Abstract
The heavy- and light-chain variable regions from a murine monoclonal antibody that recognize Pseudomonas aeruginosa serogroup O6 lipopolysaccharide (LPS) were used to generate a series of chimeric mouse-human monoclonal antibodies with identical variable regions. The murine variable-region gene segments were cloned into an immunoglobulin (Ig) cDNA expression vector that contained the human kappa light-chain and IgG1 constant regions. The IgG1 heavy-chain constant region was then replaced with the human IgG2, IgG3, IgG4, or IgA1 heavy-chain constant region. The five different expression vectors were transfected into Chinese hamster ovary cells for antibody production. The chimeric antibodies exhibited immunoreactivity and affinity similar to that of the parental murine IgG antibody toward whole cells of a serogroup O6 strain. In vitro complement deposition assays demonstrated that the chimeric IgG4 and IgA antibodies did not mediate the deposition of complement component C3 onto the surface of either purified LPS or whole bacteria. The chimeric IgG1 and IgG3 antibodies were similar in their ability to deposit C3 onto the surface of both bacteria and LPS, while IgG2 antibody was more effective at depositing C3 onto the surface of bacteria than onto purified LPS. The pattern of opsonophagocytic activity of the chimeric monoclonal antibodies was similar to that of complement deposition onto bacterial cells in that the chimeric IgG1 and IgG3 had the highest opsonic activity. Although IgG2 deposited more C3 onto the bacterial surface than did IgG4 or IgA, all three of these isotypes had low opsonic activity against the serogroup O6 target strain. This series of related antibodies will help reveal functional differences in efficacy among protective antibodies to P. aeruginosa and will be critical for defining the optimal formulation of either a vaccine for active immunization or a polyclonal intravenous IgG or monoclonal antibody cocktail for passive immunotherapy.
The difficulty of treating acute infections due to Pseudomonas aeruginosa has been amply documented (11, 12). Despite the advent of new antibiotics and therapies for infection, the efficacy of anti-pseudomonal therapy has appeared to reach its limit with currently available treatments (7, 11, 12). Overall, a major need for effective immunotherapeutic reagents to prevent or treat disease in patients at risk for these infections is apparent. Prophylactic or therapeutic immune system effectors could greatly reduce the morbidity and mortality from P. aeruginosa infection. While active immunization of most patients at risk for acute P. aeruginosa infection is unlikely to be practical due to the inability to identify these individuals early enough to immunize them, passive therapies, involving both prophylactic and therapeutic treatment, present a real opportunity to intervene in the P. aeruginosa pathogenic process.
There are still many unanswered questions about the manner in which antibody mediates protective immunity against P. aeruginosa infections and the potential of antibodies for use as prophylactic or therapeutic treatment of these infections. In addition to the specificity of the antibody variable regions for an appropriate P. aeruginosa epitope, the biologic activities associated with the Fc portion of antibodies that are most effective in protecting against P. aeruginosa infections are not well defined. The capacity to produce genetically engineered antibodies has improved the ability to investigate antibody function in vivo and in vitro and to define the antibody isotypes that are potentially the most useful for therapy of infections in humans. This report describes the construction and characterization of a set of chimeric mouse-human monoclonal antibodies (MAbs) which have identical variable regions with specificity for P. aeruginosa serogroup O6 lipopolysaccharide (LPS) and which differ in the human heavy-chain constant regions. These reagents were used to investigate some of the mechanisms of antibody-mediated protective immunity against P. aeruginosa.
MATERIALS AND METHODS
Murine variable-region genes and Ig expression vector.
The heavy- and light-chain variable regions used in the present studies were derived from MAbs, 1E1 (IgG1) and 2E12 (IgG3), respectively. These MAbs, specific for P. aeruginosa serogroup O6 LPS, were both derived by isotype switching a murine IgM MAb, IF6, specific to serogroup O6 LPS. MAbs 1E1 and 2E12 have identical heavy- and light-chain variable regions as determined from sequences of clones derived from mRNA obtained from these hybridomas (15). These particular heavy- and light-chain clones, used as templates for PCR mutagenesis to incorporate restriction sites for cloning into the TCAE 5.3 vector, were chosen at random from clones that had no PCR-induced mutations in them. The human Ig expression vector TCAE 5.3 has been described previously (17). TCAE 5.3 contains the human IgG1 heavy-chain and kappa light-chain constant region genes on the same plasmid, so that sequential transfections of heavy- and light-chain-containing plasmids with different selection markers can be avoided. The vector also has two drug resistance markers, neo and dhfr, for selection in mammalian cells, an Escherichia coli origin of replication, and an ampicillin resistance marker for replication in E. coli. Lastly, the inclusion of the dhfr gene in the vector allows amplification of the cloned Ig genes and subsequent high-level antibody production after transfection into CHO DG44 cells (gift of L Chasin, Columbia University), which have a deletion of both chromosomal copies of the dhfr gene (17). When transfected cells are grown in the presence of methotrexate, the dhfr gene and those surrounding it are duplicated on the chromosome, resulting in multiple copies of the genes encoding the MAbs.
Cloning of human IgG2, IgG3, IgG4, and IgA1 constant-region cDNAs.
Human Ig heavy-chain constant-region expression vectors were provided by Sherrie Morrison (University of California Los Angeles). Plasmids pAH4618, pAH4802, and pAH4801 contained genomic clones of the human IgG2, IgG3, and IgG4 heavy-chain constant-region genes, respectively, and lacked variable-region genes. To obtain cDNA clones of these constant-region genes, the heavy-chain variable-region gene segment from the murine hybridoma 9/5)23 was inserted in front of the heavy-chain constant regions as described by Coloma et al. (4) to allow expression of these constructs in lymphoid cells. These constructs were electroporated into murine SP2/0 cells, drug-resistant clones were selected, and mRNA was purified from these cells. First-strand cDNA was synthesized with oligo(dT) primers and avian myeloblastosis virus reverse transcriptase. The heavy-chain constant-region cDNAs were amplified by PCR with primers that hybridized to the 3′ end of the heavy-chain constant region (Hu IgG2,3 antisense and Hu IgG4 antisense [listed in Table 1]) and incorporated a termination codon and a BamHI site for cloning into the TCAE 5.3 vector. The 5′ primer used for all three human IgG constant regions incorporated an NheI site in the first two amino acids of the IgG constant regions (Hu IgG2,3,4 sense [Table 1]). The resulting PCR products were ligated into the TA cloning vector pCRII (InVitrogen, San Diego, Calif.), and the DNA was transformed into E. coli INVαf′ competent cells for cloning. Plasmid DNA from the resulting colonies was analyzed by restriction digestion followed by agarose gel electrophoresis. Plasmids carrying inserts of the correct size were sequenced to ensure that no mutations occurred during PCR amplification.
TABLE 1.
Primers used in this study
| Designation | Sequencea |
|---|---|
| V heavy antisense | 5′-AGCGCTAGCTGAGGAGACGGTGAC-3′ |
| NheI | |
| V heavy sense | 5′-GAAACGCGTGTCCACTCCCAGGTCCAGCTGCAG-3′ |
| MluI | |
| V kappa antisense | 5′-ACTCGTACGTTTTATTTCCAACTTTGTCCCC-3′ |
| BsiWI | |
| V kappa sense | 5′-GACCACGATGTGATGTTGTGATGACCCAA-3′ |
| DraIII | |
| Hu IgG2,3 antisense | 5′-GGACGGATCCTCATTTACCCGGAGACAGGGAGAG-3′ |
| BamHI | |
| Hu IgG4 antisense | 5′-GGACGGATCCTCATTTACCCAGAGACAGGGAGAG-3′ |
| BamHI | |
| Hu IgG2,3,4 sense | 5′-CTGCTAGCCACCAAGGGCCCATCSGTCTTC-3′ S=C/G |
| NheI | |
| Hu IgA1 antisense | 5′-CGAGGATCCTCAGTAGCAGGTGCCGTCCAC-3′ |
| BamHI | |
| Hu IgA1 sense | 5′-CTAAGCTAGCCCGACCAGCCCCAAGGTC-3′ |
| NheI |
Restriction sites are in boldface type. Termination codons are in italics.
The human IgA1 cDNA expression vector for expression in insect cells was provided by J. Donald Capra, University of Texas, Southwestern, Dallas, Tex. (3). To facilitate cloning into the TCAE 5.3 vector, the IgA1 cDNA was amplified by PCR with the primer set listed in Table 1, which incorporated a termination codon and a BamHI site at the 3′ end and an NheI site in the first 6 bases of the 5′ end of the CH1 domain. The PCR products were ligated as described above and amplified in E. coli, and plasmids containing the PCR products were sequenced to ensure that no mutations occurred during amplification.
Construction of the chimeric anti-LPS Ig expression vectors.
The cloned heavy- and light-chain variable-region cDNAs derived from the hybridomas as described above were used as templates for PCR with primers (Table 1) that incorporate restriction sites for ligation into the TCAE 5.3 Ig expression vector in the correct reading frame. The PCR products were first ligated into pCRII, cloned in E. coli, and sequenced again to ensure that no mutations occurred during introduction of the new restriction sites. The light- and heavy-chain variable-region products were cloned into the DraIII and BsiWI (kappa light-chain) and MluI and NheI (gamma 1 heavy-chain) sites of the Ig expression vector, respectively. The resulting completed expression vector was named pMP1 (IgG1).
Exchanging the IgG1 constant-region gene of pMP1 with IgG2, IgG3, IgG4, or IgA constant-region genes.
pMP1 was digested with NheI and BamHI to remove the human gamma 1 constant region. The plasmids containing the other constant-region genes were digested with the same enzymes. The products were separated by agarose gel electrophoresis. The band containing the expression vector portion of the pMP1 digest was cut from the gel and eluted, as were the bands containing the heavy-chain constant-region genes from the other digests. The vector and the IgG2, IgG3, IgG4, and IgA constant-region genes were then ligated in separate reactions and transformed into E. coli, and the resulting plasmids were analyzed by agarose gel electrophoresis to determine that the plasmid DNA contained the appropriately sized restriction fragments that distinguish the different heavy-chain constant-region genes.
Transfection of CHO cells for expression of the chimeric antibodies and selection of antibody-producing clones.
Plasmids pMP1 (IgG1), pMP2 (IgG2), pMP3 (IgG3), pMP4 (IgG4), and pMPA (IgA1) were separately introduced into Chinese hamster ovary (CHO) cells (DG44) by DNA-liposome-mediated transfection. Briefly, 2 μg of plasmid DNA was mixed with 8 μl of Lipofectamine (Gibco, Gaithersberg, Md.) in a final volume of 1 ml of serum-free medium. The transfection mixture was allowed to incubate for 5 h at 37°C. The cells were washed, fresh medium was added, and the cells were incubated for 48 h. The cells were harvested, resuspended in medium containing G418 (400 μg/ml; Gibco), and plated at different dilutions in 96-well plates. Plates on which colonies grew out in wells at a frequency of <30 positive wells per 96 wells (limiting dilution) were then selected for evaluation of chimeric-antibody production. Chimeric-antibody production by cells grown under antibiotic selection was measured by enzyme-linked immunosorbent assay (ELISA). Plates (Immulon II; Dynatech, Chantilly, Va.) were coated with either 2 μg of goat anti-human IgG (Fcγ specific) antibodies/ml or 2 μg of goat anti-human IgA (Fcα specific) antibodies/ml, and binding of MAbs in culture supernatants was detected with an alkaline phosphatase-conjugated goat anti-human kappa-light-chain-specific antibody (Sigma Chemical Co., St. Louis, Mo.). Confirmation that individual clones were producing IgG isotypes of the desired specificity was obtained by ELISA with murine MAbs specific for human IgG1, IgG2, IgG3, or IgG4 as described below. From these evaluations, the clones producing the largest amount of antibody were selected and replated in 96-well plates in the presence of 5 nM methotrexate (Sigma) to induce amplification of dhfr and the Ig genes. The highest producing clones were selected, and the process was repeated with 50 nM and 250 nM methotrexate. The resulting clones were then expanded for antibody production, maintaining antibiotic selection in 250 nM methotrexate.
Purification of chimeric antibodies.
Chimeric IgG antibodies were purified from tissue culture supernatants by protein G affinity chromatography. This support binds to all four human IgG subclasses. Chimeric IgA was purified by affinity chromatography with solid supports containing the lectin Jacalin.
Serologic assays.
Chimeric-antibody production was assessed by ELISA with Immulon II plates coated with 2 μg of goat anti-human IgG (Fcγ-specific) antibodies/ml or 2 μg of goat anti-human IgA (Fcα-specific) antibodies/ml and detected with an alkaline phosphatase-conjugated goat anti-human kappa-light-chain-specific antibody. The concentrations of the chimeric MAbs were determined by an antigen capture ELISA with purified human IgG1κ, IgG2κ, IgG3κ, and IgG4κ myeloma proteins and serum IgAκ antibodies of known concentrations as standards (Sigma).
Direct ELISA was used to confirm the isotype of the chimeric MAbs. Chimeric antibodies (2 μg/ml) were used to directly sensitize plates after dilution in 0.02 M phosphate buffer (pH 7.0). Chimeric IgG antibodies were detected with MAbs to subclass-specific epitopes (HP6001, anti-IgG1; HP6014, anti-IgG2; HP6058, anti-IgG3; and HP6025, anti-IgG4 [Sigma]) followed by alkaline phosphatase-conjugated anti-mouse IgG antibodies. The IgA was detected with an alkaline phosphatase-conjugated anti-human IgA antiserum.
Inhibition and competition ELISAs.
Immulon II ELISA plates were sensitized with purified P. aeruginosa serogroup O6 LPS at 2 μg/ml in 0.02 M phosphate coating buffer (0.02 M phosphate buffer [pH 7.0] containing 0.02% sodium azide) by overnight incubation at 4°C. The plates were washed three times in 0.02 M sodium phosphate-buffered saline (PBS) with 0.05% Tween 20 (PBS-T). The remaining binding sites on the plates were blocked by incubation with PBS with 1% bovine serum albumin by overnight incubation at 4°C. Chimeric antibodies (500 ng/ml) were mixed 1:1 with twice the indicated amounts of LPS for a final antibody concentration of 250 ng/ml and added to the plates. The plates were incubated for 2 h at room temperature. Bound antibody was detected with an alkaline phosphatase-conjugated goat anti-human IgG (Fcγ-specific) antibody. Similarly, ELISA plates were sensitized by overnight incubation at 4°C with serogroup O6 bacteria that were suspended after overnight growth on a Trypticase soy agar plate (BBL, Cockeysville, Md.) in phosphate coating buffer to an optical density (OD) of 0.1 at 650 nm. The plates were washed and blocked as described above for purified LPS. For the competition ELISA, bacteria were suspended in sterile 1% proteose peptone to an OD of 1.8 at 650 nm and diluted as indicated in antibody dilution buffer (PBS-T with 1% bovine serum albumin and 0.02% sodium azide). The original suspension was diluted and plated to determine the actual number of viable bacteria. The diluted bacteria were mixed 1:1 with the chimeric antibodies (final concentration, 250 ng/ml) and incubated overnight. The bacteria and bound antibody were removed by centrifugation, the supernatant was added to the ELISA plates, and the assay proceeded as described above.
Complement deposition ELISA.
Immulon II ELISA plates were sensitized with purified P. aeruginosa serogroup O6 LPS at a concentration of 10 μg/ml in 0.02 M phosphate coating buffer (pH 7.0). A second set of ELISA plates were coated with whole serogroup O6 bacteria in phosphate coating buffer. The bacteria were swabbed from an overnight Trypticase soy agar plate and suspended in coating buffer to an OD at 650 nm of 1.0, and this suspension was diluted 1:10 in phosphate coating buffer before being added to the plates. The chimeric antibodies were added to the plates at the indicated concentration and incubated for 1 h. Normal human serum was used as the complement source. The human serum was diluted 1:50 in RPMI 1640 medium and absorbed with lyophilized serogroup O6 bacteria at 1 mg/ml to remove endogenous antibodies before being added to the antibody-sensitized wells. After 15 min of incubation, the plates were washed and polyclonal rabbit anti-human C3 antiserum was added at a final dilution of 1:500. The rabbit antibodies to C3 were raised to affinity-purified human C3 as described previously (13). The rabbit antibodies bound to C3 were detected with an alkaline phosphatase-conjugated goat anti-rabbit IgG diluted 1:2,000. The OD405 was measured after incubation for 45 min at room temperature.
Opsonophagocytic killing assay.
The ability of the chimeric MAbs to mediate opsonophagocytic killing was determined as previously described (2), except that all reagents were diluted to their final concentration in RPMI 1640 medium with 10% heat-inactivated fetal bovine serum. Fresh human serum was used as a complement source at a final dilution of 1:10 after absorption for 30 min on ice with the test organism to remove the preexisting P. aeruginosa-specific antibodies. Opsonophagocytic killing assay was performed by mixing 100 μl of bacteria at 2 × 107 bacteria/ml, 100 μl of the different dilutions of MAb, 100 μl of purified fresh human polymorphonuclear leukocytes (PMNs) at 2 × 107 cells/ml as a source of phagocytic cells, and 100 μl of absorbed serum as a complement source. Duplicate tubes were used for each assay condition. Control tubes were included in each assay in which PMNs were omitted and replaced by media as described previously (15). The parental murine IgG2b MAb (2H3) and a chimeric, mucoid exopolysaccharide-specific antibody derived from 9/5)23 hybridoma (14) were used as positive and negative controls, respectively. Duplicate reaction mixtures were then incubated on a rotorack for 60 min at 37°C. Aliquots were removed from each tube, serially diluted in sterile 1% proteose peptone solution, and plated in quadruplicate on MacConkey agar plates. The plates were incubated overnight, and the colonies were counted. The percent reduction in CFU relative to that in assay tubes without added PMNs was calculated as follows: [(CFU surviving in the absence of PMNs − CFU surviving in samples with PMNs)/CFU surviving in absence of PMNs] × 100.
Statistical analysis.
The concentration of LPS or bacteria that reduced antibody binding by 50% (Inh50) in either the inhibition or the competition ELISA, along with associated confidence intervals (CI), was calculated by simple regression analysis. The confidence intervals were determined to be equivalent to P ≤ 0.05 by using the Bonferroni correction for multiple comparisons. Calculations were carried out with Statview v1.02 software (Abacus, Berkeley, Calif.) on a Macintosh computer.
RESULTS
Construction and expression of the chimeric anti-LPS antibodies.
The heavy- and light-chain variable regions used to construct the chimeric antibodies were cloned from murine hybridoma cell lines 1E1 (IgG1) and 2E12 (IgG3), respectively (15). These cell lines, derived by isotype switching of a murine IgM MAb, have identical variable regions specific for P. aeruginosa serogroup O6 LPS and have been described previously (15). ELISA analysis with MAbs specific for each of the human IgG subclasses or human IgA-specific antiserum demonstrated that the chimeric antibodies expressed the appropriate heavy-chain constant-region immunoreactivity (data not shown). Because the CHO cells producing the antibody do not synthesize the J chain component of IgM and IgA, the chimeric IgA molecules are monomeric. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis of the affinity-purified chimeric antibodies revealed bands of the appropriate sizes as deduced from the cDNA sequence for the chimeric kappa and heavy chains (data not shown).
Immunoreactivity of the chimeric antibodies.
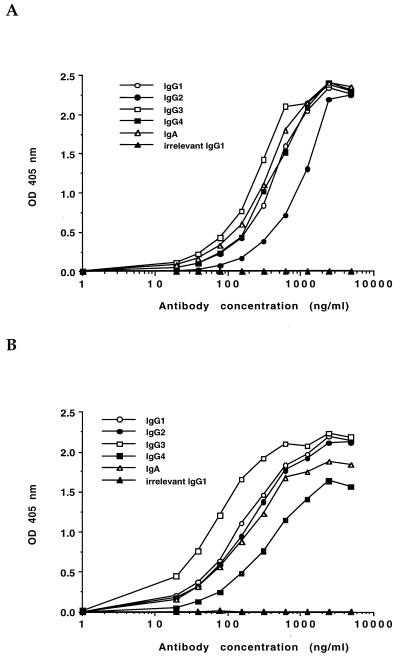
Binding activity of the chimeric antibodies was assessed by ELISA on plates coated with serogroup O6 bacteria or purified LPS. Negative controls included wells coated with either serogroup O5 bacteria or purified serogroup O5 LPS. The chimeric antibodies exhibited ELISA reactivity with purified P. aeruginosa serogroup O6 whole bacteria that was similar to that previously reported for the parental murine IgG MAbs (Fig. 1) (15). To evaluate whether the genetic manipulations involved in creating the chimeric antibodies altered the affinity of the variable regions for antigen, inhibition ELISAs were done with purified serogroup O6 LPS as the competitive inhibitor of antibody to serogroup O6 LPS bound to the plates. There was no alteration in the relative affinity of the chimeric IgG antibodies as judged by the similar Inh50 of LPS for the chimeric IgG antibodies and one of the parental murine IgG MAbs (2H3). The Inh50 for the parental murine IgG was 143 ng/ml (95% CI corrected for multiple comparisons = 45 to 450 ng/ml). The Inh50 for the chimeric IgG antibodies were as follows: human IgG1, 171 ng/ml; IgG2, 149 ng/ml; IgG3, 167 ng/ml; IgG4, 337 ng/ml; and IgA, 272 ng/ml. These values were all within the 95% CI of the parental murine IgG MAb and thus were not significantly different from the murine antibody. When the relative affinities of the chimeric IgG and IgA antibodies were measured against whole bacteria, there were also no significant differences from the parental murine IgG. The Inh50 for the bacterial competition ELISA were as follows: murine IgG2b, 1 × 107 CFU (95% CI corrected for multiple comparisons = 1.5 × 106 to 6.6 × 107 CFU); human IgG1, 5.0 × 106 CFU; IgG2, 4.8 × 106 CFU; IgG3, 2.7 × 107 CFU; IgG4, 4.3 × 106; and IgA, 2.2 × 107 CFU.
FIG. 1.
ELISA reactivities of chimeric antibodies against serogroup O6 LPS (A) or whole serogroup O6 bacteria (B). The irrelevant antibody is a human IgG1 MAb of unknown specificity. Data points represent the mean of triplicate wells. Standard deviations for all triplicates were ≤5% of the mean values.
Complement deposition.
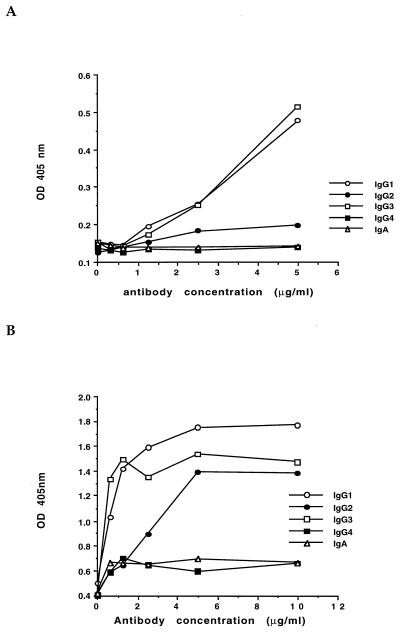
The ability of the chimeric antibodies to activate complement and deposit C3 onto the bacterial surface or onto purified LPS was measured. The chimeric IgG1 and IgG3 were similar in their ability to deposit C3 onto the surface of both bacteria and LPS, while the IgG2 antibody was somewhat less effective at depositing C3 onto the surface of bacteria and considerably less effective when assayed on purified LPS (Fig. 2). The chimeric IgG4 and IgA antibodies did not mediate the deposition of C3 onto the surface of either purified LPS or whole bacteria (Fig. 2).
FIG. 2.
Complement deposition onto either purified serogroup O6 LPS (A) or whole serogroup O6 bacteria (B). C3 deposition was measured by ELISA with a rabbit anti-human C3 antiserum. Bound C3 was measured at the indicated concentration of the chimeric antibodies added to antigen-sensitized ELISA plates. Data points represent the mean of triplicate wells. Standard deviations for all triplicates were ≤5% of the mean values.
Opsonophagocytic killing.
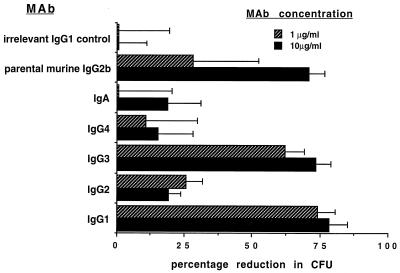
The ability of each MAb preparation to mediate in vitro opsonophagocytic killing of serogroup O6 bacteria in the presence of PMNs and complement is shown in Fig. 3. The chimeric IgG1 and IgG3 MAbs mediated a high level of killing at both antibody doses tested (10 and 1 μg/ml) compared with the results obtained for the tubes without added PMNs. There was negligible killing mediated by antibody and complement alone (in the absence of PMNs). The difference in levels of killing between the IgG1 and IgG3 antibodies was not significantly different at the 95% level (analysis of variance; Fisher probable least square difference). The levels of killing mediated by IgG1 and IgG3 were significantly greater than those mediated by IgG2, IgG4, and IgA at the 95% CI level. The negative control chimeric IgG1 antibody did not mediate killing at either dose tested, while the parental murine IgG2b mediated killing at both doses but showed a decrease in killing at the 1-μg/ml dose.
FIG. 3.
Opsonophagocytic activity of the different chimeric MAb preparations at the indicated antibody concentrations (legend), expressed as percent reduction in CFU with respect to identical tubes prepared with media added in place of PMNs. Bars represent the means, and error bars show the standard deviations.
DISCUSSION
We have described the creation of a set of variable-region-matched, isotype-switched chimeric mouse-human MAbs with specificity for P. aeruginosa serogroup O6 LPS. These variable regions are also identical in a series of isotype-switched murine MAbs (15) that have previously been shown to bind to five strains of P. aeruginosa expressing subtypes of serogroup O6 LPS (10). Thus, these antibodies recognize an O6 serogroup-specific epitope, although the antigenic determinants on all subtypes are not bound equally well (15). The Ig expression vector used in the present studies allows for the convenient exchange of the Ig heavy-chain constant-region genes once the variable-region gene segments have been introduced. The heavy-chain constant regions are all contained on an NheI-BamHI fragment that can be switched without having to reclone both variable regions into separate vectors containing the different heavy-chain constant regions.
With regard to the function of these antibodies, the genetic manipulations involved in creating the human IgG chimeric antibodies apparently did not affect the binding to bacteria or constant-region function. However, others have noted differences in antibody affinity after the genetic manipulations used to create chimeric antibodies. Zebedee et al. demonstrated that a chimeric IgG1 antibody had a greater affinity for a cryptococcal polysaccharide antigen than did the parental murine IgG1 (18). On the other hand, another chimeric IgG1 had a lower affinity compared to the parental murine IgM, which was not surprising due to loss of avidity in switching from IgM to IgG (18).
Human IgG consists of four subclasses or isotypes, IgG1 to IgG4, that differ in the effector functions that they mediate. The antibody isotype (Fc region) determines the ability of antibody-antigen complexes to direct immune responses by either activating the complement cascade or interacting with Fc receptors on phagocytic cells. With respect to complement activation, IgG1 and IgG3 are usually considered the most effective, IgG2 is less effective, and IgG4 is unable to bind C1q and activate complement (5). The ability of IgG2 to opsonize bacteria by the classical pathway of complement activation has recently been demonstrated to be dependent on the surface density of the epitope to which the antibody is directed (1). IgG2 was an efficient mediator of complement activation when the epitope density was high but was much less effective when the epitope density was low (1). Among the chimeric antibodies described in this study, IgG1 and IgG3 were efficient activators of complement whereas the activity of IgG2 was dependent on how the LPS was presented to the antibody. IgG2 showed good complement-activating ability when the LPS was presented on whole bacteria but was still less active than IgG1 and IgG3. However, when purified LPS alone was presented, the activity of IgG2 was weak. It appears that purified LPS O side chain antigens are not presented in the same way as when they are expressed on the bacteria. It could also be that the spacing of the purified LPS on ELISA plates is different, probably farther apart. These data are in accord with previous findings of Pollack et al., who demonstrated that the parental murine antibody to the chimeric antibodies described in this report recognized O-side chain antigens better on whole bacteria than on purified LPS as assessed by Western blot analysis (15).
When the opsonophagocytic activities of the chimeric antibodies were evaluated, IgG1 and IgG3 were clearly more active than the other IgG subclasses or IgA in mediating killing of this strain of bacteria. Nonetheless, the other IgG subclasses and IgA mediated a low but statistically significant level of killing. Whether these levels of killing in vitro will translate into protective immunity in vivo is currently under investigation. The low opsonophagocytic activity of the chimeric IgG2 antibody was surprising in light of the complement deposition data. These differences could be attributed to the differences in antibody-to-bacterium ratios in the ELISA and the phagocytic assay. It is hard to determine precisely how many bacteria are bound to the ELISA wells, and therefore there may have been a much higher antibody-to-bacterium ratio than in the phagocytic assay. Pollack et al. reported that a human IgG2 MAb against serogroup O6 LPS had high levels of opsonic activity in the presence of complement against a serogroup O6-expressing P. aeruginosa strain (16). It is likely that either this strain expressed higher levels of the epitope to which the MAb bound or the antibody was of higher affinity than the one reported here.
Antibody affinity also appears to play a significant role in the opsonic and protective activity of P. aeruginosa LPS-specific antibodies. The frequency of infection in previously noncolonized cystic fibrosis patients receiving an octavalent O side chain conjugate vaccine was lower in patients who responded with high-affinity LPS-specific antibodies (9). Low-affinity antibodies have also been shown to interfere with phagocytosis of P. aeruginosa (6). Serogroup O6 has five subtypes, to which the parental murine MAb reacts differently (15). Comparative opsonic activity against strains expressing these LPS subtypes is being investigated, and preliminary data indicate that the IgG2 chimeric antibody has strong opsonic activity against one of the subtypes for which it also has a higher affinity.
One problem that has plagued the development of LPS-specific immunotherapies is the difficulty in making generalizations about the protective efficacy of IgG subclasses that apply to all clinical isolates. Factors such as subtle differences in the LPS structure can affect antibody binding and affinity, potentially resulting in different protective efficacies of antibodies of the same isotype. We have previously pointed out (8) the need to evaluate immunotherapeutic agents against a variety of clinical isolates. Creation and evaluation of the IgG subclass and IgA idiotype-matched, chimeric antibodies will allow us to elucidate the complexity of the interactions of P. aeruginosa and antibodies.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grants DK51867 (to M.J.P.) and AI22535 (to G.B.P.) and a Young Investigator Award from the Cystic Fibrosis Foundation (to M.J.P.). A.A.G was supported by a NATO postdoctoral fellowship.
We thank Bert Brown, Brian Hyett, and Hannah Goldenberg for excellent technical assistance.
REFERENCES
- 1.Aase A, Michaelsen T E. Opsonophagocytic activity induced by chimeric antibodies of the four human IgG subclasses with or without help from complement. Scand J Immunol. 1994;39:581–587. doi: 10.1111/j.1365-3083.1994.tb03416.x. [DOI] [PubMed] [Google Scholar]
- 2.Ames P, DesJardins D, Pier G B. Opsonophagocytic killing activity of rabbit antibody to Pseudomonas aeruginosa mucoid exopolysaccharide. Infect Immun. 1985;49:281–285. doi: 10.1128/iai.49.2.281-285.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carayannopoulos L, Max E E, Capra J D. Recombinant human IgA expressed in insect cells. Proc Natl Acad Sci USA. 1994;91:8348–8352. doi: 10.1073/pnas.91.18.8348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coloma M J, Hastings A, Wims L A, Morrison S L. Novel vectors for the expression of antibody molecules using variable regions generated by polymerase chain reaction. J Immunol Methods. 1992;152:89–104. doi: 10.1016/0022-1759(92)90092-8. [DOI] [PubMed] [Google Scholar]
- 5.Dangl J L, Wensel T G, Morrison S L, Stryer L, Herzenberg L A, Oi V T. Segmental flexibility and complement fixation of genetically engineered chimeric human, rabbit and mouse antibodies. EMBO J. 1988;7:1989–1994. doi: 10.1002/j.1460-2075.1988.tb03037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eichler I, Joris L, Hsu Y P, Van Wye J, Bram R, Moss R. Nonopsonic antibodies in cystic fibrosis. Pseudomonas aeruginosa lipopolysaccharide-specific immunoglobulin G antibodies from infected patient sera inhibit neutrophil oxidative responses. J Clin Invest. 1989;84:1794–1804. doi: 10.1172/JCI114364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elborn J S, Shale D J, Britton J R. Cystic fibrosis: current survival and population estimates to the year 2000. Thorax. 1991;12:881–885. doi: 10.1136/thx.46.12.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hatano K, Boisot S, DesJardins D, Wright D C, Brisker J, Pier G B. Immunogenic and antigenic properties of a heptavalent high-molecular-weight O-polysaccharide vaccine derived from Pseudomonas aeruginosa. Infect Immun. 1994;62:3608–3616. doi: 10.1128/iai.62.9.3608-3616.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lang A B, Schaad U B, Rüdeberg A, Wedgewood J, Que J U, Fürer E, Cryz S J., Jr Effect of high-affinity anti-Pseudomonas aeruginosa lipopolysaccharide antibodies induced by immunization on the rate of Pseudomonas aeruginosa infection in patients with cystic fibrosis. J Pediatr. 1995;127:711–717. doi: 10.1016/s0022-3476(95)70158-3. [DOI] [PubMed] [Google Scholar]
- 10.Lanyi B, Bergan T. Serological characterization of Pseudomonas aeruginosa. Methods Microbiol. 1978;10:94–168. [Google Scholar]
- 11.Pennington J E. Pseudomonas aeruginosa immunotherapy. Eur J Clin Microbiol. 1990;9:377–380. doi: 10.1007/BF01979465. [DOI] [PubMed] [Google Scholar]
- 12.Pennington J E. Pseudomonas aeruginosa. Vaccines and immunotherapy. Infect Dis Clin North Am. 1990;4:259–270. [PubMed] [Google Scholar]
- 13.Pier G B, Grout M, DesJardins D. Complement deposition by antibodies to Pseudomonas aeruginosa mucoid exopolysaccharide (MEP) and by non-MEP specific opsonins. J Immunol. 1991;147:1869–1876. [PubMed] [Google Scholar]
- 14.Pier G B, Small G J, Warren H B. Protection against mucoid Pseudomonas aeruginosa in rodent models of endobronchial infections. Science. 1990;249:537–540. doi: 10.1126/science.2116663. [DOI] [PubMed] [Google Scholar]
- 15.Pollack M, Koles N L, Preston M J, Brown B J, Pier G B. Functional properties of isotype-switched immunoglobulin M (IgM) and IgG monoclonal antibodies to Pseudomonas aeruginosa lipopolysaccharide. Infect Immun. 1995;63:4481–4488. doi: 10.1128/iai.63.11.4481-4488.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pollack M, Tao M, Akiyama M, Pier G B, Koles N L. In vitro and in vivo functional activities of monoclonal antibodies reactive with Pseudomonas aeruginosa serogroup 6 lipopolysaccharides. Antibiot Chemother. 1991;44:163–171. doi: 10.1159/000420311. [DOI] [PubMed] [Google Scholar]
- 17.Reff M E, Carner K, Chambers K S, Chinn P C, Leonard J E, Raab R, Newman R A, Hanna N, Anderson D R. Depletion of B cells in vivo by a chimeric mouse human antibody to CD20. Blood. 1994;83:435–445. [PubMed] [Google Scholar]
- 18.Zebedee S L, Koduri R K, Mukherjee J, Mukherjee S, Lee S, Sauer D F, Scharff M D, Casadevall A. Mouse-human immunoglobulin G1 chimeric antibodies with activities against Cryptococcus neoformans. Antimicrob Agents Chemother. 1994;38:1507–1514. doi: 10.1128/aac.38.7.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]