Abstract
Background
Asthma is a common respiratory disease among both adults and children and short acting inhaled beta‐2 agonists are used widely for 'reliever' bronchodilator therapy. Long acting beta‐2 agonists (LABA) were introduced as prospective 'symptom controllers' in addition to inhaled corticosteroid 'preventer' therapy (ICS).
We originally analysed studies comparing the use of LABA with placebo in mixed populations in which only some were taking ICS and in populations not using ICS therapy. However international guidelines no longer recommend the use of LABA in people who are not taking ICS for their asthma. We are therefore no longer updating this review.
Objectives
This review aimed to determine the benefit or detriment on the primary outcome of asthma control with the regular use of LABA compared with placebo, in mixed populations in which only some were taking ICS and in populations not using ICS therapy.
Search methods
We carried out searches using the Cochrane Airways Group trial register, most recently in October 2005. We searched bibliographies of identified RCTs for additional relevant RCTs and contacted authors of identified RCTs for other published and unpublished studies.
Selection criteria
All randomised studies of at least four weeks duration, comparing a LABA given twice daily with a placebo, in chronic asthma. Selection criteria to this updated review have been altered to accommodate recently published Cochrane reviews on combination and addition of LABA to ICS therapy. Studies in which all individuals were uniformly taking ICS were excluded from this review.
Data collection and analysis
Two review authors performed data extraction and study quality assessment independently. We contacted authors of studies for missing data.
Main results
Sixty‐seven studies (representing 68 experimental comparisons) randomising 42,333 participants met the inclusion criteria. Salmeterol was used as long‐acting agent in 50 studies and formoterol fumarate in 17. The treatment period was four to nine weeks in 29 studies, and 12 to 52 weeks in 38 studies. Twenty‐four studies did not permit the use of ICS, and forty permitted either inhaled corticosteroid or cromones (in three studies this was unclear). In these studies between 22% and 92% were taking ICS, with a median of 62%. There were significant advantages to LABA treatment compared to placebo for a variety of measurements of airway calibre including morning peak expiratory flow (PEF), evening PEF and FEV1. They were associated with significantly fewer symptoms, less use of rescue medication and higher quality of life scores. This was true whether patients were taking LABA in combination with ICS or not. Findings from SMART (a recently published surveillance study) indicated significant increases in asthma related deaths, respiratory related deaths and combined asthma related deaths and life threatening experiences. The absolute increase in asthma‐related mortality was consistent with an increase of around one per 1250 patients treated with LABA for six months, but the confidence intervals are wide (from 700 to 10,000). Post‐hoc exploratory subgroups suggested that African‐Americans and those not on inhaled corticosteroids were at particular risk for the primary end‐point of death or life‐threatening asthma event. There was also a suggestion of an increase in exacerbation rate in children. Pharmacologically predicted side effects such as headache, throat irritation, tremor and nervousness were more frequent with LABA treatment.
Authors' conclusions
LABA are effective in the control of chronic asthma in the "real‐life" subject groups included. However there are potential safety issues which call into question the safety of LABA, particularly people with asthma who are not taking ICS, and it is not clear why African‐Americans were found to have significant differences in comparison to Caucasians for combined respiratory‐related death and life threatening experiences, but not for asthma‐related death.
Since the original version of this review, the US Food and Drug Agency (FDA) has added a warning that LABA should not be used to treat asthma without concurrent ICS. International guidelines only recommend the use of LABA in conjunction with ICS. Readers should consult the overviews which summarise the results of Cochrane reviews on the safety of LABAs in adults and children (Cates 2012; Cates 2014).
Keywords: Adult, Child, Humans, Adrenergic beta‐Agonists, Adrenergic beta‐Agonists/adverse effects, Adrenergic beta‐Agonists/therapeutic use, Albuterol, Albuterol/analogs & derivatives, Albuterol/therapeutic use, Asthma, Asthma/drug therapy, Bronchodilator Agents, Bronchodilator Agents/therapeutic use, Chronic Disease, Ethanolamines, Ethanolamines/therapeutic use, Quality of Life, Randomized Controlled Trials as Topic
Plain language summary
Long‐acting beta2‐agonists for chronic asthma in adults and children where background therapy contains varied or no inhaled corticosteroid
Note added in July 2014: Since the original version of this review, guidance around LABA has changed. International asthma guidelines now only recommend the use of LABA in conjunction with ICS. The US Food and Drug Agency (FDA) has issued a warning that LABA should not be used to treat asthma without concurrent ICS. The review will continue to be available for people to read on the Cochrane Library, but we will not update the review again. It is no longer considered safe to take LABA on their own without taking inhaled steroids due to harms which can occur.
Plain language summary published in 2006: In this review of studies in which patients were either not on inhaled corticosteroids, or in which some patients but not all were on inhaled corticosteroids, treatment with regular long‐acting beta‐2 agonists such as salmeterol (Serevent) or formoterol (Foradil, Oxis) in chronic asthma resulted in fewer asthma symptoms by day or night, less relief bronchodilator medication requirement, better lung function, a lower risk of acute worsening of asthma and better quality of life, but most of the evidence comes from groups in which at least some used inhaled corticosteroid therapy. There is less information on asthma control in patients who did not use a regular 'preventer medication' or in children under twelve years, but the same generally positive effects on symptoms and lung function seem to apply. We have also been particularly focused on serious adverse events, given previous concerns about potential risks, especially of death, from regular beta‐2 agonist use. A significant increase in asthma related deaths or life threatening experiences has been found in a recently published surveillance study, with an increased risk of around one event over 6 months for every thousand patients treated. This increase was mainly in African‐Americans and those not on inhaled corticosteroids, although these observations were drawn from analyses conducted after the event (post‐hoc) and as such lack the validity of pre‐defined distinctions.
Background
Asthma is a common respiratory disease among both adults and children (Pearce 2000), though there are large geographical differences in the prevalence of asthma (Janson 2001). The prevalence is increasing in many countries; estimates for asthma prevalence for adults in 1990 varied from 8.5% in Australia to 4% in the USA and 3% in the United Kingdom (Sullivan 1996) and repeat estimates in Australia in 1999 (Woods 2001) gave rates for physician diagnosed asthma of 18% while 10% had used asthma medications in the past year. As a chronic illness, asthma is responsible for significant economic costs, both direct medical costs and indirect costs. Measurements in Australia (population approximately 20 million) range from direct costs of US$250 million to US$3.6 billion for total costs. Costs per patient have been estimated at US$326 per year in Australia and US$1,043 per year in the United Kingdom (Sullivan 1996).
Pathophysiological studies over the past two decades have increasingly recognised asthma as an inflammatory airway disease. National treatment guidelines for asthma have been published in several countries including Britain, USA and Australia (BTS 1993; BTS 1995; GINA 1995 and NAC 2002). They recommend the use of ICS as 'preventer' maintenance therapy for all but mildest grades of asthma severity. In addition, bronchodilator therapy is an essential component of treatment, traditionally used for relief of symptoms as needed. The most widely used bronchodilators in asthma are inhaled beta‐2 agonists which can be divided into two groups: those with a short duration of action (2‐6 hours) which are used in a reactive 'relief' mode and those, introduced more recently, with a longer duration of action (>12 hours). The latter were introduced as prospective 'symptom controllers'. The major representatives of the short acting beta‐2 agonist agents in clinical use are salbutamol (known in North America as albuterol), terbutaline and orciprenaline, while the LABA in use are salmeterol and formoterol.
There has been controversy over the regular use of beta‐2 agonists (AAACI 1993). It was proposed that excessive use of short acting beta‐2 agonists might have contributed directly or indirectly to peaks in asthma mortality seen in the 1960's and late 1970's (Sears 1986). A study by Campbell (Campbell 1976) showed that in four Australian states there was a high correlation between the sales of adrenergic bronchodilator aerosols and the corresponding asthma mortality rates for the periods 1961‐1963 and 1964‐1966. Later, in 1989, a case control study in New Zealand linked the use of inhaled fenoterol with increased risk of death from asthma (Crane 1989). This was followed soon after by a similar study in Saskatchewan that also showed an association between excessive use of beta‐2 agonists and the risk of death or near death from asthma (Spitzer 1992).
Excessive use of beta‐2 agonists and high blood levels of beta‐2 agonists in poorly controlled asthma may contribute to deaths (Abramson 2001). This study also showed significantly less use of asthma management plans and less use of inhaled corticosteroids in fatal cases.
The development of beta‐2‐ agonists with long‐acting properties, such as salmeterol (Adkins 1997) and (e)formoterol (Bartow 1998) has provided potential pharmacological advantages over short‐acting beta‐agonists, such as prolonged bronchodilatation and protection against induced bronchospasm. However, there remain concerns about adverse effects of regular use of LABA (Devoy 1995), especially on enhancing bronchial hyper responsiveness (Cockcroft 1993), the development of tolerance to bronchodilatation (Newnham 1995), progressively reduced protection against provoking stimuli (Taylor 1997) and masking of deteriorating asthma pathology (McIvor 1998). These concerns have influenced national and international guidelines for asthma management, which stipulate that short‐acting beta‐2 agonists should be used only as needed, and not on a regular basis. LABA are in contrast generally only recommended to be taken on a regular basis.
The initial version of this review aimed to determine the benefit or detriment on the primary outcome of asthma control with the regular use of LABA compared with placebo. The review concluded that LABAs conferred benefits when given in addition to ICS therapy, and that they were safe. Several Cochrane reviews have since been published which confirm the benefit of LABA when given in addition to inhaled steroid therapy (Greenstone 2005; Ni Chroinin 2004; Ni Chroinin 2005), and so we have narrowed the focus of this update. In addition to these reviews, the publication of a large study that suggested that there was an increase in the risk of asthma related mortality, (SMART), prompted us to re‐assess the evidence base supporting the safety of LABA in chronic asthma.
Objectives
The objective of this new updated review was to compare the effects of regular inhaled LABA versus placebo in chronic asthma. The specific purpose of the review was to assess whether there are any beneficial or harmful effects from the regular use of inhaled LABA compared with placebo on the primary outcome of asthma control, assessed through measurements of airway calibre, symptoms and exacerbations of asthma. Secondary outcomes included assessment of quality of life, airway hyper‐reactivity, adverse events and the tolerance (tachyphylaxis) to bronchodilatation and bronchoprotection. Following the publication of SMART, we have also updated the review to include an estimate of the risk of death in participants given a LABA to control their asthma. Studies in which all subjects were given LABA consistently with ICS were excluded.
Methods
Criteria for considering studies for this review
Types of studies
We considered all randomised studies, both open and blinded, though double blind studies were preferable. The scoring system gave a lower score to open studies, and was used to assess the contribution of open studies, or if necessary to exclude them from analyses. Studies could be either of parallel group or crossover design.
Types of participants
We included studies in which participants had a clinical diagnosis of asthma, present for at least three months but excluded the studies already included in currently published Cochrane reviews i.e. in which all patients were on ICS (see below). Participants belonged to populations with either variable ICS rates of use, or were uniformly not on ICS. We included both adults and children. We looked specifically for evidence of reversibility of airway obstruction to short acting inhaled beta‐2 agonists as a basic diagnostic criterion. We sought to document the severity of participants' asthma at inclusion and the proportion of participants using other asthma therapies, most notably disease‐modifying agents such as ICS. Studies that included some or all participants with other pulmonary diseases, and especially smoking‐related Chronic Obstructive Pulmonary Disease (COPD) were excluded unless the results for those with asthma alone were available or could be extracted separately.
Types of interventions
Participants in one treatment arm used a LABA either salmeterol or formoterol (also known as eformoterol), administered twice daily via any inhalation device. The second treatment arm consisted of regular doses of placebo, administered in the same way. The dose, inhalational device and frequency of administration were recorded. The minimum period of treatment was changed from the two weeks in our original review to four weeks in this update, to fit with the aim of assessing regular use in a relatively chronic setting. Since the initial publication of this review, a number of reviews have been published assessing the effects of addition of ICS or combination of LABA with ICS in chronic asthma (Greenstone 2005; Ni Chroinin 2004; Ni Chroinin 2005). We have therefore modified the criteria of this review in order to address a more focused question than the one we sought to answer previously. Thus, for the 2006 update of this review, we have included studies only where a LABA was administered without a standardised co‐intervention with ICS (e.g. without standardised fixed combination therapy, where use of ICS therapy was an inclusion criterion or was uniformly provided by the study investigator). This means that we have included studies where a LABA was administered on top of usual therapy, such that a subset of participants only were using a regular ICS, provided that the regimen of maintenance ICS was not altered prior to the study. There was also a subgroup not using ICS or other disease ‐ modifying agents at all. We have combined these studies, with subdivisions into subgroups, and recorded the number of participants on "preventer" inhaled therapy where this is available (Table 1).
1. Studies by description of asthma severity and use of inhaled corticosteroids.
| Mild | % ICS Mild | Mild to moderate | %ICS M‐mod | Moderate to severe | % ICS Mod‐sev | Persistent/symptomatic | %ICS Persistent | Not reported | NR % ICS |
| Boulet 1997 | 0 | Bensch 2001 | 51 | Kraft 1997 | 0 | Bensch 2002 | 70 | Adinoff 1998 | 64 |
| Cheung 1992 | 0 | Booth 1993 | 73 | Shapiro 2000b | 0 | Busse 1998 | 65 | Busse 2004 | 64 |
| Cloosterman 2001 | 0 | Boulet 1998 | 0 | Kavuru 2000 a | 0 | D'Alonzo 1994 | 22 | ||
| Jones 1994 | 42 | Creticos 1999 | 0 | Kemp 1998 a | 60 | Garcia 2001 | 32 | ||
| Nelson 1999 | 0 | Dahl 1991 a, b | 0 | Lazarus 2001 | 0 | Hyland 1994 | NK | ||
| Prieto 2002 | 0 | Ekstrom 1998 a | 80 | Lindquist 2003 | 0 | LaForce 2005 | 60 | ||
| Rosenthal 1999 | 0 | Ekstrom 1998 b | 89 | Lockey 1999 | 62 | Nelson 1998 | 30 | ||
| Wallin 1999 | 0 | Juniper 1995 | 77 | Nathan 1999 | 0 | Pearlman 1999 | 0 | ||
| Kemp 1999 | 46 | Roberts 1999 | 0 | Ramage 1994 | 91 | ||||
| Leblanc 1996 | 80 | SLGA3014 | 50 | SAS3003 | 0 | ||||
| Levy 2005 | 74 | Weinstein 1998 | 57 | SAS3004 | 0 | ||||
| Majahan 1998 | 58 | SMART | 47 | ||||||
| Newnham 1994 | 70 | SMS40221 | NK | ||||||
| Newnham 1995 | 81 | Steffensen 1995 | 84 | ||||||
| Pearlman 1992 | 40 | Sussman 1995 | NK | ||||||
| Pleskow 2003 | 44 | von Berg 2002 | 82 | ||||||
| Schreurs 1996 | 90 | Wronska 1998 | 0 | ||||||
| Simons 1997 | 0 | ||||||||
| SLGA2004 | NK | ||||||||
| SLGL82 | NK | ||||||||
| SLMP03 | 0 | ||||||||
| Stelmach 2002 | 0 | ||||||||
| Taylor 1998 | 92 | ||||||||
| Von Berg 1998 | 50 | ||||||||
| Wolfe 2000 a,b | 30 | ||||||||
| Wolfe 2006 | 58 | ||||||||
| Zarkovic 1998 | 80 |
Types of outcome measures
Outcome measures chosen were those generally accepted as reflecting the primary outcome of asthma control. Thus the planned outcome measures were: daytime and night‐time asthma symptom scores, bronchodilator use for symptom relief, daily peak flow measurement (PEF) and its variability, clinic measurements of lung function and asthma exacerbation rates. Secondary outcomes used were: adverse events (with a particular interest in mortality and life threatening asthma events), airway hyper‐reactivity, quality of life score, global assessment of efficacy by patient and investigator, reduction in use of other asthma medication including ICS, development of tolerance to the effects of beta‐2 agonists and effects on exercise‐induced asthma.
Search methods for identification of studies
Electronic searches
The Cochrane Airways Review Group (ARG) has developed an "Asthma and Wheeze RCT" register, derived from a comprehensive search of EMBASE, MEDLINE, CINAHL. In addition hand searching of the 20 most productive respiratory care journals has been completed and relevant RCTs have been added to the register, including those published in a language other than English. Search of this database was completed using the following search strategy:
(beta and agonist*) or beta‐agonist* or bronchodilat* or salbutamol or albuterol or terbutalin* or isoproterenol or reproterol or reproterenol or rimiterol or fenoterol or ventolin or orciprenaline or metaproterenol or pirbuterol or salmeterol or eformoterol or formoterol
Searching other resources
Reference lists of all available primary studies and review articles were screened to identify potentially relevant citations. Researchers known to have an interest in the field were contacted to identify other relevant past or current studies.
Data collection and analysis
Selection of studies
From the abstracts or titles, two reviewers (JW and FR) independently reviewed literature searches to identify potentially relevant trials for full review. Searches of bibliographies and texts were carried out to identify additional studies. Using the specified criteria, inclusion of studies in the review was decided by agreement between the reviewers (JW, EHW and FR).
Data extraction and management
Methods‐study design, location and setting, method of randomisation and blinding, withdrawals/drop outs, compliance, confounders and quality score.
Participants‐ total number of subjects (N), numbers of males and females, the mean age and range, baseline severity of asthma, inclusion and exclusion criteria, ICS (or other "preventer") or not, and dose if relevant.
Interventions‐ the long‐acting beta‐2 agonist used, with dose and frequency of dosing, use of placebo drug and whether matched, the device used to administer drugs, the treatment period, the additional rescue agent used if any, any co‐intervention used during study by participants, with its dose and whether dose remained stable. Outcomes‐ which outcomes were measured, which reported, in what form and whether they were complete, the standard deviation or other measure of variability. Studies could use a variety of measures of asthma control and we specifically extracted results for the following outcomes where available: Peak expiratory flow (PEF): morning PEF: evening % Predicted PEF: morning % Predicted PEF: evening Change in PEF: morning Change in PEF: evening Amplitude of diurnal variation in PEF Forced expiratory flow in 1 second (FEV1) % Predicted FEV1 Change in FEV1 Forced Vital Capacity (FVC) Forced expiratory flow between 25‐75% of ventilatory capacity (FEF25‐75) Symptom score: whole day (24 hours) Symptom score: day time Symptom score: night time %days without asthma symptoms % nights without asthma awakenings Rescue bronchodilator use: whole day (number of doses, normally 2 puffs or 1 inhalation dry powder) Rescue bronchodilator use: day time number of doses Rescue bronchodilator use: night time number of doses Quality of life score: overall Quality of life score: symptoms Quality of life score: emotions Quality of life score: exposure to environmental stimuli Quality of life score: activity limitations Bronchial hyper reactivity Adverse events: mortality and life‐threatening events Adverse events: palpitations Adverse events: headache Adverse events: tremor Adverse events: cramps Exacerbations of asthma Overall efficacy of treatment Weaning from ICS medication or non‐steroidal 'preventer' asthma medication Tolerance to bronchodilator effects of beta‐2 agonists Tolerance to bronchoprotective effects of beta‐2 agonists on direct/indirect airway challenge Measures of airway inflammation, obtained by bronchoscopy, sputum, exhaled breath/condensate analysis
Assessment of risk of bias in included studies
The trials were scored using the Cochrane approach to assessment of allocation concealment: Grade A: Adequate concealment. Grade B: Unclear concealment. Grade C: Obviously not adequate concealment.
Each study was also assessed for validity using the method of Jadad (Jadad 1996), on a 0‐5 scale as follows: 1. Was the study described as randomised? (1=yes, 0=no) 2. Was the study described as double‐blind?(1=yes, 0=no) 3. Was there a description of withdrawals and drop outs? (1=yes, 0=no) 4. Was the method of randomisation well described and appropriate? (1=yes, 0=no) 5. Was the method of double‐blinding well described and appropriate? (1=yes, 0=no) 6. Deduct 1 point if methods of randomisation or blinding were inappropriate. Differences were resolved by discussion between the reviewers.
Dealing with missing data
Where possible we have attempted to extract and verify raw data for continuous variable endpoints for all of the studies where these are reported. However, we have come across a number of studies where means, or mean differences are reported without an associated estimate of variance (e.g. standard deviation, standard error or 95% CI). We have therefore calculated a weighted estimate of the variation for each study where this is reported, and applied this to studies where such data are missing. This is calculated based from the ratio of the sample size for each study to the pooled sample size, generating a fraction. We have multiplied the standard deviation by the fraction, and then we have summed these to generate an average standard deviation.
Data synthesis
All included trials were combined using the Review Manager. Data from parallel group and crossover studies were analysed separately.
For continuous outcomes, individual and pooled statistics were calculated as mean differences (MD) with 95% Confidence Intervals (CI), routinely using fixed effects models. However if heterogeneity was found in analyses (I square >20%, Higgins 2003), use of the random effects model was included in its investigation. Results are reported as fixed effect unless otherwise stated. Where different scales had been used to measure the outcome, the pooled standardised mean differences (SMD), with 95% Confidence Intervals (CI) were calculated. The SMD is a statistic that expresses the difference in means between the two treatment groups in units of the pooled standard deviation. This applied particularly to measures of bronchial reactivity, symptom scores and diurnal variation in PEF.
For dichotomous outcomes, individual and pooled statistics were calculated as Odds Ratios (OR) with 95% Confidence Intervals (CI), using a fixed‐effect model, with random‐effects model being used in the investigation of any heterogeneity, as above.
For pooled effects a Breslow‐Day test of heterogeneity was carried out, and a P value <0.05 was considered significant, indicating possible differences between studies. Investigation of heterogeneity included performing analyses using the domains: 1. Fixed‐effect versus random‐effects modelling. 2. Methodological quality ‐ Cochrane criteria A and B versus C ; Jadad score 3‐5 versus 1‐2. 3. Use of funnel plots to examine the effects, and investigate publication bias and look for asymmetry of effect sizes.
With regular use for at least two weeks there does not seem to be a significant difference between eformoterol used twice daily at either 6, 12 or 24 mcg dose on the development of tachyphylaxis to their bronchoprotective effects (Lipworth 1998). The effects on lung function and asthma symptom scores after 12 weeks treatment are similar for formoterol at doses of 12 and 24 mcg twice daily (Bensch 2001). Schreurs 1996 found that the lowest effective dose of eformoterol was 6 mcg twice daily on outcomes such as daily PEF, lung function, daily asthma symptoms scores and use of rescue medication. Higher doses induced a greater effect in absolute numbers for some outcomes such as PEF in the morning, with a significant difference between the 6 mcg and 24 mcg doses. Bensch 2001 showed a slightly greater frequency of adverse events with the higher dose of 24 mcg compared to 12 mcg twice daily.
The four week Dahl 1991a study showed that the lowest effective dose of salmeterol on outcomes such as PEF, asthma symptom scores, rescue bronchodilator use and nocturnal awakenings was 12.5 mcg twice daily, with dose related increases occurring with salmeterol 50 and 100 mcg twice daily. The incidence of pharmacologically predictable adverse events was greater with a dose of 100 mcg twice daily.
Fitzpatrick 1990 showed improvement in overnight PEF and sleep quality using salmeterol 50 mcg twice daily, while the 100 mcg dose also improved PEF and reduced the need for rescue bronchodilator use but did not improve sleep quality. When 100 mcg was used once daily at night (Faurschou 1994a) it was shown that there was a similar effect on nocturnal asthma symptoms compared to 50 mcg given twice daily.
Because of these differences in likely outcomes dependent on dose of LABA used, sensitivity analyses were performed for dose variations in the meta‐analysis.
Subgroup analysis and investigation of heterogeneity
Subgroup analyses were planned to assess the effect of treatment in different populations of asthmatics and the following subgroup analyses were carried out:
Asthmatics not using regular inhaled corticosteroids
Asthmatics using a variety of mixed co‐interventions
Children
Severity of asthma (mild, mild‐moderate, moderate‐severe, persistent, unclear)
Differences between subgroups were tested with an interaction test (Altman 2003).
Results
Description of studies
Results of the search
Search history is provided in Table 2. From update searches conducted between October 2002 and October 2005, 29 studies were retrieved for scrutiny. Of these 17 met the entry criteria of the review (Busse 2004; Creticos 1999; LaForce 2005; Lazarus 2001; Levy 2005; Lindqvist 2003; Pleskow 2003; SAS30003; SAS30004; SLGA2004; SLGA3014 1994; SLGL82; SLMP03; SMART; SMS40221; von Berg 2003; Wolfe 2000b). Additional data were available from the GSK clinical trials website for Kavuru 2000a; Shapiro 2000b; Dahl 1991a; Wolfe 2000a; Wolfe 2000b; Leblanc 1996; Zarkovic 1998. Data from individual studies previously published and reported as pooled data from two clinical trials were made available from the GSK website (Wolfe 2000a; Wolfe 2000b: studies: SLGA3010 and SLGA3011). Following revision of the review entry criteria, 38 studies were excluded which had previously met the entry criteria (see Table 3).
2. Search history.
| Years | Detail |
| All years ‐ October 2002 | References retrieved: 1362 Unique studies identified and assessed: 196 N failing to meet entry criteria: 111 N included: 85 (94 experimental groups) Total N: 94 |
| October 2002 ‐ October 2005 | References retrieved: 1645 Unique studies identified and assessed: 27 N failing to meet entry criteria: N included: Total N: |
3. Studies previously included (2003).
| Study ID | Reason for exclusion |
| Akinparli 1999 | 100% on ICS at baseline |
| Booth 1996 | 100% on ICS at baseline |
| Boulet 1998a | 100% on ICS at baseline |
| Boyd 1995 | 100% on ICS at baseline |
| Chuchalin 2002 | 100% on ICS at baseline |
| Faurschou 1994 | Treatment period < 4 weeks |
| Fitzgerald 1999 | 100% on ICS at baseline |
| Fitzpatrick 1990 | Treatment period < 4 weeks |
| Fuglsang 1998 | Treatment period < 4 weeks |
| Gardiner 1994 | 100% on ICS at baseline |
| Grove 1995 | 100% on ICS at baseline |
| Jartti 1998 | 100% on ICS at baseline |
| Kavuru 2000b | 100% on ICS at baseline |
| Langley 1998 | 100% on ICS at baseline |
| Langton Hewer 1995 | 100% on ICS at baseline |
| Li 1999 | 100% on ICS at baseline |
| Lipworth 1998 | 100% on ICS at baseline |
| Lipworth 2000 | 100% on ICS at baseline |
| McIvor 1998 | 100% on ICS at baseline |
| Meijer 1995 | 100% on ICS at baseline |
| Nelson 1999a | 100% on ICS at baseline |
| Nielsen 1999 | 100% on ICS at baseline |
| Nightingale 2002 | 100% on ICS at baseline |
| Norhaya 1999 | 100% on ICS at baseline |
| O'Byrne 2001 | 100% on ICS at baseline |
| Pauwels 1997 | 100% on ICS at baseline |
| Pearlman 1999 | Combination therapy versus ICS |
| Price 2002 | 100% on ICS at baseline |
| Russell 1995 | 100% on ICS at baseline |
| Self 1998 | 100% on ICS at baseline |
| Shapiro 2000a | 100% on ICS at baseline |
| Simons 1997b | 100% on ICS at baseline |
| Tan 1997 | 100% on ICS at baseline |
| van der Molen 1996 | 100% on ICS at baseline |
| Verberne 1998 | 100% on ICS at baseline |
| Wilding 1997 | 100% on ICS at baseline |
| Yates 1995 | Treatment period < 4 weeks |
| Yates 1997 | Treatment period < 4 weeks |
Included studies
Sixty‐seven published and unpublished studies representing 68 different experimental groups which contribute to the data analyses in this review (see table Characteristics of included studies).
The studies for inclusion were independently assessed and scored by at least two reviewers using the full text of the paper. Agreement on grading of quality was good. Kappa statistics showed 96% agreement on Cochrane grades and 86% on Jadad grades. After discussion between the reviewers (EHW and JAEW) and following reception of further details directly from authors or sponsors, there was full agreement on grading. A total of 159 studies were excluded for the following reasons (see table of excluded studies):
absence of a placebo group (63);
100% use of ICS throughout the study (32);
period of treatment less than 4 weeks (47);
not an RCT (five): reviews (two);
only published as an abstract without any further details available or obtainable (three);
Combination therapy versus ICS (four), or versus placebo (one);
Treatment administered at one‐off clinic visits (two).
Sixty‐three references gave additional information on the background to the review or further details on experimental designs of the included studies.
Author verification An attempt was made to contact authors for studies with data missing on relevant outcomes. A total of 24 principal authors of eligible studies were contacted. Nine authors or sponsors of studies replied with data (Busse 1998; D'Alonzo 1994; Bensch 2001; Leblanc 1996; Meijer 1995; Pauwels 1997; Pearlman 1992; Self 1998 and von Berg 1998) and fifteen have not provided any data to date or failed to reply.
Study design Fifty‐four studies were of parallel group design and 13 of cross over design.
Intervention Agents The LABA agent was salmeterol xinafoate in 49 studies and formoterol fumarate in 18 studies. Salmeterol 50 mcg given twice daily at 12 hourly intervals was used in 49 studies. Two studies compared different doses with placebo: 12.5, 50 and 100 mcg in Dahl 1991a, and 25 with 50mcg BID in SLGA3014 1994.
Formoterol fumarate was the LABA agent used in 17 studies. The dose was 24 mcg twice daily in four studies, 12 mcg twice daily in nine studies and 6 mcg twice daily in three studies. Four studies using a 24 mcg dosage had a comparison group using a different dose: Bensch 2001 and Bensch 2002 using 12 mcg, Schreurs 1996 using 6 mcg and 12 mcg,
Where data from studies were combined, the dose of LABA used was the same or equivalent i.e. salmeterol 50 mcg, formoterol 12 mcg or 24 mcg (Campbell 1999). As mentioned, sensitivity analyses were performed to assess the effect of variations in doses on the result.
The bronchodilator rescue agent used in 54 studies was a short‐acting beta‐2 agonist agent; salbutamol (albuterol) in 48 studies at doses of 200 or 400 mcg or terbutaline in 4 studies at doses of 250 or 500 mcg. In one study fenoterol was used and in additional trial the agent used was either salbutamol or terbutaline. One study permitted the use of either salbutamol or fenoterol. In one study a combination short‐acting beta‐agonist and anticholinergic was used. In three studies the rescue agent was ipratropium bromide (an anticholinergic agent), and in eight studies the agent was not stated.
Treatment period Any treatment period of four weeks or greater was specified in the revised review protocol and of the included studies the treatment periods were: four weeks (20 studies); six weeks (four studies); eight weeks (four studies); nine weeks (one study); 12 weeks (25 studies); 16 weeks (three studies); 24 weeks (three studies); 26 weeks (two studies); 36 weeks (one study); 52 weeks (five studies).
Participants Age Fifty‐six studies included adult or adolescent participants over the age of 12 and eleven included children younger than 12 years of age. Asthma severity Information on the severity of participants' asthma at entry to the study was sought from each paper. Few authors specified how the classification of participants' asthma was arrived at and consistent use of guidelines to assess severity, such as those described in the Global Strategy for Asthma Management and Prevention (GINA 1995) was used or not evident. Where authors of the included studies stated that the participants had asthma of specific grades of severity this was recorded under the categories: mild, mild ‐ moderate, moderate ‐ severe or "persistent" if asthma was described as "persistent or symptomatic". Those studies in which no details were available on the participants' asthma severity were classified as "unknown severity".
Of the 67 included studies, 28 included participants with mild ‐ moderate asthma, nine included subjects with mild asthma only and one included participants with moderate ‐ severe asthma. Eleven studies included participants with persistent or symptomatic asthma and for 18 studies the severity of participants' asthma was unknown.
Co‐interventions Studies were assessed according to the co‐intervention treatment for asthma used by subjects during the course of the study. The information was available for all but three unpublished studies (SLGA2004; SLGL82; SLMP03), and in most cases the authors specified that the dose was kept constant during the course of the study treatment period.
The majority of studies permitted a mixture of co‐intervention treatments (40 out of 67 studies). The most frequently used were ICS or cromones, but some of these studies also permitted oral steroids at low dose and theophyllines (see table 'Characteristics of included studies').
Twenty‐four studies did not permit ICS.
Table 1 details the studies in terms of their severity and concomitant steroid use.
Outcomes Not all the 68 experimental groups contributed to the analyses in the review because of lack of data. Seven studies did not contribute any data, either because none could be obtained in a suitable form from the papers or on request from the authors or sponsors, or because the outcomes in the studies could not be combined.
The results from the parallel group studies, with subjects of any age, were combined and analysed together, stratified on the basis of age (participants under 12) and maintenance therapy usage (ICS at any dose versus no ICS used). For the crossover studies, results for first period of treatment alone were not available for combination with the parallel group studies analyses. Therefore, the crossover studies were separately combined and then the two data sets compared. The results discussed below refer to studies of parallel group design, unless otherwise specified in the text.
Excluded studies
Risk of bias in included studies
The methodological quality of the majority of trials was good and on the Jadad scoring method, seven scored 5 while thirty‐three scored 4 and twenty‐three scored 3. Four studies were given the lower score of 2 as they were not blinded by intervention. Lack of information in publications on the methods of randomisation used, meant that allocation concealment could not be confirmed in many cases (although they probably were adequate), so only eight studies were graded as confirmed adequate (A), fifty‐eight were unclear (B) with one graded as obviously inadequately concealed (C).
Effects of interventions
OVERVIEW OF OUTCOMES
Measures of airway calibre showed consistent benefit from LABA treatment on morning and evening peak expiratory flow and FEV1. There were also benefits in asthma symptoms, quality of life and rescue bronchodilator usage. Major exacerbations were reduced in adults but not in children. A recently published surveillance study SMART has found a statistically significant increase in asthma‐related deaths in LABA in comparison with placebo, respiratory related deaths and the combined outcome of asthma related deaths or life threatening experiences. The increase in all cause mortality and the primary outcome of the study (combined respiratory related deaths or life threatening experiences) did not show statistical significance. The absolute increase in all of these events with LABA was in the region of one extra per thousand patients treated over 6 months, but the confidence intervals around these estimate are wide. For asthma related deaths NNT(H) is 1250 (95% CI 700 to 10,000). Serious adverse events were significantly higher with LABA when the results of three studies in children were combined.
PRIMARY OUTCOMES
AIRWAY CALIBRE ASSESSMENTS
There were statistically significant advantages to LABA treatment compared to placebo for all indices of airway calibre.
PEAK EXPIRATORY FLOW (PEF)
Morning PEF was greater in the LABA group by around 15 L/min (95% CI 11 to 19.4; 20 studies, 3682 participants), but with high levels of heterogeneity (I square 69.5%). The result remained significant with random effects modelling (23 L/min (95% CI 13.3 to 32.5). In six studies with 235 participants not using any ICS the MD was 23.08 L/min (95%CI 4.74 to 41.41). There was a comparable result from three crossover studies (38.2 L/min (95% CI 3.1 to 73.29). The majority of these studies were conducted in participants with mild and mild‐moderate asthma (4 studies (140 participants), 11 studies, (1410 participants) respectively). In the mild studies the effect of LABA was not significant with either random effects or fixed effect modelling but they included only small numbers and may be examples of Type II statistical errors. In the mild‐moderate and persistent/symptomatic studies the effect of LABA was significant with both models (mild‐moderate: FE: 16.12 L/min (95% CI 11.17 to 21.07); RE: 28.82 L/min (95% CI 14.06 to 43.59); persistent/symptomatic studies: FE/RE: 15.04 L/min (95% CI 1.95 to 28.13)).
For evening PEF the advantage to the LABA group across all studies was 12.43 L/min (95%CI 7.61 to 17.24; 14 studies, 2590 participants). In the subgroup analyses, for studies with mixed co‐interventions, the difference in favour of LABA‐treated participants was 23.81 L/min (95%CI 14.81 to 32.81). For non‐ICS users the difference was significant, but the finding was less precise (18.32 (95% CI 2.25 to 34.38), six studies, 245 participants). There was a moderate level of statistical variation in this subgroup (I square 48.1%), and when applying a random effects model the result became non‐significant (29.22 L/min (95% CI ‐9.31 to 67.74). Subgroup analyses by severity of asthma gave similar results for mild‐moderate studies (13.29 L/min (95%CI 8.12 to 18.46)). There was a significant difference in studies recruiting children less than 12 years old (change in am PEF: 12 L/min (95% CI 2 to 22), but an advantage was not evident in three studies in mild asthma with 79 participants.
When all of the studies were combined there were significantly greater changes from baseline in PEF during treatment, measured both in the morning and the evening, in the LABA group. The differences in those treated with and without ICS were similar. Change scores also indicated that LABA treatment led to significant differences compared with placebo (change in am PEF: 24.7 L/min (95% CI 22.62 to 26.79; 25 studies, 5512 participants); change in pm PEF: 15.09 L/min (95%CI 12.98 to 17.2; 22 studies, 5350 participants)). The findings remained significant when random effects modelling was applied (change in am PEF: 24.84 L/min (95%CI 20.41 to 29.27); change in pm PEF: 15.09 L/min (95%CI 12.98 to 17.2)). In studies where LABA was added to usual 'preventer' therapies, the change in am PEF was 29 L/min (95% CI 23 to 35.4; random effects model). In studies where participants were not using ICS the difference was 24 (95% CI 15 to 33; random effects model). Among the studies contributing to this result Nathan 1999; Pearlman 1999a and Rosenthal 1999 only included participants who had not used ICS for at least three months. The result from these studies alone did not show heterogeneity, MD 22.30 L/min (95%CI 16.70 to 27.89) (chi‐squared 2.26 df 2).
There was significant heterogeneity in the subgroup analyses for the change in evening PEF. In participants where LABA was added to usual therapies the subgroup effect was 17 L/min (95%CI 14 to 20) (2980 participants, 12 studies; I square 71.2%). Funnel plots and selective omission of individual studies did not fully explain the causes of heterogeneity, although omitting Jones 1994 reduced the I square statistic to 57%). This study did not provide an estimate of the variance for the published trial and so we applied an imputed standard deviation.
Subgroup analyses of change in PEF by severity of asthma confirmed advantages of similar magnitude, though in the two largest groups those with asthma of mild‐moderate and persistent/symptomatic asthma severity there was significant heterogeneity, probably related to the wide variety of co‐interventions used by participants. The random effects WMD for change in morning PEF was 25.31 L/min (95%CI 16.25 to 34.38, I square 79.9%) and for persistent/symptomatic asthma was 26.31 L/min (95%CI 17.56 to 35.06, I square 86.1%). There was a high level of statistical heterogeneity of effect size of LABAs on change in evening in mild‐moderate asthma (I square 77.6%), although random effects modelling did not alter the direction of the fixed effect (16.75 L/min (95% CI 12.03 to 21.47). For both morning and evening PEF changes there was only one study contributing to the analysis in mild asthmatics and in only two studies for moderate‐severe asthmatics, where the difference failed to achieve statistical significance.
Studies conducted in children
There was a significant advantage in treatment with LABAs in children less than 12 years old with a larger improvement seen for both change in morning PEF (MD 16 L/min (95%CI 12 to 20); four studies, 1065 children) and for change in evening PEF (15 L/min (95%CI 12 to 19); four studies, 1063 children).
FEV1
For laboratory measures of lung function, the most frequently reported measure was the forced expiratory volume in 1 second (FEV1). Overall FEV1 was higher in LABA treated participants by around 0.24 litres (95%CI 0.21 to 0.28) with a moderate level of heterogeneity (I square 45.9%). In studies where LABA was added to background therapy the effect was 0.25 L (0.21 to 0.28). Two small studies on participants not using ICS were underpowered to detect significant differences between treatment groups (MD 0.1 litres (95%CI ‐0.3 to 0.5).
Small numbers of studies in the extremes of mild or severe asthmatics, limited subgroup analyses by severity of asthma. In seven studies with 1932 participants with mild‐moderate asthma the MD was 0.22 litres (95%CI 0.16 to 0.29). Similar results were seen in five studies of crossover design with a MD at the end of treatment of 0.13 litres (95%CI 0.01 to 0.25). In one parallel group study with 354 children under 12 years the difference was 0.19 litres (95%CI 0.06 to 0.32).
LABA led to a significantly greater increase in FEV1 from baseline over that on placebo by 0.17 litres (95%CI 0.14 to 0.2); 17 studies, 3295 participants. The difference between the subgroups of studies where background therapy was used and studies with no ICS use was not significant (P = 0.269).
Two subgroups had two or more studies in them. In persistent/symptomatic asthma the mean difference was 0.2 (95 % CI 0.15 to 0.26, four studies with 1336 participants), and in moderate asthma the MD was 0.15 litres (95%CI 0.06 to 0.24, two studies with 274 participants). There was only one study each with data including subjects with either mild or severe asthma, though both gave significant results of similar magnitude to the overall outcome data.
Data on FEV1 change for children were available as % predicted FEV1 from three trials (693 children), indicating a significant difference in favour of LABA of 4% (95% CI 2 to 6).
FEV1 AUC
There was a significant difference in favour of LABA of around L‐h 2.23 L‐h (95% CI 1.71 to 2.75), seven studies, 1312 participants.
FVC, FEF 25‐75%
Results for forced vital capacity (FVC) or maximum mid expiratory flow (FEF25‐75%) were only reported in two studies using 302 subjects with asthma of mild‐moderate severity and taking a variety of co‐interventions. The differences favoured the LABA treatment group but were not statistically significant.
SYMPTOM SCORES
There were significantly fewer symptoms in the LABA group across the board on a variety of measures at the end of treatment. Scales used to measure asthma symptoms varied from 3 to 6 points and scores were generally derived as a composite based on a number of symptoms, e.g. cough, wheezing, shortness of breath and chest tightness assessed during the day and/or overnight and whether sleep was broken by asthma symptoms. The proportion of days with symptom scores of zero and nights without awakenings due to asthma were also assessed. All measures showed significant advantages in the LABA compared with placebo. All findings reported below pertain to SMDs because of the different metrics used to assess this series of outcomes.
Daytime symptoms were significantly better in LABA treated participants (‐0.34 95% CI ‐0.44 to ‐0.25; 14 studies, 1836 participants). Nocturnal symptoms were also better in LABA treated participants: SMD ‐0.54 (95% CI ‐0.64 to ‐0.45 in eight studies with 1758 participants). There was no significant difference between the subgroups analysed on the basis of including background ICS use.
Subgroup analysis of symptom score data indicated that the effect of LABAs was consistent across the groups of trials based on the classifications of severity in the review.
Symptoms fell from baseline by a greater amount during treatment with LABAs compared to placebo. The difference overall was ‐0.49 (95%CI ‐0.58 to ‐0.41) for day time symptoms in eleven studies with data reported on 2629 participants, and ‐0.54 (95%CI ‐0.87 to ‐0.22, random‐effects modelling) for night time symptoms in three studies with 823 participants. The nocturnal symptom results showed significant heterogeneity that may be due to the variations in severity of asthma and different doses of preventer drugs being used.
LABA treated participants had significantly fewer days affected by asthma (16%, 95% CI 14 to 19; nine studies, 2060 participants), and also fewer night symptoms expressed as both % nights without symptoms (10.79%, 95% CI 6.48 to 15.1; nine studies, 2093 participants), and the % nights without awakenings (15.81%, 95% CI 14.22 to 17.41; 13 studies, 3925 participants). The high level of statistical heterogeneity observed for % nights without symptoms may be partly explained by the difference between non‐ICS users and participants where LABA was added to variable usual therapies (P = 0.047). Two possible effects could explain this difference. One possibility is that in the usual therapy trials, the addition of LABA improves asthma control in the participants on stable doses of ICS whose relief from symptoms is more pronounced than it is in the participants who are given LABA alone, who do not benefit from an effective concomitant maintenance treatment. The second possibility is that since this difference is of only borderline statistical significance, it is an artefact of the numerous analyses based on multiple outcomes and subgroups identified in this review. Only one study in children was reported with the percentage of nights affected by asthma awakenings with LABA in 210 children being fewer with the MD being 6.40% (95%CI 2.11 to 10.69).
RESCUE BRONCHODILATOR USE
LABA treated participants used significantly less rescue medication than the placebo group. The rescue agent used in all studies contributing data to the analyses was a short‐acting beta‐2 agonist.
LABA reduced the requirement for rescue short‐acting beta‐agonist when expressed as absolute and change scores for 24‐hour and also day and night periods. In spite of high levels of statistical heterogeneity in these outcomes the results were significant with both fixed‐effect and random‐effects modelling (difference in SABA usage for 24 hours: ‐0.9 puffs/d, 95% CI ‐1 to ‐0.7; eight studies, 1885 participants; mean change in SABA usage over 24 hours: ‐1.2 puffs/d, 95% CI ‐1.4 to ‐1; 12 studies, 2197 participants; SABA use (day): ‐1 puffs/d, (95% CI ‐1.3 to ‐0.8; three studies, 691 participants; change in SABA use (night): ‐0.54, 95% CI ‐0.7 to ‐0.4; two studies, 633 participants).
There was significant heterogeneity found in the pooled analysis of rescue therapy use. A contributing factor in addition to variation in asthma severity and treatment may have been the different short acting beta‐2 agonist agents used, the different doses and varying inhalational devices. Heterogeneity persisted in the subgroup analyses.
Results of a similar magnitude and direction were seen for the change in rescue medication use both during the day time and at night time across the severity range. The limited data from mild and severe asthma mean that a larger evidence base in these patients is required before more reliable statistical investigation of the effects of LABA on rescue medication usage based upon disease severity can be undertaken.
EXACERBATIONS OF ASTHMA: MAJOR EXACERBATIONS
Twenty‐three studies (5995 participants) reported data on exacerbations of asthma. There was a large reduction in the odds of experiencing at least one major exacerbation during the study in the LABA group compared with placebo, OR 0.73 (95%CI 0.64 to 0.84). The definition of a major exacerbation was stated for the majority of studies. In 10 studies with 2547 adult participants a major exacerbation of asthma was defined as worsening of asthma symptoms requiring treatment in addition to the study drug and usual rescue short acting inhaled beta‐2 agonist agent. When the analysis was confined to these studies the OR was 0.64 (95%CI 0.52 to 0.79). In 10 studies with 2468 adult participants where a definition was not given or was less precise the result was similar, OR 0.59 (95%CI 0.46 to 0.76). 5 cross over studies (n=337) showed a similar but non‐significant result, OR 0.80 (95%CI 0.42 to1.54).
Paradoxically, three studies on children reported data on exacerbations and the pooled analysis actually suggested an increased risk of exacerbation, OR 1.22 (95%CI 0.92 to 1.62) though the result was not statistically significant. Based on a test of interaction, the difference between the adult and children subgroups was highly significant (P = 0.000045).
Subgroup analyses ‐ Inhaled corticosteroid (ICS) use
In 17 studies with 4439 participants with varying rates of use of 'preventer' therapy, there was a significant reduction in exacerbations in the active treatment group, OR 0.64 (95%CI 0.54 to 0.76). In six studies with 1500 participants not using ICS, the reduction in the odds of an exacerbation was similar and also significant, OR 0.71 (95%CI 0.53 to 0.96).
Subgroup analyses ‐Asthma severity
The LABA treatment group had a significantly lower risk of a major exacerbation in mild ‐ moderate asthma and persistent/symptomatic asthma, which characterised the majority of the study populations in this outcome (mild ‐ moderate asthma: OR 0.68, 95%CI 0.55 to 0.83; 10 studies with 3106 participants; persistent/symptomatic asthma: OR 0.76, 95% CI 0.63 to 0.93; eight studies, 2408 participants). The findings were re‐examined with sensitivity analysis restricted to adult studies since the paediatric data highlighted the possibility of an increase in the odds of an exacerbation in children. The resultant OR in the mild ‐ moderate subgroup of studies was lower (OR 0.46 (95% CI 0.35 to 0.6)). A single crossover study (Taylor 1998) reported a difference for the corrected rate of major exacerbations of ‐0.18 exacerbations per patient per year (95%CI ‐0.38 to 0.02) with LABA.
MINOR EXACERBATIONS OF ASTHMA
Taylor 1998 applied a somewhat onerous definition for a minor exacerbation based on fall in morning PEF, increasing symptoms and increasing use of rescue bronchodilator in a crossover study. The difference was significant (‐0.68 exacerbations per patient per year, 95%CI ‐0.95 to‐0.41).
ADVERSE EVENTS
Asthma‐related Death
Findings from SMART indicated that in participants using mixed co‐interventions (including ICS) at baseline there was a significant increase in the odds of asthma‐related death occurring in the LABA treated group (13 versus 3; RR 4.4, 1.25 to 15.3; N = 26355). This represents an absolute increase of one extra death over six months for every 1250 patients treated with LABA, but the confidence interval is wide (95% CI 700 to 10,000). The size of this difference was consistent across all the mortality and life threatening experience outcomes measured in this study, and was statistically significant for asthma related death, respiratory related death and the combined outcome of asthma‐related death and life threatening experiences, but not for all cause mortality (with or without life‐threatening experiences or the combined endpoint of respiratory‐related death or life‐threatening experiences). In those not using ICS at baseline, the number of participants suffering asthma‐related death was higher in LABA than placebo treated groups (9 versus 0, N = 14090). The published trial report did not provide an estimate of the risk ratio as the authors decided that Relative Risk should not be calculated when there were no event rates in the control group. However, we used RevMan 4.2 to calculate the Relative Risk of asthma‐related death for both the subgroups with and without ICS at baseline (using the normal adjustment of adding 0.5 to each cell when there are no events in one cell); for those taking ICS at baseline the Relative Risk is 1.34 (95% CI 0.30 to 5.97), whilst for those not taking ICS at baseline the Relative Risk is 18.98 (95% CI 1.1 to 326). The test for interaction between these subgroups does not reach significance (P= 0.08). Caution should be exercised in the interpretation of subgroup differences as patients were not randomised to ICS in this study, and data on ICS use was collected at baseline only.
A post‐hoc within study subgroup analysis by ethnicity, indicated that African‐Americans were more likely to experience a composite endpoint of respiratory ‐related death and life threatening adverse events (intubation and mechanical ventilation) than Caucasians, Relative Risk Increase 3.9 (95% CI 1.29 to 11.84). There was, however, no significant difference found in asthma‐related deaths between African‐Americans and Caucasians; results from life table analyses for African‐Americans 7 versus 1; RR 7.26(95% CI 0.89 to 58.94: N = 4685), whilst for Caucasians 6 versus 1; RR 5.82 (95% CI 0.70 to 48.37; N=18,642).
When the endpoint was broadened to incorporate respiratory‐related death there was a just significant difference in the Relative Risk of death between LABA and placebo for the total population of 2.18 (95% CI 1.07 to 4.05), N = 26355. There was no significant difference between the subgroups using ICS at baseline and those not using ICS at baseline (test for interaction P = 0.84). The increase in all‐cause mortality yielded non‐significant results (RR 1.33, 95% CI 0.76 to 2.35; three studies using the non‐ICS subgroup from SMART, N = 14534 and RR 1.37, 95% CI 0.87 to 2.14 using all participants from SMART, N = 26799).
Serious adverse events
There was a significant increase in the odds of asthma‐related serious adverse events on LABA treatment (OR 7.46, 95% CI 2.21 to 25.16; three studies, N = 895). However, the odds ratios of life‐threatening adverse events from SMART for both mixed (i.e. total) and ICS ‐ treated populations were not significantly different. LABA treatment led to a significant increase in the odds of serious adverse events where this was reported for 'total events' in three paediatric studies (OR 2.11, 1.03 to 4.31; N = 973).
Total and drug‐related adverse events
There was no significant difference between LABA and placebo in total adverse events, although the lower limit of the 95% CI only just crossed unity (OR 1.15, 95% CI 0.99 to 1.33; 18 studies, N = 3447). There was a higher instance of drug‐related adverse events occurring in LABA treated participants in mixed co‐intervention groups (OR 1.37, 95%CI 1.01 to 1.87; seven studies, N = 2130), and "nervousness" (OR 5.11, 95% CI 1.72 to 15.22;, two studies, N = 546). There were also significant differences in favour of placebo in mixed co‐intervention studies for tremor (OR 3.86, 95% CI 1.91 to 7.78; eight studies, 2257 participants), and across total populations for headache (OR 1.28, 95%CI 1.04 to 1.57; 23 studies with 5667 participants) and throat irritation (OR 1.68, 95% CI 1.10 to 2.56; eight studies, N = 1170). There was no significant different in the odds for pharyngitis, cough, cramps, myalgia/fatigue, insomnia, upper respiratory tract infection, of asthma, musculoskeletal pain or palpitations. Withdrawals All‐cause study withdrawal was less likely on LABA than on placebo treatment (OR 0.91, 95% CI 0.86 to 0.96; 19 studies, N = 30599). There was no significant difference in the likelihood of withdrawal due to adverse events between placebo and LABA (OR 1.11, 95% CI 0.93 to 1.32; 21 studies, N = 30943). Withdrawals due to lack of efficacy were significantly less frequent on LABA than on placebo (OR 0.60, 95% CI 0.53 to 0.68; 14 studies, N = 29466). There was no significant difference in withdrawal due to exacerbations of asthma (OR 0.82, 95% CI 0.46 to 1.46; seven studies, N = 1658).
SECONDARY OUTCOMES
QUALITY OF LIFE
In addition to instruments to measure general quality of life, asthma‐specific measurement of the impact of asthma on patients' quality of life is now possible, using purpose ‐designed instruments, which are of proven reliability, validity and responsiveness. These provide a robust and reliable measurement of this aspect of treatment efficacy. The asthma specific measures most often used in the studies included in this review were the Living with Asthma Questionnaire (LWAQ) by Hyland 1991 and Asthma Quality Of Life Score (AQOL) based on Juniper 1992.
Six parallel group studies (1608 participants) measured quality of life changes with treatment and reported results that could be combined for analyses. They each used the AQOL, which contains 32 questions in four domains; activity limitation, symptoms, emotional function and environmental stimuli. For the global score, there was a clinically and statistically significant advantage to the LABA treatment group compared with placebo, though on inspection there were high levels of statistical heterogeneity across the trials (I square 73.1%). The random effects MD for the improvement in global score during treatment was 0.51 (95%CI 0.42 to 0.6)).
Results for improvements in the separate QOL domains for a subset of these studies were: for activity limitations: 0.4 (95%CI 0.3 to 0.50; three trials), for symptoms: 0.73 (95%CI 0.58 to 0.87; two trials), for emotional function: 0.66 (95%CI 0.48 to 0.83; two trials) and for exposure to environmental stimuli: 0.56 (95%CI 0.42 to 0.7; two trials). There were no studies on participants not using ICS.
One crossover study (Juniper 1995) used the same instrument to assess quality of life in 140 participants on a variety of mixed co‐interventions with asthma of unclassified severity, and reported the results as differences between active and placebo treatment. There were significant advantages, similar to the combined parallel group studies results above, for the LABA group overall in the global score of 0.55 (95%CI 0.48 to 0.83) and in the four individual domains:, activity limitation 0.43 (95%CI 0.28 to 0.58); symptoms 0.65 (95%CI 0.48 to 0.83); emotional function 0.65 (95%CI 0.43 to 0.86); and exposure to environmental stimuli 0.45 (95%CI 0.29 to 0.61).
REDUCTION IN USE OF CO‐INTERVENTIONS: ANTI‐INFLAMMATORY NON‐STEROIDAL MEDICATIONS
One study (Adinoff 1998) attempted to wean subjects in both active and placebo groups from non‐steroidal asthma medications. A higher proportion of subjects (62%) treated with LABA was weaned from at least one other non steroidal medication compared to placebo (54%) but the difference was not statistically significant (OR 1.43, 95%CI 0.85 to 2.40)
GLOBAL ASSESSMENT OF EFFICACY
Four studies reported on results of the assessment of efficacy of study treatment, using scales offering the patient a range from very good or very effective to poor or poorly effective. LABA treatment was associated with significantly better assessment of efficacy by participants (OR 2.83,95% CI 2.15 to 3.74;, N = 879) There were similar results for the investigators' assessment of efficacy of study treatment (OR 8.04, 95% CI 4.63 to 13.94; two studies, N = 268).
BRONCHIAL HYPERREACTIVITY
Seven studies assessed bronchial hyperreactivity which involved inhalational challenge to the airways with solutions of methacholine or histamine, carried out 8‐12 hours after the last dose of study medication, and measured changes in "underlying" airway reactivity from baseline during treatment periods from 4‐52 weeks. There was a greater increase in PD/PC 20 i.e. a larger fall in airway reactivity in the LABA group relative to baseline, than during treatment with placebo. There was a consistent and significant difference in favour of LABA over placebo of 0.56 doubling‐doses of inhalational bronchoconstrictor (95% CI 0.30 to 0.82).
BRONCHOPROTECTION AGAINST AIRWAY CHALLENGE
Measurement of airway reactivity one hour after a dose of a beta‐2 agonist measures the bronchoprotection that it confers, presumably mainly due to physiological antagonism of the induced airway smooth muscle contraction. This was investigated in four studies for the first dose of a LABA and repeated after regular doses over periods from 2 to 8 weeks, to assess the development of "tachyphylaxis" to bronchoprotection. The degree of protection fell from the initial dose effect after regular LABA treatment. In one parallel group study with 23 participants (Cheung 1992) the difference in first dose bronchoprotection for LABA treatment compared to placebo was MD 3.94 doubling doses methacholine (95%CI 3.21 to 4.67). Similarly, in one study of crossover design (Boulet 1998b), the MD was 1.76 doubling doses (95%CI 0.81 to 2.71).
BRONCHODILATOR TOLERANCE OR TACHYPHYLAXIS ON REGULAR LABA TREATMENT
Newnham 1995 investigated tachyphylaxis to airway response to formoterol with regular treatment over 4 weeks in two crossover studies in subjects on a variety of asthma co‐interventions. The peak FEV1 increase from the dose response curve to LABA was significantly attenuated with regular use; the difference in one study was ‐0.26 litres (95%CI ‐0.43 to ‐0.09) and in the other study the results were given as 0.84 litres for active treatment compared to 1.00 litres for placebo, but the standard deviation was not available. Investigation of tachyphylaxis after regular LABA treatment to salbutamol‐induced bronchodilatation in two studies did not produce results suitable for combining in an analysis. Nelson 1999b found no significant differences in the salbutamol dose‐response curves for changes in FEV1 after treatment with salmeterol whether participants were treated with or without ICS.
EXERCISE‐INDUCED ASTHMA
Data were reported in one parallel group study (Garcia 2001) and in two crossover studies (Simons 1997b and Ramage 1994) for exercise induced asthma in subjects treated with formoterol (Garcia 2001) or salmeterol (Ramage 1994 and Simons 1997b). Regular treatment with LABAs gave protection against exercise‐induced bronchoconstriction. There was a significantly smaller fall in FEV1 (measured in litres or as a percentage) with exercise in a standard exercise test , performed 6 to 12 hours after study drug dosing, in the active treatment group compared to placebo, SMD ‐0.61 (95%CI ‐1.15 to ‐0.07) in crossover studies (n=28) and WMD ‐11.12% (95%CI ‐21.04 to ‐1.20) in one parallel group study (n=19).
Discussion
As a chronic disease, with no known cure, the accepted goals of management in chronic asthma are to minimise the adverse impact of the disease on the patient's physical and mental well being and to try, through good control, to minimise long term damage to the airways and prevent undue fixed airway obstruction thought to be due to structural "remodelling" of the airway wall. Treatment is therefore directed at improving physiological endpoints, patient‐perceived physical and mental health, and overall minimisation of disease activity and risk to health.
There have now been a number of systematic reviews on LABA use published in the Cochrane Library on asthma subjects uniformly receiving ICS. This review has attempted to summarise the remaining studies, which have included patients receiving either a variety of background disease modifying agents (usually ICS) or none. We have focused on disease control, but have also emphasised severe adverse events, and especially mortality; drug related death being the worse outcome of all. For the primary outcome of interest in this review, that of asthma control as assessed by airway calibre, asthma symptoms with rescue medication use and exacerbations of asthma, we have demonstrated that there were significant and clinically meaningful advantages to regular treatment with inhaled LABA agents compared to placebo in both adults and children, generally across the board in the subjects of interest: both those on a variable regime of preventer medication and those taking no preventer (ICS).
There were consistent advantages for FEV1, morning and evening peak expiratory flows, in patient‐assessed symptoms and in the amount of additional bronchodilator agent used in overall pooled analyses. Subgroup analyses indicated the most consistent effects to be in studies with participants using ICS or other co‐interventions regularly. Evidence from analyses on studies of asthmatics not using inhaled corticosteroids generally similar but was less consistent, though there were fewer studies in this category
The heterogeneity found in some of our analyses, especially when all studies were combined, may be explained by differences in the population of asthmatics from which participants were recruited, reflecting a large natural variation within the disease spectrum. Heterogeneity was markedly less in the analyses of studies classified by participants' use of ICS or severity of asthma. For the most part we have accepted the study authors' categorisation of "severity" of disease. This is likely to reflect a mixture of asthma features: past severe disease episodes, need for ICS at whatever dose, and current level of symptoms and lung function in spite of that dose on ICS. The concept of asthma severity is always somewhat circular and tautological, but in a robust way is helpful clinically and as a general descriptor. Although the precise definitions used might have differed in specific circumstances, it is likely that the definitions here would not have been much different to those used by national and international guidelines (BTS 1995; GINA 1995; NAC 2002) because they all arise from similar clinical concepts and shared clinical cultures and experiences. Where exactly to fit into a "severity" spectrum those described as "symptomatic" or "persistent" is perhaps rather more difficult, because it depends on context, and how aggressively their caring physician had attempted to ameliorate the symptoms with preventer therapy i.e. ICS. It is likely that they would have fitted best with the moderate ‐ severe group, and their outcomes as a group would suggest that, but we made a prospective decision to analyse them separately which was undertaken. However, overall the consistency of the results across the board and their clinically important levels make the positive results for LABA use compelling.
Evidence of deterioration in asthma control was sought in assessing occurrence of exacerbations of asthma during treatment, particularly in the light of historical concerns about regular use of beta‐2 agonist agents potentially making asthma control worse. In the event, there was a significantly decreased risk of experiencing an exacerbation of asthma in the total adult LABA treatment group, and this result was robust with differing definitions of an exacerbation. When defined precisely as worsening of asthma symptoms that required treatment in addition to the study drug and rescue bronchodilator, the OR for an exacerbation was 0.7 (95%CI 0.6 to 0.8). Although the outcome was generally consistent, it was not significant in those studies not using ICS at all or in those with either mild asthma alone or moderate ‐ severe asthma alone. A paradoxical result was obtained in studies with children alone. There was a non‐significant increase in risk of exacerbation of asthma found in five studies in which all paediatric participants used regular preventer, the majority using ICS, with cromones being used by the remainder. Why children might be different from adults needs further investigations as it could be of major concern and clinical relevance.
The lower risk of asthma exacerbations in adults is in contrast to the fears expressed in the debate over regular beta‐2 agonist use and its possible harmful effects. The finding is of interest in terms of the pathological data found in studies of airway biopsies and lavage fluid from airways taken during treatment with LABA, which have consistently shown either no change or an improvement in underlying airway inflammation on LABA treatment (Gardiner 1994, Li 1999 and Wallin 1999). LABA may enhance the effects of corticosteroid at a gene level by increasing translocation of the CS‐CS Receptor complex to the nucleus (Johnson 2004), but LABA may have independent anti‐inflammatory effects especially on the Innate Immune System (Reid 2003).
Deaths We were especially interested in the most severe potential negative outcome from LABA therapy, namely asthma‐related death and life‐threatening asthma events. Most of the data contributing to these analyses came from SMART (Nelson 2006), which recruited over 26,000 adult subjects between 1996 and 2003, 47% on ICS, before being concluded prematurely because of excess asthma deaths on LABA (Salmeterol) and difficulties in recruiting. The all‐cause deaths were low (72 in total in a 28 week study i.e. about 0.2%), and not significantly different between LABA and placebo groups. The asthma‐related death rate was lower overall than expected, by about 50%, but significantly different between groups: 13 to 3 in LABA versus placebo groups. There was a significant excess of the primary outcome of combined asthma‐related death or life threatening experience on LABA, 37 to 22 (relative risk 1.7, 95% CI 1 to 2.9), with a significant difference found between African‐Americans (who constituted 18% of this study population) and Caucasians (71%), test for interaction P = 0.02. The former as a group had more symptomatic asthma and worse lung function, but less use of ICS (38% versus 49% for Caucasians). No significant differences were found for the outcomes asthma‐related deaths or respiratory‐related deaths when African‐Americans were compared with Caucasians. Furthermore, all the excess in deaths occurred in the first three year phase of recruitment, and in particular in 1998, when recruitment was by community advertisement, rather than through the subsequent method of recruitment through doctors' clinics. The excess of deaths was also predominantly in those not on ICS. Although the finger of doubt is undoubtedly pointed at LABA for "causing" excess deaths from SMART, it may be that the excess mortality relates to poorly controlled, non‐ICS treated patients. It is possible that there could be a genetic polymorphism common in African‐Americans that makes them more pre‐disposed to severe asthma events on LABA (Hawkins 2006), but given the other features and peculiarities in this study's outcomes, it seems even more likely that danger arises if LABA is prescribed without ICS in poorly controlled subjects who should definitely be on ICS by standard guideline definitions.
For the secondary outcomes assessed, results from Quality of Life measures showed that treatment with LABA conferred clinically significant advantages at or above the minimally important level in overall score and in the separate domains of asthma symptoms, emotional function, and exposure to environmental stimuli. For activity limitation the change was just less than that defined as of minimal clinical importance, a mean difference of >= 0.5 representing the minimally clinically important change, with 1.0 and 1.5 representing moderate and maximal changes respectively (Juniper 1994).
The evidence for an effect of regular treatment with LABA in permitting weaning from non‐steroidal anti‐inflammatory asthma medication was not conclusive, but there was a significantly greater odds ratio for at least a 50% reduction in ICS dose in one study (Nielsen 1999) and a non‐significant reduction in daily ICS dose in two other studies. This was supported by a similar difference in ICS dose after weaning in a crossover study, ‐18% (95%CI ‐38 to 2) (McIvor 1998). The possibility of a reduction in need for anti‐inflammatory medication on treatment with LABA again suggests some inherent anti‐inflammatory or disease modifying effects. This may be independent of ICS use, or may be through enhancing the efficacy of ICS (Johnson 2004)
The results confirm the acceptability of treatment with LABA as assessed by both patients and investigators. Results for assessment of control of asthma as 'good' or 'very good' by patients gave significantly higher odds ratio in those using LABA compared to those using placebo. Investigators also rated efficacy with LABA as superior, though most studies did not quote the actual results.
This review specifically assessed possible pharmacologically ‐ predictable adverse effects of regular use of inhaled LABA. If beta‐2 agonists are absorbed into the systemic circulation, then stimulation of beta‐adrenergic receptors in non‐respiratory tissues will occur, in a dose‐related way i.e. dependent upon blood levels of drug achieved. These effects include a positive cardiac chronotropic (increased heart rate) and inotropic (increased force of heart contraction) effect, which may be experienced as palpitations. Skeletal muscle receptor activation will cause fine tremor of the hands, and contribute to metabolic effects including hypokalaemia, hyperglycaemia, metabolic acidosis, elevation of free fatty acids and ketones. Direct vascular effects cause systemic peripheral vasodilatation, which may precipitate headaches and cause a fall in blood pressure. For those studies that reported adverse events data, there was a small increase in the odds ratio for some for these predictable trial medication‐related adverse event occurring in the LABA group compared with the placebo group, but this was mainly for the occurrence of headache. There was no significantly increased risk found for the occurrence of palpitations, tremor or muscle cramps.
Underlying airway responsiveness was assessed to directly acting bronchoconstrictor agents such as methacholine or histamine, at a time when most of the bronchodilator effects would have dissipated, with a significant improvement in BHR during treatment, WMD 0.5 doubling doses (95%CI 0.3 to 0.7) when all studies were combined. This was similar whether participants were using ICS or not. This is reassuring in that underlying airway responsiveness is not worsened, as had been hypothesised in the "ß‐agonist debate" of the late 1980's and early 1990's. Even so the residual improvement in this late phase may not be clinically meaningful, being still less than one doubling dose, which is usually taken as the minimally significant and measurable change in PD 20 in a group.
There is ongoing debate over tachyphylaxis to LABA protection against direct airway challenge agents and against indirect agents acting through mast cell de‐granulation. The analyses confirmed that tachyphylaxis develops to the initial bronchoprotective effect of LABA on direct airway challenge with regular treatment. Even so, there was continuing clinically significant protection against direct airway challenge conferred by LABA even after regular periods of treatment.
Overall, this review gives reassuring evidence for the effectiveness of LABA used regularly in chronic asthma, and does not show any evidence that underlying control of asthma deteriorates with regular use. The evidence is based on a broad range of studies across the asthma spectrum, but is strongest for mild ‐ moderate severity and when concurrent treatment with inhaled corticosteroids is given. There was a relative paucity of studies on children with contributing data and in children a possible increase in exacerbations requires further investigation.
A lack of consistency in outcomes chosen for measurement and reported in individual studies reduced the data available for the analyses. However, the conclusions for the main clinical outcomes were based on large numbers of subjects, whereas for secondary outcomes in studies investigating the mechanisms of action of LABA the conclusions were based on smaller numbers and may thus have less weight. Heterogeneity was quite large in the analyses of some outcomes, but do not confound the conclusions.
Authors' conclusions
Implications for practice.
Long acting beta‐2 agonists (LABA) are highly effective in chronic asthma, and the evidence particularly supports their use in ICS treated asthmatics, as emphasised in current guidelines. These suggest that LABA should be used in patients who remain symptomatic in spite of the use of reasonable doses of ICS. The short acting beta‐2 agonist debate, centred mainly on epidemiological data, suggests that regular short acting beta‐2 agonist use might have risks and lead to deterioration of underlying disease. This review indicates that the overall risk of asthma‐related deaths may be increased with LABA, but the size of this increase in uncertain with a confidence interval ranging from one extra death for every 700 to 10,000 given LABA for six months.
The US Food and Drug Agency (FDA) has added a warning that LABA should not be used to treat asthma without concurrent ICS. Therefore we have decided not to carry out any further updates to this review.
Implications for research.
Future research is needed on whether LABA modify the long term accelerated decline in FEV1 in chronic asthma, especially if used in addition to ICS. Studies in children using preventer medication and adults with mild asthma not using ICS over longer periods are needed to investigate exacerbation rates and safety. LABA given regularly would seem to have disease modifying effects that cannot be explained merely by long‐acting bronchodilatation and this needs further exploration to gain insights into what pathophysiological changes occur in the airways during their use. More evidence is needed on effects of LABA on chronic inflammation and especially on airway wall remodelling, where there is a particularly marked paucity of information in such an important area.
The implications for research have been revised to reflect current evidence regarding the known benefits and harms of LABAs from other Cochrane Reviews. Readers should consult the overviews which summarise the results of Cochrane reviews on the safety of LABAs in adults and children (Cates 2012; Cates 2014).
What's new
| Date | Event | Description |
|---|---|---|
| 1 July 2014 | Amended | Statement added to abstract and plain language summary to state that the review is no longer to be updated and why. Old material, which has been superseded, has been removed from the review. |
| 1 July 2014 | Review declared as stable | LABA is no longer recommended except in addition to ICS. The editorial board of the Cochrane Airways Group in conjunction with the authors of the review have decided not to update the review any more. Readers should consult the overviews which summarise the results of Cochrane reviews on the safety of LABAs in adults and children (Cates 2012; Cates 2014). |
History
Protocol first published: Issue 2, 1999 Review first published: Issue 4, 2003
| Date | Event | Description |
|---|---|---|
| 1 August 2008 | Amended | Converted to new review format. |
| 10 November 2006 | New citation required and conclusions have changed | Substantive amendment |
Notes
The US Food and Drug Agency (FDA) has added a warning that LABA should not be used to treat asthma without concurrent ICS. Therefore we have decided not to carry out any further updates to this review.
Acknowledgements
Airways group staff especially Liz Arnold, Jane Dennis and Karen Blackhall for searches, papers retrieval, and translation. Felix Ram for help with grading studies, Chris Cates for editorial input and advice. Richard Wood‐Baker for advice and support and Denise Till of Novartis for providing unpublished data for LaForce 2005. We are grateful to staff at GSK for unpublished data and authors who responded to requests for more information.
Data and analyses
Comparison 1. Studies with parallel group design: efficacy outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Peak expiratory flow: morning | 20 | 3682 | L/min (Fixed, 95% CI) | 12.64 [8.37, 16.92] |
| 1.1 Participants using mixed cointerventions | 11 | 2826 | L/min (Fixed, 95% CI) | 21.81 [14.07, 29.55] |
| 1.2 Participants not using ICS | 6 | 235 | L/min (Fixed, 95% CI) | 19.23 [0.62, 37.84] |
| 1.3 Children <12 years | 3 | 621 | L/min (Fixed, 95% CI) | 7.73 [2.39, 13.07] |
| 2 Peak expiratory flow: evening | 14 | 2586 | L/min (Fixed, 95% CI) | 9.68 [4.79, 14.57] |
| 2.1 Participants mixed cointerventions | 8 | 2027 | L/min (Fixed, 95% CI) | 15.73 [6.28, 25.17] |
| 2.2 Subjects not using ICS (PG design) | 4 | 103 | L/min (Fixed, 95% CI) | 15.42 [‐0.82, 31.67] |
| 2.3 Children <12 years | 2 | 456 | L/min (Fixed, 95% CI) | 6.35 [0.26, 12.45] |
| 3 Change in PEF morning | 25 | 4773 | L/min (Random, 95% CI) | 25.70 [20.30, 31.09] |
| 3.1 Participants using mixed cointerventions | 12 | 2503 | L/min (Random, 95% CI) | 29.14 [21.57, 36.70] |
| 3.2 Participants not using ICS (PG design) | 8 | 1582 | L/min (Random, 95% CI) | 24.24 [14.23, 34.25] |
| 3.3 Children <12 years | 4 | 562 | L/min (Random, 95% CI) | 14.33 [8.64, 20.01] |
| 3.4 Unclear | 1 | 126 | L/min (Random, 95% CI) | 30.00 [15.87, 44.13] |
| 4 Change in PEF morning (%) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 Participants using mixed cointerventions | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Participants not using ICS | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Children <12 years | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Change in PEF evening | 22 | 4611 | L/min (Random, 95% CI) | 17.66 [12.93, 22.39] |
| 5.1 Participants using mixed cointerventions | 12 | 2744 | L/min (Random, 95% CI) | 21.32 [14.70, 27.94] |
| 5.2 Participants not using ICS | 5 | 1181 | L/min (Random, 95% CI) | 10.68 [6.38, 14.98] |
| 5.3 Children <12 years | 4 | 560 | L/min (Random, 95% CI) | 23.04 [‐3.54, 49.61] |
| 5.4 Unclear | 1 | 126 | L/min (Random, 95% CI) | 17.0 [5.02, 28.98] |
| 6 N with >/=15% increase in FEV1 | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 Participants using mixed cointerventions | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Participants not using ICS | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 Children <12 years | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.4 Unclear | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Change in PEF morning ‐percent predicted | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 Participants using mixed cointerventions | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Participants not using ICS | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 Children <12 years | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Change in PEF evening‐percent predicted | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 Participants using mixed cointerventions | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Participants not using ICS | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 Children <12 years | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Amplitude PEF: diurnal variation (l/min or %) | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 Participants using mixed cointerventions | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Participants not using ICS | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.3 Children <12 years | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 N with >/=15% increase in PEF | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10.1 Participants using mixed cointerventions | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 Participants not using ICS | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.3 Children <12 years | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Change in peak expiratory flow: % predicted | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11.1 Participants using mixed cointerventions | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.2 Participants not using ICS | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.3 Children <12 years | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 Peak expiratory flow: % predicted | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12.1 Subjects using mixed cointerventions | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.2 Participants not using ICS | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.3 Children <12 years | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 AUC‐ mean area under 12 hr serial PEF curve (% predicted) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.1 Participants using mixed cointerventions | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.2 Subjects not on ICS >12 weeks treatment | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.3 Children <12 years | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14 Change in Amplitude PEF: diurnal variation (l/min or %) | 2 | 1001 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.43 [‐0.55, ‐0.30] |
| 14.1 Participants using mixed cointerventions | 2 | 1001 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.43 [‐0.55, ‐0.30] |
| 14.2 Participants not using ICS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.3 Children <12 years | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 FEV1 | 14 | 3882 | Litres (Fixed, 95% CI) | 0.24 [0.20, 0.28] |
| 15.1 Participants using mixed cointerventions | 11 | 3458 | Litres (Fixed, 95% CI) | 0.24 [0.20, 0.28] |
| 15.2 Participants not using ICS (PG design) | 2 | 70 | Litres (Fixed, 95% CI) | 0.12 [‐0.24, 0.48] |
| 15.3 Children <12 years | 1 | 354 | Litres (Fixed, 95% CI) | 0.19 [0.06, 0.32] |
| 16 FEV1 predicted | 4 | 595 | % (Fixed, 95% CI) | 3.46 [0.20, 6.72] |
| 16.1 Participants using mixed cointerventions | 0 | 0 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 Children <12 years | 3 | 593 | % (Fixed, 95% CI) | 3.46 [0.20, 6.72] |
| 16.3 Participants not using ICS | 1 | 2 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Change in FEV (litres) | 15 | 3295 | Mean Difference (IV, Fixed, 95% CI) | 0.17 [0.13, 0.21] |
| 17.1 Participants using mixed cointerventions | 7 | 1565 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [0.14, 0.25] |
| 17.2 Participants not using ICS | 6 | 1225 | Mean Difference (IV, Fixed, 95% CI) | 0.18 [0.10, 0.25] |
| 17.3 Children <12 years | 2 | 505 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.00, 0.16] |
| 18 N with >/=15% increase in FEV1 | 2 | 344 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.90 [1.22, 2.95] |
| 18.1 Participants using mixed cointerventions | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18.2 Children <12 years | 2 | 344 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.90 [1.22, 2.95] |
| 18.3 Participants not using ICS | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19 AUC‐ mean area under 12 hr serial FEV1 curve | 7 | 1312 | L‐h (Fixed, 95% CI) | 2.23 [1.71, 2.75] |
| 19.1 Participants using mixed cointerventions | 4 | 726 | L‐h (Fixed, 95% CI) | 3.79 [2.98, 4.60] |
| 19.2 Participants not on ICS | 2 | 337 | L‐h (Fixed, 95% CI) | 2.01 [0.21, 3.81] |
| 19.3 Children <12 years | 1 | 249 | L‐h (Fixed, 95% CI) | 0.95 [0.21, 1.69] |
| 20 Change in FEV1 %predicted | 3 | 695 | Mean Difference (IV, Fixed, 95% CI) | 5.00 [1.95, 8.05] |
| 20.1 Participants using mixed cointerventions | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20.2 Participants not using ICS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20.3 Children <12 years | 3 | 695 | Mean Difference (IV, Fixed, 95% CI) | 5.00 [1.95, 8.05] |
| 21 AUC‐ mean area under 12 hr serial FEV1 curve (% predicted) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 21.1 Participants using mixed cointerventions | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 21.2 Subjects not on ICS >12 weeks treatment | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 21.3 Children <12 years | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 22 AUC‐ mean change area under 12 hr serial FEV1 curve (L‐h) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 22.1 Participants using mixed cointerventions | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 22.2 Participants not on ICS | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 22.3 Children <12 years | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 23 Fall in FEV1 post exercise (12 hrs post study drug) % | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 23.1 Participants using mixed cointerventions | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | ‐11.12 [‐21.04, ‐1.20] |
| 23.2 Participants not on ICS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23.3 Children <12 years | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24 Fall in FEV1 post exercise (pre‐medication with formoterol) % | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 24.1 Participants using mixed cointerventions | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | 5.19 [‐1.23, 11.61] |
| 24.2 Participants not on ICS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24.3 Children <12 years | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25 FEV1 12hr post dose | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 25.1 Participants using mixed cointerventions | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 25.2 Participants not using ICS | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 25.3 Children <12 years | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 26 Change in FEV1 12hr post dose | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 26.1 Participants using mixed cointerventions | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 26.2 Participants not using ICS | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 26.3 Children <12 years | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 27 Forced Vital Capacity (litres) | 2 | 302 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.14, 0.32] |
| 27.1 Participants using mixed cointerventions | 2 | 302 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.14, 0.32] |
| 27.2 Participants not using ICS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 27.3 Children <12 years | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 28 Change in Forced Vital Capacity (litres) | 5 | 626 | Mean Difference (IV, Fixed, 95% CI) | 0.13 [0.03, 0.22] |
| 28.1 Participants using mixed cointerventions | 4 | 466 | Mean Difference (IV, Fixed, 95% CI) | 0.13 [0.03, 0.22] |
| 28.2 Participants not using ICS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 28.3 Children <12 years | 1 | 160 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 29 FEF25‐75 (litres/sec) | 3 | 462 | Mean Difference (IV, Fixed, 95% CI) | 0.17 [‐0.06, 0.40] |
| 29.1 Participants using mixed cointerventions | 2 | 302 | Mean Difference (IV, Fixed, 95% CI) | 0.17 [‐0.06, 0.40] |
| 29.2 Participants not using ICS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 29.3 Children <12 years | 1 | 160 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 30 Symptom score ‐ whole day | 4 | 905 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.37 [‐0.50, ‐0.24] |
| 30.1 Participants using mixed cointerventions | 3 | 873 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.35 [‐0.49, ‐0.22] |
| 30.2 Participants all using ICS | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 30.3 Children <12 years | 1 | 32 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.93 [‐1.67, ‐0.20] |
| 31 Symptom score ‐ day time | 11 | 1995 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.33 [‐0.41, ‐0.24] |
| 31.1 Participants using mixed cointerventions | 8 | 1916 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.32 [‐0.41, ‐0.23] |
| 31.2 Participants not using ICS (PG design) | 3 | 79 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.39 [‐0.84, 0.05] |
| 31.3 Children <12 years | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 32 Symptom score ‐ night time | 9 | 1917 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.51 [‐0.60, ‐0.41] |
| 32.1 Participants using mixed cointerventions | 7 | 1851 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.51 [‐0.60, ‐0.42] |
| 32.2 Children <12 years | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 32.3 Participants not using ICS | 2 | 66 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.39 [‐0.88, 0.10] |
| 33 Change in symptom score: whole day | 2 | 333 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.60, 0.06] |
| 33.1 Participants using mixed cointerventions | 2 | 333 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.60, 0.06] |
| 33.2 Participants not using ICS | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33.3 Children <12 years | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 33.4 Unclear | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34 Change in symptom score: day time | 13 | 2629 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.48 [‐0.60, ‐0.37] |
| 34.1 Participants using mixed cointerventions | 5 | 1008 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.55 [‐0.76, ‐0.33] |
| 34.2 Participants not using ICS | 6 | 1148 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐0.54, ‐0.29] |
| 34.3 Children <12 years | 1 | 347 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 34.4 Unclear | 1 | 126 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐0.73, ‐0.03] |
| 35 Change in symptom score: night time | 4 | 823 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐0.87, ‐0.22] |
| 35.1 Participants using mixed cointerventions | 3 | 697 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.59 [‐1.03, ‐0.16] |
| 35.2 Participants not using ICS | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35.3 Children <12 years | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35.4 Unclear | 1 | 126 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐0.73, ‐0.03] |
| 36 Change in total symptom score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 36.1 Participants using mixed cointerventions | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 36.2 Participants not using ICS | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 36.3 Children <12 years | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 37 % days without rescue medication | 2 | 251 | Mean Difference (IV, Random, 95% CI) | 23.60 [12.86, 34.33] |
| 37.1 Participants using mixed cointerventions | 2 | 251 | Mean Difference (IV, Random, 95% CI) | 23.60 [12.86, 34.33] |
| 37.2 Participants not using ICS | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37.3 Children <12 years | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 38 N with <50% days free from rescue medication | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 38.1 Participants using mixed cointerventions | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 38.2 Participants not using ICS | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 38.3 Children <12 years | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 39 % days without asthma symptoms | 7 | 2254 | Mean Difference (IV, Random, 95% CI) | 15.77 [9.75, 21.79] |
| 39.1 Participants using mixed cointerventions | 7 | 2254 | Mean Difference (IV, Random, 95% CI) | 15.77 [9.75, 21.79] |
| 39.2 Participants not using ICS | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 39.3 Children <12 years | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 40 N with <50% symptom free days | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 40.1 Participants using mixed cointerventions | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 40.2 Participants not using ICS | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 40.3 Subjects children <12 years | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 41 % nighttime awakenings requiring no SABA | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 41.1 Participants using mixed cointerventions | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 41.2 Participants not using ICS | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 41.3 Children <12 years | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 41.4 Unclear | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 42 Change in nighttime awakenings | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 42.1 Participants using mixed cointerventions | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 42.2 Participants not using ICS | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 42.3 Children <12 years | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 43 N with <50% symptom free nights | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 43.1 Participants using mixed cointerventions | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 43.2 Participants not using ICS | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 43.3 Subjects children <12 years | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 44 Change in % no nighttime awakenings | 2 | 333 | Mean Difference (IV, Random, 95% CI) | 10.84 [‐0.92, 22.59] |
| 44.1 Participants using mixed cointerventions | 2 | 333 | Mean Difference (IV, Random, 95% CI) | 10.84 [‐0.92, 22.59] |
| 44.2 Participants not using ICS | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 44.3 Children <12 years | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 45 % nights without asthma awakenings | 13 | 3925 | Mean Difference (IV, Random, 95% CI) | 14.76 [9.68, 19.84] |
| 45.1 Participants using mixed cointerventions | 12 | 3715 | Mean Difference (IV, Random, 95% CI) | 15.93 [10.84, 21.02] |
| 45.2 Participants not using ICS | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 45.3 Children <12 years | 1 | 210 | Mean Difference (IV, Random, 95% CI) | 6.40 [2.11, 10.69] |
| 46 Change in % nighttime awakenings requiring no SABA | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 46.1 Participants using mixed cointerventions | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 46.2 Participants not using ICS | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 46.3 Children <12 years | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 46.4 Unclear | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 47 N with <50% nights free from rescue medication | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 47.1 Participants using mixed cointerventions | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 47.2 Participants not using ICS | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 47.3 Children <12 years | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 48 Change in % days without asthma symptoms | 13 | 2866 | Mean Difference (IV, Random, 95% CI) | 15.26 [10.51, 20.01] |
| 48.1 Participants using mixed cointerventions | 7 | 1514 | Mean Difference (IV, Random, 95% CI) | 16.33 [9.73, 22.92] |
| 48.2 Participants not using ICS | 6 | 1226 | Mean Difference (IV, Random, 95% CI) | 12.73 [8.61, 16.84] |
| 48.3 Children <12 years | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 48.4 Unclear | 1 | 126 | Mean Difference (IV, Random, 95% CI) | 16.4 [6.92, 25.88] |
| 49 Change in % nights without asthma symptoms | 10 | 2183 | Mean Difference (IV, Random, 95% CI) | 12.55 [5.53, 19.57] |
| 49.1 Participants using mixed cointerventions | 4 | 930 | Mean Difference (IV, Random, 95% CI) | 19.94 [13.64, 26.25] |
| 49.2 Participants not using ICS | 5 | 1046 | Mean Difference (IV, Random, 95% CI) | 8.10 [4.88, 11.32] |
| 49.3 Children <12 years | 1 | 207 | Mean Difference (IV, Random, 95% CI) | 5.00 [‐0.83, 10.83] |
| 50 Rescue bronchodilator use: whole day | 8 | 1885 | Mean Difference (IV, Random, 95% CI) | ‐1.17 [‐1.73, ‐0.61] |
| 50.1 Participants using mixed cointerventions | 6 | 1635 | Mean Difference (IV, Random, 95% CI) | ‐1.53 [‐1.92, ‐1.15] |
| 50.2 Participants not using ICS | 1 | 43 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.53, ‐0.07] |
| 50.3 Children <12 years | 1 | 207 | Mean Difference (IV, Random, 95% CI) | ‐0.4 [‐0.84, 0.04] |
| 51 Rescue bronchodilator use: day time | 5 | 1172 | Mean Difference (IV, Random, 95% CI) | ‐0.95 [‐1.65, ‐0.25] |
| 51.1 Participants using mixed cointerventions | 4 | 1149 | Mean Difference (IV, Random, 95% CI) | ‐1.02 [‐1.81, ‐0.22] |
| 51.2 Participants not using ICS | 1 | 23 | Mean Difference (IV, Random, 95% CI) | ‐0.56 [‐1.67, 0.55] |
| 51.3 Children <12 years | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 52 Rescue bronchodilator use: night time | 5 | 1176 | Mean Difference (IV, Random, 95% CI) | ‐0.56 [‐0.87, ‐0.25] |
| 52.1 Participants using mixed cointerventions | 4 | 1153 | Mean Difference (IV, Random, 95% CI) | ‐0.59 [‐0.91, ‐0.27] |
| 52.2 Participants not using ICS | 1 | 23 | Mean Difference (IV, Random, 95% CI) | 0.14 [‐1.30, 1.58] |
| 52.3 Children <12 years | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 53 Change in use of rescue bronchodilator/day | 3 | 691 | Mean Difference (IV, Random, 95% CI) | ‐1.03 [‐1.65, ‐0.41] |
| 53.1 Participants using mixed cointerventions | 3 | 691 | Mean Difference (IV, Random, 95% CI) | ‐1.03 [‐1.65, ‐0.41] |
| 53.2 Children <12 years | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 53.3 Participants not using ICS | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 54 Change in use of rescue bronchodilator/night | 3 | 697 | Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐0.67, ‐0.25] |
| 54.1 Participants using mixed cointerventions | 3 | 697 | Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐0.67, ‐0.25] |
| 54.2 Participants not using ICS | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 54.3 Children <12 years | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 55 Change in use of rescue bronchodilator/ whole day | 12 | 2197 | Mean Difference (IV, Random, 95% CI) | ‐1.16 [‐1.47, ‐0.85] |
| 55.1 Participants using mixed cointerventions | 5 | 842 | Mean Difference (IV, Random, 95% CI) | ‐1.35 [‐1.86, ‐0.83] |
| 55.2 Participants not using ICS | 6 | 1148 | Mean Difference (IV, Random, 95% CI) | ‐1.22 [‐1.57, ‐0.86] |
| 55.3 Children <12 years | 1 | 207 | Mean Difference (IV, Random, 95% CI) | ‐0.5 [‐0.94, ‐0.06] |
| 56 Change in % days without rescue medication | 2 | 352 | Mean Difference (IV, Random, 95% CI) | 13.0 [4.27, 21.73] |
| 56.1 Participants using mixed cointerventions | 1 | 172 | Mean Difference (IV, Random, 95% CI) | 13.0 [4.27, 21.73] |
| 56.2 Participants not using ICS | 1 | 180 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 56.3 Children <12 years | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 57 AQOL‐ Change in Quality of life score: global | 6 | 1608 | Mean Difference (IV, Fixed, 95% CI) | 0.54 [0.44, 0.64] |
| 57.1 Participants using mixed cointerventions | 4 | 1249 | Mean Difference (IV, Fixed, 95% CI) | 0.54 [0.44, 0.64] |
| 57.2 Participants not using ICS | 2 | 359 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 57.3 Children <12 years | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 58 Change in Quality of life score‐ symptoms | 2 | 916 | Mean Difference (IV, Fixed, 95% CI) | 0.73 [0.58, 0.87] |
| 58.1 Participants using mixed cointerventions | 2 | 916 | Mean Difference (IV, Fixed, 95% CI) | 0.73 [0.58, 0.87] |
| 58.2 Participants not using ICS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 58.3 Children <12 years | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 59 Change in Quality of life score: emotions | 2 | 916 | Mean Difference (IV, Fixed, 95% CI) | 0.66 [0.48, 0.83] |
| 59.1 Participants using mixed cointerventions | 2 | 916 | Mean Difference (IV, Fixed, 95% CI) | 0.66 [0.48, 0.83] |
| 59.2 Participants not using ICS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 59.3 Children <12 years | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 60 Change in Quality of life score: exposure to environmental stimuli | 2 | 916 | Mean Difference (IV, Fixed, 95% CI) | 0.56 [0.42, 0.70] |
| 60.1 Participants using mixed cointerventions | 2 | 916 | Mean Difference (IV, Fixed, 95% CI) | 0.56 [0.42, 0.70] |
| 60.2 Participants not using ICS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 60.3 Children <12 years | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 61 Change in Quality of life score: activity limitations | 3 | 1086 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [0.30, 0.50] |
| 61.1 Participants using mixed cointerventions | 2 | 916 | Mean Difference (IV, Fixed, 95% CI) | 0.41 [0.31, 0.51] |
| 61.2 Participants not using ICS | 1 | 170 | Mean Difference (IV, Fixed, 95% CI) | 0.19 [‐0.18, 0.56] |
| 61.3 Children <12 years | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 62 Mini AQLQ (Total) | 2 | AQLQ (Fixed, 95% CI) | 0.22 [0.02, 0.43] | |
| 62.1 Participants using mixed cointerventions | 2 | AQLQ (Fixed, 95% CI) | 0.22 [0.02, 0.43] | |
| 62.2 Participants not using ICS (PG design) | 0 | AQLQ (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 62.3 Children <12 years | 0 | AQLQ (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 63 Mini AQLQ (Symptoms) | 1 | AQLQ (Fixed, 95% CI) | Totals not selected | |
| 63.1 Participants using mixed cointerventions | 1 | AQLQ (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 63.2 Participants not using ICS (PG design) | 0 | AQLQ (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 63.3 Children <12 years | 0 | AQLQ (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 64 Mini AQLQ (Activity limitation) | 1 | AQLQ (Fixed, 95% CI) | Totals not selected | |
| 64.1 Participants using mixed cointerventions | 1 | AQLQ (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 64.2 Participants not using ICS (PG design) | 0 | AQLQ (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 64.3 Children <12 years | 0 | AQLQ (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 65 Mini AQLQ (Emotional function) | 1 | AQLQ (Fixed, 95% CI) | Totals not selected | |
| 65.1 Participants using mixed cointerventions | 1 | AQLQ (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 65.2 Participants not using ICS | 0 | AQLQ (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 65.3 Children <12 years | 0 | AQLQ (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 66 Mini AQLQ (Environmental stimuli) | 1 | AQLQ (Fixed, 95% CI) | Totals not selected | |
| 66.1 Participants using mixed cointerventions | 1 | AQLQ (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 66.2 Participants not using ICS | 0 | AQLQ (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 66.3 Children <12 years | 0 | AQLQ (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 67 Quality of life score: COMBINED ALL SCALES | 2 | 286 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.28, 0.08] |
| 67.1 Participants using mixed cointerventions | 1 | 79 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.28, 0.08] |
| 67.2 Subjects not using ICS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 67.3 Children <12 years | 1 | 207 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 68 Bronchial hyperreactivity‐ log PD20/PC20 methacholine or histamine | 8 | 689 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.16 [‐0.02, 0.33] |
| 68.1 Participants using mixed cointerventions | 2 | 269 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐0.19, 0.29] |
| 68.2 Children <12 years | 3 | 332 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.21 [‐0.09, 0.51] |
| 68.3 Participants not using ICS | 3 | 88 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.49 [‐0.01, 0.98] |
| 69 Bronchial hyperreactivity‐ log PD20/PC20 methacholine or histamine | 1 | 24 | Doubling doses (Fixed, 95% CI) | ‐0.01 [‐0.21, 0.19] |
| 69.1 Participants using mixed cointerventions | 0 | 0 | Doubling doses (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 69.2 Participants not using ICS | 1 | 24 | Doubling doses (Fixed, 95% CI) | ‐0.01 [‐0.21, 0.19] |
| 69.3 Children <12 years | 0 | 0 | Doubling doses (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 70 Change in BHR (end treatment vs. baseline)‐ doubling doses (DD) | 7 | 1024 | Mean Difference (IV, Fixed, 95% CI) | 0.56 [0.30, 0.82] |
| 70.1 Participants using mixed cointerventions | 2 | 277 | Mean Difference (IV, Fixed, 95% CI) | 0.45 [‐0.05, 0.95] |
| 70.2 Children <12 years | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | 0.45 [‐0.37, 1.27] |
| 70.3 Participants not using ICS | 4 | 705 | Mean Difference (IV, Fixed, 95% CI) | 0.62 [0.30, 0.95] |
| 71 Bronchoprotection to methacholine challenge(protection ratio end treatment vs. baseline)‐ doubling doses (DD) | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | 1.91 [0.88, 2.94] |
| 71.1 Participants using mixed cointerventions | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 71.2 Participants not using ICS | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | 1.91 [0.88, 2.94] |
| 71.3 Children <12 years | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 72 Bronchoprotection to methacholine challenge (protection ratio first dose treatment vs. baseline)‐ double dose | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | 3.94 [3.21, 4.67] |
| 72.1 Participants using mixed cointerventions | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 72.2 Participants not using ICS | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | 3.94 [3.21, 4.67] |
| 72.3 Children <12 years | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 73 Exacerbations asthma ‐ >1 major | 25 | 7285 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.61, 0.79] |
| 73.1 Adults‐ Similar definition of exacerbation | 11 | 3588 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.49, 0.71] |
| 73.2 Adults‐ Definition of exacerbation imprecise | 10 | 2468 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.46, 0.76] |
| 73.3 Children <12 years | 4 | 1229 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.88, 1.49] |
| 74 Exacerbations asthma ‐ >1 major(sub‐group by use of inhaled corticosteroid) | 24 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 74.1 Participants using mixed co‐interventions | 18 | 4688 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.55, 0.77] |
| 74.2 Participants not using ICS | 6 | 1500 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.53, 0.96] |
| 75 Weaned from at least 1 non steroidal asthma medication | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 75.1 Participants using mixed cointerventions | 1 | 238 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.85, 2.40] |
| 75.2 Children <12 years | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 76 Global assessment of efficacy by patient‐ very good/good | 4 | 879 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.83 [2.15, 3.74] |
| 76.1 Participants using mixed cointerventions | 3 | 695 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.34 [2.43, 4.58] |
| 76.2 Participants not using ICS | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 76.3 Children <12 years | 1 | 184 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.63 [0.91, 2.94] |
| 77 Global assessment of efficacy by investigator‐ very good/good | 2 | 268 | Odds Ratio (M‐H, Fixed, 95% CI) | 8.04 [4.63, 13.94] |
| 77.1 Participants using mixed cointerventions | 2 | 268 | Odds Ratio (M‐H, Fixed, 95% CI) | 8.04 [4.63, 13.94] |
| 77.2 Participants not using ICS | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 77.3 Children <12 years | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 78 Global assessment of efficacy by patient ‐ improved | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 78.1 Participants using mixed cointerventions | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 78.2 Participants all using ICS | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 78.3 Children <12 years | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 79 Global assessment of efficacy by patient ‐ not improved | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 79.1 Participants using mixed cointerventions | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 79.2 Participants all using ICS | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 79.3 Children <12 years | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 80 Global assessment of efficacy by investigator ‐ improved | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 80.1 Participants using mixed cointerventions | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 80.2 Participants all using ICS | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 80.3 Children <12 years | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 81 Global assessment of efficacy by investigator ‐ not improved | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 81.1 Participants using mixed cointerventions | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 81.2 Participants all using ICS | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 81.3 Children <12 years | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
1.1. Analysis.

Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 1 Peak expiratory flow: morning.
1.2. Analysis.

Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 2 Peak expiratory flow: evening.
1.3. Analysis.

Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 3 Change in PEF morning.
1.4. Analysis.
Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 4 Change in PEF morning (%).
1.5. Analysis.

Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 5 Change in PEF evening.
1.6. Analysis.
Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 6 N with >/=15% increase in FEV1.
1.7. Analysis.

Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 7 Change in PEF morning ‐percent predicted.
1.8. Analysis.

Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 8 Change in PEF evening‐percent predicted.
1.9. Analysis.

Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 9 Amplitude PEF: diurnal variation (l/min or %).
1.10. Analysis.
Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 10 N with >/=15% increase in PEF.
1.11. Analysis.
Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 11 Change in peak expiratory flow: % predicted.
1.12. Analysis.

Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 12 Peak expiratory flow: % predicted.
1.13. Analysis.

Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 13 AUC‐ mean area under 12 hr serial PEF curve (% predicted).
1.14. Analysis.

Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 14 Change in Amplitude PEF: diurnal variation (l/min or %).
1.15. Analysis.
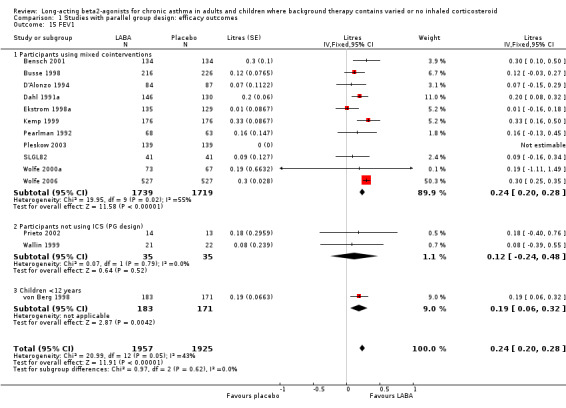
Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 15 FEV1.
1.16. Analysis.

Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 16 FEV1 predicted.
1.17. Analysis.
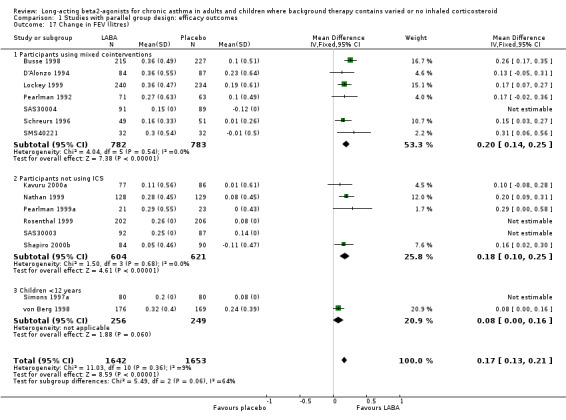
Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 17 Change in FEV (litres).
1.18. Analysis.

Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 18 N with >/=15% increase in FEV1.
1.19. Analysis.

Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 19 AUC‐ mean area under 12 hr serial FEV1 curve.
1.20. Analysis.

Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 20 Change in FEV1 %predicted.
1.21. Analysis.

Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 21 AUC‐ mean area under 12 hr serial FEV1 curve (% predicted).
1.22. Analysis.

Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 22 AUC‐ mean change area under 12 hr serial FEV1 curve (L‐h).
1.23. Analysis.
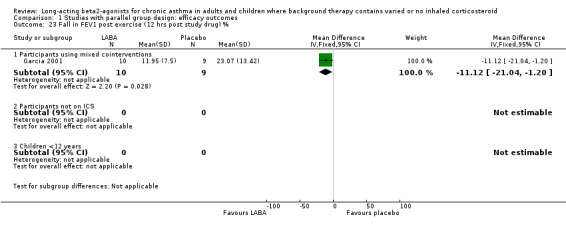
Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 23 Fall in FEV1 post exercise (12 hrs post study drug) %.
1.24. Analysis.

Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 24 Fall in FEV1 post exercise (pre‐medication with formoterol) %.
1.25. Analysis.

Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 25 FEV1 12hr post dose.
1.26. Analysis.
Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 26 Change in FEV1 12hr post dose.
1.27. Analysis.

Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 27 Forced Vital Capacity (litres).
1.28. Analysis.

Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 28 Change in Forced Vital Capacity (litres).
1.29. Analysis.

Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 29 FEF25‐75 (litres/sec).
1.30. Analysis.

Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 30 Symptom score ‐ whole day.
1.31. Analysis.

Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 31 Symptom score ‐ day time.
1.32. Analysis.
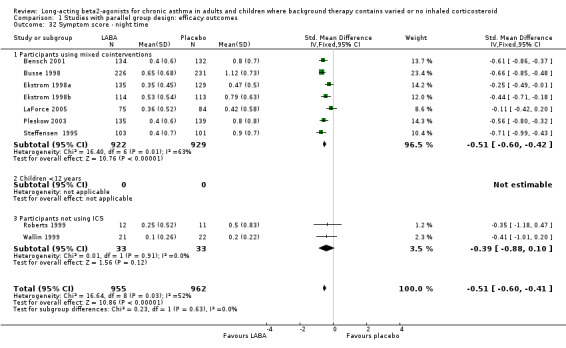
Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 32 Symptom score ‐ night time.
1.33. Analysis.

Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 33 Change in symptom score: whole day.
1.34. Analysis.
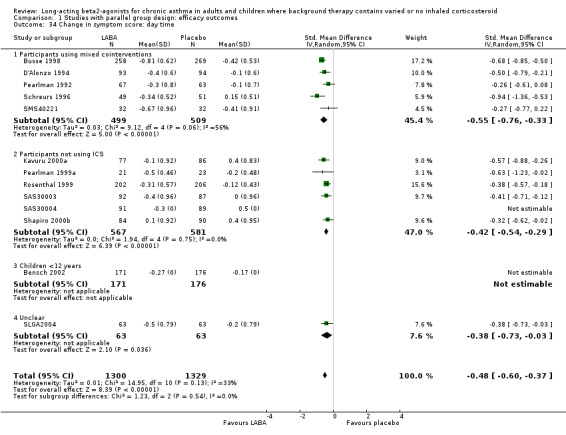
Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 34 Change in symptom score: day time.
1.35. Analysis.

Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 35 Change in symptom score: night time.
1.36. Analysis.

Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 36 Change in total symptom score.
1.37. Analysis.

Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 37 % days without rescue medication.
1.38. Analysis.

Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 38 N with <50% days free from rescue medication.
1.39. Analysis.

Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 39 % days without asthma symptoms.
1.40. Analysis.

Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 40 N with <50% symptom free days.
1.41. Analysis.
Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 41 % nighttime awakenings requiring no SABA.
1.42. Analysis.

Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 42 Change in nighttime awakenings.
1.43. Analysis.
Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 43 N with <50% symptom free nights.
1.44. Analysis.

Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 44 Change in % no nighttime awakenings.
1.45. Analysis.
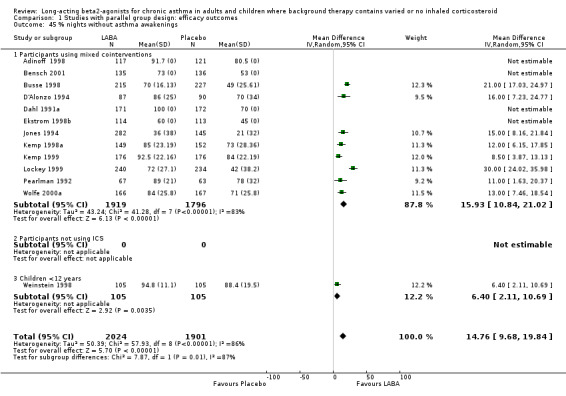
Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 45 % nights without asthma awakenings.
1.46. Analysis.
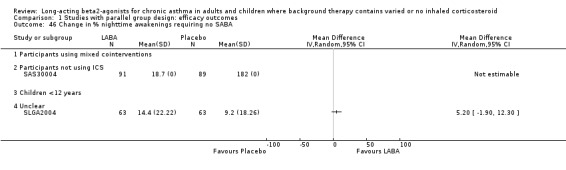
Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 46 Change in % nighttime awakenings requiring no SABA.
1.47. Analysis.
Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 47 N with <50% nights free from rescue medication.
1.48. Analysis.

Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 48 Change in % days without asthma symptoms.
1.49. Analysis.

Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 49 Change in % nights without asthma symptoms.
1.50. Analysis.

Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 50 Rescue bronchodilator use: whole day.
1.51. Analysis.

Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 51 Rescue bronchodilator use: day time.
1.52. Analysis.

Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 52 Rescue bronchodilator use: night time.
1.53. Analysis.
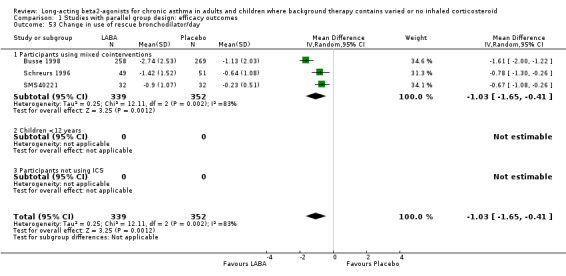
Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 53 Change in use of rescue bronchodilator/day.
1.54. Analysis.

Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 54 Change in use of rescue bronchodilator/night.
1.55. Analysis.
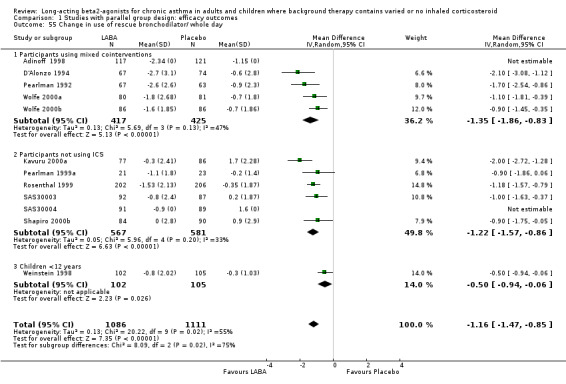
Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 55 Change in use of rescue bronchodilator/ whole day.
1.56. Analysis.

Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 56 Change in % days without rescue medication.
1.57. Analysis.

Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 57 AQOL‐ Change in Quality of life score: global.
1.58. Analysis.

Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 58 Change in Quality of life score‐ symptoms.
1.59. Analysis.

Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 59 Change in Quality of life score: emotions.
1.60. Analysis.

Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 60 Change in Quality of life score: exposure to environmental stimuli.
1.61. Analysis.

Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 61 Change in Quality of life score: activity limitations.
1.62. Analysis.
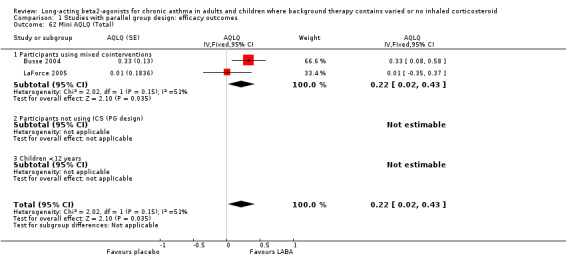
Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 62 Mini AQLQ (Total).
1.63. Analysis.

Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 63 Mini AQLQ (Symptoms).
1.64. Analysis.

Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 64 Mini AQLQ (Activity limitation).
1.65. Analysis.

Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 65 Mini AQLQ (Emotional function).
1.66. Analysis.

Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 66 Mini AQLQ (Environmental stimuli).
1.67. Analysis.

Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 67 Quality of life score: COMBINED ALL SCALES.
1.68. Analysis.

Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 68 Bronchial hyperreactivity‐ log PD20/PC20 methacholine or histamine.
1.69. Analysis.

Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 69 Bronchial hyperreactivity‐ log PD20/PC20 methacholine or histamine.
1.70. Analysis.

Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 70 Change in BHR (end treatment vs. baseline)‐ doubling doses (DD).
1.71. Analysis.
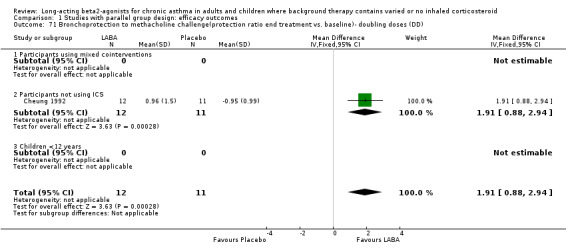
Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 71 Bronchoprotection to methacholine challenge(protection ratio end treatment vs. baseline)‐ doubling doses (DD).
1.72. Analysis.

Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 72 Bronchoprotection to methacholine challenge (protection ratio first dose treatment vs. baseline)‐ double dose.
1.73. Analysis.

Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 73 Exacerbations asthma ‐ >1 major.
1.74. Analysis.

Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 74 Exacerbations asthma ‐ >1 major(sub‐group by use of inhaled corticosteroid).
1.75. Analysis.

Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 75 Weaned from at least 1 non steroidal asthma medication.
1.76. Analysis.

Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 76 Global assessment of efficacy by patient‐ very good/good.
1.77. Analysis.

Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 77 Global assessment of efficacy by investigator‐ very good/good.
1.78. Analysis.

Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 78 Global assessment of efficacy by patient ‐ improved.
1.79. Analysis.

Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 79 Global assessment of efficacy by patient ‐ not improved.
1.80. Analysis.

Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 80 Global assessment of efficacy by investigator ‐ improved.
1.81. Analysis.

Comparison 1 Studies with parallel group design: efficacy outcomes, Outcome 81 Global assessment of efficacy by investigator ‐ not improved.
Comparison 2. Studies with parallel group design: withdrawal & safety outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death (asthma related) subgrouped by ICS at baseline | 1 | 26355 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.86 [1.19, 12.51] |
| 1.1 Participants using ICS at baseline | 1 | 12265 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.30, 5.97] |
| 1.2 Participants not using ICS at baseline | 1 | 14090 | Risk Ratio (M‐H, Fixed, 95% CI) | 18.98 [1.10, 326.01] |
| 2 Death (asthma related) subgrouped Caucasians and African Americans | 1 | 23327 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.47 [1.46, 28.68] |
| 2.1 Caucasians | 1 | 18642 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.05 [0.73, 50.30] |
| 2.2 African Americans | 1 | 4685 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.88 [0.85, 55.95] |
| 3 Death (respiratory related) | 1 | 26355 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.18 [1.07, 4.45] |
| 3.1 Participants using ICS at baseline | 1 | 12265 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.00 [0.69, 5.86] |
| 3.2 Participants not using ICS at baseline | 1 | 14090 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.33 [0.90, 6.06] |
| 4 Death (all cause) ‐ SMART non‐ICS subgroup | 3 | 14534 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.76, 2.35] |
| 4.1 Participants using mixed cointerventions | 1 | 277 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.11 [0.13, 75.68] |
| 4.2 Participants not using ICS | 1 | 14090 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.69, 2.25] |
| 4.3 Children <12 years | 1 | 167 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.04 [0.13, 73.47] |
| 5 Death (all cause) ‐ SMART all participants | 3 | 26799 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.87, 2.14] |
| 5.1 Participants using mixed cointerventions | 2 | 26632 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.85, 2.11] |
| 5.2 Participants not using ICS | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 Children <12 years | 1 | 167 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.04 [0.13, 73.47] |
| 6 SMART primary endpoint subgroups (Caucasians and African Americans) | 1 | 23327 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.49 [0.96, 2.31] |
| 6.1 Caucasians | 1 | 18642 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.62, 1.75] |
| 6.2 African Americans | 1 | 4685 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.92 [1.47, 10.43] |
| 7 Serious adverse event ‐ life threatening adverse events | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 7.1 Participants using mixed cointerventions | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Participants not using ICS | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 Children <12 years | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Serious adverse event ‐ asthma related | 3 | 895 | Odds Ratio (M‐H, Fixed, 95% CI) | 7.46 [2.21, 25.16] |
| 8.1 Participants using mixed cointerventions | 2 | 548 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.01 [0.98, 16.36] |
| 8.2 Participants not using ICS | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 Children <12 years | 1 | 347 | Odds Ratio (M‐H, Fixed, 95% CI) | 25.29 [1.48, 432.68] |
| 9 Serious adverse event related to study drug ‐ total | 4 | 973 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.11 [1.03, 4.31] |
| 9.1 Participants using mixed cointerventions | 1 | 161 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Participants not using ICS | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.3 Children <12 years | 3 | 812 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.11 [1.03, 4.31] |
| 10 Withdrawals (all reasons) | 19 | 30599 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.86, 0.96] |
| 10.1 Participants using mixed cointerventions | 10 | 28834 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.86, 0.97] |
| 10.2 Participants not using ICS | 4 | 564 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.38, 0.75] |
| 10.3 Children <12 years | 4 | 1061 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.81, 1.65] |
| 10.4 Unclear | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.37, 3.61] |
| 11 Withdrawals (adverse events) | 21 | 30943 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.93, 1.32] |
| 11.1 Participants using mixed cointerventions | 11 | 29110 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.89, 1.29] |
| 11.2 Subjects not using ICS | 5 | 674 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.31 [1.05, 5.07] |
| 11.3 Children <12 years | 4 | 1019 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.46, 1.85] |
| 11.4 Unclear | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.97 [0.17, 22.25] |
| 12 Withdrawals (asthma‐related adverse events) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12.1 Participants using mixed cointerventions | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.2 Participants not using ICS | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.4 Children <12 years | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 Withdrawals (abnormal cadiovascular test) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 13.1 Participants using mixed cointerventions | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.2 Participants not using ICS | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.3 Children <12 years | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14 Withdrawals (lack of efficacy) | 14 | 29466 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.53, 0.68] |
| 14.1 Participants using mixed cointerventions | 6 | 27883 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.54, 0.71] |
| 14.2 Participants not using ICS | 4 | 564 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.30, 0.62] |
| 14.3 Children <12 years | 4 | 1019 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.33, 1.47] |
| 15 Withdrawals (exacerbation of asthma) | 7 | 1658 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.46, 1.46] |
| 15.1 Participants using mixed cointerventions | 2 | 548 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.51, 3.10] |
| 15.2 Participants not using ICS | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.3 Children <12 years | 4 | 970 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.25, 1.29] |
| 15.4 Unclear | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.13, 7.09] |
| 16 Adverse events ‐ total | 19 | 3696 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.17 [1.02, 1.35] |
| 16.1 Participants using mixed cointerventions | 7 | 1280 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.89, 1.41] |
| 16.2 Participants not using ICS | 6 | 1008 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.94, 1.66] |
| 16.3 Children <12 years | 5 | 1268 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.96, 1.55] |
| 16.4 Unclear | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.42, 1.90] |
| 17 Adverse events ‐ any drug related | 7 | 2130 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.37 [1.01, 1.87] |
| 17.1 Participants using mixed cointerventions | 7 | 2130 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.37 [1.01, 1.87] |
| 17.2 Subjects not using ICS | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.3 Children <12 years | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Adverse events ‐ asthma related | 1 | 1041 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.60, 1.19] |
| 18.1 Participants mixed cointerventions | 1 | 1041 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.60, 1.19] |
| 18.2 Participants not using ICS | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18.3 Children <12 years | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Adverse events ‐ pharyngitis | 7 | 1419 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.94, 2.24] |
| 19.1 Participants using mixed cointerventions | 2 | 437 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.68 [0.79, 3.55] |
| 19.2 Participants not using ICS | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19.3 Children <12 years | 4 | 842 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.76, 2.25] |
| 19.4 Unclear | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.96 [0.12, 73.85] |
| 20 Adverse events ‐ cough | 13 | 2571 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.82, 1.65] |
| 20.1 Participants using mixed cointerventions | 7 | 1346 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.64, 1.86] |
| 20.2 Participants not using ICS | 2 | 203 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.26 [0.84, 12.63] |
| 20.3 Children <12 years | 3 | 882 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.64, 1.71] |
| 20.4 Unclear | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21 Adverse events ‐ nasopharyngitis | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 21.1 Participants using mixed cointerventions | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 21.2 Participants not using ICS | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 21.3 Children <12 years | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 22 Adverse events ‐ throat irritation | 8 | 1357 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.68 [1.10, 2.56] |
| 22.1 Participants using mixed cointerventions | 4 | 598 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.20 [1.17, 4.13] |
| 22.2 Subjects not using ICS | 3 | 540 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.63, 2.54] |
| 22.3 Children <12 years | 1 | 219 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.49 [0.54, 4.06] |
| 23 Adverse events ‐ upper respiratory tract infection | 12 | 2459 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.86, 1.36] |
| 23.1 Participants using mixed cointerventions | 6 | 1077 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.46, 1.01] |
| 23.2 Subjects not using ICS | 3 | 540 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.61 [0.99, 2.63] |
| 23.3 Children <12 years | 3 | 842 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.89, 1.82] |
| 24 Adverse events ‐ dyspnea | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 24.1 Participants using mixed cointerventions | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 24.2 Participants not using ICS | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 24.3 Children <12 years | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 25 Adverse events ‐ exacerbation of asthma | 5 | 1110 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.54, 1.38] |
| 25.1 Participants using mixed cointerventions | 3 | 680 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.45, 1.69] |
| 25.2 Participants not using ICS | 1 | 181 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.21 [0.13, 79.74] |
| 25.3 Children <12 years | 1 | 249 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.40, 1.58] |
| 26 Adverse events ‐ otitis media | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 26.1 Participants using mixed cointerventions | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 26.2 Participants not using ICS | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 26.3 Children <12 years | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 27 Adverse events ‐ sinus headache | 1 | 277 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.14, 7.47] |
| 27.1 Participants using mixed cointerventions | 1 | 277 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.14, 7.47] |
| 27.2 Participants not using ICS | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 27.3 Children <12 years | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 28 Adverse events ‐ pyrexia | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 28.1 Participants using mixed cointerventions | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 28.2 Participants not using ICS | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 28.3 Children <12 years | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 29 Adverse events ‐ chest pain | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 29.1 Participants using mixed cointerventions | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 29.2 Subjects not using ICS | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 29.3 Children <12 years | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 30 Adverse events ‐ abnormal cardiovascular test | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 30.1 Participants using mixed cointerventions | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 30.2 Participants not using ICS | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 30.3 Children <12 years | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 31 Adverse events ‐ palpitations | 6 | 1729 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.64, 2.89] |
| 31.1 Participants using mixed cointerventions | 6 | 1729 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.64, 2.89] |
| 31.2 Participants not using ICS | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 31.3 Children <12 years | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 32 Adverse events ‐ insomnia | 2 | 546 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.06 [0.61, 6.92] |
| 32.1 Participants using mixed cointerventions | 2 | 546 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.06 [0.61, 6.92] |
| 32.2 Participants not using ICS | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 32.3 Children <12 years | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 33 Adverse events ‐ tremor | 8 | 2257 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.86 [1.91, 7.78] |
| 33.1 Participants using mixed cointerventions | 8 | 2257 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.86 [1.91, 7.78] |
| 33.2 Participants not using ICS | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 33.3 Children <12 years | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 34 Adverse events ‐ headache | 23 | 5667 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.28 [1.04, 1.57] |
| 34.1 Participants using mixed cointerventions | 13 | 3474 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.79, 1.42] |
| 34.2 Participants not using ICS | 5 | 992 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.75 [1.12, 2.72] |
| 34.3 Children <12 years | 4 | 1061 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.95, 2.05] |
| 34.4 Unclear | 1 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.28, 6.10] |
| 35 Adverse events ‐ cramps | 3 | 892 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.56, 3.51] |
| 35.1 Participants using mixed cointerventions | 3 | 892 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.56, 3.51] |
| 35.2 Participants not using ICS | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 35.3 Children <12 years | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 36 Adverse events ‐ anxiety | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 36.1 Participants using mixed cointerventions | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 36.2 Participants not using ICS | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 36.3 Children <12 years | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 37 Adverse events ‐ nervousness | 2 | 546 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.11 [1.72, 15.22] |
| 37.1 Participants using mixed cointerventions | 2 | 546 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.11 [1.72, 15.22] |
| 37.2 Participants not using ICS | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 37.3 Children <12 years | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 38 Adverse events ‐ nausea | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 38.1 Participants using mixed cointerventions | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 38.2 Participants not using ICS | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 38.3 Children <12 years | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 39 Adverse events ‐ myalgia/fatigue | 2 | 496 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.72 [0.41, 7.24] |
| 39.1 Participants using mixed cointerventions | 1 | 277 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.24 [0.47, 38.45] |
| 39.2 Participants not using ICS | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 39.3 Children <12 years | 1 | 219 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.04, 5.60] |
| 40 Adverse events ‐ pain in limb | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 40.1 Participants using mixed cointerventions | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 40.2 Participants not using ICS | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 40.3 Children <12 years | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 41 Adverse events ‐ musculoskeletal pain | 2 | 333 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.45, 2.84] |
| 41.1 Participants using mixed cointerventions | 2 | 333 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.45, 2.84] |
| 41.2 Participants not using ICS | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 41.3 Children <12 years | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 42 Serious adverse event ‐ respiratory | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 42.1 Participants using mixed cointerventions | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 42.2 Pariticipants not using ICS | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 42.3 Children <12 years | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
2.1. Analysis.
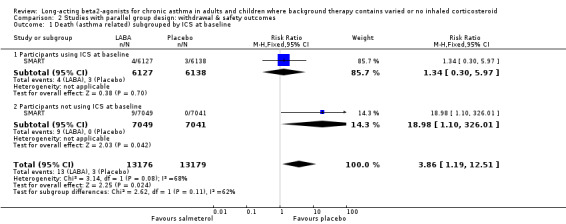
Comparison 2 Studies with parallel group design: withdrawal & safety outcomes, Outcome 1 Death (asthma related) subgrouped by ICS at baseline.
2.2. Analysis.

Comparison 2 Studies with parallel group design: withdrawal & safety outcomes, Outcome 2 Death (asthma related) subgrouped Caucasians and African Americans.
2.3. Analysis.

Comparison 2 Studies with parallel group design: withdrawal & safety outcomes, Outcome 3 Death (respiratory related).
2.4. Analysis.

Comparison 2 Studies with parallel group design: withdrawal & safety outcomes, Outcome 4 Death (all cause) ‐ SMART non‐ICS subgroup.
2.5. Analysis.

Comparison 2 Studies with parallel group design: withdrawal & safety outcomes, Outcome 5 Death (all cause) ‐ SMART all participants.
2.6. Analysis.

Comparison 2 Studies with parallel group design: withdrawal & safety outcomes, Outcome 6 SMART primary endpoint subgroups (Caucasians and African Americans).
2.7. Analysis.

Comparison 2 Studies with parallel group design: withdrawal & safety outcomes, Outcome 7 Serious adverse event ‐ life threatening adverse events.
2.8. Analysis.

Comparison 2 Studies with parallel group design: withdrawal & safety outcomes, Outcome 8 Serious adverse event ‐ asthma related.
2.9. Analysis.

Comparison 2 Studies with parallel group design: withdrawal & safety outcomes, Outcome 9 Serious adverse event related to study drug ‐ total.
2.10. Analysis.

Comparison 2 Studies with parallel group design: withdrawal & safety outcomes, Outcome 10 Withdrawals (all reasons).
2.11. Analysis.

Comparison 2 Studies with parallel group design: withdrawal & safety outcomes, Outcome 11 Withdrawals (adverse events).
2.12. Analysis.

Comparison 2 Studies with parallel group design: withdrawal & safety outcomes, Outcome 12 Withdrawals (asthma‐related adverse events).
2.13. Analysis.

Comparison 2 Studies with parallel group design: withdrawal & safety outcomes, Outcome 13 Withdrawals (abnormal cadiovascular test).
2.14. Analysis.

Comparison 2 Studies with parallel group design: withdrawal & safety outcomes, Outcome 14 Withdrawals (lack of efficacy).
2.15. Analysis.

Comparison 2 Studies with parallel group design: withdrawal & safety outcomes, Outcome 15 Withdrawals (exacerbation of asthma).
2.16. Analysis.

Comparison 2 Studies with parallel group design: withdrawal & safety outcomes, Outcome 16 Adverse events ‐ total.
2.17. Analysis.

Comparison 2 Studies with parallel group design: withdrawal & safety outcomes, Outcome 17 Adverse events ‐ any drug related.
2.18. Analysis.

Comparison 2 Studies with parallel group design: withdrawal & safety outcomes, Outcome 18 Adverse events ‐ asthma related.
2.19. Analysis.

Comparison 2 Studies with parallel group design: withdrawal & safety outcomes, Outcome 19 Adverse events ‐ pharyngitis.
2.20. Analysis.

Comparison 2 Studies with parallel group design: withdrawal & safety outcomes, Outcome 20 Adverse events ‐ cough.
2.21. Analysis.

Comparison 2 Studies with parallel group design: withdrawal & safety outcomes, Outcome 21 Adverse events ‐ nasopharyngitis.
2.22. Analysis.

Comparison 2 Studies with parallel group design: withdrawal & safety outcomes, Outcome 22 Adverse events ‐ throat irritation.
2.23. Analysis.

Comparison 2 Studies with parallel group design: withdrawal & safety outcomes, Outcome 23 Adverse events ‐ upper respiratory tract infection.
2.24. Analysis.

Comparison 2 Studies with parallel group design: withdrawal & safety outcomes, Outcome 24 Adverse events ‐ dyspnea.
2.25. Analysis.

Comparison 2 Studies with parallel group design: withdrawal & safety outcomes, Outcome 25 Adverse events ‐ exacerbation of asthma.
2.26. Analysis.

Comparison 2 Studies with parallel group design: withdrawal & safety outcomes, Outcome 26 Adverse events ‐ otitis media.
2.27. Analysis.

Comparison 2 Studies with parallel group design: withdrawal & safety outcomes, Outcome 27 Adverse events ‐ sinus headache.
2.28. Analysis.

Comparison 2 Studies with parallel group design: withdrawal & safety outcomes, Outcome 28 Adverse events ‐ pyrexia.
2.29. Analysis.

Comparison 2 Studies with parallel group design: withdrawal & safety outcomes, Outcome 29 Adverse events ‐ chest pain.
2.30. Analysis.

Comparison 2 Studies with parallel group design: withdrawal & safety outcomes, Outcome 30 Adverse events ‐ abnormal cardiovascular test.
2.31. Analysis.

Comparison 2 Studies with parallel group design: withdrawal & safety outcomes, Outcome 31 Adverse events ‐ palpitations.
2.32. Analysis.

Comparison 2 Studies with parallel group design: withdrawal & safety outcomes, Outcome 32 Adverse events ‐ insomnia.
2.33. Analysis.

Comparison 2 Studies with parallel group design: withdrawal & safety outcomes, Outcome 33 Adverse events ‐ tremor.
2.34. Analysis.

Comparison 2 Studies with parallel group design: withdrawal & safety outcomes, Outcome 34 Adverse events ‐ headache.
2.35. Analysis.

Comparison 2 Studies with parallel group design: withdrawal & safety outcomes, Outcome 35 Adverse events ‐ cramps.
2.36. Analysis.
Comparison 2 Studies with parallel group design: withdrawal & safety outcomes, Outcome 36 Adverse events ‐ anxiety.
2.37. Analysis.

Comparison 2 Studies with parallel group design: withdrawal & safety outcomes, Outcome 37 Adverse events ‐ nervousness.
2.38. Analysis.

Comparison 2 Studies with parallel group design: withdrawal & safety outcomes, Outcome 38 Adverse events ‐ nausea.
2.39. Analysis.

Comparison 2 Studies with parallel group design: withdrawal & safety outcomes, Outcome 39 Adverse events ‐ myalgia/fatigue.
2.40. Analysis.

Comparison 2 Studies with parallel group design: withdrawal & safety outcomes, Outcome 40 Adverse events ‐ pain in limb.
2.41. Analysis.

Comparison 2 Studies with parallel group design: withdrawal & safety outcomes, Outcome 41 Adverse events ‐ musculoskeletal pain.
2.42. Analysis.

Comparison 2 Studies with parallel group design: withdrawal & safety outcomes, Outcome 42 Serious adverse event ‐ respiratory.
Comparison 3. Studies by severity of asthma.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Peak expiratory flow: morning | 20 | 3682 | L/min (Fixed, 95% CI) | 15.17 [10.99, 19.36] |
| 1.1 Mild asthma | 4 | 140 | L/min (Fixed, 95% CI) | 20.74 [‐5.26, 46.73] |
| 1.2 Mild‐moderate asthma | 11 | 2410 | L/min (Fixed, 95% CI) | 16.12 [11.17, 21.07] |
| 1.3 Persistent/symptomatic | 3 | 809 | L/min (Fixed, 95% CI) | 15.04 [1.95, 28.13] |
| 1.4 Severe asthma | 0 | 0 | L/min (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.5 Unclear | 2 | 323 | L/min (Fixed, 95% CI) | 9.94 [‐0.72, 20.59] |
| 2 Peak expiratory flow: evening | 15 | 2751 | L/min (Fixed, 95% CI) | 12.29 [7.53, 17.04] |
| 2.1 Mild asthma | 3 | 79 | L/min (Fixed, 95% CI) | 35.87 [‐6.69, 78.43] |
| 2.2 Mild‐moderate asthma | 8 | 1685 | L/min (Fixed, 95% CI) | 13.29 [8.12, 18.46] |
| 2.3 Persistent/symptomatic | 2 | 661 | L/min (Fixed, 95% CI) | 3.68 [‐10.23, 17.58] |
| 2.4 Severe asthma | 0 | 0 | L/min (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.5 Unclear | 2 | 326 | L/min (Fixed, 95% CI) | 7.0 [‐22.52, 36.52] |
| 3 Change in PEF morning | 25 | 5512 | L/min (Random, 95% CI) | 24.84 [20.41, 29.27] |
| 3.1 Mild | 1 | 408 | L/min (Random, 95% CI) | 20.9 [14.62, 27.18] |
| 3.2 Mild‐moderate asthma | 9 | 2164 | L/min (Random, 95% CI) | 25.31 [16.25, 34.38] |
| 3.3 Persistent/symptomatic | 6 | 1661 | L/min (Random, 95% CI) | 26.31 [17.56, 35.06] |
| 3.4 Moderate | 2 | 274 | L/min (Random, 95% CI) | 19.11 [‐13.72, 51.93] |
| 3.5 Severe asthma | 0 | 0 | L/min (Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.6 Unclear | 7 | 1005 | L/min (Random, 95% CI) | 24.23 [18.59, 29.88] |
| 4 Change in PEF evening | 22 | 5350 | L/min (Random, 95% CI) | 16.16 [12.25, 20.07] |
| 4.1 Mild | 2 | 835 | L/min (Random, 95% CI) | 6.51 [‐5.98, 19.00] |
| 4.2 Mild‐moderate asthma | 5 | 997 | L/min (Random, 95% CI) | 17.42 [7.37, 27.47] |
| 4.3 Persistent/symptomatic | 6 | 1802 | L/min (Random, 95% CI) | 13.54 [10.19, 16.89] |
| 4.4 Moderate | 3 | 748 | L/min (Random, 95% CI) | 23.11 [2.54, 43.68] |
| 4.5 Severe asthma | 0 | 0 | L/min (Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.6 Unclear | 6 | 968 | L/min (Random, 95% CI) | 20.07 [14.13, 26.01] |
| 5 Amplitude PEF: diurnal variation (l/min or %) | 1 | 454 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.43 [‐0.62, ‐0.25] |
| 5.1 Mild‐moderate asthma | 1 | 454 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.43 [‐0.62, ‐0.25] |
| 5.2 Mild asthma | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 Severe asthma | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Change in Amplitude PEF: diurnal variation (l/min or %) | 2 | 1001 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.43 [‐0.55, ‐0.30] |
| 6.1 Mild‐moderate asthma | 2 | 1001 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.43 [‐0.55, ‐0.30] |
| 6.2 Mild asthma | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 Severe asthma | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 FEV1 | 15 | 4047 | Litres (Fixed, 95% CI) | 0.24 [0.21, 0.28] |
| 7.1 Mild | 3 | 152 | Litres (Fixed, 95% CI) | 0.10 [‐0.11, 0.30] |
| 7.2 Mild‐moderate asthma | 7 | 1932 | Litres (Fixed, 95% CI) | 0.22 [0.16, 0.29] |
| 7.3 Persistent/symptomatic | 1 | 442 | Litres (Fixed, 95% CI) | 0.12 [‐0.03, 0.27] |
| 7.4 Moderate | 0 | 0 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.5 Severe asthma | 0 | 0 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.7 Unclear | 4 | 1521 | Litres (Fixed, 95% CI) | 0.28 [0.23, 0.33] |
| 8 FEV1 predicted | 5 | 637 | % (Fixed, 95% CI) | 3.13 [0.57, 5.69] |
| 8.1 Mild | 0 | 0 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Mild‐moderate asthma | 3 | 408 | % (Fixed, 95% CI) | 3.16 [‐0.04, 6.36] |
| 8.3 Persistent/symptomatic asthma | 1 | 207 | % (Fixed, 95% CI) | 3.60 [‐0.84, 8.04] |
| 8.4 Moderate asthma | 0 | 0 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.5 Severe asthma | 0 | 0 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.6 Unclear | 1 | 22 | % (Fixed, 95% CI) | ‐3.6 [‐19.50, 12.30] |
| 9 Change in FEV (litres) | 15 | 3295 | Mean Difference (IV, Fixed, 95% CI) | 0.17 [0.14, 0.20] |
| 9.1 Mild asthma | 1 | 408 | Mean Difference (IV, Fixed, 95% CI) | 0.18 [0.08, 0.28] |
| 9.2 Mild‐moderate asthma | 2 | 505 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.00, 0.16] |
| 9.3 Persistent/symptomatic | 4 | 1336 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [0.15, 0.26] |
| 9.4 Moderate asthma | 2 | 274 | Mean Difference (IV, Fixed, 95% CI) | 0.15 [0.06, 0.24] |
| 9.5 Severe asthma | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.6 Unclear | 6 | 772 | Mean Difference (IV, Fixed, 95% CI) | 0.19 [0.12, 0.27] |
| 10 Change in FEV %predicted | 3 | 695 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.34 [0.13, 0.56] |
| 10.1 Mild‐moderate asthma | 2 | 507 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.34 [0.13, 0.56] |
| 10.2 Persistent/symptomatic asthma | 1 | 188 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.3 Severe asthma | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Forced Vital Capacity (litres) | 2 | 302 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.14, 0.32] |
| 11.1 Mild‐moderate asthma | 2 | 302 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.14, 0.32] |
| 11.2 Mild asthma | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Change in Forced Vital Capacity (litres) | 5 | 626 | Mean Difference (IV, Fixed, 95% CI) | 0.13 [0.03, 0.22] |
| 12.1 Mild‐moderate asthma | 4 | 562 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.01, 0.19] |
| 12.2 Unknown | 1 | 64 | Mean Difference (IV, Fixed, 95% CI) | 0.63 [0.26, 1.00] |
| 13 FEF25‐75 (litres/sec) | 3 | 462 | Mean Difference (IV, Fixed, 95% CI) | 0.17 [‐0.06, 0.40] |
| 13.1 Mild‐moderate asthma | 3 | 462 | Mean Difference (IV, Fixed, 95% CI) | 0.17 [‐0.06, 0.40] |
| 13.2 Mild asthma | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Symptom score‐ whole day | 4 | 905 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐0.64, ‐0.18] |
| 14.1 Mild asthma | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Mild‐moderate asthma | 3 | 873 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐0.58, ‐0.14] |
| 14.3 Moderate | 1 | 32 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.93 [‐1.67, ‐0.20] |
| 15 Symptom score ‐ day time | 11 | 1995 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.33 [‐0.41, ‐0.24] |
| 15.1 Mild asthma | 3 | 79 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.39 [‐0.84, 0.05] |
| 15.2 Mild‐moderate asthma | 1 | 264 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.28 [‐0.52, ‐0.04] |
| 15.3 Persistent/symptomatic asthma | 1 | 301 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.14 [‐0.36, 0.09] |
| 15.4 Moderate asthma | 2 | 681 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.44 [‐0.60, ‐0.29] |
| 15.5 Severe asthma | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.6 Unclear | 4 | 670 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.45, ‐0.15] |
| 16 Symptom score ‐ night time | 9 | 1917 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.48 [‐0.62, ‐0.34] |
| 16.1 Mild asthma | 2 | 66 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐0.88, 0.10] |
| 16.2 Mild‐moderate asthma | 3 | 804 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.48 [‐0.70, ‐0.25] |
| 16.3 Persistent/symptomatic asthma | 1 | 457 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐0.85, ‐0.48] |
| 16.4 Moderate asthma | 1 | 227 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐0.71, ‐0.18] |
| 16.5 Severe asthma | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.6 Unclear | 2 | 363 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐1.00, 0.18] |
| 17 Change in symptom score ‐ day time | 13 | 2629 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.48 [‐0.60, ‐0.37] |
| 17.1 Mild asthma | 1 | 408 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐0.57, ‐0.18] |
| 17.2 Mild‐moderate asthma | 2 | 226 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐1.21, ‐0.09] |
| 17.3 Persistent/symptomatic asthma | 3 | 1037 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐0.80, ‐0.50] |
| 17.4 Moderate asthma | 1 | 174 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.62, ‐0.02] |
| 17.5 Severe asthma | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.6 Unclear | 6 | 784 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐0.57, ‐0.25] |
| 18 Change in symptom score ‐ night time | 4 | 823 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐0.87, ‐0.22] |
| 18.1 Mild asthma | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.2 Mild‐moderate asthma | 2 | 226 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐1.24, ‐0.08] |
| 18.3 Moderate asthma | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.4 Persistent/symptomatic asthma | 1 | 533 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.70 [‐0.88, ‐0.53] |
| 18.5 Severe asthma | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18.6 Unclear | 1 | 64 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.50, 0.48] |
| 19 %days without asthma symptoms | 7 | 2254 | Mean Difference (IV, Random, 95% CI) | 15.77 [9.75, 21.79] |
| 19.1 Mild‐moderate asthma | 7 | 2254 | Mean Difference (IV, Random, 95% CI) | 15.77 [9.75, 21.79] |
| 19.2 Mild asthma | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19.3 Severe asthma | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20 % nights without asthma awakenings | 12 | 3687 | Mean Difference (IV, Random, 95% CI) | 16.51 [11.86, 21.15] |
| 20.1 Mild asthma | 1 | 427 | Mean Difference (IV, Random, 95% CI) | 15.00 [8.16, 21.84] |
| 20.2 Mild‐moderate asthma | 6 | 1656 | Mean Difference (IV, Random, 95% CI) | 16.32 [9.50, 23.13] |
| 20.3 Persistent/symptomatic asthma | 4 | 1427 | Mean Difference (IV, Random, 95% CI) | 17.27 [7.47, 27.07] |
| 20.4 Moderate asthma | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20.5 Severe asthma | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20.6 Unclear | 1 | 177 | Mean Difference (IV, Random, 95% CI) | 16.0 [7.23, 24.77] |
| 21 Change in %days without asthma symptoms | 9 | 2060 | Mean Difference (IV, Fixed, 95% CI) | 15.55 [12.93, 18.17] |
| 21.1 Mild asthma | 1 | 408 | Mean Difference (IV, Fixed, 95% CI) | 13.30 [7.75, 18.85] |
| 21.2 Mild‐moderate asthma | 1 | 130 | Mean Difference (IV, Fixed, 95% CI) | 17.0 [6.82, 27.18] |
| 21.3 Persistent/symptomatic asthma | 3 | 947 | Mean Difference (IV, Fixed, 95% CI) | 15.02 [11.13, 18.91] |
| 21.4 Moderate asthma | 1 | 174 | Mean Difference (IV, Fixed, 95% CI) | 10.0 [0.29, 19.71] |
| 21.5 Severe asthma | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21.6 Unclear | 3 | 401 | Mean Difference (IV, Fixed, 95% CI) | 21.31 [15.18, 27.44] |
| 22 Change in % nights without asthma symptoms | 9 | 2093 | Mean Difference (IV, Fixed, 95% CI) | 11.00 [8.67, 13.33] |
| 22.1 Mild asthma | 1 | 408 | Mean Difference (IV, Fixed, 95% CI) | 8.1 [3.91, 12.29] |
| 22.2 Mild‐moderate asthma | 1 | 130 | Mean Difference (IV, Fixed, 95% CI) | 8.0 [‐2.02, 18.02] |
| 22.3 Persistent/symptomatic asthma | 4 | 1160 | Mean Difference (IV, Fixed, 95% CI) | 13.72 [10.22, 17.21] |
| 22.4 Moderate asthma | 1 | 174 | Mean Difference (IV, Fixed, 95% CI) | 4.0 [‐5.71, 13.71] |
| 22.5 Severe asthma | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22.6 Unclear | 2 | 221 | Mean Difference (IV, Fixed, 95% CI) | 12.82 [6.58, 19.07] |
| 23 Rescue bronchodilator use: whole day | 8 | 1885 | Mean Difference (IV, Random, 95% CI) | ‐1.38 [‐2.03, ‐0.72] |
| 23.1 Mild asthma | 1 | 43 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.53, ‐0.07] |
| 23.2 Mild‐moderate asthma | 4 | 1158 | Mean Difference (IV, Random, 95% CI) | ‐1.66 [‐2.46, ‐0.86] |
| 23.3 Persistent/symptomatic asthma | 2 | 508 | Mean Difference (IV, Random, 95% CI) | ‐1.04 [‐2.31, 0.24] |
| 23.4 Moderate asthma | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23.5 Severe asthma | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23.6 Unclear | 1 | 176 | Mean Difference (IV, Random, 95% CI) | ‐2.2 [‐2.93, ‐1.47] |
| 24 Rescue bronchodilator use: day time | 5 | 1172 | Mean Difference (IV, Random, 95% CI) | ‐0.95 [‐1.65, ‐0.25] |
| 24.1 Mild asthma | 1 | 23 | Mean Difference (IV, Random, 95% CI) | ‐0.56 [‐1.67, 0.55] |
| 24.2 Mild‐moderate asthma | 2 | 491 | Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐0.80, 0.05] |
| 24.3 Persistent/symptomatic asthma | 1 | 454 | Mean Difference (IV, Random, 95% CI) | ‐1.25 [‐1.71, ‐0.79] |
| 24.4 Moderate asthma | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24.5 Severe asthma | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 24.6 Unclear | 1 | 204 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐2.53, ‐1.47] |
| 25 Rescue bronchodilator use: night time | 5 | 1176 | Mean Difference (IV, Random, 95% CI) | ‐0.56 [‐0.87, ‐0.25] |
| 25.1 Mild asthma | 1 | 23 | Mean Difference (IV, Random, 95% CI) | 0.14 [‐1.30, 1.58] |
| 25.2 Mild‐moderate asthma | 2 | 491 | Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐0.86, 0.05] |
| 25.3 Persistent/symptomatic asthma | 1 | 458 | Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐0.84, ‐0.44] |
| 25.4 Moderate asthma | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25.5 Severe asthma | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 25.6 Unclear | 1 | 204 | Mean Difference (IV, Random, 95% CI) | 1.00 [‐1.52, ‐0.48] |
| 26 Change in use of rescue bronchodilator/day | 3 | 691 | Mean Difference (IV, Random, 95% CI) | ‐0.93 [‐1.35, ‐0.52] |
| 26.1 Mild asthma | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26.2 Mild‐moderate asthma | 1 | 100 | Mean Difference (IV, Random, 95% CI) | ‐0.78 [‐1.30, ‐0.26] |
| 26.3 Persistent/symptomatic asthma | 1 | 527 | Mean Difference (IV, Random, 95% CI) | ‐1.31 [‐1.70, ‐0.92] |
| 26.4 Moderate asthma | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26.5 Severe asthma | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26.6 Unclear | 1 | 64 | Mean Difference (IV, Random, 95% CI) | ‐0.67 [‐1.08, ‐0.26] |
| 27 Change in use of rescue bronchodilator/night | 3 | 697 | Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐0.67, ‐0.25] |
| 27.1 Mild asthma | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 27.2 Mild‐moderate asthma | 2 | 633 | Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐0.72, ‐0.37] |
| 27.3 Persistent/symptomatic asthma | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 27.4 Moderate asthma | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 27.5 Severe asthma | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 27.6 Unclear | 1 | 64 | Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.56, 0.14] |
| 28 Change in use of rescue bronchodilator/ whole day | 12 | 2232 | Mean Difference (IV, Random, 95% CI) | ‐1.28 [‐1.60, ‐0.95] |
| 28.1 Mild asthma | 1 | 408 | Mean Difference (IV, Random, 95% CI) | ‐1.18 [‐1.57, ‐0.79] |
| 28.2 Mild to moderate asthma | 3 | 463 | Mean Difference (IV, Random, 95% CI) | ‐1.15 [‐1.58, ‐0.71] |
| 28.3 Persistent/symptomatic asthma | 2 | 370 | Mean Difference (IV, Random, 95% CI) | ‐1.22 [‐2.69, 0.25] |
| 28.4 Moderate asthma | 1 | 174 | Mean Difference (IV, Random, 95% CI) | ‐0.9 [‐1.75, ‐0.05] |
| 28.5 Severe asthma | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 28.6 Unclear | 5 | 817 | Mean Difference (IV, Random, 95% CI) | ‐1.50 [‐2.09, ‐0.90] |
| 29 AQOL‐ Change in Quality of life score: global | 6 | 1608 | Mean Difference (IV, Fixed, 95% CI) | 0.51 [0.42, 0.60] |
| 29.1 Mild asthma | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 29.2 Mild‐moderate asthma | 2 | 333 | Mean Difference (IV, Fixed, 95% CI) | 0.22 [‐0.01, 0.45] |
| 29.3 Persistent/symptomatic asthma | 2 | 916 | Mean Difference (IV, Fixed, 95% CI) | 0.62 [0.50, 0.73] |
| 29.4 Moderate asthma | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 29.5 Severe asthma | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 29.6 Unclear | 2 | 359 | Mean Difference (IV, Fixed, 95% CI) | 0.41 [0.22, 0.59] |
| 30 Change in Quality of life score‐ symptoms | 2 | 916 | Mean Difference (IV, Fixed, 95% CI) | 0.73 [0.58, 0.87] |
| 30.1 Mild asthma | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 30.2 Mild‐moderate asthma | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 30.3 Persistent/symptomatic asthma | 2 | 916 | Mean Difference (IV, Fixed, 95% CI) | 0.73 [0.58, 0.87] |
| 30.4 Moderate asthma | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 30.5 Severe asthma | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 30.6 Unclear | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 31 Change in Quality of life score: emotions | 2 | 916 | Mean Difference (IV, Fixed, 95% CI) | 0.66 [0.48, 0.83] |
| 31.1 Mild asthma | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 31.2 Mild‐moderate asthma | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 31.3 Persistent/symptomatic asthma | 2 | 916 | Mean Difference (IV, Fixed, 95% CI) | 0.66 [0.48, 0.83] |
| 31.4 Moderate asthma | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 31.5 Severe asthma | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 31.6 Unclear | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 32 Change in Quality of life score: exposure to environmental stimuli | 2 | 916 | Mean Difference (IV, Fixed, 95% CI) | 0.56 [0.42, 0.70] |
| 32.1 Mild asthma | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 32.2 Mild‐moderate asthma | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 32.3 Persistent/symptomatic asthma | 2 | 916 | Mean Difference (IV, Fixed, 95% CI) | 0.56 [0.42, 0.70] |
| 32.4 Moderate asthma | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 32.5 Severe asthma | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 32.6 Unclear | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 33 Change in Quality of life score: activity limitations | 2 | 916 | Mean Difference (IV, Fixed, 95% CI) | 0.41 [0.31, 0.51] |
| 33.1 Mild asthma | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 33.2 Mild‐moderate asthma | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 33.3 Persistent/symptomatic asthma | 2 | 916 | Mean Difference (IV, Fixed, 95% CI) | 0.41 [0.31, 0.51] |
| 33.4 Moderate asthma | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 33.5 Severe asthma | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 33.6 Unclear | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 34 Bronchial hyperreactivity‐ log PD20/PC20 methacholine or histamine | 8 | 689 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.00, 0.03] |
| 34.1 Mild asthma | 2 | 66 | Mean Difference (IV, Fixed, 95% CI) | 0.13 [‐0.03, 0.29] |
| 34.2 Mild‐moderate asthma | 5 | 601 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.01, 0.03] |
| 34.3 Persistent/symptomatic asthma | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 34.4 Moderate asthma | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 34.5 Severe asthma | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 34.6 Unclear | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.06, 0.26] |
| 35 Bronchodilator response to salbutamol (peak FEV1) | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 35.1 Mild‐moderate asthma | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 35.2 Mild asthma | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 35.3 Severe asthma | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 36 Exacerbations asthma (all)‐ >1 major | 25 | 7285 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.61, 0.79] |
| 36.1 Mild asthma | 2 | 835 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.44, 1.20] |
| 36.2 Mild‐moderate asthma | 10 | 3106 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.55, 0.83] |
| 36.3 Persistent/symptomatic asthma | 8 | 2408 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.63, 0.93] |
| 36.4 Moderate asthma | 1 | 181 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.28, 1.57] |
| 36.5 Severe asthma | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 36.6 Unclear | 4 | 755 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.32, 0.75] |
| 37 Global assessment of efficacy by patient‐ very good/good | 4 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 37.1 Mild asthma | 1 | 427 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.03 [1.35, 3.05] |
| 37.2 Mild‐moderate asthma | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 37.3 Persistent/symptomatic asthma | 1 | 184 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.63 [0.91, 2.94] |
| 37.4 Moderate asthma | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 37.5 Severe asthma | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 37.6 Unclear | 2 | 268 | Odds Ratio (M‐H, Fixed, 95% CI) | 7.97 [4.62, 13.74] |
3.1. Analysis.

Comparison 3 Studies by severity of asthma, Outcome 1 Peak expiratory flow: morning.
3.2. Analysis.

Comparison 3 Studies by severity of asthma, Outcome 2 Peak expiratory flow: evening.
3.3. Analysis.

Comparison 3 Studies by severity of asthma, Outcome 3 Change in PEF morning.
3.4. Analysis.

Comparison 3 Studies by severity of asthma, Outcome 4 Change in PEF evening.
3.5. Analysis.

Comparison 3 Studies by severity of asthma, Outcome 5 Amplitude PEF: diurnal variation (l/min or %).
3.6. Analysis.

Comparison 3 Studies by severity of asthma, Outcome 6 Change in Amplitude PEF: diurnal variation (l/min or %).
3.7. Analysis.

Comparison 3 Studies by severity of asthma, Outcome 7 FEV1.
3.8. Analysis.

Comparison 3 Studies by severity of asthma, Outcome 8 FEV1 predicted.
3.9. Analysis.
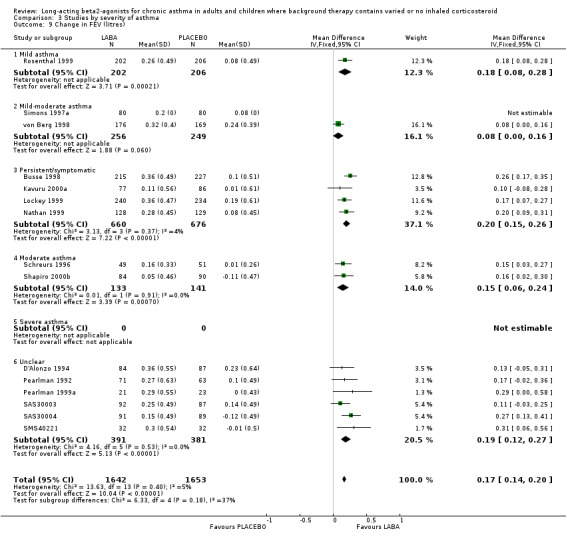
Comparison 3 Studies by severity of asthma, Outcome 9 Change in FEV (litres).
3.10. Analysis.

Comparison 3 Studies by severity of asthma, Outcome 10 Change in FEV %predicted.
3.11. Analysis.

Comparison 3 Studies by severity of asthma, Outcome 11 Forced Vital Capacity (litres).
3.12. Analysis.

Comparison 3 Studies by severity of asthma, Outcome 12 Change in Forced Vital Capacity (litres).
3.13. Analysis.

Comparison 3 Studies by severity of asthma, Outcome 13 FEF25‐75 (litres/sec).
3.14. Analysis.

Comparison 3 Studies by severity of asthma, Outcome 14 Symptom score‐ whole day.
3.15. Analysis.

Comparison 3 Studies by severity of asthma, Outcome 15 Symptom score ‐ day time.
3.16. Analysis.

Comparison 3 Studies by severity of asthma, Outcome 16 Symptom score ‐ night time.
3.17. Analysis.
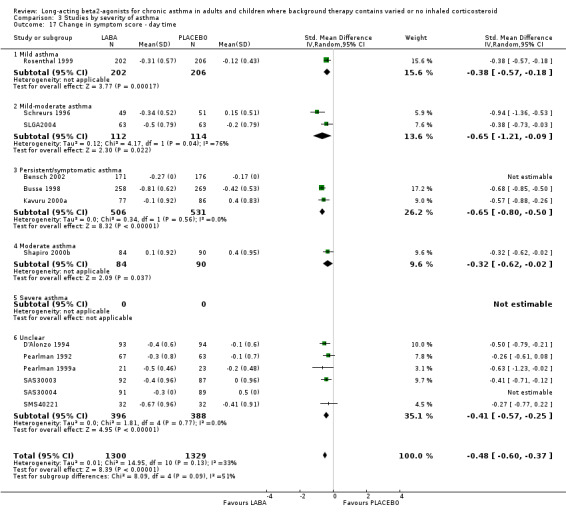
Comparison 3 Studies by severity of asthma, Outcome 17 Change in symptom score ‐ day time.
3.18. Analysis.

Comparison 3 Studies by severity of asthma, Outcome 18 Change in symptom score ‐ night time.
3.19. Analysis.

Comparison 3 Studies by severity of asthma, Outcome 19 %days without asthma symptoms.
3.20. Analysis.

Comparison 3 Studies by severity of asthma, Outcome 20 % nights without asthma awakenings.
3.21. Analysis.

Comparison 3 Studies by severity of asthma, Outcome 21 Change in %days without asthma symptoms.
3.22. Analysis.

Comparison 3 Studies by severity of asthma, Outcome 22 Change in % nights without asthma symptoms.
3.23. Analysis.

Comparison 3 Studies by severity of asthma, Outcome 23 Rescue bronchodilator use: whole day.
3.24. Analysis.

Comparison 3 Studies by severity of asthma, Outcome 24 Rescue bronchodilator use: day time.
3.25. Analysis.

Comparison 3 Studies by severity of asthma, Outcome 25 Rescue bronchodilator use: night time.
3.26. Analysis.

Comparison 3 Studies by severity of asthma, Outcome 26 Change in use of rescue bronchodilator/day.
3.27. Analysis.

Comparison 3 Studies by severity of asthma, Outcome 27 Change in use of rescue bronchodilator/night.
3.28. Analysis.

Comparison 3 Studies by severity of asthma, Outcome 28 Change in use of rescue bronchodilator/ whole day.
3.29. Analysis.
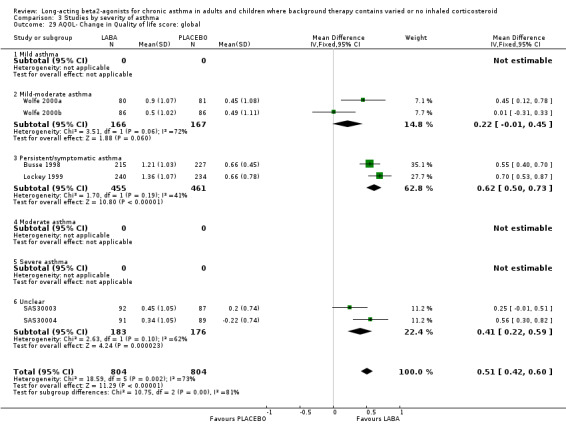
Comparison 3 Studies by severity of asthma, Outcome 29 AQOL‐ Change in Quality of life score: global.
3.30. Analysis.

Comparison 3 Studies by severity of asthma, Outcome 30 Change in Quality of life score‐ symptoms.
3.31. Analysis.

Comparison 3 Studies by severity of asthma, Outcome 31 Change in Quality of life score: emotions.
3.32. Analysis.

Comparison 3 Studies by severity of asthma, Outcome 32 Change in Quality of life score: exposure to environmental stimuli.
3.33. Analysis.
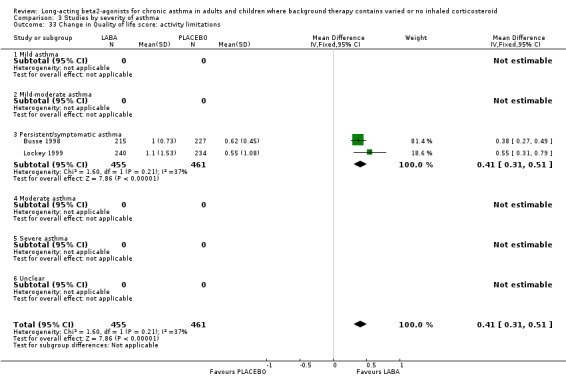
Comparison 3 Studies by severity of asthma, Outcome 33 Change in Quality of life score: activity limitations.
3.34. Analysis.

Comparison 3 Studies by severity of asthma, Outcome 34 Bronchial hyperreactivity‐ log PD20/PC20 methacholine or histamine.
3.36. Analysis.

Comparison 3 Studies by severity of asthma, Outcome 36 Exacerbations asthma (all)‐ >1 major.
3.37. Analysis.

Comparison 3 Studies by severity of asthma, Outcome 37 Global assessment of efficacy by patient‐ very good/good.
Comparison 4. Studies with crossover design.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Peak expiratory flow: morning | 5 | L/min (Fixed, 95% CI) | 38.20 [3.10, 73.29] | |
| 2 Peak expiratory flow: evening | 5 | L/min (Fixed, 95% CI) | 35.01 [‐0.78, 70.80] | |
| 3 Change in PEF morning predicted | 1 | % (Fixed, 95% CI) | 2.0 [‐1.92, 5.92] | |
| 4 Change in PEF evening predicted | 1 | % (Fixed, 95% CI) | Totals not selected | |
| 5 Change in PEF morning | 2 | L/min (Fixed, 95% CI) | 12.07 [1.75, 22.38] | |
| 6 Change in PEF evening | 2 | L/min (Fixed, 95% CI) | 5.61 [‐4.97, 16.19] | |
| 7 Amplitude PEF: diurnal variation (l/min or %) | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.2 CROSS OVER STUDIES | 1 | 56 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Change in Amplitude PEF: diurnal variation (l/min or %) | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 CROSS OVER STUDIES | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 FEV1 | 7 | Litres (Fixed, 95% CI) | 0.13 [0.01, 0.25] | |
| 10 Predicted FEV1 | 3 | % (Fixed, 95% CI) | 1.82 [‐2.86, 6.51] | |
| 11 PD20 treatment ratio | 1 | Doubling doses (Fixed, 95% CI) | 1.01 [0.36, 1.66] | |
| 12 Forced Vital Capacity (litres) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 12.1 CROSS OVER STUDIES | 2 | 790 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Change in symptom score‐ day time | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.1 CROSS OVER STUDIES | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14 Change in symptom score‐ night time | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 14.2 CROSS OVER STUDIES | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15 Days without asthma symptoms | 3 | % (Fixed, 95% CI) | 5.25 [1.30, 9.20] | |
| 16 Nights without asthma awakenings | 2 | % (Fixed, 95% CI) | 33.68 [8.96, 58.39] | |
| 17 Rescue bronchodilator use: whole day | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 17.1 CROSS OVER STUDIES | 1 | 734 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Change in use of rescue bronchodilator/day | 1 | puffs (Fixed, 95% CI) | Totals not selected | |
| 19 Change in use of rescue bronchodilator/night | 1 | puffs (Fixed, 95% CI) | Totals not selected | |
| 20 Bronchial hyperreactivity‐ log PD20/PC20 methacholine or histamine | 1 | 20 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.94, 0.81] |
| 20.2 CROSS OVER STUDIES | 1 | 20 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.94, 0.81] |
| 21 Change in BHR (end treatment vs. baseline)‐ doubling doses (DD) | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 0.77 [‐0.05, 1.59] |
| 21.1 Crossover studies | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 0.77 [‐0.05, 1.59] |
| 22 Bronchoprotection to methacholine challenge(protection ratio end treatment vs. baseline)‐ doubling doses (DD) | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 1.76 [0.81, 2.71] |
| 22.1 CROSS OVER STUDIES | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 1.76 [0.81, 2.71] |
| 23 Bronchoprotection to methacholine challenge(protection ratio first dose treatment vs. baseline)‐ DD | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 2.95 [2.15, 3.75] |
| 23.1 CROSS OVER STUDIES | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 2.95 [2.15, 3.75] |
| 24 Bronchodilator response to eformoterol (delta peak FEV1) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 24.1 CROSS OVER STUDIES | 2 | 46 | Mean Difference (IV, Fixed, 95% CI) | ‐0.26 [‐0.43, ‐0.09] |
| 25 Adverse events‐ total adverse events | 2 | 739 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.77, 1.38] |
| 26 Days with no rescue medication usage | 1 | % (Fixed, 95% CI) | Totals not selected | |
| 27 Adverse events‐ headache | 3 | 921 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.56, 1.33] |
| 27.2 CROSS OVER STUDIES | 3 | 921 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.56, 1.33] |
| 28 Adverse events‐ tremor | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 28.1 CROSS OVER STUDIES | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 29 Adverse events‐ cough | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 30 Exacerbations asthma‐ >1 major(sub‐group by use of inhaled corticosteroid) | 3 | 550 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.70, 1.67] |
| 30.4 Cross over studies | 3 | 550 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.70, 1.67] |
| 31 Rate of exacerbations asthma (number/patient/year) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 31.1 Major exacerbations | 1 | 314 | Mean Difference (IV, Fixed, 95% CI) | ‐0.18 [‐0.38, 0.02] |
| 31.2 Minor exacerbations | 1 | 314 | Mean Difference (IV, Fixed, 95% CI) | ‐0.68 [‐0.95, ‐0.41] |
| 32 Adverse events ‐ upper respiratory tract infection | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 33 Adverse events ‐ musculoskeletal pain | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 34 Global assessment of efficacy by patient‐ very good/good | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 34.2 CROSS OVER STUDIES | 1 | 56 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.5 [1.23, 16.45] |
| 35 Global assessment of efficacy by investigator‐ very good/good | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 35.2 CROSS OVER STUDIES | 1 | 56 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.33 [0.78, 14.23] |
| 36 Adverse events ‐ throat irritation | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 37 Rescue medication usage (blisters) | 1 | blisters/d (Fixed, 95% CI) | Totals not selected | |
| 38 Fall in FEV1post exercise(6‐9 hrs post study drug) % or % predicted | 1 | 24 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.51 [‐1.32, 0.31] |
| 38.2 CROSS OVER STUDIES | 1 | 24 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.51 [‐1.32, 0.31] |
| 39 Nights without symptoms | 1 | % (Fixed, 95% CI) | 4.0 [1.00, 7.00] | |
| 40 Change from baseline PD15 | 1 | Doubling doses (Fixed, 95% CI) | 2.67 [2.22, 3.12] |
4.1. Analysis.

Comparison 4 Studies with crossover design, Outcome 1 Peak expiratory flow: morning.
4.2. Analysis.

Comparison 4 Studies with crossover design, Outcome 2 Peak expiratory flow: evening.
4.3. Analysis.

Comparison 4 Studies with crossover design, Outcome 3 Change in PEF morning predicted.
4.4. Analysis.

Comparison 4 Studies with crossover design, Outcome 4 Change in PEF evening predicted.
4.5. Analysis.

Comparison 4 Studies with crossover design, Outcome 5 Change in PEF morning.
4.6. Analysis.

Comparison 4 Studies with crossover design, Outcome 6 Change in PEF evening.
4.7. Analysis.

Comparison 4 Studies with crossover design, Outcome 7 Amplitude PEF: diurnal variation (l/min or %).
4.9. Analysis.

Comparison 4 Studies with crossover design, Outcome 9 FEV1.
4.10. Analysis.
Comparison 4 Studies with crossover design, Outcome 10 Predicted FEV1.
4.11. Analysis.

Comparison 4 Studies with crossover design, Outcome 11 PD20 treatment ratio.
4.12. Analysis.

Comparison 4 Studies with crossover design, Outcome 12 Forced Vital Capacity (litres).
4.13. Analysis.

Comparison 4 Studies with crossover design, Outcome 13 Change in symptom score‐ day time.
4.14. Analysis.

Comparison 4 Studies with crossover design, Outcome 14 Change in symptom score‐ night time.
4.15. Analysis.

Comparison 4 Studies with crossover design, Outcome 15 Days without asthma symptoms.
4.16. Analysis.

Comparison 4 Studies with crossover design, Outcome 16 Nights without asthma awakenings.
4.17. Analysis.
Comparison 4 Studies with crossover design, Outcome 17 Rescue bronchodilator use: whole day.
4.18. Analysis.

Comparison 4 Studies with crossover design, Outcome 18 Change in use of rescue bronchodilator/day.
4.19. Analysis.
Comparison 4 Studies with crossover design, Outcome 19 Change in use of rescue bronchodilator/night.
4.20. Analysis.

Comparison 4 Studies with crossover design, Outcome 20 Bronchial hyperreactivity‐ log PD20/PC20 methacholine or histamine.
4.21. Analysis.

Comparison 4 Studies with crossover design, Outcome 21 Change in BHR (end treatment vs. baseline)‐ doubling doses (DD).
4.22. Analysis.

Comparison 4 Studies with crossover design, Outcome 22 Bronchoprotection to methacholine challenge(protection ratio end treatment vs. baseline)‐ doubling doses (DD).
4.23. Analysis.

Comparison 4 Studies with crossover design, Outcome 23 Bronchoprotection to methacholine challenge(protection ratio first dose treatment vs. baseline)‐ DD.
4.24. Analysis.
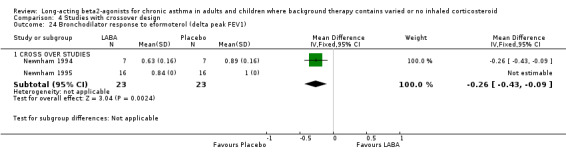
Comparison 4 Studies with crossover design, Outcome 24 Bronchodilator response to eformoterol (delta peak FEV1).
4.25. Analysis.

Comparison 4 Studies with crossover design, Outcome 25 Adverse events‐ total adverse events.
4.26. Analysis.

Comparison 4 Studies with crossover design, Outcome 26 Days with no rescue medication usage.
4.27. Analysis.

Comparison 4 Studies with crossover design, Outcome 27 Adverse events‐ headache.
4.28. Analysis.
Comparison 4 Studies with crossover design, Outcome 28 Adverse events‐ tremor.
4.29. Analysis.

Comparison 4 Studies with crossover design, Outcome 29 Adverse events‐ cough.
4.30. Analysis.

Comparison 4 Studies with crossover design, Outcome 30 Exacerbations asthma‐ >1 major(sub‐group by use of inhaled corticosteroid).
4.31. Analysis.

Comparison 4 Studies with crossover design, Outcome 31 Rate of exacerbations asthma (number/patient/year).
4.32. Analysis.

Comparison 4 Studies with crossover design, Outcome 32 Adverse events ‐ upper respiratory tract infection.
4.33. Analysis.
Comparison 4 Studies with crossover design, Outcome 33 Adverse events ‐ musculoskeletal pain.
4.34. Analysis.

Comparison 4 Studies with crossover design, Outcome 34 Global assessment of efficacy by patient‐ very good/good.
4.35. Analysis.

Comparison 4 Studies with crossover design, Outcome 35 Global assessment of efficacy by investigator‐ very good/good.
4.36. Analysis.

Comparison 4 Studies with crossover design, Outcome 36 Adverse events ‐ throat irritation.
4.37. Analysis.
Comparison 4 Studies with crossover design, Outcome 37 Rescue medication usage (blisters).
4.38. Analysis.

Comparison 4 Studies with crossover design, Outcome 38 Fall in FEV1post exercise(6‐9 hrs post study drug) % or % predicted.
4.39. Analysis.

Comparison 4 Studies with crossover design, Outcome 39 Nights without symptoms.
4.40. Analysis.

Comparison 4 Studies with crossover design, Outcome 40 Change from baseline PD15.
Comparison 5. Imputed standard deviations.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in PEF morning | 25 | 5512 | L/min (Random, 95% CI) | 24.84 [20.41, 29.27] |
| 1.1 Participants using mixed cointerventions | 12 | 2739 | L/min (Random, 95% CI) | 28.99 [22.63, 35.35] |
| 1.2 Participants not using ICS | 8 | 1582 | L/min (Random, 95% CI) | 23.57 [14.60, 32.55] |
| 1.3 Children <12 years | 4 | 1065 | L/min (Random, 95% CI) | 16.32 [12.32, 20.31] |
| 1.4 Unclear | 1 | 126 | L/min (Random, 95% CI) | 30.00 [15.87, 44.13] |
| 2 Peak expiratory flow: morning | 20 | 3682 | L/min (Fixed, 95% CI) | 15.17 [10.99, 19.36] |
| 2.1 Participants using mixed cointerventions | 11 | 2826 | L/min (Fixed, 95% CI) | 27.66 [20.40, 34.91] |
| 2.3 Participants not using ICS | 6 | 235 | L/min (Fixed, 95% CI) | 23.08 [4.74, 41.41] |
| 2.4 Children <12 years | 3 | 621 | L/min (Fixed, 95% CI) | 7.73 [2.39, 13.07] |
| 3 FEV1 | 15 | 4047 | Litres (Fixed, 95% CI) | 0.24 [0.21, 0.28] |
| 3.1 Participants using mixed cointerventions | 11 | 3458 | Litres (Fixed, 95% CI) | 0.25 [0.21, 0.29] |
| 3.2 Participants not using ICS | 2 | 70 | Litres (Fixed, 95% CI) | 0.12 [‐0.24, 0.48] |
| 3.3 Children <12 years | 2 | 519 | Litres (Fixed, 95% CI) | 0.19 [0.06, 0.32] |
| 4 Change in PEF evening | 22 | 5350 | L/min (Random, 95% CI) | 16.16 [12.25, 20.07] |
| 4.1 Participants using mixed cointerventions | 12 | 2980 | L/min (Random, 95% CI) | 18.67 [12.41, 24.93] |
| 4.2 Participants not using ICS (PG design) | 5 | 1181 | L/min (Random, 95% CI) | 10.68 [6.38, 14.98] |
| 4.3 Children <12 years | 4 | 1063 | L/min (Random, 95% CI) | 17.46 [7.69, 27.22] |
| 4.4 Unclear | 1 | 126 | L/min (Random, 95% CI) | 17.0 [5.02, 28.98] |
| 5 Change in FEV (litres) | 15 | 3295 | Mean Difference (IV, Fixed, 95% CI) | 0.17 [0.14, 0.20] |
| 5.1 Participants using mixed cointerventions | 7 | 1565 | Mean Difference (IV, Fixed, 95% CI) | 0.21 [0.16, 0.25] |
| 5.2 Participants not using ICS | 6 | 1225 | Mean Difference (IV, Fixed, 95% CI) | 0.17 [0.11, 0.22] |
| 5.3 Children <12 years | 2 | 505 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.00, 0.16] |
| 6 Change in FEV %predicted | 3 | 693 | Mean Difference (IV, Fixed, 95% CI) | 4.10 [2.22, 5.97] |
| 6.1 Participants using mixed cointerventions | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Participants not using ICS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 Children <12 years | 3 | 693 | Mean Difference (IV, Fixed, 95% CI) | 4.10 [2.22, 5.97] |
| 7 Change in % days without asthma symptoms | 9 | 2060 | Mean Difference (IV, Random, 95% CI) | 16.10 [13.64, 18.55] |
| 7.1 Participants using mixed cointerventions | 3 | 834 | Mean Difference (IV, Random, 95% CI) | 16.37 [12.65, 20.09] |
| 7.2 Participants not using ICS | 6 | 1226 | Mean Difference (IV, Random, 95% CI) | 16.02 [11.83, 20.22] |
| 7.3 Children <12 years | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Change in % nights without asthma symptoms | 9 | 2093 | Mean Difference (IV, Random, 95% CI) | 10.79 [6.48, 15.10] |
| 8.1 Participants using mixed cointerventions | 3 | 840 | Mean Difference (IV, Random, 95% CI) | 17.00 [9.27, 24.73] |
| 8.2 Children <12 years | 1 | 207 | Mean Difference (IV, Random, 95% CI) | 5.00 [‐0.83, 10.83] |
| 8.4 Participants not using ICS | 5 | 1046 | Mean Difference (IV, Random, 95% CI) | 8.64 [5.73, 11.55] |
| 9 Change in use of rescue bronchodilator/day | 3 | 691 | Mean Difference (IV, Random, 95% CI) | ‐1.03 [‐1.65, ‐0.41] |
| 9.1 Participants using mixed cointerventions | 3 | 691 | Mean Difference (IV, Random, 95% CI) | ‐1.03 [‐1.65, ‐0.41] |
| 9.2 Participants not using ICS | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.3 Children <12 years | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Peak expiratory flow: evening l/min | 11 | 2152 | Mean Difference (IV, Fixed, 95% CI) | 22.09 [13.92, 30.26] |
| 10.1 Participants using mixed cointerventions | 7 | 1866 | Mean Difference (IV, Fixed, 95% CI) | 23.46 [14.45, 32.46] |
| 10.2 Participants not using ICS | 3 | 79 | Mean Difference (IV, Fixed, 95% CI) | 35.80 [‐6.78, 78.37] |
| 10.3 Children <12 years | 1 | 207 | Mean Difference (IV, Fixed, 95% CI) | 10.5 [‐11.30, 32.30] |
| 11 Rescue bronchodilator use: whole day | 8 | 1885 | Mean Difference (IV, Random, 95% CI) | ‐1.38 [‐2.03, ‐0.72] |
| 11.1 Participants using mixed cointerventions | 6 | 1635 | Mean Difference (IV, Random, 95% CI) | ‐1.75 [‐2.28, ‐1.23] |
| 11.2 Participants not using ICS | 1 | 43 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.53, ‐0.07] |
| 11.3 Children <12 years | 1 | 207 | Mean Difference (IV, Random, 95% CI) | ‐0.4 [‐0.84, 0.04] |
| 12 Change in use of rescue bronchodilator/night | 2 | 633 | Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐0.72, ‐0.36] |
| 12.1 Participants using mixed cointerventions | 2 | 633 | Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐0.72, ‐0.36] |
| 12.2 Participants not using ICS | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.3 Children <12 years | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Change in use of rescue bronchodilator/ whole day | 12 | 2197 | Mean Difference (IV, Random, 95% CI) | ‐1.29 [‐1.62, ‐0.96] |
| 13.1 Participants using mixed cointerventions | 5 | 842 | Mean Difference (IV, Random, 95% CI) | ‐1.29 [‐1.67, ‐0.90] |
| 13.2 Participants not using ICS | 6 | 1148 | Mean Difference (IV, Random, 95% CI) | ‐1.42 [‐1.92, ‐0.92] |
| 13.3 Children <12 years | 1 | 207 | Mean Difference (IV, Random, 95% CI) | ‐0.5 [‐0.94, ‐0.06] |
| 14 FEV1 predicted | 4 | 615 | % (Fixed, 95% CI) | 3.32 [0.73, 5.91] |
| 14.1 Participants using mixed cointerventions | 0 | 0 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Participants not using ICS | 1 | 22 | % (Fixed, 95% CI) | ‐3.6 [‐19.50, 12.30] |
| 14.3 Children <12 years | 3 | 593 | % (Fixed, 95% CI) | 3.51 [0.88, 6.13] |
| 15 Peak expiratory flow: evening | 15 | 2751 | L/min (Fixed, 95% CI) | 12.29 [7.53, 17.04] |
| 15.1 Participants using mixed cointerventions | 8 | 2027 | L/min (Fixed, 95% CI) | 22.38 [13.77, 30.99] |
| 15.2 Participants not using ICS | 4 | 103 | L/min (Fixed, 95% CI) | 18.32 [2.25, 34.38] |
| 15.3 Children <12 years | 3 | 621 | L/min (Fixed, 95% CI) | 6.35 [0.26, 12.45] |
| 16 AQOL‐ Change in Quality of life score: global | 6 | 1608 | Mean Difference (IV, Fixed, 95% CI) | 0.51 [0.42, 0.60] |
| 16.1 Participants using mixed cointerventions | 4 | 1249 | Mean Difference (IV, Fixed, 95% CI) | 0.54 [0.44, 0.64] |
| 16.2 Participants not using ICS | 2 | 359 | Mean Difference (IV, Fixed, 95% CI) | 0.41 [0.22, 0.59] |
| 16.3 Children <12 years | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17 % nights without asthma awakenings | 13 | 3925 | Mean Difference (IV, Random, 95% CI) | 16.11 [11.74, 20.49] |
| 17.1 Participants using mixed cointerventions | 12 | 3715 | Mean Difference (IV, Random, 95% CI) | 17.02 [12.76, 21.29] |
| 17.2 Participants not using ICS | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.3 Children <12 years | 1 | 210 | Mean Difference (IV, Random, 95% CI) | 6.40 [2.11, 10.69] |
5.1. Analysis.

Comparison 5 Imputed standard deviations, Outcome 1 Change in PEF morning.
5.2. Analysis.
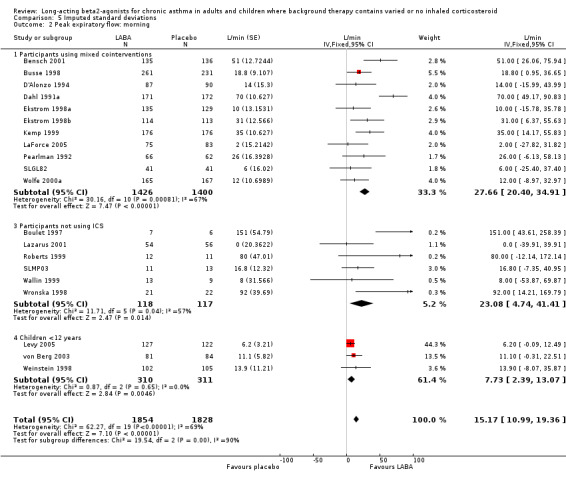
Comparison 5 Imputed standard deviations, Outcome 2 Peak expiratory flow: morning.
5.3. Analysis.
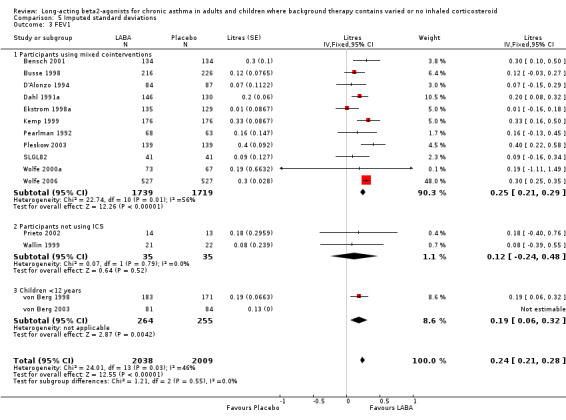
Comparison 5 Imputed standard deviations, Outcome 3 FEV1.
5.4. Analysis.

Comparison 5 Imputed standard deviations, Outcome 4 Change in PEF evening.
5.5. Analysis.

Comparison 5 Imputed standard deviations, Outcome 5 Change in FEV (litres).
5.6. Analysis.

Comparison 5 Imputed standard deviations, Outcome 6 Change in FEV %predicted.
5.7. Analysis.

Comparison 5 Imputed standard deviations, Outcome 7 Change in % days without asthma symptoms.
5.8. Analysis.

Comparison 5 Imputed standard deviations, Outcome 8 Change in % nights without asthma symptoms.
5.9. Analysis.

Comparison 5 Imputed standard deviations, Outcome 9 Change in use of rescue bronchodilator/day.
5.10. Analysis.

Comparison 5 Imputed standard deviations, Outcome 10 Peak expiratory flow: evening l/min.
5.11. Analysis.
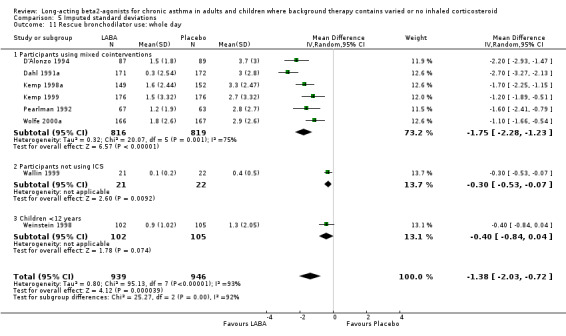
Comparison 5 Imputed standard deviations, Outcome 11 Rescue bronchodilator use: whole day.
5.12. Analysis.

Comparison 5 Imputed standard deviations, Outcome 12 Change in use of rescue bronchodilator/night.
5.13. Analysis.

Comparison 5 Imputed standard deviations, Outcome 13 Change in use of rescue bronchodilator/ whole day.
5.14. Analysis.

Comparison 5 Imputed standard deviations, Outcome 14 FEV1 predicted.
5.15. Analysis.

Comparison 5 Imputed standard deviations, Outcome 15 Peak expiratory flow: evening.
5.16. Analysis.

Comparison 5 Imputed standard deviations, Outcome 16 AQOL‐ Change in Quality of life score: global.
5.17. Analysis.

Comparison 5 Imputed standard deviations, Outcome 17 % nights without asthma awakenings.
Comparison 6. WMD archive.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Peak expiratory flow: morning l/min | 17 | 3244 | Mean Difference (IV, Fixed, 95% CI) | 21.04 [13.96, 28.13] |
| 1.1 Participants using mixed cointerventions | 11 | 2826 | Mean Difference (IV, Fixed, 95% CI) | 21.81 [14.07, 29.55] |
| 1.2 Participants not using ICS | 5 | 211 | Mean Difference (IV, Fixed, 95% CI) | 22.79 [‐6.42, 52.00] |
| 1.3 Children <12 years | 1 | 207 | Mean Difference (IV, Fixed, 95% CI) | 13.90 [‐8.08, 35.88] |
| 2 FEV1 (litres) | 11 | 2655 | Mean Difference (IV, Fixed, 95% CI) | 0.15 [0.09, 0.21] |
| 2.1 Participants using mixed cointerventions | 9 | 2136 | Mean Difference (IV, Fixed, 95% CI) | 0.14 [0.08, 0.21] |
| 2.2 Participants not using ICS | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.5 Children <12 years | 2 | 519 | Mean Difference (IV, Fixed, 95% CI) | 0.19 [0.06, 0.32] |
| 3 Peak expiratory flow: evening l/min | 13 | 2395 | Mean Difference (IV, Fixed, 95% CI) | 19.76 [12.15, 27.37] |
| 3.1 Participants using mixed cointerventions | 9 | 2109 | Mean Difference (IV, Fixed, 95% CI) | 20.49 [12.21, 28.76] |
| 3.2 Participants not using ICS | 3 | 79 | Mean Difference (IV, Fixed, 95% CI) | 35.87 [‐6.69, 78.43] |
| 3.3 Children <12 years | 1 | 207 | Mean Difference (IV, Fixed, 95% CI) | 10.5 [‐11.30, 32.30] |
| 4 Bronchial hyperreactivity‐ log PD20/PC20 methacholine or histamine | 7 | 549 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.15 [‐0.05, 0.36] |
| 4.1 Participants using mixed cointerventions | 2 | 269 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐0.19, 0.29] |
| 4.2 Participants not using ICS | 3 | 88 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.49 [‐0.01, 0.98] |
| 4.3 Children <12 years | 2 | 192 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.39 [‐0.31, 1.09] |
| 5 % Predicted FEV1 | 4 | 615 | Mean Difference (IV, Fixed, 95% CI) | 3.32 [0.73, 5.91] |
| 5.1 Participants using mixed cointerventions | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Participants not using ICS | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐3.60 [‐19.50, 12.30] |
| 5.3 Children <12 years | 3 | 593 | Mean Difference (IV, Fixed, 95% CI) | 3.51 [0.88, 6.14] |
| 6 Change in PEF morning (l/min) | 25 | 5561 | Mean Difference (IV, Random, 95% CI) | 24.64 [19.29, 29.99] |
| 6.1 Participants using mixed cointerventions | 12 | 2788 | Mean Difference (IV, Random, 95% CI) | 27.30 [19.72, 34.87] |
| 6.2 Participants not using ICS | 8 | 1582 | Mean Difference (IV, Random, 95% CI) | 24.24 [14.23, 34.24] |
| 6.3 Children <12 years | 4 | 1065 | Mean Difference (IV, Random, 95% CI) | 14.33 [8.64, 20.01] |
| 6.4 Unclear | 1 | 126 | Mean Difference (IV, Random, 95% CI) | 30.00 [15.87, 44.13] |
| 7 Change in PEF evening (l/min) | 21 | 5149 | Mean Difference (IV, Random, 95% CI) | 17.67 [12.62, 22.72] |
| 7.1 Participants using mixed cointerventions | 11 | 2779 | Mean Difference (IV, Random, 95% CI) | 21.92 [14.25, 29.59] |
| 7.2 Participants not using ICS | 5 | 1181 | Mean Difference (IV, Random, 95% CI) | 10.68 [6.37, 14.98] |
| 7.3 Children <12 years | 4 | 1063 | Mean Difference (IV, Random, 95% CI) | 23.04 [‐3.54, 49.61] |
| 7.4 Unclear | 1 | 126 | Mean Difference (IV, Random, 95% CI) | 17.0 [5.02, 28.98] |
| 8 Peak expiratory flow: morning l/min (crossover studies) | 5 | 878 | Mean Difference (IV, Fixed, 95% CI) | 43.68 [5.54, 81.82] |
| 8.1 CROSS OVER STUDIES | 5 | 878 | Mean Difference (IV, Fixed, 95% CI) | 43.68 [5.54, 81.82] |
| 9 Peak expiratory flow: evening l/min (crossover studies) | 5 | 878 | Mean Difference (IV, Fixed, 95% CI) | 39.46 [‐0.70, 79.63] |
| 9.1 CROSS OVER STUDIES | 5 | 878 | Mean Difference (IV, Fixed, 95% CI) | 39.46 [‐0.70, 79.63] |
| 10 FEV1 (litres; crossover studies) | 6 | 902 | Mean Difference (IV, Fixed, 95% CI) | 0.19 [0.02, 0.35] |
| 10.4 CROSS OVER STUDIES | 6 | 902 | Mean Difference (IV, Fixed, 95% CI) | 0.19 [0.02, 0.35] |
| 11 AUC‐ mean area under 12 hr serial FEV1 curve (L‐h) | 6 | 1063 | Mean Difference (IV, Fixed, 95% CI) | 3.49 [2.75, 4.23] |
| 11.1 Participants using mixed cointerventions | 4 | 726 | Mean Difference (IV, Fixed, 95% CI) | 3.79 [2.98, 4.60] |
| 11.2 Participants not on ICS | 2 | 337 | Mean Difference (IV, Fixed, 95% CI) | 2.01 [0.21, 3.81] |
| 11.3 Children <12 years | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Mini‐AQLQ (total score) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12.1 Participants using mixed cointerventions | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.2 Participants not using ICS | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.3 Children <12 years | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
6.1. Analysis.

Comparison 6 WMD archive, Outcome 1 Peak expiratory flow: morning l/min.
6.2. Analysis.

Comparison 6 WMD archive, Outcome 2 FEV1 (litres).
6.3. Analysis.

Comparison 6 WMD archive, Outcome 3 Peak expiratory flow: evening l/min.
6.4. Analysis.
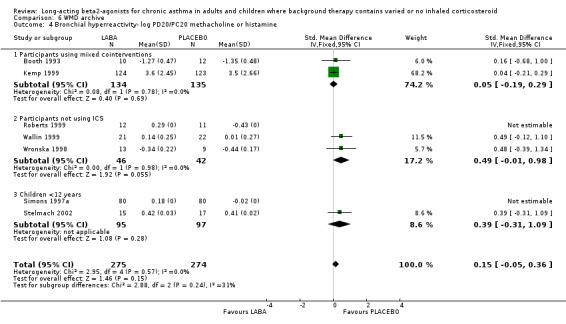
Comparison 6 WMD archive, Outcome 4 Bronchial hyperreactivity‐ log PD20/PC20 methacholine or histamine.
6.5. Analysis.

Comparison 6 WMD archive, Outcome 5 % Predicted FEV1.
6.6. Analysis.

Comparison 6 WMD archive, Outcome 6 Change in PEF morning (l/min).
6.7. Analysis.
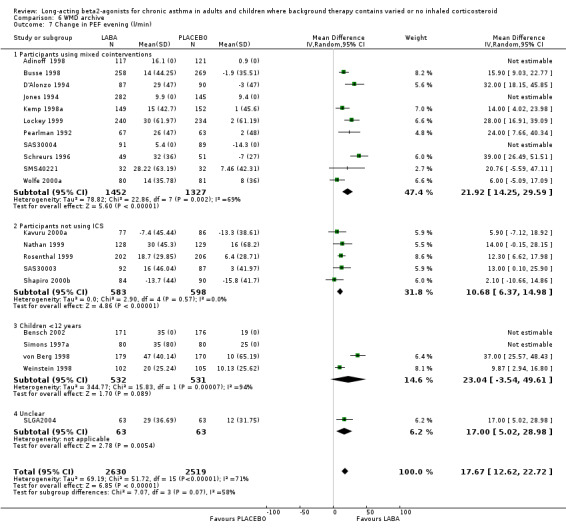
Comparison 6 WMD archive, Outcome 7 Change in PEF evening (l/min).
6.8. Analysis.

Comparison 6 WMD archive, Outcome 8 Peak expiratory flow: morning l/min (crossover studies).
6.9. Analysis.

Comparison 6 WMD archive, Outcome 9 Peak expiratory flow: evening l/min (crossover studies).
6.10. Analysis.
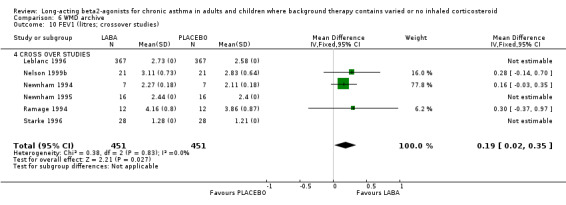
Comparison 6 WMD archive, Outcome 10 FEV1 (litres; crossover studies).
6.11. Analysis.

Comparison 6 WMD archive, Outcome 11 AUC‐ mean area under 12 hr serial FEV1 curve (L‐h).
6.12. Analysis.

Comparison 6 WMD archive, Outcome 12 Mini‐AQLQ (total score).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Adinoff 1998.
| Methods | STUDY DESIGN: Parallel group 4 groups, multicentre, 27 in USA ( majority primary care). 4‐6 weeks initially, 2 groups weaned from non steroidal asthma medications, then 36 weeks treatment RANDOMISATION: Yes, randomised in blocks 5:5:5:1 BLINDING: double blind, double dummy, matching devices WITHDRAWALS/DROP OUTS:75 withdrawals in total, 61 protocol violations, 5 lack efficacy COMPLIANCE: Not assessed CONFOUNDERS: Groups well balanced by characteristics QUALITY: Jadad 4. Cochrane B | |
| Participants | N= RANDOMISED 386 /COMPLETED 311, adult/adolescents, M=203 F=183 Mean age36.5 (range 12‐85) BASELINE SEVERITY: stable asthma, severity not stated INCLUSION : Diagnosis asthma by ATS criteria, Baseline FEV1 50‐80% predicted, >15% FEV1 reversibility to SABA. EXCLUSION: non smoker >1yr, history life threatening asthma, requiring > 2 canisters SABA/month, oral steroids or RTI < 1 month | |
| Interventions | LONG ACTING BETA AGONIST: Salmeterol 42 mcg BD PLACEBO: BD DEVICE: MDI TREATMENT PERIOD: 2‐6 weeks weaning, 36 weeks maintenance RESCUE: Albuterol prn CO‐INTERVENTIONS: ICS 64%, cromones 20%, theophylline 77% | |
| Outcomes | OUTCOMES: FEV1, PEF, Rescue use, asthma symptom score, adverse events . Exacerbations, % days with no symptoms ,% nights with no asthma awakenings | |
| Notes | Patients weaned from non steroidal asthma therapy during initial treatment period, asthma symptoms score=6 pt scale for chest tightness, wheezing, shortness of breath, coughing, exacerbation= events requiring treatment in addition to current asthma therapy | |
Bensch 2001.
| Methods | STUDY DESIGN: Parallel group. Multi centre, 26 in USA. 12 week treatment period. RANDOMISATION: Yes, no method stated. BLINDING: double blind, double dummy, placebo controlled. Matching capsules and devices. WITHDRAWALS/DROP OUTS: 83described, 35 due to adverse events. COMPLIANCE: Assessed by counting capsules and weighing canisters, >80% in all groups. CONFOUNDERS: Baseline characteristics similar for all groups. QUALITY: Jadad 4. Cochrane B | |
| Participants | N= 541 randomised, 535 ITT M=224, F=317 ADULT Mean age 35.5 yrs (SD14.6) BASELINE SEVERITY: Mild‐moderate persistent asthma. INCLUSION : Diagnosis of asthma, requiring daily use of inhaled SABA. Baseline FEV1 40‐80% predicted, >15% reversibility to inhaled SABA EXCLUSION: URTI, hospitalization/asthma exacerbation < 4 weeks, serious illness. | |
| Interventions | LONG ACTING BETA AGONIST: Formoterol 12/ 24 mcg BD SHORT ACTING BETA AGONIST: Albuterol 180 mcg QDS PLACEBO: Placebo QDS DEVICE: LABA Aerolizer DP device & SABA MDI TREATMENT PERIOD: 12weeks RESCUE: Short acting beta2 agonist‐ albuterol 90 mcg inhalation PRN CO‐INTERVENTIONS: ICS 51%, Slow release theophylline 17%. | |
| Outcomes | OUTCOMES: FEV1, FVC, FEV25‐75%, PEF, Rescue albuterol, asthma symptom score, asthma exacerbations, adverse events. | |
| Notes | Symptom Score‐ breathlessness, chest tightness, wheezing, cough; scaled 0‐4. | |
Bensch 2002.
| Methods | STUDY DESIGN: Parallel group, international paediatric multicentre (42). 2 week run‐in/ 52 week treatment period. RANDOMISATION: Randomised, no method given. BLINDING double blind, placebo controlled, no details. WITHDRAWALS/DROP OUTS: 111 post randomisation. COMPLIANCE: no details. CONFOUNDERS: groups balanced by baseline characteristics. QUALITY: Jadad 3 Cochrane B | |
| Participants | N = 601 enrolled, 518 randomised (ITT analysis population), M=342, F=194 Mean age 9 yrs (SD2) BASELINE SEVERITY: Persistent asthma on preventer. INCLUSION : Diagnosis asthma by ATS criteria, ICS or cromone treatment for 4 weeks. Baseline FEV150‐85% predicted, >15% FEV1 reversibility to SABA. EXCLUSION : Unstable asthma, URTI, OS course or exacerbation asthma within 4 weeks, QT interval on ECG >0.46sec. | |
| Interventions | LONG ACTING BETA AGONIST: Formoterol 12 mcg or 24 mcg BD PLACEBO: BD DEVICE: Aerolizer RESCUE: salbutamol PRN TREATMENT PERIOD: Part 1: 4 weeks . Part 2 : 26 weeks. CO‐INTERVENTIONS: 70% on ICS, 30% on cromones (all stable dosage) | |
| Outcomes | OUTCOMES: Part 1: Primary= AUC for FEV1 over 12 hours after morning study drug dose Secondary= PEF, FEV1, FVC, day and night ASS, rescue use, exacerbations asthma. Adverse events. | |
| Notes | Exacerbation defined as increase symptoms asthma, >4 x200mcg SABA /day, requiring OS and nebulized SABA. Data published as adjusted mean, requested actual data from authors. | |
Booth 1993.
| Methods | STUDY DESIGN: Parallel group single centre, UK, hospital setting, 2‐12 days run in/ 8 weeks treatment/ 2 weeks run out RANDOMISATION: Yes, no method stated. BLINDING: double blind, placebo controlled, matching devices WITHDRAWALS/DROP OUTS: 4 withdrawals, 3 LABA, 1 placebo COMPLIANCE: Not assessed CONFOUNDERS: Baseline characteristics similar, study performed out of pollen season, salbutamol withheld 8 hrs prior to BHR measurement QUALITY: Jadad 4. Cochrane B | |
| Participants | N= RANDOMISED 26 , M=15 F=17, adults, Mean age 40 (range 20‐64) BASELINE SEVERITY: Mild‐moderate asthma INCLUSION : Diagnosis clinically stable ,>15% FEV1 reversibility to SABA. EXCLUSION: OS within 3 months | |
| Interventions | LONG ACTING BETA AGONIST: Salmeterol 50 mcg BD PLACEBO: BD DEVICE: MDI TREATMENT PERIOD: 8 weeks RESCUE: Salbutamol 100 mcg prn CO‐INTERVENTIONS: ICS <1000mcg BDP daily in 19/26, 73% | |
| Outcomes | OUTCOMES: FEV1, PD20 methacholine, | |
| Notes | ||
Boulet 1997.
| Methods | STUDY DESIGN: Parallel group, single centre, Canada, hospital setting, 2 weeks run in/ 8 weeks treatment RANDOMISATION: Randomised treatment , code used. BLINDING: double blind, placebo controlled WITHDRAWALS/DROP OUTS: 3 withdrawals. COMPLIANCE: not stated CONFOUNDERS : Groups well balanced by characteristics, study avoided allergen season QUALITY: Jadad 4. Cochrane B | |
| Participants | N= RANDOMISED 16, 1 completed. & analysed M=5 F=8, adults, Mean age 25 (range 20‐37) BASELINE SEVERITY: mild asthma INCLUSION : Diagnosis asthma by ATS criteria, Baseline FEV1 >70% predicted. Dual response to allergen, EAR >20% fall FEV1, LAR >15% fall FEV1, atopic. EXCLUSION: URTI/ LRTI/allergen exposure <4 weeks, ICS/OS < 4 weeks, smokers | |
| Interventions | LONG ACTING BETA AGONIST: Salmeterol 50 mcg BD PLACEBO: BD DEVICE: MDI TREATMENT PERIOD: 8 weeks RESCUE: Salbutamol 100 mcg prn CO‐INTERVENTIONS: none permitted | |
| Outcomes | OUTCOMES: PEF, Rescue use, asthma symptom scores, allergen challenges, BAL, EBB, BHR | |
| Notes | PEF ‐SD for result in paper 2l/min, probable typographic error, queried with authors. Symptom Score‐ respiratory symptoms graded 11 pt scale on waking and at bed time‐ 0= nothing to 10= maximum bearable, | |
Boulet 1998b.
| Methods | STUDY DESIGN: 2 way cross over, single centre, Canada, hospital setting, 2 weeks run in/ 4 weeks treatment/ 2 weeks crossover & run out RANDOMISATION: Randomised treatment order, no method stated. BLINDING: double blind, placebo controlled WITHDRAWALS/DROP OUTS: 2 withdrawals. COMPLIANCE: checked by weighing canisters‐ result not given CONFOUNDERS : Study drug stopped 48 hrs prior to BHR measurement QUALITY: Jadad 3. Cochrane B | |
| Participants | N= RANDOMISED 16, 15 completed. & analysed M=5 F=10, adults, Mean age 31.0 (range 18‐59) BASELINE SEVERITY: moderate asthma INCLUSION : Diagnosis asthma by ATS criteria, requiring treatment for >6months. Baseline FEV1 >60% predicted. PC20 methacholine < 8mg/ml. Not using ICS regularly. EXCLUSION: URTI/ LRTI/hospitalisation <4 weeks, use of theophylline, OS, ipratropium< 4 weeks, smoking <2mths. Serious uncontrolled systemic disease or pregnancy. | |
| Interventions | LONG ACTING BETA AGONIST: Salmeterol 50 mcg BD PLACEBO: BD DEVICE: MDI TREATMENT PERIOD: 4 weeks RESCUE: Salbutamol 100 mcg prn CO‐INTERVENTIONS: none permitted | |
| Outcomes | OUTCOMES: FEV1, FVC , PEF, PC20 methacholine, Rescue use, asthma symptom scores. | |
| Notes | Symptom Score‐ respiratory symptoms graded on 6 pt scale‐ o= nothing, 1= very light, 2=light, 3= moderate, 4= severe, 5= very severe | |
Busse 1998.
| Methods | STUDY DESIGN: Parallel group, multicentre (55), USA, 2 weeks run in/ 12 weeks treatment RANDOMISATION: Randomised treatment , no method given BLINDING: double blind, double dummy, matching devices. WITHDRAWALS/DROP OUTS: 95 withdrawals, 47 active, 48 placebo. COMPLIANCE: not stated CONFOUNDERS : baseline characteristics‐ both groups similar, except morning PEF lower in active group QUALITY: Jadad 3. Cochrane B | |
| Participants | N= Randomised 538, Completed 443. M=51 F=68, adults, Mean age 36 (range 12‐80) BASELINE SEVERITY: Moderate persistent asthma. INCLUSION : Diagnosis asthma by ATS criteria, used SABA daily during 6mths. Baseline FEV1 40‐80% predicted. >15% FEV1 reversibility to SABA. Symptomatic in run in ASS>=2 EXCLUSION: URTI/ LRTI/<4 weeks, COPD, CF, unstable asthma, pregnancy, lactation, Use of OS <1mth | |
| Interventions | LONG ACTING BETA AGONIST: Salmeterol 42 mcg BD PLACEBO: BD DEVICE: MDI TREATMENT PERIOD: 12 weeks RESCUE: Salbutamol 100 mcg prn CO‐INTERVENTIONS: ICS 65%, cromones 7%, theophyllines 30%‐ all fixed doses | |
| Outcomes | OUTCOMES: FEV1, FVC, PEF, Asthma QOL score, Rescue use, asthma symptom score, % days with no symptoms ,% nights with no asthma awakenings | |
| Notes | Symptom Score‐ Night‐time 0‐3 (none ‐ so severe that no sleep possible). Day‐time 0‐3 (no symptoms ‐ symptoms so severe normal activities not possible). AQOL based on Juniper. | |
Busse 2004.
| Methods | STUDY DESIGN: Parallel group LOCATION, NUMBER OF CENTRES: USA 18 centres DURATION OF STUDY: run in= 2 weeks single blind placebo, 12 weeks treatment CONCEALMENT OF ALLOCATION: COCHRANE QUALITY SCORE: A DESCRIBED AS RANDOMISED: Yes DESCRIBED AS DOUBLE BLIND: Yes METHOD OF RANDOMISATION WELL DESCRIBED/APPROPRIATE: Yes, 1:1:1 ratio using computer generated list of random numbers METHOD OF BLINDING WELL DESCRIBED/APPROPRIATE: Yes, double dummy matched devices DESCRIPTION OF WITHDRAWALS/DROPOUTS: 30 withdrawals (10 formoterol, 3 albuterol, 13 placebo, reasons described. JADAD SCORE (5‐1): TYPE OF ANALYSIS (AVAILABLE CASE/TREATMENT RECEIVED/ ITT): ITT and per protocol COMPLIANCE: Not assessed CONFOUNDERS: Comparable baseline characteristics | |
| Participants | N SCREENED: Not reported N RANDOMISED: 239 N COMPLETED:219 M= 147 F= 92 MEAN AGE: Formoterol 39.1, placebo 36.8, albuterol 38.4 (13‐85) SEVERITY OF ASTHMA: unknown BASELINE DETAILS: duration asthma mean 22.2‐ 25.9 yrs, % pred FEV1 formoterol 63.4%, placebo 66%, albuterol 64.6%, daily rescue use: formoterol 2.52, placebo 2.55, albuterol 2.75 puffs/24hr, ICS use formoterol 65%, placebo 63%, albuterol 64%., no significant differences in symptom score or QOL score. INCLUSION CRITERIA : Persistent asthma, requiring regular or PRN bronchodilators, F EV1 % predicted = 40%, BDR = 15% or = 12% and =200ml 30 minutes after SABA 400mcg. EXCLUSION: RT infection, use of OCS, parenteral CS or hospitalisation for asthma due to exacerbation < 1 month, use of unstable anti‐inflammatory regime, corrected QT interval > 460ms, current smoker, ex‐smoker >10 PHY, serious medical condition, pregnancy, lactation, Use of oral beta blocker, non‐ potassium sparing diuretic, anti arrhythmic, tricyclic antidepressant, MAOI, NSAIDS. | |
| Interventions | 1. Formoterol 10mcg BD 2. Placebo BD 3. Albuterol 180 mcg QDS DELIVERY: DPI multidose COMBINATION INHALER FOR LABA: NO TREATMENT PERIOD: 12 weeks RESCUE: Albuterol MDI PRN (<720 mcg daily) CO‐INTERVENTIONS PERMITTED: ICS, cromolyn, montelukast, theophyllines, intra nasal ICS % on ICS: FORM group 65%, PLA group 63% | |
| Outcomes | OUTCOMES MEASURED: 12 hour AUC formoterol, Asthma QOL (mini asthma QOL questionnaire) , vital signs, blood biochemistry, ECGs, clinic spirometry, daily PEF am/pm, asthma symptoms scores, rescue use, adverse events, asthma exacerbations. FOLLOW‐UP ASSESSMENT POINTS: OUTCOMES INCLUDED IN ANALYSES SUB‐GROUPS INDENTIFIED: | |
| Notes | Mini asthma QOL‐ 4 domains of symptoms, activity limitation, emotional function, environmental stimuli. 15 Item score 1 maximum‐7 minimum, Exacerbation ‐definition asthma symptoms that did not resolve with use of study medications and thus needed additional medical treatment | |
Cheung 1992.
| Methods | STUDY DESIGN: Parallel group, single centre Netherlands, 1 week run in / 8 weeks treatment/1 weeks follow up RANDOMISATION: Randomised treatment , method not stated BLINDING: double blind, placebo controlled, identical devices. WITHDRAWALS/DROP OUTS:1 withdrawal, COMPLIANCE: measured by weighing inhaler, no difference between groups CONFOUNDERS : baseline characteristics of both groups similar, stopped study drug 36 hours prior to BHR measurement QUALITY: Jadad 4. Cochrane B | |
| Participants | N= Randomised 24, completed 23. M=11 F=13, adults, Mean age 25.1 (range 19‐36) BASELINE SEVERITY: mild asthma. INCLUSION : Diagnosis asthma by ATS criteria, atopy . Baseline FEV1 >75% predicted. >15% FEV1 reversibility to SABA. PC20 methacholine < 8mg/ml EXCLUSION: URTI/ LRTI/<2 weeks, smoking history, use of ICS/OS , theophylline/antihistamine < 6mths | |
| Interventions | LONG ACTING BETA AGONIST: Salmeterol 50 mcg BD PLACEBO: BD DEVICE: MDI via aerosol chamber TREATMENT PERIOD:8 weeks RESCUE: Albuterol 100 mcg prn up to 400 mcg/day CO‐INTERVENTIONS: none permitted | |
| Outcomes | OUTCOMES: FEV1, BHR PC20 methacholine | |
| Notes | ||
Cloostermann2001.
| Methods | STUDY DESIGN: Parallel group, single tertiary centre, Netherlands. RANDOMISATION: randomised treatment allocation, no details of method BLINDING: double blind, double dummy WITHDRAWALS/DROP OUTS: 42 during wash out stopping ICS, 17 post randomisation to study drug COMPLIANCE: assessed by weighing medication canisters CONFOUNDERS: ICS withdrawal lead to selection of milder subjects able to tolerate no ICS QUALITY: Jadad 4. Cochrane B | |
| Participants | N=258 enrolled, 204 entered run in, 162 randomised (48 SABA, 50 LABA, 47 placebo), 145 completed M= 77, F= 68 Mean age 34 yrs (SD 10) BASELINE SEVERITY: Mild allergic asthma INCLUSION : Atopic positive SPT to HDM, , clinical diagnosis asthma, baseline FEV1 > 50% predicted, FEV1 post SABA > 65% predicted, >15% reversibility to SABA, PC20 histamine < 8mg/ml EXCLUSION: Need for ICS, positive SPT to a pet still in household | |
| Interventions | SHORT ACTING BETA AGONIST: Salbutamol 200 mcg QDS PLACEBO: QDS LONG ACTING BETA AGONIST: Formoterol 12 mcg BD DEVICE: MDI TREATMENT PERIOD: 12 weeks RESCUE: combination short acting beta2 agonist/anticholinergic‐fenoterol 100 mcg/ ipratropium 40 mcg per puff PRN CO‐INTERVENTIONS: none permitted | |
| Outcomes | OUTCOMES: FEV1, BHR, PEF am /pm, ASS, rescue use, exacerbations | |
| Notes | Exacerbation= asthma deterioration treated with OS by GP, ASS= cough, wheeze, SOB, sputum, sleep disturbance, Scale 1= none ‐10 = severe | |
Creticos 1999.
| Methods | STUDY DESIGN: Parallel group LOCATION, NUMBER OF CENTRES: single in USA DURATION OF STUDY: 2 week run in, 6 months treatment CONCEALMENT OF ALLOCATION: COCHRANE QUALITY SCORE: B DESCRIBED AS RANDOMISED: Yes DESCRIBED AS DOUBLE BLIND: Yes METHOD OF RANDOMISATION WELL DESCRIBED/APPROPRIATE: no details METHOD OF BLINDING WELL DESCRIBED/APPROPRIATE: not reported DESCRIPTION OF WITHDRAWALS/DROPOUTS: not reported JADAD SCORE (5‐1): 2 TYPE OF ANALYSIS (AVAILABLE CASE/TREATMENT RECEIVED/ ITT): not reported COMPLIANCE: not reported CONFOUNDERS: no details | |
| Participants | N SCREENED: not reported N RANDOMISED: 46 N COMPLETED: 221 M= 20 F= 26 MEAN AGE: 37 (13) BASELINE DETAILS: mild‐moderate asthma. INCLUSION CRITERIA : FEV1 >65% predicted; FEV1 reversibility =12% post‐SABA; requiring rescue = 4days weekly. EXCLUSION: not reported | |
| Interventions | 1. SALMETEROL 50 mcg BD 2. Placebo BD 3. TRIAMCINOLONE 400 mcg BD 4. TRI/SALM FSC 400/50 BD DELIVERY: MDI TREATMENT PERIOD: 6 months RESCUE: Salbutamol CO‐INTERVENTIONS PERMITTED: None during treatment CO‐INTERVENTIONS : % on ICS: 0% in SALM and PLAC groups | |
| Outcomes | OUTCOMES MEASURED: airway hyper‐reactivity, rescue use, FEV1 FOLLOW‐UP ASSESSMENT POINTS: 2 monthly OUTCOMES INCLUDED IN ANALYSES: SUB‐GROUPS INDENTIFIED: None | |
| Notes | Numbers in treatment groups not given. FEV1 results only (no SD), AHR measurements not given | |
D'Alonzo 1994.
| Methods | STUDY DESIGN:Three treatment parallel group, multicentre (11) study in USA, 1‐2 weeks run in/12 weeks treatment RANDOMISATION: Yes, method not given. BLINDING:Double blind, double dummy, with 2 matching inhalers. WITHDRAWALS:42/322 , by groups‐ 15 in salmeterol , 16 in albuterol, 11in placebo CONFOUNDERS:differential rates of ICS and cromone use in regular and prn group, use of theophyllines in run in period QUALITY:Jadad=4, Cochrane B | |
| Participants | N=322 Albuterol =108, placebo=108, salmeterol =106 AGE‐ means ‐albuterol 31(14), placebo 28(12), salmeterol 29(12) SEVERITY OF ASTHMA: unknown INCLUSION: Diagnosis asthma by ATS criteria, requiring daily drug treatment for > 6 months. Baseline FEV1 50‐70% predicted, >15% FEV1 reversibility to SABA. EXCLUSION:Smokers | |
| Interventions | LONG ACTING BETA AGONIST: Salmeterol 42 mcg BD SHORT ACTING B ETA AGONIST: Albuterol 180mcg QDS PLACEBO:placebo QDS DEVICE: MDI PERIOD:12 weeks RESCUE:albuterol 90mcg prn COINTERVENTIONS: ICS ‐used by 20% on placebo , 24% on albuterol, 21% on salmeterol ORAL STEROIDS‐ not at randomisation CROMONES‐used by 9% on placebo , 6% on albuterol, 10% on salmeterol THEOPHYLIINES‐ used only during run in by 46% on placebo ,50% on albuterol, 43% on salmeterol ORAL BETA AGONISTS‐ not permitted | |
| Outcomes | OUTCOMES: FEV1, FVC, FEV25‐75%, PEF, Rescue use, asthma symptom score, symptom free days & nights, adverse events . | |
| Notes | Exacerbations defined as worsening of asthma symptoms that required treatment in addition to the study drug and rescue SABA. Symtom Score‐ composite based on individual scores for breathlessness, chest tightness, wheezing, cough. Scale : 0=none to 5= severe, activities cancelled. | |
Dahl 1991a.
| Methods | STUDY DESIGN: Parallel group multicentre (76) in 12 European countries, 3 active groups, differing doses Salmeterol. 1 week run in /4 weeks treatment/1 weeks follow up RANDOMISATION: Randomised treatment , blocks of 12 BLINDING: double blind, placebo controlled, identical devices. WITHDRAWALS/DROP OUTS:78 withdrawals COMPLIANCE: rescue inhaler MDI counter CONFOUNDERS : baseline characteristics of both groups similar, QUALITY: Jadad 5 Cochrane A | |
| Participants | N=Enrolled 1086, Randomised 692, completed 614. M=360 F=332, adults, Mean age 42 BASELINE SEVERITY: Mild‐moderate asthma INCLUSION : Diagnosis asthma clinically. Baseline FEV1 60‐90% predicted. >15% FEV1 reversibility to SABA. Symptom score >2 , diurnal variation PEF >15% on 4/7 days run in EXCLUSION: URTI/ LRTI/hospitalisation with asthma<4 weeks, use of OS >20mg/day , theophylline/antihistamine < 6mths | |
| Interventions | LONG ACTING BETA AGONIST: Salmeterol 12.5, 50, 100 mcg mcg BD PLACEBO: BD DEVICE: MDI TREATMENT PERIOD:4 weeks RESCUE: Albuterol 100 mcg prn up to 400 mcg/day CO‐INTERVENTIONS: ICS, 75%, OS <20mg/day, cromones | |
| Outcomes | OUTCOMES: FEV1, FVC, PEF, Rescue use, asthma symptom score, % days with no symptoms ,% nights with no asthma awakenings, Efficacy score‐patient and investigator rated | |
| Notes | ASS Day‐time 0‐5 (no symptoms ‐ symptoms so severe normal activities not possible) | |
Dahl 1991b.
| Methods | STUDY DESIGN: Cross over, single centre Denmark. 4 weeks treatment RANDOMISATION: Randomised treatment, method not stated BLINDING: double blind, placebo controlled WITHDRAWALS/DROP OUTS: no details COMPLIANCE: no details CONFOUNDERS : no details QUALITY: Jadad 2 Cochrane B | |
| Participants | N= Randomised 12, M= F=, adults, Mean age 32 BASELINE SEVERITY: Mild‐moderate asthma INCLUSION : Diagnosis asthma clinically. Mean Baseline FEV1 85% predicted. >15% FEV1 reversibility to SABA EXCLUSION: no details | |
| Interventions | LONG ACTING BETA AGONIST: Salmeterol 50 mcg mcg BD PLACEBO: BD DEVICE: TREATMENT PERIOD:4 weeks RESCUE: SABA CO‐INTERVENTIONS: none used | |
| Outcomes | OUTCOMES: BHR, BAL | |
| Notes | ||
Ekstrom 1998a.
| Methods | STUDY DESIGN: Parallel group, 3 treatment arms; multicentre, 25 centres Norway, Sweden, Spain, Italy. 12 weeks. RANDOMISATION: Yes, method not given. BLINDING: double blind, double dummy, matching devices. WITHDRAWALS/DROP OUTS: 38 described. 27 to asthma, 11 to adverse events. COMPLIANCE: Not assessed/ reported. CONFOUNDERS: Groups well balanced by characteristics. Excess withdrawals from SABA group, 10 subjects did not fully meet inclusion criteria. QUALITY: Jadad 4. Cochrane B | |
| Participants | N= 397 Adults, M=232 F=179 Mean age 47 (RANGE 18‐79) BASELINE SEVERITY: mild ‐ moderate asthma. INCLUSION : Diagnosis asthma by ATS criteria. Baseline FEV1 50‐80% predicted, >15% FEV1 reversibility to SABA.. EXCLUSION: none stated in paper. | |
| Interventions | LONG ACTING BETA AGONIST: Formoterol 6 mcg BD SHORT ACTING BETA AGONIST: Terbutaline 500mcg QDS PLACEBO: Placebo QDS DEVICE: Dry powder device. TEATMENT PERIOD: 12 weeks RESCUE: Terbutaline 250 mcg via turbuhaler PRN CO‐INTERVENTIONS: ICS 80%, cromones 5% ‐ stable d | |
| Outcomes | OUTCOMES: FEV1, PEF, Rescue use, asthma symptom score, adverse events. asthma deterioration | |
| Notes | Symptom Score‐ breathlessness, chest tightness, wheezing, cough. Scale :0 ‐ 3. Asthma deterioration leading to withdrawal reported. | |
Ekstrom 1998b.
| Methods | STUDY DESIGN: Parallel group, 3 treatment arms; multicentre, 28 centres Scaninavia. 12 weeks. RANDOMISATION: Yes, computer generated random order, balanced blocks.. BLINDING: double blind, double dummy, matching devices. WITHDRAWALS/DROP OUTS: 32 described, due to asthma. COMPLIANCE: Not assessed/ reported. CONFOUNDERS: Groups well balanced by characteristics. QUALITY: Jadad 5. Cochrane A | |
| Participants | N= 343 Adults, M=164 F=179 Mean age 48 (RANGE 18‐82) BASELINE SEVERITY: moderate stable asthma. INCLUSION : Diagnosis asthma by ATS criteria. Baseline FEV1 40‐80% predicted, >15% FEV1 reversibility to SABA.. EXCLUSION: none stated in paper. | |
| Interventions | LONG ACTING BETA AGONIST: Formoterol 12 mcg BD SHORT ACTING BETA AGONIST: Terbutaline 500mcg QDS PLACEBO: Placebo QDS DEVICE: Dry powder device‐ turbuhaler. TEATMENT PERIOD: 12 weeks RESCUE: Terbutaline 250 mcg via turbuhaler PRN CO‐INTERVENTIONS: ICS 89%, cromones 2% ‐ stable doses OS 2 subjects | |
| Outcomes | OUTCOMES: FEV1, PEF, Rescue use, asthma symptom score, adverse events | |
| Notes | Symptom Score‐ breathlessness, chest tightness, wheezing, cough. Scale :0 ‐ 3 | |
Garcia 2001.
| Methods | STUDY DESIGN: Parallel group single centre study, Spain, specialist hospital. 4 week treatment period after a pre‐study visit to prove exercise‐induced asthma RANDOMISATION: Randomised, no method given. BLINDING double blind, placebo controlled, masked identical devices. WITHDRAWALS/DROP OUTS: all randomised participant completed study. COMPLIANCE: no details CONFOUNDERS: groups balanced by baseline characteristics. QUALITY: Jadad 4 Cochrane B | |
| Participants | N = 104 screened, 23 enrolled, 19 randomised, 19 completed. M=8, F=11 Mean age 24.1 yrs (SD 5.6) SEVERITY OF ASTHMA: unknown INCLUSION : Clinical history of exercise induced asthma, >15% fall in FEV1 within 30 minutes exercise. EXCLUSION : Change asthma medication within 4 weeks. Use of > 2 puffs per week SABA as rescue. | |
| Interventions | LONG ACTING BETA AGONIST: Formoterol 12 mcg BD PLACEBO: BD DEVICE: MDI RESCUE: Salbutamol or terbutaline PRN via MDI TREATMENT PERIOD: 4 weeks CO‐INTERVENTIONS: Cromones, ipratropium, % on ICS 32% (6/19) ‐ stable doses | |
| Outcomes | OUTCOMES: Primary= change in bronchoprotection index (BI). Secondary= PEF | |
| Notes | BI= % FEV1 fall in challenge2 minus % FEV1 fall in challenge 2 x 100/ % FEV1 fall in challenge 1 (performed on the same day) after inhaling formoterol | |
Hyland 1994.
| Methods | STUDY DESIGN: Parallel group, multicentre, S Africa. 2 weeks run in/ 6 weeks treatment/ 2 weeks follow up RANDOMISATION: Randomised treatment, method not stated BLINDING: double blind, placebo controlled, matching devices WITHDRAWALS/DROP OUTS: 3 COMPLIANCE: no details CONFOUNDERS : no details QUALITY: Jadad 4 Cochrane B | |
| Participants | N= Randomised , completed 79, analysed 76. M=36 F=40, adults, Mean age 38.5 (range 17‐75) SEVERITY OF ASTHMA: unknown INCLUSION : Symptoms asthma and use SABA 8/14 run in days, Baseline FEV1 <75% predicted, >15% FEV1 reversibility to SABA. EXCLUSION: OS, ICS >400mcg/day, | |
| Interventions | LONG ACTING BETA AGONIST: Salmeterol 50 mcg mcg BD PLACEBO: BD DEVICE: MDI or diskhaler TREATMENT PERIOD: 4 weeks RESCUE: not stated CO‐INTERVENTIONS: ICS <400 mcg/day | |
| Outcomes | OUTCOMES: Asthma QOL score | |
| Notes | Asthma QOL score= LWAQ by Hyland | |
Jones 1994.
| Methods | STUDY DESIGN: Parallel group , multicentre UK, primary care 146 GPs, 2weeks run‐in/ 6 weeks treatment/ 2 weeks follow up. RANDOMISATION: Randomised treatment 2:1 salmeterol, method not stated BLINDING: double blind, placebo controlled, matching devices WITHDRAWALS/DROP OUTS: 85, 34 adverse events COMPLIANCE: no details CONFOUNDERS : no details. QUALITY: Jadad 3 Cochrane B | |
| Participants | N= Enrolled 669, Randomised 427, M=207 F=220, adults, Mean age 38.3 (range 18‐79) BASELINE SEVERITY: Mild asthma INCLUSION : Clinical diagnosis asthma , prescription SABA 1‐4 in past 4 months. Baseline FEV1 >75% predicted, >15% FEV1 reversibility to SABA. 8/14 run in symptoms, PEF variability or rescue use. EXCLUSION: none stated | |
| Interventions | LONG ACTING BETA AGONIST: Salmeterol 50 mcg mcg BD PLACEBO: BD DEVICE: DP diskhaler TREATMENT PERIOD: 6 weeks RESCUE: salbutamol 400mcg prn CO‐INTERVENTIONS: ICS <400 mcg/day 180/427, no ICS 247/427 % on ICS= 42% | |
| Outcomes | OUTCOMES: PEF, Rescue use, asthma symptom score Efficacy score‐patient and investigator rated | |
| Notes | Some subgroup results for ICS /noICS groups. Symptom Score‐ cough/wheeze/SOB. Night‐time 0‐3 (none ‐ so severe that no sleep possible). Day‐time 0‐3 (no symptoms ‐ symptoms so severe normal activities not possible) | |
Juniper 1995.
| Methods | STUDY DESIGN: 3 way cross over, multicentre, 14 centres Canada, 4 weeks. RANDOMISATION: Yes, no method stated. BLINDING: Double blind, double dummy, matching inhalers. WITHDRAWALS/DROP OUTS: 21 described after randomization. COMPLIANCE: >70% reported for all but 7 subjects. CONFOUNDERS: QUALITY: Jadad 4. Cochrane B | |
| Participants | N= 140 RANDOMISED. Adults, M=66 F=74 Mean age 37.5 (sd 14.5) BASELINE SEVERITY: mild ‐ moderate asthma. INCLUSION : Baseline FEV1 >60% predicted, >15% FEV1 reversibility to SABA. Symptom score >2 on 4/7 days run in EXCLUSION: Exacerbation asthma within 1 month, emergency room visit >3 months, uncontrolled illness, pregnancy. OS within 1mth. Theophylline . | |
| Interventions | LONG ACTING BETA AGONIST: Salmeterol 50 mcg BD SHORT ACTING BETA AGONIST: Salbutamol 200 mcg QDS PLACEBO: placebo QDS DEVICE: MDI. TEATMENT PERIOD: 4 weeks RESCUE:Salbutamol 100 mcg PRN CO‐INTERVENTIONS: ICS 77% . Cromones 15%, 30% none. | |
| Outcomes | OUTCOMES: FEV1, FVC, FEV25‐75%, PEF, Rescue use, asthma symptom score, symptom free days & nights, adverse events, AQOL scores. | |
| Notes | AQOL score by Juniper, measures‐symptoms, emotions, activity limitation, environment‐overall scores + individual domains | |
Kavuru 2000a.
| Methods | STUDY DESIGN: Parallel group , multicentre (42) USA, 2weeks run in/ 12 weeks treatment RANDOMISATION: Randomised treatment, method not stated BLINDING: double blind, placebo controlled, matching devices WITHDRAWALS/DROP OUTS: 80 for worsening asthma COMPLIANCE: dose counter on devices, 93‐100% adherence CONFOUNDERS : Groups well balanced by baseline characteristics QUALITY: Jadad 4 Cochrane B | |
| Participants | N= Screened 527, Randomised 356, M=190, F=166, Adolescent/ adults. Mean age 37 (range 12‐70) BASELINE SEVERITY: persistent/ symptomatic INCLUSION : Diagnosis asthma by ATS criteria >6mths, Baseline FEV1 40‐80% predicted, >15% FEV1 reversibility to SABA. EXCLUSION: Serious uncontrolled systemic disease, pregnancy, life threatening asthma, course OS <4weeks, regular OS < 6nths, smokers, ICS | |
| Interventions | LONG ACTING BETA AGONIST: Salmeterol 50 mcg mcg BD PLACEBO: BD DEVICE: DP diskhaler TREATMENT PERIOD: 12 weeks RESCUE: albuterol 100mcg prn CO‐INTERVENTIONS: none permitted | |
| Outcomes | OUTCOMES: FEV1, FVC, PEF, Rescue use, asthma symptom score, % days with no symptoms , % nights with no asthma awakenings, adverse events, Exacerbations. | |
| Notes | Symptom Score‐ cough/wheeze/SOB ‐6 pt Scale :0= none to 5= very severe Exacerbations defined as acute episode requiring emergency medical attention or hospitalization or treatment not allowed by protocol. | |
Kemp 1998a.
| Methods | STUDY DESIGN: Parallel group, multicentre, 15 centres USA, 12 weeks. RANDOMISATION: Yes, no method stated. BLINDING: Double blind, double dummy, matching inhalers. WITHDRAWALS/DROP OUTS: 41 described after randomization. COMPLIANCE: >70% reported for all but 7 subjects. CONFOUNDERS: QUALITY: Jadad 4. Cochrane B | |
| Participants | N= 451 RANDOMISED. Adults, M=262 F=189 Mean age 31 (sd 14) BASELINE SEVERITY: persistent/ symptomatic INCLUSION : Diagnosis asthma by ATS criteria. Baseline FEV1 50‐80% predicted, >15% FEV1 reversibility to SABA. Requiring daily drug treatment for > 6 months. EXCLUSION: smoking. | |
| Interventions | LONG ACTING BETA AGONIST: Salmeterol 50 mcg BD SHORT ACTING BETA AGONIST: Albuterol 180 mcg QDS PLACEBO: placebo QDS DEVICE: MDI & dry powder device. TEATMENT PERIOD: 12 weeks RESCUE:Albuterol 100 mcg PRN CO‐INTERVENTIONS: ICS 55‐70 % . Cromones 7‐12 % | |
| Outcomes | OUTCOMES: FEV1, FVC, FEV25‐75%, PEF, Rescue use, asthma symptom score, symptom free days & nights, adverse events, exacerbations. | |
| Notes | Symptom Score‐ breathlessness, chest tightness, wheezing, cough. Scale : 0‐3 Exacerbations defined as worsening of asthma symptoms that required treatment in addition to the study drug and rescue SABA. | |
Kemp 1999.
| Methods | STUDY DESIGN: Parallel group , multicentre (18) USA, 2‐4 weeks run in/ 52 weeks treatment/ 2 weeks run out RANDOMISATION: Randomised treatment, method not stated BLINDING: double blind, placebo controlled WITHDRAWALS/DROP OUTS: 87 COMPLIANCE: count unused blisters, rate > 90% both groups CONFOUNDERS : Groups well balanced by baseline characteristics QUALITY: Jadad 3 Cochrane B | |
| Participants | N= Randomised 352, M=179, F=173, Adolescent/ adults. Mean age 28.5 (range 12‐67) BASELINE SEVERITY: Mild‐moderate asthma INCLUSION : clinical diagnosis asthma > 6mths, Baseline FEV1 70‐90% predicted, >15% FEV1 reversibility to SABA. PC20 methacholine < 7.5mg/ml EXCLUSION: URTI/ LRTI <6 WKS , smoking < 12mths , | |
| Interventions | LONG ACTING BETA AGONIST: Salmeterol 50 mcg mcg BD PLACEBO: BD DEVICE: DP diskhaler TREATMENT PERIOD: 52 weeks RESCUE: albuterol 100mcg prn CO‐INTERVENTIONS: ICS ,52% active group, 40% placebo | |
| Outcomes | OUTCOMES: FEV1, FVC, PEF, asthma symptom score , Rescue use, BHR | |
| Notes | Symptom Score‐ cough/wheeze/SOB ‐6 pt Scale :0= none to 5= severe | |
Kraft 1997.
| Methods | STUDY DESIGN: 2 way cross over single centre, 6 weeks treatment/ 1 weeks wash out RANDOMISATION: Randomised treatment order, method not stated BLINDING: double blind, placebo controlled WITHDRAWALS/DROP OUTS: none occurred but 3 subjects unable to have BAL due to low FEV1 COMPLIANCE: Not assessed CONFOUNDERS : No details QUALITY: Jadad 3 Cochrane B | |
| Participants | N= Randomised 10, M=8, F=2, adults. Mean age 29.95 (SEM 1.9) BASELINE SEVERITY: Moderate to severe asthma. INCLUSION : Diagnosis asthma by ATS criteria, nocturnal symptoms. Overnight fall in PEF >20% on 4/7nights EXCLUSION: URTI/ LRTI/antibiotics <6 WKS , smoking <12mths, smoking history <5 pack years , Serious uncontrolled systemic disease, pregnancy. OS/ ICS/ cromones/ methotrexate | |
| Interventions | LONG ACTING BETA AGONIST: Salmeterol 50 mcg mcg BD PLACEBO: BD DEVICE: MDI TREATMENT PERIOD: 6 weeks RESCUE: albuterol 100mcg prn CO‐INTERVENTIONS: none permitted | |
| Outcomes | OUTCOMES: PEF, asthma symptom score , BHR, BAL | |
| Notes | Symptom Score‐ cough/wheeze/SOB ‐5 pt Scale :0= none to 4= severe | |
LaForce 2005.
| Methods | STUDY DESIGN: Parallel group LOCATION, NUMBER OF CENTRES: USA, 22 centres. DURATION OF STUDY: 12 weeks (preceded by two week run‐in). CONCEALMENT OF ALLOCATION: COCHRANE QUALITY SCORE: A DESCRIBED AS RANDOMISED: YES DESCRIBED AS DOUBLE BLIND: YES METHOD OF RANDOMISATION WELL DESCRIBED/APPROPRIATE: Computer generated sequence METHOD OF BLINDING WELL DESCRIBED/APPROPRIATE: Double‐dummy DESCRIPTION OF WITHDRAWALS/DROPOUTS: Reported (30/265: adverse events: 7; lack of efficacy: 7; withdrawal of consent: 11; others not specified) JADAD SCORE (5‐1):5 TYPE OF ANALYSIS (AVAILABLE CASE/TREATMENT RECEIVED/ ITT): ITT (LOCF) COMPLIANCE: Not assessed. CONFOUNDERS: Slightly higher number of participants taking ICS in the placebo group (65 versus 56%) | |
| Participants | N SCREENED: Not reported. N RANDOMISED: 265 N COMPLETED: 235 M= 113 F= 152 MEAN AGE: 37 SEVERITY OF ASTHMA: unknown BASELINE DETAILS: Mean FEV1 % predicted: 68; SABA usage: 2 puffs/d. INCLUSION CRITERIA: FEV1 >40% predicted; Reversibility >15% (12% if >/=200mL over baseline) EXCLUSION: Pregnant/lactating women; smoking history >10 pack years; history of malignancy; RTI or hospitalisation with exacerbation in previous month; treatment with systemic steroids. | |
| Interventions | 1. Formoterol 10mcg BID 2. Placebo 3. Salbutamol 180mcg QID DELIVERY: DPI (pMDI for salbutamol) TREATMENT PERIOD: 12 weeks RESCUE: Salbutamol (dose not specified) CO‐INTERVENTIONS PERMITTED: ICS; cromolyns; leukotriene modifiers and xanthines. CO‐INTERVENTIONS : % on ICS: LABA: 56; SABA: 56; PLA: 65 | |
| Outcomes | OUTCOMES MEASURED: AUC FEV1; am PEF; pm PEF; adverse events; symptoms; rescue beta‐agonist use; quality of life (AQLQ) FOLLOW‐UP ASSESSMENT POINTS: weeks 4 & 12 OUTCOMES INCLUDED IN ANALYSES AUC FEV1; adverse events SUB‐GROUPS INDENTIFIED: None reported | |
| Notes | Nocturnal asthma symptoms‐ 5 point scale, day time asthma symptoms: dyspnoea, chest discomfort, wheezing , cough each on 4 point scale (0‐12 total) | |
Lazarus 2001.
| Methods | STUDY DESIGN: Parallel group LOCATION, NUMBER OF CENTRES: USA, 6 ambulatory care centres (university based NIH sponsored centres) DURATION OF STUDY: 28 weeks 1997‐1999 ‐6 weeks pre‐treatment with triamcinolone, 16 weeks treatment, 6 weeks run out. CONCEALMENT OF ALLOCATION: COCHRANE QUALITY SCORE: A DESCRIBED AS RANDOMISED: Yes DESCRIBED AS DOUBLE BLIND: Yes METHOD OF RANDOMISATION WELL DESCRIBED/APPROPRIATE: stratified, internet to data centre off site. METHOD OF BLINDING WELL DESCRIBED/APPROPRIATE: triple, patients identical inhalers, clinical personnel, data entry DESCRIPTION OF WITHDRAWALS/DROPOUTS: 34 (7PLA, 13 SALM, 14 ICS) 12 in treatment period, reasons listed JADAD SCORE (5‐1): 5 TYPE OF ANALYSIS (AVAILABLE CASE/TREATMENT RECEIVED/ ITT): ITT for primary outcomes, survival data on last outcome entry COMPLIANCE: 81.1% medication CONFOUNDERS: Groups well balanced at baseline | |
| Participants | N SCREENED: 422, 361 completed run in on ICS N RANDOMISED: 339 eligible, 175 entered into LABA+ICS trial if not well controlled, 164 randomised‐ 56 placebo, 54 SALM, 54 ICS N COMPLETED: active treatment 151 (51 PLA, 48 SALM, 53 ICS) run out phase 130 (49 PLA, 41 SALM, 40 ICS) M= 39 (36%) F= 69 (64%) MEAN AGE: 31.5 (10.6) SEVERITY OF ASTHMA: persistent/ symptomatic BASELINE DETAILS: Previous ICS use 93%, rescue SABA use 0.5‐1.1p/day, ASS 0.2, AQOL median 2. INCLUSION CRITERIA : Age 12‐65, persistent asthma, FEV1 % predicted <85%, BDR = 12% increase in FEV1 after SABA if not on ICS, or FEV1 >40% predicted /FEV1 40‐80% predicted + BDR >12 % if on ICS, or if FEV1 >80% predicted PC20 < 8mg/ml methacholine. Lifetime smoking exposure < 10PH, not smoker currently, all subjects completed 6 weeks on ICS 400mcg BD prior to randomisation into PLA/SALM EXCLUSION: Pregnancy, lactation, no RTI/exacerbation <6 weeks, no regular medication except oral contraceptives or nasal beclomethasone, no serious medical illness other than asthma. | |
| Interventions | 1.SALMETEROL 50 mcg BD 2. TRIAMCINOLONE 40mcg BD 3. Placebo BD DELIVERY: MDI TREATMENT PERIOD: 16 weeks RESCUE: SABA CO‐INTERVENTIONS PERMITTED: none CO‐INTERVENTIONS : nasal ICS | |
| Outcomes | OUTCOMES MEASURED: treatment failure, asthma exacerbation, am PEF, pm PEF, FEV1, PC20, QOL, ASS, rescue, sputum markers inflammation FOLLOW‐UP ASSESSMENT POINTS: every 2‐4 weeks OUTCOMES INCLUDED IN ANALYSES: SUB‐GROUPS INDENTIFIED: None | |
| Notes | Asthma exacerbation definition‐increase cough, chest tightness, or wheezing associated with 1 or more or : change of =8 puffs/day SABA for 48 hours, =16 puffs/day SABA for 48 hours, PEF <65% reference level despite 60 minutes rescue, Symptoms despite 60 minutes treatment, requirement for OCS. Treatment failure status definition‐ requirement OCS course for exacerbation, ED visit or urgent care for asthma , hospitalisation for exacerbation, physician safety judgement | |
Leblanc 1996.
| Methods | STUDY DESIGN: 3 way cross over, multicentre 15 Canada. 12 weeks. RANDOMISATION: Yes, no method stated. BLINDING: double blind, double dummy, matching devices. WITHDRAWALS/DROP OUTS: 67 described after randomization. COMPLIANCE: 93% assessed by diary card. CONFOUNDERS: Smokers included QUALITY: Jadad 4. Cochrane B | |
| Participants | N= 367 RANDOMISED. Adults, M=164 F=203 Mean age:39 (SD 13.9) BASELINE SEVERITY: mild‐moderate asthma. INCLUSION : Baseline FEV1 >60% predicted, >15% FEV1 reversibility to SABA. Symptoms on 4/7 days run in, EXCLUSION: methylxanthines, anticholinergic, OS | |
| Interventions | LONG ACTING BETA AGONIST: Salmeterol 50 mcg BD SHORT ACTING BETA AGONIST: Salbutamol 200 mcg QDS PLACEBO: placebo QDS DEVICE: MDI. TEATMENT PERIOD: 12 weeks RESCUE: Salbutamol 100 mcg PRN CO‐INTERVENTIONS: ICS >80%, cromones 7% | |
| Outcomes | OUTCOMES: FEV1, FVC, PEF, Rescue use, asthma symptom free days & nights, adverse events . | |
| Notes | ||
Levy 2005.
| Methods | STUDY DESIGN: Parallel group LOCATION, NUMBER OF CENTRES: 22 in USA DURATION OF STUDY: 2 week run in, 12 weeks treatment CONCEALMENT OF ALLOCATION: COCHRANE QUALITY SCORE: B DESCRIBED AS RANDOMISED: Yes DESCRIBED AS DOUBLE BLIND: Yes METHOD OF RANDOMISATION WELL DESCRIBED/APPROPRIATE: not reported METHOD OF BLINDING WELL DESCRIBED/APPROPRIATE: matched placebo DESCRIPTION OF WITHDRAWALS/DROPOUTS: 22/249 reasons given JADAD SCORE (5‐1): 4 TYPE OF ANALYSIS (AVAILABLE CASE/TREATMENT RECEIVED/ ITT): ITT for efficacy variables. COMPLIANCE: not reported CONFOUNDERS: Baseline characteristics similar except higher % males in FORM group and BDR higher in PLAC group | |
| Participants | Children <13 years. N SCREENED: N/A, N RANDOMISED: 249, N COMPLETED: 227 M= 167 F= 60 MEAN AGE: 9.4 (2) BASELINE DETAILS: mild‐moderate persistent asthma, FEV1 75% predicted, asthma history 6.5 years INCLUSION CRITERIA:aged 5‐13 years, FEV1 % predicted = 50%, BDR = 15% 30 minutes after SABA 200mcg, requiring PRN bronchodilators, EXCLUSION: Change in co‐intervention or URTI or use of OCS within 1 month, significant medical condition, smoking. | |
| Interventions | 1. FORMOTEROL 10mcg BD 2. Placebo BD DELIVERY: DPI TREATMENT PERIOD: 12 weeks RESCUE: SABA albuterol PRN CO‐INTERVENTIONS PERMITTED: cromones, ICS CO‐INTERVENTIONS : % on ICS 74.8% FORM group 68.9% PLAC group | |
| Outcomes | OUTCOMES MEASURED: FEV1, exacerbations, PEF am/pm, asthma symptom scores, rescue use, AEs FOLLOW‐UP ASSESSMENT POINTS: 4 weekly OUTCOMES INCLUDED IN ANALYSES: SUB‐GROUPS INDENTIFIED: none | |
| Notes | Asthma exacerbation definition: asthma symptoms not resolving with study medication but requiring use of additional medication | |
Lindqvist 2003.
| Methods | STUDY DESIGN: Parallel group LOCATION, NUMBER OF CENTRES: single centre, Finland DURATION OF STUDY: 16 weeks with 2 week run in and 2 week follow up CONCEALMENT OF ALLOCATION: COCHRANE QUALITY SCORE: B DESCRIBED AS RANDOMISED: Yes DESCRIBED AS DOUBLE BLIND: Yes METHOD OF RANDOMISATION WELL DESCRIBED/APPROPRIATE: not reported METHOD OF BLINDING WELL DESCRIBED/APPROPRIATE: double dummy placebo controlled DESCRIPTION OF WITHDRAWALS/DROPOUTS: 2/80 reasons given JADAD SCORE (5‐1): 4 TYPE OF ANALYSIS (AVAILABLE CASE/TREATMENT RECEIVED/ ITT): efficacy population COMPLIANCE: not reported CONFOUNDERS: baseline groups similar characteristics | |
| Participants | N SCREENED: 83 N RANDOMISED: 80 N COMPLETED: 78 M= 34 F= 46 MEAN AGE: 39 SALM, 34 PLAC SEVERITY OF ASTHMA: persistent/ symptomatic BASELINE DETAILS: Asthma duration mean 4 months, FEV1 % predicted mean 80% INCLUSION CRITERIA : Asthma diagnosed within 2 years, symptomatic at enrolment, FEV1 60‐100% predicted, PD15FEV1 <0.4mg, non smokers (minimum 2 years) EXCLUSION: no ICS/ OCS within 8 WEEKS, No URTI/exacerbation use LABA or cromones within 4 weeks, no antihistamines within 2 weeks. | |
| Interventions | 1. Salmeterol 50 mcg BD 2. Placebo BD 3. Fluticasone 250 BD 4. disodium cromoglycate 5 mg QID DELIVERY: DPI TREATMENT PERIOD: 16 weeks RESCUE: SABA PRN CO‐INTERVENTIONS PERMITTED: None % on ICS 0% in SALM and PLAC groups | |
| Outcomes | OUTCOMES MEASURED: PEF am/pm, symptoms (0‐5 daytime, 0‐4 night),rescue use, FEV1, BDR, bronchoscopy inflammatory cell analysis. FOLLOW‐UP ASSESSMENT POINTS: 2 week period middle and end of treatment. OUTCOMES INCLUDED IN ANALYSES SUB‐GROUPS INDENTIFIED: none | |
| Notes | ||
Lockey 1999.
| Methods | STUDY DESIGN: Parallel group multicentre (49) USA, run as two identical studies. 2weeks run in/ 12 weeks treatment RANDOMISATION: Randomised treatment allocation, no details BLINDING: double blind, placebo controlled, matching devices WITHDRAWALS/DROP OUTS: 90 described, 54 placebo group, 36 active group COMPLIANCE: assessed from patient recordings CONFOUNDERS : Groups balanced by baseline characteristics, except active group more females, fewer ICS users, higher % predicted FEV1 QUALITY: Jadad 4 Cochrane B | |
| Participants | N= Randomised 474. M=217 F=257, adult/adolescents Mean age 39 (range 12‐76) BASELINE SEVERITY: Moderate persistent asthma. INCLUSION : Diagnosis asthma by ATS criteria >6mths, Baseline FEV1 40‐80% predicted, >15% FEV1 reversibility to SABA. Nocturnal symptoms run in 10/14 EXCLUSION: Serious uncontrolled systemic disease, pregnancy, OS change in co interventions <6wks , | |
| Interventions | LONG ACTING BETA AGONIST: Salmeterol 42 mcg BD PLACEBO: BD DEVICE: MDI TREATMENT PERIOD: 12 weeks RESCUE: albuterol 100 mcg prn CO‐INTERVENTIONS: ICS >62%, theophylline >23% | |
| Outcomes | OUTCOMES: FEV1, FVC, PEF, Asthma QOL score, Rescue use, asthma symptom score, % days with no symptoms ,% nights with no asthma awakenings, adverse events, exacerbations | |
| Notes | Exacerbations defined as worsening of asthma symptoms that required treatment in addition to the study drug and rescue SABA. Symptom Score‐ cough/wheeze/SOB. Night‐time 0‐3 (none ‐ so severe that no sleep possible). Day‐time 0‐3 (no symptoms ‐ symptoms so severe normal activities not possible | |
Mahajan 1998.
| Methods | STUDY DESIGN: Parallel group multicentre (11) USA, 12 weeks treatment RANDOMISATION: Randomised treatment allocation, no details BLINDING: double blind, placebo controlled, WITHDRAWALS/DROP OUTS: no details COMPLIANCE: no details CONFOUNDERS : Groups balanced by baseline characteristics QUALITY: Jadad 2 Cochrane B | |
| Participants | N= Randomised 207. M=142 F=65, children Mean age 8.4 (SD 2.4) BASELINE SEVERITY: Mild‐moderate asthma INCLUSION : Diagnosis asthma by ATS criteria >6mths, Baseline FEV1 60‐80% predicted, >15% FEV1 reversibility to SABA. EXCLUSION: none detailed, | |
| Interventions | LONG ACTING BETA AGONIST: Salmeterol 50 mcg BD PLACEBO: BD DEVICE: DP TREATMENT PERIOD: 12 weeks RESCUE: albuterol 100 mcg prn CO‐INTERVENTIONS: stable doses ICS >58%, cromones, theophylline, | |
| Outcomes | OUTCOMES: Asthma QOL scores ‐using FSII‐R, SLR‐C, parent activity limitation questionnaire. | |
| Notes | Functional Status II‐R, Sleep scale‐children (SLP‐C) | |
Nathan 1999.
| Methods | STUDY DESIGN: Parallel group 3 groups, multicentre (25) USA, 2 week run in/ 26 weeks treatment / 2 week run out RANDOMISATION: Randomised treatment allocation, no details BLINDING: double blind, double dummy, placebo controlled WITHDRAWALS/DROP OUTS: 81, 30 salmeterol, 23 BDP, 28 placebo COMPLIANCE: no details CONFOUNDERS : Groups well balanced by baseline characteristics QUALITY: Jadad 3 Cochrane B | |
| Participants | N= Randomised 386, M=179 F=207 adult/adolescents Mean age 30 (SD 21) SEVERITY OF ASTHMA: persistent/symptomatic INCLUSION : Diagnosis asthma by ATS criteria > 3/12, Baseline FEV1: 65‐90 % predicted. >15% FEV1 reversibility to SABA EXCLUSION: exacerbation asthma ,1mth, use of ICS/OS < 6mths, smoker, requiring > 12 puffs/day rescue SABA in run in 4/7 | |
| Interventions | LONG ACTING BETA AGONIST: Salmeterol 42 mcg BD PLACEBO: BD DEVICE: MDI, no spacer allowed TREATMENT PERIOD:26 weeks CO‐INTERVENTIONS: none permitted | |
| Outcomes | OUTCOMES: PEF ,FEV1, Rescue use, asthma symptom score, BHR , % days with no symptoms ,% nights with no asthma awakenings, Exacerbations | |
| Notes | Exacerbations defined as worsening of asthma symptoms that required treatment in addition to the study drug and rescue SABA. | |
Nelson 1998.
| Methods | STUDY DESIGN: 2 way cross over single centre USA, 1 week run in and cross over / 4 weeks treatment RANDOMISATION: Randomised treatment order allocation, no details BLINDING: double blind, placebo controlled , identical inhalers WITHDRAWALS/DROP OUTS: none occurred COMPLIANCE: no details CONFOUNDERS : subjects examined at cross over point to establish similarity of exercise response, no treatment order effect seen. QUALITY: Jadad 4 Cochrane B | |
| Participants | N= Randomised 20, M=9 F=11 adult Mean age 29 (SD 9) BASELINE SEVERITY: not described INCLUSION : Diagnosis exercise induced asthma by > 15 fall in FEV1 with exercise EXCLUSION: URTI/ LRTI/ OS 6 WKS | |
| Interventions | LONG ACTING BETA AGONIST: Salmeterol 42 mcg BD PLACEBO: BD DEVICE: MDI RESCUE: albuterol PRN TREATMENT PERIOD:4 weeks CO‐INTERVENTIONS: ICS (6/20), theophyllines: stable doses % ICS= 30% | |
| Outcomes | OUTCOMES: FEV1, FEV1 response to exercise | |
| Notes | ||
Nelson 1999b.
| Methods | STUDY DESIGN: 2 way cross over 2 centres USA, 2 week run in and 1 week cross over / 4 weeks treatment RANDOMISATION: Randomised treatment order allocation, no details BLINDING: double blind, placebo controlled , WITHDRAWALS/DROP OUTS: 6 COMPLIANCE: no details CONFOUNDERS : 14 day wash out for all beta2 agonists, none permitted during study. QUALITY: Jadad 3 Cochrane B | |
| Participants | N= Randomised 27, M=14 F=13 adult Mean age 32 (range 20‐56) BASELINE SEVERITY: Mild asthma INCLUSION : Diagnosis asthma by ATS criteria, Baseline FEV1 50‐80% predicted, >12% FEV1 reversibility to SABA. EXCLUSION: oral /inhaled beta2 agonist, use ICS/ OS | |
| Interventions | LONG ACTING BETA AGONIST: Salmeterol 42 mcg BD PLACEBO: BD DEVICE: MDI RESCUE: Ipratropium bromide PRN TREATMENT PERIOD:4 weeks CO‐INTERVENTIONS: none permitted | |
| Outcomes | OUTCOMES: FEV1, FVC, FEF 25‐75%, DRC to albuterol | |
| Notes | DRC=max FEV1 response to doubling doses inhaled albuterol | |
Newnham 1994.
| Methods | STUDY DESIGN: 2 way cross over single centre UK, 2 week run in / 4 weeks treatment RANDOMISATION: Randomised treatment order allocation, no details BLINDING: double blind, placebo controlled , matching devices WITHDRAWALS/DROP OUTS: none occurred COMPLIANCE: assessed by patient records CONFOUNDERS : No wash out at cross over. 12 hour wash‐out after study drug dose prior to DRC QUALITY: Jadad 4 Cochrane B | |
| Participants | N= Randomised 7, M=3 F= 4 adult. Mean age 34 (range 21‐52) BASELINE SEVERITY: Mild‐moderate asthma INCLUSION : Diagnosis asthma by ATS criteria, Baseline FEV1 44‐77% predicted, EXCLUSION: OS < 3mths, recent exacerbation. smokers | |
| Interventions | LONG ACTING BETA AGONIST: Formoterol 24 mcg BD PLACEBO: BD DEVICE: MDI RESCUE: Ipratropium bromide PRN TREATMENT PERIOD:4 weeks CO‐INTERVENTIONS: ICS 5/7 100‐2000mcg/day, nedocromil 1/7, theophylline % ICS=70% | |
| Outcomes | OUTCOMES: FEV1, FVC, FEF 25‐75%, DRC to albuterol, HR tremor, BP ECG | |
| Notes | ||
Newnham 1995.
| Methods | STUDY DESIGN: 2 way cross over single centre UK, 2 week run in / 4 weeks treatment RANDOMISATION: Randomised treatment order allocation, no details BLINDING: double blind, placebo controlled , matching capsules WITHDRAWALS/DROP OUTS: 2 COMPLIANCE: assessed by patient records and capsule count CONFOUNDERS : No wash out at cross over. 24 hour wash out after study drug dose prior to DRC QUALITY: Jadad 4 Cochrane B | |
| Participants | N= Randomised 18, completed 16, M=10 F= 6 adult. Mean age 33 (range 18‐53) BASELINE SEVERITY: Mild‐moderate stable asthma INCLUSION : Clinical diagnosis asthma, Baseline FEV1 40‐80% predicted, >15% FEV1 reversibility to SABA. EXCLUSION: OS/ recent exacerbation < 3mths. Smokers. Requiring > 600mcg salbutamol /day | |
| Interventions | LONG ACTING BETA AGONIST: Formoterol 24 mcg BD PLACEBO: BD DEVICE: DP capsules RESCUE: Ipratropium bromide 40mcg PRN TREATMENT PERIOD:4 weeks CO‐INTERVENTIONS: ICS 13/16 200‐2000mcg/day, theophylline 3/16‐ at constant % ICS=81% | |
| Outcomes | OUTCOMES: PEF , FEV1, FVC, FEF 25‐75%, DRC to albuterol, tremor, | |
| Notes | ||
Pearlman 1992.
| Methods | STUDY DESIGN: parallel‐group( 3), multicentre (8) in USA RANDOMIISATION: Randomised‐ means and methods not described. BLINDING: double‐blind, placebo controlled using 2 identical inhalers WITHDRAWALS: 33 DESCRIBED COMPLIANCE : Not reported in paper. CONFOUNDERS: Groups well balanced by characteristics QUALITY: Jadad score=4. COCHRANE= B | |
| Participants | N= Randomised 20, M=9 F=11 adults, Mean age 29 (SD 9) SEVERITY OF ASTHMA: unknown INCLUSION : Diagnosis exercise induced asthma by > 15 fall in FEV1 with exercise EXCLUSION: URTI/ LRTI/ OS 6 WKS | |
| Interventions | LONG ACTING BETA AGONIST : Salmeterol 42 mcg bd SHORT ACTING BETA AGONIST :Albuterol 180 mcg qds PLACEBO: placebo qds DEVICE:MDI PERIOD TREATMENT: 12 weeks RESCUE: Albuterol 90 mcg PRN COINTERVENTIONS: ICS 40%, cromones 7%, | |
| Outcomes | OUTCOMES: FEV1, FVC, PEF, Rescue use, asthma symptom score, adverse events . exacerbations | |
| Notes | Symtom Score‐ composite based on individual scores for breathlessness, chest tightness, wheezing, cough. Scale : 0=none to 5= severe, activities cancelled. Exacerbations defined as worsening of asthma symptoms that required treatment in addition to the study drug and rescue SABA. | |
Pearlman 1999a.
| Methods | STUDY DESIGN: Parallel group 6 groups, multicentre (11 centres) USA, 2 week run in / 4 weeks treatment RANDOMISATION: Randomised treatment allocation, no details BLINDING: double blind, placebo controlled , matching devices WITHDRAWALS/DROP OUTS: 7 COMPLIANCE: assessed during run in CONFOUNDERS: Groups well balanced by baseline characteristics QUALITY: Jadad 3 Cochrane B | |
| Participants | N= Randomised 44, M=24 F= 20 adult/adolescent . Mean age 32 (range 12‐62) SEVERITY OF ASTHMA: unknown INCLUSION : Diagnosis asthma by ATS criteria, stable, Baseline FEV1 50‐80% predicted,, >15% FEV1 reversibility to SABA , EXCLUSION: use of ICS/OS within 6mths, life threatening asthma, smoker, smoking history < 10pack years. | |
| Interventions | LONG ACTING BETA AGONIST: Salmeterol 42 mcg BD PLACEBO: BD DEVICE: MDI no spacer RESCUE: Albuterol prn TREATMENT PERIOD: 4 weeks CO‐INTERVENTIONS: 3 groups B= no ICS, | |
| Outcomes | OUTCOMES: PEF, FEV1, FVC, Rescue use, asthma symptom score, % days with no symptoms ,% nights with no asthma awakenings, adverse events, plasma cortisol | |
| Notes | Symptom Score‐ cough/wheeze/SOB ‐4 pt Scale :0= none to 3= severe | |
Pleskow 2003.
| Methods | STUDY DESIGN: Parallel group LOCATION, NUMBER OF CENTRES: Not clear DURATION OF STUDY: 12 weeks preceded by two week run‐in CONCEALMENT OF ALLOCATION: COCHRANE QUALITY SCORE: B DESCRIBED AS RANDOMISED: Yes DESCRIBED AS DOUBLE BLIND: Yes METHOD OF RANDOMISATION WELL DESCRIBED/APPROPRIATE: Unclear METHOD OF BLINDING WELL DESCRIBED/APPROPRIATE: Placebo inhaler devices matched to active treatments for double‐dummy technique. DESCRIPTION OF WITHDRAWALS/DROPOUTS: 484/554 completed the study (reasons for withdrawal not stated) JADAD SCORE (5‐1): 4 TYPE OF ANALYSIS (AVAILABLE CASE/TREATMENT RECEIVED/ ITT): ITT for primary outcome (FEV1 & % change in FEV1); symptoms, adverse events. Non‐ITT for remaining outcomes. COMPLIANCE: Not assessed CONFOUNDERS: Groups balanced for age, duration of asthma, height and ethnic background. | |
| Participants | Adults, N SCREENED: 707 (entered run‐in phase), N RANDOMISED: 554 , N COMPLETED: 484 M= 273 F= 281 MEAN AGE: 33.1 BASELINE DETAILS: Duration of asthma: 18 years; mild to moderate asthma INCLUSION CRITERIA: 12‐75 years; inhaled SABA daily for previous two months; FEV1 between 40‐80% predicted; =15% increase in FEV1 post‐SABA; CXR consistent with asthma prior to visit 1 EXCLUSION: Pregnant women/women child‐bearing potential who did not have reliable form of contraception; significant coronary heart disease; prior MI; uncontrolled hypertension; diabetes; convulsive disorder; intolerance of beta‐agonists; URTI within one month of study entry; hospitalisation for acute asthma within one month of study entry or during run‐in; non‐compliance to medical regimes; parenteral/oral steroids in month prior to visit 1; newly instituted/modified ICS therapy (including discontinuation); disodium cromoglycate; oral/inhaled anticholinergics; desensitization therapy; recent use of astemizole; use of theophylline in month prior to visit 1; use of antiarrhythmics; use of Prozac; vaccination with live virus in month prior to visit 1; weight 35% above or 25% predicted; significant smoking history; malignancy | |
| Interventions | 1. Formoterol 12mcg BD 2. Formoterol 24mcg BD 3. Short‐acting beta‐agonist four times daily 4. Placebo DELIVERY: MDI TREATMENT PERIOD: 12 weeks RESCUE: Salbutamol 90mcg prn CO‐INTERVENTIONS PERMITTED: ICS, theophylline SR. CO‐INTERVENTIONS: SABA prn % on ICS: Review by Gunkel gives pooled % with study 40 as 47.3%, Pleskow 44% ICS (45% FORM 12, 43% FORM 24, 49% PLA | |
| Outcomes | OUTCOMES MEASURED: % change in FEV1 L; FEV1 AUC; exacerbations (Defined as requirement for nebulised SABA); symptoms; FEV1 AUC; am PEF; deaths; ECG FOLLOW‐UP ASSESSMENT POINTS: 0, 2, 4, 8 & 12weeks OUTCOMES INCLUDED IN ANALYSES: combined asthma score; exacerbations; adverse events; withdrawal due to adverse events; deaths; ECG (number of category 4 abnormal counts) SUB‐GROUPS INDENTIFIED: Not reported. | |
| Notes | FORNDA 20831_41 published as Pleskow 2003 | |
Prieto 2002.
| Methods | STUDY DESIGN: Parallel group single centre study, Spain outpatient allergy clinic. 5‐7 day run in / 6 week treatment period RANDOMISATION: Randomised, no details BLINDING double blind, placebo controlled, no details WITHDRAWALS/DROP OUTS: 3 described. COMPLIANCE: weighing used canisters, result 83% active group, 90% placebo. CONFOUNDERS: participants not sensitized to other perennial allergens. QUALITY: Jadad 3 Cochrane B | |
| Participants | N = 30 randomised, 27 completed. M= 7, F= 20 Mean age 39.5 yrs range 17‐55. BASELINE SEVERITY: Seasonal mild asthma only. INCLUSION : Clinical diagnosis seasonal asthma, Baseline FEV1 >80% predicted, positive skin prick test to pollen allergen and negative to perennial allergens. EXCLUSION : Ever smoker, perennial asthma, COPD, URTI within 4 weeks, regular use of asthma therapy. | |
| Interventions | LONG ACTING BETA AGONIST: Salmeterol 50mcg BD PLACEBO: BD DEVICE: MDI via spacer RESCUE: Albuterol via MDI TREATMENT PERIOD: 6 weeks CO‐INTERVENTIONS: none permitted | |
| Outcomes | OUTCOMES: BHR PC20 methacholine, PC20 AMP, exhaled nitric oxide level, FEV1 | |
| Notes | ||
Ramage 1994.
| Methods | STUDY DESIGN: 2 way cross over single centre UK, 1 week run in / 4 weeks treatment/ 1 week wash out at cross over RANDOMISATION: Randomised treatment allocation, randomisation blocks balanced BLINDING: double blind, placebo controlled , WITHDRAWALS/DROP OUTS: none occurred COMPLIANCE: No details CONFOUNDERS: No details QUALITY: Jadad 4 Cochrane A | |
| Participants | N= Randomised 12, M=8 F= 4 adult. Mean age 25.8 (range 18‐50) SEVERITY OF ASTHMA: unknown INCLUSION : Clinical diagnosis asthma, Baseline FEV1 > 65% predicted; >20% fall in FEV1 with exercise , EXCLUSION: exacerbation/ change in asthma medication < 4wks, smoker URTI/ LRTI/hospitalisation/ OCS < 4 WKS , unstable asthma, smoking | |
| Interventions | LONG ACTING BETA AGONIST: Salmeterol 50 mcg BD PLACEBO: BD DEVICE: MDI RESCUE: Salbutamol 100 mcg prn TREATMENT PERIOD: 4 weeks CO‐INTERVENTIONS: ICS 11/12, cromone 1/12, theophylline %ICS 91% | |
| Outcomes | OUTCOMES: Max fall in FEV1 with exercise | |
| Notes | ||
Roberts 1999.
| Methods | STUDY DESIGN: Parallel group single centre UK, 2 week run in / 6 weeks treatment RANDOMISATION: Randomised treatment allocation, no details BLINDING: double blind, placebo controlled , matching devices. WITHDRAWALS/DROP OUTS: none occurred COMPLIANCE: No details CONFOUNDERS: Groups well balanced by baseline characteristics QUALITY: Jadad 4 Cochrane B | |
| Participants | N= Randomised 26, M=14 F= 12 adult. Mean age 31 (range 18‐55) BASELINE SEVERITY: symptomatic/persistent INCLUSION : Clinical diagnosis asthma, atopic, requiring SABA as sole treatment. Diurnal variation PEF >15% or ASS >2 or using SABA 7/14 days run in EXCLUSION:ICS / OCS < 4 week, methylxanthines, cromones, anticholinergic, antihistamines | |
| Interventions | LONG ACTING BETA AGONIST: Salmeterol 50 mcg BD PLACEBO: BD DEVICE: MDI RESCUE: Salbutamol 100 mcg prn TREATMENT PERIOD: 6 weeks CO‐INTERVENTIONS: none permitted | |
| Outcomes | OUTCOMES: PEF, Rescue use, asthma symptom score, EBB. BAL. BHR | |
| Notes | Symptom Score‐ cough/wheeze/SOB. Night‐time 5 pt: 0‐4 (none ‐ so severe that no sleep possible). Day‐time 5 pt: 0‐4 (no symptoms ‐ symptoms so severe normal activities not possible) | |
Rosenthal 1999.
| Methods | STUDY DESIGN: 2 Parallel group studies, multi‐centre (31)USA, 2 week run in / 24 weeks treatment/ 4 weeks run out RANDOMISATION: Randomised treatment allocation, no details BLINDING: double blind, placebo controlled , blinded devices. WITHDRAWALS/DROP OUTS: 87 after randomisation COMPLIANCE: No details CONFOUNDERS: Groups well balanced by baseline characteristics QUALITY: Jadad 4 Cochrane B | |
| Participants | N= Randomised 408, M=241 F= 167 adult/adolescent. Mean age 28 (range 12‐65) BASELINE SEVERITY: Mild asthma INCLUSION : Clinical diagnosis asthma, Baseline FEV1 >70% predicted, >15% FEV1 reversibility to SABA.BHR PD20 <512mcg or 7.5mg/ml methacholine EXCLUSION: URTI/ LRTI < 6 WKS hospitalisation < 12wks Serious uncontrolled systemic disease | |
| Interventions | LONG ACTING BETA AGONIST: Salmeterol 42 mcg BD PLACEBO: BD DEVICE: MDI RESCUE: Albuterol 100 mcg prn TREATMENT PERIOD: 24 weeks CO‐INTERVENTIONS: none permitted | |
| Outcomes | OUTCOMES: PEF, FEV1, FVC, Rescue use, asthma symptom score. BHR. . Exacerbations | |
| Notes | Symptom Score‐ cough/wheeze/SOB. Night‐time 4 pt: 0‐3 (none ‐ so severe that no sleep possible). Day‐time 5 pt: 0‐4 (no symptoms ‐ symptoms so severe normal activities not possible) Exacerbations defined as worsening of asthma symptoms that required treatment in addition to the study drug and rescue SABA. If ICS/OS required subject withdrawn | |
SAS30003.
| Methods | STUDY DESIGN: Parallel group LOCATION, NUMBER OF CENTRES: 37 centres in North and Central America DURATION OF STUDY: 12 weeks CONCEALMENT OF ALLOCATION: COCHRANE QUALITY SCORE: B DESCRIBED AS RANDOMISED: Yes DESCRIBED AS DOUBLE BLIND: Yes METHOD OF RANDOMISATION WELL DESCRIBED/APPROPRIATE: Not reported METHOD OF BLINDING WELL DESCRIBED/APPROPRIATE: Not reported DESCRIPTION OF WITHDRAWALS/DROPOUTS: 81/360 JADAD SCORE (5‐1): 3 TYPE OF ANALYSIS (AVAILABLE CASE/TREATMENT RECEIVED/ ITT): ITT COMPLIANCE: Not reported. CONFOUNDERS: Baseline characteristics similar. | |
| Participants | N SCREENED: Not reported. N RANDOMISED: 360 N COMPLETED: 279 M= 159 F= 201 MEAN AGE: 33 years BASELINE DETAILS: FEV1 2.3L; SEVERITY OF ASTHMA: unknown, severity of asthma not described INCLUSION CRITERIA: =12 years of age; ATS defined asthma; FEV1 40‐85% predicted; FEV1 reversibility =15% post‐SABA; participants not treated with ICS were to have required SABA prn for one week prior to visit 1, and those with 7 day total symptom score =7 for 7 days prior to visit 2; participants previously treated with salmeterol were required to have been treated for one week prior to visit 1. EXCLUSION: ICS treatment which had been altered or initiated within previous three months. | |
| Interventions | 1. Salmeterol 42mcg BID 2. Placebo 3. Fluticasone/salmeterol combination 88/42mcg BID 4. Fluticasone 88mcg BID DELIVERY: MDI TREATMENT PERIOD: 12 weeks RESCUE: Salbutamol dose unspecified prn CO‐INTERVENTIONS PERMITTED: ICS; SABA prn CO‐INTERVENTIONS: as above % on ICS: 0% in PLA and SALM | |
| Outcomes | OUTCOMES MEASURED: Withdrawals; Change in am FEV1; change in am PEF; change in pm PEF; Change in rescue medication usage; change in nighttime awakenings; change in daily asthma symptom score; change in AQLQ FOLLOW‐UP ASSESSMENT POINTS: 12 weeks OUTCOMES INCLUDED IN ANALYSES: Withdrawals; change in am PEF; change in pm PEF; Change in rescue medication usage; change in nighttime awakenings; change in daily asthma symptom score SUB‐GROUPS INDENTIFIED: Participants using ICS; participants not using ICS (stratified data not reported) | |
| Notes | ||
SAS30004.
| Methods | STUDY DESIGN: Parallel group LOCATION, NUMBER OF CENTRES: 45 centres in USA DURATION OF STUDY: 12 weeks CONCEALMENT OF ALLOCATION: COCHRANE QUALITY SCORE: B DESCRIBED AS RANDOMISED: Yes DESCRIBED AS DOUBLE BLIND: Yes METHOD OF RANDOMISATION WELL DESCRIBED/APPROPRIATE: Not reported METHOD OF BLINDING WELL DESCRIBED/APPROPRIATE: Not reported DESCRIPTION OF WITHDRAWALS/DROPOUTS: 122/365 JADAD SCORE (5‐1): 3 TYPE OF ANALYSIS (AVAILABLE CASE/TREATMENT RECEIVED/ ITT): ITT COMPLIANCE: Not assessed CONFOUNDERS: Baseline characteristics similar | |
| Participants | N SCREENED: Not reported N RANDOMISED: 365 N COMPLETED: 243 M= 145 F= 220 MEAN AGE: 39 BASELINE DETAILS: Baseline FEV1 2.2L; SEVERITY OF ASTHMA: unknown INCLUSION CRITERIA: =12 years of age; ATS defined asthma; asthma inadequately controlled with ICS; FEV1 40‐85% predicted; FEV1 =15% reversibility; treatment with ICS prior to visit 1 EXCLUSION: None reported | |
| Interventions | 1. Salmeterol 42mcg BID 2. Placebo 3. Fluticasone 220mcg BID 4. Fluticasone/salmeterol combination 220/42mcg BID DELIVERY: MDI TREATMENT PERIOD: 12 weeks RESCUE: SABA prn CO‐INTERVENTIONS PERMITTED: SABA prn CO‐INTERVENTIONS: SABA prn % on ICS: 0 | |
| Outcomes | OUTCOMES MEASURED: Withdrawal due to worsening asthma; change in FEV1 L; Change in am PEF; Change in pm PEF; Change in SABA usage; Change in % days with no SABA; Change in % nights with no awakening; Change in symptom scores; % change in symptom scores; Change in AQLQ; withdrawals; adverse events FOLLOW‐UP ASSESSMENT POINTS: 12 weeks OUTCOMES INCLUDED IN ANALYSES: Withdrawal due to worsening asthma; withdrawals; adverse events SUB‐GROUPS INDENTIFIED: Not reported | |
| Notes | ||
Schreurs 1996.
| Methods | STUDY DESIGN: Parallel group, 4 groups, multi‐centre (15) Netherlands, hospital setting, 1 week run in / 4 weeks treatment RANDOMISATION: Randomised treatment allocation, no details BLINDING: double blind, placebo controlled , WITHDRAWALS/DROP OUTS: 28 COMPLIANCE: No details CONFOUNDERS: Groups well balanced by baseline characteristics QUALITY: Jadad 3 Cochrane B | |
| Participants | N=Enrolled 236 Randomised 220 completed 194. M=134 F= 60 adults. Mean age 47 (SD 15) BASELINE SEVERITY: Moderate asthma. INCLUSION : Diagnosis asthma by ATS criteria 6 months. Baseline FEV1 40‐70% predicted, >15% FEV1 reversibility to SABA. EXCLUSION: URTI/ LRTI < 4 WKS, pronounced seasonal allergy, Serious uncontrolled systemic disease, OS, theophyllines, anticholinergics | |
| Interventions | LONG ACTING BETA AGONIST: Formoterol 6mcg, 12 mcg or 24 mcg BD PLACEBO: BD DEVICE: DP turbuhaler RESCUE: terbutaline 50 mcg prn TREATMENT PERIOD: 4 weeks CO‐INTERVENTIONS: ICS 90%, cromones; at stable dose | |
| Outcomes | OUTCOMES: PEF, FEV1, Rescue use, asthma symptom score, diurnal variation PEF, | |
| Notes | Symptom Score‐ cough/wheeze/SOB ‐4 pt Scale :0= none to 3= severe | |
Shapiro 2000b.
| Methods | STUDY DESIGN: Parallel group, multi‐ centre (42) study in USA, 2 week run in / 12 weeks treatment RANDOMISATION: Randomised treatment allocation, no details BLINDING: double blind, placebo controlled , WITHDRAWALS/DROP OUTS: 13 active = FP, 22 placebo + FP COMPLIANCE: by medication count .>91% all groups CONFOUNDERS: Groups well balanced by baseline characteristics QUALITY: Jadad 3 Cochrane B | |
| Participants | N=screened 484 Randomised 181 M=78 F= 103 adults/adolescents. Mean age 38 (range 12‐69) BASELINE SEVERITY: moderate stable asthma on regular ICS INCLUSION : Diagnosis asthma by ATS criteria > 6mths Using ICS regularly for >3mths pre study . Baseline FEV1 >75% predicted, >15% FEV1 reversibility to SABA. EXCLUSION: unstable asthma‐ requiring > 12 puffs /day albuterol, OCS for exacerbation < 4 WKS, regular OS within 6mths, nocturnal asthma > 3/week, smoker, smoking history > 10 pack years | |
| Interventions | LONG ACTING BETA AGONIST: Salmeterol 50 mcg BD PLACEBO: BD DEVICE: DP diskhaler RESCUE: albuterol 100 mcg prn TREATMENT PERIOD: 12 weeks CO‐INTERVENTIONS: none permitted | |
| Outcomes | OUTCOMES: Exacerbation of asthma leading to withdrawal, PEF, FEV1, Rescue use, asthma symptom score, 12 hr serial FEV1, nocturnal asthma awakenings | |
| Notes | Exacerbations defined as clinical worsening of asthma symptoms that require Emergency room treatment, hospitalization or treatment in addition to the study drug and rescue SABA. Symptom Score‐ cough/wheeze/SOB. 5 pt: 0‐4 (no symptoms ‐ symptoms so severe normal activities not possible) | |
Simons 1997a.
| Methods | STUDY DESIGN: Parallel group, 3 treatment groups, multicentre Canada, 2 week run in/ 52 weeks treatment / 2 week run out RANDOMISATION: Randomised treatment allocation, no details BLINDING: double blind, placebo controlled , WITHDRAWALS/DROP OUTS: 60 COMPLIANCE: by counting unused blisters. 99% all subjects had compliance >75% CONFOUNDERS: Groups well balanced by baseline characteristics QUALITY: Jadad 3 Cochrane B | |
| Participants | N enrolled= 315 Randomised 241 M=140 F= 101 children. Mean age 9.3 (range 6‐14) BASELINE SEVERITY: Mild‐moderate persistent asthma INCLUSION : Clinical stable asthma, atopic, well controlled, Baseline FEV1 >70% predicted, >10% FEV1 reversibility to SABA. PC20 methacholine < 8mg/ml EXCLUSION use ICS/ OS for >4 weeks previously, OS/ICS within 3mths. exacerbation < 12 WKS, | |
| Interventions | LONG ACTING BETA AGONIST: Salmeterol 50 mcg BD PLACEBO: BD DEVICE: DP diskus RESCUE: albuterol 200 mcg prn TREATMENT PERIOD: 4 weeks CO‐INTERVENTIONS: ICS not permitted (3rd group given BDP 400mcg/day) | |
| Outcomes | OUTCOMES: FEV1, FVC, FEV25‐75%, PEF, Rescue use, days missing school due to asthma , BHR, % days with no rescue use ,% nights with no asthma awakenings, adverse events | |
| Notes | Exacerbations defined as worsening of asthma symptoms that required treatment in addition to the study drug and rescue SABA. | |
SLGA2004.
| Methods | STUDY DESIGN: Parallel group LOCATION, NUMBER OF CENTRES: 12 in USA DURATION OF STUDY: 4 weeks CONCEALMENT OF ALLOCATION: COCHRANE QUALITY SCORE: B DESCRIBED AS RANDOMISED: Yes DESCRIBED AS DOUBLE BLIND: Yes METHOD OF RANDOMISATION WELL DESCRIBED/APPROPRIATE: not reported METHOD OF BLINDING WELL DESCRIBED/APPROPRIATE: not reported DESCRIPTION OF WITHDRAWALS/DROPOUTS: 15/210 JADAD SCORE (5‐1): 3 TYPE OF ANALYSIS (AVAILABLE CASE/TREATMENT RECEIVED/ ITT): ITT for safety data, efficacy population excluded WD prior to visit 3 and protocol deviations COMPLIANCE: Not reported CONFOUNDERS: Baseline characteristics similar | |
| Participants | N SCREENED: not reported N RANDOMISED: 210 N COMPLETED: 195 M= 135 F= 75 MEAN AGE: 32 (14) BASELINE DETAILS: Mild‐moderate asthma requiring therapy during previous 6 months INCLUSION CRITERIA: =12 years of age; ATS defined asthma; FEV1 50‐85% predicted; FEV1 reversibility =15% post‐SABA EXCLUSION: serious uncontrolled illness, current smokers, > 10 pack year history smoking | |
| Interventions | 1.Salmeterol 50mcg BD MDPI 2.Salmeterol 50 mcg BD Diskhaler 3.Placebo BD TREATMENT PERIOD: 4 weeks RESCUE: SABA PRN CO‐INTERVENTIONS PERMITTED: not specified CO‐INTERVENTIONS : not reported % on ICS not reported | |
| Outcomes | OUTCOMES MEASURED: 12 hour serial FEV1, 12 hour serial FEF 25‐75%, am/pm PEF, rescue use, awakenings, symptoms, QOL using SF36, ALQL, sleep scale FOLLOW‐UP ASSESSMENT POINTS: 4 weeks OUTCOMES INCLUDED IN ANALYSES SUB‐GROUPS INDENTIFIED: none | |
| Notes | ||
SLGA3014 1994.
| Methods | STUDY DESIGN: Parallel group LOCATION, NUMBER OF CENTRES: 36 centres in USA. DURATION OF STUDY: CONCEALMENT OF ALLOCATION: COCHRANE QUALITY SCORE: B DESCRIBED AS RANDOMISED: Yes DESCRIBED AS DOUBLE BLIND: Yes METHOD OF RANDOMISATION WELL DESCRIBED/APPROPRIATE: Not reported METHOD OF BLINDING WELL DESCRIBED/APPROPRIATE: Yes (double‐dummy) DESCRIPTION OF WITHDRAWALS/DROPOUTS: 59/449 (lack of efficacy: 11; adverse event: 8; failure to return: 7; other: 33). JADAD SCORE (5‐1): 4. TYPE OF ANALYSIS (AVAILABLE CASE/TREATMENT RECEIVED/ ITT): Non‐ITT for efficacy outcomes; ITT for safety outcomes. COMPLIANCE: Not assessed. CONFOUNDERS: Baseline characteristics not adequately presented. | |
| Participants | N SCREENED: 756 N RANDOMISED: 449 N COMPLETED: 390 M= 61% F= 39% MEAN AGE: 8 years (range 4‐11) BASELINE DETAILS: Caucasian 78%, Black 12%, Mean PEFR 80% predicted; mean FEV1 70% predicted; 65% report nocturnal symptoms; 80% report that symptoms interfere with activities one day per week SEVERITY OF ASTHMA: persistent INCLUSION CRITERIA: PEFR (4‐5 years)/FEV1 (6‐11 years 45‐80% predicted) EXCLUSION: Not reported. | |
| Interventions | 1. Salmeterol 25mcg BID 2. Salmeterol 50mcg BID 3. Placebo DELIVERY: DPI TREATMENT PERIOD: 12 weeks (no run‐in described) RESCUE: SABA prn CO‐INTERVENTIONS PERMITTED: ICS, cromolyn, nedocromil CO‐INTERVENTIONS: cromolyn/nedocromil: 25% % on ICS: approx 50% | |
| Outcomes | OUTCOMES MEASURED: Serial PEFR % predicted; PEFR AUC; serial FEV1 % predicted; FEV1 AUC; change in post‐dose PEFR % predicted; change in post‐dose FEV1 % predicted; mean change in am PEFR; change in daily asthma symptom score; change in % nights with no awakenings; withdrawals; adverse events; FOLLOW‐UP ASSESSMENT POINTS: Day 1, weeks 6 & 12 OUTCOMES INCLUDED IN ANALYSES: Withdrawals; adverse events SUB‐GROUPS INDENTIFIED: <9 years versus >9 years. | |
| Notes | Symptom score 1= no symptoms at all, 2= symptoms with little or no discomfort, unrestricted activity, 3=symptoms occurred, were sometimes annoying or affected routine activity, 4= symptoms occurring even at rest, were annoying or affected activity | |
SLGL82.
| Methods | STUDY DESIGN: Parallel group LOCATION, NUMBER OF CENTRES: Thailand, 4 centres DURATION OF STUDY: 4 weeks CONCEALMENT OF ALLOCATION: COCHRANE QUALITY SCORE: B DESCRIBED AS RANDOMISED: Yes DESCRIBED AS DOUBLE BLIND: Yes METHOD OF RANDOMISATION WELL DESCRIBED/APPROPRIATE: Not reported METHOD OF BLINDING WELL DESCRIBED/APPROPRIATE: Not reported. DESCRIPTION OF WITHDRAWALS/DROPOUTS: 7/90 (Adverse events: 5; other reasons: 2) JADAD SCORE (5‐1): 3 TYPE OF ANALYSIS (AVAILABLE CASE/TREATMENT RECEIVED/ ITT): Available case for am & pm PEFR; ITT for other outcomes COMPLIANCE: Not assessed. CONFOUNDERS: Symptom scores higher in placebo group for night symptoms; | |
| Participants | Adults, SCREENED: Not reported , N RANDOMISED: 90 , N COMPLETED: 82 M= 30 F= 52 MEAN AGE: 43 BASELINE DETAILS: Mild asthma: 21; moderate asthma: 67 (criteria unspecified); FEV1: 1.73L INCLUSION CRITERIA: FEV1 >50% predicted; PEFR during last 7 days of run‐in >50% predicted; FEV1 15% reversibility post‐SABA; day/night symptom score =2 EXCLUSION: <12 years; serious uncontrolled disease; lower RTI; hospitalisation due to airways disease; maintenance dose of prednisolone <20mcg/d; intolerance of beta‐agonist; current treatment with beta‐agonist. | |
| Interventions | 1. Salmeterol 50mcg BID 2. Placebo DELIVERY: MDI TREATMENT PERIOD: 4 weeks RESCUE: Salbutamol (dose not specified) CO‐INTERVENTIONS PERMITTED: oral steroids up to 20mcg/d CO‐INTERVENTIONS: Not stated. % on ICS: Not reported. | |
| Outcomes | OUTCOMES MEASURED: Symptoms; rescue medication usage; FEV1; PEFR; adverse events FOLLOW‐UP ASSESSMENT POINTS: 2 & 4 weeks. OUTCOMES INCLUDED IN ANALYSES: Symptoms; rescue medication usage; FEV1; PEFR; adverse events SUB‐GROUPS INDENTIFIED: Not reported. | |
| Notes | ||
SLMP03.
| Methods | STUDY DESIGN: Parallel group LOCATION, NUMBER OF CENTRES: Italy, one centre. DURATION OF STUDY: 8 weeks CONCEALMENT OF ALLOCATION: COCHRANE QUALITY SCORE: B DESCRIBED AS RANDOMISED: Yes DESCRIBED AS DOUBLE BLIND: Yes METHOD OF RANDOMISATION WELL DESCRIBED/APPROPRIATE: Not reported METHOD OF BLINDING WELL DESCRIBED/APPROPRIATE: Same inhaler device use for both treatments. DESCRIPTION OF WITHDRAWALS/DROPOUTS: 15/24 (reasons not specified) JADAD SCORE (5‐1): 4 TYPE OF ANALYSIS (AVAILABLE CASE/TREATMENT RECEIVED/ ITT): ITT COMPLIANCE: Not assessed CONFOUNDERS: Characteristics similar. | |
| Participants | N SCREENED: Not reported. N RANDOMISED: 24 N COMPLETED: 9 M= 16 F= 8 MEAN AGE: 9.6 years (SD 2.7) BASELINE DETAILS: am PEFR: 325L/min; pm PEFR 334L/min; median % nights free from asthma: 100; median % days free from asthma: 90. INCLUSION CRITERIA: 6‐14 years; mild to moderate asthma (criteria not specified); recent asthma attacks despite treatment with beta‐agonists; sensitivity to methacholine. EXCLUSION: Seasonal allergic asthma; treatment with topical steroids, theophylline, salmeterol, anticholinergic agents, beta‐blockers or cromones. | |
| Interventions | 1. Salmeterol 50mcg BID 2. Placebo DELIVERY: DPI TREATMENT PERIOD: 8 weeks RESCUE: Salbutamol (dose not specified). CO‐INTERVENTIONS PERMITTED: Rescue medication (SABA prn) CO‐INTERVENTIONS: Not reported. % on ICS:0% | |
| Outcomes | OUTCOMES MEASURED: PD20; am PEFR; pm PEFR; symptoms & rescue medication usage. FOLLOW‐UP ASSESSMENT POINTS: 8 weeks OUTCOMES INCLUDED IN ANALYSES: PD20; am PEFR; pm PEFR; symptoms & rescue medication usage SUB‐GROUPS INDENTIFIED: Not reported. | |
| Notes | ||
SMART.
| Methods | STUDY DESIGN: Parallel group LOCATION, NUMBER OF CENTRES: USA, 6163 centres DURATION OF STUDY: 28 weeks CONCEALMENT OF ALLOCATION: COCHRANE QUALITY SCORE: B DESCRIBED AS RANDOMISED: Yes DESCRIBED AS DOUBLE BLIND: Yes METHOD OF RANDOMISATION WELL DESCRIBED/APPROPRIATE: Not reported. METHOD OF BLINDING WELL DESCRIBED/APPROPRIATE: Treatments administered through same delivery device. DESCRIPTION OF WITHDRAWALS/DROPOUTS: 6102/26355 (adverse events: 244; lack of efficacy: 878; other reasons: 4974) JADAD SCORE (5‐1): 4 TYPE OF ANALYSIS (AVAILABLE CASE/TREATMENT RECEIVED/ ITT): ITT COMPLIANCE: VAS measured (0‐10, 10=most doses taken): 7.9 CONFOUNDERS: Baseline characteristics similar. | |
| Participants | N SCREENED: Not reported. N RANDOMISED: 26,355 N COMPLETED: 19128 M= 9389 F= 16671 MEAN AGE: 39 BASELINE DETAILS: Mean PEFR: 355L/min; PEFR predicted: 84%; duration of asthma: 16.3 years SEVERITY OF ASTHMA: unknown INCLUSION CRITERIA: =12 years of age; diagnosis of asthma (clinical investigator); receiving current prescription of asthma medication. EXCLUSION: Prior use of LABA; pregnancy or lactation; concurrent disease that may pose a risk to the participant; sensitivity to long‐acting beta‐agonists; current ß‐blocker use. | |
| Interventions | 1. Salmeterol 42mcg BID 2. Placebo DELIVERY: MDI TREATMENT PERIOD: 52 weeks RESCUE: Salbutamol (dose not reported). CO‐INTERVENTIONS PERMITTED: usual asthma therapy CO‐INTERVENTIONS: ICS, methylxanthines, leukotriene agents % on ICS: 47% | |
| Outcomes | OUTCOMES MEASURED: Mortality; adverse events. FOLLOW‐UP ASSESSMENT POINTS: 28 weeks. OUTCOMES INCLUDED IN ANALYSES: Mortality; adverse events. SUB‐GROUPS INDENTIFIED: Caucasian/African American participants; ICS at baseline/no ICS at baseline. | |
| Notes | Exacerbations defined as worsening of asthma symptoms that required treatment in addition to the study drug and rescue SABA. Symptom Score‐ cough/wheeze/SOB. Night‐time 0‐3 (none ‐ so severe that no sleep possible). Day‐time 0‐3 (no symptoms ‐ symptoms so severe normal activities not possible | |
SMS40221.
| Methods | STUDY DESIGN: Parallel group LOCATION, NUMBER OF CENTRES: Taiwan, one centre. DURATION OF STUDY: 4 weeks CONCEALMENT OF ALLOCATION: COCHRANE QUALITY SCORE: B DESCRIBED AS RANDOMISED: Yes DESCRIBED AS DOUBLE BLIND: Yes METHOD OF RANDOMISATION WELL DESCRIBED/APPROPRIATE: Not reported METHOD OF BLINDING WELL DESCRIBED/APPROPRIATE: Both treatments delivered by the same device. DESCRIPTION OF WITHDRAWALS/DROPOUTS: 13/64 (adverse events: 3; lack of efficacy: 6; other reasons: 4) JADAD SCORE (5‐1): 4 TYPE OF ANALYSIS (AVAILABLE CASE/TREATMENT RECEIVED/ ITT): ITT COMPLIANCE: Not assessed. CONFOUNDERS: Baseline data not available (all data reported as change). | |
| Participants | N SCREENED: Not reported N RANDOMISED: 64 N COMPLETED: 51 M= 21 F= 43 MEAN AGE: 48 BASELINE DETAILS: Not reported. SEVERITY OF ASTHMA: unknown INCLUSION CRITERIA: >18 years; FEV1 % predicted >50; increase in FEV1 >15% predicted post 400mcg fenoterol or >20% post 800mcg fenoterol; symptom score of at least 2 on three of last 7 days of run‐in period. EXCLUSION: Serious uncontrolled disease; lower RTI; hospitalisation with acute exacerbation of airways disease in previous two weeks; OCS in excess of 20mcg/d. | |
| Interventions | 1. Salmeterol 50mcg BID 2. Placebo DELIVERY: DPI TREATMENT PERIOD: 4 weeks preceded by run‐in (duration not specified). RESCUE: Fenoterol (dose not specified). CO‐INTERVENTIONS PERMITTED: Fenoterol; OCS up to 20mcg/d CO‐INTERVENTIONS: SABA prn % on ICS: Not reported. | |
| Outcomes | OUTCOMES MEASURED: Change in am PEFR (L/min); change in pm PEFR (L/min); change in FEV1 (L); change in FVC (L); change in symptoms; physician rated assessment; participant rated assessment; adverse events. FOLLOW‐UP ASSESSMENT POINTS: 4 weeks. OUTCOMES INCLUDED IN ANALYSES: Change in am PEFR (L/min); change in pm PEFR (L/min); change in FEV1 (L); change in FVC (L); change in symptoms; physician rated assessment; participant rated assessment; adverse events. SUB‐GROUPS INDENTIFIED: Not reported. | |
| Notes | ||
Starke 1996.
| Methods | STUDY DESIGN: 2 way cross over single centre UK, 2 week run in/ 4 week treatment period/ 7‐10 day run out RANDOMISATION: Randomised treatment allocation, no details BLINDING: double blind, placebo controlled ,matching devices WITHDRAWALS/DROP OUTS: 6 COMPLIANCE: no details CONFOUNDERS: data from weeks 2‐4 treatment analysed QUALITY: Jadad 4 Cochrane B | |
| Participants | N Randomised 28 M=15 F= 13 older adults Median age 74 (range 64‐88) BASELINE SEVERITY: not specified INCLUSION : over 64 years age. >15% diurnal variation PEF or symptoms of airflow limitation > 4/7 run in days, able to use MDI with or without a spacer/adapter. EXCLUSION: LRTI/hospitalisation asthma with 4 weeks, use of methylxanthines, Serious uncontrolled systemic disease | |
| Interventions | LONG ACTING BETA AGONIST: Salmeterol 50 mcg BD PLACEBO: BD DEVICE: MDI +/‐ spacer RESCUE: Salbutamol 100 mcg prn TREATMENT PERIOD: 4 weeks CO‐INTERVENTIONS: ICS , OS, cromones, ketotifen, anticholinergics‐ all at stable dose | |
| Outcomes | OUTCOMES: FEV1, PEF, Rescue use, asthma symptom score. Efficacy score‐patient and investigator rated, adverse events | |
| Notes | Symptom Score‐ cough/wheeze/SOB. 6 pt: 0‐5 (no symptoms ‐ symptoms so severe normal activities not possible) | |
Steffensen 1995.
| Methods | STUDY DESIGN: Parallel group ( 3 treatment groups)‐multicentre , 20 Scandinavia. 2 week run in/ 12 weeks. RANDOMISATION: Yes, no method stated. BLINDING: double blind, double dummy, matching devices. WITHDRAWALS/DROP OUTS: 42 described after randomization. COMPLIANCE:. Not assessed CONFOUNDERS: Groups well balanced by characteristics QUALITY: Jadad 4. Cochrane B | |
| Participants | N= 362 ENROLLED, 304 RANDOMISED 204 to LABA or placebo). ADULT , M=98 F=106 Mean age: 48.5 (SD 14) BASELINE SEVERITY: not specified, clinically stable. INCLUSION : Baseline FEV1 >40% predicted, >15% FEV1 reversibility to SABA. Requiring SABA. EXCLUSION: unstable asthma, altered dose medication. | |
| Interventions | LONG ACTING BETA AGONIST: Formoterol 12 mcg BD SHORT ACTING BETA AGONIST: Salbutamol 400 mcg QDS PLACEBO: placebo QDS DEVICE: Dry powder device. TEATMENT PERIOD: 12 weeks RESCUE: Salbutamol 100 mcg PRN CO‐INTERVENTIONS: ICS 84%, OS 6%, cromones stable dose | |
| Outcomes | OUTCOMES: FEV1, FVC, PEF, Rescue use, asthma symptom score. adverse events, including asthma exacerbations. Eficacy rating | |
| Notes | Symptom Score‐ Day: 0=none, to 3=very severe symptoms. night: 0=none to 3=almost no sleep due to asthma. No definition of asthma exacerbation recorded as an adverse event given. | |
Stelmach 2002.
| Methods | STUDY DESIGN: Parallel group single centre study, Poland, university centre. 4 week run in (off all treatment except Ipratropium) / 4 week treatment period RANDOMISATION: Randomised, computer generated schedule. BLINDING double blind, placebo controlled, matching capsules. WITHDRAWALS/DROP OUTS: 2 in placebo group with exacerbations. COMPLIANCE: no details CONFOUNDERS: groups balanced by baseline characteristics. QUALITY: Jadad 5 Cochrane A | |
| Participants | N = 34 randomised, 32 completed. M=19, F= 13 Mean age 11.9 yrs (SD 1.5) BASELINE SEVERITY: Moderate persistent asthma GINA guidelines. INCLUSION : Clinical diagnosis asthma > 6 months, , >15% FEV1 reversibility to SABA. Atopic EXCLUSION : Exacerbation or hospitalisation for asthma or change asthma medication or URTI within 4 weeks. Serious medical illness. | |
| Interventions | LONG ACTING BETA AGONIST: Formoterol 12 mcg BD PLACEBO: BD DEVICE: DP capsules RESCUE: Salbutamol PRN via MDI TREATMENT PERIOD: 6 weeks CO‐INTERVENTIONS: none permitted | |
| Outcomes | OUTCOMES: Primary= serum levels of inflammatory markers, ECP, sIL‐2R, IL‐4, ICAM‐1, IgE. Secondary= BHR PC20 histamine, FEV1, ASS. | |
| Notes | ||
Sussman 1995.
| Methods | STUDY DESIGN: 2 way cross over single centre UK, 2‐4 week run in/ 4‐6 week treatment period RANDOMISATION: Randomised treatment allocation, no details BLINDING: double blind, placebo controlled , WITHDRAWALS/DROP OUTS: 2 COMPLIANCE: no details CONFOUNDERS: no details QUALITY: Jadad 3 Cochrane B | |
| Participants | N Randomised 18 M=12 F= 6 adults Mean age not stated (range 18‐60) BASELINE SEVERITY: moderate INCLUSION : over 18 years age. v Baseline FEV1 >60% predicted, >15% FEV1 reversibility to SABA >15% diurnal variation EXCLUSION: no details | |
| Interventions | LONG ACTING BETA AGONIST: Eformoterol 24 mcg BD PLACEBO: BD DEVICE: DP capsules RESCUE: Ipratropium 80 mcg prn TREATMENT PERIOD: 4‐6 weeks CO‐INTERVENTIONS: ICS , salbutamol, theophylline | |
| Outcomes | OUTCOMES: DRC to formoterol, PEF, Rescue use,tremor, serum potassium | |
| Notes | ||
Taylor 1998.
| Methods | STUDY DESIGN:3 way cross over, 2 centre, New Zealand. 4 week run in/ 24 weeks treatment/ 4 week run out. RANDOMISATION: Yes, no method stated. BLINDING: double blind, double dummy, matching devices. WITHDRAWALS/DROP OUTS: 35 described after randomization and 8 protocol violators. COMPLIANCE:. Assessed by counting blisters, >87% CONFOUNDERS: Not analysed by ITT, Efficacy analysis similar. QUALITY: Jadad 4. Cochrane B | |
| Participants | N= ENROLLED189, RANDOMISED 165, completed/analysed 157 . ADULT , M=73 F=92 Mean age: 38 (range 18‐64) BASELINE SEVERITY: Mild‐moderate asthma INCLUSION : >15% FEV1 reversibility to SABA. PC20 methacholine <8mg/ml. EXCLUSION: current smokers, requiring OS or theophyllines, SABA requirement > 10 puffs/day, significant illness. | |
| Interventions | LONG ACTING BETA AGONIST: Salmeterol 50 mcg BD SHORT ACTING BETA AGONIST: Salbutamol 400 mcg QDS PLACEBO: placebo QDS DEVICE: Dry powder device‐ diskhaler. TEATMENT PERIOD: 24 weeks RESCUE: Salbutamol 100 mcg PRN CO‐INTERVENTIONS: None 8%, ICS 92%, cromones stable dose | |
| Outcomes | OUTCOMES: FEV1, FVC, PEF, Rescue use, exacerbations, daily asthma score. | |
| Notes | Exacerbations‐ Major = daily asthma score 3, PEF 40‐60% predicted, requiring OS. Minor‐ Daily asthma score 2, PEF 61‐75% predicted, increased rescue use. | |
von Berg 1998.
| Methods | STUDY DESIGN: Parallel group, multicentre (57) in 11 .countries, 2 week run in on / 52 week treatment with 2 week period off treatment at 6mths to assess BHR RANDOMISATION: Randomised treatment allocation, method not stated BLINDING: double blind, placebo controlled , WITHDRAWALS/DROP OUTS: 66 COMPLIANCE: checked verbally CONFOUNDERS: Groups well balanced by baseline characteristics QUALITY: Jadad 3 Cochrane B | |
| Participants | N Enrolled 627, Randomised 426, completed 360, M=262 F= 164 children Mean age 10 (range 5‐15) BASELINE SEVERITY: Mild‐moderate asthma INCLUSION : Clinical diagnosis asthma, >15% FEV1 reversibility to SABA or diurnal variation PEF > 15%, am PEF<85% best in run in. EXCLUSION : URTI/ LRTI/hospitalisation / changed asthma medication <4 weeks, requiring OS/ anticholinergics/ methylxanthines at entry | |
| Interventions | LONG ACTING BETA AGONIST: Salmeterol 50 mcg BD PLACEBO: BD DEVICE: DP diskhaler RESCUE: Salbutamol 100 mcg prn TREATMENT PERIOD: 52 weeks CO‐INTERVENTIONS: ICS 50%, cromones 22% | |
| Outcomes | OUTCOMES: PEF, FEV1, Rescue use, asthma symptoms, Exacerbations, BHR, % days with no symptoms ,% nights with no asthma awakenings | |
| Notes | Exacerbations defined as worsening of asthma symptoms that required treatment in addition to the study drug and rescue SABA. no details on symptom score. | |
von Berg 2003.
| Methods | STUDY DESIGN: Parallel group, LOCATION, NUMBER OF CENTRES: multi centre (32) in Europe DURATION OF STUDY: 2 weeks run in, 12 weeks treatment CONCEALMENT OF ALLOCATION: COCHRANE QUALITY SCORE: A DESCRIBED AS RANDOMISED: Yes DESCRIBED AS DOUBLE BLIND: Yes METHOD OF RANDOMISATION WELL DESCRIBED/APPROPRIATE: computer generated schedule by consecutive patient number, ratio 1:1:1 METHOD OF BLINDING WELL DESCRIBED/APPROPRIATE: Yes, identical devices DESCRIPTION OF WITHDRAWALS/DROPOUTS: 23 mainly due to asthma deterioration (7 placebo, 5 FM 4.5mcg, 2 FM 9mcg) JADAD SCORE (5‐1): 5 TYPE OF ANALYSIS (AVAILABLE CASE/TREATMENT RECEIVED/ ITT): ITT COMPLIANCE: no details CONFOUNDERS: 3 treatment groups well balanced for baseline characteristics and asthma histories | |
| Participants | N SCREENED: 331 N RANDOMISED: 248 N COMPLETED: 225 M= 161 F= 87 MEAN AGE: 11 (6‐17) SEVERITY OF ASTHMA: unknown BASELINE DETAILS: mean duration asthma 6 yrs (1‐16), FEV1 % predicted 80%, BRD 15%, ICS use in run in 82%. INCLUSION CRITERIA : 6‐17 years, diagnosis asthma ATS guidelines = 6 months, FEV1 % predicted =40%, receiving anti‐inflammatory agents (ICS, OCS, nedocromil, cromones) at stable dose for 30 days, able to use turbuhaler, BDR either increase in FEV1 =15%, or in % predicted FEV1 =9%, or am PEF =15% 4/8 days run in. EXCLUSION: Use of astemizole = 60days, regular nasal ICS or antihistamines = 30 days, immunotherapy =90 days, significant seasonal asthma or allergy, significant medical condition. | |
| Interventions | 1. Formoterol 9 mcg BD 2. Formoterol 4.5 mcg BD 3. placebo DELIVERY: turbuhaler COMBINATION INHALER FOR LABA: No TREATMENT PERIOD: 12 weeks RESCUE: terbutaline 250 mcg PRN CO‐INTERVENTIONS PERMITTED: ICS, cromones, nedocromil CO‐INTERVENTIONS : ICS 82% | |
| Outcomes | OUTCOMES MEASURED: PEF am/pm, FEV1, ASS. sleep disturbance, AEs, blood biochemistry FOLLOW‐UP ASSESSMENT POINTS: baseline, 4,8,12 weeks OUTCOMES INCLUDED IN ANALYSES: SUB‐GROUPS INDENTIFIED: None | |
| Notes | ASS: symptoms 4 pt scale 0 none ‐3 affecting daily activities or affecting sleep | |
Wallin 1999.
| Methods | STUDY DESIGN: Parallel group, 3 treatment groups 2 active 1 placebo, multicentre (3) in Sweden, 2 ‐4 week run in / 9 week treatment RANDOMISATION: Randomised treatment allocation, method not stated BLINDING: double blind, double dummy, placebo controlled , matched devices WITHDRAWALS/DROP OUTS: 4 COMPLIANCE: no details CONFOUNDERS: study conducted outside pollen season, same investigator performed bronchoscopies. QUALITY: Jadad 4 Cochrane B | |
| Participants | N Randomised 64, completed 360, M=36 F= 28 adults Mean age not given (range 18‐48) BASELINE SEVERITY: Mild intermittent persistent asthma INCLUSION : Clinical diagnosis asthma using SABA PRN as maintenance treatment, atopic, Baseline FEV1 >70% predicted, PC20 methacholine 0.1‐6 mg/ml EXCLUSION : URTI <4 weeks, smoking within 5 years | |
| Interventions | LONG ACTING BETA AGONIST: Formoterol 24 mcg BD PLACEBO: BD ICS GROUP: budesonide 400mcg BD DEVICE: DP Aerolizer or MDI RESCUE: Salbutamol 100 mcg prn TREATMENT PERIOD: 9 weeks CO‐INTERVENTIONS: none permitted | |
| Outcomes | OUTCOMES: PEF, FEV1, Rescue use, asthma symptom score, BHR, EBB, BAL | |
| Notes | Symptom Score‐ 4 pt, day or night time ‐ Scale :0= none to 3= severe. | |
Weinstein 1998.
| Methods | STUDY DESIGN: Parallel group, multicentre (11) USA, 1‐2 week run in on single blind placebo / 12 week treatment RANDOMISATION: Randomised treatment allocation, method not stated BLINDING: double blind, double dummy, placebo controlled , WITHDRAWALS/DROP OUTS: 20, 4 lack efficacy, 6 adverse events, 2 exacerbations COMPLIANCE: no details CONFOUNDERS: Groups well balanced by baseline characteristics QUALITY: Jadad 3 Cochrane B | |
| Participants | N SCREENED: 240 N Randomised 207, M=142 F= 65 children Mean age 8.4 (range 4‐11) BASELINE SEVERITY: persistent asthma, severity not stated INCLUSION : Diagnosis asthma by ATS criteria, Baseline FEV150‐80% predicted, >15% FEV1 reversibility to SABA. EXCLUSION : URTI/ LRTI/hospitalisation <4 weeks, OS, anticholinergics, theophyllines, antihistamines, tobacco exposure, Serious uncontrolled systemic disease | |
| Interventions | LONG ACTING BETA AGONIST: Salmeterol 50 mcg BD PLACEBO: BD DEVICE: DP Rotadiscs RESCUE: albuterol 100 mcg prn TREATMENT PERIOD: 12 weeks CO‐INTERVENTIONS: ICS 57%, cromones 32% , immunotherapy continued at same dose | |
| Outcomes | OUTCOMES: PEF, FEV1, Rescue use, asthma symptoms, night asthma awakenings, Exacerbations, adverse events | |
| Notes | Exacerbations defined as worsening of asthma symptoms that required treatment in addition to the study drug and rescue SABA. | |
Wolfe 2000a.
| Methods | STUDY DESIGN: Parallel group 3, 2 forms of salmeterol, powder and aerosol and placebo, multicentre (27) USA 2 week run in period / 12 week treatment period RANDOMISATION: Randomised treatment allocation, computer generated BLINDING: double blind, double dummy, placebo controlled WITHDRAWALS/DROP OUTS: 103 COMPLIANCE: no details CONFOUNDERS: Groups well balanced by baseline characteristics QUALITY: Jadad 3 Cochrane B | |
| Participants | N Randomised 498, M=235 F= 263 adults/ adolescents Mean age 33 (range 12‐79) BASELINE SEVERITY: Mild‐moderate asthma INCLUSION : Diagnosis asthma by ATS criteria 6 months requiring pharmacotherapy. Baseline FEV1>85% predicted, >15% FEV1 reversibility to SABA. EXCLUSION : URTI/ LRTI/exacerbation <6 weeks, smoker or >10 year pack history | |
| Interventions | LONG ACTING BETA AGONIST: Salmeterol 50 mcg BD either accuhaler or 42 mcg aerosol PLACEBO: BD aerosol or accuhaler DEVICE: MDI or accuhaler RESCUE: albuterol 100 mcg prn TREATMENT PERIOD: 12 weeks CO‐INTERVENTIONS: ICS >30% all groups, cromones continued at same dose | |
| Outcomes | OUTCOMES: PEF, FEV1, Rescue use, asthma symptom score, frequency nocturnal asthma, % days with no symptoms, adverse events, | |
| Notes | ASS‐ no details in paper | |
Wolfe 2000b.
| Methods | As for Wolfe 2000a | |
| Participants | As for Wolfe 2000a | |
| Interventions | As for Wolfe 2000a | |
| Outcomes | As for Wolfe 2000a | |
| Notes | Study data available as SLGA3011. Entered as Wolfe 2000b as the published paper reported pooled data from two clinical trials (SLGA3010/SLGA3011) | |
Wolfe 2006.
| Methods | STUDY DESIGN: Parallel group LOCATION, NUMBER OF CENTRES: 194 cited for screening. DURATION OF STUDY: 16 weeks (preceded by two week run‐in) CONCEALMENT OF ALLOCATION: COCHRANE QUALITY SCORE: B DESCRIBED AS RANDOMISED: YES. DESCRIBED AS DOUBLE BLIND: YES ‐ an open label arm was also included as a parallel group to the blinded assessments. METHOD OF RANDOMISATION WELL DESCRIBED/APPROPRIATE: Not reported. METHOD OF BLINDING WELL DESCRIBED/APPROPRIATE: Double‐blind. Devices not described as identical. DESCRIPTION OF WITHDRAWALS/DROPOUTS: 294/2083 (Adverse events: 104; data missing: 8; lack of efficacy: 52; protocol violation: 43; withdrawn consent: 114; loss to follow‐up: 43; admin problem: 17) JADAD SCORE (5‐1): 3. TYPE OF ANALYSIS (AVAILABLE CASE/TREATMENT RECEIVED/ ITT): ITT. COMPLIANCE: Not reported. CONFOUNDERS: Similar characteristics at baseline. | |
| Participants | Adults, SCREENED: 3820, N RANDOMISED: 2085 , N COMPLETED: 1791 M= 936 F= 1149 MEAN AGE: 38.11 SEVERITY OF ASTHMA: unknown BASELINE DETAILS: Moderate asthma (68‐70% predicted) INCLUSION CRITERIA: Use of inhaled beta‐agonist for two months prior to study entry; FEV1 reversibility >/=12% predicted post‐SABA; CXR consistent with asthma in 18 months prior to study entry (including run‐in) EXCLUSION: Hospitalisation within one month/treatment of exacerbation of asthma in month prior to study entry; evidence/ array of other systemic diseases; pregnancy/failure to use of reliable contraception; change to ongoing medication used to treat chronic asthma | |
| Interventions | 1. Formoterol 12mcg BID 2. Placebo 3. Formoterol 24mcg BID 4. Open label formoterol 12mcg BID (with up to two uses prn per day) DELIVERY: DPI (Aerolizer) TREATMENT PERIOD: 16 weeks RESCUE: SABA prn CO‐INTERVENTIONS PERMITTED: ICS; leukotriene antagonists; disodium cromoglycate. CO‐INTERVENTIONS: None as standard % on ICS: 57.8 | |
| Outcomes | OUTCOMES MEASURED: Post‐dose FEV1; adverse events; exacerbations (use of oral steroids) FOLLOW‐UP ASSESSMENT POINTS: 4, 8, 12 & 16 weeks. OUTCOMES INCLUDED IN ANALYSES: Absolute FEV1; adverse events SUB‐GROUPS INDENTIFIED: Regular ICS throughout the study; Regular ICS added after baseline; no regular ICS during the study; regular anti‐inflammatory medication throughout the study; regular anti‐inflammatory medication added after the study; no regular anti‐inflammatory medication during the study. | |
| Notes | ||
Wronska 1998.
| Methods | STUDY DESIGN: Parallel group single centre Poland, 1 week run in period / 4 week treatment period RANDOMISATION: Randomised treatment allocation, no details BLINDING: double blind, no details WITHDRAWALS/DROP OUTS: no details COMPLIANCE: no details CONFOUNDERS: significant difference in baseline BHR, salmeterol group more responsive QUALITY: Jadad 2 Cochrane C | |
| Participants | N Randomised 22, M=9 F= 13 adults Mean age 34 (SD 12) BASELINE SEVERITY: No details on severity of asthma INCLUSION : Clinical diagnosis asthma . EXCLUSION : use ICS <4 weeks, | |
| Interventions | LONG ACTING BETA AGONIST: Salmeterol 50 mcg BD PLACEBO: BD DEVICE: no details RESCUE: Salbutamol or fenoterol prn TREATMENT PERIOD: 4 weeks CO‐INTERVENTIONS: none on ICS | |
| Outcomes | OUTCOMES: PEF, FEV1, asthma symptom score, BHR | |
| Notes | Symptom Score‐ 6 pt, day 5 pt night time ‐ Scale :0= none to 4/5= most severe. | |
Zarkovic 1998.
| Methods | STUDY DESIGN: 2 way cross over multicentre (14) UK and Austria 2 week run in period / 26 week treatment period/ 2 week wash out at crossover RANDOMISATION: Randomised treatment allocation, no details BLINDING: double blind, double dummy , no details WITHDRAWALS/DROP OUTS:23 COMPLIANCE: no details CONFOUNDERS: no details QUALITY: Jadad 3 Cochrane B | |
| Participants | N Enrolled 153 Randomised 91, M=59 F= 32 children Mean age 11 (range 6‐15) BASELINE SEVERITY: Mild‐moderate asthma INCLUSION : Clinical diagnosis asthma 12mths requiring SABA, Baseline PEF>85% predicted, >15% FEV1 reversibility to SABA. EXCLUSION : URTI/ LRTI/exacerbation <4 weeks, unstable asthma, OS, theophyllines, anticholinergics | |
| Interventions | LONG ACTING BETA AGONIST: Salmeterol 50 mcg BD PLACEBO: BD DEVICE: DP diskhaler RESCUE: Salbutamol 200 mcg prn TREATMENT PERIOD26 weeks CO‐INTERVENTIONS: ICS >80%, cromones 4% | |
| Outcomes | OUTCOMES: PEF, FEV1, Rescue use, asthma symptom score, % days with no symptoms, ,% nights with no asthma awakenings. Exacerbations, BHR | |
| Notes | Exacerbations defined as worsening of asthma symptoms that required treatment in addition to the study drug and rescue SABA. ASS‐ no details on scale | |
ITT= Intention to treat population (number of participants in analyses of results in a study). ATS= American Thoracic Society, BTS= British Thoracic Society, GINA= Global Initiative for Asthma, ASS=asthma symptom score. OS= oral corticosteroids. ICS= Inhaled corticosteroids. LABA= long‐acting beta‐2 agonist. SABA= Short‐acting beta‐2 agonist. Short‐acting beta‐2 agonist salbutamol known in USA as albuterol‐ dose measured at mouthpiece 90 mcg = 100mcg from inhaler . Minimum acceptable dose ICS =(MAD). COAD= chronic obstructive airways disease. Bronchial hyperreactivity = BHR (measured as PD20 or PC20 of direct agents methacholine and histamine or indirect agents AMP). BAL= bronchoalveolar lavage, EBB= endobronchial biopsy, BDP = beclomethasone dipropionate, BUD= budesonide , FP= fluticasone propionate. Cromones = sodium cromoglycate, nedocromil sodium . MDI= metered dose inhaler. DP= dry powder. AQOL score = Asthma quality of life score, based on Juniper 1992 (Juniper EF, Guyatt GH. Evaluation of impairment of health related quality of life in asthma: development of a questionnaire for use in clinical trials. Thorax 1992;47:76‐83) value of 1 =maximum impairment, value 7= no impairment. Results 0.5=small change, 1.0= moderate change, 1.5= large change. LWAQ= Living with asthma questionnaire (Hyland M. The living with asthma questionnaire. Respir Med 1991;85 supp:13‐16.), lower score indicates better quality of life. CAQC= Childhood Asthma Questionnaire Form C, produces 5 sub scale scores, including self reported severity ( the frequency of 5 symptoms of severity of asthma), distress (feelings about these symptoms) , active quality of living (frequency and enjoyment of activities including swimming, sports, outdoor play). Christie M, French D, Sowden A, West A. The development of child centred, disease specific questionnaires for living with asthma. Psychosomatic Medicine 1993;55:541‐8. SF‐36= Generic Health related quality of life questionnaire FS II‐R : Stein R, Jessup D. Functional status II(R): a measure of child health status. Med Care 1990;28:1041‐55. SLR‐C: Boyer G, Aaronson G, Meltzer E, LaForce C, Grossman J, Yancey S. Enhancement of a general quality of life scale: validation of a sleep scale. J Allergy Clin Immunol 1992;89:186. ECP= eosinophilic cationic protein, sIL‐2R= soluble receptor of interlukin‐4, ICAM‐1= level of soluble intercellular adhesion molecule‐1, immunoglobulin E= IgE
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Akpinarli 1999 | 100% on ICS at baseline |
| Arvidsson 1989 | No placebo group |
| Arvidsson 1991 | No placebo group |
| Aziz 1999 | Treatment period < 4 weeks |
| Aziz 1998 | Treatment period < 4 weeks |
| Aziz 1998a | Treatment period < 4 weeks |
| Baki 1998 | Treatment period < 4 weeks |
| Baumgarten 2002 | Not RCT, No placebo group |
| Beach 1993 | No placebo group |
| Becker 1989 | Treatment period < 4 weeks |
| Becker 1993 | No placebo group |
| Bhagat 1995 | Treatment period < 4 weeks |
| Bishop 1994 | Treatment period < 4 weeks |
| Blake 1992 | Treatment period < 4 weeks |
| Boner 1992 | No placebo group |
| Booth 1996 | 100% on ICS at baseline |
| Boulet Laviol 1997 | No placebo group |
| Boulet 1998a | ICS in all treatment groups |
| Boulet 2001 | Treatment period < 4 weeks |
| Bowers 1997 | Review |
| Boyd 1995 | 100% on ICS at baseline |
| Brambilla 1994 | No placebo group |
| Brambilla 2003 | No placebo group |
| Britton 1992 | No placebo group |
| Byrnes 1996 | No placebo group |
| Byrnes 2000 | No placebo group |
| Campbell 1999 | No placebo group |
| Campbell 2000 | No placebo group |
| Carlsen 1995 | Treatment period < 4 weeks |
| Castle 1993 | No placebo group |
| Charpin 1992 | No placebo group |
| Chuchalin 2002 | Combination therapy versus placebo |
| Clark 1993 | Not RCT‐ case reports |
| Clauzel 1998 | No placebo group |
| Cockcroft 1997 | Treatment period < 4 weeks |
| Cox 1996 | No placebo group |
| D'Urzo 2001 | 100% on ICS at baseline |
| D5125C00344 | 100% on ICS at baseline |
| De Carli 1995 | No placebo group |
| De Oliveira 1998 | No placebo group |
| Derom 1992 | Treatment period < 4 weeks |
| Di Lorenzo 1995 | No placebo group |
| Droszcz 1999 | No placebo group, subjects COPD and Asthma |
| Faurschou 1992 | No placebo group |
| Faurschou 1994a | Treatment period < 4 weeks |
| Faurschou 1996 | No placebo group |
| Faurschou 1997 | Abstract only, full details not available |
| FitzGerald 1999 | 100% on ICS at baseline |
| Fitzpatrick 1990 | Treatment period < 4 weeks |
| Fuglsang 1998 | Treatment period < 4 weeks |
| Fuller 1993 | No placebo group |
| Gardiner 1994 | 100% on ICS at baseline |
| Giannini 1995 | Treatment period < 4 weeks |
| Giannini 1999 | Treatment period < 4 weeks |
| Gong 1996 | Treatment period < 4 weeks |
| Goodwin 1996 | No placebo group |
| Green 1002 | Treatment period < 4 weeks |
| Greening 1994 | No placebo group |
| Grove 1995 | 100% on ICS at baseline |
| Grove 1996 | Treatment period < 4 weeks |
| Hacki 1993 | No placebo group |
| Hekking 1990 | No placebo group |
| Hermansson 1995 | No placebo group |
| Jartti 1998 | 100% on ICS at baseline |
| Jenkins 1991 | letter‐ not RCT |
| Kalra 1996 | Treatment period < 4 weeks |
| Kavuru 2000b | Combination therapy versus ICS |
| Kemp 1993 | Treatment period < 4 weeks |
| Kemp 1994 | Treatment period < 4 weeks |
| Kemp 1998b | 100% on ICS at baseline |
| Kesten 1991 | No placebo group |
| Kesten 1992 | No placebo group |
| Kozlik‐Feldmann 1996 | No placebo group |
| Lai 1995 | No placebo group |
| Langley 1998 | 100% on ICS at baseline |
| Langton Hewer 1995 | 100% on ICS at baseline |
| Lenney 1995 | No placebo group |
| Li 1999 | 100% on ICS at baseline |
| Lipworth 1998 | 100% on ICS at baseline |
| Lipworth 1999 | Treatment period < 4 weeks |
| Lipworth 2000a | 100% on ICS at baseline |
| Lipworth 2000b | Treatment period < 4 weeks |
| Lotvall 1992 | No placebo group |
| Lundback 1993 | No placebo group |
| Malo 1992 | Cohort study‐ not RCT |
| Mann 1996 | No placebo group |
| Martin 1999 | No placebo group |
| McIvor 1998 | 100% on ICS at baseline |
| Meijer 1995 | 100% on ICS at baseline |
| Midgren 1992 | No placebo group |
| Molimard 2001 | No placebo group |
| Nelson 1999a | 100% on ICS at baseline |
| Newnham 1993 | Treatment period < 4 weeks |
| Nichol 1990 | Treatment period < 4 weeks |
| Nielsen 1999 | 100% on ICS at baseline |
| Nightingale 2002 | 100% on ICS at baseline |
| Nix 1990 | Treatment period < 4 weeks |
| Norhaya 1999 | 100% on ICS at baseline |
| Nowak 1992 | Treatment period < 4 weeks |
| O'Byrne 2001 | Combination therapy versus ICS |
| Palmer 1992 | No placebo group |
| Pauwels 1997 | 100% on ICS at baseline |
| Pearlman 1999 | Combination therapy versus ICS. |
| Pederson 1993 | Treatment period < 4 weeks |
| Price 2002 | 100% on ICS at baseline |
| Quebe‐Fehling 1996 | Treatment period < 4 weeks |
| Rabe 1993 | Treatment period < 4 weeks |
| Ramsdale 1991 | Treatment period < 4 weeks |
| Rhee 1997 | No placebo group |
| Ringbaek 1996 | No placebo group |
| Rosenhall 1990 | No placebo group |
| Russell 1995 | 100% on ICS at baseline |
| Ruttenvan Molken1995 | No placebo group |
| Schaaning 1996 | No placebo group |
| Scherner 2004 | Treatment period < 4 weeks |
| Self 1998 | 100% on ICS at baseline |
| Shapiro 2000a | Combination therapy versus ICS |
| Shepherd 1991 | Letter ‐ not RCT |
| Sichletidis 1993 | No placebo group |
| Siergiejko 1998 | No placebo group |
| Simons 1992 | Treatment period < 4 weeks |
| Simons 1997b | 100% on ICS at baseline |
| SLGA2016 | Treatment administered as one‐offs at clinic visits |
| SLGB4011R | Treatment period < 4 weeks |
| SMO30003 | Treatment administered as one‐offs at clinic visits |
| SMS30035 | Treatment period < 4 weeks |
| Smyth 1993 | Treatment period < 4 weeks |
| Sprogoe‐Jakobsen1992 | No placebo group |
| Staehr 1995 | No placebo group |
| Steffensen 1996 | No placebo group |
| Tan 1997 | 100% on ICS at baseline |
| Tattersfield 2001 | No placebo group, not regular use of LABA |
| Taylor 1992 | No placebo group |
| Taylor Jensen 1997 | Treatment period < 4 weeks |
| Thomson 1998 | No placebo group |
| Totterman 1998 | No placebo group |
| Turner 1998 | Treatment period < 4 weeks |
| Twentyman 1990 | Treatment period < 4 weeks |
| Ullman 1988 | No placebo group |
| Van der Molen 1996 | 100% on ICS at baseline |
| van der Woude 2001 | Treatment period < 4 weeks |
| Venables 1992 | No placebo group |
| Verberne 1991 | Abstract only‐ details not available |
| Verberne 1996 | No placebo group |
| Verberne 1997 | No placebo group |
| Verberne 1998 | 100% on ICS at baseline |
| Verberne 2000 | Review‐ not RCT |
| Verini 1998 | Treatment period < 4 weeks |
| Wallaert 1999 | No placebo group |
| Walters 1992 | Abstract only‐ details not available |
| Weinstein 1997 | Treatment period < 4 weeks |
| Wenzel 1998 | No placebo group |
| Wilding 1997 | 100% on ICS at baseline |
| Wolfe 1995 | No placebo group |
| Wong 1997 | Treatment period < 4 weeks |
| Woolcock 1996 | No placebo group |
| Yates 1995 | Treatment period < 4 weeks |
| Yates 1997 | Treatment period < 4 weeks |
| Zellweger 1994 | No placebo group, Treatment period < 4 weeks |
| Zimmerman 2004 | 100% on ICS at baseline |
Contributions of authors
Julia Walters carried out initial selection and retrieval of studies, grading, data extraction, correspondence with authors, analyses and writing the first draft of the review. Haydn Walters assisted with study selection, grading, analyses and writing first and subsequent drafts. Peter Gibson assisted with protocol development and editorial revision of drafts of the review. Toby Lasserson assisted with the 2006 update of the review, by extracting, entering and analysing data, converting estimates from WMD to GIV outcomes where appropriate, imputing standard deviations and writing up the results.
Sources of support
Internal sources
No sources of support supplied
External sources
Commonwealth Department of Health and Aging, Australia.
Declarations of interest
E H Walters has taken part in collaborative clinical pharmacology studies with a number of pharmaceutical companies including GSK, Astra Zeneca, Pfizer, Boehringer, Schering Plough, SKB, and Novartis. He has, in the past, held consultancies with GSK, Pfizer and Zeneca. He has had sponsorship to meetings from a number of the companies listed over the past 15 years. PG Gibson has taken part in collaborative clinical studies with a number of pharmaceutical companies including GSK, AstraZeneca, Boerhinger Ingelheim, and Aventis. He has attended meetings sponsored by a number of companies.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Adinoff 1998 {published data only}
- Adinoff A, Schwartz H, Rickard K, Yancey S, Swearingen B. Salmeterol compared with current therapies in chronic asthma. Journal of Family Practice 1998;47(4):278‐84. [PubMed] [Google Scholar]
Bensch 2001 {published data only}
- Bensch G, Lapidus R, Levine B, Lumry W, Yegen U, Kiselev P, et al. A randomized, 12‐week, double‐blind, placebo‐controlled study comparing formoterol dry powder inhaler with albuterol metered‐dose inhaler. Annals of Allergy Asthma & Immunology 2001;86(1):19‐27. [DOI] [PubMed] [Google Scholar]
- Della Cioppa G, Kiseley P, Matos D, Yegen I, Townley RG. QID Albuterol worsens peak flow variability in asthma whereas BID Formoterol does not [abstract]. Annals of Allergy 1998;80:88. [Google Scholar]
- FORNDA 20831_40. A twelve week, double‐blind, parallel group trial comparing the safety, tolerability and efficacy of formoterol dry powder capsules for inhalation delivered by a single‐dose inhaler versus albuterol metered dose inhaler device versus placebo in patients with mild to moderate asthma. www.fda.gov 2001.
- Mann M, Chowdhury B, Sullivan E, Nicklas R, Anthracite R, Meyer RJ. Serious asthma exacerbations in asthmatics treated with high‐dose formoterol. Chest 2003;124(1):70‐4. [DOI] [PubMed] [Google Scholar]
Bensch 2002 {published data only}
- Bensch G, Berger WE, Blokhin BM, Socolovsky AL, Thomson MH, Till MD, et al. One‐year efficacy and safety of inhaled formoterol dry powder in children with persistent asthma. Annals of Allergy, Asthma, & Immunology 2002;89(2):180‐90. [DOI] [PubMed] [Google Scholar]
- Mann M, Chowdhury B, Sullivan E, Nicklas R, Anthracite R, Meyer RJ. Serious asthma exacerbations in asthmatics treated with high‐dose formoterol. Chest 2003;124(1):70‐4. [DOI] [PubMed] [Google Scholar]
Booth 1993 {published data only}
- Booth H, Fishwick K, Harkawat R, Devereux G, Hendrick DJ, Walters EH. Changes in methacholine induced bronchoconstriction with the long acting beta2 agonist salmeterol in mild to moderate asthmatic patients. Thorax 1993;48(11):1121‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boulet 1997 {published data only}
- Boulet LP, Laviolette M, Boucher S, et al. A Twelve week comparison of salmeterol and salbutamol in the treatment of mild to moderate asthma‐ a Canadian multicentre study. Journal of Allergy & Clinical Immunology 1997;99(1 Pt 1):13‐21. [DOI] [PubMed] [Google Scholar]
Boulet 1998b {published data only}
- Boulet L, Cartier A, Milot J, Cote J, Malo JL, Laviolette M. Tolerance to the protective effects of salmeterol on methacholine‐induced bronchoconstriction‐ influence of inhaled corticosteroids. European Respiratory Journal 1998;11(5):1091‐7. [DOI] [PubMed] [Google Scholar]
Busse 1998 {published data only}
- Busse WW, Casale TB, Murray JJ, Petrocella V, Cox F, Rickard K. Efficacy, safety, and impact on quality of life of salmeterol in patients with moderate persistent asthma. American Journal of Managed Care 1998;4(11):1579‐87. [PubMed] [Google Scholar]
Busse 2004 {published data only}
- Busse W, Levine B, Andriano K, Lavecchia C, Yegen U. Efficacy, tolerability, and effect on asthma‐related quality of life of formoterol bid via multidose dry powder inhaler and albuterol QID via metered dose inhaler in patients with persistent asthma: a multicenter, randomized, double‐blind, double‐dummy, placebo‐controlled, parallel‐group study. Clnical Therapeutics 2004;26(10):1587‐98. [DOI] [PubMed] [Google Scholar]
Cheung 1992 {published data only}
- Cheung D, Timmers MC, Zwinderman AH, et al. Long term effects of a long acting beta adrenoceptor agonist in patients with mild asthma. New England Journal of Medicine 1992;327(17):1198‐1203. [DOI] [PubMed] [Google Scholar]
Cloostermann2001 {published data only}
- Cloosterman SG, Bijl‐Hofland ID, Herwaarden CL, Akkermans RP, Elshout FJ, Folgering HT, et al. A placebo‐controlled clinical trial of regular monotherapy with short‐acting and long‐acting beta(2)‐agonists in allergic asthmatic patients. Chest 2001;119(5):1306‐15. [DOI] [PubMed] [Google Scholar]
- Cloosterman SG, Schermer TR, Bijl‐Hofland ID, Heide S, Brunekreef B, Elshout FJ, et al. Effects of house dust mite avoidance measures on Der p 1 concentrations and clinical condition of mild adult house dust mite‐allergic asthmatic patients, using no inhaled steroids. Clinical & Experimental Allergy 1999;29(10):1336‐46. [DOI] [PubMed] [Google Scholar]
- Schayck CP, Cloosterman SG, Bijl‐Hofland ID, Hoogen H, Folgering HT, Weel C. Is the increase in bronchial responsiveness or FEV1 shortly after cessation of beta2‐agonists reflecting a real deterioration of the disease in allergic asthmatic patients? A comparison between short‐acting and long‐acting beta2‐agonists. Respiratory Medicine 2002;96(3):155‐62. [DOI] [PubMed] [Google Scholar]
Creticos 1999 {published data only}
- Creticos PS, Freidhoff LR, Bernstein DI, Chu T, Khattignavong AP, Pasatiempo AM, et al. Comparison of an inhaled corticosteroid (triamcinolone acetonide) to a long‐acting bronchodilator (salmeterol), the combination, and placebo in mild‐moderate adult asthmatic patients. International Archives of Allergy and Immunology 1999;118(2‐4):345‐6. [DOI] [PubMed] [Google Scholar]
D'Alonzo 1994 {published data only}
- Arledge T, Liddle D, Stahl E, Rossing T. Salmeterol does not cause tolerance during long term asthma therapy. Journal of Allergy & Clinical Immunology 1996;98(6 Pt 1):1116‐9. [DOI] [PubMed] [Google Scholar]
- D'Alonzo GE. Efficacy of inhaled salmeterol in the treatment of asthma. European Respiratory Review 1995;5:128‐32. [Google Scholar]
- D'Alonzo GE, Nathan RA, Henchowicz MD. Salmeterol xinafoate as maintenance therapy compared with albuterol in patients with asthma. JAMA 1994;271(18):1412‐6. [PubMed] [Google Scholar]
- Nathan R, Seltzer J, Kemp J, Chervinsky P, Alexander W, Liddle R, et al. Safety of salmeterol in the maintenance treatment of asthma. Annals of Allergy, Asthma, & Immunology 1995;75(3):243‐8. [PubMed] [Google Scholar]
Dahl 1991a {published data only}
- Dahl R, Earnshaw JS, Palmer JBD. Salmeterol‐a 4 week study of a long acting beta2 adrenoceptor agonist for the treatment of reversible airways disease. European Respiratory Journal 1991;4(10):1178‐84. [PubMed] [Google Scholar]
- Faurschou P. Chronic dose‐ranging studies with salmeterol. European Respiratory Review 1991;1(4):282‐7. [Google Scholar]
Dahl 1991b {published data only}
- Dahl R, Pedersen B, Venge P. Bronchoalveolar lavage studies. European Respiratory Review 1991;1:272‐5. [Google Scholar]
Ekstrom 1998a {published data only}
- Ekstrom T, Ringdal N, Sobradillo V, Runnerstrom E, Soliman S. Low‐dose formoterol Turbuhaler(TM) (Oxis(TM)) bid, a 3‐month placebo‐controlled comparison with terbutaline (qid). Respiratory Medicine 1998;92(8):1040‐5. [DOI] [PubMed] [Google Scholar]
Ekstrom 1998b {published data only}
- Ekstrom T, Ringdal N, Tukiainen P, Runnerstrom E, Soliman S. A 3 month comparison of formoterol with terbutaline via turbuhaler‐A placebo controlled study. Annals of Allergy, Asthma, & Immunology 1998;81(3):225‐30. [DOI] [PubMed] [Google Scholar]
Garcia 2001 {published data only}
- Garcia R, Guerra P, Feo F, Galindo P, Gomez E, Borja J, et al. Tachyphylaxis following regular use of formoterol in exercise‐induced bronchospasm. Journal of Investigational Allergology & Clinical Immunology 2001;11(3):176‐82. [PubMed] [Google Scholar]
Hyland 1994 {published data only}
- Hyland ME, Kenyon CAP, Jacobs P. Sensitivity of quality of life domains and constructs to longitudinal change in a clinical trial comparing salmeterol and placebo in asthmatics. Quality of Life Research 1994;3(2):121‐6. [DOI] [PubMed] [Google Scholar]
Jones 1994 {published data only}
- Jones K. Salmeterol xinafoate in the treatment of mild to moderate asthma in primary care‐UK Study Group. Thorax 1994;49(10):971‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Juniper 1995 {published data only}
- Juniper EF, Johnston P, Borkhoff C, Haukioja A. A multicentre comparison of salmeterol and salbutamol on asthma‐specific quality of life [abstract]. Quality of Life Research 1994;3:83. [Google Scholar]
- Juniper EF, Johnston PR, Borkhoff CM, Guyatt G, Boulet L‐P, Haukioja A. Quality of life in asthma clinical trials‐a comparison of salmeterol and salbutamol. American Journal of Respiratory & Critical Care Medicine 1995;151(1):66‐70. [DOI] [PubMed] [Google Scholar]
Kavuru 2000a {published data only}
- Edwards T, Gross G, Mitchell D, Chervinsky P, Woodring A, Baitinger L, et al. The salmeterol xinafoate/fluticasone propionate dry powder combination product via diskus(r) inhaler improves asthma control compared to salmeterol xinafoate or fluticasone propionate dry powder alone. American Journal of Respiratory and Critical Care Medicine 1998;157(3 Suppl):A414. [Google Scholar]
- Kavuru M, Melamed J, Gross G, LaForce C, House K, Prillaman B, et al. Salmeterol and fluticasone propionate combined in a new powder inhalation device for the treatment of asthma‐A randomized, double‐blind, placebo‐controlled trial. Journal of Allergy & Clinical Immunology 2000;105(6):1108‐16. [DOI] [PubMed] [Google Scholar]
- SFCA3002. A randomized, double‐blind, parallel‐group trial evaluating safety and efficacy of salmeterol 50mcg BID and fluticasone propionate 100mcg BID individually and in combination and placebo in subjects with asthma. http://ctr.gsk.co.uk 2005.
Kemp 1998a {published data only}
- Kemp J, Wolfe J, Grady J, LaForce C, Stahl E, Arlidge T, et al. Salmeterol powder compared with albuterol aerosol as maintenance therapy for asthma in adolescent and adult patients. Clinical Therapeutics 1998;20(2):270‐82. [DOI] [PubMed] [Google Scholar]
- Wolfe J, LaForce C, Chervinsky P, Galant S, Lumry W, Noonan M, et al. Inhaled salmeterol powder compared with albuterol aerosol given regularly or as needed for asthma (abstract). Annals of Allergy, Asthma and Immunology 1995;74:52. [Google Scholar]
Kemp 1999 {published data only}
- Chervinsky P, Goldberg P, Galant S, Wang Y, Arledge T, Welch MB, et al. Long‐term cardiovascular safety of salmeterol powder pharmacotherapy in adolescent and adult patients with chronic persistent asthma‐A randomized clinical trial. Chest 1999;115(3):642‐8. [DOI] [PubMed] [Google Scholar]
- Kemp J, DeGraff A, Pearlman D, Chervinsky P, Galant S, Goldberg P, et al. A 1‐year study of salmeterol powder on pulmonary function and hyperresponsiveness to methacholine. Journal of Allergy & Clinical Immunology 1999;104(6):1189‐97. [DOI] [PubMed] [Google Scholar]
- Rosenthal R, Chervinsky P, DeGraaf A, et al. Long term regular treatment with salmeterol is effective in protecting against bronchial hyperresponsiveness as measured by methacholine challenge in asthmatics. Journal of Allergy and Clinical Immunology 1997;99(1 Pt 2):323s. [Google Scholar]
Kraft 1997 {published data only}
- Kraft M, Wenzel SE, Bettinger CM, Martin RJ. The effect of salmeterol on nocturnal symptoms, airway function and inflammation in asthma. Chest 1997;111(5):1249‐54. [DOI] [PubMed] [Google Scholar]
LaForce 2005 {published data only}
- LaForce C, Prenner BM, Andriano K, Lavecchia C, Yegen U. Efficacy and safety of formoterol delivered via a new multidose dry powder inhaler (Certihaler) in adolescents and adults with persistent asthma. Journal of Asthma 2005;42(2):101‐6. [PubMed] [Google Scholar]
Lazarus 2001 {published data only}
- Lazarus SC, Boushey H, Fahy JV, Chinchilli VM, Lemanske RF, Sorkness CA, et al. Long‐acting ß2‐agonist monotherapy versus continued therapy with inhaled corticosteroids in patients with persistent asthma: a randomized controlled trial. Journal of the American Medical Association 2001;285(20):2583‐93. [DOI] [PubMed] [Google Scholar]
Leblanc 1996 {published data only}
- Leblanc P, Knight A, Kreisman H, Borkhoff CM, Johnston PR. A placebo controlled crossover comparison of salmeterol and salbutamol in patients with asthma. American Journal of Respiratory & Critical Care Medicine 1996;154(2 Pt 1):324‐8. [DOI] [PubMed] [Google Scholar]
Levy 2005 {published data only}
- Levy R, Pinnas J, Milgrom H, Smith J, Yegen U. Safety and efficacy in children with persistent asthma treated with formoterol 10 mcg BID delivered via Certihaler: a novel multidose dry‐powder inhaler. Pediatric Asthma, Allergy, and Immunology 2005;18(1):25‐35. [Google Scholar]
Lindqvist 2003 {published and unpublished data}
- Lindqvist A, Karjalainen EM, Laitinen LA, Kava T, Altraja A, Pulkkinen M, et al. Salmeterol resolves airway obstruction but does not possess anti‐eosinophil efficacy in newly diagnosed asthma: a randomized, double‐blind, parallel group biopsy study comparing the effects of salmeterol, fluticasone propionate, and disodium cromoglycate. Journal of Allergy & Clinical Immunology 2003;112(1):23‐8. [DOI] [PubMed] [Google Scholar]
Lockey 1999 {published data only}
- Lockey R, DuBuske L, Friedman B, Petrocella V, Cox F, Rickard K. Nocturnal Asthma. Effect of Salmeterol on Quality of Life and Clinical Outcomes. Chest 1999;115(3):666‐73. [DOI] [PubMed] [Google Scholar]
Mahajan 1998 {published data only}
- Mahajan P, Stahl E, Arledge T. Quality of life in pediatric asthma patients treated with salmeterol and impact on the daily activities of their parents. Pediatric Asthma Allergy & Immunology 1998;12(1):21‐8. [Google Scholar]
Nathan 1999 {published data only}
- Nathan R, Pinnas J, Schwartz H, Grossman J, Yancey S, Emmett A, et al. A six‐month, placebo‐controlled comparison of the safety and efficacy of salmeterol or beclomethasone for persistent asthma. Annals of Allergy 1999;82(6):521‐9. [DOI] [PubMed] [Google Scholar]
Nelson 1998 {published data only}
- Nelson J, Strauss L, Skowronski M, Ciufo R, Novak R, McFadden EJ. Effect of long‐term salmeterol treatment on exercise‐induced asthma. New England Journal of Medicine 1998;339(3):141‐6. [DOI] [PubMed] [Google Scholar]
Nelson 1999b {published data only}
- Nelson H, Berkowitz R, Tinkelman D, Emmett A, Rickard K, Yancey S. Lack of Subsensitivity to Albuterol After Treatment with Salmeterol in Patients with Asthma. American Journal of Respiratory & Critical Care Medicine 1999;159(5 Pt 1):1556‐61. [DOI] [PubMed] [Google Scholar]
- SLGA5020. A single‐center, randomized, double‐blind, cross‐over clinical trial to examine sub‐sensitivity in adult subjects with asthma receiving salmeterol xinafoate 42mcg BID and placebo BID. www.ctr.gsk.co.uk.
Newnham 1994 {published data only}
- Newnham DM, McDevitt DG, Lipworth BJ. Bronchodilator sub‐sensitivity after chronic dosing with eformoterol in patients with asthma. American Journal of Medicine 1994;97(1):29‐37. [DOI] [PubMed] [Google Scholar]
Newnham 1995 {published data only}
- Newnham D, Grove A, McDevitt D, Lipworth B. Subsensitivity of bronchodilator and systemic beta 2 adrenoceptor responses after regular twice daily treatment with eformoterol dry powder in asthmatic patients. Thorax 1995;50(5):497‐504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan KS, Hall IP, Dewar JC, Dow E, Lipworth BJ. Association between beta2‐adrenoceptor polymorphism and susceptibility to bronchodilator desensitisation in moderately severe stable asthmatics. Lancet 1997;350(9083):995‐9. [DOI] [PubMed] [Google Scholar]
Pearlman 1992 {published data only}
- Arledge T, Liddle D, Stahl E, Rossing T. Salmeterol does not cause tolerance during long term asthma therapy. Journal of Allergy & Clinical Immunology 1996;98(6 Pt 1):1116‐9. [DOI] [PubMed] [Google Scholar]
- Nathan R, Seltzer J, Kemp J, Chervinsky P, Alexander W, Liddle R, et al. Safety of salmeterol in the maintenance treatment of asthma. Annals of Allergy, Asthma, & Immunology 1995;75(3):243‐8. [PubMed] [Google Scholar]
- Pearlman DS. Long‐acting beta 2‐agonist salmeterol compared with albuterol in maintenance asthma therapy. Annals of Allergy Asthma & Immunology 1995;75(2):180‐4. [PubMed] [Google Scholar]
- Pearlman DS, Chervinsky P, LaForce C, Seltzer JM, Southern DL, Kemp JP, et al. A comparison of salmeterol with albuterol in the treatment of mild‐to‐moderate asthma. New England Journal Of Medicine 1992;327(20):1420‐5. [DOI] [PubMed] [Google Scholar]
- Pearlman DS, Liddle R. Controlling asthma symptoms: Salmeterol compared with salbutamol in large‐scale multicentre studies. European Respiratory Review 1994;4(21):301‐5. [Google Scholar]
Pearlman 1999a {published data only}
- Pearlman D, Stricker W, Weinstein S, Gross G, Chervinsky P, Woodring A, et al. Inhaled salmeterol and fluticasone: A study comparing monotherapy and combination therapy in asthma. Annals of Allergy 1999;82(3):257‐65. [DOI] [PubMed] [Google Scholar]
Pleskow 2003 {published data only}
- FORNDA 20831_41. A twelve week, double‐blind, parallel group trial comparing the safety, tolerability and efficacy of formoterol dry powder capsules for inhalation delivered by a single‐dose inhaler versus albuterol metered dose inhaler device versus placebo in patients with mild to moderate asthma. www.fda.gov 2001.
- Mann M, Chowdhury B, Sullivan E, Nicklas R, Anthracite R, Meyer RJ. Serious asthma exacerbations in asthmatics treated with high‐dose formoterol. Chest 2003;124(1):70‐4. [DOI] [PubMed] [Google Scholar]
- Pleskow W, LaForce C, Yegen Ü, Matos D, Della Cioppa G. Formoterol Delivered via the Dry Powder Aerolizer® Inhaler Versus Albuterol MDI and Placebo in Mild‐to‐Moderate Asthma: A Randomized, Double‐Blind, Double‐Dummy Trial. Journal of Asthma 2003;40(5):505‐14. [DOI] [PubMed] [Google Scholar]
Prieto 2002 {published data only}
- Prieto L, Gutierrez V, Torres V, Uixera S, Marin J. Effect of salmeterol on seasonal changes in airway responsiveness and exhaled nitric oxide in pollen‐sensitive asthmatic subjects. Chest 2002;122(3):798‐805. [DOI] [PubMed] [Google Scholar]
Ramage 1994 {published data only}
- Ramage L, Cree IA, Dhillon DP. Comparison of salmeterol with placebo in mild asthma: effect on peripheral blood phagocyte function and cytokine levels. International Archives of Allergy & Immunology 1994;105(2):181‐4. [DOI] [PubMed] [Google Scholar]
- Ramage L, Lipworth BJ, Ingram CG, et al. Reduced protection against exercise induced bronchoconstriction after chronic dosing with salmeterol. Respiratory Medicine 1994;88(5):363‐8. [DOI] [PubMed] [Google Scholar]
Roberts 1999 {published data only}
- Roberts B, Bradding P, Holgate S. Effects of a six week course of salmeterol on bronchial reactivity. Thorax 1992;47:230P. [Google Scholar]
- Roberts J, Bradding P, Britten K, Walls A, Wilson S, Gratziou C, et al. The long‐acting beta2‐agonist salmeterol xinafoate: effects on airway inflammation in asthma. European Respiratory Journal 1999;14(2):275‐82. [DOI] [PubMed] [Google Scholar]
Rosenthal 1999 {published data only}
- Rosenthal R, Busse W, Kemp J, Baker J, Kalberg C, Emmett A, et al. Effect of long‐term salmeterol therapy compared with as‐needed albuterol use on airway hyperresponsiveness. Chest 1999;116(3):595‐602. [DOI] [PubMed] [Google Scholar]
SAS30003 {unpublished data only}
- SAS30003. A stratified, randomized, double‐blind, placebo‐controlled, parallel‐group, 12‐week trial evaluating the safety and efficacy of the salmeterol/fluticasone propionate combination in HFA 134a MDI, 42/88mcg BID, and salmeterol in propellant 11/12 MDI, 42mcg BID, fluticasone propionate in propellant 11/12 MDI, 88mcg BID, and placebo propellant HFA 134a MDI in adult and adolescent subjects with asthma. www.ctr.gsk.co.uk.
SAS30004 {published data only}
- Edin HM, Payne E, Herrle MR, Schoaf L, Mather DB, Scott CA, et al. Salmeterol/fluticasone propionate combination via HFA MDI improves quality of life. Journal of Allergy & Clinical Immunology 2001;107(2):S246. [Google Scholar]
- Nathan RA, Mitchell D, Condemi J, Heller A, Schoaf L, Herrle M, et al. Cardiovascular and hypothalamic‐pituitary‐adrenal axis safety of fluticasone propionate/salmeterol HFA MDI in adolescent and adult patients with asthma. American Journal for Respiratory and Critical Care Medicine 2001;163(5):A863. [Google Scholar]
- Pearlman DS, Kent E, Lanz MJ, Peden D, Baitinger L, Herrle M, et al. Fluticasone propionate/salmeterol HFA MDI has a rapid onset of effect in asthmatics treated with short or long‐acting beta‐agonists (BA) or inhaled corticosteroids (ICS). Amercian Journal of Respiratory and Critical Care Medicine 2001;163(5):A865. [Google Scholar]
- Rooklin A, Elkayam D, Weiler J, Windom H, Schoaf L, Scott C, et al. The fluticasone propionate/salmeterol HFA MDI is significantly more efficacious in treating asthma than placebo HFA MDI, fluticasone propionate CFC MDI or salmeterol CFC MDI. Journal of Allergy and Clinicial Immunology 2001;107(2):100s. [Google Scholar]
- SAS30004. A randomized, double‐blind, placebo‐controlled, parallel‐group 12‐week trial evaluating the safety and efficacy of the salmeterol/fluticasone propionate combination in GR106642X MDI, 50/250mcg BID, and salmeterol in propellant 11/12 MDI, 50mcg BID, fluticasone propionate in propellant 11/12 MDI, 250mcg BID, and placebo propellant GR106642X MDI in adult and adolescent subjects with asthma. www.ctr.gsk.co.uk 2004.
Schreurs 1996 {published data only}
- Schreurs A, Sinninghe Damste H, Graaff C, Greefhorst A. A dose‐response study with formoterol Turbuhaler as maintenance therapy in asthmatic patients. European Respiratory Journal 1996;9(8):1678‐83. [DOI] [PubMed] [Google Scholar]
Shapiro 2000b {published data only}
- Aggarwal SK, Frith LJ, Ho M, Weeks T, Ho SY. Fluticasone propionate/salmeterol delivered from a single inhaler demonstrates synergistic benefits in asthma. Amercian Journal of Respiratory and Critical Care Medicine 2003;167(7):A890. [Google Scholar]
- Bateman ED, Frith L, Braunstein GL. Achieving guideline‐based asthma control ‐ does the patient benefit?. European Respiratory Journal 2002;20(3):588‐95. [DOI] [PubMed] [Google Scholar]
- Bateman ED, Frith L, Ho M. Guideline‐based asthma control reduces maximally the impact of upon patient‐assessed quality of life. Amercian Journal of Respiratory and Critical Care Medicine 2002;165(8):A44. [Google Scholar]
- Cook CK, Prillaman BA, House KW, Rickard KA, Shah TP. Concurrent use of salmeterol/fluticasone propionate Diskus powder combination product and fluticasone propionate aqueous nasal spray does not adversely affect HPA‐axis function. Annals of Allergy, Asthma and Immunology 2001;86(1):98. [Google Scholar]
- Dorinsky P, Yancey S, Kral K, Emmett A, House K, Prillaman B, et al. Asthma control with salmeterol/fluticasone propionate (50/250mcg) dry powder combination Diskus has a faster onset of effect compared with salmeterol or fluticasone propionate in patients with asthma. Journal Allergy & Clinical Immunology 2000;161(Suppl 1):A195. [Google Scholar]
- Edin H, Prillaman B, Baitinger LA, House KW, Shah TP. Improved ability to perform strenuous activities after treatment with fluticasone/salmeterol combination. Amercian Journal of Respiratory and Critical Care Medicine 2002;165(8):A112. [Google Scholar]
- Lumry W, Windhom H, Mendelson L, Pedinoff A, Prillaman B, Baitinger L, et al. The salmeterol/fluticasone propionate (50/250mcg) dry powder combination Diskus has a faster onset of effect compared with salmeterol or fluticasone propionate in patients with asthma. Journal Allergy & Clinical Immunology 1999;103(1 Pt 2):S132. [Google Scholar]
- Lundback B, Pieters WR, Johansson G, Palmqvist M, Price MJ, Sondhi S, et al. Cost‐effectiveness analyses of salmeterol/fluticasone propionate combination product and fluticasone propionate in patients with asthma 1: Introduction and Overview. Pharmacoeconomics 1999;16(Suppl 2):1‐8. [Google Scholar]
- Markham A, Adkins JC. Inhaled salmeterol/fluticasone propionate combination: A pharmacoeconomic review of its use in the management of asthma. Pharmacoeconomics 2000;18(6):591‐608. [DOI] [PubMed] [Google Scholar]
- McCarthy TP, Edin H, House K, Vandermeer AK. Quality of life and asthma control assessment in patients previously treated with inhaled corticosteroids (ICS), treated with salmeterol/fluticasone combination (SFC) metered dose inhaler (MDI). Thorax 2001;56(Suppl 3):64. [Google Scholar]
- McCarthy TP, Edin HM, House K, Vandermeer AK, Scott C. Low dose salmeterol/fluticasone propionate combination (SFC) via metered dose inhaler (MDI) improves asthma control and quality of life in patients not well controlled on inhaled steroids. European Respiratory Journal 2002;20(Suppl 38):47s. [Google Scholar]
- Nathan RA, Dorinsky P, Carranza Rosenzweig JR, Shah T, Edin H, Prillaman B. Improved ability to perform strenuous exercises after treatment with fluticasone/salmeterol combination in patients with persistent asthma. Journal of Asthma 2003;40(7):815‐22. [DOI] [PubMed] [Google Scholar]
- Palmqvist M, Price MJ, Sondhi S, et al. Cost‐effectiveness analysis of salmeterol/fluticasone propionate 50/250mcg vs fluticasone propionate 250mcg in adults and adolescents with asthma. 4: Results. Pharmacoeconomics 1999;16(Suppl 2):23‐8. [Google Scholar]
- Pearlman D, Baitinger L, Woodring A, Prillaman B, House K, Shah T. Salmeterol 50mcg/fluticasone propionate 250mcg diskus combination product demonstrates improvements in lung function regardless of baseline corticosteroid therapy. American Journal of Respiratory and Critical Care Medicine 2000;161(Suppl 3):A196. [DOI] [PubMed] [Google Scholar]
- Pieters WR, Lundback B, Johansson G, Palmqvist M, Price MJ, Sondhi S, et al. Cost‐effectiveness analyses of salmeterol/fluticasone propionate combination product and fluticasone propionate in patients with asthma 2: Study Methodlogies. Pharmacoeconomics 1999;16(Suppl 2):9‐14. [Google Scholar]
- Reese PR, Mahajan P, Woodring A. Salmeterol/fluticasone propionate combination product improves quality of life in asthma patients. European Respiratory Journal 1998;12(Suppl 28):35s. [Google Scholar]
- SFCA3003. A randomized, double‐blind, parallel‐group trial evaluating safety and efficacy of Salmeterol 50mcg BID and Fluticasone Propionate 250mcg BID individually and in combination and placebo in subjects with asthma. http://ctr.gsk.co.uk 2005.
- Shapiro G, Lumry W, Wolfe J, Given J, White M, Woodring A, et al. Combined salmeterol 50 mug and fluticasone propionate 250 in the diskus device for the treatment of asthma. American Journal of Respiratory & Critical Care Medicine 2000;161(2 Pt 1):527‐34. [DOI] [PubMed] [Google Scholar]
- Stempel DA. Salmeterol/fluticasone combination product in asthma ‐ An evaluation of its cost‐effectiveness vs fluticasone ‐ foreword. Pharmacoeconmics 1999;16(Suppl 2):U6‐7. [PubMed] [Google Scholar]
- White MV, Shapiro G, Taylor J, Dunn A, Woodring L, Baitinger B, et al. The salmeterol xinafoate/fluticasone propionate dry powder combination product via diskus r inhaler improves asthma control compared to the individual products in patients previously treated with inhaled corticosteroids. American Journal of Respiratory and Critical Medicine 1999;159(3):A635. [Google Scholar]
Simons 1997a {published data only}
- Malozowski S, Stadel BV, Pian LP. Comparison of beclomethasone, salmeterol, and placebo in children with asthma. New England Journal of Medicine 1998; Vol. 339, issue 10:704‐5. [DOI] [PubMed]
- Simons FER. A comparison of Beclomethasone, salmeterol and placebo in children with asthma. New England Journal of Medicine 1997;337(23):1659‐65. [DOI] [PubMed] [Google Scholar]
- Simons FER, et al. A one year placebo controlled study of the efficacy and safety of beclomethasone DP versus salmeterol in glucocorticoid naive children with asthma. Journal of Allergy & Clinical Immunology 1997;99:S402. [Google Scholar]
SLGA2004 {unpublished data only}
- SLGA2004. A randomized, double‐blind, double‐dummy, placebo‐controlled, comparative clinical trial of salmeterol xinafoate via multi‐dose powder inhaler versus salmeterol xinafoate via Diskhaler for four weeks in adolescent and adult subjects with mild‐to‐moderate asthma.. http://ctr.gsk.co.uk 2006.
SLGA3014 1994 {unpublished data only}
- SLGA3014. www.fda.gov 2001.
SLGL82 {published data only}
- SLGL82. A double‐blind parallel group study of inhaled salmeterol in asthmatic patients. www.ctr.gsk.co.uk 2006.
SLMP03 {unpublished data only}
- SLMP03. A single centre, randomised, double‐blind, parallel group, placebo controlled study, to evaluate the effect of inhaled salmeterol xinafoate (50micrograms bd from a Diskhaler) on variations in bronchoconstriction induced by methacholine, in paediatric patients with mild to moderate asthma. www.ctr.gsk.co.uk 2006.
SMART {published data only}
- Knobil K, Yancey S, Kral K, Rickard K. Salmeterol Multicentre Asthma Research Trial (SMART): Results from an interim analysis. Chest 2003;124:335s. [Google Scholar]
- Nelson HS, Weiss ST, Bleecker ER, Yancey SW, Dorinsky PM, the SMART Study Group. The Salmeterol Multicenter Asthma Research Trial: A Comparison of Usual Pharmacotherapy for Asthma or Usual Pharmacotherapy Plus Salmeterol. Chest 2006;129(1):15‐26. [DOI] [PubMed] [Google Scholar]
- SLGA5011 SMART. SMART: a double‐blind, randomized, placebo‐controlled surveillance study of asthma event outcomes in subjects receiving either usual pharmacotherapy of asthma or usual pharmacotherapy plus salmeterol 42mcg twice daily. http://www.ctr.gsk.co.uk 2006. [Google Scholar]
SMS40221 {unpublished data only}
- SMS40221. Comparing the efficacy and safety of inhaled salmeterol 50mcg BID and placebo via diskus in patients with reversible airways obstruction. www.ctr.gsk.co.uk 2005.
Starke 1996 {published data only}
- Starke I, Luce P. The efficacy and safety of inhaled salmeterol 50 microg bd in older patients with reversible airflow obstruction. Age & Ageing 1996;25(1):67‐71. [DOI] [PubMed] [Google Scholar]
Steffensen 1995 {published data only}
- Steffensen I, Faurschou P. Formoterol as inhalation powder in the treatment of patients with reversible obstructive lung diseases. A 3‐month placebo‐controlled comparison of the effects of formoterol and salbutamol, followed by a 12‐month period with formoterol alone. Ugeskrift for Laeger 1996;158(49):7092‐6. [PubMed] [Google Scholar]
- Steffensen I, Faurschou P, Riska H, Rostrup J, Wegener T. Inhaled formoterol dry powder in the treatment of patients with reversible obstructive airway disease. A 3‐month, placebo‐controlled comparison of the efficacy and safety of formoterol and salbutamol, followed by a 12‐month trial with formoterol. Allergy 1995;50(8):657‐63. [DOI] [PubMed] [Google Scholar]
Stelmach 2002 {published data only}
- Stelmach I, Gorski P, Jerzynska J, Stelmach W, Majak P, Kuna P. A randomized, double‐blind trial of the effect of treatment with formoterol on clinical and inflammatory parameters of asthma in children. Annals of Allergy, Asthma, & Immunology 2002;89(1):67‐73. [DOI] [PubMed] [Google Scholar]
Sussman 1995 {published data only}
- Sussman HS. Continuous eformoterol and beta 2‐receptor responsiveness. British Journal of Clinical Practice 1995;81 Suppl:16‐7. [PubMed] [Google Scholar]
Taylor 1998 {published data only}
- Taylor DR, Drazen JM, Herbison GP, Yandava CN, Hancox RJ, Town GI. Asthma exacerbations during long term beta agonist use: influence of beta(2) adrenoceptor polymorphism. Thorax 2000;55(9):762‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DR, Town GI, Herbison GP, Boothman‐Burrell D, et al. Asthma control during long‐term treatment with regular inhaled salbutamol and salmeterol. Thorax 1998;53(9):744‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
von Berg 1998 {published data only}
- Berg A, Blic J, Rosa M, et al. A comparison of regular salmeterol vs 'as required' salbutamol therapy in asthmatic children. Respiratory Medicine 1998;92(2):292‐9. [DOI] [PubMed] [Google Scholar]
- Berg A, Blic J, Rosa M, et al. Regular salbutamol xinafoate compared with salbutamol as required in children with moderate asthma. American Journal of Respiratory & Critical Care Medicine 1996;4:A76. [Google Scholar]
von Berg 2003 {published data only}
- Berg A, Papageorgiou Saxoni F, Wille S, Carrillo T, Kattamis C, Helms PJ. Efficacy and tolerability of formoterol Turbuhaler in children. International Journal of Clinical Practice 2003;57(10):852‐6. [PubMed] [Google Scholar]
Wallin 1999 {published data only}
- Wallin A, Sandstrom T, Soderberg M, Howarth P, Lundback B, Della‐Cioppa G, et al. The effects of regular inhaled formoterol, budesonide, and placebo on mucosal inflammation and clinical indices in mild asthma. American Journal of Respiratory & Critical Care Medicine 1999;159(1):79‐86. [DOI] [PubMed] [Google Scholar]
Weinstein 1998 {published data only}
- Pearlman DS, Bronsky E, Chervinsky P, et al. Inhaled salmeterol powder compared with placebo administered over 12 weeks to children with mild to moderate asthma. American Journal of Respiratory & Critical Care Medicine 1996;153(4):A76. [Google Scholar]
- SLD390. www.fda.gov 2001.
- Weinstein S, Pearlman D, Bronsky E, Byrne A, Arledge T, Liddle R, et al. Efficacy of salmeterol xinafoate powder in children with chronic persistent asthma. Annals of Allergy, Asthma, & Immunology 1998;81(1):51‐8. [DOI] [PubMed] [Google Scholar]
Wolfe 2000a {published and unpublished data}
- SLGA3010. A randomized, double‐blind, double‐dummy, comparative clinical trial of salmeterol 50mcg via the Diskus and salmeterol 50mcg via the metered‐dose inhaler versus placebo for 12 weeks in adolescent and adult subjects with mild‐to‐moderate asthma. www.ctr.gsk.co.uk.
- Wolfe J, Kreitzer S, Chervinsky P, Lawrence M, Wang Y, Reilly D, et al. Comparison of powder and aerosol formulations of salmeterol in the treatment of asthma. Annals of Allergy 2000;84(3):334‐40. [DOI] [PubMed] [Google Scholar]
Wolfe 2000b {published and unpublished data}
- SLGA3011. A randomized, double‐blind, double‐dummy, comparative clinical trial of salmeterol 50mcg via the Diskus and salmeterol 50mcg via the metered‐dose inhaler versus placebo for twelve weeks in adolescent and adult subjects with mild‐to‐moderate asthma.. www.ctr.gsk.co.uk.
- Wolfe J, Kreitzer S, Chervinsky P, Lawrence M, Wang Y, Reilly D, et al. Comparison of powder and aerosol formulations of salmeterol in the treatment of asthma. Annals of Allergy 2000;84(3):334‐40. [DOI] [PubMed] [Google Scholar]
Wolfe 2006 {published data only}
- FOR258D2307. www.fda.gov 2004.
- Lapidus RJ, Wolfe J, Greos LS, Friedman, Orevillo C, Ziehmer B, et al. Formoterol 24 mcg provides effective bronchodilation and is well tolerated in patients with persistent stable asthma [Abstract]. Journal of Allergy & Clinical Immunology 2005;115(2 Suppl):S150. [Google Scholar]
- Wolfe J, Laforce C, Friedman B, Sokol W, Till D, Della Cioppa G, et al. Formoterol, 24 microg bid, and serious asthma exacerbations: similar rates compared with formoterol, 12 microg bid, with and without extra doses taken on demand, and placebo. Chest 2006;129(1):27‐38. [DOI] [PubMed] [Google Scholar]
Wronska 1998 {published data only}
- Wronska J, Chazan R, Mazurek J, Droszcz W. Treatment with salmeterol and quality of life in patients with asthma. Pneumonologia i Alergologia Polska 1998;66(3‐4):193‐7. [PubMed] [Google Scholar]
Zarkovic 1998 {published data only}
- Gotz MH, Taak NK. The efficacy and safety of inhaled salmeterol (50mcg bd) compared with salbutamol (200mcg prn) in children with asthma. European Respiratory Journal 1995;8 Suppl 19:517s. [Google Scholar]
- SMS30013. A multicentre, randomised, double‐blind, crossover study to investigate the efficacy and safety of inhaled salmeterol xinafoate (50µg twice daily from the Diskhaler) compared with placebo (from the Diskhaler) with salbutamol (200µg to use ‘as required’ from the Diskhaler) in children with asthma.. www.ctr.gsk.co.uk.
- Zarkovic J, Gotz MH, Holgate ST, Taak NK. Effect of long‐term regular salmeterol treatment in children with moderate asthma. Clinical Drug Investigation 1998;15(3):169‐75. [Google Scholar]
References to studies excluded from this review
Akpinarli 1999 {published data only}
- Akpinarli A, Tuncer A, Saraclar Y, Sekerel B, Kalayci O. Effect of formoterol on clinical parameters and lung functions in patients with bronchial asthma: A randomised controlled trial. Archives of Disease in Childhood 1999;81(1):45‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Arvidsson 1989 {published data only}
- Arvidsson P, Larsson S, Lofdahl CG, Melander B, Wahlander L, Svedmyr N. Formoterol, a new long acting bronchodilator for inhalation. European Respiratory Journal 1989;2(4):325‐30. [PubMed] [Google Scholar]
Arvidsson 1991 {published data only}
- Arvidsson P, Larsson S, Lofdahl CG, Melander B, Svedmyr N, Wahlander L. Inhaled formoterol during one year in asthma: a comparison with salbutamol. European Respiratory Journal 1991;4(10):1168‐73. [PubMed] [Google Scholar]
Aziz 1998 {published data only}
- Aziz I, Hall IP, McFarlane LC, Lipworth BJ. Beta 2‐adrenceptor regulation and bronchodilator sensitivity after regular treatment with formoterol in subjects with stable asthma. Journal of Allergy & Clinical Immunology 1998;101(3):337‐41. [DOI] [PubMed] [Google Scholar]
Aziz 1998a {published data only}
- Aziz I, Tan KS, Hall IP, Devlin MM, Lipworth BJ. Subsensitivity to bronchoprotection against adenosine monophosphate challenge following regular once‐daily formoterol. European Respiratory Journal 1998;12(3):580‐4. [DOI] [PubMed] [Google Scholar]
Aziz 1999 {published data only}
- Aziz I, Lipworth BJ. A bolus of inhaled budesonide rapidly reverses airway sub‐sensitivity and beta2‐adrenoceptor down‐regulation after regular inhaled formoterol. Chest 1999;115(3):623‐8. [DOI] [PubMed] [Google Scholar]
Baki 1998 {published data only}
- Baki A, Karaguzel G. Short‐term effects of budesonide, nedocromil sodium and salmeterol on bronchial hyperresponsiveness in childhood asthma. Acta Paediatrica Japan 1998;40(3):247‐51. [DOI] [PubMed] [Google Scholar]
Baumgarten 2002 {published data only}
- Baumgarten C, Geldszus R, Behre U, Peslis N, Trautmann M. Initial treatment of symptomatic mild to moderate bronchial asthma with the salmeterol/fluticasone propionate (50/250 microg) combination product (SAS 40023). European Journal of Medical Research 2002;7(1):1‐7. [PubMed] [Google Scholar]
Beach 1993 {published data only}
- Beach JR, Young CL, Harkawat R, Gardiner PV, Avery AJ, Coward GA, et al. Effect on airway responsiveness of six weeks treatment with salmeterol. Pulmonary Pharmacology 1993;6(2):155‐7. [DOI] [PubMed] [Google Scholar]
Becker 1989 {published data only}
- Becker AB, Simons F. Formoterol, a new long‐acting selective beta 2‐adrenergic receptor agonist: double‐blind comparison with salbutamol and placebo in children with asthma. Journal of Allergy & Clinical Immunology 1989;84(6):891‐5. [DOI] [PubMed] [Google Scholar]
Becker 1993 {published data only}
- Becker AB, Simons FER. Formoterol, a new beta2 agonist, improves chronic airway hyperresponsiveness in children with asthma. Immunology and Allergy practice 1993;14(7):18‐9. [Google Scholar]
Bhagat 1995 {published data only}
- Bhagat R, Kalra S, Swystun VA, Cockcroft DW. Rapid onset of tolerance to the bronchoprotective effect of salmeterol. Chest 1995;108(5):1235‐9. [DOI] [PubMed] [Google Scholar]
Bishop 1994 {published data only}
- Bishop AL, Chervinsky P, Liddle R, et al. Salmeterol for treatment of reversible obstructive airways disease in children. Journal of Allergy & Clinical Immunology 1994;93:248. [Google Scholar]
Blake 1992 {published data only}
- Blake K, Pearlman D, Scott C, Wang Y, Stahl E, Arledge T. Prevention of exercise‐induced bronchospasm in pediatric asthma patients: a comparison of salmeterol powder with albuterol. Annals of Allergy, Asthma, & Immunology 1999;82(2):205‐11. [DOI] [PubMed] [Google Scholar]
Boner 1992 {published data only}
- Boner A. Salmeterol: long term studies in children. European Respiratory Journal 1992;5:318s. [Google Scholar]
Booth 1996 {published data only}
- Booth H, Bish R, Walters J, Whitehead F, Walters EH. Salmeterol tachyphylaxis in steroid treated asthmatic subjects. Thorax 1996;51(11):1100‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boulet 1998a {published data only}
- Boulet L, Cartier A, Milot J, Cote J, Malo JL, Laviolette M. Tolerance to the protective effects of salmeterol on methacholine‐induced bronchoconstriction‐ influence of inhaled corticosteroids. European Respiratory Journal 1998;11(5):1091‐7. [DOI] [PubMed] [Google Scholar]
Boulet 2001 {published data only}
- Boulet LP, Chakir J, Milot J, Boutet M, Laviolette M. Effect of salmeterol on allergen‐induced airway inflammation in mild allergic asthma. Clinical & Experimental Allergy 2001;31(3):430‐7. [DOI] [PubMed] [Google Scholar]
Boulet Laviol 1997 {published data only}
- Boulet LP, Laviolette M, Boucher S, et al. A twelve‐ week comparison of salmeterol and salbutamol in the treatment of mild‐to‐moderate asthma: a Canadian multicentre study. Journal of Allergy & Clinical Immunology 1997;99(1 Pt 1):13‐21. [DOI] [PubMed] [Google Scholar]
Bowers 1997 {published data only}
- Bowers BW, Cox FM, Kalberg C, et al. The impact of salmeterol on asthma specific quality of life in patients reporting nocturnal asthma awakenings due to asthma. Annals of Allergy, Asthma, & Immunology 1997;78:110. [Google Scholar]
Boyd 1995 {published data only}
- Boyd G. Salmeterol xinafoate in asthmatic patients under consideration for maintenance oral corticosteroid therapy‐ UK Study Group. European Respiratory Journal 1995;8(9):1494‐8. [PubMed] [Google Scholar]
Brambilla 1994 {published data only}
- Brambilla C, Chastang C, Georges D, et al. Salmeterol compared with slow release terbutaline in nocturnal asthma: a multi center randomised double blind double dummy sequential clinical trial. Allergy 1994;49(6):421‐6. [DOI] [PubMed] [Google Scholar]
Brambilla 2003 {published data only}
- Brambilla C, Gros V, Bourdeix I. Formoterol 12 microg BID administered via single‐dose dry powder inhaler in adults with asthma suboptimally controlled with salmeterol or on‐demand salbutamol: a multicenter, randomized, open‐label, parallel‐group study. Clinical Therapeutics 2003;25(7):2022‐36. [DOI] [PubMed] [Google Scholar]
Britton 1992 {published data only}
- Britton MC, Earnshaw JS, Palmer JBD. A twelve month comparison of salmeterol with salbutamol in asthmatic patients. European Respiratory Journal 1992;5(9):1062‐7. [PubMed] [Google Scholar]
Byrnes 1996 {published data only}
- Byrnes CA, Weber EA, Moorat A, et al. A Comparison of salmeterol 50mcg bd and 100mcg bd with salbutamol 200mcg qds in paediatric asthma. American Journal of Respiratory & Critical Care Medicine 1996;153(4):A408. [Google Scholar]
Byrnes 2000 {published data only}
- Byrnes C, Shrewsbury S, Barnes PJ, Bush A. Salmeterol in paediatric asthma. Thorax 2000;55(9):780‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Campbell 1999 {published data only}
- Campbell LM, Anderson TJ, Parashchak MR, Burke CM, Watson SA, Turbitt ML. A comparison of the efficacy of long‐acting beta 2‐agonists: eformoterol via Turbohaler and salmeterol via pressurized metered dose inhaler or Accuhaler, in mild to moderate asthmatics. Respiratory Medicine 1999;93(7):236‐44. [PubMed] [Google Scholar]
Campbell 2000 {published data only}
- Campbell LM, Berggren F, Emmas C. The cost effectiveness of eformoterol via Turbohaler(TM) and salmeterol via pressurised metered dose inhaler and metered dose powder inhaler in mild to moderate asthma. Journal of Medical Economics 2000;3:49‐60. [Google Scholar]
Carlsen 1995 {published data only}
- Carlsen K, Roksund O, Olsholt K, Nja F, Leegaard J, Bratten G. Overnight protection by inhaled salmeterol on exercise‐induced asthma in children. European Respiratory Journal 1995;8(11):1852‐5. [DOI] [PubMed] [Google Scholar]
Castle 1993 {published data only}
- Castle W, Fuller R, Hall J. Serevent nationwide surveillance study:comparison of salmeterol with salbutamol in asthmatic patients who require regular bronchodilator treatment. British Medical Journal 1993;306(6884):1034‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Charpin 1992 {published data only}
- Charpin D, Vervloet D. Quality of life in moderate chronic asthma: salmeterol versus current treatments. Allergy 1992;47(12 Suppl):230. [Google Scholar]
Chuchalin 2002 {published data only}
- Chuchalin A, Svensson K, Stahl E, Ovcharenko SI, Goriachkina LA, Sidorenko IV, et al. A health‐related quality‐of‐life comparison of formoterol (Oxis) Turbuhaler plus budesonide (Pulmicort) Turbuhaler with budesonide Turbuhaler alone and noncorticosteroid treatment in asthma: a randomized clinical study in Russia. Respiration 2002;69(5):427‐33. [DOI] [PubMed] [Google Scholar]
- Chuchalin AG, Ovcharenko SI, Goriachkina LA, Sidorenko IV, Tsoi AN. The safety and efficacy of formoterol (Oxis) turbuhaler plus budesonide (Pulmicort) turbuhaler in mild to moderate asthma: a comparison with budesonide Turbuhaler alone and current non‐corticosteroid therapy in Russia. International Journal of Clinical Practice 2002;56(1):15‐20. [PubMed] [Google Scholar]
Clark 1993 {published data only}
- Clark CE, Ferguson AD, Siddorn J. Respiratory arrests in young asthmatics on salmeterol. Respiratory Medicine 1993;87(3):227‐8. [DOI] [PubMed] [Google Scholar]
Clauzel 1998 {published data only}
- Clauzel AM, Molimard M, Gros V, Lepere E, Febvre N, Michel FB. Use of formoterol dry powder administered for three months via a single dose inhaler in 1,380 asthmatic patients. Journal of Investigational Allergology & Clinical Immunology 1998;8(5):265‐70. [PubMed] [Google Scholar]
Cockcroft 1997 {published data only}
- Cockcroft DW, Swystun VA, Bhagat R. Salmeterol and airway response to allergen. Canadian Respiratory Journal 1997;4:37‐40. [Google Scholar]
Cox 1996 {published data only}
- Cox F, Goodwin B, Wenzel S, Rickard K, Kalberg C, Emmett A. Asthma specific quality of life in patients treated with salmeterol or albuterol. Journal of Allergy & Clinical Immunology 1996;97:256. [Google Scholar]
D'Urzo 2001 {published data only}
- D'Urzo AD, Chapman KR, Cartier A, Hargreave FE, Fitzgerald M, Tesarowski D. Effectiveness and safety of salmeterol in non‐specialist practice settings. Chest 2001;119(3):714‐9. [DOI] [PubMed] [Google Scholar]
D5125C00344 {unpublished data only}
- D5125C00344. A 3‐month, multi‐centre, double‐blind, double‐dummy, randomised, parallel group, phase III study to investigate the efficacy and safety of formoterol HFA pMDI compared with placebo and Oxis Turbuhaler in subjects with asthma. www.astrazeneca.com 2005.
De Carli 1995 {published data only}
- Carli G, Arpinelli F, Irvine SH, Bamfi F, Ravinetto R, Recchia G. Change of quality of life induced by salmeterol and salbutamol in adult asthmatic patients. Therapie 1995;50 Suppl:N118. [Google Scholar]
De Oliveira 1998 {published data only}
- Oliveira M, Jardim J, Faresin S, Lucas S, Nery L. Efficacy of and tolerance to salmeterol compared to salbutamol in patients with bronchial asthma. Revista da Associacao Medica Brasileira 1998;44(3):169‐75. [PubMed] [Google Scholar]
Derom 1992 {published data only}
- Derom EY, Pauwels RA, Straten MEF. The effect of inhaled salmeterol on methacholine responsiveness in subjects with asthma up to 12 hours. Journal of Allergy & Clinical Immunology 1992;89(4):811‐5. [DOI] [PubMed] [Google Scholar]
Di Lorenzo 1995 {published data only}
- Di‐Lorenzo G, Morici G, Norrito F, Mansueto P, Melluso M, Purello‐D'Ambrosio F, et al. Comparison of the effects of salmeterol and salbutamol on clinical activity and eosinophil cationic protein serum levels during the pollen season in atopic asthmatics. Clinical & Experimental Allergy 1995;25(10):951‐6. [DOI] [PubMed] [Google Scholar]
Droszcz 1999 {published data only}
- Droszcz P, Droszcz W. Results of 30 days and 12 months treatment with salmeterol in patients with asthma and COPD. Polski Merkuriusz Lekarski 1999;6:266‐7. [PubMed] [Google Scholar]
Faurschou 1992 {published data only}
- Faurschou P. Salmeterol and Salbutamol: long term efficacy and safety. European Respiratory Journal 1992;5:317‐8. [Google Scholar]
Faurschou 1994a {published data only}
- Faurschou P, Engel AM, Haanaes OC. Salmeterol in two different doses in the treatment of nocturnal bronchial asthma poorly controlled by other therapies. Allergy 1994;10(827‐32). [DOI] [PubMed] [Google Scholar]
Faurschou 1996 {published data only}
- Faurschou P, Steffensen I, Jacques L. Effect of addition of inhaled salmeterol to the treatment of moderate‐to‐severe asthmatics uncontrolled on high‐dose inhaled steroids. European Respiratory Journal 1996;9(9):1885‐90. [DOI] [PubMed] [Google Scholar]
Faurschou 1997 {published data only}
- Faurschou P, Dahl R, Jeffery P, et al. Comparison of the anti‐inflammatory effects of fluticasone and salmeterol in asthma‐ A placebo controlled, DB CO with bronchoscopy, bronchial methacholine provocation and lavage [abstract]. European Respiratory Journal 1997;10:243s. [Google Scholar]
FitzGerald 1999 {published data only}
- FitzGerald J, Chapman K, Della Cioppa G, Stubbing D, Fairbarn M, Till M, et al. Sustained bronchoprotection, bronchodilatation, and symptom control during regular formoterol use in asthma of moderate or greater severity. Journal of Allergy & Clinical Immunology 1999;103(31):427‐35. [DOI] [PubMed] [Google Scholar]
Fitzpatrick 1990 {published data only}
- Fitzpatrick MF, MacKay T, Driver H, Douglas H. Salmeterol in nocturnal asthma‐a double blind , placebo controlled trial of a long acting inhaled beta agonist. British Medical Journal 1990;301(6765):1365‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fuglsang 1998 {published data only}
- Fuglsang G, Vikre‐Jorgensen J, Agertoft L, Pedersen S. Effect of salmeterol treatment on nitric oxide level in exhaled air and dose‐response to terbutaline in children with mild asthma. Pediatric Pulmonology 1998;25(5):314‐21. [DOI] [PubMed] [Google Scholar]
Fuller 1993 {published data only}
- Fuller R, Castle W, Palmer J, Hull J. Bronchodilator treatment in asthma. British Medical Journal 1993;306(6892):1611. [Google Scholar]
Gardiner 1994 {published data only}
- Gardiner PV, Ward C, Booth H, et al. Effect of eight weeks treatment with salmeterol on bronchoalveolar lavage inflammatory indices in asthmatics. American Journal of Respiratory & Critical Care Medicine 1994;150(4):1006‐11. [DOI] [PubMed] [Google Scholar]
Giannini 1995 {published data only}
- Giannini D, Carletti A, Dente FL, et al. Tolerance to salbutamol in allergen induced bronchoconstriction. American Journal of Respiratory & Critical Care Medicine 1995;151:A39. [Google Scholar]
Giannini 1999 {published data only}
- Giannini D, Bacci E, Dente FL, Franco A, Vagagginin B, Testi R, et al. Inhaled beclomethasone dipropionate reverts tolerance to the protective effect of salmeterol on allergen challenge. Chest 1999;115(3):629‐34. [DOI] [PubMed] [Google Scholar]
Gong 1996 {published data only}
- Gong H, Linn WS, Shamou DA, et al. Effect of inhaled salmeterol on sulphur dioxide induced bronchoconstriction in asthmatic subjects. Chest 1996;110(5):1229‐35. [DOI] [PubMed] [Google Scholar]
Goodwin 1996 {published data only}
- Goodwin R, Cox F, Lumry W, et al. The impact of salmeterol versus albuterol on disease specific quality of life in mild to moderate asthmatics. Journal of Allergy & Clinical Immunology 1996;97(3):256. [Google Scholar]
Green 1002 {published data only}
- Green C, Price J. Prevention of exercise induced asthma by inhaled salmeterol xinafoate. Archives of Disease in Childhood 1992;67(8):1014‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Greening 1994 {published data only}
- Greening AP, Ind PW, Northfield M, Shaw G. Added salmeterol versus higher dose corticosteroid in asthma patients with symptoms on existing inhaled corticosteroid. Lancet 1994;344(8917):219‐24. [DOI] [PubMed] [Google Scholar]
Grove 1995 {published data only}
- Grove A, Lipworth BJ. Bronchodilator sub‐sensitivity to salbutamol after twice daily salmeterol in asthmatic patients. Lancet 1995;346(8969):201‐6. [DOI] [PubMed] [Google Scholar]
Grove 1996 {published data only}
- Grove A, Lipworth BJ. Effects of prior treatment with salmeterol and formoterol on airway and systemic beta 2 responses to formoterol. Thorax 1996;51(6):585‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hacki 1993 {published data only}
- Hacki M, Ritter A, Medici T. Formoterol: three years therapy in asthma. European Respiratory Journal 1993;6 Suppl 17:529s. [Google Scholar]
Hekking 1990 {published data only}
- Hekking P, Maesen F, Greefhorst A, et al. Long term efficacy of formoterol compared with salbutamol. Lung 1990;168 Suppl:76‐82. [DOI] [PubMed] [Google Scholar]
Hermansson 1995 {published data only}
- Hermansson B, Jenkins R. A 4‐week comparison of salmeterol and terbutaline in adult asthma. Allergy 1995;50(7):551‐8. [DOI] [PubMed] [Google Scholar]
Jartti 1998 {published data only}
- Jartti TT, Kaila TJ, Tahvanainen KU, Kuusela TA, Vanto TT, Valimaki IA. Altered cardiovascular autonomic regulation after salmeterol treatment in asthmatic children. Clinical Physiology 1998;18(4):345‐53. [DOI] [PubMed] [Google Scholar]
Jenkins 1991 {published data only}
- Jenkins M, Hilton C, Kock J, Palmer J. Exacerbations of asthma in patients on salmeterol. Lancet 1991;337(8746):913‐4. [DOI] [PubMed] [Google Scholar]
Kalra 1996 {published data only}
- Kalra S, Swystun V, Bhagat R, Cockcroft DW. Inhaled corticosteroids do not prevent the development of tolerance to the bronchoprotective effect of salmeterol. Chest 1996;109(4):953‐6. [DOI] [PubMed] [Google Scholar]
Kavuru 2000b {published data only}
- Kavuru M, Melamed J, Gross G, LaForce C, House K, Prillaman B, et al. Salmeterol and fluticasone propionate combined in a new powder inhalation device for the treatment of asthma ‐ a randomized, double‐blind, placebo‐controlled trial. Journal of Allergy & Clinical Immunology 2000;105(6):1108‐16. [DOI] [PubMed] [Google Scholar]
Kemp 1993 {published data only}
- Kemp J, Bukstein D, Busse W. Effective salmeterol, salbutamol and placebo in the prevention of exercise induced bronchospasm. Chest 1993;104 Suppl:10s. [Google Scholar]
Kemp 1994 {published data only}
- Kemp JP, Dockhorn RJ, Busse WW, et al. Prolonged effect of inhaled salmeterol against exercise induced bronchoconstriction after chronic dosing with salmeterol. American Journal of Respiratory & Critical Care Medicine 1994;150(6 Pt 1):1612‐5. [DOI] [PubMed] [Google Scholar]
Kemp 1998b {published data only}
- Kemp J, Cook D, Incaudo G, Corren J, Kalberg C, Emmett A, et al. Salmeterol improves quality of life in patients with asthma requiring inhaled corticosteroids‐ Salmeterol Quality of Life Study Group. Journal of Allergy & Clinical Immunology 1998;101(2 Pt 1):188‐95. [DOI] [PubMed] [Google Scholar]
Kesten 1991 {published data only}
- Kesten S, Chapman KR, Broder I, et al. A three month comparison of inhaled formoterol versus four times daily inhaled albuterol in the management of stable asthma. American Review of Respiratory Disease 1991;144(3 Pt 1):622‐5. [DOI] [PubMed] [Google Scholar]
Kesten 1992 {published data only}
- Kesten S, Chapman KR, Broder I, Cartier A, Hyland RH, Knight A, et al. Sustained improvement in asthma with long‐term use of formoterol fumarate. Annals of Allergy 1992;69(5):415‐20. [PubMed] [Google Scholar]
Kozlik‐Feldmann 1996 {published data only}
- Kozlik‐Feldmann R, Berg A, Berdel D, Reinhardt D. Long‐term effects of formoterol and salbutamol on bronchial hyperreactivity and beta‐adrenoceptor density on lymphocytes in children with bronchial asthma. European Journal of Medical Research 1996;1(10):465‐70. [PubMed] [Google Scholar]
Lai 1995 {published data only}
- Lai C, Chan C, Ho S, Hui A, Lai K. Inhaled salmeterol and albuterol in asthmatic patients receiving high‐dose inhaled corticosteroids. Chest 1995;108(1):36‐40. [DOI] [PubMed] [Google Scholar]
Langley 1998 {published data only}
- Langley SJ, Masterson CM, Batty EP, Woodcock A. Bronchodilator response to salbutamol after chronic dosing with salmeterol or placebo. European Respiratory Journal 1998;11(5):1081‐5. [DOI] [PubMed] [Google Scholar]
Langton Hewer 1995 {published data only}
- Langton Hewer S, Hobbs J, French D, Lenney W. Pilgrim's progress: the effect of salmeterol in older children with chronic severe asthma. Respiratory Medicine 1995;89(6):435‐40. [DOI] [PubMed] [Google Scholar]
Lenney 1995 {published data only}
- Lenney W, Pedersen S, Boner AL, Ebbutt A, Jenkins M. Efficacy and safety of salmeterol in childhood asthma. European Journal of Pediatrics 1995;154(12):983‐90. [DOI] [PubMed] [Google Scholar]
Li 1999 {published data only}
- Li X, Bamford T, Ward C, et al. Influence of salmeterol on eosinophil inflammation in bronchial biopsies from asthmatics on inhaled steroid. European Respiratory Journal 1998;12 Suppl 28:155s. [Google Scholar]
- Li X, Ward C, Thien F, Bish R, Bamford T, Bao X, et al. An antiinflammatory effect of salmeterol, a long‐acting beta2 agonist, assessed in airway biopsies and bronchoalveolar lavage in asthma. American Journal of Respiratory & Critical Care Medicine 1999;160(5 Pt 1):1493‐9. [DOI] [PubMed] [Google Scholar]
Lipworth 1998 {published data only}
- Lipworth B, Tan S, Devlin M, Aiken T, Baker R, Hendrick D. Effects of treatment with formoterol on bronchoprotection against methacholine. American Journal of Medicine 1998;104(5):431‐8. [DOI] [PubMed] [Google Scholar]
Lipworth 1999 {published data only}
- Lipworth BJ, Aziz I. A high dose of albuterol does not overcome bronchoprotective sub‐sensitivity in asthmatic subjects deceiving regular salmeterol or formoterol. Journal of Allergy & Clinical Immunology 1999;103(1 Pt 1):88‐92. [DOI] [PubMed] [Google Scholar]
Lipworth 2000a {published data only}
- Lipworth B, Aziz I. Bronchodilator response to albuterol after regular formoterol and effects of acute corticosteroid administration. Chest 2000;117(1):156‐62. [DOI] [PubMed] [Google Scholar]
Lipworth 2000b {published data only}
- Lipworth BJ, Dempsey OJ, Aziz I. Functional antagonism with formoterol and salmeterol in asthmatic patients expressing the homozygous Glycine‐16 beta2 adrenoceptor polymorphism. Chest 2000;118(2):321‐8. [DOI] [PubMed] [Google Scholar]
Lotvall 1992 {published data only}
- Lotvall J, Lunde H, Ullman A, Tornquist H, Svedmyr N. Twelve months, treatment with inhaled salmeterol in asthmatic patients. effects on b2receptor function and inflammatory cells. Allergy 1992;47(5):477‐83. [DOI] [PubMed] [Google Scholar]
Lundback 1993 {published data only}
- Lundback B, Rawlinson DW, Palmer JBD. Twelve month comparison of salmeterol and salbutamol as dry powder formulations in asthmatic patients. Thorax 1993;48(2):148‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Malo 1992 {published data only}
- Malo J‐L, Ghezzo H, Trudeau C. Salmeterol a new inhaled beta2 agonist, has a longer blocking effect than albuterol on hyperventilation induced bronchoconstriction. Journal of Allergy & Clinical Immunology 1992;89(2):567‐74. [DOI] [PubMed] [Google Scholar]
Mann 1996 {published data only}
- Mann RD, Kubota K, Pearce G, et al. Salmeterol: a study by prescription event monitoring in a UK cohort of 15,407 patients. Journal of Clinical Epidemiology 1996;49(2):247‐50. [DOI] [PubMed] [Google Scholar]
Martin 1999 {published data only}
- Martin R, Kraft M, Beaucher W, et al. Comparative study of extended release albuterol sulfate and long‐acting inhaled salmeterol xinafoate in the treatment of nocturnal asthma. Annals of Allergy, Asthma, & Immunology 1999;83(2):121‐6. [DOI] [PubMed] [Google Scholar]
McIvor 1998 {published data only}
- Mcivor RA, Pizzichini E, Turner MO, et al. Potential masking effects of salmeterol on airway inflammation in asthma. American Journal of Respiratory & Critical Care Medicine 1998;158(3):924‐30. [DOI] [PubMed] [Google Scholar]
Meijer 1995 {published data only}
- Meijer GG, Postma DS, Mulder PGH, et al. Long term circadian effects of salmeterol in asthmatic children treated with inhaled corticosteroids. American Journal of Respiratory & Critical Care Medicine 1995;152(6 Pt 1):1887‐92. [DOI] [PubMed] [Google Scholar]
Midgren 1992 {published data only}
- Midgren B, Melander B, Persson G. Formoterol, a new long acting beta2 agonist, inhaled twice daily in stable asthmatic subjects. Chest 1992;101(4):1019‐22. [DOI] [PubMed] [Google Scholar]
Molimard 2001 {published data only}
- Molimard M, Bourcereau V, LeGros V, Bourdeix I, Leynadier F, Duroux P. Comparison between formoterol 12mcg BID and on‐demand salbutamol in moderate persistent asthma. Respiratory Medicine 2001;95(1):64‐70. [DOI] [PubMed] [Google Scholar]
Nelson 1999a {published data only}
- Nelson H, Berkowitz R, Tinkelman D, Emmett A, Rickard K, Yancey S. Lack of sub‐sensitivity to albuterol after treatment with salmeterol in patients with asthma. American Journal of Respiratory & Critical Care Medicine 1999;159(5 Pt 1):1556‐61. [DOI] [PubMed] [Google Scholar]
- SLGA5020. A single‐center, randomized, double‐blind, cross‐over clinical trial to examine sub‐sensitivity in adult subjects with asthma receiving salmeterol xinafoate 42mcg BID and placebo BID. www.ctr.gsk.co.uk.
Newnham 1993 {published data only}
- Newnham DM, Ingram CG, Earnshaw J, et al. Salmeterol provides prolonged protection against exercise induced bronchoconstriction in a majority of subjects with mild stable asthma. Respiratory Medicine 1993;87(6):439‐44. [DOI] [PubMed] [Google Scholar]
Nichol 1990 {published data only}
- Nichol G, Nix A, Barnes P, Chung K. Prolonged attenuation of BHR to methacholine by long acting b2adrenoceptor agonist formoterol. American Review of Respiratory Disease 1990;141:A 210. [Google Scholar]
Nielsen 1999 {published data only}
- Nielsen L, Pedersen B, Faurschou P, Madsen F, Wilcke J, Dahl R. Salmeterol reduces the need for inhaled corticosteroid in steroid‐dependent asthmatics. Respiratory Medicine 1999;93(12):863‐8. [DOI] [PubMed] [Google Scholar]
Nightingale 2002 {published data only}
- Nightingale JA, Rogers DF, Barnes PJ. Comparison of the effects of salmeterol and formoterol in patients with severe asthma. Chest 2002;121(5):1401‐6. [DOI] [PubMed] [Google Scholar]
Nix 1990 {published data only}
- Nix A, Nichol G, Robson A, Barnes P, Chung K. Effect of formoterol, a long‐lasting beta2‐adrenoceptor agonist, against methacholine‐induced bronchoconstriction. British Journal of Clinical Pharmacology 1990;29(3):321‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Norhaya 1999 {published data only}
- Norhaya M, Yap T, Zainudin B. Addition of inhaled salmeterol to inhaled corticosteroids in patients with poorly controlled nocturnal asthma. Respirology 1999;4(1):77‐81. [DOI] [PubMed] [Google Scholar]
Nowak 1992 {published data only}
- Nowak D, Jorres R, Rabe KF, et al. Salmeterol protects against hyperventilation induced bronchoconstriction over 12 hours. European Journal of Clinical Pharmacology 1992;43(6):591‐5. [DOI] [PubMed] [Google Scholar]
O'Byrne 2001 {published data only}
- O'Byrne PM, Barnes P, Rodriguez‐Roisin R, Runnerstrom E, Sandstrom T, Svensson K, et al. Low dose inhaled budesonide and formoterol in mild persistent asthma: the OPTIMA randomized trial. American Journal of Respiratory & Critical Care Medicine 2001;164(8 Pt 1):1392‐7. [DOI] [PubMed] [Google Scholar]
Palmer 1992 {published data only}
- Palmer JBD, Stuart AM, Shepherd GL, Viskum K. Inhaled salmeterol in the treatment of patients with moderate to severe ROAD‐ a 3 month comparison of the efficacy and safety of bd salmeterol (100mcg) and salmeterol (50mcg). Respiratory Medicine 1992;86(5):409‐17. [DOI] [PubMed] [Google Scholar]
Pauwels 1997 {published data only}
- Andersson F, Stahl E, Barnes P, Lofdahl C, O'Byrne P, Pauwels R, et al. Adding formoterol to budesonide in moderate asthma‐health economic results from the FACET study. Respiratory Medicine 2001;95(6):505‐12. [DOI] [PubMed] [Google Scholar]
- Juniper E, Svensson K, O'Byrne P, Barnes P, Bauer C, Lofdahl C‐G, et al. Asthma quality of life during 1 year of treatment with budesonide with or without formoterol. European Respiratory Journal 1999;14(5):1038‐43. [DOI] [PubMed] [Google Scholar]
- Pauwels RA, Lofdahl CG, Postma DS, et al. Effect of inhaled formoterol and budesonide on exacerbations of asthma. New England Journal of Medicine 1997;337(20):1405‐11. [DOI] [PubMed] [Google Scholar]
- Tattersfield A, Postma D, Barnes P, Svensson K, Bauer C, O'Byrne P, et al. Exacerbations of asthma‐a descriptive study of 425 severe exacerbations‐ The FACET International Study Group. American Journal of Respiratory & Critical Care Medicine 1999;160(2):594‐9. [DOI] [PubMed] [Google Scholar]
Pearlman 1999 {published data only}
- Pearlman D, Stricker W, Weinstein S, Gross G, Chervinsky P, Woodring A, et al. Inhaled salmeterol and fluticasone: A study comparing monotherapy and combination therapy in asthma. Annals of Allergy 1999;82(3):257‐65. [DOI] [PubMed] [Google Scholar]
Pederson 1993 {published data only}
- Pedersen B, Dahl R, Larsen BB, et al. The effect of salmeterol on the early and late phase reaction to bronchial allergen and post challenge variation in BHR, blood eosinophils, serum ECP and serum eosinophil protein X. Allergy 1993;48(5):377‐82. [DOI] [PubMed] [Google Scholar]
Price 2002 {published data only}
- Price D, Dutchman D, Mawson A, Bodalia B, Duggan S, Todd P. Early asthma control and maintenance with eformoterol following reduction of inhaled corticosteroid dose. Thorax 2002;57(9):791‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Quebe‐Fehling 1996 {published data only}
- Quebe‐Fehling E, Brambilla R, Bromly CL, Fishwick K, Walters EH, Hendrick D. The duration of action of inhaled formoterol dry powder. British Journal of Clinical Practice 1996;50(8):446‐9. [PubMed] [Google Scholar]
Rabe 1993 {published data only}
- Rabe KF, Jorres R, Nowak D, et al. Comparison of the effects of salmeterol and formoterol on airway tone and responsiveness over 24 hours in bronchial asthma. American Review of Respiratory Disease 1993;147(6 Pt 1):1436‐41. [DOI] [PubMed] [Google Scholar]
Ramsdale 1991 {published data only}
- Ramsdale E, Otis J, Kline P, Gontovnick L, Hargreave F, O'Byrne P. Prolonged protection against methacholine induced bronchoconstriction by the inhaled b2 agonist formoterol. American Review of Respiratory Disease 1991;143(5 Pt 1):998‐1001. [DOI] [PubMed] [Google Scholar]
Rhee 1997 {published data only}
- Rhee YK. A comparison of salmeterol with salbutamol inhalation in treatment of mild to moderate asthma. Tuberculosis and Respiratory Diseases 1997;44(4):815‐21. [Google Scholar]
Ringbaek 1996 {published data only}
- Ringbaek TJ, Soes‐Petersen U, Christensen M, Iversen ET, Rasmussen FV. Salmeterol compared with salbutamol controlled release in patients with moderate bronchial asthma. Ugeskrift for Laeger 1996;158(27):3940‐3. [PubMed] [Google Scholar]
Rosenhall 1990 {published data only}
- Rosenhall L, Sandstrom T, Wallin A. Formoterol, a long acting inhaled b2 agonist, twice daily for 1 year in asthmatic patients. American Review of Respiratory Disease 1990;141:A 210. [Google Scholar]
Russell 1995 {published data only}
- Russell G, Williams DAJ, Weller P, Price JF. Salmeterol xinafoate in children on high dose inhaled steroids. Annals of Allergy, Asthma and Immunology 1995;75(5):423‐8. [PubMed] [Google Scholar]
Ruttenvan Molken1995 {published data only}
- Rutten‐van C M, Custers F, van‐Doorslater EKA, et al. Comparison of performance of four instruments in evaluating the effects of salmeterol on asthma quality of life. European Respiratory Journal 1995;8(6):888‐98. [PubMed] [Google Scholar]
Schaaning 1996 {published data only}
- Schaanning J, Vilsvik J, Henriksen AH. Efficacy and duration of salmeterol in protecting against exercise induced bronchoconstriction. Annals of Allergy, Asthma, & Immunology 1996;76(1):57‐60. [DOI] [PubMed] [Google Scholar]
Scherner 2004 {published data only}
- Schermer TR, Hoff WJ, Greefhorst AP, Creemers JP, Sips AP, Westbroek J, et al. Profiles of measured and perceived bronchodilation. A placebo‐controlled cross‐over trial comparing formoterol and salmeterol in moderate persistent asthma. Pulmonary Pharmacology & Therapeutics 2004;17(4):205‐12. [DOI] [PubMed] [Google Scholar]
Self 1998 {published data only}
- Self T, Rumbak MJ, Kelso T, Eberle L, Abou‐Shala N, Learned CC, et al. Does salmeterol facilitate 'step‐down' therapy in patients with asthma receiving moderate to high doses of inhaled corticosteroids?. Current Therapeutic Research, Clinical & Experimental 1998;59:803‐11. [Google Scholar]
Shapiro 2000a {published data only}
- Shapiro G, Lumry W, Wolfe J, Given J, White M, Woodring A, et al. Combined salmeterol 50 mug and fluticasone propionate 250 in the diskus device for the treatment of asthma. American Journal of Respiratory & Critical Care Medicine 2000;161(2 Pt 1):527‐34. [DOI] [PubMed] [Google Scholar]
Shepherd 1991 {published data only}
- Shepherd G, Jenkins W, Alexander J. Asthma exacerbations in patients taking regular salmeterol, or salbutamol for symptoms. Lancet 1991;337(8754):1424. [DOI] [PubMed] [Google Scholar]
Sichletidis 1993 {published data only}
- Sichletidis L, Daskalopoulou E, Kyriakis G, et al. Comparative efficacy of salbutamol and salmeterol in exercise induced asthma. Journal of International Medical Research 1993;21(2):81‐8. [DOI] [PubMed] [Google Scholar]
Siergiejko 1998 {published data only}
- Siergiejko Z, Zietkowsk1 Z, Rogalewska A, Chyrek‐Borowska S. Clinical evaluation of 12 week salmeterol therapy in allergic asthma patients. Pneumonologia i Alergologia Polska 1998;66(9‐10):450‐5. [PubMed] [Google Scholar]
Simons 1992 {published data only}
- Simons F, Soni N, Watson W, Becker A. Bronchodilator and bronchoprotective effects of salmeterol in young patients with asthma. Journal of Allergy & Clinical Immunology 1992;90(5):840‐6. [DOI] [PubMed] [Google Scholar]
Simons 1997b {published data only}
- Simons FER, Gerstner TV, Cheang MS. Tolerance to the bronchoprotective effect of salmeterol in adolescents with exercise‐induced asthma using concurrent inhaled glucocorticoid treatment. Pediatrics. Pediatrics 1997;99(5):655‐9. [DOI] [PubMed] [Google Scholar]
SLGA2016 {unpublished data only}
- SLGA2016. www.fda.gov 2001.
SLGB4011R {unpublished data only}
- SLGB4011R. www.ctr.gsk.co.uk 2005.
SMO30003 {unpublished data only}
- SMO30003. A single‐centre, randomised, placebo‐controlled, double‐blind, 3‐way crossover proof‐of‐concept study to evaluate the effect of reduced fine particle mass (FPM) from the salmeterol 50mg GR106642X metered dose inhaler compared with salmeterol 50mg P11/12 metered dose inhaler on the peak bronchodilatory effect and bronchoprotection against methacholine‐induced bronchoconstriction. www.ctr.gsk.co.uk 2005.
SMS30035 {published data only}
- SMS30035. A double‐blind, three‐way crossover study comparing GR33343G (Salmeterol) 25µg bd, salmeterol 50µg bd and placebo in the treatment of childhood asthma.. www.ctr.gsk.co.uk 2006.
Smyth 1993 {published data only}
- Smyth ET, Pavord ID, Wong C, Wisniewiski ASZ, Williams J, Tattersfield AE. Interaction and dose equivalence of salbutamol and salmeterol in patients with asthma. British Medical Journal 1993;306(6877):543‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sprogoe‐Jakobsen1992 {published data only}
- Sprogoe‐Jakobsen U, Viktrup L, Davidsen O, Viskum K. Bronchial asthma treated with long‐acting beta 2 agonist. Comparison between formoterol (12 mu/g) inhaled twice daily and salbutamol (200 mu/g) inhaled 4 times daily. Ugeskr‐Laeger 1992;154(3):325‐8. [PubMed] [Google Scholar]
Staehr 1995 {published data only}
- Staehr P, Vestbo I. Salmeterol improves control in asthmatic patients treated in general practice. A comparative study of salmeterol and salbutamol in patients with mild to moderate asthma. Ugeskrift for Laeger 1995;157(1):36‐40. [PubMed] [Google Scholar]
Steffensen 1996 {published data only}
- Steffensen I, Faurschou P. Formoterol as inhalation powder in the treatment of patients with reversible obstructive lung diseases. A 3‐month placebo‐controlled comparison of the effects of formoterol and salbutamol, followed by a 12‐month period with formoterol alone. Ugeskrift for Laeger 1996;158(49):7092‐6. [PubMed] [Google Scholar]
Tan 1997 {published data only}
- Tan KS, Grove A, McLean A, Gnosspelius Y, Hall IP, Lipworth BJ. Systemic corticosteroid rapidly reverses bronchodilator sub‐sensitivity induced by formoterol in asthmatic patients. American Journal of Respiratory & Critical Care Medicine 1997;156(1):28‐35. [DOI] [PubMed] [Google Scholar]
- Tan KS, Hall IP, Dewar JC, Dow E, Lipworth BJ. Association between beta2‐adrenoceptor polymorphism and susceptibility to bronchodilator desensitisation in moderately severe stable asthmatics. Lancet 1997;350(9083):995‐9. [DOI] [PubMed] [Google Scholar]
Tattersfield 2001 {published data only}
- Tattersfield A, Lofdahl C‐G, Postma D, Eivindson A, Schreurs A, Rasidikis A, et al. Comparison of formoterol and terbutaline for as‐needed treatment of asthma: as randomised trial. The Lancet 2001;357(9252):257‐61. [DOI] [PubMed] [Google Scholar]
Taylor 1992 {published data only}
- Taylor IK, O'Shaugnessy KM, Choudry NB, et al. A comparative study in atopic subjects with asthma of the effects of salmeterol and salbutamol on allergen induced bronchoconstriction, increase in AR ,increase in urinary leukotriene E4 excretion. Journal of Allergy & Clinical Immunology 1992;89(2):575‐83. [DOI] [PubMed] [Google Scholar]
Taylor Jensen 1997 {published data only}
- Taylor DA, Jensen MW, Aikman SL, Harris JG, Barnes PJ, O'Connor BJ. Comparison of salmeterol and albuterol‐induced bronchoprotection against adenosine monophosphate and histamine in mild asthma. American Journal of Respiratory & Critical Care Medicine 1997;156(6):1731‐7. [DOI] [PubMed] [Google Scholar]
Thomson 1998 {published data only}
- Thomson N, Angus R, Quebe‐Fehling E, Brambilla R. Efficacy and tolerability of formoterol in elderly patients with reversible obstructive airways disease. Respiratory Medicine 1998;92(3):562‐7. [DOI] [PubMed] [Google Scholar]
Totterman 1998 {published data only}
- Totterman KJ, Huhti L, Sutinen E, Backman R, Pietinalho A, Falck M, et al. Tolerability to high doses of formoterol and terbutaline via Turbuhaler(TM) for 3 days in stable asthmatic patients. European Respiratory Journal 1998;12(3):573‐9. [DOI] [PubMed] [Google Scholar]
Turner 1998 {published data only}
- Turner M, Johnston P, Pizzichini E, et al. Anti‐inflammatory effects of salmeterol compared with beclomethasone in eosinophilic mild exacerbations of asthma: a randomized, placebo controlled trial. Canadian Respiratory Journal 1998;5(4):261‐8. [DOI] [PubMed] [Google Scholar]
Twentyman 1990 {published data only}
- Twentyman OP, Finnerty JP, Harris A, et al. Protection against allergen induced asthma by salmeterol. Lancet 1990;336(8727):1338‐42. [DOI] [PubMed] [Google Scholar]
Ullman 1988 {published data only}
- Ullman A, Svedymr N. Salmeterol a new long acting inhaled beta agonist: comparison with salbutamol in adult asthmatic patients. Thorax 1988;43(9):674‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van der Molen 1996 {published data only}
- Molen T, Postma DS, Schreurs AJM, Bosveld HEP, Sears MR, Jong B. Discriminative aspects of two generic and two asthma‐specific instruments: relation with symptoms, bronchodilator use and lung function in patients with mild asthma. Quality of Life Research 1997;6(4):353‐61. [DOI] [PubMed] [Google Scholar]
- Molen T, Postma DS, Turner MO, Jong BM, Sears MR. Effects of the long acting beta agonist formoterol on asthma control in asthmatic patients using inhaled corticosteroids. Thorax 1996;52(6):535‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molen T, Sears MR, Graaff CS, Postma DS, Jong B. Quality of life during formoterol treatment: comparison between asthma‐specific and generic questionnaires. European Respiratory Journal 1998;12(1):30‐4. [DOI] [PubMed] [Google Scholar]
van der Woude 2001 {published data only}
- Woude HJ, Winter TH, Aalbers R. Decreased bronchodilating effect of salbutamol in relieving methacholine induced moderate to severe bronchoconstriction during high dose treatment with long acting beta2 agonists. Thorax 2001;56(7):529‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Venables 1992 {published data only}
- Venables TA. A multicentre study in general practice to compare the efficacy and tolerability of inhaled salmeterol and terbutaline in the treatment of asthma. British Journal of Clinical Research 1992;3:125‐36. [Google Scholar]
Verberne 1991 {published data only}
- Verberne A, Lenney W, Kerribyjn K. A 3 way crossover study comparing twice daily dosing of salmeterol 25mcg and 50mcg with placebo in children with mild to moderate reversible airways disease [abstract]. American Review of Respiratory Disease 1991;143 Suppl 2:20. [Google Scholar]
Verberne 1996 {published data only}
- Verberne AAPH, Hop WCJ, Creyghton FBM, et al. Airway responsiveness after a single dose of salmeterol and during four months of treatment in children with asthma. Journal of Allergy & Clinical Immunology 1996;97(4):938‐46. [DOI] [PubMed] [Google Scholar]
Verberne 1997 {published data only}
- Verberne A, Frost C, Roorda R, Laag H, Kerrebijn K. One year treatment with salmeterol compared with beclomethasone in children with asthma. The Dutch Paediatric Asthma Study Group. American Journal of Respiratory & Critical Care Medicine 1997;156(3 Pt 1):688‐95. [DOI] [PubMed] [Google Scholar]
Verberne 1998 {published data only}
- Verberne AA, Frost C, Duiverman EJ, et al. Addition of salmeterol versus doubling the dose of beclomethasone in children with asthma‐The Dutch Asthma Study Group. American Journal of Respiratory & Critical Care Medicine 1998;158(1):213‐9. [DOI] [PubMed] [Google Scholar]
Verberne 2000 {published data only}
- Verberne A, Jongste J. Long‐acting beta2‐agonists in childhood asthma: Don't change a winning team (yet). Pediatric Pulmonology 2000;29(3):169‐71. [DOI] [PubMed] [Google Scholar]
Verini 1998 {published data only}
- Verini M, Verrotti A, Greco R, Chiarelli F. Comparison of the bronchodilator effect of inhaled short‐ and long‐acting beta2‐agonists in children with bronchial asthma. A randomised trial. Clinical Drug Investigation 1998;16(1):19‐224. [DOI] [PubMed] [Google Scholar]
Wallaert 1999 {published data only}
- Wallaert B, Brun P, Ostinelli J, Murciano D, Champel F, Blaive B, et al. A comparison of two long acting beta agonists, oral bambuterol and inhaled salmeterol, in the treatment of asthmatics with nocturnal symptoms. Respiratory Medicine 1999;93(1):33‐8. [DOI] [PubMed] [Google Scholar]
Walters 1992 {published data only}
- Walters H. Quality of life in long term studies [abstract]. European Respiratory Journal 1992;5:318s. [Google Scholar]
Weinstein 1997 {published data only}
- Weinstein S, Chervinsky P, Pollard SJ, Bronsky EA, Nathan RA, Prenner B, et al. A one‐week dose‐ranging study of inhaled salmeterol in children with asthma. Journal of Asthma 1997;34(1):43‐52. [DOI] [PubMed] [Google Scholar]
Wenzel 1998 {published data only}
- Wenzel SE, Lumry W, Manning M, Kalberg C, Cox F, Emmett A, et al. Efficacy, safety, and effects on quality of life of salmeterol versus albuterol in patients with mild to moderate persistent asthma. Annals of Allergy 1998;80(6):463‐70. [DOI] [PubMed] [Google Scholar]
Wilding 1997 {published data only}
- Wilding P, Clark M, Thompson J, et al. The effect of long term treatment with salmeterol on asthma control‐a double blind randomised crossover study. British Medical Journal 1997;314(7092):1441‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wolfe 1995 {published data only}
- Wolfe J, LaForce C, Chervinsky P, Galant S, Lumry W, Noonan M, et al. Inhaled salmeterol powder compared with albuterol aerosol given regularly or as needed for asthma. Annals of Allergy, Asthma and Immunology 1995;74:52. [Google Scholar]
Wong 1997 {published data only}
- Wong AG, O'Shaughnessy AD, Walker CM, Sears MR. Effects of long‐acting and short‐acting beta‐agonists on methacholine dose‐response curves in asthmatics. European Respiratory Journal 1997;10(2):330‐6. [DOI] [PubMed] [Google Scholar]
Woolcock 1996 {published data only}
- Woolcock A, Lundback B, Ringdal N, et al. Comparison of the addition of salmeterol to inhaled steroids with the doubling of the dose of inhaled steroids. American Journal of Respiratory & Critical Care Medicine 1996;153(5):1481‐8. [DOI] [PubMed] [Google Scholar]
Yates 1995 {published data only}
- Yates DH, Sussman HS, Shaw MJ, Barnes PJ, Chung KF. Regular formoterol treatment in mild asthma. Effect on bronchial responsiveness during and after treatment. American Journal of Respiratory & Critical Care Medicine 1995;152(4 Pt 1):1170‐4. [DOI] [PubMed] [Google Scholar]
Yates 1997 {published data only}
- Yates DH, Worsdell M, Barnes PJ. Effect of regular salmeterol treatment on albuterol‐induced bronchoprotection in mild asthma. American Journal of Respiratory & Critical Care Medicine 1997;156(3):988‐91. [DOI] [PubMed] [Google Scholar]
Zellweger 1994 {published data only}
- Zellweger JP, Plenninger M, Ruff P. 24 hour protective effect of salmeterol 50mg v formoterol 24 mg against methacholine induced bronchoconstriction in mild to moderate asthmatic patients: a randomised double blind crossover single dose trial. European Respiratory Journal 1994;7 Suppl 18:422s. [Google Scholar]
Zimmerman 2004 {published data only}
- Zimmerman B, D'Urzo A, Berube D. Efficacy and safety of formoterol Turbuhaler when added to inhaled corticosteroid treatment in children with asthma. Pediatric Pulmonology 2004;37(2):122‐7. [DOI] [PubMed] [Google Scholar]
Additional references
AAACI 1993
- American Academy of Allergy and Clinical Immunology. Inhaled beta2 agonist in asthma. Journal of Allergy & Clinical Immunology 1993;91(6):1234‐7. [PubMed] [Google Scholar]
Abramson 2001
- Abramson M, Bailey M, Couper F, Driver J, Drummer O, Forbes A, et al. Are asthma medications and management related to deaths from asthma?. American Journal of Respiratory & Critical Care Medicine 2001;163(1):12‐8. [DOI] [PubMed] [Google Scholar]
Adcock 1996
- Adcock I, Stevens D, Barnes P. Interactions of glucocorticoids and beta2 agonists. European Respiratory Journal 1996;9(1):160‐9. [DOI] [PubMed] [Google Scholar]
Adkins 1997
- Adkins JC, McTavish D. Salmeterol. A review of its pharmacological properties and clinical efficacy in the management of children with asthma. Drugs 1997;54(2):331‐54. [DOI] [PubMed] [Google Scholar]
Altman 2003
- Altman DG, Bland JM. Statistics notes: Interaction revisited: the difference between two estimates. British Medical Journal 2003;326(7382):219. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bartow 1998
- Bartow RA, Brogen RN. Formoterol. An update of its pharmacological properties and therapeutic efficacy in the management of asthma. Drugs 1998;55(2):303‐22. [DOI] [PubMed] [Google Scholar]
Beasley 1999
- Beasley R, Pearce N, Crane J, Burgess C. Beta‐agonists: What is the evidence that their use increases the risk of asthma morbidity and mortality?. Journal of Allergy & Clinical Immunology 1999;104(2 Pt 2):18S‐30S. [DOI] [PubMed] [Google Scholar]
Bisgaard 2000
- Bisgaard H. Long‐acting beta(2)‐agonists in management of childhood asthma: A critical review of the literature. Pediatric Pulmonology 2000;29(3):221‐34. [DOI] [PubMed] [Google Scholar]
Boulet 1994
- Boulet LP. Long versus short acting beta agonists. Drugs 1994;47(2):207‐22. [DOI] [PubMed] [Google Scholar]
Boyer 1992
- Boyer G, Aaronson G, Meltzer E, LaForce C, Grossman J, Yancey S. Enhancement of a general quality of life scale: validation of a sleep scale [abstract]. Journal of Allergy & Clinical Immunology 1992;89:186. [Google Scholar]
BTS 1993
- British Thoracic Society et al. Guidelines on the management of asthma. Thorax 1993;48(2 Suppl):S1‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
BTS 1995
- British Thoracic Society et al. The British guidelines on asthma management 1995 review and position statement. Thorax 1997;52 Suppl 1:S1‐S20. [Google Scholar]
BTS summary 1993
- British Thoracic Society. Guidelines for the Management of Asthma: a Summary. British Medical Journal 1993;306(6880):776‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Campbell 1976
- Campbell A. Mortality from asthma and bronchodilator aerosols. Medical Journal of Australia 1976;1(12):386‐91. [PubMed] [Google Scholar]
Cates 2012
- Cates CJ, Oleszczuk M, Stovold E, Wieland LS. Safety of regular formoterol or salmeterol in children with asthma: an overview of Cochrane reviews. Cochrane Database of Systematic Reviews 2012, Issue 10. [DOI: 10.1002/14651858.CD010005.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cates 2014
- Cates C J, Wieland L S, Oleszczuk M, Kew K M. Safety of regular formoterol or salmeterol in adults with asthma: an overview of Cochrane reviews. Cochrane Database of Systematic Reviews 2014, Issue 2. [10.1002/14651858.CD010314.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Christie 1993
- Christie M, French D, Sowden A, West A. The development of child centred, disease specific questionnaires for living with asthma. Psychosomatic Medicine 1993;55(6):541‐8. [DOI] [PubMed] [Google Scholar]
Cockcroft 1993
- Cockcroft DW, McParland CP, Britto SA. Regular inhaled salbutamol and airway responsiveness to allergen. Lancet 1993;342(8875):833‐7. [DOI] [PubMed] [Google Scholar]
Cockcroft 1996
- Cockcroft DW, Swystun VA. Functional antagonism produced by inhaled beta agonists. Thorax 1996;51(10):1051‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Crane 1989
- Crane J, Pearce N, Flatt A, et al. Prescribed fenoterol and death from asthma in New Zealand 1981‐1983. A case control study. Lancet 1989;1(8644):917‐22. [DOI] [PubMed] [Google Scholar]
D'Urzo 1997
- D'Urzo AD. Long acting beta agonists. Role in primary care asthma treatment. Canadian Family Physician 1997;43:1773‐7. [PMC free article] [PubMed] [Google Scholar]
Devoy 1995
- Skorodin MS. Asthma mortality and beta agonists. Chest 1995;108(6):1768. [DOI] [PubMed] [Google Scholar]
Devoy Fuller 1995
- Devoy MA, Fuller RW, Palmer JB. Are there any detrimental effects of the use of inhaled long‐acting beta 2‐agonists in the treatment of asthma?. Chest 1995;107(4):1116‐24. [DOI] [PubMed] [Google Scholar]
Faurschou 1994
- Faurschou P, Engel AM, Haanaes OC. Salmeterol in two different doses in the treatment of nocturnal bronchial asthma poorly controlled by other therapies. Allergy 1994;49(10):827‐32. [DOI] [PubMed] [Google Scholar]
French 1994
- French DJ, Christie MJ, Sowden AJ. The reproducibility of the Childhood Asthma Questionnaires: measures of quality of life for children with asthma aged 4‐16 yrs. Quality of Life Research 1994;3(3):215‐24. [DOI] [PubMed] [Google Scholar]
Fuller 1995
- Fuller R. Safety of salmeterol in the treatment of asthma. European Respiratory Review 1995;5(27):133‐7. [Google Scholar]
Giannini 2000
- Giannini D, Franco A, Bacci E, Dente FL, Taccola M, Vagaggini B, et al. The protective effect of salbutamol inhaled using different devices on methacholine bronchoconstriction. Chest 2000;117(5):1319‐23. [DOI] [PubMed] [Google Scholar]
GINA 1995
- National Heart Lung and Blood Institute/WHO. Global strategy for asthma management and prevention. Bethesda: National Institutes of Health, 1995. [Google Scholar]
GINA 2000
- Bousquet J. Global initiative for asthma (GINA) and its objectives. Clinical & Experimental Allergy 2000;30 Suppl 1:S2‐5. [DOI] [PubMed] [Google Scholar]
Greenstone 2005
- Greenstone IR, Ni Chroinin M, Masse V, Danish A, Magdalinos H, Zhang X, et al. Combination of inhaled long‐acting beta2‐agonists and inhaled steroids versus higher dose of inhaled steroids in children and adults with persistent asthma (Cochrane Review). Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI] [PubMed] [Google Scholar]
Hawkins 2006
- Hawkins GA, Tantisira K, Meyers DA, Ampleford EJ, Klanderman B, Liggett SB, et al. Sequence, Haplotype and Association Analysis of ADR{beta}2 in Multi‐Ethnic Asthma Case/Control Subjects. American Journal of Respiratory and Critical Care Medicine 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. British Medical Journal 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Howarth 2000
- Howarth PH, Beckett P, Dahl R. The effect of long‐acting beta2‐agonists on airway inflammation in asthmatic patients. Respiratory Medicine 2000;94 Suppl F:S22‐5. [DOI] [PubMed] [Google Scholar]
Hyland 1991
- Hyland ME. The Living with Asthma Questionnaire. Respiratory Medicine 1991;85 Suppl B:13‐6. [DOI] [PubMed] [Google Scholar]
Jadad 1996
- Jadad A, Moore R, Carroll D, Jenkinson C, Reynolds J, Gavaghan D, et al. Assessing the quality of reports of randomised control trials: is blinding really necessary?. Controlled Clinical Trials 1996;17(1):1‐12. [DOI] [PubMed] [Google Scholar]
Janson 2001
- Janson C, Anto J, Burney P, et al. The European community respiratory health survey: what are the main results so far?. European Respiratory Journal 2001;18(3):598‐611. [DOI] [PubMed] [Google Scholar]
Johnson 2004
- Johnson M. Interactions between corticosteroids and beta2‐agonists in asthma and chronic obstructive pulmonary disease. Proceedings of the American Thoracic Society 2004;1(3):200‐6. [DOI] [PubMed] [Google Scholar]
Juniper 1992
- Juniper EF, Guyatt GH. Evaluation of impairment of health related quality of life in asthma: development of a questionnaire for use in clinical trials. Thorax 1992;47(2):76‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Juniper 1994
- Juniper EF, Guyatt GH, Willan A, Griffith L. Determining a minimal important change in a disease‐specific quality of life questionnaire. Journal of Clinical Epidemiology 1994;47(1):81‐7. [DOI] [PubMed] [Google Scholar]
Kips 2001
- Kips J, Pauwels R. Long‐acting inhaled beta(2)‐agonist therapy in asthma. American Journal of Respiratory & Critical Care Medicine 2001;164(6):923‐32. [DOI] [PubMed] [Google Scholar]
Lai 1989
- Lai CKW, Twentyman OP, Holgate ST. The effect of an increase in inhaled allergen dose after rimiterol hydrobromide on the occurrence and magnitude of the late asthmatic response and the associated change in nonspecific bronchial hyperresponsiveness. American Review of Respiratory Disease 1989;140(4):917‐23. [DOI] [PubMed] [Google Scholar]
Lipworth 1999a
- Lipworth BJ, Hall IP, Aziz I, Tan KS, Wheatley A. Beta2‐adrenoceptor polymorphism and bronchoprotective sensitivity with regular short and long‐acting beta2‐agonist therapy. Clinical Science 1999;96(3):253‐9. [PubMed] [Google Scholar]
Lipworth Hall 1999
- Lipworth B, Hall I, Tan S, Aziz I, Coutie W. Effects of genetic polymorphism on ex vivo and in vivo function of beta2‐adrenoceptors in asthmatic patients. Chest 1999;115(2):324‐8. [DOI] [PubMed] [Google Scholar]
Moore 1996
- Moore RH, Khan A, DIckey B. Long‐acting inhaled beta2‐agonists in asthma therapy. Chest 1996;113(4):1095‐8. [DOI] [PubMed] [Google Scholar]
NAC 2002
- National Asthma Campaign. Asthma management handbook. Melbourne: National Asthma Council Australia Ltd, 2002. [Google Scholar]
Ni Chroinin 2004
- Ni Chroinin M, Greenstone IR, Ducharme FM. Addition of inhaled long‐acting beta2‐agonists to inhaled steroids as first line therapy for persistent asthma in steroid‐naive adults. The Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD005307.pub2] [DOI] [PubMed] [Google Scholar]
Ni Chroinin 2005
- Ni Chroinin M, Greenstone IR, Danish A, Magdolinos H, Masse V, Zhang X, et al. Long‐acting beta2‐agonists versus placebo in addition to inhaled corticosteroids in children and adults with chronic asthma (Cochrane Review). Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI] [PubMed] [Google Scholar]
Page 1993
- Page CP. An explanation of the asthma paradox. American Review of Respiratory Disease 1993;147(6 Pt 2):S29‐32. [DOI] [PubMed] [Google Scholar]
Palmquist 1997
- Palmqvist M, Persson G, Lazer L, Rosenborg J, Larsson P, Lotvall J. Inhaled dry‐powder formoterol and salmeterol in asthmatic patients: onset of action, duration of effect and potency. European Respiratory Journal 1997;10(11):2484‐9. [DOI] [PubMed] [Google Scholar]
Partridge 2000
- Partridge M, Fabbri L, Chung K. Delivering effective asthma care‐ how do we implement asthma guidelines?. European Respiratory Journal 2000;15(2):235‐7. [DOI] [PubMed] [Google Scholar]
Pearce 2000
- Pearce N, Sunyer J, Cheng S, et al. Comparison of asthma prevalence in the ISAAC and the ECRHS. ISAAC Steering Committee and the European Community Respiratory Health Survey. International Study of Asthma and Allergies in Childhood. European Journal of Respiratory Medicine 2000;16(3):420‐6. [DOI] [PubMed] [Google Scholar]
Reid 2003
- Reid DW, Ward C, Wang N, Zheng L, Bish R, Orsida B, et al. Possible anti‐inflammatory effect of salmeterol against interleukin‐8 and neutrophil activation in asthma in vivo. European Respiratory Journal 2003;21(6):994‐9. [DOI] [PubMed] [Google Scholar]
Salpeter 2006
- Salpeter SR, Buckley NS, Ormiston TM, Salpeter EE. Meta‐analysis: effect of long‐acting beta‐agonists on severe asthma exacerbations and asthma‐related deaths. Ann Intern Med 2006;144(12):904‐12. [DOI] [PubMed] [Google Scholar]
Sears 1986
- Sears M, Rea H, Rothwell R, O'Donnell T, Holst P, Gillies A, et al. Asthma mortality: comparison between New Zealand and England. British Medical Journal 1986;293(6558):1342‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sears 1998
- Sears MR. Asthma treatment: inhaled beta‐agonists. Canadian Respiratory Journal 1998;5 Suppl A:54A‐9A. [PubMed] [Google Scholar]
Selroos 1998
- Selroos O. The pharmacologic and clinical properties of Oxis (formoterol) Turbuhaler. Allergy 1998;53(42 Suppl):14‐9. [DOI] [PubMed] [Google Scholar]
Shale 1994
- Shale DJ. Respiratory arrests in young asthmatics on salmeterol. Respiratory Medicine 1994;88(1):75‐6. [DOI] [PubMed] [Google Scholar]
Shrewsbury 2002
- Shrewsbury S, Pyke S, Sears M. MIASMA: asthma exacerbation reduction with salmeterol. Chest 2002;121(3):1002‐4. [DOI] [PubMed] [Google Scholar]
Sont 1999
- Sont JK, Willems LN, Bel EH, Krieken JH, Vandenbroucke JP, Sterk PJ. Clinical control and histopathologic outcome of asthma when using airway hyperresponsiveness as an additional guide to long‐term treatment. The AMPUL Study Group. American Journal of Respiratory & Critical Care Medicine 1999;159(4 Pt 1):1043‐51. [DOI] [PubMed] [Google Scholar]
Spitzer 1992
- Spitzer WO, Suissa S, Ernst P, Horwitz RI, Habbick B, et al. The use of beta‐agonists and the risk of death and near death from asthma. New England Journal of Medicine 1992;326(8):501‐6. [DOI] [PubMed] [Google Scholar]
Stein 1990
- Stein R, Jessup D. Functional status II(R). A measure of child health status. Medical Care 1990;28(11):1041‐55. [DOI] [PubMed] [Google Scholar]
Sullivan 1996
- Sullivan S, Elixhauser A, Buist A, Luce B, Eisenberg J, Weiss K. National Asthma Education and Prevention Program working group report on the cost effectiveness of asthma care. Program working group report on the cost effectiveness of asthma care. American Journal of Respiratory & Critical Care Medicine 1996;154(3 Pt 2):S84‐95. [DOI] [PubMed] [Google Scholar]
Tattersfield 1993
- Tattersfield AE. Clinical pharmacology of long‐acting beta‐receptor agonists. Life Sciences 1993;52(26):2151‐9. [DOI] [PubMed] [Google Scholar]
Taylor 1997
- Taylor D, Jensen M, Aikman S, Harris J, Barnes PJ, O'Connor B. Comparison of salmeterol and albuterol induced bronchoprotection against AMP and histamine in mild asthma. American Journal of Respiratory & Critical Care Medicine 1997;156(6):1731‐7. [DOI] [PubMed] [Google Scholar]
Taylor 2000
- Taylor DR, Hancox RJ. Interactions between corticosteroids and beta agonists. Thorax 2000;55(7):595‐602. [DOI] [PMC free article] [PubMed] [Google Scholar]
TSANZguidelines 1989
- Woolcock A. Rubinfeld AR. Seale JP. Landau LL. Antic R. Mitchell C, et al. Thoracic society of Australia and New Zealand. Asthma management plan. Medical Journal of Australia 1989;151(11‐12):650‐3. [PubMed] [Google Scholar]
Van der Molen 1997
- Molen T, Postma DS, Schreurs AJM, Bosveld HEP, Sears MR, Meyboom de Jong B. Discriminative aspects of two generic and two asthma‐specific instruments: relation with symptoms, bronchodilator use and lung function in patients with mild asthma. Quality of Life Research 1997;6(4):353‐61. [DOI] [PubMed] [Google Scholar]
van Noord 1996
- Noord J, Smeets J, Raaijmakers J, Bommer A, Maesen F. Salmeterol versus formoterol in patients with moderately severe asthma: onset and duration of action. European Respiratory Journal 1996;9(8):1684‐8. [DOI] [PubMed] [Google Scholar]
Van Schayck 2000
- Schayck C. Do long‐acting beta2‐adrenergic agonists deserve a different place in guidelines for the treatment of asthma and COPD?. European Respiratory Journal 2000;15(4):631‐4. [DOI] [PubMed] [Google Scholar]
Verberne 1995
- Verberne AAPH, McCormack EM, Fuller R, et al. An overview of nine clinical trials of salmeterol in an asthmatic population. European Respiratory Journal 1995;8(Suppl 19):156S. [DOI] [PubMed] [Google Scholar]
Vervloet 1998
- Vervloet D, Ekstrom T, Pela R, Duce Gracia F, Kopp C, Silvert BD, et al. A 6‐month comparison between formoterol and salmeterol in patients with reversible obstructive airways disease. Respiratory Medicine 1998;92(6):836‐42. [DOI] [PubMed] [Google Scholar]
Weinberger 1995
- Weinberger M. Salmeterol for the treatment of asthma. Annals of Allergy, Asthma and Immunology 1995;75(3):209‐11. [PubMed] [Google Scholar]
Woods 2001
- Woods R, Walters E, Wharton C, Watson N, Abramson M. The rising prevalence of asthma in young Melbourne adults is associated with improvement in treatment. Annals of Allergy, Asthma, & Immunology 2001;87(2):117‐23. [DOI] [PubMed] [Google Scholar]


