Abstract
Rabies is a fatal zoonotic, neurological disease caused by rabies lyssavirus (RABV) and other lyssaviruses. In this study, we established novel serological neutralizing tests (NT) based on vesicular stomatitis virus pseudotypes possessing all 18 known lyssavirus glycoproteins. Applying this system to comparative NT against rabbit sera immunized with current RABV vaccines, we showed that the current RABV vaccines fail to elicit sufficient neutralizing antibodies against lyssaviruses other than to those in phylogroup I. Furthermore, comparative NT against rabbit antisera for 18 lyssavirus glycoproteins showed glycoproteins of some lyssaviruses elicited neutralizing antibodies against a broad range of lyssaviruses. This novel testing system will be useful to comprehensively detect antibodies against lyssaviruses and evaluate their cross-reactivities for developing a future broad-protective vaccine.
Keywords: cross-reactivity, lyssavirus, pseudotype, rabies, vaccine
Rabies is a neglected infectious disease that is responsible for an estimated 59,000 human deaths worldwide each year [26]. The disease in terrestrial animals and humans is primarily caused by the classical rabies lyssavirus (RABV), which is classified under the Genus Lyssavirus within the Subfamily Alpharhabdovirinae, belonging to the Family Rhabdoviridae in the Order Mononegavirales [24]. Once clinical symptoms of rabies appear, the disease is almost invariably fatal [45]. Since the 1950s, numerous lyssaviruses related to RABV have been identified. All lyssaviruses cause neurological disease in mice when infected intracranially under laboratory conditions [3, 19]. To date, 17 lyssavirus species have been documented: RABV, Lagos bat lyssavirus (LBV) in 1956, Mokola lyssavirus (MOKV) in 1968, Duvenhage lyssavirus (DUVV) in 1970, European bat lyssavirus 1 (EBLV-1) in 1977, European bat lyssavirus 2 (EBLV-2) in 1986, Aravan lyssavirus (ARAV) in 1991, Australian bat lyssavirus (ABLV) in 1996, Khujand lyssavirus (KHUV) in 2001, West Caucasian bat lyssavirus (WCBV) and Irkut lyssavirus (IRKV) in 2002, Shimoni bat lyssavirus (SHIBV) and Ikoma lyssavirus (IKOV) in 2009, Bokeloh bat lyssavirus (BBLV) in 2010, Lleida bat lyssavirus (LLEBV) in 2012, Gannoruwa bat lyssavirus (GBLV) in 2016, and Taiwan bat lyssavirus (TWBLV) in 2018 [2, 25, 30]. These viruses are officially recognized by the International Committee on Taxonomy of Viruses [24]. In addition, Kotalahti bat lyssavirus (KBLV) has been recently discovered from a dead Brandt’s bat (Myotis brandtii) in Eastern Finland as a novel lyssavirus [6]. Of these 18 lyssaviruses, 16, (not MOKV or IKOV) have been isolated from bat species [43]. MOKV has been isolated from rodent species [10, 44] and IKOV from the African civet [38]. Until now, at least seven lyssaviruses, RABV, ABLV, DUVV, EBLV-1, EBLV-2, IRKV, and MOKV, have been responsible for fatal infections in humans [42]. While instances of human infection by lyssaviruses other than RABV are rare, they are fatal and the real number of cases is unknown because of limited surveillance and misdiagnosis [8, 37].
Lyssaviruses can be classified into two phylogroups by their genomic sequences [1]. Phylogroup I consists of RABV, ABLV, ARAV, BBLV, DUVV, EBLV-1, EBLV-2, GBLV, IRKV, KBLV, KHUV, and TWBLV, and phylogroup II includes LBV, MOKV, and SHIBV. However, WCBV, IKOV, and LLEBV are unclassified. Historically, research has primarily focused on the cross-reactivity of RABV vaccine immune sera against other lyssaviruses [21]. These investigations have demonstrated that RABV vaccines do not offer protection against other phylogroup lyssaviruses. Consequently, the search for vaccine antigens effective against new lyssaviruses has become imperative. However, there has been limited exploration of cross-reactivity using immune sera tailored to each specific lyssavirus [27]. In our previous study, cross-neutralization activities using only 5 lyssaviruses were compared, suggesting limited cross-reactivities among lyssaviruses [31]. To further validate cross-reactivities among lyssaviruses in detail, comprehensive neutralization assays using all lyssaviruses would need to be conducted, however, it is very difficult to obtain all the viruses to be tested. Therefore, in this study, cross-reactivities among all 18 lyssaviruses were examined using a panel of vesicular stomatitis viruses (VSVs) pseudotyped with all 18 lyssavirus glycoproteins. These tools enabled us to perform neutralization tests (NTs) to conduct a comprehensive analysis of cross-reactivities for the entire range of known lyssaviruses.
First, expression plasmids, each containing a lyssaviral glycoprotein gene, were constructed as described our recent study [31]. Briefly, complete open reading frames encoding glycoproteins of RABV-SRV9 strain (Accession number, AF499686), ARAV (EF614259), BBLV (JF311903), DUVV (JN986749), EBLV-1 (KP241939), EBLV-2 (EF157977), GBLV (KU244266), IRKV (JX442979), KBLV (LR994545), KHUV (EF614261), TWBLV (MF472710), LBV (EU259198), MOKV (NC_006429), SHIBV (GU170201), WCBV (EF614258), IKOV (JX193798), and LLEBV (KY006983) were artificially synthesized (Azenta, Chelmsford, MA, USA) and cloned into the expression plasmid, pCAGGS [40]. The expression plasmid encoding the ABLV (AF426298) glycoprotein was kindly provided by Prof. Christopher C. Broder, Department of Microbiology and Immunology, Uniformed Services University, USA. The NT based on the pseudotyped VSV (VSVp) was developed using VSV pseudotyped with lyssaviral glycoprotein and expressing secreted alkaline phosphatase (SEAP) as a biomarker. The VSVps were generated as previously reported [23, 32]. Briefly, plasmids expressing each glycoprotein were transfected into 80% confluent HEK293T cells using polyethylenimine (PEI) (Thermo Fisher Scientific, Waltham, MA, USA). On two days post-transfection, VSVΔG-SEAP, a recombinant VSV whose G gene was replaced by the SEAP gene was inoculated at a multiplicity of infection of 1. VSVΔG-SEAP was kindly provided by Dr. Y. Matsuura, Osaka University, Japan. After 24 hr, the culture supernatants including each VSVp were collected and filtered through a 0.45 µm syringe filter (MERCK, Darmstadt, Germany) to remove cell debris, and stored at −80°C until use. Each VSVp was named based on its pseudotyped glycoprotein, e.g., VSVp-RABV. The titration of each VSVp was determined by a SEAP reporter assay using substrate solution (SIGMAFAST p-Nitrophenyl Phosphate Tablets, Thermo Fisher Scientific). The NT with each VSVp was performed as previously reported [32]. The neutralization titers are represented as the serum dilution that reduced VSVp infectivity by 75% (IC75) compared with no-serum control. IC75 was calculated by CompuSyn software (ComboSyn Inc., Paramus, NJ, USA). The VSVps for EBLV-1 and IKOV did not yield measurable titers, prompting the creation of chimeric glycoproteins. These chimeric envelope glycoproteins were engineered by fusing the ectodomains and transmembrane domains of the EBLV-1 and IKOV envelope glycoproteins with the cytoplasmic domain from the VSV glycoprotein. The expression plasmid encoding the VSV glycoprotein (AJ318514) was kindly provided by Dr. S. Fukushi, Department of Virology I, National Institute of Infectious Diseases, Japan [23].
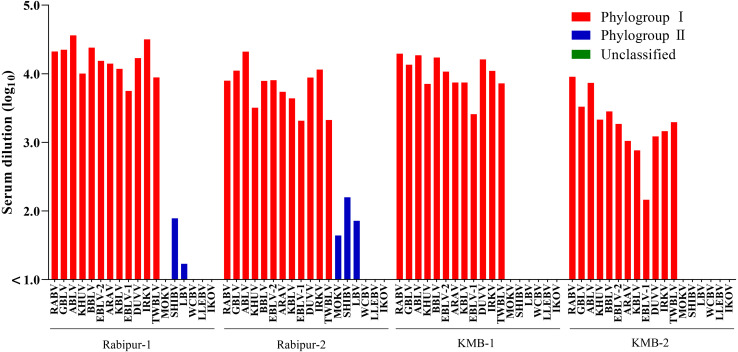
To examine the cross-protective activities of human and animal RABV vaccines against lyssaviruses, comparative NT was conducted between all the VSVps and a panel of rabbit antisera generated by RABV vaccination in our previous study [31]: Briefly, the results of NTs using VSVps indicated that the sera from rabbits immunized with the human vaccine, Rabipur, had high neutralization titers against RABV. These sera also displayed cross neutralizing reactions against other phylogroup I lyssaviruses, with titers within a four-fold range of those against RABV (Fig. 1, Table 1). In contrast, neutralization titers against phylogroup II and unclassified lyssaviruses were over 100 times lower than those against RABV, or below the detection limit. Sera from rabbits immunized with the animal vaccine, KMB, showed a trend similar to that of Rabipur-immunized rabbit sera (Fig. 1, Table 1). These findings indicate that the current rabies vaccines are effective at inducing high serum neutralization titers against lyssaviruses in phylogroup I but have limited to no efficacy against phylogroup II and unclassified lyssaviruses.
Fig. 1.
Comparison of neutralizing titers of Rabies lyssavirus (RABV) vaccine-immunized rabbit sera against 18 lyssaviruses. Serum neutralization tests using sera from two rabbits immunized with human RABV vaccine (Rabipur-1 and -2) and two rabbits immunized with animal RABV vaccine (KMB-1 and -2) were conducted against vesicular stomatitis viruses pseudotyped with 18 lyssaviruses. The titers are shown as the geometric mean of two independent experiments.
Table 1. Neutralization titers against 18 pseudotyped virus.
| Rabbit antiserum | Phylogroup | Neutralization titers against VSVp (IC75) |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I |
II |
Unclassified |
|||||||||||||||||
| RABV | GBLV | ABLV | KHUV | BBLV | EBLV-2 | ARAV | KBLV | EBLV-1 | DUVV | IRKV | TWBLV | MOKV | SHIBV | LBV | WCBV | LLEBV | IKOV | ||
| RABV-G | I | 2,187 | 726 | 2,475 | 444 | 951 | 1,348 | 468 | 450 | 89 | 1,211 | 1,989 | 135 | <10 | <10 | <10 | <10 | <10 | <10 |
| GBLV-G | I | 10,399 | 23,970 | 40,105 | 11,897 | 22,308 | 4,739 | 5,312 | 7,632 | 874 | 20 | 3,662 | 8,174 | 49 | 520 | 30 | 213 | <10 | <10 |
| ABLV-G | I | 7,671 | 37,844 | 81,190 | 19,883 | 12,229 | 8,733 | 7,664 | 9,583 | 2,229 | 7,026 | 10,193 | 456 | 760 | 792 | 470 | <10 | <10 | <10 |
| KHUV-G | I | 487 | 3,985 | 9,228 | 11,342 | 1,954 | 1,455 | 1,931 | 3,501 | 155 | 51 | 443 | 166 | 352 | 937 | 228 | <10 | <10 | <10 |
| BBLV-G | I | 4,489 | 24,544 | 37,041 | 16,180 | 32,422 | 11,573 | 11,660 | 12,695 | 3,026 | 15,360 | 11,753 | 7,289 | 93 | 191 | 330 | <10 | <10 | <10 |
| EBLV-2-G | I | 1,743 | 12,916 | 34,401 | 9,532 | 9,250 | 13,190 | 10,699 | 6,968 | 5,588 | 1,402 | 3,263 | 749 | 272 | 1,264 | 294 | 38 | <10 | <10 |
| ARAV-G | I | 5,052 | 9,050 | 16,112 | 5,086 | 6,592 | 3,763 | 15,416 | 4,985 | 2,014 | 4,187 | 9,732 | 5,531 | <10 | 26 | 14 | <10 | <10 | <10 |
| KBLV-G | I | 1,082 | 4,902 | 16,737 | 3,787 | 2,358 | 3,208 | 1,518 | 8,643 | 370 | 777 | 421 | 1,169 | <10 | <10 | 12 | <10 | <10 | <10 |
| EBLV-1-G | I | 970 | 2,524 | 5,805 | 4,365 | 3,410 | 3,088 | 2,266 | 3,563 | 4,012 | 2,145 | 5,894 | 1,191 | 688 | 950 | 412 | 27 | <10 | <10 |
| DUVV-G | I | 4,880 | 19,159 | 59,446 | 38,658 | 29,820 | 47,743 | 37,519 | 35,864 | 17,659 | 116,600 | 60,585 | 2,325 | 43 | 353 | 164 | <10 | <10 | <10 |
| IRKV-G | I | 1,363 | 1,288 | 2,705 | 1,559 | 1,278 | 1,338 | 733 | 1,149 | 620 | 1,583 | 7,878 | 756 | 53 | 10 | 167 | <10 | <10 | <10 |
| TWBLV-G | I | 5,886 | 14,278 | 20,533 | 5,981 | 10,028 | 6,499 | 5,640 | 9,130 | 1,821 | 5,301 | 6,321 | 44,684 | 104 | 92 | 87 | <10 | <10 | <10 |
| MOKV-G | II | 956 | 959 | 7,125 | 3,146 | 570 | 1,592 | 529 | 1,657 | 30 | 32 | 74 | 299 | 201,967 | 4,311 | 2,924 | 67 | <10 | 24 |
| SHIBV-G | II | 14,254 | 43,130 | 64,102 | 43,625 | 12,745 | 3,711 | 9,606 | 10,610 | 888 | 604 | 11,297 | 911 | 36,372 | 512,586 | 73,811 | <10 | <10 | <10 |
| LBV-G | II | 166 | 553 | 1,491 | 278 | 223 | 123 | 33 | 272 | 20 | 29 | 195 | 63 | 154 | 4,697 | 5,103 | <10 | <10 | 42 |
| WCBV-G | Unclassified | 148 | 1,559 | 3,716 | 2,067 | 2,437 | 2,910 | 86 | 617 | 359 | 40 | 177 | 48 | 70 | 45 | 138 | 8,116 | <10 | 45 |
| LLEBV-G | Unclassified | <10 | <10 | <10 | 23 | 56 | 295 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | 39,418 | 2,867 |
| IKOV-G | Unclassified | <10 | <10 | <10 | <10 | <10 | 31 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | 327 | 12 | <10 | 17 | 22,752 |
| Rabipur-1 | I (vaccine) | 21,066 | 22,289 | 36,116 | 10,046 | 23,967 | 15,403 | 14,030 | 11,803 | 5,624 | 16,880 | 31,772 | 8,860 | <10 | 78 | 17 | <10 | <10 | <10 |
| Rabipur-2 | I (vaccine) | 7,887 | 11,055 | 20,947 | 3,193 | 7,848 | 8,059 | 5,446 | 4,386 | 2,067 | 8,791 | 11,494 | 2,120 | 44 | 158 | 72 | <10 | <10 | <10 |
| KMB-1 | I (vaccine) | 19,532 | 13,531 | 18,577 | 7,089 | 17,253 | 10,750 | 7,458 | 7,471 | 2,578 | 16,155 | 11,008 | 7,247 | <10 | <10 | <10 | <10 | <10 | <10 |
| KMB-2 | I (vaccine) | 9,004 | 3,295 | 7,359 | 2,143 | 2,821 | 1,854 | 1,052 | 766 | 146 | 1,223 | 1,456 | 1,965 | <10 | <10 | <10 | <10 | <10 | <10 |
The abbreviations for the 18 pseudotyped viruses (VSVp) and antisera against each lyssavirus glycoprotein (-G) are listed below: RABV, Rabies lyssavirus; GBLV, Gannoruwa bat lyssavirus; ABLV, Australian bat lyssavirus; KHUV, Khujand lyssavirus; BBLV, Bokeloh bat lyssavirus; EBLV-2, European bat lyssavirus 2; ARAV, Aravan lyssavirus; KBLV, Kotalahti bat lyssavirus; EBLV-1, European bat lyssavirus 1; DUVV, Duvenhage lyssavirus; IRKV, Irkut lyssavirus; TWBLV, Taiwan bat lyssavirus; MOKV, Mokola lyssavirus; SHIBV, Shimoni bat lyssavirus; LBV, Lagos bat lyssavirus; WCBV, West Caucasian bat lyssavirus; LLEBV, Lleida bat lyssavirus; IKOV, Ikoma lyssavirus. The sera of the rabbits immunized with human rabies vaccine (Rabipur) and animal rabies vaccine (KMB) were also used.
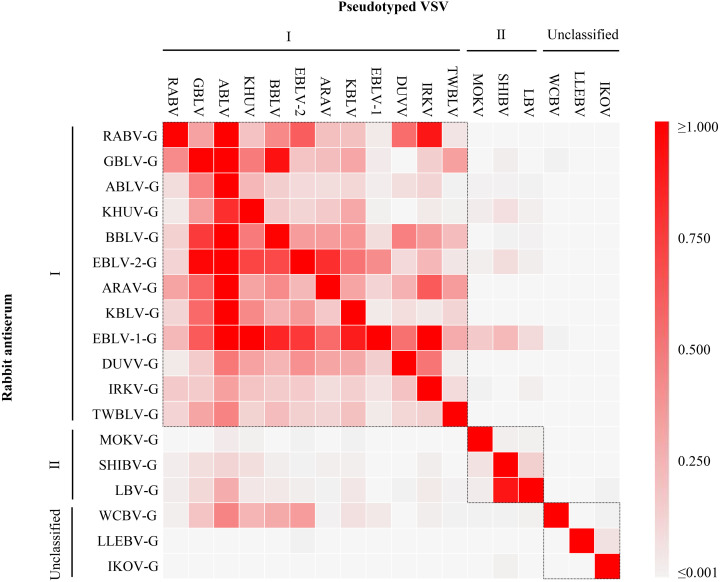
Next, to investigate whether any lyssavirus glycoproteins can induce broad protective antibodies, cross neutralization reactivity among lyssaviruses was comprehensively analyzed using 18 VSVp and polyclonal anti-glycoprotein sera. These antisera were generated in our previous study [31]. Briefly, eighteen female Japanese white rabbits were inoculated six times with a glycoprotein-encoding expression plasmid (one glycoprotein-encoding plasmid per rabbit). For each inoculation, 400 µg of the expression plasmid was mixed with 100 µg of PEI in Opti-MEM (Thermo Fisher Scientific), and the mixture was administered to the rabbits at two-week intervals [33]. Blood samples were collected for serum two weeks following the final injection (permission numbers: 120146, 121128, and 122165). Anti-RABV glycoprotein (CVS-11 strain) rabbit serum was prepared as previously described [29]. NTs using the VSVps with rabbit sera against the glycoproteins of all 18 lyssaviruses revealed specific patterns of cross-reactivity according to their phylogroups: sera directed against glycoproteins from phylogroup I lyssaviruses exhibited high neutralization titers against VSVps of the same phylogroup, yet they showed reduced neutralizing ability against phylogroup II. Notably, the neutralizing titers against VSVps of unclassified lyssaviruses, WCBV, LLEBV, and IKOV, were nearly undetectable (Fig. 2, Table 1). Similarly, antisera against phylogroup II glycoproteins showed strong neutralization against their corresponding VSVps but weaker neutralization against phylogroup I. Almost no neutralizing activity was observed against the VSVps of the unclassified lyssaviruses. In contrast, within the same phylogroup, some discrepancies in cross-reactivity were observed: rabbit sera against GBLV and KHUV glycoproteins did not neutralize VSVp-DUVV, even though they belong to the same phylogroup I. Similarly, antiserum against MOKV glycoprotein exhibited limited cross-reactivity with VSVp-SHIBV and LBV, which are part of phylogroup II. Interestingly, antisera against EBLV-1 glycoprotein demonstrated a broad neutralization capacity, affecting both phylogroup I and II VSVps. In contrast, VSVps of unclassified lyssaviruses showed unique reactivity: antisera against IKOV and LLEBV glycoproteins showed almost no cross-reactivity with any of the VSVps tested. Interestingly, the antiserum against WCBV glycoprotein, despite being an unclassified lyssavirus, was capable of neutralizing several VSVps of phylogroup I (Fig. 2, Table 1).
Fig. 2.
Summary of cross-reactivity in serum neutralization tests among 18 lyssaviruses. The relative neutralizing titers were compared in all combinations between 18 pseudotyped viruses (VSVps) and 18 rabbit sera against each lyssavirus glycoprotein (-G). The relative neutralizing titers of each combination were calculated as the ratio of the neutralizing antibody titer against the corresponding VSVp set as 1.000, and illustrated by the color gradient: red signifying high cross-reactivity to white denoting no cross-reactivity. Phylogroups are enclosed with dotted line.
This comprehensive study highlights the challenges and the innovations needed to evaluating the cross-reactivity of lyssaviruses. Conventional methods, such as the Rapid Fluorescent Focus Inhibition Test and the Fluorescent Antibody Virus Neutralization test, which are considered gold standards by the World Health Organization and The World Organisation for Animal Health [7, 39, 47] are time-consuming, and require biosafety level−3 facilities and expensive reagents, such as fluorescent antibodies. Pseudotyped rabies viruses with either green fluorescent protein or luciferase as a biomarker have been successfully generated and employed for high-throughput screening [5, 48]. The pseudotyped virus expressing SEAP as a biomarker, which we utilized in this study, can be quantified using an absorbance system, such as ELISA, and it offers a straightforward and cost-effective alternative. In a recent investigation related to SARS-CoV-2, the pseudotyped virus neutralization antibody titers were regarded as the most reliable indicator of vaccine efficacy and protection, primarily because of the remarkable sensitivity of NT-based pseudotyped virus [4].
In this study, even the hyperimmune sera generated with six RABV vaccinations, failed to exhibit cross-reactivity with phylogroup II and unclassified lyssavirus, indicating the limitation of RABV vaccination against lyssavirus infections. In a previous report, cats with a history of three RABV vaccinations were infected with LBV belonging phylogroup II. The cats were euthanized after a 3-day illness characterized by neurological symptoms [16]. In addition, most sera from humans inoculated with the RABV vaccine did not possess virus neutralization activity against lyssaviruses belonging to different phylogroups [11, 36]. These findings indicate that existing pre-exposure prophylaxis (PrEP) and post-exposure prophylaxis (PEP) measures for use with RABV vaccines may not be effective in preventing infections caused by phylogroup II and unclassified lyssaviruses.
The comprehensive NT in this study offers critical insights into the complexity of lyssavirus immunology and the risk of relying solely on genetic homology for predicting antigenic cross-reactivity. While lyssaviruses within the same phylogroup generally exhibit a relatively high degree of glycoprotein amino acid homology [14], the observed lack of cross-neutralization among certain lyssaviruses (e.g., GBLV, KHUV, and DUVV) despite being in the same phylogroup indicates that small variations in glycoprotein amino acids, especially in neutralizing epitopes, can lead to significant changes in antigenic structure. While the precise locations of neutralizing epitopes in RABV glycoprotein (RABV-G) have been identified using techniques such as mutagenesis and monoclonal antibodies (mAbs), the locations of the neutralizing epitopes in other lyssavirus glycoproteins are only inferred based on their known positions in RABV-G [12, 34]. Considering the difficulty to predict cross-reactivity among lyssaviruses solely on amino acid sequence homology of whole glycoproteins, further studies on detailed analysis of antigenic structures of each lyssavirus are expected.
Lyssaviruses other than RABV have been reported in a limited number of human infections. MOKV from phylogroup II was responsible for an infection in an infant that led to a fatal outcome. Furthermore, infections with neurological signs in companion animals have also been reported, including cases of LBV from phylogroup II affecting dogs and cats [9], and WCBV, an unclassified strain, infecting cats [35]. It has become clear that “rabies free” countries [18], have endemic lyssaviruses circulating within bat populations, such as ABLV in Australia and EBLV-1, 2 in the UK [17, 41, 46]. In both nations humans have died from lyssavirus infection as a result of bat bites [20, 22]. These matters highlight the need for the development of pan-lyssavirus vaccines capable of providing protection against all lyssaviruses.
The lyssavirus glycoprotein is instrumental in triggering the production of neutralizing antibodies [28]. Notably, monoclonal antibodies (mAbs) from individuals who received the RABV vaccine have recently been isolated, and some of these mAbs have demonstrated broad-spectrum neutralization activity against various lyssaviruses [11, 28]. Additionally, there have been reports on the immunogenicity of chimeric glycoproteins possessing neutralizing epitope sites of the G protein of MOKV or LBV (phylogroup II) and RABV-G (phylogroup I) in various combinations, which succeeded in acquiring broad cross-reactivities against both phylogroups I and II [13, 15]. This suggested that the detailed analysis of the reactivity of each lyssavirus glycoprotein other than RABV-G could be utilized to develop broad-reactive vaccines. Our findings of broad-spectrum neutralization by EBLV-1 and WCBV antisera are particularly promising, as they may be able to guide the development of a pan-lyssavirus vaccine. The identification of glycoproteins that elicit cross-protective antibodies may serve as the basis for a next-generation vaccine design that would offer protection against a range of lyssaviruses, not just RABV. This novel testing system will be useful to comprehensively detect antibodies against lyssaviruses and evaluate their cross-reactivities for developing a future broad-protective vaccine.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Acknowledgments
This study was supported by grants from the Japan Agency for Medical Research and Development (grant no. JP22fk0108141 and JP23fk0108615), and a Health Labor Sciences Research Grant (grant no. 22HA1005).
REFERENCES
- 1.Badrane H, Bahloul C, Perrin P, Tordo N. 2001. Evidence of two Lyssavirus phylogroups with distinct pathogenicity and immunogenicity. J Virol 75: 3268–3276. doi: 10.1128/JVI.75.7.3268-3276.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banyard AC, Evans JS, Luo TR, Fooks AR. 2014. Lyssaviruses and bats: emergence and zoonotic threat. Viruses 6: 2974–2990. doi: 10.3390/v6082974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banyard AC, Selden D, Wu G, Thorne L, Jennings D, Marston D, Finke S, Freuling CM, Müller T, Echevarría JE, Fooks AR. 2018. Isolation, antigenicity and immunogenicity of Lleida bat lyssavirus. J Gen Virol 99: 1590–1599. doi: 10.1099/jgv.0.001068 [DOI] [PubMed] [Google Scholar]
- 4.Benkeser D, Montefiori DC, McDermott AB, Fong Y, Janes HE, Deng W, Zhou H, Houchens CR, Martins K, Jayashankar L, Castellino F, Flach B, Lin BC, O’Connell S, McDanal C, Eaton A, Sarzotti-Kelsoe M, Lu Y, Yu C, Borate B, van der Laan LWP, Hejazi NS, Kenny A, Carone M, Williamson BD, Garver J, Altonen E, Rudge T, Huynh C, Miller J, El Sahly HM, Baden LR, Frey S, Malkin E, Spector SA, Andrasik MP, Kublin JG, Corey L, Neuzil KM, Carpp LN, Pajon R, Follmann D, Donis RO, Koup RA, Gilbert PB. Immune Assays Moderna Inc. Coronavirus Vaccine Prevention Network (CoVPN)/Coronavirus Efficacy (COVE) United States Government (USG)/CoVPN Biostatistics Teams.2023. Comparing antibody assays as correlates of protection against COVID-19 in the COVE mRNA-1273 vaccine efficacy trial. Sci Transl Med 15: eade9078. doi: 10.1126/scitranslmed.ade9078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cai M, Liu H, Jiang F, Sun Y, Wang W, An Y, Zhang M, Li X, Liu D, Li Y, Yu Y, Huang W, Wang Y. 2022. Analysis of the evolution, infectivity and antigenicity of circulating rabies virus strains. Emerg Microbes Infect 11: 1474–1487. doi: 10.1080/22221751.2022.2078742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calvelage S, Tammiranta N, Nokireki T, Gadd T, Eggerbauer E, Zaeck LM, Potratz M, Wylezich C, Höper D, Müller T, Finke S, Freuling CM. 2021. Genetic and antigenetic characterization of the novel kotalahti bat lyssavirus (KBLV). Viruses 13: 69. doi: 10.3390/v13010069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ciconello FN, Katz ISS, Fernandes ER, Guedes F, Silva SR. 2022. A comparative review of serological assays for the detection of rabies virus-specific antibodies. Acta Trop 226: 106254. doi: 10.1016/j.actatropica.2021.106254 [DOI] [PubMed] [Google Scholar]
- 8.Cleaveland S, Fèvre EM, Kaare M, Coleman PG. 2002. Estimating human rabies mortality in the United Republic of Tanzania from dog bite injuries. Bull World Health Organ 80: 304–310. [PMC free article] [PubMed] [Google Scholar]
- 9.Coertse J, Geldenhuys M, le Roux K, Markotter W. 2021. Lagos bat virus, an under-reported rabies-related lyssavirus. Viruses 13: 576. doi: 10.3390/v13040576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coertse J, Markotter W, le Roux K, Stewart D, Sabeta CT, Nel LH. 2017. New isolations of the rabies-related Mokola virus from South Africa. BMC Vet Res 13: 37. doi: 10.1186/s12917-017-0948-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Benedictis P, Minola A, Rota Nodari E, Aiello R, Zecchin B, Salomoni A, Foglierini M, Agatic G, Vanzetta F, Lavenir R, Lepelletier A, Bentley E, Weiss R, Cattoli G, Capua I, Sallusto F, Wright E, Lanzavecchia A, Bourhy H, Corti D. 2016. Development of broad-spectrum human monoclonal antibodies for rabies post-exposure prophylaxis. EMBO Mol Med 8: 407–421. doi: 10.15252/emmm.201505986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dietzschold B, Gore M, Casali P, Ueki Y, Rupprecht CE, Notkins AL, Koprowski H. 1990. Biological characterization of human monoclonal antibodies to rabies virus. J Virol 64: 3087–3090. doi: 10.1128/jvi.64.6.3087-3090.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evans JS, Selden D, Wu G, Wright E, Horton DL, Fooks AR, Banyard AC. 2018. Antigenic site changes in the rabies virus glycoprotein dictates functionality and neutralizing capability against divergent lyssaviruses. J Gen Virol 99: 169–180. doi: 10.1099/jgv.0.000998 [DOI] [PubMed] [Google Scholar]
- 14.Evans JS, Horton DL, Easton AJ, Fooks AR, Banyard AC. 2012. Rabies virus vaccines: is there a need for a pan-lyssavirus vaccine? Vaccine 30: 7447–7454. doi: 10.1016/j.vaccine.2012.10.015 [DOI] [PubMed] [Google Scholar]
- 15.Fisher CR, Lowe DE, Smith TG, Yang Y, Hutson CL, Wirblich C, Cingolani G, Schnell MJ. 2020. Lyssavirus Vaccine with a Chimeric Glycoprotein Protects across Phylogroups. Cell Rep 32: 107920. doi: 10.1016/j.celrep.2020.107920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foggin CM. 1988. Rabies and Rabies-Related Viruses in Zimbabwe: Historical, Virological and Ecological Aspects. pp. 186–221. University of Zimbabwe, Harare. [Google Scholar]
- 17.Folly AJ, Marston DA, Golding M, Shukla S, Wilkie R, Lean FZX, Núñez A, Worledge L, Aegerter J, Banyard AC, Fooks AR, Johnson N, McElhinney LM. 2021. Incursion of European bat lyssavirus 1 (EBLV-1) in serotine bats in the United Kingdom. Viruses 13: 1979. doi: 10.3390/v13101979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fooks AR, Banyard AC, Horton DL, Johnson N, McElhinney LM, Jackson AC. 2014. Current status of rabies and prospects for elimination. Lancet 384: 1389–1399. doi: 10.1016/S0140-6736(13)62707-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fooks AR, Cliquet F, Finke S, Freuling C, Hemachudha T, Mani RS, Müller T, Nadin-Davis S, Picard-Meyer E, Wilde H, Banyard AC. 2017. Rabies. Nat Rev Dis Primers 3: 17091. doi: 10.1038/nrdp.2017.91 [DOI] [PubMed] [Google Scholar]
- 20.Fooks AR, McElhinney LM, Pounder DJ, Finnegan CJ, Mansfield K, Johnson N, Brookes SM, Parsons G, White K, McIntyre PG, Nathwani D. 2003. Case report: isolation of a European bat lyssavirus type 2a from a fatal human case of rabies encephalitis. J Med Virol 71: 281–289. doi: 10.1002/jmv.10481 [DOI] [PubMed] [Google Scholar]
- 21.Fooks AR, Shipley R, Markotter W, Tordo N, Freuling CM, Müller T, McElhinney LM, Banyard AC, Rupprecht CE. 2021. Renewed public health threat from emerging lyssaviruses. Viruses 13: 1769. doi: 10.3390/v13091769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fraser GC, Hooper PT, Lunt RA, Gould AR, Gleeson LJ, Hyatt AD, Russell GM, Kattenbelt JA. 1996. Encephalitis caused by a Lyssavirus in fruit bats in Australia. Emerg Infect Dis 2: 327–331. doi: 10.3201/eid0204.960408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fukushi S, Watanabe R, Taguchi F. 2008. Pseudotyped vesicular stomatitis virus for analysis of virus entry mediated by SARS coronavirus spike proteins. Methods Mol Biol 454: 331–338. doi: 10.1007/978-1-59745-181-9_23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Genus: Lyssavirus. ICTV.https://ictv.global/report/chapter/rhabdoviridae/rhabdoviridae/lyssavirus [accessed on November 10, 2023].
- 25.Gunawardena PS, Marston DA, Ellis RJ, Wise EL, Karawita AC, Breed AC, McElhinney LM, Johnson N, Banyard AC, Fooks AR. 2016. Lyssavirus in Indian flying foxes, Sri Lanka. Emerg Infect Dis 22: 1456–1459. doi: 10.3201/eid2208.151986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hampson K, Coudeville L, Lembo T, Sambo M, Kieffer A, Attlan M, Barrat J, Blanton JD, Briggs DJ, Cleaveland S, Costa P, Freuling CM, Hiby E, Knopf L, Leanes F, Meslin FX, Metlin A, Miranda ME, Müller T, Nel LH, Recuenco S, Rupprecht CE, Schumacher C, Taylor L, Vigilato MA, Zinsstag J, Dushoff J. Global Alliance for Rabies Control Partners for Rabies Prevention.2015. Estimating the global burden of endemic canine rabies. PLoS Negl Trop Dis 9: e0003709. doi: 10.1371/journal.pntd.0003709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanlon CA, Kuzmin IV, Blanton JD, Weldon WC, Manangan JS, Rupprecht CE. 2005. Efficacy of rabies biologics against new lyssaviruses from Eurasia. Virus Res 111: 44–54. doi: 10.1016/j.virusres.2005.03.009 [DOI] [PubMed] [Google Scholar]
- 28.Hellert J, Buchrieser J, Larrous F, Minola A, de Melo GD, Soriaga L, England P, Haouz A, Telenti A, Schwartz O, Corti D, Bourhy H, Rey FA. 2020. Structure of the prefusion-locking broadly neutralizing antibody RVC20 bound to the rabies virus glycoprotein. Nat Commun 11: 596. doi: 10.1038/s41467-020-14398-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hotta K, Motoi Y, Okutani A, Kaku Y, Noguchi A, Inoue S, Yamada A. 2007. Role of GPI-anchored NCAM-120 in rabies virus infection. Microbes Infect 9: 167–174. doi: 10.1016/j.micinf.2006.11.003 [DOI] [PubMed] [Google Scholar]
- 30.Hu SC, Hsu CL, Lee MS, Tu YC, Chang JC, Wu CH, Lee SH, Ting LJ, Tsai KR, Cheng MC, Tu WJ, Hsu WC. 2018. Lyssavirus in Japanese Pipistrelle, Taiwan. Emerg Infect Dis 24: 782–785. doi: 10.3201/eid2404.171696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inoue Y, Kaku Y, Harada M, Ishijima K, Kuroda Y, Tatemoto K, Mendoza VM, Nishino A, Yamamoto T, Inoue S, Matsuu A, Maeda K. 2023. Cross-neutralization activities of antibodies against 18 lyssavirus glycoproteins. Jpn J Infect Dis (In press). [DOI] [PubMed] [Google Scholar]
- 32.Kaku Y, Noguchi A, Marsh GA, Barr JA, Okutani A, Hotta K, Bazartseren B, Fukushi S, Broder CC, Yamada A, Inoue S, Wang LF. 2012. Second generation of pseudotype-based serum neutralization assay for Nipah virus antibodies: sensitive and high-throughput analysis utilizing secreted alkaline phosphatase. J Virol Methods 179: 226–232. doi: 10.1016/j.jviromet.2011.11.003 [DOI] [PubMed] [Google Scholar]
- 33.Kaku Y, Noguchi A, Marsh GA, McEachern JA, Okutani A, Hotta K, Bazartseren B, Fukushi S, Broder CC, Yamada A, Inoue S, Wang LF. 2009. A neutralization test for specific detection of Nipah virus antibodies using pseudotyped vesicular stomatitis virus expressing green fluorescent protein. J Virol Methods 160: 7–13. doi: 10.1016/j.jviromet.2009.04.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lafon M, Wiktor TJ, Macfarlan RI. 1983. Antigenic sites on the CVS rabies virus glycoprotein: analysis with monoclonal antibodies. J Gen Virol 64: 843–851. doi: 10.1099/0022-1317-64-4-843 [DOI] [PubMed] [Google Scholar]
- 35.Leopardi S, Barneschi E, Manna G, Zecchin B, Priori P, Drzewnioková P, Festa F, Lombardo A, Parca F, Scaravelli D, Maroni Ponti A, De Benedictis P. 2021. Spillover of West caucasian bat lyssavirus (WCBV) in a domestic cat and Westward expansion in the palearctic region. Viruses 13: 2064. doi: 10.3390/v13102064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malerczyk C, Freuling C, Gniel D, Giesen A, Selhorst T, Müller T. 2014. Cross-neutralization of antibodies induced by vaccination with Purified Chick Embryo Cell Vaccine (PCECV) against different Lyssavirus species. Hum Vaccin Immunother 10: 2799–2804. doi: 10.4161/21645515.2014.972741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mallewa M, Fooks AR, Banda D, Chikungwa P, Mankhambo L, Molyneux E, Molyneux ME, Solomon T. 2007. Rabies encephalitis in malaria-endemic area, Malawi, Africa. Emerg Infect Dis 13: 136–139. doi: 10.3201/eid1301.060810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marston DA, Ellis RJ, Horton DL, Kuzmin IV, Wise EL, McElhinney LM, Banyard AC, Ngeleja C, Keyyu J, Cleaveland S, Lembo T, Rupprecht CE, Fooks AR. 2012. Complete genome sequence of Ikoma lyssavirus. J Virol 86: 10242–10243. doi: 10.1128/JVI.01628-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moore SM, Hanlon CA. 2010. Rabies-specific antibodies: measuring surrogates of protection against a fatal disease. PLoS Negl Trop Dis 4: e595. doi: 10.1371/journal.pntd.0000595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Niwa H, Yamamura K, Miyazaki J. 1991. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108: 193–199. doi: 10.1016/0378-1119(91)90434-D [DOI] [PubMed] [Google Scholar]
- 41.Prada D, Boyd V, Baker M, Jackson B, O’Dea M. 2019. Insights into Australian bat lyssavirus in insectivorous bats of Western Australia. Trop Med Infect Dis 4: 46. doi: 10.3390/tropicalmed4010046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shepherd JG, Davis C, Streicker DG, Thomson EC. 2023. Emerging rhabdoviruses and human infection. Biology (Basel) 12: 878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shipley R, Wright E, Selden D, Wu G, Aegerter J, Fooks AR, Banyard AC. 2019. Bats and viruses: emergence of novel lyssaviruses and association of bats with viral zoonoses in the EU. Trop Med Infect Dis 4: 31. doi: 10.3390/tropicalmed4010031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shope RE, Murphy FA, Harrison AK, Causey OR, Kemp GE, Simpson DI, Moore DL. 1970. Two African viruses serologically and morphologically related to rabies virus. J Virol 6: 690–692. doi: 10.1128/jvi.6.5.690-692.1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Warrell MJ, Warrell DA. 2015. Rabies: the clinical features, management and prevention of the classic zoonosis. Clin Med (Lond) 15: 78–81. doi: 10.7861/clinmedicine.14-6-78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wise EL, Marston DA, Banyard AC, Goharriz H, Selden D, Maclaren N, Goddard T, Johnson N, McElhinney LM, Brouwer A, Aegerter JN, Smith GC, Horton DL, Breed AC, Fooks AR. 2017. Passive surveillance of United Kingdom bats for lyssaviruses (2005–2015). Epidemiol Infect 145: 2445–2457. doi: 10.1017/S0950268817001455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.World Health Organization.2018. Rabies vaccines: WHO position paper, April 2018-recommendations. Vaccine 36: 5500–5503. doi: 10.1016/j.vaccine.2018.06.061 [DOI] [PubMed] [Google Scholar]
- 48.Wright E, Temperton NJ, Marston DA, McElhinney LM, Fooks AR, Weiss RA. 2008. Investigating antibody neutralization of lyssaviruses using lentiviral pseudotypes: a cross-species comparison. J Gen Virol 89: 2204–2213. doi: 10.1099/vir.0.2008/000349-0 [DOI] [PMC free article] [PubMed] [Google Scholar]