Abstract
Background
A growing evidence base supports the use of autologous haematopoietic stem cell transplantation (aHSCT) for treatment of relapsing-remitting multiple sclerosis (RRMS), but it has not yet been integrated into most national clinical guidelines. The objective of this study was to assess efficacy and safety when aHSCT is implemented in routine healthcare.
Methods
We assessed 231 patients and the final analysis included 174 RRMS patients who were treated with aHSCT in Sweden before 1 January 2020. Efficacy was evaluated by performing a retrospective analysis of prospectively collected data from the Swedish MS registry. Procedure-related safety was assessed by analysing data from electronic patient records covering a period of 100 days following aHSCT.
Results
With a median follow-up time of 5.5 (IQR: 3.4–7.5) years, the Kaplan-Meier estimate for no evidence of disease activity was 73% (95% CI 66% to 81%) at 5 years and 65% (95% CI 57% to 75%) at 10 years. Out of the 149 patients with baseline disability, 80 (54%) improved, 55 (37%) were stable and 14 (9%) deteriorated. The mean number of adverse events per patient was 1.7 (±SD: 1.5) for grade 3 events and 0.06 (±SD: 0.3) for grade 4 events. Febrile neutropenia was the most common adverse event, affecting 68% of patients. There was no treatment-related mortality.
Conclusions
Treatment with aHSCT for RRMS is associated with freedom from disease activity in a majority of patients, with acceptable adverse events. This procedure should be considered a standard of care for patients with highly active RRMS.
Keywords: MULTIPLE SCLEROSIS, HAEMATOLOGY, CLINICAL NEUROLOGY
WHAT IS ALREADY KNOWN ON THIS TOPIC
Autologous haematopoietic stem cell transplantation (aHSCT) is an emerging treatment option for relapsing-remitting multiple sclerosis (RRMS) patients. To date, only one randomised clinical trial has compared aHSCT with standard disease-modifying treatment (DMT) for RRMS. This trial demonstrated a significant advantage of aHSCT over standard DMT in terms of time to progression and neurological disability after 2 years. Moreover, there were no recorded Common Terminology Criteria for Adverse Events grade 4 adverse events or instances of treatment-related mortality. However, it remains uncertain whether these results can be translated into routine healthcare.
WHAT THIS STUDY ADDS
This study supports and strengthens the evidence from the sole randomised controlled trial on aHSCT for RRMS conducted thus far. More than half of the participants experienced improved disability, and approximately two-thirds displayed no evidence of disease activity over a 10-year period. The incidence of severe adverse events was low, and there was no record of treatment-related mortality, suggesting that aHSCT can be safely implemented within routine healthcare.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
aHSCT has the potential to benefit a larger number of MS patients and should be considered a standard of care for highly active MS. Further research is needed to identify the specific patient populations that would derive the most benefit from aHSCT.
Introduction
Multiple sclerosis (MS) is a leading cause of permanent neurological disability in young adults.1 The prevailing theory posits that MS is an autoimmune inflammatory disease mediated by B and T lymphocytes,2 resulting in demyelination, gliosis and axonal degeneration in the central nervous system (CNS).3 4 The most frequently observed disease course at onset is relapsing-remitting MS (RRMS), characterised by distinct inflammatory episodes in the CNS that cause varying degrees of residual disability. Over time, RRMS typically transitions into a secondary progressive (SPMS) disease course marked by neurodegeneration and disability accumulation. Natural history studies estimate that the median time from RRMS onset to secondary progression is approximately 19 years.5
Current disease-modifying treatments (DMTs) primarily focus on reducing inflammation to prevent the formation of MS plaques and clinical relapses. However, the extent to which DMTs slow disability accumulation and delay the transition to SPMS remains unknown. It has been proposed that around half of disability worsening in RRMS occurs without an associated relapse. Such progression independent of relapse activity is common; it occurs frequently in early MS and persists even with highly effective MS therapies.6 An increasingly used treatment goal is to maintain no evidence of disease activity (NEDA), which encompasses the absence of new relapses, new or enlarged lesions on MRI, and confirmed disability worsening (CDW).7 8
High-dose chemotherapy followed by autologous haematopoietic stem cell transplantation (aHSCT) has been used to treat MS since the 1990s. The goal of aHSCT is to reset the immune system by eliminating autoreactive lymphocytes, in order to induce long-term remission.9 Growing evidence supports the efficacy and safety of aHSCT, with two-thirds of treated patients maintaining NEDA up to 4–5 years post-treatment.10 11 It has also been reported that more than half of patients improve in disability outcomes after aHSCT.12 13 The treatment-related mortality rate following aHSCT is estimated at 0.2%–0.3%.11 14 15 The timing of aHSCT is crucial, as it is significantly more effective in RRMS than in progressive forms of MS.11 15 Efficacy and safety have improved in recent years due to increasing experience, improved patient selection, and optimised conditioning regimens.16 The Swedish Board of Health and Welfare approved aHSCT for MS in 2016, but in most countries, it has not yet been integrated into clinical guidelines. The outcome of aHSCT for RRMS in broader use outside clinical trials remains undetermined.
The objective of this multicentre retrospective cohort study was to assess the efficacy and safety of aHSCT for RRMS when implemented in a setting of routine healthcare.
Methods
Data collection
Patients were identified using the Swedish MS registry (SMSreg) and local European Society for Blood and Marrow Transplantation (EBMT) registries at the seven transplantation centres throughout Sweden. The SMSreg is a nationwide registry that has been amassing prospective data on MS patients since the mid-1990s. It currently has an estimated coverage of >80% overall and nearly 100% for patients with advanced therapy such as aHSCT.17
Data were extracted from the SMSreg and electronic patient records. A neurologist at each transplantation centre retrospectively reviewed disease course and all outcome data in the SMSreg to ensure their validity. A haematologist collected safety data by systematically analysing medical records from the time of stem cell mobilisation to 3 months following aHSCT. All severe adverse events (AEs), defined as AE of grade 3 or higher, were documented in accordance with version 5.0 of the Common Terminology Criteria for Adverse Events.18 Anaemia, leucopenia, neutropenia and thrombocytopaenia as well as transient alopecia and amenorrhoea were expected during the first weeks after aHSCT and were not included. A comprehensive overview of all data is given in online supplemental section 1. The study protocol is available in online supplemental file 2.
jnnp-2023-331864supp001.pdf (134KB, pdf)
jnnp-2023-331864supp002.pdf (318.5KB, pdf)
Inclusion criteria
Inclusion criteria were diagnosis of MS according to the revised McDonald criteria,19 with a relapsing-remitting disease course,20 and aHSCT performed for MS at any of the seven Swedish transplantation centres before 1 January 2020.
Exclusion criteria
Exclusion criteria were progressive MS (primary progressive MS or SPMS) according to Lublin et al 20 at the time of aHSCT, that the patient did not consent to reporting of data to the EBMT register or failure to meet the minimal dataset. The minimal dataset covered disease course at the time of transplantation, date of transplantation, data on conditioning regimen and at least one follow-up visit (unless early death before first follow-up visit) with data on clinical assessment using the Kurtzke Expanded Disability Status Scale (EDSS) and neuroradiological assessment with MRI.21
Endpoints
The primary endpoints were NEDA at 5 years and treatment-related mortality. Secondary endpoints were NEDA at 3 and 10 years; CDW, relapse-free survival and MRI event-free survival at 3, 5 and 10 years; annualised relapse rate after aHSCT; proportion of patients with confirmed disability improvement; and EDSS change between baseline and follow-up at 1, 2 and 3 years. The frequency and grade of severe AEs were used to estimate the safety of the procedure, limited to within 100 days of aHSCT in order to restrict information bias. Definitions of these endpoints are given in online supplemental section 2.
Procedures
Mobilisation
Stem cells were mobilised using a combination of cyclophosphamide 2 g/m2 and granulocyte-colony-stimulating factor (G-CSF) 5 µg/kg subcutaneously starting on day 5 or 6 until stem cell harvest.
Harvest
Haematopoietic stem cells were harvested by apheresis of peripheral blood. A minimum of 2.0×106 CD34+ cells/kg were harvested and cryopreserved, with no ex vivo manipulation.
Conditioning
Two conditioning regimens were used; BEAM-antithymocyte globulin (ATG) and Cy-ATG. The BEAM-ATG comprised carmustine 300 mg/m2, etoposide 800 mg/m2, cytarabine arabinoside 3200 mg/m2, melphalan 140 mg/m2 and ATG from rabbit (rATG) 10 mg/kg and was given during 7 days. This protocol was mainly used during the first few years of the study period and was then replaced by Cy-ATG at all centres. The Cy-ATG protocol was given during 5 days and included cyclophosphamide 200 mg/kg, rATG 10 mg/kg and 5000 mg methylprednisolone, including tapering after stem cell infusion, and hyperhydration and uromitexan to prevent haemorrhagic cystitis. There was a minimum wash-out time of 48 and 24 hours from the last chemotherapy administration to the reinfusion of the autologous stem cells for BEAM-ATG and Cy-ATG, respectively.
Antimicrobial prophylaxis
Oral ciprofloxacin was used to prevent bacterial infection during the neutropenic phase, except for eight patients who received prophylactic intravenous antibiotics. Prophylaxis against herpes simplex virus and Pneumocystis jiroveci was given for a minimum of 3 months following aHSCT. Monitoring for reactivation of cytomegalovirus (CMV) and Epstein-Barr virus (EBV) was performed for patients with positive serology.
Supportive care
If needed, all patients were given filtered and irradiated blood products until their lymphocyte counts exceeded 1.0×109/L.
Statistical analysis
Statistical analysis was performed with V.3.5.3 of R, using the packages ggplot2, survival, fBasics, ggpubr, moments, survminer, plotrix, grid, gridExtra, lattice and devtools. Data were summarised using frequencies for categorical variables, medians (IQR) for discrete variables and time data unless inappropriate due to rare events, and means (±SD:) for continuous variables. The Mann-Whitney test was used to determine statistically significant differences between two groups, Fisher’s exact test was used to determine statistically significant differences between proportions, and the Wilcoxon signed rank test was used to determine statistically significant differences between two time points. Survival was estimated using Kaplan-Meier plots (95% CI). A two-tailed p<0.05 was considered statistically significant.
Results
Patient characteristics
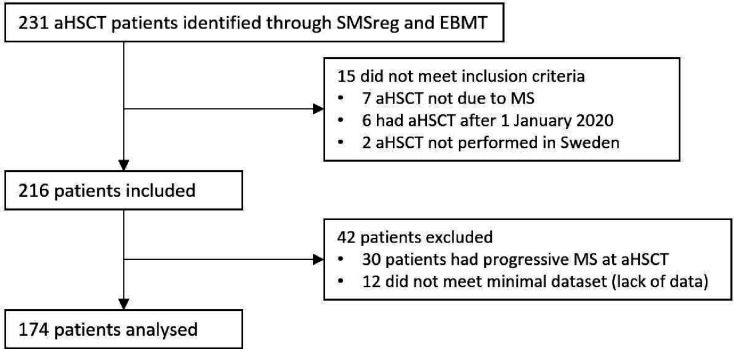
Data were exported from the SMSreg on 22 May 2022. We evaluated 231 patients for participation in the study, of whom 174 were included in the final analysis. Fifteen patients did not meet the inclusion criteria and were not analysed further, 30 patients had progressive MS at aHSCT and 12 did not fulfil the minimal dataset; these 42 patients were excluded from the study (figure 1). Baseline patient characteristics are shown in table 1.
Figure 1.
In this study, a total of 231 patients were evaluated for potential inclusion, with 174 ultimately being incorporated into the final analysis. Fifteen patients were excluded as they failed to meet the inclusion criteria, which required a diagnosis of multiple sclerosis (MS), a relapsing-remitting disease course and autologous haematopoietic stem cell transplantation (aHSCT) performed for MS at any of the seven Swedish transplantation centres before 1 January, 2020. Thirty patients had progressive MS at aHSCT and were excluded. An additional 12 did not fulfil the requirements for the minimal dataset, which were data on disease course at the time of transplantation, date of transplantation, data on conditioning regimen and at least one follow-up visit. EBMT, European Society for Blood and Marrow Transplantation; SMSreg, Swedish MS registry.
Table 1.
Baseline patient characteristics
| n=174 | No of patients (%) |
| Age in years (range: 9–58) | |
| 0–9 | 1 (0.6) |
| 10–19 | 6 (3.4) |
| 20–29 | 70 (40) |
| 30–39 | 68 (39) |
| 40–49 | 26 (15) |
| 50–59 | 3 (1.7) |
| Sex | |
| Female | 112 (64) |
| Male | 62 (36) |
| Comorbidities* | |
| Depression | 7 (4.0) |
| Obesity | 5 (2.9) |
| Asthma | 5 (2.9) |
| Bipolar disorder | 4 (2.3) |
| Anxiety disorder | 4 (2.3) |
| Mb Crohn | 3 (1.7) |
| Hypertension | 3 (1.7) |
| Psoriasis | 3 (1.7) |
| Prior malignancy† | 2 (1.1) |
| Diabetes mellitus | 2 (1.1) |
| Chronic renal disease | 2 (1.1) |
| Rheumatoid arthritis | 2 (1.1) |
| Prior deep vein thrombosis | 2 (1.1) |
| Thyrotoxicosis | 2 (1.1) |
| Ankylosing spondylitis | 2 (1.1) |
| Irritable bowel syndrome | 2 (1.1) |
| No comorbidity | 122 (70) |
| EDSS at aHSCT‡ | |
| 0–1.5 | 23 (13) |
| 2–3.5 | 88 (51) |
| 4–5.5 | 38 (22) |
| 6–6.5 | 16 (9.2) |
| 7–9.5 | 8 (4.6) |
| Gadolinium-enhancing lesions at aHSCT§ | |
| 0 | 91 (57) |
| 1–9 | 44 (28) |
| 10–20 | 11 (6.9) |
| >20 | 13 (8.2) |
*Comorbidities with a frequency of more than 1%.
†Two cases of breast cancer.
‡Data missing for one patient.
§Fifteen patients did not have a contrast-enhanced MRI scan at baseline.
aHSCT, autologous haematopoietic stem cell transplantation; EDSS, Expanded Disability Status Scale.
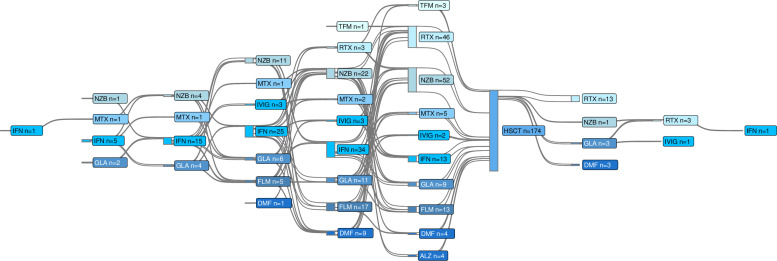
The first patient was treated on 25 May 2004. Median age at aHSCT was 31 years (IQR: 26–36) and 64% of the patients were women. Median disease duration was 3.4 years (IQR: 1.0–6.9). The patients had received a median of 2 (IQR: 1–3) DMTs prior to aHSCT, and 23 patients were previously untreated. The median follow-up time was 5.5 years (IQR: 3.4–7.5). A total of 2435 follow-up visits with EDSS scoring and 1785 MRI scans were analysed, with a cumulative follow-up time amounting to 1034 years. After a median of 2.9 years (IQR: 2.1–4.4), 20 patients (11%) received DMT after aHSCT (figure 2). After a median of 4.1 years (IQR: 1.8–5.9) years, 10 patients transitioned from RRMS to SPMS.
Figure 2.
Current and previous treatments. This Sankey diagram shows disease-modifying treatments used before and after autologous haematopoietic stem cell transplantation (aHSCT). Twenty-three patients had not used any disease-modifying treatment prior to aHSCT. ALZ, alemtuzumab; DMF, dimethyl fumarate; FLM, fingolimod; GLA, glatiramer acetate; IFN, interferon; IVIG, intravenous IgG; MTX, mitoxantrone; NZB, natalizumab; RTX, rituximab; TMF, teriflunomide.
Procedures
All patients were mobilised with cyclophosphamide+G CSF. For the conditioning, BEAM-ATG was used in 33 patients and Cy-ATG in 141 patients. The last patient receiving BEAM-ATG was treated in 2015. The median time to engraftment (defined as absolute neutrophil count ≥0.5 and thrombocyte counts >20 and rising, without transfusion of thrombocytes) was 12 days (IQR: 11–13.5). The median time of hospitalisation for aHSCT was 20 days (IQR: 19–22), calculated from the day of admission to the hospital to the day of discharge. Twenty-eight patients received G-CSF in the first week following aHSCT. There was a mean decrease in body weight of 2.2 (±SD: 2.1) kg and a median loss of plasma albumin of 7.5 (5–11) g/L during hospitalisation for aHSCT.
Primary endpoints
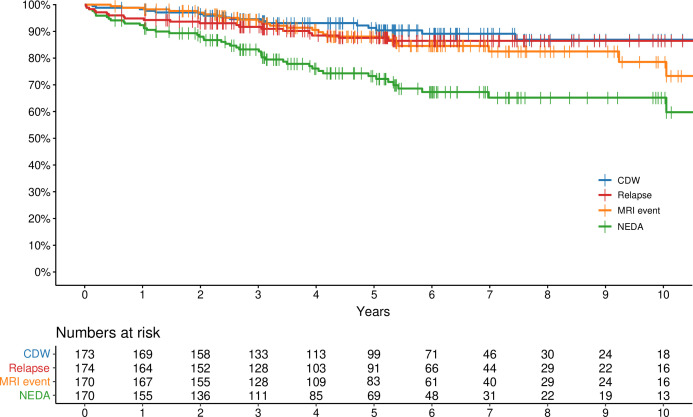
The Kaplan-Meier estimate of NEDA at 5 years was 73% (95% CI 63% to 81%). There was no treatment-related mortality (figure 3).
Figure 3.
Primary and secondary endpoints. Kaplan-Meier curves for the primary endpoint: no evidence of disease activity (NEDA), and for the secondary endpoints: freedom from MRI events, freedom from clinical relapses and freedom from confirmed disability worsening (CDW).
Secondary endpoints
Clinical relapses, MRI events and CDW
The Kaplan-Meier estimates of clinical relapses, MRI events and CDW at different time points are presented in figure 3 and table 2. Notably, all instances of CDW occurred independent of relapses, and there was no relapse-associated worsening. An ad hoc analysis was made to determine the further clinical course in the 48 patients who displayed evidence of disease activity after AHSCT. A new baseline was set after the first instance of disease activity. After a median follow-up time of 6.2 years (IQR: 5.1–7.4), 5 patients exhibited CDW, 12 suffered from a clinical relapse and 16 patients had an MRI event. The Kaplan-Meier estimate of NEDA was 62% (95% CI 49% to 79%) 5 years after rebaselining.
Table 2.
Kaplan-Meier estimates of NEDA and freedom from clinical relapses, MRI events and CDW by year
| Year | Clinical relapse | MRI event | CDW | NEDA | ||||
| KM | 95% CI | KM | 95% CI | KM | 95% CI | KM | 95% CI | |
| 1 | 95% | 92% to 98% | 99% | 97% to 100% | 98% | 96% to 100% | 92% | 88% to 96% |
| 2 | 92% | 89% to 96% | 97% | 94% to 100% | 96% | 94% to 99% | 87% | 82% to 93% |
| 3 | 91% | 87% to 96% | 94% | 91% to 98% | 95% | 91% to 98% | 83% | 77% to 89% |
| 5 | 87% | 82% to 93% | 88% | 83% to 94% | 91% | 87% to 96% | 73% | 66% to 80% |
| 10 | 86% | 80% to 92% | 79% | 69% to 90% | 87% | 80% to 94% | 65% | 56% to 74% |
CDW, confirmed disability worsening; KM, Kaplan-Meier estimate; NEDA, no evidence of disease activity.
Annualised relapse rate
The annualised relapse rate was 1.7 (±SD: 1.9) in the year prior to aHSCT and 0.035 (±SD: 0.12) during the follow-up period (p<0.0001).
Proportion of patients with confirmed disability improvement
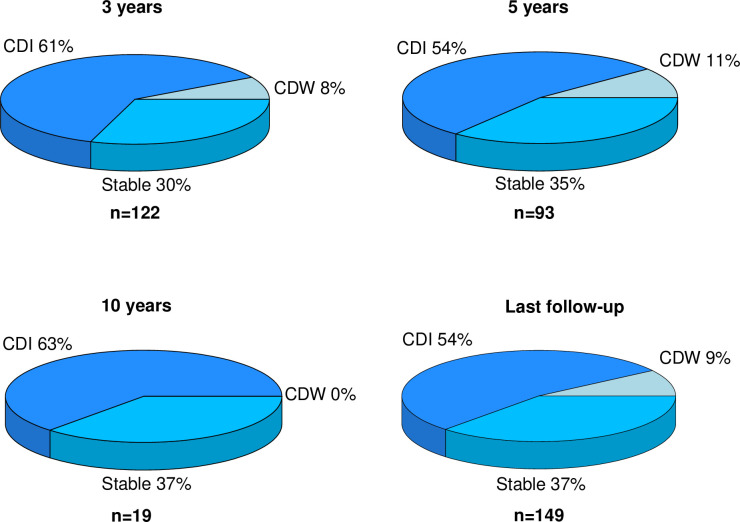
Of the 149 patients with any degree of disability at baseline (EDSS≥2), 80 (54%) improved in disability, 55 (37%) were stable and 14 (9%) deteriorated at the end of follow-up. The proportions of patients with changes in disability at different time points are presented in figure 4.
Figure 4.
Changes in disability over time. Proportions of patients with confirmed disability improvement (CDI), stable disability and confirmed disability worsening (CDW) at different timepoints. Scores on the Kurtzke Expanded Disability Status Scale (EDSS) at baseline were compared with the EDSS scores at 3, 5 and 10 years as well as with the EDSS scores at last follow-up. Only patients with some degree of disability (EDSS≥2) were taken into account for this analysis.
EDSS change
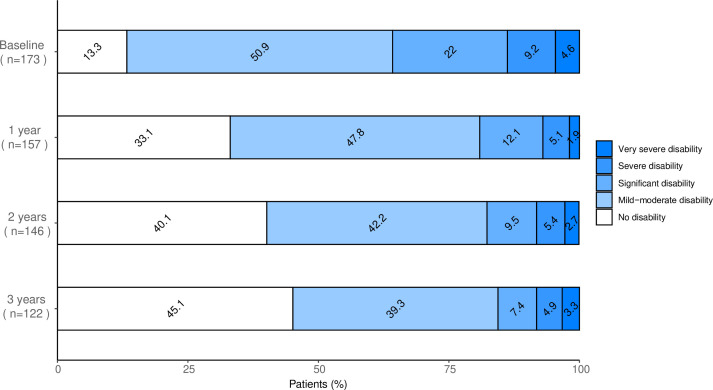
The median EDSS at baseline was 3.5 (IQR: 2–4). The evolution of EDSS over the first 3 years is shown in figure 5. At the last follow-up, the median EDSS was 2 (IQR: 1–3.5), significantly lower than at baseline (p<0.0001).
Figure 5.
Proportions of patients with different levels of disability over time. Disability strata defined using the Kurtzke Expanded Disability Status Scale (EDSS) from baseline and over the first 3 years after autologous haematopoietic stem cell transplantation. No disability was defined as EDSS 0–1.5, mild-to-moderate disability as EDSS 2–3.5, significant disability as EDSS 4–5.5, severe disability as EDSS 6–6.5 and very severe disability as EDSS 7–9.5.
Safety
To assess the safety of aHSCT, we analysed mortality, need for intensive care and severe AEs. The mean number of severe AEs per patient was 1.7 (±SD: 1.5) for grade 3 events and 0.06 (±SD: 0.3) for grade 4 events. Thirty patients (18%) did not experience any severe AE (those directly related to the procedure excluded). The frequencies of all severe AEs are presented in table 3.
Table 3.
Severe adverse events by frequency
| n=174 | Grade 3 (%) | Grade 4 (%) |
| Febrile neutropenia* | 119 (68.4) | 6 (3.4) |
| Hypokalaemia† | 31 (17.8) | – |
| Nausea | 14 (8.0) | – |
| Serum sickness | 11 (6.3) | – |
| Oral mucositis | 9 (5.1) | – |
| Diarrhoea | 9 (5.1) | – |
| Elevated transaminases | 9 (5.1) | – |
| Hypoalbuminaemia | 7 (4.0) | – |
| Hypotension | 6 (3.4) | – |
| Fatigue | 5 (2.9) | – |
| Anorexia | 5 (2.9) | – |
| Hyperglycaemia | 5 (2.9) | – |
| Pericarditis | 2 (1.1) | 1 (0.6) |
| Depression | 2 (1.1) | 1 (0.6) |
| Thromboembolic event | 2 (1.1) | 1 (0.6) |
| Skin/soft tissue infection (non-neutropenic)‡ | 3 (1.7) | – |
| Vascular access thrombosis | 3 (1.7) | – |
| Pneumonia (non-neutropenic)‡ | 3 (1.7) | – |
| Catheter-related infection (non-neutropenic)‡ | 3 (1.7) | – |
| Cytokine release syndrome | 3 (1.7) | – |
| Myalgia | 3 (1.7) | – |
| Vomiting | 3 (1.7) | – |
| Elevated gamma-GT | 3 (1.7) | – |
| Heart failure | 1 (0.6) | 1 (0.6) |
| Hyponatraemia | 1 (0.6) | 1 (0.6) |
| Sepsis (non-neutropenic)‡ | 2 (1.1) | – |
| Infectious enterocolitis (non-neutropenic)‡ | 2 (1.1) | – |
| Fever | 2 (1.1) | – |
| Seizures | 2 (1.1) | – |
| Urticaria | 2 (1.1) | – |
| Syncope | 2 (1.1) | – |
| Abdominal pain | 2 (1.1) | – |
| Postherpetic pain | 2 (1.1) | – |
| CMV reactivation | 1 (0.6) | – |
| EBV reactivation | 1 (0.6) | – |
| Varicella zoster | 1 (0.6) | |
| Pyelonephritis (non-neutropenic)‡ | 1 (0.6) | – |
| Non-infectious enterocolitis | 1 (0.6) | – |
| Atrial fibrillation | 1 (0.6) | – |
| Hypoxia | 1 (0.6) | – |
| Pulmonary infiltrates | 1 (0.6) | – |
| Interstitial oedema in lungs | 1 (0.6) | – |
| Renal insufficiency | 1 (0.6) | – |
| Allergic reaction | 1 (0.6) | – |
| Leucocytosis | 1 (0.6) | – |
| Immunological thrombocytopaenia | 1 (0.6) | – |
| Mania | 1 (0.6) | – |
| Hallucinations | 1 (0.6) | – |
| Hemichorea | 1 (0.6) | – |
| Skeletal pain | 1 (0.6) | – |
| Genital herpes simplex infection | 1 (0.6) | – |
| Vaginal haemorrhage | 1 (0.6) | – |
| Elevated alkaline phosphatase | 1 (0.6) | – |
All grades 3 and 4 adverse events according to CTCAE V.5.0 for all patients from start of mobilisation to day +100 after aHSCT. Anaemia, neutropenia, leucopenia and thrombocytopaenia as well as transient alopecia and amenorrhoea were expected during the first weeks after aHSCT, and were excluded. Neurological adverse events assessed as manifestations of MS were not included. There were no grade 5 adverse events.
*Febrile neutropenia comprises all episodes of fever (according to CTCAE V.5.0) regardless of clinical infection occurring during the neutropenic phase following stem cell mobilisation and conditioning.
†Hypokalaemia was associated with furosemide treatment after hyperhydration in 15 patients.
‡Occurring outside the neutropenic phase.
aHSCT, autologous haematopoietic stem cell transplantation; CMV, cytomegalovirus; CTCAE, Common Terminology Criteria for Adverse Events; EBV, Epstein-Barr virus.
Overall mortality
One patient in the cohort died during the follow-up period. The deceased patient had pre-existing depression and a history of a suicide attempt. The cause of death was attributed to suicide precipitated by substance abuse. The death occurred more than 6 years after aHSCT, and this event was deemed unrelated to the treatment.
Intensive care
Five patients were admitted for intensive care, with a median duration of 2 days (range: 1–2). The reasons for intensive care were correction of hyponatraemia (n=2), sepsis and hypoxia (n=1), febrile neutropenia and hypotonia (n=1), and pulmonary embolism in combination with perimyocarditis with a transient decrease in left ventricle ejection fraction to 30% (n=1).
Febrile neutropenia
Febrile neutropenia was the most frequently observed AE linked to aHSCT, affecting 125 patients (72%) Intravenous antibiotics were administered to 138 patients (79%).
Bacterial infections
Bacterial infection was verified through culture in 61 patients (35%) at any time from stem cell mobilisation to day+100. The most common bacterial species were Escherichia coli (n=20 patients), alpha streptococci (n=8) and coagulase negative streptococci (n=6). The most common clinical infections were septicaemia (n=20), urinary tract infections (n=17), and venous catheter-related infections (n=4).
Common AEs
Other common AEs included hypokalaemia, which affected 31 patients (18%) and was associated with the use of hyperhydration and furosemide in half of the cases. There were no recorded arrhythmias. Nausea interfering with dietary intake and requiring parenteral nutrition occurred in 14 patients (8.0%). ATG-associated serum sickness requiring steroids or intravenous fluids affected 11 patients (6.3%). Thromboembolic events occurred in eight patients (4.6%): deep vein thrombosis (n=5), vascular access thrombosis (n=2) and pulmonary embolism (n=1). One case of autoimmune disease (immunological thrombocytopenic purpura) occurred in the first 100 days following aHSCT.
There were few associations between AEs and pre-existing comorbidities. The only grade 4 psychiatric AE happened in a patient with pre-existing bipolar disorder and the only grade 4 thromboembolic event occurred in a patient with heterozygote activated protein C resistance. Gastrointestinal AEs with grade 3 diarrhoea occurred in one patient with pre-existing Crohn’s disease and in another who had undergone a gastric by-pass procedure.
EBV and CMV
None of the patients developed EBV-related or CMV-related disease. In 59 patients, at least one PCR test showed detectable EBV levels, while 49 patients had PCR tests positive for CMV. In the majority of cases, low levels of EBV and CMV reactivations (below 900 copies/mL) were detected in a single blood sample from asymptomatic patients. Twenty-one patients had measurable EBV levels in at least two samples, but only one received rituximab treatment as a preventive measure. In the case of CMV, eight patients had persistent, measurable levels in at least two samples. Five of these patients received pre-emptive oral treatment for CMV, while one patient required intravenous therapy. Data were unavailable for five patients.
Other viral and fungal infections
Other viral infections were verified in 23 patients (13%). Respiratory viruses (rhinoviruses, adenoviruses and coronaviruses) were found in eight patients. Four patients tested positive for BK virus, with three cases detected exclusively in urine and one case found in both urine and plasma. All four of these patients had undergone cyclophosphamide conditioning. Notably, no instances of haemorrhagic cystitis were observed. Herpes zoster reactivation was documented in three patients. In the full cohort, only 1.7% (n=3 patients) had a confirmed localised fungal infection. Two of these patients presented with oral candidiasis, while the third had vaginal candidiasis. Importantly, no cases of invasive fungal infection were seen.
Discussion
A key finding of this cohort study of 174 RRMS patients is that treatment with aHSCT was followed by maintenance of NEDA over 5 years in 73% of patients, without compromising safety. There was no treatment-related mortality and AEs were manageable. These findings support what is currently the only randomised controlled trial of aHSCT for RRMS,12 suggesting that the results are generalisable to routine healthcare settings.
One of the advantages of aHSCT is that it is a one-time treatment, allowing for comparison with immune replacement therapies such as alemtuzumab and cladribine. While direct trial comparisons are generally discouraged, factors such as age, disease duration, annualised relapse rate and the percentage of patients with gadolinium-enhancing lesions at baseline were reasonably comparable between the CARE-MS trials and our study. In the CLARITY trial, however, patients were older, had longer disease durations and a lower percentage of participants had gadolinium-enhancing lesions at baseline, suggesting a slightly less inflammatory disease. Disability was lower in both CARE-MS trials and CLARITY, indicating that patients in our trial had more severe disease. The proportion of patients maintaining NEDA over 2 years (88%) was considerably higher than that reported in the CARE-MS I and II studies of alemtuzumab (32%–39%)22 23 and the CLARITY study of cladribine (47%).24 A previous study comparing outcomes of patients treated with aHSCT or alemtuzumab at two Swedish centres reported similar results, with the Kaplan-Meier estimate of NEDA at 3 years being 88% for aHSCT and 37% for alemtuzumab.13
A substantial number of patients were followed for more than 5 years. At the 10-year mark, 65% of patients still maintained NEDA and 88% did not experience disability worsening. These results indicate a durable response, further emphasised by the low number of patients requiring additional treatment after aHSCT and the low conversion rate from RRMS to SPMS. These figures are slightly more favourable than those reported in a study of Italian patients treated with the BEAM conditioning regimen,25 which may reflect differences in disease severity and emphasises the importance of early intervention.
The indication for aHSCT in Sweden evolved during the study period. Initially considered a rescue treatment, reserved for patients with the most aggressive forms of MS where other treatment options were considered futile.26 The indication was later changed to include patients with active disease despite adequate course of treatment with at least one DMT or with rapidly evolving severe RRMS defined by at least two disabling relapses in 1 year with evidence of MRI disease activity, similar to the European Medicines Agency label for natalizumab. Consequently, this group represents individuals with a poor long-term prognosis; nevertheless, aHSCT was associated with an improvement in EDSS at group level. Nearly half of the patients were free from disability 3 years after aHSCT, a significant increase from the 13% who had no disability at baseline. Over half of those with disability at baseline improved after aHSCT, and only 9% had worsened at the last follow-up. This improvement distinguishes aHSCT from standard DMTs. Although this was an observational study where parts of the improvement can be explained by regression to the mean, the proportion of patients with improvement in EDSS was very similar to that reported in the randomised controlled Multiple Sclerosis International Stem Cell Transplant trial.12
The comprehensive analysis of medical records allowed us to obtain high-resolution safety data, presenting the full range of severe AEs directly related to the procedure. All AEs could be managed using standard procedures at a tertiary referral hospital. Several commonly anticipated AEs, such as hypokalaemia resulting from loop diuretic use, serum sickness after rATG administration and elevated hepatic transaminases following cyclophosphamide treatment, were all resolved before hospital discharge. Hypokalaemia episodes did not lead to any observed arrhythmias. Only six patients (3.4%) experienced grade 4 AEs, which occurred evenly throughout the study period.
The most frequent severe AEs were febrile neutropenia and infectious complications, both of which are generally expected during the transient impairment of the immune system after conditioning. We observed a higher incidence of febrile neutropenia compared with most previous studies. One potential explanation could be that oral ciprofloxacin, instead of intravenous antibiotics, is used for bacterial prophylaxis after aHSCT in Sweden. Eight patients in this study received prophylaxis with intravenous antibiotics and had comparable rates of febrile neutropenia, but none of them had any positive bacterial cultures. We defined febrile neutropenia as measurements of fever during neutropenia, and not, as in several previous studies, as positive bacterial cultures. Conversely, earlier research indicated a high prevalence of EBV and CMV reactivations following aHSCT for MS,27 28 while our study found a very low frequency of clinically relevant EBV and CMV reactivations despite active monitoring. Taking together data from our and previous studies, although varying types of infections are unavoidable in conjunction with aHSCT, we believe that the low risk of severe complications should not deter patients from undergoing this procedure in situations where the clinical benefits are considered to be high.
Acknowledging a low capture rate of AEs by review of medical records, we deliberately chose not to gather data on AEs beyond the initial 3 months. For reference, we recently published a registry linkage study on a largely overlapping patient cohort treated with aHSCT (n=139), and compared safety outcomes with patients treated with alemtuzumab (n=132) and a large reference population (n=2486) treated with regular DMTs.29 Data from SMSreg were linked to a series of national demographic and health registers with excellent coverage, including the mortality register, cancer register and national patient register. The study revealed a moderately elevated risk of infections requiring hospital care with aHSCT, also beyond the first 6 months. Thyroid disease was also more common compared with the reference population, but significantly lower than with alemtuzumab. Importantly, no malignancies were noted in the aHSCT group. Apart from the same fatal case reported here, there were no additional mortalities with aHSCT, compared with four with alemtuzumab.29
This study was an observational cohort study, inherently limited by the absence of a control group. Some data may have been missing, potentially leading to underreporting, primarily of AEs. To ensure data accuracy in SMSreg, an on-site neurologist cross-verified each patient’s register data with their medical health records. When any patient’s medical record is accessed in Sweden, the patient’s vital status is automatically synchronised with the Swedish Tax Agency’s records, which are updated daily. Consequently, we have complete and up-to-date mortality data for all patients, regardless of their last follow-up visit, and hence can confirm that no patient in the study cohort died from COVID-19 during the recent pandemic. The absence of a control group precludes definitive conclusions about the effect size in comparison to other RRMS treatments and makes it impossible to estimate the magnitude of the regression to the mean. Conversely, notable strengths of this study are the near-complete coverage, high data density and granularity of the SMSreg.
In summary, our findings demonstrate that aHSCT for RRMS is feasible within regular healthcare and can be performed without compromising safety. Our study corroborates the results observed in the only randomised controlled trial conducted to date.12 We believe that aHSCT could benefit a greater number of MS patients and should be included as a standard of care for highly active MS.
Acknowledgments
This study was presented as an abstract and oral presentation at the European Society for Blood and Marrow Transplantation 49th Annual Meeting, Paris, 23-26 April 2023.
Footnotes
Contributors: JB is the guarantor of the study and accepts full responsibility for the work and/or the conduct of the study, had access to the data, and controlled the decision to publish. The study was conceptualised by JB. The methodology was planned by JB, KC, PL, HC and TS. Funding was acquired by JB and TS. Data were collected by JB, TS, PL, SE, EA, SL, EI, JF, JL, NA, JM, NL and AD. Data were validated by JB, TS, EI, JF, JL, NA, JM, NL and FP. Data curation and data analysis were performed by JB, CZ and TS. Supervision and coordination of the study were performed by JB and TS. Visualisations were made by JB, CZ and TS. The original draft of the manuscript was written by JB and TS. Reviewing and editing were performed by all coauthors.
Funding: The study was funded by the Center for Clinical Research Dalarna with grant numbers CKFUU-936620 and CKFUU-976388, the Marianne and Marcus Wallenberg Foundation, Region Stockholm, the Swedish Research Council, the Swedish Society for Medical Research (2020-02700), the Swedish Society of Medicine and Uppsala‐Örebro Regional Research Council (RFR-967904).
Disclaimer: None of the funders had any role in the study design, the collection, analysis, or interpretation of data, the writing of the manuscript, or the decision on where to publish the article.
Competing interests: EI has received speakers fee from Merck and honoraria from advisory boards for Sanofi-Aventis, Biogen and Merck. FP previously received research grants from Merck KGaA, Janssen and UCB outside this study. FP has received payment for expert testimony from Novartis. FP has participated in Data Monitoring Committee for clinical trials from Chugai, Lundbeck and Roche. JM has received lecture honorarium from Merck. NL has received honoraria from Sanofi. All other individual authors declare that there is no conflict of interest.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. Deidentified individual participant data supporting the findings presented in this article, including text, tables, figures and appendices, will be accessible alongside the study protocol for a period of 5 years, starting 9 months after the article’s publication. Researchers with a sound proposal can request access to this data by contacting joachim.burman@uu.se. To obtain access, those requesting data will need to sign a data access agreement.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and the Ethical Review Authority in Sweden granted approval for the study on 14 April 2021 (ref: 2021-01530). As this is a retrospective observational study, a specific consent for this study was not obtained, but every patient involved in the study gave their written consent, permitting their data to be reported to the European Society for Blood and Marrow Transplantation (EBMT) registry. Although participation in national quality registries like SMSreg is obligatory for Swedish citizens receiving publicly funded healthcare, patients retain the option to opt out of research conducted using data from these registers.
References
- 1. Noseworthy JH, Lucchinetti C, Rodriguez M, et al. Multiple sclerosis. N Engl J Med 2000;343:938–52. 10.1056/NEJM200009283431307 [DOI] [PubMed] [Google Scholar]
- 2. Weiner HL. Multiple sclerosis is an inflammatory T-cell-mediated autoimmune disease. Arch Neurol 2004;61:1613–5. 10.1001/archneur.61.10.1613 [DOI] [PubMed] [Google Scholar]
- 3. Compston A, Coles A. Multiple sclerosis. Lancet 2008;372:1502–17. 10.1016/S0140-6736(08)61620-7 [DOI] [PubMed] [Google Scholar]
- 4. Dendrou CA, Fugger L, Friese MA. Immunopathology of multiple sclerosis. Nat Rev Immunol 2015;15:545–58. 10.1038/nri3871 [DOI] [PubMed] [Google Scholar]
- 5. Rovaris M, Confavreux C, Furlan R, et al. Secondary progressive multiple sclerosis: current knowledge and future challenges. Lancet Neurol 2006;5:343–54. 10.1016/S1474-4422(06)70410-0 [DOI] [PubMed] [Google Scholar]
- 6. Lublin FD, Häring DA, Ganjgahi H, et al. How patients with multiple sclerosis acquire disability. Brain 2022;145:3147–61. 10.1093/brain/awac016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Smith AL, Cohen JA, Hua LH. Therapeutic targets for multiple sclerosis: current treatment goals and future directions. Neurotherapeutics 2017;14:952–60. 10.1007/s13311-017-0548-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nixon R, Bergvall N, Tomic D, et al. No evidence of disease activity: indirect comparisons of oral therapies for the treatment of relapsing-remitting multiple sclerosis. Adv Ther 2014;31:1134–54. 10.1007/s12325-014-0167-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Muraro PA, Martin R, Mancardi GL, et al. Autologous haematopoietic stem cell transplantation for treatment of multiple sclerosis. Nat Rev Neurol 2017;13:391–405. 10.1038/nrneurol.2017.81 [DOI] [PubMed] [Google Scholar]
- 10. Sormani MP, Muraro PA, Saccardi R, et al. NEDA status in highly active MS can be more easily obtained with autologous hematopoietic stem cell transplantation than other drugs. Mult Scler 2017;23:201–4. 10.1177/1352458516645670 [DOI] [PubMed] [Google Scholar]
- 11. Muraro PA, Pasquini M, Atkins HL, et al. Long-term outcomes after autologous hematopoietic stem cell transplantation for multiple sclerosis. JAMA Neurol 2017;74:459–69. 10.1001/jamaneurol.2016.5867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Burt RK, Balabanov R, Burman J, et al. Effect of nonmyeloablative hematopoietic stem cell transplantation vs continued disease-modifying therapy on disease progression in patients with relapsing-remitting multiple sclerosis: a randomized clinical trial. JAMA 2019;321:165–74. 10.1001/jama.2018.18743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhukovsky C, Sandgren S, Silfverberg T, et al. Autologous haematopoietic stem cell transplantation compared with alemtuzumab for relapsing-remitting multiple sclerosis: an observational study. J Neurol Neurosurg Psychiatry 2021;92:189–94. 10.1136/jnnp-2020-323992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sharrack B, Saccardi R, Alexander T, et al. Autologous haematopoietic stem cell transplantation and other cellular therapy in multiple sclerosis and immune-mediated neurological diseases: updated guidelines and recommendations from the EBMT autoimmune diseases working party (ADWP) and the joint accreditation committee of EBMT and ISCT (JACIE). Bone Marrow Transplant 2020;55:283–306. 10.1038/s41409-019-0684-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Burt RK, Han X, Quigley K, et al. Real-world application of autologous hematopoietic stem cell transplantation in 507 patients with multiple sclerosis. J Neurol 2022;269:2513–26. 10.1007/s00415-021-10820-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Das J, Sharrack B, Snowden JA. Correction to: autologous haematopoietic stem cell transplantation in multiple sclerosis: a review of current literature and future directions for transplant haematologists and oncologists. Curr Hematol Malig Rep 2019;14:136. 10.1007/s11899-019-00506-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hillert J, Stawiarz L. The Swedish MS registry – clinical support tool and scientific resource. Acta Neurol Scand 2015;132:11–9. 10.1111/ane.12425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. National Institutes of Health, National Cancer Institute . Common terminology criteria for adverse events (CTCAE) V5.0: US Department of health and human services. 2017. Available: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf
- 19. Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 2018;17:162–73. 10.1016/S1474-4422(17)30470-2 [DOI] [PubMed] [Google Scholar]
- 20. Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology 2014;83:278–86. 10.1212/WNL.0000000000000560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983;33:1444–52. 10.1212/wnl.33.11.1444 [DOI] [PubMed] [Google Scholar]
- 22. Coles AJ, Twyman CL, Arnold DL, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet 2012;380:1829–39. 10.1016/S0140-6736(12)61768-1 [DOI] [PubMed] [Google Scholar]
- 23. Cohen JA, Coles AJ, Arnold DL, et al. Alemtuzumab versus interferon beta 1A as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet 2012;380:1819–28. 10.1016/S0140-6736(12)61769-3 [DOI] [PubMed] [Google Scholar]
- 24. Giovannoni G, Cook S, Rammohan K, et al. Sustained disease-activity-free status in patients with relapsing-remitting multiple sclerosis treated with cladribine tablets in the CLARITY study: a post-hoc and subgroup analysis. Lancet Neurol 2011;10:329–37. 10.1016/S1474-4422(11)70023-0 [DOI] [PubMed] [Google Scholar]
- 25. Boffa G, Massacesi L, Inglese M, et al. Long-term clinical outcomes of hematopoietic stem cell transplantation in multiple sclerosis. Neurology 2021. 10.1212/WNL.0000000000011461 [DOI] [PubMed] [Google Scholar]
- 26. Burman J, Iacobaeus E, Svenningsson A, et al. Autologous haematopoietic stem cell transplantation for aggressive multiple sclerosis: the Swedish experience. J Neurol Neurosurg Psychiatry 2014;85:1116–21. 10.1136/jnnp-2013-307207 [DOI] [PubMed] [Google Scholar]
- 27. Nash RA, Dansey R, Storek J, et al. Epstein-Barr virus-associated posttransplantation lymphoproliferative disorder after high-dose immunosuppressive therapy and Autologous Cd34-selected hematopoietic stem cell transplantation for severe autoimmune diseases. Biol Blood Marrow Transplant 2003;9:583–91. 10.1016/s1083-8791(03)00228-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mehra V, Rhone E, Widya S, et al. Epstein-Barr virus and monoclonal gammopathy of clinical significance in autologous stem cell transplantation for multiple sclerosis. Clin Infect Dis 2019;69:1757–63. 10.1093/cid/ciz047 [DOI] [PubMed] [Google Scholar]
- 29. Alping P, Burman J, Lycke J, et al. Safety of alemtuzumab and autologous hematopoietic stem cell transplantation compared to noninduction therapies for multiple sclerosis. Neurology 2021;96:e1574–84. 10.1212/WNL.0000000000011545 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jnnp-2023-331864supp001.pdf (134KB, pdf)
jnnp-2023-331864supp002.pdf (318.5KB, pdf)
Data Availability Statement
Data are available on reasonable request. Deidentified individual participant data supporting the findings presented in this article, including text, tables, figures and appendices, will be accessible alongside the study protocol for a period of 5 years, starting 9 months after the article’s publication. Researchers with a sound proposal can request access to this data by contacting joachim.burman@uu.se. To obtain access, those requesting data will need to sign a data access agreement.