Abstract
Proteins secreted into the culture medium by Mycobacterium tuberculosis are thought to play an important role in the development of protective immune responses. In this report, we describe the molecular cloning of a novel, low-molecular-weight antigen (MTB12) secreted by M. tuberculosis. Sequence analysis of the MTB12 gene indicates that the protein is initially synthesized as a 16.6-kDa precursor protein containing a 48-amino-acid hydrophobic leader sequence. The mature, fully processed form of MTB12 protein found in culture filtrates has a molecular mass of 12.5 kDa. MTB12 protein constitutes a major component of the M. tuberculosis culture supernatant and appears to be at least as abundant as several other well-characterized culture filtrate proteins, including members of the 85B complex. MTB12 is encoded by a single-copy gene which is present in both virulent and avirulent strains of the M. tuberculosis complex, the BCG strain of M. bovis, and M. leprae. Recombinant MTB12 containing an N-terminal six-histidine tag was expressed in Escherichia coli and purified by affinity chromatography. Recombinant MTB12 protein elicited in vitro proliferative responses from the peripheral blood mononuclear cells of a number of purified protein derivative-positive (PPD+) human donors but not from PPD− donors.
Tuberculosis remains one of the world’s most serious health threats, with approximately 2 billion people infected worldwide and an estimated 2.9 million deaths due to tuberculosis annually (20). The recent increase in the incidence of tuberculosis, particularly antibiotic-resistant tuberculosis, underscores the need for an effective vaccine against this important disease (19). The only vaccine currently in use is the live, attenuated strain of Mycobacterium bovis, bacillus Calmette-Guérin (BCG), that was derived in the early 1920s (6, 7). Although vaccination with BCG is widely practiced worldwide, its efficacy is reported to vary considerably among different clinical trials and geographically distinct populations. A recent review of all previous controlled clinical trials concluded that vaccination with BCG reduced the overall risk of tuberculosis by approximately 50% (8).
Recently, emphasis has been placed on the apparent requirement for live mycobacterial organisms for effective vaccination against tuberculosis and the hypothesis that proteins released by live bacilli during an extended latent infection are particularly important for the generation of protective immune responses (for a review, see reference 25). Accordingly, a significant body of literature addressing the characterization and identification of proteins that are secreted by M. tuberculosis has been compiled (for reviews, see references 25 and 32). Culture filtrate proteins (CFP) obtained from in vitro-cultivated M. tuberculosis are highly antigenic in terms of their capacity to stimulate in vitro proliferation and cytokine production from T cells of infected mice, guinea pigs, and purified protein derivative-positive (PPD+) human donors (4, 25, 27, 35). Furthermore, various preparations of CFP have been shown to offer some degree of protection when used as vaccines in animal models of tuberculosis (2, 17, 26, 27). Therefore, CFP is considered to be an important source of candidate antigens for a potential subunit vaccine against tuberculosis. Although a number of components of CFP have been isolated, cloned, and extensively characterized, a recent analysis of CFP by two-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) revealed that CFP is comprised of up to 205 distinct proteins (32). We are currently characterizing culture supernatants of M. tuberculosis with the aim of identifying novel antigenic proteins. In this report, we describe the identification, molecular cloning, and expression of a novel, low-molecular-weight antigen (MTB12) from M. tuberculosis culture supernatants. MTB12 is a highly abundant component of M. tuberculosis culture supernatant that is readily detectable in Coomassie blue-stained gels of CFP. The MTB12 gene is present in virulent and avirulent strains of the M. tuberculosis complex, M. leprae, and M. bovis (BCG), and recombinant MTB12 protein elicits in vitro responses from the peripheral blood mononuclear cells (PBMC) of PPD+ human donors.
MATERIALS AND METHODS
Strains.
M. tuberculosis H37Ra, H37Rv, and Erdman were provided by Sean Skerritt (Seattle VA Hospital). M. tuberculosis strain C is a clinical isolate provided by Lee Riley (University of California, Berkeley). Pelleted samples of M. bovis BCG and M. leprae were kindly provided by Paul Tan (Genesis Corp.). Mycobacterial genomic DNA was prepared as previously described (18). Genomic DNA from M. tuberculosis H37Ra and H37Rv was fragmented for library generation by using either partial digestion with Sau3A (H37Rv library) or sonication (H37Ra library). In both cases, DNA fragments in a size range of 300 to 4,000 bp were blunted with Klenow polymerase, ligated to EcoRI adapters, and subcloned into EcoRI-predigested λZAP bacteriophage arms as specified by the manufacturer (Stratagene). Phage were packaged by using Gigapack II packaging extracts (Stratagene) as recommended by the manufacturer.
HPLC purification of CFP.
Concentrated CFP of M. tuberculosis Erdman was provided by John Belisle (Colorado State University) and purified by a two-step process. CFP was initially fractionated by high-pressure liquid chromatography (HPLC) on a 4.6- by 25-cm Aquapore C18 column (Brownlee) at a flow rate of 1 ml/min with a 0 to 60% acetonitrile gradient in 30 min. One of the major peaks resolved by this method was shown by protein sequence analysis to be a mixture of proteins and was therefore subjected to further purification using microbore HPLC. The sample was resolved on a 1.1- by 100-mm Aquapore C18 column (Brownlee) at a flow rate of 80 μl/min with a 20 to 70% acetonitrile gradient in 70 min. Peak fractions from the microbore HPLC were loaded onto biobrene-treated glass fiber filters (Perkin-Elmer/Applied Biosystems). The loaded filters were then placed in a Procise 494 protein sequencer (Perkin-Elmer/Applied Biosystems) and sequenced from the amino terminus, using traditional Edman chemistry.
Cloning of the M. tuberculosis MTB12 gene.
The M. leprae homolog of the MTB12 gene was amplified from M. leprae genomic DNA by PCR. PCR primers (5′-ATGAAATCCATCGCCACCTATGCA-3′ and 5′-TCAACGCCCCGCGGCCTGCAACAG-3′) were designed based on sequence obtained from GenBank accession no. U00016_13. The PCR program consisted of 30 cycles of 94°C for 1 min, 55°C for 1 min, and 72°C for 1 min. A single amplification product of the expected size (495 bp) was subcloned into the pCR vector (Invitrogen), and the insert identity was confirmed by DNA sequence analysis. The cloned amplification product was reisolated by digestion with EcoRI and agarose gel purification and was labeled to high specific activity (∼109 cpm/μg) with [α-32P]dCTP, using the random primer method (14). This probe was used to screen an M. tuberculosis H37Rv genomic library prepared in the λZAPII vector (Stratagene). Approximately 40,000 PFU were screened by plaque hybridization. Filters were washed to a final stringency of 0.2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) at 65°C. Hybridizing plaques were purified to homogeneity by two subsequent rounds of low-density plaque screening, and Bluescript phagemids were excised from positive clones as specified by the manufacturer (Stratagene). Sequence analysis revealed that one of the clones contained the complete M. tuberculosis MTB12 open reading frame (ORF) plus 1.2 kb of 5′ untranslated sequence and 2 kb of 3′ untranslated sequence.
For serological screening, a polyclonal rabbit antiserum was raised against the concentrated culture filtrates of M. tuberculosis by injecting rabbits with 200 μg of protein in incomplete Freund’s adjuvant plus 100 μg of N-acetylmuramyl-l-alanyl-d-isoglutamine (MDP) (Pierce). Serum was collected after two subsequent boosts given at approximately 6-week intervals. This serum was preabsorbed against total Escherichia coli protein and was used to screen an M. tuberculosis H37Ra genomic expression library (described above). An alkaline phosphatase-conjugated goat anti-rabbit secondary antibody (Zymed) plus nitroblue tetrazolium–5-bromo-4-chloro-3-indolylphosphate (GibcoBRL) was used to detect immunoreactive plaques.
Expression and purification of recombinant MTB12.
Recombinant proteins corresponding to the full-length M. tuberculosis MTB12 and mature protein (lacking signal sequence) were prepared by using the pET17b E. coli high-level expression system (Novagen). MTB12 DNA was amplified by PCR using the primers 5′-AATTACATATGCATCACCATCACCATCACACCGGTAGTTTGAACCAAACGCAC-3′ and the M13 universal primer (full-length protein) or 5′- CAATTACATATGCATCACCATCACCATCACGACCCGGCATCCGCCCCT GAC-3′ and 5′-CATGGAATTCTCAGTTCCCTGCGGCCTGCAGCAA-3′ (mature protein). The 5′ oligonucleotides contained an NdeI restriction site preceding the ATG initiation codon (underlined) followed by nucleotide sequences encoding six histidines (bold) and internal sequences derived from the MTB12 genomic DNA (italic). The resultant PCR products were digested with NdeI and EcoRI and subcloned into the pET17b vector digested with NdeI and EcoRI. Ligation products were initially transformed into E. coli XL1-Blue competent cells (Stratagene) and were subsequently transformed into E. coli BL-21(pLysE) host cells (Novagen) for expression. Five hundred-milliliter cultures of recombinant E. coli were induced to express recombinant MTB12 (rMTB12) by the addition of 1 mM 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (IPTG) at mid-log phase of growth. Growth was continued for 3 h, and bacteria were pelleted and lysed in 20 ml of lysis buffer (10 mM Tris [pH 8.0], 2 mM phenylmethylsulfonyl fluoride, 20 μg of leupeptin per ml, one Complete protease inhibitor tablet [Boehringer Mannheim] per 25 ml) by rapid freeze-thaw followed by brief sonication. Lysates were separated into a soluble protein fraction and an inclusion body fraction by a 10-min centrifugation at 10,000 × g. The soluble fraction was made up to a final concentration of 100 mM Na2HPO4 (pH 8.0)–10 mM Tris (pH 8.0)–8 M urea, and recombinant protein containing the N-terminal histidine tag was purified by using Ni-nitrilotriacetic acid (NTA) resin (Qiagen) according to the manufacturer’s protocols. Briefly, Ni-NTA resin was washed in lysis buffer and added to the soluble E. coli lysate fraction. Binding was conducted with constant mixing for 1 h at 4°C. Ni-NTA resin containing the bound protein was washed with binding buffer (pH 6.3) and eluted in binding buffer containing 350 mM imidazole. The eluted material was dialyzed against three changes of phosphate-buffered saline (PBS), sterile filtered, and stored at −20°C. The purified recombinant proteins were shown by SDS-PAGE analysis to be free of any significant amount of E. coli protein (see Fig. 4). Recombinant proteins were assayed for endotoxin contamination by using the Limulus assay (BioWhittaker) and were shown to contain <10 endotoxin units/mg. A polyclonal rabbit antiserum was raised against recombinant mature MTB12 by injecting rabbits with 200 μg of protein in IFA plus 100 μg of MDP (Pierce). Serum was collected after two subsequent boosts given at approximately 6-week intervals.
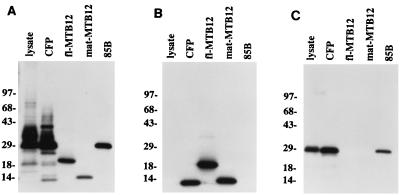
FIG. 4.
Immunoblot analyses of MTB12. Western blots containing M. tuberculosis lysate (2.5 μg), CFP (2.5 μg), recombinant mature MTB12 (50 ng), full-length MTB12 (Fl-MTB12; 50 ng), and 85B (50 ng) were incubated with anti-CFP (A), anti-MTB12 (B), or anti-85B (C) polyclonal rabbit antisera. Immunoreactive proteins were detected by using [125I]protein A followed by autoradiography. Positions of size markers are indicated in kilodaltons.
Immunoblot analysis of rMTB12.
M. tuberculosis H37Rv total lysate or CFP (2.5 μg of each) as well as 50 ng of the indicated recombinant protein (full-length MTB12, mature MTB12, or 85B) were separated by SDS-PAGE on 12% gels and transferred to nitrocellulose by using a semidry transfer apparatus (Bio-Rad). Blots, in triplicate, were blocked for a minimum of 1 h with PBS–0.1% Tween 20 containing 1% bovine serum albumin and were probed with anti-CFP, anti-MTB12, and anti-85B polyclonal rabbit antisera diluted 1:400 in PBS–0.1% Tween 20 as indicated. Reactivity was assessed as previously described (31), using [125I]protein A followed by autoradiography. For amino acid sequence determination, CFP was separated by SDS-PAGE on a 12% gel and transferred in triplicate to a polyvinylidene difluoride (PVDF) membrane. Membrane strips were stained with Coomassie blue to detect protein bands. Specific bands were excised from the stained strip and subjected to N-terminal sequence analysis using a Procise 494 protein sequencer (Perkin-Elmer/Applied Biosystems). A second and a third strip were completely destained in methanol and were analyzed by Western blotting using an anti-MTB12 polyclonal rabbit antiserum or corresponding preimmune serum as described above.
Immunological reactivity of rMTB12.
Proteins isolated from CFP by microbore HPLC and recombinant MTB12 proteins were assayed for the ability to elicit in vitro proliferative responses from whole PBMC of healthy PPD− and PPD+ donors. PBMC were obtained from heparinized blood by Ficoll gradient centrifugation or by leukapheresis. PBMC (2 × 105 well) were incubated in 96-well round-bottom plates (Costar) in medium only (RPMI 1640 with 10% pooled human serum) or in medium containing specific antigens at the indicated concentrations. Plates were cultured for 5 days at 37°C in 5% CO2 and were pulsed with 1 μCi of [3H]thymidine (Amersham) for the final 18 h. Cells were harvested onto filter mats and counted in a Matrix 9600 direct beta counter (Packard).
Nucleotide sequence accession number.
The nucleotide and the deduced amino acid sequences of MTB12 have been entered in the GenBank database under accession no. AF062036.
RESULTS
Purification of MTB12 from M. tuberculosis CFP.
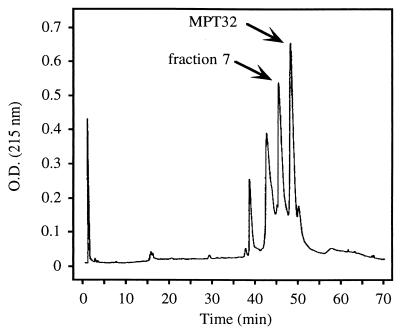
The CFP of M. tuberculosis has been repeatedly shown to be rich in immunologically reactive protein antigens (25, 35). To identify novel antigenic components of this material, we fractionated CFP by C18 reverse-phase HPLC followed by microbore HPLC and characterized protein peaks eluting from the second fractionation step by N-terminal amino acid sequencing. Peak fractions were also assayed for the ability to elicit in vitro proliferative responses from the PBMC of PPD+ human donors. We focused our efforts on a single peak (Fig. 1, fraction 7) that eluted just prior to the 45/47-kDa secreted antigen MPT32 (21). Fraction 7 contained a single protein with an N-terminal se- quence (DPASAPDVPTAAQLTSLLNSLADPNVSFA) that was not present in any previously reported secreted protein of M. tuberculosis. In addition, this fraction elicited in vitro proliferative responses in the PBMC from two of two PPD+ donors but not from PPD− donors (Table 1).
FIG. 1.
Chromatogram of M. tuberculosis CFP fractionated by conventional HPLC (C18) followed by microbore HPLC (C18) and eluted with a 20 to 70% acetonitrile gradient. Peak fractions were analyzed by N-terminal amino acid sequencing. The peak labeled fraction 7 was found to contain a protein with novel N-terminal sequence. The peak labeled MPT32 was found to correspond to the 45/47-kDa secreted antigen (21).
TABLE 1.
In vitro proliferative responses of PBMC obtained from PPD+ or PPD− donors in the presence of M. tuberculosis HPLC fraction 7
| Fraction 7 (μg/ml) | Mean [3H]thymidine incorporation (cpm) of triplicate wells
|
|||
|---|---|---|---|---|
| PPD+
|
PPD−
|
|||
| D7 | D131 | D10 | D22 | |
| 5 | 2,260 (11.3a) | 285 (3.8) | 198 (1.3) | 191 (1.8) |
| 1 | 834 (4.2) | 184 (2.4) | 211 (1.3) | 128 (1.2) |
| 0.2 | 464 (2.3) | 82 (1.1) | 198 (1.3) | 92 (0.9) |
| 0 | 200 | 76 | 158 | 105 |
Stimulation index (mean cpm incorporated in the presence of antigen divided by mean cpm incorporated in the absence of antigen).
Molecular cloning of the gene encoding MTB12.
A search of the gene data banks with the N-terminal sequence obtained from fraction 7 indicated that this sequence was derived from a novel protein of M. tuberculosis. However, the search did reveal homology with the product of a putative ORF from the M. leprae genome sequencing project (accession no. U00016_13). The predicted amino acid sequence of the M. leprae ORF contained a region that was identical to the fraction 7 N-terminal sequence at 21 of 29 residues. To facilitate the isolation of the M. tuberculosis fraction 7 gene, the M. leprae homolog was amplified from M. leprae genomic DNA by PCR and was used as a probe to screen an M. tuberculosis H37Rv λZAP genomic library by plaque hybridization. Five positive phage clones were obtained. Sequence analysis revealed that the insert of one of these clones contained a 507-bp ORF encoding a 168-amino-acid protein with a predicted molecular mass of 16.6 kDa (Fig. 2A). Residues 49 to 78 of the predicted protein sequence had 100% identity with the N-terminal sequence obtained from the microbore HPLC fraction 7. These residues were preceded by a stretch of 48 highly hydrophobic amino acids that are presumed to constitute an N-terminal signal sequence, directing the transport of the protein to the extracellular space. Thus, the N-terminal sequence of the protein identified as HPLC fraction 7 likely represents the amino terminus of the mature fully processed protein found in the CFP. The mature, processed protein had a predicted molecular mass of 12.5 kDa and a pI of 5.03. Based on the size of the mature protein, we have designated this protein MTB12. Similarity between the M. tuberculosis MTB12 protein and the M. leprae homolog was significant: 107 of 168 (63.7%) amino acid identity and 22 conservative substitutions (Fig. 2B). The most significant difference between the two species was a four-residue deletion within the putative signal sequence region of the M. leprae homolog. Other conservative and nonconservative substitutions occurred throughout the protein and were not restricted to any particular region. Further database searching did not reveal the presence of any structural domains that might provide evidence of the biological function of MTB12.
FIG. 2.
(A) Nucleotide sequence and predicted amino acid sequence of the M. tuberculosis MTB12 gene. Those amino acids comprising the putative leader sequence (absent from the mature protein) are shown in italics. The amino acid residues determined during N-terminal sequence analysis of HPLC fraction 7 are shown in bold. The position of the β-galactosidase–MTB12 fusion in clone Ra-1 (isolated during a library screen using anti-CFP polyclonal antiserum) is indicated by an arrow at position 64. (B) Comparison of the amino acid sequence of the M. tuberculosis MTB12 protein and the predicted protein sequence of the M. leprae homolog (GenBank accession no. U00016_13). Identical amino acids are shown with a shaded background. The amino acid residues determined during N-terminal sequence analysis are indicated by a horizontal line above the MTB12 sequence.
The MTB12 gene was also isolated by using a serological screening approach that was being performed concurrently to the biochemical characterization of M. tuberculosis CFP. In this second approach, a polyclonal rabbit antiserum generated against total M. tuberculosis CFP was used to screen an M. tuberculosis H37Ra genomic expression library. Of the 27 clones thus isolated, two contained inserts corresponding to MTB12. One of these clones (designated Ra-1) contained the complete MTB12 ORF (including the signal sequence) plus 63 bp of 5′ untranslated sequence in frame with the vector-encoded β-galactosidase. This clone expressed a 21-kDa fusion protein that exhibited strong immunoreactivity with the anti-CFP antiserum (data not shown). A number of other clones corresponding to previously characterized secreted proteins, including 85B (9), 45/47-kDa secreted antigen (21), and MPT64 (24), were also isolated by using this antiserum.
The organization of the MTB12 gene in various members of the M. tuberculosis complex was analyzed by Southern blotting. A probe comprising the insert of clone Ra-1 (containing the complete MTB12 coding region plus a small amount of 5′ and 3′ untranslated sequence) was hybridized to a blot containing PstI-digested genomic DNAs from M. tuberculosis H37Ra, H37Rv, and Erdman; a recent M. tuberculosis clinical isolate referred to as strain C; and the M. bovis BCG. Hybridizing bands of approximately 1.0 and 1.6 kbp were observed in all strains (data not shown). Sequence analysis of the 3.6-kbp genomic clone from which the M. tuberculosis MTB12 gene was isolated revealed the presence of three PstI restriction sites in the vicinity of the MTB12 ORF. The first site was located near the 3′ end of the MTB12 ORF, and the remaining two sites were located 988 bp upstream and 1,579 bp downstream of the first PstI site. Thus, the sizes of the predicted PstI restriction fragments were in complete agreement with the hybridization pattern observed during Southern blot analysis of genomic DNAs. Southern blot hybridizations to genomic DNAs from a panel of other mycobacterial species (using the same stringency conditions) were negative (data not shown), suggesting that these species either lack an MTB12 homolog or contain a homolog with sufficient sequence divergence to avoid detection at the stringencies used (0.1× SSC at 65°C).
Recognition of rMTB12 protein by human PBMC.
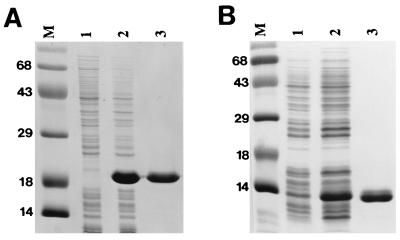
rMTB12 protein containing an N-terminal six-histidine affinity tag was expressed in the pET17b E. coli high-level expression system (Novagen). Two recombinant proteins were prepared, one corresponding to the mature protein (lacking a signal sequence) and the other corresponding to the full-length MTB12 protein expressed by clone Ra-1 (containing the leader sequence plus an additional 21 residues derived from the 5′ untranslated region). Recombinant proteins were purified to homogeneity by Ni-NTA affinity chromatography and were shown by SDS-PAGE to be free of contaminating E. coli protein (Fig. 3). Recombinant MTB12 protein was assayed for the ability to elicit in vitro proliferative responses from PPD+ and PPD− human donor PBMC. Although proliferative responses to recombinant protein were weaker than the responses elicited by total CFP, recombinant MTB12 consistently elicited in vitro proliferation from a number of PPD+ donors (Table 2). Of the 10 PPD+ donors tested, 4 responded to recombinant MTB12 protein with a stimulation index of between 4 and 12. Recombinant MTB12 protein did not elicit substantial proliferation from any of the six PPD− individuals tested. Viability of all PBMC was confirmed by proliferation in the presence of CFP (PPD+ individuals), tetanus toxoid (PPD− individuals), and phytohemagglutinin (data not shown). Interestingly, full-length recombinant MTB12 protein elicited a slightly stronger response than did mature protein in several of the MTB12-responsive donors (data not shown), suggesting that amino acids comprising the leader sequence may contribute to MTB12-specific immune response.
FIG. 3.
Expression and purification of rMTB12 proteins. Recombinant proteins corresponding to full-length MTB12 protein (A) or mature MTB12 protein lacking the leader sequence (B), both containing amino-terminal six-histidine tags, were expressed by using the pET17b E. coli high-level expression system. Lysates of uninduced cultures (lane 1), IPTG-induced cultures (lane 2), and affinity-purified recombinant protein (lane 3) were fractionated by SDS-PAGE and stained with Coomassie blue. Positions of size markers (lane M) are indicated in kilodaltons.
TABLE 2.
In vitro proliferative responses of PBMC obtained from PPD− or PPD+ human donors in the presence of recombinant MTB12 protein
| Patient | Mean [3H]thymidine incorporation (cpm) of triplicate wells ± SEM with indicated recall antigen
|
||||
|---|---|---|---|---|---|
| Medium only | CFP (5 μg/ml) | rMTB12
|
Tetanus toxoid (5 μg/ml) | ||
| 5 μg/ml | 2 μg/ml | ||||
| PPD− | |||||
| D63 | 1,655 ± 100 | 1,548 ± 79 (0.9a) | 1,341 ± 118 (0.8) | NDb | 11,653 ± 1,000 (7.0) |
| D142 | 97 ± 50 | 1,975 ± 1,127 (20.3) | 157 ± 32 (1.6) | ND | 19,350 ± 1,668 (199.5) |
| D67 | 192 ± 60 | 950 ± 380 (4.9) | 266 ± 82 (1.4) | ND | 18,946 ± 643 (98.7) |
| D172 | 1,199 ± 366 | 1,480 ± 425 (1.2) | 1,133 ± 231 (0.9) | ND | 37,151 ± 4,310 (30.9) |
| D106 | 101 ± 44 | 2,647 ± 626 (26.2) | 234 ± 107 (2.3) | ND | 5,793 ± 1,190 (57.4) |
| D212 | 38 ± 9 | 734 ± 520 (19.3) | 138 ± 67 (3.6) | ND | 653 ± 361 (17.2) |
| PPD+ | |||||
| D7 | 681 ± 448 | 38,107 ± 2,133 (56) | 4,408 ± 2,773 (6.5) | 2,506 ± 689 (3.7) | ND |
| D62 | 483 ± 160 | 32,275 ± 7,799 (66.8) | 1,914 ± 978 (4) | 1,612 ± 817 (3.3) | ND |
| D103 | 446 ± 252 | 24,875 ± 2,572 (55.8) | 5,228 ± 1,506 (11.7) | 997 ± 313 (2.2) | ND |
| D131 | 477 ± 191 | 26,536 ± 1,151 (55.6) | 1,731 ± 1,286 (3.6) | 502 ± 142 (1.1) | ND |
| D160 | 1,383 ± 773 | 37,427 ± 8,234 (27.1) | 5,091 ± 1,910 (3.7) | 1,762 ± 58 (1.3) | ND |
| D184 | 155 ± 60 | 32,523 ± 6,351 (210) | 449 ± 155 (2.9) | 1,656 ± 1,118 (10.7) | ND |
| D201 | 414 ± 148 | 41,361 ± 3,500 (99.9) | 542 ± 242 (1.3) | 483 ± 189 (1.2) | ND |
| D-Ro | 452 ± 162 | 53,004 ± 790 (117.3) | 310 ± 69 (0.7) | 303 ± 101 (0.7) | ND |
| D-Ed | 1,063 ± 1,117 | 18,821 ± 1,941 (17.7) | 3,448 ± 1,375 (3.2) | 1,478 ± 385 (1.4) | ND |
| D-Em | 1,178 ± 114 | 41,046 ± 4,402 (34.8) | 1,156 ± 292 (0.9) | 1,718 ± 487 (1.5) | ND |
Stimulation index (mean cpm incorporated in the presence of antigen divided by mean cpm incorporated in the absence of antigen).
ND, not determined.
Western blot analysis of endogenous MTB12 protein in M. tuberculosis.
Polyclonal rabbit antisera raised against total CFP or against recombinant mature MTB12 protein were used to probe Western blots containing M. tuberculosis lysate and CFP (Fig. 4). As expected, antiserum raised against total CFP reacted strongly with a number of bands in CFP and in crude lysate (Fig. 4A). This same antiserum also recognized the full-length and mature recombinant MTB12 proteins, 85B, and a low-molecular-mass (12-kDa) band in the CFP lane. The 12-kDa CFP protein migrated slightly faster than mature recombinant MTB12 protein and was therefore presumed to represent the fully processed endogenous MTB12 protein lacking the N-terminal six-histidine tag of the recombinant. This interpretation was confirmed by probing a duplicate Western blot with a rabbit polyclonal anti-MTB12 antiserum (Fig. 4B). This serum reacted strongly with both the full-length and mature forms of recombinant MTB12 protein but was specific for only the single low-molecular-mass (12-kDa) protein in the M. tuberculosis CFP. Once again, the immunoreactive protein in CFP migrated slightly faster than recombinant mature MTB12 protein, implying that this band corresponded to the endogenous MTB12 mature protein lacking the N-terminal six-histidine tag. Interestingly, the anti-MTB12 antiserum did not detect any proteins in the M. tuberculosis lysate. This latter finding suggested that the MTB12 protein is rapidly processed and exported from the bacilli after synthesis. To confirm that the lack of detectable MTB12 in lysate was the result of rapid export and not degradation of the M. tuberculosis lysate, a third blot was probed with a rabbit polyclonal antiserum raised against the M. tuberculosis secreted protein 85B (9). This antiserum recognized recombinant 85B, native 85B in CFP, and a single band corresponding to 85B in the lysate (Fig. 4C), confirming the integrity of the lysate. Together, these results suggest that MTB12 has a very short intracellular half-life and that it is exported more rapidly than another extracellular protein, 85B. Interestingly, only the mature, fully processed form of 85B protein was detected in lysate, indicating that leader sequences of both MTB12 and 85B are rapidly removed after synthesis.
The intensity of the signal in the Western blot of M. tuberculosis CFP probed with anti-MTB12 antiserum prompted us to determine the abundance of MTB12 in CFP relative to other well-characterized CFP proteins. CFP was separated by one-dimensional SDS-PAGE, transferred to a PVDF membrane by Western blotting, and stained with Coomassie blue. A triplet of proteins with the same approximate molecular weight as mature MTB12 was readily visible after staining (Fig. 5, lane 1). The middle band of the triplet was determined to be MTB12 according to two different criteria. First, this band (on a duplicate blot) was reactive with the polyclonal anti-MTB12 antiserum by Western blotting (Fig. 5, lane 2) but not with the corresponding preimmune serum (Fig. 5, lane 3). Second, the identity of a number of predominant CFP proteins was determined by N-terminal sequencing of bands excised from the Coomassie blue-stained PVDF membrane. The middle band of the triplet contained the N-terminal sequence of mature MTB12, whereas the lower band of the triplet corresponded to GroES. The uppermost band of the triplet could not be identified, as it was blocked at the N terminus. The locations of three other abundant CFP proteins (85B complex, MPT64, and GroEL) that were identified by N-terminal sequencing are indicated at the right in Fig. 5. Together, these data suggest that MTB12 is a highly abundant component of M. tuberculosis CFP and that MTB12 is at least as abundant as several other well-characterized CFP proteins.
FIG. 5.
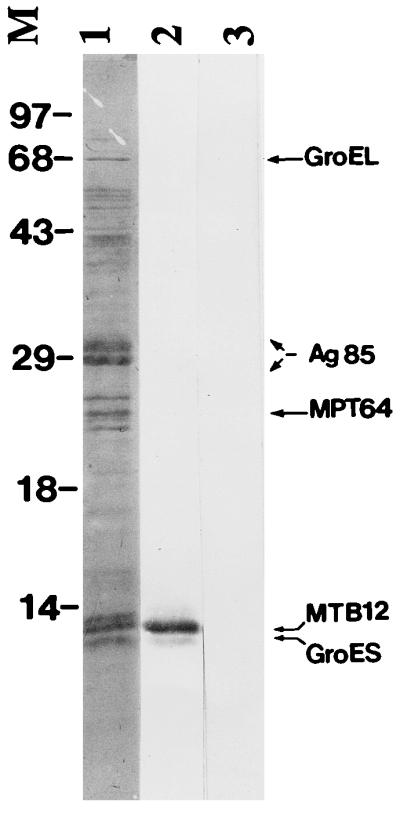
Relative abundance of MTB12 in M. tuberculosis culture filtrates. M. tuberculosis CFP was separated by SDS-PAGE, transferred to a PVDF membrane by Western blotting and stained with Coomassie blue (lane 1). Bands corresponding to the most highly abundant proteins were excised and characterized by N-terminal amino acid sequencing. Identities of these proteins are indicated where known. A pair of duplicate strips were destained in 100% methanol, blocked with 1% bovine serum albumin and probed with a rabbit polyclonal anti-MTB12 antiserum (lane 2) or corresponding preimmune serum (lane 3). The middle band of the triplet migrating at approximately 12 kDa was shown by both amino acid sequencing and Western blot analysis to be MTB12. Positions of size markers (M) are indicated in kilodaltons.
DISCUSSION
In this study, a novel protein present in M. tuberculosis CFP was identified, cloned, and characterized in terms of abundance and immunological reactivity. The crude CFP from M. tuberculosis has been extensively characterized as a rich source of antigens that elicit protective responses in various models of tuberculosis (2, 26). Furthermore, it has been speculated that active synthesis and secretion of CFP components are responsible for the greater efficacy of vaccination using live attenuated mycobacteria than of vaccination using killed organisms. Consequently, the CFP is currently being characterized by a number of labs in an effort to define specific proteins that may be useful as subunit vaccine reagents. The MTB12 protein reported herein is a highly abundant component of M. tuberculosis CFP that appears to be actively secreted. The presence of a consensus signal sequence at the N terminus and the removal of this sequence from the mature, extracellular form of the protein confirm that MTB12 is indeed targeted to the extracellular space by M. tuberculosis. However, it is not clear from this analysis whether the MTB12 protein is released directly into the culture medium or whether it represents a surface protein that is fortuitously shed into the culture medium by membrane turnover. The lack of a recognizable lipid attachment site and the inability to detect MTB12 protein in M. tuberculosis lysate preparations suggest that the membrane turnover hypothesis is less likely. Also, the MTB12 protein contains two consensus sites for N-linked glycosylation. Although there is limited evidence to suggest that glycosylation does occur in mycobacteria (11–13, 15), only O-linked glycosylation has been reported to date.
It is intriguing that MTB12 has not been previously identified as a component of CFP considering the apparent abundance of the protein. Various preparations of CFP are known to vary in composition dependent on the culture media used (3, 10, 23, 32) and the growth phase of the culture (i.e., early log versus late log phase) (3). Therefore, it is possible that secretion of MTB12 is dependent on one or both of these parameters. The CFP analyzed in this study corresponded to filtrate proteins of a late-log-phase culture grown in GAS medium (32). Also, mature MTB12 is found in close proximity to the GroES protein after one-dimensional SDS-PAGE and has a pI (5.03) which is similar to that determined for GroES (32). It is therefore plausible that the MTB12 protein has been previously overlooked due to its proximity to the well-characterized GroES protein.
During preparation of this report, a search of a recently released database containing portions of the M. tuberculosis genome revealed the presence of a cosmid (MTCY27) that harbored the complete MTB12 ORF (sequence accession no. MTCY27.04). Analysis of the cosmid sequence in the region of the MTB12 ORF confirmed that digestion with PstI would produce the 1- and 1.6-kbp hybridizing bands that we observed by Southern blotting. Although the complete M. tuberculosis genome is not yet available, this result also suggests that MTB12 is likely to be encoded by a single-copy gene. Furthermore, our Southern blot results indicate that these same two PstI sites are conserved in other strains of M. tuberculosis and in M. bovis, implying that the MTB12 gene is conserved among members of the M. tuberculosis complex. Interestingly, database searches also revealed homology between MTB12 and the predicted ORF of a distal cosmid (MTCY261.12). Despite significant sequence divergence between these two ORFs (40% overall sequence identity), they encode proteins that are almost identical in size, and like MTB12, this related protein appears to have an N-terminal signal sequence that would presumably lead to export into the extracellular space. The functions of both MTB12 and this related protein are unknown, and a search of the SWISSPRO databanks with the MTB12 sequence did not reveal the presence of any obvious structural domains. However, the inability to detect MTB12 protein in M. tuberculosis lysates suggests that it is rapidly transported out of the bacillus after synthesis and is therefore likely to be functionally active only in the extracellular space.
Recognition of recombinant MTB12 protein by the PBMC of healthy, disease-free, PPD+ donors provides intriguing evidence that MTB12 may play a role during the development of protective immune responses against M. tuberculosis. In addition, there is considerable evidence to suggest strong cellular immune responses are developed against other abundant, secreted proteins of M. tuberculosis, including members of the 85B complex (22, 28, 29), the 38-kDa antigen (33), the Apa (Tb45/47) protein (21, 30), and others. There is a high likelihood that secreted proteins such as these will be processed and presented in the context of major histocompatibility complex (MHC) class II molecules, resulting in the in vivo stimulation of CD4+ T cells. These molecules therefore represent likely candidates for vaccine development since priming of an appropriate cellular response in a naive individual would be expected to influence the outcome of a subsequent infection. We are currently assessing MTB12-specific immune responses in a broader array of donor types, including patients in various states of disease progression, healthy household contacts, and BCG recipients. In addition, we are investigating MTB12-specific immune responses in murine models of tuberculosis.
Comparison of the antigenicity of recombinant mature MTB12 with that of the equivalent full-length protein indicated that for some human donors the full-length MTB12 protein elicited a slightly stronger in vitro response than did mature protein (data not shown). This finding suggests that the hydrophobic leader sequence, which is normally cleaved during processing, may contribute to the antigenicity of the full-length molecule. We have also observed a similar phenomenon in mouse immunization experiments (unpublished data). Although leader sequence peptides derived from endogenously synthesized proteins are known to be bound and presented by classical (16, 34) and nonclassical (1, 5) class I MHC molecules, presentation of exogenous leader sequence peptides by class I or class II MHC molecules has not been well described. Thus, it would be interesting to determine the relative contributions of CD4+ and CD8+ T cells during the augmented proliferative response observed in the presence of full-length MTB12 protein.
The abundance of MTB12 in culture supernatants together with the immunological data presented herein suggests that MTB12 may have potential value as a subunit vaccine component to protect against infection by M. tuberculosis. We are currently assessing the protective capability of MTB12 vaccination in murine models of tuberculosis, using both recombinant protein in conjunction with specific adjuvants and DNA vaccine approaches.
ACKNOWLEDGMENTS
We thank John Belisle for providing M. tuberculosis CFP and Teresa Bement for performing human PBMC proliferation assays.
S.M.J. was supported in part by NIH training grant 5T32HD07233. J.R.W. and Y.A.W.S. were supported in part by fellowships from the Medical Research Council of Canada.
REFERENCES
- 1.Aldrich C J, De Cloux A, Woods A S, Cotter R J, Soloski M J, Forman J. Identification of a Tap-dependent leader peptide recognized by alloreactive T cells specific for a class Ib antigen. Cell. 1994;79:649–658. doi: 10.1016/0092-8674(94)90550-9. [DOI] [PubMed] [Google Scholar]
- 2.Andersen P. Effective vaccination of mice against Mycobacterium tuberculosis infection with a soluble mixture of secreted mycobacterial proteins. Infect Immun. 1994;62:2536–2544. doi: 10.1128/iai.62.6.2536-2544.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersen P, Askgaard D, Ljungqvist L, Bennedsen J, Heron I. Proteins released from Mycobacterium tuberculosis during growth. Infect Immun. 1991;59:1905–1910. doi: 10.1128/iai.59.6.1905-1910.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boesen H, Jensen B N, Wilcke T, Andersen P. Human T-cell responses to secreted antigen fractions of Mycobacterium tuberculosis. Infect Immun. 1995;63:1491–1497. doi: 10.1128/iai.63.4.1491-1497.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braud V, Jones E Y, McMichael A. The human major histocompatibility complex class Ib molecule HLA-E binds signal sequence-derived peptides with primary anchor residues at positions 2 and 9. Eur J Immunol. 1997;27:1164–1169. doi: 10.1002/eji.1830270517. [DOI] [PubMed] [Google Scholar]
- 6.Brewer T F, Colditz G A. Relationship between bacille Calmette-Guerin (BCG) strains and the efficacy of BCG vaccine in the prevention of tuberculosis. Clin Infect Dis. 1995;20:126–135. doi: 10.1093/clinids/20.1.126. [DOI] [PubMed] [Google Scholar]
- 7.Colditz G A, Berkey C S, Mosteller F, Brewer T F, Wilson M E, Burdick E, Fineberg H V. The efficacy of bacillus Calmette-Guerin vaccination of newborns and infants in the prevention of tuberculosis: meta-analyses of the published literature. Pediatrics. 1995;96:29–35. [PubMed] [Google Scholar]
- 8.Colditz G A, Brewer T F, Berkey C S, Wilson M E, Burdick E, Fineberg H V, Mosteller F. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA. 1994;271:698–702. [PubMed] [Google Scholar]
- 9.Content J, de la Cuvellerie A, De Wit L, Vincent-Levy-Frebault V, Ooms J, De Bruyn J. The genes coding for the antigen 85 complexes of Mycobacterium tuberculosis and Mycobacterium bovis BCG are members of a gene family: cloning, sequence determination, and genomic organization of the gene coding for antigen 85-C of M. tuberculosis. Infect Immun. 1991;59:3205–3212. doi: 10.1128/iai.59.9.3205-3212.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Bruyn J, Bosmans R, Nyabenda J, Van Vooren J P. Effect of zinc deficiency on the appearance of two immunodominant protein antigens (32 kDa and 65 kDa) in culture filtrates of mycobacteria. J Gen Microbiol. 1989;135:79–84. doi: 10.1099/00221287-135-1-79. [DOI] [PubMed] [Google Scholar]
- 11.Dobos K M, Khoo K H, Swiderek K M, Brennan P J, Belisle J T. Definition of the full extent of glycosylation of the 45-kilodalton glycoprotein of Mycobacterium tuberculosis. J Bacteriol. 1996;178:2498–2506. doi: 10.1128/jb.178.9.2498-2506.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dobos K M, Swiderek K, Khoo K H, Brennan P J, Belisle J T. Evidence for glycosylation sites on the 45-kilodalton glycoprotein of Mycobacterium tuberculosis. Infect Immun. 1995;63:2846–2853. doi: 10.1128/iai.63.8.2846-2853.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Espitia C, Mancilla R. Identification, isolation and partial characterization of Mycobacterium tuberculosis glycoprotein antigens. Clin Exp Immunol. 1989;77:378–383. [PMC free article] [PubMed] [Google Scholar]
- 14.Feinberg A P, Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983;132:6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- 15.Garbe T, Harris D, Vordermeier M, Lathigra R, Ivanyi J, Young D. Expression of the Mycobacterium tuberculosis 19-kilodalton antigen in Mycobacterium smegmatis: immunological analysis and evidence of glycosylation. Infect Immun. 1993;61:260–267. doi: 10.1128/iai.61.1.260-267.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henderson R A, Michel H, Sakaguchi K, Shabanowitz J, Appella E, Hunt D F, Engelhard V H. HLA-A2.1-associated peptides from a mutant cell line: a second pathway of antigen presentation. Science. 1992;255:1264–1266. doi: 10.1126/science.1546329. [DOI] [PubMed] [Google Scholar]
- 17.Hubbard R D, Flory C M, Collins F M. Immunization of mice with mycobacterial culture filtrate proteins. Clin Exp Immunol. 1992;87:94–98. doi: 10.1111/j.1365-2249.1992.tb06419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hurley S S, Splitter G A, Welch R A. Rapid lysis technique for mycobacterial species. J Clin Microbiol. 1987;25:2227–2229. doi: 10.1128/jcm.25.11.2227-2229.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacobs R F. Multiple-drug-resistant tuberculosis. Clin Infect Dis. 1994;19:1–10. doi: 10.1093/clinids/19.1.1. [DOI] [PubMed] [Google Scholar]
- 20.Kochi A. The global tuberculosis situation and the new control strategy of the World Health Organization. Tubercle. 1991;72:1–6. doi: 10.1016/0041-3879(91)90017-m. . (Editorial.) [DOI] [PubMed] [Google Scholar]
- 21.Laqueyrerie A, Militzer P, Romain F, Eiglmeier K, Cole S, Marchal G. Cloning, sequencing, and expression of the apa gene coding for the Mycobacterium tuberculosis 45/47-kilodalton secreted antigen complex. Infect Immun. 1995;63:4003–4010. doi: 10.1128/iai.63.10.4003-4010.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Launois P, De Leys R, Niang M N, Drowart A, Andrien M, Dierckx P, Cartel J L, Sarthou J L, Van Vooren J P, Huygen K. T-cell-epitope mapping of the major secreted mycobacterial antigen Ag85A in tuberculosis and leprosy. Infect Immun. 1994;62:3679–3687. doi: 10.1128/iai.62.9.3679-3687.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagai S, Wiker H G, Harboe M, Kinomoto M. Isolation and partial characterization of major protein antigens in the culture fluid of Mycobacterium tuberculosis. Infect Immun. 1991;59:372–382. doi: 10.1128/iai.59.1.372-382.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oettinger T, Andersen A B. Cloning and B-cell-epitope mapping of MPT64 from Mycobacterium tuberculosis H37Rv. Infect Immun. 1994;62:2058–2064. doi: 10.1128/iai.62.5.2058-2064.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Orme I M, Andersen P, Boom W H. T cell response to Mycobacterium tuberculosis. J Infect Dis. 1993;167:1481–1497. doi: 10.1093/infdis/167.6.1481. [DOI] [PubMed] [Google Scholar]
- 26.Pal P G, Horwitz M A. Immunization with extracellular proteins of Mycobacterium tuberculosis induces cell-mediated immune responses and substantial protective immunity in a guinea pig model of pulmonary tuberculosis. Infect Immun. 1992;60:4781–4792. doi: 10.1128/iai.60.11.4781-4792.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roberts A D, Sonnenberg M G, Ordway D J, Furney S K, Brennan P J, Belisle J T, Orme I M. Characteristics of protective immunity engendered by vaccination of mice with purified culture filtrate protein antigens of Mycobacterium tuberculosis. Immunology. 1995;85:502–508. [PMC free article] [PubMed] [Google Scholar]
- 28.Roche P W, Peake P W, Billman-Jacobe H, Doran T, Britton W J. T-cell determinants and antibody binding sites on the major mycobacterial secretory protein MPB59 of Mycobacterium bovis. Infect Immun. 1994;62:5319–5326. doi: 10.1128/iai.62.12.5319-5326.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roche P W, Triccas J A, Avery D T, Fifis T, Billman-Jacobe H, Britton W J. Differential T cell responses to mycobacteria-secreted proteins distinguish vaccination with bacille Calmette-Guerin from infection with Mycobacterium tuberculosis. J Infect Dis. 1994;170:1326–1330. doi: 10.1093/infdis/170.5.1326. [DOI] [PubMed] [Google Scholar]
- 30.Romain F, Laqueyrerie A, Militzer P, Pescher P, Chavarot P, Lagranderie M, Auregan G, Gheorghiu M, Marchal G. Identification of a Mycobacterium bovis BCG 45/47-kilodalton antigen complex, an immunodominant target for antibody response after immunization with living bacteria. Infect Immun. 1993;61:742–750. doi: 10.1128/iai.61.2.742-750.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Skeiky Y A, Benson D R, Parsons M, Elkon K B, Reed S G. Cloning and expression of Trypanosoma cruzi ribosomal protein P0 and epitope analysis of anti-P0 autoantibodies in Chagas’ disease patients. J Exp Med. 1992;176:201–211. doi: 10.1084/jem.176.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sonnenberg M G, Belisle J T. Definition of Mycobacterium tuberculosis culture filtrate proteins by two-dimensional polyacrylamide gel electrophoresis, N-terminal amino acid sequencing, and electrospray mass spectrometry. Infect Immun. 1997;65:4515–4524. doi: 10.1128/iai.65.11.4515-4524.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Torres M, Herrera T, Villareal H, Rich E A, Sada E. Cytokine profiles for peripheral blood lymphocytes from patients with active pulmonary tuberculosis and healthy household contacts in response to the 30-kilodalton antigen of Mycobacterium tuberculosis. Infect Immun. 1998;66:176–180. doi: 10.1128/iai.66.1.176-180.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei M L, Cresswell P. HLA-A2 molecules in an antigen-processing mutant cell contain signal sequence-derived peptides. Nature. 1992;356:443–446. doi: 10.1038/356443a0. [DOI] [PubMed] [Google Scholar]
- 35.Young D B, Kaufmann S H, Hermans P W, Thole J E. Mycobacterial protein antigens: a compilation. Mol Microbiol. 1992;6:133–145. doi: 10.1111/j.1365-2958.1992.tb01994.x. [DOI] [PubMed] [Google Scholar]