Abstract
The administration of medications to children has been a challenge for parents and caregivers for generations. Pharmaceutical companies have often overcome the difficulties of weight-based dosing and the inability of most young children to swallow solid dosage forms by creating oral liquids. While oral liquids offer advantages in terms of dose flexibility, swallowability, and ease of administration for young children and patients with enteral tubes, they have been plagued by issues such as taste, volume, and texture, to name a few. While the recommendations for broader use of oral syringes can help with the issue of measuring accuracy and incremental dosing, the issues of poor taste and frequently unacceptable volumes for doses remain a problem. New oral dosage forms which have begun to enter the United States marketplace have the potential to improve adherence and acceptability of oral medications for children, but come with their own unique challenges.
Keywords: drug delivery systems, excipients, pediatrics, taste
Medications intended for use in the pediatric population should be formulated in a dosage form that is acceptable to children in doses that are easily measured and appropriate for their age and weight. Solid oral dosage forms such as tablets and capsules are often difficult for young children to swallow and are usually not available in dosages that are appropriate. Extemporaneous compounding can help with this issue at times, but is limited by the availability of a published recipe and compounding resources. Oral liquids can be helpful and easy to swallow but have other challenges, as outlined below. Some medications, having been formulated with adults in mind, require using creative solutions, such as the use of intravenous dexamethasone for oral administration due to the dilute concentration and high alcohol content of dexamethasone oral solution. Nifedipine capsules are pierced with a needle and syringe to withdraw pediatric doses (see Figure 1). Manipulating oral dosage forms has long been an essential component of pediatric nursing and pharmaceutical care.
Figure 1.
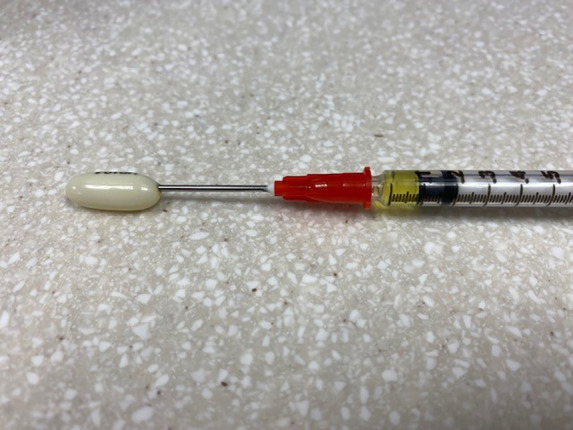
Nifedipine capsule pierced with a needle and syringe for dose removal.
Since the Best Pharmaceuticals for Children Act (BPCA) became law in 2002 and the Pediatric Research Equity Act (PREA) followed in 2003, pharmaceutical companies have new incentives and requirements to develop their medications for children, if the medication is for a disease that exists in children. More recently, the Research to Accelerate Cures and Equity (RACE) for Children Act of 2017 ensures that drugs for adult cancers which have similar molecular targets in children will be studied for those pediatric indications. BPCA, PREA, and RACE have helped to motivate earlier and more thoughtful pediatric trials and corresponding dosage forms for relevant age groups. In a “Guidance for Industry” from the US Food and Drug Administration (FDA) published in 2020, sponsors are advised to provide plans for development of an age-appropriate formulation if the adult formulation is not appropriate for pediatric patients.1 While many of these new medications have been developed in the traditional liquid form, some have been approved and others are in development which utilize novel dosage forms. This review will serve to summarize the current state of oral medications for children, describe newer oral dosage forms, and examine the devices designed to measure and administer these medications.
Oral Liquids: Advantages and Challenges
Oral liquids have long been the dosage form of choice for pediatric drug formulations. They solve the swallowability problem of solid oral dosage forms and in addition can help to overcome administration issues with geriatric patients and those with enteral feeding tubes. The dose flexibility offered by oral liquids when used with oral syringes allows for doses that would not otherwise be attainable with most solid dosage forms. Oral liquids will likely always comprise the majority of oral medications for children, but they do come with some challenges, which are outlined in this section.
Taste and Smell. Active pharmaceutical ingredients (APIs) are frequently bitter in taste, and often present a patient acceptance challenge when incorporated into liquid dosage forms. While tablet and capsule formulations can help to mask some of the taste issues, these solid dosage forms are generally not tolerated by children younger than 8 years and are often not accepted even into the teenage years.
Liquid formulations include additional ingredients other than the API; these excipients include sweeteners, flavoring agents, preservatives, suspending agents, and coloring agents.2 Children are naturally more sensitive to bitter tastes than adults,3 thus the addition of acids, sugars, and salt to liquid formulations can assist in palatability as they help to mask bitter tastes.4 Despite these efforts at improving the taste of liquid medications, taste remains a challenge in the administration of oral liquid medications to children. In a study by McDonald et al,5 when caregivers were surveyed about the reasons they have difficulty in administering medications to children, the most common reason was the taste of the medication, followed by the medication being in a formulation that the child could not take. Taste will remain a challenge with oral liquid medications unless better taste masking technologies and strategies can be developed. Smell can also be a challenge, as many oral liquids have poor smells. A common example is clindamycin, which is very difficult to administer if the child is able to smell the medication prior to administration. Oral syringes can help overcome this by containing the medication until administration by mouth.
Texture. Children are more sensitive to differences in the texture of medications, in addition to flavors. In a study involving interviews of 221 caregivers of children 0 to 18 years and 57 children ages 12 to 18 years by Venables et al,6 texture affected 8% of medications studied and was a significant predictor of medication refusal. Many suspensions are gritty in nature, and this can be highly objectionable for pediatric patients. A common example where this may affect patient acceptability is if a prescriber chooses to change a patient from amoxicillin to amoxicillin with clavulanate based on bacterial susceptibility or the patient’s clinical course. While amoxicillin is generally sweet tasting and thick but smooth in mouth feel, amoxicillin with clavulanate is gritty in nature and somewhat “chalky.” This can lead to dose refusal and subsequent therapeutic failure.
Volume. Challenges with taste are further complicated by the concentration and corresponding volume of doses that many medications require. Table 1 lists a variety of medications which are commonly used in the pediatric population, and the volume of common doses for 3 different weight categories. There is no guidance on which volumes of oral liquid medications are acceptable for different ages and patient weights, but in the author’s opinion any amount greater than 2 mL for an infant or toddler and 5 mL for a young child (age 3 to 7 years) is predictably problematic. As demonstrated by the doses shown in Table 1, most of the examples exceed these volumes. In 2011 the European Medicines Agency (EMA) released a draft guideline on development of medicines for pediatric use which gave a maximum volume of 5 mL for children younger than 4 years of age and 10 mL for children between 4 and 12 years.7 Comments were received from pharmaceutical companies, individuals, government agencies, and professional organizations and these recommendations were subsequently deleted from the current version, which was published in 2013.8
Table 1.
Commonly Used Medications, Concentrations, and Dose Volumes for Pediatric Patients
| Medication | Concentration | Weight-Based Dose |
10 kg Patient
(Around 12 Months of Age) |
20 kg Dose
(Around 5 Years of Age) |
30 kg
(Around 10 Years of Age) |
|---|---|---|---|---|---|
| Amoxicillin | 400 mg / 5 mL | 45 mg/kg/dose given twice daily | 450 mg = 5.6 mL | 900 mg = 11.25 mL | 1350 mg = 16.8 mL |
| Cefpodoxime | 100 mg / 5 mL | 5 mg/kg/dose given twice daily | 50 mg = 2.5 mL | 100 mg = 5 mL | 150 mg = 7.5 mL |
| Cephalexin | 250 mg / 5 mL | 25 mg/kg/dose given twice daily | 250 mg = 5 mL | 500 mg = 10 mL | 750 mg = 15 mL |
| Clindamycin | 75 mg / 5 mL | 10 mg/kg/dose given three times daily | 100 mg = 6.7 mL | 200 mg = 13.3 mL | 300 mg = 20 mL |
| Dexamethasone | 1 mg/10mL | 0.6 mg/kg/dose | 6 mg = 60 mL | 12 mg = 120 mL | 18 mg = 180 mL |
| Oseltamivir | 6 mg / 1 mL | 30, 45, 60, and 75 mg per dose based on weight categories; dose given twice daily | 30 mg = 5 mL | 45 mg = 7.5 mL | 60 mg = 10 mL |
| Vigabatrin | 500 mg / 10 mL (500 mg powder sachet is dissolved in 10 mL water by the caregiver at the time of dose administration) | 50 mg/kg/dose for children 1 month – 2 years; standard dose for weight categories in children 2 years and older | 500 mg/10 mL | 650 mg/13 mL | 1000 mg/20 mL |
While different administration techniques exist, such as administering small amounts over time, the ability of the caregiver to administer a complete dose decreases as the volume gets larger. Larger volume doses inevitably bring up the question from caregivers regarding mixing oral liquid doses in breast milk, infant formula, or food. This technique is generally not advisable as compatibility with these items is variable and some of the dose may be lost should the child not finish the feeding. When mixing liquid drug doses with food it is recommended to use only a small amount of food to mix the medication in so that the entire dose is consumed. Compatibility with the food should also be considered when mixing medications, most notably with calcium-containing foods such as yogurt and acidic foods such as apple sauce. Compatibility issues are challenging for pharmaceutical companies who must consider common foods from different parts of the world, as the ingredients of yogurt and even the mineral content of water are different.
Dose Frequency. Dosing frequency adds to the patient acceptability issues of taste, smell, and texture. Many common pediatric medications, such as clindamycin, cephalexin, and metronidazole, require administration multiple times per day, often for 7 to 10 days or longer. The frequency and total number of doses can be extremely problematic for caregivers. Extended-release tablets and capsules, which often allow for less frequent dose administration, are generally too large for young children to swallow and are usually only available in adult dosages.
Excipients. Excipient content of medications for children has long been a concern, and 7 excipients appear on the Key Potentially Inappropriate Drugs in PediatricS (KIDs) List—a reference of drugs and excipients which are potentially inappropriate for children.9 Included on this list are ethanol and benzyl alcohol. Benzyl alcohol is a preservative which is present in many parenteral and oral medications and in large doses may produce gasping syndrome in neonates.10,11
Ethanol is an excipient in some oral liquid medications and is used to enhance solubility of the API. While not commonly used, its presence in some important medications for children warrants attention. The alcohol content and extreme volume of doses of dexamethasone elixir (see Table 1) have led to its avoidance in practice and the use of the injectable dexamethasone administered orally in the hospital environment.12 Phenobarbital oral solution, which is between 13.5% and 15% ethanol depending on formulation, is commonly used in young infants for the treatment of seizures, particularly in the neonatal intensive care unit. FDA regulations state that for over the counter (OTC) medications intended for children under 6 years of age, alcohol content should not exceed 0.5%.13 An equivalent regulation does not exist for prescription medications, however the fact that the alcohol content of phenobarbital elixir is 30 times the recommended amount for OTC medications should warrant concern. While a recipe exists to extemporaneously compound phenobarbital without alcohol,14 the logistics of this can be difficult, especially in smaller hospitals or rural areas where a compounding pharmacy may be difficult to find.
As previously mentioned, sweeteners are frequently used for taste-masking purposes, but their use may also increase the risk of dental carries.15 In contrast to the positive taste-masking that can be achieved with sweeteners, if a medication is too sweet it may become very desirable and lead to a higher poisoning risk, thus the risks and benefits of taste must be weighed when formulating oral liquid medications in this population. Table 2 contains a list of common excipients in pediatric dosage forms and their problems.
Table 2.
Common problematic excipients in pediatric formulations
| Excipient | Potential Problems for Pediatric Patients | Examples of Medications Formulated With the Excipient |
|---|---|---|
| Benzyl Alcohol | Gasping syndrome, seizures, paralysis | Diazepam, furosemide, tobramycin, midazolam, lorazepam, trimethoprim/sulfamethoxazole |
| Ethanol | CNS depression, hypoglycemia | Dexamethasone, phenobarbital |
In an effort to provide a central source for information about the safety of excipients in the pediatric population, the European Paediatric Formulation Initiative (EuPFI) created the “Safety & Toxicity of Excipients for Paediatrics” or “STEP” database.16 Efforts such as these highlight the need for special consideration and attention to the unique safety challenges of formulating medications for children.
Measuring Accuracy. While oral liquid medications offer the benefit of flexible dosing—a necessary component of any medication in the pediatric population due to changes in patient body weight over time—this introduces the potential hazard of measuring liquids inappropriately. Historically, the use of household teaspoons and tablespoons was routinely recommended, hence the fact that many oral liquid concentrations are listed in milligrams per 5 mL (a practice which the author believes should be abandoned), however the variability in volume of these spoons as well as the potential for spillage makes them less than ideal. Studies measuring caregivers’ ability to accurately measure appropriate doses consistently demonstrate the superiority of the oral syringe, although it is often found to be more difficult to use.17–19 Competent oral syringe use can be enhanced by education from the pharmacist. In 2015 The American Academy of Pediatrics recommended the use of the oral syringe as the most appropriate device for measuring and administering liquid medications to children.20 Unfortunately, prescription medications are often not packaged with measuring devices, leaving it to the caregiver to independently obtain an appropriate device for the child’s dose21 which adds to the cost of the prescription for the family, typically 2 to 10 dollars, depending on the syringe and package size. In the author’s experience, retail pharmacies often fail to counsel patients about the most appropriate measuring device for the patient’s medication(s) and parents are left to figure this out on their own. One new oral syringe adapter, the MediFrida, offers a new solution for caregivers of infants by providing a pacifier with a channel that allows attachment of an oral syringe22 although the syringe provided in the package only includes volumetric markings for common acetaminophen and ibuprofen doses. The medication passes through the channel and into the infant’s cheek, potentially helping to reduce spitting problems and bypassing some taste receptors.
In addition to the need to measure accurately, bottles of liquid medications often do not contain enough overfill to account for drug loss on the sides of the bottle. In the author’s experience, on 2 separate occasions a bottle of amoxicillin that should have lasted for 10 days of therapy only lasted for 9. Part of this problem is the lack of a bottle adapter to connect the syringe to, such that the oral syringe can’t reach the liquid in the bottle after a few days of therapy, meaning the caregiver must tip the bottle carefully or pour the medication into a separate, smaller container (see Figure 2). Additionally, since oral syringes and bottle adapters are generally not provided, and not covered by insurance, this may add to the cost of therapy for the family. In addition to measuring difficulties, many oral liquid medications are suspensions which require adequate shaking immediately prior to administration. Failure to shake the suspension well enough can lead to subtherapeutic dosing at the beginning of the bottle and supratherapeutic dosing later in the course.
Figure 2.

Oral syringe use where the syringe is unable to reach the liquid medication during the last half of a course of therapy.
Osmolality. Osmolality of medications can be very relevant when administering oral liquids to infants, particularly in premature infants. A study published in 2021 found that more than 86% of oral liquids commonly administered in this population were greater than the recommended osmolality of 500 mOsm/kg.23 This increased osmolality can potentially lead to adverse outcomes including bloating, abdominal pain, and diarrhea that may evolve to more serious adverse effects including electrolyte abnormalities and necrotizing enterocolitis. Thus, the osmolality of the oral dosage form along with the volume needed to be administered often needs to be considered when planning dosing and feeding timing and their frequency. The study from Shah et al23 provides a comprehensive review and listing of the osmolality of many medications important in pediatric practice; some commonly used medications such as multivitamins, acetaminophen, and oral sodium chloride liquid had osmolalities of greater than 5,000 mOsm/kg.
Shelf Life. Many currently available oral liquids are available as powders in bottles which are then mixed into suspensions by the addition of water. Once the water is added, a beyond use date is assigned, which is typically 30 days or less. United States Pharmacopeia chapter 795 (USP <795>), covers nonsterile pharmaceutical compounding. Proposed revisions include a maximum beyond use date of 35 days for preserved aqueous dosage forms. Short beyond use dates such as this can lead to drug waste and the necessity for caregivers to refill medications more frequently, in particular those for chronic use.24
Other Currently Available Oral Dosage Forms
While oral liquids comprise the majority of oral medications given to children, other options exist for some medications. Chewable tablets have been an option for medications such as acetaminophen, montelukast, aspirin, phenytoin, and carbamazepine, for many years. In recent years, an extended-release chewable methylphenidate product, QuilliChew ER (methylphenidate) has been approved,25 offering an extended-release option despite its chewable formulation. Chewable tablets offer the advantage of swallowability, but still struggle with potential taste, texture, and dose flexibility problems.
Soluble/disintegrating tablets, similar to chewable tablets, offer the advantage of swallowability but again may pose texture and taste challenges for children. They also may not be available in pediatric doses. Examples of medications currently available as soluble tablets are lansoprazole, loratadine, olanzapine, and ondansetron.
Current Solutions to Oral Dosage Form Issues
Taste Masking. As noted above, taste masking is a common method of overcoming the poor taste of bitter drugs. A common technique is to add sugar and/or flavoring. Decreasing the pH by adding acids such as citric acid can also improve palatability, particularly for children.4 Unfortunately both acids and sugar can contribute to the formation of dental carries and are thus not ideal for children.4 Coating drug powders, often with cellulose, can also help to improve palatability upon administration.26
Flavoring agents are available on the market to assist with medication taste. These are more commonly available in the outpatient environment and can often assist in patient acceptance of poor tasting medications. A commonly available product, FlavoRx, provides flavoring agents for many frequently used oral liquid medications.27 The child can choose which flavor they prefer from the list of flavor options for their medication. Medication-specific recipes are provided to the pharmacy to ensure that stability and shelf life are not affected. While not all medications have flavoring options, this type of flavoring may assist in a child’s acceptance of an otherwise poor-tasting medication. Parents and caregivers may also use commercially available food such as chocolate syrup to improve taste. This technique may help some children but also carries the risk of contributing to dental carries similar to flavoring. All sweeteners should also be considered when administering medications to patients with diabetes, as they may significantly affect blood glucose levels. Mixing medications with foods or drinks, while common, is often untested and it is unknown how many of these drugs may perform when administered with various foods and drinks.28
A relatively novel technique to overcome poor taste is with the use of bitter blockers. Historically the term “bitter blocker” has been used to describe any additive which improves the taste of a medicine. More recently it has been defined as a compound which interacts with the molecular pathway of bitterness at a taste-cell level.29 Most bitter tastes are due to interactions with the “taste 2 receptor family” or TAS2R located on the tongue. Most bitter tasting medications interact with multiple TAS2R receptors, thus a specific bitter blocker will only work if it antagonizes one of those receptors and also will likely only decrease the bitter taste rather than eliminate it. As bitter blockers are excipients, determination of the safety of these in the intended patient population is vitally important, as described previously. While bitter blockers are not included in any currently available medications, their use may pose a desirable “taste neutral” advantage as they prevent or decrease bitter tastes without sweetening the medications thus avoiding differences in taste preferences.
Extemporaneous Compounding. Historically, the lack of available pediatric friendly dosage forms has led to the need for extemporaneous compounding of solid oral dosage forms into oral suspensions for pediatric use. While several oral liquids have been approved in recent years (amlodipine, lisinopril, enalapril, topiramate, zonisamide, etc), the need for extemporaneous compounding remains. Compounding pharmacies can provide options for pediatric patients whose medication is only available as a solid dosage form, or for medications which contain an unacceptable excipient, such as carbohydrates for patients on a ketogenic diet. Despite advances in pediatric oral dosage forms, compounding pharmacies continue to offer solutions for dosage form challenges.
Novel Oral Dosage Forms
Multiparticulates. The term “multiparticulate” is frequently used to describe many of the novel solid oral dosage forms and includes oral granules, pellets, mini-tablets, sprinkle capsules, and oral powders.30 “Granules” and “mini-tablets” are sometimes used interchangeably and there is currently no set definition or standard to define them. These dosage forms offer the advantage of dose flexibility while maintaining the shelf life and potential taste masking advantages of a traditional tablet. In general, mini-tablets are defined as having a diameter of between 2 and 4 mm. See Figure 3 for an example of mini-tablets. Doses may be adjusted by changing the number of mini-tablets or the number or strength of the granule sachets.
Figure 3.

Mini-tablets.
Acceptability of these dosage forms to infants and young children has been the subject of research studies over the past several years. The European Medicines Agency requires that acceptability of the size and shape of a tablet must be justified and supported by clinical evidence.8 In one of the earliest studies to examine the acceptability of mini-tablets in children, Spomer et al31 enrolled 60 children aged 0.5 to 6 years old in a 2-way crossover pilot study where each child received either an uncoated 2-mm mini-tablet with a beverage or 3 mL of a 15% glucose syrup, after which they received the other formulation. While statistical differences could not be found due to the small population size, there were no differences found between the acceptability of either dosage form in any of the age groups, and formulation refusal was higher for the syrup. A study published in 2013 investigated the acceptability of uncoated and coated mini-tablets compared to 3 mL of syrup in a crossover study of 306 patients aged 6 months to 5 years.32 They found that the acceptability of the uncoated mini-tablets was statistically significantly better than that for the syrup. In addition, the ability of the patients to swallow the uncoated mini-tablets was also higher than that for the syrup. None of the patients inhaled the uncoated mini-tablets or the syrup. Another study of children aged 1 to 4 years investigated the acceptability of suspensions, syrups, powders, and mini-tablets in a cross-over design of 148 patients. Acceptability was measured using a visual analog scale, and the mini-tablets demonstrated significantly higher acceptability than each of the 3 comparators.33
While suitability of mini-tablets in young children has been readily accepted, their utility in the neonatal and infant population has been questioned due to concerns about safety and swallowability. Caregiver and parental fears regarding choking and aspiration of these small solid dosage forms are understandable. Despite this, recent studies have found that this dosage form could work well for this age group. In a study in Japan of children in 2 age groups, 6 to 11 months and 12 to 24 months, investigators found that patients aged 6 to 11 months had a significantly easier time swallowing mini-tablets than fine granules and liquids.34 Children 12 to 23 months had no significant difference in swallowing mini-tablets compared to fine granules and liquids. In a study of 151 neonates, defined as between 2 and 28 days of life, the swallowability and acceptability of 2mm uncoated mini-tablets was compared in a crossover study design to 0.5 mL of syrup.35 All patients accepted both the mini-tablets as well as the syrup, demonstrating 100% acceptability. The mini-tablets demonstrated a higher rate of swallowability than the syrup. Importantly no adverse effects such as aspiration or coughing were reported.
Several examples of these novel dosage forms are currently available in the United States. Ivacaftor (Kalydeco) and Lumacaftor/Ivacaftor (Orkambi) for cystic fibrosis are available both as tablets for older children and adults and as oral granules in packets for children ages 4 months through 5 years (Ivacaftor) and 2 years through 5 years (Lumacaftor/Ivacaftor).36,37 Hydrocortisone (Alkindi Sprinkle) is now available as an oral granule in capsules for neonates through age <17 years.38 The capsules are opened so that the oral granules may be put in a small amount of food for administration; the granules may also be swallowed without food. Dabigatran (Pradaxa) was approved in 2021 as an oral pellet that can also be mixed in a small amount of food for administration to children 3 months to <12 years.39 These novel dosage forms often come with steep price tags, but most companies offer either coupons or medication assistance programs.
Modified Release Oral Liquids. One method to partially overcome the problem of dosing frequency is the development of dosage forms with modified release formulations which allow for less frequent dosing. Methods of accomplishing a modified release in an oral liquid include the use of ion exchange resins.40 These drug complexes are then either incorporated into microcapsules or lipospheres, or they are simply suspended in a vehicle. A recently approved example of this is Quillivant XR which is a once daily methylphenidate liquid formulation. Spray drying can also be used to accomplish modified release by enclosing the drug in a polymeric shell and then suspending in liquid. ZMax, an extended-release azithromycin oral suspension, was a one dose modified release liquid that utilized the spray drying technique, though it has been discontinued in the United States. It should also be noted that these novel modified release oral liquids open up the concept of modified release to children who are not able to swallow large tablets or capsules.
Pharmacokinetic Differences Between Dosage Forms. When considering the use of novel dosage forms in clinical settings, a discussion of the effect of drug formulation on its pharmacokinetics is of paramount importance. It is ideal if different oral formulations have similar absorption (rate and extent of absorption) characteristics assuring similar pharmacokinetics and pharmacodynamics to ensure easier prescribing. If a prescriber wishes to change a patient from a tablet to a liquid or vice versa, they generally expect that equivalent doses will provide equivalent systemic exposure, unfortunately this is not always the case. In 2017 the first liquid formulation of spironolactone was approved by the FDA. This liquid formulation results in 15% to 37% higher serum concentrations than spironolactone tablets.41 Fortunately spironolactone has a wide therapeutic index, thus the clinical effect of this difference is minimal—however this illustrates the point that equivalent doses do not always result in equivalent pharmacokinetics. In 1 study that investigated bioequivalence profiles of pediatric formulations, 40% of pediatric formulations were not bioequivalent to the adult reference product.42 When assessing new dosage forms for the pediatric population, pharmacists must consider the product’s pharmacokinetics in the specific population for which the product is intended.
Other Challenges—Tubes. While multiparticulates offer advantages for oral drug administration, there will clearly be challenges for those patients with enteral feeding tubes, including temporary nasogastric, nasoduodenal, and nasojejunal tubes, or surgically placed gastrostomy tubes. The importance of this is demonstrated by the recent release of an FDA draft guidance on in vitro testing and labeling of oral drugs which are administered via enteral feeding tubes.43 While tube diameter is a common consideration when choosing a specific tube for a patient, the internal diameter is not provided by most manufacturers, making testing of drug formulations administered through these tubes challenging. An additional consideration for administering solid dosage forms through tubes is the amount of liquid required to flush the medication through the tube, which is dependent on both the tube length and the medication itself. The potential for medications to adhere to the luminal surface of these tubes leading to improper dosing as well as tube obstruction are important considerations.
Devices and Packaging for Novel Oral Dosage Forms. The question of how to accurately measure multiparticulate doses and package them in an easy-to-use manner remains a challenge for which several potential solutions have been proposed. One issue that drug developers must contend with is creating a solution to measure many potential doses that is easy for caregivers with a variety of health literacy levels to use. In addition, use in hospitals will require the ability to accurately measure many doses and then dispense them one dose at a time, preferentially with a barcode on the label.
Stick packs are packets generally made of foil, in which various doses can be packaged and then dispensed. These offer the advantage of a pre-measured dose and portability, while the unit dose nature of this packaging is helpful to hospitals and long-term/extended care facilities who dispense medications one dose at a time. Issues for manufacturers include the reliability of packaging machinery to accurately and consistently measure and package the correct dose, and the need for multiple dose sizes. Dabigatran oral pellets are available in 6 “packet” sizes, with 23 weight ranges and doses in the corresponding dosing table.39 A large number of packet sizes adds to the complexity and difficulty of production. In addition, this creates stocking difficulties for retail and hospital pharmacies.
Several measuring devices for granules and mini-tablets are in development. The Sympfiny syringe (Figure 4) is similar in design to an oral syringe, but the dose is pre-set and “locked” into place, after which the device is attached to the bottle and the granules fall by gravity into the syringe. Devices such as the IQ-dose (Figure 5) are designed to attach to the bottle of mini-tablets. The user then sets the prescribed number of tablets and tips the bottle to fill the holes with the specified number of mini-tablets. The mini-tablets are then poured onto a spoon or mixed with a soft food. A straw device has also been developed; each straw contains a dose of a multiparticulate medication and the dose is administered by the child sipping a liquid through the straw.
Figure 4.
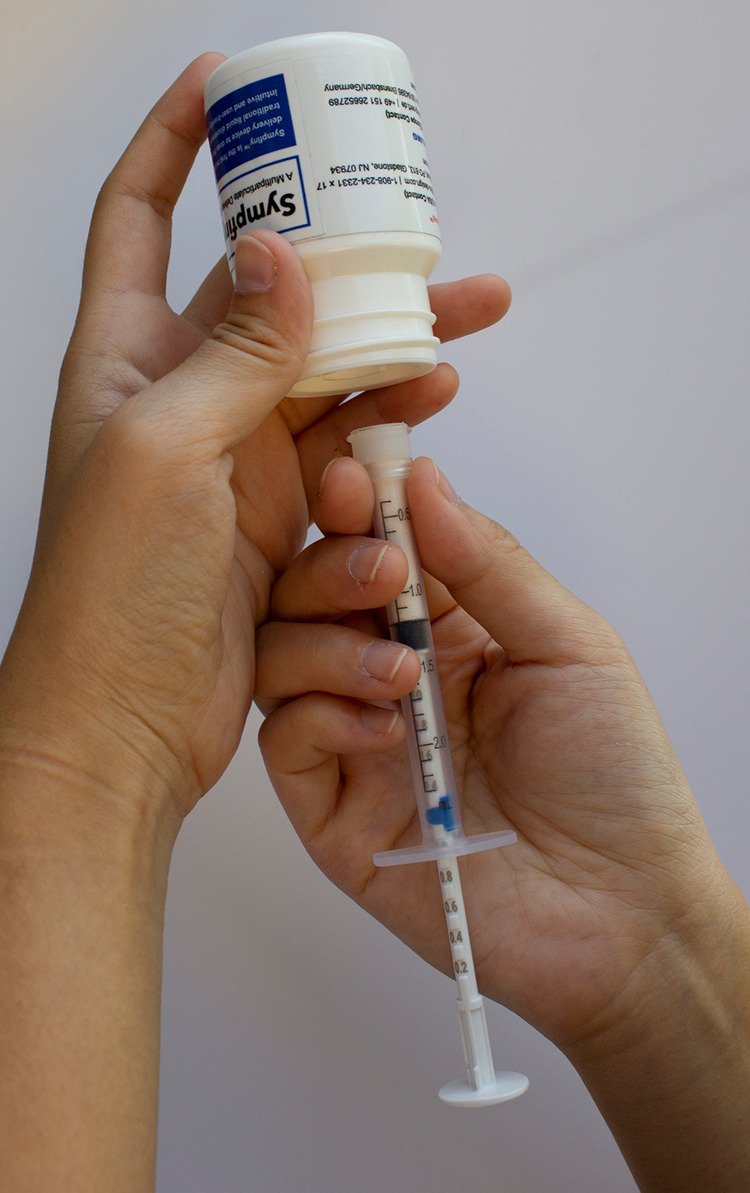
Sympfiny Syringe, Patent Pending, HS Design with permission.
Figure 5.

IQ-dose Mini-tablet Dispenser. Patent holder: SGH Healthcaring with permission.
Importance of Real World Clinical Experience
While studies thus far have demonstrated that novel solid oral dosage forms are acceptable even to young infants, the transition to the home or hospital environment is an important area that has yet to be explored.44 While an infant might readily swallow mini-tablets in a clinical trial setting, expecting a parent to accurately measure out a dose and then prepare and administer it to an infant is another situation entirely. Preparation and safety measures for hospital administration should also be explored, as hospital regulations and processes are vastly different from home use, requiring single dose dispensing with bar codes.
Global Challenges
The unique needs of developing countries should not be overlooked. The World Health Organization’s “Make Medicines Child Size” initiative has outlined goals for improving the availability and simplicity of medications for children. While much of the initiative focuses on specific diseases such as HIV and tuberculosis, there is also a focus on the need for better dosage forms that enable safe, flexible dosing, have long shelf lives, and can be easily transported. “Flexible solid dosage forms” are the recommended dosage form because of their ability to measure a range of doses and their superior shelf life. These include mini-tablets as well as oro-dispersable tablets that can be made to prepare an oral liquid. The shift towards solid dosage forms will likely benefit children from around the world.
Conclusion
Oral liquid medications have long been the standard oral dosage form for children and continue to offer the advantages of dose flexibility and ease of swallowability for pediatric patients. The continued development of commercially available oral liquids has helped to decrease the reliance on extemporaneous compounding for pediatric medications. Challenges of oral liquids include taste, volume, texture, excipients, and shelf life. Taste masking strategies such as flavoring agents can help with taste, and bitter blockers show promise as well. Novel solid oral dosage forms may help overcome some of the issues encountered with oral liquids, however their wide acceptance by healthcare practitioners and caregivers has yet to be seen. Challenges include the development of accurate measuring devices, or appropriately sized stick packs. Finally, while new drugs are being developed into these novel dosage forms, the effect this will have on older drugs—which make up the majority of medications used in pediatrics—is unknown. Will we see a new dexamethasone or will we have to keep drawing up IV solutions? Will we continue to pierce nifedipine capsules with a syringe and needle to withdraw the contents and measure out a partial dose? Pediatric healthcare practitioners are trained with an arsenal of tricks to manipulate dosage forms to fit our patients’ needs. Let’s work together to make sure our patients will benefit from the recent developments in oral dosage forms.
ABBREVIATIONS
- API
Active Pharmaceutical Ingredient;
- BPCA
Best Pharmaceuticals for Children Act;
- EMA
European Medicines Agency;
- EuPFI
European Paediatric Formulations Initiative;
- FDA
US Food and Drug Administration;
- OTC
Over The Counter;
- PREA
Pediatric Research Equity Act;
- RACE
Research to Accelerate Cures and Equity Act;
- USP
United States Pharmacopeia
Footnotes
Disclosure. The author declares no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria.
References
- 1.US Food and Drug Administration Pediatric Study Plans: Content of and Process for Submitting Initial Pediatric Study Plans and Amended Initial Pediatric Study Plans, Guidance for Industry. Accessed December 15, 2023. https://www.fda.gov/media/86340/download.
- 2.Pawar S, Kumar A. Issues in the formulation of drugs for oral use in children: role of excipients. Paediatr Drugs. 2002;4(6):371–379. doi: 10.2165/00128072-200204060-00004. [DOI] [PubMed] [Google Scholar]
- 3.Mennella JA, Bobowski NK. The sweetness and bitterness of childhood: Insights from basic research on taste preferences. Physiol Behav. 2015;152(pt B):502–507. doi: 10.1016/j.physbeh.2015.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mennella JA, Spector AC, Reed DR, Coldwell SE. The bad taste of medicines: overview of basic research on bitter taste. Clin Ther. 2013;35(8):1225–1246. doi: 10.1016/j.clinthera.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McDonald D, Kimler K, Robinson C, et al. Medication administration to children: The caregiver perspective. Poster presented at: The European Paediatric Formulations Initiative Conference; September 2018. London, United Kingdom [Google Scholar]
- 6.Venables R, Batchelor H, Hodson J et al. Determination of formulation factors that affect oral medicines acceptability in a domiciliary paediatric population. Int J Pharm. 2015;480(1–2):55–62. doi: 10.1016/j.ijpharm.2015.01.023. [DOI] [PubMed] [Google Scholar]
- 7.European Medicines Agency Guideline on pharmaceutical development of medicines for paediatric use. Accessed December 15, 2023. https://www.ema.europa.eu/documents/scientific-guideline/draft-guideline-pharmaceutical-development-medicines-paediatric-use_en-0.pdf.
- 8.European Medicines Agency Guideline on pharmaceutical development of medicines for paediatric use. Accessed December 15, 2023. https://www.ema.europa.eu/documents/scientific-guideline/guideline-pharmaceutical-development-medicines-paediatric-use_en.pdf.
- 9.Meyers RS, Thackray J, Matson KL et al. Key potentially inappropriate drugs in pediatrics: the KIDs list. J Pediatr Pharmacol Ther. 2020;25(3):175–191. doi: 10.5863/1551-6776-25.3.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown WJ, Buist NR, Gipson HT et al. Fatal benzyl alcohol poisoning in a neonatal intensive care unit. Lancet. 1982;1(8283):1250. doi: 10.1016/s0140-6736(82)92377-7. [DOI] [PubMed] [Google Scholar]
- 11.Gershanik J, Boecler B, Ensley H et al. The gasping syndrome and benzyl alcohol poisoning. N Engl J Med. 1982;307(22):1384–1388. doi: 10.1056/NEJM198211253072206. [DOI] [PubMed] [Google Scholar]
- 12.McCallister A, So TY, Stewart J. Evaluation of the efficacy of a onetime injectable dexamethasone administered orally in the pediatric emergency department for asthma exacerbation. J Pediatr Pharmacol Ther. 2017;22(5):326–331. doi: 10.5863/1551-6776-22.5.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.US Food and Drug Administration Over-The-Counter drug products intended for oral ingestion that contain alcohol Fed Regis 19896158629–58630. Codified at 21 CFR §328 [Google Scholar]
- 14.Cober MP, Johnson CE. Stability of an extemporaneously prepared alcohol-free phenobarbital suspension. Am J Health Syst Pharm. 2007;64(6):644–646. doi: 10.2146/ajhp060107. [DOI] [PubMed] [Google Scholar]
- 15.Feigal RJ, Jensen ME, Mensing CA. Dental caries potential of liquid medications. Pediatrics. 1981;68(3):416–419. [PubMed] [Google Scholar]
- 16.European Paediatric Formulation Initiative Safety & toxicity of excipients for paediatrics. Accessed December 15, 2023. http://www.eupfi.org/step-database-info/
- 17.Sobhani P, Christopherson J, Ambrose PJ, Corelli RL. Accuracy of oral liquid measuring devices: comparison of dosing cup and oral dosing syringe. Ann Pharmacother. 2008;42(1):46–52. doi: 10.1345/aph.1K420. [DOI] [PubMed] [Google Scholar]
- 18.Beckett VL, Tyson LD, Carroll D et al. Accurately administering oral medication to children isn’t child’s play. Arch Dis Child. 2012;97(9):838–841. doi: 10.1136/archdischild-2012-301850. [DOI] [PubMed] [Google Scholar]
- 19.Yin HS, Parker RM, Sanders LM et al. Liquid medication errors and dosing tools: a randomized controlled experiment. Pediatrics. 2016;138(4):e20160357. doi: 10.1542/peds.2016-0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.American Academy of Pediatrics , Committee on Drugs. Metric units and the preferred dosing of orally administered liquid medications Pediatrics 20151354784–787. [Google Scholar]
- 21.Johnson A, Meyers R. Evaluation of measuring devices packaged with prescription oral liquid medications. J Pediatr Pharmacol Ther. 2016;21(1):75–80. doi: 10.5863/1551-6776-21.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frida MediFrida. Accessed December 15, 2023. https://frida.com/products/medifrida. [Google Scholar]
- 23.Shah DD, Kuzmov A, Clausen D et al. Osmolality of commonly used oral medications in the neonatal intensive care unit. J Pediatr Pharmacol Ther. 2021;26(2):172–178. doi: 10.5863/1551-6776-26.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.United States Pharmacopeia Proposed updates to USP general chapters <795> and <797>. Accessed December 15, 2023. https://go.usp.org/Proposed_2021_Revisions_795_797.
- 25.QuilliChew ER [package insert] Monmouth Junction, NJ: Tris Pharma; June 2021. [Google Scholar]
- 26.Sohi H, Sultana Y, Khar RK. Taste masking technologies in oral pharmaceuticals: recent developments and approaches. Drug Dev Ind Pharm. 2004;30(5):429–448. doi: 10.1081/ddc-120037477. [DOI] [PubMed] [Google Scholar]
- 27.FlavoRx Accessed December 15, 2023. https://www.flavorx.com/
- 28.Martir J, Flanagan T, Mann J, Fotaki N. Co-administration of paediatric medicines with food and drinks in the context of their physicochemical properties-a global perspective on practices and recommendations. AAPS J. 2020;22(2):54. doi: 10.1208/s12248-020-0432-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andrews D, Salunke S, Cram A et al. Bitter-blockers as a taste masking strategy: a systematic review towards their utility in pharmaceuticals. Eur J Pharm Biopharm. 2021;158:35–51. doi: 10.1016/j.ejpb.2020.10.017. [DOI] [PubMed] [Google Scholar]
- 30.Strickley RG. Pediatric oral formulations: an updated review of commercially available pediatric oral formulations since 2007. J Pharm Sci. 2019;108(4):1335–1365. doi: 10.1016/j.xphs.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 31.Spomer N, Klingmann V, Stoltenberg I et al. Acceptance of uncoated mini-tablets in young children: results from a prospective exploratory cross-over study. Arch Dis Child. 2012;97(3):283–286. doi: 10.1136/archdischild-2011-300958. [DOI] [PubMed] [Google Scholar]
- 32.Klingmann V, Spomer N, Lerch C et al. Favorable acceptance of mini-tablets compared with syrup: a randomized controlled trial in infants and preschool children. J Pediatr. 2013;163(6):1728–1732.e1. doi: 10.1016/j.jpeds.2013.07.014. 721. [DOI] [PubMed] [Google Scholar]
- 33.van Riet-Nales DA, de Neef BJ, Schobben AF et al. Acceptability of different oral formulations in infants and preschool children. Arch Dis Child. 2013;98(9):725–731. doi: 10.1136/archdischild-2012-303303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mitsui N, Hida N, Kamiya T et al. Swallowability of minitablets among children aged 6-23 months: an exploratory, randomized crossover study. Pharmaceutics. 2022;14(1):198. doi: 10.3390/pharmaceutics14010198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klingmann V, Seitz A, Meissner T et al. Acceptability of uncoated mini-tablets in neonates–a randomized controlled trial. J Pediatr. 2015;167(4):893–896.e892. doi: 10.1016/j.jpeds.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 36.Kalydeco [package insert] Boston, MA: Vertex Pharmaceuticals Incorporated; Dec, 2020. [Google Scholar]
- 37.Orkambi [package insert] Boston, MA: Vertex Pharmaceuticals Incorporated; Jul, 2019. [Google Scholar]
- 38.Alkindi Sprinkle [package insert] Deer Park, IL: Eton Pharmaceuticals, Inc.; February 2022 [Google Scholar]
- 39.Pradaxa [package insert] Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc.; June 2021 [Google Scholar]
- 40.Trofimiuk M, Wasilewska K, Winnicka K. How to modify drug release in paediatric dosage forms? Novel technologies and modern approaches with regard to children’s population. Int J Mol Sci. 2019;20(13):3200. doi: 10.3390/ijms20133200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carospir [package insert] Farmville, NC: CMP Pharma, Inc.; August 2017 [Google Scholar]
- 42.Batchelor HK, Marriott JF. Formulations for children: problems and solutions. Br J Clin Pharmacol. 2015;79(3):405–418. doi: 10.1111/bcp.12268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.US Food and Drug Administration Oral products administered via enteral feeding tube: in vitro testing and labeling recommendations. Accessed December 15, 2023. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/oral-drug-products-administered-enteral-feeding-tube-in-vitro-testing-and-labeling-recommendations.
- 44.Walsh J, Schaufelberger D, Iurian S et al. Path towards efficient paediatric formulation development based on partnering with clinical pharmacologists and clinicians, a conect4children expert group white paper. Br J Clin Pharmacol. 2022;88(12):5034–5051. doi: 10.1111/bcp.14989. [DOI] [PubMed] [Google Scholar]


