Summary
Background
Human Immunodeficiency Virus (HIV)-exposed uninfected (HEU) infants have a higher burden of infectious diseases related morbidity and mortality compared with HIV-unexposed uninfected (HUU). Immunization of pregnant women living with HIV (PWLWH) could reduce the severity and burden of infectious diseases for HEU in early infancy.
Methods
We conducted a systematic review of safety and immunogenicity of vaccines administered to PWLWH and meta-analyses to test the overall effect of immunogenicity comparing pregnant women without HIV (PWWH) to PWLWH. We searched MEDLINE, Embase, Web of Science, Virtual Health Library and Cochrane databases in accordance with PRISMA guidelines for randomized controlled trials and observational studies. Review articles, case series, conference abstracts, and animal studies were excluded. Studies were included from inception to 6th September 2023, with no language restrictions. Random effects meta-analyses were performed for immunogenicity using Review manager (RevMan) analysis software version 5.4.1, Geometric Mean Titer (GMT) values were transformed to obtain the mean and standard deviation within RevMan, the effect size was computed and reported as mean difference with respective 95% confidence intervals. The review was registered with PROSPERO CRD42021289081.
Findings
We included 12 articles, comprising 3744 pregnant women, 1714 were PWLWH given either influenza, pneumococcal or an investigational Group B streptococcal (GBS) vaccine. Five studies described safety outcomes, and no increase in adverse events was reported in PWLWH compared to PWWH. The GMT increase from baseline to 28–35 weeks post vaccination in HA units ranged from 12.4 (95% CI: 9.84–14.9) to 238.8 (95% CI: 0.35–477.9). Meta-analyses of influenza vaccines showed the pooled geometric mean difference in Hemagglutination Inhibition (HAI) titers post vaccination was 56.01 (95% CI: 45.01–67.01), p < 0.001. The increase was less in PWLWH when compared with PWWH: −141.76 (95% CI: −194.96, −88.55), p < 0.001.
Interpretation
There are limited data on the safety and immunogenicity of vaccines given to PWLWH making policy consideration in this group difficult when new vaccines are introduced. With new vaccines on the horizon, PWLWH need to be included in studies to promote vaccine confidence for this special population.
Funding
This work was funded by Medical Research Council Joint Clinical Trials Round 9 [MR/T004983/1].
Keywords: HIV, Pregnancy, Vaccines, Safety, Immunogenicity
Research in context.
Evidence before this study
Previous reports suggest that maternal vaccines given to pregnant women living with HIV (PWLWH) are likely to be less immunogenic than in pregnant women without HIV (PWWH). Individual studies that compared maternal vaccines in PWLWH compared to PWWH documented the need for a deeper understanding of the difference in immunogenic responses to vaccines in this group. No systematic review and meta-analyses have previously reported on the safety and immunogenicity of maternal vaccines given to this special group.
We electronically searched MEDLINE, Embase, Web of Science, Virtual Health Library and Cochrane data bases from inception to 31st January 2022, we re-run the search from 1st February 2022 to 6th September 2023 with no language restrictions. The search was done in accordance with PRISMA reporting guidelines for randomized controlled trials and observational studies reporting on the safety and immunogenicity of vaccines given to pregnant women living with HIV. Review articles, case series, conference abstracts, letters and animal studies were excluded. Search terms were: (Pregnan∗ [MeSH] OR matern∗ [MeSH] OR expectan∗ [MeSH]) AND (HIV OR AIDS) AND (Vaccin∗ [MeSH] OR immun∗[MeSH]). Random effect model of meta-analyses was performed for immunogenicity using Review manager (RevMan) analysis software version 5.4.1. Risk of bias assessment was done in RevMan. The inverse variance-weighted average method was used to estimate the pooled mean difference and test for overall effect(Z). Statistical heterogeneity between studies was measured using I-square (I2).
Added value of this study
Our review provides a comprehensive assessment of the safety and immunogenicity of influenza, pneumococcal and investigational Group B streptococcal given to PWLWH and found no difference in vaccine safety in PWLWH compared to PWWH. We identified a significant increase in antibody concentration four weeks post vaccination, however the increase was lower in PWLWH when compared to PWWH.
Implications of all the available evidence
With new vaccines under consideration for administration during pregnancy, vaccine developers, researchers, policy makers and health service providers need to explore new avenues to enhance vaccine confidence in special groups like PWLWH. These may require different vaccine formulations or schedules to keep PWLWH and their infants protected. Our findings demonstrate potential conundrums for vaccine policy in countries with a high HIV burden and highlights the need for PWLWH to be included in trials of maternal vaccines to ensure confidence in this group.
Introduction
Globally, improved access to lifelong combined antiretroviral therapy (cART) has markedly contributed to the elimination of mother to child transmission of HIV(EMTCT).1 In 2021, approximately 38.4 million people (54% female) were living with HIV worldwide. Each week around 4900 women aged 15–24 years became infected, of which 4000 were in Sub-Saharan Africa (SSA).2 According to the World Health Organization (WHO), in 2021 there were an average of 1.3 million (1.0–1.6million) pregnant women living with HIV(PWLWH) with 81% (63–97%) on cART drugs.3
There are an estimated 15.4 million children who are HIV-exposed but uninfected (HEU). The majority of these live in low-and-middle income countries (LMICs) particularly SSA, where the HIV burden is highest.4,5 Higher infection related morbidity, impaired growth patterns, and higher mortality rates are reported in HEU infants compared with HIV-unexposed uninfected infants (HUU).4,6, 7, 8 In infancy, the burden of infections is higher for HEU than HUU particularly in the first six months of life; this is partly attributed to the low levels of placentally transferred antibodies from PWLWH compared to pregnant women without HIV (PWWH).9, 10, 11 A cohort study following up infants born to PWLWH compared with PWWH in Tanzania, reported an increased risk of cough (RR,1.28; 95% CI,1.03–1.29), fever (RR,1.16; 95% CI,1.03–1.29), unscheduled outpatient visits (RR,1.74; 95% CI,1.35–2.25) and hospitalization (RR,3.56; 95% CI,1.80–7.05) in HEU infants compared with HUU.12
The WHO recommends tetanus vaccines for use during pregnancy and evidence reviewed from clinical trials on the safety and immunogenicity of other maternal vaccines including influenza and Tdap concluded that these are safe for use in pregnancy.13 Several new vaccines are under development respiratory syncytial virus (RSV), group B streptococcus (GBS) and cytomegalovirus (CMV).14 However, data concerning safety and immunogenicity are scarce in pregnant women, who are usually excluded from early phase clinical trials. Efficient maternal vaccination programs for PWLWH could reduce the burden of infectious diseases in HEU. However, HIV is usually an exclusion criterion in clinical vaccine trials which impacts countries’ ability to provide guidance on vaccine administration once a vaccine is licensed, this may affect vaccine confidence and uptake in PWLWH.
The objective of this study was to describe the safety and immunogenicity of vaccines administered in PWLWH to reduce the associated morbidity and mortality due to infectious diseases in HEU.
Methods
Search strategy
We searched MEDLINE, Embase, Web of Science, Virtual Health Library and Cochrane Library databases for randomized controlled trials (RCTs) and observational studies reporting on the safety and immunogenicity of vaccines given to PWLWH, with a comparator group of PWWH (full search strategy available in Supplementary Table S1). Additional searches were done by forward citation. Search terms: (Pregnan∗ [MeSH] OR matern∗ [MeSH] OR expectan∗ [MeSH]) AND (HIV OR AIDS) AND (Vaccin∗ [MeSH] OR immun∗[MeSH]). The search was conducted from inception to 31st January 2022 and rerun from 1st February 2022 to 6th September 2023 using the same search terms. We had no language restrictions and abstracts were translated using Google translate if none of the authors spoke the language of the manuscript. The study was registered on the International Prospective Register of Systematic reviews (PROSPERO), registration number: CRD42021289081. The study protocol is available online.
Study selection and data collection
Eligible studies were those where a vaccine was given during pregnancy to PWLWH and those that had a comparator group of women who were HIV-uninfected; randomized controlled trials and observational studies were included. Review articles, case series, conference abstracts, letters and animal studies were excluded. The references for included studies were imported from databases into Endnote version 20, Clarivate, USA. Duplicate articles were identified and removed. The initial title and abstract screening were carried out independently by two authors (EN, JC), identifying articles for full review and potential inclusion. Rayyan enterprise software (rayyan.ai) a web application screening tool for systematic review,15 was used to assess full articles for inclusion. Studies were rated “include” or “exclude” using the Rayyan program. Any differences in rating were resolved by discussion with the senior author (KLD).
A data extraction form was developed using a spreadsheet program developed by Microsoft-excel. The following data were extracted for each included study: author and date, study design, setting, population, maternal HIV status and treatment if available, formulation of vaccine given, gestational age at which the vaccine was given, route of vaccine administration, number of doses given and dosing schedule followed, any solicited and unsolicited adverse events with level of severity recorded, time intervals post-vaccination at which samples were collected for immunogenicity analysis, the immunogenicity primary outcome and its measurement. Data for meta-analysis was extracted from articles which reported on PWLWH with a comparator PWWH. The extracted information (Supplementary Table S3), included study identifier details, number of participants enrolled in the study and those included in the immunogenicity analysis, HIV sero-status, type of influenza vaccine by subtype (A/H1N1, A/H3N2 and B/Victoria/Yamagata) and doses given. The antibody concentrations in Geometric Mean Titers (GMT) along with their 95% confidence intervals were extracted for baseline and post vaccination (28–35 days) periods. Data are reported according to PRISMA guidelines.
Risk of bias assessment
The risk of bias assessment was done by three authors (EN, VT, JO), Review Manager version 5.4.1(RevMan) was used to assess the methods, participants, interventions and outcomes for risk of bias in all the included RCTs Using the RevMan criteria we assessed studies for Random sequence generation, allocation concealment, blinding of study participants and personnel, blinding of outcome assessment, incomplete outcome data and selective reporting (Supplementary Table S2). The methodological quality of Non-RCTs was assessed using the Newcastle-Ottawa Scale,16 with scores under three categories including selection, comparability and outcomes (Supplementary Table S5). Any disagreements were resolved by consensus in consultation with KLD.
Statistics
Our meta-analyses explored the outcome variable of immunogenicity following receipt of influenza vaccine and how this is influenced by the HIV status in pregnant women.
We performed meta-analyses for the immunogenicity of vaccines given to PWLWH using a random effect model; the inverse variance method was used to estimate the pooled mean difference pre and post vaccination in PWLWH, and test the overall effect(Z), with 95% confidence intervals and p-value at 5% level of significance across studies. A comparison of mean differences for post-vaccination immunogenicity response between PWLWH and PWWH was also conducted.
Prior to analysis, the GMT values were transformed to obtain the mean and standard deviation. The level of statistical heterogenicity was assessed using I2 statistic. The group specific analyses included HIV status as well as vaccine subtype (A/H1N1, A/H3N2 and B/Victoria/Yamagata). We opted for subgroup analyses over meta-regression due to the small number of studies included in this study. All included immunogenicity results were reported on the same scale.
We assessed for publication bias using funnel plot asymmetry (Supplementary Fig. S1). All Statistical analyses were done with Review Manager version 5.4.1.
Role of funding source
The work was supported by the MRC Joint Clinical Trials Round 9 [MR/T004983/1]. The funding body played no role in the design of the study and the collection, analysis or interpretation of data or in writing of the manuscript.
Results
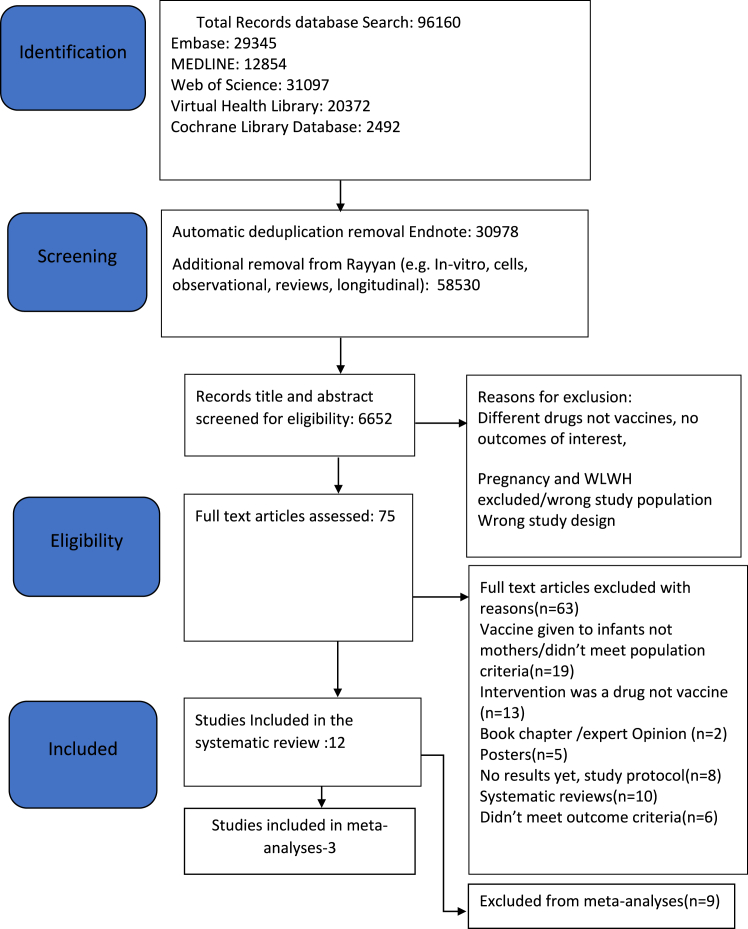
We identified 96,160 articles through the literature search, 75 full articles were assessed and 12 met the inclusion criteria. These studies comprised 3744 pregnant women, of which 1714 were PWLWH (Table 1). A total of three investigational vaccines in different formulations were identified in our review: pneumococcal, influenza, and group B streptococcus vaccines (Table 1). From the twelve studies we included in the review, seven were RCTs and five were observational studies. Nine of the studies were from low-and-middle-income-countries (LMICs) (South Africa [n = 5], Brazil [n = 3] and Malawi [n = 1], and there were three additional studies from the USA (Fig. 1 PRISMA diagram).
Table 1.
Characteristics of studies included in the systematic review and meta-analyses: safety and immunogenicity of vaccines administered to pregnant women living with HIV.
| Authors and country | Study setting/design | Inclusion criteria | Total sample size/(specified number PWLWH) | Type of vaccine given and formulation | Gestational age (GA) at vaccination and number of doses given | Reactogenicity: Solicited adverse events (AEs) | Safety: Maternal and fetal serious unsolicited AEs | Time intervals–sample collection for antibody measurements | Immunogenicity primary outcome and measure | Immunogenicity result summary |
|---|---|---|---|---|---|---|---|---|---|---|
| Almeida, V.D.C. et al., 2009; Brazil | Two referral services hospitals; Prospective Cohort | All pregnant women with HIV infection | 46 (46 PWLWH) | 23-valent pneumococcal polysaccharide vaccine (Pneumo-23) 25 μg of each capsular polysaccharide (serotypes 1, 3, 5, 6 B, 9 V and 14) | GA 32–34 weeks, one dose | Mild local reactions in 6.8% (n = 44) of women (local reactions: pain, edema and erythema) | 4.4% (n = 46) of enrolled infants diagnosed with HIV within a month of birth | Mothers: pre-vaccination, at delivery. Infants: at birth, 1,2,3 and 6 months |
Quantification of IgG antibodies against serotypes (1,3,5,6 B, 9 V and 14) | 1.5–2.9-fold increase in antibody titers. Frequency of ≥2-fold response 22.7–63.6%. Except serotype 3 which did not generate a response. |
| Richardson, K. and Weinberg, A.,2011; USA | Single site; Prospective open-label cohort study | Pregnant women with and without HIV infection | 38 (20 PWLWH, 18 HIV-uninfected) | Trivalent inactivated influenza vaccine (IIV3) antigens varied according to year | GA not specified, one dose given | Not applicable | Not applicable | Baseline, 6 weeks post vaccination, 12 weeks post delivery | Comparison of: Hemagglutination inhibition titers (HAI), Cell-mediated immunity (CMI) assays, flow cytometry |
HAI: significantly lower response in PWLWH for influenza A; equally low for influenza B. CMI: no significant vaccine response in either group Cytometry: significantly greater increase in CD4 subtypes in infected women |
| Abzug, M. et al., 2013; USA | 31 US International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) group sites | Pregnant women with HIV infection | 127 (127 PWLWH) | 30 μg unadjuvanted, inactivated pH1N1 | GA 14–34 weeks, two doses 21 days apart | 3.1% (n = 127) reported local and systemic reactions (local reactions: pain, tenderness, erythema, pruritis; systemic: headache, rhinorrhea, chills) | One fetal death 26 weeks of undetermined etiology. | Mothers: at entry (pre-vaccination), 21 days after dose 1, 10 and 21 days after dose 2 Infants: at delivery, 3 and 6 months |
Complete response (both seroprotection (≥40HAI titer) and seroresponse (≥4-fold increase from baseline)) measured by HAI | Complete response rate was 61% after dose 1 and 65% post dose 2. Seroprotection attained by 66% and 75% post doses 1 and 2 respectively. 59% mothers and 12% infants had seroprotective titers 6 months post delivery |
| Madhi, S.A. et al., 2014; South Africa | Four Antenatal Clinics (ANCs); Double-blind placebo controlled randomized controlled trial (RCT) | Pregnant women with and without HIV infection | 2116 (194 PWLWH) | Trivalent Inactivated Influenza Vaccine (IIV3) VAXIGRIP 15 μg each of A (H1N1), A (H3N2), B(Victoria) or placebo | GA 20–36 weeks | PWLWH analyzable for reactogenicity (n = 97): severe local event in 4.1% and 18.6% severe systemic event. PWWH(n = 181) severe local events-5% and 15% severe systemic | Preterm births-204,8 fetal deaths, 24 stillbirths, 22 infant and 4 maternal deaths | Baseline (pre-vaccination) and 1-month post vaccination | Vaccine-specific seroconversion: Increase in HAI to ≥1:40 if initially ≤1:10, or ≥4-fold increase if initially ≥1:10 | Seroconversion in HIV-uninfected vs HIV-infected treatment arms: A (H1N1) 72.5% vs 42.9%; A (H3N2) 64.8% vs 35.7%; B(Victoria) 92.3% vs 40.0% |
| Nunes, M.C. et al., 2015; South Africa | One ANC; RCT (Sub-study of Madhi et al., 2014) | Pregnant women with HIV and without HIV infection | 198 (98 HIV uninfected and 100 PWLWH) | Trivalent inactivated influenza vaccine (IIV3) VAXIGRIP 15 μg each of A (H1N1), A (H3N2), B(Victoria) | GA not specified, one dose given | Not applicable | Not applicable | Mothers: pre-vaccination, 1-month postvaccination, at delivery, 24 weeks post delivery Infants: within 1 week of birth, 8,16 and 24 weeks of age |
Antibody quantification and persistence; seroconversion (≥1:40HAI and ≥4-fold increase) HAI | Seroconversion at 1 month in HIV-uninfected vs HIV-infected: A (H1N1) 70.0% vs 40.3%; A (H3N2) 63.3% vs 35.7%; B(Victoria) 91.7% vs 40.0%. At 24 weeks, the HIV-uninfected group maintained increased titers to A (H3N2) and B. HIV-infected titers returned to pre-vaccine levels. |
| Weinberg, A. et al., 2015; USA | 31 US IMPAACT sites; Nested-exploratory descriptive study (Sub-study of Abzug et al., 2013) | All pregnant women with HIV infection | 119 PWLWH (57 in sub-study) | Double strength (30 μg) unadjuvanted inactivated pH1N1 vaccine | GA 14–34 weeks, two doses given 21–28 days apart | Not applicable | Not applicable | At entry, pre-vaccination, before dose 2 (anticipated peak primary antibody response); 10–14 days post dose 2: peak anamnestic response | Quantification of HAI, CMI assays, IgG/IgA FluoroSpot, B/T-cell phenotyping | pH1N1 titers significantly increased post dose 1. IgG secreting cells significantly increased post dose 1. IFNy effector T-cells tended to decrease post vaccine. Granzyme B cells had marginal significant increase post dose 1. |
| Heyderman, R. et al., 2016; Malawi/South Africa | Two ANCs; Prospective open-label cohort study | Pregnant women with and without HIV infection, and their infants | 270 women (90 HIV uninfected, 89 PWLWH and CD4 count >350, 91 PWLWH and CD4 count >50 and ≤ 350); 266 infants (87; 88; 91 in respective groups) |
Glycoconjugate GBS vaccine (5 μg each of serotypes Ia, Ib, III) | GA 24–35, one dose given | 1.14% and 4% (n = 175) of PWLWH reported a severe local and systemic reaction respectively compared to 4.4% and 10% (n = 90) of PWWH | 1 maternal death, 1 stillbirth, 7 infant deaths. | Mothers: 15- and 31-days post vaccination and at delivery. Infants: at delivery, day 42. |
Placental transfer of GBS serotype-specific antibodies, and maternal antibodies. IgG concentration measured by specific ELISA protocol. | Antibody concentration higher post-vaccine in all groups. Higher response in HIV-uninfected than HIV-infected women. No difference between high/low CD4 count groups. |
| Nunes, M. C. et al., 2018; South Africa | Four ANCs; Double-blind placebo-controlled RCT. (Sub-study of Madhi et al., 2014) | Pregnant women with and without HIV infection | 155 (75 HIV uninfected and 80 PWLWH) | IIV3 VAXIGRIP 15 μg each of A (H1N1), A (H3N2), B(Victoria) | GA 20–36 weeks | Not applicable | Not applicable | Pre-vaccination, 1-month post vaccination | Comparison of Microneutralization and HAI assays | Microneutralization assay more sensitive, giving fold increases of 2–3 times higher than HAI |
| Dhar N. et al., 2020; South Africa | Placebo controlled RCT. (Stored Samples from Madhi et al., 2014) | Pregnant women with and without HIV infection | 140 PWLWH (77 IIV3 and 68 Placebo), 145 HIV uninfected (68 IIV3 and 77 placebo) | IIV3 VAXIGRIP 15 μg each of A (H1N1), A (H3N2), B(Victoria) or placebo | GA 20–36 weeks | Not applicable | Not applicable | Pre-vaccination, 1-month post vaccination | Comparison of H1/stalk IgG and HAI assays, analysis with regard to infection rate | H1/stalk IgG and HAI higher in PWWH vs PWLWH at all points. H1/stalk increased 2.24-fold vs 1.79-fold after vaccine; HAI increased 5.1–11.3-fold vs 2.3–3.4-fold. |
| Nunes, M.C. et al., 2020; South Africa | Seven ANCs; Double-blind RCT | All Pregnant women with HIV infection | 800 PWLWH | IIV3 containing 15 μg of A/H1N1, A/H3N2, B/Yamagata. Randomized to receive single dose, double dose, or two single doses one month apart (266:265:269 women respectively) | GA 12–36 weeks | PWLWH(n = 772); local reactions greater in double than single dose (47.8% vs 38.1%). Severe systemic reaction greater in single than double dose (10.4% vs 5.5%) after dose 1. Severe local reaction-6% and severe systemic-8.8% | 136 preterm births, 21 fetal deaths, 22 infant and 4 maternal deaths | Pre-vaccination, 1-month post vaccination | Seroconversion rate (≥1/40HAI titers after vaccination with ≥4-fold increase) | Double dose more immunogenic vs single or two single doses. Seroconversion for A/H1N1, A/H3N2, B/Yamagata in double dose group: 65%, 52%, 29%; single dose: 49%, 41%, 18%; two single doses: 52%, 47%, 23%. |
| Weinberg, A. et al., 2021; Brazil | Eight out-patient clinics; Double-blind placebo controlled RCT | All pregnant women with HIV infection | 347 PWLWH | Conjugated and Polysaccharide Pneumococcal vaccines (PCV-10 and PPV-23) or placebo | GA ≥14 and < 34 weeks | Adverse events similar across treatment groups, except injection site and grade 2 systemic reactions more frequent in treatment arms. | Low rate of preterm birth in PCV-10 group (2%, vs 13% and 12% in PPV-23 and placebo). | Mothers: Baseline, 4 weeks post vaccination, at delivery, 24 weeks postpartum. Infants: at birth, 8, 16, 24 weeks |
Mothers: seroresponse, defined as ≥2-fold increase in anti-PNC antibody concentration measured by ELISA against ≥5 of 7 measured serotypes | Seroresponse rates PCV-10 65%; PPV-23 65%; placebo 0%. Seroresponse and differences persisted at delivery and 24 weeks postpartum. |
| Duarte, G. et al., 2022; Brazil | 8 sites; Double-blind placebo-controlled RCT. (Stored samples from Weinberg et al., 2021) | All pregnant women with HIV infection | 346 PWLWH | PCV-10, PPV-23, and placebo; one dose given in pregnancy. Postpartum, placebo receipts received a single dose of either PCV-10 or PPV-23. | GA ≥14 and < 34 weeks | Not applicable | Not applicable | Pre-vaccination, 1 month post vaccination | Anti-pneumococcal antibody concentrations for 8 serotypes; memory B- and T- cell responses against 1 serotype for subset | Antibody concentrations robustly increased across groups, but generally lower antepartum than postpartum, and lower in PCV-10 than PPV-23. No appreciable increase in memory response in any group. |
A total number of 3744 pregnant women were involved in the 12 studies, 1714 of these were PWLWH, as indicated above (Table 1) some of the immunogenicity studies were nested within bigger studies, samples analyzed were from the main studies hence these women are counted once.
CMI = Cell mediated immunity. GA = Gestational age. HAI = Hemagglutination inhibition titers. PWLWH = Pregnant women living with HIV. PCV = Conjugated Pneumococcal Vaccine. PPV = Polysaccharide Pneumococcal Vaccine. IIV3 = Trivalent Inactivated Influenza Vaccine.
Fig. 1.
PRISMA flow diagram-studies on the safety and immunogenicity of vaccines given to pregnant women living with HIV.
We included all 12 studies in our systematic review, of which five reported safety outcomes and all the 12 reported immunogenicity outcomes.
Five studies comprising 3456 pregnant women (1250 PWLWH and 2206 PWWH) reported on vaccine safety.17, 18, 19, 20, 21 For PWLWH with analyzable reactogenicity data the study by Madhi et al.17 reported at least one severe local reaction (redness, swelling, hardness and/or bruising) was reported in 4.1% (4.1; 1.1–10.2) and 18.6% (18.6; 12.0–27.5) had at least one severe systemic reaction (headache, weakness, fever, rigors, joint pains, muscle pain and/or increased sweating) these were reported within seven days post-vaccination. In comparison to PWWH, 5% (5.0; 2.5–9.3) reported at least one severe local reaction and 15% (15; 10.4–20.9) reported at least one severe systemic reaction (Supplementary Table S4). One study,21 reported an increased rate of injection site reactions with administration of double dose influenza vaccine in PWLWH.
Preterm births were the most common SAE reported,17,21 these occurred more commonly in PWLWH compared to PWWH, at 17% (n = 800) and 10% (n = 1062) respectively.
There were five studies that assessed immunogenicity for influenza, pneumococcal and Group B Streptococcal (GBS) vaccines.9,10,17,18,21 These all reported higher antibody titers or concentrations, 4 weeks post vaccination in PWLWH compared with baseline. However, the antibody responses were lower when compared with PWWH.
Meta-analyses results
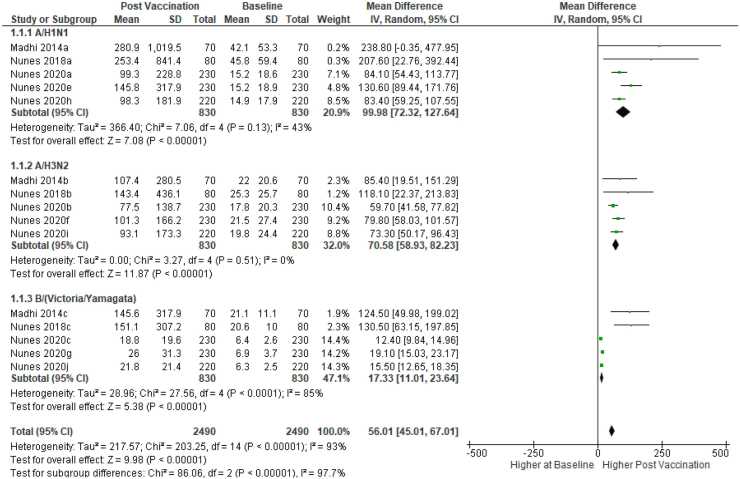
Three studies,11,17,21 on Influenza vaccine had sufficient comparable data for meta-analyses stratified by the vaccine subtype (A/H1N1, A/H3N2 and B/Victoria/Yamagata). The pooled mean difference in antibody concentration pre-vaccination compared to 28–35 days post vaccination in PWLWH demonstrated a significant increase in antibody concentration post vaccination in all the three studies (Fig. 2).
Fig. 2.
Forest plot of pooled mean difference in antibody geometric mean titers between vaccine sub groups at days 28–35 post vaccination and baseline. GMT = Geometric mean titers. IV = Inverse variance.95% CI = Ninety five percent Confidence Interval. SD = Standard deviation. HIV = Human Immunodeficiency Virus. Three influenza vaccine subgroups include; A/H1NI, A/H3N2 and B (Victoria/Yamagata). Using a random effect model (Random): the inverse variance (IV) was used to estimate the pooled mean difference of GMT pre-vaccination (baseline) and 28–35 days post-vaccination.
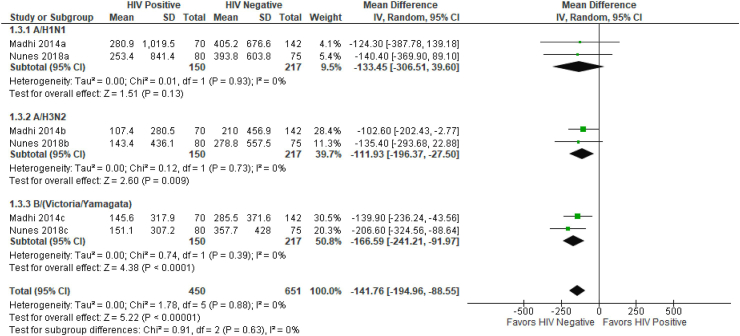
However comparing PWLWH to PWWH, two studies11,17 had comparable immunogenicity data for meta-analyses. The pooled mean difference in antibody concentration following influenza vaccination at 28–35 days post vaccination was significantly higher among PWWH compared to PWLWH (mean difference −141.8 (95% CI: −194.9, −88.6) (I2 = 0%, τ2 = 217.6, p < 0.0001). The largest difference was observed among women who received B strains (−166 [95% CI: −241.2, −91.9]) while the smallest difference was among those who received A/H3N1 (−111.9 [95% CI: −196.4, −27.5]) (Fig. 3).
Fig. 3.
Forest plot of pooled mean difference of geometric mean titers post vaccination comparing PWLWH to PWWH. GMT = Geometric Mean Titers. IV = Inverse variance. Random = Random effects model. 95% CI = Ninety five percent Confidence Interval. SD = Standard deviation. HIV = Human Immunodeficiency Virus. PWLWH = Pregnant women living with HIV. PWWH = Pregnant women without HIV. Three influenza vaccine subgroups were included in the analysis; A/H1NI, A/H3N2 and B(Victoria/Yamagata).
Risk of bias
The overall risk of bias using RevMan for RCTs assessing selection, performance, detection, attrition and reporting bias was low however the blinding of outcome assessment was rated unclear risk (Supplementary Table S2).
The score for risk of bias in observational studies using the NOS varied across studies; one study20 scored 9/9 and others scored between 7 and 8, the overall total score was 7.8/9 (Supplementary Table S5).
The level of statistical heterogenicity assessed using I2 statistic, Chi2 = 0.74, df = 5(p = 0.88); I2 = 0.
The reporting bias was assessed using a funnel plot (Supplementary Fig. S1), the data points were within the line of symmetry showing no reporting bias for the studies included in the analysis.
Discussion
This systematic review and meta-analysis is the first to describe the safety and immunogenicity of vaccines given to PWLWH. Overall published data are lacking; however, by searching national research databases, secondary analyses, and data from clinical trial databases we have been able to include comparable data from 12 studies, across four countries including three LMICs. Our findings show that vaccines given to PWLWH were generally safe with no major difference in the occurrence of adverse events reported between HIV positive and the HIV negative pregnant women. This supports the WHO and other recommendations regarding the safety of vaccines licensed for use during pregnancy.22 Events like preterm births are generally reported to be higher in PWLWH compared to PWWH, with some studies reporting the prevalence of spontaneous preterm births to be three times higher in PWLWH than the general population.23,24 The findings from our review are not different from these previous studies with preterm births reported more in PWLWH compared to PWWH.
Immunogenicity findings showed an increase in antibody titers and/or concentrations for PWLWH irrespective of the vaccine administered, dose and schedule followed, indicating that immunization boosts immunity in this group although both the overall response and placental transfer are lower in PWLWH than PWWH. The time intervals at which this immunogenic response was measured varied across studies so comparisons for meta-analyzes could only be done for influenza vaccine.
PWLWH are often excluded from vaccine clinical trials, leading to a vicious cycle of limited data and thus confidence in vaccines in this special group, as was evidenced in the initial stages of the COVID-19 pandemic and other infectious disease outbreaks.25, 26, 27 There is still a paucity of data comparing maternal vaccine outcomes in PWLWH following vaccination against highly infectious diseases, despite evidence that they can be safely included in clinical trials.28,29
The lack of randomized clinical trial data on influenza vaccine for meta-analyses in special groups has been previously documented by Alexander Domnich et al. whose comprehensive review majorly focused on observational studies, noting that RCTs were very uncommon.30 Although his review focused on the elderly, poor to modest immunogenic response was confirmed in the meta-analyses when traditional inactivated influenza vaccine was compared to adjuvanted one. The need to generate more evidence on the efficacy and effectiveness of influenza vaccine in special populations still remains.31 Previous systematic reviews and meta-analyses on influenza vaccine given during pregnancy reported on only three RCTs and the efficacy and effectiveness majorly assessed the prevention of influenza confirmed illness in children. No data was reported specific to PWLWH. The benefit of reducing severe influenza disease and hospitalization following vaccination was highlighted irrespective of the subtype or formulation of vaccine given and geographical location.30,32,33
Our meta-analyses of influenza vaccine show that although there is an increase in antibody concentration four weeks post vaccination in both PWLWH and PWWH groups, the increase is smaller in the PWLWH. In order to protect infants who are HEU from vaccine preventable diseases through maternal immunization, administration of vaccines which elicit a good immunogenic response in PWLWH is important.34
The use of antiretroviral therapy (ART) in the treatment of HIV has been reported to contribute to improved immunologic responses post vaccination in HEU infants.35, 36, 37 In a study evaluating both qualitative and quantitative antibody responses to pneumococcal (PCV)and Haemophilus influenza type b (HibCV) conjugate vaccines, children living with HIV(CLWH) were reported to have lower functional antibody titers however vaccines were equally effective in preventing invasive bacterial disease in both the CLWH and HUU.35 Despite the need to explore different formulations and timing schedules for maternal vaccination in PWLWH, the timing of these studies is of essence. There is paucity of data on immunogenic responses post vaccination for PWLWH in the era of cART use, with no justifiable reasons for further delays before studies are done. New maternal vaccines like the bivalent RSV prefusion F protein-based vaccine (RSVpreF) have successfully undergone phase 3 clinical trials and are shown to be safe and effective in preventing severe RSV-associated lower respiratory tract infections and hospitalization, PWLWH were excluded and representation from LMICs was limited.38,39 The exclusion of PWLWH from such studies results in exclusion from licensure for such special groups.
Our systematic review identified studies of safety and immunogenicity in only 1714 PWLWH which is <1% of all PWLWH and we found data for only a limited number of vaccines. We have previously highlighted the equity issue of excluding pregnant women from vaccine clinical trials, and our current review highlights a pressing need to include PWLWH in trials of future investigational vaccines to provide better data and licensure for use in this group.
The limitations of our study include the few studies that we found that include PWLWH, thus our data is from a low percentage of the global population of PWLWH. The findings from the systematic review relied heavily on the methodological quality of included studies. However, the majority were rated as having a low level of potential bias, which mitigated this somewhat. Due to the diversity of immunological tests, vaccines studied and variability in measurements for immunogenicity across studies, we were only able to pool the results from three studies in the meta-analysis. We found that the grading of reactogenicity and reporting of adverse events differed across studies and was not uniform, making meta-analyses impossible. These complexities and difficulties around maternal vaccine safety monitoring have been reported in a previous study conducted in a hospital setting,31 and the WHO has made concerted effort to advocate for the utilization of the Global Alignment of immunization safety assessment in pregnancy (GAIA) case definitions.32,33 This is critical especially since none of the studies in our review used the GAIA criteria to assess safety and no study has used them to assess safety in PWLWH. None of the vaccine related reporting of AEs or lower immunogenicity response reporting for PWLWH has resulted in this population being excluded from the use of maternal vaccines.
As new vaccines are introduced into the antenatal immunization schedule, including Respiratory Syncytial Virus (RSV) and combined acellular pertussis (DTaP), there is a critical need to plan ahead for more standardized data on maternal vaccine safety and immunogenicity in special sub-populations such as PWLWH. This is vital for populations in geographical locations where the burden of HIV disease is high and vaccine need is greatest. The availability of uniform accurate data and standardized definitions will improve maternal vaccine confidence especially in special sub-populations such as PWLWH who may require different vaccine formulations or schedules to keep themselves and their infants protected.
Contributors
Eve Nakabembe (EN), Jo Cooper (JC), Kyle Amaral (KA), Valerie Tusubira (VT), Yingfen Hsia (YH), Bahaa Abu-Raya (BA), Musa Sekikubo (MSe), Annettee Nakimuli (AN), Prof Manish Sadarangani (MSa), Prof Kirsty Le Doare (KLD).
EN, JC, MSa and KLD participated in conceptualization, study design, literature search and data curation.
EN, VT, YH and KLD participated in the data curation and formal analysis.
All authors participated in the visualization, data interpretation, writing, reviewing and editing of the manuscript, they have read and agreed to the submission of this version of the manuscript. All authors had full access to the data in the study and made the decision to submit for publication. EN, JC, KA, VT and KLD accessed and verified the data underlying the study.
Data sharing statement
The data collected for the study is available on application to the corresponding author.
Declaration of interests
EN received clinical research stipend from MRC Joint Clinical Trials Round 9 [MR/T004983/1].
KLD is funded by a United Kingdom Research Institute Future Leaders Fellowship.
KLD has been an investigator for projects funded by Pfizer and Minervax. All funds have been paid to her institution and she has not received personal payments of any kind.
MSa is supported via salary awards from the BC Children’s Hospital Foundation and Michael Smith Health Research BC.
MSa has been an investigator on projects funded by GlaxoSmithKline, Merck, Moderna, Pfizer,Sanofi-Pasteur,Seqirus, Symvivo and VBI vaccines. All funds have been paid to his institute, and he has not received any personal payments.
BA has received honoraria for participation in meetings organized by Sanofi, relating to pertussis and RSV.
All other authors report no conflict of interest.
Acknowledgements
United Kingdom Research Institute Future Leaders Fellowship.
Medical Research Council Joint Clinical Trials Round 9 [MR/T004983/1].
BC Children’s Hospital Foundation and Michael Smith Health Research BC.
We would like to acknowledge Dr. Joseph Ouma (RIP) who worked as the study statistician on this project and who passed away suddenly before we were able to publish this work.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2024.102448.
Appendix A. Supplementary data
References
- 1.Evans C., Chasekwa B., Ntozini R., et al. Mortality, human immunodeficiency virus (HIV) transmission, and growth in children exposed to HIV in rural Zimbabwe. Clin Infect Dis. 2021;72(4):586–594. doi: 10.1093/cid/ciaa076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.UNAIDS . UNAIDS Fact sheet. 2022. Global HIV statistics. [Google Scholar]
- 3.WHO . WHO; 2022. HIV,Prevention of mother to child transmission. [Google Scholar]
- 4.Slogrove A.L., Powis K.M., Cotton M.F. Human immunodeficiency virus-exposed uninfected infants: surviving and thriving or overlooked by success? Clin Infect Dis. 2019;68(12):2156–2158. doi: 10.1093/cid/ciy1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nyemba D.C., Kalk E., Vinikoor M.J., et al. Growth patterns of infants with in-utero HIV and ARV exposure in cape town, South Africa and lusaka, Zambia. BMC Publ Health. 2022;22:1–14. doi: 10.1186/s12889-021-12476-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taron-Brocard C., Le Chenadec J., Faye A., et al. Increased risk of serious bacterial infections due to maternal immunosuppression in HIV-exposed uninfected infants in a European country. Clin Infect Dis. 2014;59(9):1332–1345. doi: 10.1093/cid/ciu586. [DOI] [PubMed] [Google Scholar]
- 7.Wedderburn C.J., Weldon E., Bertran-Cobo C., et al. Early neurodevelopment of HIV-exposed uninfected children in the era of antiretroviral therapy: a systematic review and meta-analysis. Lancet Child Adolesc Health. 2022;6(6):393–408. doi: 10.1016/S2352-4642(22)00071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Powis K.M., Smeaton L., Hughes M.D., et al. In-utero triple antiretroviral exposure associated with decreased growth among HIV-exposed uninfected infants in Botswana. AIDS. 2016;30(2):211. doi: 10.1097/QAD.0000000000000895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jallow S., Agosti Y., Kgagudi P., et al. Impaired transplacental transfer of respiratory syncytial virus-neutralizing antibodies in human immunodeficiency virus-infected versus -uninfected pregnant women. Clin Infect Dis. 2019;69(1):151–154. doi: 10.1093/cid/ciy1071. [DOI] [PubMed] [Google Scholar]
- 10.Richardson K., Weinberg A. Reduced immunogenicity of influenza vaccines in HIV-infected compared with uninfected pregnant women is associated with regulatory T cells. Aids. 2011;25(5):595–602. doi: 10.1097/QAD.0b013e32834411a8. [DOI] [PubMed] [Google Scholar]
- 11.Nunes M.C., Weinberg A., Cutland C.L., et al. Neutralization and hemagglutination-inhibition antibodies following influenza vaccination of HIV-infected and HIV-uninfected pregnant women. PLoS One. 2018;13(12) doi: 10.1371/journal.pone.0210124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Locks L.M., Manji K.P., Kupka R., et al. High burden of morbidity and mortality but not growth failure in infants exposed to but uninfected with human immunodeficiency virus in Tanzania. J Pediatr. 2017;180:191–199.e2. doi: 10.1016/j.jpeds.2016.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Organization WH . 2014. Safety of immunization during pregnancy: a review of the evidence: global advisory committee on vaccine safety. [Google Scholar]
- 14.Maertens K., Orije M.R.P., Van Damme P., Leuridan E. Vaccination during pregnancy: current and possible future recommendations. Eur J Pediatr. 2020;179:235–242. doi: 10.1007/s00431-019-03563-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ouzzani M., Hammady H., Fedorowicz Z., Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5:1–10. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wells G.A., Shea B., O’Connell D., et al. 2000. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. [Google Scholar]
- 17.Madhi S.A., Cutland C.L., Kuwanda L., et al. Influenza vaccination of pregnant women and protection of their infants. N Engl J Med. 2014;371(10):918–931. doi: 10.1056/NEJMoa1401480. [DOI] [PubMed] [Google Scholar]
- 18.Almeida V.D.C., Mussi-Pinhata M.M., De Souza C.B.S., et al. Immunogenicity of 23-valent pneumococcal polysaccharide vaccine in HIV-infected pregnant women and kinetics of passively acquired antibodies in young infants. Vaccine. 2009;27(29):3856–3861. doi: 10.1016/j.vaccine.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 19.Abzug M.J., Nachman S.A., Muresan P., et al. Safety and immunogenicity of 2009 pH1N1 vaccination in HIV-infected pregnant women. Clin Infect Dis. 2013;56(10):1488–1497. doi: 10.1093/cid/cit057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heyderman R.S., Madhi S.A., French N., et al. Group B streptococcus vaccination in pregnant women with or without HIV in Africa: a non-randomised phase 2, open-label, multicentre trial. Lancet Infect Dis. 2016;16(5):546–555. doi: 10.1016/S1473-3099(15)00484-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nunes M.C., Cutland C.L., Moultrie A., et al. Immunogenicity and safety of different dosing schedules of trivalent inactivated influenza vaccine in pregnant women with HIV: a randomised controlled trial. Lancet HIV. 2020;7(2):e91–e103. doi: 10.1016/S2352-3018(19)30322-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arora M., Lakshmi R. Vaccines - safety in pregnancy. Best Pract Res Clin Obstet Gynaecol. 2021;76:23–40. doi: 10.1016/j.bpobgyn.2021.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Albert A.Y.K., Elwood C., Wagner E.C., et al. Investigation of factors associated with spontaneous preterm birth in pregnant women living with HIV. AIDS. 2020;34(5):719–727. doi: 10.1097/QAD.0000000000002464. [DOI] [PubMed] [Google Scholar]
- 24.Zack R.M., Golan J., Aboud S., Msamanga G., Spiegelman D., Fawzi W. Risk factors for preterm birth among HIV-infected Tanzanian women: a prospective study. Obstet Gynecol Int. 2014;2014 doi: 10.1155/2014/261689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yan Z., Yang M., Lai C.-L. COVID-19 vaccinations: a comprehensive review of their safety and efficacy in special populations. Vaccines. 2021;9(10):1097. doi: 10.3390/vaccines9101097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lai C.-C., Chen I.-T., Chao C.-M., Lee P.-I., Ko W.-C., Hsueh P.-R. COVID-19 vaccines: concerns beyond protective efficacy and safety. Expet Rev Vaccine. 2021;20(8):1013–1025. doi: 10.1080/14760584.2021.1949293. [DOI] [PubMed] [Google Scholar]
- 27.Schwartz D.A. Clinical trials and administration of Zika virus vaccine in pregnant women: lessons (that should have been) learned from excluding immunization with the Ebola vaccine during pregnancy and lactation. Vaccines. 2018;6(4):81. doi: 10.3390/vaccines6040081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haddad L.B., Jamieson D.J., Rasmussen S.A. Pregnant women and the Ebola crisis. N Engl J Med. 2018;379(26):2492–2493. doi: 10.1056/NEJMp1814020. [DOI] [PubMed] [Google Scholar]
- 29.Nunes M.C., Madhi S.A. COVID-19 vaccines in pregnancy. Trends Mol Med. 2022;28(8):662–680. doi: 10.1016/j.molmed.2022.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Domnich A., Arata L., Amicizia D., Puig-Barberà J., Gasparini R., Panatto D. Effectiveness of MF59-adjuvanted seasonal influenza vaccine in the elderly: a systematic review and meta-analysis. Vaccine. 2017;35(4):513–520. doi: 10.1016/j.vaccine.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 31.Carmona A., Latorre Tejerina M., Martínez Sebastián A., et al. Risk measurement of perinatal and neonatal morbidity characteristics and applicability of GAIA case definitions: results and lessons learnt of a hospital-based prospective cohort study in the valencia region (2019–2020) Int J Environ Res Publ Health. 2022;19(12):7132. doi: 10.3390/ijerph19127132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bonhoeffer J., Kochhar S., Hirschfeld S., et al. Global alignment of immunization safety assessment in pregnancy–the GAIA project. Vaccine. 2016;34(49):5993–5997. doi: 10.1016/j.vaccine.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 33.Stuurman A.L., Riera M., Lamprianou S., et al. Vaccine safety surveillance in pregnancy in low-and middle-income countries using GAIA case definitions: a feasibility assessment. Vaccine. 2018;36(45):6736–6743. doi: 10.1016/j.vaccine.2018.09.033. [DOI] [PubMed] [Google Scholar]
- 34.Dangor Z., Nunes M.C., Kwatra G., Lala S.G., Madhi S.A. Vaccination of HIV-infected pregnant women: implications for protection of their young infants. Trop Dis Travel Med Vaccines. 2017;3(1):1–8. doi: 10.1186/s40794-016-0044-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Madhi S.A., Izu A., Violari A., et al. Effect of HIV-exposure and timing of antiretroviral treatment initiation in children living with HIV on antibody persistence and memory responses to Haemophilus influenzae type b and pneumococcal polysaccharide-protein conjugate vaccines. Vaccine. 2020;38(12):2651–2659. doi: 10.1016/j.vaccine.2020.02.026. [DOI] [PubMed] [Google Scholar]
- 36.Simani O.E., Izu A., Violari A., et al. Effect of HIV-1 exposure and antiretroviral treatment strategies in HIV-infected children on immunogenicity of vaccines during infancy. AIDS. 2014;28(4):531–541. doi: 10.1097/QAD.0000000000000127. [DOI] [PubMed] [Google Scholar]
- 37.Jones C.E., Naidoo S., De Beer C., Esser M., Kampmann B., Hesseling A.C. Maternal HIV infection and antibody responses against vaccine-preventable diseases in uninfected infants. JAMA. 2011;305(6):576–584. doi: 10.1001/jama.2011.100. [DOI] [PubMed] [Google Scholar]
- 38.Kampmann B., Madhi S.A., Munjal I., et al. Bivalent prefusion F vaccine in pregnancy to prevent RSV illness in infants. N Engl J Med. 2023;388(16):1451–1464. doi: 10.1056/NEJMoa2216480. [DOI] [PubMed] [Google Scholar]
- 39.Madhi S.A., Polack F.P., Piedra P.A., et al. Respiratory syncytial virus vaccination during pregnancy and effects in infants. N Engl J Med. 2020;383(5):426–439. doi: 10.1056/NEJMoa1908380. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.