Abstract
Secondary metabolites (SMs) are the primary source of therapeutics and lead chemicals in medicine. They have been especially important in the creation of effective cures for conditions such as cancer, malaria, bacterial and fungal infections, neurological and cardiovascular problems, and autoimmune illnesses. In the present study, Aspergillus pseudodeflectus AUMC 15761 was demonstrated to use wheat bran in solid state fermentation (SSF) at optimum conditions (pH 7.0 at 30 °C after 10 days of incubation and using sodium nitrate as a nitrogen source) to produce methyl ferulate and oleic acid with significant antioxidant and antibacterial properties. Gas chromatography-mass spectrometry (GC–MS) analysis of the crude methanol extract revealed eleven peaks that indicated the most common chemical components. Purification of methyl ferulate and oleic acid was carried out by column chromatography, and both compounds were identified by in-depth spectroscopic analysis, including 1D and 2D NMR and HR-ESI–MS. DPPH activity increased as the sample concentration increased. IC50 values of both compounds obtained were 73.213 ± 11.20 and 104.178 ± 9.53 µM, respectively. Also, the MIC value for methyl ferulate against Bacillus subtilis and Staphylococcus aureus was 0.31 mg/mL, while the corresponding MIC values for oleic acid were 1.25 mg/mL and 0.62 mg/mL for both bacterial strains, respectively. Molecular modeling calculations were carried out to reveal the binding mode of methyl ferulate and oleic acid within the binding site of the crucial proteins of Staphylococcus aureus. The docking results were found to be well correlated with the experimental data.
Subject terms: Biotechnology, Microbiology
Introduction
Currently, infectious diseases related to bacteria, fungi, and viruses represent a significant issue for global health1. Worldwide, acute respiratory infections (ARIs), intestinal infections, HIV/AIDS, tuberculosis, and malaria annually account for roughly 4, 3, 1.8, 0.7, and 1.3 million deaths, respectively2,3. Rising microbial resistance to conventional medication has had an significant impact on research into novel substances that demonstrate broad-spectrum antibacterial activity4.
Secondary metabolites are synthesized by a range of plants, animals, and microorganisms. Fungi, which are particularly prolific sources of bioactive secondary metabolites5, continue to be among the most important organisms investigated for therapeutic agents and lead compounds in medicine. They have been especially important in the development of effective therapies for cancer, malaria, bacterial and fungal infections, neurological and cardiovascular diseases, and autoimmune disorders6.
Fungi create a broad array of secondary metabolites, some of which are harmful to humans, plants, animals, and the environment7. In contrast, they also serve as a reliable source for the creation of a number of beneficial products, including enzymes8–10, biodiesel11, fatty acids12, and mycotoxins13, as well as being used to make pharmaceutical, agrochemical, and cosmetic commodities. Despite the fact that many secondary metabolites generated from fungi have been discovered previously, there are still many others that have yet to be discovered. Only a few secondary metabolites have been recognized from fungi, although there have been more than species of 150,000 fungi identified thus far (Species Fungorum database). This is because it is difficult to find and recognize new secondary metabolites7.
Methyl ferulate, which was first detected from the medicinal plant Stemona tuberosa, has the ability to cross cell membranes and enter the brain; it also exhibits anti-free radical characteristics14,15. Methyl ferulate has also been found in a diverse range of fruits such as oranges and tomatoes, and in some cereals such as rice and corn16–19. Because of its low toxicity and diminishing oxidation activity, it has been utilized effectively as a food additive20,21 in cosmetics, as well as in health care and skin care products14. Oleic acid is a natural product that has recently become commonly used to prevent food from oxidizing and it also has great antimicrobial potential value against many fungi and bacteria22,23. Oleic acid, which makes up around 80% of the total fatty acids in virgin olive oil24, is used to decrease cholesterol and reduce inflammation in order to avoid heart diseases25–27.
Globally, there is a huge accumulation of agricultural and industrial waste. Nevertheless, because these wastes usually include a substantial content of carbohydrates, minerals, and proteins along with cellulose (30–40%), hemicellulose (20–40%), and lignin (20–30%)28, they should not be regarded as "wastes" but rather as "raw materials" for other industrial processes29. Notwithstanding the fact that they are currently underutilized in Egypt, a variety of agro-industrial wastes have been employed as substrates in the solid state fermentation process due to their availability, low cost, environmental friendliness, prolonged shelf life, and simplicity of downstream processing30,31. As a result, the present study focused on the production of methyl ferulate and oleic acid by Aspergillus pseudodeflectus AUMC 15761 from wheat bran under solid state fermentation conditions as well as on methods to purify, identify, and employ these metabolites as antioxidant and antibacterial agents. In addition, the binding mode of the two obtained compounds within the binding site of the crucial proteins of Staphylococcus aureus was investigated using molecular modeling computations.
Results
Morphological and molecular identification of Aspergillus pseudodeflectus strain
The morphological characteristics of the Aspergillus strain used in this study shared the identical features of A. pseudodeflectus as having radiate, brown conidial heads. Stipes 35–200 × 2.5–3.5 µm. Vesicles globose to clavate, 4–12 µm. Conidia globose to ellipsoidal, brown, 3.5–5 µm. Hülle cells absent (Fig. 1).
Figure 1.
Aspergillus pseudodeflectus AUMC 15761 (A–C), 7—day-old colonies on Cz, MEA, and CYA at 25 °C. (D–F), Conidiophores and biseriate, columnar conidial heads. (Scale bars = 10 µm).
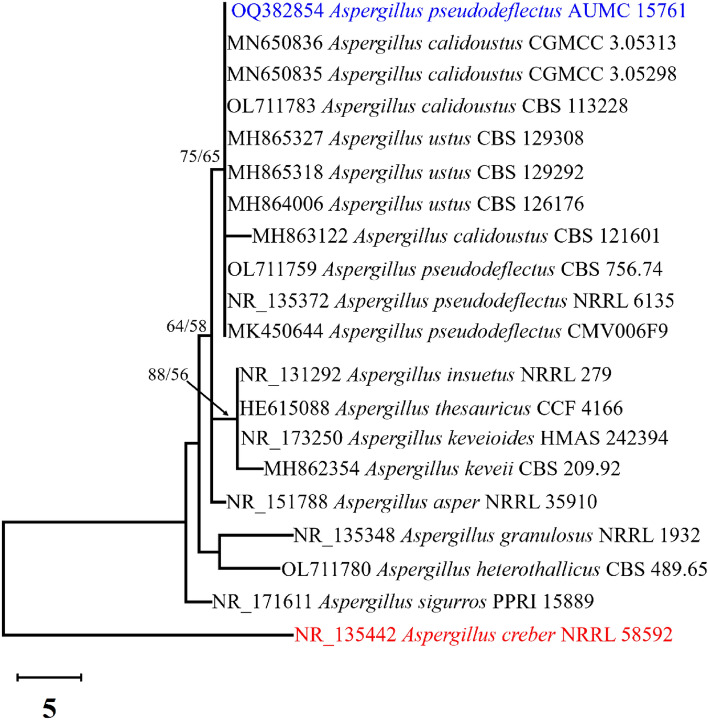
Phylogenetic analysis based on ITS sequencing was employed to confirm the identification of the strain. The final ITS data set contained 20 sequences that produced 616 characters, of which 505 characters could be correctly aligned, 46 characters were counted as variable, and 8 as informative. The Tamura 3-parameter using a discrete Gamma distribution (T92 + G) was the perfect model used to represent the relationship among taxa. The maximum Parsimony method yielded 10 trees, the most parsimonious of which (Fig. 2) has a tree length of 61, the highest log likelihood of − 1180.74, consistency index of 0.733333, retention index of 0.818182, and a composite index of 0.600000 is shown in Fig. 2.
Figure 2.
The most parsimonious phylogenetic tree obtained from a heuristic search (1000 replications) of the ITS sequence of A. pseudodeflectus AUMC 15761 (in blue) compared to other closely similar ITS sequences belonging to genus Aspergillus: section Usti in GenBank. Bootstrap support values for ML/MP ≥ 50% are indicated near the respective nodes. The tree is rooted to Aspergillus creber BRRL 5892 as an outgroup (in red).
Production of secondary metabolites by Aspergillus pseudodeflectus AUMC 15761 utilizing lignocellulosic wastes
The five lignocellulosic wastes (barley bran, date palm leaves, orange peels, rice straw, and wheat bran) used in SSF were fermented in different proportions by A. pseudodeflectus AUMC 15761. Wheat bran produced the most powerful crude extract that exhibited the largest inhibition zone against the examined strains. Following Escherichia. coli in terms of severity of impact were Bacillus subtilis, Staphylococcus aureus, and Staphylococcus epidermidis (Fig. 3).
Figure 3.
Antibacterial activity (as inhibition zone), of the crude extract of different lignocellulosic wastes fermented by A. pseudodeflectus AUMC 15761 under SSF. Mean values (± SD) on the bars of the graph with different letters are significantly different (p ≤ 0.05; n = 3).
GC–MS analysis
The GC–MS analysis of the methanol extract was carried out to evaluate its potential components since wheat bran extract was determined to be the most promising. Based on the retention time, molecular weight, and fragmentation pattern of the most prominent chemicals, the current results revealed eleven peaks. These were shown to have retention times of 23.222, 27.429, and 29.769 min, respectively, for trans-Ferulic acid, 3-(2, 5-Dimethoxyphenyl) propionic acid, and oleic acid (Table 1; Fig. 4).
Table 1.
GC–MS spectral analysis of the chemical compounds detected in the methanol extract of wheat bran fermented by A. pseudodeflectus AUMC 15761 under SSF.
| Retention time (min) | Name of the compound | Molecular weight | Molecular formula |
|---|---|---|---|
| 37.318 | 1,8-dihydroxy-3-methoxy-6-methylAnthraquinone | 270.28 | C16H14O4 |
| 9.023 | 2-Hydroxycyclopent-2-en-1-one | 98.1 | C5H6O2 |
| 27.429 | 3-(2,5-Dimethoxyphenyl)propionic acid | 210.23 | C11H14O4 |
| 27.310 | 3-(3',5'-Dimethoxy-4'-hydroxyphenyl)-E2-propenal | 212.24 | C11H16O4 |
| 23.849 | 4-((1E)-3-Hydroxy-1-propenyl)-2- methoxyphenol | 180.2 | C10H12O3 |
| 13.065 | 4-Vinylphenol | 120.15 | C8H8O |
| 14.141 | Erythritol | 122.12 | C4H10O4 |
| 23.220 | trans- Ferulic acid | 194.18 | C10H10O4 |
| 10.775 | Glycerin | 92.09 | C3H8O3 |
| 26.865 | Hexadecanoic acid | 256.42 | C16H32O2 |
| 29.769 | Oleic acid | 282.5 | C18H34O2 |
Figure 4.
GC–MS chromatogram of the methanol extract wheat bran fermented by A. pseudodeflectus AUMC 15761 under SSF.
Optimization of production conditions of the bio-active secondary metabolites using wheat bran
Based on a one factor at a time (OFAT) analysis, the results obtained revealed that A. pseudodeflectus AUMC 15761 could produce the most bio-active secondary metabolites with the greatest effect against the tested bacteria at pH 7.0 using sodium nitrate as a nitrogen supply after 10 days of incubation at 30 °C. These optimal conditions were found to cause the greatest inhibition of the four tested bacterial strains. The inhibition zones were 20.5 ± 1.3, 37.9 ± 1.7, 27.0 ± 1.8, and 19.0 ± 1.7 mm for B. subtilis, E. coli, S. aureus, and S. epidermidis, respectively (Fig. 5).
Figure 5.
Optimization of fermentation conditions of the bio-active secondary metabolites using wheat bran fermented by A. pseudodeflectus AUMC 15761 under SSF. Mean values (± SD) for bars on the graph with different letters are significantly different (p ≤ 0.05; n = 3).
Production and purification of bio-active secondary metabolites by column chromatography
Aspergillus pseudodeflectus AUMC 15761 could ferment 500 g of wheat bran in SSF, and 90.0 g (18%) of crude extract were produced. Using n-hexane, dichloromethane (DCM), and 0–100% gradients of MeOH in the DCM solvent system, twelve fractions (F1–F12) were obtained after fractionation by means of the VLC column (Table 2), Fraction F3 (750 mg), which was eluted by DCM: MeOH (95: 5) had the highest inhibition (23.0 ± 1.8, 32.9 ± 1.6, 42.5 ± 1.4, and 39.4 ± 1.5 mm) against B. subtilis, E. coli, S. aureus, and S. epidermidis, respectively. Consequently, it was subjected to purification by silica gel open column (1 × 100 cm). Nine sub-fractions (F3S1–F3S9) were subsequently produced as a result, with F3S8 (40 mg) and F3S4 (100 mg) being the two most active. Further purification using a 0.5 × 25 cm open column of both fractions, produced a pure compound 2 (40 mg) from F3S4, while F3S8 required a final purification step by preparative TLC plates (60 PF254) to obtain the pure compounds 1 (8.0 mg).
Table 2.
The antibacterial potential of the fractions obtained by VLC column of wheat bran fermented by A. pseudodeflectus AUMC 15761 under SSF.
| Fractions | Tested microbes | |||
|---|---|---|---|---|
| B. subtilis | E. coli | S. aureus | S. epidermidis | |
| F1- n-hexane | 17.0 ± 1.8fg | 0i | 0i | 0i |
| F2- DCM | 0i | 0i | 17.5 ± 1.5fg | 0i |
| F3 | 23.0 ± 1.8e | 32.9 ± 1.6c | 42.5 ± 1.4a | 39.4 ± 1.5b |
| F4 | 0i | 0i | 0i | 0i |
| F5 | 16.5 ± 1.2gh | 31.5 ± 1.7d | 30.5 ± 1.5d | 33.4 ± 1.6c |
| F6 | 18.3 ± 1.3f | 15.7 ± 0.9h | 17.5 ± 1.2fg | 0i |
| F7 | 0i | 0i | 0i | 0i |
| F8 | 0i | 0i | 0i | 0i |
| F9 | 0i | 0i | 0i | 0i |
| F10 | 0i | 0i | 0i | 0i |
| F11 | 0i | 0i | 0i | 0i |
| F12 | 0i | 0i | 0i | 0i |
*Mean values (± SD) with different letters are significantly different (p ≤ 0.05; n = 3).
HR-ESI MS and NMR spectroscopic analysis
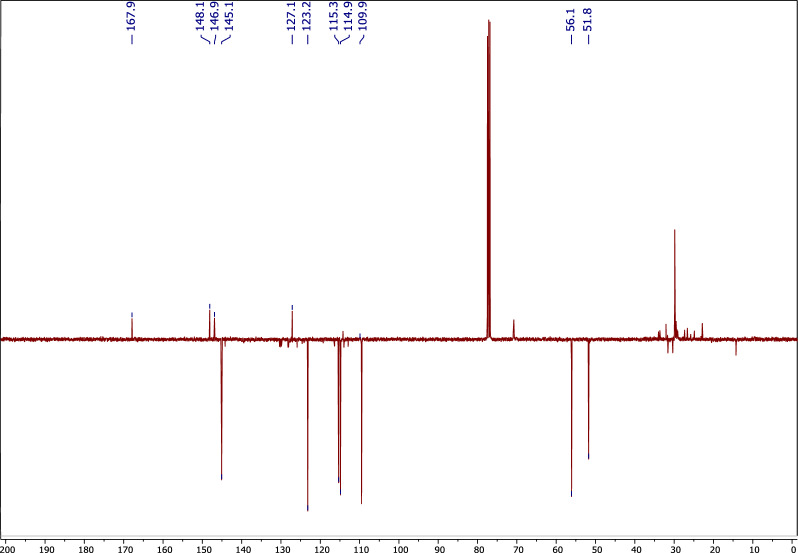
Compound 1 was isolated from A. pseudodeflectus AUMC 15761 as an off-white powder (8.0 mg), suggesting a molecular formula of C11H12O4 as deduced from its HR-ESI–MS spectrum (Fig. 6) which exhibited a [M−H]− peak at m/z 207.0664 and a [M−H + H2O]− peak at m/z 224.9995. The APT NMR spectrum of 1 (Table 3; Fig. 7), along with the HSQC analysis, confirmed the presence of 11 carbon atoms. These showed four quaternary carbons (including one carbonyl ester resonates at δC 167.8), five methines (including an olefinic double bond at δC 145.1 and 115.3), and two methoxy methyls (δC 51.7 and 56.0).
Figure 6.
The HR-ESI–MS spectrum of compound 1.
Table 3.
1H, 13C, and 2D NMR spectroscopic data of methyl ferulate (1) measured in CDCl3 (400 and 100 MHz, respectively).
| No | δH, (J in Hz) | δC, type | 1H–1H COSY | HMBC |
|---|---|---|---|---|
| 1 | – | 127.1, s | – | |
| 2 | 7.02, 1H, d (2.0) | 109.9, d | – | C-4, C-6, C-7 |
| 3 | – | 146.9, s | – | |
| 4 | – | 148.1, s | – | |
| 5 | 6.91, 1H, d (8.0) | 114.8, d | H-6 | C-1, C-3 |
| 6 | 7.06, 1H, dd (2.0, 8.0) | 123.1, d | H-5 | C-2, C-4 |
| 7 | 7.63, 1H, d (16.0) | 145.1, d | H-8 | C-2, C-6, C-8, C-9 |
| 8 | 7.28, 1H, d (16.0) | 115.3, d | H-7 | C-1, C-9 |
| 9 | – | 167.8, s | – | |
| 10 | 3.79, 3H, s | 51.7, q | – | C-9 |
| 11 | 3.92, 3H, s | 56.0, q | – | C-3 |
Figure 7.
APT-NMR spectrum of compound 1 (100 MHz, CDCl3).
The 1H NMR spectrum of 1 (Table 3; Fig. 8), confirmed the presence of an olefinic double bond at δH 7.63 (1H, d, J = 16.0 Hz) and δH 7.28 (1H, d, J = 16.0 Hz). The HSQC correlation between the rest three methines and their corresponding protons [δC 109.9/δH 7.02 (1H, d, J = 2.0 Hz), δC 114.8/δH 6.91 (1H, d, J = 8.0 Hz), and δC 123.1/δH 7.06 (1H, dd, J = 2.0, 8.0 Hz)], along with the rest three quaternaries, revealed the presence of a tri-substituted benzene ring (Table 3; Fig. 9). The olefinic proton resonates at δH 7.28 and showed 1H-1H cosy correlations to the olefinic proton at δH 7.63 (Table 3; Fig. 10) and HMBC correlations with both the carbonyl carbon at δC 167.8 and the aromatic carbon at δC 127.1, indicating that 1 is a cinnamic acid derivative (Table 3; Fig. 11). The HMBC correlations between the methoxy protons [δH 3.79 (3H, s)] and the carbonyl carbon (δC 167.8) confirmed that 1 is a cinnamic acid methyl ester derivative. The other methoxy group at δH 3.92 (3H, s) showed a HMBC correlation with the quaternary aromatic carbon δC 146.9, and was downfield of the quaternary carbon δC 148.1, indicating that 1 is a ferulic acid methyl ester. The stereochemistry of the double bond was determined to be trans by the large coupling constant of its olefinic protons [δH 7.28 (1H, d, J = 16.0 Hz), at δH 7.63 (1H, d, J = 16.0 Hz)]. Based on this cumulative analysis, the structure of 1 was established as methyl ferulate (Fig. 12).
Figure 8.
1H-NMR spectrum of compound 1 (400 MHz, CDCl3).
Figure 9.
HSQC spectrum of compound 1 (400 MHz, CDCl3).
Figure 10.
1H–1H COSY spectrum of compound 1 (400 MHz, CDCl3).
Figure 11.
HMBC spectrum of compound 1 (400 MHz, CDCl3).
Figure 12.
Chemical structure of methyl trans-ferulate (1) and oleic acid (2) produced by A. pseudodeflectus AUMC 15761 on wheat bran under SSF.
Compound 2 was isolated from A. pseudodeflectus AUMC 15761 as a colorless oil (40.0 mg). HR-ESI–MS (Fig. 13) suggested the molecular formula as C18H34O2 according to the mass peak [M + H]+ at m/z 283.2641. The 1H-NMR spectrum (Table 4; Fig. 14) exhibited a protons signal at δH 5.34 (2H, m, H-9, H–10) and δH 2.01 (4H, m, H–8, H–11), assignable to olefinic protons and allylic protons, respectively. The spectrum also revealed a methylene group α to carbonyl functionality (δH 2.34, 2H, t, J = 7.6, H-2). This was also substantiated by the APT NMR spectrum of 2 (Table 4; Fig. 15) which revealed the presence of a carbonyl carbon signal (δC 180.4, C–1), two olefinic carbon signals (δC 130.2 and 129.9, C–9, C–10), a group of methylene carbons resonances at δC 22.8–34.2, and finally a primary methyl group signal (δC 14.3, C–18), all of which were in agreement with a monounsaturated fatty acid. Thus, by comparing the 1H, 13C-NMR, and mass data of compound 2, oleic acid was therefore determined to be the substance involved (Fig. 12).
Figure 13.
HR-ESI–MS spectrum of compound 2.
Table 4.
1H, 13C-NMR spectroscopic data of compound 2.
| No | δH, (J in Hz) | δC, type |
|---|---|---|
| 1 | 180.4, s | |
| 2 | 2.34, 2H, t, (7.6) | 34.2, t |
| 3 | 1.64, 2H, m | 24.8, t |
| 4–7, 12–17 | 1.25, 34H, m | 22.8–32.1, t |
| 8, 11 | 2.01, 4H, m | 27.3, 27.4, t |
| 9,10 | 5.34, 2H, m | 129.9, 130.2, d |
| 18 | 0.87, 3H, t, (7.2) | 14.3, q |
Figure 14.
1H-NMR spectrum of compound 2 (400 MHz, CDCl3).
Figure 15.
APT-NMR spectrum of compound 2 (100 MHz, CDCl3).
Antioxidant activity of methyl ferulate and oleic acid produced by Aspergillus pseudodeflectus AUMC 15761
The results of the current study revealed two antioxidant substances—methyl ferulate and oleic acid. The DPPH activity increased as the sample concentration increased (Fig. 16A), with IC50 values of 73.213 ± 11.20 and 104.178 ± 9.53 µM were significantly greater (p < 0.05) respectively, for both substances, as compared to the value (60.299 ± 4.769 µM) of ascorbic acid (Fig. 16B; Table 5).
Figure 16.
(A) Antioxidant activity (% DPPH) and (B) IC50 of methyl ferulate and oleic acid produced by A. pseudodeflectus AUMC 15761 compared to ascorbic acid as standard.
Table 5.
The t-Test of pairwise comparison between methyl ferulate and oleic acid compared to ascorbic acid.
| Methyl ferulate | Oleic acid | Ascorbic acid | |
|---|---|---|---|
| Mean | 73.213 | 104.178 | 60.299 |
| Variance | 125.440 | 90.821 | 22.743 |
| Observations | 3.000 | 3.000 | 3.000 |
| Pooled variance | 108.130 | 56.782 | |
| Hypothesized mean difference | 0.000 | 0.000 | |
| Df | 4.000 | 4.000 | |
| t Stat | -3.647 | 7.132 | |
| P(T < = t) one-tail | 0.011 | 0.001 | |
| t Critical one-tail | 2.132 | 2.132 | |
| P(T < = t) two-tail | 0.022 | 0.002 |
Antibacterial activity of methyl ferulate and oleic acid
In the present study, the antibacterial tests against B. subtilis and S. aureus demonstrated a significant antibacterial impact for both methyl ferulate and oleic acid. The MIC of methyl ferulate against the two bacterial strains was 0.31 mg/mL, while the MICs of pure oleic acid against both strains were 1.25 and 0.62 mg/mL, respectively (Tables 6, 7; Fig. 17).
Table 6.
Antibacterial activity of pure methyl ferulate produced by Aspergillus pseudodeflectus AUMC 15761 from wheat bran in SSF compared to the Bacitracin antibacterial standard.
| Tested bacteria | Purified Methyl ferulate (mg/mL) | Mean of Bacitracin (10 U) (MB) | P value | R value | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 | 2.5 | 1.25 | 0.62 | 0.31 | 0.15 | ||||||||||
| M | %MB | M | %MB | M | %MB | M | %MB | M | %MB | M | %MB | ||||
| B. subtilis | 15.43 | 1.08 | 14.7 | 1.03 | 13.5 | 0.94 | 12.2 | 0.85 | 11.3 | 0.79 | 0 | – | 14.33 | 0.326 | 0.92 |
| E. coli | 0 | – | 0 | – | 0 | – | 0 | – | 0 | – | 0 | – | 0 | – | – |
| S. aureus | 20.27 | 0.97 | 18.23 | 0.87 | 16.5 | 0.79 | 15.23 | 0.73 | 14.6 | 0.69 | 0 | – | 21 | 0.337 | 0.98 |
| S. epidermidis | 0 | – | 0 | – | 0 | – | 0 | – | 0 | – | 0 | – | 0 | – | – |
For the data above, r equals 0.92, 0.98. This represents a very strong positive correlation. M = mean; %MB = mean value divided by the standard value.
Table 7.
Antibacterial activity of pure oleic acid produced by A. pseudodeflectus AUMC 15761 from wheat bran in SSF compared to the Bacitracin antibacterial standard.
| Tested bacteria | Purified oleic acid (mg/mL) | Mean of Bacitracin (10 U) (MB) | P value | R value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 | 2.5 | 1.25 | 0.62 | 0.31 | |||||||||
| M | %MB | M | %MB | M | %MB | M | %MB | M | %MB | ||||
| B. subtilis | 11.5 | 0.8 | 9.67 | 0.67 | 8.33 | 0.58 | 0 | – | 0 | – | 14.33 | 0.41 | 0.833 |
| E. coli | 0 | – | 0 | – | 0 | – | 0 | – | 0 | – | 0 | – | – |
| S. aureus | 17.73 | 0.84 | 12.53 | 0.6 | 9.6 | 0.46 | 8.47 | 0.4 | 0 | – | 21 | 0.582 | 0.883 |
| S. epidermidis | 0 | – | 0 | – | 0 | – | 0 | – | 0 | – | 0 | – | – |
For the data provided above, r equals 0.833, 0.883. This represents a very strong positive correlation.
*Statistically significant at p < 0.05 (**) highly statistically significant at p < 0.01.
R = zero (no linear association between the variables or no consistent linear component to that relationship);R = 1 (perfect positive linear relationship between the variables); 0 < R < 1 (positive linear trend and the sampledindividuals are scattered around the line of best fit; the smaller the absolute value of R the less well the data canbe visualized by a single linear relationship. M = mean; %MB = mean value divided by the standard value.
Figure 17.
Antibacterial activity of the pure methyl ferulate and oleic acid produced by A. pseudodeflectus AUMC 15761 from wheat bran in SSF on (A,E), B. subtilis (B,F), E. coli (C,G), S. aureus (D,H) and, S. epidermidis, respectively (1 = 5 µg/mL, 2 = 2.5 µg/mL, 3 = 1.25 µg/mL, 4 = 0.62 µg/mL, and 5 = 0.31 µg/mL).
R = zero (no linear association between the variables or no consistent linear component to that relationship); R = 1 (perfect positive linear relationship between the variables); 0 < R < 1 (positive linear trend and the sampled individuals are scattered around the line of best fit; the smaller the absolute value of R the less well the data can be visualized by a single linear relationship. M = mean; %MB = mean value divided by the standard value.
Docking computations
In order to recognize target proteins for the antibacterial activity of the two compounds obtained in the present study, four various protein targets of Staphylococcus aureus were investigated, including dihydrofolate reductase (PDB code: 5ISP32), pyruvate kinase (PDB code: 5OE333), and sortase A (PDB code: 2MLM34). Before generation of data, the performance of the AutoDock 4.2.6 software was evaluated via the re-docking of the co-crystalized inhibitors towards their targets. Based on the data illustrated in Fig. 18, the anticipated binding modes were essentially identical to their native structures with RMSD values less than 1.0 Å, displaying the strong accuracy of the utilized technique.
Figure 18.
(i), Superimposed structures of the experimental mode (in cyan) and the anticipated docking pose (in grey) (ii), 2D representation of the predicted binding modes of the co-crystalized ligands with the active site of different protein targets of Staphylococcus aureus.
Using the assessed protocol, the docking pose of oleic acid and methyl ferulate with dihydrofolate reductase, pyruvate kinase, and sortase A was predicted and presented in Fig. 18. As illustrated in Fig. 19, oleic acid and methyl ferulate exhibited multiple H-bonds and additional interactions involving vdW (van der Waals), pi-based, and hydrophobic interactions. More precisely, oleic acid displayed an eminent docking score towards dihydrofolate reductase compared to methyl ferulate, with values of − 6.3 and − 5.7 kcal/mol, respectively (Table 8). Inspection the binding mode of oleic acid demonstrated that 2 H-bonds with GLN19 (1.78 Å) and LEU20 (2.18 Å). However, methyl ferulate exhibited 3 H-bonds with SER49 (1.68 Å), ALA7 (1.98 Å), and TYR98 (2.44 Å).
Figure 19.
2D molecular interaction of the obtained compounds towards the active site of different protein targets of Staphylococcus aureus.
Table 8.
Computed docking scores of the obtained compounds towards different protein targets of Staphylococcus aureus.
| Compound | Docking score (kcal/mol) | ||
|---|---|---|---|
| Dihydrofolate reductase | Pyruvate kinase | Sortase A | |
| Oleic acid | − 6.3 | − 6.3 | − 5.2 |
| Methyl ferulate | − 5.7 | − 5.7 | − 4.9 |
For pyruvate kinase, oleic acid, and methyl ferulate, the exposed docking scores had values of − 6.3 and − 5.7 kcal/mol, respectively. Oleic acid formed a hydrogen bond with GLY307 (2.10 Å). In comparison, methyl ferulate exhibited four hydrogen bonds with HIS394 (2.26 Å), GLY302 (2.01 Å), GLY279 (1.88 Å), and GLY300 (2.16 Å). Sortase A, oleic acid and methyl ferulate displayed good docking scores with values of − 5.2 and − 4.9 kcal/mol, respectively. Oleic acid exhibited three hydrogen bonds with THR106 (1.99 Å), ARG139 (2.97 Å), and TRP136 (1.90 Å). On the other hand, methyl ferulate established two H-bonds with GLY61 (2.12 Å) and CYS126 (3.03 Å).
Discussion
Secondary metabolites created by fungi have been shown to be a wonderful source of new drugs, biofuels, industrial chemicals, food additives, and feed additives35. Penicillins, lovastatin, echinocandin B, and cyclosporine A offer examples of how important it is to investigate fungal sources for novel pharmaceuticals36. Aromatic compounds, amino acids, fatty acids, butanolides, butenolides, cytochalasans, macrolides, naphthalenones, pyrones, and terpenes are only a few of the structural types of metabolites that fungi produce36,37. There have been 15600 fungal metabolites identified from species of Alternaria, Aspergillus, Claviceps, Fusarium, and Penicillium38.
In the present study, A. pseudodeflectus AUMC 15761 was identified based on sequencing of the ITS region. The fungus utilized wheat bran as a substrate in SSF to produce, for the first time, methyl ferulate and oleic acid, as confirmed by an antibacterial bioassay. Methyl ferulate was first reported from the plant Stemona tuberosa14,15 and then re-isolated from Coriolopsis aspera39,40, Morinda citrifolia41, and Kigelia. africana42. The Aspergillus ustus group has been found to produce several active metabolites such as the three novel isoquinoline alkaloids, TMC-120A-C (1–3) (furo [3, 2-h] isoquinoline-type) discovered from A. ustus TC 111843; averufin, versicolorine C, along with austalides O and J have been isolated from different strains of A. ustus44; A newly isochroman derivative, pseudodeflectusin (9-hydroxy-7-methyl-2-(methylethylidine)-furano [3, 2-H] isochroman-3-one), was discovered from Aspergillus pseudodeflectus45; Drimane sesquiterpenoids 1, 15 were isolated from Aspergillus pseudodeflectus strains F-1860, F-186144; Ophiobolin K and 6-epi-ophiobolin K, have been developed from A. calidoustus UFMGCB 410746; Drimane sesquiterpenoids 1, 2, 7, and 15, TMC-120A-C, desferritriacetylfusigen and TMC-120 derivative 1, have been found in several strains of A. calidoustus44.
In addition, several bio-active secondary metabolites have been produced by many species of fungi in SmF or SSF47, such as penicillins and cephalosporins isolated from Penicillium and Acremonium, respectively48,49; feruloyl esterase produced by Aspergillus terreus GA2 using maize bran50; methyl ferulate from the fruiting bodies of Coriolopsis aspera40; festuclavine 2 produced by A. fumigatus51; cyclosporine 41, which exhibits broad spectrum of antifungal activity52, derived from Toplypocladium inflatum53; geranylgeraniol, farnesol, hexacosanol, oleic acid, and squalene synthesized by Colletotrichum coccodes54; 1-Octacosanol produced by Phyllosticta capitalensis55; a set of peptaibols, that are extremely potent growth inhibitors of several species of fungi, including the plant pathogens Alternaria alternata, Phoma cucurbitaceum, Fusarium spp., as well as the human pathogen A. fumigatus, have been reported from Trichoderma reesei56; and Echinocandin B 38 from Aspergillus nidulans57.
The most potent antibacterial crude metaboliate from Aspergillus pseudodeflectus AUMC 15761’s was produced after 10 days of fermentation utilizing wheat bran in SSF at pH 7.0 and 30 °C, using sodium nitrate as a nitrogen source. The pH of the growth medium and other physical factors, such as the incubation temperature, were found to have a substantial impact on the production of secondary metabolites, with synthesis rapidly decreasing on either side of an optimal point. By changing the degree of dissociation of different molecules in the media, the quantity of hydrogen or hydroxyl ions may have direct or indirect effects on a cell. As a result, variations in pH have an effect on the solubility and dissociation of intermediate products as well as the activity of microbial enzymes58,59.
One of the unsaturated fatty acids formed by the reaction of palmitic and stearic acids is oleic acid. Moreover, enzymatic activity can convert saturated fatty acids such as stearic acid and palmitic acid into oleic acid60. In the present study, the methanolic extract of wheat bran fermented by A. pseudodeflectus AUMC 15761 was utilized to isolate oleic acid. Saturated fatty acids and monounsaturated fatty acids, including palmitic and oleic acids, occurred in abundance in the fatty acid profiles of Mucor circinelloides URM 4140, M. hiemalis URM 4144, and Penicillium citrinum URM 412661. Oleic acid, also known as 9-octadecenoic acid, is a healthy kind of omega-9 unsaturated fatty acid that is very useful for people's health22. Unsaturated fatty acids do indeed lower cholesterol by activating cholesterol acetyltransferase, as is widely known. Cancer, cardiovascular, autoimmune, Parkinson's, Alzheimer's, inflammatory, and hypertensive illnesses are all treated effectively with fatty acids. These compounds have been employed as an anticancer treatment because they may cause cancer cells to undergo apoptosis and regulate the cell membrane22.
Aspergillus terreus, Claviceps purpurea, Tolyposporium sp., Mortierella alpina, and Mortierella isabellina are a few examples of species of fungi that may collect lipids. Although several fungi may produce lipids, the majority of fungi are studied primarily for their capacity to create specific lipids such as docosahexaeneoic acid (DHA), gamma-linolenic acid (GLA), eicosapentaenoic acid (EPA), and arachidonic acid (ARA)62.
Bio-guided isolation of the secondary metabolites of the methanol extract of A. pseudodeflectus AUMC 15761 led to the purification of two active compounds—methyl ferulate and oleic acid. Their structures were determined by comparing their NMR and HR-EI-Ms data with that available from the literature15,39,42. It is noteworthy that this is the first report of producing oleic acid and methyl ferulate from A. pseudodeflectus.
Methyl ferulate and oleic acid in this study were found to have a significant antibacterial potential against gram positive bacteria, showing the best activity against B. subtilis and S. aureus, while E. coli and S. epidermidis were not affected by either compound in the present study. In the study, the MICs for methyl ferulate and oleic acid against B. subtilis and S. aureus were 79% and 69%, respectively, compared to the bacitracin standard, with MICs of 79% and 69%, and 58% and 40%, respectively. Similarly, the antibacterial activity of methyl ferulate against Shigella putrefaciens was determined63. Certain oils, including rose essential oil, have been proven to have antibacterial properties against S. aureus, and the efficacy against gram positive S. aureus was observed to be less than that of rifampin and gentamicin, with negligible MIC values64. Because of the observed sensitivity of gram-positive bacteria to the presence of one phenolic hydroxyl group in methyl ferulate, its antibacterial mechanism was taken into consideration42,65. Although the exact mechanism of the antibacterial activity of fatty acids is unknown, it is thought that their functional nature is connected to the permeability, membrane disruption, and fatal changes in the bacterial cytoplasm. As a result, rupture or alteration of the membrane-dependent conduction systems may occur22,66. Escherichia coli, a normally resistant bacterium, becomes very susceptible to the bactericidal effects of fatty acids if the lipopolysaccharide outer membrane is destroyed using ethylenediaminetetraacetic acid. As gram-negative bacteria, they are protected by their outer lipid membrane from the corrosive effects of oleic acid67.
Methyl ferulate has been reported to have antioxidant activity (% DPPH), with IC50 values of 73.213 11.20 µM, respectively41. Considering that, the principal mode of action of phenolic antioxidants is believed to be the scavenging of free radicals68. Due to the presence of one phenolic hydroxyl group in methyl ferulate, its antioxidant mechanism was taken into consideration42,65. Antioxidant activity (% DPPH) of OA with IC50 values of 104.178 ± 9.53 µM has been reported in the literature69. Methy ferulate is a new natural antibacterial agent with strong efficacy and low toxicity. It has great potential for applications in food preservation63. Oleic acid, which accounts for about 80% of the total fatty acids in virgin olive oil, has recently become an often used substance to protect food from oxidizing24.
Oleic acid and methyl ferulate both had positive docking results against several Staphylococcus aureus protein targets, based on the docking results. The development of hydrogen bonding interactions with the active site of the Staphylococcus aureus target proteins under investigation may be responsible for the high docking scores of these two compounds. These findings shed additional light on the significance of the chemicals that have been identified as potential candidates for antibacterial medication.
Materials and methods
Materials and chemicals
For the extraction, fractionation, and column chromatography, the organic solvents used were supplied by El-Nasr Pharmaceutical and Chemical Co. (ADWIC), Egypt. The deuterated chloroform (CDCl3) used for NMR analysis was purchased from Sigma-Aldrich. TLC pre-coated plates (F254 & PF254) and silica gel for column chromatography (70–230 and 230–400 Mesh) were provided by Merck (Darmstadt, Germany).
Strain isolation and preservation
Using the dilution plate technique70, the strain for this investigation was isolated from a soil sample collected from Egypt's Aswan Governorate. Before adding Czapek's Dox agar (CzA) to Petri plates, the soil solution was appropriately diluted. The cultures were then maintained for two weeks at 25 °C. To create pure cultures of the fungus, the developed colonies were purified on CzA utilizing the single spore isolation technique71.
Morphological and molecular identification of the Aspergillus strain
For morphological identification of the strain of Aspergillus, the fungus was inoculated on Malt Extract Agar (MEA), Czapek’s Yeast Autolysate Agar (CYA), and CzA72, and incubated for 7 days at 25 °C. The fungus in this study was morphologically identified on the basis of its macroscopic and microscopic characteristics, following the relevant key of Aspergillus: section Usti73. This strain was deposited and designated as AUMC 15761 in the culture collection of the Assiut University Mycological Centre. DNA isolation was carried out74, and the PCR reaction was performed by SolGent Co., Ltd (Daejeon, South Korea) using SolGent EF-Taq and the universal primers ITS1 and ITS475. DNASTAR (version 5.05) was used to generate the contiguous sequences of the species of Aspergillus used in this investigation. There are 20 sequences in the overall ITS dataset that were used for phylogenetic analysis, consisting of one outgroup sequence for Aspergillus creber NRRL 58,592 (NR_135442), the sequence for Aspergillus pseudodeflectus (AUMC 15761 in this manuscript), and 18 sequences from the genus Aspergillus: section Usti acquired from GenBank. MAFFT (version 6.861b)76 with the default settings was used to align all sequences. Optimization of the alignment gaps and sparse uninformative characters was conducted by BMGE77. Maximum-likelihood (ML) and maximum-parsimony (MP) phylogenetic analyses were carried out using MEGA X (version 10.2.6)78, and 1000 replications79 were employed to assess the robustness of the most parsimonious trees. The ideal nucleotide substitution model for ML analysis was identified using Modeltest 3.7's Akaike Information Criterion (AIC)80. After editing, the tree was saved in TIF format81.
Inoculum
Aspergillus pseudodeflectus AUMC 15761 was grown for 7 days on MEA at 25 °C and a spore solution (prepared in 10% tween 80) containing 1.5 × 108 spores/mL was used to inoculate the substrate (5 mL/10 g substrate)82.
Solid state fermentation (SSF) of lignocellulosic wastes
In order to determine how the selected isolate of A. pseudodeflectus produced an antibacterial chemical, five samples of agricultural waste were studied. The fermentation materials included barley bran (BB), date palm leaves (DPL), orange peels (OP), rice straw (RS), and wheat bran (WB). They were purchased from local marketplaces in Egypt's Assiut Governorate. Before being reduced to a size of 500 µm, they were cleaned with tap water to get rid of dirt and other impurities. As part of the pretreatment procedure, the samples were treated with 1.0% NaOH, thoroughly filtered, and then washed with tap water. They were then dried at 60 °C83. In order to determine how the selected A. pseudodeflectus produced an antibacterial chemical, five samples of agricultural waste were studied. Separate Erlenmeyer flasks (500 mL) were filled with 10 g of the lignocellulosic material, and the residue was then wetted down by 88% with a citrate buffer (pH 5.0). The flasks were next incubated for seven days at 30 °C.
Extraction of bio-active compounds
Following the incubation time, the flask contents underwent 48 h of oven drying at 60 °C. The mycelial mat and solid substrate were stirred in 50 mL of methanol for 2 h at 180 rpm in each flask. The clear supernatant was obtained after centrifugation (10,000 rpm at 4 °C for 10 min). The volume of the methanol extract was then reduced by a rotary evaporator (Heidolph: Model reddot winner 2020; Germany). The sample was lyophilized into a powder using a freeze dryer (VirTis: Model 6 KBTES-55, NY; USA)84.
Antibacterial effect of the crude extracts
The agricultural waste-derived crude extract residue from each sample was dissolved in dimethyl sulfoxide (DMSO) at a 10% concentration. The antibacterial efficacy of the crude extract was assessed using the agar well diffusion technique85, using 50 µL in each 5 mm well for Escherichia coli ATCC 8739, Bacillus subtilis ATCC 6633, Staphylococcus aureus ATCC 6538, and Staphylococcus epidermidis ATCC 12228. Bacitracin (10 U) and Piperacillin/Tazobactam 10: 1 (110 µg/disc), served as a positive control. This test was performed after each stage of purification.
GC–MS analysis
This analysis was carried out at the Analytical Chemistry Unit (ACAL), Faculty of Science, Assiut University, Egypt. Before being injected into a GC–MS device (7890A-5975B; Thermo Scientific GC/MS; model ISQ; USA), with a nonpolar HP-5MS Capillary Standard column (30 × 0.25 × 0.25) mm, 0.5 g of the sample residue was dissolved in 5 mL of methanol and centrifuged for 15 min (10,000 rpm and 5 °C). The following was the cycle's parameters: oven program on at 120 °C for 5 min, 30 °C/min rising to 265 °C for 25 min, then 50 °C/min increased to 280 °C for 5 min; run duration 48 min; post run 260 °C for 2 min; flow program 0.5 mL/min. for 10.9 min., and then 1 mL/min for 30 min. Equilibration time was 0.5 min, and the maximum temperature 280°C84.
Optimization of bio-active secondary metabolites production by A. pseudodeflectus AUMC 15761
For maximization of secondary metabolites production, the respective pH (4.0, 5.0, 6.0, 7.0, and 8.0), temperature (20, 25, 30, 35, and 40 °C), nitrogen source (peptone, yeast extract, sodium nitrate, ammonium chloride, and ammonium sulphate, each at 0.2%), incubation time (2, 4, 6, 8, up to 14 days) were adjusted using one factor at a time (OFAT)84. For the testing, a 10 g amount of wheat bran was placed in 500 mL Erlenmeyer flasks. Following the incubation time, the bio-active Secondary metabolites were extracted as described above and then used in further tests.
Production of bio-active secondary metabolites by A. pseudodeflectus AUMC 15761 utilizing wheat bran in SSF
Ten g portions of wheat bran were separately placed into 500 mL Erlenmeyer conical flasks, and the spore suspension (1.5 × 108 spore/mL) was prepared from a seven-day-old A. pseudodeflectus AUMC 15761 culture and added to each flask in a volume of 5.0 mL were used to inoculate the residue. The flasks were then kept under optimal production cultural conditions. Following the incubation time, the flask contents were extracted, and the dried extract was applied throughout the purification process.
Purification of the secondary metabolites by column chromatography
Sample preparation for column chromatography
The residue was combined with an equivalent weight of silica gel powder and a trace amount of methanol was added to create a slurry prior to each step of purification. The extract was placed onto a vacuum liquid chromatography (VLC) column for fractionation after being dried and slurred. The last stage of purification was carried out by adding the obtained fractions to silica gel column chromatography84.
Vacuum liquid chromatography (VLC)
With 900 g of silica gel (230–400 Mesh), the entire crude extract was fractionated using a VLC column (5.0 × 120 cm). The n-hexane, Dichloromethane (DCM) and 0–100% gradients of DCM in MeOH (by adding 10.0% MeOH each time) were used in the fractionation process. A low-pressure evaporator was used to dry out the solvents after collecting a total of 250 mL of elutes. Fractions that produced the strongest antibacterial properties and have comparable spots were combined, condensed, and dried for use in the further purification process.
Thin layer chromatograph (TLC)
TLC was carried out on pre-coated silica gel F254 plates. A series of solvents of increasing polarities were used for developing the spots. For visualization of the spots, the plates were subjected to a UV inspection (at 365 and 254 nm) and then sprayed with 10% (v/v) H2SO4 in methanol, dried using a hot air drier, and heated to 110°C84.
Final purification of the active secondary metabolites
The fraction containing active chemicals was subsequently chromatographed on an open column (1.0 × 100 cm) equipped with a 35 g silica gel (70–230 Mesh). It was eluted in n–hexane gradients with 0–20% EtOAc (adding 1.0% EtOAc each time). TLC was used to detect 25 mL elutes using three mobile systems (n–hexane: EtOAc: 95: 5, 90: 10, and 80: 20). After combining and drying similar elutes, nine subfractions were obtained. The subfractions containing the most active compounds were uploaded over a second column (0.5 × 25 cm) packed with 15 g silica gel and a solvent system of n–hexane: acetone (95: 5) was used for elution. A 15 mL elutes were collected, subjected to TLC, and those containing nearly pure compounds were combined. Following the method of Siddiqui, et al.86, they were finally purified using preparative TLC plates (60 PF254). The antibacterial test for the purified compound was performed as described above.
Spectroscopic NMR
The analysis was completed at the Micro-Analytical Unit (MAU) of Cairo University's School of Pharmacy in Giza, Egypt. Bruker Avance III HD 400 and 100 MHz spectrometers (Bruker Biospin, Rheinstetten, Germany) and NMR software Topspin 3.2 pl 6 were used to produce the 1H and 13C-NMR spectra. The internal reference standard was tetramethylsilane (TMS). An LTQ Orbitrap XL spectrometer was used to obtain the HR-ESI–MS data (Thermo Fisher Scientific; USA).
DPPH radical scavenging activity
Using the methods described by Yen and Duh87, a freshly prepared methanol solution containing 0.004% (w/v) of the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical was refrigerated at 10 °C in the dark. Pure compound concentrations of 100, 50, 25, 12.5, 6.25, 3.125, 1.56, and 0.78 µM methanol were utilized for the pure synthesized sample, respectively. The absorbance at 515 nm was estimated using a 40 µL fraction of the sample-containing methanol solution combined with 3 mL of the DPPH solution (Milton Roy, Spectronic 1201; Canada). The decrease in the absorbance at 515 nm was monitored continuously until it stabilized, with data obtained at 1 min intervals for 16 min. The absorbance of the DPPH radical without an antioxidant was measured, as was the absorbance of ascorbic acid, a reference chemical. The percentage inhibition (PI) of the DPPH radical was estimated using the Eq. (1):
| 1 |
where AC is the control absorbance at time zero and AT is the sample absorbance plus DPPH at 16 min. The dose–response curve graphic plots were used to estimate the 50% inhibitory concentration (IC50), which is the concentration necessary to inhibit the DPPH radical by 50%. The experiment was carried out in triplicate.
Computational methods
The crystal structures of dihydrofolate reductase (PDB code: 5ISP32), pyruvate kinase (PDB code: 5OE333), and sortase A (PDB code: 2MLM34) were obtained and utilized as templates for all in-silico computations. To prepare these proteins, all inhibitors, ions, and water molecules were removed. Modeller software was employed to construct all missing residues88. In addition, the H + + website was utilized to inspect the protonation states of the studied targets89. All missing hydrogens were added. The structure of the investigated inhibitors was manually built. Prior to docking computations, all inhibitors were minimized using the MMFF94S force field within SZYBKI software90,91. The atomic charges of the obtained compounds were determined utilizing the Gasteiger method92. All docking calculations were conducted using AutoDock4.2.6 software93. Based on the AutoDock protocol, the investigated targets were saved in pdpqt format94. The number of genetic algorithm (GA) runs was adjusted to 250. Moreover, energy evaluations (eval) were set to 25,000,000. The default settings of the other docking parameters were utilized. The grid box was set out to include the active site for all investigated targets.
Statistical analysis
The experimental data were recorded as an average value and SD, and each test was done in three replicates. The Duncan's multiple range test was performed after the analysis of variance (ANOVA: two-factor with replication) and (t-Test: Two-Sample Assuming Equal Variances) on the data95,96.
Conclusions
In this study, Aspergillus pseudodeflectus AUMC 15761 used wheat bran in SSF to produce methyl ferulate and oleic acid for the first time, both of which have strong antibacterial and antioxidant properties. The most prevalent chemical components were determined using gas chromatography-mass spectrometry (GC–MS) analysis of the crude methanol extract. An in-depth spectroscopic investigation was used to identify methyl ferulate and oleic acid, which were then purified using column chromatography. Significant antioxidant and antibacterial properties of the two compounds against Bacillus subtilis and Staphylococcus aureus, respectively, were detected. These findings were ditto analyzed in-silico utilizing the molecular docking technique. According to docking computations, methyl ferulate, and oleic acid demonstrated good antibacterial activity.
Acknowledgements
Prof. Dr. M. T. Said is greatly appreciated for his kind help in all statistical analyses.
Author contributions
All authors participated equally to data analysis, authoring, and revising the article. The final version of the manuscript has been reviewed and approved by all authors.
Funding
Open access funding is provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This research was also funded by Researchers Supporting Project number (RSP2024R364), King Saud University, Riyadh, Saudi Arabia.
Data availability
All data related to this manuscript are incorporated in the manuscript only.
Competing interests
The authors declare no competing interests.
Footnotes
The original online version of this Article was revised: The Funding section in the original version of this Article was incomplete. It now reads "Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This research was also funded by Researchers Supporting Project number (RSP2024R364), King Saud University, Riyadh, Saudi Arabia.”.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
4/8/2024
A Correction to this paper has been published: 10.1038/s41598-024-58336-9
Contributor Information
Fuad Ameen, Email: fuadameen@ksu.edu.sa.
Osama Abdel-Hafeez Mohamed Al-Bedak, Email: osamaalbedak@science.au.edu.eg.
References
- 1.Li D, Rui Y-X, Guo S-D, Luan F, Liu R, Zeng N. Ferulic acid: A review of its pharmacology, pharmacokinetics and derivatives. Life Sci. 2021;284:119921. doi: 10.1016/j.lfs.2021.119921. [DOI] [PubMed] [Google Scholar]
- 2.Lozano, R. et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet380, 2095–2128 (2012). [DOI] [PMC free article] [PubMed]
- 3.Tang Y, Hao J, Fan C, Cao X. Preparative separation of high-purity trans-and cis-ferulic acid from wheat bran by pH-zone-refining counter-current chromatography. J. Chromatogr. A. 2021;1636:461772. doi: 10.1016/j.chroma.2020.461772. [DOI] [PubMed] [Google Scholar]
- 4.Malheiro JF, Maillard J-Y, Borges F, Simões M. Evaluation of cinnamaldehyde and cinnamic acid derivatives in microbial growth control. Int. Biodeter. Biodegrad. 2019;141:71–78. doi: 10.1016/j.ibiod.2018.06.003. [DOI] [Google Scholar]
- 5.Pelaez F. Biological activities of fungal metabolites. Handb. Ind. Mycol. 2004;22:41–92. [Google Scholar]
- 6.Newman DJ, Cragg GM. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016;79:629–661. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- 7.Hawksworth, D., Lücking, R. Fungal diversity revisited: 2.2 to 3.8 million species. Microbiol. Spectr.5, FUNK-0052-2016 (2017). [DOI] [PMC free article] [PubMed]
- 8.Ismail MA, Moubasher AH, Mohamed RA, Al-Bedak OA. Agro–industrial residues as alternative sources for cellulases and xylanases production and purification of xylanase produced by Aspergillus flavus AUMC 10331 isolated from extreme habitat. Curr. Res. Environ. Appl. Mycol. 2018;8:313–322. doi: 10.5943/cream/8/3/3. [DOI] [Google Scholar]
- 9.AL-Kolaibe, A. M., Moharram, A. M. & Al-Bedak, O. A. Worthwhile enzyme production and eco-friendly bioconversion of three agricultural residues by Aspergillus curvatus and Aspergillus gaarensis, promising enzyme-producers isolated from extreme environment. J. Basic Appl. Mycol.12, 1–14 (2021).
- 10.Zhao CH, Liu X, Zhan T, He J. Production of cellulase by Trichoderma reesei from pretreated straw and furfural residues. RSC Adv. 2018;8:36233–36238. doi: 10.1039/C8RA05936E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Otari SV, Patel SK, Kalia VC, Lee J-K. One-step hydrothermal synthesis of magnetic rice straw for effective lipase immobilization and its application in esterification reaction. Bioresour. Technol. 2020;302:122887. doi: 10.1016/j.biortech.2020.122887. [DOI] [PubMed] [Google Scholar]
- 12.Kothri, M. et al. Microbial sources of polyunsaturated fatty acids (PUFAs) and the prospect of organic residues and wastes as growth media for PUFA-producing microorganisms. FEMS Microbiol. Lett.367, fnaa028 (2020). [DOI] [PubMed]
- 13.Fumagalli F, Ottoboni M, Pinotti L, Cheli F. Integrated mycotoxin management system in the feed supply chain: Innovative approaches. Toxins. 2021;13:572. doi: 10.3390/toxins13080572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sultana, R. Ferulic acid ethyl ester as a potential therapy in neurodegenerative disorders. Biochim. Biophys. Acta 1822, 748–752 (2012). [DOI] [PubMed]
- 15.Phuong NTM, Cuong TT, Quang DN. Anti-inflammatory activity of methyl ferulate isolated from Stemona tuberosa Lour. Asian Pac. J. Trop. Med. 2014;7:S327–S331. doi: 10.1016/S1995-7645(14)60254-6. [DOI] [PubMed] [Google Scholar]
- 16.Pinheiro PG, Santiago GMP, da Silva FEF, de Araújo ACJ, de Oliveira CRT, Freitas PR, Rocha JE, de Araújo Neto JB, da Silva MMC, Tintino SR. Ferulic acid derivatives inhibiting Staphylococcus aureus tetK and MsrA efflux pumps. Biotechnol. Rep. 2022;34:e00717. doi: 10.1016/j.btre.2022.e00717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D Archivio, M. et al. Polyphenols, dietary sources and bioavailability. Annali-Istituto Superiore di Sanita43, 348 (2007). [PubMed]
- 18.Srinivasan M, Sudheer AR, Menon VP. Ferulic acid: Therapeutic potential through its antioxidant property. J. Clin. Biochem. Nutr. 2007;40:92–100. doi: 10.3164/jcbn.40.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ou S, Kwok KC. Ferulic acid: Pharmaceutical functions, preparation and applications in foods. J. Sci. Food Agric. 2004;84:1261–1269. doi: 10.1002/jsfa.1873. [DOI] [Google Scholar]
- 20.Kikuzaki H, Hisamoto M, Hirose K, Akiyama K, Taniguchi H. Antioxidant properties of ferulic acid and its related compounds. J. Agric. Food Chem. 2002;50:2161–2168. doi: 10.1021/jf011348w. [DOI] [PubMed] [Google Scholar]
- 21.Moussouni, S. et al. Crude peroxidase from onion solid waste as a tool for organic synthesis. Part II: Oxidative dimerization–cyclization of methyl p-coumarate, methyl caffeate and methyl ferulate. Tetrahedron Lett.52, 1165–1168 (2011).
- 22.Ghavam M, Afzali A, Manca ML. Chemotype of damask rose with oleic acid (9 octadecenoic acid) and its antimicrobial effectiveness. Sci. Rep. 2021;11:1–7. doi: 10.1038/s41598-021-87604-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gochev, V. et al. Comparative evaluation of antimicrobial activity and composition of rose oils from various geographic origins, in particular Bulgarian rose oil. Nat. Prod. Commun.3, 1934578X0800300706 (2008).
- 24.Beltrán, G., Del Rio, C., Sánchez, S. & Martínez, L. Influence of harvest date and crop yield on the fatty acid composition of virgin olive oils from cv. Picual. J. Agric. Food Chem.52, 3434–3440 (2004). [DOI] [PubMed]
- 25.Food, U., Administration, D. FDA Completes Review of Qualified Health Claim Petition for Oleic Acid and the Risk of Coronary Heart Disease (2018).
- 26.Barbour JA, Howe PR, Buckley JD, Bryan J, Coates AM. Effect of 12 weeks high oleic peanut consumption on cardio-metabolic risk factors and body composition. Nutrients. 2015;7:7381–7398. doi: 10.3390/nu7095343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cater NB, Heller HJ, Denke MA. Comparison of the effects of medium-chain triacylglycerols, palm oil, and high oleic acid sunflower oil on plasma triacylglycerol fatty acids and lipid and lipoprotein concentrations in humans. Am. J. Clin. Nutr. 1997;65:41–45. doi: 10.1093/ajcn/65.1.41. [DOI] [PubMed] [Google Scholar]
- 28.Kim J-Y, Lee HW, Lee SM, Jae J, Park Y-K. Overview of the recent advances in lignocellulose liquefaction for producing biofuels, bio-based materials and chemicals. Bioresour. Technol. 2019;279:373–384. doi: 10.1016/j.biortech.2019.01.055. [DOI] [PubMed] [Google Scholar]
- 29.Mussatto, S. I., Ballesteros, L. F., Martins, S. & Teixeira, J. A. Use of agro-industrial wastes in solid-state fermentation processes. Industrial Waste274 (2012).
- 30.Praveen, V. & Savitha, J. Solid state fermentation: an effective method for lovastatin production by fungi over submerged fermentation. E3 J. Biotechnol. Pharm. Res. 3, 15–21 (2012).
- 31.Javed S, Azeem M, Mahmood S, Al-Anazi KM, Farah MA, Ali S, Ali B. Biotransformation of agricultural wastes into lovastatin and optimization of a fermentation process using response surface methodology (RSM) Agronomy. 2022;12:2848. doi: 10.3390/agronomy12112848. [DOI] [Google Scholar]
- 32.Reeve, S. M. et al. Charged propargyl-linked antifolates reveal mechanisms of antifolate resistance and inhibit trimethoprim-resistant MRSA strains possessing clinically relevant mutations. J. Med. Chem.59, 6493–6500. 10.1021/acs.jmedchem.6b00688 (2016). [DOI] [PMC free article] [PubMed]
- 33.Witzgall, F., Ewert, W. & Blankenfeldt, W. Structures of the N-terminal domain of PqsA in complex with anthraniloyl- and 6-fluoroanthraniloyl-AMP: Substrate activation in Pseudomonas quinolone signal (PQS). Biosynthesis18, 2045–2055, (2017). [DOI] [PubMed]
- 34.Zhulenkovs D, Rudevica Z, Jaudzems K, Turks M, Leonchiks A. Discovery and structure–activity relationship studies of irreversible benzisothiazolinone-based inhibitors against Staphylococcus aureus sortase A transpeptidase. Bioorg. Med. Chem. 2014;22:5988–6003. doi: 10.1016/j.bmc.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 35.Keller NP. Fungal secondary metabolism: regulation, function and drug discovery. Nat. Rev. Microbiol. 2019;17:167–180. doi: 10.1038/s41579-018-0121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Evidente A, Kornienko A, Cimmino A, Andolfi A, Lefranc F, Mathieu V, Kiss R. Fungal metabolites with anticancer activity. Nat. Prod. Rep. 2014;31:617–627. doi: 10.1039/C3NP70078J. [DOI] [PubMed] [Google Scholar]
- 37.Cole J, Jarvis B, Schweikert M. Secondary Fungal Metabolites. Academic Press; 2003. [Google Scholar]
- 38.Sulyok M, Stadler D, Steiner D, Krska R. Validation of an LC-MS/MS-based dilute-and-shoot approach for the quantification of> 500 mycotoxins and other secondary metabolites in food crops: Challenges and solutions. Anal. Bioanal. Chem. 2020;412:2607–2620. doi: 10.1007/s00216-020-02489-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thuan NN, Ngan NT, Kuo P-C, Trinh NTN, Thien LT, Mai DS, Tuan NN, Tan LV, Thang TD. Secondary metabolites from the fruiting bodies of Coriolopsis aspera in Vietnam and their bioactivities. Chem. Nat. Compd. 2021;57:1104–1106. doi: 10.1007/s10600-021-03559-9. [DOI] [Google Scholar]
- 40.Woo K, Lee K. CAS: 528: DC% 2BC3sXhslSmsLbP. Nat. Prod. Sci. 2013;19:221. [Google Scholar]
- 41.Kwon YS, Kim CM. Antioxidant constituents from the stem of Sorghum bicolor. Arch. Pharm. Res. 2003;26:535–539. doi: 10.1007/BF02976877. [DOI] [PubMed] [Google Scholar]
- 42.Ilmiawati, A., Anggraini, D., Syahbirin, G., Rahayu, D. & Sugita, P. In AIP Conference Proceedings. 030009 (AIP Publishing LLC).
- 43.Kohno J, Hiramatsu H, Nishio M, Sakurai M, Okuda T, Komatsubara S. Structures of TMC-120A, B and C, novel isoquinoline alkaloids from Aspergillus ustus TC 1118. Tetrahedron. 1999;55:11247–11252. doi: 10.1016/S0040-4020(99)00648-1. [DOI] [Google Scholar]
- 44.Kozlovskii A, Antipova T, Zhelifonova V, Baskunov B, Ivanushkina N, Kochkina G, Ozerskaya S. Secondary metabolites of fungi of the Usti section, genus Aspergillus and their application in chemosystematics. Microbiology. 2017;86:176–182. doi: 10.1134/S0026261717020114. [DOI] [PubMed] [Google Scholar]
- 45.Ogawa A, Murakami C, Kamisuki S, Kuriyama I, Yoshida H, Sugawara F, Mizushina Y. Pseudodeflectusin, a novel isochroman derivative from Aspergillus pseudodeflectus a parasite of the sea weed, Sargassum fusiform, as a selective human cancer cytotoxin. Bioorg. Med. Chem. Lett. 2004;14:3539–3543. doi: 10.1016/j.bmcl.2004.04.050. [DOI] [PubMed] [Google Scholar]
- 46.Rodrigues de Carvalho, C. et al. Biological activities of ophiobolin K and 6-epi-ophiobolin K produced by the endophytic fungus Aspergillus calidoustus. Nat. Prod. Res.30, 478–481 (2016). [DOI] [PubMed]
- 47.Asther M, Haon M, Roussos S, Record E, Delattre M, Lesage-Meessen L, Labat M, Asther M. Feruloyl esterase from Aspergillus niger: A comparison of the production in solid state and submerged fermentation. Process Biochem. 2002;38:685–691. doi: 10.1016/S0032-9592(02)00196-6. [DOI] [Google Scholar]
- 48.Gaynes R. The discovery of penicillin: New insights after more than 75 years of clinical use. Emerg. Infect. Dis. 2017;23:849. doi: 10.3201/eid2305.161556. [DOI] [Google Scholar]
- 49.Bo, G. Giuseppe Brotzu and the discovery of cephalosporins. Clin. Microbiol. Infect.6, 6–8 (2000). [DOI] [PubMed]
- 50.Kumar CG, Kamle A, Mongolla P, Joseph J. Parametric optimization of feruloyl esterase production from Aspergillus terreus strain GA2 isolated from tropical agro-ecosystems cultivating sweet sorghum. J. Microbiol. Biotechnol. 2011;21:947–953. doi: 10.4014/jmb.1104.04014. [DOI] [PubMed] [Google Scholar]
- 51.Keller NP, Turner G, Bennett JW. Fungal secondary metabolism—from biochemistry to genomics. Nat. Rev. Microbiol. 2005;3:937–947. doi: 10.1038/nrmicro1286. [DOI] [PubMed] [Google Scholar]
- 52.Yang X, Feng P, Yin Y, Bushley K, Spatafora JW, Wang C. Cyclosporine biosynthesis in Tolypocladium inflatum benefits fungal adaptation to the environment. MBio. 2018;9:e01211–01218. doi: 10.1128/mBio.01211-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brookman JL, Denning DW. Molecular genetics in Aspergillus fumigatus. Curr. Opin. Microbiol. 2000;3:468–474. doi: 10.1016/S1369-5274(00)00124-7. [DOI] [PubMed] [Google Scholar]
- 54.Talukdar, R., Wary, S., Mili, C., Roy, S. & Tayung, K. Antimicrobial secondary metabolites obtained from endophytic fungi inhabiting healthy leaf tissues of Houttuynia cordata Thunb., an ethnomedicinal plant of Northeast India. J. Appl. Pharm. Sci.10, 099–106 (2020).
- 55.Sengupta, S., Nandi, I., Bhattacharyya, D. & Ghosh, M. Anti-oxidant and anti-bacterial properties of 1-Octacosanol isolated from rice Bran Wax. J. Plant Biochem. Physiol.6, 206 (2018).
- 56.Marik T, Tyagi C, Balázs D, Urbán P, Szepesi Á, Bakacsy L, Endre G, Rakk D, Szekeres A, Andersson MA. Structural diversity and bioactivities of peptaibol compounds from the Longibrachiatum clade of the filamentous fungal genus Trichoderma. Front. Microbiol. 2019;10:1434. doi: 10.3389/fmicb.2019.01434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nyfeler, R. & Keller-Schierlein, W. Metabolites of microorganisms Echinocandin B, a novel polypeptide-antibiotic from Aspergillus nidulans var. echinulatus: isolation and structural components. Helvetica Chim. Acta57, 2459–2477 (1974). [DOI] [PubMed]
- 58.Kalyani, P., Botsa, S. M., Laxmi, K. D. & Anil, S. Optimization of cultural conditions for biomass and antibacterial metabolite production by Aspergillus fumigatus strain MF1. Hybrid Adv.2, 100016 (2023).
- 59.El-Sayed AS, Shindia AA, Ali GS, Yassin MA, Hussein H, Awad SA, Ammar HA. Production and bioprocess optimization of antitumor Epothilone B analogue from Aspergillus fumigatus, endophyte of Catharanthus roseus, with response surface methodology. Enzyme Microb. Technol. 2021;143:109718. doi: 10.1016/j.enzmictec.2020.109718. [DOI] [PubMed] [Google Scholar]
- 60.Ghavam M, Azarnivand H, Akhbari M. Examining of the quality and quantity of active ingredients of Smirnova iranica Sabeti in different habitats. Health Biotechnol. Biophar. 2018;1:21–27. [Google Scholar]
- 61.Rivaldi JD, Carvalho AKF, da Conceição LRV, de Castro HF. Assessing the potential of fatty acids produced by filamentous fungi as feedstock for biodiesel production. Prep. Biochem. Biotechnol. 2017;47:970–976. doi: 10.1080/10826068.2017.1365246. [DOI] [PubMed] [Google Scholar]
- 62.Thevenieau F, Nicaud J-M. Microorganisms as sources of oils. Ocl. 2013;20:D603. doi: 10.1051/ocl/2013034. [DOI] [Google Scholar]
- 63.Li T, Shen Y, Chen H, Xu Y, Wang D, Cui F, Han Y, Li J. Antibacterial properties of coaxial spinning membrane of methyl ferulate/zein and its preservation effect on sea bass. Foods. 2021;10:2385. doi: 10.3390/foods10102385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jirovetz L, Eller G, Buchbauer G, Schmidt E, Denkova Z, Stoyanova A, Nikolova R, Geissler M. Chemical composition, antimicrobial activities and odor descriptions of some essential oils with characteristic floral-rosy scent and of their principal aroma compounds. Recent Res. Dev. Agron. Hortic. 2006;2:1–12. [Google Scholar]
- 65.Merkl, R., Hrádková, I., Filip, V. & Šmidrkal, J. Antimicrobial and antioxidant properties of phenolic acids alkyl esters. Czech J. Food Sci.28, 275–279 (2010).
- 66.Dufour M, Manson JM, Bremer PJ, Dufour J-P, Cook GM, Simmonds RS. Characterization of monolaurin resistance in Enterococcus faecalis. Appl. Environ. Microbiol. 2007;73:5507–5515. doi: 10.1128/AEM.01013-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Speert DP, Wannamaker LW, Gray ED, Clawson CC. Bactericidal effect of oleic acid on group A streptococci: Mechanism of action. Infect. Immunity. 1979;26:1202–1210. doi: 10.1128/iai.26.3.1202-1210.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Karamac, M., Bucinski, A., Pegg, R. B. & Amarowicz, R. Antioxidant and antiradical activity of ferulates. Czech J. Food Sci.-UZPI (Czech Republic) (2002).
- 69.Reddy KVK, Naidu KA. Oleic acid, hydroxytyrosol and n-3 fatty acids collectively modulate colitis through reduction of oxidative stress and IL-8 synthesis; in vitro and in vivo studies. Int. Immunopharmacol. 2016;35:29–42. doi: 10.1016/j.intimp.2016.03.019. [DOI] [PubMed] [Google Scholar]
- 70.Warcup J. The soil-plate method for isolation of fungi from soil. Nature. 1950;166:117–118. doi: 10.1038/166117b0. [DOI] [PubMed] [Google Scholar]
- 71.Choi, Y.-W., Hyde, K. D. & Ho, W. Single spore isolation of fungi. Fungal diversity (1999).
- 72.Smith, D. & Onions, A. H. The Preservation and Maintenance of Living Fungi (CAB International, 1994).
- 73.Houbraken J, Due M, Varga J, Meijer M, Frisvad JC, Samson RA. Polyphasic taxonomy of Aspergillus section Usti. Stud. Mycol. 2007;59:107–128. doi: 10.3114/sim.2007.59.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moubasher AH, Ismail MA, Al-Bedak OA, Mohamed RA. Ramophialophora chlamydospora, a new species from an alkaline lake of Wadi-El-Natron, Egypt. Asian J. Mycol. 2019;2:110–117. doi: 10.5943/ajom/2/1/5. [DOI] [Google Scholar]
- 75.Al-Bedak OA, Moubasher AH. Aspergillus gaarensis, a new addition to section Circumdati from soil of Lake El-Gaar in Wadi-El-Natron, Egypt. Stud. Fungi. 2020;5:59–65. doi: 10.5943/sif/5/1/5. [DOI] [Google Scholar]
- 76.Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Criscuolo A, Gribaldo S. BMGE (Block Mapping and Gathering with Entropy): A new software for selection of phylogenetic informative regions from multiple sequence alignments. BMC Evol. Biol. 2010;10:210. doi: 10.1186/1471-2148-10-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Felsenstein J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.2307/2408678. [DOI] [PubMed] [Google Scholar]
- 80.Posada D, Crandall KA. Modeltest: Testing the model of DNA substitution. Bioinformatics. 1998;14:817–818. doi: 10.1093/bioinformatics/14.9.817. [DOI] [PubMed] [Google Scholar]
- 81.Al-Bedak OA, Teaima EA, Ali E, Said M, Shalaby E, Moharram ZA. Impact of fumigation with phosphine on viability of wheat grains stored for six months at two levels of moisture content, in addition to description of four new records of associated fungi and assessment of their potential for enzymatic production. J. Basic Appl. Mycol. 2020;11:77–97. [Google Scholar]
- 82.Ramadan AM, Ahmed M, Shehata RM, El-Sheikh HH, Zidan SA, Al-Bedak OA. GC-MS analysis, antifungal, and antioxidant activity of rice straw-based extract produced by Aspergillus tubingensis AUMC 15759 in solid state fermentation. Egypt. Acad. J. Biol. Sci. G. 2023;15:43–60. [Google Scholar]
- 83.Azeem M, Arshad M, Mahmood S, Abrar S, Zahoor AF, Javed S, Tariq B, Hayyat K. Development of one pot strategy for hyper production and in vivo evaluation of lovastatin. Molecules. 2020;25:4380. doi: 10.3390/molecules25194380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ramadan AM, Shehata RM, El-Sheikh HH, Ameen F, Stephenson SL, Zidan SA, Al-Bedak OA. Exploitation of sugarcane bagasse and environmentally sustainable production, purification, characterization, and application of lovastatin by Aspergillus terreus AUMC 15760 under solid-state conditions. Molecules. 2023;28:4048. doi: 10.3390/molecules28104048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chaman S, Sharma G, Reshi AK. Study of antimicrobial properties of Catharanthus roseus by agar well diffusion method. Int. Res. J. Pharm. Appl. Sci. 2013;3:65–68. [Google Scholar]
- 86.Siddiqui BS, Gulzar T, Begum S, Afshan F, Sattar FA. Insecticidal amides from fruits of Piper nigrum Linn. Nat. Prod. Res. 2005;19:143–150. doi: 10.1080/14786410410001704750. [DOI] [PubMed] [Google Scholar]
- 87.Yen GC, Duh PD. Scavenging effect of methanolic extracts of peanut hulls on free-radical and active-oxygen species. J. Agric. Food Chem. 1994;42:629–632. doi: 10.1021/jf00039a005. [DOI] [Google Scholar]
- 88.Martí-Renom MA, Stuart AC, Fiser A, Sánchez R, Melo F, Šali A. Comparative protein structure modeling of genes and genomes. Annu. Rev. Biophys. Biomol. Struct. 2000;29:291–325. doi: 10.1146/annurev.biophys.29.1.291. [DOI] [PubMed] [Google Scholar]
- 89.Gordon JC, Myers JB, Folta T, Shoja V, Heath LS, Onufriev A. H++: A server for estimating pKas and adding missing hydrogens to macromolecules. Nucleic Acids Res. 2005;33:W368–W371. doi: 10.1093/nar/gki464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Halgren TAMMFFVI. MMFF94s option for energy minimization studies. J. Comput. Chem. 1999;20:720–729. doi: 10.1002/(SICI)1096-987X(199905)20:7<720::AID-JCC7>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 91.SZYBKI 1.9.0.3 (OpenEye Scientific Software, 2016).
- 92.Gasteiger J, Marsili M. Iterative partial equalization of orbital electronegativity: A rapid access to atomic charges. Tetrahedron. 1980;36:3219–3228. doi: 10.1016/0040-4020(80)80168-2. [DOI] [Google Scholar]
- 93.Huey R, Morris GM. Using AutoDock 4 with AutoDocktools: a tutorial. The Scripps Research Institute; 2008. pp. 54–56. [Google Scholar]
- 94.Forli S, Huey R, Pique ME, Sanner MF, Goodsell DS, Olson AJ. Computational protein-ligand docking and virtual drug screening with the AutoDock suite. Nat. Protoc. 2016;11:905–919. doi: 10.1038/nprot.2016.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.St L, Wold S. Analysis of variance (ANOVA) Chemom. Intell. Lab. Syst. 1989;6:259–272. doi: 10.1016/0169-7439(89)80095-4. [DOI] [Google Scholar]
- 96.Arthur, J. T. Test Two Sample Assuming Equal Variances in Excel (2009).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data related to this manuscript are incorporated in the manuscript only.