Abstract
Background
The ANGIOCAT trial showed a clinical benefit of direct to angiography suite (DTAS) for patients with large vessel occlusion (LVO) stroke admitted within 6 hours after symptom onset in decreased hospital workflow time and improved clinical outcome. However, the impact of DTAS implementation on hospital costs is unknown. This economic evaluation aims to assess the cost-utility of DTAS from the provider (hospital) perspective.
Methods
A cost-utility analysis was applied to compare DTAS with the standard direct to CT (DTCT) suite approach using direct cost and health outcomes data. The time horizon was 90 days. One-way sensitivity analysis as well as probabilistic sensitivity analysis was performed, varying the model parameters by ±25%. Measures included costs, quality-adjusted life years, and incremental cost-effectiveness ratios. Health outcomes, classified according to the modified Rankin Scale, were obtained from the ANGIOCAT trial. Respective utilities were obtained from the literature.
Results
DTAS is the dominant strategy. The incremental cost-effectiveness ratio is −€89 110 (−$97 600) with cost saving per patient of –€2848 (–$3120). The improved clinical outcome is directly related with a decrease in costs for the hospital, mainly due to the decrease in costs of hospital stay, improved clinical outcome and fewer complications.
Conclusions
For patients with LVO admitted within 6 hours after symptom onset, the DTAS not only improves clinical outcome but also decreases the costs (dominant option) compared with the standard DTCT. Multicentric international randomized clinical trials are ongoing to determine the replicability of our findings.
Keywords: stroke, economics, thrombectomy, angiography
WHAT IS ALREADY KNOWN ON THIS TOPIC
The direct to angiography suite (DTAS) approach for patients with large vessel occlusion (LVO) stroke has been found to be an effective and safe tool to reduce in-hospital delays and potentially improve clinical outcomes. With the growing implementation of DTAS in stroke centers around the world, the health economic impact of this strategy has become an important topic and was investigated in this study.
WHAT THIS STUDY ADDS
This study shows that DTAS is a dominant protocol compared with the direct to CT (DTCT) suite protocol for the treatment of patients with a suspected LVO stroke on admission.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Implementation of DTAS not only improves clinical outcome but also decreases the costs of hospital care for the treatment of patients with an LVO admitted within 6 hours after symptom onset.
Introduction
In 2019, stroke was the second leading cause of death (accountable for 11.6% of total deaths globally) and the third leading cause of combined death and disability.1 In Spain, there were 101 845 incident cases and 29 646 death counts related to stroke in 2016.2 The economic burden of stroke is substantial, especially when taking into account productivity costs (eg, mortality losses, morbidity losses, social care, etc) in addition to the direct healthcare costs of the acute and chronic treatments.3 In 2017, the stroke-related healthcare costs in Spain were estimated to be €1.7 billion, accounting for 1.68% of the total healthcare expenditure with inpatient hospital care being the main contributor (€569 million, 34%).3 The total costs of stroke for society in Spain in 2017, including productivity losses, was estimated at €3.6 billion, accounting for 0.3% of gross domestic product.3
Although mechanical thrombectomy candidates are a minority of all stroke patients, the development of this therapy became a paradigm shift after the results of five pivotal trials in 2015.
Timely reperfusion is the fundamental principle underlying successful reperfusion therapy for stroke,4 and time from symptom onset to reperfusion (OTR) is the main modifiable predictor of functional outcome.5
Therefore, establishing quick and efficient imaging and workflow protocols that can save time while allowing appropriate selection of patients for therapy is paramount. Similarly, to the percutaneous coronary intervention protocols in acute myocardial infarction, several case–control studies have shown that transporting stroke patients directly to the angiosuite is safe, feasible and results in a significant reduction in median door to treatment times down to 16 min and potentially higher chances of good outcomes.4 6–9 Several meta-analyses10 11 and one randomized clinical trial (ANGIOCAT trial) have recently confirmed these data.12
Direct to angiography suite (DTAS) protocols require the immediate availability of the angiography suite and stroke team, and although clinical data must be confirmed in multicenter ongoing trials, DTAS is the most effective time-shortening strategy published so far. The growing implementation of DTAS in many stroke centers around the world and the potential cost of this strategy has become an important topic.
Our aim is to explore the cost-effectiveness of DTAS compared with the usual in-hospital stroke pathway based on data from the ANGIOCAT trial.
Methods
Study design
The ANGIOCAT trial was a prospective, open, randomized clinical trial (NCT04001738) with blinded assessment of the primary end point by an independent investigator. A total of 174 patients were randomly assigned to follow either the DTAS (89 patients) or the conventional workflow (85 patients) to assess the indication of endovascular thrombectomy (EVT), and the primary outcome favored the DTAS workflow by a decrease in disability across the range of the modified Rankin Scale (mRS) (adjusted common OR 2.2, 95% CI 1.2 to 4.1, P=0.009).12
For this analysis, costs of hospital care were based on costing of input consumed during treatment (‘microcosting’) in the Vall d’Hebron hospital in 2018. The following hospital care costs were collected (before and after discharge): (1) hospital stay; (2) laboratory; (3) mechanical thrombectomy procedure; (4) intravenous tissue plasminogen activator (IV-tPA) for intravenous thrombolysis; (5) other.
Description of modeling approach
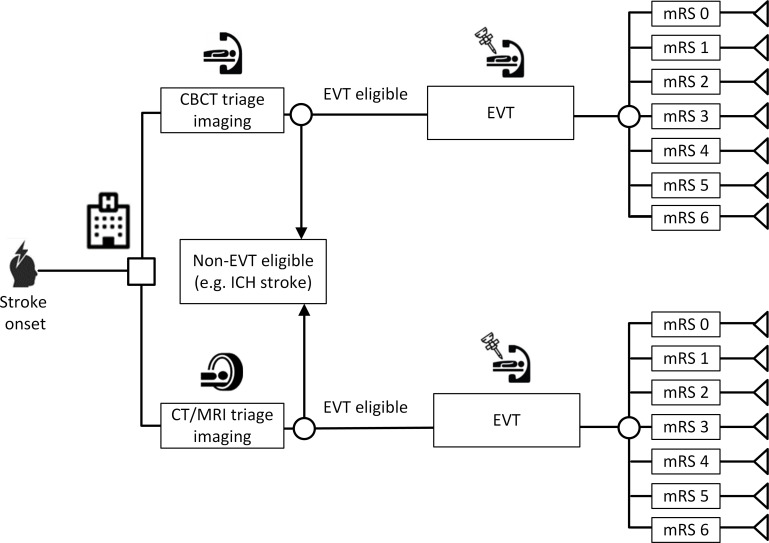
The purpose of this analysis was to compare the expected incremental cost and outcomes for ischemic stroke patients who take the DTAS route using cone-beam CT (CBCT) versus ischemic stroke patients who follow the traditional conventional imaging approach. To achieve this, a decision tree was constructed (figure 1) and a cost-utility analysis was carried out. A cost-utility analysis is a type of economic evaluation to compare costs and outcomes of alternative interventions. Outcomes are measured as quality-adjusted life years (QALYs) which consider the quality of life (health utilities) and quantity of life (life years).
Figure 1.
Decision tree that was used to model cost-utility of DTAS (top branch) versus the conventional workflow (bottom branch). CBCT, cone-beam CT; DTAS, direct to angiography suite; EVT, endovascular thrombectomy; ICH, intracerebral hemorrhage; mRS, modified Rankin Scale.
The incremental costs and incremental outcomes (QALYs) are then combined into a single metric, namely the incremental cost per QALY gained (or incremental cost-effectiveness ratio). Using QALYs as a standardized measure of effectiveness allows comparison across disease areas which is particularly useful in decision-making.
In order to add rigor to the analysis, a probabilistic sensitivity analysis (PSA) is conducted to quantify the level of confidence in the result of the evaluation, in relation to uncertainty in the model inputs. The PSA therefore addresses uncertainties associated with input values of an economic model which is constructed based on input from other studies. Details about the input parameters and sensitivity analysis of the evaluation are provided below.
The analysis was carried out from the Spanish healthcare provider perspective. QALYs based on the mRS were used as a measure of effectiveness. The time horizon was 90 days. This study was conducted according to the13 13 reporting guidelines13 (see online supplemental table 1).
jnis-2023-020275supp001.pdf (805.4KB, pdf)
Unit costs and resource utilization
As the analysis was conducted from a provider perspective, costs relative to each mRS were included and estimated by taking an average from direct hospital costs calculated in Euros (€). The following hospital care cost were collected (before and after discharge): (1) hospital stay; (2) laboratory; (3) procedure; (4) IV-tPA for intravenous thrombolysis; (5) other (consumables, rehabilitation and personnel costs). A summary of the unit costs related to the mRS scores (excluding costs relating to CBCT and dedicated angiosuite setup) is presented in online supplemental figure 1.
The cost of CBCT was based on the acquisition cost of the machine and room (angiosuite) setup to have a room idle to receive emergency stroke patients without delay. A discounted annual equivalent cost was calculated by taking into consideration the lifespan of the machine (10 years), discount rate (3.5%) and procedural volume per year (200 cases). The annual acquisition cost per case was calculated to be €1202 equating to €301 for 90 days.
The cost was considered as incremental to CT imaging for conservative purposes. For the analysis, it was assumed a dedicated room was needed.
Health state utility values and calculation of QALYs
Clinical outcomes were measured as QALYs based on an average of utility data derived from three literature studies.14–16 This is a standardized measure that takes into account the quality and quantity of life lived—a year in perfect health would equate to 1 QALY. The quality aspect of the QALY was based on the mRS. The mRS scores ranged from 0 to 6 (0 being the best and 6 the worst (death)). Costs and outcomes were considered over 90 days.
Sensitivity analysis
In order to account for any uncertainty in the model, a one-way sensitivity analysis was conducted in the form of a tornado diagram. Model parameters were varied by ±25% so the impact of each parameter on the incremental cost could be seen.
As the DTAS requires capital expenditure with a dedicated angiosuite, the cost per case is dependent on procedural volume. As such, a break-even analysis was carried out to determine the minimum number of procedures required for the net cost impact to be zero.
A PSA was also undertaken to further assess the uncertainty and subsequently add more rigor to the analysis. The PSA was run in the form of a Monte Carlo simulation whereby the model was run a large number of times. The model variable type and subsequent distribution determined the inputs for the PSA (table 1). As confidence intervals for the variables were not available, a 25% variance was used. Using outputs from the simulations, the net monetary benefit was calculated to determine the probability of cost-effectiveness and different willingness to pay thresholds. In line with the National Institute for Health and Clinical Excellence (NICE) guidance, a €22 000 threshold was used.17 18 The PSA simulation outputs also provided the ability to generate a cost-effectiveness plane to show which quadrant(s) the outputs fell into.
Table 1.
Model inputs used in probabilistic sensitivity analysis
| Model variable | Distribution | Value and confidence intervals |
| % of patients that have thrombectomy | Beta | DTAS: 83% (62% to 100%) DTCT: 86% (64% to 100%) |
| Utility values: mRS 0–6 | Beta | mRS 0 (functional independence): 0.93 (0.7 to 1). mRS 1 (functional independence): 0.88 (0.66 to 1). mRS 2 (functional independence): 0.78 (0.59 to 0.98). mRS 3 (disabled): 0.58 (0.44 to 0.73). mRS 4 (disabled): 0.37 (0.28 to 0.46). mRS 5 (disabled): 0.09 (0.07 to 0.11). mRS 6 (death): 0 |
| Patient distributions according to utilities – DTAS | Beta | mRS 0 (functional independence): 8% (6% to 10%). mRS 1 (functional independence): 20% (15% to 25%). mRS 2 (functional independence): 15% (11% to 19%). mRS 3 (disabled): 15% (11% to 19%). mRS 4 (disabled): 14% (10% to 17%). mRS 5 (disabled): 7% (5% to 8%). mRS 6 (death): 22% (16% to 27%) |
| Patient distributions according to utilities – DTCT | Beta | mRS 0 (functional independence): 1% (1% to 2%). mRS 1 (functional independence): 11% (8% to 14%). mRS 2 (functional independence): 15% (11% to 19%). mRS 3 (disabled): 19% (14% to 24%). mRS 4 (disabled): 10% (7% to 12%). mRS 5 (disabled): 10% (7% to 12%). mRS 6 (death): 34% (26% to 43%) |
| CBCT and angiosuite cost | Gamma | €2 000 000 (€1 500 000 to €2 500 000) |
| CT cost | Gamma | 0 |
| Costs related to mRS – DTAS | Gamma | mRS 0 (functional independence): €11 536 (€8652 to €14,420). mRS 1 (functional independence): €16 286 (€12 214 to €20 357). mRS 2 (functional independence): €12 665 (€9 499 to €15 831). mRS 3 (disabled): €13 784 (€10 338 to €17 230). mRS 4 (disabled): €26 315 (€19 736 to €32 894). mRS 5 (disabled): €26 295 (€19 721 to €32 869). mRS 6 (death): €17 250 (€12 937 to €21 562) |
| Costs related to mRS – DTCT | mRS 0 (functional independence): €13 669 (€10 252 to €17 086). mRS 1 (functional independence): €15 951 (€11 963 to €19 938). mRS 2 (functional independence): €19 494 (€14 620 to €24 367). mRS 3 (disabled): €19 074 (€14 306 to €23 843). mRS 4 (disabled): €26 266 (€19 699 to €32 832). mRS 5 (disabled): €31 530 (€23 647 to €39 412). mRS 6 (death): €18 189 (€13 642 to €22 736) |
€1∼$1.10.
CBCT, cone-beam CT; DTAS, direct to angiography suite; DTCT, direct to CT; mRS, modified Rankin Scale.
A cost-effectiveness acceptability curve was subsequently created using calculated net monetary benefit values from each iteration. This was done to determine the probability of CBCT DTAS being cost-effective at a range of willingness to pay thresholds.
Results
Deterministic analysis
The deterministic analysis showed that the DTAS pathway using CBCT is both cost saving (€2848) and provides better patient outcomes (additional 0.032 QALYs) when compared with the traditional pathway of stopping for conventional imaging. This means DTAS with CBCT is dominant over the conventional pathway. The incremental cost-effectiveness ratio was calculated to be –€89 110.
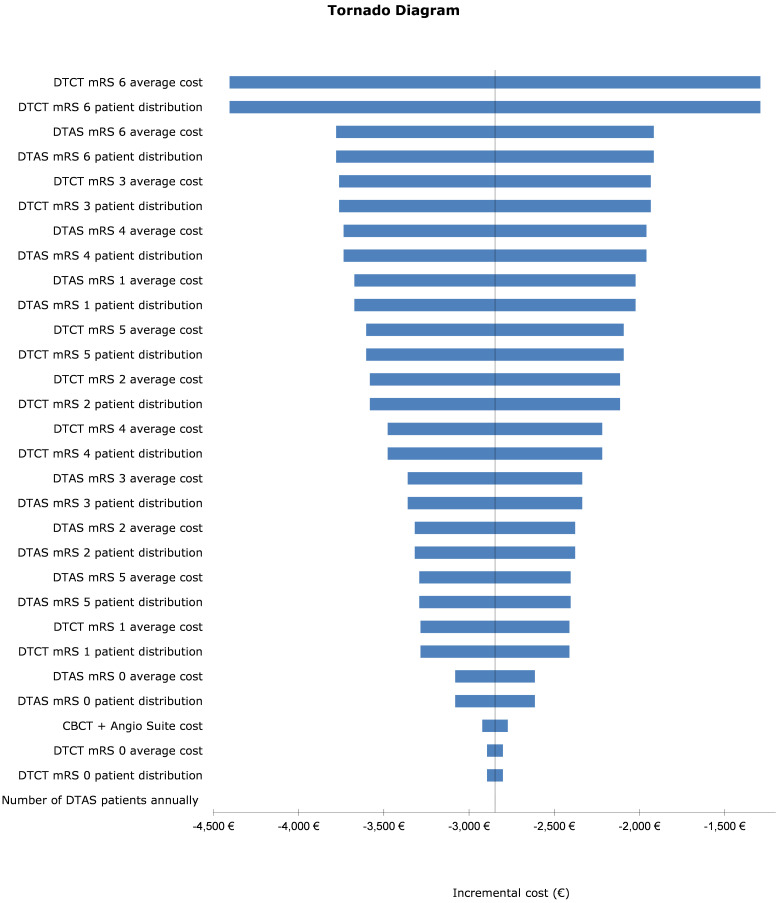
One-way sensitivity analysis
Results for the one-way sensitivity analysis in the form of a Tornado diagram are presented in figure 2. As the deterministic analysis identified the intervention to be dominant, only the incremental cost (instead of the incremental cost effectiveness ratio which is a summary of the cost-effectiveness of a healthcare intervention) was used as the central measure of variability. The bars in the diagram show the extent of the cost-effectiveness impact when a ±25% variation is applied to each of the variables. Those that had the largest impact are portrayed on the top; the cost attributed to patients in the direct to CT (DTCT) arm who died (mRS 6) had the biggest impact. When the value of this variable was decreased by 25%, it still resulted in a cost saving of €1290.
Figure 2.
Results for the one-way sensitivity analysis in the form of a Tornado diagram. CBCT, cone-beam CT; DTAS, direct to angiography suite; DTCT, direct to CT; mRS, modified Rankin Scale. €1∼$1.10.
To examine the impact of mRS 6 in the DTCT arm further, a one-way sensitivity analysis was undertaken in the form of varying the cost of mRS 6 in different increments to see the resulting impact on the cost saving. Online supplemental table 2 shows the results of this analysis.
Break-even analysis
The annual volume of procedures whereby the net impact on cost was zero was calculated to be 76 procedures (the minimum amount of procedures required annually for the pathway to be considered as cost saving). When the number of procedures is increased, the cost-effectiveness is improved and therefore high-volume providers would benefit from the CBCT DTAS pathway the most.
Probabilistic sensitivity analysis
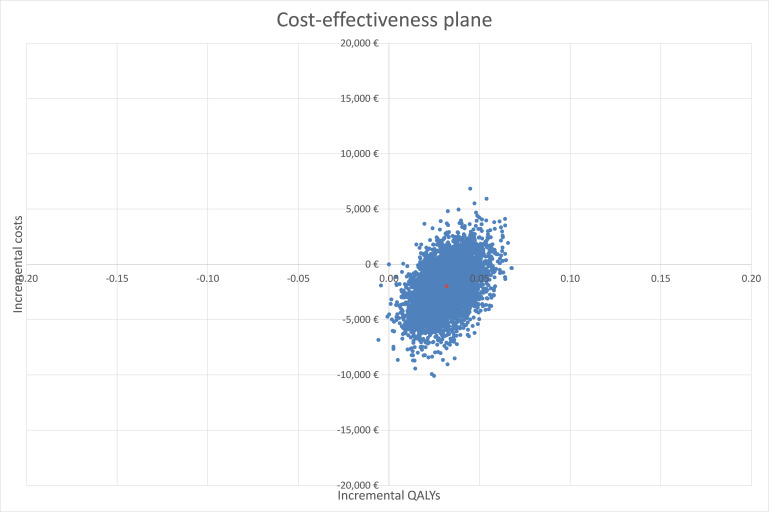
A Monte Carlo simulation of 5000 iterations was undertaken for the PSA and showed dominance of the CBCT DTAS pathway over the current standard of DTCT. The average cost saving was calculated to be €1983 and an additional 0.032 QALYs gained per patient.
Plots of the 5000 iterations of paired incremental costs and QALYs are shown in figure 3. Most of the plots fall into the south-east quadrant (cost saving and more effective), further signifying the dominance of the CBCT DTAS pathway and reducing the uncertainty around the resulting conclusion.
Figure 3.
Plot of the 5000 iterations of paired incremental costs and quality-adjusted life years (QALYs). €1∼$1.10.
Iterations from the simulation were also used to f+orm a cost-effectiveness acceptability curve (CEAC) which determines the probability CBCT DTAS is cost-effective relative to DCTC at a range of willingness to pay thresholds (how much one is willing to pay for an additional QALY gain).
A €22 000 threshold was adopted. The proportion of simulations that portrayed CBCT DTAS as being cost-effective (below €22 000 threshold) was 91.4%. Online supplemental figure 2 shows the probability of CBCT DTAS being cost-effective at different thresholds. Unsurprisingly, as the threshold limit increases, the probability of CBCT DTAS being cost-effective increases.
Discussion
Following the recent evolution of acute stroke management, DTAS has been discovered as an effective and safe tool to reduce in-hospital delays and probably improve clinical outcomes after the publication of several clinical experiences and the ANGIOCAT trial. The cost associated with those medical advances is one of the most important issues to argue against its application. Our study shows that DTAS is a cost-effective protocol even taking into account the cost of investing in a new angiosuite dedicated to acute stroke attention.
There are two main reasons for cost reduction associated with DTAS: improving functional outcome and minimizing imaging protocol in patients with a suspected large vessel occlusion (LVO) acute stroke.
In the ANGIOCAT trial, functional outcome improvement was driven by reducing in-hospital delays and increasing the rate of patients who undergo endovascular treatment. Time from admission to reperfusion has been repeatedly associated with functional outcome and the effect of DTAS reducing this is beyond doubt the reason after the consistent evidence published in the last few years. In the ANGIOCAT trial, although the DTCT group presented a remarkably low median door to puncture time of 42 min, the same interval was only 18 min when the DTAS was followed, similar than previously published in pilot and collaborative studies.7 9 10 17–19 On the other hand, the ANGIOCAT trial is the first study which compared protocols in patients with a confirmed LVO whether or not they received mechanical thrombectomy, and showed that DTAS was associated with an increased rate of EVT (100% of DTAS vs 89% of DTCT patients). The benefits of the DTAS workflow observed in the ANGIOCAT trial in terms of reduction of long-term disability have been previously published.12 Moreover, the present study confirms an improvement in patient quality of life, showing that 0.032 QALYs were gained for each additional patient that underwent DTAS instead of DTCT.
Length of stay and healthcare costs after initial treatment for acute stroke are associated with 90-day mRS.20 Similar to other cost-effectiveness studies related to acute stroke management, the reduction in disability presented the highest impact in terms of costs in our study as shown in the Tornado diagram. Since the distribution of disability is shifted towards lower scores in DTAS patients the total costs are lower. However, we also observed that for each disability score as determined by the mRS scale, the cost for DTAS patients was lower than for DTCT patients (online supplemental figure 1). A possible explanation would be a faster recovery and achievement of final mRS score among DTAS patients, and therefore healthcare costs during the 90 days after stroke. This hypothesis is supported by previous studies in which DTAS was associated with a higher rate of early dramatic clinical recovery.21
In order to efficiently and consistently adopt a DTAS protocol, institutions should be able to provide immediate availability of the angiosuite and the team without interfering with the daily activity scheduled in the angiosuite. To ensure the clinical benefits of DTAS to a majority of patients admitted with a suspected acute stroke due to LVO, implementation of new angiosuites primarily dedicated to triage and treatment of these patients has been proposed.9
The return of the initial investment depends on the number of patients who will benefit from the new workflow. In this study, break-even was achieved with an annual number of 76 patients undergoing the DTAS protocol—taking into consideration the 10-year lifespan of the angiosuite. At present most large comprehensive stroke centers perform over 200 thrombectomies per year, meaning that even if DTAS could only be applied to a proportion of all treated patients the investment can be recouped.
A recent study showed that DTAS is cost-effective from a healthcare system perspective by including a lifetime time horizon, mainly due to long-term costs related to disability.22 In this study we focused on hospital-related costs during the acute and sub-acute phase until 90 days post-procedure, with the aim to provide a contextualized assessment relevant for the hospital setting. Health technology assessments from the hospital perspective can be a valuable tool in the hospital’s decision-making process of optimal resource allocation for health technology investments. In addition, by using hospital-specific clinical and cost data, a tailored hospital budget impact analysis can be generated. Although the major cost savings following DTAS implementation can be expected from long-term cost savings related to patient disability,22 this study shows that DTAS decreases acute hospital costs and is already cost-effective when considering a relatively short time horizon of 90 days.
Our model may be considered as a conservative approach since we included the necessary investment for a newly dedicated angiosuite. Most previously published studies were developed in the absence of such a dedicated angiosuite, a scenario that might also be possible. However, if the benefit of DTAS will definitively be confirmed in ongoing multicentric randomized trials,23 24 the number of potential candidate patients in which DTAS cannot be performed must be reduced to the lowest to guarantee equity in the access to best clinical practices.
Our study has been performed in a single center of the Spanish public healthcare system with longstanding experience in performing DTAS at the beginning of the trial (>200 DTAS patients since 2016). The study was performed with a robust EMS pre-hospital assessment with clinical severity tools, round-the clock 365 days a year on-site stroke neurology, and a single payor healthcare system. Although some of these elements may not be determinant, all may influence the reproducibility of these results which should be evaluated in the setting of other multicenter studies including different healthcare systems. Nevertheless, our study includes data from real-world patients and outcomes from a randomized clinical trial resulting in high quality scientific evidence.
Conclusion
For patients with LVO admitted within 6 hours after symptom onset, the DTAS workflow not only improves clinical outcome,12 but also decreases the costs (dominant option) compared with the standard DTCT suite. Multicentric international randomized clinical trials are ongoing to determine the replicability of our findings.
Footnotes
Twitter: @marcriboj
Contributors: All authors made substantial contributions to the conception or design of the study; or the acquisition, analysis, modeling or interpretation of the data. Additionally, all authors were involved in drafting and revising the manuscript. Finally, all authors gave their final approval of the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. TK is the guarantor.
Funding: The research was supported by Philips.
Competing interests: AT reported receiving personal fees from Anaconda Biomed, Balt, Medtronic, Perflow, and Stryker outside the submitted work. MR reported receiving personal fees from Anaconda Biomed, AptaTargets, Cerenovus, Johnson & Johnson, Medtronic, Methinks, Philips, Sanofi, Stryker, and Rapid AI outside the submitted work and is co-principal investigator of the WE-TRUST trial (NCT04701684) funded by Philips. HVB, SV, CG and TK are Philips employees.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1. GBD 2019 Stroke Collaborators . Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol 2021;20:795–820. 10.1016/S1474-4422(21)00252-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. GBD 2016 Stroke Collaborators . Global, regional, and national burden of stroke, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2019;18:439–58. 10.1016/S1474-4422(19)30034-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Luengo-Fernandez R, Violato M, Candio P, et al. Economic burden of stroke across Europe: a population-based cost analysis. Eur Stroke J 2020;5:17–25. 10.1177/2396987319883160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ribo M, Molina CA, Cobo E, et al. Association between time to reperfusion and outcome is primarily driven by the time from imaging to reperfusion. Stroke 2016;47:999–1004. 10.1161/STROKEAHA.115.011721 [DOI] [PubMed] [Google Scholar]
- 5. Saver JL, Goyal M, van der Lugt A, et al. Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: a meta-analysis. JAMA 2016;316:1279–88. 10.1001/jama.2016.13647 [DOI] [PubMed] [Google Scholar]
- 6. Brehm A, Tsogkas I, Maier IL, et al. One-stop management with perfusion for transfer patients with stroke due to a large-vessel occlusion: feasibility and effects on in-hospital times. AJNR Am J Neuroradiol 2019;40:1330–4. 10.3174/ajnr.A6129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jadhav AP, Kenmuir CL, Aghaebrahim A, et al. Interfacility transfer directly to the neuroangiography suite in acute ischemic stroke patients undergoing thrombectomy. Stroke 2017;48:1884–9. 10.1161/STROKEAHA.117.016946 [DOI] [PubMed] [Google Scholar]
- 8. Psychogios M-N, Behme D, Schregel K, et al. One-stop management of acute stroke patients: minimizing door-to-reperfusion times. Stroke 2017;48:3152–5. 10.1161/STROKEAHA.117.018077 [DOI] [PubMed] [Google Scholar]
- 9. Psychogios M-N, Maier IL, Tsogkas I, et al. One-stop management of 230 consecutive acute stroke patients: report of procedural times and clinical outcome. J Clin Med 2019;8:2185. 10.3390/jcm8122185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Galecio-Castillo M, Vivanco-Suarez J, Zevallos CB, et al. Direct to angiosuite strategy versus standard workflow triage for endovascular therapy: systematic review and meta-analysis. J Neurointerv Surg 2022. 10.1136/neurintsurg-2022-018895 [Epub ahead of print 16 Jun 2022]. [DOI] [PubMed] [Google Scholar]
- 11. Mohammaden MH, Doheim MF, Elfil M, et al. Direct to angiosuite versus conventional imaging in suspected large vessel occlusion: a systemic review and meta-analysis. Stroke 2022;53:2478–87. 10.1161/STROKEAHA.121.038221 [DOI] [PubMed] [Google Scholar]
- 12. Requena M, Olivé-Gadea M, Muchada M, et al. Direct to angiography suite without stopping for computed tomography imaging for patients with acute stroke: a randomized clinical trial. JAMA Neurol 2021;78:1099–107. 10.1001/jamaneurol.2021.2385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Husereau D, Drummond M, Augustovski F, et al. Consolidated health economic evaluation reporting standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. BJOG 2022;129:336–44. 10.1111/1471-0528.17012 [DOI] [PubMed] [Google Scholar]
- 14. Ali M, MacIsaac R, Quinn TJ, et al. Dependency and health utilities in stroke: data to inform cost-effectiveness analyses. Eur Stroke J 2017;2:70–6. 10.1177/2396987316683780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dijkland SA, Voormolen DC, Venema E, et al. Utility-weighted modified Rankin scale as primary outcome in stroke trials: a simulation study. Stroke 2018;49:965–71. 10.1161/STROKEAHA.117.020194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rebchuk AD, O’Neill ZR, Szefer EK, et al. Health utility weighting of the modified Rankin scale: a systematic review and meta-analysis. JAMA Netw Open 2020;3:e203767. 10.1001/jamanetworkopen.2020.3767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McCabe C, Claxton K, Culyer AJ. The NICE cost-effectiveness threshold: what it is and what that means. Pharmacoeconomics 2008;26:733–44. 10.2165/00019053-200826090-00004 [DOI] [PubMed] [Google Scholar]
- 18. Vallejo-Torres L, García-Lorenzo B, Serrano-Aguilar P. Estimating a cost-effectiveness threshold for the Spanish NHS. Health Econ 2018;27:746–61. 10.1002/hec.3633 [DOI] [PubMed] [Google Scholar]
- 19. Mendez AA, Farooqui M, Dajles A, et al. Direct transfer to angiosuite triage strategy for patients undergoing mechanical thrombectomy in a rural setting. SVIN 2021;1:e000124. 10.1161/SVIN.121.000124 [DOI] [Google Scholar]
- 20. Dawson J, Lees JS, Chang T-P, et al. Association between disability measures and healthcare costs after initial treatment for acute stroke. Stroke 2007;38:1893–8. 10.1161/STROKEAHA.106.472381 [DOI] [PubMed] [Google Scholar]
- 21. Ribo M, Boned S, Rubiera M, et al. Direct transfer to angiosuite to reduce door-to-puncture time in thrombectomy for acute stroke. J Neurointerv Surg 2018;10:221–4. 10.1136/neurintsurg-2017-013038 [DOI] [PubMed] [Google Scholar]
- 22. Requena M, Seguel-Ravest V, Vilaseca-Jolonch A, et al. Evaluating the cost-utility of a direct transfer to angiosuite protocol within 6 H of symptom onset in suspected large vessel occlusion patients. J Med Econ 2022;25:1076–84. 10.1080/13696998.2022.2113221 [DOI] [PubMed] [Google Scholar]
- 23. DIRECTANGIO . Effect of direct transfer to angiosuite on functional outcome in severe acute stroke, ClinicalTrials.gov, U.S. National Library of Medicine, identifier: NCT03969511. n.d. Available: https://clinicaltrials.gov/ct2/show/NCT03969511
- 24. WE-TRUST . Workflow optimization to reduce time to endovascular reperfusion for ultra-fast stroke treatment, ClinicalTrials.gov, U.S. National Library of Medicine, identifier: NCT04701684. n.d. Available: https://clinicaltrials.gov/ct2/show/NCT04701684
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jnis-2023-020275supp001.pdf (805.4KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.