Summary
Background
Gastrointestinal cancers account for a quarter of the global cancer incidence and a third of cancer-related deaths. We sought to estimate the lifetime risks of developing and dying from gastrointestinal cancers at the country, world region, and global levels in 2020.
Methods
For this population-based systematic analysis, we obtained estimates of gastrointestinal cancer incidence and mortality rates from GLOBOCAN for 185 countries, alongside all-cause mortality and population data from the UN. Countries were categorised into quartiles of the Human Development Index (HDI). The lifetime risk of gastrointestinal cancers was estimated with a standard method that adjusts for multiple primaries, taking into account competing risks of death from causes other than cancer and life expectancy.
Findings
The global lifetime risks of developing and dying from gastrointestinal cancers from birth to death was 8·20% (95% CI 8·18–8·21) and 6·17% (6·16–6·18) in 2020. For men, the risk of developing gastrointestinal cancers was 9·53% (95% CI 9·51–9·55) and of dying from them 7·23% (7·22–7·25); for women, the risk of developing gastrointestinal cancers was 6·84% (6·82–6·85) and of dying from them 5·09% (5·08–5·10). Colorectal cancer presented the highest risk, accounting for 38·5% of the total lifetime risk of developing, and 28·2% of dying from, gastrointestinal cancers, followed by cancers of the stomach, liver, oesophagus, pancreas, and gallbladder. Eastern Asia has the highest lifetime risks for cancers of the stomach, liver, oesophagus, and gallbladder, Australia and New Zealand for colorectal cancer, and Western Europe for pancreatic cancer. The lifetime risk of gastrointestinal cancers increased consistently with increasing level of HDI; however, high HDI countries (the third HDI quartile) had the highest death risk.
Interpretation
The global lifetime risk of gastrointestinal cancers translates to one in 12 people developing, and one in 16 people dying from, gastrointestinal cancers. The identified high risk and observed disparities across countries warrants context-specific targeted gastrointestinal cancer control and health systems planning.
Funding
Beijing Nova Program, CAMS Innovation Fund for Medical Sciences, and Talent Incentive Program of Cancer Hospital, CAMS (Hope Star).
Introduction
Few studies have assessed the cumulative effect of gastrointestinal cancers, including oesophageal, gastric, liver, colorectal, pancreatic, and gallbladder cancer, in terms of cancer-specific incidence and mortality from a lifetime risk perspective,1, 2 despite these cancers accounting for a quarter of all cancer cases and a third of cancer-related deaths worldwide.3 The lifetime risk of developing or dying from gastrointestinal cancers refers to the chance a person has, over the course of one's lifetime (from birth to death), of developing or dying from gastrointestinal cancer. The metric assesses the burden from a longitudinal perspective of the cumulative probability of developing or dying from gastrointestinal cancers over a lifetime, taking into account both demographic changes and competing risks from causes of death other than gastrointestinal cancer. Together with other indicators, lifetime risk can aid a complete understanding and interpretation of the profile of gastrointestinal cancers worldwide and in so doing, inform future health-care planning given competing priorities and health-care constraints.
In the past three decades, as a result of a combination of factors—ie, rapid globalisation, demographic trends, a changing prevalence and distribution of key risk factors, alongside innovations in cancer diagnosis and treatment, and improving health-care infrastructure—striking variations in the incidence and mortality of predominant gastrointestinal cancer types are seen across world regions and individual countries.3 To better understand the cumulative burden of gastrointestinal cancers, we sought to estimate the lifetime and age-conditional probabilities of developing and dying from six types of gastrointestinal cancers in 185 countries at the global, regional, and country levels using data from the Global Cancer Observatory (GLOBOCAN) 2020. The results seek to inform national prevention and control strategies tailored to the cancer-specific lifetime risks of gastrointestinal cancers within world regions, ultimately to reduce the burden within existing resources and systems worldwide.
Research in context.
Evidence before this study
We searched PubMed using no language or date restrictions for articles before Dec 31, 2022, using the terms (gastrointestinal cancer* OR GI cancer* OR esophageal cancer* OR oesophageal cancer* OR esophagus cancer* OR oesophagus cancer* OR gastric cancer* OR stomach cancer* OR colorectal cancer* OR colorectum cancer* OR liver cancer* OR pancreatic cancer* OR pancreas cancer* OR gallbladder cancer) AND ((lifetime risk) OR (lifetime probability)). We found that the cumulative risk of gastrointestinal cancer incidence or mortality for those aged 0–74 years was widely used as a surrogate indicator to assess the lifetime risk of developing or dying from gastrointestinal cancer for the past six decades, which could significantly underestimate the risk in populations in which life expectancy was relatively high or the competing risks of deaths from causes other than gastrointestinal cancer were relatively low. Previous studies on lifetime risk of developing or dying from gastrointestinal cancer have been limited to one cancer registry or one country, or focusing on a single cancer type; no studies systematically explore the lifetime risk of gastrointestinal cancer. With the ageing and longevity of the global population, there are more than 100 countries reporting a life expectancy higher than 74 years in 2020, thus it is imperative to comparatively assess the lifetime risk of developing or dying from gastrointestinal cancer globally and the disparities across countries by using an updated comprehensive indicator, to aid for long-term global health system planning for gastrointestinal cancer.
Added value of this study
To our knowledge, this study is the first to quantify the risk of developing or dying from gastrointestinal cancers across 185 countries at the global, regional, and national levels using a standardised method that adjusts for multiple primaries, accounts for competing risks of death from non-gastrointestinal cancer causes, and incorporates life expectancy data. We estimated that one in 12 people will develop, and one in 16 people will die from, gastrointestinal cancers during their lifetime, with the highest risk for colorectal cancer, followed by cancers of the stomach, liver, oesophagus, pancreas, and gallbladder. Eastern Asia has the highest lifetime risks for cancers of the stomach, liver, oesophagus, and gallbladder, Australia and New Zealand for colorectal cancer, and Western Europe for pancreas cancer.
Implications of all the available evidence
Countries with a very high Human Development Index (HDI) have a higher risk of developing and dying from gastrointestinal cancers than low HDI countries, and the narrower gap in the mortality rates compared with that of incidence highlights the importance of access to and affordability of health-care, along with the crucial role of national health policy decisions. The apparent heterogeneity in lifetime risks across regions and countries indicates that it is no longer appropriate to consider gastrointestinal cancer as simple entities and that there is an urgent need to establish a context-specific, targeted cancer prevention plan for different gastrointestinal cancer types in each country.
Methods
Data sources
Six major gastrointestinal cancers were classified according to the 10th edition of the International Classification of Diseases: oesophagus (C15), stomach (C16), colorectum (C18–21), liver (C22), gallbladder (C23), and pancreas (C25). New cases and deaths from the six major gastrointestinal cancers were obtained from the GLOBOCAN estimates for 2020, developed and disseminated by the International Agency for Research on Cancer (IARC), by sex and 5-year age groups (from 0–4 to ≥85 years) and for comparison with other cancers, also obtained from GLOBOCAN.4, 5 Population and all-cause mortality data were obtained from the UN World Population Prospects 2019.6 We classified the world regions according to the 20 aggregated geographic regions, as defined by the UN Population Division.4 In addition, we categorised the world regions and countries into quartiles according to the Human Development Index (HDI) of the UN Development Programme to capture human progress and the wellbeing of countries by measuring three dimensions of human development: health, education, and income.7
Statistical methods
The adjusted for multiple primaries (AMP) method1, 2 was used to calculate the lifetime risk, which takes into account deaths from other causes, and corrects for the inclusion of multiple primary cancers in the incidence rates. We used gastrointestinal cancer incidence, mortality, and all-cause mortality rates by 5-year age groups to estimate the lifetime risk of developing or dying from gastrointestinal cancers by sex at different ages, which represents the probability of developing or dying from gastrointestinal cancers from that age onwards. Further details of the methods of lifetime risk are provided in the appendix (pp 1–4).
We calculated the lifetime risk of developing or dying from gastrointestinal cancers overall, and by the gastrointestinal cancer type and sex. Estimates were made at the global, world regional, and national levels, and by HDI quartiles and different age intervals. We then compared the lifetime risk of gastrointestinal cancer with other common cancer types, to help assess the burden from a public health and clinical health-care perspective. In sensitivity analyses, we compared the lifetime risks of gastrointestinal cancers with the cumulative risks of gastrointestinal cancers for the age group of 0–74 years, a traditional indicator of cancer burden that can be calculated directly as a surrogate for the lifetime risk that is unadjusted for competing risks from non-cancer causes, multiple primaries, and life expectancy.1, 2, 8 The absolute numbers of cases and deaths related to gastrointestinal cancer, all-cause deaths, and population were merged by five-year age group and sex in each country to obtain the pooled lifetime risk at aggregated levels. The 95% CI was calculated by assuming a Poisson distribution for cancer incidence and mortality, using the corresponding variance. All analyses were done with SAS software, version 9.4. World maps were created with the QGIS software, version 3.61.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or the writing of the report.
Results
In 2020, the estimated global lifetime risk (ie, from birth to death) of developing and dying from gastrointestinal cancers was 8·20% (95% CI 8·18–8·21) and 6·17% (95% CI 6·16–6·18), respectively (table). Colorectal cancer presented the highest risk, accounting for 38·5% and 28·2% of the total lifetime risks of developing and dying from gastrointestinal cancers, respectively, followed by cancers of the stomach, liver, oesophagus, pancreas, and gallbladder. In general, the estimates of lifetime risks of developing and dying from gastrointestinal cancers were higher in men than in women (figure 1), and positively associated with increasing levels of human development. The lifetime risk of developing gastrointestinal cancers ranged from 11·39% (95% CI 11·37–11·41) in very high HDI countries to 2·61% (2·58–2·65) in low HDI countries, and the lifetime risk of dying from gastrointestinal cancers ranged from 7·29% (95% CI 7·27–7·30) to 2·31% (2·28–2·35). However, the lifetime risks of developing and dying from cancers of stomach, liver, and oesophagus (but not the colorectum, pancreas, or gallbladder) were higher in high HDI countries than those in very high HDI countries, and there were also higher lifetime risks of dying from total gastrointestinal cancers in high HDI countries (8·42% [95% CI 8·40–8·44]) relative to very high HDI countries (7·29% [7·27–7·30]). The lifetime risks of developing and dying from pancreatic cancer were substantially higher in very high HDI countries than other HDI country groups. Among low and medium HDI countries, liver cancer (0·63% [0·62–0·65]) and oesophageal cancer (0·61% [0·60–0·62]), respectively, had the highest lifetime death risks.
Table.
Lifetime risks of developing and dying from gastrointestinal cancers by sex and level of human development in 2020
| Colorectum | Stomach | Liver | Oesophagus | Pancreas | Gallbladder | Gastrointestinal cancers | |
|---|---|---|---|---|---|---|---|
| Gastrointestinal cancer development in the total population | |||||||
| World | 3·16 (3·15–3·17) | 1·77 (1·76–1·77) | 1·36 (1·35–1·36) | 0·93 (0·93–0·93) | 0·89 (0·88–0·89) | 0·20 (0·19–0·20) | 8·20 (8·18–8·21) |
| Very high HDI country | 5·53 (5·52–5·55) | 2·23 (2·22–2·24) | 1·28 (1·28–1·29) | 0·65 (0·64–0·65) | 1·72 (1·71–1·72) | 0·23 (0·23–0·23) | 11·39 (11·37–11·41) |
| High HDI country | 3·28 (3·27–3·29) | 2·36 (2·35–2·37) | 2·10 (2·09–2·10) | 1·46 (1·45–1·46) | 0·82 (0·81–0·83) | 0·20 (0·19–0·20) | 10·09 (10·07–10·11) |
| Medium HDI country | 0·65 (0·65–0·66) | 0·62 (0·61–0·63) | 0·41 (0·41–0·42) | 0·66 (0·66–0·67) | 0·14 (0·14–0·15) | 0·21 (0·20–0·21) | 2·69 (2·67–2·70) |
| Low HDI country | 0·80 (0·78–0·81) | 0·53 (0·51–0·54) | 0·65 (0·63–0·66) | 0·37 (0·36–0·38) | 0·21 (0·20–0·22) | 0·07 (0·06–0·07) | 2·61 (2·58–2·65) |
| Gastrointestinal cancer deaths in the total population | |||||||
| World | 1·74 (1·73–1·74) | 1·30 (1·29–1·30) | 1·27 (1·26–1·27) | 0·87 (0·87–0·87) | 0·85 (0·85–0·85) | 0·15 (0·14–0·15) | 6·17 (6·16–6·18) |
| Very high HDI country | 2·70 (2·69–2·71) | 1·24 (1·23–1·24) | 1·08 (1·07–1·08) | 0·52 (0·51–0·52) | 1·60 (1·60–1·61) | 0·15 (0·15–0·15) | 7·29 (7·27–7·30) |
| High HDI country | 2·00 (1·99–2·01) | 1·99 (1·98–1·99) | 2·03 (2·02–2·04) | 1·44 (1·44–1·45) | 0·81 (0·80–0·81) | 0·15 (0·15–0·16) | 8·42 (8·40–8·44) |
| Medium HDI country | 0·41 (0·41–0·42) | 0·55 (0·54–0·56) | 0·39 (0·38–0·39) | 0·61 (0·60–0·62) | 0·14 (0·13–0·14) | 0·16 (0·16–0·16) | 2·26 (2·25–2·27) |
| Low HDI country | 0·59 (0·58–0·61) | 0·47 (0·45–0·48) | 0·63 (0·62–0·65) | 0·36 (0·34–0·37) | 0·20 (0·19–0·21) | 0·06 (0·05–0·06) | 2·31 (2·28–2·35) |
| Gastrointestinal cancer development in the male population | |||||||
| World | 3·36 (3·35–3·37) | 2·27 (2·26–2·28) | 1·79 (1·78–1·79) | 1·24 (1·24–1·25) | 0·88 (0·87–0·88) | 0·14 (0·13–0·14) | 9·53 (9·51–9·55) |
| Very high HDI country | 5·99 (5·96–6·01) | 2·90 (2·89–2·92) | 1·70 (1·68–1·71) | 1·01 (1·00–1·01) | 1·71 (1·70–1·73) | 0·17 (0·16–0·17) | 13·11 (13·08–13·14) |
| High HDI country | 3·46 (3·44–3·48) | 3·05 (3·03–3·06) | 2·78 (2·76–2·79) | 1·89 (1·88–1·90) | 0·82 (0·81–0·83) | 0·14 (0·14–0·14) | 11·98 (11·94–12·01) |
| Medium HDI country | 0·74 (0·73–0·76) | 0·77 (0·76–0·78) | 0·52 (0·51–0·53) | 0·79 (0·78–0·80) | 0·17 (0·16–0·17) | 0·13 (0·12–0·13) | 3·12 (3·09–3·14) |
| Low HDI country | 0·81 (0·78–0·84) | 0·59 (0·57–0·62) | 0·80 (0·78–0·83) | 0·40 (0·38–0·42) | 0·21 (0·20–0·23) | 0·05 (0·04–0·06) | 2·89 (2·83–2·94) |
| Gastrointestinal cancer deaths in the male population | |||||||
| World | 1·83 (1·82–1·84) | 1·65 (1·64–1·65) | 1·66 (1·66–1·67) | 1·16 (1·15–1·16) | 0·84 (0·83–0·84) | 0·10 (0·10–0·10) | 7·23 (7·22–7·25) |
| Very high HDI country | 2·90 (2·88–2·91) | 1·55 (1·54–1·56) | 1·40 (1·39–1·41) | 0·80 (0·79–0·80) | 1·61 (1·59–1·62) | 0·11 (0·11–0·11) | 8·36 (8·33–8·39) |
| High HDI country | 2·09 (2·07–2·11) | 2·55 (2·53–2·57) | 2·69 (2·67–2·70) | 1·87 (1·85–1·88) | 0·80 (0·79–0·81) | 0·11 (0·11–0·12) | 10·11 (10·08–10·14) |
| Medium HDI country | 0·47 (0·46–0·48) | 0·69 (0·68–0·71) | 0·49 (0·48–0·50) | 0·73 (0·72–0·74) | 0·16 (0·15–0·16) | 0·10 (0·09–0·10) | 2·65 (2·63–2·67) |
| Low HDI country | 0·60 (0·58–0·63) | 0·53 (0·50–0·55) | 0·78 (0·75–0·81) | 0·39 (0·37–0·41) | 0·21 (0·19–0·23) | 0·04 (0·04–0·05) | 2·58 (2·52–2·63) |
| Gastrointestinal cancer development in the female population | |||||||
| World | 2·97 (2·96–2·97) | 1·26 (1·25–1·26) | 0·92 (0·91–0·92) | 0·61 (0·60–0·61) | 0·90 (0·89–0·90) | 0·26 (0·26–0·26) | 6·84 (6·82–6·85) |
| Very high HDI country | 5·12 (5·10–5·13) | 1·60 (1·59–1·61) | 0·89 (0·88–0·90) | 0·31 (0·30–0·31) | 1·72 (1·71–1·73) | 0·29 (0·28–0·29) | 9·75 (9·73–9·78) |
| High HDI | 3·09 (3·07–3·10) | 1·63 (1·62–1·65) | 1·39 (1·38–1·40) | 1·00 (1·00–1·01) | 0·82 (0·82–0·83) | 0·25 (0·25–0·26) | 8·12 (8·10–8·14) |
| Medium HDI country | 0·55 (0·55–0·56) | 0·46 (0·45–0·46) | 0·29 (0·29–0·30) | 0·52 (0·52–0·53) | 0·12 (0·11–0·12) | 0·29 (0·29–0·30) | 2·23 (2·21–2·25) |
| Low HDI country | 0·79 (0·76–0·81) | 0·46 (0·44–0·48) | 0·50 (0·48–0·52) | 0·33 (0·32–0·35) | 0·20 (0·18–0·21) | 0·08 (0·07–0·09) | 2·36 (2·32–2·40) |
| Gastrointestinal cancer deaths in the female population | |||||||
| World | 1·65 (1·64–1·66) | 0·94 (0·94–0·95) | 0·86 (0·86–0·87) | 0·58 (0·58–0·58) | 0·86 (0·86–0·87) | 0·19 (0·19–0·19) | 5·09 (5·08–5·10) |
| Very high HDI country | 2·53 (2·52–2·54) | 0·95 (0·94–0·95) | 0·77 (0·76–0·78) | 0·25 (0·25–0·26) | 1·61 (1·60–1·61) | 0·19 (0·19–0·20) | 6·30 (6·28–6·32) |
| High HDI country | 1·90 (1·89–1·91) | 1·40 (1·39–1·41) | 1·35 (1·34–1·36) | 1·00 (1·00–1·01) | 0·81 (0·81–0·82) | 0·20 (0·19–0·20) | 6·66 (6·64–6·69) |
| Medium HDI country | 0·35 (0·34–0·35) | 0·40 (0·39–0·41) | 0·28 (0·27–0·28) | 0·48 (0·48–0·49) | 0·11 (0·11–0·12) | 0·23 (0·22–0·23) | 1·85 (1·83–1·86) |
| Low HDI country | 0·59 (0·56–0·61) | 0·41 (0·39–0·43) | 0·48 (0·47–0·50) | 0·32 (0·31–0·34) | 0·19 (0·18–0·21) | 0·07 (0·06–0·08) | 2·07 (2·03–2·11) |
Data are % (95% CI). HDI=Human Development Index.
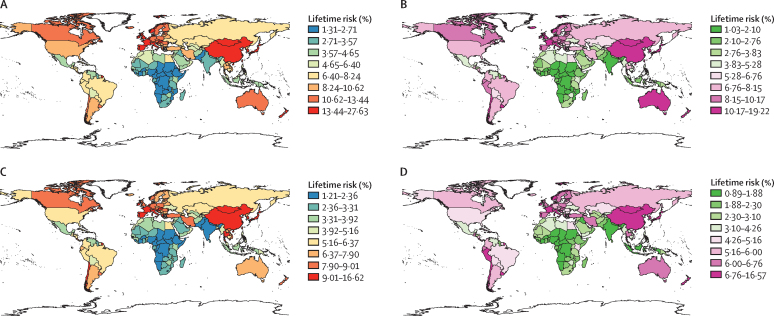
Figure 1.
Lifetime risks of developing or dying from gastrointestinal cancer in 2020, by sex
(A) Lifetime risk of developing gastrointestinal cancer for males in 2020. (B) Lifetime risk of developing gastrointestinal cancer for females in 2020. (C) Lifetime risk of dying from gastrointestinal cancer for males in 2020. (D) Lifetime risk of dying from gastrointestinal cancer for females in 2020.
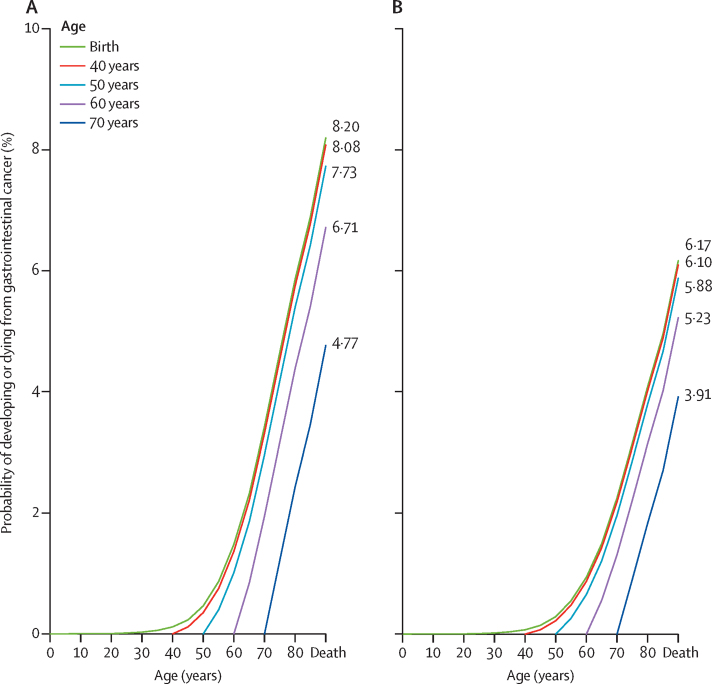
The risk of developing gastrointestinal cancers from birth to age 40 years was extremely low, globally, resulting in similar accrued risk from birth to death as from the age of 40 years to death (8·08% [8·06–8·10]) for both sexes. A similar pattern was seen for the risk of dying from gastrointestinal cancers. In general, a decreasing trend was observed by age, with a remaining risk of 4·77% (95% CI 4·75–4·78) for developing and 3·91% (3·90–3·93) for dying from gastrointestinal cancers from the age of 70 years (figure 2; appendix p 5).
Figure 2.
Lifetime risks of developing or dying from gastrointestinal cancer within selected age intervals in 2020, both sexes
(A) Developing gastrointestinal cancer. (B) Dying from gastrointestinal cancer. The curves represent the probabilities of developing or dying from gastrointestinal cancer from an age free of gastrointestinal cancer to a specific age range. The age-conditional risk of gastrointestinal cancer is available in the appendix (p 5).
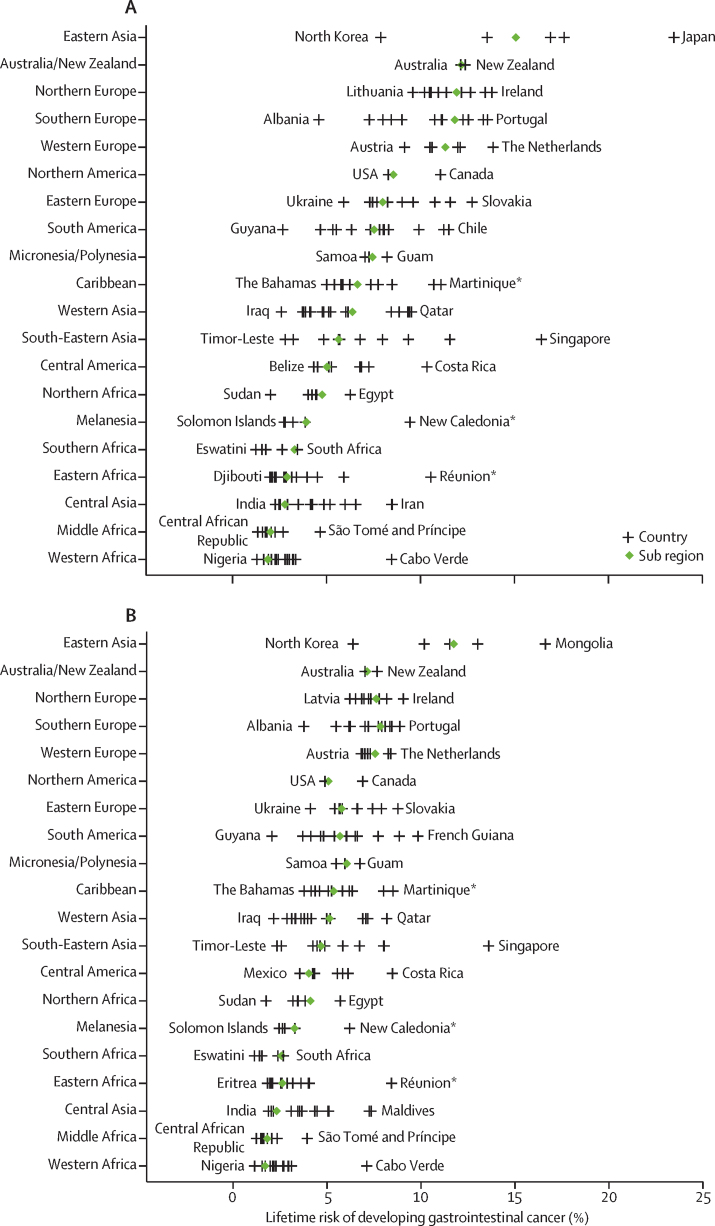
There were marked discrepancies in lifetime risks between countries within world regions for different gastrointestinal cancer types (appendix pp 6–10). We observed the highest lifetime risk of developing (15·08% [95% CI 15·05–15·10]) and dying from (11·75% [11·72–11·77]) gastrointestinal cancers in Eastern Asia, and the lowest risk in Western Africa (developing 1·88% [1·83–1·93]; dying 1·71% [1·66–1·76]). At the country level, Japan (23·48% [23·41–23·56]) presented the highest risk of developing gastrointestinal cancers and Mongolia (16·62% [15·37–17·87]) presented the highest risk of dying from gastrointestinal cancers. Eswatini showed the lowest risk of developing (1·24% [0·69–1·79]) and dying from (1·14% [0·59–1·68]) gastrointestinal cancers (figure 3; appendix pp 11–52).
Figure 3.
Lifetime risks of developing or dying from gastrointestinal cancer in 2020 by region and country, both sexes
(A) Developing gastrointestinal cancer. (B) Dying from gastrointestinal cancer. Green diamonds indicate the average lifetime risk of gastrointestinal cancer in each selected geographic region. Vertical lines represent the estimated lifetime risk of gastrointestinal cancer in each selected country, with the name of the country representing the one with the lowest or highest risk within that geographic region. Details of the results of the statistical test comparing lifetime risk at the geographic regional level with the global average level are available in the appendix (pp 6–10). *French Overseas Territory.
The cancer-specific analyses showed significant variations by geographic region. The regions with the highest lifetime risk of developing a specific gastrointestinal cancer type were Eastern Asia for stomach, liver, oesophagus, and gallbladder cancer, Australia and New Zealand and Southern Europe for colorectal cancer, and Western Europe for pancreatic cancer. The regions with the lowest lifetime risk of developing a specific gastrointestinal cancer type were Western Africa for oesophageal and colorectal cancer, Southern Africa for stomach cancer, Central Asia for liver cancer, and Middle Africa for pancreas and gallbladder cancer (appendix pp 73–78).
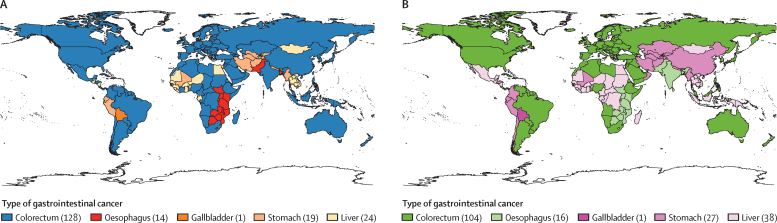
The lifetime risks of developing and dying from the most common gastrointestinal cancer type in 185 countries, overall and by sex, are available (appendix pp 53–66). Considerable global heterogeneity in the leading gastrointestinal cancer types was observed at the national level (figure 4). Specifically, colorectal cancer was the leading gastrointestinal cancer type with the highest lifetime incidence and mortality risk in 128 and 104 countries, followed by liver cancer in 24 and 38 countries, stomach cancer in 19 and 27 countries, and oesophageal cancer in 14 and 16 countries, respectively. Gallbladder cancer was the most common gastrointestinal cancer type in Bolivia and Chile. There was similar heterogeneity in gastrointestinal cancer type by sex and country (appendix pp 11–52).
Figure 4.
Distribution of the leading gastrointestinal cancer type with the highest lifetime incidence or death risk in 2020, both sexes
(A) Lifetime incidence risk. (B) Lifetime death risk. Number in parentheses indicates the number of countries for which that cancer is the highest risk among other gastrointestinal cancers.
We estimated the lifetime risk of gastrointestinal cancer compared with the other cancer types (appendix p 67). The lifetime risk of developing gastrointestinal cancer (8·20% [95% CI 8·18–8·21]) surpassed the risk of female breast cancer (5·90% [5·89–5·91]), male genital cancer (comprising of penis, prostate, and testis, 4·88% [4·87–4·89]), and respiratory cancer (including larynx, trachea, bronchus and lung, 3·87% [3·86–3·88]), contributing to approximately one-third of the total lifetime cancer development risk worldwide (an estimated 25·10% [25·08–25·11]). Similarly, we observe a global lifetime risk of 16·50% (95% CI 16·48–16·51) for the total cancer death. Gastrointestinal cancer presents the highest lifetime risk of death (6·17% [6·16–6·18]), followed by respiratory cancer (3·21% [3·20–3·21]) and female breast cancer (2·13% [2·12–2·13]).
Correlations between the lifetime risks and the cumulative risks of developing and dying from gastrointestinal cancers over the age range of 0–74 years are available (appendix p 79). We found marked differences in these two indicators according to variations in life expectancy.
It was estimated that the cumulative risk for those aged 0–74 years overestimated the lifetime risk of developing or dying from gastrointestinal cancer in 24 of 85 countries where life expectancy was younger than 74 years. By contrast, uniformly higher lifetime risks of developing and dying from gastrointestinal cancers compared with the cumulative risk for those aged 0–74 years were observed in 99 of 100 countries with life expectancy older than 74 years (appendix pp 68–72). For example, in Japan, where the reported life expectancy is 85 years, the cumulative risks of developing (11·31% [11·23–11·38]) and dying from (3·68% [3·64–3·73]) gastrointestinal cancer for those aged 0–74 years were significantly lower than the lifetime risk of developing (23·48% [23·41–23·56]) and dying from (13·02% [12·96–13·07]) gastrointestinal cancer. However, in Malawi, where the average life expectancy is only 65·6 years, we observed an overestimated cumulative risk for those aged 0–74 years compared with the lifetime risk of developing (4·11% [3·81–4·42] vs 3·40% [3·14–3·65]) and dying from (3·83% [3·54–4·13] vs 3·18% [2·93–3·43]) gastrointestinal cancer.
Discussion
Gastrointestinal cancer comprises approximately one-third of the global lifetime cancer risk, with a high lifetime mortality to incidence risk ratio of 0·75. The risk was largely driven by ages after 40 years and was higher in men than women. Regions with the highest lifetime risk include Eastern Asia for stomach, liver, oesophageal, and gallbladder cancers, Australia and New Zealand for colorectal cancer, and Western Europe for pancreatic cancer. Colorectal cancer was the predominant type of gastrointestinal cancer with the highest lifetime risk in 128 countries, followed by cancers of liver, stomach, and oesophagus. The lifetime risk of gastrointestinal cancers increased with increasing levels of human development, with the exception of high HDI countries, which had the highest lifetime risk of death from gastrointestinal cancer. The identified disparities in the lifetime risk of gastrointestinal cancer across the world indicate unequal distributions of socioeconomic resources, varied levels of risk factor management, and inequitable accessibility to health-care services among nations.
The lifetime risks calculated here for developing and dying from gastrointestinal cancers from birth to death were higher than the previous estimates of the cumulative risk of gastrointestinal cancers for ages of 0–74 years.5 This aligns with previous research, which showed that the cumulative risk might underestimate the risk in populations in which life expectancy was relatively high or the competing risks of death from other causes were relatively low.1, 2, 8 The cumulative risk for those aged 0–74 years is conditional on survival to age 74 years, and does not depend on the probability of death and life expectancy within a population, so it is used to make direct comparisons of cancer risk between different populations. It can be used as an approximation of the lifetime risk when the average life expectancy of the population is close to age 74 years. However, in 2020, there were 100 countries with a reported a life expectancy greater than 74 years, in which the cumulative risk for ages 0–74 years would underestimate the lifetime risk. In contrast to fixing the upper age limit at 74 years, the lifetime risk calculated by the AMP method takes into account the gastrointestinal cancer burden beyond the age of 74 years up to the life expectancy, as well as the multiple primary gastrointestinal cancers and the competing risk of death from causes other than gastrointestinal cancer. Thus, the observed differences between these two indicators can be expected given the global nature of population ageing and the swift socioeconomic advancements of the past few decades. Moreover, predominance of colorectal cancer in 70% of countries is a marker of the transitions in the cancer profile worldwide, with an ongoing shift from infection-related cancers to those associated with rapid social and economic change.3, 9 These transitions were largely driven by the extensive changes in the prevalence of important underlying risk factors, including but not limited to infectious agents, smoking, alcohol consumption, obesity, unhealthy diet, and physical inactivity.3, 10, 11, 12, 13, 14 Moreover, we found a generally higher lifetime risk of gastrointestinal cancers in men than in women, with the exception of gallbladder cancer. In addition to differences in the distributions of the above mentioned risk factors, differences in metabolism (eg, cholesterol) and sex hormones might contribute to the sex disparities in gallbladder cancer.15
The lifetime risk of developing gastrointestinal cancers increased consistently with increasing HDI levels. However, the risk of dying from gastrointestinal cancers was higher in high HDI countries than in very high HDI countries. This was due to a greater risk of dying from stomach, liver, and oesophageal cancers in high HDI countries than in very high HDI countries. We observed a marginally higher lifetime risk of dying from gastrointestinal cancers in low HDI countries than in medium HDI countries, due to a greater contribution of colorectal, liver, and pancreatic cancers in low HDI countries than in medium HDI countries. We observed a 4·4-times higher risk of developing, and 3·2-times higher risk of dying from, gastrointestinal cancers in very high HDI countries compared with low HDI countries. The narrower gap in the lifetime risk of gastrointestinal cancer mortality between very high and low HDI countries, compared with that of gastrointestinal cancer incidence, highlights the importance of the access to and affordability of health care, as well as the crucial role of national health policy decisions.
Extremely high ratios of lifetime mortality to incidence risk were found for oesophageal, liver, and pancreatic cancers, of around 0·95, compared with ratios of around 0·75 for stomach and gallbladder cancers, and 0·55 for colorectal cancer. These figures relate to the fact that most gastrointestinal cancers, including oesophageal, pancreatic, gastric, liver, and gallbladder cancers, are difficult to detect early, and curative treatment options are either resource-limited or unavailable at the time of diagnosis.3, 15, 16, 17 Thus, effective strategies are essential to reduce the burden of gastrointestinal cancers, including the reduction of exposure to common risk factors, the development of tailored gastrointestinal cancer screening programmes for high-risk populations, improving access, affordability, and outcomes of cancer treatment, and leveraging technology to improve cancer prevention and control.18
We observed significant differences in lifetime risk between regions and countries by type of gastrointestinal cancers. Colorectal and pancreatic cancers showed parallel elevated lifetime risks in countries with a higher HDI, suggesting a common role for lifestyle changes and increased detection through organised or opportunistic cancer screening.
In particular, people living in Eastern Asia had the highest lifetime risks of stomach, liver, and oesophageal cancer, reflecting a long-standing high prevalence of some major risk factors in specific countries, including smoking, excessive alcohol consumption (eg, in Mongolia), Helicobacter pylori infection (eg, in China), hepatitis B virus infection (eg, in China and Mongolia), hepatitis C virus infection (eg, in Japan and Mongolia), and exposure to aflatoxincontaminated foods (eg, in China, Japan, South Korea, and Mongolia) and liver flukes (eg, in Thailand).11, 12, 13, 19, 20 Other factors related to metabolic conditions and nutritional status, such as excessive bodyweight, unhealthy dietary patterns, and diabetes, also need to be carefully considered when planning targeted national health systems and cancer control programmes in these countries.21, 22, 23, 24 We identified a considerably elevated lifetime risk of oesophageal squamous cell carcinoma in a geographical band running along the Great Rift Valley of East Africa and the Indian Ocean coast, including countries such as Kenya, Tanzania, Malawi, and Uganda. Although the cause has not been fully elucidated, potential risk factors include alcohol and tobacco consumption, indoor air pollution, a nutrient-deficient diet, consumption of hot drinks or food, exposure to ruminant animals, poor oral health, opium use, consumption of pickled foods and salted tea, intake of fermented milk, and exposure to nitrosamines, among others.25, 26, 27 In addition, although an average low lifetime risk (0·20% [95% CI 0·19–0·20]) of developing gallbladder cancer was found worldwide, the risk was particularly high in Bolivia (1·57% [1·43–1·71]) and Chile (1·25% [1·11–1·32]), supporting the role of genetic factors (eg, Native American ancestry) and country-specific factors in modulating cancer risk.15
We found that the lifetime risk of gastrointestinal cancer exceeded that of female breast cancer, male genital cancer (comprising penis, prostate, and testis), and respiratory cancer (including larynx, tracheae, bronchus, and lung), contributing to approximately one-third of the global lifetime risk of developing and dying from cancer. These data are informative to enhance public health messaging on the heavy burden of gastrointestinal cancer and to improve resource planning for gastrointestinal cancer control by health-care providers and commissioners. It might also assist clinicians and patients in comparing the lifetime risk of gastrointestinal cancer to other challenging health risks, and enable the establishment of a comprehensive and personalised health-care programme.
This study has several strengths. First, we systematically used representative cancer data from the GLOBOCAN 2020 database and death data from WHO, thus enabling comparable lifetime risk estimates for gastrointestinal cancers worldwide.1, 2 Second, the AMP method used here to estimate the lifetime risk of gastrointestinal cancers can effectively deduct the overestimation of the incidence risk from the multiple primary cancers and take into account the competing risks of death from other causes, as well as life expectancy. The traditional indicator of cumulative incidence risk for those aged 0–74 years underestimates cancer risk in populations in which the life expectancy is relatively high, or the competing risks of death from other causes is relatively low.28 Considering the growing cancer burden accompanied with increasing life expectancy and population ageing,23, 29, 30, 31, 32, 33 the lifetime risks calculated here provide an up-to-date and intuitive indicator for comparing gastrointestinal cancer burdens across populations. Third, we systematically compared the lifetime risk of developing and dying from different types of gastrointestinal cancers across countries. These results highlight the extent to which different types of gastrointestinal cancer threaten public health, and help to identify the priority gastrointestinal cancer type in each country to assist in the formulation of future prevention and control strategies.34
There are also several limitations. First, the GLOBOCAN national estimates are derived from registry data (both incidence and survival) and vital statistics (mortality), and their accuracy are dependent on the availability and quality of the source information in each country. There remain some deficiencies in the quality and coverage of incidence data in several regions, particularly in Central and South America, Asia-Pacific, and Africa. Only about one-third of the world's population has robust official national mortality statistics, with a marked absence in vital registration systems in Africa and several Asian regions. As a result, the national estimates of lifetime risk presented here, particularly in transitioning countries, should be interpreted with caution.5, 35 Second, the present analysis focuses on cross-sectional estimates of the lifetime risk of gastrointestinal cancer in 2020, the most recent year for which IARC's global estimates are available, so trends in the lifetime risk of gastrointestinal cancer could not be assessed. Third, the lifetime risks presented here were estimated by applying gastrointestinal cancer incidence, mortality, and all-cause mortality rates at different ages in 2020 as if they applied to a cohort as they aged. This method represents a reasonable and necessary simplification within the scope of this study, given the inability of obtaining actual incidence and mortality from the entire lifetime of individuals in a real-world longitudinal cohort. Thus, it should be noted that as the distributions of lifestyle and behavioural risk factors continue to shift in transitioned and transitioning countries, subsequent trends in the incidence rates of specific gastrointestinal cancers are likely to change in direction and magnitude,9, 36 and hence the lifetime risks of gastrointestinal cancers can change. Fourth, the estimates presented here do not reflect the effect of the COVID-19 pandemic, as they are based on extrapolations of cancer data collected in earlier years, before the pandemic.
In 2020, the global lifetime risk of gastrointestinal cancers translates approximately to one in 12 people developing, and one in 16 people dying from, gastrointestinal cancers. The apparent heterogeneity in lifetime risks across regions and countries indicates that there is an urgent need to establish context-specific, targeted cancer prevention plans for different gastrointestinal cancer types according to their underlying cause and causal pathways. Country-specific cancer control plans focusing on population education, risk factor control, tailored cancer screening programmes for high-risk populations, and improvements in the availability and affordability of appropriate health care and treatment are essential to reduce the burden of these commonly occurring yet potentially fatal gastrointestinal cancers.18
Data sharing
Aggregated cancer incidence and mortality data are freely available online at https://gco.iarc.fr/today/. The population, all-cause mortality, and life expectancy data are freely available at https://population.un.org/wpp/. Other requests for the full dataset can be made to the corresponding author, Wen-Qiang Wei, at weiwq@cicams.ac.cn.
Declaration of interests
We declare no competing interests.
Acknowledgments
Acknowledgments
Funding was provided by Beijing Nova Program (Z201100006820069), CAMS Innovation Fund for Medical Sciences (2021-I2M-1-023), and Talent Incentive Program of Cancer Hospital, CAMS (Hope Star).
Editorial note: The Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Contributors
SW contributed to the conceptualisation, methodology, software, formal analysis, writing of the original draft, and funding acquisition. RZ contributed to the methodology, software, formal analysis, and reviewing and editing. JL contributed to the methodology, visualisation, and reviewing and editing. HZ, LL, RC, KS, and BH contributed to the reviewing and editing of the manuscript. FB contributed to the methodology, resources, and reviewing and editing. WW contributed to the conceptualisation, project administration, supervision, and reviewing and editing. JH contributed to the resources, supervision, and reviewing and editing. SW and RZ had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Supplementary Material
References
- 1.Ahmad AS, Ormiston-Smith N, Sasieni PD. Trends in the lifetime risk of developing cancer in Great Britain: comparison of risk for those born from 1930 to 1960. Br J Cancer. 2015;112:943–947. doi: 10.1038/bjc.2014.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sasieni PD, Shelton J, Ormiston-Smith N, Thomson CS, Silcocks PB. What is the lifetime risk of developing cancer?: the effect of adjusting for multiple primaries. Br J Cancer. 2011;105:460–465. doi: 10.1038/bjc.2011.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnold M, Abnet CC, Neale RE, et al. Global burden of 5 major types of gastrointestinal cancer. Gastroenterology. 2020;159:335–349. doi: 10.1053/j.gastro.2020.02.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 5.Ferlay J, Ervik M, Lam F, et al. Global Cancer Observatory: cancer today. 2020. https://gco.iarc.fr/today
- 6.UN. Department of Economic and Social Affairs/Population Dynamics World Population Prospects. 2019. https://population.un.org/wpp/Download/Archive/Standard
- 7.Cullen J, Schwartz MD, Lawrence WF, Selby JV, Mandelblatt JS. Short-term impact of cancer prevention and screening activities on quality of life. J Clin Oncol. 2004;22:943–952. doi: 10.1200/JCO.2004.05.191. [DOI] [PubMed] [Google Scholar]
- 8.Day NE. Cancer incidence in five continents. Cumulative rate and cumulative risk. IARC Sci Publ. 1992;120:862–864. [PubMed] [Google Scholar]
- 9.Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683–691. doi: 10.1136/gutjnl-2015-310912. [DOI] [PubMed] [Google Scholar]
- 10.GBD 2019 Colorectal Cancer Collaborators Global, regional, and national burden of colorectal cancer and its risk factors, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol Hepatol. 2022;7:627–647. doi: 10.1016/S2468-1253(22)00044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.GBD 2017 Oesophageal Cancer Collaborators The global, regional, and national burden of oesophageal cancer and its attributable risk factors in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5:582–597. doi: 10.1016/S2468-1253(20)30007-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.GBD 2017 Stomach Cancer Collaborators The global, regional, and national burden of stomach cancer in 195 countries, 1990–2017: a systematic analysis for the Global Burden of Disease study 2017. Lancet Gastroenterol Hepatol. 2020;5:42–54. doi: 10.1016/S2468-1253(19)30328-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akinyemiju T, Abera S, Ahmed M, et al. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: results from the Global Burden of Disease Study 2015. JAMA Oncol. 2017;3:1683–1691. doi: 10.1001/jamaoncol.2017.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keum N, Giovannucci E. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol. 2019;16:713–732. doi: 10.1038/s41575-019-0189-8. [DOI] [PubMed] [Google Scholar]
- 15.Roa JC, García P, Kapoor VK, Maithel SK, Javle M, Koshiol J. Gallbladder cancer. Nat Rev Dis Primers. 2022;8:69. doi: 10.1038/s41572-022-00398-y. [DOI] [PubMed] [Google Scholar]
- 16.Aslanian HR, Lee JH, Canto MI. AGA clinical practice update on pancreas cancer screening in high-risk individuals: expert review. Gastroenterology. 2020;159:358–362. doi: 10.1053/j.gastro.2020.03.088. [DOI] [PubMed] [Google Scholar]
- 17.Zeng H, Ran X, An L, et al. Disparities in stage at diagnosis for five common cancers in China: a multicentre, hospital-based, observational study. Lancet Public Health. 2021;6:e877–e887. doi: 10.1016/S2468-2667(21)00157-2. [DOI] [PubMed] [Google Scholar]
- 18.Pramesh CS, Badwe RA, Bhoo-Pathy N, et al. Priorities for cancer research in low- and middle-income countries: a global perspective. Nat Med. 2022;28:649–657. doi: 10.1038/s41591-022-01738-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 20.Huang J, Lucero-Prisno DE, Zhang L, et al. Updated epidemiology of gastrointestinal cancers in East Asia. Nat Rev Gastroenterol Hepatol. 2023;20:271–287. doi: 10.1038/s41575-022-00726-3. [DOI] [PubMed] [Google Scholar]
- 21.Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391:1023–1075. doi: 10.1016/S0140-6736(17)33326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allemani C, Weir HK, Carreira H, et al. Global surveillance of cancer survival 1995–2009: analysis of individual data for 25 676 887 patients from 279 population-based registries in 67 countries (CONCORD-2) Lancet. 2015;385:977–1010. doi: 10.1016/S0140-6736(14)62038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bray F, Jemal A, Grey N, Ferlay J, Forman D. Global cancer transitions according to the Human Development Index (2008–2030): a population-based study. Lancet Oncol. 2012;13:790–801. doi: 10.1016/S1470-2045(12)70211-5. [DOI] [PubMed] [Google Scholar]
- 24.de Martel C, Georges D, Bray F, Ferlay J, Clifford GM. Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Glob Health. 2020;8:e180–e190. doi: 10.1016/S2214-109X(19)30488-7. [DOI] [PubMed] [Google Scholar]
- 25.Abnet CC, Arnold M, Wei WQ. Epidemiology of esophageal squamous cell carcinoma. Gastroenterology. 2018;154:360–373. doi: 10.1053/j.gastro.2017.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murphy G, McCormack V, Abedi-Ardekani B, et al. International cancer seminars: a focus on esophageal squamous cell carcinoma. Ann Oncol. 2017;28:2086–2093. doi: 10.1093/annonc/mdx279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Middleton DRS, Mmbaga BT, Menya D, et al. Alcohol consumption and oesophageal squamous cell cancer risk in east Africa: findings from the large multicentre ESCCAPE case-control study in Kenya, Tanzania, and Malawi. Lancet Glob Health. 2022;10:e236–e245. doi: 10.1016/S2214-109X(21)00506-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schouten LJ, Straatman H, Kiemeney LA, Verbeek AL. Cancer incidence: life table risk versus cumulative risk. J Epidemiol Community Health. 1994;48:596–600. doi: 10.1136/jech.48.6.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neupane S, Bray F, Auvinen A. National economic and development indicators and international variation in prostate cancer incidence and mortality: an ecological analysis. World J Urol. 2017;35:851–858. doi: 10.1007/s00345-016-1953-9. [DOI] [PubMed] [Google Scholar]
- 30.Arnold M, Rentería E, Conway DI, Bray F, Van Ourti T, Soerjomataram I. Inequalities in cancer incidence and mortality across medium to highly developed countries in the twenty-first century. Cancer Causes Control. 2016;27:999–1007. doi: 10.1007/s10552-016-0777-7. [DOI] [PubMed] [Google Scholar]
- 31.Ho JY, Hendi AS. Recent trends in life expectancy across high income countries: retrospective observational study. BMJ. 2018;362 doi: 10.1136/bmj.k2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.GBD 2016 Mortality Collaborators Global, regional, and national under-5 mortality, adult mortality, age-specific mortality, and life expectancy, 1970–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1084–1150. doi: 10.1016/S0140-6736(17)31833-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.GBD 2017 Mortality Collaborators Global, regional, and national age-sex-specific mortality and life expectancy, 1950–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1684–1735. doi: 10.1016/S0140-6736(18)31891-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Atun R, Cavalli F. The global fight against cancer: challenges and opportunities. Lancet. 2018;391:412–413. doi: 10.1016/S0140-6736(18)30156-9. [DOI] [PubMed] [Google Scholar]
- 35.Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941–1953. doi: 10.1002/ijc.31937. [DOI] [PubMed] [Google Scholar]
- 36.Arnold M, Park JY, Camargo MC, Lunet N, Forman D, Soerjomataram I. Is gastric cancer becoming a rare disease? A global assessment of predicted incidence trends to 2035. Gut. 2020;69:823–829. doi: 10.1136/gutjnl-2019-320234. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Aggregated cancer incidence and mortality data are freely available online at https://gco.iarc.fr/today/. The population, all-cause mortality, and life expectancy data are freely available at https://population.un.org/wpp/. Other requests for the full dataset can be made to the corresponding author, Wen-Qiang Wei, at weiwq@cicams.ac.cn.