Abstract
The transferrin binding protein genes (tbpA and tbpB) from two strains of Moraxella catarrhalis have been cloned and sequenced. The genomic organization of the M. catarrhalis transferrin binding protein genes is unique among known bacteria in that tbpA precedes tbpB and there is a third gene located between them. The deduced sequences of the M. catarrhalis TbpA proteins from two strains were 98% identical, while those of the TbpB proteins from the same strains were 63% identical and 70% similar. The third gene, tentatively called orf3, encodes a protein of approximately 58 kDa that is 98% identical between the two strains. The tbpB genes from four additional strains of M. catarrhalis were cloned and sequenced, and two potential families of TbpB proteins were identified based on sequence similarities. Recombinant TbpA (rTbpA), rTbpB, and rORF3 proteins were expressed in Escherichia coli and purified. rTbpB was shown to retain its ability to bind human transferrin after transfer to a membrane, but neither rTbpA nor rORF3 did. Monospecific anti-rTbpA and anti-rTbpB antibodies were generated and used for immunoblot analysis, which demonstrated that epitopes of M. catarrhalis TbpA and TbpB were antigenically conserved and that there was constitutive expression of the tbp genes. In the absence of an appropriate animal model, anti-rTbpA and anti-rTbpB antibodies were tested for their bactericidal activities. The anti-rTbpA antiserum was not bactericidal, but anti-rTbpB antisera were found to kill heterologous strains within the same family. Thus, if bactericidal ability is clinically relevant, a vaccine comprising multiple rTbpB antigens may protect against M. catarrhalis disease.
In recent years, Moraxella (Branhamella) catarrhalis has gained recognition as a significant human pathogen (for reviews, see references 5, 9, and 24). It has been identified as a cause of bacteremia, epiglottitis, meningitis, otitis media, and pneumonia in children, adults, and the elderly. M. catarrhalis is the third leading cause of otitis media in children, responsible for about 20% of disease, following Streptococcus pneumoniae and nontypeable Haemophilus influenzae. In adults and the elderly, M. catarrhalis is mainly associated with chronic respiratory ailments such as bronchitis or pneumonia, where it exacerbates the disease. Invasive diseases such as bacteremia and meningitis are less common but can be fatal (7, 18, 23).
Approximately 70% of children will experience at least one bout of otitis media by the time they are 3 years old, with many children having multiple episodes (36). The peak incidence of otitis media occurs in children between 1 and 2 years of age, at a time when their language skills are developing. Recurrent or chronic otitis media can lead to deafness, speech impairment, or learning disabilities. Treatments include antibiotics or surgery to remove tonsils and adenoids or the insertion of tympanostomy tubes. The estimated cost of these primary treatments is about $2 billion dollars per year in the United States alone (3), with secondary costs such as speech therapy and special education classes costing billions more per year. In addition, most strains of M. catarrhalis are resistant to β-lactam antibiotics such as the penicillins, although treatment with cephalosporin, macrolide, and tetracycline antibiotics has been successful (8, 25). The need for an effective otitis media vaccine is obvious.
Bacteria have evolved several mechanisms to overcome host iron restriction, including the use of siderophores and iron binding proteins such as transferrin, lactoferrin, hemin, and hemoglobin binding proteins. To obtain iron from host iron binding proteins, M. catarrhalis utilizes both transferrin and lactoferrin binding proteins (31). Other characterized bacterial transferrin receptors are composed of two proteins, transferrin binding protein A (TbpA) and transferrin binding protein B (TbpB). In vivo, both TbpA and TbpB bind human transferrin, but only TbpB will still bind transferrin after sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and electroblotting (31). The tbpA and tbpB genes, encoding the TbpA and TbpB proteins, from strains of Actinobacillus pleuropneumoniae, H. influenzae, Neisseria gonorrhoeae, N. meningitidis, and Pasteurella haemolytica have been cloned and sequenced (2, 6, 10, 11, 20, 22, 27). The TbpA proteins are generally highly conserved within a species, while the TbpB proteins tend to be more variable. For N. meningitidis, it was found that the TbpB proteins could be separated into two families based on sequence and antigenicity (28); however, there are common antigenic domains in the TbpB proteins from N. meningitidis, N. gonorrhoeae, H. influenzae, and A. pleuropneumoniae (17, 33). Furthermore, TbpBs from N. meningitidis, H. influenzae, and A. pleuropneumoniae have been demonstrated to be protective antigens in various animal challenge models (1, 21, 22, 29).
In this report, we describe the cloning and sequence analysis of the genes encoding the Tbps from M. catarrhalis. Recombinant Tbps (rTbps) were expressed in Escherichia coli, and the purified proteins were used to immunize animals. It was found that rTbpB elicited bactericidal antibodies and could therefore represent a potential antigen for inclusion in a vaccine against M. catarrhalis.
MATERIALS AND METHODS
Recombinant DNA techniques.
Restriction endonucleases were purchased from Boehringer Mannheim (Laval, Quebec, Canada), New England Biolabs (Mississauga, Ontario, Canada), Bethesda Research Laboratories, or Pharmacia and were used according to the manufacturers’ specifications. Oligonucleotides were synthesized on an ABI model 380B DNA synthesizer and purified by chromatography (Oligonucleotide Purification Cartridge; Perkin-Elmer, Foster City, Calif.). To facilitate the cloning of some of the tbp fragments, additional restriction enzyme sites for ClaI, MstII, SfiI, and AvrII were introduced between the SalI and HindIII sites of pBluescript.SK, generating plasmid pSKMA. Other recombinant DNA methods were performed as specified by Sambrook et al. (30).
Bacterial strains and media.
M. catarrhalis 3 and 4223 were clinical isolates provided by T. Murphy (State University of New York, Buffalo); strains Q8 and R1 were gifts from M. Bergeron (University of Laval, Montreal, Quebec, Canada); strain LES-1 was obtained from L. Stenfors (University of Tromso, Tromso, Finland); strain VH-9 was obtained from V. Howie (University of Texas, Galveston); strains H-04 and M35 were obtained from G. D. Campbell (Louisiana State University, Shreveport); and strain ATCC 25240 was purchased from the American Type Culture Collection (Rockville, Md.). M. catarrhalis strains were maintained on Mueller-Hinton agar (Becton Dickinson, Cockeysville, Md.) or grown in brain heart infusion medium (BHI; Difco, Detroit, Mich.), with or without the addition of ethylenediamine-di(O-hydroxyphenylacetic acid) (EDDA; Sigma, St. Louis, Mo.) as described previously (15). E. coli strains were grown in YT (Difco) or NZCYM (Becton Dickinson) medium supplemented with 100 μg of ampicillin per ml as required.
Purification of native TbpA and TbpB and generation of antisera.
Native TbpA and TbpB were individually purified from M. catarrhalis 4223 by affinity chromatography using human transferrin immobilized on Sepharose as described previously (41). Guinea pigs (Hartley outbred; Charles River, Quebec, Quebec, Canada) were immunized intramuscularly on day 1 with 5 μg of purified protein emulsified in complete Freund’s adjuvant (Difco) and were boosted on days 14 and 29 with the same dose of protein emulsified in incomplete Freund’s adjuvant (Difco). Blood was collected on day 42.
Cloning the M. catarrhalis tbp genes.
Chromosomal DNA and EMBL3 libraries were prepared as described previously (22). Briefly, chromosomal DNA from M. catarrhalis 4223, Q8, and M35 was partially digested with Sau3AI and size fractionated. Fragments of 15 to 23 kb were ligated with BamHI-digested EMBL3 arms (Promega, Madison, Wis.) and packaged according to the manufacturer’s instructions. The 4223 library, grown in the presence of 200 μM EDDA, was screened with anti-TbpA and anti-TbpB antibodies, and three putative clones that reacted with both antisera were identified. The production of the appropriate protein bands was confirmed by reactivity of the antisera with electroblotted protein obtained from trichloroacetic acid-precipitated culture lysates.
Phage clone LM3-24 was found to contain a 13.2-kb SalI insert of the strain 4223 tbp locus. To localize the tbpA and tbpB genes, restriction enzyme and Southern blot analyses were performed. For tbpA, degenerate primers were designed based on conserved sequences found in other bacterial TbpA proteins, and a 300-bp probe was generated by PCR amplification (primers 223 and 224 [26]). For tbpB, a degenerate antisense oligonucleotide primer was synthesized based on a conserved sequence found in other bacterial TbpB proteins. The tbpB oligonucleotide probe was E G G F Y G P 5′ GAA/G GGX GGX TTC/T TAC/T GGX CCX 3′ 3′ CTC/T CCA/G CCA/G AAA ATA/G CCA/G GGA/T 5′
Part of the strain 4223 tbpA gene was localized to a 3.8-kb HindIII fragment which was subcloned into pACYC177 (New England Biolabs), generating plasmid pLEM3. The remainder of the 4223 tbpA gene was subcloned into pACYC177 as a 1.6-kb HindIII fragment, generating plasmid pLEM25. The tbpB gene was localized to a 5.5-kb NheI-SalI fragment, which was cloned into pBR328, generating plasmid pLEM23. The remainder of the intergenic sequence was cloned on two fragments: a 3.1-kb AvrII fragment cloned into pSKMA, generating plasmid DS-1698-1-1, and a 3.0-kb AvrII-ClaI fragment cloned into pSKMA, generating plasmid DS-1754-1. Figure 1A illustrates the restriction map and subclones of the 4223 transferrin receptor locus.
FIG. 1.
M. catarrhalis tbp locus and subclones of the 4223 (A) and Q8 (B) genomic clones. Restriction enzymes: Av, AvaII; Ar, AvrI; B, BglI; C; ClaI; H, HindIII; N, NheI; S, SalI.
The strain Q8 phage library was probed with two [α-32P]dCTP-labeled oligonucleotide probes that differed by four nucleotides (underlined) and were based on the TbpA-specific sequence IRDLTRYDPG: I R D L T R Y D P G ATT CGC GAC TTA ACA CGC TAT GAC CCT GGCATT CGT GAT TTA ACT CGC TAT GAC CCT GGT
Phage clone SLRD-A contained a 13-kb SalI insert of the Q8 tbp locus, and the tbpA and tbpB genes were localized by restriction enzyme and Southern blot analyses. Fragments were subcloned into pSKMA: plasmid pSLRD1 contains a 1.6-kb SalI-AvrII insert, plasmid pSLRD2 contains a 2-kb AvrII fragment, plasmid pSLRD4 contains a 4.1-kb AvrII insert, pSLRD3 contains a 2.2-kb AvrII-EcoRI fragment, and plasmid pSLRD5 contains a 3.8-kb EcoRI-PstI insert. Figure 1B illustrates the restriction map and subclones of the Q8 transferrin receptor locus.
The M35 phage library was screened with a digoxigenin-labeled (Boehringer Mannheim) 4223 tbpA gene probe. Phage clone M35-2.3 was found to contain a 13-kb insert of the M35 tbp genes. The tbpB gene was localized to a 7.5-kb NheI-SalI fragment by restriction enzyme and Southern blot analyses and was subcloned into pBR328, generating plasmid pLEM40.
PCR amplification of M. catarrhalis tbpB genes from additional strains.
The tbpB gene was PCR amplified from three additional M. catarrhalis strains (3, LES-1, and R1), using oligonucleotide primers based on the sequences 5′-GATGGGATAAGCACGCCCTACTT-3′ (sense) and 5′-CCCATCAGCCAAACAAACATTGTGT-3′ (antisense), which are found in the intergenic regions surrounding 4223 tbpB.
PCR amplification was performed in buffer containing 10 mM Tris-HCl (pH 8.9), 25 mM KCl, 5 mM (NH4)2SO4, and 2 mM MgSO4. Each 100-μl reaction mixture contained 10 ng of chromosomal DNA, 1 μg of each primer, 2.5 U of Pwo DNA polymerase (Boehringer Mannheim), and 0.2 mM deoxynucleoside triphosphates (Perkin-Elmer). The cycling conditions were 25 cycles of 95°C for 30 s, 45°C for 1.0 min, and 72°C for 2.0 min, followed by a 10-min elongation at 72°C. Specific 2.4-kb fragments were amplified, and DNA was purified for direct sequencing by agarose gel extraction, using a Geneclean kit (Bio 101 Inc., Vista, Calif.).
Sequencing of the M. catarrhalis tbp genes.
Plasmid DNA for sequencing was prepared by using a Qiagen (Chatsworth, Calif.) Plasmid Midi kit. To ensure a complete sequence, phage DNA was used to sequence across the joins of subclones. DNA samples were sequenced with an ABI model 373A DNA sequencer, using dye terminator chemistry. Oligonucleotide primers 17 to 25 bases in length were used to sequence both strands of the genes.
Construction of clones expressing rTbpA or rTbpB.
Plasmids pLEM3 and pLEM25 contain the strain 4223 tbpA gene (Fig. 1A). pLEM 3 was digested with BglI and HindIII to generate a 1.8-kb fragment comprising most of tbpA but without the extreme 5′ sequence. Oligonucleotides were synthesized to re-create the first 61 bases of the tbpA gene to the BglI site, and an NdeI site was added at the 5′ end for cloning purposes. The NdeI-BglI oligonucleotides were ligated with the BglI-HindIII fragment and cloned into plasmid pT7-7 (35) that had been digested with NdeI and HindIII, generating plasmid pLEM27. pLEM25 was digested with HindIII to excise the 1.6-kb 3′ fragment of tbpA, which was inserted into pLEM27 that had been digested with HindIII and dephosphorylated. DNA from the resulting plasmid, pLEM29, was used to transform E. coli BL21(DE3) cells. Since the 4223 and Q8 tbpA genes were so similar, only the 4223 rTbpA protein was expressed.
Constructs that would express the M. catarrhalis rTbpB proteins with or without their lipoprotein signal sequences were generated. Plasmid pLEM23 contains most of the 4223 tbpB gene, excluding the extreme 5′ end. Oligonucleotides were synthesized to re-create the first 58 bp of the tbpB gene encoding the mature TbpB protein up to an NheI site, and an NdeI site was added at the 5′ end for cloning purposes. pLEM23 was digested with NheI and ClaI, excising a 1.0-kb fragment of tbpB which was ligated with the oligonucleotides and pT7-7 that had been digested with NdeI and ClaI. The resulting plasmid, pLEM31, thus contains the 5′ half of 4223 tbpB encoding the mature protein. Oligonucleotides were synthesized to re-create the ∼104-bp extreme 3′ end of tbpB from ClaI to an AvaII site, and a BamHI site was added for cloning purposes. pLEM23 was digested with ClaI and AvaII, and the 0.9-kb fragment was ligated with the AvaII-BamHI oligonucleotides and inserted into pT7-7 that had been digested with BamHI and ClaI, generating plasmid pLEM32. The 1.0-kb NdeI-ClaI fragment of pLEM31 and the 1.0-kb ClaI-BamHI fragment of pLEM32 were ligated into pT7-7 that had been digested with NdeI and BamHI. The resulting plasmid, pLEM33, thus contains the full-length strain 4223 tbpB gene encoding the mature TbpB protein under the control of the T7 promoter. To express the strain 4223 tbpB gene encoding the lipoprotein, pLEM33 was digested with NdeI and NheI to excise the ∼58-bp 5′ fragment, and NdeI-NheI oligonucleotides encoding the lipoprotein leader sequence were substituted. The resulting plasmid was designated pLEM37. Similar schemes were used to generate the strain Q8 tbpB expression plasmids pSLRD35A and pSLRD35B, which express the lipoprotein and mature protein, respectively.
Expression and purification of recombinant proteins.
Plasmid DNA was purified by using a Qiagen Plasmid Midi kit and was used to transform E. coli BL21(DE3) cells (Novagen, Madison, Wis.) by electroporation. Overnight cultures were grown in YT broth containing ampicillin, and a 1:50 inoculum was grown to an optical density at 578 nm (OD578) of 0.3 before induction with 400 μM isopropyl-β-d-thiogalactopyranoside for 3 h. rTbpA, rTbpB, and rORF3 were produced as inclusion bodies in E. coli and were purified by the same process. To purify rTbpA, cells from 500-ml culture were resuspended in 50 ml of 50 mM Tris-HCl (pH 8.0) containing 0.1 M NaCl and 5 mM 4-(2-aminoethyl)-benzenesulfonylfluoride protease inhibitor (Calbiochem, La Jolla, Calif.) and disrupted by sonication (three 10-min pulses, 70% duty circle). The suspension was centrifuged at 20,000 × g for 30 min, and the pellet was extracted with 50 ml of 50 mM Tris-HCl (pH 8.0) containing 0.5% Triton X-100 and 10 mM EDTA. The sample was centrifuged as described above, and the resultant pellet was further extracted with 50 ml of 50 mM Tris-HCl (pH 8.0) containing 2 M urea and 5 mM dithiothreitol (DTT). Centrifuged as above, the resultant pellet contained inclusion bodies of rTbpA. The protein was solubilized in 50 mM Tris-HCl (pH 8.0) containing 6 M guanidine hydrochloride and 5 mM DTT. The sample was centrifuged for clarification, and the supernatant was purified on a Superdex 200 gel filtration column equilibrated in 50 mM Tris-HCl (pH 8.0) containing 2 M guanidine hydrochloride and 5 mM DTT. The fractions were analyzed by SDS-PAGE; those containing rTbpA were pooled, and Triton X-100 was added to a final concentration of 0.1%. The sample was dialyzed overnight at 4°C against 50 mM Tris-HCl (pH 8.0) and clarified by centrifugation for 30 min, and the supernatant was stored at −20°C.
Expression of the 4223 and Q8 rTbpB lipoproteins was ∼20% of total E. coli protein expression, which allowed for their ready purification, whereas expression of the mature proteins was negligible, and so their purification was not pursued. The extraction of inclusion bodies and subsequent purification of the 4223 and Q8 rTbpB proteins were performed as described for the rTbpA proteins. The 4223 rORF3 protein was produced as ∼10% of total E. coli proteins and was purified as described for rTbpA.
Transferrin binding assay.
The transferrin binding activity of rTbpA, rTbpB, and rORF3 was assessed by the method of Schryvers and Lee (31), with modifications. Briefly, purified recombinant protein was subjected to discontinuous electrophoresis through SDS–12.5% polyacrylamide gels. The proteins were electrophoretically transferred to a polyvinylidene difluoride (PVDF) membrane (Millipore, Bedford, Mass.) and incubated with horseradish peroxidase (HRP)-conjugated human transferrin (1:50 dilution; Jackson ImmunoResearch Labs Inc., Mississauga, Ontario, Canada) at 4°C overnight. LumiGLO substrate (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, Md.) was used for chemiluminescent detection of HRP activity according to the manufacturer’s instructions.
Immunization of animals and immunoassays.
Groups of five BALB/c mice (Charles River) were injected three times subcutaneously on days 1, 29, and 43 with purified rTbpB (0.3 to 10 μg) in the presence or absence of AIPO4 (1.5 mg per dose). Blood samples were taken on days 14, 28, 42, and 56 for analyzing the anti-rTbpB antibody titers by enzyme-linked immunosorbent assay (ELISA).
Groups of two guinea pigs (Hartley outbred; Charles River) were immunized intramuscularly with 5-μg doses of purified rTbpA or rTbpB emulsified in complete or incomplete Freund’s adjuvant as described above. Anti-Tbp antibody titers in guinea pig immune sera were determined by antigen-specific ELISAs as previously described (40). Microtiter wells (Nunc-MAXISORB, Nunc, Roskilde, Denmark) were coated with 50 μl of protein (0.5 μg ml−1). The reactive titer of an antiserum was defined as the reciprocal of the highest dilution consistently showing a twofold increase in absorbance at 450 nm over that obtained with the preimmune serum samples.
Whole-cell ELISAs.
M. catarrhalis 4223 was grown in the presence of EDDA as described above. Cell pellets were collected by centrifugation, washed with phosphate-buffered saline (PBS), and resuspended in 50 mM carbonate-bicarbonate buffer (pH 9.6). The OD490 of the suspension was adjusted to 0.5, and 200 μl of a 1:100 dilution of whole bacteria was used to coat microtiter wells. The plates were air dried at 37°C overnight and then blocked with PBS–0.1% bovine serum albumin at 37°C for 1 h (250 μl per well). After three washes with PBS–0.1% Tween 20, 200 μl of antiserum at an appropriate dilution (in PBS–0.1% gelatin) was added to the wells and further incubated at 37°C for 2 h. Affinity-purified F(ab′)2 fragment of donkey anti-guinea pig immunoglobulin G (heavy plus light chain) antibodies conjugated to HRP (Jackson ImmunoResearch Laboratories) was used as the reporter. The reactions were developed by using tetramethylbenzidine-H2O2 (ADI), and absorbancies were measured at 450 nm (using 540 nm as a reference wavelength) in a Flow Multiskan MCC microplate reader (ICN Biomedicals).
Antigenic conservation of Tbps in M. catarrhalis.
M. catarrhalis strains were grown in BHI medium with or without 25 μM EDDA. Sample concentrations were standardized, and whole-cell lysates were separated by SDS-PAGE and electrophoretically transferred to PVDF membranes. Guinea pig anti-4223 rTbpA or anti-4223 rTbpB antibody was used as primary antibody, and HRP-conjugated goat anti-guinea pig immunoglobulin G antibody was used as secondary antibody. Approximately 90 M. catarrhalis strains, obtained from North America or Finland, were tested for their antigenic reactivities.
Bactericidal antibody activity.
The bactericidal antibody assay was performed as previously described (39). Briefly, the M. catarrhalis strains were grown to an OD578 of 0.5 in BHI medium containing 25 μM EDDA. The bacteria were diluted so that approximately 150 to 450 CFU was added to each reaction. Guinea pig anti-rTbpA or anti-rTbpB antiserum and prebleed serum controls were heated to 56°C for 30 min to inactivate endogenous complement and were diluted 1:64 with Veronal buffer containing 0.1% bovine serum albumin (VBS) (39). Guinea pig complement (Biowhittaker, Walkersville, Md.) was diluted 1:10 in VBS. Aliquots of 25 μl each of diluted antiserum, bacteria, and complement were added to duplicate wells of a 96-well microtiter plate (Nunc). The plates were incubated at 37°C for 60 min, with gentle shaking at 70 rpm on a rotary platform; 50 μl of each reaction mixture was plated onto Mueller-Hinton agar plates (Becton Dickinson), which were incubated at 37°C for 24 h and then at room temperature for 24 h before the bacteria were counted. Antisera were determined to be bactericidal if ≥50% of bacteria were killed compared with preimmune serum controls. Each assay was repeated at least twice in duplicate. Anti-4223 rTbpB antisera from two guinea pigs gave identical results at 1:64 dilution; however, only one of two anti-Q8 rTbpB antisera exhibited bactericidal activity at 1:64 dilution. Statistical analysis was performed by the Mann-Whitney rank sum test.
To assess the potential bactericidal activity of preimmune sera, samples containing bacteria, complement, and preimmune sera were compared with those containing only bacteria and complement. No differences were detected between the two groups, indicating that preimmune sera were not bactericidal for the strains tested. An additional control included bacteria with antisera but no complement. No bactericidal activity was observed, indicating that there was no antibody-mediated clumping of bacteria resulting in false activity.
Nucleotide sequence accession numbers.
The nucleotide sequences of the genes in the tbp loci from M. catarrhalis 4223 and Q8 have been deposited at GenBank and assigned accession no. AF039312 and AF039315, respectively. The accession numbers for the nucleotide sequences of the tbpB genes from M. catarrhalis 3, LES-1, M35, and R1 are AF039311, AF039313, AF039314, and AF039316, respectively.
RESULTS
Cloning of the M. catarrhalis tbp genes.
The genomic tbp genes were cloned from a phage expression library of M. catarrhalis 4223, using monospecific anti-TbpA and anti-TbpB antisera. The genomic tbp genes were cloned from additional phage libraries of M. catarrhalis Q8 and M35, using DNA probes. The phage clones contained approximately 13-kb inserts which had very similar restriction enzyme maps. The entire insert could not be cloned into plasmids, and so multiple subclones of the 4223 and Q8 tbp genes encompassing the whole sequence were generated (Fig. 1). The M35 tbpB gene was subcloned and the tbpB genes from three additional M. catarrhalis strains (3, LES-1, and R1) were amplified by PCR using primers derived from the flanking regions of 4223 tbpB.
Analysis of the nucleotide sequence of the tbp genes.
The 4223- and Q8-derived tbp genes were sequenced in their entirety and were found to include three complete genes and two partial genes, found at each end of the 13-kb inserts. The arrangement of the M. catarrhalis tbp genes was unique compared with that of other known bacterial tbp operons, in that the tbpA gene preceded the tbpB gene, with an intergenic distance of approximately 2.8 kb. A third open reading frame, designated orf3, was found to be located between tbpA and tbpB. The tbpA, tbpB, and orf3 genes are approximately 3.2, 2.1, and 1.5 kb, respectively, in length. The distance between tbpA and orf3 is about 1 kb, and that between orf3 and tbpB is approximately 273 bp. Putative promoter elements could be identified upstream of all three genes (Fig. 2A). We did not find a complete Fur (ferric uptake regulator)-binding sequence overlapping the −10 region of the promoter, such as identified in other bacterial tbpB promoters (Fig. 2B).
FIG. 2.
Nucleotide sequences of the promoter regions of M. catarrhalis 4223 tbpA, orf3, and tbpB genes. (A) Putative promoter elements for tbpA, orf3, and tbpB. RBS, ribosome-binding site. (B) Comparison of Fur-binding sites from E. coli (13), N. meningitidis tbpB (20), H. influenzae tbpB (11), and M. catarrhalis 4223 tbpB. Dots indicate residues identical to the E. coli consensus sequence, and underlining indicates dyad symmetry.
Analysis of the deduced amino acid sequences of the Tbps.
The deduced strain 4223 and strain Q8 TbpA proteins are 1,074 and 1,070 residues, respectively, in length and are 98% identical (Fig. 3). The encoded 4223 and Q8 TbpA proteins have molecular masses of 119.4 and 118.9 kDa, respectively. The M. catarrhalis TbpA protein sequences are somewhat larger than sequences of other known TbpA proteins, but there is still about 40% identity and 52 to 53% similarity with the H. influenzae, N. gonorrhoeae, and N. meningitidis TbpA proteins, as illustrated in Fig. 3 (6, 20, 22). The main difference between the M. catarrhalis TbpA and other TbpA proteins appears to be an approximately 112-residue insert in the M. catarrhalis proteins between residues 572 and 684. There is another, smaller insert of 19 residues located between residues 800 and 819.
FIG. 3.
Alignment of the deduced TbpA protein sequences for M. catarrhalis 4223 and Q8, N. meningitidis B16B6 and M982 (20), N. gonorrhoeae FA19 (6), and H. influenzae type b Eagan (22). Dots indicate identical residues, and dashes have been inserted to maximize sequence alignment.
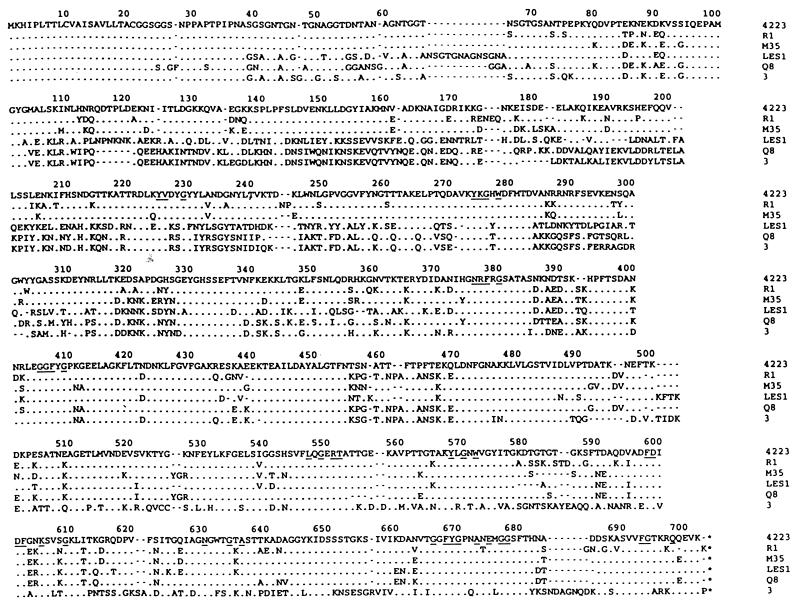
An alignment of the six deduced M. catarrhalis TbpB proteins is shown in Fig. 4. The 4223 and Q8 TbpB proteins are 63% identical and 70% similar and have molecular masses of 75.5 and 76.6 kDa, respectively. The M35 and R1 TbpB proteins have masses of 76.5 and 77.0 kDa, respectively, and are more closely related to 4223 TbpB, with 83 to 86% identity and 88 to 90% similarity. The TbpB protein from strain LES-1 is 76.8 kDa and is 63% identical to both the 4223 and Q8 proteins. The strain 3 TbpB protein is 76.9 kDa and is more closely related to the Q8 TbpB, with 71% identity to Q8 but only 51% identity to 4223. It is also apparent from Fig. 4 that most of the heterogeneity between the TbpBs resides in the N-terminal half of the protein. There is a consensus signal sequence for lipoproteins (38), and it is predicted that Cys21 is the N-terminal residue of the mature lipoprotein. Residues that are conserved among the TbpB proteins from A. pleuropneumoniae, H. influenzae, N. gonorrhoeae, N. meningitidis, P. haemolytica, and M. catarrhalis are underlined in Fig. 4 (27). The level of identity with the TbpBs from other species is very low and is found in relatively short stretches throughout the sequence.
FIG. 4.
Alignment of the deduced TbpB sequences from M. catarrhalis 4223, Q8, 3, LES-1, M35, and R1. Dots indicate identical residues, and dashes have been inserted for maximum alignment of the sequences. Residues conserved among TbpB proteins from A. pleuropneumoniae, H. influenzae, M. catarrhalis, N. gonorrhoeae, N. meningitidis, and P. haemolytica are underlined.
The orf3 gene encodes proteins of 58.1 and 57.9 kDa from M. catarrhalis 4223 and Q8, respectively, and these are 98% identical. They contain a carboxy-terminal phenylalanine residue that is often associated with a membrane location (34), although a typical consensus signal sequence cannot be identified. A comparison with the protein databases revealed little homology between the ORF3 protein and known proteins.
Expression, purification, and characterization of recombinant proteins.
The 4223 rTbpA, 4223 rTbpB, Q8 rTbpB, and 4223 rORF3 proteins were produced as inclusion bodies in E. coli, using the inducible T7 promoter system. The rTbpA and rORF3 proteins were produced at ∼10% of total protein, while the rTbpB protein (with lipoprotein leader) was produced at about 20% of total protein. The proteins have been purified by similar methods which involve enrichment for the inclusion bodies after sonic disruption of the cells, solubilization of the inclusion bodies, and then by Superdex 200 chromatography (Fig. 5A). The recombinant proteins were further characterized for their ability to bind to human transferrin after transfer to a PVDF membrane as described for the N. meningitidis and H. influenzae rTbpB proteins (22, 37). Both the 4223 and Q8 rTbpB proteins bound human transferrin (Fig. 5B), but under the same conditions, rTbpA and rORF3 did not bind (data not shown).
FIG. 5.
Purification of the recombinant TbpA and TbpB proteins (A) and transferrin binding of rTbpB proteins (B). Lane 1, E. coli cells expressing 4223 rTbpA; lane 2, purified rTbpA; lane 3, E. coli cells expressing 4223 rTbpB; lane 4, purified 4223 rTbpB; E. coli cells expressing Q8 rTbpB; lane 5, purified Q8 rTbpB. Proteins were transferred to a PVDF membrane and incubated with human transferrin conjugated to HRP.
Antigenic conservation and bactericidal antibody activities.
Hyperimmune sera directed against the purified rTbpA or rTbpB proteins were raised in guinea pigs. As shown in Table 1, the recombinant TbpA and TbpB proteins generated high-titer antibodies. On immunoblot analysis, the anti-rTbpA antiserum recognized specific proteins in all of the 32 M. catarrhalis strains tested, and both the anti-4223 rTbpB and anti-Q8 rTbpB antisera recognized specific proteins in all of approximately 90 M. catarrhalis strains tested. Representative immunoblot data for samples grown under iron-replete or iron-deficient conditions and probed with anti-rTbpA or anti-rTbpB antiserum are presented in Fig. 6.
TABLE 1.
ELISA titers for guinea pig anti-rTbp antisera
| Coated antigen (strain) | Titera
|
||
|---|---|---|---|
| Anti-4223 rTbpA | Anti-4223 rTbpB | Anti-Q8 rTbpB | |
| rTbpA (4223) | 614,400 | ||
| rTbpB (4223) | 1,638,400 | 204,800 | |
| rTbpB (Q8) | 1,638,400 | 1,638,400 | |
Anti-rTbp antibody titers in sera collected after three immunizations were determined by ELISA. The reactive titer was defined as the reciprocal of the dilution consistently showing a twofold increase in absorbance over that obtained with the prebleed serum sample. Values are average titers from two guinea pigs.
FIG. 6.
Antigenic conservation of TbpA and TbpB in M. catarrhalis strains. Bacteria were grown in BHI medium with (+) or without (−) EDDA, and whole-cell lysates were probed with anti-4223 rTbpA or anti-4223 rTbpB antibody. (A) Immunoblot with anti-4223 rTbpA; (B) immunoblot with anti-4223 rTbpB. Lanes represent the following strains: 1, 4223; 2, VH-9; 3, M35; 4, H-04; 5, ATCC 25240; 6, R1; 7, Q8; 8, LES-1; and 9, 3.
The bactericidal antibody activities of anti-rTbpA and anti-rTbpB antisera were assessed at an antibody dilution of 1:64, and an arbitrary cutoff of ≥50% killing was chosen as significant. Anti-4223 rTbpA antibody was not bactericidal against either the homologous strain or strain Q8 and was not tested further. Anti-4223 rTbpB antibody was bactericidal against the homologous strain and five of eight heterologous strains, while anti-Q8 rTbpB antibody killed two heterologous strains (Table 2).
TABLE 2.
Bactericidal antibody activities of guinea pig anti-rTbpB antisera
| Strain | Locale | Source | Bactericidal antibody activitya
|
|
|---|---|---|---|---|
| Anti-4223 rTbpB | Anti-Q8 rTbpB | |||
| 4223 | New York | MEFb | 99.9* | 0 |
| M35 | Quebec | MEF | 92.3* | 0 |
| R1 | Quebec | Bronchial secretion | 87.5* | 7.0 |
| LES-1 | Finland | MEF | 12.5 | 51.0* |
| Q8 | Quebec | Sputum | 16.5 | 99.5* |
| 3 | New York | Sputum | 23.0* | 34.0* |
| VH-9 | Texas | MEF | 92.8* | 0 |
| H-04 | Nova Scotia | MEF | 85.0* | 98.7* |
| ATCC 25240 | 81.3* | 0 | ||
Killing by antiserum diluted 1:64 compared to prebleed controls in which killing is assumed to be 0%. *, statistical significance compared to prebleed controls (P < 0.05) in the Mann-Whitney rank sum test.
MEF, middle ear fluid.
DISCUSSION
The bacterial tbp loci from A. pleuropneumoniae, H. influenzae, N. gonorrhoeae, N. meningitidis, and P. haemolytica have been cloned and sequenced, and the gene arrangement has always been found to be tbpB followed by tbpA (2, 10, 11, 20, 22, 27). The distance between the two genes has ranged from as little as 13 bp for H. influenzae to 87 bp for N. meningitidis (20, 22). A single promoter sequence has been identified upstream of the tbpB gene, suggesting that the genes form an operon. In addition, the polar effects of transcriptional terminators in the N. gonorrhoeae and N. meningitidis tbpB genes provide direct evidence for an operonic arrangement (2, 19). In M. catarrhalis, there is a unique gene arrangement of tbpA-orf3-tbpB, and there are three potential promoter sequences suggesting possible independent transcription of the genes. This unexpected gene arrangement has been confirmed in three independent clinical isolates of M. catarrhalis, strains 4223, Q8, and M35, indicating that it was not an artifact of cloning. Recently, Ogunnariwo et al. (26) found that the degenerate primers designed to PCR amplify the junctional region between tbpB and tbpA worked in all species tested except M. catarrhalis, M. bovis, and M. lacunata. The lack of a typical tbpB-tbpA gene arrangement in M. catarrhalis demonstrated in this study provides a logical explanation for these findings and also suggests that this situation may apply for the other Moraxella species.
In many bacterial species, the product of the fur gene represses the transcription of iron-regulated genes. A consensus sequence for Fur binding has been determined for several E. coli promoters (13), and Fur-binding sequences overlapping the −10 regions of the N. meningitidis and H. influenzae tbpB promoters have been identified (11, 20). However, only a short homologous sequence can be identified in the −10 region of the M. catarrhalis tbpB promoter, and there is very limited dyad symmetry (Fig. 2). These data suggest that expression of the M. catarrhalis Tbps may be regulated by a novel mechanism.
Consistent with other bacterial Tbps, the M. catarrhalis TbpA proteins from strains 4223 and Q8 are highly conserved, while the corresponding TbpB proteins are more variable. While the M. catarrhalis TbpA proteins are very similar to each other, they are longer than TbpA proteins from other species, the main differences being two inserts of about 112 and 19 residues in the central region of each of the proteins (Fig. 3). Compared to a proposed TbpA/LbpA topology model (12), the inserts are found in the seventh and ninth extracellular loops, respectively. The 112-residue insert contains an extra two cysteine residues and two potential transmembrane sequences located at 587 to 596 and 608 to 617, which could result in extra periplasmic and extracellular loops. When the sequences of the TbpB proteins from six M. catarrhalis strains were compared, they appeared to separate into two groups, one more closely related to strain 4223, which included strains R1 and M35, with the other family comprised of strains Q8 and 3. The TbpB protein from strain LES-1 was equally similar to both families. The six strains from which the tbpB genes were cloned were chosen for their geographic diversity and anatomic source (Table 2). Strains 4223 and 3 are from Buffalo, N.Y.; strains M35, Q8, and R1 are from Montreal, Quebec, Canada; and strain LES-1 is from Finland. Strains 4223, LES-1, and M35 were all derived from patients with otitis media, while strains 3, R1, and Q8 were from sputum or bronchial secretions. There appears to be no correlation between the geographic or anatomic source of the organism and the TbpB sequence. Compared to the TbpB proteins from other organisms, there is limited homology found scattered throughout the sequence in short motifs (27). The conservation of these sequences among the TbpB proteins from several species suggests that they serve a functional or structural role. It is interesting that only the M. catarrhalis 3 TbpB protein contains the VCCSNLEHLKFG motif (conserved residues underlined) found in all other TbpB proteins.
The recombinant TbpA and TbpB proteins were expressed in good yield from E. coli, with the rTbpB proteins expressed at approximately 20% of total protein. Although the M. catarrhalis TbpB protein was designed to be expressed as the mature lipoprotein, it was found that the recombinant protein was expressed as inclusion bodies and was not associated with the membrane. There are two possible explanations for this: first, the M. catarrhalis signal sequence does not function in E. coli; and second, the high expression level of the recombinant protein dictates that the host produces it in inclusion bodies. In this respect, the observation that A. pleuropneumoniae rTbpB was found in both an outer membrane and an inclusion body fraction during high-level expression (10) may indicate that the latter explanation is a greater contributing factor.
Since no other bacterial transferrin receptors had been shown to include a third protein, we wished to determine whether the ORF3 protein was part of the transferrin binding complex for M. catarrhalis. Although the M. catarrhalis rTbpB proteins retained the ability to bind human transferrin after electroblotting, as has been demonstrated for the N. meningitidis and H. influenzae rTbpB proteins (22, 37), neither rTbpA nor rORF3 did. To determine whether the orf3 gene was iron repressible, we had planned to generate specific anti-rORF3 antisera and perform immunoblot analyses of strains grown with and without EDDA. However, although we were able to express rORF3 protein in good yield from E. coli, we were unable to generate antibodies in guinea pigs after five immunizations, and the protein was not studied further (data not shown).
The rTbpA and rTbpB proteins were both found to be highly immunogenic, and polyclonal antibodies were used to assess antigenic conservation and bactericidal antibody activity. By immunoblot analysis, specific ∼115-kDa TbpA and ∼80-kDa TbpB proteins were identified in all strains examined. Both TbpA and TbpB were found to be produced constitutively when grown in BHI medium under iron-sufficient conditions (Fig. 6). However, in some strains it appeared that the expression of TbpA (samples 1, 7, and 9) and/or TbpB (samples 1 to 3 and 6 to 8) could be increased under iron-limiting conditions, i.e., the addition of the iron chelator EDDA. This effect could be reversed upon addition of exogenous iron (data not shown). The immunoblot results are consistent with the earlier finding of Schryvers and Lee (31), who used dot bots to show that M. catarrhalis expressed low levels of transferrin and lactoferrin binding proteins under iron-sufficient conditions. Holland et al. (16) have demonstrated that fresh clinical isolates of H. influenzae type b expressed Tbps constitutively, although passaged laboratory strains expressed iron-regulated Tbps. Hardie et al. (14) have also demonstrated constitutive expression of Tbp in most invasive H. influenzae type b and approximately 40% of commensal Haemophilus species that bound transferrin.
An approximately 80-kDa M. catarrhalis outer membrane protein, designated OMP B1, has been identified in patient sera from adults with bronchiectasis or children with otitis media (4, 32). This protein was shown to bind transferrin and, judging from its molecular weight, would therefore appear to be TbpB. More extensive studies on the human immune response to the transferrin receptor proteins indicate that there is a strong reactivity against TbpB but little or no reactivity against TbpA (30a).
There is currently no animal model of otitis media caused by M. catarrhalis, and so bactericidal antibody activity was used as a surrogate assay to assess candidate vaccine antigens, on the assumption that bactericidal antibody activity may correlate with clearance or protection. M. catarrhalis strains have a tendency to aggregate or clump, and using the naturally nonclumping strain Q8 or a spontaneous nonclumping mutant of strain 4223, termed RH408, we had previously developed a bactericidal antibody assay for M. catarrhalis (39). By expanding the assay to include aggregating strains, we have been able to use it to screen candidate vaccine antigens. The anti-rTbpA antibody did not kill its homologous strain and was not pursued further. TbpA is a transmembrane protein, and it was possible that we had not re-formed the native conformation after purification from inclusion bodies and the antibody was unable to recognize the native TbpA protein in intact bacteria. However, we have demonstrated that both anti-rTbpA and anti-rTbpB antibodies recognize intact bacteria in a whole-cell ELISA with antibody titers of 400 to 1,600, suggesting that this is not the reason for the lack of bactericidal antibody activity observed with anti-rTbpA (data not shown). The anti-4223 rTbpB antibody was bactericidal against the homologous strain and, with an arbitrary cutoff of ≥50% killing, was also bactericidal for five of eight heterologous strains. From our sequence data, strains 4223, M35, and R1 appeared to be closely related, and the bactericidal antibody data showed that anti-4223 rTbpB killed strains 4223, M35, and R1. It also killed strains VH-9, H-04 and ATCC 25240, suggesting that these latter strains might have TbpB proteins with sequences similar to that of 4223 TbpB. The anti-Q8 rTbpB antibody was considerably poorer at killing heterologous strains, killing only strains H-04 and LES-1. When the bactericidal antibody activities were quantitated by increasing antibody dilutions, it was found that anti-Q8 rTbpB antibody was much less bactericidal against strain Q8 than anti-4223 rTbpB antibody was against strain 4223. Anti-4223 rTbpB antibody was still capable of killing 50% of 4223 cells at an antibody dilution of about 1:12,000, whereas anti-Q8 rTbpB antibody killed 50% of Q8 cells at a maximum dilution of about 1:100. It is not clear why the anti-Q8 rTbpB antibody has such relatively low bactericidal antibody activity, but it is interesting that anti-Q8 rTbpB antibody killed LES-1 and the more potent anti-4223 rTbpB antibody did not. The identification of two putative families of M. catarrhalis TbpB proteins based on genetic and antigenic properties is reminiscent of the N. meningitidis TbpB proteins, for which two families have been identified (28). Strain H-04 is unique in that it was killed by both anti-rTbpB antisera. It would be interesting to determine the tbpB gene sequence from this strain and to investigate whether anti-H-04 rTbpB antiserum can kill M. catarrhalis strains from both putative families.
In summary, we have cloned and sequenced the transferrin receptor locus from M. catarrhalis and demonstrated that it has a unique organization that includes a third gene. Our data indicate that there are at least two possible families of M. catarrhalis TbpB proteins that can be identified on the basis of their sequences. Using a bactericidal antibody assay to screen, our data demonstrate that antibody raised to a rTbpB protein from one family was able to kill other members of the family but was unable to kill members of the second family. If protection against M. catarrhalis can be afforded by the generation of bactericidal antibodies in the host, then the TbpB proteins represent good candidate vaccine antigens.
ACKNOWLEDGMENTS
We thank Bill Bradley for synthesis of oligonucleotides and Diane England for DNA sequencing. We also acknowledge the excellent technical assistance of Debbie Coleman, Manjit Haer, Deon Persaud, and Wan Xu-Li.
REFERENCES
- 1.Ala’Aldeen D A A. Transferrin receptors of Neisseria meningitidis: promising candidates for a broadly cross-protective vaccine. J Med Microbiol. 1996;44:237–243. doi: 10.1099/00222615-44-4-237. [DOI] [PubMed] [Google Scholar]
- 2.Anderson J E, Sparling P F, Cornelissen C N. Gonococcal transferrin-binding protein 2 facilitates but is not essential for transferrin utilization. J Bacteriol. 1994;176:3162–3170. doi: 10.1128/jb.176.11.3162-3170.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bluestone C D. Modern management of otitis media. Pediatr Clin North Am. 1989;36:1371–1377. doi: 10.1016/s0031-3955(16)36794-3. [DOI] [PubMed] [Google Scholar]
- 4.Campagnari A A, Ducey T F, Rebmann C A. Outer membrane protein B1, an iron-repressible protein conserved in the outer membrane of Moraxella (Branhamella) catarrhalis, binds human transferrin. Infect Immun. 1996;64:3920–3924. doi: 10.1128/iai.64.9.3920-3924.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Catlin B W. Branhamella catarrhalis: an organism gaining respect as a pathogen. Clin Microbiol Rev. 1990;3:293–320. doi: 10.1128/cmr.3.4.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cornelissen C N, Biswas G D, Tsai J, Paruchuri D K, Thompson S A, Sparling P F. Gonococcal transferrin-binding protein 1 is required for transferrin utilization and is homologous to TonB-dependent outer membrane receptors. J Bacteriol. 1992;174:5788–5797. doi: 10.1128/jb.174.18.5788-5797.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daoud A, Abuekteish F, Masaadeh H. Neonatal meningitidis due to Moraxella catarrhalis and review of the literature. Ann Trop Paediatr. 1996;16:199–201. doi: 10.1080/02724936.1996.11747826. [DOI] [PubMed] [Google Scholar]
- 8.Doern G V, Brueggemann A B, Pierce G, Hogan T, Holley H P, Jr, Rauch A. Prevalence of antimicrobial resistance among 723 outpatient clinical isolates of Moraxella catarrhalis in the United States in 1994 and 1995: results of a 30-center national surveillance study. Antimicrob Agents Chemother. 1996;40:2884–2886. doi: 10.1128/aac.40.12.2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Enright M C, McKenzie H. Moraxella (Branhamella) catarrhalis—clinical and molecular aspects of a rediscovered pathogen. J Med Microbiol. 1997;46:360–371. doi: 10.1099/00222615-46-5-360. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez G C, Yu R-H, Rosteck P, Schryvers A B. Sequence, genetic analysis, and expression of Actinobacillus pleuropneumoniae transferrin receptor genes. Microbiology. 1995;141:2405–2416. doi: 10.1099/13500872-141-10-2405. [DOI] [PubMed] [Google Scholar]
- 11.Gray-Owen S D, Loosmore S, Schryvers A B. Identification and characterization of genes encoding the human transferrin-binding proteins from Haemophilus influenzae. Infect Immun. 1995;63:1201–1210. doi: 10.1128/iai.63.4.1201-1210.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gray-Owen S D, Schryvers A B. Bacterial transferrin and lactoferrin receptors. Trends Microbiol. 1996;4:185–191. doi: 10.1016/0966-842x(96)10025-1. [DOI] [PubMed] [Google Scholar]
- 13.Griggs D W, Konisky J. Mechanism for iron-regulated transcription of the Escherichia coli cir gene: metal-dependent binding of Fur protein to the promoters. J Bacteriol. 1989;171:1048–1054. doi: 10.1128/jb.171.2.1048-1054.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hardie K R, Adams R A, Towner K J. Transferrin-binding ability of invasive and commensal isolates of Haemophilus spp. J Med Microbiol. 1993;39:218–224. doi: 10.1099/00222615-39-3-218. [DOI] [PubMed] [Google Scholar]
- 15.Harkness R E, Guimond M-J, McBey B-A, Klein M H, Percy D H, Croy B A. Branhamella catarrhalis pathogenesis in SCID and SCID/beige mice. APMIS. 1993;101:805–810. [PubMed] [Google Scholar]
- 16.Holland J, Langford P R, Towner K J, Williams P. Evidence for in vivo expression of transferrin-binding proteins in Haemophilus influenzae type b. Infect Immun. 1992;60:2986–2991. doi: 10.1128/iai.60.7.2986-2991.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holland J, Parsons T R, Hasan A A, Cook S M, Stevenson P, Griffiths E, Williams P. Conservation and antigenic cross-reactivity of the transferrin-binding proteins of Haemophilus influenzae, Actinobacillus pleuropneumoniae and Neisseria meningitidis. Microbiology. 1996;142:3505–3513. doi: 10.1099/13500872-142-12-3505. [DOI] [PubMed] [Google Scholar]
- 18.Ioannidis J P A, Worthington M, Griffiths J K, Snydman D R. Spectrum and significance of bacteremia due to Moraxella catarrhalis. Clin Infect Dis. 1995;21:390–397. doi: 10.1093/clinids/21.2.390. [DOI] [PubMed] [Google Scholar]
- 19.Irwin S W, Averil N, Cheng C Y, Schryvers A B. Preparation and analysis of isogenic mutants in the transferrin receptor protein genes, tbpA and tbpB, from Neisseria meningitidis. Mol Microbiol. 1993;8:1125–1133. doi: 10.1111/j.1365-2958.1993.tb01657.x. [DOI] [PubMed] [Google Scholar]
- 20.Legrain M, Mazarin V, Irwin S W, Bouchon B, Quentin-Millet M-J, Jacobs E, Schryvers A B. Cloning and characterization of Neisseria meningitidis genes encoding the transferrin-binding proteins Tbp1 and Tbp2. Gene. 1993;130:73–80. doi: 10.1016/0378-1119(93)90348-7. [DOI] [PubMed] [Google Scholar]
- 21.Lissolo L, Maitre-Wilmotte G, Dumas P, Mignon M, Danve B, Quentin-Millet M-J. Evaluation of transferrin-binding protein 2 within the transferrin-binding protein complex as a potential antigen for future meningococcal vaccines. Infect Immun. 1995;63:884–890. doi: 10.1128/iai.63.3.884-890.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loosmore S M, Yang Y-P, Coleman D C, Shortreed J M, England D M, Harkness R E, Chong P S-C, Klein M H. Cloning and expression of the Haemophilus influenzae transferrin receptor genes. Mol Microbiol. 1996;19:575–586. doi: 10.1046/j.1365-2958.1996.406943.x. [DOI] [PubMed] [Google Scholar]
- 23.Meyer G A, Shope T R, Waecker N J, Jr, Lanningham F H. Moraxella (Branhamella) catarrhalis bacteremia in children. A report of two cases and review of the literature. Clin Pediatr. 1995;34:146–150. doi: 10.1177/000992289503400305. [DOI] [PubMed] [Google Scholar]
- 24.Murphy T F. Branhamella catarrhalis: epidemiology, surface antigenic structure, and immune response. Microbiol Rev. 1996;60:267–279. doi: 10.1128/mr.60.2.267-279.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nissinen A, Gronroos P, Huovinen P, Herva E, Katila M-L, Klaukka T, Kontiainen S, Liimatainen O, Oinonen S, Makela P H. Development of β-lactamase-mediated resistance to penicillin in middle-ear isolates of Moraxella catarrhalis in Finnish children, 1978–1993. Clin Infect Dis. 1995;21:1193–1196. doi: 10.1093/clinids/21.5.1193. [DOI] [PubMed] [Google Scholar]
- 26.Ogunnariwo J A, Schryvers A B. Rapid identification and cloning of bacterial transferrin and lactoferrin receptor protein genes. J Bacteriol. 1996;178:7326–7328. doi: 10.1128/jb.178.24.7326-7328.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ogunnariwo J A, Woo T K W, Lo R Y C, Gonzalez G C, Schryvers A B. Characterization of the Pasteurella haemolytica transferrin receptor genes and the recombinant receptor proteins. Microb Pathog. 1997;23:273–284. doi: 10.1006/mpat.1997.0156. [DOI] [PubMed] [Google Scholar]
- 28.Rokbi B, Mazarin V, Maitre-Wilmotte G, Quentin-Millet M-J. Identification of two major families of transferrin receptors among Neisseria meningitidis strains based on antigenic and genomic features. FEMS Microbiol Lett. 1993;110:51–58. doi: 10.1111/j.1574-6968.1993.tb06294.x. [DOI] [PubMed] [Google Scholar]
- 29.Rossi-Campos A, Anderson C, Gerlach G-F, Klashinsky S, Potter A A, Willson P J. Immunization of pigs against Actinobacillus pleuropneumoniae with two recombinant protein preparations. Vaccine. 1992;10:512–518. doi: 10.1016/0264-410x(92)90349-o. [DOI] [PubMed] [Google Scholar]
- 30.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 30a.Schryvers, A. B. Unpublished data.
- 31.Schryvers A B, Lee B C. Comparative analysis of the transferrin and lactoferrin binding proteins in the family Neisseriaceae. Can J Microbiol. 1989;35:409–415. doi: 10.1139/m89-063. [DOI] [PubMed] [Google Scholar]
- 32.Sethi S, Hill S L, Murphy T F. Serum antibodies to outer membrane proteins (OMPs) for Moraxella (Branhamella) catarrhalis in patients with bronchiectasis: identification of OMP B1 as an important antigen. Infect Immun. 1995;63:1516–1520. doi: 10.1128/iai.63.4.1516-1520.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stevenson P, Williams P, Griffiths E. Common antigenic domains in transferrin-binding protein 2 of Neisseria meningitidis, Neisseria gonorrhoeae, and Haemophilus influenzae type b. Infect Immun. 1992;60:2391–2396. doi: 10.1128/iai.60.6.2391-2396.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Struyve M, Moons M, Tommassen J. Carboxy-terminal phenylalanine is essential for the correct assembly of a bacterial outer membrane protein. J Mol Biol. 1991;218:141–148. doi: 10.1016/0022-2836(91)90880-f. [DOI] [PubMed] [Google Scholar]
- 35.Tabor S, Richardson S S. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci USA. 1985;82:1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Teele D, Klein J, Rosner B the Greater Boston Otitis Media Study Group. Epidemiology of otitis media during the first seven years of life in children in greater Boston: a prospective, cohort study. J Infect Dis. 1989;160:83–94. doi: 10.1093/infdis/160.1.83. [DOI] [PubMed] [Google Scholar]
- 37.Vonder Haar R A, Legrain M, Kolbe H V J, Jacobs E. Characterization of a highly structured domain in Tbp2 from Neisseria meningitidis involved in binding to human transferrin. J Bacteriol. 1994;176:6207–6213. doi: 10.1128/jb.176.20.6207-6213.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu H C, Tokunaga M. Biogenesis of lipoproteins in bacteria. Curr Top Microbiol Immunol. 1986;125:127–157. doi: 10.1007/978-3-642-71251-7_9. [DOI] [PubMed] [Google Scholar]
- 39.Yang Y P, Myers L E, McGuinness U, Chong P, Kwok Y, Klein M H, Harkness R E. The major outer membrane protein, CD, extracted from Moraxella (Branhamella) catarrhalis is a potential vaccine antigen that induces bactericidal antibodies. FEMS Immunol Med Microbiol. 1997;17:187–199. doi: 10.1111/j.1574-695X.1997.tb01012.x. [DOI] [PubMed] [Google Scholar]
- 40.Yang Y P, Munson R S, Jr, Grass S, Chong P, Harkness R E, Gissoni L, James O, Kwok Y, Klein M H. Effect of lipid modification on the physicochemical, structural, antigenic and immunoprotective properties of Haemophilus influenzae outer membrane protein P6. Vaccine. 1997;15:976–987. doi: 10.1016/s0264-410x(96)00296-4. [DOI] [PubMed] [Google Scholar]
- 41.Yu R-H, Schryvers A B. The interaction between human transferrin and transferrin binding protein 2 from Moraxella (Branhamella) catarrhalis differs from that of other human pathogens. Microb Pathog. 1993;15:433–445. doi: 10.1006/mpat.1993.1092. [DOI] [PubMed] [Google Scholar]