Abstract
Purpose
The aim of the study was to identify the characteristics of patients infected with coronavirus disease 2019 (COVID-19) and to determine risk factors for COVID-19 infection in Japanese patients.
Patients and Methods
We conducted a single-center retrospective observational study in Japanese adult patients (≥20 years) who visited Kenwakai Hospital (Nagano Japan). We analyzed data of 378 patients (mean age, 75 ± 14 years; men, 54%) from the hospital’s electronic information system. COVID-19 was diagnosed by polymerase-chain reaction. Patients were divided into 2 groups based on diagnosis of COVID-19.
Results
Patients infected with COVID-19 showed significantly higher rates of men (69.8 vs 51.6%, P = 0.025) than uninfected control patients. After adjustment for possible confounding factors, COVID-19 infection was significantly associated with BUN (odds ratio [OR], 1.02; 95% confidence interval [CI], 1.01–1.03) and serum creatinine (Scr) (OR, 1.14; 95% CI, 1.05–1.24). This association was observed in men (BUN, P = 0.012; Scr, P = 0.012), but not in women (BUN, P = 0.43; Scr, P = 0.54).
Conclusion
BUN and Scr are potential risk factors for infection of COVID-19 in Japanese patients, particularly in men. Our results suggest that renal parameters might be important in Japanese male patients for the early detection of COVID-19 infection.
Keywords: adult patients, COVID-19, risk factors, kidney function, Japanese
Introduction
The coronavirus disease 2019 (COVID-19), which is caused by SARS-CoV-2, was first reported in Wuhan, China, in December 2019.1 COVID-19 has become a worldwide pandemic, causing more than 5 million deaths as of October 2021. COVID-19 infection causes not only respiratory disorder but also disease to other organs such as neurologic manifestations, loss of smell and taste, and myositis.2 The degree of severity ranges from asymptomatic or mild symptomatic symptom to critical condition.3 In addition, even a few months after recovery, more than one-third patients showed late-onset symptoms including muscle aches, joint pains, or ataxia as well as defect of memory, depression, insomnia.4,5 Then, it is important to identify risk factors related to COVID-19 infection.
In a report from Chinese center, age is one important risk factor for COVID-19 infection.3 Importantly, age is also associated with mortality of Chinese patients infected with COVID-19.6 Gender difference is reported to be another risk factor for COVID-19 infection in an Italian retrospective study.7 A cohort study demonstrated that susceptibility to COVID-19 infection had significantly higher risk in individuals with depressive disorder than those without it.8 Ethnicity is possible to be involved in susceptibility to COVID-19 infection. Black ethnic population shows more susceptible to COVID-19.9 This is partly because that they have a high percentage of abnormal expression of angiotensin-converting enzyme 2 (ACE 2), which contributes to the host receptors for SARS-CoV-2. On the other hand, a meta-analysis showed that ACE 2 polymorphism did not exhibit an association with COVID-19 infection risk.10 Few studies have examined the risk factor for COVID-19 infection in Japanese patients. Here, we present a retrospective analysis of clinical characteristics of Japanese patients in an effort to identify risk factors associated with COVID-19 infection.
Materials and Methods
Subjects and Data Collection
This was a single-center retrospective observational cohort study, which was approved by the Ethics Committee of Kenwakai Hospital (No. 2023004) and Osaka Medical and Pharmaceutical University (No. 2023–061) and was conducted in accordance with the Declaration of Helsinki. We recruited eligible patients (≥20 years) who visited Kenwakai Hospital (Nagano, Japan) between 1 January 2019 and 28 February 2023. We excluded patients without information of BMI, smoking status, or blood pressure (BP). Finally, the remaining 378 patients were included in the present analyses (Figure 1).
Figure 1.
Flow chart describing the identification of the study cohort.
Abbreviation: BMI, body mass index.
In the daily practice, COVID-19 was diagnosed by polymerase-chain reaction (PCR). The electronic medical chart was carried out to collect data of patients infected with COVID-19 and non-infected with it. Collected data comprised demographical data, clinical laboratory data, diagnosis, and medication history. Patients were divided into 2 groups based on diagnosis of COVID-19: patients infected with COVID-19 (n = 43) and those without it (n = 335). The renal function was estimated by the following equation of estimated glomerular filtration rate (eGFR).11
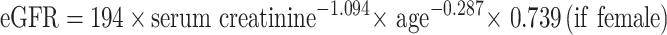 |
Statistical Analysis
Continuous variables were expressed as means ± standard deviation (SD) or medians (interquartile ranges). The significance was tested using the Student's t-test or the Mann–Whitney U-test. Categorical variables were expressed as numbers (%) and compared using the chi-squared test. Odds ratios (ORs) and 95% confidence intervals (CIs) were analyzed using logistic regression models. Significance was defined as a P value <0.05 (two-tailed). All statistical analyses were performed using SPSS for Windows software (ver. 19.0; SPSS Inc., Tokyo, Japan).
Results
Patients’ Characteristics
A total of 522 adult patients visited to Kenwakai Hospital between 1 January 2019 and 28 February 2023. In accordance with the inclusion criteria, 378 patients were eligible for the analysis cohort (Figure 1). The characteristics of patients are shown in Table 1. In our study cohort, the mean age was 75 years and 54% were men. Regarding the type of pharmacotherapy, 84 patients (22%), 147 (39%), 294 (78%), 54 (14%), 159 (42%), 5 (1.3%), 59 (16%), and 6 (1.6%) received anti-diabetic drugs, anti-hyperlipidemic drugs, anti-hypertensive drugs, anticoagulant and antiplatelet drugs, angiotensin II receptor blocker (ARB), angiotensin-converting enzyme (ACE) inhibitors, aspirin, or dexamethasone, respectively. Among 378 patients, 43 (11.4%) were diagnosed with COVID-19 infection by PCR confirmed tests for the SARS-CoV-2 virus.
Table 1.
Characteristics of Patients
| Variables | Total (n = 378) | Covid (+) | Covid (-) | P-value |
|---|---|---|---|---|
| n = 43 | n = 335 | |||
| Age, years | 75 ± 14 | 74 ± 14 | 75 ± 14 | 0.94 |
| Men, n (%) | 203 (54) | 30 (69.8) | 173 (51.6) | 0.025 |
| BMI, kg/m2 | 22 ± 3.9 | 22 ± 3.8 | 22 ± 3.9 | 0.96 |
| Smoking, n (%) | 28 (7.4) | 1 (2.3) | 27 (8.1) | 0.15 |
| Comorbidities | ||||
| Diabetes, n (%) | 145 (38) | 18 (42) | 127 (38) | 0.62 |
| Heart failure, n (%) | 98 (26) | 14 (33) | 84 (25) | 0.29 |
| Cerebral infarction, n (%) | 85 (22) | 10 (23) | 75 (22) | 0.90 |
| Cerebral hemorrhage, n (%) | 29 (7.7) | 5 (12) | 24 (7.2) | 0.22 |
| Cancer, n (%) | 53 (14) | 6 (14) | 47 (14) | 0.99 |
| Hypertension, n (%) | 133 (35) | 16 (37) | 117 (35) | 0.77 |
| Dementia, n (%) | 17 (4.5) | 0 (0) | 17 (5.1) | 0.12 |
| Pneumonia, n (%) | 56 (15) | 5 (12) | 51 (15) | 0.53 |
| Dialysis, n (%) | 179 (47) | 26 (60) | 153 (46) | 0.067 |
| Medications | ||||
| Anti-diabetic drugs, n (%) | 84 (22) | 11 (26) | 73 (22) | 0.57 |
| Anti-hyperlipidemic drugs, n (%) | 147 (39) | 17 (40) | 130 (39) | 0.93 |
| Anti-hypertensive drugs, n (%) | 294 (78) | 32 (74) | 262 (78) | 0.57 |
| Anticoagulant and antiplatelet drugs, n (%) | 54 (14) | 8 (19) | 46 (14) | 0.39 |
| ARB, n (%) | 159 (42) | 19 (44) | 140 (42) | 0.77 |
| ACE inhibitors, n (%) | 5 (1.3) | 1 (2.3) | 4 (1.2) | 0.46 |
| Aspirin, n (%) | 59 (16) | 10 (23) | 49 (15) | 0.14 |
| Dexamethasone, n (%) | 6 (1.6) | 1 (2.3) | 5 (1.5) | 0.52 |
| Systolic blood pressure, mmHg | 139 ± 24 | 140 ± 24 | 139 ± 24 | 0.67 |
| Diastolic blood pressure, mmHg | 77 ± 14 | 78 ± 16 | 77 ± 14 | 0.78 |
| Laboratory data | ||||
| Serum Zn, μg/dL | 68 ± 16 | 69 ± 18 | 68 ± 16 | 0.76 |
| Total protein, g/dL | 6.4 ± 0.6 | 6.3 ± 0.49 | 6.4 ± 0.66 | 0.45 |
| Albumin, g/dL | 3.6 ± 0.5 | 3.5 ± 0.44 | 3.6 ± 0.51 | 0.38 |
| T-bil, mg/dL | 0.6 ± 0.3 | 0.52 ± 0.26 | 0.57 ± 0.29 | 0.29 |
| AST, U/L | 20 ± 19 | 18 ± 12 | 20 ± 20 | 0.067 |
| ALT, U/L | 16 ± 15 | 18 ± 21 | 16 ± 14 | 0.66 |
| LDH, U/L | 205 ± 56 | 190 ± 45 | 207 ± 57 | 0.048 |
| Uric acid, mg/dL | 6.5 ± 2.0 | 7.0 ± 1.8 | 6.4 ± 2.0 | 0.077 |
| BUN, mg/dL | 39 ± 26 | 51 ± 41 | 37 ± 24 | 0.020 |
| Scr, mg/dL | 5.1 ± 4.8 | 6.9 ± 5.3 | 4.9 ± 4.7 | 0.011 |
| eGFR, mL/min/1.73 m2 | 36 ± 34 | 27 ± 32 | 37 ± 34 | 0.041 |
| Serum sodium, mEq/L | 140 ± 3.5 | 138 ± 4.6 | 140 ± 3.2 | 0.037 |
| Serum potassium, mEq/L | 4.4 ± 0.6 | 4.5 ± 0.54 | 4.4 ± 0.60 | 0.054 |
| Serum chloride, mEq/L | 104 ± 4.0 | 102 ± 4.2 | 104 ± 4.0 | 0.013 |
| Total cholesterol, mg/dL | 165 ± 35 | 156 ± 27 | 166 ± 36 | 0.16 |
| Triglyceride, mg/dL | 125 ± 86 | 130 ± 72 | 124 ± 87 | 0.29 |
| HDL cholesterol, mg/dL | 55 ± 18 | 47 ± 14 | 56 ± 18 | 0.003 |
| LDL cholesterol, mg/dL | 95 ± 28 | 95 ± 24 | 95 ± 28 | 0.99 |
| β2-microglobulin, mg/L | 26 ± 7.4 | 28 ± 7.0 | 25 ± 7.4 | 0.016 |
| Glucose, mg/dL | 136 ± 46 | 143 ± 51 | 135 ± 46 | 0.36 |
| BNP, pg/mL | 197 ± 350 | 290 ± 501 | 185 ± 325 | 0.25 |
| WBC, 103/μL | 6.2 ± 3.5 | 6.9 ± 2.2 | 6.1 ± 3.7 | 0.2 |
| RBC, 106/μL | 3.8 ± 0.65 | 3.8 ± 0.61 | 3.8 ± 0.65 | 0.75 |
| Hemoglobin, g/dL | 12 ± 1.8 | 12 ± 1.7 | 12 ± 1.9 | 0.76 |
| Hct, % | 35 ± 4.5 | 36 ± 4.4 | 35 ± 4.5 | 0.039 |
| PLT, 103/μL | 209 ± 72 | 210 ± 61 | 209 ± 73 | 0.9 |
Abbreviations: ACE, angiotensin-converting enzyme; ALB, albumin; ALT, alanine transaminase; ARB, angiotensin II receptor blocker; AST, aspartate transaminase; BMI, body mass index; BNP, brain natriuretic peptide; BUN, blood urea nitrogen; eGFR, estimated glomerular filtration rate; Hct, hematocrit; Ht, hematocrit; LDH, lactate dehydrogenase; LDL, low density lipoprotein; HDL, high density lipoprotein; RBC, red blood cells; PLT, platelet; Scr, serum creatinine; T-Bil, total bilirubin; TP, total protein; WBC, white blood cells; Zn, zinc.
Risk Factors Associated with COVID-19 Infection
As shown in Table 1, there were no significant differences in comorbidities, use of medications, and BP between patients infected with COVID-19 and uninfected control patients. As for laboratory data, patients infected with COVID-19 exhibited significantly lower lactate dehydrogenase (LDH), eGFR, serum sodium, serum chloride, and high-density lipoprotein (HDL) cholesterol and higher blood urea nitrogen (BUN), Scr, β2-microglobulin, and hematocrit (Hct) than uninfected control patients.
Next, we analyzed multivariate logistic regression analysis of risk factors for COVID-19 infection (Table 2). The risk for infection of COVID-19 was significantly increased in men (OR, 2.47; 95% CI, 1.18–5.16; P = 0.017), BUN (OR, 1.02; 95% CI, 1.01–1.03; P = 0.002), and Scr (OR, 1.14; 95% CI, 1.05–1.24; P = 0.002) after adjustments for possible confounding factor. Conversely, the risk for infection of COVID-19 was significantly decreased in serum sodium (OR, 0.90; 95% CI, 0.82–0.97; P = 0.008), serum chloride (OR, 0.91; 95% CI, 0.84–0.98; P = 0.012), and HDL cholesterol (OR, 0.96; 95% CI, 0.94–0.99; P = 0.003).
Table 2.
Multivariate Logistic Regression Analysis of Risk Factors for COVID-19
| Variables | Unadjusted OR (95% CI) | P-value | Adjusted OR (95% CI) | P-value |
|---|---|---|---|---|
| Sex (Men) | 2.16 (1.09–4.29) | 0.028 | 2.47 (1.18–5.16) | 0.017 |
| Dialysis | 1.82 (0.95–3.48) | 0.07 | ||
| AST, U/L | 0.99 (0.95–1.02) | 0.40 | ||
| LDH, U/L | 0.99 (0.99–1.00) | 0.08 | ||
| Uric acid, mg/dL | 1.16 (0.98–1.37) | 0.078 | ||
| BUN, mg/dL | 1.02 (1.01–1.03) | 0.003 | 1.02 (1.01–1.03) | 0.002 |
| Scr, mg/dL | 1.09 (1.02–1.16) | 0.01 | 1.14 (1.05–1.24) | 0.002 |
| eGFR, mL/min/1.73 m2 | 0.99 (0.98–1.00) | 0.09 | ||
| Serum sodium, mEq/L | 0.89 (0.83–0.97) | 0.008 | 0.90 (0.82–0.97) | 0.008 |
| Serum chloride, mEq/L | 0.91 (0.84–0.98) | 0.009 | 0.91 (0.84–0.98) | 0.012 |
| HDL cholesterol, mg/dL | 0.97 (0.95–0.99) | 0.006 | 0.96 (0.94–0.99) | 0.003 |
| β2-microglobulin, mg/L | 1.06 (0.998–1.14) | 0.059 | ||
| Hct, % | 1.08 (0.99–1.18) | 0.082 |
Notes: Data are adjusted for age, BMI, smoking, use of antihyperlipidemic drug, use of antidiabetic drugs, and use of antihypertensive drugs.
Abbreviations: BUN, blood urea nitrogen; LDH, lactate dehydrogenase; OR, odds ratio; 95% CI, 95% confidence interval.
Next, stratified analyses according to sex were performed (Figure 2). High values in BUN (OR, 1.02; 95% CI, 1.01–1.04; P = 0.012) and Scr (OR, 1.14; 95% CI, 1.03–1.27; P = 0.012), and low value in HDL cholesterol (OR, 0.96; 95% CI, 0.92–0.99; P = 0.015) were significantly associated with COVID-19 infection in men, but not in women (BUN, P = 0.43; Scr, P = 0.54; HDL cholesterol, P = 0.33). On the other hand, the risk of COVID-19 infection significantly decreased along with increase in serum sodium (OR, 0.84; 95% CI, 0.74–0.97; P = 0.013) and chloride (OR, 0.83; 95% CI, 0.72–0.96; P = 0.01) in women, but not in men (serum sodium, P = 0.13; serum chloride, P = 0.25).
Figure 2.
Odds ratios (ORs) and 95% confidence intervals (CIs) for the risk of COVID-19 infection. Data were adjusted for age, BMI, smoking status, use of antihyperlipidemic drugs, use of antidiabetic drugs, and use of antihypertensive drugs. The solid squares are centered on the point estimate, and the horizontal lines extending from squares represent 95% CIs.
Discussion
In the present study, we demonstrated that high levels in BUN and Scr were risk factors for COVID-19 infection after adjusted for possible confounding factors (age, BMI, smoking, use of antihyperlipidemic drugs, use of antidiabetic drugs, and use of antihypertensive drugs). In addition, the risk for COVID-19 infection significantly decreased along with increase in serum sodium, serum chloride, and HDL cholesterol, and this significant association was unchanged even after adjusting for possible confounding factors (age, BMI, smoking, use of antihyperlipidemic drugs, use of antidiabetic drugs, and use of antihypertensive drugs). Of note, high levels in BUN and Scr were associated with COVID-19 infection in men, but not in women. Low values in serum sodium and serum chloride in women and low value in HDL cholesterol in men were more susceptible in COVID-19 infection. Thus, to the best of our knowledge, this is the first study to reveal factors differently involved with COVID-19 infection by sex in Japanese patients and identify the potential relevance of kidney function with COVID-19 infection in Japanese male patients.
Several studies have reported that advanced age, male, hypertension, diabetes mellitus, a history of cerebrovascular or cardiovascular disease, chronic obstructive pulmonary disease, and cancer significantly contributed to prognosis of COVID-19.12,13 Age is a significant factor affecting the susceptibility to COVID-19.14 A meta-analysis revealed that patients aged over 70 years were at a higher risk of COVID-19 infection (relative risk, 1.65; 95% CI, 1.50–1.81).15 Our study population consisted of elderly patients (75 ± 14 years old), and we found that 11.4% of adult patients had COVID-19 infection. Elderly patients tend to be subjected with diabetes mellitus, take anti-hypertensive medications, and have a history of cerebrovascular or cardiovascular diseases. In the present study, however, there were no significant differences in these variables between two groups.
Our results showed that men were significantly related to COVID-19 infection. This is consistent with a meta-analysis showed that men have a statistically significant higher risk of being diagnosed with COVID-19 than women (relative risk, 1.08; 95% CI, 1.03–1.12).15 Of note, the risk factors for COVID-19 differed between men and women: high levels in BUN and Scr were significantly associated with COVID-19 infection in men, but not in women. However, the exact cause of kidney dysfunction remains unclear. One possible explanation is pre-existing chronic kidney disease (CKD). Abnormal regulation of the innate and adaptive immune system is accelerated by CKD.16 As progression of CKD, many patients require dialysis, which is related to persistent inflammation.17 Indeed, we found in the present study that the prevalence of dialysis tended to be high rate of patients with COVID-19 (60%). It is possible that inflammation might occur in CKD patients and such patients might be subjected with COVID-19 infection.
HDL-cholesterol modulates inflammation.18,19 Several studies have reported the association of HDL-cholesterol with COVID-19 infection.20,21 In addition, HDL-associated phospholipids, total cholesterol, and ApoA-I protein levels are important determinants in discrimination between patients with COVID-19 pneumonia and non-COVID-19 controls. Consistent with these reports, our study showed that HDL-cholesterol is inversely associated with COVID-19 infection even adjusted for possible confounding factors. Interestingly, this relationship was observed in men, but not in women.
Hyponatremia occurs in approximately one-third of patients with pneumonia.22 Hyponatremia in COVID-19 is reported to be observed in patients at admission.23,24 In addition, COVID-19 infection was detected in patients with hyponatremia with an elevated risk for in-hospital mortality and sepsis.25,26 In the present study, we found that serum sodium was significantly lower in patients with COVID-19 infection than uninfected control patients. This relationship was observed in women, but not in men.
Our study revealed that there was no significant difference in serum Zn between patients infected with COVID-19 and controls. Previous studies demonstrated that status of some micronutrients such as vitamins A, C, D, and E, as well as copper (Cu), zinc (Zn), and selenium (Se) has been linked with COVID-19 infection and disease severity.27 Baarz et al reported that six days of zinc supplementation in elderly zinc-deficient patients raised serum zinc level and suppressed IL-2 expression, suggesting an improvement in immune function.28 Their results suggested that identifying those individuals who might benefit from immediate zinc supplementation such as acute infection is important. The relationship between zinc and COVID-19, including how zinc deficiency affects the severity of COVID-19 and whether zinc supplements can improve clinical outcomes, is currently under investigation and recommends against using zinc supplementation above the recommended dietary allowance for the prevention of COVID-19.29 Although one should always consider the individual case, it can be deduced from current recommendations and risk assessments of the NIH, European Food Safety Authority (EFSA), and other health authorities that it seems more beneficial for health to constantly administer zinc in a low dose.30
Limitations
This study had some limitations. First, this was a retrospective study with a relatively small population. Second, all data were from one hospital in Japan, so the findings may not be generalizable to other populations. Third, there is too large a difference between the study groups which consisted of case group (n = 43) and control group (n = 335). Finally, genetic factors could play a role in the COVID-19 infection, so future study will be needed to examine the effects of different genetic variants on the diagnosis and severity of COVID-19 in patients.
Conclusion
In conclusion, we found that high levels of BUN and Scr were associated with COVID-19 infection in men, but not in women. Low values of serum sodium and serum chloride in women and low value of HDL cholesterol in men were more susceptible in COVID-19 infection. This is the first study to reveal factors differently involved with COVID-19 infection by sex in Japanese patients and identify the potential relevance of kidney function with COVID-19 infection in Japanese male patients. Further studies are needed with larger populations to confirm our findings and to assess the utility of risk factors for COVID-19 infection in Japanese patients.
Acknowledgments
The authors would like to sincerely appreciate all investigators for their help and support.
Funding Statement
This work was supported by JSPS KAKENHI (grant no. JP21K06722).
Data Sharing Statement
All data relevant to the study are included in the article.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 2020;55(3):105924. doi: 10.1016/j.ijantimicag.2020.105924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) Outbreak in China: summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- 4.Jaywant A, Vanderlind WM, Alexopoulos GS, Fridman CB, Perlis RH, Gunning FM. Frequency and profile of objective cognitive deficits in hospitalized patients recovering from COVID-19. Neuropsychopharmacology. 2021;46(13):2235–2240. doi: 10.1038/s41386-021-00978-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220–232. doi: 10.1016/S0140-6736(20)32656-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ambrosino I, Barbagelata E, Ortona E, et al. Gender differences in patients with COVID-19: a narrative review. Monaldi Arch Chest Dis. 2020;90:2. doi: 10.4081/monaldi.2020.1389 [DOI] [PubMed] [Google Scholar]
- 8.Nasirpour N, Esmailzadehha N, Hajebi A, Savari E, Ghanbari B, Motevalian A. Preexisting depression and COVID-19: a cohort study on the risk of susceptibility and hospitalization. BMC Psychiatry. 2023;23(1):942. doi: 10.1186/s12888-023-05438-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khunti K, Singh AK, Pareek M, Hanif W. Is ethnicity linked to incidence or outcomes of covid-19? BMJ. 2020;369:m1548. doi: 10.1136/bmj.m1548 [DOI] [PubMed] [Google Scholar]
- 10.Luoyi H, Yan P, Qihong F, Anand V. Relationship between angiotensin-converting enzyme insertion/deletion polymorphism and the risk of COVID-19: a meta-analysis. J Renin Angiot Aldost Syst. 2023;2023:3431612. doi: 10.1155/2023/3431612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53(6):982–992. doi: 10.1053/j.ajkd.2008.12.034 [DOI] [PubMed] [Google Scholar]
- 12.Fang X, Li S, Yu H, et al. Epidemiological, comorbidity factors with severity and prognosis of COVID-19: a systematic review and meta-analysis. Aging. 2020;12(13):12493–12503. doi: 10.18632/aging.103579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng Z, Peng F, Xu B, et al. Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis. J Infect. 2020;81(2):e16–e25. doi: 10.1016/j.jinf.2020.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ayoub HH, Chemaitelly H, Mumtaz GR, et al. Characterizing key attributes of COVID-19 transmission dynamics in China’s original outbreak: model-based estimations. Global Epidemiol. 2020;2:100042. doi: 10.1016/j.gloepi.2020.100042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pijls BG, Jolani S, Atherley A, et al. Demographic risk factors for COVID-19 infection, severity, ICU admission and death: a meta-analysis of 59 studies. BMJ open. 2021;11(1):e044640. doi: 10.1136/bmjopen-2020-044640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huck DM, Buckley LF, Chandraker A, Blankstein R, Weber B. Targeting pharmacotherapies for inflammatory and cardiorenal endpoints in kidney disease. J Cardiovasc Pharmacol. In press 2024. doi: 10.1097/FJC.0000000000001482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kadatane SP, Satariano M, Massey M, Mongan K, Raina R. The Role of Inflammation in CKD. Cells. 2023;12(12):1581. doi: 10.3390/cells12121581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewington S, Whitlock G; Prospective Studies C. Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet. 2007;370(9602):1829–1839. doi: 10.1016/S0140-6736(07)61778-4 [DOI] [PubMed] [Google Scholar]
- 19.van der Steeg WA, Holme I, Boekholdt SM, et al. High-density lipoprotein cholesterol, high-density lipoprotein particle size, and apolipoprotein A-I: significance for cardiovascular risk: the IDEAL and EPIC-Norfolk studies. J Am Coll Cardiol. 2008;51(6):634–642. doi: 10.1016/j.jacc.2007.09.060 [DOI] [PubMed] [Google Scholar]
- 20.Wang G, Zhang Q, Zhao X, et al. Low high-density lipoprotein level is correlated with the severity of COVID-19 patients: an observational study. Lipids Health Dis. 2020;19(1):204. doi: 10.1186/s12944-020-01382-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feingold KR. The bidirectional link between HDL and COVID-19 infections. J Lipid Res. 2021;62:100067. doi: 10.1016/j.jlr.2021.100067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nair V, Niederman MS, Masani N, Fishbane S. Hyponatremia in community-acquired pneumonia. Am j Nephrol. 2007;27(2):184–190. doi: 10.1159/000100866 [DOI] [PubMed] [Google Scholar]
- 23.Berni A, Malandrino D, Parenti G, Maggi M, Poggesi L, Peri A. Hyponatremia, IL-6, and SARS-CoV-2 (COVID-19) infection: may all fit together? J Endocrinol Invest. 2020;43(8):1137–1139. doi: 10.1007/s40618-020-01301-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frontera JA, Valdes E, Huang J, et al. Prevalence and impact of hyponatremia in patients with coronavirus disease 2019 in New York City. Crit Care Med. 2020;48(12):e1211–e1217. doi: 10.1097/CCM.0000000000004605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu W, Lv X, Li C, et al. Disorders of sodium balance and its clinical implications in COVID-19 patients: a multicenter retrospective study. Int Emerg Med. 2021;16(4):853–862. doi: 10.1007/s11739-020-02515-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tezcan ME, Dogan Gokce G, Sen N, Zorlutuna Kaymak N, Ozer RS. Baseline electrolyte abnormalities would be related to poor prognosis in hospitalized coronavirus disease 2019 patients. New Microb New Infect. 2020;37:100753. doi: 10.1016/j.nmni.2020.100753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bae M, Kim H. Mini-Review on the Roles of Vitamin C, Vitamin D, and Selenium in the Immune System against COVID-19. Molecules. 2020;25(22):5346. doi: 10.3390/molecules25225346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Y, Zhang H, Yuan G, et al. The impact of circadian rhythms on the immune response to influenza vaccination in middle-aged and older adults (IMPROVE): a randomised controlled trial. Immun Ageing. 2022;19(1):46. doi: 10.1186/s12979-022-00304-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guidelines ZC-T. Available from: https://wwwcovid19treatmentguidelinesnihgov/therapies/supplements/zinc/. Accessed January 17, 2024.
- 30.Wolf J, Rink L. Handbook on the Toxicology of Metals. 5th ed. Elsevier; 2022:963–984. [Google Scholar]




