Abstract
Background
Breast cancer (BC) is the most common cancer among women worldwide. We conducted a systematic review and meta-analysis to cover the existing research gap and contribute to existing knowledge to provide both researchers and clinicians with a better profile on the topic and consequently help improve the quality of life (QoL) of patients with BC.
Methods
A comprehensive review of original articles published in English from January 2000 to October 2021 from databases including Embase, Scopus, PubMed and Web of Science was conducted based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement.
Results
Based on the meta-regression which examined a total of 9012 patients with BC, the QoL score calculated by EORTC QLQ-C30 was 64.72 (95% CI 59.24 to 70.20), while the score obtained from FACT-B was 84.39 (95% CI 64.24 to 104.54) and the scores from QLQ-BR23 and SF-36 were 66.33 (95% CI 62.76 to 69.90) and 57.23 (95% CI 47.65 to 66.82), respectively. A meta-analysis affirmed a significant direct relationship between the QoL score of patients with BC and their age (p=0.03). The results also revealed that the QoL scores of patients who had completed treatment were higher than those who were currently under treatment.
Conclusion
The present systematic review identified several factors that affect the QoL of women with BC worldwide and provided several implications for developing policy interventions to effectively improve the QoL of women with BC. In this way, clinicians can sufficiently give advice to their patients with the purpose of improving their QoL.
PROSPERO registration number CRD42022309791.
Keywords: quality of life, cancer, breast
Key messages.
What was already known?
Breast cancer is the most common cancer among women worldwide, in such a way that in 2019 over 268 000 new cases were diagnosed, with nearly 42 000 of women dying from the disease on the same year.
What are the new findings?
Based on the meta-regression which examined a total of 9012 patients with breast cancer, the quality of life score calculated by EORTC QLQ-C30 was 64.72 (95% CI 59.24 to 70.20), while the score obtained from FACT-B was 84.39 (95% CI 64.24 to 104.54) and the scores from QLQ-BR23 and SF-36 were 66.33 (95% CI 62.76 to 69.90) and 57.23 (95% CI 47.65 to 66.82), respectively.
What is their significance?
Our review identified several factors associated with quality of life.
The findings provide scientific evidence to build an inclusive multidimensional plan which encompasses the identified factors to improve the level of quality of life of breast cancer survivors.
Introduction
Breast cancer (BC) is the most common cancer among women worldwide, in such a way that in 2019 over 268 000 new cases were diagnosed, with nearly 42 000 of women dying from the disease on the same year.1 Due to the significantly increasing rate of incidence of BC and the related technological advances in diagnosis and treatment, patients’ survival outcomes have been achieved.2 However, in addition to survival, quality of life (QoL) has been regarded as a key outcome indicator in patients with BC, particularly in cancer survivorship research and clinical trials.3 4
In fact, the diagnosis of BC causes significant physical, mental and economic consequences to the patients and their families, which consequently require an important change in a person’s natural lifestyle and even the dynamism of family members.5 Disease symptoms, negative psychological effects including anxiety, stress, fear and depression, decreased level of perceived life expectancy, and the potential adverse side effects of the disease are the main domains of QoL in different stages of the disease from non-invasive BC to completing the course of treatment, or even receiving palliative care in patients with advanced cancer.6 Thus, QoL is a multidimensional concept which encompasses physical, psychological and social well-being. Many definitions have been given for QoL; however, according to the WHO, QoL is defined as a person’s perception and satisfaction with life and their general appraisal of their level of functional well-being.7 As women are the most important members of the family, their QoL can not only affect their survival, but also the cohesion of their family’s structure. Accordingly, evidence has shown that psychosocial problems could double the severity of the physical symptoms of the disease, particularly in patients upon diagnosis who typically feel their treatment symptoms are devastating and intolerable.6 Thus, highlighting the significance of patients’ QoL following BC diagnosis is essential, and its improvement should be mentioned as one of the key objectives in the BC treatment procedure.8
Furthermore, it is necessary to determine the influencing factors of QoL to allow patients to successfully transition to a survival status and adjust themselves to the stressful events of the disease. Many factors have been affirmed to affect the QoL of patients with BC. For example, age, disease stage, economic issues, daily work-life challenges, and the medical and psychosomatic features of patients with BC, including pain, stress, anxiety and depression, in addition to declining self-efficacy and diminishing social relationships, have been mentioned in several studies.9–15
There are several useful articles reporting the status of QoL of patients with BC along with its associated factors. However, studies on factors influencing QoL in these patients focusing on different geographical regions, stage of treatment, age and study period are scarce. Identification of influencing factors could assist healthcare professionals in developing efficient health approaches and promoting the QoL of patients with BC worldwide. Therefore, we conducted a systematic review and meta-analysis to cover the existing research gap and contribute to existing knowledge to provide both researchers and clinicians with a better profile on the topic and consequently help improve the QoL of patients with BC.
Methods
Registration and reporting
This systematic review was registered with PROSPERO in 2022 (CRD42022309791; available at https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022309791) and was reported based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement.16
Databases and search terms
A comprehensive review of original articles published in English from January 2000 to October 2021 from databases including Embase, Scopus, PubMed and Web of Science was conducted with the following search terms: (Breast Neoplasm[Title]) OR (Breast Tumors[Title])) OR (Breast Tumor[Title])) OR (Breast Cancer[Title])) OR (Mammary Cancer[Title])) OR (Mammary Cancers[Title])) OR (Malignant Neoplasm of Breast[Title])) OR (Breast Malignant Neoplasm[Title])) OR (Breast Malignant Neoplasms[Title])) OR (Malignant Tumor of Breast[Title])) OR (Breast Malignant Tumor[Title])) OR (Breast Malignant Tumors[Title])) OR (Cancer of Breast[Title])) OR (Cancer of the Breast[Title])) OR (Human Mammary Carcinomas[Title])) OR (Human Mammary Neoplasm[Title])) OR (Human Mammary Neoplasms[Title])) OR (Breast Carcinoma[Title])) OR (Breast Carcinomas[Title])) AND (Life quality[Title]) OR (Health Related Quality of Life[Title])) OR (Health-Related Quality of Life[Title])) OR (HRQOL[Title]) OR (quality of life[Title])).
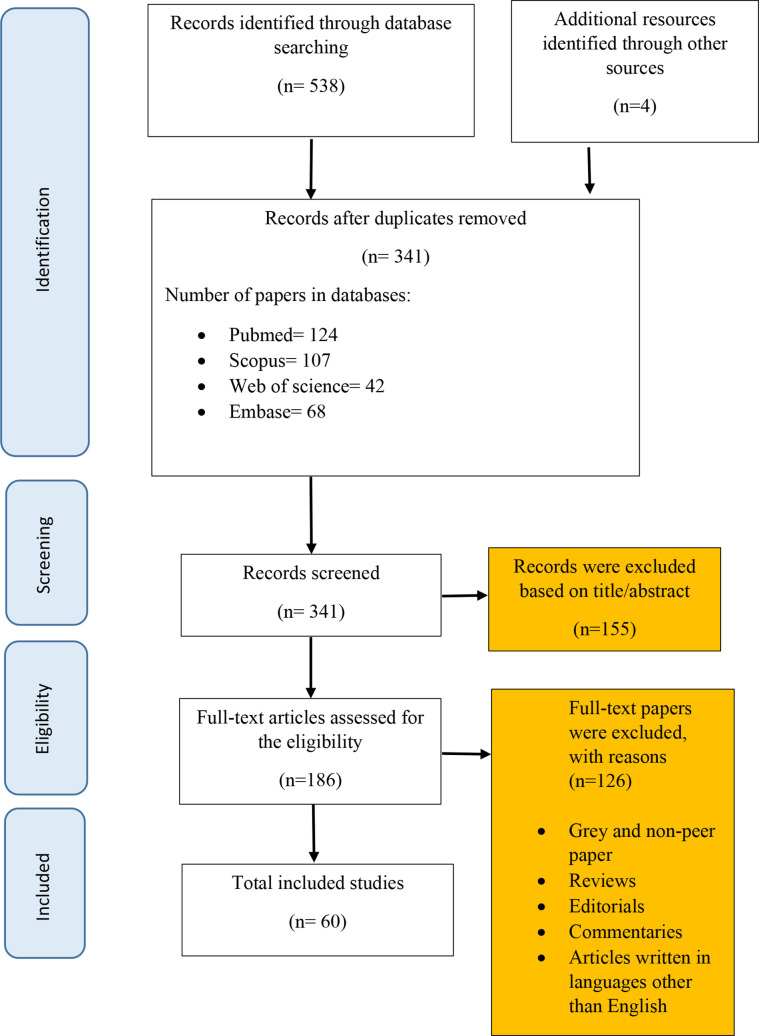
From searching the electronic databases, 545 articles were identified. After importing the papers to the EndNote software, duplicates were removed, resulting in 341 articles. Two independent research members then screened the titles and abstracts of the remaining papers to check for data relevancy. This process led to 186 articles. Accordingly, studies which included data on QoL of patients with BC or its determinants were considered for further review. Conference abstracts were also searched and the references of the included articles were examined for inclusion as additional references. Finally, applying the inclusion and exclusion criteria resulted in 60 studies for inclusion in the research (figure 1).
Figure 1.
Flow diagram of the review process (PRISMA). PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Inclusion criteria
Studies with quantitative data on the rate of QoL of patients with BC or its contributing factors were included in the review. Different types of studies, including cross-sectional, prospective, case study, case series and cohort studies, with available full text in English and published between January 2000 and October 2021 were also considered.
Exclusion criteria
Other types of studies including interventional studies, case–control, reviews, reports, commentaries, letters to the editor and books were excluded. Additionally, studies focusing on diagnosis or therapeutic approaches and medication therapies were excluded from the review.
Data collection tools
Based on the initial review, it was found that in most of the literature four main questionnaires were used to score the QoL of patients with BC. Three of them (namely, EORTC QLQ-C30, QLQ-BR23 and SF-36) had similar scales, ranging from 0 to 100, where higher scores are associated with higher prevalence.17 The QLQ-C30 scale evaluates global health and QoL and encompasses 30 items, with three parts on symptoms (nausea and vomiting, pain, and fatigue) and five sections on functional items (physical, emotional, social, role and cognitive).18 19 On the other hand, the FACT-B tool is a 27-item questionnaire which measures physical, emotional, functional, social or family well-being using a different scale, ranging from 0 to 144; the closer the score is to 144, the better the QoL.20
Quality assessment
The Newcastle-Ottawa Scale (NOS) was used to evaluate the quality of papers which have been reviewed in our research. In the process of quality assessment, two independent research members participated. In case of any discrepancy, the conflicting issue was resolved by consulting with a third reviewer. The NOS allocates a maximum of 9 points for the minimum risk of bias in three areas of exposure/outcome ascertainment, selection of study groups and comparability. This scoring includes 4 points for selection of study groups, 2 points for comparability of groups and 3 points for ascertainment of exposure and outcomes. Scores of 0 or 1 in the selection area, 0 in the comparability area, and 0 or 1 in the outcome/exposure area indicate that the article has poor quality. Accordingly, the lowest and highest NOS scores for each of the papers could be 0 and 10, respectively. Thus, in this review, an article with a score below 4 is regarded to have a low level of quality.21
Data extraction
To extract data we applied a preliminary data extraction form through which we extracted information such as authors’ name, publication date, geographical region, data collection tool, study design, study population, sampling method, risk of bias, and outcome measures including prevalence of QoL and its associated factors.
Statistical analysis
We used random-effects model to estimate the pooled QoL of patients with BC. The findings were presented on a forest plot at a confidence level of 95%. According to the sample size and publication year, the I2 test of heterogeneity was carried out along with a meta-regression analysis. Afterwards, a subgroup analysis was carried out based on age, stage of treatment, geographical region and publication year. Data were analysed using the Comprehensive Meta-Analysis and R software.
Results
Overview
Based on the meta-regression which examined a total of 9012 patients with BC, the QoL score calculated by EORTC QLQ-C30 was 64.72 (95% CI 59.24 to 70.20), while the score obtained from FACT-B was 84.39 (95% CI 64.24 to 104.54) and the scores from QLQ-BR23 and SF-36 were 66.33 (95% CI 62.76 to 69.90) and 57.23 (95% CI 47.65 to 66.82), respectively (table 1).
Table 1.
Meta-analysis based on questionnaires
| Groups | Studies (n) | Effect size and 95% CI | Test of null (two-tailed) | |||||
| Point estimate | SE | Variance | Lower limit | Upper limit | Z value | P value | ||
| EORTC QLQ-C30 | 26 | 64.72 | 2.79 | 7.81 | 59.24 | 70.20 | 23.16 | 0.00 |
| FACT- B | 17 | 84.39 | 10.28 | 105.70 | 64.24 | 104.54 | 8.21 | 0.00 |
| QLQ-BR23 | 8 | 66.33 | 1.82 | 3.32 | 62.76 | 69.90 | 36.41 | 0.00 |
| SF-36 | 9 | 57.23 | 4.89 | 23.91 | 47.65 | 66.82 | 11.70 | 0.00 |
Subgroup analysis of continents and WHO regions
The results of the analysis based on three questionnaires (EORTC QLQ-C30, QLQ-BR23 and SF-36) showed that the lowest QoL scores respectively belonged to South America at 52.04 (95% CI 29.14 to 64.94), followed by Africa at 58.69 (95% CI 56.85 to 60.54). Based on the FACT-B questionnaire, South America at 74.31 (95% CI 82.2 to 94.9) and North America at 80.53 (95% CI 35.01 to 126.05) relatively had the lowest QoL score for patients with BC (table 2).
Table 2.
Meta-analysis based on continents and WHO regions
| Groups | Tools | Continents | Effect size and 95% CI | Test of null (two-tailed) | |||||
| Point estimate | SE | Variance | Lower limit | Upper limit | Z value | P value | |||
| Continents | Other tools | Africa | 58.69 | 0.94 | 0.89 | 56.85 | 60.54 | 62.38 | 0.00 |
| Asia | 65.57 | 3.66 | 13.39 | 58.40 | 72.75 | 17.92 | 0.00 | ||
| Europe | 63.21 | 3.93 | 15.46 | 55.50 | 70.92 | 16.08 | 0.00 | ||
| North America | 63.75 | 0.87 | 0.76 | 62.04 | 65.46 | 72.96 | 0.00 | ||
| South America | 52.04 | 1.48 | 2.19 | 29.14 | 64.94 | 21.66 | 0.00 | ||
| FACT-B | Africa | 85.39 | 1.84 | 3.37 | 91.79 | 98.99 | 51.95 | 0.00 | |
| Asia | 89.26 | 6.23 | 38.85 | 77.04 | 101.48 | 14.32 | 0.00 | ||
| Europe | 88.61 | 36.20 | 1310.21 | 3.36 | 145.25 | 2.05 | 0.04 | ||
| North America | 80.53 | 23.23 | 539.44 | 35.01 | 126.05 | 3.47 | 0.00 | ||
| South America | 74.31 | 3.26 | 10.64 | 82.20 | 94.99 | 27.17 | 0.00 | ||
| WHO regions | Other tools | AFRO | 58.69 | 0.94 | 0.89 | 56.85 | 60.54 | 62.38 | 0.00 |
| AMRO | 63.75 | 0.87 | 0.76 | 62.04 | 65.46 | 72.96 | 0.00 | ||
| EMRO | 65.84 | 2.92 | 8.53 | 60.11 | 71.56 | 22.54 | 0.00 | ||
| EURO | 62.72 | 3.68 | 13.58 | 55.50 | 69.94 | 17.02 | 0.00 | ||
| SEARO | 67.96 | 7.25 | 52.62 | 53.75 | 82.18 | 9.37 | 0.00 | ||
| WPRO | 63.22 | 5.80 | 33.64 | 51.85 | 74.59 | 10.90 | 0.00 | ||
| FACT-B | AFRO | 95.39 | 1.84 | 3.37 | 91.79 | 98.99 | 51.95 | 0.00 | |
| AMRO | 82.97 | 18.63 | 347.06 | 46.46 | 119.49 | 4.45 | 0.00 | ||
| EMRO | 68.83 | 1.11 | 1.23 | 66.66 | 71.00 | 62.14 | 0.00 | ||
| EURO | 74.31 | 36.20 | 1310.21 | 3.36 | 145.25 | 2.05 | 0.04 | ||
| WPRO | 93.39 | 5.00 | 24.97 | 83.60 | 103.18 | 18.69 | 0.00 | ||
In addition, related findings based on the different WHO regions showed that the lowest QoL was observed in AFRO at 58.69 (95% CI 56.85 to 60.54), and based on the FACT-B questionnaire EMRO at 68.83 (95% CI 66.6 to 71) got the lowest score (table 2).
Meta-analysis of different stages of treatment
To enrich the review findings, we divided patients into two categories: those who were under treatment and those who have completed the treatment procedure. The results revealed that patients in the second group had higher QoL scores compared with the former (table 3).
Table 3.
Meta-analysis based on stage of treatment
| Tools | Groups | Studies (n) | Effect size and 95% CI | Test of null (two-tailed) | |||||
| Point estimate | SE | Variance | Lower limit | Upper limit | Z value | P value | |||
| Other tools | Treated | 21 | 64.19 | 4.21 | 17.69 | 55.95 | 72.44 | 15.26 | 0.00 |
| Under treatment | 22 | 62.77 | 2.14 | 4.60 | 58.57 | 66.98 | 29.28 | 0.00 | |
| FACT-B | Treated | 11 | 84.35 | 12.76 | 162.73 | 59.35 | 109.35 | 6.61 | 0.00 |
| Under treatment | 6 | 84.46 | 22.39 | 501.33 | 40.58 | 128.35 | 3.77 | 0.00 | |
Meta-analysis based on questionnaire items
We analysed all items of all four questionnaires to obtain the score of each item. Based on the findings in EORTC QLQ-C30, the items cognitive functioning and social functioning had the highest scores with 77.42 (95% CI 74.76 to 80.07) and 76.19 (95% CI 72.62 to 79.76), respectively. The item diarrhoea had the lowest score with 12.82 (95% CI 9.45 to 16.19). In QLQ-BR23, the item body image had the highest score with 59.14 (95% CI 40.60 to 77.68), while the item breast-related symptoms had the lowest score with 16.90 (95% CI 10.47 to 23.33). In SF-36, similar to EORTC QLQ-C30, the item social functioning had the highest score with 73.96 (95% CI 55.27 to 92.65). Finally, in FACT-B, all items approximately had the same value, with no significant differences between them (table 4).
Table 4.
Meta-analysis based on questionnaire items
| Questionnaires | Items | Effect size and 95% CI | Test of null (two-tailed) | |||||
| Point estimate | SE | Variance | Lower limit | Upper limit | Z value | P value | ||
| EORTC QLQ-C30 | Physical functioning | 77.33 | 1.55 | 2.41 | 74.29 | 80.37 | 49.86 | 0.00 |
| Role functioning | 70.80 | 2.25 | 5.07 | 66.39 | 75.21 | 31.44 | 0.00 | |
| Emotional functioning | 70.54 | 1.14 | 1.30 | 68.31 | 72.77 | 61.87 | 0.00 | |
| Cognitive functioning | 77.42 | 1.35 | 1.83 | 74.76 | 80.07 | 57.20 | 0.00 | |
| Social functioning | 76.19 | 1.82 | 3.32 | 72.62 | 79.76 | 41.84 | 0.00 | |
| Fatigue | 34.22 | 3.40 | 11.59 | 27.54 | 40.89 | 10.05 | 0.00 | |
| Pain | 25.63 | 1.32 | 1.75 | 23.04 | 28.22 | 19.40 | 0.00 | |
| Dyspnoea | 19.71 | 1.47 | 2.18 | 16.82 | 22.60 | 13.36 | 0.00 | |
| Insomnia | 31.24 | 1.87 | 3.51 | 27.56 | 34.91 | 16.66 | 0.00 | |
| Appetite loss | 20.97 | 1.95 | 3.79 | 17.15 | 24.78 | 10.77 | 0.00 | |
| Constipation | 19.90 | 1.99 | 3.96 | 16.00 | 23.80 | 10.00 | 0.00 | |
| Diarrhoea | 12.82 | 1.72 | 2.95 | 9.45 | 16.19 | 7.46 | 0.00 | |
| Financial problems | 30.56 | 3.47 | 12.02 | 23.76 | 37.35 | 8.81 | 0.00 | |
| QLQ-BR23 | Body image | 59.14 | 9.46 | 89.44 | 40.60 | 77.68 | 6.25 | 0.00 |
| Sexual performance | 29.62 | 4.35 | 18.94 | 21.09 | 38.14 | 6.81 | 0.00 | |
| Sexual satisfaction | 47.31 | 2.42 | 5.86 | 42.57 | 52.05 | 19.55 | 0.00 | |
| Future prospects | 44.40 | 2.94 | 8.66 | 38.63 | 50.17 | 15.09 | 0.00 | |
| Adverse reactions to treatment | 21.60 | 2.76 | 7.65 | 16.18 | 27.02 | 7.81 | 0.00 | |
| Breast-related symptoms | 16.90 | 3.28 | 10.76 | 10.47 | 23.33 | 5.15 | 0.00 | |
| Arm-related symptoms | 18.48 | 4.06 | 16.47 | 10.53 | 26.44 | 4.55 | 0.00 | |
| Hair loss | 41.04 | 5.33 | 28.41 | 30.60 | 51.49 | 7.70 | 0.00 | |
| SF-36 | Physical functioning | 63.42 | 5.54 | 30.71 | 52.56 | 74.28 | 11.44 | 0.00 |
| Role limitations due to physical health | 67.79 | 8.71 | 75.93 | 50.71 | 84.87 | 7.78 | 0.00 | |
| Role limitations due to emotional problems | 69.17 | 10.21 | 104.23 | 49.16 | 89.18 | 6.78 | 0.00 | |
| Energy/fatigue | 45.49 | 21.32 | 454.42 | 3.71 | 87.27 | 2.13 | 0.03 | |
| Emotional well-being (mental health) | 54.56 | 7.83 | 61.26 | 39.22 | 69.90 | 6.97 | 0.00 | |
| Social functioning | 73.96 | 9.53 | 90.89 | 55.27 | 92.65 | 7.76 | 0.00 | |
| Pain | 57.85 | 15.62 | 243.98 | 27.24 | 88.47 | 3.70 | 0.00 | |
| FACT-B | Physical well-being | 19.06 | 0.70 | 0.48 | 17.70 | 20.43 | 27.43 | 0.00 |
| Social well-being | 19.90 | 0.93 | 0.87 | 18.07 | 21.73 | 21.34 | 0.00 | |
| Emotional well-being | 18.59 | 0.64 | 0.41 | 15.33 | 19.85 | 25.78 | 0.00 | |
| Functional well-being | 18.02 | 0.88 | 0.78 | 16.29 | 19.75 | 20.43 | 0.00 | |
| Breast cancer | 20.42 | 0.77 | 0.59 | 19.92 | 24.92 | 30.58 | 0.00 | |
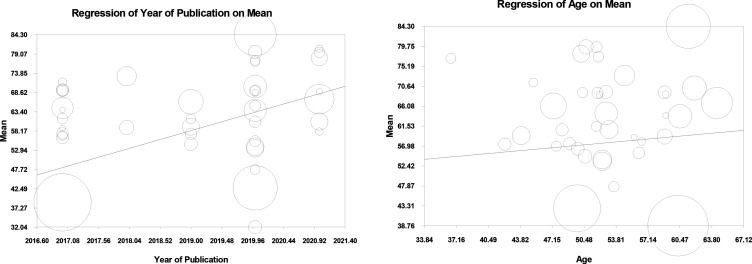
Meta-regression based on publication year
The meta-regression analysis by year of publication depicted a significant direct relationship between QoL score and passing of time. In fact, per unit of increase in the publication year, the QoL score augmented by 5 points (figure 2).
Figure 2.
Meta-regression based on year of publication and age.
Subgroup analysis of age
A meta-analysis affirmed a significant direct relationship between the QoL score of patients with BC and their age (p=0.03), so that a unit of increase in a patient’s age led to an augmentation in the patient’s QoL by 0.19 (figure 2).
Discussion
Overview
The main objective of this systematic review and meta-analysis was to combine the results of existing literature exploring QoL scores and identifying its determinants in women with BC worldwide. After an all-embracing review, we identified 60 articles that met our inclusion criteria. The vast majority of these studies used EORTC QLQ-C30, QLQ-BR23, SF-36 and FACT-B questionnaires to calculate QoL scores. Our review identified several factors associated with QoL. These findings provide scientific evidence to build an inclusive multidimensional plan which encompasses the identified factors to improve the level of QoL of BC survivors.
Total score
The average total score of QoL reported in our review was dependent on the questionnaires used in the included studies, ranging from 57.23 to 84.39. The scores were lower than the scores reported for Chinese patients with BC undergoing chemotherapy (average score=93.9) and much lower than the scores reported for Austrian patients with BC who had just completed their treatment procedure, with a mean score of 108.1.22 23 The differences between the scores obtained might be due to the diverse study population in terms of age groups. Most of the literature affirmed that patients with BC in their 50s and 60s had higher QoL compared with those in their 30s and 40s.24 In line with the results of three studies, our review found that QoL score was directly affected in older patients, so that younger patients with BC experienced poorer QoL than their older counterparts.25–30 Therefore, healthcare providers should give more attention and support to younger women, who need a wide-ranging sympathetic plan and follow-ups as psychosocial support systems will help them successfully complete their treatment regimen and attain psychological health in a shorter possible time. Other reasons for existing inconsistencies in the QoL score might be explained by the heterogeneity of samples and study population, as well as the low statistical power in some of the research due to the small sample size. More importantly, some of the literature highlighted that the difference in QoL scores might be due to the different reference value manuals for assessing QoL in patients with BC. For example, EORTC QLQ-C30 is mainly based on pretreatment QoL data. Thus, it can be concluded that evaluating the QoL of patients at different stages of treatment, including surgery, radiotherapy and chemotherapy, can potentially affect the scores obtained.
Regional score
In terms of geographical region, the mean QoL score of patients with BC living in Ethiopia, Morocco, Nigeria and Nepal was lower than studies conducted in the countries of other continents, including Asia, Australia and America.31–37 Furthermore, several studies characterised the QoL of patients with BC in the entire WHO region. A systematic review in patients from the Eastern Mediterranean region revealed that the global QoL score ranged between 31.1 and 75.6.5 Comparisons show that the mean score for the global QoL domains in our review was higher, indicating better QoL worldwide. These discrepancies might be due to differences in patients’ sociodemographic characteristics. For instance, the majority of Ethiopian population belongs to lower socioeconomic class, where a significant portion of income goes to usual domestic expenditures and where the population cannot afford treatments such as chemotherapy and radiotherapy.
Stages of disease
Regarding treatment-related factors, QoL was negatively affected by advanced stages of the disease and its devastating symptoms and side effects. For example, chemotherapy is significantly associated with poorer QoL in women with BC. These patients are more likely to be diagnosed with advanced stage cancer and might experience higher levels of exhaustion, pain, stress and probably other rigorous psychological side effects, consequently decreasing their QoL.38 Other therapeutic approaches offered to patients in the early stages of the disease are not expected to be connected with advanced stage disease; therefore, they are less likely to adversely influence patients’ QoL. Intriguingly, one of the studies revealed that complementary medicine, such as spiritual remedy and herbal therapy, was associated with better QoL, physical functioning and social well-being.39 However, as this type of medication is frequently used in the Middle East countries, related findings might not be applicable to all countries with different cultures with the intention of adding them into therapeutic care approaches for patients with BC.
Other variables
Generally, multiple clinical factors such as cancer stage, time since diagnosis and disease duration were found to be associated with QoL. Literature affirmed that QoL was negatively affected by chemotherapy and mastectomy, while it was reported to be directly influenced by hormone therapy, early treatment and breast reconstruction surgery.40 Thus, the perception of body image with regard to body weight and actual body mass index in patients with BC after chemotherapy or mastectomy is a significant factor since distorted body weight perception might influence biopsychosocial functioning.41 In addition, early diagnosis and medical interventions to improve both physical and psychological well-being of patients with BC were mentioned as useful strategies to improve patients’ QoL.41 42 Although most of the physical symptoms caused by the disease treatment procedures are recovered after a while, the inability to remember, learn new things, concentrate or make decisions, as well as the struggle with a variety of physical and mental disorders, including insomnia, fatigue, stress and anxiety, may continue in patients with BC even years after the end of treatment. Such difficulties cause negative emotions in patients and harmfully influence their psychological condition.43 44 Hence, systematic symptom management interventions are essential in patients with BC who are adjusting themselves to normal lifestyle.
Limitations
Our review has a number of limitations which need to be considered with caution when interpreting the results. First, despite the rigorous search strategy with no restrictions on publication year, our review was limited to studies published in English. Second, lack of data from some geographical regions limited our ability to perform subgroup analysis for different regions. Third, our review lacked data on comparison of QoL in patients with BC in terms of type of surgery performed within the breast. Furthermore, it did not consider the influence of different types of therapy on QoL. Fourth, most of the included studies did not report key clinical variables that could affect QoL, such as the mean time since diagnosis or the precise stage of the disease, despite the fact that it is a reasonable expectation that can change during the continuum of care and that can affect patients’ QoL.
Conclusion
The present systematic review identified several factors that affect the QoL of women with BC worldwide and provided several implications for developing policy interventions to effectively improve the QoL of women with BC. The implementation of QoL assessment tool within a clinical setting can help in the diagnosis, prognosis, patient monitoring, clinical decision-making, treatment and necessary follow-ups as it provides precise assessments of patients’ physical, mental, functional and social well-being and provides them with adequate knowledge about their own care. In this way, clinicians can sufficiently give advice to their patients with the purpose of improving their QoL.
Footnotes
Correction notice: This article has been updated since it was first published. The article type has been changed to Systematic review.
Contributors: Conception and design of study: SRao, AG, FPK, AJB. Acquisition of data: FB, FPK, SRao, MS, FZ, NR, ZBC, BA, SD, MZ, HS, FDnK, NV. Analysis and/or interpretation of data: BSH, AG, EA. Drafting the manuscript: MMi, AG, AJB. Revising the manuscript critically for important intellectual content: AG. Approval of the version of the manuscript to be published: SRao, AG, SRaf, AJB.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not required.
References
- 1. Society AC. Breast cancer facts & figures 2019–2020. American Cancer Society, 2019: 1–44. [Google Scholar]
- 2. Cardoso F, Kyriakides S, Ohno S, et al. Early breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up†. Ann Oncol 2019;30:1194–220. 10.1093/annonc/mdz173 [DOI] [PubMed] [Google Scholar]
- 3. van Leeuwen M, Husson O, Alberti P, et al. Understanding the quality of life (QOL) issues in survivors of cancer: towards the development of an EORTC QOL cancer survivorship questionnaire. Health Qual Life Outcomes 2018;16:1–15. 10.1186/s12955-018-0920-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bottomley A, Reijneveld JC, Koller M, et al. Current state of quality of life and patient-reported outcomes research. Eur J Cancer 2019;121:55–63. 10.1016/j.ejca.2019.08.016 [DOI] [PubMed] [Google Scholar]
- 5. Gonzalez L, Bardach A, Palacios A, et al. Health-Related quality of life in patients with breast cancer in Latin America and the Caribbean: a systematic review and meta-analysis. Oncologist 2021;26:e794–806. 10.1002/onco.13709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Richardson LC, Wang W, Hartzema AG, et al. The role of health-related quality of life in early discontinuation of chemotherapy for breast cancer. Breast J 2007;13:581–7. 10.1111/j.1524-4741.2007.00512.x [DOI] [PubMed] [Google Scholar]
- 7. Organization, W.H . WHOQOL: Measuring quality of life, 2014. https://www.who.int/tools/whoqol#:~:text=WHO%20defines%20Quality%20of%20Life,%2C%20expectations%2C%20standards%20and%20concerns [Google Scholar]
- 8. Tigeneh W. Pattern of cancer in Tikur Anbessa specialized hospital oncology center in Ethiopia from 1998 to 2010. Int J cancer Res Mol Mech 2015;1:1–5. [Google Scholar]
- 9. Ekwueme DU, Trogdon JG. The economics of breast cancer in younger women in the U.S.: the present and future. Am J Prev Med 2016;50:249–54. 10.1016/j.amepre.2015.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Keim-Malpass J, Levine B, Danhauer SC. Work-rRelated perceptions and quality of life among breast cancer survivors. Psychooncology 2016;25:873–6. 10.1002/pon.3951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kwan ML, Ergas IJ, Somkin CP, et al. Quality of life among women recently diagnosed with invasive breast cancer: the pathways study. Breast Cancer Res Treat 2010;123:507–24. 10.1007/s10549-010-0764-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hutter N, Vogel B, Alexander T, et al. Are depression and anxiety determinants or indicators of quality of life in breast cancer patients? Psychol Health Med 2013;18:412–9. 10.1080/13548506.2012.736624 [DOI] [PubMed] [Google Scholar]
- 13. Leung J, Pachana NA, McLaughlin D. Social support and health-related quality of life in women with breast cancer: a longitudinal study. Psychooncology 2014;23:1014–20. 10.1002/pon.3523 [DOI] [PubMed] [Google Scholar]
- 14. Papadopoulou C, Kotronoulas G, Schneider A, et al. Patient-Reported self-efficacy, anxiety, and health-related quality of life during chemotherapy: results from a longitudinal study. Oncol Nurs Forum 2017;44:127–36. 10.1188/17.ONF.127-136 [DOI] [PubMed] [Google Scholar]
- 15. Herschbach P, Berg P, Waadt S, et al. Group psychotherapy of dysfunctional fear of progression in patients with chronic arthritis or cancer. Psychother Psychosom 2010;79:31–8. 10.1159/000254903 [DOI] [PubMed] [Google Scholar]
- 16. Zhang X, Tan R, Lam WC, et al. PRISMA (preferred reporting items for systematic reviews and meta-analyses) extension for Chinese herbal medicines 2020 (PRISMA-CHM 2020). Am J Chin Med 2020;48:1279–313. 10.1142/S0192415X20500639 [DOI] [PubMed] [Google Scholar]
- 17. Brandini da Silva FC, José da Silva J, Sarri AJ, et al. Comprehensive validation study of quality-of-life questionnaire using objective clinical measures: breast cancer treatment outcome scale (BCTOS), Brazilian Portuguese version. Clin Breast Cancer 2019;19:e85–100. 10.1016/j.clbc.2018.10.004 [DOI] [PubMed] [Google Scholar]
- 18. Aaronson NK, Ahmedzai S, Bergman B, et al. The European organization for research and treatment of cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365–76. 10.1093/jnci/85.5.365 [DOI] [PubMed] [Google Scholar]
- 19. Kindts I, Laenen A, van den Akker M, et al. Proms following breast-conserving therapy for breast cancer: results from a prospective longitudinal monocentric study. Support Care Cancer 2019;27:4123–32. 10.1007/s00520-019-04698-0 [DOI] [PubMed] [Google Scholar]
- 20. Zhu X-Y, Li Z, Chen C, et al. Physical therapies for psychosomatic symptoms and quality of life induced by aromatase inhibitors in breast cancer patients: a systematic review and meta-analysis. Front Oncol 2021;11:745280. 10.3389/fonc.2021.745280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Peterson J. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2. Ottawa: Ottawa Hospital Research Institute, 2011: 1–12. [Google Scholar]
- 22. Ai Z-P, Gao X-L, Li J-F. Changing trends and influencing factors of the quality of life of chemotherapy patients with breast cancer. Chin Nurs Res 2017;4:18–23. 10.1016/j.cnre.2017.03.006 [DOI] [Google Scholar]
- 23. Brennan ME, Butow P, Spillane AJ, et al. Patient-reported quality of life, unmet needs and care coordination outcomes: moving toward targeted breast cancer survivorship care planning. Asia Pac J Clin Oncol 2016;12:e323–31. 10.1111/ajco.12254 [DOI] [PubMed] [Google Scholar]
- 24. Kroenke CH, Kwan ML, Neugut AI, et al. Social networks, social support mechanisms, and quality of life after breast cancer diagnosis. Breast Cancer Res Treat 2013;139:515–27. 10.1007/s10549-013-2477-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ahmed AE. The predictors of poor quality of life in a sample of Saudi women with breast cancer. Breast Cancer: Targets and Therapy 2017;9:51. 10.2147/BCTT.S125206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Akin S, Can G, Durna Z, et al. The quality of life and self-efficacy of Turkish breast cancer patients undergoing chemotherapy. Eur J Oncol Nurs 2008;12:449–56. 10.1016/j.ejon.2008.07.006 [DOI] [PubMed] [Google Scholar]
- 27. Najafi F, Zendehdel K, Mirzania M, et al. Self-reported versus proxy reported quality of life for breast cancer patients in the Islamic Republic of Iran. East Mediterr Health J 2016;22:786–93. 10.26719/2016.22.11.786 [DOI] [PubMed] [Google Scholar]
- 28. Pehlivan S, Kuzhan A, Yildirim Y, et al. Comfort and quality of life in patients with breast cancer undergoing radiation therapy. J Buon 2016;21:549–55. [PubMed] [Google Scholar]
- 29. Safa A, Rahemi Z, Aghajani M. Quality of life in patients with breast cancer in Kashan: a cross-sectional study. Life Science Journal 2014;11:141–5. [Google Scholar]
- 30. Homaee Shandiz F, Karimi FZ, Khosravi Anbaran Z, Shandiz FH, et al. Investigating the quality of life and the related factors in Iranian women with breast cancer. Asian Pac J Cancer Prev 2017;18:2089-2092. 10.22034/APJCP.2017.18.8.2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Grabsch B, Clarke DM, Love A, et al. Psychological morbidity and quality of life in women with advanced breast cancer: a cross-sectional survey. Palliat Support Care 2006;4:47–56. 10.1017/S1478951506060068 [DOI] [PubMed] [Google Scholar]
- 32. Safaee A, Moghimi-Dehkordi B, Zeighami B, et al. Predictors of quality of life in breast cancer patients under chemotherapy. Indian J Cancer 2008;45:107. 10.4103/0019-509X.44066 [DOI] [PubMed] [Google Scholar]
- 33. Høyer M, Johansson B, Nordin K, et al. Health-related quality of life among women with breast cancer - a population-based study. Acta Oncol 2011;50:1015–26. 10.3109/0284186X.2011.577446 [DOI] [PubMed] [Google Scholar]
- 34. Jassim GA, Whitford DL. Quality of life of Bahraini women with breast cancer: a cross sectional study. BMC Cancer 2013;13:1–14. 10.1186/1471-2407-13-212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lôbo SA, et al. Quality of life in women with breast cancer undergoing chemotherapy. Acta Paulista de Enfermagem 2014;27:554–9. 10.1590/1982-0194201400090 [DOI] [Google Scholar]
- 36. Dubashi B, Vidhubala E, Cyriac S, et al. Quality of life among younger women with breast cancer: study from a tertiary cancer Institute in South India. Indian J Cancer 2010;47:142. 10.4103/0019-509X.63005 [DOI] [PubMed] [Google Scholar]
- 37. Nakitare SK. Health related quality of life of breast cancer patients at Kenyatta national Hospital. Kenya: University of Nairobi, 2012. [Google Scholar]
- 38. Meeske KA, Patel SK, Palmer SN, et al. Factors associated with health-related quality of life in pediatric cancer survivors. Pediatr Blood Cancer 2007;49:298–305. 10.1002/pbc.20923 [DOI] [PubMed] [Google Scholar]
- 39. Albabtain H, Alwhaibi M, Alburaikan K, et al. Quality of life and complementary and alternative medicine use among women with breast cancer. Saudi Pharm J 2018;26:416–21. 10.1016/j.jsps.2017.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sinaei F, Zendehdel K, Adili M, et al. Association between breast reconstruction surgery and quality of life in Iranian breast cancer patients. Acta Med Iran 2017;55:35–41. [PubMed] [Google Scholar]
- 41. Nikmanesh Z, Shirazi M, Farazinezhad F. Examining the predictive role of emotional self-regulation in quality of life and perception of suffering among patients with breast cancer. Middle East J Cancer 2017;8:93–101. [Google Scholar]
- 42. Moradi R, et al. Investigating the relationship between self-efficacy and quality of life in breast cancer patients receiving chemical therapy. Bmj 2017;6:358. 10.15562/bmj.v6i1.358 [DOI] [Google Scholar]
- 43. Nesvold I-L, Reinertsen KV, Fosså SD, et al. The relation between arm/shoulder problems and quality of life in breast cancer survivors: a cross-sectional and longitudinal study. J Cancer Surviv 2011;5:62–72. 10.1007/s11764-010-0156-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schmidt M, Weyer-Elberich V, Hengstler JG, et al. Prognostic impact of CD4-positive T cell subsets in early breast cancer: a study based on the FinHer trial patient population. Breast Cancer Res 2018;20:1–10. 10.1186/s13058-018-0942-x [DOI] [PMC free article] [PubMed] [Google Scholar]