Abstract
Individual trials of abemaciclib, palbociclib, and ribociclib show a similar impact on progression-free survival yet differing statistical significance for overall survival (OS). A robust comparative evaluation of OS, safety, and tolerability of the three drugs is warranted. A systematic literature search identified phase 3 randomized clinical trials reporting OS of CDK4/6 inhibitors (CDK4/6i) in combination with endocrine therapy in ER-positive/HER2-negative advanced breast cancer. Trial-level data on OS and common and serious adverse events (AE) were extracted for each drug. In the absence of direct comparisons, a network meta-analysis was performed to evaluate pairwise comparative efficacy, safety, and tolerability of each of the CDK4/6i. Seven studies comprising of 4415 patients met the inclusion criteria. Median follow-up was 73.3 months (range: 48.7–97.2 months). There were no statistically significant differences in OS between any of the CDK4/6i. Compared to palbociclib, ribociclib and abemaciclib both showed significantly higher GI toxicity (grade 1–2 vomiting OR 1.87 [95% CI 1.37–2.56] and OR 2.27 [95% CI 1.59–3.23] respectively). Compared to palbociclib, abemaciclib was associated with more grade 3–4 diarrhea OR 118.06 [95% CI 7.28–1915.32]. In contrast, palbociclib was associated with significantly more neutropenia than ribociclib and abemaciclib but significantly lower risk of grade 3–4 infections. Abemaciclib had significantly less grade 3–4 transaminitis and grade 3–4 neutropenia than ribociclib. Treatment discontinuation and death due to AE were significantly higher with abemaciclib than palbociclib and ribociclib. There is no statistically significant difference in OS between CDK4/6i despite differing statistical significance levels of individual trials. Real-world data analyses may help to identify if there is a meaningful inter-drug difference in efficacy. Significant differences between CDK4/6i are observed for safety and tolerability outcomes.
Subject terms: Breast cancer, Targeted therapies
Introduction
Inhibition of cyclin dependent kinase 4 (CDK4) and cyclin dependant kinase 6 (CDK6) in combination with endocrine therapy is the first-line standard of care for hormone receptor positive and erb-B2-negative (HR+/HER2−) locally advanced or metastatic breast cancer (MBC)1–3. In phase III trials, CDK4/6 inhibitors trials, palbociclib, ribociclib, and abemaciclib have shown a consistent improvement in progression-free survival (PFS) when combined with an aromatase inhibitor (AI), fulvestrant, or tamoxifen4,5 with the hazard ratios for PFS ranging between 0.50 and 0.59. In contrast, while individual trials for ribociclib with endocrine therapy have reported statistically significant improvement in overall survival (OS), such improvements have not been reported for palbociclib or abemaciclib6–11. This is reflected in the National Comprehensive Cancer Network guidelines where ribociclib is the only category 1 preferred first-line treatment option for HR+/HER2− MBC in combination with an AI; whereas both abemaciclib and ribociclib are category 1 preferred first-line in combination with fulvestrant3.
CDK4/6 inhibitors can be associated with significant symptom burden that may limit tolerability and impact patients’ health-related quality of life12. In a pooled analysis of clinical trials, more than 70% of older patients had their treatment dose reduced and more than 15% discontinued treatment13. Tolerability is a key metric for CDK4/6 inhibitors given the duration of treatment can extend over 2 years, especially when used in the first-line setting.
A robust analysis of both relative efficacy and relative tolerability is therefore of interest to help clinicians and patients make informed decisions about the optimal agent to be used.
Methods
Search strategy and study selection
A network meta-analysis, registered in PROSPERO (registration number CRD42023392416) was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines (PRISMA)14. Inclusion criteria comprised phase 3 randomized controlled trials (RCTs) in which patients with HR+/HER2− metastatic breast cancer were treated with a CDK4/6 inhibitor in combination with endocrine therapy (AI, fulvestrant or tamoxifen) compared to endocrine therapy alone in the first or second-line setting. There was no limitation on year or language of publication. Meta-analyses, single-arm trials, and observational studies were excluded. Only studies of human subjects were included. When more than one publication was identified for the same clinical trial, data from the most recent or complete report were included.
A search strategy was constructed using ClinicalTrials.gov. Titles and abstracts identified by these strategies were screened independently by two reviewers (C.K. and E.A.) for inclusion; disagreements were resolved by consensus. The following variables from all eligible manuscripts were extracted: year of publication, median duration of follow-up, study sample size and the treatment in the experimental and control groups. For each approved CDK4/6 inhibitor, data was extracted on efficacy and on pre-specified common and serious treatment related adverse events. For efficacy outcomes, the study-reported hazard ratios (HR) and respective 95% confidence intervals (CI) for overall survival (OS) were extracted. For safety and tolerability, the data extracted included treatment-related death, treatment discontinuation due to adverse event and selected adverse events (AEs). For hematological toxicities, data were extracted on grade 3–4 neutropenia, anemia, and thrombocytopenia. For GI toxicities, data were extracted on both grade 1–2 and grade 3–4 diarrhea, nausea, and vomiting. Additional data was extracted on grade 1–2 stomatitis, grade 1–2 fatigue and/or asthenia, grade 3–4 venous thromboembolism (VTE), grade 3–4 transaminitis, grade 3–4 dyspnea and/or cough, grade 3–4 infection, grade 3–4 prolonged QT and grade 1–2 alopecia. The number of events and the number of patients at risk were extracted individually for both the CDK4/6 inhibitor and control groups in each trial. Outcome measures were obtained from the most recently published manuscripts and cross-referenced with data in the clinicaltrials.gov registry to ensure consistency.
Data synthesis and statistical analysis
When more than one study reported data for either efficacy or safety and tolerability outcomes, these were pooled in a meta-analysis using RevMan 5.4 (Cochrane Collaboration, Copenhagen, Denmark). For efficacy, HR for OS and associated 95% CI were pooled using generic inverse variance. For toxicity profile, the odds ratio (OR) and associated standard error (SE) for each adverse event were calculated relative to endocrine therapy alone using the Mantel–Haenszel method. Pooling was performed using fixed effects modeling irrespective of statistical heterogeneity. Due to the expected differences in endocrine therapy and patient characteristics between studies, analyses were performed separately for each endocrine therapy backbone (AI/tamoxifen or fulvestrant) to compare ribociclib and abemaciclib to palbociclib. Then a network meta-analysis was performed using WINBUGS within Microsoft Excel (Microsoft Corp, Redmond WA). A post-hoc sensitivity meta-analysis was also performed repeating the analysis utilizing post-hoc data from one trial in which there were substantial missing data. Statistical tests were two-sided, and statistical significance was defined as p < 0.05. No correction was made for multiple statistical testing.
Ethics approval
This study was exempt from ethics board approval since it used publicly available data exclusively.
Results
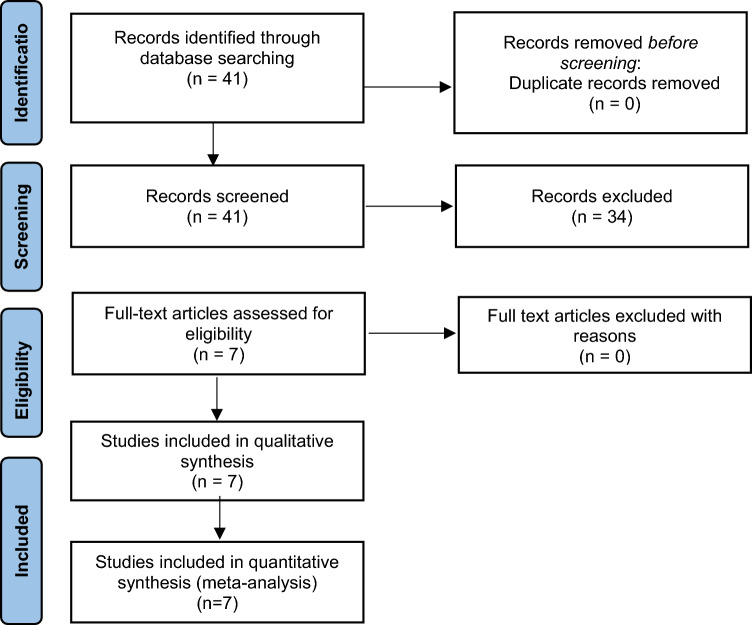
The study selection schema is shown in Fig. 1. Seven phase III RCTs were included in the analysis including PALOMA-2, PALOMA-3, MONALEESA-2, MONALEESA-3, MONALEESA-7, MONARCH-2, and MONARCH-36–11,15,16. In total, the analysis comprised of 4415 patients, of which 2718 patients received a CDK4/6 inhibitor (1153 ribociclib, 791 palbociclib,774 abemaciclib). In 4 RCTs (1441 patients), the endocrine therapy backbone was an AI or tamoxifen and in 3 RCTs (1277 patients) it was fulvestrant. The median follow-up was 70.2 months (range: 48.7–97.2 months). Characteristics of the studies are outlined in Table 1.
Figure 1.
PRISMA flow diagram.
Table 1.
Characteristics of included studies.
| Study characteristics | CDK4/6i with AI or tamoxifen | CDK4/6i with fulvestrant | |||||
|---|---|---|---|---|---|---|---|
| PAL-2 | MONALEESA-2 | MONALEESA-7 | MONARCH-3 | PALOMA-3 | MOLANEESA-3 | MONARCH 2 | |
| Year of initial publication | 2015 | 2016 | 2018 | 2017 | 2016 | 2018 | 2017 |
| Year of updated data | 2022 | 2022 | 2022 | 2023 | 2021 | 2021 | 2020 |
| Total number of patients | 666 | 668 | 672 | 493 | 521 | 726 | 669 |
| Line | 1st | 1st | 1st and 2nd line (after chemotherapy) | 1st | Progression after ET (adjuvant or 1st line) | 1st and 2nd line | Progression after ET (neo/adjuvant or 1st line) |
| Menopausal status | Post | Post | Pre | Post | Pre/post | Post | Pre/post |
| Median follow-up (months) | 90 | 80 | 53.5 | 97.2 | 73.3 | 56.3 | 48.7 |
| CDK4/6i | Palbociclib | Ribociclib | Ribociclib | Abemaciclib | Palbociclib | Ribociclib | Abemaciclib |
| Median OS in placebo + endocrine arm (months | 51.2 | 51.4 | 48.0 | 54.5 | 28 | 41.5 | 37.3 |
| Median OS in CDK4/6I + endocrine arm (months | 53.9 | 63.9 | 58.7 | 66.8 | 34.8 | 53.7 | 46.7 |
| Reported HR for OS | 0.956 | 0.76 | 0.76 | 0.804 | 0.814 | 0.73 | 0.757 |
| Reported 95% CI for HR of OS | 0.777–1.177 | 0.63–0.93 | 0.61–0.96 | 0.637–1.015 | 0.644–1.029 | 0.59–0.90 | 0.606–0.945 |
Efficacy
In the meta-analysis of the CDK4/6 inhibitors with an AI backbone, palbociclib had a non-significantly worse OS compared to ribociclib and abemaciclib (HR 1.26 [95% CI 0.88–1.80, p = 0.21] and 1.19 [95% CI 0.80–1.76, p = 0.39]) respectively. There were no differences in OS with ribociclib compared to abemaciclib (HR 1.06 [95% CI 0.80–1.41, p = 0.70]). For the fulvestrant backbone, palbociclib had similar OS compared to both ribociclib and abemaciclib; HR 1.12 (95% CI 0.75–1.66, p = 0.59) and 1.08 (95% CI 0.72–1.61, p = 0.73) respectively. Similarly, there were no differences in OS between ribociclib and abemaciclib (HR 0.96 [95% CI 0.66–1.42, p = 0.85]). Table 2 summarizes all indirect comparisons between the 3 different CDK4/6 inhibitors (forest plots for these analyses are shown in the Supplementary File.
Table 2.
Differences in OS between the CDK4/6i with any ET or AI backbone with the PALOMA-2 sensitivity analysis.
| AI backbone | |||
|---|---|---|---|
| Control | Palbociclib | Ribociclib | Abemaciclib |
| AI backbone | |||
| Palbociclib | – | 0.79 (0.56, 1.14), p = 0.21 | 0.84 (0.57, 1.24), p = 0.39 |
| Ribociclib | 1.26 (0.88, 1.80), p = 0.21 | – | 1.06 (0.80, 1.41), p = 0.70 |
| Abemaciclib | 1.19 (0.80, 1.76), p = 0.39 | 0.95 (0.71, 1.26), p = 0.70 | – |
| Fulvestrant backbone | |||
| Palbociclib | – | 0.90 (0.60, 1.33), p = 0.59 | 0.93 (0.62, 1.40), p = 0.73 |
| Ribociclib | 1.12 (0.75, 1.66), p = 0.59 | – | 1.04 (0.71, 1.52), p = 0.85 |
| Abemaciclib | 1.08 (0.72, 1.61), p = 0.73 | 0.96 (0.66, 1.42), p = 0.85 | – |
| PALOMA-2 sensitivity analysis | |||
|---|---|---|---|
| Control | Palbociclib | Ribociclib | Abemaciclib |
| Palbociclib | – | 0.87 (0.61, 1.25), p = 0.46 | 0.93 (0.63, 1.37), p = 0.70 |
| Ribociclib | 1.14 (0.80, 1.63), p = 0.46 | – | 1.06 (0.80, 1.41), p = 0.70 |
| Abemaciclib | 1.08 (0.73, 1.60), p = 0.70 | 0.95 (0.71, 1.26), p = 0.70 | – |
HR (95% CI), p value.
In the PALOMA-2 trial, OS data was missing in 13% of the participants in the experimental arm and 21% in the control arm. In the post-hoc analysis utilizing data from the PALOMA-2 trial which excluded missing data, there was a smaller magnitude association with worse OS with palbociclib compared to ribociclib and abemaciclib (HR 1.14 [95% CI 0.80–1.63, p = 0.46] and 1.08 [95% CI 0.73, 1.60 p = 0.70] respectively). This lower magnitude effect remained statistically non-significant.
Safety and tolerability
Differences in safety and tolerability were observed between the 3 different CDK4/6 inhibitors (see Table 3). When assessing the AI/tamoxifen backbone, compared to palbociclib, abemaciclib had significantly more GI toxicity including more grade 1–2 vomiting and grade 1–2 diarrhea. Grade 3–4 neutropenia was significantly lower with abemaciclib however grade 3–4 infections were significantly higher. Grade 3-transaminitis was also higher with abemaciclib. Compared to palbociclib, ribociclib had significantly more GI toxicity with more grade 1–2 nausea, more grade 1–2 vomiting, grade 3–4 vomiting and grade 3–4 transaminitis. In comparison to ribociclib, abemaciclib had significantly more diarrhea of any grade and more grade-3–4 anemia. When assessing the fulvestrant backbone, compared to palbociclib, abemaciclib had significantly more GI toxicity including all grade nausea, grade 1–2 vomiting, grade 1–2 vomiting, grade 3–4 diarrhea. Abemaciclib had less grade 3–4 neutropenia than palbociclib but more grade 3–4 infections. Furthermore grade 3–4 dyspnea/pneumonitis was higher with abemaciclib. Compared to palbociclib, ribociclib had significantly more grade 3–4 QT prolongation and grade 3–4 transaminitis. Furthermore, ribociclib had more GI toxicity than palbociclib including more grade 1–2 nausea, grade 1–2 vomiting, and grade 1–2 diarrhea. Ribociclib had less grade 1–2 fatigue/asthenia than palbociclib, less grade 3–4 neutropenia, but more grade 3–4 infections.
Table 3.
Adverse events between the CDK4/6i with any ET or AI backbone.
| With AI | With fulvestrant | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Abemaciclib vs palbociclib (control) | ||||||
| Anemia grade 3–4 | 1.32 | 0.73–2.39 | 0.352 | 3.34 | 1.65–6.78 | 0.001 |
| Neutropenia grade 3–4 | 0.17 | 0.13–0.24 | < 0.001 | 0.23 | 0.17–0.31 | < 0.001 |
| Neuropathy grade 3–4 | 0.45 | 0.02–11.12 | 0.627 | 3.93 | 0.19–82.14 | 0.376 |
| Prolonged QT grade 3–4 | 0.45 | 0.02–11.12 | 0.627 | 0.78 | 0.05–12.54 | 0.859 |
| Transaminitis grade 3–4 | 7.55 | 2.57–22.21 | < 0.001 | 2.54 | 1.0–6.44 | 0.050 |
| Nausea grade 1–2 | 1.25 | 0.93–1.67 | 0.139 | 1.81 | 1.35–2.42 | < 0.001 |
| Nausea grade 3–4 | 5.49 | 0.61–49.32 | 0.129 | 20.11 | 1.19–340.89 | 0.038 |
| Vomiting grade 1–2 | 2.27 | 1.59–3.23 | < 0.001 | 1.95 | 1.37–2.78 | 0.000 |
| Vomiting grade 3–4 | 3.43 | 0.66–17.8 | 0.143 | 3.15 | 0.35–28.3 | 0.306 |
| Diarrhea grade 1–2 | 7.56 | 5.48–10.44 | < 0.001 | 9.69 | 6.95–13.49 | < 0.001 |
| Diarrhea grade 3–4 | 7.65 | 3.15–18.55 | < 0.001 | 118.06 | 7.28–1915.32 | 0.001 |
| Stomatitis grade 1–2 | 0.81 | 0.53–1.23 | 0.330 | 1.52 | 1.01–2.29 | 0.045 |
| Alopecia grade 1–2 | 0.78 | 0.57–1.07 | 0.121 | 1.03 | 0.71–1.5 | 0.876 |
| Fatigue/asthenia grade 1–2 | 1.18 | 0.88–1.58 | 0.269 | 1.09 | 0.81–1.45 | 0.569 |
| Dyspnea/pneumonitis grade 3–4 | 1.92 | 0.6–6.11 | 0.272 | 11.28 | 1.48–86.2 | 0.019 |
| Infection grade 3–4 | 8.54 | 3.27–22.32 | < 0.001 | 4.61 | 1.76–12.07 | 0.002 |
| VTE grade 3–4 | 3.11 | 0.95–10.2 | 0.061 | 1.78 | 0.54–5.82 | 0.343 |
| Discontinuation due to AE | 1.84 | 1.2–2.83 | 0.005 | 2.49 | 1.34–4.64 | 0.004 |
| Treatment-related death | 1.52 | 0.63–3.6 | 0.352 | 15.18 | 0.88–261.71 | 0.061 |
| Ribociclib vs palbociclib (control) | ||||||
| Anemia grade 3–4 | 0.68 | 0.38–1.21 | 0.194 | 1.37 | 0.63–2.99 | 0.427 |
| Neutropenia grade 3–4 | 0.4 | 0.31–0.51 | < 0.001 | 0.73 | 0.55–0.97 | 0.039 |
| Neuropathy grade 3–4 | 0.22 | 0.01–5.43 | 0.355 | 5.03 | 0.26–97.76 | 0.286 |
| Prolonged QT grade 3–4 | 4.01 | 0.48–33.42 | 0.199 | 11.03 | 11.45–83.87 | 0.020 |
| Transaminitis grade 3–4 | 14.73 | 5.35–40.52 | < 0.001 | 8.94 | 3.83–20.88 | < 0.001 |
| Nausea grade 1–2 | 1.34 | 1.05–1.72 | 0.019 | 1.63 | 1.22–2.17 | 0.001 |
| Nausea grade 3–4 | 7.41 | 0.95–57.57 | 0.056 | 10.88 | 0.62–191.08 | 0.103 |
| Vomiting grade 1–2 | 1.87 | 1.37–2.56 | < 0.001 | 1.71 | 1.2–2.42 | 0.003 |
| Vomiting grade 3–4 | 5.42 | 1.24–23.67 | 0.025 | 5.06 | 0.62–41.31 | 0.130 |
| Diarrhea grade 1–2 | 1.15 | 0.88–1.51 | 0.315 | 1.45 | 1.05–2.01 | 0.024 |
| Diarrhea grade 3–4 | 1.11 | 0.4–3.07 | 0.841 | 5.03 | 0.26–97.76 | 0.286 |
| Stomatitis grade 1–2 | 0.83 | 0.59–1.17 | 0.288 | 0.82 | 0.53–1.27 | 0.374 |
| Alopecia grade 1–2 | 0.76 | 0.58–0.98 | 0.034 | 1.13 | 0.79–1.63 | 0.513 |
| Fatigue/asthenia grade 1–2 | 1.11 | 0.86–1.42 | 0.406 | 0.73 | 0.54–0.98 | 0.036 |
| Dyspnea/pneumonitis grade 3–4 | 1.88 | 0.67–5.24 | 0.227 | 5.79 | 0.72–46.54 | 0.099 |
| Infection grade 3–4 | 3.84 | 1.47–10.01 | 0.006 | 5.64 | 2.19–14.51 | < 0.001 |
| VTE grade 3–4 | 1.33 | 0.4–4.45 | 0.644 | 1.99 | 0.63–6.29 | 0.241 |
| Discontinuation due to AE | 0.8 | 0.53–1.22 | 0.300 | 2.31 | 1.24–4.29 | 0.008 |
| Treatment-related death | 0.59 | 0.24–1.47 | 0.257 | 3.59 | 0.17–74.97 | 0.410 |
| Abemaciclib vs ribociclib (control) | ||||||
| Anemia grade 3–4 | 1.95 | 1.09–3.49 | 0.025 | 2.44 | 1.39–4.27 | 0.002 |
| Neutropenia grade 3–4 | 0.44 | 0.33–0.59 | < 0.001 | 0.32 | 0.24–0.42 | < 0.001 |
| Neuropathy grade 3–4 | 2.04 | 0.04–130.26 | 0.737 | 0.73 | 0.12–4.38 | 0.731 |
| Prolonged QT grade 3–4 | 0.16 | 0.01–2.77 | 0.208 | 0.07 | 0.01–0.54 | 0.011 |
| Transaminitis grade 3–4 | 0.15 | 0.31–0.85 | 0.032 | 0.28 | 0.17–0.48 | < 0.001 |
| Nausea grade 1–2 | 0.93 | 0.71–1.22 | 0.600 | 1.11 | 0.86–1.44 | 0.432 |
| Nausea grade 3–4 | 0.74 | 0.23–2.34 | 0.608 | 1.9 | 0.74–4.88 | 0.182 |
| Vomiting grade 1–2 | 1.12 | 0.9–1.63 | 0.554 | 1.14 | 0.85–1.53 | 0.383 |
| Vomiting grade 3–4 | 0.63 | 0.23–1.75 | 0.375 | 0.62 | 0.18–2.14 | 0.450 |
| Diarrhea grade 1–2 | 6.55 | 4.87–88 | < 0.001 | 6.68 | 5.01–8.91 | < 0.001 |
| Diarrhea grade 3–4 | 6.9 | 3.34–14.26 | < 0.001 | 27.16 | 8.47–87.14 | < 0.001 |
| Stomatitis grade 1–2 | 0.97 | 0.65–1.45 | 0.882 | 1.86 | 1.26–2.74 | 0.002 |
| Alopecia grade 1–2 | 1.02 | 0.76–1.38 | 0.898 | 0.91 | 0.65–1.27 | 0.579 |
| Fatigue/asthenia grade 1–2 | 1.06 | 0.81–1.4 | 0.681 | 1.49 | 1.13–1.96 | 0.005 |
| Dyspnea/pneumonitis grade 3–4 | 1.02 | 0.41–2.56 | 0.966 | 1.95 | 0.81–4.69 | 0.136 |
| Infection grade 3–4 | 2.23 | 1.3–3.81 | 0.003 | 0.82 | 0.49–1.36 | 0.442 |
| VTE grade 3–4 | 2.34 | 0.89–6.12 | 0.083 | 0.89 | 0.37–2.8 | 0.842 |
| Discontinuation due to AE | 2.3 | 1.53–3.45 | < 0.001 | 1.08 | 0.69–1.68 | 0.733 |
| Treatment-related death | 2.55 | 1.05–6.22 | 0.039 | 5.01 | 1.08–23.32 | 0.040 |
Significant OR bolded.
Compared to ribociclib and palbociclib, abemaciclib had more treatment discontinuation secondary to adverse events. There was no significant difference between ribociclib and palbociclib. Treatment-related death was higher with abemaciclib compared to other CDK4/6 inhibitors (see Table 3). This association was statistically significant for the comparison between abemaciclib and ribociclib and approached but did not meet statistical significance significant for the comparison between abemaciclib and palbociclib.
Discussion
Three CDK4/6 inhibitors have been approved for use in combination with endocrine therapy for HR+/HER− MBC. While all have shown superiority over endocrine therapy alone, the relative efficacy, safety and tolerability is unknown as no head-to-head trials have been performed. PFS effects have been very consistent for all CDK4/6i trials, with HR ranging between 0.50 and 0.59 and with meta-analyses not suggesting any statistically significant or clinically meaningful differences in PFS between drugs17. Therefore, the main markers of differentiation in the efficacy of drugs have been measured by OS. In this study, we performed a network meta-analysis to indirectly evaluate the differences in OS and safety profile of these agents. Our results show that efficacy differences in OS between the three agents are non-significant, and in most cases, effect sizes are not clinically meaningful irrespective of statistical significance. However, as expected, marked differences in safety and tolerability were identified.
While no statistically significant difference in OS was observed between the 3 CDK4/6 inhibitors, there was a non-significant association with shorter OS benefit with palbociclib than the other CDK4/6 inhibitors. The reasons for this are unclear but may reflect trial design rather than inter-drug differences. The OS analysis for PALOMA-2 was limited by a substantial proportion of missing data. OS was missing in 13% of the participants in the experimental arm and 21% in the control arm. In a post-hoc sensitivity analysis of the PALOMA-2 trial excluding participants with missing OS data, larger magnitude relative (HR 0.87 vs 0.96) and absolute effects (difference in median OS 7 vs 2.7 months) were observed. However, as expected, with the loss of power associated with any sensitivity analysis, the effect remained non-significant15,18. Using these post-hoc data in our meta-analysis resulted in lower magnitude effects for OS between palbociclib and other CDK4/6 inhibitors. These effects remained non-significant and based on thresholds recommended by the American Society of Clinical Oncology, were of borderline clinical meaningfulness19.
Another notable difference between these trials relates to the potential for informative censoring. The difference between study arms in the proportion of patients who were censored for reasons other than end of follow-up (e.g. premature loss to follow up due to AEs or withdrawal of consent) was higher with ribociclib than with palbociclib studies (> 5% in MONALEESA-2 versus < 1% in PALOMA-2). The reasons for unbalanced censoring are unclear, but may impact both the cross-trial comparison of different CDK4/6 inhibitors and meta-analytic comparisons20,21.
Consistent with prior reports, substantial differences in safety and tolerability were observed between the different CDK4/6 inhibitors22. In general, compared to ribociclib and abemaciclib, palbociclib showed more frequent hematological toxicity, but less frequent gastrointestinal toxicity. Patients with HR+/HER2− MBC report shortness of breath, fatigue, pain and vomiting as the most bothersome symptoms affecting their quality of life23. Furthermore, in a meta-analysis of phase 3 breast cancer trials, patients reporting more diarrhea had lower health-related quality of life and worse physical function24. Discussing side effect differences between drugs is an important method to increase patients’ satisfaction and increase adherence to treatment25. Of note, there were more treatment-related deaths reported with abemaciclib than with other CDK4/6 inhibitors, although this observation was only statistically significant in the comparison of abemaciclib with ribociclib. This finding should be interpreted with caution given that it is possible that these deaths may be related to the breast cancer despite not meeting imaging criteria for progression. It can be difficult to distinguish treatment-related from disease-related causes of death especially among patients with breast cancer who do not have disease which is measurable by Response Evaluation Criteria in Solid Tumors (RECIST) criteria26. However, with data suggesting that mechanisms of resistance to CDK4/6 inhibitors seem uniform between the different agents, the higher odds of non-cancer deaths with abemaciclib relative to placebo compared to other CDK4/6 inhibitors is an important observation27.
Our study has some limitations. This is a literature-based network meta-analysis rather than using individual patient data. Although the included studies were generally homogenous, there were differences in endocrine therapy backbone, patient populations (e.g. menopausal status) and as detailed above there was concern for post-randomization differences such as missing data and potential for unbalanced informative censoring among some studies. To address inter-study heterogeneity, our analysis compared studies with the same endocrine therapy backbone which would limit heterogeneity, but results in a smaller sample size for comparison and consequently reduced statistical power. There is therefore an incomplete ability to assess the assumptions of transitivity. However, in the absence of direct comparisons, assessment of relative efficacy, safety, and tolerability therefore needs to be based on indirect comparisons ideally based on network meta-analytic methods as utilized in this study. The main limitation of a network meta-analysis in this setting is that unlike in individual patient data analysis where the unit of analysis is an individual study participant, in a meta-analysis, the unit of analysis is each individual trial. With only seven trials included, statistical power is reduced and this may decrease the certainty of our analysis.
In summary, despite differences between trial effect sizes and statistical significance, in this network meta-analysis, there was no statistically significant difference in OS between the different CDK4/6 inhibitors. Significant differences between CDK4/6i were observed for safety and tolerability outcomes. Real-world data analyses may help to identify if a there is a meaningful inter-drug difference in efficacy, safety or tolerability.
Supplementary Information
Author contributions
C.K. and E.A. contributed equally to the study design, data analysis and writing the main manuscript text. All authors reviewed the manuscript.
Data availability
All data available upon request to corresponding author, E. Amir.
Competing interests
E. Amir: Honoraria from Novartis, Seagen, Gilead and AstraZeneca outside the submitted work. Michelle Nadler: Honoraria from Novartis and Exact Sciences outside the submitted work.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-53151-8.
References
- 1.Burstein HJ, Somerfield MR, Barton DL, Dorris A, Fallowfield LJ, Jain D, Johnston SRD, Korde LA, Litton JK, Macrae ER, et al. Endocrine treatment and targeted therapy for hormone receptor-positive, human epidermal growth factor receptor 2–negative metastatic breast cancer: ASCO guideline update. J. Clin. Oncol. 2021;39:3959–3977. doi: 10.1200/JCO.21.01392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gennari A, André F, Barrios CH, Cortés J, de Azambuja E, DeMichele A, Dent R, Fenlon D, Gligorov J, Hurvitz SA, et al. ESMO clinical practice guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2021;32:1475–1495. doi: 10.1016/j.annonc.2021.09.019. [DOI] [PubMed] [Google Scholar]
- 3.NCCN Guidelines Version 4.2023 Breast Cancer. NCCN Clin. Pract. Guidel. Oncol. (2023).
- 4.Hortobagyi GN, Stemmer SM, Burris HA, Yap Y-S, Sonke GS, Paluch-Shimon S, Campone M, Blackwell KL, André F, Winer EP, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N. Engl. J. Med. 2016;375:1738–1748. doi: 10.1056/NEJMoa1609709. [DOI] [PubMed] [Google Scholar]
- 5.Finn RS, Martin M, Rugo HS, Jones S, Im S-A, Gelmon K, Harbeck N, Lipatov ON, Walshe JM, Moulder S, et al. Palbociclib and letrozole in advanced breast cancer. N. Engl. J. Med. 2016;375:1925–1936. doi: 10.1056/NEJMoa1607303. [DOI] [PubMed] [Google Scholar]
- 6.Slamon DJ, Neven P, Chia SKL, Jerusalem GHM, De Laurentiis M, Im S-A, Petrakova K, Bianchi GV, Martin M, Nusch A, et al. Updated overall survival (OS) results from the phase III MONALEESA-3 trial of postmenopausal patients (pts) with HR+/HER2− advanced breast cancer (ABC) treated with fulvestrant (FUL) ± ribociclib (RIB) J. Clin. Oncol. 2021;39:1001. doi: 10.1200/JCO.2021.39.15_suppl.1001. [DOI] [Google Scholar]
- 7.Hortobagyi GN, Stemmer SM, Burris HA, Yap Y-S, Sonke GS, Hart L, Campone M, Petrakova K, Winer EP, Janni W, et al. Overall survival with ribociclib plus letrozole in advanced breast cancer. N. Engl. J. Med. 2022;386:942–950. doi: 10.1056/NEJMoa2114663. [DOI] [PubMed] [Google Scholar]
- 8.Lu Y-S, Im S-A, Colleoni M, Franke F, Bardia A, Cardoso F, Harbeck N, Hurvitz S, Chow L, Sohn J, et al. Updated overall survival of ribociclib plus endocrine therapy versus endocrine therapy alone in pre- and perimenopausal patients with HR+/HER2− advanced breast cancer in MONALEESA-7: A phase III randomized clinical trial. J. Am. Assoc. Cancer Res. 2022;28:851–859. doi: 10.1158/1078-0432.CCR-21-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sledge GW, Jr, Toi M, Neven P, Sohn J, Inoue K, Pivot X, Burdaeva O, Okera M, Masuda N, Kaufman PA, et al. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor-positive, ERBB2-negative breast cancer that progressed on endocrine therapy—MONARCH 2: A randomized clinical trial. JAMA Oncol. 2020;6:116–124. doi: 10.1001/jamaoncol.2019.4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnston S, Martin M, Di Leo A, Im S-A, Awada A, Forrester T, Frenzel M, Hardebeck MC, Cox J, Barriga S, et al. MONARCH 3 final PFS: A randomized study of abemaciclib as initial therapy for advanced breast cancer. NPJ Breast Cancer. 2019;5:5. doi: 10.1038/s41523-018-0097-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goetz, P. M. et al. Abstract GS01–12: MONARCH 3: Final overall survival results of abemaciclib plus a nonsteroidal aromatase inhibitor as first-line therapy for HR+, HER2− advanced breast cancer. in San Antonio Breast Cancer Conference 2023
- 12.Oswald LB, Arredondo B, Kadono M, Martinez-Tyson D, Meade CD, Penedo F, Antoni MH, Soliman H, Costa RLB, Jim HSL. A mixed-methods study of cyclin-dependent kinase 4 and 6 inhibitor symptom burden and quality of life among metastatic breast cancer patients and providers. Cancer Med. 2021;10:4823–4831. doi: 10.1002/cam4.4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howie LJ, Singh H, Bloomquist E, Wedam S, Amiri-Kordestani L, Tang S, Sridhara R, Sanchez J, Prowell TM, Kluetz PG, et al. Outcomes of older women with hormone receptor-positive, human epidermal growth factor receptor-negative metastatic breast cancer treated with a CDK4/6 inhibitor and an aromatase inhibitor: An FDA pooled analysis. J. Am. Soc. Clin. Oncol. 2019;37:3475–3483. doi: 10.1200/JCO.18.02217. [DOI] [PubMed] [Google Scholar]
- 14.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finn RS, Rugo HS, Dieras VC, Harbeck N, Im S-A, Gelmon KA, Walshe JM, Martin M, Chavez Mac Gregor M, Bananis E, et al. Overall survival (OS) with first-line palbociclib plus letrozole (PAL+LET) versus placebo plus letrozole (PBO+LET) in women with estrogen receptor–positive/human epidermal growth factor receptor 2–negative advanced breast cancer (ER+/HER2− ABC): Analyses from PALOMA-2. J. Clin. Oncol. 2022;40:1003. doi: 10.1200/JCO.2022.40.17_suppl.LBA1003. [DOI] [Google Scholar]
- 16.Cristofanilli M, Turner NC, Bondarenko I, Ro J, Im S-A, Masuda N, Colleoni M, DeMichele A, Loi S, Verma S, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17:425–439. doi: 10.1016/S1470-2045(15)00613-0. [DOI] [PubMed] [Google Scholar]
- 17.Desnoyers A, Nadler MB, Kumar V, Saleh R, Amir E. Comparison of treatment-related adverse events of different Cyclin-dependent kinase 4/6 inhibitors in metastatic breast cancer: A network meta-analysis. Cancer Treat. Rev. 2020;90:102086. doi: 10.1016/j.ctrv.2020.102086. [DOI] [PubMed] [Google Scholar]
- 18.Helwick, C. Paloma-2: No overall survival benefit reported with palbociclib/letrozole in advanced breast cancer. ASCO Post (2022).
- 19.Ellis LM, Bernstein DS, Voest EE, Berlin JD, Sargent D, Cortazar P, Garrett-Mayer E, Herbst RS, Lilenbaum RC, Sima C, et al. American Society of Clinical Oncology perspective: Raising the bar for clinical trials by defining clinically meaningful outcomes. J. Am. Soc. Clin. Oncol. 2014;32:1277–1280. doi: 10.1200/JCO.2013.53.8009. [DOI] [PubMed] [Google Scholar]
- 20.Templeton AJ, Ace O, Amir E, Vera-Badillo F, Ocana A, Pond GR, Tannock IF. Influence of censoring on conclusions of trials for women with metastatic breast cancer. Eur. J. Cancer. 2015;51:721–724. doi: 10.1016/j.ejca.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 21.Templeton AJ, Amir E, Tannock IF. Informative censoring: A neglected cause of bias in oncology trials. Nat. Rev. Clin. Oncol. 2020;17:327–328. doi: 10.1038/s41571-020-0368-0. [DOI] [PubMed] [Google Scholar]
- 22.Desnoyers A, Nadler MB, Kumar V, Saleh R, Amir E. Comparison of treatment-related adverse events of different Cyclin-dependent kinase 4/6 inhibitors in metastatic breast cancer: A network. Cancer Treat. Rev. 2022;90:102086. doi: 10.1016/j.ctrv.2020.102086. [DOI] [PubMed] [Google Scholar]
- 23.Galipeau N, Klooster B, Krohe M, Tang DH, Revicki DA, Cella D. Understanding key symptoms, side effects, and impacts of HR+/HER2− advanced breast cancer: Qualitative study findings. J. Patient Rep. Outcomes. 2019;3:10. doi: 10.1186/s41687-019-0098-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen T-Y, King-Kallimanis B, Merzoug L, Fiero M, Gao JJ, Beaver JA, Bhatnagar V, Kluetz PG. Patient-reported diarrhea impact on physical functioning and quality of life in clinical trial data submitted to the US food and drug administration. J. Clin. Oncol. 2020;38:e19105. doi: 10.1200/JCO.2020.38.15_suppl.e19105. [DOI] [Google Scholar]
- 25.Josfeld L, Keinki C, Pammer C, Zomorodbakhsch B, Hübner J. Cancer patients’ perspective on shared decision-making and decision aids in oncology. J. Cancer Res. Clin. Oncol. 2021;147:1725–1732. doi: 10.1007/s00432-021-03579-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur. J. Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 27.Stanciu I-M, Parosanu AI, Orlov-Slavu C, Iaciu IC, Popa AM, Olaru CM, Pirlog CF, Vrabie RC, Nitipir C. Mechanisms of resistance to CDK4/6 inhibitors and predictive biomarkers of response in HR+/HER2− metastatic breast cancer: A review of the literature. Diagnostics. 2023 doi: 10.3390/diagnostics13050987. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data available upon request to corresponding author, E. Amir.