Abstract
Gamma interferon (IFN-γ)-activated macrophages are believed to play a key role in resistance to Babesia bovis through parasite suppression by macrophage secretory products. However, relatively little is known about interactions between this intraerythrocytic parasite and the macrophages of its bovine host. In this study, we examined the in vitro effect of intact and fractionated B. bovis merozoites on bovine macrophage nitric oxide (NO) production. In the presence of IFN-γ, B. bovis merozoites stimulated NO production, as indicated by the presence of increased l-arginine-dependent nitrite (NO2−) levels in culture supernatants of macrophages isolated from several cattle. The merozoite crude membrane (CM) fraction stimulated greater production of NO, in a dose-dependent manner, than did the merozoite homogenate or the soluble, cytosolic high-speed supernatant fraction. Stimulation of NO production by CM was enhanced by as little as 1 U of IFN-γ per ml of culture medium. Upregulation of inducible NO synthase mRNA in bovine macrophages by either B. bovis-parasitized erythrocytes and IFN-γ or CM was also observed. B. bovis-specific T-helper lymphocyte culture supernatants, all of which contained IFN-γ, were also found to induce l-arginine-dependent NO2− production. Supernatants that induced the highest levels of NO also contained biologically active TNF. These results show that B. bovis merozoites and antigen-stimulated B. bovis-immune T cells can induce the production of NO, a molecule implicated in both protection and pathologic changes associated with hemoprotozoan parasite infections.
Bovine babesiosis is an economically important tick-borne disease of cattle that is caused by intraerythrocytic apicomplexan parasites of the genus Babesia. Babesia bovis-infected erythrocytes undergo sequestration by attachment to capillary endothelium in a manner reminiscent of the most severe form of human malaria, caused by Plasmodium falciparum; this results in organ damage, cerebral dysfunction, and pulmonary edema (58). It has been hypothesized that the severe organ abnormalities that occur during acute B. bovis infection, similar to those observed during experimental malaria, are mediated in part by inflammatory cytokines, including gamma interferon (IFN-γ) and tumor necrosis factor (TNF), and nitric oxide (NO) (58).
Although activation of macrophages could lead to immunopathological consequences, macrophages are also believed to be important for immunity to B. bovis and other intraerythrocytic parasites via removal of parasitized erythrocytes by phagocytosis and as antigen-presenting cells (APC) for T-helper (Th) lymphocytes. Furthermore, macrophage secretory products have been shown to inhibit the growth of P. falciparum and B. bovis in vitro (24, 34). When generated by chemical donors or activated macrophages in vitro, NO and its reactive nitrogen intermediate derivatives were shown to inhibit intracellular parasites including Leishmania major, P. falciparum, and B. bovis (22, 23, 27, 40, 53, 54).
Studies in mice with B. microti, P. yoelii, P. vinckei, and P. chabaudi demonstrated that IFN-γ, TNF-α, and TNF-β are important components of immunity to these parasites (3, 15, 32, 44, 47). IFN-γ facilitates the phagocytosis of P. falciparum-infected erythrocytes by human macrophages (33, 35). In addition, parasite-specific, IFN-γ-producing human CD4+ Th-cell clones inhibited the growth of P. falciparum in the presence of adherent peripheral blood mononuclear cells (PBMC) in vitro (18, 37). The protective role of TNF-α appears to depend on the timing of its appearance, which may partially explain the paradoxical roles of TNF in protection and immunopathology. In resistant C57BL/6 mice, TNF-α appeared early during P. chabaudi infection, whereas in susceptible A/J mice, high levels of TNF-α were observed only late in infection, just preceding death (25).
Experiments performed during the past decade showed that malarial parasites and secreted toxins induced TNF-α in human and murine macrophages and inducible NO synthase (iNOS) in murine macrophages. Macrophages exposed in vitro to P. yoelii, P. berghei, or P. falciparum produced TNF-α, which was enhanced by IFN-γ (4, 36, 50, 51). Similarly, P. falciparum extract, in the presence of IFN-γ, stimulated murine macrophages to produce both TNF-α and NO (31, 41). However, the interpretation of these results has been questioned by the recent discovery that many continuously cultured strains of P. falciparum are contaminated with Mycoplasma species (56). Mycoplasma organisms induce TNF-α and other inflammatory mediators in murine, human, and bovine macrophages (28–30, 56), and experiments with Mycoplasma-free P. falciparum are being repeated to verify that the induction of inflammatory cytokines and NO was due to the parasite itself (43).
Several observations support the hypothesis that IFN-γ produced by effector Th cells plays a key role in protection against babesiosis (10). In cattle, IFN-γ regulates B-cell synthesis of the opsonizing immunoglobulin G2 subclass (17). Parasite-specific Th-cell clones isolated from B. bovis-immune cattle produce IFN-γ and TNF (11, 14). Bovine macrophages have upregulated expression of iNOS when activated by IFN-γ in the presence of bacterial LPS (2) or TNF-α (20). Thus, the induction of macrophage iNOS by either B. bovis extracts or antigen-activated T cells would be indicative of macrophage activation and a potential babesiacidal effector mechanism. Conversely, overproduction of NO could also contribute to the pathologic changes associated with infection, including cerebral babesiosis.
This study was undertaken to determine if bovine macrophages produce NO when exposed to B. bovis in the presence or absence of IFN-γ. Potential Mycoplasma contamination of B. bovis cultures was ruled out by a PCR-based assay. We report, for the first time, that bovine macrophages produce NO following in vitro exposure to B. bovis merozoites or a membrane-enriched fraction. The effect of different concentrations of either IFN-γ or B. bovis on NO production was also examined. Furthermore, the functional relevance of B. bovis-specific Th cells that produce IFN-γ and TNF was evaluated by the determining the ability of Th-cell supernatants to stimulate NO production. Induction of iNOS was confirmed by demonstrating reduced nitrite (NO2−) levels in the presence of NG-monomethyl-l-arginine (l-NMMA) and enhanced levels of iNOS steady-state mRNA in macrophages cultured with B. bovis.
MATERIALS AND METHODS
Parasite cultivation and antigen preparation.
The Mexico strain of B. bovis was cultured in bovine erythrocytes under microaerophilic conditions and crude antigens were prepared as previously described (8). CM antigen, which contains pelleted parasite organelles and parasite membranes, was separated from soluble, cytosolic high-speed supernatant (HSS) by lysing the merozoites by two passages through a French pressure cell (SLM Instruments, Inc., Urbana, Ill.) at 1,500 lb/in2 to obtain a homogenate (H) and then by centrifugation at 145,000 × g for 1 h. Control CM antigen was prepared by hypotonic lysis and centrifugation from uninfected bovine erythrocytes (RBC) from the same donor used to cultivate B. bovis. Protein concentrations of parasite antigens were determined by the Bradford assay (Bio-Rad, Hercules, Calif.) with a bovine serum albumin standard. Working dilutions of B. bovis CM and recombinant bovine IFN-γ in complete RPMI tested negative for endotoxin by the Endotect Limlulus amebocyte lysate assay (ICN, Aurora, Ohio), which has a sensitivity of 0.06 to 0.1 ng/ml. In addition, more than 25 samples of B. bovis HSS and CM fractions collected over a period of 4 years and 4 preparations of purified B. bovis DNA were shown to be negative for Mycoplasma by PCR with the Mycoplasma PCR primer set (Stratagene, La Jolla, Calif.) as specified by the manufacturer.
Culture of bovine macrophages.
Macrophages were isolated from PBMC by a modification of a previously described method (38). Peripheral blood was collected from the jugular vein into 60-ml syringes containing 2 ml of 0.5 M EDTA (Gibco BRL, Grand Island, N.Y.) and centrifuged at 1,200 × g for 30 min. Buffy coats were collected and diluted to 30 to 35 ml with 2 volumes of Hanks balanced salt solution (Gibco BRL) containing 2 mM EDTA (HBSS-EDTA), underlayered with 15 ml of Histopaque-1077 (Sigma, St. Louis, Mo.) or Lymphoprep (Nycomed, Oslo, Norway), and centrifuged at 900 × g for 20 min. PBMC were then removed from the interface between the plasma and Ficoll solution, pooled, diluted at least 1:3 with HBSS-EDTA, and centrifuged at 500 × g for 15 min. The pellets containing PBMC were then washed repeatedly at 250 × g until the supernatants were clear, resuspended in complete RPMI 1640 medium, placed in 100-mm polystyrene petri dishes (Becton Dickinson, Franklin Lakes, N.J.) at 1 × 107 to 2 × 107 PBMC/ml (10 ml/dish), and incubated for 1 to 3 h at 37°C in 5% CO2 in air. Nonadherent cells were removed by washing the plates three times with prewarmed (37°C) complete RPMI 1640 medium (8), which contained 10% heat-inactivated fetal bovine serum (HyClone, Logan, Utah), and adherent cells were cultured in 10 ml of complete RPMI at 37°C for an additional 6 to 8 days to allow maturation into macrophages. After approximately 1 week in culture, adherent macrophages were washed once with prewarmed complete RPMI and once with Mg2+- and Ca2+-containing HBSS, and removed by vortexing following incubation at 37°C for 1 h in 0.5 mM EDTA in Mg2+- and Ca2+-free HBSS.
Stimulation of macrophages in vitro.
Macrophages were diluted to 106 cells/ml, and 100 μl/well was plated into quadruplicate wells of 96-well, flat-bottom tissue culture plates (Costar, Cambridge, Mass.) for NO2− assays. Alternatively, cells (3 × 106 cells) were plated into six-well plates for RNA isolation. The cells were left to adhere overnight at 37°C in 5% CO2 in air. The culture medium was then removed and replaced with complete RPMI alone or containing the indicated amounts of antigen prepared from B. bovis-infected or uninfected RBC, which included B. bovis H, CM, or HSS and RBC CM. As a positive control for induction of iNOS, macrophages were also cultured with lipopolysaccharide (LPS) from Escherichia coli O55:B5 (Sigma). Cultures were performed with or without recombinant bovine IFN-γ (Ciba-Geigy; kindly provided by Lorne Babiuk, VIDO, Saskatoon, Canada) and/or 250 μM l-NMMA (CalBiochem, San Diego, Calif.). Macrophages were also incubated with B. bovis-infected RBC which contained approximately 4 or 10% parasitized RBC (PPE) at a 2.5% final packed-cell volume (PCV). As a negative control, uninfected RBC from the same donor cow used for parasite culture were also added at the same final PCV. Supernatants harvested from Th-cell lines specific for B. bovis were also added to macrophages at a final dilution of 75% (vol/vol).
NO2− detection by the Griess reaction.
Macrophage culture supernatants were transferred (50 μl/well) to new 96-well, flat-bottom plates, 50 μl of 1% (wt/vol) sulfanilamide (Sigma) in 2.5% H3PO4 and then 50 μl of 0.1% (wt/vol) naphthylethylenediamine dihydrochloride (Sigma) in 2.5% H3PO4 were added to the supernatants, and the absorbance at 540 nm (A540) was compared to a NaNO2 standard curve. Preliminary studies demonstrated that NO2− measurement by the Griess reaction was optimal after the macrophages were cultured with the appropriate stimulus for 96 h. The results are presented as the mean micromolar concentration of NO2− in quadruplicate cultures ± 1 standard deviation (SD). Student’s one-tailed t test was used to determine statistically significant differences in NO2− production.
Analysis of iNOS mRNA by reverse transcription-PCR.
Total cellular RNA was isolated from macrophage cultures by using the TRIzol reagent RNA isolation method as specified by the manufacturer (Gibco BRL). RNA purity was assessed by the A260/A280 ratio, and integrity was verified by agarose gel electrophoresis. Total cellular RNA (1 μg) was reverse transcribed in a 20-μl reaction mixture containing 5 mM MgCl2, 1× PCR buffer, 1 mM deoxynucleoside triphosphates, 1 U of RNase inhibitor, 2.5 μM oligo(dT)16, and 50 U of reverse transcriptase (RT) (Perkin-Elmer, Branchburg, N.J.). The reactions, performed in a PCR system 2400 thermal cycler (Perkin-Elmer), were performed under the following incubation conditions: 25°C for 10 min, 42°C for 15 min, and 99°C for 5 min; they were repeated at least once. Identical reactions were performed for each sample without RT to verify the absence of genomic DNA.
PCR primers, purchased from Gibco BRL, included bovine iNOS sense primer (5′-TAGAGGAACATCTGGCCAGG-3′) and antisense primer (5′-TGGCAGGGTCCCCTCTGATG-3′), which amplify a 372-bp product (2), and bovine β-actin sense primer (5′-ACCAACTGGGACGACATGGAG-3′) and antisense primer (5′-GCATTTGCGGTGGACAATGGA-3′), which amplify an 890-bp product (16). The actin primer sequences were kindly provided by Gary Splitter, Department of Animal Health and Biomedical Sciences, University of Wisconsin, Madison, Wisconsin. PCRs for bovine iNOS were performed with a 1:50 dilution of the DNA product (5 ng of RNA equivalent). Equivalence of the template was assessed by parallel amplification of bovine β-actin. PCRs for bovine β-actin were performed with a 1:5,000 dilution of the DNA product (0.05 ng of RNA equivalent). Other components of the 50-μl reaction mixture included 2.5 mM MgCl2, 1× PCR buffer, 0.4 mM deoxynucleoside triphosphates, and 1 U of AmpliTaq Gold (Perkin-Elmer). Activation of AmpliTaq Gold required a 10-min preamplification incubation at 94°C, after which cDNA was amplified for 35 cycles consisting of 94°C for 1 min, 60°C for 1 min, and 72°C for 2 min. The final extension was allowed to continue for 10 min. Each PCR product (25 μl) was electrophoresed on a 1% agarose gel containing 0.5 μg of ethidium bromide per ml. PCR products were visualized under UV light and analyzed with the IS1000 gel imaging system (Alpha Innotech, San Leandro, Calif.). Negative controls included simultaneous amplification of reaction mixtures without RT or without template.
T-cell lines and proliferation.
Cell lines were established by culturing 4 × 106 PBMC in 1.5 ml of complete RPMI 1640 medium with B. bovis (Mexico) CM antigen (25 μg/ml) per well in 24-well plates (Costar) as described previously (7). T lymphocytes were subcultured under the same conditions weekly for up to 10 weeks at a density of 5 × 105 cells/well with CM antigen and 2 × 106 irradiated (3,000 rads) autologous PBMC as a source of APC. As previously shown (7), the B. bovis-specific T-cell lines used in the studies reported here contained predominantly CD4+ T cells (48) as shown by flow cytometry analysis. Proliferation assays were performed essentially as described previously (7) with 3 × 104 T cells, 2 × 105 APC, and 1 to 25 μg of antigen per ml; the antigen consisted of either B. bovis CM or control uninfected RBC membranes prepared from the same source of RBC used for in vitro cultivation of B. bovis. The assays were conducted in triplicate, and the mixtures were radiolabeled with 0.25 μCi of [3H]thymidine (Dupont New England Nuclear, Boston, Mass.) during the final 6 h of culture, harvested, and counted in a liquid scintillation counter. The results are presented as the mean cpm ± 1 SD of triplicate cultures.
IFN-γ ELISA and TNF bioassays.
Supernatants were collected from B. bovis-specific CD4+ T-cell lines derived from animals C97, G3, and G6 in culture for 6 weeks (animal G3) or 10 weeks (animals C97 and G6) and after 7 days of stimulation with B. bovis antigen and APC. Supernatants were centrifuged to pellet cellular debris and assayed for IFN-γ with a commercial enzyme-linked immunosorbent assay (ELISA) kit (IDEXX Laboratories, Westbrook, Maine) as specified by the manufacturer. IFN-γ protein was estimated from a standard curve derived with a dilution series of a cloned Th-cell culture supernatant that was shown to contain 400 U of IFN-γ per ml by the vesicular stomatitis virus cytopathic effect reduction assay with Madin-Darby bovine kidney cells (13). Recombinant bovine IFN-γ similarly assayed for vesicular stomatitis virus neutralization contained approximately 0.6 U of biological activity per ng of protein (6).
A biological assay to measure bovine TNF activity by using murine WEHI-164 cells and recombinant TNF-α as a standard has been described previously (11). Cytopathicity was determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) dye reduction assay. TNF titers in the supernatants were compared with a standard murine recombinant TNF-α (Genzyme, Cambridge, Mass.) that had a reported activity of 2 × 106 U/ml and in our assay had a titer of 4.5 × 105 U/ml.
RESULTS
Mycoplasma detection assay.
Aliquots of more than 25 B. bovis CM or HSS preparations and 4 samples of purified B. bovis DNA were assayed for Mycoplasma contamination by PCR with primers that amplify DNA from several different species of Mycoplasma. All the samples were negative, demonstrating that the cultured B. bovis parasites used in these experiments were free of Mycoplasma (data not shown).
l-Arginine-dependent production of NO2− by bovine macrophages.
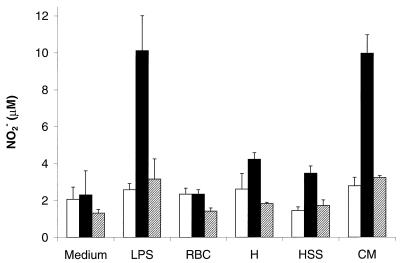
To determine whether B. bovis could stimulate NO production by bovine macrophages, peripheral blood monocyte-derived macrophages were isolated from four cattle and stimulated for 96 h with either LPS or B. bovis antigens with or without bovine IFN-γ. NO production was determined by measurement of NO2−, a stable derivative of NO, levels in culture supernatants by the Griess assay. Although the level of NO2− produced varied among the animals, macrophages isolated from all cattle and stimulated with IFN-γ (500 U/ml) and either LPS (2 μg/ml) or B. bovis CM antigen (100 μg of protein/ml) generated significantly greater levels of NO2− than did macrophages cultured with either IFN-γ or CM alone (Table 1). The NO2− concentration in supernatants from macrophages cultured with IFN-γ plus H, HSS, CM, or LPS were all found to be significantly greater (P < 0.05) than that in supernatants from macrophages cultured with medium, RBC, IFN-γ, or RBC plus IFN-γ (Fig. 1). In this experiment, NO2− production by macrophages incubated with CM and IFN-γ was comparable to that produced by macrophages stimulated by LPS and IFN-γ and was significantly greater (P < 0.001) than that produced by macrophages stimulated with IFN-γ and either H or HSS. Culturing macrophages with or without LPS, RBC, B. bovis, or IFN-γ alone or with RBC plus IFN-γ failed to induce NO2− production. Addition of the l-arginine analog l-NMMA (250 μM) abrogated NO2− generation by macrophages stimulated with IFN-γ in combination with LPS or the B. bovis crude antigen preparations.
TABLE 1.
Macrophages isolated from cattle generate NO in response to B. bovis CM and IFN-γ
| Animal | NO2− concn (μM) in supernatants of macrophages cultured witha:
|
||||
|---|---|---|---|---|---|
| Medium | CM | IFN-γ | CM + IFN-γb | LPS + IFN-γ | |
| G1 | 2.7 ± 0.3 | 5.5 ± 0.7 | 3.5 ± 0.7 | 34.5 ± 5.2** | 63.7 ± 2.9 |
| G4 | 1.9 ± 0.2 | 3.9 ± 1.0 | 4.9 ± 1.4 | 40.8 ± 3.2* | 44.5 ± 6.2 |
| G5 | 1.9 ± 1.1 | 5.2 ± 0.4 | 2.3 ± 0.5 | 10.8 ± 1.2* | 15.3 ± 5.3 |
| G7 | 3.6 ± 0.3 | 11.0 ± 2.0 | 5.6 ± 1.5 | 78.4 ± 9.6** | 94.6 ± 2.4 |
Macrophages were cultured for 96 h in complete RPMI 1640 medium alone or containing CM (100 μg/ml), IFN-γ (500 U/ml), CM plus IFN-γ, or LPS (2 μg/ml) plus IFN-γ. The results are shown as the mean NO2− levels for quadruplicate cultures ± 1 SD.
NO2− levels in supernatants from macrophages cultured with CM plus IFN-γ were all significantly greater (*, P < 0.005; **, P < 0.001) than those in supernatants from macrophages cultured with either IFN-γ or CM alone, by Student’s one-tailed t test.
FIG. 1.
l-Arginine-dependent production of NO2− by macrophages exposed to different B. bovis preparations and IFN-γ. Bovine macrophages were exposed to 100 μg of protein per ml of H, HSS, or CM fractions of B. bovis merozoites or a CM fraction of uninfected RBC, either alone (open bars), with 500 U of IFN-γ per ml (solid bars), or with IFN-γ and 250 μM l-NMMA (hatched bars). Cells treated with medium or LPS (2 μg per ml) served as negative and positive controls, respectively. NO2− levels were determined by the Griess assay. The results are presented as the mean NO2− concentrations for quadruplicate cultures and 1 SD.
NO production by bovine macrophages stimulated with B. bovis and IFN-γ is dose dependent.
To determine if the production of NO was dependent on the concentration of either B. bovis CM or IFN-γ, bovine macrophages were stimulated with 6.25 to 200 μg of CM per ml in the presence of 0, 100, 500, or 1,000 U of IFN-γ per ml (Fig. 2A) or serial dilutions of IFN-γ (ranging from 0 to 1,000 U/ml) in the presence of 100 μg of B. bovis CM per ml (Fig. 2B and C). NO2− production increased proportionally with increasing concentrations of CM when combined with a single concentration of IFN-γ (Fig. 2A). However, there was no apparent difference in NO2− production by macrophages exposed to one concentration of CM and 100, 500, or 1,000 U of IFN-γ per ml. In a separate experiment, similar levels of NO2− were induced by CM and 8 to 1,000 U of IFN-γ per ml whereas CM alone or CM plus IFN-γ plus l-NMMA failed to induce NO2− (Fig. 2B). To further define the minimal amount of IFN-γ required for costimulation with CM, IFN-γ was added from 0.03 to 8.0 U/ml and a dose-dependent effect was achieved (Fig. 2C). These data suggested that very little, and perhaps a biologically relevant amount of, IFN-γ is required to enhance NO production in B. bovis-stimulated macrophages.
FIG. 2.
NO2− production by macrophages is dependent on the concentration of B. bovis CM and IFN-γ. (A) Macrophages were stimulated with 6.25 to 200 μg of CM per ml alone (diamonds) or in the presence of 100 (circles), 500 (triangles), or 1,000 (squares) U of IFN-γ per ml. (B) Macrophages were incubated with B. bovis CM (100 μg per ml) and 0 to 1,000 U of IFN-γ per ml (diamonds), IFN-γ alone (circles), or CM plus IFN-γ in the presence of 250 μM l-NMMA (squares). (C) Macrophages were stimulated with B. bovis CM (100 μg per ml) in the presence of 0.03 to 8 U of IFN-γ per ml. The results are presented as the mean NO2− concentrations for quadruplicate cultures.
B. bovis induces the expression of iNOS mRNA.
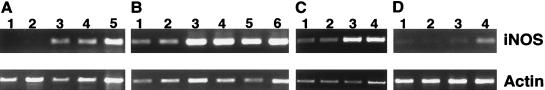
To confirm the enhanced production of NO in macrophages stimulated with B. bovis CM and IFN-γ, steady-state mRNA for iNOS was examined in cells cultured with B. bovis CM, B. bovis-infected RBC, or uninfected RBC. Reverse transcription-PCR analysis revealed that iNOS mRNA expression was detected in macrophages cultured for 6 h with B. bovis CM, CM plus IFN-γ, or LPS plus IFN-γ but was not detected in cells cultured with medium or IFN-γ alone (Fig. 3A). iNOS mRNA levels were higher in macrophages cultured with B. bovis CM and IFN-γ for 6 or 24 h than in macrophages cultured with IFN-γ alone (Fig. 3A and B). Furthermore, the iNOS mRNA levels were higher in macrophages cultured for 24 h with CM alone than in macrophages cultured with medium (Fig. 3B). Similar results were obtained with macrophages cultured with B. bovis CM with or without IFN-γ for 2 and 48 h (data not shown). Although CM alone did not induce NO2− production, there was no detectable difference in the level of iNOS mRNA between cells cultured with CM alone and cells cultured with CM plus IFN-γ as determined by densitometry image analysis and comparison with actin data (data not shown). When macrophages were examined after 24 h of culture with B. bovis CM, the effect of IFN-γ on cells stimulated with either LPS or CM was also not apparent (Fig. 3B). To confirm the ability of babesial parasites to induce iNOS, freshly harvested parasitized RBC or control, cultured uninfected RBC from the same donor animal were used as the stimulus in 6- or 24-h macrophage cultures (Fig. 3C and D). Macrophages cultured for 6 h had low levels of iNOS steady-state mRNA in the presence of medium or RBC and IFN-γ (Fig. 3C). Compared with medium, culture with infected RBC alone resulted in an approximately eightfold increase in the level of steady-state iNOS mRNA, and compared with uninfected RBC plus IFN-γ, culture with infected RBC plus IFN-γ resulted in an approximate fourfold increase in the level of steady-state iNOS mRNA (Fig. 3C). In a separate experiment, culture of macrophages for 24 h revealed increased iNOS transcript levels only in the presence of infected RBC and IFN-γ, and uninfected RBC again did not stimulate iNOS. The differences in baseline levels of iNOS expressed by macrophages cultured for either 6 or 24 h in the absence of Babesia are probably due to differing activation states of the cultures, which were obtained from different donor animals (G4, G5, and G6) at different times.
FIG. 3.
Expression of iNOS mRNA after exposure to B. bovis. Total RNA was isolated from macrophages, reverse transcribed, and amplified by PCR with iNOS- or actin-specific primers. (A) Macrophages from animal G6 were cultured for 6 h with medium (lane 1), 500 U of IFN-γ per ml (lane 2), 2 μg of LPS per ml plus IFN-γ (lane 3), 100 μg of B. bovis CM per ml (lane 4), or B. bovis CM plus IFN-γ (lane 5). (B) Macrophages from animal G5 were cultured for 24 h with medium (lane 1), 500 U of IFN-γ per ml (lane 2), 2 μg of LPS per ml (lane 3), LPS plus IFN-γ (lane 4), 100 μg of B. bovis CM per ml (lane 5), or B. bovis CM plus IFN-γ (lane 6). (C) Macrophages from animal G4 were cultured for 6 h with medium (lane 1), uninfected RBC (10% PCV) plus 50 U of IFN-γ per ml (lane 2), B. bovis-infected RBC (10% PCV, 10% PPE) (lane 3), or B. bovis-infected RBC plus IFN-γ (lane 4). (D) Macrophages from animal G6 were cultured for 24 h with uninfected RBC (2.5% PCV) (lane 1), uninfected RBC plus 500 U of IFN-γ per ml (lane 2), B. bovis-infected RBC (2.5% PCV, 4% PPE) (lane 3), or B. bovis-infected RBC plus IFN-γ (lane 4). The reverse transcription-PCR products for iNOS (372 bp) and actin (890 bp) were visualized on ethidium bromide-stained agarose gels, and the relative band intensities were derived by densitometry image analysis and normalization to actin.
Macrophage stimulation by Th-cell culture supernatants.
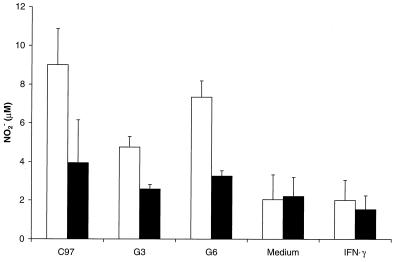
B. bovis-specific CD4+ Th-cell lines produce both IFN-γ and TNF-α (11). To determine whether the secreted products of antigen-stimulated B. bovis-specific CD4+ T cells could activate macrophages to produce NO, macrophages were cultured for 96 h with supernatants harvested from T-cell lines stimulated with B. bovis CM and APC. The cell lines, in culture for 6 weeks (G3) or 10 weeks (C97 and G6), responded specifically by proliferation in response to B. bovis CM antigen compared with control uninfected RBC antigen (Table 2). NO2− production by macrophages cultured with supernatants from all three Th-cell lines was significantly greater (P < 0.05, by the Student t test) than that induced by either medium or IFN-γ (1,000 U/ml) alone (Fig. 4). Addition of l-NMMA significantly inhibited NO2− production (P < 0.05), demonstrating that the NO2− levels were generated by macrophages and were not due to NO2− present in the T-cell supernatants themselves. l-NMMA had no effect on NO2− production by macrophages cultured in medium or IFN-γ alone. Supernatants of B. bovis-specific Th-cell lines from animals C97, G3, and G6 contained 151, 17, and 154 U of IFN-γ per ml and 9, <1, and 11 U of TNF per ml, respectively (Table 2). Interestingly, supernatants from cell lines that induced the highest levels of NO (C97 and G6) also had the highest levels of IFN-γ and TNF.
TABLE 2.
Proliferation and IFN-γ production by B. bovis-stimulated Th-cell lines
| T-cell linea | Radioactivity (cpm) incorporated by Th-cell lines stimulated withb:
|
Concn (U/ml) of cytokinesc:
|
||
|---|---|---|---|---|
| RBC CM | B. bovis CM | IFN-γ | TNF | |
| C97 | 3,109 ± 1,924 | 19,982 ± 679 | 151 | 9 |
| G3 | 1,626 ± 24 | 35,292 ± 1,299 | 17 | <1 |
| G6 | 152 ± 33 | 105,033 ± 307 | 154 | 11 |
T-cell lines were maintained in culture for 6 weeks (G3) or 10 weeks (C97 and G6) by weekly stimulation with 25 μg of B. bovis CM antigen per ml and autologous APC. At 7 days after stimulation, the cell lines were tested in a proliferation assay.
A total of 3 × 104 T cells and 2 × 105 irradiated APC were cultured for 3 days with 25 μg of B. bovis CM or control uninfected RBC CM antigen per ml, radiolabeled, and counted. The results are mean radioactivity ± 1SD.
Supernatants were harvested from the same cell lines in culture for a total of 6 weeks (G3) or 10 weeks (C97 and G6), 7 days after stimulation with antigen and APC, and were assayed for IFN-γ and TNF.
FIG. 4.
NO production by macrophages exposed to culture supernatants of B. bovis-specific Th-cell lines. NO2− levels were determined by the Griess assay of culture supernatants from macrophages that were cultured for 96 h with B. bovis-specific T-cell culture supernatants (75%, vol/vol) obtained from C97, G3, and G6 cell lines. Controls consisted of complete RPMI 1640 culture medium or 1,000 U of IFN-γ per ml, and each treatment was performed in the absence (open bars) or presence (solid bars) of 250 μM l-NMMA. Results are shown as the mean NO2− levels for quadruplicate cultures and 1 SD.
DISCUSSION
This report is the first to demonstrate the induction of iNOS and production of NO in bovine macrophages by the protozoal parasite B. bovis. Mycoplasma contamination of our cultures was ruled out (56). While NO2− levels were significantly increased in culture supernatants of CM- plus IFN-γ-stimulated macrophages isolated from each animal tested, there was evidence of donor-dependent variation in NO production (Table 1), as described previously (1). Variation from experiment to experiment in the levels of NO2− produced after stimulation with either CM or LPS was also observed and probably reflects the different activation states of these primary cell cultures. Bovine iNOS activity was detected at some level when macrophages were exposed to IFN-γ in combination with each B. bovis merozoite preparation tested. However, the highest levels of NO2− were present in supernatants of macrophages cultured with CM and IFN-γ, indicating that the stimulatory B. bovis component may be insoluble and/or closely associated with merozoite membranes, organelles, or vesicles that are not disrupted by lysis in the French press. The source of NO2− detected by the Griess reaction was confirmed to be l-arginine through inhibition of NO2− generation by the l-arginine analog l-NMMA. The upregulation of iNOS by B. bovis CM and infected RBC was also confirmed at the transcriptional level.
Activation of bovine macrophages by IFN-γ produced by parasite-specific Th1 and Th0 lymphocytes (11) is hypothesized to be important for B. bovis parasite clearance after challenge infection of immune hosts (9, 12), but the mechanism of parasite inhibition by activated macrophages has not been determined. Phagocytosis of parasitized RBC is one way in which activated macrophages may remove parasites from the host (26), and preliminary results indicated that macrophages activated by adherence to plastic alone inhibited parasite replication (45). Macrophage secretory products were also reportedly toxic for P. falciparum and B. bovis parasites in vitro (24, 34). P. falciparum was shown to induce TNF-α in both human and murine macrophages (4, 5, 36, 39, 50–52) and NO production in murine macrophages in vitro (41). However, recent evidence that P. falciparum cultures are commonly contaminated with Mycoplasma (56), a prokaryote known to induce TNF-α and other inflammatory molecules in macrophages (28–30, 56), indicates that the interpretation of these earlier studies will require further experimentation (43). The secretory products that directly inhibit B. bovis in vitro do not include TNF-α itself (49), but NO and its reactive nitrogen intermediate derivatives were inhibitory for both B. bovis and P. falciparum in vitro (24, 27, 40, 53). Thus, the present study provides a link between the observed effector function of NO and its induction by B. bovis parasites and specific Th-cell products.
The induction of iNOS by CM occurred in the absence of IFN-γ, and addition of exogenous IFN-γ did not appear to increase the level of mRNA, whereas NO2− production was significantly enhanced when macrophages were cocultured with CM and IFN-γ. These differences probably reflect the different assay sensitivities, and RT-PCR is useful only for demonstrating relative differences in mRNA levels. Bovine macrophages stimulated with viruses or gram-positive bacteria also had upregulated levels of iNOS or NO in the absence of IFN-γ (1, 2). Of interest was the relatively low, and perhaps physiologically relevant, level of IFN-γ (1 U/ml) required to enhance NO generation in the presence of B. bovis CM. The supernatants obtained from antigen-stimulated Th-cell lines all contained titers of IFN-γ sufficient to induce NO production by macrophages. These supernatants, which were diluted before being added to the macrophages, could have contained no more than 20 μg of CM antigen per ml, a concentration that was suboptimal for stimulating NO when combined with IFN-γ (Fig. 2A). Furthermore, recombinant bovine IFN-γ alone did not induce NO production by macrophages in this or other studies (2). Together, these data suggest that other products in addition to residual antigen, such as TNF-α and/or TNF-β present in the Th-cell supernatants, may be mediating macrophage costimulation. In support of this possibility, the presence of biologically active TNF in these supernatants correlated with NO production. Furthermore, others have demonstrated that recombinant TNF-α plus IFN-γ can upregulate both iNOS and NO2− in bovine (20) and murine (39) macrophages. Thus, our results are consistent with the possibilities that IFN-γ and TNF produced by parasite-specific Th cells can stimulate NO production in the presence of small amounts of antigen and that B. bovis merozoite antigen, either alone or with IFN-γ, can induce iNOS indirectly through stimulation of macrophage TNF-α.
NO is a molecule with a diverse array of biological functions, including immunoregulatory ones (57). Recently, endogenous expression of NO was shown to be required for transcriptional upregulation of the IL-12 p40 subunit, but not the p35 subunit, in murine macrophages activated by IFN-γ (42). Since IL-12 is one of the key cytokines required for enhanced IFN-γ production by NK cells and T cells and for priming of a type 1 Th-cell response (55), activation of macrophages by protozoal parasites and their products may contribute to the induction of a type 1 immune response, provided that IL-12 p35 is sufficiently expressed to form the biologically active heterodimer. This type of immune response could then lead to either enhanced parasite clearance or pathologic consequences, the latter of which were found in a murine malaria model to be dependent on TNF-α and IFN-γ (21). Alternatively, selective production of IL-12 p40 could result in the formation of p40 homodimers, which can theoretically antagonize functional IL-12 heterodimeric activity and interfere with induction and activation of type 1 immune responses (19). Preliminary results in our laboratory showed an upregulation of TNF-α and both IL-12 p35 and p40 subunits in macrophages cultured with B. bovis CM and IFN-γ (46). Thus, NO production by bovine monocyte-derived macrophages following exposure to B. bovis is indicative of an activation state that reflects a complex host-parasite interaction.
While NO production has been induced in murine macrophages by an extract of the human parasite P. falciparum (41), the present study is, to our knowledge, the first to demonstrate induction of iNOS by an apicomplexan parasite in monocyte-derived macrophages from a nonrodent species. Studies to investigate the potential link between upregulation of macrophage iNOS and cytokines IL-12 and TNF-α following exposure to B. bovis, to elucidate the roles of NO and IL-12 in the initiation of the immune response to B. bovis, and to define the mechanisms by which activated macrophages inhibit B. bovis replication are under way. Identification of the parasite products that induce the production of NO and inflammatory cytokines and that are enriched in the CM fraction may also prove useful in the design of vaccines for B. bovis and related hemoparasites.
ACKNOWLEDGMENTS
We thank Sue Ellen Chantler, Kim Kegerreis, Debby Alperin, and Daming Zhu for technical assistance and Tien-min Chou and Bill Davis for their advice during the course of this work.
This research was supported by National Institutes of Health NIAID grant R01-AI30136.
REFERENCES
- 1.Adler H, Frech B, Meier P, Jungi T W, Peterhans E. Noncytopathic strains of bovine viral diarrhea virus prime bovine bone marrow-derived macrophages for enhanced generation of nitric oxide. Biochem Biophys Res Commun. 1994;202:1562–1568. doi: 10.1006/bbrc.1994.2109. [DOI] [PubMed] [Google Scholar]
- 2.Adler H, Frech B, Thony M, Pfister H, Peterhans E, Jungi T W. Inducible nitric oxide synthase in cattle. Differential cytokine regulation of nitric oxide synthase in bovine and murine macrophages. J Immunol. 1995;154:4710–4718. [PubMed] [Google Scholar]
- 3.Amante F H, Good M F. Prolonged Th1-like response generated by a Plasmodium yoelii-specific T cell clone allows complete clearance of infection in reconstituted mice. Parasite Immunol. 1997;19:111–126. doi: 10.1046/j.1365-3024.1997.d01-187.x. [DOI] [PubMed] [Google Scholar]
- 4.Bate C A W, Taverne J, Playfair J H L. Malarial parasites induce TNF production by macrophages. Immunology. 1988;64:227–231. [PMC free article] [PubMed] [Google Scholar]
- 5.Bate C A W, Taverne J, Playfair J H L. Soluble malarial antigens are toxic and induce the production of tumor necrosis factor in vivo. Immunology. 1989;66:600–605. [PMC free article] [PubMed] [Google Scholar]
- 6.Beyer J C, Stich R W, Brown W C, Cheevers W P. Cloning and expression of caprine interferon-gamma. Gene. 1998;210:103–108. doi: 10.1016/s0378-1119(98)00046-8. [DOI] [PubMed] [Google Scholar]
- 7.Brown W C, Logan K S. Babesia bovis: bovine helper T cell lines reactive with soluble and membrane antigens of merozoites. Exp Parasitol. 1992;74:188–199. doi: 10.1016/0014-4894(92)90046-d. [DOI] [PubMed] [Google Scholar]
- 8.Brown W C, Logan K S, Wagner G G, Tetzlaff C L. Cell-mediated immune responses to Babesia bovis merozoite antigens in cattle following infection with tick-derived or cultured parasites. Infect Immun. 1991;59:2418–2426. doi: 10.1128/iai.59.7.2418-2426.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown W C, Rice-Ficht A C. Use of helper T cells to identify potential vaccine antigens of Babesia bovis. Parasitol Today. 1994;10:145–149. doi: 10.1016/0169-4758(94)90265-8. [DOI] [PubMed] [Google Scholar]
- 10.Brown W C, Woods V M, Chitko-McKown C G, Hash S M, Rice-Ficht A C. Interleukin-10 is expressed by bovine type 1 helper, type 2 helper, and unrestricted parasite-specific T-cell clones and inhibits proliferation of all three subsets in an accessory-cell-dependent manner. Infect Immun. 1994;62:4697–4708. doi: 10.1128/iai.62.11.4697-4708.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown W C, Woods V M, Dobbelaere D A E, Logan K S. Heterogeneity in cytokine profiles of Babesia bovis-specific bovine CD4+ T-cell clones activated in vitro. Infect Immun. 1993;61:3273–3281. doi: 10.1128/iai.61.8.3273-3281.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown W C, Zhao S, Logan K S, Grab D J, Rice-Ficht A C. Identification of candidate vaccine antigens of bovine hemoparasites Theileria parva and Babesia bovis by use of helper T cell clones. Vet Parasitol. 1995;57:189–203. doi: 10.1016/0304-4017(94)03120-l. [DOI] [PubMed] [Google Scholar]
- 13.Brown W C, Zhao S, Rice-Ficht A C, Logan K S, Woods V M. Bovine helper T cell clones recognize five distinct epitopes on Babesia bovis merozoite antigens. Infect Immun. 1992;60:4364–4372. doi: 10.1128/iai.60.10.4364-4372.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown W C, Estes D M. Type I and type II responses in cattle and their regulation. In: Schijns V E C J, Horzinek M, editors. Cytokines in veterinary medicine. Wallingford, United Kingdom: CAB International; 1997. pp. 15–33. [Google Scholar]
- 15.Clark I A, Hunt N H, Butcher G A, Cowden W B. Inhibition of murine malaria (Plasmodium chabaudi) in vivo by recombinant interferon-gamma or tumor necrosis factor, and its enhancement by butylated hydroxyanisole. J Immunol. 1987;139:3493–3496. [PubMed] [Google Scholar]
- 16.Degen J L, Neubauer M G, Degen S J, Seyfried C E, Morris D R. Regulation of protein synthesis in mitogen-activated bovine lymphocytes. Analysis of actin-specific and total mRNA accumulation and utilization. J Biol Chem. 1983;258:12153–12162. [PubMed] [Google Scholar]
- 17.Estes D M, Closser N M, Allen G K. IFN-gamma stimulates IgG2 production from bovine B cells costimulated with anti-μ and mitogen. Cell Immunol. 1994;154:287–295. doi: 10.1006/cimm.1994.1078. [DOI] [PubMed] [Google Scholar]
- 18.Fell A H, Currier J, Good M F. Inhibition of Plasmodium falciparum growth in vitro by CD4+ and CD8+ T cells from non-exposed donors. Parasite Immunol. 1994;16:579–586. doi: 10.1111/j.1365-3024.1994.tb00313.x. [DOI] [PubMed] [Google Scholar]
- 19.Gillessen S, Carvajal D, Ling P, Podlaski F J, Stremlo D L, Familletti P C, Gubler U, Presky D H, Stern A S, Gately M K. Mouse interleukin-12 (IL-12) p40 homodimer: a potent IL-12 antagonist. Eur J Immunol. 1995;25:200–206. doi: 10.1002/eji.1830250133. [DOI] [PubMed] [Google Scholar]
- 20.Goff W L, Johnson W C, Wyatt C R, Cluff C W. Assessment of bovine mononuclear phagocytes and neutrophils for induced l-arginine-dependent nitric oxide production. Vet Immunol Immunopathol. 1996;55:45–62. doi: 10.1016/s0165-2427(96)05629-2. [DOI] [PubMed] [Google Scholar]
- 21.Grau G E, Piguet P-F, Vassalli P, Lambert P-H. Tumor-necrosis factor and other cytokines in cerebral malaria: experimental and clinical data. Immunol Rev. 1989;112:49–70. doi: 10.1111/j.1600-065x.1989.tb00552.x. [DOI] [PubMed] [Google Scholar]
- 22.Green S J, Mellouk S, Hoffman S L, Meltzer M S, Nacy C A. Cellular mechanisms of nonspecific immunity to intracellular infection: cytokine-induced synthesis of toxic nitrogen oxides from l-arginine by macrophages and hepatocytes. Immunol Lett. 1990;25:15–19. doi: 10.1016/0165-2478(90)90083-3. [DOI] [PubMed] [Google Scholar]
- 23.Green S J, Meltzer M S, Hibbs J B, Jr, Nacy C A. Activated macrophages destroy intracellular Leishmania major amastigotes by an l-arginine-dependent killing mechanism. J Immunol. 1990;144:278–283. [PubMed] [Google Scholar]
- 24.Gyan B, Troye-Blomberg M, Perlmann P, Bjorkman A. Human monocytes cultured with and without interferon-gamma inhibit Plasmodium falciparum parasite growth in vitro via secretion of reactive nitrogen intermediates. Parasite Immunol. 1994;16:371–375. doi: 10.1111/j.1365-3024.1994.tb00362.x. [DOI] [PubMed] [Google Scholar]
- 25.Jacobs P, Radzioch D, Stevenson M M. A Th1-associated increase in tumor necrosis factor alpha expression in the spleen correlates with resistance to blood-stage malaria in mice. Infect Immun. 1996;64:535–541. doi: 10.1128/iai.64.2.535-541.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacobson R H, Parrodi F, Wright I G, Fitzgerald C J, Dobson C. Babesia bovis: in vitro phagocytosis promoted by immune serum and by antibodies produced against protective antigens. Parasitol Res. 1993;79:221–226. doi: 10.1007/BF00931896. [DOI] [PubMed] [Google Scholar]
- 27.Johnson W C, Cluff C W, Goff W L, Wyatt C R. Reactive oxygen and nitrogen intermediates and products from polyamine degradation are babesiacidal in vitro. Ann N Y Acad Sci. 1996;791:136–147. doi: 10.1111/j.1749-6632.1996.tb53520.x. [DOI] [PubMed] [Google Scholar]
- 28.Jungi T W, Krampe M, Sileghem M, Griot C, Nicolet J. Differential and strain-specific triggering of bovine alveolar macrophage effector functions by mycoplasmas. Microb Pathog. 1996;21:487–98. doi: 10.1006/mpat.1996.0078. [DOI] [PubMed] [Google Scholar]
- 29.Kita M, Ohmoto Y, Hirai Y, Yamaguchi N, Imanishi J. Induction of cytokines in human peripheral blood mononuclear cells by mycoplasmas. Microbiol Immunol. 1992;36:507–516. doi: 10.1111/j.1348-0421.1992.tb02048.x. [DOI] [PubMed] [Google Scholar]
- 30.Kostyal D A, Butler G H, Beezhold D H. Mycoplasma hyorhinis molecules that induce tumor necrosis factor alpha secretion by human monocytes. Infect Immun. 1995;63:3858–3863. doi: 10.1128/iai.63.10.3858-3863.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kremsner P G, Nussler A, Neifer S, Chaves M F, Bienzle U, Senaldi G, Grau G E. Malaria antigen and cytokine-induced production of reactive nitrogen intermediates by murine macrophages: no relevance to the development of experimental cerebral malaria. Immunology. 1993;78:286–290. [PMC free article] [PubMed] [Google Scholar]
- 32.Kumar S, Miller L H. Cellular mechanisms in immunity to blood stage infection. Immunol Lett. 1990;25:109–114. doi: 10.1016/0165-2478(90)90100-5. [DOI] [PubMed] [Google Scholar]
- 33.Kumaratilake L M, Ferrante A. IL-4 inhibits macrophage-mediated killing of Plasmodium falciparum in vitro. A possible parasite-immune evasion mechanism. J Immunol. 1992;149:194–199. [PubMed] [Google Scholar]
- 34.Montealegre F, Levy M G, Ristic M, James M A. Growth inhibition of Babesia bovis in culture by secretions from bovine mononuclear phagocytes. Infect Immun. 1985;50:523–526. doi: 10.1128/iai.50.2.523-526.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ockenhouse C F, Schulman S, Shear H L. Induction of crisis forms in the human malaria parasite Plasmodium falciparum by gamma-interferon-activated, monocyte-derived macrophages. J Immunol. 1984;133:1601–1608. [PubMed] [Google Scholar]
- 36.Picot S, Peyron F, Vuillez J-P, Barbe G, Marsh K, Ambroise-Thomas P. Tumor necrosis factor production by human macrophages stimulated in vitro by Plasmodium falciparum. Infect Immun. 1990;58:214–216. doi: 10.1128/iai.58.1.214-216.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Quakyi I A, Currier J, Fell A, Taylor D W, Roberts T, Houghten R A, England R D, Berzofsky J A, Miller L H, Good M F. Analysis of human T cell clones specific for conserved peptide sequences within malaria proteins. Paucity of clones responsive to intact parasites. J Immunol. 1994;153:2082–2092. [PubMed] [Google Scholar]
- 38.Rafie-Kolpin M, Essenberg R C, Wyckoff J H., III Identification and comparison of macrophage-induced proteins and proteins induced under various stress conditions in Brucella abortus. Infect Immun. 1996;64:5274–5283. doi: 10.1128/iai.64.12.5274-5283.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rockett K A, Awburn M M, Aggarwal B B, Cowden W B, Clark I A. In vivo induction of nitrite and nitrate by tumor necrosis factor, lymphotoxin, and interleukin-1: possible roles in malaria. Infect Immun. 1992;60:3725–3730. doi: 10.1128/iai.60.9.3725-3730.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rockett K A, Awburn M M, Cowden W B, Clark I A. Killing of Plasmodium falciparum in vitro by nitric oxide derivatives. Infect Immun. 1991;59:3280–3283. doi: 10.1128/iai.59.9.3280-3283.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rockett K A, Kwiatkowski D, Bate C A W, Awburn M M, Rockett E J, Clark I A. In vitro induction of nitric oxide by an extract of Plasmodium falciparum. J Infect. 1996;32:187–196. doi: 10.1016/s0163-4453(96)80018-1. [DOI] [PubMed] [Google Scholar]
- 42.Rothe H, Hartmann B, Geerlings P, Kolb H. Interleukin-12 gene-expression of macrophages is regulated by nitric oxide. Biochem Biophys Res Commun. 1996;224:159–163. doi: 10.1006/bbrc.1996.1000. [DOI] [PubMed] [Google Scholar]
- 43.Row J A, Scragg I, Kwiatkowski D, Ferguson D J, Carucci D J, Newbold C I. British Society for Parasitology Ninth Malaria Meeting. 1997. Mycoplasma contamination of Plasmodium falciparum cultures: detection, eradication, and implications, abstr. P24a. [DOI] [PubMed] [Google Scholar]
- 44.Ruebush M J, Hanson W L. Transfer of immunity to Babesia microti of human origin using T lymphocytes in mice. Cell Immunol. 1980;52:255–265. doi: 10.1016/0008-8749(80)90347-0. [DOI] [PubMed] [Google Scholar]
- 45.Shoda, L. K. M., R. W. Stich, and W. C. Brown. Unpublished observations.
- 46.Shoda L K M, Stich R W, Dreewes M, Brown W C. Second Woods Hole Immunoparasitology Meeting. 1998. Induction of IL-12 p35, IL-12 p40, TNF-α, and iNOS in bovine macrophages by Babesia bovis or fractionated antigen, abstr. 61. [Google Scholar]
- 47.Stevenson M M, Tam M F, Nowotarski M. Role of interferon-gamma and tumor necrosis factor in host resistance to Plasmodium chabaudi AS. Immunol Lett. 1990;25:115–121. doi: 10.1016/0165-2478(90)90101-u. [DOI] [PubMed] [Google Scholar]
- 48.Stich, R. W., A. C. Rice-Ficht, W. Tuo and W. C. Brown. Babesia bovis: common protein fractions recognized by oligoclonal B. bovis-specific CD4+ T cell lines from genetically diverse cattle. Submitted for publication. [DOI] [PubMed]
- 49.Tambrallo L J, Buening G M, McLaughlin R M. The effect of neutrophils, tumor necrosis factor, and granulocyte macrophage/colony stimulating factor on Babesia bovis and Babesia bigemina in culture. Vet Parasitol. 1992;43:177–188. doi: 10.1016/0304-4017(92)90159-7. [DOI] [PubMed] [Google Scholar]
- 50.Taverne J, Bate C A W, Kwiatkowski D, Jakobsen P H, Playfair J H L. Two soluble antigens of Plasmodium falciparum induce tumor necrosis factor release from macrophages. Infect Immun. 1990;58:2923–2928. doi: 10.1128/iai.58.9.2923-2928.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taverne J, Bate C A W, Playfair J H L. Malaria exoantigens induce TNF, are toxic and are blocked by T-independent antibody. Immunol Lett. 1990;25:207–212. doi: 10.1016/0165-2478(90)90116-8. [DOI] [PubMed] [Google Scholar]
- 52.Taverne J, Bate C A W, Sarkar D A, Meager A, Rook G A, Playfair J H L. Human and murine macrophages produce TNF in response to soluble antigens of Plasmodium falciparum. Parasite Immunol. 1990;12:33–43. doi: 10.1111/j.1365-3024.1990.tb00934.x. [DOI] [PubMed] [Google Scholar]
- 53.Taylor-Robinson A W. Antimalarial activity of nitric oxide: cytostasis and cytotoxicity towards Plasmodium falciparum. Biochem Soc Trans. 1997;25:262S. doi: 10.1042/bst025262s. [DOI] [PubMed] [Google Scholar]
- 54.Taylor-Robinson A W, Phillips R S, Severn A, Moncada S, Liew F Y. The role of TH1 and TH2 cells in a rodent malaria infection. Science. 1993;260:1931–1934. doi: 10.1126/science.8100366. [DOI] [PubMed] [Google Scholar]
- 55.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 56.Turrini F, Giribaldi G, Valente E, Arese P. Mycoplasma contamination of Plasmodium cultures—a case study of parasite parasitism. Parasitol Today. 1997;13:367–368. doi: 10.1016/s0169-4758(97)01088-0. [DOI] [PubMed] [Google Scholar]
- 57.Wei X Q, Charles I G, Smith A, Ure J, Feng G J, Huang F P, Xu D, Muller W, Moncada S, Liew F Y. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature. 1995;375:408–411. doi: 10.1038/375408a0. [DOI] [PubMed] [Google Scholar]
- 58.Wright I G, Goodger B V, Clark I A. Immunopathophysiology of Babesia bovis and Plasmodium falciparum infections. Parasitol Today. 1988;4:214–218. doi: 10.1016/0169-4758(88)90161-5. [DOI] [PubMed] [Google Scholar]