Abstract
Fibroblast growth factor-21 (FGF21) is an intercellular signaling molecule secreted by metabolic organs, including skeletal muscle, in response to intracellular stress. FGF21 crosses the blood–brain barrier and acts via the nervous system to coordinate aspects of the adaptive starvation response, including increased lipolysis, gluconeogenesis, fatty acid oxidation, and activation of the hypothalamic–pituitary–adrenocortical (HPA) axis. Given its beneficial effects for hepatic lipid metabolism, pharmaceutical FGF21 analogues are used in clinical trials treatment of fatty liver disease. We predicted pharmacologic treatment with FGF21 increases HPA axis activity and skeletal muscle glucocorticoid signaling and induces skeletal muscle atrophy in mice. Here we found a short course of systemic FGF21 treatment decreased muscle protein synthesis and reduced tibialis anterior weight; this was driven primarily by its effect in female mice. Similarly, intracerebroventricular FGF21 reduced tibialis anterior muscle fiber cross-sectional area; this was more apparent among female mice than male littermates. In agreement with the reduced muscle mass, the topmost enriched metabolic pathways in plasma collected from FGF21-treated females were related to amino acid metabolism, and the relative abundance of plasma proteinogenic amino acids was increased up to 3-fold. FGF21 treatment increased hypothalamic Crh mRNA, plasma corticosterone, and adrenal weight, and increased expression of glucocorticoid receptor target genes known to reduce muscle protein synthesis and/or promote degradation. Given the proposed use of FGF21 analogues for the treatment of metabolic disease, the study is both physiologically relevant and may have important clinical implications.
Keywords: FGF21, stress, HPA axis, glucocorticoid, muscle, protein restriction
An adequate supply of amino acids is needed for essential physiological processes, including protein synthesis, hormone and neurotransmitter production, and fuel metabolism (1). In the face of dietary protein or caloric restriction, and even during starvation, circulating concentrations of amino acids remain remarkably stable (2, 3). The ability to maintain circulating amino acid concentrations, across a range of environmental and internal perturbations, highlights the presence of physiologic mechanisms that control systemic amino acid homeostasis. We and others find that changes in feeding behavior and macronutrient selection represent such a mechanism (4-10). And when behavioral modifications are unavailable or insufficient, structural and functional proteins of the body can be accessed to provide amino acids in times of need (11).
Skeletal muscle serves as the largest accessible reservoir of protein and amino acids in the body. In response to starvation or other metabolic stressors, the skeletal muscle protein pool can be accessed to provide amino acids for use as fuel or for cellular maintenance and repair (12). To accommodate these demands, the dynamic balance between protein synthesis and protein degradation is modified to favor muscle atrophy; this metabolic adaptation to starvation is largely accomplished by actions of the hypothalamic–pituitary–adrenal (HPA) axis (12, 13). Glucocorticoid receptor (GR) signaling in skeletal muscle upregulates the expression of key transcription factors leading to muscle catabolism, including Krüpple-like factor-15 (Klf15) and the forkhead box proteins (Foxos) (14-18). Additionally, GR activity represses protein synthesis by increasing gene expression for Regulated in development and DNA damage response-1 (Redd1). Both Redd1 and Klf15 inhibit the master regulator of protein synthesis, mechanistic target of rapamycin complex-1 (mTORC1) (16, 19). The cumulative effect of GR activation is increased free amino acid availability in skeletal muscle and plasma. Chronic exposure to excess glucocorticoids thereby leads to skeletal muscle atrophy (13, 20-22).
Fibroblast growth factor-21 (FGF21) is an intercellular signaling molecule that is produced by various organs and tissues in response to intracellular stress (23). It was initially identified as a hepatokine, secreted into circulation by the liver during a prolonged fast (23-25). Newer data revealed that protein or amino acid deficit, rather than fasting or starvation per se, drives FGF21 secretion (26). Multiple physiologic effects of FGF21 are thought to arise from its signaling via the FGF-receptor 1 (FGFR1), a receptor tyrosine kinase, and its obligatory coreceptor β-klotho (KLB) (27). FGFR1 has low expression in liver (28). Accordingly, liver FGF21 is thought to act in an endocrine manner to coordinate several aspects of the adaptive starvation response (29, 30), including lipolysis (31) and gluconeogenesis (32), together with activation of the HPA axis (32, 33). Collectively, these data support the possibility that FGF21 also increases systemic amino acid availability by accessing skeletal muscle protein and amino acids via the HPA axis.
FGF21 is a promising therapeutic target for the treatment of hepatic steatosis, because it potently reduces liver triglycerides in mice, monkeys, and humans (23, 25, 34-37). Recent work by ourselves and others revealed that the benefit of FGF21 for hepatic lipid metabolism is sex dependent. FGF21 decreased liver triglycerides in obese male mice, but this was abrogated among obese female littermates (38, 39). Thus, sex is an important biological variable influencing FGF21 physiology. Similarly, it is well-established that sex is an important variable influencing stress physiology. Both basal HPA axis tone and the glucocorticoid response to stressors is increased in female rodents and women compared with male counterparts (40-42). In addition, female skeletal muscle is more sensitive to glucocorticoid-induced atrophy (12, 43).
Here we tested the hypothesis that pharmacologic administration of FGF21 activates the HPA axis, resulting in increased plasma amino acids and skeletal muscle atrophy. Because aspects of FGF21 and HPA axis biology are sex dependent, we included both male and female mice in our experimental design. The study is both physiologically relevant and may have important implications for the proposed clinical use of FGF21 analogues in the treatment of metabolic disease.
Material and Methods
Animals
All animal experiments were approved by the Institutional Care and Use Committees of the University of California, Davis. Age-matched male and female C57BL/6J mice (n = 3-13/group) were obtained from the Jackson Laboratory or were bred in-house, for up to 2 generations removed from founders obtained from the Jackson Laboratory. Because therapeutic interest in pharmacologic FGF21 stems from its beneficial effects for obesity-associated metabolic disease, all experiments used diet-induced obese (DIO) mice.
FGF21 Administration
For systemic administration, mice received 0.2 mg/kg/day FGF21 or an equivalent volume of saline. Twice daily intraperitoneal (IP) injections (0.1 mg/kg/injection) were performed for 12 days. We chose this dose because it is the median effective dose for glucose lowering in male mice (44). In our hands, it is effective to improve glucose tolerance in both sexes, and to reduce body fat and liver triglycerides in male mice (38). The twice-daily dosing was modeled after another study, to better maintain plasma concentrations of the hormone (44). In 1 study, we used once daily subcutaneous injections (0.2 mg/kg/injection) for 1, 4, 8, 15, and 32 days to measure protein turnover, to minimize stress from repeated injections in the longer study. Mice were single-housed on a 12-hour light, 12-hour dark cycle in a temperature (20-22 °C) and humidity-controlled vivarium with ad libitum access to food and water.
For intracerebroventricular (ICV) administration, mice received continuous infusion of FGF21 (0.4 μg/day) or an equivalent volume of saline for 13 days. Other investigators have observed physiologic effects using ICV doses that vary from 0.4 to 1.0 μg/day (10, 31, 32, 45-47). We chose 0.4 μg/day because it is effective to activate brain FGF–receptor signaling in rodents, without appreciable efflux to the periphery (45). First, anesthetized mice were fitted with an ICV cannula (Brain Infusion Kit 3, Alzet Corporation, Palo Alto, CA) fitted to an Alzet osmotic minipump (0.25 μL/hour, model 1002), which administered either FGF21 or saline continuously for 13 days. Brain infusion cannulas were placed using a stereotaxic apparatus (Kopf Instruments, Kujunga, CA) with tips in the lateral cerebral ventricle using the following coordinates: 0.7 mm posterior to bregma, 1.2 mm lateral to the midsagittal suture, and to a depth of 2.5 mm from the brain surface. The connected minipumps were inserted in a subcutaneous pocket on the mouse's flank, and the cannulas were secured to the skull and wound closed using vetbond (3M, St. Paul, MN). The cannulas were secured by glue. The animals received 5 mg/kg meloxicam after surgery and on the first 2 postoperative days. The location of the cannula was confirmed at the end of the experiment by methylene blue staining.
Diets
High-fat diet (D12492) was manufactured by Research Diets (New Brunswick, NJ) and based on the American Institute of Nutrition Rodent Diets growth formula, AIN-93G (48) containing 20% kcal from protein, 20% kcal from carbohydrate, and 60% kcal from fat. We maintained the mice on high-fat diet for 6 to 9 weeks prior to beginning the experiments to increase body fat by approximately 20% in adult males and females (see 6, 49, 50).
Tissue Collection
Mice were euthanized in counterbalanced order by pentobarbital injection in the home room, beginning at ZT4, 2 hours following the last IP injection of FGF21 or saline. To minimize stress, tissues were collected in a nearby room along the same hallway. Whole trunk blood was collected in chilled EDTA-coated tubes either following decapitation or using cardiac puncture and centrifuged at 3000g for 15 minutes at 4 °C. Plasma was aliquoted and stored for later use at −80 °C. The tibialis anterior (TA) muscle, gastrocnemius-soleus complex, and inguinal white adipose tissues were dissected free of connective tissue, weighed, and frozen in dry ice-cooled isopentane. Muscles from the right limb were pinned to cork board prior to freezing for histological use.
Muscle Fiber Cross-sectional Area
We cut serial cross-sections (10 μm) from the TA and soleus using a Leica CM 3050S cryostat. To determine cross-sectional area (CSA), we fixed TA muscle sections in cold acetone for 5 minutes at −20 °C, followed by 3 5-minute washes with phosphate-buffered saline with 0.1% Tween 20. We then incubated the sections with mouse-on-mouse block (MKB-2213 VECTOR laboratories) in phosphate-buffered saline (60 μL:2.5 mL) for 1 hour at room temperature (RT). We blocked sections in 5% normal goat serum in phosphate-buffered saline with 0.1% Tween 20 (blocking buffer) for 30 minutes at RT and then incubated in primary antibody for laminin protein (1:500, Sigma catalog no. L9393, RRID:AB_477163) overnight at 4 °C. After incubation in primary antibody, we incubated sections in goat–antirabbit AlexaFluor 647 secondary antibody for 30 minutes at RT (RRID:AB_2535812), and then coverslipped using ProLong Gold Antifade reagent (Life Technologies, catalog no. P36930). We imaged the slides using Leica DFC300 light microscope at 2× objective and analyzed using CellPose in ImageJ. Fibers from 4 regions of a single section were analyzed per muscle, per animal.
Stable Isotope Labeling & Protein Synthesis
Newly synthesized proteins were labeled using deuterium oxide (D2O) according to previously published guidelines (51, 52). Briefly, prior to starting chronic FGF21 injections, mice were first given an IP injection of 99% D2O equivalent to 5% of the body water pool. Mice were subsequently provided ad libitum access to 8% D2O enriched drinking water for the duration of the experiment. Male and female mice were sacrificed at 1, 4, 8, 15, and 32 days after initial bolus of D2O (n = 3/sex/treatment/time point) (53).
At euthanasia, muscles were weighed and rapidly frozen in liquid nitrogen. For analysis of tracer enrichment, TA was powdered using a liquid nitrogen-cooled mortar and pestle. Tissue was homogenized and fractionated to obtain the myofibrillar protein fraction and plasma was prepared as described previously (51, 54) to determine the precursor pool enrichment. Derivatized alanine was analyzed on an Agilent 7890A gas chromatograph coupled to an Agilent 5975 mass spectrometer as previously described (54-56). The deuterium enrichments of both the protein (product) and the precursor were used to calculate the fraction new: fraction new = Eproduct/Eprecursor, where the Eproduct is the enrichment (E) of protein-bound alanine and Eprecursor is the calculated maximum alanine enrichment from equilibration of the body water pool. The fraction new data were then plotted across the time points to calculate k (1/day) using a 1-phase association (53).
Fibroblast Growth Factor-21
Endotoxin-free, recombinant human FGF21 (hFGF21; ProSpecBio, Rehovot, Israel) was first dissolved in H2O, according to the manufacturer's instructions, and was further diluted in 0.9% sterile saline.
Gene Expression
Tissue was collected from the mice at euthanasia, after 12 days of IP FGF21 or saline treatment, and frozen in isopentane for later analysis. The muscles were first powdered using a mortar and pestle chilled with liquid nitrogen and homogenized in QIAzol Lysis Buffer using a Bead Beater. Total RNA was isolated from the homogenate using the RNeasy mini kit. Complementary DNA was synthesized using the high-capacity complementary DNA reverse transcription kit (Life Technologies/ThermoFisher, Carlsbad, CA). Gene expression analysis was performed in duplicate with TaqMan gene expression assays and utilizing a Roche LightCycler 480. Samples with poor technical replicates (>0.5 Ct difference) were excluded from further analysis. Expression of DNA damage-inducible transcript 4 (aka REDD1) (Ddit4; Mm00512504_g1), corticotropin-releasing hormone (Crh; Mm01293920_s1), beta-klotho (Klb; Mm00473122_m1), fibroblast growth factor receptor 1 (Fgfr1; Mm00438930_m1), forkhead box 01 (Foxo1; Mm00490671_m1), muscle RING-finger protein-1 (Trim63; Mm01185221_m1), muscle atrophy F-box (Fbxo32; Mm00499523_m1), and Krüppel-like factor-15 (Klf15; Mm00517792_m1), was normalized to the housekeeping genes 18S rRNA (Rn18s; Mm04277571_s1) or (Gapdh; Mm99999915_g1) using the 2ΔΔCt method. For the hypothalamus, we used the housekeeping gene Rn18s, which was stably expressed between the sexes and between the treatment groups. For skeletal muscle, expression of Rn18s (and several other housekeeping genes) was considerably different between males and females. Ultimately, we chose Gapdh, which was more stably expressed. Nonetheless, a slight sex difference persisted, and for this reason gene expression is expressed relative to the saline-treated controls for each sex.
Plasma Hormones
Circulating corticosterone and insulin-like growth factor-1 (IGF-1) were measured by the University of California, Davis Mouse Metabolic Phenotyping Center (MMPC). IGF-1 was measured from trunk blood collected at euthanasia using an enzyme-linked immunosorbent assay (R & D Systems, Minneapolis, MN; RRID:AB_2783729, https://scicrunch.org/resolver/AB_2783729). Corticosterone was measured using radioimmunoassay (MP Biomedicals, Orangeburg, NY). For the IP injection study, corticosterone was measured from trunk blood collected at euthanasia. For the ICV study, blood was collected from the tip of the tail vein within 3 minutes of first disturbing the animal's cage, in counterbalanced order, just prior to euthanasia. For both assays, samples flagged by the MMPC for poor quality were removed from further analysis.
Plasma Metabolomics
Plasma was submitted to the West Coast Metabolomics Center (WCMC) at the University of California, Davis for untargeted primary metabolite analysis by automated liner exchange cold injection system gas chromatography time of flight mass spectroscopy. Data were acquired as previously described (38, 57). In total, 306 metabolites were quantified and, of those, 101 were identified as known compounds by the WCMC. Analyses were limited to identified compounds. Metabolomics data were analyzed using MetaboAnalyst 6.0 (https://www.metaboanalyst.ca/) and the MetaboAnalystR package (version 3.3.0) in R (version 4.3.0). Sample concentrations in plasma collected from saline or FGF21-treated mice, separated by sex, were normalized using sum normalization and log-transformed data were scaled using mean centering before using the provided KEGG compound identifiers to perform either partial least squares discriminant analysis or KEGG pathway quantitative enrichment analysis. Output from MetaboAnalyst was visualized using GraphPad Prism. Metabolomics data are available at the NIH Common Fund's National Metabolomics Workbench data repository (https://www.metabolomicsworkbench.org) under assigned Study ID ST 002746 (58). The data can be accessed directly via their Project DOI (http://dx.doi.org/10.21228/M8ST56). This work is supported by NIH grant U2C-DK119886.
Statistical Analysis
The remaining data were analyzed using GraphPad Prism (GraphPad Software, La Jolla, CA) and/or SigmaStat (Systat Softward, San Jose, CA) by t-test or 2-way analysis of variance. Planned comparisons were made using the Tukey honestly significant difference test. Data are plotted as means ± standard error of the mean unless otherwise noted.
Results
FGF21 and Energy Balance
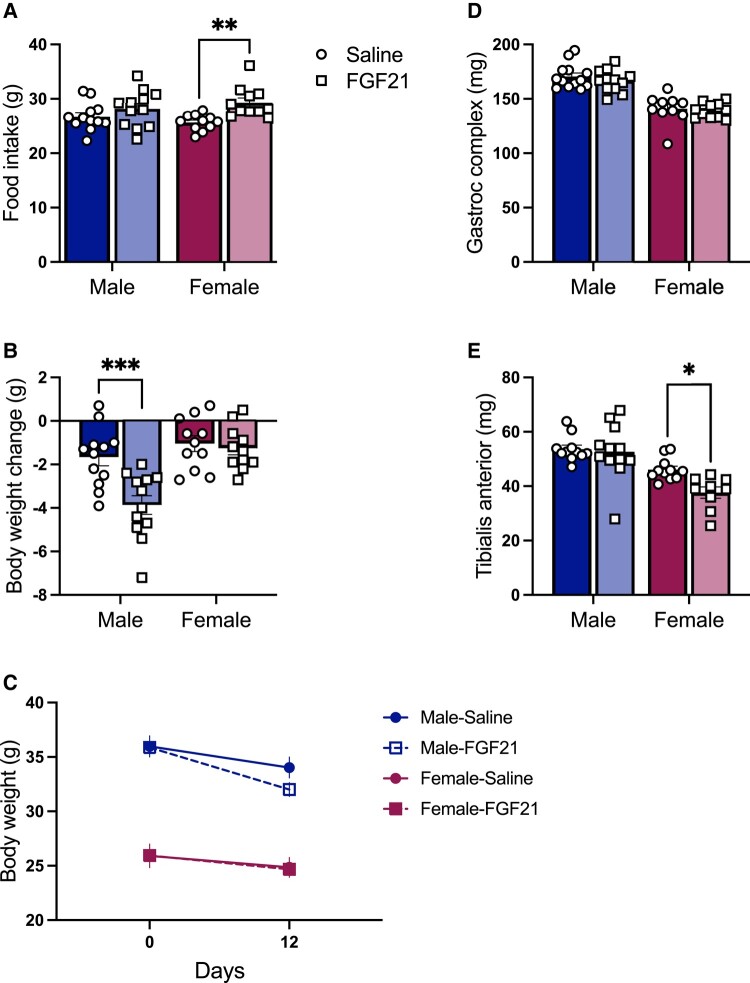
The extensive literature reports that FGF21 reduces body weight and body fat in male mice via increased energy expenditure, and this is often associated with increased food intake (29, 34, 47, 59). In agreement with this literature, mice treated with IP FGF21 (0.2 mg/kg/day) consumed more calories than saline-treated controls over the 12 days of treatment (P treatment < .01) (Fig. 1A). The effect of FGF21 on body weight and body fat loss depends on sex, such that FGF21-induced body weight and fat loss is more apparent in male mice than in female littermates (38). Likewise in this study, FGF21-treated mice lost weight compared with saline-treated controls, in a sex-dependent manner: P (treatment × sex) < .01 (Fig. 1B); P (time × treatment × sex) < .05 (Fig. 1C). FGF21-induced weight loss was more apparent in males (Tukey, P < .001).
Figure 1.
FGF21-induced muscle atrophy in female mice. IP FGF21-treated mice (0.2 mg/kg/day for 12 days) ate more than saline-treated controls (A) (P [treatment] < .01). FGF21 caused body weight loss, in a sex-dependent manner: (B) P [treatment × sex] < .01) and (C) P (time × treatment × sex) < .05). FGF21 had no effect on gastrocnemius–soleus complex weight (D), but it significantly decreased wet weight of the tibialis anterior (E): P (treatment) < .01). Tukey's HSD post hoc test. *P < .05, **P < .01; ***P < .001; N = 9-13/group.
FGF21 and Skeletal Muscle Atrophy
To determine the effect of chronic IP FGF21 treatment on skeletal muscle mass, mice were euthanized, and we collected and weighed the gastrocnemius–soleus complex and TA muscles. Muscle mass was smaller in female mice than in male littermates, as expected given their smaller body size (P [sex] < .001) (Fig. 1C and 1D). There was no effect of FGF21 treatment on gastrocnemius–soleus complex weight (Fig. 1C). However, TA weight was significantly less in FGF21-treated groups (P [treatment] < .05), and the statistical significance was primarily driven by the effect of FGF21 in female mice (Tukey, P < .05) (Fig. 1D).
FGF21 and Plasma Metabolites
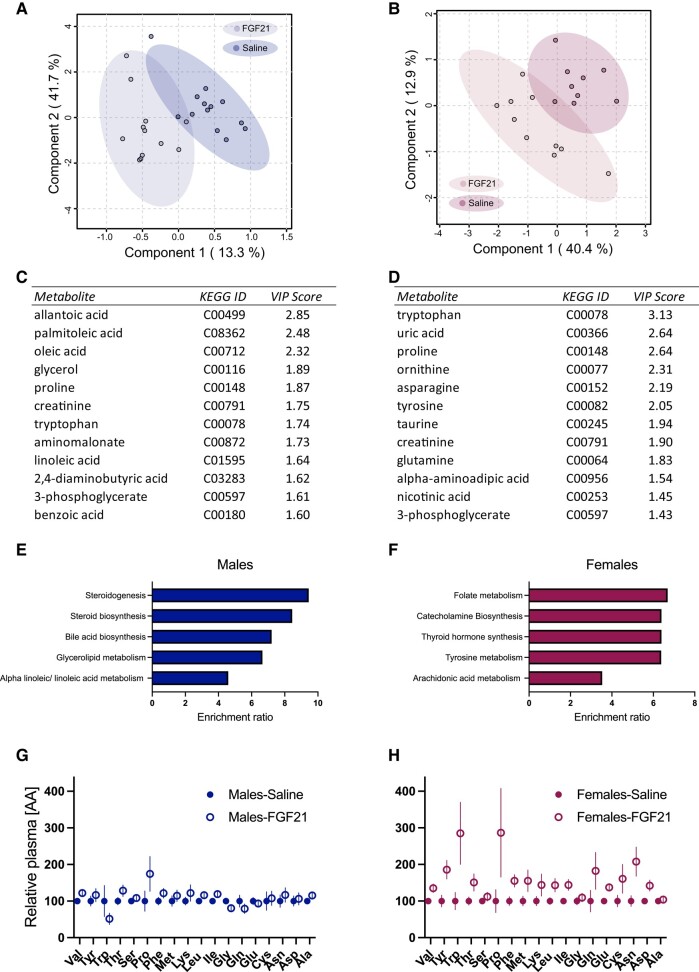
To identify metabolic pathways affected by FGF21, we analyzed untargeted “primary metabolites” (carbohydrates and sugar phosphates, amino acids, hydroxyl acids, free fatty acids, purines, pyrimidines, aromatics, exposome-derived chemicals) in plasma from DIO mice treated for 12 days with IP FGF21 (0.2 mg/kg/day) or saline. Partial least squares discriminant analysis showed clear separation between saline- and FGF21-treated mice in both sexes (Fig. 2A and 2B). In agreement with the effect of FGF21 to induce muscle atrophy in female mice, the top individual metabolites important for distinguishing between treatment groups in females were amino acids or metabolites involved in amino acid metabolism/biosynthesis (Fig. 2D). In agreement with our recent report that the effect of FGF21 to reduce hepatic and adipose tissue triglycerides is more apparent in male mice than in female littermates (38), the top individual metabolites important for distinguishing between treatment groups in males were fatty acids and glycerol, followed by amino acids (Fig. 2C). KEGG pathway quantitative enrichment analysis revealed 4 of the top 5 most enriched pathways in females were related to amino acid metabolism (Fig. 2F). These included “Folate metabolism,” “Catecholamine biosynthesis,” “Thyroid hormone synthesis,” “Tyrosine metabolism,” and “Arachidonic acid metabolism.” The top 5 most enriched pathways in males were related to lipid metabolism (Fig. 2E). These included “Steroidogenesis,” “Steroid biosynthesis,” “Bile acid biosynthesis,” “Glycerolipid metabolism,” and “Alpha linolenic acid and linoleic acid metabolism.” Lastly, FGF21-treated female mice had greater relative concentration of the proteinogenic amino acids in plasma than saline-treated controls (P [treatment] < .05) (Fig. 2H). Thus, pharmacologic treatment with FGF21 alters amino acid metabolism and increases plasma amino acid availability in female mice.
Figure 2.
FGF21 and the plasma metabolome. Partial least squares discriminant analysis (PLS-DA) 2D scores plots of plasma metabolites from FGF21 (vs saline) treated male (A) and female (B) mice. Variable Importance in Projection (VIP) scores (component 1) from the top 12 most relevant metabolites in the PLS-DA analysis from male (C) and female (D) mice. The top 5 most enriched KEGG pathways from FGF21 (vs saline) treated males were related to lipid metabolism (E). Four of the top 5 most enriched KEGG pathways from FGF21 (vs saline) treated females were related to amino acid metabolism (F). FGF21 increased the relative abundance of proteinogenic amino acids in plasma, and this was more apparent in females (G) (P [treatment] < .05) compared with male littermates (H). N = 9-13/group.
FGF21 Treatment and the HPA Axis
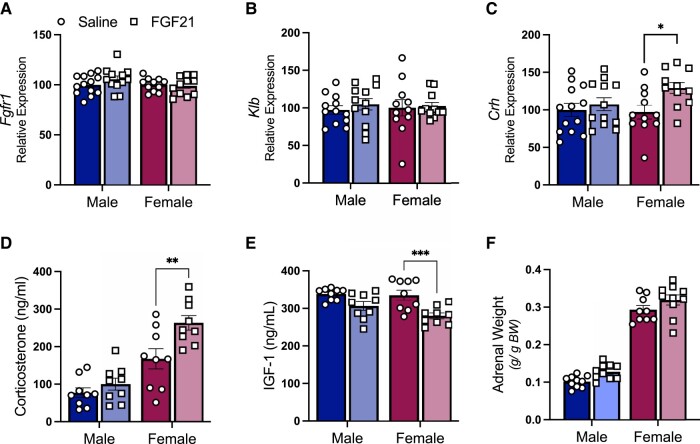
Because chronic glucocorticoid exposure both induces muscle atrophy and increases circulating amino acids, we measured indices of HPA axis activity. First, we dissected hypothalamus from mice treated for 12 days with IP FGF21 (0.2 mg/kg/day) or saline. To confirm hypothalamic expression of the FGF21 receptor complex, we measured mRNA for Fgfr1 and Klb. Expression of these genes was not affected by sex or by FGF21 treatment (Fig. 3A and 3B). FGF21-treated mice showed greater expression of hypothalamic Crh mRNA (P [treatment] < .05), and the statistical significance was driven primarily by the effect in female mice (Tukey post hoc P < .05) (Fig. 3C). Next, we measured corticosterone in plasma, by radioimmunoassay. As expected, female mice had higher plasma corticosterone than male littermates (P [treatment] < .001). FGF21-treated mice showed higher circulating corticosterone than controls (P [treatment] < .01) (Fig. 3D), and this effect was more apparent in female mice (Tukey post hoc P < .01). Corticosterone suppresses plasma IGF-1, which we also observed (P [treatment] < .001) (Fig. 3E). Again, the statistical significance was driven primarily from the effect in female mice (Tukey post hoc, P < .001). As an index of chronic HPA axis activity (33, 60-62), we dissected, cleaned, and weighed the adrenal glands. The absolute weight of the adrenal glands was significantly greater in females (P [sex] < .001), as expected (63), and it tended to be increased by FGF21 treatment (P [treatment] < .06), (1.82 mg ± 0.06 vs 2.21 mg ± 0.13 and 4.14 mg ± 0.15 vs 4.22 mg ± 0.07, for males and females, respectively). When corrected for body weight, female mice had larger adrenal glands than male littermates (P [sex] < .001), and FGF21-treated mice had larger adrenal glands than saline-treated controls (P [treatment] < .05) (Fig. 3F). Together these findings are consistent with increased HPA axis activity in FGF21-treated mice, which is more apparent among females.
Figure 3.
FGF21 increased HPA axis activity. FGF21–receptor complex mRNAs (Fgfr1 and Klb) were expressed in male and female hypothalamus and were not affected by IP FGF21 treatment (0.2 mg/kg/day for 12 days) (A, B). FGF21-treated mice had increased expression of corticotropin-releasing hormone (Crh), and increased plasma corticosterone (C-D) (P [treatment] < .05). FGF21 reduced plasma insulin-like growth factor 1 (IGF-1) (E) (P [treatment] < .001). FGF21-treated mice had larger adrenal glands (F) (P [treatment] < .001). Tukey's HSD post hoc test, *P < .05, **P < .01, ***P < .001; N = 9-13/group.
Intracerebroventricular Administration of FGF21, Glucocorticoids, and Muscle Atrophy
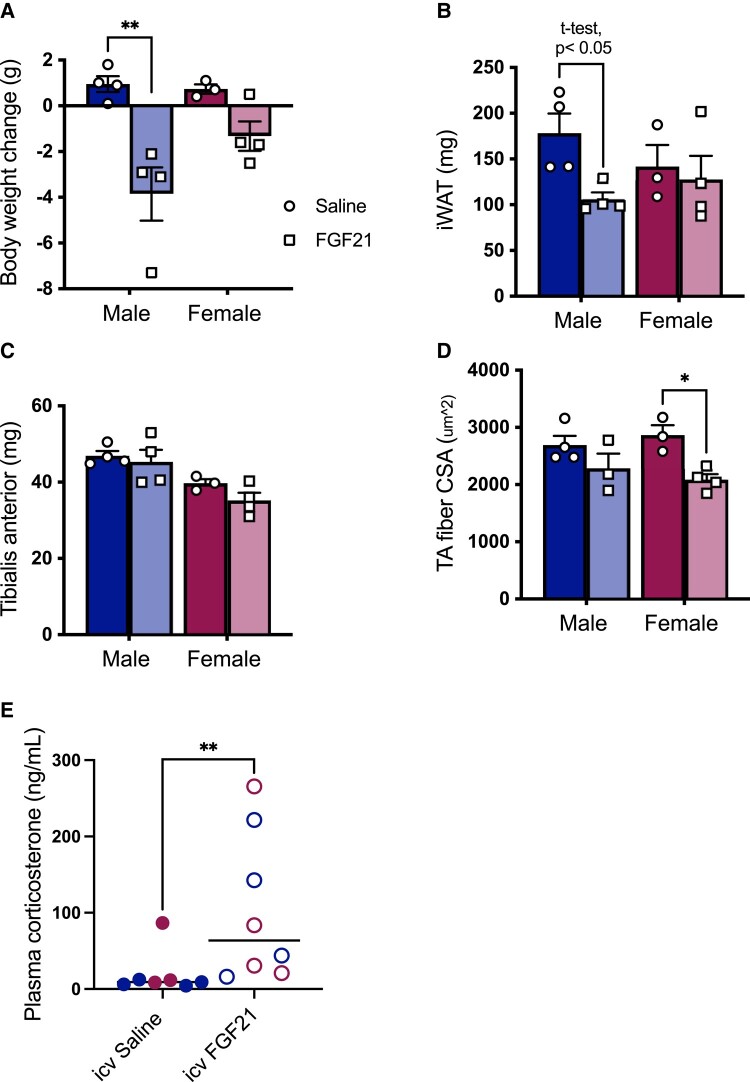
FGF21 acts directly in the hypothalamus to activate the HPA axis and increase plasma glucocorticoids (32, 33). To isolate the cell nonautonomous effects of FGF21 on skeletal muscle, we delivered FGF21 or its saline vehicle directly to the brain of DIO male and female littermates via osmotic pump to the lateral ventricle (0.4 μg/day), for 13 days. As before, FGF21-treated mice lost more weight than saline-treated controls (P [treatment] < .01) (Fig 4A) and the statistical significance was driven primarily by the effect in males (Tukey, P < .05). FGF21-treated male mice had less fat in the inguinal depot than saline-treated controls (t-test, P < .05) (Fig 4B). As expected, female mice had smaller TA muscles than male mice (P [sex] < .001) (Fig 4C). ICV FGF21-treated mice had smaller muscle fiber CSA than saline-treated controls (P [treatment] < .01) (Fig 4D), which was more apparent in female mice (Tukey, P < .05). Lastly, ICV FGF21-treated mice had increased plasma corticosterone compared with saline-treated controls (Mann–Whitney test, P < .01 (Fig 4E)).
Figure 4.
Intracerebroventricular FGF21 reduced muscle fiber cross-sectional area in female mice. FGF21-treated mice (0.4 µg/day ICV for 13 days) lost more weight than saline-treated littermates (A) (P [treatment] < .01). FGF21-treated males had smaller inguinal white adipose tissue (iWAT) depots than saline-treated controls (B) (t-test). Females had smaller tibialis anterior (TA) muscles than males (C) (P [sex] < .001). FGF21 reduced muscle fiber cross-sectional area (CSA) (D) (P [treatment] < .01), and it increased plasma corticosterone (E) (Mann–Whitney test [P < .01], females in red, males in blue). Tukey's HSD post hoc test, *P < .05, **P < .01, N = 3-4/group.
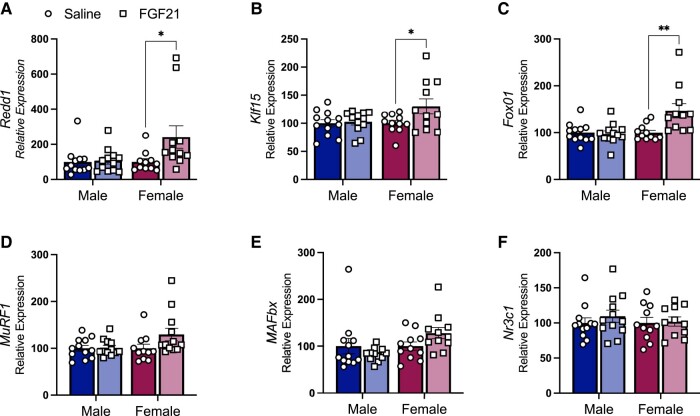
FGF21 Treatment Increases Transcription of Glucocorticoid Target Genes in TA Muscle
GRs act as a transcription factor for gene networks that induce skeletal muscle atrophy. GR activity increases the transcription of genes that reduce muscle protein synthesis and promote degradation (12, 15, 22). We measured mRNA for GR target genes in TA collected from the mice in Fig. 1. FGF21-treated mice had greater expression of mRNA for DNA damage-inducible transcript-4 (aka Ddit4 or Redd1) (P [treatment] < .05) and Klf15 (P [treatment] < .05) (Fig. 5A and 5B), which reduces muscle protein synthesis by inhibiting mTORC1 (16). FGF21-treated mice had greater relative expression of mRNA for Forkhead-box O1 (Foxo1), and this was sex-dependent (P [treatment × sex] < .01) (Fig. 5C). All these outcomes were more apparent among female mice (Tukey post hoc P < .05). In turn, FoxO1 and KLF15 regulate the transcription of the E3 ligases muscle RING-finger protein-1 (MuRF1) and muscle atrophy F-box (MAFbx), which are a part of the ubiquitin–proteasome system, the primary intracellular degradation pathway (12). However, there was no significant effect of FGF21 treatment on either of these downstream targets (Fig. 5D and 5E). There was no effect of FGF21 on skeletal muscle GR mRNA (Fig. 5F). Collectively, these data support a mechanistic role for skeletal muscle GR-signaling and decreased protein synthesis in FGF21-induced skeletal muscle atrophy in female mice.
Figure 5.
IP FGF21 increased the expression of GR target genes in skeletal muscle of female mice. FGF21 (0.2 mg/kg/day for 12 days) increased the expression of GR target genes Redd1 and Klf15 (A, B) (P ([treatment] < .05). FGF21 increased the relative expression of GR target gene Foxo1 in a sex-dependent manner (C) (P [treatment × sex] < .01). There was no significant effect of FGF21 on expression of FOXO1 target genes Murf1 and Mafbx (D, E). There was no significant effect of FGF21 treatment on glucocorticoid receptor (Nr3c1) expression (F). Tukey's HSD post hoc test: *P < .05, **P < .01; N = 9-13/group.
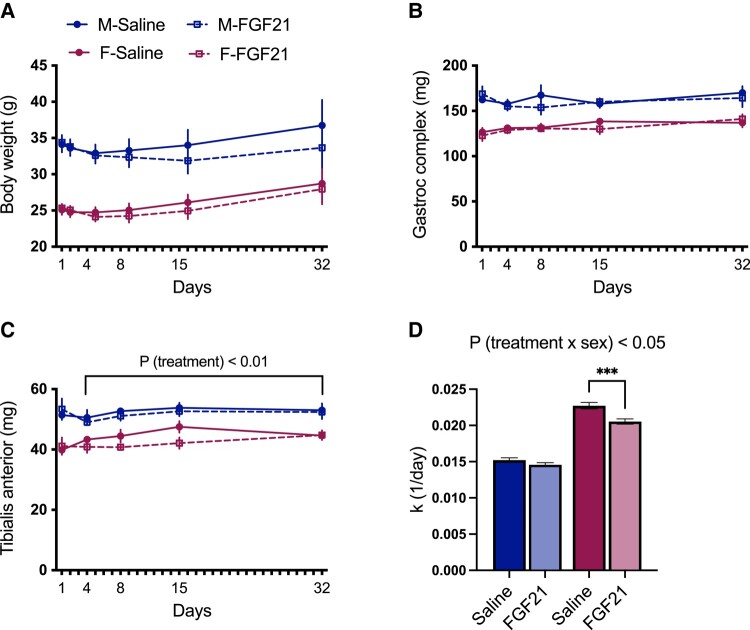
FGF21 Treatment Decreases Muscle Protein Synthesis
To directly measure the effect of FGF21 on muscle protein synthesis we performed stable isotope labeling using D2O (53). Female mice were lighter than male littermates (P [sex] < .001) (Fig. 6A). Accordingly, female mice had significantly smaller muscles (P [sex] < .001) (Fig. 6B and 6C). For mice treated with FGF21 or saline for 4 or more days, there was a significant effect of treatment to decrease TA weight (P [treatment] < .01) (Fig. 6C). The rate of protein synthesis was greater in female mice than in males (P [sex] < .001). The effect of FGF21 treatment depended on sex (P [treatment × sex] < .05), such that FGF21 significantly decreased the rate of muscle protein synthesis in female TA (Tukey's post hoc, P < .05) (Fig. 6D). These findings support that pharmacologic treatment with FGF21 reduces muscle protein synthesis, and that female mice are more sensitive to this outcome than male littermates.
Figure 6.
FGF21 decreased muscle protein synthesis in female mice. Mice were treated with FGF21 (0.2 mg/kg/day) or saline for 1, 4, 15, or 32 days, while we performed stable isotope labeling with deuterium oxide to measure protein turnover. Female mice (maroon) were lighter than males (blue) (A) (P [sex] < .001). Female mice had significantly smaller gastrocnemius (B) and tibialis anterior (C) muscles compared with males (P [sex] < .001). Mice treated with FGF21 for >4 days had decreased TA weight (C) (P [treatment] < .01). The effect of FGF21 treatment on the rate of protein synthesis depended on sex (D) (P [treatment × sex] < .05), such that FGF21 treatment significantly reduced the rate of muscle protein synthesis in female TA (Tukey's HSD post hoc test, P < .05). n = 3-6 mice per sex/treatment/timepoint.
Discussion
FGF21 is secreted in response to dietary amino acid restriction or imbalance, and other nutritional and metabolic stressors. In turn, FGF21 acts via the nervous system to increase amino acid availability by altering feeding behavior, and shifting macronutrient selection towards increased consumption of dietary protein (6, 8, 10, 64). Here we report that pharmacologic administration of FGF21 also increased systemic amino acids by accessing skeletal muscle, together with activation of the HPA axis and GR target genes in skeletal muscle. These findings provide additional insight into the regulation of protein and amino acid homeostasis by FGF21.
In 3 independent experiments, a short course of treatment with FGF21 either decreased the wet weight of the TA muscle, or reduced TA muscle fiber CSA, in female mice. We used 0.2 mg/kg IP or subcutaneous FGF21 daily. This is a relatively low dose, since most preclinical experiments deliver 1 mg/kg/day intraperitoneally, but it is nonetheless effective to cause body weight and body fat loss, and to reduce hepatic steatosis in male mice, and it improves glucose tolerance in both sexes (38, 44). Because of these beneficial effects on lipid and carbohydrate metabolism, several FGF21 analogues have been developed for human use in treating cardiometabolic diseases (23, 35-37)—highlighting the potential translational relevance of our findings. FGF21 analogues are clinically effective to improve fatty liver, though they have performed poorly for body fat loss and glucose control in humans. Changes in muscle mass have not typically been reported. In one study, the FGF21 analogue efruxifermin, which is engineered for increased affinity at FGFR2c and FGFR3c in addition to FGFR1c, appears to cause loss of lean body mass. When delivered at the highest dose, it significantly decreased body weight by an average 3.3 kg while significantly increasing body fat by an average 2.3% (65). Another FGF21 analogue, pegbelfermin, reduced lean muscle mass in male cynomolgus monkeys by 12% and reduced lean area by 15%. Female monkeys were not included in the study design (66). FGF21 significantly decreased total lean mass in several other preclinical studies, but this was not explored further (34, 66-70). Conversely, in another study, when a very low dose of FGF21 (0.1 mg/kg) was administered to male mice for 7 days, no effect on skeletal muscle mass or fiber CSA was observed (71). Considering the present findings, future clinical trials should explicitly consider the effect of FGF21 analogues on muscle mass, with the explicit inclusion of sex as a biological variable potentially influencing this outcome.
To begin understanding the potential physiologic and molecular mechanisms by which pharmacologic FGF21 promotes muscle atrophy, we measured activation of the HPA axis. FGF21 can cross the blood–brain barrier and is present in both human and rodent cerebrospinal fluid (72, 73). Moreover, FGFR1 is expressed in CRH neurons of the paraventricular nucleus of the hypothalamus (32). Previous studies by us and others show FGF21 activates the HPA axis (31-33). Intra-paraventricular nucleus injection of recombinant FGF21 increases Crh mRNA, plasma adrenocorticotropic hormone, and corticosterone (32). Accordingly, the FGF21-treated mice in this study had greater hypothalamic Crh mRNA expression, despite no differences in either Fgfr1 or Klb, and circulating corticosterone was significantly increased. FGF21 treatment induced adrenal hypertrophy, indicating chronic stimulation of the adrenal cortex by adrenocorticotropic hormone. Chronic activation of the HPA axis, or chronic treatment with synthetic glucocorticoids, causes skeletal muscle atrophy and weakness via muscle GR signaling (22). Type 2B “fast” muscle fibers are particularly sensitive to glucocorticoids, due to their higher expression of GR. The TA muscle has a high percentage of type 2B fibers, and is more sensitive to glucocorticoid-induced atrophy (13), perhaps contributing to the more robust effect of FGF21 on TA vs the gastrocnemius–soleus complex. Notably, salivary cortisol was not altered by efruxifermin treatment (65). While salivary cortisol is a reliable indicator of HPA axis activity, its values are responsive to a variety of influences and especially to acute environmental stressors, and negative data can be difficult to interpret (74). Determining potential acute and chronic effects of pharmacologic FGF21 on HPA axis activity in people will require additional studies focused on this endpoint, with carefully controlled experimental design.
We found FGF21-induced HPA activation was mirrored by increased expression of FoxO1, Redd1, and Klf15. These genes are direct and downstream targets of the GR in TA and are thought to decrease protein synthesis by inhibiting mTOR (22, 75). When we used stable isotope labeling to directly measure protein synthesis across 32 days of FGF21 treatment, we found FGF21 treated female mice had a decreased rate of muscle protein synthesis. The ∼11% decrease in protein synthesis is consistent with the 12% (Fig. 6) to 22% (Fig. 1) reduction in TA mass observed after 2 weeks of FGF21. Even smaller differences can have important consequences, if sustained. For instance, muscle mass decreases approximately 3% to 8% per decade after the age of 30, eventually leading to sarcopenia in old age (76). Thus, longer pharmacologic (and physiologic) studies are needed. Nonetheless, these outcomes are consistent with a noncell-autonomous mechanism by which FGF21 induces muscle atrophy, but loss of function experiments will be necessary to explicitly determine the role of GRs.
In these experiments, the effects of pharmacologic FGF21 to reduce mass of the TA muscle, decrease protein synthesis, activate the HPA axis, and increase plasma amino acids were preferentially observed among female mice compared with male littermates. In a recent study, we found the effects of pharmacologic FGF21 to increase lipid catabolism were also sex dependent, but regarding lipid catabolism, male mice were more affected than female littermates (38). When we analyzed metabolic pathways associated with FGF21 treatment, we found the 5 most differently enriched KEGG pathways in females were associated with amino acid metabolism while the 5 most differently enriched KEGG pathways in males were associated with lipid metabolism. Taken together, these outcomes are consistent with the idea that protein sparing during metabolic stress depends on lipid fuel availability (77, 78). That is, greater sensitivity to FGF21-induced lipid catabolism in males may be protein sparing. Related to this, the present study was conducted in DIO mice to model the proposed clinical use of FGF21 analogues. To the extent increased lipid fuel availability is protein sparing, effects of FGF21 on muscle atrophy may be more pronounced in lean individuals. Further empirical research will be needed to determine this.
Because FGF21 acts in the brain to cause body weight and body fat loss and to increase plasma glucocorticoids in (male) rodents (31-33, 45), we reasoned that ICV administration of FGF21 would recapitulate its systemic effects. We used a very low dose that was previously demonstrated to increase HPA axis activity (32), and does not result in meaningful efflux to the periphery (45). After a short course of continuous ICV administration, FGF21-treated mice lost more weight than saline-treated controls, and this was driven primarily by its effect in males. Likewise, FGF21-treated male mice, but not female littermates, had smaller inguinal white fat pads than controls. In this experiment there was no significant effect of FGF21 treatment on muscle mass, possibly reflecting the smaller sample size. However histological analysis of fiber CSA showed that FGF21-treated mice had smaller muscle fibers, which was driven primarily by its effect in females. The findings mirror effects observed with systemic treatment, again raising the possibility that greater sensitivity to FGF21-induced lipid catabolism in males may be protein sparing.
Our findings agree with a long literature demonstrating the HPA axis response to stressors are more pronounced in females (41, 42), and reports that the effect of glucocorticoids on muscle atrophy are more pronounced in skeletal muscle of females compared with males (43, 79). This sex difference likely results from sex differences in circulating androgens (80, 81). For instance, early work from Mayer and Rosen showed competitive inhibition of the GR by androgens contributes to their anabolic effect in skeletal muscle (82). Thus, castration increased the binding of labeled dexamethasone to GR in male rats. When both castration and adrenalectomy were performed, there was an inverse relationship between the amount of labeled dexamethasone bound to the GR and the amount of testosterone administered to the animal (83). Future studies will be needed determine the relative contributions of sex hormones and/or chromosomes to these differential outcomes.
The current work focused only on effects of pharmacologic FGF21; when and how endogenously produced FGF21 may affect skeletal muscle mass is also an important topic that requires further investigation. FGF21 was initially identified as a hepatokine, but it can be secreted by other metabolic organs and tissues, including skeletal muscle, in response to intracellular stress (84-87). For instance, in a recent study, Fgf21 mRNA from skeletal muscle was increased roughly 4-fold during a prolonged fast (88) and skeletal muscle–specific FGF21-null mice were partly protected against fasting-induced fat loss and muscle atrophy—with parallel differences in muscle protein synthesis and autophagy pathways. Sex of the animals was not reported in that study (88). Likewise, plasma FGF21 is increased, and skeletal muscle mass is decreased in mice lacking the mitochondrial protein Optic Atrophy 1 (OPA1), and these outcomes were diminished among mice lacking both Fgf21 and Opa1 in muscle (89). That study used both males and females, but the sex of animals in any given experiment was not reported. Thus, endogenous skeletal muscle FGF21 contributes to muscle atrophy in some conditions. It is not known if this represents a paracrine or endocrine action, since the first order KLB:FGFR-expressing cells mediating these outcomes were not yet identified. Similarly, the current study did not identify the key FGFR:KLB-expressing cells mediating effects of pharmacologic FGF21 nor did it determine the relative importance of FGFR1 vs other FGFR isoforms (23).
In humans, plasma FGF21 is acutely increased after binge drinking (90) or following the consumption of fructose and other simple sugars (91), and it is chronically increased among individuals with diabetes, metabolic and alcoholic fatty liver disease, sarcopenia, and mitochondrial myopathies. These physiologic conditions are all associated with muscle atrophy (92-96), but it is not yet known if the increased FGF21 represents a mechanism for muscle loss in these conditions (eg, alcoholic myopathy) or is simply a biomarker. These are important topics for future research.
In conclusion, we find pharmacologic FGF21 decreases muscle protein synthesis, induces skeletal muscle atrophy, and increases plasma amino acids in female mice. FGF21 is secreted downstream of the intracellular stress response pathway and is thought to act as a neuroendocrine signal of dietary protein and/or amino acid restriction (26). In turn, we (6, 8) and others (10) have recently shown that FGF21 can alter feeding behavior and macronutrient selection to increase the consumption of protein, restoring systemic homeostasis. The present studies identify an additional mechanism by which FGF21 can increase systemic amino acid availability—by accessing skeletal muscle. These findings highlight a potential physiologic role for FGF21 in systemic protein metabolism, and they may inform the proposed clinical use of FGF21 analogues for the treatment of cardiometabolic disease.
Acknowledgments
We thank Fredrick F. Peelor (OMRF) and Yi-Je Chen (UC Davis) for excellent technical assistance.
Abbreviations
- CRH
corticotropin-releasing hormone
- CSA
cross-sectional area
- DIO
diet-induced obese
- FGF21
fibroblast growth factor-21
- FGFR
fibroblast growth factor receptor
- Foxo
forkhead box protein
- GR
glucocorticoid receptor
- HPA
hypothalamic–pituitary–adrenocortical
- ICV
intracerebroventricular
- IGF-1
insulin-like growth factor-1
- IP
intraperitoneal
- KLB
β-klotho
- Klf15
Krüpple-like factor-15
- Redd1
regulated in development and DNA damage response-1
- TA
tibialis anterior
Contributor Information
Karlton R Larson, Department of Neurobiology, Physiology, and Behavior, College of Biological Sciences, University of California Davis, Davis, CA 95616, USA.
Devi Jayakrishnan, Department of Neurobiology, Physiology, and Behavior, College of Biological Sciences, University of California Davis, Davis, CA 95616, USA.
Karla A Soto Sauza, Department of Neurobiology, Physiology, and Behavior, College of Biological Sciences, University of California Davis, Davis, CA 95616, USA.
Michael L Goodson, Department of Neurobiology, Physiology, and Behavior, College of Biological Sciences, University of California Davis, Davis, CA 95616, USA.
Aki T Chaffin, Department of Neurobiology, Physiology, and Behavior, College of Biological Sciences, University of California Davis, Davis, CA 95616, USA.
Arik Davidyan, Department of Neurobiology, Physiology, and Behavior, College of Biological Sciences, University of California Davis, Davis, CA 95616, USA; Department of Biological Sciences, California State University Sacramento, Sacramento, CA 95819, USA.
Suraj Pathak, Department of Neurobiology, Physiology, and Behavior, College of Biological Sciences, University of California Davis, Davis, CA 95616, USA.
Yanbin Fang, Department of Neurobiology, Physiology, and Behavior, College of Biological Sciences, University of California Davis, Davis, CA 95616, USA.
Diego Gonzalez Magaña, Department of Neurobiology, Physiology, and Behavior, College of Biological Sciences, University of California Davis, Davis, CA 95616, USA.
Benjamin F Miller, Aging & Metabolism Program, Oklahoma Medical Research Foundation, Oklahoma City, OK 73104, USA; Oklahoma City Veterans Affairs Medical Center, Oklahoma City, OK 73104, USA.
Karen K Ryan, Department of Neurobiology, Physiology, and Behavior, College of Biological Sciences, University of California Davis, Davis, CA 95616, USA.
Funding
This work was supported by the National Institutes of Health R01DK121035 to K.K.R., and F31DK124080 to A.T.C. A.T.C. was supported by the National Center for Advancing Translational Sciences, NIH, through grant number UL1 TR001860 and linked award TL1 TR001861. K.A.S.S. was supported by the National Institute of General Medical Sciences of the NIH T32GM007377. D.J. was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases funded training program in Musculoskeletal Health Research T32AR079099. Sequencing was carried out at the UC Davis Genome Center DNA Technologies and Expression Analysis Core, supported by NIH Shared Instrumentation Grant 1S10OD010786-01.
Disclosures
The authors have nothing to disclose.
Data Availability
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Bröer S, Bröer A. Amino acid homeostasis and signalling in mammalian cells and organisms. Biochem J. 2017;474(12):1935‐1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Felig P, Owen OE, Wahren J, Cahill GF Jr. Amino acid metabolism during prolonged starvation. J Clin Invest. 1969;48(3):584‐594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kalhan SC, Uppal SO, Moorman JL, et al. Metabolic and genomic response to dietary isocaloric protein restriction in the rat. J Biol Chem. 2011;286(7):5266‐5277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. DiBattista D, Holder MD. Enhanced preference for a protein-containing diet in response to dietary protein restriction. Appetite. 1998;30(3):237‐254. [DOI] [PubMed] [Google Scholar]
- 5. Hill CM, Qualls-Creekmore E, Berthoud HR, et al. FGF21 and the physiological regulation of macronutrient preference. Endocrinology. 2020;161(3):bqaa019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Larson KR, Chaffin ATB, Goodson ML, Fang Y, Ryan KK. Fibroblast growth factor-21 controls dietary protein intake in male mice. Endocrinology. 2019;160(5):1069‐1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Morrison CD, Reed SD, Henagan TM. Homeostatic regulation of protein intake: in search of a mechanism. Am J Physiol Regul Integr Comp Physiol. 2012;302(8):R917‐R928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wu C-T, Larson KR, Goodson ML, Ryan KK. Fibroblast growth factor 21 and dietary macronutrient intake in female mice. Physiol Behav. 2022;257:113995. [DOI] [PubMed] [Google Scholar]
- 9. Solon-Biet SM, Cogger VC, Pulpitel T, et al. Defining the nutritional and metabolic context of FGF21 using the geometric framework. Cell Metab. 2016;24(4):555‐565. [DOI] [PubMed] [Google Scholar]
- 10. Hill CM, Laeger T, Dehner M, et al. FGF21 signals protein Status to the brain and adaptively regulates food choice and metabolism. Cell Rep. 2019;27(10):2934‐2947.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kamata S, Yamamoto J, Kamijo K, et al. Dietary deprivation of each essential amino acid induces differential systemic adaptive responses in mice. Mol Nutr Food Res. 2014;58(6):1309‐1321. [DOI] [PubMed] [Google Scholar]
- 12. Bodine SC, Furlow JD. Glucocorticoids and skeletal muscle. Adv Exp Med Biol. 2015;872:145‐176. [DOI] [PubMed] [Google Scholar]
- 13. Braun TP, Marks DL. The regulation of muscle mass by endogenous glucocorticoids. Front Physiol. 2015;6:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bodine SC, Latres E, Baumhueter S, et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294(5547):1704‐1708. [DOI] [PubMed] [Google Scholar]
- 15. Sandri M, Sandri C, Gilbert A, et al. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004;117(3):399‐412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shimizu N, Yoshikawa N, Ito N, et al. Crosstalk between glucocorticoid receptor and nutritional sensor mTOR in skeletal muscle. Cell Metab. 2011;13(2):170‐182. [DOI] [PubMed] [Google Scholar]
- 17. Waddell DS, Baehr LM, van den Brandt J, et al. The glucocorticoid receptor and FOXO1 synergistically activate the skeletal muscle atrophy-associated MuRF1 gene. Am J Physiol Endocrinol Metab. 2008;295(4):E785‐E797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Watson ML, Baehr LM, Reichardt HM, Tuckermann JP, Bodine SC, Furlow JD. A cell-autonomous role for the glucocorticoid receptor in skeletal muscle atrophy induced by systemic glucocorticoid exposure. Am J Physiol Endocrinol Metab. 2012;302(10):E1210‐E1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang H, Kubica N, Ellisen LW, Jefferson LS, Kimball SR. Dexamethasone represses signaling through the mammalian target of rapamycin in muscle cells by enhancing expression of REDD1. J Biol Chem. 2006;281(51):39128‐39134. [DOI] [PubMed] [Google Scholar]
- 20. Kaplan SA, Shimizu CS. Effects of cortisol on amino acids in skeletal muscle and plasma. Endocrinology. 1963;72(2):267‐272. [DOI] [PubMed] [Google Scholar]
- 21. Tipton KD, Hamilton DL, Gallagher IJ. Assessing the role of muscle protein breakdown in response to nutrition and exercise in humans. Sports Med. 2018;48(Suppl 1):53‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kuo T, Harris CA, Wang J-C. Metabolic functions of glucocorticoid receptor in skeletal muscle. Mol Cell Endocrinol. 2013;380(1-2):79‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fisher FM, Maratos-Flier E. Understanding the physiology of FGF21. Annu Rev Physiol. 2016;78(1):223‐241. [DOI] [PubMed] [Google Scholar]
- 24. Nishimura T, Nakatake Y, Konishi M, Itoh N. Identification of a novel FGF, FGF-21, preferentially expressed in the liver. Biochim Biophys Acta. 2000;1492(1):203‐206. [DOI] [PubMed] [Google Scholar]
- 25. Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab. 2007;5(6):426‐437. [DOI] [PubMed] [Google Scholar]
- 26. Laeger T, Henagan TM, Albarado DC, et al. FGF21 is an endocrine signal of protein restriction. J Clin Invest. 2014;124(9):3913‐3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ogawa Y, Kurosu H, Yamamoto M, et al. Βklotho is required for metabolic activity of fibroblast growth factor 21. Proc Natl Acad Sci U S A. 2007;104(18):7432‐7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fon Tacer K, Bookout AL, Ding X, et al. Research resource: comprehensive expression atlas of the fibroblast growth factor system in adult mouse. Mol Endocrinol. 2010;24(10):2050‐2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Potthoff MJ, Inagaki T, Satapati S, et al. FGF21 induces PGC-1alpha and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proc Natl Acad Sci U S A. 2009;106(26):10853‐10858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Inagaki T, Dutchak P, Zhao G, et al. Endocrine regulation of the fasting response by PPARα-mediated induction of fibroblast growth factor 21. Cell Metab. 2007;5(6):415‐425. [DOI] [PubMed] [Google Scholar]
- 31. Owen BM, Ding X, Morgan DA, et al. FGF21 acts centrally to induce sympathetic nerve activity, energy expenditure, and weight loss. Cell Metab. 2014;20(4):670‐677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liang Q, Zhong L, Zhang J, et al. FGF21 maintains glucose homeostasis by mediating the cross talk between liver and brain during prolonged fasting. Diabetes. 2014;63(12):4064‐4075. [DOI] [PubMed] [Google Scholar]
- 33. Ryan KK, Packard AEB, Larson KR, et al. Dietary manipulations that induce ketosis activate the HPA axis in male rats and mice: a potential role for fibroblast growth factor-21. Endocrinology. 2018;159(1):400‐413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Xu J, Lloyd DJ, Hale C, et al. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes. 2009;58(1):250‐259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sanyal A, Charles ED, Neuschwander-Tetri BA, et al. Pegbelfermin (BMS-986036), a PEGylated fibroblast growth factor 21 analogue, in patients with non-alcoholic steatohepatitis: a randomised, double-blind, placebo-controlled, phase 2a trial. Lancet. 2019;392(10165):2705‐2717. [DOI] [PubMed] [Google Scholar]
- 36. Bao L, Yin J, Gao W, Wang Q, Yao W, Gao X. A long-acting FGF21 alleviates hepatic steatosis and inflammation in a mouse model of non-alcoholic steatohepatitis partly through an FGF21-adiponectin-IL17A pathway. Br J Pharmacol. 2018;175(16):3379‐3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lee JH, Kang YE, Chang JY, et al. An engineered FGF21 variant, LY2405319, can prevent non-alcoholic steatohepatitis by enhancing hepatic mitochondrial function. Am J Transl Res. 2016;8(11):4750‐4763. [PMC free article] [PubMed] [Google Scholar]
- 38. Chaffin AT, Larson KR, Huang K-P, et al. FGF21 controls hepatic lipid metabolism via sex-dependent interorgan crosstalk. JCI Insight. 2022;7(19):e155848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Makarova E, Kazantseva A, Dubinina A, et al. Fibroblast growth factor 21 (FGF21) administration sex-specifically affects blood insulin levels and liver steatosis in obese ay mice. Cells. 2021;10(12):3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rincón-Cortés M, Herman JP, Lupien S, Maguire J, Shansky RM. Stress: influence of sex, reproductive status and gender. Neurobiol Stress. 2019;10:100155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kitay JI. Sex differences in adrenal cortical secretion in the rat. Endocrinology. 1961;68(5):818‐824. [DOI] [PubMed] [Google Scholar]
- 42. Goel N, Workman JL, Lee TT, Innala L, Viau V. Sex differences in the HPA axis. Compr Physiol. 2014;4(3):1121‐1155. [DOI] [PubMed] [Google Scholar]
- 43. Baehr L, Tunzi M, Bodine S. Muscle hypertrophy is associated with increases in proteasome activity that is independent of MuRF1 and MAFbx expression. Front Physiol. 2014;5:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hale C, Chen MM, Stanislaus S, et al. Lack of overt FGF21 resistance in two mouse models of obesity and insulin resistance. Endocrinology. 2012;153(1):69‐80. [DOI] [PubMed] [Google Scholar]
- 45. Douris N, Stevanovic DM, Fisher FM, et al. Central fibroblast growth factor 21 browns white fat via sympathetic action in male mice. Endocrinology. 2015;156(7):2470‐2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Claflin KE, Sullivan AI, Naber MC, et al. Pharmacological FGF21 signals to glutamatergic neurons to enhance leptin action and lower body weight during obesity. Mol Metab. 2022;64:101564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sarruf DA, Thaler JP, Morton GJ, et al. Fibroblast growth factor 21 action in the brain increases energy expenditure and insulin sensitivity in obese rats. Diabetes. 2010;59(7):1817‐1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Reeves PG, Nielsen FH, Fahey GC Jr. AIN-93 purified diets for laboratory rodents: final report of the American institute of nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123(11):1939‐1951. [DOI] [PubMed] [Google Scholar]
- 49. Zore T, Palafox M, Reue K. Sex differences in obesity, lipid metabolism, and inflammation-A role for the sex chromosomes? Mol Metab. 2018;15:35‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Salinero AE, Anderson BM, Zuloaga KL. Sex differences in the metabolic effects of diet-induced obesity vary by age of onset. Int J Obes (Lond). 2018;42(5):1088‐1091. [DOI] [PubMed] [Google Scholar]
- 51. Miller BF, Robinson MM, Reuland DJ, et al. Calorie restriction does not increase short-term or long-term protein synthesis. J Gerontol A Biol Sci. 2013;68(5):530‐538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Miller BF, Reid JJ, Price JC, Lin H-JL, Atherton PJ, Smith K. CORP: the use of deuterated water for the measurement of protein synthesis. J Appl Physiol (1985). 2020;128(5):1163‐1176. [DOI] [PubMed] [Google Scholar]
- 53. Abbott CB, Lawrence MM, Kobak KA, et al. A novel stable isotope approach demonstrates surprising degree of age-related decline in skeletal muscle collagen proteostasis. Function (Oxf). 2021;2(4):zqab028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Drake JC, Bruns DR, Peelor FF III, et al. Long-lived Snell dwarf mice display increased proteostatic mechanisms that are not dependent on decreased mTORC1 activity. Aging Cell. 2015;14(3):474‐482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lawrence MM, Van Pelt DW, Confides AL, et al. Massage as a mechanotherapy promotes skeletal muscle protein and ribosomal turnover but does not mitigate muscle atrophy during disuse in adult rats. Acta Physiol (Oxf). 2020;229(3):e13460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Miller BF, Hamilton KL, Majeed ZR, et al. Enhanced skeletal muscle regrowth and remodelling in massaged and contralateral non-massaged hindlimb. J Physiol. 2018;596(1):83‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Edgar R, Domrachev M, Lash AE. Gene expression omnibus: nCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002;30(1):207‐210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sud M, Fahy E, Cotter D, et al. Metabolomics Workbench: an international repository for metabolomics data and metadata, metabolite standards, protocols, tutorials and training, and analysis tools. Nucleic Acids Res. 2016;44(D1):D463‐D470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Coskun T, Bina HA, Schneider MA, et al. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology. 2008;149(12):6018‐6027. [DOI] [PubMed] [Google Scholar]
- 60. Ulrich-Lai YM, Figueiredo HF, Ostrander MM, Choi DC, Engeland WC, Herman JP. Chronic stress induces adrenal hyperplasia and hypertrophy in a subregion-specific manner. Am J Physiol Endocrinol Metab. 2006;291(5):E965‐E973. [DOI] [PubMed] [Google Scholar]
- 61. Chaffin AT-B, Fang Y, Larson KR, Mul JD, Ryan KK. Sex-dependent effects of MC4R genotype on HPA axis tone: implications for stress-associated cardiometabolic disease. Stress. 2019;22(5):571‐580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Goodson ML, Packard AEB, Buesing DR, et al. Chronic stress and Rosiglitazone increase indices of vascular stiffness in male rats. Physiol Behav. 2017;172:16‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Malendowicz LK. Sex differences in adrenocortical structure and function. VI. Long-term effect of gonadectomy and testosterone or estradiol replacement on rat adrenal cortex. Endokrinologie. 1980;75(3):311‐323. [PubMed] [Google Scholar]
- 64. Wu C-T, Chaffin AT, Ryan KK. Fibroblast growth factor 21 facilitates the homeostatic control of feeding behavior. J Clin Med. 2022;11(3):580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Harrison SA, Ruane PJ, Freilich BL, et al. Efruxifermin in non-alcoholic steatohepatitis: a randomized, double-blind, placebo-controlled, phase 2a trial. Nat Med. 2021;27(7):1262‐1271. [DOI] [PubMed] [Google Scholar]
- 66. Thompson KE, Guillot M, Graziano MJ, Mangipudy RS, Chadwick KD. Pegbelfermin, a PEGylated FGF21 analogue, has pharmacology without bone toxicity after 1-year dosing in skeletally-mature monkeys. Toxicol Appl Pharmacol. 2021;428:115673. [DOI] [PubMed] [Google Scholar]
- 67. Laeger T, Baumeier C, Wilhelmi I, Würfel J, Kamitz A, Schürmann A. FGF21 improves glucose homeostasis in an obese diabetes-prone mouse model independent of body fat changes. Diabetologia. 2017;60(11):2274‐2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Emanuelli B, Vienberg SG, Smyth G, et al. Interplay between FGF21 and insulin action in the liver regulates metabolism. J Clin Invest. 2014;124(2):515‐527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Stanislaus S, Hecht R, Yie J, et al. A novel Fc-FGF21 with improved resistance to proteolysis, increased affinity toward β-klotho, and enhanced efficacy in mice and cynomolgus monkeys. Endocrinology. 2017;158(5):1314‐1327. [DOI] [PubMed] [Google Scholar]
- 70. Liu C, Schönke M, Zhou E, et al. Pharmacological treatment with FGF21 strongly improves plasma cholesterol metabolism to reduce atherosclerosis. Cardiovasc Res. 2022;118(2):489‐502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Benoit B, Meugnier E, Castelli M, et al. Fibroblast growth factor 19 regulates skeletal muscle mass and ameliorates muscle wasting in mice. Nat Med. 2017;23(8):990‐996. [DOI] [PubMed] [Google Scholar]
- 72. Lewis JE, Ebling FJP, Samms RJ, Tsintzas K. Going back to the biology of FGF21: new insights. Trends Endocrinol Metab. 2019;30(8):491‐504. [DOI] [PubMed] [Google Scholar]
- 73. Hsuchou H, Pan W, Kastin AJ. The fasting polypeptide FGF21 can enter brain from blood. Peptides. 2007;28(12):2382‐2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hellhammer DH, Wüst S, Kudielka BM. Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology. 2009;34(2):163‐171. [DOI] [PubMed] [Google Scholar]
- 75. Brugarolas J, Lei K, Hurley RL, et al. Regulation of mTOR function in response to hypoxia by REDD1 and the TSC1/TSC2 tumor suppressor complex. Genes Dev. 2004;18(23):2893‐2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Volpi E, Nazemi R, Fujita S. Muscle tissue changes with aging. Curr Opin Clin Nutr Metab Care. 2004;7(4):405‐410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lowell BB, Goodman MN. Protein sparing in skeletal muscle during prolonged starvation. Dependence on lipid fuel availability. Diabetes. 1987;36(1):14‐19. [DOI] [PubMed] [Google Scholar]
- 78. Jagoe RT, Engelen MPKJ. Muscle wasting and changes in muscle protein metabolism in chronic obstructive pulmonary disease. Eur Respir J Suppl. 2003;46(Supplement 46):52s‐63s. [DOI] [PubMed] [Google Scholar]
- 79. Bodine SC, Furlow JD. Glucocorticoids and skeletal muscle. In: Wang J-C, Harris C eds. Glucocorticoid Signaling: From Molecules to Mice to Man. Advances in Experimental Medicine and Biology. Springer; 2015:145‐176. [DOI] [PubMed] [Google Scholar]
- 80. Brouillette J, Rivard K, Lizotte E, Fiset C. Sex and strain differences in adult mouse cardiac repolarization: importance of androgens. Cardiovasc Res. 2005;65(1):148‐157. [DOI] [PubMed] [Google Scholar]
- 81. Handelsman DJ, Hirschberg AL, Bermon S. Circulating testosterone as the hormonal basis of sex differences in athletic performance. Endocr Rev. 2018;39(5):803‐829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Mayer M, Rosen F. Interaction of glucocorticoids and androgens with skeletal muscle. Metab Clin Exp. 1977;26(8):937‐962. [DOI] [PubMed] [Google Scholar]
- 83. Mayer M, Rosen F. Interaction of anabolic steroids with glucocorticoid receptor sites in rat muscle cytosol. Am J Physiol. 1975;229(5):1381‐1386. [DOI] [PubMed] [Google Scholar]
- 84. Keipert S, Ost M, Johann K, et al. Skeletal muscle mitochondrial uncoupling drives endocrine cross-talk through the induction of FGF21 as a myokine. Am J Physiol Endocrinol Metab. 2014;306(5):E469‐E482. [DOI] [PubMed] [Google Scholar]
- 85. Miyake M, Nomura A, Ogura A, et al. Skeletal muscle-specific eukaryotic translation initiation factor 2α phosphorylation controls amino acid metabolism and fibroblast growth factor 21-mediated non-cell-autonomous energy metabolism. FASEB J. 2016;30(2):798‐812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kim KH, Jeong YT, Oh H, et al. Autophagy deficiency leads to protection from obesity and insulin resistance by inducing Fgf21 as a mitokine. Nat Med. 2013;19(1):83‐92. [DOI] [PubMed] [Google Scholar]
- 87. Guridi M, Tintignac LA, Lin S, Kupr B, Castets P, Rüegg MA. Activation of mTORC1 in skeletal muscle regulates whole-body metabolism through FGF21. Sci Signal. 2015;8(402):ra113. [DOI] [PubMed] [Google Scholar]
- 88. Oost LJ, Kustermann M, Armani A, Blaauw B, Romanello V. Fibroblast growth factor 21 controls mitophagy and muscle mass. J Cachexia Sarcopenia Muscle. 2019;10(3):630‐642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Tezze C, Romanello V, Desbats MA, et al. Age-Associated loss of OPA1 in muscle impacts muscle mass, metabolic homeostasis, systemic inflammation, and epithelial senescence. Cell Metab. 2017;25(6):1374‐1389.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Desai BN, Singhal G, Watanabe M, et al. Fibroblast growth factor 21 (FGF21) is robustly induced by ethanol and has a protective role in ethanol associated liver injury. Mol Metab. 2017;6(11):1395‐1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Dushay JR, Toschi E, Mitten EK, Fisher FM, Herman MA, Maratos-Flier E. Fructose ingestion acutely stimulates circulating FGF21 levels in humans. Mol Metab. 2015;4(1):51‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Koo BK, Kim D, Joo SK, et al. Sarcopenia is an independent risk factor for non-alcoholic steatohepatitis and significant fibrosis. J Hepatol. 2017;66(1):123‐131. [DOI] [PubMed] [Google Scholar]
- 93. Mesinovic J, Zengin A, De Courten B, Ebeling PR, Scott D. Sarcopenia and type 2 diabetes mellitus: a bidirectional relationship. Diabetes Metab Syndr Obes. 2019;12:1057‐1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Hill C, Tallis J. Is obesity a risk factor for skeletal muscle ageing? Aging (Albany NY). 2019;11(8):2183‐2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kim G, Kim JH. Impact of skeletal muscle mass on metabolic health. Endocrinol Metab. 2020;35(1):1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Pacifico J, Geerlings MAJ, Reijnierse EM, Phassouliotis C, Lim WK, Maier AB. Prevalence of sarcopenia as a comorbid disease: a systematic review and meta-analysis. Exp Gerontol. 2020;131:110801. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.