Abstract
Previous studies have implicated ferric reduction in the iron uptake pathway of the opportunistic pathogen Cryptococcus neoformans. Here we studied iron uptake directly, using 55Fe in the presence of reductants. Uptake was linear with respect to time and number of yeast cells. The plot of uptake versus concentration exhibited a steep rise up to about 1 μM, a plateau between 1 and 25 μM, and a second steep rise above 25 μM, consistent with high- and low-affinity uptake systems. A Km for high-affinity uptake was estimated to be 0.6 μM Fe(II); 1 μM was used for standardized uptake assays. At this concentration, the uptake rate was 110 ± 3 pmol/106 cells/h. Iron repletion (15 μM) and copper starvation drastically decreased high-affinity iron uptake. Incubation at 0°C or in the presence of 2 mM KCN abolished high-affinity iron uptake, suggesting that uptake requires metabolic energy. When exogenous reducing agents were not supplied and the culture was washed free of secreted reductants, uptake was reduced by 46%; the remaining uptake activity presumably was dependent upon the cell membrane ferric reductase. Further decreases in free Fe(II) levels achieved by trapping with bathophenanthroline disulfonate or reoxidizing with potassium nitrosodisulfonate reduced iron uptake very drastically, suggesting that it is the Fe(II) species which is transported by the high-affinity transporter. The uptake of Fe was stimulated two- to threefold by deferoxamine, but this increment could be abolished by copper starvation or inhibition of the ferric reductase by Pt, indicating that Fe solubilized by this molecule also entered the reductive iron uptake pathway.
Iron is required for oxidoreductase enzymes in almost all living organisms. Although iron is widely distributed in the environment, under neutral pH and aerobic conditions oxidation of iron to poorly soluble Fe(III) causes ambient concentrations to be very low. Microbes mobilize environmental iron for uptake according to one of three basic strategies: by secreting iron-chelating chemicals, by reducing insoluble environmental Fe(III) to soluble Fe(II), or by expressing receptors and deferration mechanisms for iron-containing proteins. The most widely studied microbial iron uptake systems use secreted hydroxamate or catecholate Fe(III) chelators, termed siderophores, and cell surface receptors for the ferrated complex (21). One such siderophore, deferoxamine (Desferal; Ciba-Geigy), is manufactured by fermentation and is used clinically to mobilize trivalent metals. Saccharomyces cerevisiae (16), Listeria monocytogenes (5), Legionella pneumophila (14), and higher plants (18, 23) use the second strategy, reducing Fe(III) to Fe(II) prior to uptake of the iron. The obligate pathogens Neisseria gonorrhoeae and Neisseria meningitidis use the third strategy, binding and deferrating human transferrin and lactoferrin (19); Hemophilus influenzae scavenges iron directly from heme and hemoglobin (24).
Iron uptake mechanisms in pathogenic fungi are incompletely understood. Hydroxamate siderophores have been detected in culture supernatants of Histoplasma capsulatum (2). Rhizopus species express surface receptors for ferri-deferoxamine but do not secrete hydroxamate siderophores (1); thus, deferoxamine represents an iron uptake-related vitamin in Rhizopus, as it does in the pathogenic bacterium Yersinia enterocolitica (25). In Cryptococcus neoformans, deferoxamine stimulates growth in the presence of iron starvation, but this fungus does not secrete Fe(III) chelators, nor does it express a specific transferrin receptor (12, 13). Rather, C. neoformans and Candida albicans reduce extracellular Fe(III) to the much more soluble form, Fe(II), like S. cerevisiae, and are presumed to transport nascent Fe(II) into their cells (20, 22).
In what has been termed nutritional immunity, vertebrates use unsaturated transferrins to withhold their iron from microbes and respond to infections by further sequestering their stores of iron, even to the point of anemia (3). This inference is supported by the observation that saturation with physiologic iron chelators leads to opportunistic infections (28). It has been hypothesized that the administration of therapeutic iron-sequestering agents may further protect host iron stores and thereby alleviate infections. Such compounds are being actively sought, but currently the only chelator which can be safely administered is deferoxamine, a microbial hydroxamate siderophore. One might expect that this compound would sequester iron and block iron uptake by microbes which lack receptors for the iron chelate; however, the compound also might be predicted to stimulate uptake in microbes which express such receptors. Indeed, it has been found that the administration of deferoxamine alleviates malaria (9) but promotes opportunistic infections by Rhizopus species (1). Thus, microbial iron uptake mechanisms can powerfully affect the host-parasite balance, and understanding them has the potential to lead to better management of infections.
C. neoformans is the etiologic agent of a meningitis which complicates many immunodeficiency diseases, including 10 to 20% of those resulting from the human immunodeficiency virus (17). Although the symptoms of cryptococcosis can often be suppressed by treatment with antifungal agents, in most cases the infection is incurable and lifelong treatment is required (29). Because control of iron uptake has decided the outcome of infections in the model cases cited above, we investigated iron uptake in C. neoformans with the hope of being able to bolster nutritional immunity. We previously described two mechanisms by which this organism reduces extracellular iron: expression of a cell membrane ferric reductase and secretion of the nonspecific reductant 3-hydroxyanthranilic acid (22). In the present report, we describe iron uptake physiologically. We chose to use a serotype D strain because of the availability of both meiotic and molecular analyses in the serotype D cryptococcal system.
MATERIALS AND METHODS
Yeast strains and culture conditions.
We used strain B3501, MATα, serotype D, obtained from the National Institutes of Health culture collection. Cultures were stored on brain heart agar slants, from which liquid cultures were started in 2% glucose–2% yeast extract (GYE; Becton Dickinson and Co.) broth. Limited-iron medium (LIM) contained, per liter, 20 g of glucose, 5 g of asparagine, 400 mg of K2HPO4, 100 mg of MgSO4 · 7H2O, 50 mg of CaCl2 · 2H2O, 1 mg of thiamine, 57 μg of boric acid, 396 μg of CuSO4 · 5H2O, 72 μg of MnCl2 · 4H2O, 4.2 mg of ZnCl2, and 37 μg of (NH4)6Mo7O24 · 4H2O. It was buffered with 50 mM 2-(N-morpholino)ethanesulfonic acid adjusted to pH 6.0 with NaOH. Salts of polyvalent metals were dissolved in water treated with Chelex-100 (Bio-Rad) and filter sterilized. Other components were purified over a Chelex-100 column and filter sterilized. Cultures in defined medium were started from stationary-phase cultures in GYE broth, washed twice with LIM, and inoculated at a density of 106 cells/ml. All glassware was soaked in Citranox acid detergent overnight and rinsed with distilled, deionized water before use with LIM. Iron repletion was accomplished by adding the desired concentration of Fe(III)-hydroxyethylenediaminetriacetic acid (FeHEDTA) to LIM. All cultures were grown at room temperature with agitation at 200 rpm. Platinum inhibition was achieved by use of LIM saturated with platinum(II) chloride; the concentration was determined by atomic absorption spectroscopy (Galbraith Laboratories, Knoxville, Tenn.).
Iron uptake assays.
Cells were grown in LIM with or without various concentrations of FeHEDTA to the late growth phase (<2 × 107 cells/ml) or to the stationary phase. The cells were washed twice and diluted in LIM to a concentration of 4 × 106 cells/ml. To assay ferrous iron uptake, an equal volume of LIM containing 100 mM ascorbate and 1 mM dithiothreitol (DTT) at pH 6.0 (LAD) was added to 5 ml of cells. To assay Fe(III) uptake, an equal volume of LIM (without reductants) was added to 5 ml of cells. Iron was added to the cultures at the desired concentration as FeHEDTA diluted in LAD [for Fe(II)] or LIM [for Fe(III)] containing 55FeCl3 (0.2 μCi; New England Nuclear Corp.). Immediately upon the addition of iron, a 3-ml initial-time sample was filtered through a GF/A glass microfiber filter (Whatman) and washed twice with 10 ml of 0.1 M disodium EDTA (pH 6.0). Cultures were incubated with agitation at 150 rpm and room temperature for 20 min, after which a second 3-ml sample was obtained. Counts on filters were determined with 10 ml of scintillation fluid (Biosafe II; Research Products International) by use of a model 1410 liquid scintillation counter (Wallac). Uptake was calculated by subtracting the counts per minute of the initial sample from that of the 20-min sample and comparing the result to an internal standard. Deferoxamine mesylate was a gift from the Ciba Corporation. Statistical comparisons were done with Student’s t test.
RESULTS AND DISCUSSION
Kinetics of Fe(II) uptake.
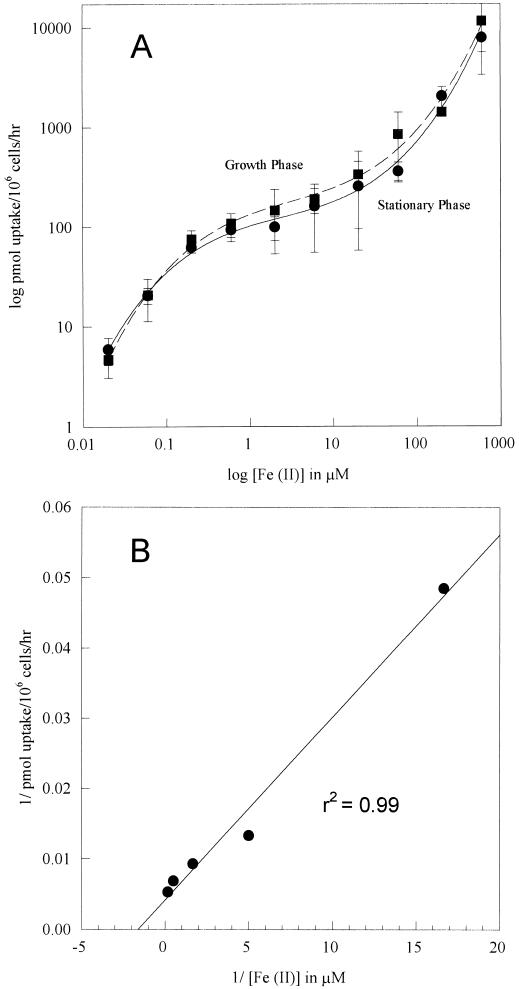
The uptake of Fe(II) was studied with 55Fe in the presence of the reductants DTT and ascorbate. Uptake was linear with respect to time for 40 min and linear with respect to number of cells assayed for up to 107 cells/ml (data not shown). Suspensions of C. neoformans grown in LIM were assayed for uptake in various concentrations of Fe(II). Each concentration was studied in triplicate, several experiments were performed, and a typical experiment is shown in Fig. 1. A log-log plot of uptake versus concentration shows a steeply sloping portion well below 1 μM, a shallowly sloping, relative plateau between 1 and 25 μM, and a second steeply sloping portion above 25 μM (Fig. 1A). This pattern is consistent with a combination of high- and low-affinity uptake systems, as described for S. cerevisiae (6, 7), and indicates saturation of the high-affinity uptake system in the range of 1 to 5 μM. A double-reciprocal plot indicated a Km of approximately 0.6 μM for the high-affinity system (Fig. 1B; data for a growth-phase culture are shown). Clearly, the high-affinity uptake system is tuned to concentrate ambient iron in the range of 0.1 to 1 μM very efficiently. Because it is very difficult to reduce the iron content of growth media below 100 nM, these data seem to explain why C. neoformans grows readily, even in iron-depleted media; in order to observe the failure of growth due to iron limitation, we found it necessary to use an avid synthetic chelator of Fe(II) or Fe(III) (12, 13).
FIG. 1.
Fe(II) uptake by growth-phase and stationary-phase cells. (A) Velocity depicted as a function of concentration. A logarithmic plot was used in order to encompass a 5-log-unit variation in Fe(II) concentrations. Error bars indicate standard deviations. (B) Double-reciprocal plot of low-concentration data from an exponentially growing culture. The experiment was performed three times, and representative results are shown.
We chose 1 μM Fe(II) as the standard concentration for assaying the high-affinity system in order to minimize the effect of changes in substrate concentration. At 1 μM, the uptake of Fe(II) was 110 ± 3 pmol/106 cells/h, close to the Vmax for the high-affinity system. When the temperature of incubation was reduced to 4°C, high-affinity uptake was 96% inhibited (27 ± 5 versus 1 ± 0 pmol/106 cells/h; the experiment was performed in triplicate), indicating that active respiratory metabolism is required for transport. That inference was supported by the finding that 2 mM KCN inhibited uptake by 79% (38 ± 8 versus 8 ± 1 pmol/106 cells/h; the experiment was performed in triplicate). These results are very similar to those obtained for S. cerevisiae, except that for the latter, the Km for high-affinity iron uptake is somewhat lower (0.15 μM) (7).
Regulation of ferrous transport.
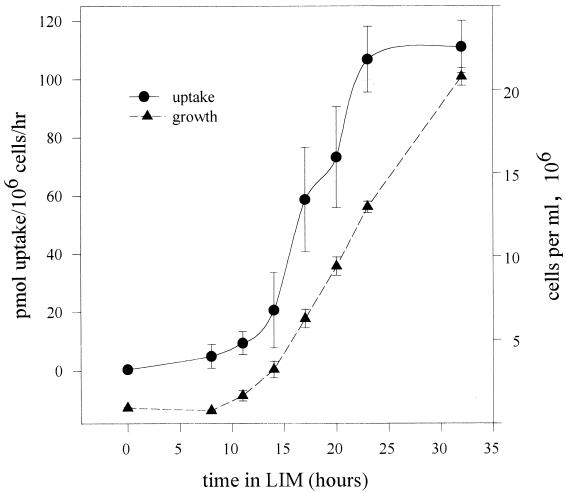
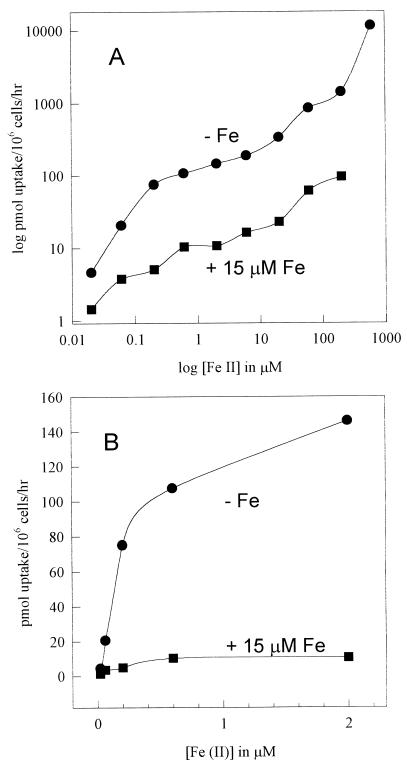
Cultures in the late growth phase (24-h incubation time) and in the stationary phase (72-h incubation time) in LIM exhibited comparable uptake activities at all concentrations of Fe(II) studied (Fig. 1). Cultures transferred from GYE (approximately 25 μM Fe) to LIM (approximately 0.2 μM Fe) did not immediately express the high-affinity (low-substrate-concentration) uptake system; such cultures began to express that uptake system only after 10 h of growth in LIM, reaching maximum activity at about 24 h (Fig. 2; an experiment with duplicate time points is shown). This result suggests that the high-affinity uptake system was regulated by iron in the culture and that the culture had outgrown the iron stores left over from the complex medium. Indeed, we observed that uptake activity was down regulated 50% after 24 h of growth in LIM containing 1 μM Fe(III) (data not shown) and that both high-affinity and low-affinity (high-concentration) uptake systems were down regulated 90% by growth in 15 μM Fe(III), as shown in Fig. 3 [uptake was studied once at a range of 55Fe(II) concentrations; the result was confirmed by four uptake studies at 1 μM 55Fe(II), each performed in triplicate]. Thus, the high-affinity uptake system is potentially resistant to iron overloading at up to 25 μM, partly because of the saturation phenomenon, whereby increases in iron concentrations from 1 to 25 μM result in very little incremental uptake, and partly because the regulation of uptake activity (presumably genetic regulation of expression of the ferrous transporter) begins at about 1 μM ambient iron.
FIG. 2.
Expression of high-affinity uptake system following transfer from GYE medium to LIM medium. Cells from a stationary-phase culture in GYE were inoculated at 106/ml and agitated. Growth was monitored optically at 700 nm, and high-affinity Fe(II) uptake was measured periodically. Results represent averages of three separate timed cultures assayed in duplicate. Error bars indicate standard deviations.
FIG. 3.
Effect of Fe(III) in growth medium upon Fe(II) uptake activity. Cells were grown in LIM with or without (−Fe) 15 μM Fe, washed in LIM, and assayed for Fe(II) uptake at various concentrations. (A) Double-logarithm plot. (B) Arithmetic plot of low-concentration data.
Given the sensitivity of the high-affinity iron uptake system to regulation by only 15 μM Fe, the utility of high-velocity iron uptake at high concentrations (25 to 200 μM) is not at all clear, yet above 25 μM an upward curve in the concentration-velocity plot suggests a contribution by a low-affinity, high-velocity uptake mechanism (Fig. 1, 3, and 4). The low-affinity system concentrated approximately 1 nmol/106 cells/h at 200 μM Fe(II), and the lack of a second plateau in the plot suggests that the low-affinity mechanism is not saturable. This second system appeared to be down regulated during growth by the same relatively low concentration of iron (15 μM) as that which regulated the high-affinity system (Fig. 3A). Thus, if low-affinity uptake were not also regulated, the iron-replete plot should have converged upon the iron-depleted plot at high iron concentrations in the log-log plot, since the constant equal to the saturated high-affinity uptake system should represent a diminishing fraction of the increasing logarithms. Accordingly, both high- and low-affinity systems appeared to have been down regulated 1 log unit by 15 μM Fe(III). Curiously, at 200 μM Fe(II), the low-affinity system of the high-iron culture transported about as much iron (100 pmol/106 cells/h) as the Vmax for the maximally induced high-affinity system of the low-iron culture, making regulation of the high-affinity system seem futile. One can assert that an Fe(II) concentration of 100 μM may not be physiologic, but even with aerobic cultures we have observed such a concentration for a constitutive ferric reductase regulatory mutant (11).
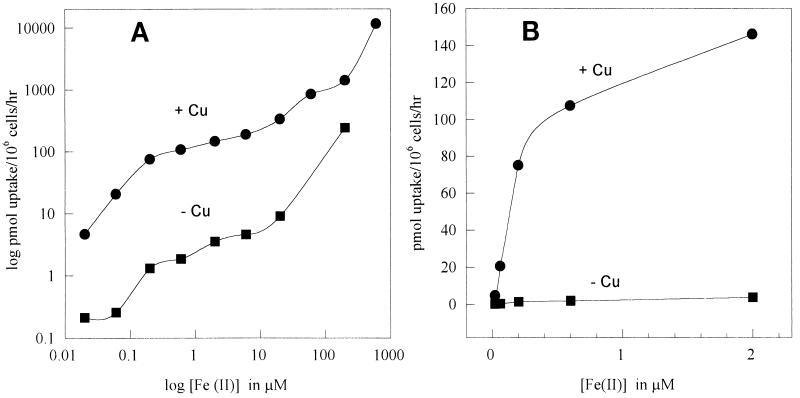
FIG. 4.
Effect of culture copper deprivation upon Fe(II) uptake. Cells were grown in LIM containing 1.6 μM CuSO4 (+Cu) or in LIM formulated without CuSO4 (−Cu). (A) Double-logarithm plot. (B) Arithmetic plot of low-concentration data.
Dependence upon exogenous copper.
Perhaps the most striking finding for the S. cerevisiae system has been the dependence of iron uptake upon a copper-containing protein thought to function as an oxidase (4). Indeed, there is evidence for the direct association of a copper-containing protein with the ferrous transporter in S. cerevisiae, and it is inferred that copper-containing oxidases may be widely required for eukaryotic iron transport (15, 26). Nascent Fe(II) released from the cell membrane ferric reductase is presumed to be reoxidized by the copper-containing protein at the time of transport into the cell; thus, the main uptake pathway may perhaps be called “reductive/oxidative.” We assayed Fe(II) uptake in copper-starved C. neoformans cultures. Cryptococcal LIM normally contains 1.6 μM Cu(II). When this copper was not supplied, 24-h cultures reached optical densities 10% lower than those of cultures containing 1.6 μM Cu(II). Under these conditions, the high-affinity system was 90% suppressed, as shown in Fig. 4 (uptake was studied once at a range of concentrations; the result was confirmed by an uptake study performed in triplicate at 1 mM 55Fe). Thus, it seems likely that a copper-containing factor is also required for high-affinity iron uptake in C. neoformans.
Specificity for Fe(II) of the iron uptake step.
Iron assimilation (iron-dependent growth) in C. neoformans appears to require the reduction of Fe(III) to Fe(II) as a first step (13, 22, 27). We defined two main mechanisms for the reduction of extracellular Fe(III) prior to uptake. The first is a plasma membrane ferric reductase, and the second consists of secreted ferric reductants, one of which has been identified as 3-hydroxyanthranilate. Expression of the enzyme and secretion of the reductant are both down regulated by environmental iron, a finding which supports the above hypothesis (22). In our uptake assays, we usually fixed iron in the Fe(II) state with the exogenous reducing agents ascorbic acid and DTT. We tested whether reduction is required for physiologic iron uptake. When a 24-h growth-phase culture was assayed for the uptake of 1 μM Fe(III) without the exogenous reductants DTT and ascorbic acid but still in the presence of its culture supernatants, the uptake rate was only slightly lower than that in the presence of the exogenous reductants (no reductants, 118 ± 26 pmol/106 cells/h; reductants, 144 ± 38; three experiments were performed in triplicate; P = 0.3, not significant). However, washing the cells twice in fresh LIM resulted in a 46% decrease in iron uptake, presumably by the removal of secreted 3-hydroxyanthranilate (washed, no exogenous reductants, 60 ± 16 pmol/106 cells/h; exogenous reductants, 111 ± 8; three experiments were performed in triplicate; P = 0.05). The substantial remaining uptake activity presumably depended upon the reduction of extracellular Fe(III) by the cell membrane ferric reductase (22). This hypothesis was tested in further experiments with washed cells without exogenous reductants (Table 1, experiment A). Bathophenanthroline disulfonate (BPDS) (1 mM), an avid chelator of Fe(II), was found to abolish iron uptake quantitatively; the mild oxidant potassium nitrosodisulfonate (1 mM) inhibited the uptake of iron by 94%, although at this concentration it did not inhibit growth. Thus, reduction of Fe(III) to Fe(II) appeared to be essential for high-affinity uptake. Interestingly, the velocities at which physiologic reduction and uptake occur are very different, for the reduction of Fe(III) can occur in a burst at 60 nmol/106 cells/h (23) while the maximum velocity for Fe(II) uptake is 120 pmol/106 cells/h, about 500-fold lower. Thus, it is possible for Fe(II) to accumulate in the extracellular fluid, where it may represent a pool of reducing equivalents available for electrochemical work, such as the reduction of melanin (11).
TABLE 1.
Iron uptake in the presence of various reagentsa
| Expt | Reagent | Uptake, pmol/106 cells/h (mean ± SD) | P (vs no reagent) |
|---|---|---|---|
| A | None | 128 ± 32 | |
| BPDS (1 mM) | 0 ± 0 | <0.01 | |
| (KSO3)2 NO (1 mM)b | 8 ± 1 | <0.01 | |
| B | None | 92 ± 15 | |
| DFOc (1 μM) | 188 ± 14 | <0.01 | |
| DFO (1 μM) and Pt (5.6 μM) | 104 ± 15 | 0.23 |
Uptake was studied with LIM containing 1 μM Fe(II). Data represent two experiments performed in triplicate.
Potassium nitrosodisulfonate (Fremy’s salt).
DFO, deferoxamine.
Stimulation of iron uptake by deferoxamine.
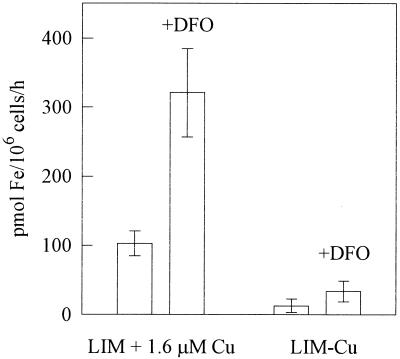
Although C. neoformans does not secrete siderophores, its response to them is of considerable interest. We have observed that deferoxamine relieves iron starvation caused by the avid synthetic chelator ethylenediaminedi(o-hydroxyphenylacetic acid) (12), while others have found deferoxamine to exacerbate experimental cryptococcosis in the guinea pig (1). Thus, C. neoformans behaves as though it expresses a receptor for Fe(III)-deferoxamine. We tested the effect of 1 μM deferoxamine mesylate (added 10 min prior to the experiment) upon the uptake of Fe(III) by 24-h growth-phase cultures of C. neoformans. The experiment was performed without exogenous reductants because deferoxamine is specific for Fe(III). The threefold stimulation observed (Fig. 5) did not seem attributable to the nonspecific solubilization of Fe(III) by deferoxamine, because iron was always used with equimolar HEDTA, a synthetic iron chelator and solubilizer. We tested whether deferoxamine-stimulated uptake was independent of the copper-dependent uptake pathway but found that deferoxamine-stimulated uptake was 90% abolished by growth without copper (Fig. 5). Thus, deferoxamine-mediated uptake apparently proceeds via the copper-dependent uptake pathway.
FIG. 5.
Stimulation of iron uptake by 1 μM deferoxamine (DFO). Cultures grown in LIM with and without (Lim−Cu) 1.6 μM CuSO4 were washed and assayed for iron uptake without exogenous reductants. The results are averages of two experiments performed in triplicate. Error bars show standard deviations.
We also considered the relationship of deferoxamine to the iron reduction step. The fate of Fe(III) in the S. cerevisiae uptake model is believed to be reduction to Fe(II) by the cell membrane ferric reductase, followed by reoxidation by the copper-containing uptake-linked oxidase during transport (4, 15, 26). Assuming an equivalent pathway in C. neoformans, Fe(III) complexed by deferoxamine may enter the uptake pathway either prior to the reductive step or following reoxidation. Since the ferric reductase is subject to inhibition by Pt(II)Cl2 (6), inhibition of deferoxamine-mediated iron uptake by Pt(II) would suggest that deferoxamine does not allow Fe(III) to bypass the reductive step, while the maintenance of deferoxamine-stimulated uptake in the presence of Pt inhibition of the reductase would suggest the donation of Fe(III) by deferoxamine after the copper-mediated oxidase step. We found approximately 80% inhibition of the cryptococcal ferric reductase by 4 μM Pt(II); when we performed uptake experiments with 5.6 μM Pt(II), deferoxamine-stimulated uptake was inhibited by Pt (Table 1, experiment B). This result suggests that the input of Fe(III) by deferoxamine precedes the enzyme-mediated ferric reduction step. Moreover, because deferoxamine-stimulated iron uptake is abolished by copper starvation (see previous paragraph), it is doubly unlikely that the Fe(III)-deferoxamine complex bypasses the copper-dependent oxidase step. Thus, a deferoxamine receptor protein may be associated with the plasma membrane ferric reductase. Alternatively, deferoxamine may simply be more efficient than FeHEDTA at presenting Fe(III) to the ferric reductase. The deferoxamine stimulation of iron uptake that we observed with C. neoformans is similar to observations made with Rhizopus species, which cause opportunistic infections in deferoxamine-treated patients (1). It is not clear why cases of deferoxamine-associated cryptococcosis have not likewise been reported.
ACKNOWLEDGMENT
This work was supported by the Department of Veterans Affairs.
REFERENCES
- 1.Boelaert J R, de Locht M, van Cutsem J, Kerrels V, Cantinieaux B, Verdonck A, van Landuyt H W, Schneider Y-J. Mucormycosis during deferoxamine therapy is a siderophore-mediated infection. In vitro and in vivo studies. J Clin Invest. 1993;91:1979–1986. doi: 10.1172/JCI116419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burt W R. Identification of coprogen B and its breakdown products from Histoplasma capsulatum. Infect Immun. 1982;35:990–996. doi: 10.1128/iai.35.3.990-996.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cartwright G E, Wintrobe M M. The anemia of infection. Adv Intern Med. 1952;5:165. [PubMed] [Google Scholar]
- 4.Dancis A, Yuan D S, Haile D, Askwith C, Eide D, Moehle C, Kaplan J, Klausner R D. Molecular characterization of a copper transport protein in S. cerevisiae: an unexpected role for copper in iron transport. Cell. 1994;76:393–402. doi: 10.1016/0092-8674(94)90345-x. [DOI] [PubMed] [Google Scholar]
- 5.Deneer H G, Healey V, Boychuk I. Reduction of exogenous ferric iron by a surface-associated ferric reductase of Listeria spp. Microbiology. 1995;141:1985–1992. doi: 10.1099/13500872-141-8-1985. [DOI] [PubMed] [Google Scholar]
- 6.Dix D R, Bridgham J T, Broderius M A, Byersdorfer C A, Eide D J. The FET4 gene encodes the low affinity Fe(II) transport protein of Saccharomyces cerevisiae. J Biol Chem. 1994;269:26092–26099. [PubMed] [Google Scholar]
- 7.Eide D, Davis-Kapan S, Jordan I, Sipe D, Kaplan J. Regulation of iron uptake in Saccharomyces cerevisiae. J Biol Chem. 1992;267:20774–20781. [PubMed] [Google Scholar]
- 8.Emery H S, Shelburne C P, Bowman J P, Fallon P G, Schultz C A, Jacobson E S. Genetic study of oxygen resistance and melanization in Cryptococcus neoformans. Infect Immun. 1994;62:5694–5697. doi: 10.1128/iai.62.12.5694-5697.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gordeuk V, Thuma P, Brittenham G, McLaren C, Parry D, Backenstose A, Biemba G, Msiska R, Holmes L, McKinley E, Vargas L, Gilkeson R, Poltera A A. Effect of iron chelation therapy on recovery from deep coma in children with cerebral malaria. N Engl J Med. 1992;327:1473–1477. doi: 10.1056/NEJM199211193272101. [DOI] [PubMed] [Google Scholar]
- 10.Jacobson E S, Emery H S. Catecholamine uptake, melanization, and oxygen toxicity in Cryptococcus neoformans. J Bacteriol. 1991;173:401–403. doi: 10.1128/jb.173.1.401-403.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacobson E S, Hong J D. Redox buffering by melanin and Fe(II) in Cryptococcus neoformans. J Bacteriol. 1997;179:5340–5346. doi: 10.1128/jb.179.17.5340-5346.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobson E S, Petro M J. Extracellular iron chelation in Cryptococcus neoformans. J Med Vet Mycol. 1987;25:415–418. [PubMed] [Google Scholar]
- 13.Jacobson E S, Vartivarian S E. Iron assimilation in Cryptococcus neoformans. J Med Vet Mycol. 1992;30:443–450. [PubMed] [Google Scholar]
- 14.Johnson W, Varner L, Poch M. Acquisition of iron by Legionella pneumophila: role of iron reductase. Infect Immun. 1991;59:2376–2381. doi: 10.1128/iai.59.7.2376-2381.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaplan J, O’Halloran T V. Iron metabolism in eukaryotes: Mars and Venus at it again. Science. 1996;271:1510–1512. doi: 10.1126/science.271.5255.1510. [DOI] [PubMed] [Google Scholar]
- 16.LeSuisse E, Raguzzi F, Crichton R R. Iron uptake by the yeast Saccharomyces cerevisiae: involvement of a reduction step. J Gen Microbiol. 1987;133:3229–3236. doi: 10.1099/00221287-133-11-3229. [DOI] [PubMed] [Google Scholar]
- 17.Levitz S M. The ecology of Cryptococcus neoformans and the epidemiology of cryptococcosis. Rev Infect Dis. 1991;13:1163–1169. doi: 10.1093/clinids/13.6.1163. [DOI] [PubMed] [Google Scholar]
- 18.Marschner H, Romheld V, Kissel M. Different strategies in higher plants in mobilization and uptake of iron. J Plant Nutr. 1986;9:695–713. [Google Scholar]
- 19.Mickelsen P A, Sparling P F. Ability of Neisseria gonorrhoeae, Neisseria meningitidis, and commensal Neisseria species to obtain iron from transferrin and iron compounds. Infect Immun. 1981;33:555–564. doi: 10.1128/iai.33.2.555-564.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morrissey J A, Williams P H, Cashmore A M. Candida albicans has a cell-associated ferric-reductase activity which is regulated in response to levels of iron and copper. Microbiology. 1996;142:485–492. doi: 10.1099/13500872-142-3-485. [DOI] [PubMed] [Google Scholar]
- 21.Neilands J B. Microbial iron compounds. Annu Rev Biochem. 1981;50:715–731. doi: 10.1146/annurev.bi.50.070181.003435. [DOI] [PubMed] [Google Scholar]
- 22.Nyhus K J, Jacobson E S. Ferric reduction by Cryptococcus neoformans. Infect Immun. 1996;65:434–438. doi: 10.1128/iai.65.2.434-438.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olsen R A, Brown J C, Bennett J H, Blume D. Reduction of Fe3+ as it relates to Fe chlorosis. J Plant Nutr. 1982;5:433–445. [Google Scholar]
- 24.Pidcock K A, Wooten J A, Daley B A, Stull T L. Iron acquisition by Haemophilus influenzae. Infect Immun. 1988;56:721–725. doi: 10.1128/iai.56.4.721-725.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robins-Browne R M, Prpic J K. Effects of iron and desferrioxamine on infections with Yersinia enterocolitica. Infect Immun. 1985;47:774–779. doi: 10.1128/iai.47.3.774-779.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stearman R, Yuan D S, Yamaguchi-Iwai Y, Klausner R D, Dancis A. A permease-oxidase complex involved in high-affinity iron uptake in yeast. Science. 1996;271:1552–1557. doi: 10.1126/science.271.5255.1552. [DOI] [PubMed] [Google Scholar]
- 27.Vartivarian S E, Cowart R E, Anaissie E J, Tashiro T, Sprigg H A. Iron acquisition by Cryptococcus neoformans. J Med Vet Mycol. 1995;33:151–156. [PubMed] [Google Scholar]
- 28.Weinberg E D. Iron and infection. Microbiol Rev. 1978;42:45–66. doi: 10.1128/mr.42.1.45-66.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zugar A, Louis E, Holzman R S, et al. Cryptococcal disease in patients with the acquired immunodeficiency syndrome: diagnostic features and outcome of treatment. Ann Intern Med. 1986;104:234–240. doi: 10.7326/0003-4819-104-2-234. [DOI] [PubMed] [Google Scholar]