Abstract
Background
Intronic GAA repeat expansions in the fibroblast growth factor 14 gene (FGF14) have recently been identified as a common cause of ataxia with potential phenotypic overlap with RFC1-related cerebellar ataxia, neuropathy and vestibular areflexia syndrome (CANVAS). Our objective was to report on the frequency of intronic FGF14 GAA repeat expansions in patients with an unexplained CANVAS-like phenotype.
Methods
We recruited 45 patients negative for biallelic RFC1 repeat expansions with a combination of cerebellar ataxia plus peripheral neuropathy and/or bilateral vestibulopathy (BVP), and genotyped the FGF14 repeat locus. Phenotypic features of GAA-FGF14-positive versus GAA-FGF14-negative patients were compared.
Results
Frequency of FGF14 GAA repeat expansions was 38% (17/45) in the entire cohort, 38% (5/13) in the subgroup with cerebellar ataxia plus polyneuropathy, 43% (9/21) in the subgroup with cerebellar ataxia plus BVP and 27% (3/11) in patients with all three features. BVP was observed in 75% (12/16) of GAA-FGF14-positive patients. Polyneuropathy was at most mild and of mixed sensorimotor type in six of eight GAA-FGF14-positive patients. Family history of ataxia (59% vs 15%; p=0.007) was significantly more frequent and permanent cerebellar dysarthria (12% vs 54%; p=0.009) significantly less frequent in GAA-FGF14-positive than in GAA-FGF14-negative patients. Age at onset was inversely correlated to the size of the repeat expansion (Pearson’s r, −0.67; R2=0.45; p=0.0031).
Conclusions
GAA-FGF14-related disease is a common cause of cerebellar ataxia with polyneuropathy and/or BVP, and should be included in the differential diagnosis of RFC1 CANVAS and disease spectrum.
Keywords: CEREBELLAR ATAXIA, NEUROPATHY, NEUROGENETICS, VERTIGO, MOVEMENT DISORDERS
Introduction
Dominantly inherited intronic GAA repeat expansions in the fibroblast growth factor 14 gene (FGF14) have recently been shown to be a common cause of hereditary ataxia (GAA-FGF14-related disease; spinocerebellar ataxia 27B (MIM: 620 174)).1 2 Initial observations of cerebellar ataxia and bilateral vestibulopathy (BVP) in a subset of patients carrying an FGF14 GAA repeat expansion suggested partial phenotypic overlap between GAA-FGF14-related disease and cerebellar ataxia, neuropathy and vestibular areflexia syndrome (CANVAS).1 2 Biallelic intronic pentanucleotide repeat expansions in the replication factor C subunit 1 gene (RFC1) are a frequent cause of CANVAS, accounting for 70% to 100% of cases in various series.3 4 Phenotypic analysis of RFC1-positive patients has shown that CANVAS is not a strictly delineated disease entity but rather a phenotypic cluster occurring along a continuum of variable involvement of the cerebellar, sensory and vestibular systems.5–8 While biallelic RFC1 repeat expansions are the main cause of CANVAS-spectrum disease, other causative genes are yet to be identified, especially in the subgroup of patients with partial features of CANVAS.4 9
Here, we studied the frequency of FGF14 GAA repeat expansions in patients with a combination of cerebellar ataxia plus peripheral neuropathy and/or BVP negative for biallelic RFC1 repeat expansions, and report on the phenotypic spectrum of GAA-FGF14-positive patients.
Methods
Patient enrollment
Forty-five index patients with neurodegenerative ataxia for which an underlying genetic cause had not yet been identified were recruited from seven different centres in Europe (France: 1, Germany: 4, Spain: 1, UK: 1 centre). To be eligible for inclusion in the study, patients needed to have cerebellar ataxia plus polyneuropathy confirmed by nerve conduction studies (excluding focal entrapment neuropathies) and/or BVP evidenced by reduced bilateral vestibulo-ocular reflex by bedside head impulse test or video head impulse test (vHIT); and negative results on screening for biallelic RFC1 repeat expansions. The bedside head impulse test, performed by experienced neurologists with expertise in ataxia, was available in 38 of 45 (84%) patients, the vHIT was available in 21 of 45 (47%) patients and either test was available in 39 of 45 (87%) patients. Results of brain MRI and nerve conduction studies were available for review in 82% (37/45) and 80% (36/45) of patients, respectively. Deep phenotyping was performed through review of medical records and, when possible, patient re-evaluation using a standardised data sheet for both GAA-FGF14-positive and GAA-FGF14-negative patients.
Genetic screening for RFC1 and FGF14 repeat expansions
Screening for RFC1 repeat expansions was performed as described previously.3 The FGF14 repeat locus was genotyped by long-range PCR. Repeat sizes were measured by capillary electrophoresis of fluorescent long-range PCR amplification products, as described previously.10 Results of fragment length analysis were confirmed by agarose gel electrophoresis of PCR amplification products. Patients who had large amplification products by PCR underwent bidirectional repeat-primed PCRs targeting the 5’-end and the 3’-end of the locus to ascertain the presence of a GAA repeat expansion.10 Expansions of at least 250 GAA repeat units were considered pathogenic.1 2
Data availability
Individual deidentified patient data may be shared at the request of any qualified investigator on reasonable request.
Results
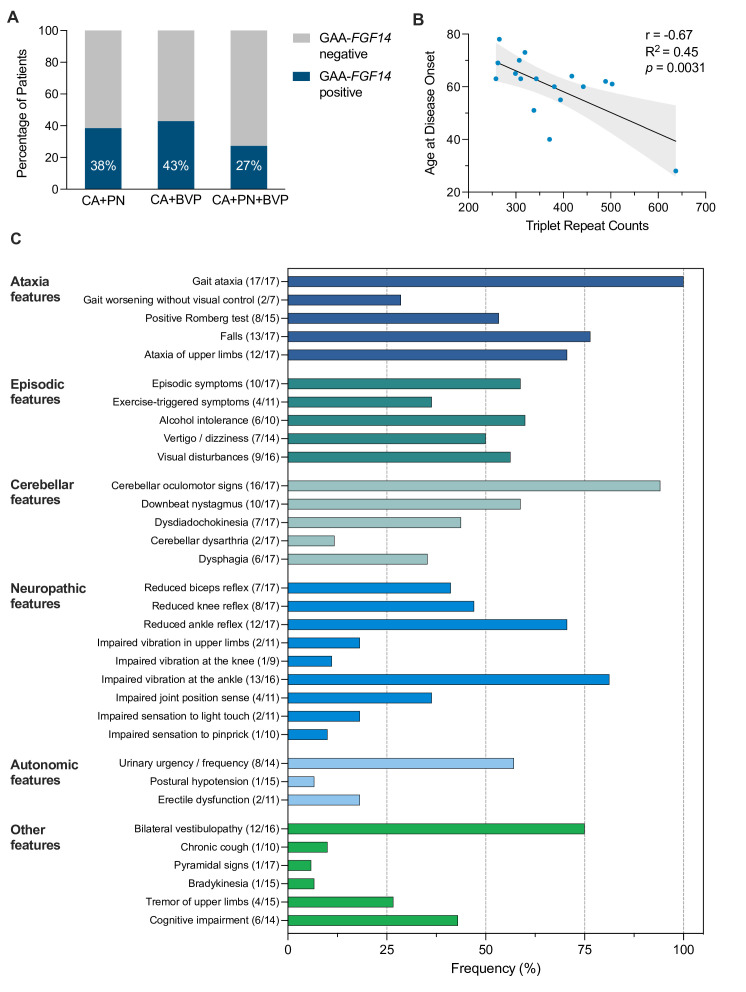
Of the 45 patients enrolled in this study, 17 (38%) carried a heterozygous FGF14 (GAA)≥250 repeat expansion (median size of expansion, 343 repeat units; range, 258–637 repeat units). Repeat expansions were present in 38% of patients with cerebellar ataxia plus polyneuropathy (5/13), 43% of patients with cerebellar ataxia plus BVP (9/21) and 27% of patients with all three features (3/11) (figure 1A). While no patient met the proposed diagnostic criteria for clinically probable or definite CANVAS, 1 GAA-FGF14-positive and 2 GAA-FGF14-negative patients fulfilled the criteria for clinically possible CANVAS.9
Figure 1.
Frequency of the FGF14 GAA repeat expansion, age at onset correlation and clinical features of GAA-FGF14-positive patients. (A) Percentage of patients who carried an FGF14 (GAA)≥250 repeat expansion in the subgroups with (1) cerebellar ataxia plus polyneuropathy (CA+PN) (5 of 13 patients), (2) cerebellar ataxia plus bilateral vestibulopathy (CA+BVP) (9 of 21) and (3) cerebellar ataxia plus polyneuropathy and bilateral vestibulopathy (CA+PN+ BVP) (3 of 11). (B) Inverse correlation between size of the FGF14 repeat expansion and age at disease onset in 17 patients (Pearson’s r, −0.67; R2=0.45; p=0.0031). The grey area displays the 95% CI. Simple linear regression fitting (slope, −0.079 and intercept, 89.84) suggests that age at onset decreases by about 3.96 years (95% CI: 1.56 to 6.37 years) for every increment of 50 GAA repeats above the pathogenic threshold of 250 repeat units. (C) Frequency of individual phenotypic features in 17 GAA-FGF14-positive patients. Numbers in brackets indicate the number of affected patients over the total number of patients assessed for this feature. FGF14, fibroblast growth factor 14 gene.
Median age of onset was 63 years (range, 28–78 years) in the GAA-FGF14-positive cohort. We observed an inverse correlation between the age at onset and the size of the repeat expansion (17 patients; Pearson’s r, −0.67; R2=0.45; p=0.0031) (figure 1B). Clinical cerebellar features predominantly included gait ataxia (100%), cerebellar oculomotor signs (94%) and upper limb ataxia (71%) (figure 1C). Brain MRI of 10 patients showed cerebellar atrophy (10/13; 77%), which was limited to the vermis in 3 patients and extended to the hemispheres in 7 patients. The vHIT confirmed bilateral vestibular hypofunction in all eight patients in whom it was performed. Chronic cough was rarely observed in GAA-FGF14-positive patients (1/10; 10%). Of the eight patients with polyneuropathy confirmed by nerve conduction studies, two had mild length-dependent sensory axonal neuropathy (2/8; 25%) and six had mild mixed sensorimotor axonal neuropathy (6/8; 75%). Mild distal muscle weakness and/or atrophy of the lower extremities was observed in three of six patients with sensorimotor neuropathy. Alternative causes of neuropathy were not identified. The polyneuropathy was limited to the lower extremities in five patients and was generalised in three patients. None had electrodiagnostic evidence of sensory neuronopathy, a hallmark of RFC1-related disease.11 Otherwise unexplained urinary urgency was present in 57% of patients, suggesting that autonomic dysfunction might be a feature of GAA-FGF14-related disease. Walking aids were used by 50% of patients (8/16) after an average disease duration of 10.8 years, whereas use of a wheelchair was rare and occurred after long-standing disease (~20 years) in two patients (2/16; 12%). Treatment with 4-aminopyridine resulted in objective and/or subjective improvement in ataxia in four of five (80%) patients.
Table 1 presents the baseline characteristics of the GAA-FGF14-positive and GAA-FGF14-negative cohorts. Comparison of all clinical features in the two cohorts revealed significantly less frequent permanent cerebellar dysarthria (2/17; 12% vs 14/26; 54%; Fisher’s exact test p=0.009) and non-significantly more frequent episodic symptoms (10/17; 59% vs 7/26; 27%; Fisher’s exact test p=0.06) in GAA-FGF14-positive compared with GAA-FGF14-negative patients. Family history of ataxia, which was positive in 59% of GAA-FGF14-positive patients, was significantly more frequent in GAA-FGF14-positive compared with GAA-FGF14-negative patients (59% vs 15%; Fisher’s exact test, p=0.007).
Table 1.
Characteristics of the GAA-FGF14-positive and GAA-FGF14-negative patients
| GAA-FGF14-positive (n=17)* |
GAA-FGF14-negative (n=28)† |
|
| Male sex—no. (%) | 13 (76) | 13 (46) |
| Triplet repeat count of the larger allele | 343 (258–637) | 62 (8–247) |
| Age at disease onset—years | 63 (28–78) | 60 (15–80) |
| Age at onset of gait ataxia—years | 63 (37–78) | 61 (30–80) |
| Disease duration—years | 14 (4–24) | 8 (2–56) |
| Age at last examination—years | 77 (44–86) | 77 (49–91) |
| Positive family history—no./total no. (%) | 10/17 (59) | 4/26 (15) |
| Presenting symptoms at disease onset—no. (%)‡ | ||
| Gait unsteadiness | 14 (82) | 26 (93) |
| Vertigo or dizziness | 7 (41) | 5 (18) |
| Visual disturbances (diplopia, oscillopsia, blurring) | 3 (18) | 1 (4) |
| Episodic dysarthria | 3 (18) | 0 (0) |
| Sensory symptoms | 0 (0) | 2 (7) |
| Phenotypic classification—no. (%) | ||
| Cerebellar ataxia plus polyneuropathy | 5 (29) | 8 (29) |
| Cerebellar ataxia plus bilateral vestibulopathy | 9 (52) | 12 (43) |
| Cerebellar ataxia plus polyneuropathy and bilateral vestibulopathy | 3 (18) | 8 (29) |
| Ancillary tests—no. (%) | ||
| Brain MRI | 13 (76) | 24 (86) |
| Nerve conduction studies | 15 (88) | 21 (75) |
| Video head impulse test | 8 (47) | 13 (46) |
Unless specified, data are reported as median (range).
*Data on vestibular system function were missing for one patient.
†Data on vestibular system function were missing for five patients.
‡Patients may present with multiple symptoms at disease onset.
FGF14, fibroblast growth factor 14 gene.
Discussion
Our study demonstrates that FGF14 GAA repeat expansions are common in patients negative for biallelic RFC1 repeat expansions presenting with a combination of cerebellar ataxia plus polyneuropathy and/or BVP. Compared with European cohorts of late-onset ataxia in which the frequency of GAA-FGF14 ataxia is 10–18%,1 2 the frequency of 38% observed in this cohort suggests that FGF14 repeat expansions are enriched in patients partially fulfilling criteria for CANVAS. These results may suggest a combined vulnerability of the cerebellar, peripheral nerve and vestibular systems in GAA-FGF14-related disease. Our study thus confirms and extends previous findings showing that BVP is part of the phenotypic spectrum of GAA-FGF14-related disease.1 2 Our estimate of the frequency of BVP in GAA-FGF14-related disease may even represent an underestimate, as only a relatively small proportion of patients underwent vHIT. Moreover, given the inclusion criteria of our study, the true prevalence of BVP in unselected cohorts of GAA-FGF14-positive patients fully assessed with vHIT remains to be established. Although the prevalence of vestibular impairment in spinocerebellar ataxias has not been well studied, this feature is not specific to GAA-FGF14-related disease, as it is found with variable frequency in other inherited ataxias such as RFC1-related disease (87–90%),5 6 Friedreich ataxia (53–55%)12 13 and spinocerebellar ataxia 3 (57–100%).14–16
Despite phenotypic overlap between RFC1-related disease and GAA-FGF14-related disease, certain features may help differentiate these disorders. Chronic cough, a prevalent feature in RFC1-related disease,5 6 was uncommon in our cohort. While motor neuropathy is typically absent or minimal in RFC1-positive patients,5 11 17 it co-occurred with sensory neuropathy in six of eight GAA-FGF14 patients. Our findings also suggest that episodic symptoms—which were common in previously reported cohorts1—are a frequent feature in GAA-FGF14-positive patients, which may help to discriminate these patients from RFC1-positive patients in whom episodic symptoms are rare. Finally, the pattern of inheritance, which is autosomal dominant in GAA-FGF14-related disease and autosomal recessive in RFC1-related disease, may help differentiating both disorders, although acknowledging that in comparison with other dominant spinocerebellar ataxias18 a substantial proportion of patients with GAA-FGF14-related disease present sporadically (15–50%, depending on cohorts)1 or with seemingly recessive inheritance.
Limitations of this study include its small cohort size and the fact that only 29% (13/45) of patients underwent brain MRI, nerve conduction studies and vHIT. Since bedside head impulse test has a sensitivity of less than 70% for detecting vestibulopathy compared with vHIT,19 a systematic assessment of the vestibular function in phenotypically unselected GAA-FGF14-positive cohorts using vHIT will be necessary to fully define the frequency of vestibular hypofunction in GAA-FGF14-related disease in future studies. Larger natural history studies are needed to fully define the phenotypic spectrum of GAA-FGF14-related disease (for first in-depth phenotype and progression study, see Wilke et al 20) and to assess its frequency in patients meeting the proposed diagnostic criteria for clinically definite CANVAS negative for biallelic RFC1 repeat expansions. Such studies will also be critical to evaluate the degree to which polyneuropathy is pathologically related to GAA-FGF14-related disease—a late-onset disorder—rather than an age-related process, given its high prevalence in the general elderly population.21
In conclusion, we showed that FGF14 GAA repeat expansions are a common cause of cerebellar ataxia plus polyneuropathy and/or BVP in patients negative for biallelic RFC1 repeat expansions, thus expanding the phenotypic spectrum of this recently described disorder. Our results further suggest that GAA-FGF14-related disease should be included in the differential diagnosis of RFC1 CANVAS and disease spectrum.
Acknowledgments
We thank the patients and their families for participating in this study, and Madeleine Wacker (Synofzik Lab) for preparing some of the samples. We thank Mr Felix Heindl (Munich) for help with clinical data collection of some of the patients, Dr Annette Hartmann (Medical University of Vienna) for preparation and handling of samples and related information and Miss Natalia Dominik (University College London) for technical assistance with RFC1 genotyping. DP holds a Fellowship award from the Canadian Institutes of Health Research (CIHR).
Footnotes
Twitter: @ChristelDepienn
DP and CW contributed equally.
Contributors: Design or conceptualisation of the study: DP, CW, BB and MSy. Acquisition of data: DP, CW, AT, SN, RC, M-JD, HG-M, MA, TW, JF, DT, CD, DR, JG, MMR, PG, BB, HH, LS, MSt, AC and MSy. Analysis or interpretation of the data: DP, CW and MSy. Drafting or revising the manuscript for intellectual content: DP, CW, AT, SN, RC, M-JD, HG-M, MA, TW, JF, DT, CD, DR, JG, MMR, PG, BB, HH, LS, MSt, AC and MSy.
Funding: This work was supported, in part, by the Clinician Scientist program ‘PRECISE.net’ funded by the Else Kröner-Fresenius-Stiftung (to CW, AT and MSy), the grant 779257 ‘Solve-RD’ from the European’s Union Horizon 2020 research and innovation programme (to HH and MSy) and the grant 01EO 1401 by the German Federal Ministry of Education and Research (BMBF) (to MSt). This project was also supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) No 441409627, as part of the PROSPAX consortium under the frame of EJP RD, the European Joint Programme on Rare Diseases, under the EJP RD COFUND-EJP N° 825575 (to MSy and BB). This work was also supported by the Wellcome Trust and the UK Medical Research Council (MRC) (to HH), and by the Fondation Groupe Monaco and the grant PT79418 from the Montreal General Hospital Foundation (to BB). This work was also supported by the National Institute for Health Research University College London Hospitals Biomedical Research Centre UCLH and the North Thames CRN (to PG). PG and HG-M work at University College London Hospitals/University College London, which receives a proportion of funding from the Department of Health’s National Institute for Health Research Biomedical Research Centre’s funding scheme. PG received funding from the Medical Research Council (MR/N028767/1) and CureSCA3 in support of HG-M work. AC was supported by the Medical Research Council (MR/T001712/1), Fondazione Cariplo (grant n. 2019-1836), the Inherited Neuropathy Consortium, and Fondazione Regionale per la Ricerca Biomedica (Regione Lombardia, project ID 1751723). The funders had no role in the conduct of this study.
Competing interests: DP reports no disclosures. CW reports no disclosures. AT reports no disclosures. SN reports no disclosures. RC reports no disclosures. M-JD reports no disclosures. HG-M reports no disclosures. MA has received consultancy honoraria from Merz, Ipsen Pharmaceuticals, Orkyn, AbbVie, Reata, Ever Pharma, all unrelated to the present manuscript. TW has received consultancy honoraria from Ipsen Pharmaceuticals and AbbVie, all unrelated to the present manuscript. JF reports no disclosures. DT reports no disclosures. CD reports no disclosures. DR has received grant/research support from Janssen and Lundbeck; he has served as a consultant or on advisory boards for AC Immune, Janssen, Roche and Rovi and he has served on speakers bureaus of Janssen and Pharmagenetix. He also received honoraria from Gerot Lannacher, Janssen and Pharmagenetix, and travel support from Angelini and Janssen, all unrelated to the present manuscript. JG reports no disclosures. MMR reports no disclosures. PG reports no disclosures. BB reports no disclosures. HH reports no disclosures. LS reports no disclosures. MSt reports no disclosures. AC reports no disclosures. MSy has received consultancy honoraria from Janssen, Ionis, Orphazyme, Servier, Reata, GenOrph and AviadoBio, all unrelated to the present manuscript.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The institutional review boards of the University of Tübingen (598/2011BO1) and the University College London (07/Q0512/26) approved this study. Participants gave informed consent to participate in the study before taking part.
References
- 1. Pellerin D, Danzi MC, Wilke C, et al. Deep intronic FGF14 GAA repeat expansion in late-onset cerebellar ataxia . N Engl J Med 2023;388:128–41. 10.1056/NEJMoa2207406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rafehi H, Read J, Szmulewicz DJ, et al. An intronic GAA repeat expansion in FGF14 causes the autosomal-dominant adult-onset ataxia SCA50/ATX-FGF14. Am J Hum Genet 2023;110:1018. 10.1016/j.ajhg.2023.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cortese A, Simone R, Sullivan R, et al. Biallelic expansion of an intronic repeat in RFC1 is a common cause of late-onset ataxia. Nat Genet 2019;51:649–58. 10.1038/s41588-019-0372-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Davies K, Szmulewicz DJ, Corben LA, et al. RFC1-related disease: molecular and clinical insights. Neurol Genet 2022;8:e200016. 10.1212/NXG.0000000000200016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Traschütz A, Cortese A, Reich S, et al. Natural history, phenotypic spectrum, and discriminative features of multisystemic RFC1 disease. Neurology 2021;96:e1369–82. 10.1212/WNL.0000000000011528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cortese A, Tozza S, Yau WY, et al. Cerebellar ataxia, neuropathy, vestibular areflexia syndrome due to RFC1 repeat expansion. Brain 2020;143:480–90. 10.1093/brain/awz418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gisatulin M, Dobricic V, Zühlke C, et al. Clinical spectrum of the pentanucleotide repeat expansion in the RFC1 gene in ataxia syndromes. Neurology 2020;95:e2912–23. 10.1212/WNL.0000000000010744 [DOI] [PubMed] [Google Scholar]
- 8. Traschütz A, Wilke C, Haack TB, et al. Sensory axonal neuropathy in RFC1-disease: tip of the iceberg of broad subclinical multisystemic neurodegeneration. Brain 2022;145:e6–9. 10.1093/brain/awac003 [DOI] [PubMed] [Google Scholar]
- 9. Szmulewicz DJ, Roberts L, McLean CA, et al. Proposed diagnostic criteria for cerebellar ataxia with neuropathy and vestibular areflexia syndrome (CANVAS). Neurol Clin Pract 2016;6:61–8. 10.1212/CPJ.0000000000000215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bonnet C, Pellerin D, Roth V, et al. Optimized testing strategy for the diagnosis of GAA-FGF14 ataxia/spinocerebellar ataxia 27B. Sci Rep 2023;13:9737. 10.1038/s41598-023-36654-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Currò R, Salvalaggio A, Tozza S, et al. RFC1 expansions are a common cause of idiopathic sensory neuropathy . Brain 2021;144:1542–50. 10.1093/brain/awab072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Maudoux A, Teissier N, Francois M, et al. Vestibular impact of Friedreich ataxia in early onset patients. Cerebellum Ataxias 2020;7:6. 10.1186/s40673-020-00115-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zeigelboim BS, Mesti JC, Fonseca VR, et al. Otoneurological abnormalities in patients with Friedreich’s ataxia. Int Arch Otorhinolaryngol 2017;21:79–85. 10.1055/s-0036-1572529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gordon CR, Joffe V, Vainstein G, et al. Vestibulo-ocular arreflexia in families with spinocerebellar ataxia type 3 (Machado-Joseph disease). J Neurol Neurosurg Psychiatry 2003;74:1403–6. 10.1136/jnnp.74.10.1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Buttner N, Geschwind D, Jen JC, et al. Oculomotor phenotypes in autosomal dominant Ataxias. Arch Neurol 1998;55:1353–7. 10.1001/archneur.55.10.1353 [DOI] [PubMed] [Google Scholar]
- 16. Bürk K, Fetter M, Abele M, et al. Autosomal dominant cerebellar ataxia type I: oculomotor abnormalities in families with SCA1, SCA2, and SCA3. J Neurol 1999;246:789–97. 10.1007/s004150050456 [DOI] [PubMed] [Google Scholar]
- 17. Huin V, Coarelli G, Guemy C, et al. Motor neuron pathology in CANVAS due to RFC1 expansions. Brain 2022;145:2121–32. 10.1093/brain/awab449 [DOI] [PubMed] [Google Scholar]
- 18. Klockgether T. Sporadic ataxia with adult onset: classification and diagnostic criteria. Lancet Neurol 2010;9:94–104. 10.1016/S1474-4422(09)70305-9 [DOI] [PubMed] [Google Scholar]
- 19. Yip CW, Glaser M, Frenzel C, et al. Comparison of the bedside head-impulse test with the video head-impulse test in a clinical practice setting: a prospective study of 500 outpatients. Front Neurol 2016;7:58. 10.3389/fneur.2016.00058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wilke C, Pellerin D, Mengel D, et al. GAA-FGF14 ataxia (SCA27B): phenotypic profile, natural history progression and 4-aminopyridine treatment response. Brain 2023:awad157. 10.1093/brain/awad157 [DOI] [PubMed] [Google Scholar]
- 21. Hanewinckel R, Drenthen J, van Oijen M, et al. Prevalence of polyneuropathy in the general middle-aged and elderly population. Neurology 2016;87:1892–8. 10.1212/WNL.0000000000003293 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Individual deidentified patient data may be shared at the request of any qualified investigator on reasonable request.