Abstract
Shiga toxins 1 (Stx1) and 2 (Stx2) are encoded by toxin-converting bacteriophages of Stx-producing Escherichia coli (STEC), and so far two Stx1- and one Stx2-converting phages have been isolated from two STEC strains (A. D. O’Brien, J. W. Newlands, S. F. Miller, R. K. Holmes, H. W. Smith, and S. B. Formal, Science 226:694–696, 1984). In this study, we isolated two Stx2-converting phages, designated Stx2Φ-I and Stx2Φ-II, from two clinical strains of STEC associated with the outbreaks in Japan in 1996 and found that Stx2Φ-I resembled 933W, the previously reported Stx2-converting phage, in its infective properties for E. coli K-12 strain C600 while Stx2Φ-II was distinct from them. The sizes of the plaques of Stx2Φ-I and Stx2Φ-II in C600 were different; the former was larger than the latter. The restriction maps of Stx2Φ-I and Stx2Φ-II were not identical; rather, Stx2Φ-II DNA was approximately 3 kb larger than Stx2Φ-I DNA. Furthermore, Stx2Φ-I and Stx2Φ-II showed different phage immunity, with Stx2Φ-I and 933W belonging to the same group. Infection of C600 by Stx2Φ-I or 933W was affected by environmental osmolarity differently from that by Stx2Φ-II. When C600 was grown under conditions of high osmolarity, the infectivity of Stx2Φ-I and 933W was greatly decreased compared with that of Stx2Φ-II. Examination of the plating efficiency of the three phages for the defined mutations in C600 revealed that the efficiency of Stx2Φ-I and 933W for the fadL mutant decreased to less than 10−7 compared with that for C600 whereas the efficiency of Stx2Φ-II decreased to 0.1% of that for C600. In contrast, while the plating efficiency of Stx2Φ-II for the lamB mutant decreased to a low level (0.05% of that for C600), the efficiencies of Stx2Φ-I and 933W were not changed. This was confirmed by the phage neutralization experiments with isolated outer membrane fractions from C600, fadL mutant, or lamB mutant or the purified His6-tagged FadL and LamB proteins. Based on the data, we concluded that FadL acts as the receptor for Stx2Φ-I and Stx2Φ-II whereas LamB acts as the receptor only for Stx2Φ-II.
Shiga toxin-producing Escherichia coli (STEC) strains are associated with diarrhea, hemorrhagic colitis, and the hemolytic-uremic syndrome (HUS) in humans. Serotype O157:H7 constitutes the main STEC causative serotype, but other serotypes such as O26:H11 are also involved (24). The frequency with which STEC infections are being reported continues to increase, probably reflecting both a greater interest in this pathogen and a real increase in its incidence and geographic spread (10). In Japan, an epidemic STEC O157:H7 infection started with a large outbreak in primary schools in Okayama in 1996, which involved 468 patients, 2 of whom developed HUS and died (52). This was followed by a large outbreak of STEC O157:H7 infection in primary schools in Osaka in 1996, affecting more than 6,000 children (52).
STEC isolated from patients with hemorrhagic colitis produces two immunologically distinct toxins that are cytotoxic to Vero cells (47, 49). One of the two Vero toxins is physicochemically, biologically, and immunologically identical to Shiga toxin from Shigella dysenteriae type 1 (32) and is known as Shiga toxin 1 (Stx1), while the other is immunologically unrelated to Shiga toxin (49, 58) and is known as Shiga toxin 2 (Stx2). Both Stx1 and Stx2 have been purified and their physicochemical and biological properties have been reported (32, 58). It has been proposed that the E. coli O157:H− strain E32511 produces yet another distinct toxin resembling the Stx2 family (14). Two variants of Stx2 have been well characterized (9, 54). The variants, designated SLT-IIv (Stx2e) and SLT-IIva, are cytotoxic for Vero and HeLa cells. SLT-IIv (Stx2e) is the causative agent of edema disease in pigs (25, 54), while SLT-IIva has been associated with diarrhea in a human infant (21).
Several investigators have reported that production of Stx1 and Stx2 in E. coli is conferred by toxin-converting bacteriophages (33, 47–49). The Stx1 encoded on the phages was first demonstrated in E. coli O26:H11 strain H19 (47, 48). Strain H19 was lysogenized by two Stx-converting phages, designated H19A and H19B, which possess different host ranges. Phage H19B is related to coliphage lambda and carries the stx1 operon far from the attachment site, indicating that it became bacteriophage associated in the distant past (19). O’Brien et al. reported that another STEC O157:H7 strain, 933, was lysogenized with two Stx-converting phages, designated 933J and 933W; phage 933J was indistinguishable from the Stx-converting phage H19A of E. coli H19 (33). They also reported that E. coli 933 makes both Stx1 and Stx2 and that Stx1 is encoded by phage 933J while Stx2 is encoded by phage 933W (49). Subsequently, we also reported that STEC O157:H7 strain 83-1386 or J-2 was lysogenized with Stx1- or Stx2-converting phage and that the phages were similar to those of H19B and 933W, respectively (23, 59). In summary, two Stx1-converting phages, represented by H19B and 933J, and one Stx2-converting phage, represented by 933W, have been reported in the literature to date (35).
Interestingly, it has been reported that none of the Stx-producing S. dysenteriae type I strains or Stx2 variant-producing E. coli strains possess the toxin-converting phages. In fact, DNA hybridization analysis with probes specific for toxin-converting phage DNA revealed that several STEC and edema disease-causing strains, but not Shigella strains, possessed H19A- or 933W-related phage sequences, although the mechanism of infection of the Stx-converting phages in E. coli strains remains obscure (31). Therefore, these studies led us to investigate whether the production of Stx1 or Stx2 from STEC strains isolated from the outbreaks in Japan are associated with Stx-converting phages.
In this study, we isolated two distinct Stx2-converting phages, designated Stx2Φ-I and Stx2Φ-II, from clinical strains of STEC associated with the outbreak in Japan in 1996. Examination of the phage plaque sizes, the restriction endonuclease maps, the specificity of lysogenic immunity, the osmotic regulation of infection, and the receptors indicated that Stx2Φ-I and Stx2Φ-II are different in their infective properties. The possibility of diverse host E. coli strains acquiring Stx2-converting phages and its implications are discussed.
MATERIALS AND METHODS
Bacterial strains, growth media, and plasmid construction.
The bacterial strains and plasmids used in this study are listed in Table 1. The growth media used were Luria-Bertani (LB) broth prepared with half the usual amount of NaCl and supplemented with 2.5 mM CaCl2. When necessary, ampicillin, kanamycin, tetracycline, or chloramphenicol was added at 100, 30, 50, or 50 μg/ml, respectively. pMAW302 and pMAW320 were constructed by ligating an 1,800-bp EcoRI fragment encompassing the nucleotides from position 303 upstream of the 5′ end of fadL to position 153 downstream from the 3′ end (4), which was amplified by PCR with the primers 5′-GAATTCCGGAAAGTGCTGCTCCAGTTGTTAA-3′ and 5′-GAATTCCTGTGGATACCGCTTATTGATTTGA-3′. Both primers contained an EcoRI site. pMAW301 was constructed by ligating a 1,600-bp EcoRI fragment encompassing the nucleotides from position 100 upstream of the 5′ end of lamB to position 160 downstream from the 3′ end (5), which was amplified by PCR with the primers 5′-GAATTCTCGACTGCATAAGGAGCCGGGCGTT-3′ and 5′-GAATTCTTCAGATAATGACAACCTGTTTTTTA-3′. Both primers contained an EcoRI site.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype and relevant characteristic(s) | Source or reference |
|---|---|---|
| Strains | ||
| C600 | supE44 hsdR thi-1 thr-1 leuB6 lacY1 tonA21 | 41 |
| XL1-Blue | Host strain for cloning | Strategene |
| Okayama O-27 | Clinical isolate of E. coli O157:H7 | T. Takeda |
| Morioka V526 | Clinical isolate of E. coli O157:H7 | T. Takeda |
| Sakai V443 | Clinical isolate of E. coli O157:H7 | T. Takeda |
| Osaka V743 | Clinical isolate of E. coli O157:H7 | T. Takeda |
| Yokohama 97.4-C | Clinical isolate of E. coli O157:H7 | T. Takeda |
| C600(Stx2Φ-I) | C600 lysogenized with Stx2Φ-I; Stx2+ | This study |
| C600(Stx2Φ-II) | C600 lysogenized with Stx2Φ-II; Stx2+ | This study |
| C600(933W) | C600 lysogenized with phage 933W; Stx2+ | A. D. O’Brien |
| RAM191 | ΔompC178 zei-198::Tn10 | 28 |
| RK4786 | ompF::Tn5 | 15 |
| RS3338 | fadL71::Tn10 fadR | 37 |
| GS20 | lamB20::Tn5 | T. J. Silhavy |
| MW47 | C600 lamB20::Tn5 transductant of GS20 donor | This study |
| MW72 | C600 fadL71::Tn10 transductant of RS3338 donor | This study |
| MW78 | MW72 lamB20::Tn5 transductant of GS20 donor | This study |
| Plasmids | ||
| pUC119 | Cloning vector | Takara |
| pMAW301 | Vector pUC119 cloned C600 lamB gene | This study |
| pMAW302 | Vector pUC119 cloned C600 fadL gene | This study |
| pSU18 | pACYC184-derived cloning vector | 3 |
| pMAW320 | Vector pSU18 cloned C600 fadL gene | This study |
| pQE30 | Vector for His6-tagged recombinant protein | Qiagen |
| pMAW311 | Vector pQE30 cloned C600 fadL gene | This study |
| pMAW312 | Vector pQE30 cloned C600 lamB gene | This study |
Genetic procedures.
Transformation was performed as described by Dagert and Erlich (6). Generalized transduction with bacteriophage P1 was carried out as described by Miller (27).
Preparation of Stx2-converting phages from STEC strains.
The method for the preparation of Stx2-converting phages from STEC strains has been described previously (23, 59). Each STEC strain was grown at 37°C for 2 h in LB broth supplemented with 2.5 mM CaCl2. The cells were harvested by centrifugation, suspended in saline, and irradiated with UV light. The irradiated bacteria were inoculated into the same medium and incubated at 37°C for 2 h. A few drops of chloroform was added to the culture, and the supernatant obtained by centrifugation was sterilized by being passed through a Millipore (Bedford, Mass.) filter membrane (pore size, 0.45 μm). The sterilized supernatant was mixed with a fresh culture of E. coli C600, added to LB soft agar, and poured onto an LB hard-agar plate. Lysogenized bacteria were isolated from each plaque formed on the plate, and the stx2 gene of each lysogenized strain was determined by colony hybridization with an stx2 gene probe (59).
Colony screening by stx gene probe hybridization.
Lysogenized bacteria were cultured on nylon membranes (GeneScreen hybridization transfer membranes [NEN Research Products, Boston, Mass.]) placed over LB agar plates. The plates were incubated at 37°C until bacterial colonies reached approximately 1 mm in diameter. The bacterial colonies on the nylon membrane were lysed, the chromosomal DNA was denatured by published procedures (8), and the nylon membranes were analyzed by Southern hybridization with digoxigenin-labeled stx1 or stx2 gene probes, which contained an intact stx1 or stx2 gene, respectively (23, 59). Southern hybridization was performed by following the instruction manual provided with the DNA labeling and detection kit (Boehringer, Mannheim, Germany). Among the lysogenized strains screened, lysogenized E. coli C600(Stx2Φ-I) and C600(Stx2Φ-II), which were isolated from Okayama O-27 and Morioka V526, respectively, were used to isolate Stx2Φ-I and Stx2Φ-II.
ELISA.
A previously developed sensitive enzyme-linked immunosorbent assay (ELISA) (36) that can detect as little 50 pg of Stx2 per ml was used for the toxin assay. Filter-sterilized culture supernatants and bacterial lysates obtained by sonication were tested by the ELISA. Polystyrene beads coated with anti-Stx2 were used as the solid phase. Fab′ of anti-Stx2 conjugated with horseradish peroxidase by the maleimide method (20) was used as the second antibody, and the substrate was tetramethylbenzidine.
Assay for cytotoxicity.
Assay for cytotoxicity to Vero cells was performed as described previously (32). Briefly, Vero cells were grown in Eagle’s minimal essential medium supplemented with 2% fetal calf serum, 0.4% glucose, 0.15% sodium bicarbonate, and 0.003% phenol red. Cytotoxicity was assayed in wells of a Falcon microtiter plate (Becton Dickinson, Franklin Lakes, N.J.). About 104 cells in 0.15 ml of growth medium were seeded into each well. Filter-sterilized culture supernatants and bacterial lysates obtained by sonication were tested for cytotoxicity. Test samples (0.02 ml) were added to each well and incubated under 5% CO2 in air at 37°C. The cells were observed microscopically for 7 days. The cytotoxic titers were determined by using 10-fold serial dilutions; the highest toxin dilution that caused lysis of 50% of the cell monolayer was taken as the titer of each toxin.
Restriction mapping and cloning.
Restriction endonucleases were purchased from Takara Shuzo (Tokyo, Japan), and digestions were performed by following the instructions of the manufacturer. Phage lambda DNA digested with HindIII or a 1-kb DNA ladder (GIBCO BRL, Rockville, Md.) was used as a molecular weight standard. The phage DNA was digested with BamHI or XhoI. These digested fragments were ligated with BamHI or XhoI-digested pBluescript (Stratagene, La Jolla, Calif.) and were transformed into E. coli XLI-Blue. Finally, all digested fragments were cloned. Restriction mapping was performed by a variety of methods, including double digestion, digestion of isolated fragments, Southern hybridization, and partial sequencing.
DNA sequencing.
Nucleotide sequencing was carried out by the chain termination technique of Sanger et al. (44) with the dye terminator kit (Applied Biosystems Inc., Norwalk, Conn.). The universal T3 and T7 primers and synthetic oligonucleotide primers were used for sequencing in a walking strategy. Oligonucleotide primers for sequencing were synthesized on an Oligo1000M DNA synthesizer (Beckman, Palo Alto, Calif.) and purified by reverse-phase high-pressure liquid chromatography (Waters, Milford, Mass.). The DNA sequence was obtained with an ABI 377 automated sequencer (Applied Biosystems Inc.).
Phage sensitivity testing and phage neutralization.
Phage sensitivity was quantitated by placing the test culture, which was incubated with 10-fold-serially diluted portions of the phage, in an overlay on LB agar plates, and the plates were incubated at 37°C for 18 h. The PFU were calculated by counting the plaque numbers (41).
(i) Infection of Stx2-converting phage under high-osmolarity conditions.
Strain C600 was cultured in LB broth samples supplemented with various concentrations of NaCl or sucrose, which were used as high-osmolarity media. The bacterial culture (5 × 107 cells) was incubated with 5 × 103 PFU of Stx2-converting phage in a volume of 0.2 ml. After incubation at 37°C for 60 min, the samples were plated to determine the infecting phage.
(ii) Stx2-converting phage neutralization by the outer membrane fraction.
Outer membrane were prepared from 50 ml of cells grown to an optical density at 600 nm of 1.0 in LB broth essentially by the method of Manning et al. (26). The insoluble fraction obtained after extraction of whole-cell envelopes with 2% Triton X-100 and 5 mM MgCl2 was called the outer membrane fraction, and it was suspended in distilled water and stored at −20°C. The outer membrane fraction (100 μg/ml) was incubated with 5 × 103 PFU of Stx2-converting phage in a volume of 1 ml. After incubation at 37°C for 60 min, 0.2-ml samples were plated with strain C600 to determine the plaque number of surviving phage.
(iii) Stx2-converting phage neutralization by His6-tagged FadL or LamB protein.
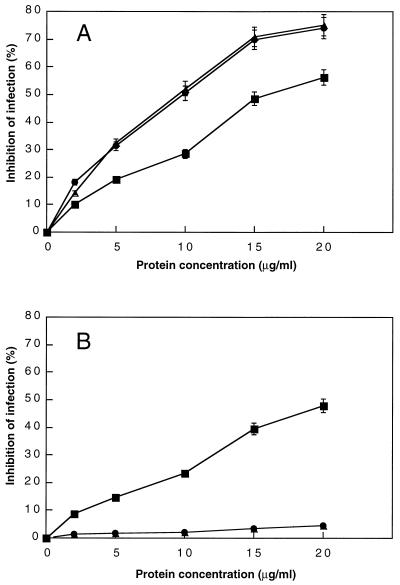
A fusion protein purification was performed as described previously (53). A fusion protein of FadL and LamB tagged with six histidine residues at the N terminus was constructed by using the QIAexpress system with pQE-30 plasmid (Qiagen, Valencia, Calif.). The FadL-LamB fusion protein purified by Ni-nitrilotriacetic acid chromatography (Qiagen) was used for the Stx2-converting phage neutralization experiments described in Fig. 5. Purified fusion protein was incubated with 5 × 103 PFU of Stx2-converting phage in a volume of 1 ml. After incubation at 37°C for 60 min, 0.2-ml samples were plated with strain C600 to determine the plaque number of surviving phage.
FIG. 5.
Effect of FadL and LamB on infection of E. coli C600 by Stx2-converting phages. Stx2Φ-I (•), Stx2Φ-II (■), and 933W (▴) (5 × 103 PFU) were treated with various concentrations of purified His6-tagged FadL (A) or LamB (B) fusion protein in a volume of 1 ml. After incubation at 37°C for 60 min, 0.2-ml samples were plated with strain C600 to determine the inhibition of phage infection. The data shown are the means of triplicate experiments.
RESULTS
Isolation of Stx2-converting phages from STEC strains.
Five STEC O157:H7 strains associated with different outbreaks in Japan in 1996 (Table 1) were used to search for Stx2-converting phages. Each of the representative strains used produced large amounts of Stx1 and Stx2 (data not shown). In a typical experiment, approximately 500 plaques formed on a plate seeded with C600 by UV induction from a STEC strain such as Morioka V526. To isolate Stx1- or Stx2-converting phages, we excised some of the plaques, purified them on LB agar plates, and examined them for the presence of stx1 or stx2 genes by colony hybridization with stx1 or stx2 gene probes (see Materials and Methods). The yield of plaques that gave rise to stx1- and stx2-positive clones obtained from Okayama O-27 or Yokohama 97.4-C was 2 to 10%, and the yield of plaques from Morioka V526 was 5 to 10%. Interestingly, the morphology of the plaques which gave rise to the stx1-positive clones was uniform, while those which gave the stx2-positive clones included plaques with diameters of 1 and 3 mm (Fig. 1). We therefore designated the two representative phages which hybridized with the stx2 probe Stx2Φ-I (3-mm plaque) and Stx2Φ-II (1-mm plaque), and these were derived from Okayama O-27 and Morioka V526, respectively (Fig. 1).
FIG. 1.
Forms of plaques on a lawn of E. coli C600 infected with Stx2Φ-I and Stx2Φ-II. The C600 culture (5 × 107 cells) was incubated with 5 × 103 PFU of Stx2Φ-I (A) or Stx2Φ-II (B) at 37°C for 60 min. These mixtures were plated to determine the form of the phages and incubated at 37°C for 18 h. Bar, 10 mm.
Characterization of Stx2-converting phages.
To assess the Stx2 activity in Stx2Φ-I and Stx2Φ-II, levels of Stx2 protein present in the whole bacterial lysates or culture supernatants of C600 lysogenized with Stx2Φ-I or Stx2Φ-II were investigated by the polystyrene bead ELISA with anti-Stx2 antibody or by the cytotoxicity assay using Vero cells. As shown in Table 2, similar amounts of Stx2 were expressed from C600(Stx2Φ-I) and C600(Stx2Φ-II), although the level of intracellular Stx2 production was half of the level in Okayama O-27 or Morioka V526 cells. Although some Stx2 was secreted from the two C600 lysogens into the culture supernatants, the level of each was 1/10 of that secreted from the parental O157:H7 strains. This result was also reproducible in the Vero cell cytotoxicity assay; however, the levels of Stx2 secreted into the culture supernatants of C600(Stx2Φ-I) and C600(Stx2Φ-II) were further reduced compared with those of Okayama O-27 and Morioka V526 (Table 2). These data suggest that Stx2Φ-I and Stx2Φ-II encode Stx2-associated toxin and that in Okayama O-27 or Morioka V526, the Stx2 protein can be secreted into the culture supernatants more efficiently than in the respective lysogenized C600 strains.
TABLE 2.
Stx2 production and cytotoxicity of original and Stx2-converting phage-lysogenized strains
| Strain | Stx2 production (ng/ml) by:
|
CD50c (Vero cell assay) for:
|
||
|---|---|---|---|---|
| Cella | Supb | Cell | Sup | |
| Okayama O-27 | 62 | 122 | 105 | 107 |
| Morioka V526 | 74 | 120 | 106 | 107 |
| C600 | NDd | ND | <10 | <10 |
| C600(Stx2Φ-I) | 32 | 12 | 104 | 103 |
| C600(Stx2Φ-II) | 28 | 10 | 104 | 103 |
Supernatant obtained by centrifugation of cell sonicate of an overnight culture.
Supernatant of an overnight culture.
CD50, highest toxin dilution that caused lysis of 50% of the cell monolayer.
ND, not detectable.
Relationship of Stx2-converting phages to 933W.
To assess the relationship between Stx2Φ-I or Stx2Φ-II and 933W, which is the sole Stx2-converting phage known that has been isolated from O157:H7 (933 strain) (33), we examined the three phages for their lysogenic immunity. As shown in Table 3, Stx2Φ-I and Stx2Φ-II showed no immunity to each other whereas Stx2Φ-I, but not Stx2Φ-II, showed immunity to 933W, suggesting that Stx2Φ-I is closely related to 933W but not to Stx2Φ-II.
TABLE 3.
Capacity of Stx2-converting phages to form plaques on various E. coli hosts
| Strain | Phage titer
|
||
|---|---|---|---|
| Stx2Φ-I | Stx2Φ-II | 933W | |
| C600 | 2.4 × 107 | 2.6 × 107 | 3.9 × 107 |
| C600(Stx2Φ-I) | NDa | 2.0 × 107 | ND |
| C600(Stx2Φ-II) | 2.5 × 107 | ND | 3.3 × 107 |
| C600(933W) | ND | 2.5 × 107 | ND |
ND, not detectable.
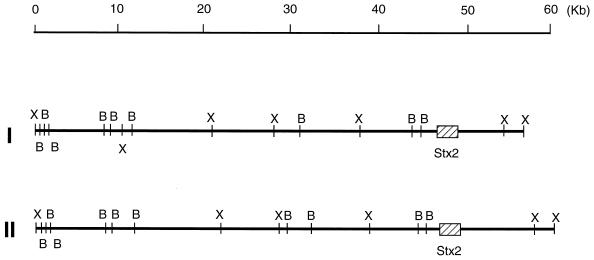
To determine the structures of Stx2Φ-I and Stx2Φ-II, we constructed restriction maps of the phage DNA including the location of the two stx2 genes as described in Materials and Methods. As shown in Fig. 2, although the restriction maps are similar, the full length of Stx2Φ-II was 3 kb longer than Stx2Φ-I, which resulted mostly from the differences of each of the BamHI-XhoI fragments containing the stx2 gene. In this study, we sequenced the two stx2 genes in Stx2Φ-I and Stx2Φ-II and found that the two stx2 sequences were identical to each other and that they were also identical to the stx2 gene from 933W (11, 21, 22, 54). The open reading frames containing the stx2 gene of Stx2Φ-I and Stx2Φ-II were located downstream of p gene of phage λ, as is the case for the stx2 gene in 933W (7, 45).
FIG. 2.
Restriction endonuclease maps of Stx2Φ-I (I) and Stx2Φ-II (II). Areas of the stx2 gene are indicated by solid boxes. Abbreviations: B, BamHI; X, XhoI.
Effect of osmolarity on phage infection.
To further characterize Stx2Φ-II, we examined whether infection of E. coli K-12 by the phages was affected by changes in the osmolarity of LB broth, since the infection efficiency of Stx-converting phages has been shown to be affected by the osmolarity of the culture medium for growing the indicator E. coli strain (33). C600 was grown in LB broth containing various concentrations of NaCl (0 to 0.6 M) or sucrose (0 to 30%), the bacteria were infected with 5 × 103 PFU of Stx2-converting phages for 1 h in the media, and the numbers of infected phages were measured as described in Materials and Methods. As shown in Fig. 3, the infection efficiency of Stx2Φ-I, Stx2Φ-II, or 933W decreased as the concentration of NaCl (Fig. 3A) or sucrose (Fig. 3B) increased. Therefore, the effects of an increase in the osmolarity on the infection efficiency of Stx2Φ-I and 933W are similar and are greater than the effects on the infection efficiency of Stx2Φ-II.
FIG. 3.
Effect of osmolarity on the infection of Stx2-converting phages. E. coli C600 was cultured in LB broth supplemented with various concentrations of NaCl (A) or sucrose (B), and the bacterial culture (5 × 107 cells) was incubated with 5 × 103 PFU of Stx2Φ-I (•), Stx2Φ-II (■), or 933W (▴) in a volume of 0.2 ml. After incubation at 37°C for 60 min, the samples were plated and incubated at 37°C for 18 h to determine phage infectivity. The data shown are the means of triplicate experiments.
Involvement of outer membrane-associated proteins of E. coli in Stx2Φ-II infection.
Stx-converting phages are lambdoid phages (7, 18, 19, 45), and therefore the involvement of the lamB gene (coding for the LamB protein) in the infection of C600 by Stx2Φ-II was checked. The lamB20::Tn5 mutation of GS20 was introduced by P1 phages into C600, and one of the lamB20::Tn5 transductants, designated MW47, was examined for phage sensitivity. Although MW47 (lamB) was sensitive to Stx2Φ-I and 933W, its sensitivity to Stx2Φ-II was greatly diminished (to 0.1% of that to Stx2Φ-I and 933W) (Table 4). The reduced efficiency of Stx2Φ-II to infect MW47 (lamB) was restored upon introduction of pMAW301 (the cloned lamB gene in pUC119), suggesting that the LamB protein is important for Stx2Φ-II infection of C600.
TABLE 4.
Average plating efficiencies of Stx2-converting phage
| Strain | Genotype and characteristics | Phage titer
|
||
|---|---|---|---|---|
| Stx2Φ-I | Stx2Φ-II | 933W | ||
| C600 | Reference | 2.4 × 107 | 2.6 × 107 | 3.9 × 107 |
| MW47 | lamB | 2.2 × 107 | 3.1 × 103 | 3.2 × 107 |
| MW47(pMAW301) | lamB+ | 2.3 × 107 | 2.8 × 107 | 2.9 × 107 |
| RAM191 | ompC | 1.9 × 107 | 1.9 × 107 | 2.8 × 107 |
| RK4786 | ompF | 2.0 × 107 | 2.2 × 107 | 3.4 × 107 |
| MW72 | fadL | NDa | 7.7 × 103 | ND |
| MW72(pMAW302) | fadL+ | 2.7 × 107 | 2.6 × 107 | 2.9 × 107 |
| MW78 | fadL lamB | ND | ND | ND |
| MW78(pMAW320) | fadL+ lamB | 1.2 × 107 | 9.9 × 103 | 1.5 × 107 |
| MW78(pMAW301) | fadL lamB+ | ND | 1.2 × 104 | ND |
| MW78(pMAW320, pMAW301) | fadL+ lamB+ | 8.2 × 107 | 9.3 × 107 | 8.4 × 107 |
ND, not detectable.
We reasoned that the expression of the phage receptor could be osmolarity regulated, since the infection of C600 by Stx2Φ-I and 933W was greatly affected by changing the osmolarity of the medium used to grow C600. Since the outer membrane porin proteins such as OmpF and OmpC are known to be the receptors of phage T2 and T4, respectively (13, 16, 30) and FadL is known to be the receptor of phage T2 (31), the effects of defined mutations of ompC, ompF, or fadL in E. coli on infection of the three converting phages were examined. Examination of the sensitivity of RAM191 ΔompC178 zei-198::Tn10 (28) and RK4786 ompF::Tn5 (15) mutants to the three Stx2-converting phages showed no significant difference among the phages, suggesting that OmpC and OmpF were not involved in phage infection. In contrast, when a fadL mutation was introduced into C600(MW72), the bacteria become resistant to Stx2Φ-I and 933W and partly resistant to Stx2Φ-II (Table 4). The introduction of pMAW302 (a cloned fadL gene) into MW72 (fadL) restored its sensitivity to Stx2Φ-I and 933W, clearly indicating that the FadL protein plays a crucial role in the infection of E. coli by Stx2Φ-I and 933W.
To further investigate the contribution of FadL and LamB proteins in the infection of E. coli by Stx2-converting phage, a fadL-lamB double mutation was constructed in C600 (MW78 [fadL lamB]) and examined for the extent of resistance to Stx2Φ-II. As shown in Table 4, the sensitivity of MW78 (fadL lamB) to Stx2Φ-II disappeared, but it was almost completely restored upon introduction of both plasmid pMAW320 (a cloned fadL gene in pSU18) and pMAW301 (a cloned lamB gene in pUC119). Introduction of pMAW320 (a cloned fadL gene in pSU18) alone into MW78 (fadL lamB) restored the sensitivity to Stx2Φ-I and 933W but not Stx2Φ-II (Table 4), while the introduction of pMAW301 (a cloned lamB gene in pUC119) into MW78 (fadL lamB) only partly restored the sensitivity to Stx2Φ-II.
Contribution of FadL and LamB protein to Stx2-converting phage infection.
Table 4 shows that a fadL mutation had a significant effect on the efficiency of infection of Stx2-converting phages and that a lamB mutation affected Stx2Φ-II infection of E. coli. This was further demonstrated by using a phage neutralization experiment. Outer membrane fractions prepared from each of the strains C600, MW72 (fadL), MW47 (lamB), and MW78 (fadL lamB) were incubated with Stx2-converting phage at 37°C for 60 min, the phages were plated with C600, and the ineffective phage numbers were assayed as described in Materials and Methods. The outer membrane fraction from C600 efficiently inactivated the infectivity of Stx2Φ-I, Stx2Φ-II, and 933W (Fig. 4, column A). However, the outer membrane fraction from strain MW72 (fadL) was unable to inactivate Stx2Φ-I and 933W (97 and 98% surviving phages, respectively) (column B). Under the same conditions, the outer membrane fraction from MW47 (lamB) could inactivate Stx2Φ-I and 933W completely (column D). In contrast, the outer membrane fractions from strain MW72 (fadL) and MW47 (lamB) could partially inactivate Stx2Φ-II (59 and 46% surviving phages, respectively) (column B or D), and the outer membrane fraction from strain MW78 (fadL lamB) was unable to neutralize the infectivity of all the three phages (column F). Furthermore, upon addition of the His-tagged FadL protein to the phage solutions, the infective capacity of Stx2Φ-I and 933W decreased with increases of the protein concentration. In fact, at a concentration of 20 μg/ml, the inhibition of the ineffective capacity of Stx2Φ-I and 933W to C600 reached the maximum point, at which 75% of the original phage infectivity (zero) was blocked by the His-tagged FadL protein (Fig. 5A). In contrast, inhibition of the infective capacity of Stx2Φ-II decreased partially upon addition of the His-tagged FadL or LamB protein (56% for His-tagged FadL and 47% for LamB) (Fig. 5). These results strongly suggest that the receptor for Stx2Φ-I and 933W was the FadL protein and that for Stx2Φ-II consisted of the both FadL and LamB proteins.
FIG. 4.
Neutralization of Stx2-converting phages by the outer membrane fractions of various bacterial strains. The insoluble fraction obtained after extraction of whole-cell envelopes with 2% Triton X-100 and 5 mM MgCl2 is the outer membrane fraction. The outer membrane fraction (100 μg/ml) was incubated with 5 × 103 PFU of Stx2Φ-I (lanes 1), Stx2Φ-II (lanes 2), and 933W (lanes 3) in a volume of 1 ml. After incubation at 37°C for 60 min, 0.2-ml samples were plated with strain C600 to determine the plaque number of surviving phage. The data shown are the means of triplicate experiments. The strains from which the outer membrane fraction was extracted are as follows: A, C600; B, MW72; C, MW72(pMAW302); D, MW47; E, MW47(pMAW301); F, MW78; G, MW78(pMAW320, pMAW301).
DISCUSSION
Stx1 and Stx2 are the major virulence factors of STEC and are responsible for the symptoms of bloody diarrhea and HUS. The stx1 and stx2 genes so far reported have been found as a part of the lambdoid phage genome, thus allowing the toxin genes to spread easily from one E. coli strain to another. It is therefore important to investigate the distribution of Stx-converting phages in STEC strains associated with outbreaks and to understand their infective properties. In this study, we attempted to isolate Stx-converting phages from five STEC strains that were associated with different outbreaks in Japan in 1996 and succeeded in identifying two different types of Stx2-converting phages, designated Stx2Φ-I and Stx2Φ-II.
Detailed characterization of Stx2Φ-I and Stx2Φ-II showed that the stx2 gene of Stx2Φ-I and Stx2Φ-II was identical to that of 933W, the sole known Stx2-converting phage (34). Furthermore, it was found that Stx2Φ-I is closely related to 933W but not to Stx2Φ-II and that Stx2Φ-II is a novel Stx2-converting phage. Our conclusion was drawn from the following results. The plaques of Stx2Φ-II developed in C600 were significantly smaller than those of Stx2Φ-I, for which the morphology of plaques resembled that of 933W. Although the full length of the Stx2Φ-I genome was similar to that of 933W (7, 34, 45, 49), it was approximately 3 kb smaller than that of Stx2Φ-II (Fig. 2). The phage infection immunity displayed by Stx2Φ-I, Stx2Φ-II, and 933W revealed that Stx2Φ-I and 933W belong to the same immunity group but that Stx2Φ-II does not. In support of this view, the effect of changes in the osmolarity of the medium on the infection of C600 by Stx2Φ-I or 933W was different from that on infection by Stx2Φ-II (Fig. 5). The differences would result from different expression of the outer membrane proteins used as the phage receptors on the host E. coli K-12 in media with different osmolarities. In fact, the infectivity of Stx2Φ-I and 933W was strongly dependent upon the FadL protein expressed on C600, while that of Stx2Φ-II was dependent on both LamB and FadL expression (Table 4). The different proteins involved in the phage infection of E. coli were also confirmed by performing phage neutralization experiments with outer membrane fractions of the isogenic E. coli strains with defined lamB, fadL, or lamB fadL mutations or by using the purified His6-tagged FadL or LamB protein. These data clearly indicate that Stx2Φ-I and Stx2Φ-II are not identical to each other but have diverged considerably.
Most double-stranded-DNA-tailed bacteriophages of enteric bacteria have been assigned to one of several phage groups or so-called quasi-species, where phages of the same group are considered to have a common gene pool. Common to the tailed phages is a highly structured genome composed of a specifically ordered set of genetic modules (43). The order of the functional genetic modules is conserved among members of a phage group and sometimes also between unrelated phage groups. A recent study of 12 lambdoid phage genomes illustrated that an individual phage genome can be considered to be a particular combination of “alleles” of genetic modules that are available in the gene pool of that phage group (17). Two recently published studies documented a surprisingly promiscuous exchange of gene segments across phage group boundaries (12, 42). In this context, it is tempting to speculate that the different requirements of the outer membrane of E. coli K-12, as mentioned above, would be reflected by different compositions of the tail proteins, since the FadL and LamB proteins have been shown to be the receptors for the T2 and λ phages, respectively (29, 40). Taking the above notion into account, we assumed that recombination of tail fiber gene segments between the Stx2-converting phages and other phages such as T2 or λ phages could have occurred. Rearrangement of the tail fibers of Stx2-converting phages with those of some other phages through exchange of part of the tail genes would be beneficial for enhancing the phage host range, resulting in the emergence of various STEC strains.
In this study, we observed that secretion of Stx2 into the culture supernatant from C600 lysogenized with Stx2Φ-I or Stx2Φ-II was greatly diminished compared with that from the STEC strains Okayama O-29 and Morioka V526, as determined by ELISA with anti-Stx2 antibody (Table 2). This is reminiscent of our previous report that secretion of Stx2 from C600 carrying a cloned stx2 gene was greatly reduced compared with that from the parental STEC strain (60). Thus, these data suggest that the functions required for the secretion of Stx2 are not encoded by the phage itself but, rather, may be encoded by the chromosome of STEC. Although the precise secretion system still remains to be elucidated, it is possible that the genes required for the secretion of Stx2 protein are located on some chromosomal region unique to the STEC strains. Indeed, the recent whole genomic sequencing of some of the STEC strains has indicated that STEC strains possess at least 1.5 Mb of additional DNA sequence compared to E. coli K-12 (13a). Alternatively, the increased secretion of Stx2 from the STEC strains examined in this study may have resulted from the additional copy of stx2 genes, as indicated by Schmitt et al. (46), who showed that STEC strains had two copies of the stx2 gene. Indeed, the STEC strains examined in this study also possess two copies of the stx2 gene, one in Stx2Φ-I or Stx2Φ-II and the other located somewhere in the chromosome (data not shown). In any case, we must await the results of further studies to elucidate the precise secretion system involved in Stx2 secretion from STEC strains.
STEC strains associated with bloody diarrhea and HUS can be detected by different methods. The methods that have been used to subtype E. coli O157:H7 strains include Stx genotype determination (31), plasmid profile analysis (38, 55), multilocus enzyme electrophoresis (56), antimicrobial susceptibility (50), bacteriophage typing (1), random amplified polymorphic DNA fingerprinting (51), and genomic DNA restriction fragment length polymorphism analysis (39). In addition, pulsed-field gel electrophoresis has recently been successfully used in the determination of the molecular epidemiology of STEC strains (2). We have also developed a detection method for STEC strains that involves a sensitive bead ELISA and PCR with common and specific primers for various Stxs (57). The detailed information on Stx2Φ-I or Stx2Φ-II provided by this study would be useful for epidemiological studies in the future. By applying the facts to the epidemiological classification of STEC strains, we can successfully identify the origin of each outbreak by tracing the route of the food contamination. In this sense, the development of a monitoring system for the distribution of Stx-converting phages in members of the Enterobacteriaceae should be an important step. In this context, we are currently sequencing the whole genomes of Stx2Φ-I and Stx2Φ-II and seeking phage-specific sequences to develop diagnostic DNA probes.
ACKNOWLEDGMENTS
We thank G. Balakrish Nair for critical reading of the manuscript, Tae Takeda for providing STEC strains, and Hideo Hayashi for valuable discussion.
M. Watarai is the recipient of a Research Fellowship of the Japan Society for the Promotion of Science for Young Scientists. This study was supported by the Organization for Pharmaceutical Safety and Research (OPSR).
REFERENCES
- 1.Ahmed R, Bopp C, Borczyk A, Kasatiya S. Phage-typing scheme for Escherichia coli serotype O157:H7. J Infect Dis. 1987;155:806–809. doi: 10.1093/infdis/155.4.806. [DOI] [PubMed] [Google Scholar]
- 2.Barrett T J, Lior H, Green J H, Khaharia R, Wellis J G, Bell B P, Greene K D, Lewis J, Griffin P M. Laboratory investigation of a multistate food-borne outbreak of Escherichia coli O157:H7 by using pulsed-field gel electrophoresis and phage typing. J Clin Microbiol. 1994;32:1013–1017. doi: 10.1128/jcm.32.12.3013-3017.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartolomé B, Jubete Y, Martinez E, de la Cruz F. Construction and properties of a family of pACYC184-derived cloning vectors compatible with pBR322 and its derivatives. Gene. 1991;102:75–78. doi: 10.1016/0378-1119(91)90541-i. [DOI] [PubMed] [Google Scholar]
- 4.Black P N. Primary sequence of the Escherichia coli fadL gene encoding an outer membrane protein required for long-chain fatty acid transport. J Bacteriol. 1991;173:435–442. doi: 10.1128/jb.173.2.435-442.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clement J M, Hofnung M. Gene sequence of the lambda receptor, an outer membrane protein of E. coli K12. Cell. 1981;27:507–514. doi: 10.1016/0092-8674(81)90392-5. [DOI] [PubMed] [Google Scholar]
- 6.Dagert M, Erlich S D. Prolonged incubation in calcium chloride improves the competence of Escherichia coli. Gene. 1979;6:23–28. doi: 10.1016/0378-1119(79)90082-9. [DOI] [PubMed] [Google Scholar]
- 7.Datz M, Janetzki-Mittmann C, Franke S, Gunzer F, Schmidt H, Karch H. Analysis of the enterohemorrhagic Escherichia coli O157:H7 DNA region containing lambdoid phage gene p and Shiga-like toxin structural genes. Appl Environ Microbiol. 1996;62:791–797. doi: 10.1128/aem.62.3.791-797.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis L G, Dibner M D, Battey J F. Basic methods in molecular biology. New York, N.Y: Elsevier Science Publishing Co.; 1986. [Google Scholar]
- 9.Gannon V P J, Teerling C, Masrei S A, Gyles C L. Molecular cloning and nucleotide sequence of another variant of the Escherichia coli Shiga-like toxin II family. J Gen Microbiol. 1990;136:1125–1135. doi: 10.1099/00221287-136-6-1125. [DOI] [PubMed] [Google Scholar]
- 10.Griffin P M, Tauxe R V. The epidemiology of infections caused by Escherichia coli O157:H7, other enterohemorrhagic E. coli, and the associated hemolytic uremic syndrome. Epidemiol Rev. 1991;13:60–98. doi: 10.1093/oxfordjournals.epirev.a036079. [DOI] [PubMed] [Google Scholar]
- 11.Gyles C L, DeGrandis S A, MacKenzie C, Brunton J L. Cloning and nucleotide sequence analysis of the genes determining verocytotoxin production in a porcine edema disease isolate of Escherichia coli. Microb Pathog. 1988;5:419–426. doi: 10.1016/0882-4010(88)90003-4. [DOI] [PubMed] [Google Scholar]
- 12.Haggard-Ljungquist E, Halling C, Calendar R. DNA sequences of the tail fiber genes of bacteriophage P2: evidence for horizontal transfer of tail fiber genes among unrelated bacteriophages. J Bacteriol. 1992;174:1462–1477. doi: 10.1128/jb.174.5.1462-1477.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hantke K. Major outer membrane proteins of E. coli K12 serve as receptors for the phages T2 (protein 1) and 434 (protein 1) Mol Gen Genet. 1978;164:131–135. doi: 10.1007/BF00267377. [DOI] [PubMed] [Google Scholar]
- 13a.Hayashi, H., and H. Shinagawa. Personal communication.
- 14.Head S C, Karmali M A, Roscoe M E, Petric M, Strockbine N A, Wachsmuth I K. Serological differences between verocytotoxin 2 and Shiga-like toxin II. Lancet. 1988;ii:571. doi: 10.1016/s0140-6736(88)90228-0. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 15.Heller K, Mann B J, Kadner R J. Cloning and expression of the gene for the vitamin B12 receptor protein in the outer membrane of Escherichia coli. J Bacteriol. 1985;161:896–903. doi: 10.1128/jb.161.3.896-903.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henning U, Jahn K. Two-component nature of bacteriophage T4 receptor activity in Escherichia coli K-12. J Bacteriol. 1979;137:664–666. doi: 10.1128/jb.137.1.664-666.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Highton P J, Chang Y, Myers R J. Evidence for the exchange of segments between genomes during the evolution of lambdoid bacteriophages. Mol Microbiol. 1990;4:1329–1340. doi: 10.1111/j.1365-2958.1990.tb00712.x. [DOI] [PubMed] [Google Scholar]
- 18.Huang A, De Grandis S, Friesen J, Karmali M, Petric M, Congi R, Brunton J L. Cloning and expression of the genes specifying Shiga-like toxin production in Escherichia coli H19. J Bacteriol. 1986;166:375–379. doi: 10.1128/jb.166.2.375-379.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang A, Friesen J, Brunton J L. Characterization of a bacteriophage that carries the genes for production of Shiga-like toxin 1 in Escherichia coli. J Bacteriol. 1987;169:4308–4312. doi: 10.1128/jb.169.9.4308-4312.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Imagawa M, Yoshitake S, Ishikawa E, Niitsu Y, Urushizaki I, Kanazawa R, Tachibana S, Nakazawa N, Ogawa H. Development of a highly sensitive sandwich enzyme immunoassay for human ferritin using affinity-purified anti-ferritin labelled with beta-d-galactosidase from Escherichia coli. Clin Chim Acta. 1982;121:277–289. doi: 10.1016/0009-8981(82)90237-6. [DOI] [PubMed] [Google Scholar]
- 21.Ito H, Terai A, Kurazono H, Takeda Y, Nishibuchi M. Cloning and nucleotide sequencing of Vero toxin 2 variant genes from the Escherichia coli O91:H21 isolated from a patient with the hemolytic uremic syndrome. Microb Pathog. 1990;8:47–60. doi: 10.1016/0882-4010(90)90007-d. [DOI] [PubMed] [Google Scholar]
- 22.Jackson M P, Neill R J, O’Brien A D, Holmes R K, Newland J W. Nucleotide sequence analysis and comparison of the structural genes for Shiga-like toxin I and Shiga-like toxin II encoded by bacteriophages from Escherichia coli 933. FEMS Microbiol Lett. 1987;44:109–114. doi: 10.1016/0882-4010(87)90106-9. [DOI] [PubMed] [Google Scholar]
- 23.Kurazono H, Sasakawa C, Yoshikawa M, Takeda Y. Cloning of a Vero toxin (VT1, Shiga-like toxin I) gene from a VT1-converting phage isolated from Escherichia coli O157:H7. FEMS Microbiol Lett. 1987;48:23–26. [Google Scholar]
- 24.Levine M M. Escherichia coli that cause diarrhea: enterotoxigenic, enteropathogenic, enteroinvasive, enterohemorrhagic, and enteroadherent. J Infect Dis. 1987;155:377–389. doi: 10.1093/infdis/155.3.377. [DOI] [PubMed] [Google Scholar]
- 25.MacLeod D L, Gyles C L. Purification and characterization of an Escherichia coli Shiga-like toxin II variant. Infect Immun. 1990;58:1232–1239. doi: 10.1128/iai.58.5.1232-1239.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manning P A, Beutin L, Achtman M. Outer membrane of Escherichia coli: properties of the F sex factor traT protein which is involved in surface exculusion. J Bacteriol. 1980;142:285–294. doi: 10.1128/jb.142.1.285-294.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 28.Misra R, Benson S A. Isolation and characterization of OmpC porin mutants with altered pore properties. J Bacteriol. 1988;170:528–533. doi: 10.1128/jb.170.2.528-533.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morona R, Henning U. New locus (ttr) in Escherichia coli K-12 affecting sensitivity to bacteriophage T2 and growth on oleate as the sole carbon source. J Bacteriol. 1986;168:534–540. doi: 10.1128/jb.168.2.534-540.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mutoh N, Furukawa H, Mizushima S. Role of lipopolysaccharide and outer membrane protein of Escherichia coli K-12 in the receptor activity for bacteriophage T4. J Bacteriol. 1978;136:693–699. doi: 10.1128/jb.136.2.693-699.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Newland J W, Neill R J. DNA probes for Shiga-like toxins I and II and for toxin-converting phages. J Clin Microbiol. 1988;26:1292–1297. doi: 10.1128/jcm.26.7.1292-1297.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Noda M, Yutsudo T, Nakabayashi N, Hirayama T, Takeda Y. Purification and some properties of Shiga-like toxin from Escherichia coli O157:H7 that is immunologically identical to Shiga toxin. Microb Pathog. 1987;2:339–349. doi: 10.1016/0882-4010(87)90076-3. [DOI] [PubMed] [Google Scholar]
- 33.O’Brien A D, Newland J W, Miller S F, Holmes R K, Smith H W, Formal S B. Shiga-like toxin-cinverting phages from Escherichia coli strains that cause hemorrhagic colitis or infantile diarrhea. Science. 1984;226:694–696. doi: 10.1126/science.6387911. [DOI] [PubMed] [Google Scholar]
- 34.O’Brien A D, Marques L R M, Kerry C F, Newland J W, Holmes R K. Shiga-like toxin converting phage of enterohemorrhagic Escherichia coli strain 933. Microb Pathog. 1989;6:381–390. doi: 10.1016/0882-4010(89)90080-6. [DOI] [PubMed] [Google Scholar]
- 35.O’Brien A D, Tesh V L, Donohue-Rolfe A, Jackson M P, Olsnes S, Sandvig K, Lindberg A A, Keusch G T. Shiga toxin: biochemistry, genetics, mode of action, and role in pathogenesis. Curr Top Microbiol Immunol. 1992;180:65–94. doi: 10.1007/978-3-642-77238-2_4. [DOI] [PubMed] [Google Scholar]
- 36.Oku Y, Uesaka Y, Hirayama T, Takeda Y. Development of a highly sensitive bead-ELISA to detect bacterial protein toxins. Microbiol Immunol. 1988;32:807–816. doi: 10.1111/j.1348-0421.1988.tb01442.x. [DOI] [PubMed] [Google Scholar]
- 37.Oliver D B. Identification of five new essential genes involved in the synthesis of a secreted protein in Escherichia coli. J Bacteriol. 1985;161:285–291. doi: 10.1128/jb.161.1.285-291.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ostroff S M, Tarr P I, Neill M A, Lewins J H, Hargrett-Bean N, Kobayashi J M. Toxin genotypes and plasmid profiles as determinants of systemic sequelae in Escherichia coli O157:H7 infection. J Infect Dis. 1989;160:994–998. doi: 10.1093/infdis/160.6.994. [DOI] [PubMed] [Google Scholar]
- 39.Paros M, Tarr P I, Kim H, Besser T E, Hancock D D. A comparison of human and bovine Escherichia coli O157:H7 isolates by toxin genotype, plasmid profile, and bacteriophage lambda-restriction fragment length polymorphism profile. J Infect Dis. 1993;168:1300–1303. doi: 10.1093/infdis/168.5.1300. [DOI] [PubMed] [Google Scholar]
- 40.Randall-Hazelbauer L, Schwartz M. Isolation of the bacteriophage lambda receptor from Escherichia coli. J Bacteriol. 1973;116:1436–1446. doi: 10.1128/jb.116.3.1436-1446.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 42.Sandmeier H, Iida S, Arber W. DNA inversion regions Min of plasmid p15B and Cin of bacteriophage P1: evolution of bacteriophage tail fiber genes. J Bacteriol. 1992;174:3936–3944. doi: 10.1128/jb.174.12.3936-3944.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sandmeier H. Acquisition and rearrangement of sequence motifs in the evolution of bacteriophage tail fibres. Mol Microbiol. 1994;12:343–350. doi: 10.1111/j.1365-2958.1994.tb01023.x. [DOI] [PubMed] [Google Scholar]
- 44.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmidt H, Scheef J, Janetzki-Mittmann C, Datz M, Karch H. An ileX tRNA gene is located close to the Shiga toxin II operon in enterohemorrhagic Escherichia coli O157 and non-O157 strains. FEMS Microbiol Lett. 1997;149:39–44. doi: 10.1111/j.1574-6968.1997.tb10305.x. [DOI] [PubMed] [Google Scholar]
- 46.Schmitt C K, McKee M L, O’Brien A D. Two copies of Shiga-like toxin II-related genes common in enterohemorrhagic Escherichia coli strains are responsible for the antigenic heterogeneity of the O157:H− strain E32511. Infect Immun. 1991;59:1065–1073. doi: 10.1128/iai.59.3.1065-1073.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scotland S M, Smith H R, Rowe B. Two distinct toxins active on Vero cells from Escherichia coli O157. Lancet. 1985;ii:885–886. doi: 10.1016/s0140-6736(85)90146-1. [DOI] [PubMed] [Google Scholar]
- 48.Smith H W, Green P, Parsell Z. Vero cell toxins in Escherichia coli and related bacteria: transfer by phage and conjugation and toxic action in laboratory animals, chickens, and pigs. J Gen Microbiol. 1983;129:3121–3137. doi: 10.1099/00221287-129-10-3121. [DOI] [PubMed] [Google Scholar]
- 49.Strockbine N A, Marques L R M, Newland J W, Smith H W, Holmes R K, O’Brien A D. Two toxin-converting phages from Escherichia coli O157:H7 strain 933 encode antigenically distinct toxins with similar biologic activities. Infect Immun. 1986;53:135–140. doi: 10.1128/iai.53.1.135-140.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Swerdlow D L, Woodruff B A, Brady R C, Griffin P M, Tippen S, Donnell H D, Geldreich E, Payne B J, Meyer A, Wells J G, Greene K D, Bright M, Bean N H, Blake P A. A waterborne outbreak in Missouri of Escherichia coli O157:H7 associated with bloody diarrhea and death. Ann Intern Med. 1992;117:812–818. doi: 10.7326/0003-4819-117-10-812. [DOI] [PubMed] [Google Scholar]
- 51.Wang G, Whittam T S, Berg C M, Berg D E. RAPD (arbitrary primer) PCR is more sensitive than multilocus enzyme electrophoresis for distinguishing related bacterial strains. Nucleic Acids Res. 1993;2157:5930–5933. doi: 10.1093/nar/21.25.5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Watanabe H, Wada A, Inagaki Y. Outbreaks of enterohaemorrhagic Escherichia coli O157:H7 infection by two different genotype strains in Japan, 1996. Lancet. 1996;348:831–832. doi: 10.1016/s0140-6736(05)65257-9. [DOI] [PubMed] [Google Scholar]
- 53.Watarai M, Tobe T, Yoshikawa M, Sasakawa C. Contact of Shigella with host cells triggers release of Ipa invasins and is an essential function of invasiveness. EMBO J. 1995;14:2461–2470. doi: 10.1002/j.1460-2075.1995.tb07243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weinstein D L, Jackson M P, Samuel J E, Holmes R K, O’Brien A D. Cloning and sequencing of a Shiga-like toxin II variant from an Escherichia coli strain responsible for edema disease of swine. J Bacteriol. 1988;170:4223–4230. doi: 10.1128/jb.170.9.4223-4230.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wells J G, Davis B R, Wachsmuth I K, Riley L W, Remis R S, Sokolow R, Morris G K. Laboratory investigation of hemorrhagic colitis associated with a rare Escherichia coli serotype. J Clin Microbiol. 1983;18:512–520. doi: 10.1128/jcm.18.3.512-520.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Whittam T S, Wachsmuth I K, Wilson R A. Genetic evidence of clonal descent of Escherichia coli O157:H7 associated with hemorrhagic colitis and hemolytic uremic syndrome. J Infect Dis. 1988;157:1124–1133. doi: 10.1093/infdis/157.6.1124. [DOI] [PubMed] [Google Scholar]
- 57.Yamasaki S, Lin S, Shirai H, Terai A, Oku Y, Ito H, Ohmura M, Karasawa T, Tsukamoto T, Kurazono H, Takeda Y. Typing of verotoxins by DNA hybridization with poly- and oligonucleotide probes, a bead-enzyme-linked immunosorbent assay, and polymerase chain reaction. Microbiol Immunol. 1996;40:345–352. doi: 10.1111/j.1348-0421.1996.tb01078.x. [DOI] [PubMed] [Google Scholar]
- 58.Yutsudo T, Nakabayashi N, Hirayama T, Takeda Y. Purification and some properties of a Vero toxin from Escherichia coli O157:H7 that is immunologically unrelated to Shiga toxin. Microb Pathog. 1987;3:21–30. doi: 10.1016/0882-4010(87)90034-9. [DOI] [PubMed] [Google Scholar]
- 59.Yutsudo T, Kurazono H, Sasakawa C, Yoshikawa M, Takeda Y. Cloning of a Vero toxin (VT2) gene from a VT2-converting phage isolated from Escherichia coli O157:H7. FEMS Microbiol Lett. 1987;48:273–276. [Google Scholar]