Abstract
Objectives
To determine the baseline trends in the total birth prevalence of neural tube defects (NTDs) in England (2000–2019) to enable the impact of folic acid fortification of non-wholemeal wheat flour to be monitored.
Design
Population-based, observational study using congenital anomaly (CA) registration data for England curated by the National Congenital Anomaly and Rare Disease Registration Service (NCARDRS).
Setting
Regions of England with active registration in the time period.
Participants
Babies that were liveborn or stillborn and pregnancies that resulted in a termination of pregnancy or a late miscarriage (20–23 weeks’ gestation) with an NTD.
Main outcome measures
Total birth prevalence of anencephaly, spina bifida and all NTDs in England. Poisson regression analysis was used to evaluate time trends with regional register as a random effect. The progress of national registration across England was assessed.
Results
There were 4541 NTD pregnancies out of 3 637 842 births in England; 1982 anencephaly and 2127 spina bifida. NTD prevalence was 12.5 (95% CI 12.1 to 12.9) per 10 000 total births. NTD prevalence per 10 000 total births was significantly higher in 2015–2019 (13.6, 95% CI 12.9 to 14.4) compared with 2010–2014 (12.1, 95% CI 11.7 to 12.5). An increasing trend in NTDs overall was detected (incidence rate ratio (IRR) 1.01, 1.00 to 1.02), although further analysis determined this effect was confined to 2015–2019 (compared against 2000–2004, IRR 1.14, 1.04 to 1.24). The birth prevalence of anencephaly reflected this pattern. The prevalence of spina bifida remained relatively stable over time.
Conclusions
Baseline NTD prevalence for England has been established. National and standardised CA registration is in place, facilitating the systematic and consistent monitoring of pre-fortification and post-fortification NTD trends and evaluating the impact of fortification on NTD prevalence.
Keywords: Epidemiology, Paediatrics, Child Health
WHAT IS ALREADY KNOWN ON THIS TOPIC
The association between folic acid and neural tube defects (NTDs) is well-established and women have been advised to supplement their diets with folic acid before conception.
Red blood cell folate concentrations have been dropping in women of childbearing age.
Mandatory fortification of non-wholemeal wheat flour will be implemented in the UK.
WHAT THIS STUDY ADDS
Baseline levels of NTDs have been established using a 20-year trend (2000–2019) from which the impact of folic acid fortification can be evaluated.
No reduction in NTDs over the 20-year period was detected, and for NTDs as a group, and anencephaly specifically, prevalence increased after 2015.
The increase in prevalence, recorded primarily for anencephaly, coincides with improvements in data collection and standardisation since the centralisation of registration.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
We have demonstrated that an effective and consistent monitoring and surveillance system is in place nationally for NTDs prior to the implementation of fortification.
This will enable the impact of folic acid fortification of non-wholemeal wheat flour on NTD prevalence to be evaluated.
Introduction
Neural tube defects (NTDs) are congenital anomalies (CAs) of the brain and spine, resulting from incomplete closure of the embryonic neural tube, that can be life-threatening or result in long-term severe disability.1 Low folate levels increase the risk of a pregnancy being affected by an NTD (anencephaly, spina bifida and encephalocele) but while pregnant women have been advised to supplement their diet with folic acid prior to and around conception for 30 years,2 uptake has been low3 4 and there has been little recent change in NTD prevalence in Europe.5 6
Between 2008 and 2019, red blood cell (RBC) folate concentrations in women of childbearing age in the UK fell by 31%, leaving approximately 89% of women at increased risk of a folate-sensitive NTD.7 The USA, Canada, Australia, New Zealand and countries across South America have introduced mandatory fortification of foods with folic acid, achieving reductions in NTD prevalence of between 15% and 61%.8 9 To date, no country in Europe has introduced a mandatory folic acid fortification programme, despite estimates that folic acid fortification would prevent the occurrence of NTDs in up to 1000 pregnancies a year.10 In September 2021, the UK government announced the mandatory fortification of non-wholemeal wheat flour with folic acid to reduce the prevalence of NTDs.11 12
CA registration enables the surveillance of CAs and provides an effective framework for the evaluation of population-level interventions. Established in 2015, the National Congenital Anomaly and Rare Disease Registration Service (NCARDRS) curates and analyses individual data on pregnancies, fetuses, babies, children and adults with CAs and rare diseases in England.13 National registration of CAs has been in place since 2018.14 Prior to 2015, CAs were recorded by regional registries operating across some areas of England, covering up to 32% of births.
In this study, we assessed the trends in the total birth prevalence of NTDs as a group and separately for anencephaly and spina bifida, in England by using data from legacy regional registries active for the period 2000–2015 and combining this with data from NCARDRS from 2015 to 2019. Data presented are the baseline from which the impact of folic acid fortification on NTDs can be evaluated.
Methods
Data sources
Confirmed/probable NTDs identified in utero, at birth or in childhood were extracted from the respective data management systems and deduplicated. Data for all registries were collected in accordance with guidance from EUROCAT.15 Babies that were liveborn or stillborn or pregnancies that resulted in a termination of pregnancy or late miscarriage (20–23 weeks’ gestation) were included. Data were extracted on 17 June 2021.
Cases with a diagnostic code corresponding to an NTD according to the International Classification of Diseases and Related Health Problems, 10th revision (ICD-10) were selected (Q00, Q01 and Q05). This included spina bifida with and without hydrocephaly. Babies with multiple NTDs were counted once overall but were counted in each NTD subtype when reported separately.
The number of total births (live births and stillbirths) per year by region was extracted using Office for National Statistics data.
Trend analysis
We defined NTD birth prevalence as the total number of fetuses and babies with an NTD per 10 000 total births (live and stillbirths) with 95% CIs calculated using the Poisson distribution.
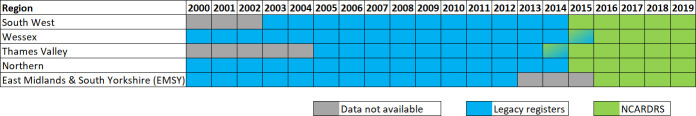
To establish a stable trend without the influence of the developing ascertainment of new regions, the analysis was restricted to the five regions with at least 10 years of consecutive data collection prior to the formation of NCARDRS in 2015 (figure 1).
Figure 1.
Years of data and regions included in the trend and statistical analysis. NCARDRS, National Congenital Anomaly and Rare Disease Registration Service.
The ‘England’ prevalence trend was calculated by dividing the total number of fetuses and babies with an NTD with the total number of births in regions with active CA registration, for NTDs as a group and for anencephaly and spina bifida. The number of encephalocele cases was low so time trends were not plotted. Total prevalence time trends were summarised by using restricted cubic splines.6 A spline is a piecewise polynomial where the x-axis is split into various intervals and a polynomial is fitted to each interval. This analysis considers a cubic spline which consists of cubic polynomials fitted to different intervals for birth year. Preparation of the trends and fitting of splines were performed in R Studio.
Statistical analysis
Differences between rates were formally tested using two-sided exact significance tests. An association with time was investigated as a continuous variable in a Poisson regression analysis with regional registry as a random effect to account for heterogeneity between the registries. This analysis was performed using the entire dataset (2000–2019), and then using the data from 2000 to 2014 to assess if an increasing or decreasing trend was already detectable prior to the formation of NCARDRS.
Additionally, a non-linear relationship with time was investigated by categorising the data in four equal time periods of 5 years (2000–2004, 2005–2009, 2010–2014 and 2015–2019). Data collection was not in place across all five regions for the period 2000–2004, and to account for this, and developing ascertainment in the early years of a register, the reference period was rotated in turn. Incidence rate ratios (IRR) and 95% CI were used to estimate the size of the effect. Power calculations were performed to compare two independent proportions using a χ2 test assuming a 15–20% reduction in NTD prevalence10 and the average births per year for the five regions between 2015 and 2019. Significance was assumed at the α=0.05 level. Statistical analyses were conducted using Stata V.15.
Assessment of NCARDRS national ascertainment
The progress of NTD prevalence in regions of NCARDRS completely new to registration (North West, London and the South East and East of England) was assessed by comparing NTD, anencephaly and spina bifida prevalence in these regions from 2018 to 2019 with prevalence in more established regions that were in operation prior to the formation of NCARDRS using χ2 tests. Established regions included the five used to estimate the prevalence trend (Thames Valley, Wessex, Northern, South West ; 2015–2019 and EMSY; 2016-2019) and two regions that had been collecting data to varying degrees prior to the formation of NCARDRS (West Midlands and Yorkshire and Humber, 2016–2019). Static data at point of annual reporting was used to reduce the bias of the continued accumulation of data over time.
Patient involvement
Patients were not involved in setting the research question or the outcome measures nor were they involved in design and implementation of the study.
Results
Trends in total NTD prevalence
The proportion of total births in England covered by active CA registration and included in this analysis ranged from approximately 15% to 32% (figure 1).
There were 4541 NTD pregnancies in 3 637 842 births over the five registers in England (2000–2019). Of the total NTD cases, 43.7% (n=1982) had anencephaly, 46.8% had spina bifida (n=2127) and 10.9% (n=499) had encephalocele (table 1). More than one subtype of NTD was recorded in 1.4% (n=63) of cases. Where the sex was known, 48.9% (n=1351) were male (table 1).
Table 1.
The number* and subtype of NTDs across the legacy registers† and NCARDRS for EMSY, Northern, South West, Thames Valley and Wessex from 2000 to 2019
| Legacy regional registers† (2000–2014) | NCARDRS (2015–2019)* |
Total | |
| NTD subgroup | |||
| Anencephaly | 1378 | 604 | 1982 |
| Spina bifida | 1546 | 581 | 2127 |
| Encephalocele | 356 | 143 | 499 |
| All cases with an NTD* | 3234 | 1307 | 4541 |
| Sex | |||
| Male | 998 (30.9%) | 353 (27.0%) | 1351 |
| Female | 1031 (31.9%) | 356 (27.2%) | 1387 |
| Intersex | 24 (0.7%) | 0 (0.0%) | 24 |
| Not known | 1181 (36.5%) | 598 (45.8%) | 1779 |
| Birth outcome | |||
| Live birth | 520 (16.1%) | 227 (17.4%) | 747 |
| Stillbirth | 89 (2.8%) | 20 (1.5%) | 109 |
| Termination | 2585 (79.9%) | 1016 (77.8%) | 3601 |
| Miscarriage (20–24 weeks) | 37 (1.1%) | 30 (2.3%) | 67 |
| Unknown | 3 (0.1%) | 14 (1.0%) | 17 |
| Region | |||
| EMSY | 1046 (32.3%) | 359 (27.5%) | 1405 |
| Northern | 711 (22.0%) | 219 (16.8%) | 930 |
| South West | 681 (21.1%) | 329 (25.2%) | 1010 |
| Thames Valley | 311 (9.6%) | 199 (15.2%) | 510 |
| Wessex | 485 (15.0%) | 201 (15.4%) | 686 |
| Total | 3234 | 1307 | 4541 |
A small number of cases for 2015 were collected by the legacy registers, and some cases prior to 2015 were collected by NCARDRS.
*The number of cases of each NTD subtype does not sum to the total number of cases of NTDs as 63 cases (1.4%) had more than one NTD and are included in each appropriate category.
†Legacy refers to those registers in existence prior to the transition to NCARDRS with sufficient data quality to be included in the trend.
EMSY, East Midlands and South Yorkshire; NCARDRS, National Congenital Anomaly and Rare Disease Registration Service; NTD, neural tube defect.
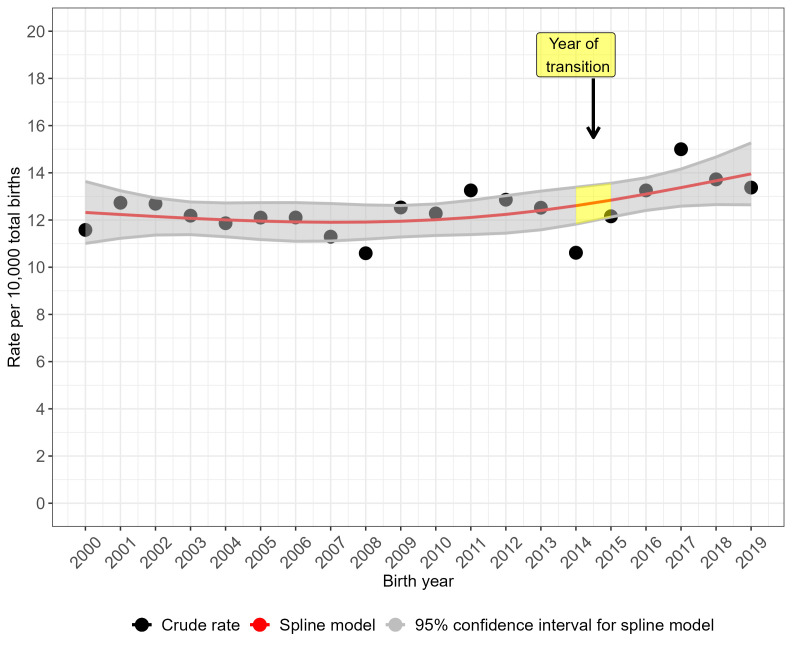
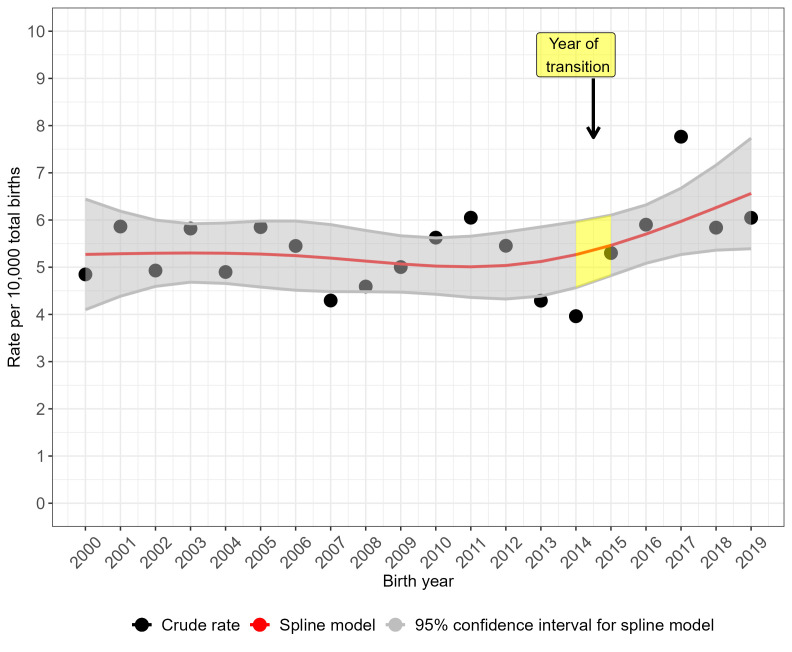
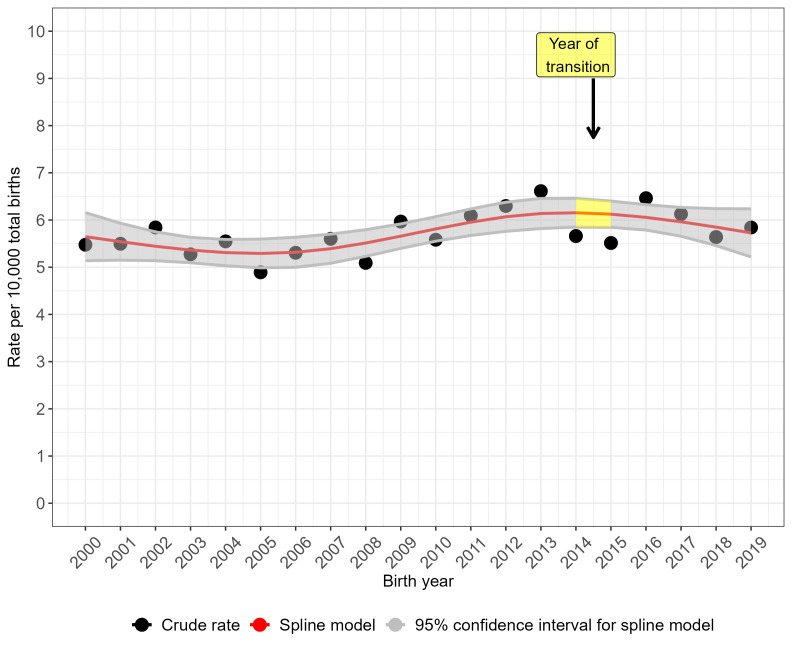
The crude prevalence rate for NTDs overall (figure 2) and for anencephaly (figure 3) peaked in 2017 but the prevalence rate was more stable over time for spina bifida (figure 4). Spina bifida crude prevalence peaked at 6.6 per 10 000 total births in 2013, followed by a fall in 2014 and then recovery. Crude prevalence for all three condition groups fell between 2014 and 2015, coinciding with the disruption of data collection during transition of the legacy regional registries’ data to NCARDRS in 2015. The cubic spline model shows rising prevalence from 2015 for NTDs as a group (figure 2) and more sharply for anencephaly (figure 3). For spina bifida, the spline model shows increasing prevalence until 2015, and then a stabilising, slightly downward trend (figure 4).
Figure 2.
Trends in the total prevalence of neural tube defects in England, 2000–2019: cubic spline estimates with 95% CIs and crude rates of pooled data for five regions with active congenital anomaly registration (East Midlands and South Yorkshire, Northern, South West, Thames Valley, Wessex).
Figure 3.
Trends in the total prevalence of anencephaly in England, 2000–2019: cubic spline estimates with 95% CIs and crude rates of pooled data for five regions with active congenital anomaly registration (East Midlands and South Yorkshire, Northern, South West, Thames Valley, Wessex).
Figure 4.
Trends in the total prevalence of spina bifida in England, 2000–2019: cubic spline estimates with 95% CIs and crude rates of pooled data for five regions with active congenital anomaly registration (East Midlands and South Yorkshire, Northern, South West, Thames Valley, Wessex).
Table 2 presents prevalence rates for NTDs and subtypes overall and table 3 presents this data by region for legacy (pre-2015) and NCARDRS data collection (2015–2019). Between 2000 and 2014, the combined rate of NTD per 10 000 births in England was 12.1 cases (95% CI 11.7 to 12.5), rising to 13.6 (95% CI 12.9 to 14.4) in 2015–2019 (p=0.004). The rate of anencephaly per 10 000 total births rose significantly from 5.2 (95% CI 4.9 to 5.4) between 2000 and 2014 to 6.2 (95% CI 5.7 to 6.8) between 2015 and 2019 (p=0.001). There was no difference in the rate of spina bifida in 2015–2019 compared with 2000–2014 (p=0.28).
Table 2.
Total prevalence of NTDs per 10 000 total births (±95% CIs) in the combined active legacy* regional registers (2000–2014) and NCARDRS for the periods of reporting (2015–2019)
| Data source | Cases | Total births (live births and stillbirths) | Prevalence per 10 000 total births (95% CI) |
| All NTDs | |||
| 2000–2014 | 3239 | 2 679 981 | 12.1 (11.7 to 12.5) |
| 2015–2019 | 1302 | 957 861 | 13.6 (12.9 to 14.4) |
| Total (2000–2019) | 4541 | 3 637 842 | 12.5 (12.1 to 12.9) |
| Anencephaly | |||
| 2000–2014 | 1385 | 2 679 981 | 5.2 (4.9 to 5.4) |
| 2015–2019 | 597 | 957 861 | 6.2 (5.7 to 6.8) |
| Total (2000–2019) | 1982 | 5.5 (2.2 to 5.7) | |
| Spina bifida | |||
| 2000–2014 | 1540 | 2 679 981 | 5.7 (5.5 to 6.0) |
| 2015–2019 | 587 | 957 861 | 6.1 (5.6 to 6.6) |
| Total (2000–2019) | 2127 | 5.9 (5.6 to 6.1) |
*Legacy refers to those regional registers in existence prior to the transition to NCARDRS. We have used a cut-off of 2015—some cases in 2015 were collected by the legacy registers, and some cases in 2014 were collected by NCARDRS.
NCARDRS, National Congenital Anomaly and Rare Disease Registration Service; NTD, neural tube defect.
Table 3.
Total prevalence of NTDs per 10 000 total births (±95% CIs) by region, as registered by regional registers (2000–2014) and NCARDRS for the periods of reporting (2015–2019)
| Regional register | Time period | NTD prevalence | Anencephaly prevalence | Spina bifida prevalence |
| EMSY (legacy) | 2000–2013 | 11.86 (11.16 to 12.60) | 4.93 (4.48 to 5.42) | 5.72 (5.23 to 6.24) |
| EMSY (NCARDRS) | 2016–2019 | 12.78 (11.49 to 14.17) | 6.52 (5.61 to 7.53) | 5.27 (4.46 to 6.19) |
| Northern (legacy) | 2000–2014 | 14.83 (13.76 to 15.96) | 6.01 (5.34 to 6.74) | 7.36 (6.61 to 8.17) |
| Northern (NCARDRS) | 2015–2019 | 14.22 (12.39 to 16.24) | 6.75 (5.51 to 8.18) | 6.03 (4.86 to 7.39) |
| South West (legacy) | 2003–2014 | 11.57 (10.72 to 12.48) | 4.50 (3.98 to 5.08) | 5.74 (5.15 to 6.39) |
| South West (NCARDRS) | 2015–2019 | 13.83 (12.37 to 15.40) | 6.05 (5.10 to 7.12) | 6.35 (5.37 to 7.44) |
| Thames Valley (legacy) | 2005–2014 | 10.77 (9.63 to 12.01) | 4.60 (3.87 to 5.44) | 4.90 (4.14 to 5.76) |
| Thames Valley (NCARDRS) | 2015–2019 | 13.16 (11.34 to 15.18) | 5.63 (4.46 to 7.01) | 5.70 (4.53 to 7.08) |
| Wessex (legacy) | 2000–2014 | 11.09 (10.12 to 12.14) | 5.99 (5.28 to 6.77) | 3.96 (3.39 to 4.60) |
| Wessex (NCARDRS) | 2015–2019 | 14.55 (12.65 to 16.66) | 6.03 (4.83 to 7.43) | 6.79 (5.51 to 8.27) |
*Legacy refers to those regional registers in existence prior to the transition to NCARDRS. We have used a cut-off of 2015—some cases in 2015 were collected by the legacy registers and some cases in 2014 were collected by NCARDRS.
EMSY, East Midlands and South Yorkshire; NCARDRS, National Congenital Anomaly and Rare Disease Registration Service; NTD, neural tube defect.
Random effects Poisson regression models
Table 4 displays the results of the random effects Poisson regression models that take into account heterogeneities across registries in estimating the time trends. For NTDs overall, the rate of increase per year was small but significant (IRR 1.01, 95% CI 1.00 to 1.02; p=0.001) between 2000 and 2019 (table 4), with no significant association with time prior to the transition to NCARDRS (2000–2014) (1.003, 95% CI 0.99 to 1.01, p=0.47). Examining the association with time in 5-year periods, the rate of NTDs was significantly higher than baseline (2000–2004) only after 2015, with the adjusted rate in 2015–2019; 13.6% (3.9% to 24.2%) higher compared with 2000–2004 (table 4). When the reference period was rotated in turn, the rate for the most recent period (2015–2019) was significantly higher than for all other time periods, although this effect was weakest when compared with the time-period immediately prior; the 2015–2019 prevalence rate was 9.5% (95% CI 1.3% to 18.5%; p=0.023) higher compared with 2010–2015; 16.0% (95% CI 7.5% to 25.4%; p<0.001) higher than in 2005–2009.
Table 4.
Incidence rate ratios (IRRs) produced by random effects Poisson regression models for the trend over time for different time periods for the total number of babies with NTDs, anencephaly and spina bifida reported in the regions Northern, EMSY, Wessex, Thames Valley and South West
| Model | Time period | IRR (95% CI) | P value |
| NTDs | |||
| Overall | 2000–2019 | 1.01 (1.004 to 1.02) | 0.001 |
| Legacy only | 2000–2014 | 1.003 (0.99 to 1.01) | 0.47 |
| 5-year time blocks | 2000–2004 | Ref. | |
| 2005–2009 | 0.98 (0.90 to 1.07) | 0.64 | |
| 2010–2014 | 1.04 (0.95 to 1.14) | 0.44 | |
| 2015–2019 | 1.14 (1.04 to 1.24) | 0.005 | |
| Anencephaly | |||
| Overall | 2000–2019 | 1.01 (1.003 to 1.02) | 0.005 |
| Legacy only | 2000–2014 | 0.996 (0.98 to 1.01) | 0.54 |
| 5-year time blocks | 2000–2004 | Ref. | |
| 2005–2009 | 0.97 (0.85 to 1.12) | 0.72 | |
| 2010–2014 | 1.02 (0.88 to 1.17) | 0.82 | |
| 2015–2019 | 1.21 (1.06 to 1.38) | 0.005 | |
| Spina bifida | |||
| Overall | 2000–2019 | 1.01 (1.0004 to 1.02) | 0.04 |
| Legacy only | 2000–2014 | 1.02 (1.002 to 1.03) | 0.03 |
| 5-year time blocks | 2000–2004 | Ref. | |
| 2005–2009 | 0.99 (0.87 to 1.13) | 0.89 | |
| 2010–2014 | 1.11 (0.97 to 1.27) | 0.12 | |
| 2015–2019 | 1.10 (0.96 to 1.25) | 0.17 |
*Poisson regression model also included region, set as a random term.
†Legacy refers to those registers in existence prior to the transition to NCARDRS.
EMSY, East Midlands and South Yorkshire; NCARDRS, National Congenital Anomaly and Rare Disease Registration Service; NTD, neural tube defect.
The trend for anencephaly was similar to the trend for NTDs overall; while there was an increasing trend of around 1% per year between 2000 and 2019, this was not significant between 2000 and 2014. The time-period analysis showed that the prevalence between 2015 and 2019 was significantly higher than the other three time-periods (table 4).
For spina bifida, there was a statistically significant, slow but linear annual increase of around 1% per year from 2000 to 2019, that was also present prior to 2015 (2000–2014) (table 4). However, no 5-year period had a significantly higher or lower prevalence of spina bifida than another (table 4).
Prevalence in new versus existing regions of NCARDRS: stability of prevalence and ascertainment
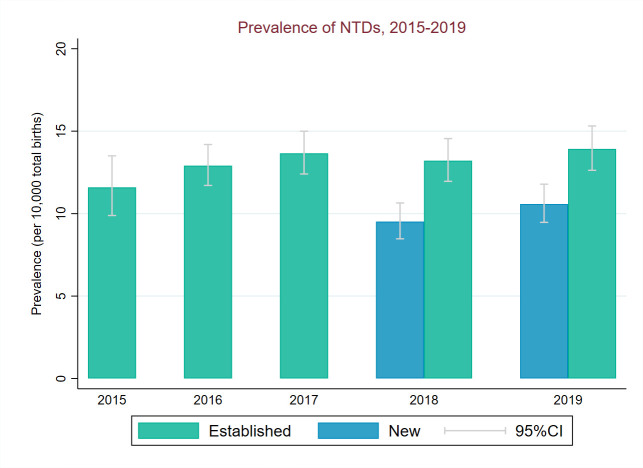
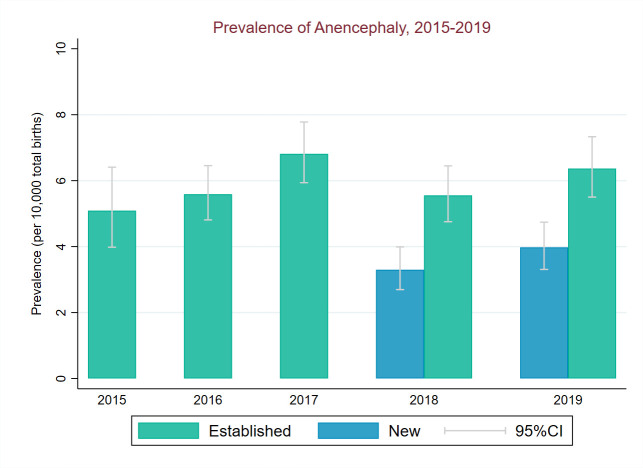
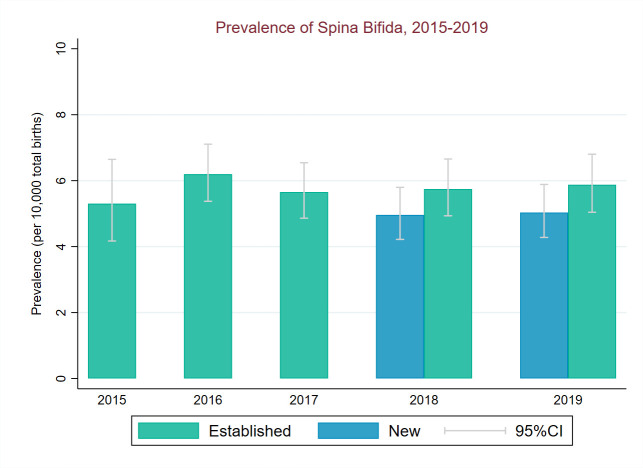
NTD total prevalence (2018–2019) in regions new to CA registration was significantly below prevalence in the established regions for all NTDs (χ2=32.7, p<0.001). This was driven by a lower prevalence of anencephaly in new regions compared with established regions (χ2=35.1, p<0.001)) (figures 5–7). Spina bifida prevalence (2018–2019) was similar in new and established regions (p=0.51, figure 7).
Figure 5.
Total birth prevalence of NTDs between 2015 and 2019 for established and new regions of National Congenital Anomaly and Rare Disease Registration Service. NTDs, neural tube defects.
Figure 6.
Total birth prevalence of anencephaly between 2015 and 2019 for established and new regions of National Congenital Anomaly and Rare Disease Registration Service.
Figure 7.
Total birth prevalence of spina bifida between 2015 and 2019 for established and new regions of National Congenital Anomaly and Rare Disease Registration Service.
Statistical power analysis
Using the average annual total births for the five regions of 191 527 and an NTD prevalence of 13.6 per 10 000 total births (2015–2019), the statistical power to calculate a 20% reduction in prevalence comparing the year after implementation to the preceding year was 0.67; for a 15% reduction it was 0.49. However, at current birth rates of approximately 600K total births per year in England, the statistical power to detect a 20% or 15% difference after 1 year using the national registration dataset increased to 0.99 and 0.88, respectively.
Discussion
Our study demonstrates that NTD prevalence in England increased between 2000 and 2019 but the rate of increase was not linear; for NTDs overall and for anencephaly, the rate in the period 2015–2019 was significantly higher than any previous 5-year period. This increase coincides with the formation of NCARDRS, seems driven by an increase in anencephaly prevalence and so could be a result of improvements in data collection after the centralisation of CA registration in England. NCARDRS evaluates and monitors the effectiveness of the NHS Fetal Anomaly Screening Programme (FASP) and anomalies included in this programme are subjected to enhanced registration and active case ascertainment.16 Anencephaly and spina bifida are auditable conditions for the FASP. Anencephaly is a severe NTD that is often detected at the first trimester and pregnancies rarely result in a live birth,17 making this condition more difficult to ascertain, so much so that the rate of anencephaly is used to measure data quality across registries.18 A true increase in NTD prevalence would also be expected to be observed in spina bifida prevalence, and this is not the case. Nevertheless, a long-term downward trend in RBC folate concentrations in women of childbearing age in the UK has been established10 and a relationship between higher NTD prevalence as a consequence of lower RBC folate levels cannot be discounted.
Strengths and limitations of study
This is a large study evaluating a 20-year trend for England, combining and standardising data collected by regional registries and the NCARDRS. Only data from regions of England with well-established CA registers were included to ensure consistency and reduce the impact of developing ascertainment in new regions. The heterogeneities across registries were accounted for in the statistical models but prior to 2015 there were differences in the collection of data in different registries. The centralisation of CA registration in England has resulted in the standardisation of sources of ascertainment, disease coding and confirmation criteria nationally. Only 2 years of data were available to assess the expansion of CA registration in regions new to registration, and while ascertainment of anencephaly is lower than established regions, that gap is closing.
Comparisons with other studies
The most recent published estimates for England range from 10.4 (Thames Valley, 1998–2017) to 13.5 (North England, 2000–2017) per 10 000 births and 14.1 per 10 000 births in Wales (1998–2017) were used to estimate the number of NTD pregnancies that might be prevented with fortification.10 Our study indicates NTD prevalence in England to be slightly higher making it likely that folic acid supplementation would prevent even more NTDs.10 19
Despite long-standing advice for women who may become pregnant to supplement their diet with folic acid, no reduction in the prevalence of NTDs in England was observed. This is consistent with the findings from other studies in Europe5 6 20 an in the UK.21 In Europe, from 1991 to 2001, annual NTD prevalence increased by between 1% and 7% in 1995–1999 and decreased by between 1% and 5% between 1999 and 2003, with stable rates thereafter until 2012.6
Evaluations of folic acid fortification have often relied on retrospective data collection from hospitals which do not include terminations or fetal losses,8 are based on regional data22 or birth certificate reports.23 The importance of rigorous national monitoring systems in the assessment of the impact of mandatory folic acid fortification is clear.22 The establishment of NCARDRS, and the expansion of CA registration nationally, provides a unique opportunity to robustly monitor and evaluate the impact of the fortification on CA outcomes with high statistical power. Additionally, systems are in place to monitor the impact of folic acid fortification on other folic acid-sensitive conditions such as oro-facial cleft and cardiac anomalies.24
Conclusions
The mandatory fortification of non-wholemeal flour with folic acid is a welcome policy change. We have presented a baseline estimate from which the impact of fortification on NTD birth prevalence can be measured and demonstrated that an effective and consistent monitoring and surveillance system is in place nationally, prior to the implementation of fortification allowing the impact of this intervention to be evaluated.
Acknowledgments
Many thanks to Adenike Adesanya for assistance with literature searching. Thanks to the NCARDRS and legacy registers registration teams and the notifiers from around the country for their care and attention registering cases. This work uses data that has been provided by patients, the NHS and other health care organisations as part of patient care and support. The data is collated, maintained and quality assured by the National Congenital Anomaly and Rare Disease Registration Service, which is part of NHS England.
Footnotes
Twitter: @KarenLuyt
Contributors: Study was designed by JMB, SS, JR, AT, GS and AC. Analysis was performed by JB, DM and TH. JB drafted the manuscript. All authors reviewed and commented on the manuscript. JMB is the guarantor of this manuscript and accepts full responsibilty for the work and conduct of the study, had access to the data, and controlled the decision to publish.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available upon reasonable request. Study data may be made available to accredited researchers with the correct legal permissions by submitting a request to the Data Access Request Service (DARS) of NHS England (https://digital.nhs.uk/services/data-access-request-service-dars).
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants but specific ethical approval was not required. The NDRS has legal permission to collect patient-level data and to use it to protect the health of the population. This permission is given under legal instructions known as Directions, from the Secretary of State for Health and Social Care, under section 254 of the Health and Social Care Act 2012 (2012 Act).
References
- 1. Greene NDE, Copp AJ. Neural tube defects. Annu Rev Neurosci 2014;37:221–42. 10.1146/annurev-neuro-062012-170354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Prevention of neural tube defects: results of the medical research council vitamin study. Lancet 1991;338:131–7. 10.1016/0140-6736(91)90133-A [DOI] [PubMed] [Google Scholar]
- 3. Public Health England. Health of women before and during pregnancy: health behaviours, risk factors and inequalities; 2019.
- 4. Toivonen KI, Lacroix E, Flynn M, et al. Folic acid supplementation during the preconception period: a systematic review and meta-analysis. Prev Med 2018;114:1–17. 10.1016/j.ypmed.2018.05.023 [DOI] [PubMed] [Google Scholar]
- 5. Morris JK, Springett AL, Greenlees R, et al. Trends in congenital anomalies in Europe from 1980 to 2012. PLoS One 2018;13:e0194986. 10.1371/journal.pone.0194986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Khoshnood B, Loane M, de Walle H, et al. Long term trends in prevalence of neural tube defects in Europe: population based study. BMJ 2015;351:h5949. 10.1136/bmj.h5949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Public Health England. National diet and nutrition survey. years 1 to 9 of the rolling programme (2008/2009–2016/2017): time trend and income analyses; 2019.
- 8. Castillo-Lancellotti C, Tur JA, Uauy R. Impact of folic acid fortification of flour on neural tube defects: a systematic review. Public Health Nutr 2013;16:901–11. 10.1017/S1368980012003576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Thurston L, Borman B, Bower C. Mandatory fortification with folic acid for the prevention of neural tube defects: a case study of Australia and New Zealand. Childs Nerv Syst 2023;39:1737–41. 10.1007/s00381-022-05823-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Morris JK, Addor M-C, Ballardini E, et al. Prevention of neural tube defects in Europe: a public health failure. Front Pediatr 2021;9:647038. 10.3389/fped.2021.647038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Folic acid added to flour to prevent spinal conditions in babies [press release]. 2021. Available: https://www.gov.uk/government/news/folic-acid-added-to-flour-to-prevent-brain-and-spinal-conditions-in-foetuses
- 12. Haggarty P. UK introduces folic acid fortification of flour to prevent neural tube defects. Lancet 2021;398:1199–201. 10.1016/S0140-6736(21)02134-6 [DOI] [PubMed] [Google Scholar]
- 13. Stevens S, Miller N, Rashbass J. Development and progress of the National congenital anomaly and rare disease registration service. Arch Dis Child 2018;103:215–7. 10.1136/archdischild-2017-312833 [DOI] [PubMed] [Google Scholar]
- 14. NCARDRS . National Congenital Anomaly and Rare Disease Registration Service Congenital Anomaly Statistics Report 2018. Report No.: PHE publications gateway number: GW-1445. UK Government: Public Health England; 2020. [Google Scholar]
- 15. EUROCAT . EUROCAT guide 1.4: instruction for the registration of congenital anomalies. University of Ulster; 2013. [Google Scholar]
- 16. Aldridge N, Pandya P, Rankin J, et al. Fetal anomaly ultrasound scan detection rates in England for 14 physical conditions; a three-year national cohort study; 2022.
- 17. Copp AJ, Greene NDE. Neural tube defects--disorders of neurulation and related embryonic processes. Wiley Interdiscip Rev Dev Biol 2013;2:213–27. 10.1002/wdev.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Loane M, Dolk H, Garne E, et al. Paper 3: EUROCAT data quality indicators for population-based registries of congenital anomalies. Birth Defects Res A Clin Mol Teratol 2011;91 Suppl 1:S23–30. 10.1002/bdra.20779 [DOI] [PubMed] [Google Scholar]
- 19. Mayer CD, Craig L, Horgan G. Stochastic Modelling to estimate the potential impact of Fortification of flour with folic acid in the UK. Food Standards Scotland; 2017. [Google Scholar]
- 20. Morris JK, Wellesley DG, Barisic I, et al. Epidemiology of congenital cerebral anomalies in Europe: a multicentre, population-based EUROCAT study. Arch Dis Child 2019;104:1181–7. 10.1136/archdischild-2018-316733 [DOI] [PubMed] [Google Scholar]
- 21. Morris JK, Rankin J, Draper ES, et al. Prevention of neural tube defects in the UK: a missed opportunity. Arch Dis Child 2016;101:604–7. 10.1136/archdischild-2015-309226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hilder L. Neural tube defects in Australia, 2007–2011: before and after mandatory folic acid Fortification. National Perinatal Epidemiology and Statistics Unit, University of New South Wales; 2016. [Google Scholar]
- 23. Honein MA, Paulozzi LJ, Mathews TJ, et al. Impact of folic acid fortification of the US food supply on the occurrence of neural tube defects. JAMA 2001;285:2981. 10.1001/jama.285.23.2981 [DOI] [PubMed] [Google Scholar]
- 24. Ingrid Goh Y, Bollano E, Einarson TR, et al. Prenatal multivitamin supplementation and rates of congenital anomalies: a meta-analysis. J Obstet Gynaecol Can 2006;28:680–9. 10.1016/S1701-2163(16)32227-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request. Study data may be made available to accredited researchers with the correct legal permissions by submitting a request to the Data Access Request Service (DARS) of NHS England (https://digital.nhs.uk/services/data-access-request-service-dars).