H. Tumor necrosis factor and disease severity in children with falciparum malaria. N Engl J Med. 1989;320:1586–1589. doi: 10.1056/NEJM198906153202404. [DOI] [PubMed] [Google Scholar]
- 20.Grau G E, Piguet P F, Vassalli P, Lambert P-H. Tumor necrosis factor and other cytokines in cerebral malaria: experimental and clinical data. Immunol Rev. 1989;112:49–70. doi: 10.1111/j.1600-065x.1989.tb00552.x. [DOI] [PubMed] [Google Scholar]
- 21.Grau G E, Bieler G, De Kossodo S, Tacchini-Cottier F, Vassalli P, Piguet P F, Lambert P-H. Significance of cytokine production and adhesion molecules in malarial immunopathology. Immunol Lett. 1990;25:189–194. doi: 10.1016/0165-2478(90)90113-5. [DOI] [PubMed] [Google Scholar]
- 22.Grau G E, Pointaire P, Piguet P F, Vesin C, Rosen H, Stamenkovic I, Takei F, Vassalli P. Late administration of monoclonal antibody to leukocyte function-antigen 1 abrogates incipient murine cerebral malaria. Eur J Immunol. 1991;21:2265–2267. doi: 10.1002/eji.1830210939. [DOI] [PubMed] [Google Scholar]
- 23.Grau G E, de Kossodo S. Cerebral malaria: mediators, mechanical obstruction or more. Parasitol Today. 1994;10:408–410. doi: 10.1016/0169-4758(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 24.Greenberg J. Differences in the course of Plasmodium berghei infections in some hybrid and backcross mice. Am J Trop Med Hyg. 1955;5:19–28. doi: 10.4269/ajtmh.1956.5.19. [DOI] [PubMed] [Google Scholar]
- 25.Greenberg J, Kendrick L P. Parasitaemia and survival in inbred strains of mice infected with Plasmodium berghei. J Parasitol. 1956;43:413–420. [PubMed] [Google Scholar]
- 26.Greenberg J, Kendrick L P. Some characteristics of Plasmodium berghei passed within inbred strains of mice. J Parasitol. 1957;43:420–428. [PubMed] [Google Scholar]
- 27.Hermsen C C, Crommert J V D, Sauerwein R W, Eling W C M. Circulating tumor necrosis factor α is not involved in the development of cerebral malaria in Plasmodium berghei-infected C57BL mice. Parasite Immunol. 1997;19:571–577. doi: 10.1046/j.1365-3024.1997.d01-175.x. [DOI] [PubMed] [Google Scholar]
- 28.Hill A V S, Allsopp C E M, Kwiatkowski D, Anstey M, Twumasi P, Rowe P A, Bennett S, McMichael A J, Townsend A R, Greenwood B M. Common West African HLA Ag are associated with protection from severe malaria. Nature. 1991;352:595–600. doi: 10.1038/352595a0. [DOI] [PubMed] [Google Scholar]
- 29.Jennings V M, Actor J K, Lal A A, Hunter R L. Cytokine profile suggesting that murine cerebral malaria is an encephalitis. Infect Immun. 1997;65:4883–4887. doi: 10.1128/iai.65.11.4883-4887.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kern P, Hemmer C J, Gruss H-J, Dietrich M. Elevated tumor necrosis factor alpha and interleukin-6 serum levels as markers for complicated Plasmodium falciparum malaria. Am J Med. 1989;87:139–143. doi: 10.1016/s0002-9343(89)80688-6. [DOI] [PubMed] [Google Scholar]
- 31.Kwiatkowski D, Hill A V S, Sambou L, Twumasi P, Castracane J, Manogue K, Cerami A, Brewster D, Greenwood B M. TNF concentration in fatal cerebral, non-fatal cerebral, and uncomplicated Plasmodium falciparum malaria. Lancet. 1990;336:1201–1204. doi: 10.1016/0140-6736(90)92827-5. [DOI] [PubMed] [Google Scholar]
- 32.Mackey L J, Hochmann A, June C H, Contreras C E, Lambert P-H. Immunological aspects of Plasmodium berghei infection in five strains of mice. II. Immunopathology of cerebral and tissue lesions during the infection. Clin Exp Immunol. 1980;42:412–420. [PMC free article] [PubMed] [Google Scholar]
- 33.Maegraith B. Other pathological processes in malaria. Bull W H O. 1974;50:187–193. [PMC free article] [PubMed] [Google Scholar]
- 34.Neill A L, Hunt N H. Pathology of fatal and resolving Plasmodium berghei cerebral malaria in mice. Parasitology. 1992;105:165–175. doi: 10.1017/s0031182000074072. [DOI] [PubMed] [Google Scholar]
- 35.Patnaik J K, Das B S, Mishra S K, Mohanty S, Satpathy S K, Mohanty D. Vascular clogging, mononuclear cell margination, and enhanced vascular permeability in the pathogenesis of human cerebral malaria. Am J Trop Med Hyg. 1994;51:642–647. [PubMed] [Google Scholar]
- 36.Polder T H, Eling W M C, Curfs J H A J, Jerusalem C R, Wijers-Rouw M. Ultrastructural changes in the blood-brain barrier of mice infected with Plasmodium berghei. Acta Leiden. 1992;60:31–46. [PubMed] [Google Scholar]
- 37.Porta J, Carota A, Pizzolato G P, Wildi E, Wildmer M C, Marguiraz C, Grau G E. Immunopathological changes in human cerebral malaria. Clin Neuropathol. 1993;12:142–146. [PubMed] [Google Scholar]
- 38.Rest J. Cerebral malaria in inbred mice. I. A new model and its pathology. Trans R Soc Trop Med Hyg. 1982;76:410–415. doi: 10.1016/0035-9203(82)90203-6. [DOI] [PubMed] [Google Scholar]
- 39.Roberts D J, Craig A G, Berendt A R, Pinches R, Nash G, Marsh K, Newbold C I. Rapid switching to multiple antigenic and adhesive phenotypes in malaria. Nature. 1992;357:689–692. doi: 10.1038/357689a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rudin W, Eugster H-P, Bordmann G, Bonato J, Müller M, Yamage M, Ryffel B. Resistance to cerebral malaria in tumor necrosis factor-α/β-deficient mice is associated with a reduction of intercellular adhesion molecule-1 up-regulation and T helper type 1 response. Am J Pathol. 1997;150:257–266. [PMC free article] [PubMed] [Google Scholar]
- 41.Russel A S, June C H. On the nature of early mortality in murine malaria. J Parasitol. 1980;66:1065–1066. [PubMed] [Google Scholar]
- 42.Smith J D, Chitnis C E, Craig A G, Roberts D J, Hudson-Taylor D E, Peterson D S, Pinches R, Newbold C I, Miller L H. Switches in expression of Plasmodium falciparum var genes correlate with changes in antigenic and cytoadherent phenotypes of infected erythrocytes. Cell. 1995;82:101–113. doi: 10.1016/0092-8674(95)90056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Su X S, Heatwole V M, Wertheimer S P, Guinet F, Herrfeldt J A, Peterson D S, Ravetch J A, Wellems T E. The large diverse gene family var encodes proteins in cytoadherence and antigenic variation of Plasmodium falciparum-infected erythrocytes. Cell. 1995;82:89–100. doi: 10.1016/0092-8674(95)90055-1. [DOI] [PubMed] [Google Scholar]
- 44.Wery M, Weyn J, Timperman G, Hendrix L. Observations on the virulence and the antigenic characters of cloned and uncloned lines of the ANKA isolate of Plasmodium berghei. 1. Production of recrudescent parasitaemias in immunized mice. Ann Soc Belge Med Trop. 1979;59:347–360. [PubMed] [Google Scholar]
- 45.White N, Ho M. The pathophysiology of malaria. Adv Parasitol. 1992;31:84–173. doi: 10.1016/s0065-308x(08)60021-4. [DOI] [PubMed] [Google Scholar]