Abstract
Following invasion into the host cell, the protozoan Toxoplasma gondii secretes a variety of proteins that modify the parasitophorous vacuole. Within the vacuole, the 28-kDa dense granule protein known as GRA2 is specifically targeted to the tubulovesicular network which forms connections with the vacuolar membrane. To investigate the importance of GRA2, we derived from strain RH a mutant T. gondii line in which GRA2 was disrupted by replacement with the marker Ble (selecting for phleomycin resistance). The Δgra2 mutant invaded and grew normally in both fibroblasts and macrophages in vitro; however, it was less virulent during acute infection in mice. The survival rate of mice inoculated with Δgra2 was significantly higher; some infected mice survived the acute infection, whereas all mice infected with the wild-type strain RH succumbed to early death. Chronic infection by Δgra2 was detected by positive serology, immunohistochemical detection of parasites and cysts in the brain, and reisolation of parasites by bioassay at 6 weeks postinfection. Thus, absence of GRA2 partially attenuates the virulence of T. gondii during the acute phase of infection and allows for establishment of chronic infection by the otherwise highly virulent RH strain. These results establish that GRA2 plays an important role during in vivo infection and provide a potential model for examining acute pathogenesis by T. gondii.
Toxoplasmosis is a widespread infection caused by the intracellular protozoan T. gondii. The natural infection is typified by a brief acute phase followed by an asymptomatic chronic infection. In the highly susceptible mouse host, acute infection by the virulent strain RH is characterized by a rapid dissemination of the parasite to the lungs and ultimately to the central nervous system, leading to death by pneumonia or encephalitis within 10 to 12 days postinoculation (13). In more resistant hosts, acute infection is generally benign and progresses rapidly into a chronic phase characterized by parasite encystment in various tissues, including the central nervous system and muscles (17). Tissue cysts cause little pathology and stimulate a protective immunity against reinfection, presumably by occasional cyst rupture and release of parasite antigens (18). Immunity to toxoplasmosis is mediated by a vigorous cell-mediated response that relies on production of interleukin-12 (IL-12), gamma interferon (IFN-γ), and tumor necrosis factor alpha (TNF-α) (12, 19, 23). In immunodeficient patients, failure of the immune system to control the balance between encystment and reactivation, especially in the central nervous system, often leads to toxoplasmic encephalitis (27).
Within the infected cell, the rapidly multiplying vegetative forms of T. gondii (tachyzoites) reside within a fusion-resistant compartment called the parasitophorous vacuole (24, 30). Following invasion of the host cell, the parasitophorous vacuole is modified by secretion of parasite proteins from several prominent organelles called rhoptries and dense granules (8, 16). Additionally, the parasite secretes multilamellar vesicles from its posterior end which assemble into a network of membranous tubules connected to the parasitophorous vacuole membrane (39). At least eight distinct dense-granule proteins (GRA proteins) are released within the vacuole, where they preferentially associate with the vacuolar membrane or with the network membrane (reviewed in reference 9).
The GRA proteins were first described as components of the excretory/secretory antigens released by the parasite when incubated with serum (11). These proteins may be important protective antigens since they are secreted in abundance and are major components of both the vacuole surrounding tachyzoites and the cyst wall surrounding the more slowing growing bradyzoites (reviewed in reference 9). GRA2 is expressed by both tachyzoite and bradyzoite stages, and immunization with purified GRA2 has been shown to induce both a vigorous antibody response and T-cell response during the chronic infection (5, 31, 34). Immunological responses to GRA2 may be important in controlling infection, as immunization with the native protein partially protects mice against acute toxoplasmosis (36).
To investigate the role of GRA2 in intracellular survival and during infection in mice, we constructed a T. gondii mutant line that lacks expression of GRA2 and analyzed the in vitro and in vivo characteristics of this knockout mutant.
MATERIALS AND METHODS
Plasmids.
The pGRA2/Ble/GRA2(8.9) construct was derived from plasmid pGRA2/Ble (29), which contains 810 bp of the 5′ flanking region of GRA2 in frame with the phleomycin resistance (Ble) selectable marker and followed by 425 bp of the 3′ region from the SAG1 gene (7). The PacI-PstI fragment corresponding to the 3′ region of SAG1 in plasmid pGRA2/Ble (29) was replaced with a 400-bp fragment corresponding to the 3′ untranslated region of GRA2 that was amplified by PCR (sense oligonucleotide, 5′ TGCTTAATTAAGACTACGACGAAAGTGATGCGC 3′; antisense oligonucleotide, 5′ TGCGGATCCGTCGACTGGAACTACGGTGTTTC 3′) from a cosmid clone containing GRA2 (provided by Steve Parmley, Palo Alto Medical Foundation, Palo Alto, Calif.). The 3′ region of GRA2 was further extended by cloning of a 2-kb SalI-HindIII fragment isolated from plasmid pG43 (28) into the SalI-XbaI sites, thus providing a 2.4-kb 3′ GRA2 region downstream of the ble gene. The 5′ GRA2 region was also extended by restriction cloning of a 2.4-kb fragment from the cosmid containing GRA2 into the PstI-XhoI sites of the construct, thus giving a 3.2-kb 5′ GRA2 region upstream of ble.
Parasite manipulations.
T. gondii tachyzoites of strain RH (obtained from E. Pfefferkorn, Dartmouth College, Hanover, N.H.) were propagated in human foreskin fibroblast (HFF) monolayers in Dulbecco’s modified minimal essential medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 1 mM glutamine, 10 mM HEPES, and 10 μg of gentamicin/ml.
Parasites were transformed by electroporation using 1.6 × 108 cells combined with 50 μg of circular plasmid in the first transfection experiment or 300 μg of circular plasmid in the second transfection, selected for phleomycin resistance, and cloned by limiting dilutions in 96-well microtiter plates (29). Clones that no longer expressed GRA2 were identified on a dot blot probed with the anti-GRA2 monoclonal antibody (MAb) TG17-179 (10). Knockout clones were complemented by cotransfection of 107 tachyzoites with 100 μg of the circular plasmid pG43 mixed with 10 μg of the circular plasmid TUB5/CAT (provided by D. Soldati, Zentrum für Molekulare Biologie des Universität Heidelberg, Heidelberg, Germany) and selected for chloramphenicol resistance as described previously (25).
Genetic analyses.
T. gondii genomic DNA was digested with either NcoI or NsiI (New England Biolabs Inc., Beverly, Mass.), electrophoresed in agarose gels, transferred to nylon membranes, and hybridized at high stringency with specific probes as described previously (29). The probe corresponding to the NsiI-PacI fragment of the ble gene was amplified by PCR (sense oligonucleotide, 5′ TGCATGCATGACCAAGCGACGCCCAAC 3′; antisense oligonucleotide, 5′ GCATTAATTAAGAGATGCCTGCAAGCAATTC 3′) from the pSAG1/Ble template (29). A probe corresponding to the 544-bp SalI-BstEII fragment within the GRA2 gene was purified by restriction digest from pG43; it spans most of the GRA2 coding sequence and intron (28). Probes were labeled with [α-32P]dCTP by using a random primed labeling kit (Boehringer Mannheim, Indianapolis, Ind.).
Western blotting.
Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose by semidry electrophoresis. Membranes were blocked, incubated with the anti-GRA2 MAb TG17-179 (10), rinsed, and incubated with peroxidase-conjugated goat secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, Pa.). A rabbit anti-T. gondii actin serum (14) was used as an internal control of the quantity of proteins loaded in each lane. Signals were detected by using an enhanced chemiluminescence system (Pierce Chemical Co., Rockford, Ill.).
Uracil incorporation assay.
T. gondii growth in HFF cells was measured by selective incorporation of [3H]uracil (ICN Pharmaceuticals Inc., Costa Mesa, Calif.) (33). HFF monolayers grown in 24-well plates were inoculated in triplicate with freshly lysed tachyzoites resuspended in 1 ml of minimum essential medium containing 3% dialyzed FBS, 1 mM glutamine, 10 mM HEPES, and 10 μg of gentamicin/ml and supplemented with 1 μCi of [3H]uracil 24 h before harvesting. Monolayers infected with 2 × 106, 2 × 104, or 5 × 103 parasites and lysed 24, 72, or 120 h, respectively, after inoculation; nucleic acids were precipitated with trichloroacetic acid, and incorporation of the radioactive metabolite was assessed by scintillation spectroscopy. Data are expressed as the means of two separate experiments ± standard errors of the means.
Macrophage culture, activation, and invasion assays.
Bone marrow-derived monocyte cultures were isolated from the femurs of CD1 outbred mice (Charles River Laboratories, Wilmington, Mass.) and grown on bacterial-grade petri dishes (Sarstedt, Newton, N.C.) for 7 days in DMEM–20% L929 cell-conditioned medium–10% FBS–10 μg of gentamicin/ml. For use in experiments, 1- to 2-week-old macrophages were dislodged with cold calcium- and magnesium-free phosphate-buffered saline and plated on LabTek slides (Fisher, Pittsburgh, Pa.) at 2 × 105 cells/ml in fresh medium overnight to establish monolayers at 50% confluency. Macrophages were activated by incubation with recombinant mouse IFN-γ (60 U/ml; Gibco BRL, Gaithersburg, Md.) and lipopolysaccharide (LPS; 20 ng/ml; Sigma Chemical Co., St. Louis, Mo.) for 16 h prior to T. gondii infection. For invasion assays, 3 × 106 parasites were delivered to macrophage monolayers in 0.15 ml of invasion medium by settling for 1 min in a 37°C water bath. Following the 1-min pulse, monolayers were washed extensively to remove nonadherent parasites and recultured in fresh medium without IFN-γ or LPS. Monolayers were fixed after 1 h and stained with rabbit anti-P30 to identify parasites and anti-LAMP1 (MAb 1D4B) to evaluate lysosome fusion. The number of parasites per 100 macrophages was determined from three or more counts of 300 to 500 host cells, and the percentage of LAMP1-positive vacuoles was determined from three or more counts of approximately 25 vacuoles each. Parallel cultures of nonactivated and activated macrophages were fixed at 20 or 40 h postinvasion to evaluate parasite replication. The average number of parasites per vacuole was determined from three or more counts of 300 to 500 host cells. Results are reported as means ± standard deviations from a representative experiment.
Restriction fragment length polymorphism (RFLP) identification and genetic mapping of the GRA2 gene.
A 1,100-bp fragment encompassing majority of the GRA2 gene and 3′ untranslated region was amplified by PCR using sense (5′ACGGCCATGGCCGAGTTTTCCGGAGTTGTTAAC3′) and antisense (5′TGCGGATCCGTCGACTGGACCTACGGTGTTTG3′) oligonucleotides. The GRA2 locus was amplified from genomic DNAs of the type II strain PLK and the type III strain CEP, digested with restriction endonucleases, electrophoresed in agarose gels, and stained with ethidium bromide.
In vivo experiments.
Female outbred CD1 mice (Charles River) were inoculated by intraperitoneal (i.p.) injection of 101 freshly harvested tachyzoite parasites. One month postinfection, the number of survivors was recorded and their T. gondii serology was tested by Western blotting against an RH tachyzoite lysate. For statistical analyses, the χ2 test was used to calculate the survival rate of mice inoculated with wild-type strain RH (expected) versus the survival rate of mice inoculated with the knockout mutants (observed) (df = 1). Seropositive survivors were sacrificed 6 weeks after injection, and one half of the brain was homogenized and inoculated into a single female outbred CD1 mouse. After 6 to 8 days, the peritoneal fluid was harvested and inoculated onto HFF monolayers to recover parasites in culture (22). The other half of the brain was fixed in 4% formalin in PBS, rinsed, dehydrated, and embedded in paraffin. Serial sections were rehydrated, stained with either a rabbit serum recognizing the bradyzoite-specific antigen BAG5 (dilution of 1:1,000; provided by L. Weiss, Albert Einstein College of Medicine, New York, N.Y.) or the rabbit serum anti-SAG1 (dilution of 1:1,000; provided by L. H. Kasper, Dartmouth Medical School, Hanover, N.H.), detected by using a Vectastain Elite ABC kit (Vector Laboratories, Burlingame, Calif.), and counterstained with hematoxylin. Sections of a mouse brain chronically infected with the type III strain CEP (22) were used as positive controls for the presence of cysts. Slides were mounted in PBS-glycerol (50/50) and examined on a Zeiss Axioscope.
RESULTS
Construction of the Δgra2 knockout and restoration by complementation.
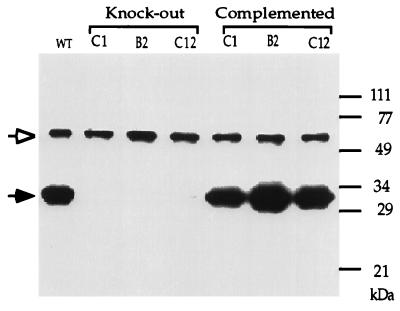
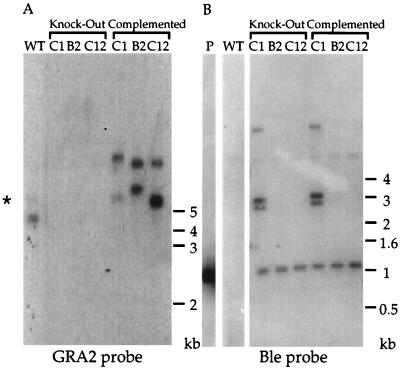
The GRA2 gene exists as a single copy within the haploid genome of T. gondii (28). To disrupt this gene by homologous recombination, a plasmid containing the positive selectable marker Ble flanked by 3.2 kb of the upstream and 2.4 kb of the downstream genomic regions of the GRA2 gene was electroporated into freshly harvested tachyzoites (Fig. 1). Eighteen of 117 Ble-resistant transformants isolated from two independent transfections failed to express GRA2 when analyzed by Western blotting (Fig. 2 and data not shown). Three clones were arbitrarily selected for further analysis: clone C12 from the first transfection and clones C1 and B2 from the second transfection. A genomic probe corresponding to the coding sequence of GRA2 including the intron (Fig. 1) failed to hybridize to DNA from these three clones, demonstrating that targeted disruption of the open reading frame had occurred (Fig. 3A). Hybridization with a probe corresponding to the ble coding sequence showed that clones B2 and C12 had integrated a single copy of the ble gene whereas clone C1 had integrated four copies, one at the GRA2 locus and three extra copies, presumably at different sites in the genome (Fig. 3B). Although the selection was performed on the entire pool of electroporated parasites and therefore did not prevent isolation of siblings, the genotypic differences observed between clones C1 and B2 indicated that they originated from independent events (Fig. 3B). Collectively, these results establish that all three clones represent knockouts of the GRA2 gene, which are hence designated genotypically as Δgra2 and phenotypically as Gra2−.
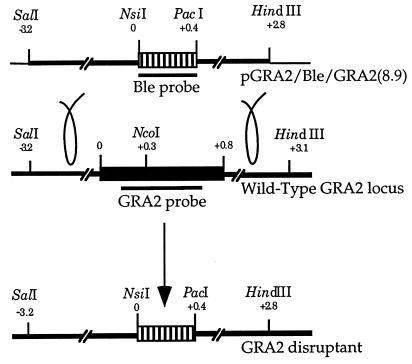
FIG. 1.
Diagram of the targeting construct and genomic locus involved in construction of Δgra2 knockout parasites. Plasmid pGRA2/Ble/GRA2(8.9), used to transform the parental RH line, contains 3.2 kb of 5′ GRA2 flanking sequence and 2.4 kb of 3′ GRA2 flanking sequence. Striped boxes represent the Ble selectable marker; the black box represents the GRA2 coding sequence; thick black lines represent the 5′ and 3′ flanking sequences of the GRA2 gene; thin black lines symbolize the plasmid vector pBluescript; loops symbolize the double crossover between the plasmid and the chromosomal GRA2 locus. Probes used to hybridize the Southern blots in Fig. 3 are represented by the thick black lines beneath the coding sequences.
FIG. 2.
Western blot analysis showing that the knockout lines failed to express GRA2 and that complementation restored expression of the protein. WT, wild-type parental T. gondii strain RH. Knockout clones C1 and B2 originated from transformation using 300 μg of plasmid DNA, whereas clone C12 originated from transformation using 50 μg of plasmid DNA. T. gondii lysates corresponding to approximately 5 × 105 parasites were loaded in each lane. The membrane was probed with both MAb Tg17-179 against GRA2 (solid arrowhead) and a rabbit serum against T. gondii actin (open arrowhead) as an internal control of the quantity of protein loaded in each lane.
FIG. 3.
Genomic analysis of the Δgra2 knockout and complemented clones. (A) Southern blot analysis showing that the knockout clones lack the GRA2 open reading frame, in contrast to the wild type (WT). The knockout clones were complemented by nonhomologous integration of plasmid pG43 into the T. gondii genome. Genomic DNA was digested with NcoI and hybridized with the GRA2 genomic fragment (SalI-BstEII) of 544 bp corresponding to most of the GRA2 open reading frame. Two fragments (a 4.5-kb fragment and a faintly hybridizing 5.4-kb fragment indicated by *) are detected in the wild type; in the complemented clones these fragments are of different sizes, reflecting their integration into different genomic locations. (B) Knockout clones B2 and C12 were derived from a single integration of the ble gene at the GRA2 locus, whereas knockout clone C1 integrated three additional copies of the selectable marker. Genomic DNA was digested with NsiI and hybridized with a 480-bp fragment (NsiI-PacI) corresponding to the ble open reading frame. Digestion of plasmid pGRA2/Ble/GRA2(8.9) (P) liberates a hybridizing fragment of 1 kb, due to the presence of an NsiI site in the 3′ flanking region of GRA2.
Restoration of GRA2 expression was accomplished by transforming knockout parasites with plasmid pG43, which contains the entire coding sequence of the GRA2 gene flanked by 2.4 kb of each of the 5′ and 3′ flanking regions. These complemented transformants were shown by Western blotting to reexpress GRA2 at levels comparable to those for the wild-type (Fig. 2). Genomic Southern analysis of the complemented knockout clones confirmed that integration of the GRA2 gene had occurred elsewhere in the genome, leaving the Ble cassette unaltered (Fig. 3B).
Lack of GRA2 does not alter the in vitro T. gondii growth rate.
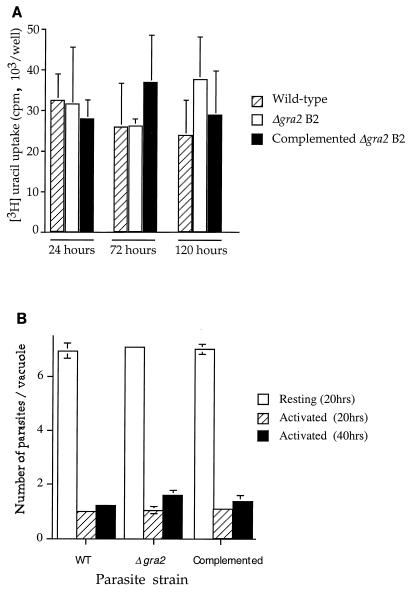
Preliminary results indicated that the three independent knockout clones C1, B2, and C12 grew similarly in HFF cells (data not shown). Subsequently, we chose the Δgra2 clone B2, as well as a complemented clone derived from this knockout but which expressed wild-type levels of GRA2, for use in both in vitro and in vivo experiments reported in this study. To determine if the lack of GRA2 had an effect on growth rate, proliferation was monitored by selective [3H]uracil incorporation by the parasite (33). Wild-type strain RH, Δgra2 clone B2, and the complemented clone showed similar levels of [3H]uracil incorporation when assayed 24, 72, or 120 h after inoculation (Fig. 4A). Although the incorporation levels at different time points appear equal in Fig. 4A, they actually reflect exponential growth, as the input inoculum was varied to prevent overgrowth during the assay (see Materials and Methods).
FIG. 4.
Proliferation of wild-type and of Δgra2 mutant parasites in vitro. (A) The wild type, mutant, and complemented clone showed similar levels of [3H]uracil incorporation at 24, 72, and 120 h after inoculation of HFF cells. Monolayers were challenged with 2 × 106, 2 × 104, and 5 × 103 parasites, pulsed with [3H]uracil for the final 24 h, and harvested at 24, 72, and 120 h, respectively. (B) Growth in macrophages as determined by counting the average number of parasites per vacuole at 20 or 40 h postinoculation. Similar growth rates were observed for the wild type, mutant, and complemented clone in normal macrophages, while activated macrophages suppressed their growth to similar extents.
We also examined the survival of the mutant in both normal and activated bone marrow-derived macrophages cultured in vitro. The wild type, Δgra2 clone, and complemented clone showed the same level of invasion into normal macrophages, as determined by both counting the number of parasites per 100 cells and calculating the average level of lysosome fusion (approximately 5% in all groups) (data not shown). These results indicate the Δgra2 mutant is able to invade and survive normally in macrophages during the first hour after infection. We also examined the proliferation of the mutant and wild-type strains in normal and activated macrophages. Similar levels of replication of the wild type, mutant, and complemented clone were detected in normal macrophages, and they were equally suppressed in activated macrophages (Fig. 4B). Taken together, these results indicate that lack of GRA2 did not alter the in vitro growth characteristics of T. gondii.
Lack of GRA2 causes partial attenuation of RH strain.
Intraperitoneal infection of mice with the type I strain RH is always lethal within 8 to 12 days, regardless of the mouse strain used (22). Consequently, the establishment of chronic infection and encystment of the parasite in mouse tissues does not occur with strain RH in the absence of chemotherapy. At a low inoculum (101 or fewer tachyzoites), some mice survive RH challenge; however, this is presumably due to the low viability of the inoculum. Inoculation with 104 or fewer dead tachyzoites is insufficient to induce seroconversion due to the low antigen dose (data not shown). Therefore, a positive serology in mice surviving inoculation was used as an indicator of infection.
To investigate if lack of GRA2 had any effect on T. gondii virulence, CD1 mice were inoculated i.p. with 101 tachyzoites, and survivors were tested for anti-T. gondii serology 1 month after inoculation. Inoculation with the wild-type strain RH resulted in death of all but 12 of 45 animals (27% survival) (Table 1). In marked contrast, 23 (51%) of 45 mice inoculated with the B2 knockout clone survived (Table 1). Complementation of the B2 clone restored the virulence of the parasites, which again exhibited a higher rate of mortality (17% survival) (Table 1). As expected, none of the 12 mice surviving RH challenge had been infected, as confirmed by their negative serology, while somewhat surprisingly, 10 of those surviving inoculation with the B2 knockout clone were serologically positive (Table 1). Importantly, none of the five mice surviving inoculation with the complemented B2 clone had a positive T. gondii serology (Table 1). Despite the fact that none of the mice surviving inoculation with the C12 clone were serologically positive, this clone was also significantly less virulent, with 12 (48%) of 25 mice surviving inoculation (Table 1). The increased survival of mice inoculated with the B2 or C12 clone was not due to overall lower viability of these isolates, as confirmed by their ability to form plaques on monolayers that was indistinguishable from the ability of wild-type strain RH (data not shown). Collectively, these results indicate that the absence of GRA2 decreases acute virulence of T. gondii in mice such that animals often survive an otherwise lethal infection with strain RH.
TABLE 1.
Disruption of GRA2 allows mice to survive acute infection with T. gondii RH
| T. gondii line | Survivorsa | Seropositivity of survivorsb |
|---|---|---|
| Wild type (RH) | 12/45 (27) | 0/12 |
| gra2 B2 knockout | 23/45 (51)c | 10/23 |
| gra2 C12 knockout | 12/25 (48)c | 0/12 |
| Complemented B2 clone | 6/35 (17) | 0/6 |
Numerators represent numbers of surviving mice 4 weeks after inoculation; denominators represent the total number of mice inoculated in the course of four separate experiments; numbers in parentheses represent the percentage of surviving mice.
Numerators represent numbers of surviving mice which were positive for T. gondii serology; denominators represent the total number of mice surviving in the course of four separate experiments.
Statistically significant difference versus wild-type strain RH or the complemented clone (P < 0.05).
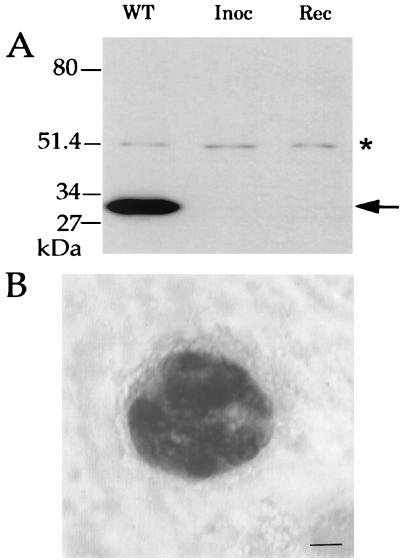
While the above results indicate that Δgra2 is capable of causing a nonlethal infection, they do not establish if surviving mice had cleared the parasite or remained chronically infected. To determine if the Δgra2 clone B2 was capable of forming latent infection in mice, the brains from four seropositive mice originally inoculated with the B2 clone were isolated 6 weeks after inoculation and separated into two parts. One part of each brain was homogenized and inoculated into the peritoneal cavity of a CD1 mouse; peritoneal exudate was harvested 8 days later and cultured in vitro. Parasites were recovered from one of the four seropositive mice, while parasites were not recovered from the remaining three. Western blot analysis confirmed that these recovered parasites did not express GRA2 (Fig. 5A). Restriction analysis of the SAG1 gene amplified from their genomic DNA showed that they contained the type I allele (22), confirming they were not due to contamination with a genetically distinct, less virulent lineage (data not shown).
FIG. 5.
(A) Western blot analysis of parasites recovered from a mouse chronically infected with Δgra2 parasites. Recovered parasites (Rec) lacked expression of GRA2, as did the original knockout clone that was injected (Inoc) and the wild-type line (WT). The membrane was probed with both MAb Tg17-179 against GRA2 (solid arrowhead) and a rabbit serum against T. gondii actin (asterisk) as an internal control of the quantity of protein in each lane. (B) Immunohistochemical staining of BAG5 in a cyst-like structure within the brain of a mouse chronically infected with Δgra2. Scale bar: 3 mm.
Following acute infection, T. gondii differentiates into a slow-growing cyst stage called bradyzoite that expresses a number of stage-specific antigens, including the small heat shock protein BAG5 (2, 32). To evaluate the presence of chronic infections, the remaining portion of the brains from the four seropositive mice were sectioned and immunohistochemically stained for the presence of either the bradyzoite-specific antigen BAG5 or the tachyzoite-specific antigen SAG1. BAG5-positive cyst-like structures and numerous immunoreactive BAG5-positive foci of parasites were detected in sections of brain examined from the single mouse that was positive by bioassay (Fig. 5B). No SAG1-reactive parasites were detected in approximately 20 sections of this same brain that were stained in parallel (data not shown). No BAG5- or SAG1-positive cells were detected in approximately 20 sections examined each for the remaining brains of the three seropositive mice (data not shown). Collectively, these results demonstrate that infection with the Δgra2 knockout clone is capable of leading to chronic infection and formation of cyst-like structures in the brains of mice, although some surviving mice appear to clear the infection.
Mapping of GRA2 to chromosome X.
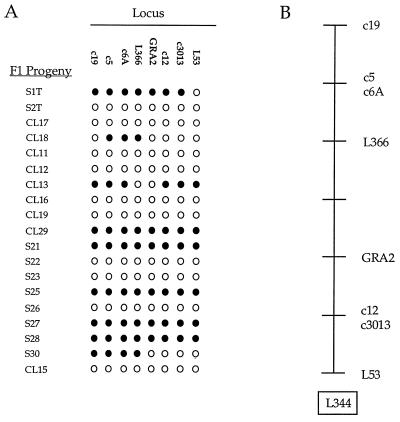
Previous studies have indicated that a locus associated with acute virulence lies on chromosome VIII in T. gondii (22). To map the chromosomal location of GRA2, genomic DNAs from strains CEP and PLK were amplified and screened with restriction enzymes to detect polymorphisms at the GRA2 locus (see Materials and Methods). An RFLP was identified by restriction digestion with the enzyme MnlI: CEP contains an additional site that cleaves a 500-bp fragment observed in PLK into two smaller fragments of approximately 150 and 350 bp (data not shown). We then analyzed the segregation of this MnlI RFLP among 19 recombinant progeny from a previously described genetic cross (38). The resulting segregation pattern for GRA2 alleles indicated it is located on chromosome X (Fig. 6A). Because additional crossovers are detected by inclusion of GRA2, the map of chromosome X has been redrawn to attain the most parsimonious arrangement of markers on this chromosome (Fig. 6B).
FIG. 6.
Segregation pattern of GRA2 and adjacent genes located on chromosome X as determined by RFLP analysis of 19 recombinant progeny. Open circles represent type III alleles; closed circles represent type II alleles. (B) Linear map of chromosome X depicting the most parsimonious arrangement of markers. One node = 5.25 map units.
DISCUSSION
We report here the construction and phenotypic analysis of a T. gondii knockout mutant that lacks expression of the dense-granule protein GRA2. This mutant was obtained by gene replacement of the GRA2 open reading frame with a single copy of the Ble selectable marker, as confirmed by Southern blotting. Western blot analysis confirmed that the recombinants failed to express GRA2, a 28-kDa glycoprotein that belongs to a family of parasite proteins stored in electron-dense organelles and secreted into the parasitophorous vacuole following invasion. Although dense-granule proteins are suspected to play a role in intravacuolar survival of the parasite, recovery of Δgra2 mutants which displayed normal growth indicates that the GRA2 protein is not essential under normal culture conditions in vitro. The GRA proteins are also expressed during the chronic phase of infection, where they are incorporated into the cyst wall (41). Importantly, phenotypic analysis of the Δgra2 mutant showed that despite being able to differentiate normally, the mutant exhibited decreased virulence in mice, allowing establishment of chronic infection by an otherwise highly virulent lineage.
Intraperitoneal inoculation with tachyzoites of the parental strain RH (type I) leads to an acute infection that is lethal within 12 days postinoculation in the mouse model (22). This phenotype of high acute virulence is shared by a subset of T. gondii strains that comprise a clonal subpopulation designated type I strains (37). The basis of their high acute virulence is incompletely understood but presumably results from the relatively few genetic differences between this clonal grouping and the closely related but distinct types II and III strains, which are considerably less virulent in mice (21, 22). When inoculated i.p. with wild-type strain RH, surviving mice are observed only with a very low inoculum and they remain seronegative, indicating they were never infected, presumably due to less than 100% viability of the parasites (22). Similarly, in the present study, mice infected with strain RH died from acute infection, while mice surviving inoculation with strain RH remained seronegative. In contrast, inoculation with similar low doses of the Δgra2 B2 clone led to seropositive survivors, indicating that acute infection had occurred but was controlled. It is not known if all 10 surviving mice that were seropositive harbored chronic infection, as parasites were reisolated from only one of four animals that were analyzed by bioassay. Furthermore, direct evidence of the presence of bradyzoites expressing BAG5 was obtained only for this bioassay-positive mouse. Previous studies have indicated that bioassay is the most sensitive and reliable means of detecting chronic infections (15). While we cannot rule out the possibility of chronic infections that were below the level of detection, the absence of recoverable parasites in three of four mice subjected to bioassay suggests that the infection was self-limiting or eliminated by the immune response in these animals. A second knockout clone, C12, also was generated by a single insertion/replacement event to delete the GRA2 locus. Like the B2 clones, it was significantly less virulent in terms of acute deaths; however, it did not result in seropositive survivors. The reasons for the differences between B2 and C12 clones remain unknown but may relate to individual variation in the parental population of RH strain used to generate the mutants. Nonetheless, both the increased survival and the seropositivity of survivors inoculated with B2 were a result of the absence of GRA2 since the complemented clone was fully restored to wild-type virulence in both attributes.
Strain RH does not normally form cysts spontaneously in mice in the absence of chemotherapy to prevent death. However, this strain can be induced to express bradyzoite-specific proteins in vitro by altering the cell culture conditions (40), and it forms cysts in resistant hosts such as rats (26). We observed a similar frequency of stage conversion to parasites expressing the bradyzoite-specific antigen BAG5 in the Δgra2 mutant and the wild-type strain RH under in vitro conditions that favor bradyzoite formation (unpublished data). Consequently, Δgra2 parasites do not appear to have a defect in stage conversion.
BAG5 is a low-molecular-weight heat shock protein that is expressed early in the differentiation of bradyzoites in vitro and during cyst formation in vivo (4). Mutant parasites in which BAG5 expression has been disrupted grow normally in vitro and in vivo, indicating it is not essential during infection in mice (3). Here we have used BAG5 as a positive marker for differentiation to bradyzoites and demonstrated that the Δgra2 clone was capable of forming cysts in vivo, as recognized by BAG5-positive staining of sections from infected mouse brain. These same cysts failed to stain with antibodies for the tachyzoite antigen SAG1, indicating they had fully differentiated into bradyzoites. Consequently, the absence of GRA2 which resulted in greater survival of mice following acute infection does not appear to influence differentiation.
The parasite genetic factors that control acute virulence are largely unknown, although preliminary evidence implicates a locus near SAG1 (the gene encoding the major tachyzoite surface protein) on chromosome VIII (22). This SAG1-associated locus mediates a strain-specific difference in pathogenicity between the acutely virulent type I strains like RH and type II and III strains which are nonvirulent. The contribution of GRA2 to pathogenesis is likely unrelated to this phenomenon, since there are no apparent strain-specific, virulence-associated polymorphisms in GRA2 and it maps to chromosome X rather than chromosome VIII. GRA2 is secreted into the parasitophorous vacuole during tachyzoite growth and is a major component of the cyst wall during bradyzoite growth, suggesting that it may play a role in acquisition of nutrients from the host cell. Although no apparent difference in growth rate was observed in vitro, increased survival of mice challenged with Δgra2 suggests the growth rate and/or dissemination of this mutant is restricted in vivo. Comprehensive studies on the pathogenesis of the gram-negative bacterial pathogens of the genus Salmonella have identified numerous examples of niche-specific nutrient limitations that become apparent in vivo, despite apparently normal growth in vitro (20). Thus, the observed role of GRA2 in survival within the host may depend on specific requirements for nutrient acquisition that are unique to the in vivo environment(s). Infection of mice with the virulent RH strain of T. gondii is normally characterized by an early involvement of lungs and subsequent dissemination to the central nervous system (13). However, the actual cause of death in these animals remains undefined and could occur from acute inflammatory responses in one or more tissues. Further studies on in vivo infections by Δgra2 parasites will be necessary to investigate the basis of its decreased virulence.
Despite the previous findings that immunization with purified GRA2 induces an antigen-specific protective response (36), its role during the acute infection, where early responses are controlled by innate immunity, has not been investigated. Parasite secretory proteins, in particular GRA proteins, constitute the major part of circulating antigens in the plasma of mice 24 h postinfection (1). Whether these antigens are actively secreted or the result of parasite lysis in the bloodstream during the acute infection is unknown, but their presence in the serum may be important during the early immune responses. The initial infection by T. gondii induces a cascade of proinflammatory cytokines, including IL-12, TNF-α, and IFN-γ, that collectively regulate resistance to acute toxoplasmosis (12, 19, 23). Several mechanisms of antitoxoplasmal activity, including cytokine activation of macrophages to kill or inhibit parasite growth, cytolysis of infected host cells, and antibody-mediated opsonization of parasites that leads to intracellular destruction, have been well described. The defect in Δgra2 parasites is not related simply to survival within macrophages, as similar rates of infection and growth were observed for both wild-type and mutant parasites in vitro. However, the increased survival of mice inoculated with Δgra2 null mutants suggest that the innate or acquired immune response may be more effective at controlling proliferation in vivo. Thus, it is possible that differences exist in the monokine/cytokine responses elicited by this mutant when it encounters macrophages or other immune effector cells. The survival of mice following initial infection by the B2 null mutant, which does not express GRA2, provides a model to study both innate and acquired immunity during infection by a virulent lineage of T. gondii.
ACKNOWLEDGMENTS
We thank M. F. Cesbron-Delauw, L. H. Kasper, S. Parmley, D. Soldati, and L. M. Weiss for providing reagents, Marinella Messina for the cloning of initial constructs of GRA2/Ble/GRA2, Amy Crawford for cell culture, and E. Labruyère-Dadaglio for fruitful discussions throughout the work.
This work was supported by National Institutes of Health grant AI 36629.
REFERENCES
- 1.Asai T, Kim T, Kobayashi M, Kojima S. Detection of nucleoside triphosphate hydrolase as a circulating antigen in sera of mice infected with Toxoplasma gondii. Infect Immun. 1987;55:1332–1335. doi: 10.1128/iai.55.5.1332-1335.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bohne W, Gross U, Ferguson D J P, Hessemann J. Cloning and characterization of a bradyzoite-specifically expressed gene (hsp30/bag1) of Toxoplasma gondii, related to genes encoding small heat-shock proteins of plants. Mol Microbiol. 1995;16:1221–1230. doi: 10.1111/j.1365-2958.1995.tb02344.x. [DOI] [PubMed] [Google Scholar]
- 3.Bohne W, Hunter C A, White M W, Ferguson D J P, Gross U. Targeted disruption of the bradyzoite-specific gene BAG1 does not prevent tissue cyst formation in Toxoplasma gondii. Mol Biochem Parasitol. 1998;92:291–301. doi: 10.1016/s0166-6851(97)00236-3. [DOI] [PubMed] [Google Scholar]
- 4.Bohne W, Wirsing A, Gross U. Bradyzoite-specific gene expression in Toxoplasma gondii requires minimal genomic elements. Mol Biochem Parasitol. 1997;85:89–98. doi: 10.1016/s0166-6851(96)02814-9. [DOI] [PubMed] [Google Scholar]
- 5.Brinkmann V, Remington J S, Sharma S. Vaccination of mice with the protective F3G3 antigen of Toxoplasma gondii activates CD4+ but not CD8+ cells and induces Toxoplasma specific IgG antibody. Mol Immunol. 1993;30:353–358. doi: 10.1016/0161-5890(93)90064-i. [DOI] [PubMed] [Google Scholar]
- 6.Brusca J S, Radolf J D. Isolation of integral membrane proteins by phase partitioning with Triton X-114. Methods Enzymol. 1994;228:182–193. doi: 10.1016/0076-6879(94)28019-3. [DOI] [PubMed] [Google Scholar]
- 7.Burg J L, Perlman D, Kasper L H, Ware P L, Boothroyd J C. Molecular analysis of the gene encoding the major surface antigen of Toxoplasma gondii. J Immunol. 1988;141:3584–3591. [PubMed] [Google Scholar]
- 8.Carruthers V B, Sibley L D. Sequential protein secretion from three distinct organelles of Toxoplasma gondii accompanies invasion of human fibroblasts. Eur J Cell Biol. 1997;73:114–123. [PubMed] [Google Scholar]
- 9.Cesbron-Delauw M F. Dense granule organelles of Toxoplasma gondii: their role in the host-parasite relationship. Parasitol Today. 1994;10:293–296. doi: 10.1016/0169-4758(94)90078-7. [DOI] [PubMed] [Google Scholar]
- 10.Charif H, Darcy F, Torpier G, Cesbron-Delauw M F, Capron A. Toxoplasma gondii: characterization and localization of antigens secreted from tachyzoites. Exp Parasitol. 1990;71:114–124. doi: 10.1016/0014-4894(90)90014-4. [DOI] [PubMed] [Google Scholar]
- 11.Darcy F, Deslee D, Santoro F, Charif H, Auriault C, Decoster A, Duquesne V, Capron A. Induction of a protective anti-body dependent response against toxoplasmosis by in vitro excreted/secreted antigens from tachyzoites of Toxoplasma gondii. Parasite Immunol. 1988;10:553–567. doi: 10.1111/j.1365-3024.1988.tb00242.x. [DOI] [PubMed] [Google Scholar]
- 12.Denkers E Y, Scharton-Kersten T, Gazzinelli R T, Yap G, Charest H, Sher A. Cell-mediated immunity to Toxoplasma gondii: redundant and required mechanisms revealed by studies in gene knockout mice. In: Kaufmann S H E, editor. Host response to intracellular pathogens. New York, N.Y: Chapman & Hall; 1997. pp. 167–181. [Google Scholar]
- 13.Derouin F, Garin Y J F. Toxoplasma gondii: blood and tissue kinetics during acute and chronic infections in mice. Exp Parasitol. 1991;73:460–468. doi: 10.1016/0014-4894(91)90070-d. [DOI] [PubMed] [Google Scholar]
- 14.Dobrowolski J M, Niesman I R, Sibley L D. Actin in Toxoplasma gondii is encoded by a single-copy gene, ACT1 and exists primarily in a globular form. Cell Motil Cytoskel. 1997;37:253–262. doi: 10.1002/(SICI)1097-0169(1997)37:3<253::AID-CM7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 15.Dubey J P, Thulliez P, Powell E C. Toxoplasma gondii in Iowa sows: comparison of antibody titers to isolation of T. gondii by bioassays in mice and cats. J Parasitol. 1995;81:48–53. [PubMed] [Google Scholar]
- 16.Dubremetz J F, Achbarou A, Bermudes D, Joiner K A. Kinetics and pattern of organelle exocytosis during Toxoplasma gondii host-cell interaction. Parasitol Res. 1993;79:401–408. doi: 10.1007/BF00931830. [DOI] [PubMed] [Google Scholar]
- 17.Frenkel J K, Dubey J P. Toxoplasmosis and its prevention in cats and man. J Infect Dis. 1972;126:664–673. doi: 10.1093/infdis/126.6.664. [DOI] [PubMed] [Google Scholar]
- 18.Frenkel J K, Escajadillo A. Cyst rupture as a pathogenic mechanism of toxoplasmic encephalitis. Am J Trop Med Hyg. 1987;36:517–522. doi: 10.4269/ajtmh.1987.36.517. [DOI] [PubMed] [Google Scholar]
- 19.Gazzinelli R T, Amichay D, Sharton-Kersten T, Grunwald E, Farber J M, Sher A. Role of macrophage-derived cytokines in the induction and regulation of cell-mediated immunity to Toxoplasma gondii. Curr Top Microbiol Immunol. 1996;219:126–139. doi: 10.1007/978-3-642-51014-4_12. [DOI] [PubMed] [Google Scholar]
- 20.Groisman E A, Ochman H. How to become a pathogen. Trends Microbiol. 1994;2:289–294. doi: 10.1016/0966-842x(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 21.Howe D K, Sibley L D. Toxoplasma gondii comprises three clonal lineages: correlation of parasite genotype with human disease. J Infect Dis. 1995;172:1561–1566. doi: 10.1093/infdis/172.6.1561. [DOI] [PubMed] [Google Scholar]
- 22.Howe D K, Summers B C, Sibley L D. Acute virulence in mice is associated with markers on chromosome VIII in Toxoplasma gondii. Infect Immun. 1996;64:5193–5198. doi: 10.1128/iai.64.12.5193-5198.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hunter C A, Suzuki Y, Subaste C S, Remington J S. Cells and cytokines in resistance to Toxoplasma gondii. Curr Top Microbiol Immunol. 1996;219:113–125. doi: 10.1007/978-3-642-51014-4_11. [DOI] [PubMed] [Google Scholar]
- 24.Joiner K A, Furhman S A, Miettinen H M, Kasper L H, Mellman I. Toxoplasma gondii: fusion competence of parasitophorous vacuoles in Fc-receptor transfected fibroblasts. Science. 1990;249:641–646. doi: 10.1126/science.2200126. [DOI] [PubMed] [Google Scholar]
- 25.Kim K, Soldati D, Boothroyd J C. Gene replacement in Toxoplasma gondii with chloramphenicol acetyltransferase as selectable marker. Science. 1993;262:911–914. doi: 10.1126/science.8235614. [DOI] [PubMed] [Google Scholar]
- 26.Lecomte V, Chumpitazi B F F, Pasquier B, Ambroise-Thomas P, Santoro F. Brain-tissue cysts in rats infected with the RH stain of Toxoplasma gondii. Parasitol Res. 1992;78:267–269. doi: 10.1007/BF00931740. [DOI] [PubMed] [Google Scholar]
- 27.Luft B J, Hafner R, Korzun A H, Leport C, Antoniskis D, Bosler E M, Bourland D D, Uttamchandani R, Fuhrer J, Jacobson J. Toxoplasmic encephalitis in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1993;329:995–1000. doi: 10.1056/NEJM199309303291403. [DOI] [PubMed] [Google Scholar]
- 28.Mercier C, Lecordier L, Darcy F, Deslee D, Murray A, Tourvielle B, Maes P, Capron A, Cesbron-Delauw M F. Toxoplasma gondii: molecular characterization of a dense granule antigen (GRA2) associated with the network of the parasitophorous vacuole. Mol Biochem Parasitol. 1993;58:71–82. doi: 10.1016/0166-6851(93)90092-c. [DOI] [PubMed] [Google Scholar]
- 29.Messina M, Niesman I R, Mercier C, Sibley L D. Stable DNA transformation of Toxoplasma gondii using phleomycin selection. Gene. 1995;165:213–217. doi: 10.1016/0378-1119(95)00548-k. [DOI] [PubMed] [Google Scholar]
- 30.Mordue D G, Sibley L D. Intracellular fate of vacuoles containing Toxoplasma gondii is determined at the time of formation and depends on the mechanism of entry. J Immunol. 1997;159:4452–4459. [PubMed] [Google Scholar]
- 31.Murray A, Mercier C, Decoster A, Lecordier L, Capron A, Cesbron-Delauw M F. Multiple B-cell epitopes in a recombinant GRA2 secreted antigen of Toxoplasma gondii. Appl Parasitol. 1993;34:235–244. [PubMed] [Google Scholar]
- 32.Parmley S F, Weiss L M, Yang S. Cloning of a bradyzoite-specific gene of Toxoplasma gondii encoding a cytoplasmic antigen. Mol Biochem Parasitol. 1995;73:253–257. doi: 10.1016/0166-6851(95)00100-f. [DOI] [PubMed] [Google Scholar]
- 33.Pfefferkorn E R, Pfefferkorn L C. Specific labeling of intracellular Toxoplasma gondii with uracil. J Protozool. 1977;24:449–453. doi: 10.1111/j.1550-7408.1977.tb04774.x. [DOI] [PubMed] [Google Scholar]
- 34.Pistoia V, Facchetti P, Ghiotto F, Cesbron-Delauw M F, Prigione I. Characterization of human T cell clones specific for Toxoplasma gondii. Curr Top Microbiol Immunol. 1996;219:165–173. doi: 10.1007/978-3-642-51014-4_15. [DOI] [PubMed] [Google Scholar]
- 35.Potasman I, Araujo F G, Desmonts G, Remington J S. Analysis of Toxoplasma gondii antigens recognized by human sera obtained before and after acute infection. J Infect Dis. 1986;154:650–657. doi: 10.1093/infdis/154.4.650. [DOI] [PubMed] [Google Scholar]
- 36.Sharma S D, Araujo F G, Remington J S. Toxoplasma antigen isolated by affinity chromatography with monoclonal antibody protects mice against lethal infection with Toxoplasma gondii. J Immunol. 1984;133:2818–2820. [PubMed] [Google Scholar]
- 37.Sibley L D, Boothroyd J C. Virulent strains of Toxoplasma gondii comprise a single clonal lineage. Nature (London) 1992;359:82–85. doi: 10.1038/359082a0. [DOI] [PubMed] [Google Scholar]
- 38.Sibley L D, LeBlanc A J, Pfefferkorn E R, Boothroyd J C. Generation of a restriction fragment length polymorphism linkage map for Toxoplasma gondii. Genetics. 1992;132:1003–1015. doi: 10.1093/genetics/132.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sibley L D, Niesman I R, Parmley S F, Cesbron-Delauw M F. Regulated secretion of multi-lamellar vesicles leads to formation of a tubulo-vesicular network in host cell vacuoles occupied by Toxoplasma gondii. J Cell Sci. 1995;108:1669–1677. doi: 10.1242/jcs.108.4.1669. [DOI] [PubMed] [Google Scholar]
- 40.Soete M, Camus D, Dubremetz J F. Experimental induction of bradyzoite-specific antigen expression and cyst formation by the RH strain of Toxoplasma gondii in vitro. Exp Parasitol. 1994;78:361–370. doi: 10.1006/expr.1994.1039. [DOI] [PubMed] [Google Scholar]
- 41.Torpier G, Charif H, Darcy F, Liu J, Dardé M L, Capron A. Toxoplasma gondii: differential localization of antigens secreted from encysted bradyzoites. Exp Parasitol. 1993;77:13–22. doi: 10.1006/expr.1993.1056. [DOI] [PubMed] [Google Scholar]