Abstract
Many pathogenic Escherichia coli produce the toxin alpha-hemolysin (Hly), and lipopolysaccharide (LPS), interleukin-1 (IL-1), and tumor necrosis factor (TNF) have all been recognized as important effector molecules during infections by gram-negative organisms. Despite the characterization of many in vitro effects of hemolysin, no direct relationship has been established between hemolysin, LPS, proinflammatory cytokine production, and E. coli-induced mortality. Previously, we have shown in vivo that hemolysin elicits a distinct IL-1α spike by 4 h into a lethal hemolytic E. coli infection. Using three transformed E. coli strains, WAF108, WAF270, and WAH540 (which produce no Hly [Hlynull], acylated Hly [Hlyactive], or nonacylated Hly [Hlyinactive], respectively), we sought to determine the specific roles of hemolysin acylation, LPS, IL-1, and TNF in mediating the lethality of E. coli infection in mice. WAF270 was 100% lethal in BALB/c, C3H/HeJ, and C57BL/6 mice; in mice pretreated with antibody to the type 1 IL-1 receptor; in type 1 IL-1 receptor-deficient mice; and in dual (type 1 IL-1 receptor-type 1 TNF receptor)-deficient mice at doses which were nonlethal (0%) with both WAF108 and WAH540. At lethal doses, WAF270 killed by 6 ± 2.3 h while WAF108 and WAH540 killed at 36 ± 9.4 and 36 ± 13.8 h, respectively. These differences in mortality were not due to IL-1 or TNF release, and the enhanced expression of LPS, which corresponded to Hly expression, was not likely the primary factor causing mortality. We demonstrate that bacterial fatty acid acylation of hemolysin is required in order for it to elicit IL-1 release by monocytes and to confer its virulence on E. coli.
Escherichia coli remains the most common gram-negative bacterial species isolated from infections in hospitalized patients (17), and despite significant advances in antimicrobial therapy and critical care technology, mortality from sepsis caused by gram-negative organisms remains 40% or higher (26, 44, 58, 63). Pathogenic E. coli organisms make a variety of virulence factors, and among them are the group of toxic proteins called RTX (repeats in toxin) cytolysins. Alpha-hemolysin (Hly) is the prototype RTX cytolysin (31). At least 40% of pathogenic E. coli organisms produce it, while most fecal isolates do not (6, 11, 27, 29, 49, 50, 51). Production of Hly is associated with enhanced E. coli virulence (35, 59, 60). It remains unclear (i) what effector mechanism(s) mediates its lethality, (ii) whether Hly fatty acid acylation is critical to Hly’s clinically relevant functions (e.g., causing cell lysis, eliciting interleukin-1 (IL-1), and mediating death), and (iii) whether Hly-associated lipopolysaccharide (LPS) is responsible for the enhanced virulence of hemolytic E. coli (LPS is believed to be bound to Hly [9, 10, 55]). To study these issues, three transformed E. coli strains (WAF108, WAF270, and WAH540), differing only in their ability to produce and secrete functionally active Hly, as defined by its ability to lyse erythrocytes in vitro, were used. WAF108 produces no Hly (Hlynull), WAF270 produces hemolytic, fully acylated Hly (Hlyactive), and WAH540, a newly constructed strain, produces full-length, nonacylated, functionally inactive Hly (Hlyinactive) (its genetic construct encodes the same peptide sequence as that of the Hly of WAF270 [8, 61]).
We have previously shown that live Hly-producing E. coli WAF270 is lethal at 108 CFU intraperitoneally (i.p.) and elicits a distinct IL-1α spike 4 to 6 h after infection while nonhemolytic E. coli WAF108 does not elicit this IL-1α spike and is nonlethal at the same inoculum dose (35). Tumor necrosis factor (TNF) blockade with antibodies to TNF-α and TNF-β failed to abrogate the lethality of WAF270 in this study (35). Although Hly is known to cause IL-1β release from monocytes in vitro (5), the relationships between E. coli, Hly, the acylation state of Hly, host IL-1 release, and mortality are unclear.
Despite the characterization of many LPS-mediated responses (19, 22, 24, 30, 40, 43, 45, 52, 64), the contribution of the proinflammatory cytokines to the toxicity and host clearance of live gram-negative bacterial infections remains ill defined. Efforts at using anti-LPS monoclonal antibody (MAb), anti-TNF MAb, and IL-1 receptor antagonists to treat sepsis caused by gram-negative organisms in human and large-animal studies have been discouraging (26, 44, 58, 63). Alternatively, IL-1R1 knockout (KO) mice were partially resistant to lethal E. coli peritonitis (1). In the present study, we hypothesized that Hly-provoked IL-1 signaling with or without TNF signaling was responsible for the enhanced lethality of Hly-producing E. coli. We also proposed that the nonacylated Hly of WAH540 would not activate monocytes, elicit IL-1β, or enhance the lethality of E. coli as the acylated Hly of WAF270 would, implying that both the hemolytic and the nonhemolytic activities of Hly depend on its fatty acid acylation via expression of the hlyC locus.
MATERIALS AND METHODS
Bacteria.
The human fecal isolate E. coli J198 (O22 ColV− Hly−) (59) and the laboratory E. coli strains WAM783 (DH1 transformed with pSF4000ΔBamHI [8, 60]), WAF108 (O22 Hlynull, ampicillin resistant [Ampr], chloramphenicol resistant [Cmr]) (57), WAF270 (O22 Hlyactive Cmr) (35), and WAH540 (O22 Hlyinactive Cmr) were used in the following studies.
Bacteria were grown in tryptic soy broth at 37°C for 6 or 9 h unless otherwise stated. Ampicillin (100 μg/ml) was added for growing WAF108, and chloramphenicol (20 μg/ml) was added for growing WAM783, WAF108, WAF270, and WAH540. The bacteria were washed, and concentrations were estimated by measuring absorbances at 600 nm (A600) and confirmed by plating on tryptic soy agar with 5% sheep blood overnight (to verify the presence or absence of hemolysis).
WAF108, WAF270, and WAH540, all transformants of J198, are genetically identical except for the presence of slightly modified constructs of the plasmid pSF4000 containing the Hly determinant, hlyCABD (59). The hlyA locus encodes the secreted Hly protein (23, 33), while hlyC encodes posttranslational modifications (covalent fatty acid acylation of lysine residues) activating the protein (28, 32, 37, 54). The hlyB and -D loci encode transport genes facilitating translocation of Hly (7, 41). WAF108 contains the plasmid pSF4000:Tn1 and produces no Hly (Hlynull) (59). The transposon Tn1 is inserted into the hlyA locus. WAF270 contains the complete pSF4000, enabling production and secretion of fully modified Hly (61). WAH540 contains a pSF4000 plasmid in which the only BamHI fragment is deleted (8). This fragment is approximately 2.9 kb in length and includes the first 483 bp of the hlyC locus (633 bp total). This deletion has no effect on the expression of downstream coding segments, since the complete hlyA locus is expressed. WAH540 was constructed by electroporating pSF4000ΔBamHI (isolated from E. coli WAM783 [8] with a plasmid kit [Qiagen, Inc., Chatsworth, Calif.] according to the instructions of the manufacturer) into E. coli J198 at a field strength of 14 kV/cm and a pulse length of 5 ms, as previously described (13, 21). This enabled the production and secretion of full-length, nonacylated Hly (Hlyinactive) by new transformants. Transformants displaying restriction patterns in agreement with the predicted restriction map for pSF4000ΔBamHI (8, 60, 61) were assayed for expression of the Hly gene and hemolytic activity. A single transformant which expressed the highest level of Hlyinactive among the transformants was isolated and denoted WAH540. Linearized (digested) plasmid from WAH540 showed a single band of approximately 12.3 kb, matching the linearized stock plasmid from WAM783. The restriction pattern of the putative E. coli WAH540 matched the predicted restriction map of pSF4000ΔBamHI (8, 60, 61).
Hemolysin gene expression and hemolytic assay.
Culture supernatants of WAF108, WAF270, and WAH540 were clarified with 0.2-μm-pore-size filters and partially purified by ultrafiltration through Centricon 30 centrifugation filters (Amicon, Beverly, Mass.). Bacterial products (Hly and others) were partially purified from 9-h cultures containing 6 × 1010 CFU in 25 cm3 of medium into ending volumes of approximately 500 μl. Partially purified supernatant preparations from equivalently sized cultures were separated by sodium dodecyl sulfate–7.5% polyacrylamide gel electrophoresis. After protein transfer, immunoprobing was performed with the murine anti-Hly MAbs B7 and G8 (gifts of R. A. Welch) as previously described (12, 42). Supernatants from 9-h bacterial cultures were filtered and diluted with 10 mM CaCl2–150 mM NaCl in a microtiter plate. Sheep erythrocytes (RBC) were washed and resuspended to 2% (wt/vol) in CaCl2-saline, and 100 μl was added to each well. Following a 1-h incubation at 37°C, unlysed cells were pelleted and the percent lysis was calculated by measuring the A415 of the supernatant, as previously described (60).
LPS O22 purification and characterization.
Whole bacteria were lyophilized and crushed into powder. By a modified phenol-water extraction method (2), LPS was extracted from each strain. Each purified LPS was lyophilized, and its purity was confirmed by sodium dodecyl sulfate–14% polyacrylamide gel electrophoresis, spectral analysis (A245–290), and agarose gel electrophoresis. The LPS serotype was confirmed by immunoblot analysis with rabbit anti-LPS O22 antiserum (E. coli Reference Center, Pennsylvania State University) as previously described (12, 47). Endotoxin was quantified by a chromogenic Limulus amebocyte lysate (LAL) (BioWhittaker, Inc., Walkersville, Md.) assay according to the instructions of the manufacturer. Whole bacteria (10, 102, 103, or 104 CFU) and culture supernatants from cultures diluted to contain equal amounts of bacteria (as determined by overnight plating) were assayed for total LPS content. Aliquots of these samples were also compared by immunoprobing with rabbit anti-LPS O22 antiserum and by densitometry (12, 34, 47). Endotoxin bioactivities were compared by measuring splenocyte proliferation and TNF-α release. Naive BALB/c murine splenocytes (2 × 105) were stimulated for 24 h with 5 μg of purified LPS O22/ml, derived from WAF108, WAF270, and WAH540 in RPMI–10% fetal calf serum (FCS). The cell cultures were then pulsed with [3H]thymidine (56), and the cells were harvested and counted for β scintillation. Similarly, human peripheral blood mononuclear cells (PBMC) (2 × 105) were stimulated for 6 to 24 h with 1 to 10 ng of LPS O22/ml, and the supernatants were harvested for TNF-α quantitation by enzyme-linked immunosorbent assay (ELISA) with anti-human TNF-α MAb (Pharmingen, San Diego, Calif.) (36).
Bacterium-induced monocyte IL-1β release.
Human PBMC were separated by sodium diatrizoate polysucrose gradient (Histopaque-1077; Sigma), washed, and then incubated with viable E. coli WAF108, WAF270, or WAH540 at bacteria-to-monocyte ratios of 0.04 to 4,000 CFU/monocyte for 90 min at 37°C in RPMI–10% FCS, as previously described (5). Fresh cell supernatant IL-1β determinations were made by a sandwich ELISA technique with anti-human IL-1β MAbs (Endogen, Cambridge, Mass.) according to the manufacturer’s specifications.
Animals.
Male and female BALB/c, C57BL/6 (Taconic, Germantown, N.Y., and Hilltop Lab Animals, Inc., Scottsdale, Pa.), C3H/HeJ (Jackson, Bar Harbor, Maine), and receptor-deficient mice (described below) were housed in a pathogen-free environment and fed lab chow and water ad libitum according to National Research Council standards. All procedures were approved by the University of Virginia Animal Use Committee.
Mice deficient in the expression of either the type 1 IL-1 receptor (IL-1R1 KO) or the type 1 TNF receptor (TNFR1 KO) were generated by gene targeting through homologous recombination in murine embryonic stem cells, as described previously (8, 38, 39). Transgenic homozygous IL-1R1 KO animals were subsequently bred through multiple generations prior to their use in this study. Homozygous IL-1R1 KO and TNFR1 KO animals were mated to produce doubly heterozygous mutant mice. Doubly heterozygous mutants were then mated to produce doubly homozygous mutants, dual (IL-1R1–TNFR1) KOs. All animals used were genotyped for the IL-1R1 and TNFR1 alleles by PCR (39).
Bacterial dose responses, bacterial kinetics, and serum cytokine responses.
BALB/c, C57BL/6, C3H/HeJ, and KO mice were given doses ranging from 107 to 5 × 109 CFU of E. coli WAF108, WAF270, or WAH540 in 0.15 M NaCl i.p. The 50% lethal dose (LD50) and LD100 for each transformant (calculated by the Reed-Muench method [62]) were determined for BALB/c and C57BL/6 mice. To determine the LD100 of endotoxin alone (independent of other bacterial products), BALB/c mice were challenged i.p. with doses of 1 to 60 mg of LPS O22/kg of body weight. In vivo growth-elimination kinetics were then established by injecting i.p. a sublethal dose (107 CFU) and a lethal dose (108 CFU for WAF270 or 3 × 109 CFU for WAF108 and WAH540). Diluents of homogenized lung, liver, spleen, and kidney tissue and both blood and peritoneal lavage fluid were collected from the animals 4 and 18 h after injection and plated on blood agar with the respective antibiotic. When indicated, serum samples for cytokine determinations were obtained from the mice at 90 min and 5 and 10 h after bacterial challenge. Serum samples were stored at −80°C until being assayed. Serum IL-1β and TNF-α levels were determined with ELISA kits (Genzyme Diagnostics, Cambridge, Mass.) according to the instructions of the manufacturer.
IL-1 receptor blockade prior to bacterial challenge.
BALB/c and C57BL/6 mice were treated intravenously with 200 μg of hamster anti-mouse monoclonal immunoglobulin G (IgG) anti-IL-1R1 antibody (M147; a gift of J. Sims, Immunex Corp.) (46, 57) or 200 μg of Syrian hamster anti-mouse IgG (Jackson ImmunoResearch Laboratories, Inc., West Grove, Pa.) in 100 μl of phosphate-buffered saline–10% murine serum 2 h prior to bacterial infection. The dose used to block IL-1R1 in vivo was based on the work of Rogers et al., who used the identical antibody in a similar murine system (46). The mice were then inoculated i.p. with 5 × 108 CFU of WAF108, WAF270, or WAH540.
Statistics.
Parametric data were compared by analysis of variance (ANOVA) and then post hoc by Tukey’s honestly significant difference tests. Nonparametic data were compared by χ2 analyses followed by Pearson χ2 and Fisher exact tests, with Bonferroni corrections for multiple comparisons. Survival statistics were also compared by product-limit (Kaplan-Meier) analysis when applicable.
RESULTS
Characterization of E. coli WAH540 and nonacylated Hlyinactive.
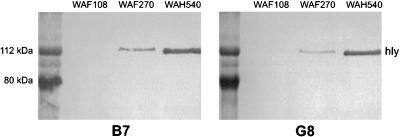
All transformants had nearly equivalent growth kinetics in vitro. WAF270, but not WAF108 or WAH540, displayed significant hemolytic activity on blood agar cultures. Quantitative hemolytic assays confirmed WAF270’s hemolytic activity (100 and 10% RBC lysis occurred at 16- and 2,096-fold supernatant dilutions, respectively). WAF108 and WAH540 supernatants caused no RBC lysis. Immunoblots of bacterial culture supernatants revealed Hly (active or inactive) expression from WAF270 and WAH540 but none from WAF108, as predicted (Fig. 1). WAH540 generated more Hlyinactive per CFU of bacteria than WAF270 generated Hlyactive. The relative amount of LPS O22 expression by these bacterial transformants correlated with the amount of Hly expressed. Chromogenic quantitative LAL assays confirmed that E. coli WAH540 produces more LPS per CFU than WAF270, which produces more than WAF108: WAH540, WAF270, and WAF108 contained 0.95 (±0.02), 0.76 (±0.01), and 0.58 (±0.02) pg of LPS/102 CFU, respectively, and released 0.27 (±0.07), 0.08 (±0.003), and 0.02 (±0.0001) pg/ml of diluted supernatant, respectively (P ≤ 0.002 by ANOVA and post hoc analyses). Densitometric analysis of LPS immunoblots (targeting the O22 polysaccharide) of whole bacteria and supernatants from equivalently sized cultures confirmed these relative differences in LPS expression (Fig. 2). The in vitro cellular stimulatory effects of the LPS produced by the three transformants were equivalent, as measured by the proliferative capacity of murine splenocytes ([3H]thymidine uptake [data not shown]) and TNF-α release of human PBMC (Fig. 3). In vitro exposure of human PBMC to live WAF270 elicited markedly higher levels of IL-1β secretion than exposure to live WAF108 or WAH540 (Fig. 4); this is similar to what other hemolytic E. coli strains have been shown to elicit (5).
FIG. 1.
Immunoblots of filtered culture supernatants of WAF108, WAF270, and WAH540 with two MAbs against Hly, B7 and G8. Protein mass markers appear on the left in each blot.
FIG. 2.
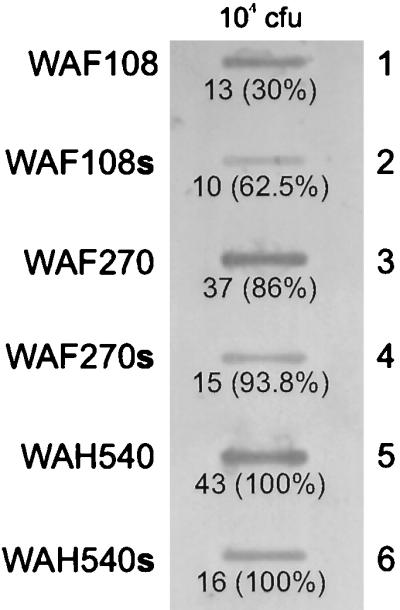
Semiquantitative LPS immunoblot with rabbit anti-LPS O22 antiserum of 104 CFU of whole E. coli cells (rows 1, 3, and 5) and supernatants (s) from equivalently sized E. coli cultures diluted 1:104 (rows 2, 4, and 6). The relative densitometric values (and percentages) are shown below each blot; 100% corresponds to the blot of whole bacterial or supernatant samples (WAH540 and WAH540s) with the highest density, to which the others are compared.
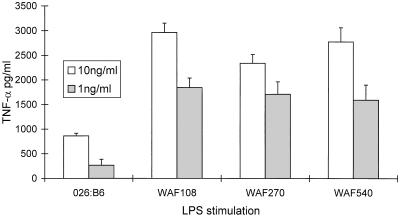
FIG. 3.
Supernatant TNF-α ELISA. Human PBMC (2 × 105, in triplicate) were stimulated for 24 h in RPMI-10% FCS with 1 or 10 ng of purified LPS O26:B6 or LPS O22/ml, purified from E. coli WAF108, WAF270, or WAH540, and the supernatants were assayed for TNF-α. Error bars indicate significant errors of the means. There were no differences in TNF-α secretion between transformants when the cells were stimulated for less time (i.e., 6 to 12 h).
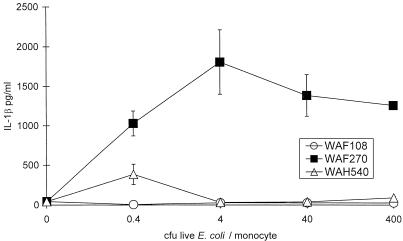
FIG. 4.
Human monocyte IL-1β release in response to viable E. coli mutants after 90 min of exposure in vitro. Note that WAF270 induced significantly higher levels of IL-1β secretion at all ratios of bacteria to monocytes. P ≤ 0.0003 by ANOVA; error bars indicate standard errors of the means.
Animal studies.
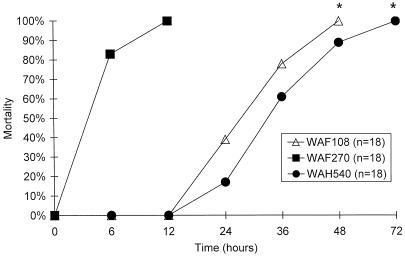
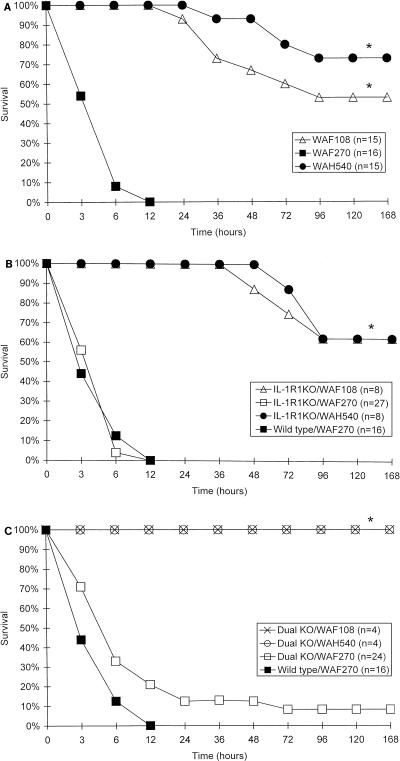
BALB/c and C57BL/6 mice responded similarly to each E. coli transformant (WAF108, WAF270, and WAH540). E. coli WAF270 was significantly more lethal than WAF108 and WAH540 when given at equal doses over the range of 5 × 107 to 5 × 109 CFU. The LD50 and LD100 were estimated at 6.5 (±1) × 107 and 1.1 (±0.5) × 108 CFU for WAF270, 7 (±1.5) × 108 and 3 (±1.5) × 109 CFU for WAF108, and 8 (±1.5) × 108 and 4 (±1.5) × 109 CFU for WAH540, respectively. A dose of 108 CFU of WAF270 was 100% lethal, but 108 CFU of WAF108 or WAH540 was nonlethal (0%). At their respective lethal doses, WAF270 caused death significantly more rapidly than WAF108 and WAH540 (Fig. 5). WAF270 was also lethal at significantly lower doses than WAF108 or WAH540 in C3H/HeJ mice: e.g., 5 × 108 CFU of WAF270 was 100% lethal, while the same dose of WAF108 or WAH540 was nonlethal (n = 8 to 10/group). Challenge (i.p.) with purified LPS O22 caused 100% mortality at a dose of 40 mg/kg 48 h after injection. The amount of LPS contained within an LD100 inoculum of WAF270, determined as described above by the LAL assay, was 0.76 μg/108 CFU, representing an initial LPS load over 103-fold less than the LD100 of purified LPS O22. Four or 18 h after lethal or sublethal bacterial challenge, the amount of live bacteria recoverable from the peritoneum, lung, liver, kidney, spleen, and blood was equivalent for all three E. coli transformants (1 to 10% recovery relative to the initial inoculum, depending on the organ site).
FIG. 5.
Times of death after i.p. infection with LD100 of WAF108, WAF270, or WAH540 for C57BL/6 mice. n, number per group; P = 0.000001 by Kaplan-Meier analysis.
Bacterial challenge with each E. coli transformant elicited significant TNF-α secretion by 90 min after challenge (Table 1) in a manner similar to that caused by LPS O22 when given alone (data not shown). Serum IL-1β was detectable by 90 min in mice challenged with WAF108, WAF270, or WAH540. The serum IL-1β level decreased by 5 h in WAF108- and WAH540-challenged animals but increased in WAF270-challenged animals (Table 1).
TABLE 1.
Serum cytokine levels in wild-type (C57BL/6) mice after E. coli challengea
| E. coli strain | Serum TNF-α level (pg/ml)
|
Serum IL-1β level (pg/ml)
|
||
|---|---|---|---|---|
| 90 min | 5 h | 90 min | 5 h | |
| WAF108 | 4,670 ± 790 (5)* | 1,630 ± 200 (5)* | 830 ± 200 (3) | 290 ± 100 (3)† |
| WAF270 | 3,910 ± 520 (4) | 1,190 ± 160 (2) | 1,170 ± 200 (2) | 1,610 ± 790 (2)† |
| WAH540 | 4,570 ± 370 (5)* | 1,040 ± 140 (5)* | 700 ± 70 (3) | 510 ± 100 (4) |
C57BL/6 mice were challenged i.p. with 5 × 108 CFU of WAF108, WAF270, or WAH540. The data are given as means ± standard errors of the means, with the number of mice tested per group in parentheses. Each serum sample was tested in duplicate. TNF-α and IL-1β levels were significantly different among groups, as compared by ANOVA (P < 0.000001 and P < 0.04, respectively). (Post hoc honestly significant difference tests revealed significant differences between certain groups: ∗, comparing 90-min and 5-h time points; †, comparing bacterial strains.)
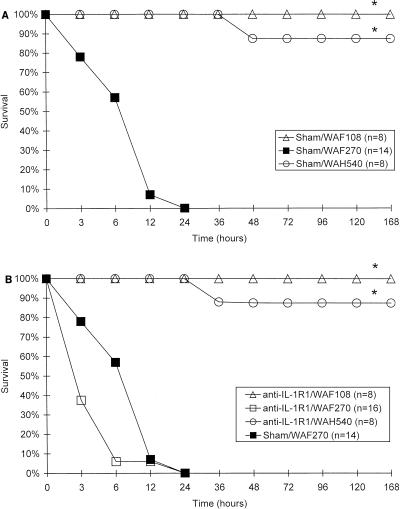
Anti-IL-1R1 MAb did not alter the lethality of E. coli WAF270 (Fig. 6). Similarly, WAF270 was significantly more lethal than WAF108 and WAH540 in both IL-1R1 and dual-KO mice at the same doses, as was demonstrated in wild-type mice (Fig. 7). There was no significant difference between the lethalities of individual bacterial transformants (WAF108, WAF270, or WAH540) in IL-1R1 KO mice and those in matched wild-type mice at doses from 7 × 107 to 5 × 108 CFU; there were only slight differences between lethalities in dual (IL-1R1–TNFR1)-KO mice challenged with WAF108 or WAH540 and those in matched wild-type mice at these doses.
FIG. 6.
Survival curves as a function of time following i.p. bacterial challenge (5 × 108 CFU) in wild-type (C57BL/6) mice pretreated with anti-mouse IgG (Sham) (A) or anti-IL-1R1 MAb (B). n, number/group; ∗, P < 0.000001 and α < 0.0001, by χ2 analysis and post hoc Fisher exact tests, respectively. Note that the abscissas are not on a linear scale.
FIG. 7.
Survival curves as a function of time following i.p. bacterial challenge (5 × 108 CFU) in wild-type (A), IL-1R1 KO (B), and dual (IL-1R1–TNFR1)-KO (C) mice with WAF108, WAF270, or WAH540. n, number per group; ∗, P < 0.000001 and α < 0.0001, by χ2 analysis and post hoc Fisher exact tests, respectively. Note that the abscissas are not on a linear scale.
DISCUSSION
Investigators continue to develop simple models to explain bacterial pathogenesis, but it is becoming clearer that host-bacterial interactions are extraordinarily complex, so that attempts at targeting human therapies toward specific effector molecules (e.g., TNF and IL-1) or bacterial products (e.g., LPS) within the framework of these paradigms have been discouraging (26, 44, 48, 58, 63). We postulate that in order for these types of approaches to be effective the therapies will need to be multitargeted and relatively specific to the type of pathogen. Hly is another potential target. It is well characterized biochemically and has a wide range of effects both in vivo and in vitro, including lysis of erythrocytes, fibroblasts, leukocytes, and epithelial and endothelial cells (14, 15, 53); cellular ATP depletion (5); promotion of superoxide anion release (4); and inhibition of macrophage antigen presentation (18). In this study we have increased our understanding of the pathogenesis of hemolytic E. coli infections.
We demonstrate that fatty acid acylation of Hly is necessary in order for the live hemolytic E. coli transformant, WAF270, to elicit IL-1β from monocytes in vitro, to cause hemolysis, and to enhance its virulence in vivo. The two nonhemolytic E. coli transformants, WAF108 and WAH540, which produce no Hly (Hlynull) and nonacylated Hly (Hlyinactive), respectively, required doses more than 10-fold that of WAF270, which produces fully acylated Hly (Hlyactive), in order to induce mortality. The WAF270 transformant elicited an IL-1β (and an IL-1α [35]) secretion pattern both in vivo and via monocytes in vitro that was markedly different from that elicited by either WAF108 or WAH540. The hemolytic transformant elicited high levels of IL-1, while the nonhemolytic strains elicited only low levels in patterns similar to those elicited by endotoxin challenge. Hlyactive (from WAF270) and Hlyinactive (from WAH540) had identical electrophoretic properties and were recognized by two anti-Hly MAbs which bind to distinct epitopes of Hly, although Hlyinactive is nonhemolytic and Hlyactive is very hemolytic. Therefore, we conclude that fatty acid acylation of Hly via expression of the hlyC locus is critical to the function of Hly and the virulence of hemolytic E. coli.
Demonstrating the lethal potential of purified Hly alone has been difficult due to its instability ex vivo and its association with LPS. In prior studies, where we compared the lethal potential of partially purified culture supernatants from WAF108, WAF270, and WAH540 cultures, WAF270 supernatant appeared to be more lethal than the others (unpublished results). In the present study we recognized that concentrates of the culture supernatants contain large amounts of LPS bound to Hly. The partial purification process itself reduced the hemolytic activity of the preparations and probably changed the conformation of Hly (9, 10, 20, 55). Thus, it is likely that Hlyactive or Hlyinactive is expressed in conjunction with LPS in the respective transformant, WAF270 or WAH540.
We have demonstrated that these transformants express antigenically and functionally identical endotoxins, but they express different quantities of LPS. It appears that the amount of LPS expressed by a transformant correlates with the relative differences in Hlynull, Hlyactive, or Hlyinactive production by the transformant. In other words, WAH540 expresses the most Hlyinactive and consequently the most LPS. This was confirmed by both LAL and immunoblot assays. Despite the association between Hly and LPS expression, we demonstrated that the release of Hly-associated LPS does not seem to be primarily responsible for the enhanced lethality of Hly-producing E. coli (WAF270), since WAH540 (which expresses more LPS) is actually considerably less virulent than WAF270 and equivalent in virulence to WAF108 (which expresses the least LPS). The LPS expressed by each transformant has the same stimulatory effect in vitro, and thus, their endotoxins seem equally potent. Furthermore, at their respective LD100s, an inoculum of WAF270 contains markedly less LPS O22 than does an inoculum of WAF108 or WAH540 (0.76 μg versus 5.8 or 9.5 μg, respectively). At equal doses of 108 CFU/mouse in which the initial inoculum of WAF270, WAF108, or WAH540 contained similar amounts of LPS (0.76, 0.58, and 0.95 μg, respectively), only challenge with WAF270 caused mortality. Additionally, equivalent amounts of each specific transformant were recoverable from the lung, spleen, liver, kidney, blood, and peritoneum during the acute phase of a lethal infection, suggesting that the transformants have similar in vivo growth-elimination kinetics, but only WAF270 caused early mortality (<8 h). WAF108 and WAH540 caused late mortality (36 to 72 h), similar to the lethality induced by challenge with LPS O22 alone (48 h). Finally, endotoxin-tolerant C3H/HeJ mice responded to WAF270 in a manner similar to that of the endotoxin-sensitive strains (BALB/c and C57BL/6). Collectively, these data suggest that LPS is not the primary factor responsible for hemolytic WAF270-induced lethality at LD100 or less. The increased lethality of WAF270 appears to be directly related to the expression of acylated Hly.
Both IL-1 and TNF-α have detrimental effects which correlate with the severity of certain types of infections in animal models. However, they do not appear to be critical mediators of the lethality of hemolytic E. coli infection in our model. Because of the associated IL-1α spike recognized during WAF270 infection (35), we initially hypothesized that IL-1 signaling (with or without TNF signaling) was the effector mechanism mediating the lethality of hemolytic E. coli. In the present study we show that WAF270 challenge similarly elicited a sustained level of IL-1β secretion. However, we demonstrate that neither preventing IL-1 signaling with IL-1R1 MAb blockade in wild-type mice nor challenging IL-1R1-deficient mice altered the lethality of hemolytic E. coli (WAF270). The lethality profiles of WAF108 and WAH540 were also similar in wild-type and KO animals. Thus, mortality from any of the E. coli transformants does not appear to be mediated via signaling through IL-1R1.
Significant TNF-α secretion was also demonstrated after challenge with either WAF108, WAF270, or WAH540 at 90 min, as would be expected. These elevated serum TNF-α levels decreased by 5 h after challenge. In our model, TNF’s role in Hly-producing E. coli challenges did not appear to be different than in non-Hly-producing E. coli challenges, because WAF108, WAF270, and WAH540 all elicited similar TNF-α secretory profiles. TNF-α and -β antibody blockade previously failed to alter WAF270-induced mortality (35), and now we demonstrate that dually (IL-1R1–TNFR1) deficient mice were not protected from WAF270-induced mortality. Collectively, the results of these studies support the notion that there is no correlation between IL-1 or TNF signaling and hemolytic E. coli WAF270-induced mortality.
The mechanism by which Hly leads to enhanced lethality of E. coli remains ill defined. It is now clear, however, that fatty acid acylation of the lysine residues of Hly, via expression of the hlyC locus, is absolutely critical to facilitating both its toxicity and its rendering of IL-1 secretion. It is perplexing that despite the correlation between Hly expression and IL-1 secretion, the prevention of IL-1 and/or TNF signaling does not protect animals from acute hemolytic E. coli infection as it has in other models of nonhemolytic E. coli infection (1) or Listeria infection (46). Necropsy studies of animals infected with WAF270 have not been helpful in identifying a cause of death, demonstrating only a mild-to-moderate hepatic infiltration of neutrophils (unpublished results).
We have learned that animals infected with WAF270 and a sterile stool adjuvant actually recruit significantly fewer leukocytes to the peritoneum during WAF270 infection than during WAF108 infection. A much greater percentage of the leukocytes that are recovered from WAF270-infected animals are nonviable compared to those isolated from WAF108-infected animals (unpublished data). Thus, the mechanism by which Hly enhances the lethality of E. coli may be explained, in part, by its effects on neutrophil recruitment, degranulation, and cell viability. In vitro studies corroborate the hypothesis that Hly has profound effects on neutrophils (e.g., increased granule formation [3], increased neutrophilic chemiluminescence [16], increased release of toxic free radicals [4], and diminished bactericidal activity of neutrophils [50]), although a relationship between Hly’s effect on neutrophils and Hly-induced mortality remains to be determined. Finally, we have demonstrated that pretreatment with killed bacteria well before the generation of an anamnestic response (as early as 2 days before live bacterial challenge) completely protects animals from an otherwise-lethal WAF270 challenge, again suggesting that upregulating nonspecific immune responses (e.g., neutrophilic recruitment and function) may protect against this rapidly fatal bacterial infection (25). Collectively, these studies provoke the suspicion that nonspecific immune mechanisms like granulocyte recruitment and effector functions may be particularly relevant during hemolytic E. coli infections, and they support the notion that the deleterious effects of hemolysin and hemolytic bacteria may be preventable or suppressible in clinical settings.
ACKNOWLEDGMENTS
T. G. Gleason was supported by Public Health Service, National Research Service Award 1F32AI09482-01A1.
E. coli J198, WAF108, and WAF270 and anti-Hly MAbs B7 and G8 were kindly provided, with technical guidance, by R. A. Welch (University of Wisconsin). E. coli WAM783 was a gift of E. L. Hewlett (University of Virginia). Murine IL-1R1 MAb (MAb M147) and rabbit anti-LPS O22 antiserum were kindly provided by J. Sims (Immunex Corp., Seattle, Wash.) and the E. coli Reference Center (Pennsylvania State University), respectively. We also thank C. Hahn for his technical guidance on the bacterial electroporation techniques described.
REFERENCES
- 1.Acton R D, Dahlberg P S, Uknis M E, Klaerner G, Fink G S, Norman J G, Dunn D L. Differential sensitivity to Escherichia coli infection in mice lacking tumor necrosis factor p55 or interleukin-1 p80 receptors. Arch Surg. 1996;131:1216–1221. doi: 10.1001/archsurg.1996.01430230098017. [DOI] [PubMed] [Google Scholar]
- 2.Apicella M A, Griffiss J M, Schneider H. Isolation and characterization of lipopolysaccharides, lipooligosaccharides, and lipid A. Methods Enzymol. 1994;235:242–252. doi: 10.1016/0076-6879(94)35145-7. [DOI] [PubMed] [Google Scholar]
- 3.Bhakdi S, Greulich S, Muhly M, Eberspacher B, Becker H, Thiele A, Hugo F. Potent leukocidal action of Escherichia coli hemolysin mediated by permeabilization of target cell membranes. J Exp Med. 1989;169:737–754. doi: 10.1084/jem.169.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhakdi S, Martin E. Superoxide generation by human neutrophils induced by low doses of Escherichia coli hemolysin. Infect Immun. 1991;59:2955–2962. doi: 10.1128/iai.59.9.2955-2962.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhakdi S, Muhly M, Korom S, Schmidt G. Effects of Escherichia coli hemolysin on human monocytes, cytocidal action and stimulation of interleukin-1 release. J Clin Invest. 1990;85:1746–1753. doi: 10.1172/JCI114631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blanco M, Blanco J E, Alonso M P, Blanco J. Virulence factors and O groups of Escherichia coli strains isolated from cultures of blood specimens from urosepsis and non-urosepsis patients. Microbiologia. 1994;10:249–256. [PubMed] [Google Scholar]
- 7.Blight M A, Holland I B. Structure and function of haemolysin B, P-glycoprotein and other members of a novel family of membrane translocators. Mol Microbiol. 1990;4:873–880. doi: 10.1111/j.1365-2958.1990.tb00660.x. [DOI] [PubMed] [Google Scholar]
- 8.Boehm D F, Welch R A, Snyder I S. Domains of Escherichia coli hemolysin (HlyA) involved in binding of calcium and erythrocyte membranes. Infect Immun. 1990;58:1959–1964. doi: 10.1128/iai.58.6.1959-1964.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bohach G A, Snyder I S. Chemical and immunological analysis of the complex structure of Escherichia coli alpha-hemolysin. J Bacteriol. 1985;164:1071–1080. doi: 10.1128/jb.164.3.1071-1080.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bohach G A, Snyder I S. Composition of affinity-purified alpha-hemolysin of Escherichia coli. Infect Immun. 1986;53:435–437. doi: 10.1128/iai.53.2.435-437.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brauner A, Katouli M, Ostenson C G. P-fimbriation and haemolysin production are the most important virulence factors in diabetic patients with Escherichia coli bacteraemia: a multivariate statistical analysis of seven bacterial virulence factors. J Infect. 1995;31:27–31. doi: 10.1016/s0163-4453(95)91271-1. [DOI] [PubMed] [Google Scholar]
- 12.Burnette W N. Western blotting: electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981;112:195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- 13.Calvin N M, Hanawalt P C. High-efficiency transformation of bacterial cells by electroporation. J Bacteriol. 1988;170:2796–2801. doi: 10.1128/jb.170.6.2796-2801.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cavalieri S J, Bohach G A, Snyder I S. Escherichia coli alpha-hemolysin: characteristics and probable role of pathogenicity. Microbiol Rev. 1984;48:326–343. doi: 10.1128/mr.48.4.326-343.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cavalieri S J, Snyder I S. Cytotoxic activity of partially purified Escherichia coli alpha haemolysin. J Med Microbiol. 1982;15:11–21. doi: 10.1099/00222615-15-1-11. [DOI] [PubMed] [Google Scholar]
- 16.Cavalieri S J, Snyder I S. Effect of Escherichia coli alpha-hemolysin on human peripheral leukocyte function in vitro. Infect Immun. 1982;37:966–974. doi: 10.1128/iai.37.3.966-974.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. National Nosocomial Infections Surveillance (NNIS) report, data summary from Oct. 1986–Apr. 1996, issued May 1996. A report from the National Nosocomial Infections Surveillance (NNIS) System. Am J Infect Control. 1996;24:380–388. [PubMed] [Google Scholar]
- 18.Cluff C W, Garcia M, Ziegler H K. Intracellular hemolysin-producing Listeria monocytogenes strains inhibit macrophage-mediated antigen processing. Infect Immun. 1990;58:3601–3612. doi: 10.1128/iai.58.11.3601-3612.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colotta F, Saccani S, Giri J G, Dower S K, Sims J E, Introna M, Mantovani A. Regulated expression and release of the IL-1 decoy receptor in human mononuclear phagocytes. J Immunol. 1996;156:2534–2541. [PubMed] [Google Scholar]
- 20.Czuprynski C J, Welch R A. Biologic effects of RTX toxins: the possible role of lipopolysaccharide. Trends Microbiol. 1995;3:480–483. doi: 10.1016/s0966-842x(00)89016-2. [DOI] [PubMed] [Google Scholar]
- 21.Dower W W J, Miller J F, Ragsdale C W. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 1988;16:6127–6145. doi: 10.1093/nar/16.13.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fantuzzi G, Zheng H, Faggioni R, Benign F, Ghezzi P, Sipe J D, Shaw A R, Dinarello C A. Effect of endotoxin in IL-1β-deficient mice. J Immunol. 1996;157:291–296. [PubMed] [Google Scholar]
- 23.Felmlee T, Pellet S, Welch R A. Nucleotide sequence of an Escherichia coli chromosomal hemolysin. J Bacteriol. 1985;163:94–105. doi: 10.1128/jb.163.1.94-105.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giri J G, Wells J, Dower S K, McCall C E, Guzman R N, Slack J, Bird T A, Shanebeck K, Grabstein K H, Sims J E, Alderson M R. Elevated levels of shed type II IL-1 receptor in sepsis. Potential role for type II receptor in regulation of IL-1 responses. J Immunol. 1994;153:5802–5809. [PubMed] [Google Scholar]
- 25.Gleason T G, Sawyer R G, Houlgrave C W, Pruett T L. Owen H. Wangensteen Surgical Forum of the 83rd Annual Clinical Congress 1997. Chicago, Ill: American College of Surgeons; 1997. Killed bacterial preparations protect against live bacterial infection better than endotoxin: tolerance is not unique to endotoxin; pp. 67–70. [Google Scholar]
- 26.The HA-1A Sepsis Study Group. Treatment of gram-negative bacteremia and septic shock with HA-1A human monoclonal antibody against endotoxin: a randomized, double-blind placebo-controlled trial. N Engl J Med. 1991;324:429–436. doi: 10.1056/NEJM199102143240701. [DOI] [PubMed] [Google Scholar]
- 27.Ikaheimo R, Siitonen A, Karkkainen U, Makela P H. Virulence characteristics of Escherichia coli in nosocomial urinary tract infection. Clin Infect Dis. 1993;16:785–791. doi: 10.1093/clind/16.6.785. [DOI] [PubMed] [Google Scholar]
- 28.Issartel J-P, Koronakis V, Hughes C. Activation of Escherichia coli prohaemolysin to the mature toxin by acyl carrier protein-dependent fatty acylation. Nature. 1991;351:759–761. doi: 10.1038/351759a0. [DOI] [PubMed] [Google Scholar]
- 29.Korhonen T K, Valtonen M V, Parkkinen J, Vaisanen-Rhen V, Finne J, Orskov F, Orskov I, Svenson S B, Makela P H. Serotypes, hemolysin production, and receptor recognition of Escherichia coli strains associated with neonatal sepsis and meningitis. Infect Immun. 1985;48:486–491. doi: 10.1128/iai.48.2.486-491.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li P, Allen H, Banerjee S, Franklin S, Herzog L, Johnston C, McDowell J, Paskind M, Rodman L, Salfeld J, Twone E, Tracey D, Warwell S, Wei F-Y, Wong W, Kamen R, Seshadri T. Mice deficient in IL-1b-converting enzyme are defective in production of mature IL-1b and resistant to endotoxic shock. Cell. 1995;80:401–411. doi: 10.1016/0092-8674(95)90490-5. [DOI] [PubMed] [Google Scholar]
- 31.Lobo A L, Welch R A. Identification and assay of RTX family of cytolysins. Methods Enzymol. 1994;235:667–678. doi: 10.1016/0076-6879(94)35180-5. [DOI] [PubMed] [Google Scholar]
- 32.Ludwig A, Garcia F, Bauer S, Jarchau T, Benz R, Hoppe J, Goebel W. Analysis of the in vivo activation of hemolysin (HlyA) from Escherichia coli. J Bacteriol. 1996;178:5422–5430. doi: 10.1128/jb.178.18.5422-5430.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mackman N, Nicaud J-M, Gray L, Holland I B. Genetical and functional organization of the Escherichia coli haemolysin determinant 2001. Mol Gen Genet. 1985;201:282–288. doi: 10.1007/BF00425672. [DOI] [PubMed] [Google Scholar]
- 34.Malek A M, Izumo S, Alper S L. Quantitative densitometric analysis using a commercially available handheld CCD digital camera. BioTechniques. 1997;22:1150–1153. [PubMed] [Google Scholar]
- 35.May A K, Sawyer R G, Gleason T, Whitworth A, Pruett T L. In vivo cytokine response to Escherichia coli alpha-hemolysin determined with genetically engineered hemolytic and nonhemolytic E. coli variants. Infect Immun. 1996;64:2167–2171. doi: 10.1128/iai.64.6.2167-2171.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Molloy R G, O’Riordain M, Holzheimer R, Nestor M, Collins K, Mannick J A, Rodrick M L. Mechanisms of increased tumor necrosis factor after thermal injury. J Immunol. 1993;151:2142–2149. [PubMed] [Google Scholar]
- 37.Nicaud J-M, Mackman N, Gray L, Holland I B. Characterization of HlyC and mechanism of activation and secretion of hemolysin from E. coli 2001. FEBS Lett. 1985;187:339–344. doi: 10.1016/0014-5793(85)81272-2. [DOI] [PubMed] [Google Scholar]
- 38.Norman J G, Fink G, Franz M, Guffey J, Carter G, Davison B, Sexton C, Glaccum M. Active interleukin-1 receptor required for maximal progression of acute pancreatitis. Ann Surg. 1996;223:163–169. doi: 10.1097/00000658-199602000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Norman J G, Fink G W, Sexton C, Carter G. Transgenic animals demonstrate a role for the IL-1 receptor in regulating IL-1beta gene expression at steady-state and during systemic stress induced by acute pancreatitis. J Surg Res. 1996;63:231–236. doi: 10.1006/jsre.1996.0253. [DOI] [PubMed] [Google Scholar]
- 40.Oldenburg H S A, Pruitt J H, Lazarus D D, Rogy M A, Chizzonite R, Lowry S F, Moldawer L L. Interleukin 1 binding to its type I, but not type II receptor, modulates the in vivo acute phase response. Cytokine. 1995;7:510–516. doi: 10.1006/cyto.1995.0069. [DOI] [PubMed] [Google Scholar]
- 41.Oropeza-Wekerle R L, Speth W, Imhof B, Gentschev I, Goebel W. Translocation and compartmentalization of Escherichia coli hemolysin (HlyA) J Bacteriol. 1990;172:3711–3717. doi: 10.1128/jb.172.7.3711-3717.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pellett S, Boehm D F, Snyder I S, Rowe G, Welch R A. Characterization of monoclonal antibodies against the Escherichia coli hemolysin. Infect Immun. 1990;58:822–827. doi: 10.1128/iai.58.3.822-827.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pfeffer K, Matsuyama T, Kundig T M, Wakeman A, Kishihara K, Shahinian A, Wiegmann K, Ohashi P S, Knonke M, Mak T W. Mice deficient for the 55kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell. 1993;73:457–467. doi: 10.1016/0092-8674(93)90134-c. [DOI] [PubMed] [Google Scholar]
- 44.Phase III rhIL-1ra Sepsis Syndrome Study Group. Recombinant human interleukin-1 receptor antagonist in the treatment of patients with sepsis syndrome: results from a randomized, double-blind, placebo-controlled trial. JAMA. 1994;271:1836–1843. . (Erratum, 272:1170, 1994.) [PubMed] [Google Scholar]
- 45.Pruitt J H, Welborn M B, Edwards P D, Harward T R, Seeger J W, Martin T D, Smith C, Kenney J A, Wesdorp R I, Meijer S, Cuesta M A, Abouhanze A, Copeland III E M, Giri J, Sims J E, Moldawer L L, Oldenburg H S. Increased soluble interleukin-1 type II receptor concentrations in postoperative patients and in patients with sepsis syndrome. Blood. 1996;87:3282–3288. [PubMed] [Google Scholar]
- 46.Rogers H W, Sheehan K C F, Brunt L M, Dower S K, Unanue E R, Schreiber R D. Interleukin 1 participates in the development of anti-Listeria responses in normal and SCID mice. Proc Natl Acad Sci USA. 1992;89:1011–1015. doi: 10.1073/pnas.89.3.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sandhu G S, Eckloff B W, Kline B C. Chemiluminescent substrates increase sensitivity of antigen detection in Western blots. BioTechniques. 1991;11:14–16. [PubMed] [Google Scholar]
- 48.Saravolatz L D, Wherry J C, Spooner C, Markowitz N, Allfred R, Remick K, Fournel M, Pennington J E. Clinical safety, tolerability and pharmacokinetics of murine monoclonal antibody to human tumor necrosis factor alpha. J Infect Dis. 1994;169:214–217. doi: 10.1093/infdis/169.1.214. [DOI] [PubMed] [Google Scholar]
- 49.Saxen H, Tarkka E, Hannikainen P, Nikku R, Rautio M, Siitonen A. Escherichia coli and appendicitis: phenotypic characteristics of E. coli isolates from inflamed and noninflamed appendices. Clin Infect Dis. 1996;23:1038–1042. doi: 10.1093/clinids/23.5.1038. [DOI] [PubMed] [Google Scholar]
- 50.Siegried L, Puzova H, Kmetova M, Kerestesova A. Killing of alpha-haemolytic and non-haemolytic Escherichia coli strains in human serum and polymorphonuclear leucocytes. J Med Microbiol. 1992;37:3–7. doi: 10.1099/00222615-37-1-3. [DOI] [PubMed] [Google Scholar]
- 51.Siitonen A, Takala A, Ratiner Y A, Pere A, Makela P H. Invasive Escherichia coli infections in children: bacterial characteristics in different age groups and clinical entities. Pediatr Infect Dis. 1993;12:606–612. doi: 10.1097/00006454-199307000-00012. [DOI] [PubMed] [Google Scholar]
- 52.Sims J E, Gayle M A, Slack J L, Alderson M R, Bird T A, Giri J G, Colotta F, Re F, Mantovani A, Shanebeck K, Grabstein K H, Dower S K. Interleukin 1 signaling occurs via the type I receptor. Proc Natl Acad Sci USA. 1993;90:6155–6159. doi: 10.1073/pnas.90.13.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Snyder I S, Koch N A. Production and characteristics of hemolysins of Escherichia coli. J Bacteriol. 1966;91:763–767. doi: 10.1128/jb.91.2.763-767.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stanley P, Packman L, Koronakis V, Hughes C. Fatty acylation of two internal lysine residues required for the toxic activity of Escherichia coli hemolysin. Science. 1994;266:1992–1996. doi: 10.1126/science.7801126. [DOI] [PubMed] [Google Scholar]
- 55.Stanley P L, Diaz P, Bailey M J, Gygi D, Juarez A, Hughes C. Loss of activity in the secreted form of Escherichia coli haemolysin caused by an rfaP lesion in core lipopolysaccharide assembly. Mol Microbiol. 1993;10:781–787. doi: 10.1111/j.1365-2958.1993.tb00948.x. [DOI] [PubMed] [Google Scholar]
- 56.Strong D M, Ahmed A A, Thurman G B, Sell K W. In vitro stimulation of murine spleen cells using a microculture system and a multiple automated sample harvester. J Immunol Methods. 1973;2:279–287. doi: 10.1016/0022-1759(73)90054-9. [DOI] [PubMed] [Google Scholar]
- 57.Theodos C M, Shankar A, Glasebrook A L, Roeder W D, Titus R G. The effect of treating with anti-interleukin-1 receptor antibody on the course of experimental murine cutaneous leishmaniasis. Parasite Immunol. 1994;16:571–577. doi: 10.1111/j.1365-3024.1994.tb00312.x. [DOI] [PubMed] [Google Scholar]
- 58.The TNF-α Mab Sepsis Study Group. Efficacy and safety of monoclonal antibody to human tumor necrosis factor α in patients with sepsis syndrome: a randomized, controlled, double-blind, multicenter clinical trial. JAMA. 1995;273:934–941. [PubMed] [Google Scholar]
- 59.Welch R A, Dellinger E P, Minshew B, Falkow S. Haemolysin contributes to virulence of extra-intestinal E. coli infections. Nature. 1981;294:665–667. doi: 10.1038/294665a0. [DOI] [PubMed] [Google Scholar]
- 60.Welch R A, Falkow S. Characterization of Escherichia coli hemolysins conferring quantitative differences in virulence. Infect Immun. 1984;43:156–160. doi: 10.1128/iai.43.1.156-160.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Welch R A, Hull R, Falkow S. Molecular cloning and physical characterization of a chromosomal hemolysin from Escherichia coli. Infect Immun. 1983;42:178–186. doi: 10.1128/iai.42.1.178-186.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Welkos S, O’Brien A. Determination of median lethal and infectious doses in animal model systems. Methods Enzymol. 1994;235:29–39. doi: 10.1016/0076-6879(94)35128-7. [DOI] [PubMed] [Google Scholar]
- 63.The XOMA Sepsis Study Group. A controlled clinical trial of E5 murine monoclonal IgM antibody to endotoxin in the treatment of gram-negative sepsis. JAMA. 1991;266:1097–1102. [PubMed] [Google Scholar]
- 64.Zheng H, Fletcher D, Kozak W, Jiang M, Hofmann K, Conn C A, Soszynski D, Grabiec C, Trumbauer M E, Shaw A, Kostura M J, Stevens K, Rosen H, North R J, Chen H Y, Tocci M J, Kluger M J, Van der Ploeg L H T. Resistance to fever induction and impaired acute-phase response in interleukin-1β deficient mice. Immunity. 1995;3:9–19. doi: 10.1016/1074-7613(95)90154-x. [DOI] [PubMed] [Google Scholar]