Abstract
Gingival inflammation, bacterial infection, alveolar bone destruction, and subsequent tooth loss are characteristic features of periodontal disease, but the precise mechanisms of bone loss are poorly understood. Most animal models of the disease require injury to gingival tissues or teeth, and the effects of microorganisms are thus complicated by host responses to tissue destruction. To determine whether three putative periodontal pathogens, Porphyromonas gingivalis, Campylobacter rectus, and Fusobacterium nucleatum, could cause localized bone resorption in vivo in the absence of tissue injury, we injected live or heat-killed preparations of these microorganisms into the subcutaneous tissues overlying the calvaria of normal mice once daily for 6 days and then examined the bones histologically. We found that all three microorganisms (both live and heat killed) stimulated bone resorption and that the strain of F. nucleatum used appeared to be the strongest inducer of osteoclast activity. Treatment of the mice concomitantly with indomethacin reduced but did not completely inhibit bone resorption by these microorganisms, suggesting that their effects were mediated, in part, by arachidonic acid metabolites (e.g., prostaglandins). Our findings indicate that these potential pathogens can stimulate bone resorption locally when placed beside a bone surface in vivo in the absence of prior tissue injury and support a role for them in the pathogenesis of bone loss around teeth in periodontitis.
Periodontal disease is a common inflammatory disorder that often leads to irreversible alveolar bone resorption and tooth loss. It begins as a mixed bacterial infection in the gingiva surrounding teeth (18, 34) and leads subsequently to loss of attachment of the periodontal ligament, which anchors teeth to the surrounding bone. Since there are minimal systemic effects of periodontitis, it is likely that the bone and soft tissue destruction around affected teeth results from the local release of inflammatory mediators secondary to bacterial infection (8, 10, 17, 21, 38). Several potential periodontal pathogens have been studied, and of these, Porphyromonas gingivalis, Campylobacter rectus, Actinobacillus actinomycetemcomitans, and Fusobacterium nucleatum are considered to represent a significant portion of the pathogenic microbiota (7, 10, 21, 40). They possess or can induce in host cells several factors, such as lipopolysaccharide (LPS) (37), interleukin-1 (IL-1) (9), IL-6 (28, 29), tumor necrosis factor (31), surface-associated proteins (27), fimbriae (12), vesicles, toxins, and enzymes (30), which are thought to cause, directly or indirectly, irreversible loss of periodontal supportive tissues.
We showed previously that P. gingivalis (13) and C. rectus (14) can cause soft tissue destruction following injection of viable bacteria into the mid-dorsal subcutaneous (s.c.) tissue of normal mice. Others have shown that a variety of bacterial products from some of these microorganisms can stimulate osteoclast formation (26) and bone resorption in organ cultures of rodent bone (17, 19, 20, 25, 38, 39). To date, however, there have been no reports of any individual putative periodontal pathogen causing bone resorption in an in vivo model without damage to soft tissues or bone before the introduction of microorganisms.
To address this question, we injected potential periodontal pathogens into the s.c. tissues overlying the calvaria of normal mice using a model that we had developed previously to examine the in vivo effects of potential osteoclast-stimulating factors (1, 2). We hypothesized that this model would be amenable to evaluating host-bacterium interactions which contribute to bone resorption in vivo. We found that P. gingivalis, C. rectus, and F. nucleatum stimulated bone resorption in this model and that the effects were mediated, in part, by arachidonate metabolites.
MATERIALS AND METHODS
Animals.
Female ICR Swiss mice (Harlan Laboratories, Indianapolis, Ind.) weighing 20 to 25 g were housed in isolator cages in an American Association for Accreditation of Laboratory Animal Care-accredited animal facility at the University of Texas Health Science Center at San Antonio. Autoclaved TEKLAD chow (Sprague-Dawley Co., Madison, Wis.) and water were provided ad libitum.
Microorganisms.
We chose to study three potential periodontopathogenic bacteria: P. gingivalis W50 (13), C. rectus 576 (14), and F. nucleatum T18 (16). The bacteria were grown on prereduced Trypticase soy agar plates enriched with 5% (vol/vol) sheep blood (ETSA) in an anaerobic chamber (85% N2, 5% CO2, 10% H2). P. gingivalis and C. rectus were cultured for 72 h while F. nucleatum was cultured for 24 h on these plates.
All bacterial manipulations were carried out with Coy anaerobic chambers to ensure maximum viability. The cells were harvested aseptically with a sterile cotton applicator soaked in reduced transport fluid (RTF) (36) and immediately suspended in RTF. A sample was diluted up to 1/1,000, the optical density was measured at 600 nm (Beckman DV-65 spectrophotometer), and the bacterial cell concentration was determined by use of strain-specific growth curves. The stock suspension was then either diluted with RTF or centrifuged at 7,000 × g for 6 min, and a portion of the supernatant was removed to obtain the desired concentration. Bacterial cell suspensions were transported in anaerobic gas-filled vacuum vials and were used within 15 to 30 min of preparation. Previous studies with vital dyes (15) demonstrated that >95% of bacteria treated in this way are viable at the time of injection.
Heat-killed bacteria were prepared by placing 200 μl of bacterial cell suspension in a sealed tube and heating it to 85°C for 10 min. Samples of both live and heat-killed bacterial cell suspensions were plated on ETSA and cultured for 24 to 72 h to determine their viability and/or purity.
Injection of bacterial suspensions.
Live and heat-killed bacteria (2 × 106 to 2 × 109 in 10 μl of RTF) or RTF was injected into the s.c. tissue overlying the parietal bone on the right side of the calvarium of mice by use of a Hamilton (Reno, Nev.) syringe once daily for 6 days. The mice were euthanatized 8 h after the last injection, and the calvaria were removed for histological assessment. Based on preliminary experiments, the number of injected bacteria needed to evoke a resorptive response ranged from 2 × 106 to 2 × 109 for C. rectus and F. nucleatum and 2 × 107 to 2 × 109 for P. gingivalis. Generally, five mice were used for each dose of the bacteria and three mice were used for evaluation of the RTF control.
To determine whether any of the observed effects were prostaglandin mediated, mice (five to nine per group) injected with 2 × 109 bacteria of each species were also given s.c. injections of either indomethacin (40 μg in phosphate-buffered saline [PBS] three times daily) or PBS into the flank beginning 2 h before the first bacterial injection and continuing until 4 h after the last injection. This dosage schedule has been shown to be effective in previous studies (1) and was the highest dose of indomethacin that could be given without causing animal sickness or death over the duration of the experiment. None of the treated mice exhibited general signs of sickness during the experiment. A control group was injected with RTF over the calvarium and given indomethacin s.c.
Bone histology.
The calvaria were fixed in 10% phosphate-buffered formalin and decalcified in 14% EDTA. The anterior half of the frontal bones and most of the occipital bones were trimmed off, and the parietal bones were cut coronally. These half calvaria were then embedded in paraffin with the cut edges at the bottom of the cassettes, and four nonconsecutive levels were cut, providing eight coronal sections through each calvarium. These sections (5 μm thick) were stained with hematoxylin and eosin. Histomorphometric analysis of the following variables was carried out on the two sections from each calvarium which contained the largest number of bone marrow spaces and thus the greatest length of bone surface available for assessment of resorption by use of the Bioquant image analysis system (R&M Biometrics, Nashville, Tenn.) and a digitizing tablet: total bone area between the sagittal suture and the temporalis muscle insertion and osteoclast number (expressed per square millimeter of total bone area) (1). Additional sections were cut at the four levels in each calvarium, and sections from mice treated with each type of bacterium or vehicle were stained by the Gram stain method for the detection of bacteria which might have survived in the soft tissue and bone.
Statistical analyses.
Statistical analyses were carried out with Minitab (State College, Pa.) statistical software. Values for all variables for control groups of mice were found similar by one-way analysis of variance and were pooled for comparison with those for treatment groups. Differences between control groups and individual treatment groups were compared with Student’s t test. Differences between dose responses were tested by two-way analysis of variance with dose and microorganism as the independent variables. In groups in which values did not have a normal distribution, the data were log transformed prior to analysis.
RESULTS
Soft tissue swelling occurred at the injection site within 24 h of the first injection and increased in size throughout the experiment in almost all of the mice injected with bacteria but not in the controls. This finding was most pronounced in animals given the highest numbers of bacteria and was barely detectable in those given the lowest numbers. Two mice given the highest dose of P. gingivalis developed abscesses and ulceration of the overlying skin and were excluded from the study. No difference in the extent of soft tissue swelling was observed between mice given live bacteria and those given heat-killed bacteria. The mice did not display any systemic effects of the injections, maintained normal weight and behavior, and had no evidence of spread of infection to other sites, as determined at autopsy.
Histological examination revealed edema and a mixed inflammatory infiltrate of variable intensity in the soft tissues overlying the calvaria; this infiltrate consisted of polymorphonuclear leukocytes, lymphocytes, and macrophages. Microabscesses (Fig. 1) and areas of soft tissue necrosis were evident in sections from mice given the highest numbers of bacteria, and where the necrosis abutted the bone, there was necrosis of the underlying periosteal cells. No bacteria were seen in Gram-stained calvarial sections from mice with either a pronounced or a mild soft tissue inflammatory infiltrate. Thus, we assumed that the bacteria were removed by host cells during the 8 h between the last injection and euthanasia.
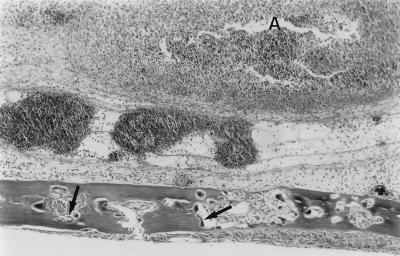
FIG. 1.
Effects of local injections of F. nucleatum on murine calvarial bone and soft tissue. Live F. nucleatum bacteria (2 × 107) were injected once daily for 6 days into the s.c. tissues overlying the calvaria of mice, and the animals were sacrificed 24 h later. Edema and abscesses (A) developed in the soft tissue overlying the calvarium of this mouse injected with F. nucleatum. In addition, increased numbers of osteoclasts (arrows) were seen inside the calvarium, and these caused an increase in the size of the bone marrow spaces (see Fig. 2C for comparison) due to increased endosteal bone resorption. Hematoxylin-eosin stain was used.
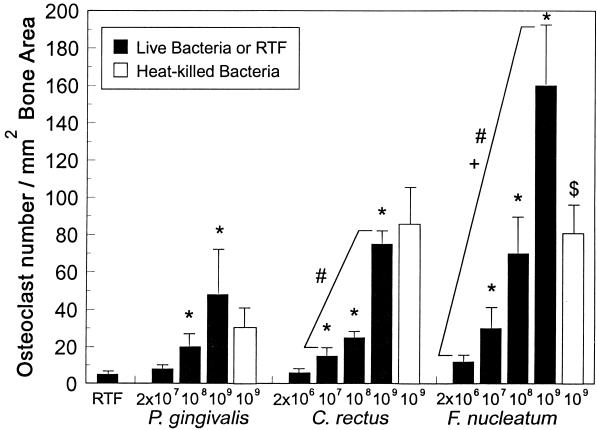
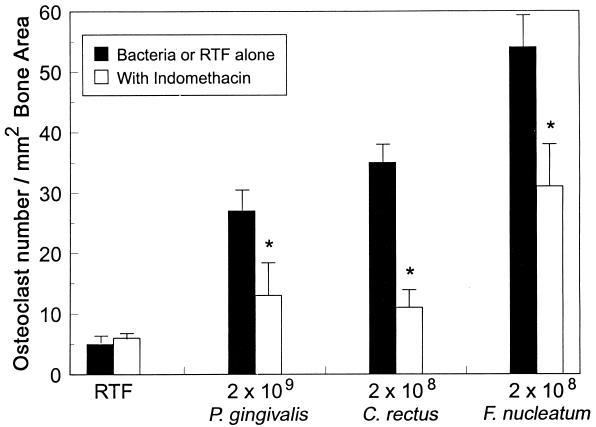
Increased numbers of osteoclasts were seen inside the calvaria of mice treated with the highest numbers of all three bacteria, and the effects are illustrated in Fig. 2; however, the intensity of the resorption and the numbers of osteoclasts seen varied in response to similar numbers of injected microorganisms (Fig. 2). Thus, although each bacterial species produced a dose-dependent increase in osteoclast numbers within the calvaria (Fig. 3), within the context of the species examined, F. nucleatum was found to be the most potent inducer of osteoclasts and P. gingivalis was found to be the least potent inducer. At lower doses, osteoclasts tended to be confined to the endosteal marrow surfaces, particularly in mice given P. gingivalis (Fig. 2); at higher doses, they were also present on the upper periosteal surface, consistent with more aggressive resorption. In many places, osteoclasts had eroded through the full thickness of the bone into the overlying periosteal tissues, particularly when the highest numbers of microorganisms were injected. Both live and heat-killed P. gingivalis and C. rectus were equally effective in stimulating osteoclast production (Fig. 3). However, heat-killed F. nucleatum stimulated the production of significantly fewer osteoclasts than live F. nucleatum. Indomethacin treatment of mice given numbers of bacteria eliciting similar levels of bone resorption (2 × 109 for P. gingivalis and 2 × 108 for C. rectus and F. nucleatum) resulted in a significant reduction in osteoclast numbers (Fig. 4). The intensity and extent of the soft tissue inflammatory infiltrate, however, were histologically similar in bacterially challenged mice treated with indomethacin or PBS.
FIG. 2.
Effects of local injections of F. nucleatum and P. gingivalis on mouse calvaria. Live bacteria were injected once daily for 6 days into the s.c. tissues overlying the calvaria of mice, and the animals were sacrificed 24 h later. (A) Increased numbers of osteoclasts (arrows) were seen in the bone marrow spaces, which were enlarged as a result of the increased osteoclast activity in the calvarium of a mouse injected with 2 × 107 F. nucleatum bacteria. (B) In contrast, only occasional osteoclasts (arrows) were seen in the bone marrow spaces of the calvarium of a mouse injected with 2 × 107 P. gingivalis bacteria, and few of these marrow spaces were enlarged. These appearances (B) are similar to those of the calvarium of a control mouse (C) given daily injections of the vehicle (RTF). A moderately heavy acute inflammatory infiltrate and moderate edema are present in the soft tissues overlying the calvaria of the mice injected with bacteria. Mild edema and a few inflammatory cells are present in the soft tissue overlying the calvarium of the control mouse. Hematoxylin-eosin stain was used.
FIG. 3.
Effects of injections of live or heat-killed bacteria on osteoclast numbers in mouse calvaria. Organisms were injected once daily for 6 days at the doses indicated. The mice were sacrificed 24 h later, and osteoclast numbers in decalcified calvarial sections were counted. Values are means ± standard errors of means. ∗, Significantly different from mean value for vehicle-treated (RTF) mice (P < 0.05). #, Dose response significantly different from that to P. gingivalis (P < 0.01). +, Dose response significantly different from that to C. rectus (P < 0.001). $, Significantly different from mean value for mice treated with live F. nucleatum bacteria (P < 0.01).
FIG. 4.
Effects of indomethacin and live bacteria on osteoclast numbers in mouse calvaria. Injections of indomethacin (40 μg in PBS) were given s.c. every 8 h for 6 days, beginning 2 h before the first bacterial injection and continuing until 4 h after the last injection in groups of mice (n = 5 for bacteria alone; n = 5, n = 6, and n = 7 for indomethacin with P. gingivalis, F. nucleatum, and C. rectus, respectively; n = 8 and n = 9 for RTF alone and RTF with indomethacin, respectively). Values are means ± standard errors of means. All values for the bacterium-injected mice (with and without indomethacin) were significantly different from values for the vehicle-treated (RTF) mice (P < 0.05). ∗, Significantly different from values for mice given bacteria alone (P < 0.01).
The osteoclast-stimulating effects of the bacterial injections appeared to be confined locally to the superior part of the calvaria at the site where the microorganisms were injected, because we saw no increase in osteoclast numbers within the bone marrow cavities inside the temporal bones, which are attached to the lateral borders of the parietal bones. Injected microorganisms can spread over the surface of the parietal bones because the skin is very loosely attached. However, firm attachment of the temporalis muscle prevents the spread of microorganisms to the temporal bones.
DISCUSSION
Periodontitis is an important oral infection that occurs around the teeth of up to 40% of U.S. citizens (5) and, like osteoporosis, is often quoted as a potentially cytokine-mediated cause of significant bone loss. Numerous potential pathogens have been isolated from the pockets of inflammation in the gingiva adjacent to affected teeth of patients with periodontal disease (22), and of these, P. gingivalis has frequently been considered to be responsible for causing the associated bone loss in adults (6). Surprisingly, although all three of the potential periodontal pathogens that we chose to study stimulated bone resorption, P. gingivalis elicited the least osteoclast-stimulating activity in our in vivo calvarial bone resorption model. The number of microorganisms that we injected (2 × 106 to 2 × 109) is relatively small in comparison to the number of microorganisms required to cause soft tissue-destructive inflammatory lesions following injection into the flanks of normal mice (>5 × 109) (4, 13) and is within the range of the level of colonization in the gingival sulci of patients with periodontitis (35). Thus, the effects that we observed on osteoclasts in the calvaria of mice in the present study, particularly with the lower numbers of microorganisms, are unlikely to have been due to overwhelming infection.
Increased bone resorption and localized osteolysis are well-established features of infections occurring inside bones (osteomyelitis) as a result of pathogenic microorganisms, such as gram-positive staphylococci (24) and streptococci and gram-negative Escherichia coli, Proteus spp., and Haemophilus influenzae (23). The activation of osteoclasts in such infections is likely to be mediated by cytokines and inflammatory mediators released locally by host cells in response to bacterial cell products. It is widely believed that similar mechanisms lead to alveolar bone loss around affected teeth in patients with periodontitis, with the potential for tooth loss. Gingival fibroblasts have been reported to increase prostaglandin E2 and IL-1 production when cultured with LPS from P. gingivalis (33), and IL-1β, tumor necrosis factor alpha, and IL-6, which stimulate bone resorption in vivo (3, 11, 32), are found in gingival crevicular fluid (9, 31).
The dramatic increase in osteoclast numbers and the full-thickness calvarial bone resorption defects that we observed in response to all three bacteria were similar to the effects that we observed in the calvaria of mice following local injections of IL-1 (1). In those experiments, we saw resorption defects through the full thickness of calvarial bones 4 days after the last of three daily injections of IL-1. This time corresponds to the day of experiment termination following local calvarial injections of bacteria for 6 days in the present study. The local osteoclast-stimulating effect seen 4 days after the IL-1 injections was prevented by concomitant treatment with indomethacin and thus appeared to be eicosanoid dependent (e.g., prostaglandins) (1). Treatment of mice with indomethacin in the present study significantly attenuated the increase in osteoclast numbers and activity; thus, the changes observed in the calvaria were likely to have been partly eicosanoid mediated. Although the dose of indomethacin given (40 mg three times daily for 7 days) was less than half that which we administered previously to prevent IL-1-induced local bone resorption in mice (1), it was the highest dose that we could administer for 7 days in the present study, since higher doses (60 mg or more three times daily) caused morbidity or mortality in the mice. Thus, we believe that the dose was sufficiently high to have reached therapeutic levels in blood and to have accounted for the reduced bone resorption.
Our observation that preparations of heat-killed P. gingivalis and C. rectus stimulated bone resorption to the same degree as live organisms suggests that the effects are likely to be due either to direct stimulation of osteoclasts by bacterial cell products or to indirect stimulation of osteoclasts by products, such as cytokines, released locally by host cells, rather than to the release of osteoclast-stimulating factors by live bacteria during the infection. This observation was not uniform, however, since live F. nucleatum elicited significantly greater osteoclast numbers than heat-killed bacteria. The basis for this difference is not obvious; however, it is clear that the lipopolysaccharides of many oral microorganisms vary in their biologic activities and are quite different from the classic LPS molecules of the Enterobacteriaceae. We propose that heat-killed bacteria primarily elicit host responses via LPS or heat-stable polysaccharide components. One inference is that the LPS from F. nucleatum was less active in our model system than LPS components from the other microorganisms examined. Our previous studies with local calvarial injections of IL-1 (1) suggested that a cascade of inflammatory mediators may be activated during IL-1 exposure and that these mediators may be responsible for the subsequent dramatic osteolytic defects. Thus, localized bacterial gingival infection could lead to the initiation of a cascade of inflammatory events that lead to alveolar bone loss that may or may not require the continuous presence of live bacteria. The in vivo model described in this paper will permit further studies with purified or partially purified bacterial cell products and inhibitors of known cytokines to explore the mechanisms that lead to localized tooth loss.
To our knowledge, this is the first report of bone resorption being caused in vivo in a dose-dependent monoinfection by microorganisms which have been identified in the gingival pockets of affected teeth in patients with periodontitis. Our findings raise the prospect that specific antimicrobial or antiosteoclast therapy could be evaluated in this model with the goal of preventing the establishment of bone loss in individuals at high risk of developing periodontitis or of abrogating the progression of the bone destruction that can result in the loss of otherwise healthy teeth in patients with established periodontitis.
ACKNOWLEDGMENTS
This research was supported by U.S. Public Health Service grants DE-07267 and DE-08569 from the National Institute for Dental Research.
REFERENCES
- 1.Boyce B F, Aufdemorte T B, Garrett I R, Yates A J P, Mundy G R. Effects of interleukin-1 on bone turnover in normal mice. Endocrinology. 1989;125:1142–1150. doi: 10.1210/endo-125-3-1142. [DOI] [PubMed] [Google Scholar]
- 2.Boyce B F, Yoneda T, Lowe C, Soriano P, Mundy G R. Requirement of pp60c-src expression for osteoclasts to form ruffled borders and resorb bone in mice. J Clin Invest. 1992;90:1622–1627. doi: 10.1172/JCI116032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De La Mata J, Uy H L, Guise T A, Story B, Boyce B F, Mundy G R, Roodman G D. IL-6 enhances hypercalcemia and bone resorption mediated by PTHrP in vivo. J Clin Invest. 1995;95:2846–2852. doi: 10.1172/JCI117990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ebersole J L, Kesavalu L, Schneider S, Machen R L, Holt S C. Comparative virulence of periodontopathogens in a mouse abscess model. Oral Dis. 1995;1:115–128. doi: 10.1111/j.1601-0825.1995.tb00174.x. [DOI] [PubMed] [Google Scholar]
- 5.Fox C H. New considerations in the prevalence of periodontal disease. Curr Opin Dent. 1992;2:5–11. [PubMed] [Google Scholar]
- 6.Haffajee A D, Socransky S S. Microbial etiological agents of destructive periodontal diseases. Periodontology 2000. 1994;5:78–111. doi: 10.1111/j.1600-0757.1994.tb00020.x. [DOI] [PubMed] [Google Scholar]
- 7.Holt S C, Ebersole J L, Felton J, Brunsvold M, Kornman K S. Implantation of Bacteroides gingivalis in non-human primates initiates progression of periodontitis. Science. 1988;239:55–57. doi: 10.1126/science.3336774. [DOI] [PubMed] [Google Scholar]
- 8.Hopps R M, Sismey-Durrant H J. Mechanisms of alveolar bone loss in periodontal disease. In: Hamada S, Holt S C, McGhee J R, editors. Periodontal disease: pathogens and host immune response. Tokyo, Japan: Quintessence Publishing Co., Ltd.; 1991. pp. 307–320. [Google Scholar]
- 9.Hou L T, Liu C M, Rossomando E F. Crevicular interleukin-1b in moderate and severe periodontitis patients and the effect of phase I periodontal treatment. J Clin Periodontol. 1995;22:162–167. doi: 10.1111/j.1600-051x.1995.tb00128.x. [DOI] [PubMed] [Google Scholar]
- 10.Ishihara Y, Nishihara T, Maki E, Noguchi T, Koga T. Role of interleukin-1 and prostaglandin in in vitro bone resorption induced by Actinobacillus actinomycetemcomitans lipopolysaccharide. J Periodontal Res. 1991;26:155–160. doi: 10.1111/j.1600-0765.1991.tb01639.x. [DOI] [PubMed] [Google Scholar]
- 11.Johnson R A, Boyce B F, Mundy G R, Roodman G D. Tumors producing human tumor necrosis factor induce hypercalcemia and osteoclastic bone resorption in nude mice. Endocrinology. 1989;124:1424–1427. doi: 10.1210/endo-124-3-1424. [DOI] [PubMed] [Google Scholar]
- 12.Kawata Y, Hanazawa S, Amano S, Murakami Y, Matsumoto T, Nishida K, Kitano S. Porphyromonas gingivalis fimbriae stimulate bone resorption in vitro. Infect Immun. 1994;62:3012–3016. doi: 10.1128/iai.62.7.3012-3016.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kesavalu L, Ebersole J L, Machen R L, Holt S C. Porphyromonas gingivalis virulence in mice: induction of immunity to bacterial components. Infect Immun. 1992;60:1455–1464. doi: 10.1128/iai.60.4.1455-1464.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kesavalu L, Holt S C, Crawley R R, Borinski R, Ebersole J L. Virulence of Wolinella recta in a murine abscess model. Infect Immun. 1991;59:2806–2817. doi: 10.1128/iai.59.8.2806-2817.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kesavalu L, Holt S C, Ebersole J L. Hydrolytic enzymes of Porphyromonas gingivalis: in vitro distribution and functions. Int J Oral Biol. 1997;22:255–267. [Google Scholar]
- 16.Kinder S, Holt S C. Characterization of coaggregation between Bacteroides gingivalis T22 and Fusobacterium nucleatum T18. Infect Immun. 1989;57:3425–3433. doi: 10.1128/iai.57.11.3425-3433.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirby A C, Meghji S, Nair S P, White P, Reddi K, Nishihara T, Nakashimi K, Willis A C, Sim R, Wilson M, Henderson B. The potent bone-resorbing mediator of Actinobacillus actinomycetemcomitans is homologous to the molecular chaperone GroEL. J Clin Invest. 1995;96:1185–1194. doi: 10.1172/JCI118150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liakoni H, Barber P, Newman H N. Bacterial penetration of pocket soft tissues in chronic adult and juvenile periodontitis cases. An ultrastructural study. J Clin Periodontol. 1987;14:22–28. doi: 10.1111/j.1600-051x.1987.tb01508.x. [DOI] [PubMed] [Google Scholar]
- 19.Meghji S, Henderson B, Wilson M. Higher-titer antisera from patients with periodontal disease inhibit bacterial capsule-induced bone breakdown. J Periodontal Res. 1993;28:115–121. doi: 10.1111/j.1600-0765.1993.tb01058.x. [DOI] [PubMed] [Google Scholar]
- 20.Meghji S, Wilson M, Ivanyi L, Harris M. Comparison of the osteolytic activity and serum antibodies to capsules of gram negative bacteria. J Dent Res. 1990;69:980. [Google Scholar]
- 21.Meghji S, Wilson M, Barber P, Henderson B. Bone resorbing activity of surface-associated material of Actinobacillus actinomycetemcomitans and Eikenella corrodens. J Med Microbiol. 1994;41:197–203. doi: 10.1099/00222615-41-3-197. [DOI] [PubMed] [Google Scholar]
- 22.Moore W E C, Moore L V H. The bacteria of periodontal diseases. Periodontology 2000. 1994;5:66–77. doi: 10.1111/j.1600-0757.1994.tb00019.x. [DOI] [PubMed] [Google Scholar]
- 23.Nair S, Meghji S, Wilson M, Reddi K, White P, Henderson B. Bacterially induced bone destruction: mechanisms and misconceptions. Infect Immun. 1996;64:2371–2380. doi: 10.1128/iai.64.7.2371-2380.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nair S, Song Y, Meghji S, Reddi K, Harris M, Ross A, Poole S, Wilson M, Henderson B. Surface-associated proteins from Staphylococcus aureus demonstrate potent bone resorbing activity. J Bone Min Res. 1995;10:726–734. doi: 10.1002/jbmr.5650100509. [DOI] [PubMed] [Google Scholar]
- 25.Nishihara T, Ohsaki Y, Ueda N, Saito N, Mundy G R. Mouse interleukin-1 receptor antagonist induced by Actinobacillus actinomycetemcomitans lipopolysaccharide blocks the effects of interleukin-1 on bone resorption and osteoclast-like cell formation. Infect Immun. 1994;62:390–397. doi: 10.1128/iai.62.2.390-397.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishihara T, Ueda N, Amano K, Ishihara Y, Hayakawa H, Kuroyanagi T, Ohsaki Y, Nagata K, Noguchi T. Actinobacillus actinomycetemcomitans Y4 capsular-polysaccharide-like polysaccharide promotes osteoclast-like cell formation by interleukin-1α production in mouse marrow cultures. Infect Immun. 1995;63:1893–1898. doi: 10.1128/iai.63.5.1893-1898.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reddi K, Meghji S, Wilson M, Henderson B. Comparison of the osteolytic activity of surface-associated proteins of bacteria implicated in periodontal disease. Oral Dis. 1995;1:26–31. doi: 10.1111/j.1601-0825.1995.tb00153.x. [DOI] [PubMed] [Google Scholar]
- 28.Reddi K, Poole S, Nair S, Meghji S, Henderson B, Wilson M. Lipid A-associated proteins from periodontopathogenic bacteria induce interleukin-6 production by human gingival fibroblasts and monocytes. FEMS Immunol Med Microbiol. 1995;11:137–144. doi: 10.1111/j.1574-695X.1995.tb00100.x. [DOI] [PubMed] [Google Scholar]
- 29.Reinhardt R A, Masada M P, Kaldahl W B, DuBois L M, Kornman K S, Choi J I, Kalkwarf K L, Allison A C. Gingival fluid IL-1 and IL-6 levels in refractory periodontitis. J Clin Periodontol. 1993;20:225–331. doi: 10.1111/j.1600-051x.1993.tb00348.x. [DOI] [PubMed] [Google Scholar]
- 30.Rifkin B R, Vernillo A T, Golub L M. Blocking periodontal disease progression by inhibiting tissue-destructive enzymes: a potential therapeutic role for tetracyclines and their chemically-modified analogs. J Periodontol. 1993;64:819–827. doi: 10.1902/jop.1993.64.8s.819. [DOI] [PubMed] [Google Scholar]
- 31.Rossomando E F, White L. A novel method for the detection of TNF-α in gingival crevicular fluid. J Periodontol. 1993;64:445–449. [PubMed] [Google Scholar]
- 32.Sabatini M, Boyce B F, Aufdemorte T, Bonewald L, Mundy G R. Infusions of recombinant human interleukins 1α and 1β cause hypercalcemia in normal mice. Proc Natl Acad Sci USA. 1988;85:5235–5239. doi: 10.1073/pnas.85.14.5235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sismey-Durrant H J, Hopps R M. Effect of lipopolysaccharide from Porphyromonas gingivalis on prostaglandin E2 and interleukin-1-β release from rat periosteal and human gingival fibroblasts in vitro. Oral Microbiol Immunol. 1991;6:378–380. doi: 10.1111/j.1399-302x.1991.tb00510.x. [DOI] [PubMed] [Google Scholar]
- 34.Slots J, Genco R J. Black pigmented bacteroides species, Capnocytophaga species and Actinobacillus actinomycetemcomitans in human periodontal disease: virulence factors in colonization, survival and tissue destruction. J Dent Res. 1984;63:412–421. doi: 10.1177/00220345840630031101. [DOI] [PubMed] [Google Scholar]
- 35.Socransky S S, Smith C, Martin L, Paster B J, Dewhirst F E, Levin A E. “Checkerboard” DNA-DNA hybridization. BioTechniques. 1994;17:788–792. [PubMed] [Google Scholar]
- 36.Syed S A, Loesche W J. Survival of human dental plaque flora in various transport media. Appl Microbiol. 1972;24:638–644. doi: 10.1128/am.24.4.638-644.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilson M. Biological activities of lipopolysaccharides from oral bacteria and their relevance to the pathogenesis of chronic periodontitis. Sci Prog. 1995;78:19–34. [PubMed] [Google Scholar]
- 38.Wilson M, Kamin S, Harvey W. Bone resorbing activity of purified capsular material from Actinobacillus actinomycetemcomitans. J Periodontal Res. 1985;20:484–491. doi: 10.1111/j.1600-0765.1985.tb00831.x. [DOI] [PubMed] [Google Scholar]
- 39.Wilson M, Meghji S, Barber P, Henderson B. Biological activities of surface-associated material from Porphyromonas gingivalis. FEMS Immunol Med Microbiol. 1993;6:147–155. doi: 10.1111/j.1574-695X.1993.tb00317.x. [DOI] [PubMed] [Google Scholar]
- 40.Zambon J J, Christersson L A, Genco R J. Diagnosis and treatment of localized juvenile periodontitis. J Am Dent Assoc. 1986;113:295–299. doi: 10.14219/jada.archive.1986.0152. [DOI] [PubMed] [Google Scholar]