Abstract
Background:
With addiction rates and opioid deaths increasing, health care providers are obligated to help stem the opioid crisis. As limited studies examine the comparative effectiveness of fixed-dose combination nonopioid analgesia to opioid-containing analgesia, a comparative effectiveness study was planned and refined by conducting a pilot study.
Methods:
The Opioid Analgesic Reduction Study (OARS) pilot, a stratified, randomized, multisite, double-blind clinical trial, was designed to test technology and procedures to be used in the full OARS trial. Participants engaged in the full protocol, enabling the collection of OARS outcome data. Eligible participants reporting to 1 of 5 sites for partial or full bony impacted mandibular third molar extraction were stratified by biologic sex and randomized to 1 of 2 treatment groups, OPIOID or NONOPIOID. OPIOID participants were provided 20 doses of hydrocodone 5 mg/acetaminophen 300 mg. NONOPIOID participants were provided 20 doses of ibuprofen 400 mg/acetaminophen 500 mg. OARS outcomes data, including pain experience, adverse effects, sleep quality, pain interference, overall satisfaction, and remaining opioid tablets available for diversion, were collected via surveys, electronic medication bottles, eDiary, and activity/sleep monitor.
Results:
Fifty-three participants were randomized with 50 completing the OARS pilot protocol. Across all outcome pain domains, in all but 1 time period, NONOPIOID was better in managing pain than OPIOID (P < 0.05 level). Other outcomes suggest less pain interference, less adverse events, better sleep quality, better overall satisfaction, and fewer opioid-containing tablets available for diversion.
Discussion:
Results suggest patients requiring impacted mandibular third molar extraction would benefit from fixed-dose combination nonopioid analgesia.
Knowledge Transfer Statement:
Study results suggest fixed-dose nonopioid combination ibuprofen 400 mg/acetaminophen 500 mg is superior to opioid-containing analgesic (hydrocodone 5 mg/acetaminophen 500 mg). This knowledge should inform surgeons and patients in the selection of postsurgical analgesia.
Keywords: pain, clinical trial, oral and maxillofacial surgery, hydrocodone, ibuprofen, acetaminophen
Introduction
Exacerbated by the COVID-19 pandemic, the opioid crisis has reached epic proportions, with 75% of total overdose deaths in 2020 involving an opioid (Centers for Disease Control and Prevention 2021a, 2021b). As addiction and associated opioid-related deaths increase, all health care professionals, including dentists, are called to stem the crisis (Denisco et al. 2011; Somerman and Volkow 2018; Suda et al. 2020; Chua et al. 2021; Heron et al. 2022). Despite public health efforts, dentists continue to include opioids in their armamentarium for management of postsurgical pain. While the number of routine opioid prescriptions following dental procedures has declined, dentistry accounted for 23% of all opioid prescriptions written for children (Okunev et al. 2021). Third molar extraction remains a direct pathway for young adults to gain access to legal opioids (Manchikanti et al. 2012). Understanding that the introduction to opioids at a young age is known to be a risk factor for addiction (McCabe et al. 2013; McCabe et al. 2020), it is prudent that researchers find suitable alternatives to opioids in the management of severe postoperative dental pain.
Within current pain research, studies suggest nonopioid-containing analgesic alternatives are effective in managing postsurgical pain (Mehlisch and Frakes 1984; Mehlisch et al. 1990; Barkin 2001; Hyllested et al. 2002; Menhinick et al. 2004; Mehlisch, Aspley, Daniels, and Bandy 2010; Mehlisch, Aspley, Daniels, Southerden, et al. 2010; Ong et al. 2010; Daniels et al. 2011), with combination analgesic therapy superior to noncombination analgesic therapy (Hyllested et al. 2002; Mehlisch 2002; Bailey et al. 2013; Derry et al. 2013; Moore, Derry, et al. 2015; Moore, Wiffen, et al. 2015; Moore et al. 2018). Furthermore, studies demonstrate opioids are not superior to nonopioids (Daniels et al. 2011; Best et al. 2017; Chang et al. 2017; Bijur et al. 2021). Often using the impacted third molar pain model, published studies tend to focus on placebo-controlled studies or studies that follow participant pain relief for a limited amount of time postsurgery, generally 8 to 24 h (Moore, Derry, et al. 2015). Missing, however, are prospective double-blind randomized trials that compare a combination of nonopioids to the most commonly prescribed opioid-containing analgesic, hydrocodone with acetaminophen, and follow participants for the entire postoperative period. To address this gap, the Opioid Analgesic Reduction Study (OARS) was designed. OARS is a multicenter trial planned to demonstrate whether a combination of over-the-counter analgesics is as effective in controlling postoperative pain as compared to the most commonly prescribed combination opioid analgesic (Feldman et al. 2022).
As the trial design employed a multifaceted, electronic infrastructure, it became apparent a pilot study would be invaluable to test protocols, feasibility, compatibility, and connectivity of data collection devices. Conducted at 5 clinical sites, the pilot followed the full study protocol as a double-blind randomized trial enabling site-based personnel to gain experience with the protocol prior to full study initiation.
While the primary outcomes of the pilot study focused on study feasibility and procedures, superiority analysis of biologic and perception outcomes (i.e., pain experience, pain interference with daily function, sleep quality, and satisfaction) was performed. Given the urgency and epidemic nature of the opioid crisis, this article was prepared to report results of the pilot study, specifically examining the following hypotheses:
The combination of acetaminophen and ibuprofen (NONOPIOID) is superior to the most commonly prescribed opioid analgesic, hydrocodone and acetaminophen (OPIOID), with respect to pain management, pain interference, sleep quality, and overall satisfaction.
Patients receiving NONOPIOID medication experience fewer and less severe adverse events than patients receiving OPIOIDs.
Patients receiving 5 d of opioid-containing analgesics have unused tablets/capsules remaining after their acute pain episode has been resolved.
Methods
Human Subject Approval
Human subject approval was obtained prior to recruitment and enrollment of any participants. All referenced surveys and pilot studies were approved by the Rutgers University Institutional Review Board (DHHS Federal Wide Assurance Identifier FWA00003913 approved study IDs Pro2019002262 and Pro2020001891). This trial was registered at clinicaltrials.gov as NCT05283499.
Design
A stratified, randomized, multisite, double-blind study was designed for subjects undergoing surgical removal of partial or full bony impacted third molars. Participants were stratified by biologic sex as reported by the participant and then randomized to the OPIOID group or the NONOPIOID group. There were no significant changes to trial design.
Interventions
OPIOID group participants were provided 20 doses of hydrocodone 5 mg/acetaminophen 300 mg. NONOPIOID group participants were provided 20 doses of ibuprofen 400 mg/acetaminophen 500 mg.
Study Procedures
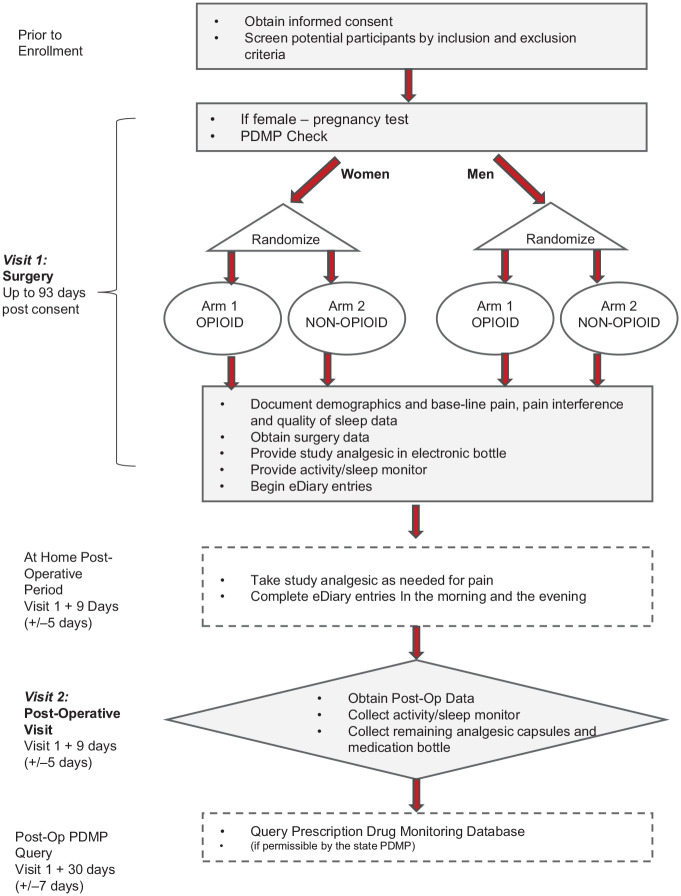
Figure 1 illustrates the study procedure timeline.
Figure 1.
Schedule of events, consisting of 2 visits after consent and preliminary eligibility determination. This figure formating is acceptable in gray/black.
Written informed consent was obtained from each participant and initial eligibility determined during visit 0. On the same day or up to 93 days later, participants returned for impacted mandibular third molar extraction surgery. During this surgical visit (visit 1), eligibility was finalized after a negative pregnancy test for female participants and a Prescription Drug Monitoring Program (PDMP) query run for all participants to ensure the participant had not been issued an opioid prescription within the past 6 mo. Prior to surgery, the preoperative survey was completed, which recorded the participant’s current pain levels, sleep quality, and daily activity. Surgical treatment plans were not influenced by study participation as any number of third molars could be extracted as long as at least 1 mandibular partial or full bony impacted molar was being extracted. After surgery, the research coordinator interviewed the surgeon to collect surgical information, including tooth numbers and level of impaction, surgery length, type of anesthesia, and medications used during surgery (antibiotics, opioids, and anti-inflammatory agents). At the conclusion of surgery, participants were instructed to take their first dose of assigned analgesic if accompanied by an escort or as soon as they got home if there was no escort. While the first dose was required, subsequent doses were recommended every 4 to 6 h as needed for pain.
During the postoperative period, electronic medication bottles recorded medication usage by weight change in real time. Participants were asked to complete an electronic diary twice daily, upon waking and prior to going to sleep. Entries were made via the participant’s smartphone after receiving a text message that included a link to a REDCap survey, which provided a structured eDiary entry. EDiary entries included pain levels and experienced adverse events. In addition, during evening entries, participants recorded pain interference (ability to carry out normal daily activities). As part of the morning entries, participants were asked about the quality of the previous night’s sleep.
The postoperative visit marked the end of the postoperative period and occurred between days 5 and 15. During the postoperative visit, participants had a postoperative exam; returned all study materials, including medication bottles and unused analgesic; and completed a postoperative survey, which collected information related to current pain levels, sleep quality, pain interference, adverse events, and satisfaction with their assigned analgesic.
Participants
Men and nonpregnant women presenting to a clinical site for partial or full bony impacted mandibular third molar surgery who were at least 18 y of age, were English speaking, and had provided consent were offered the opportunity to enroll. Participants had to be in good health; had to be able to take ibuprofen, paracetamol (acetaminophen), and hydrocodone; and could not have had an opioid prescription in the last 6 mo or a history of more than 1 opioid prescription. In addition, the participant’s social history was reviewed and could not include consuming 3 or more alcoholic drinks daily and/or history of alcoholism, drug or alcohol abuse, or family history of drug or alcohol abuse in a first-degree relative. All clinical sites were in academic health centers, including Rutgers University in Newark, New Jersey; University of Illinois in Chicago, Illinois; University of Maryland in Baltimore, Maryland; University of Michigan in Ann Arbor, Michigan; and University of Rochester in Rochester, New York.
Randomization
Randomization was performed within each site, stratified by biological sex by the statistician at the Statistical and Data Management Core. Thus, unique sequential participant IDs were generated, one for each site/sex-stratified group. Randomized assignment to either OPIOID or NONOPIOID was performed at a 1:1 ratio with a block of 4 containing 2 opioid and 2 nonopioid assignments. Participants were assigned participant IDs and randomly assigned a group based upon the order they reported to the clinical site for their surgical visit (visit 1).
Blinding
Participant kits were prepared by study staff at the OARS Clinical Protocol Coordinating Core, located within the Rutgers School of Dental Medicine. While OARS Core staff were unblinded to prepare study packages, all clinical site staff, including the site director, research coordinator, and surgeon and participants, were blinded.
Each participant kit consisted of 2 electronic bottles containing assigned analgesic, an activity monitor, study instructions, and postoperative instructions. Analgesic blinding was performed by over-encapsulating study analgesic products. An OPIOD kit contained hydrocodone 5 mg/acetaminophen 300 mg over-encapsulated into size DB-AA orange capsules, and the second bottle contained a placebo over-encapsuled into size 00 white capsules. A NONOPIOID kit contained 1 electronic bottle with ibuprofen 400 mg over-encapsuled into size orange DB-AA capsules, and the second bottle contained acetaminophen 500 mg over-encapsuled into size 00 white capsules. By providing both OPIOID and NONOPIOID participants a bottle of brown capsules and a bottle of white capsules, all participants received the same postoperative instructions.
Outcomes
While primary outcomes for the pilot study examined feasibility and procedures, the analysis was based on the primary and secondary clinical and behavioral outcomes identified for the full OARS trial. Primary outcomes for the OARS trial include pain levels using the numeric rating scale (Ferreira-Valente et al. 2011), Brief Pain Inventory (BPI; Kean et al. 2016), and overall satisfaction using the questions from the Pain Treatment Satisfaction Scale (PTSS; Evans et al. 2004). Secondary outcomes include adverse events (Gilron et al. 2019), sleep quality assessed by Numeric Rating Scale (NRS; Zisapel and Nir 2003; Cappelleri et al. 2009) and Pittsburgh Sleep Questionnaire (PSQ-3; Ayearst et al. 2012), pain interference assessed by Patient Report Outcomes Measures Information System (PROMIS-6b; Kean et al. 2016), and potential opioid diversion (McCabe et al. 2013; McCabe et al. 2020). Table 1 describes the primary and secondary outcome measures. There were no changes to these trial outcomes after the pilot was commenced.
Table 1.
Outcome Measures.
| Outcome Measure Source | Measure Description | Collection Method and Timing |
|---|---|---|
|
Pain experience (primary)
• Brief Pain Inventory (BPI) |
Four-item survey asking subjects to rate their worst pain, least pain, average pain, and current pain using a 11-point numeric rating scale (where 0 = no pain and 10 = worst pain) | • Preoperative survey • Patient-reported eDiary entries for first 7 evenings and 7 mornings during the postoperative period • Postoperative survey |
|
Pain control satisfaction (primary)
• Pain Treatment Satisfaction Scale (PTSS) |
Six questions from the PTSS. Four items asked subjects to rate on a 5-point Likert scale (where 1 = very satisfied, 2 = satisfied, 3 = neither satisfied or dissatisfied, 4 = dissatisfied, and 5 = very dissatisfied) the time it took for the pain medication to work, the amount of pain relief provided by their pain medication, the duration of pain relief provided by their pain medication, and overall satisfaction with their pain medication. A fifth item using a 5-point Likert scale (where 1 = generally exceeds my expectations, 2 = somewhat exceeds my expectations, 3 = meets my expectations, 4 = does not quite meet my expectations, and 5 = does not meet my expectations at all) asks subjects to rate whether the level of pain relief met their expectations. The sixth item using a 5-point Likert scale (where 1 = definitely yes, 2 = probably yes, 3 = I don’t know, 4 = probably not, and 5 = definitely not) requests subjects to rate whether their pain medication could have been more effective. | • Postoperative survey |
|
Ability to sleep
• Pittsburgh Sleep Questionnaire (PSQ-3) • Numeric Rating Scale (NRS) |
PSQ-3: 3-item survey asking subjects whether they had trouble falling asleep, were awakened by pain during the night, and whether they were awakened by pain in the morning (yes = 1/no = 0 response). NRS: Overall rating of a subject’s overall quality of sleep using the 11-point numeric scale where 0 = excellent and 10 = very poor. |
• Preoperative survey • Patient-reported eDiary entries for first 7 mornings during the postoperative period |
|
Pain interference
• Patient Report Outcomes Measures Information System (PROMIS-6b) |
Six-item survey using a 5-point Likert scale. Five items (using a Likert scale where 1 = not all, 2 = a little bit, 3 = quite a bit, 5 = very much) include how much did pain interfere with a subject’s enjoyment of life, ability to concentrate, day-to-day activities, recreational activities, and doing tasks away from home. A sixth item asking subjects if pain kept them from socializing with others used a 5-point Likert scale where 1 = never, 2 = rarely, 3 = sometimes, 4 = often, and 5 = always. | • Preoperative survey • Patient-reported eDiary entries for first 7 evenings during the postoperative period • Postoperative survey |
| Adverse events | Fifteen of the most common adverse events experienced when taking hydrocodone, ibuprofen, and acetaminophen | • Preoperative survey • Patient-reported eDiary entries for first 7 evenings and 7 mornings during the postoperative period • Postoperative survey |
Sample Size
Sample size was determined to assess the precision of the estimated mean score of pain level, measured by the BPI (Kean et al. 2016), using a 11-point NRS (0 = no pain and 10 = worst pain). Specifically, we determined n = 25 patients per treatment condition such that the 95% confidence interval (CI) for the mean score for each treatment condition was within the ±1.5 (Doleman et al. 2021) range, assuming the standard deviation of the pain levels was 3.6 (Chang et al. 2017) or less. Sample size was also based upon a minimum number of participants considered sufficient for user testing of OARS procedures and technology systems. Rutgers had conducted a previous pilot to develop the protocol with 34 participants. Based upon this experience and the desire to test the refined protocol and REDCap OARS system at all study sites, it was determined that at least 20 participants should be enrolled at Rutgers, followed by an additional 8 participants at each site, as the study protocol required investigational product to be shipped in quantities of 8 participants at a time to the non-Rutgers sites. By guaranteeing 4 participants being randomly assigned to OPIOID and 4 participants randomly assigned to NONOPIOID, the DEA 222 form could be accurately completed without breaking the blinded nature of the study.
Interim Analysis
There was no plan for any interim analyses.
Stopping Guidelines
Stopping guidelines included any fatality or 2 instances of the same serious adverse event possibly related to investigational product.
Statistical Analysis
An intention-to-treat analysis was performed. All patients who were enrolled and randomized were included in the analyses. Descriptive statistics were provided to summarize the patient demographics and selected baseline characteristics. To account for the repeated-measures design and the correlations within study site, mixed-model analysis was used to compare continuous outcomes, including pain levels, sleep quality, and pain interference scores. Random-effects logistic regression analysis was used to compare the binary responses (yes/no) (e.g., responses for each subscale of the PSQ-3). Day (day 1 of treatment, days 2 to 7 postsurgery), group (NONOPIOID vs. OPIOID), and day-by-group interactions were included in the statistical models as fixed effects. Patients and sites were adjusted using nested random effects to account for the possibly stronger within-patient than within-site correlations in the data. Marginal Poisson log-linear model (Heagerty and Zeger 2000) was used to compare the number of adverse events. Linear contrasts were constructed to estimate and compare these outcomes over the first 24 h, 48 h, 72 h, and 7 days between the NONOPIOID versus OPIOID groups. Patient satisfaction, measured at the postoperative visit, was assessed using mixed-model analysis with study site as a random effect to account for the possible within-site correlation. All regression analyses adjusted for the number of extracted mandibular full bony impacted third molars as a covariate due to its imbalanced distribution between treatment groups. Potential for diversions was assessed by summary statistics and compared using the Wilcoxon test due to skewed distributions. Statistical significance was defined as P < 0.05 for each comparison. All statistical analyses were performed using SAS v.9.4 (SAS Institute).
Subgroup Analysis and Adjusted Analyses
Subgroup analyses were not performed. Descriptive analysis revealed an uneven distribution of the number of extracted mandibular full bony impacted third molars between treatment groups. All regression analyses (i.e., mixed-model, random-effect logistic, and marginal Poisson log-linear models) adjusted for this difference by including the number of extracted mandibular full bony impacted third molars as a covariate, as pain associated with mandibular full bony impacted third molar extractions is expected to be different from pain associated with mandibular partial bony impacted third molars.
All analyses were based upon 25 participants in each group by original group assignment.
Results
Participant Flow
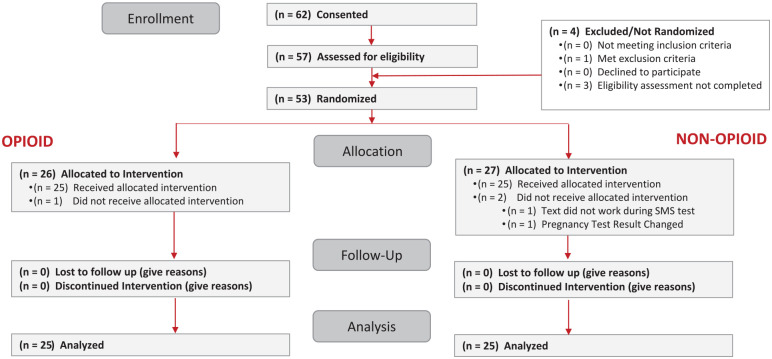
The Consolidated Standards of Reporting Trials (CONSORT) diagram for the pilot study is provided in Figure 2. Sixty-two participants were consented, and 53 were randomized, with 25 in each group undergoing impacted mandibular third molar surgery, receiving investigational product, and completing the protocol. Study analysis was based upon these 50 participants.
Figure 2.
Consolidated Standards of Reporting Trials (CONSORT) diagram. Sixty-two subjects were consented, with 57 undergoing eligibility determination. Fifty-three were randomized, and 50 completed the protocol. This figure is acceptable in gray/black.
Recruitment
Recruitment was begun at Rutgers University so that any significant procedural issues could be addressed prior to training research coordinators at each of the other clinical sites. After 20 participants were successfully enrolled and protocols completed at Rutgers, enrollment began at each site of the other clinical sites. Recruitment began at the Rutgers University site on January 6, 2020, with the last postoperative visit completed on March 12, 2021. All 1-mo PDMP follow-up checks were completed by April 7, 2021. Enrollment was stopped at Rutgers University after 20 participants completed their postoperative visit, as minimum planned enrollment had been achieved and feasibility determined. Enrollment at the 4 other clinical sites was concluded once the planned enrollment of 8 participants was completed.
Demographics
The number of participants enrolled at each site along with the participant demographics by random group assignment is presented in Table 2. Fifty-two percent of participants were female. One-third of the participants were African American, and one-quarter were Hispanic. There were no significant differences between OPIOID and NONOPIOID groups for age, biologic sex, race, or ethnicity.
Table 2.
Subject Demographics and Surgical Parameters.
| Demographics | All (n = 50) | NONOPIOID (n = 25) | OPIOID (n = 25) | P Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Median (IQR) | Range | Mean (SD) | Median (IQR) | Range | Mean (SD) | Median (IQR) | Range | Mean (SD) | t Test | |
| Current age, y | 24 (21, 29) | 19–42 | 25.5 (5.3) | 23 (21, 27) | 19–42 | 25.3 (5.7) | 27 (21, 29) | 19–38 | 25.7 (4.9) | 0.787 |
| Biologic sex, n (%) | χ2 | |||||||||
| Female | 25 (50) | 12 (48) | 13 (52) | 0.777 | ||||||
| Race, n (%) | 0.189 | |||||||||
|
African American
Caucasian Hawaiian or Pacific Islander Asian Two or more races Do not want to report |
17 (34) 14 (28) 1 (2) 7 (14) 4 (8) 7 (14) |
7 (28) 11 (44) 1 (4) 3 (12) 1 (4) 2 (8) |
10 (40) 3 (12) 0 (0) 4 (16) 3 (12) 5 (20) |
|||||||
| Hispanic | 13 (26) | 7 (28) | 6 (24) | 0.359 | ||||||
|
Site, n (%)
Chicago Maryland Michigan Rochester Rutgers |
8 (16) 8 (16) 8 (16) 6 (12) 20 (40) |
4 (16) 4 (16) 4 (16) 3 (12) 10 (40) |
4 (16) 4 (16) 4 (16) 3 (12) 10 (40) |
NA | ||||||
| Preoperative Pain Levels | Median (IQR) | Range | Mean (SD) | Median (IQR) | Range | Mean (SD) | Median (IQR) | Range | Mean (SD) | Wilcoxon Rank Sum |
| Least pain a | 1.5 (0, 4) | 0–8 | 2.3 (2.5) | 1 (0, 3) | 0–8 | 2.0 (2.5) | 2 (0, 5) | 0–8 | 2.6 (2.6) | 0.366 |
| Average pain a | 0 (0, 2) | 0–8 | 1.2 (1.9) | 0 (0, 1) | 0–4 | 0.9 (1.4) | 0 (0, 3) | 0–8 | 1.6 (2.3) | 0.308 |
| Pain now being felt a | 1 (0, 2.5) | 0–9 | 1.7 (2.3) | 1 (0, 2) | 0–9 | 1.7 (2.4) | 1 (0, 3) | 0–8 | 1.8 (2.3) | 0.702 |
| Worst pain a | 3 (0, 6) | 0–9 | 3.1 (3.0) | 1 (0, 6) | 0–9 | 2.7 (3.0) | 3 (0, 6) | 0–8 | 3.5 (2.9) | 0.402 |
| Surgical Parameters | ||||||||||
| All (n = 50) | NONOPIOID (n = 25) | OPIOID (n = 25) | P Value | |||||||
| Surgical Treatment Duration | Median (IQR) | Range | Mean (SD) | Median (IQR) | Range | Mean (SD) | Median (IQR) | Range | Mean (SD) | Wilcoxon Rank Sum |
| Time, min | 30 (20, 45) | 10–90 | 33.4 (18.3) | 25 (15, 40) | 10–60 | 29.1 (15.8) | 30 (25, 60) | 15–90 | 38.2 (20.2) | 0.102 a |
| Teeth extracted | n (%) | n (%) | n (%) | χ2 P Value | ||||||
|
Tooth 1
Tooth 16 Tooth 17 Tooth 32 |
27 (54) 30 (60) 45 (90) 41 (82) |
13 (52) 16 (64) 23 (92) 19 (76) |
14 (56) 14 (56) 22 (88) 22 (88) |
0.777 0.564 0.637 0.269 |
||||||
| Number of Teeth Extracted | Median (IQR) | Range | Mean (SD) | Median (IQR) | Range | Mean (SD) | Median (IQR) | Range | Mean (SD) | Wilcoxon Rank Sum |
|
No. of maxillary third molars
No. of mandibular third molars Total No. of third molars No. of full bony impacted third molars extracted |
1.5 (0, 2) 2 (1, 2) 3.5 (2, 4) 0 (0, 1) |
0–2 1–2 1–4 0–2 |
1.1 (0.9) 1.7 (0.5) 2.9 (1.2) 0.6 (0.8) |
1 (0, 2) 2 (1, 2) 3 (2, 4) 0 (0, 1) |
0–2 1–2 1–4 0–2 |
1.2 (0.9) 1.7 (0.5) 2.8 (1.2) 0.3 (0.6) |
2 (0, 2) 2 (2, 2) 4 (2, 4) 1 (0, 2) |
0–2 1–2 1–4 0–2 |
1.1 (1.0) 1.8 (0.4) 2.9 (1.3) 0.8 (0.9) |
0.932 0.541 0.924 0.012 |
| Anesthesia/Analgesia Used during Surgery | n (%) | n (%) | n (%) | χ2 P Value | ||||||
|
Local
Oral/enteral Conscious sedation Nitrous oxide General anesthesia |
44 (88) 0 (0) 8 (16) 2 (4) 16 (32) |
23 (92) 0 (0) 4 (16) 1 (4) 7 (28) |
21 (84) 0 (0) 4 (16) 1 (4) 9 (36) |
0.384 0.999 0.999 0.999 0.544 |
||||||
| Other Pharmaceutical Used | n (%) | n (%) | n (%) | χ2 P Value | ||||||
| AntibioticsAnti-inflammatory | 21 (42) 12 (24) |
9 (36) 5 (20) |
12 (48) 7 (28) |
0.390 0.507 |
||||||
IQR, interquartile range; NA, not applicable as sites were provided designated enrollment targets.
Numeric Rating Scale where 0 = no pain and 10 = worst pain.
Baseline Pain and Surgical Parameters
Table 2 also reports pain levels collected prior to surgery and surgical procedure data collected after surgery completion. Data collected included BPI, BPI pain level components, surgery time, number of third molars extracted, number of full bony third mandibular extractions, anesthesia type, and whether antibiotics or anti-inflammatory agents were administered. While there were no statistically significant differences at the P < 0.05 level with regard to baseline pain levels, specific teeth extracted, surgical time, type of anesthesia, and administration of antibiotics and/or anti-inflammatory agents, there was a significant difference in the number full bony impacted mandibular third molar extractions, with the OPIOID group experiencing a greater number of full bony mandibular impacted extractions (mean = 0.8, SD = 0.9) than the NONOPIOD group (mean = 0.3, SD = .6) with P = 0.012.
Outcome Measures
Table 3 contains the outcomes measures, which were collected via eDiary entries and postoperative survey. Results are reported for the following 4 time frames: first 24 h, first 48 h, first 72 h, and first 7 days.
Table 3.
OARS Pilot Trial Outcomes.
| Pain Experience | ||||||
|---|---|---|---|---|---|---|
| Time Period | NONOPIOID | OPIOID | Difference (Mixed-Model Analysis) | Effect Size | ||
| Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | P Value | Cohen’s d | ||
| Brief Pain Index a | First 24 h First 48 h First 72 h Day 1 to day 7 |
4.4 (3.4, 5.4) 3.9 (2.9, 4.9) 3.6 (2.7, 4.6) 2.9 (2.1, 3.7) |
5.9 (4.7, 7.0) 5.4 (4.3, 6.6) 5.2 (4.1, 6.4) 4.4 (3.5, 5.2) |
−1.5 (−2.6, −0.4) −1.5 (−2.6, −0.5) −1.6 (−2.6, −0.6) −1.5 (−2.5, −0.5) |
0.010 0.005 0.003 0.004 |
0.62 0.66 0.67 0.58 |
| Average pain b | First 24 h First 48 h First 72 h Day 1 to day 7 |
4.1 (2.9, 5.4) 3.6 (2.4, 4.8) 3.3 (2.1, 4.6) 2.5 (1.3, 3.7) |
5.9 (4.5, 7.3) 5.4 (4.0, 6.8) 5.0 (3.6, 6.4) 4.0 (2.8, 5.9) |
−1.8 (−3.1, −0.5) −1.7 (−3.0, −0.5) −1.7 (−2.9, −0.5) −1.5 (−2.7, −0.3) |
0.006 0.006 0.006 0.019 |
0.68 0.69 0.68 0.58 |
| Least pain b | First 24 h First 48 h First 72 h Day 1 to day 7 |
2.8 (1.8, 3.9) 2.4 (1.4, 3.4) 2.2 (1.2, 3.2) 1.5 (0.6, 2.5) |
4.3 (3.1, 5.5) 4.0 (2.9, 5.2) 3.8 (2.6, 5.0) 3.1 (2.1, 4.0) |
−1.5 (−2.7, −0.3) −1.7 (−2.8, −0.5) −1.6 (−2.8, −0.5) −1.5 (−2.7, −0.4) |
0.018 0.006 0.005 0.007 |
0.61 0.73 0.74 0.70 |
| Pain now being felt b | First 24 h First 48 h First 72 h Day 1 to day 7 |
4.5 (3.2, 5.8) 3.7 (2.4, 5.0) 3.3 (2.0, 4.6) 2.5 (1.3, 3.7) |
5.8 (4.3, 7.3) 5.1 (3.6, 6.5) 4.7 (3.2, 6.2) 3.8 (2.5, 5.0) |
−1.4 (−2.7, 0.0) −1.4 (−2.7, −0.1) −1.4 (−2.7, −0.1) −1.3 (−2.5, −0.0) |
0.051 0.037 0.030 0.044 |
0.48 0.52 0.54 0.50 |
| Worst pain b | First 24 h First 48 h First 72 h Day 1 to day 7 |
5.4 (3.8, 6.9) 4.9 (3.4, 6.4) 4.6 (3.1, 6.0) 3.5 (2.1, 5.0) |
7.2 (5.5, 8.9) 6.7 (5.1, 8.4) 6.4 (4.7, 8.1) 5.0 (3.6, 6.5) |
−1.9 (−3.3, −0.5) −1.8 (−3.2, −0.5) −1.8 (−3.2, −0.5) −1.5 (−2.8, −0.2) |
0.010 0.008 0.007 0.030 |
0.60 0.62 0.63 0.50 |
| Satisfaction | ||||||
| NONOPIOID | OPIOID | Difference (Mixed-Model Analysis) | Effect Size | |||
| Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | P Value | Cohen’s d | ||
| Time for medication to take effect c | 2.0 (1.5, 2.5) | 2.2 (1.8, 2.6) | −0.2 (−0.7, 0.3) | 0.434 | 0.14 | |
| Level of pain relief c | 1.9 (1.3, 2.4) | 1.9 (1.5, 2.4) | −0.1 (−0.5, 0.4) | 0.831 | 0.03 | |
| Duration of pain relief c | 2.0 (1.4, 2.5) | 2.1 (1.6, 2.5) | −0.1 (−0.6, 0.4) | 0.678 | 0.07 | |
| Overall satisfaction c | 2.0 (1.5, 2.5) | 2.2 (1.8, 2.6) | −0.2 (−0.8, 0.4) | 0.561 | 0.12 | |
| Sleep Quality | ||||||
| NONOPIOID | OPIOID | Difference (Mixed-Model Analysis) | Effect Size | |||
| Time Period | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | P Value | Cohen’s d | |
| Sum of PSQ-3 Sleep Interference Questions d | First 24 h First 48 h First 72 h Day 1 to day 7 |
0.9 (0.45, 1.39) 0.9 (0.51, 1.34) 1.0 (0.63, 1.41) 0.8 (0.38, 1.13) |
2.0 (1.4, 2.5) 1.6 (1.2, 2.1) 1.5 (1.0, 1.9) 1.2 (0.8, 1.5) |
−1.0 (−1.61, −0.5) −0.7 (−1.2, −0.2) −0.5 (−0.9, 0.0) −0.4 (−0.8, 0.0) |
0.001 0.006 0.056 0.073 |
1.06 0.92 0.64 0.54 |
| NONOPIOID | OPIOID | Difference (Mixed-Model Analysis) | Effect Size | |||
| Time Period | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | P Value | Cohen’s d | |
| Overall quality of sleep e | First 24 h First 48 h First 72 h Day 1 to day 7 |
3.7 (2.4, 5.2) 3.4 (2.1, 4.7) 3.3 (2.1, 4.6) 2.9 (1.7, 4.1) |
5.4 (3.9, 7.0) 5.2 (3.8, 6.7) 4.9 (3.5, 6.3) 4.4 (3.2, 5.6) |
−1.7 (−3.2, −0.1) −1.8 (−3.2, −0.4) −1.5 (−2.8, −0.2) −1.5 (−2.7, −0.3) |
0.034 0.011 0.021 0.019 |
0.69 0.79 0.70 0.70 |
| Pain Interference | ||||||
| NONOPIOID | OPIOID | Difference (Mixed-Model Analysis) | Effect Size | |||
| Time Period | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | P Value | Cohen’s d | |
| Raw PROMIS-6b score f | First 24 h First 48 h First 72 h Day 1 to day 7 |
15.9 (13.0, 18.8) 13.8 (11.2, 16.4) 12.9 (10.4, 15.4) 11.4 (9.0, 13.7) |
19.7 (16.3, 23.1) 17.6 (14.4, 20.7) 16.7 (13.7, 19.8) 14.3 (11.9, 16.6) |
−3.8 (−7.7, 0.2) −3.8 (−7.4, −0.2) −3.9 (−7.3, −0.4) −2.9 (−6.3, 0.6) |
0.065 0.041 0.029 0.091 |
0.42 0.48 0.55 0.55 |
| Adverse Events | NONOPIOID | OPIOID | Comparison (Random-Effects Logistic Regression Analysis) | |||
| Time Period | % Yes (95% CI) | % Yes (95% CI) | Odds Ratio (95% CI) | P Value | ||
| % Subjects answering yes to any event | First 24 h First 48 h First 72 h Day 1 to day 7 |
69 (41, 87) 60 (34, 81) 47 (24, 71) 40 (22, 63) |
84 (62, 94) 80 (58, 92) 75 (51, 89) 57 (35, 77) |
0.6 (0.1, 3.0) 0.5 (0.1, 2.2) 0.4 (0.1, 1.7) 0.5 (0.2, 1.6) |
0.526 0.362 0.214 0.253 |
|
| NONOPIOID | OPIOID | Comparison (Marginal Log-Linear Poisson Regression Analysis) | ||||
| Time Period | Mean (95% CI) | Mean (95% CI) | Ratio of Means (95% CI) | P Value | ||
| No. of events per subject g | First 24 h First 48 h First 72 h Day 1 to day 7 |
1.5 (1.1, 2.0) 1.0 (0.7, 1.4) 0.8 (0.5, 1.0) 0.7 (0.6, 1.0) |
1.9 (1.3, 2.9) 1.8 (1.3, 2.7) 1.7 (1.2, 2.6) 1.4 (0.9, 2.2) |
1.0 (0.5, 1.7) 0.7 (0.4, 1.2) 0.5 (0.3, 0.9) 0.5 (0.3, 0.9) |
0.882 0.187 0.031 0.024 |
|
| NONOPIOID | OPIOID | Difference (Mixed-Model Analysis) | Effect Size | |||
| Time Period | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | P Value | Cohen’s d | |
| Adverse event severity h | First 24 h First 48 h First 72 h Day 1 to day 7 |
1.9 (0.6, 3.2) 1.3 (0.1, 2.6) 1.0 (−0.3, 2.2) 1.3 (0.1, 2.5) |
2.7 (1.1, 4.2) 2.2 (0.7, 3.7) 2.0 (0.5, 3.5) 2.4 (1.2, 3.6) |
−0.7 (−2.4, 1.0) −0.9 (−2.5, 0.7) −1.0 (−2.6, 0.6) −1.1 (−2.6, 0.5) |
0.398 0.287 0.214 0.167 |
0.38 0.39 0.43 0.51 |
CI, confidence interval; NRS, Numeric Rating Scale; PROMIS-6b, Patient Report Outcomes Measures Information System; PSQ-3, Pittsburgh Sleep Questionnaire.
Mean NRS rating for the 4 component questions where 0 = no pain and 10 = worst pain (a lower score indicates a more effective analgesic).
NRS scale where 0 = no pain and 10 = worst pain.
Likert scale where 1 = very satisfied, 2 = satisfied, 3 = neither satisfied or dissatisfied, 4 = dissatisfied, and 5 = very dissatisfied (a lower score indicates a more effective analgesic).
Sum of 3-item questionnaire where 1 = yes, 0 = no (possible range = 0 to 3).
NRS where 0 = excellent and 10 = very poor.
Sum of 6 questions on a Likert scale. Five items using a Likert scale (where 1 = not all, 2 = a little bit, 3=somewhat, 4=quite a bit, and 5 = very much) include how much did pain interfere with a subject’s enjoyment of life, ability to concentrate, day-to-day activities, recreational activities, and doing tasks away from home. A sixth item asking subjects if pain kept them from socializing with others used a 5-point Likert scale where 1 = never, 2 = rarely, 3 = sometimes, 4 = often, and 5 = always (possible range = 6 to 30).
Sum of the number of adverse events per time period with a response of mild, moderate or severe.
Average number of events per reporting period × severity rating where 0 = none, 1 = mild, 2 = moderate, and 3 = severe (possible range = 0 to 48).
BPI was highest during the first 24 h and decreased during each 24-h period. For all time periods, OPIOID experienced statistically significant pain at the P < 0.05 level. For all but 1 BPI pain component rating at 1 time period (average pain, least pain, worst pain, and pain right now), OPIOID participants’ differences were statistically significant at the P < 0.05 level with the remaining “pain right now” rating difference for day 1 through day 7 marginally statistically significant at the P < 0.05 level (P = 0.051). There were significant differences between OPIOID and NONOPIOID, with participants using the ibuprofen/acetaminophen combination analgesic experiencing less pain than participants using the hydrocodone/acetaminophen combination analgesic. Adjusted mean differences were 1.4 to 1.8 rating units on a pain scale from 0 to 10, reflecting a 14% to 18% difference.
When asked about their satisfaction with pain relief onset, level of pain relief, duration of pain relief, and overall satisfaction with the analgesic provided, no statistically significant differences were found at the P < 0.05 level, although nonopioid satisfaction ratings reflected higher levels of satisfaction among all domains than opioid participant satisfaction ratings. Ratings used a 5-point Likert scale with 1 = very satisfied and 5 = very dissatisfied.
Participant rating of overall quality of sleep was statistically significant at the P < 0.05 level, with NONOPIOID participants rating higher sleep quality for all 4 time periods. The 3-question PSQ-3, which consists of questions about not being able to fall asleep due to pain, being woken up in the middle of the night with pain, and waking up in the morning with pain, reflects poorer-quality sleep for the first 24, 48, and 72 h by OPIOID participants than for NONOPIOD participants, although only the first 24- and 48-h time periods are statistically significant at the P < 0.05 level. The first 72-h time period and the 1- to 7-d time period are not statistically significant at the P < 0.05 level. When asked about overall quality of sleep, sleep NRS ratings for NONOPIOID were, on average, 1.5 to 1.8 rating units better (15%–18%) on a sleep quality scale from 0 to 10, with 0 = best quality sleep and 10 = worst quality sleep. All time period differences were statistically significant at the P < 0.05 level.
With regard to pain interfering with normal daily activities, participants assigned to NONOPIOID indicated superior ability to perform all aspects of daily activity (enjoyment of life, ability to concentrate, perform daily activities, engage in recreational activities, and doing tasks away from home), as reflected by the PROMIS-6b questionnaire. Two time periods, first 48 h and first 72 h, were significant at the P < 0.05 level, and the remaining 2 time periods, first 24 h and first 7 days, were not significant at the P < 0.05 level.
Adverse events included participants experiencing analgesic side effects (i.e., nausea, vomiting, fatigue, inability to concentrate, euphoria, headache, diarrhea, constipation, rashes). These adverse events were evaluated in 3 ways: looking at the percentage of participants in each reporting period indicating at least 1 symptom summed up over all reporting time periods, counting the number of symptoms (number of events) reported by the participants over the course of the reporting periods, and adding up the severity of all symptoms over the reporting time periods. Using all 3 methodologies, opioid participants experienced a greater number of adverse events with greater severity, although most of these differences were not statistically significant at the P < 0.05 level. For example, 69% (95% CI, 41%, 87%) of NONOPIOID participants reported at least 1 adverse event in the first 24 h as compared to 84% (95% CI, 62%, 94%) of the OPIOID participants. In the first 72 h, 47% (95% CI, 24%, 71%) of NONOPIOID participants reported at least 1 adverse event as compared to 75% (95% CI, 51%, 89%) of the OPIOID participants. The lack of statistical significance is likely due to the small sample size within each group in experiencing adverse events, as evidenced by the large 95% confidence intervals.
Last, capsules returned during the postoperative visit reflect capsules that are available for diversion (use by other individuals). On average, 6.3 opioid-containing tablets or capsules would remain available for diversion for each patient prescribed 20 tablets of hydrocodone had study investigational product not been collected.
There were no unanticipated problems and no serious adverse events, and none of the subjects residing in states allowing a PDMP follow-up query had filled a subsequent opioid-containing prescription 1 mo after surgery. Participants residing in states not allowing a PDMP follow-up a notice were sent via e-mail informing them of opioid addiction counseling availability. No participants sought out counseling.
Discussion
The OARS pilot study was successful confirming trial feasibility as patients reporting to oral and maxillofacial surgery clinics for impacted mandibular third molar extractions were able to be recruited as study participants.
Even with a small sample size of 50 participants, we find that patients requiring impacted (partial or full bony) mandibular third molar surgery had less postoperative pain, better quality of sleep, and better ability to engage in their daily activities for the immediate postsurgery days if the fixed-dose combination of over-the-counter analgesics was prescribed rather than an opioid-containing analgesic. Our study also revealed a significant number of leftover opioid-containing tablets available for diversion when 20 tablets were routinely prescribed for postoperative impacted third molar extraction surgery.
In all dimensions (pain experience, sleep quality, pain interference with daily activities, and overall satisfaction), the nonopioid appeared to perform better than the opioid in most comparisons. Prescribing a nonopioid alternative would put fewer opioid tablets in circulation and reduce the possibility of opioid diversion. Because the aim of this pilot study is to assess feasibility, we did not power our study to test between-treatment differences for statistical significance and determined the sample size based on the precision of the effect size estimates. With the small sample size of n = 25 patients per treatment group, we defined the statistical significance by P < 0.05 for each test. The multiple test adjustment will be deferred to the rigorously planned large trial that is currently ongoing. If we were to apply the Bonferroni correction in this pilot study in testing the pain difference measured by BPI, the primary outcome, the adjusted α level for each test would be 0.0125, after adjusting for 4 tests (first 24, 48, 72 h and days 1 to 7) and keeping the overall α level at 5%. After this adjustment, the between-group difference in BPI in all 4 tests (i.e., the first 24, 48, 72 h and days 1 to 7) remained statistically significant (all P values <0.0125). We noted that not all differences in the submeasurements of BPI (average, least, right now, worst) remained statistically significant based on the adjusted cutoff (P < 0.0125). Similarly, for the other secondary outcomes (e.g., sleep quality assessment), not all of them showed statistically significant differences. We will perform rigorous comparisons with proper multiple testing adjustment in the large trial when the sample size is sufficiently powered to test the treatment effect.
While the preliminary data are promising, the small sample size limits the ability to do subgroup analysis. Even with randomization, the pilot study yielded an OPIOID group who underwent a greater number of full bony impacted third molar extractions. The preliminary data from this pilot may be generalizable given the gender, race, and ethnicity distributions, but other demographic and baseline variables such as education level, socioeconomic status, medical/dental history, and pain tolerance were not examined.
Our study found large and significant differences in pain levels, with NONOPIOID experiencing significantly less postoperative pain. Even if there were no differences between OPIOID and NONOPIOID pain levels, ibuprofen/acetaminophen should be the first choice in the management of moderate to severe postoperative oral surgical pain.
Author Contributions
C.A. Feldman, J. Fredericks-Younger, contributed to conception and design, data acquisition, analysis, and interpretation, drafted the manuscript; P.J. Desjardins, D.H. Fine, contributed to conception and design, data interpretation, critically revised the manuscript; H. Malmstrom, M. Miloro, G. Warburton, contributed to conception and design, data acquisition and interpretation, critically revised the manuscript; B. Ward, V. Ziccardi, S.-E. Lu, contributed to conception and design data analysis and interpretation, critically revised the manuscript; P. Greenberg, T. Andrews, P.B. Matheson, contributed to design, data analysis and interpretation, critically revised the manuscript.
Acknowledgments
The authors thank Julie Chapman-Greene, Rita Cacciato, Susan Ferguson, Jennifer Lay-Luskin, Megan Thompson, Jane Phillips, and Yosmery Garcia, without whose efforts this project would never have been possible.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Supported by National Institute of Dental and Craniofacial Research. (NIDCR stands for the National Institute of Craniofacial Research) of the National Institutes of Health awards under award numbers UG3DE028860 and UH3DE028860. The opinions and assertions in this article are those of the authors and are not to be construed as necessarily representing the views of the organizations the authors are affiliated with, the organizations that participate as network regions, or the National Institutes of Health.
ORCID iD: C.A. Feldman  https://orcid.org/0000-0001-8004-4473
https://orcid.org/0000-0001-8004-4473
References
- Ayearst LE, Harsanyi Z, Michalko KJ. 2012. The Pain and Sleep Questionnaire Three-Item Index (PSQ-3): A reliable and valid measure of the impact of pain on sleep in chronic nonmalignant pain of various etiologies. Pain Res Manage. 17(4):281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey E, Worthington HV, van Wijk A, Yates JM, Coulthard P, Afzal Z. 2013. Ibuprofen and/or paracetamol (acetaminophen) for pain relief after surgical removal of lower wisdom teeth. Cochrane Database Syst Rev. 12:CD004624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkin RL. 2001. Acetaminophen, aspirin, or ibuprofen in combination analgesic products. Am J Ther. 8(6):433–442. [DOI] [PubMed] [Google Scholar]
- Best AD, De Silva RK, Thomson WM, Tong DC, Cameron CM, De Silva HL. 2017. Efficacy of codeine when added to paracetamol (acetaminophen) and ibuprofen for relief of postoperative pain after surgical removal of impacted third molars: a double-blinded randomized control trial. J Oral Maxillofac Surg. 75(10):2063–2069. [DOI] [PubMed] [Google Scholar]
- Bijur PE, Friedman BW, Irizarry E, Chang AK, Gallagher EJ. 2021. A randomized trial comparing the efficacy of five oral analgesics for treatment of acute musculoskeletal extremity pain in the emergency department. Ann Emerg Med. 77(3):345–356. [DOI] [PubMed] [Google Scholar]
- Cappelleri JC, Bushmakin AG, McDermott AM, Sadosky AB, Petrie CD, Martin S. 2009. Psychometric properties of a single-item scale to assess sleep quality among individuals with fibromyalgia. Health Qual Life Outcomes. 7(1):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. 2021. a. Drug overdose deaths | drug overdose | CDC Injury Center [accessed 2022 Nov 21]. https://www.cdc.gov/drugoverdose/index.html
- Centers for Disease Control and Prevention. 2021. b. Drug overdose deaths in the U.S. top 100,000 annually [accessed 2022 Nov 21]. https://www.cdc.gov/nchs/pressroom/nchs_press_releases/2021/20211117.htm
- Chang AK, Bijur PE, Esses D, Barnaby DP, Baer J. 2017. Effect of a single dose of oral opioid and nonopioid analgesics on acute extremity pain in the emergency department: a randomized clinical trial. JAMA. 318(17):1661–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua K-P, Hu H-M, Waljee JF, Brummett CM, Nalliah RP. 2021. Opioid prescribing patterns by dental procedure among US publicly and privately insured patients, 2013 through 2018. J Am Dent Assoc. 152(4):309–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels SE, Goulder MA, Aspley S, Reader S. 2011. A randomised, five-parallel-group, placebo-controlled trial comparing the efficacy and tolerability of analgesic combinations including a novel single-tablet combination of ibuprofen/paracetamol for postoperative dental pain. Pain. 152(3):632–642. [DOI] [PubMed] [Google Scholar]
- Denisco RC, Kenna GA, O’Neil MG, Kulich RJ, Moore PA, Kane WT, Mehta NR, Hersh EV, Katz NP. 2011. Prevention of prescription opioid abuse. J Am Dent Assoc. 142(7):800–810. [DOI] [PubMed] [Google Scholar]
- Derry CJ, Derry S, Moore RA. 2013. Single dose oral ibuprofen plus paracetamol (acetaminophen) for acute postoperative pain. Cochrane Database Syst Rev. 6:CD010210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doleman B, Leonardi-Bee J, Heinink TP, Boyd-Carson H, Carrick L, Mandalia R, Lund JN, Williams JP. 2021. Pre-emptive and preventive NSAIDs for postoperative pain in adults undergoing all types of surgery. Cochrane Database Syst Rev. 6(6):CD012978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans CJ, Trudeau E, Mertzanis P, Marquis P, Peña BM, Wong J, Mayne T. 2004. Development and validation of the Pain Treatment Satisfaction Scale (PTSS): a patient satisfaction questionnaire for use in patients with chronic or acute pain. Pain. 112(3):254–266. [DOI] [PubMed] [Google Scholar]
- Feldman CA, Fredericks-Younger J, Lu S-E, Desjardins PJ, Malmstrom H, Miloro M, Warburton G, Ward B, Ziccardi V, Fine D. 2022. The Opioid Analgesic Reduction Study (OARS)—a comparison of opioid vs. non-opioid combination analgesics for management of post-surgical pain: a double-blind randomized clinical trial. Trials. 23(1):160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira-Valente MA, Pais-Ribeiro JL, Jensen MP. 2011. Validity of four pain intensity rating scales. Pain. 152(10):2399–2404. [DOI] [PubMed] [Google Scholar]
- Gilron I, Carr DB, Desjardins PJ, Kehlet H. 2019. Current methods and challenges for acute pain clinical trials. PAIN Rep. 4(3):e647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heagerty PJ, Zeger SL. 2000. Marginalized multilevel models and likelihood inference (with comments and a rejoinder by the authors). Stat Sci. 15(1):1–26. [Google Scholar]
- Heron MJ, Nwokorie NA, O’Connor B, Brown RS, Fugh-Berman A. 2022. Survey of opioid prescribing among dentists indicates need for more effective education regarding pain management. J Am Dent Assoc. 153(2):110–119. [DOI] [PubMed] [Google Scholar]
- Hyllested M, Jones S, Pedersen JL, Kehlet H. 2002. Comparative effect of paracetamol, NSAIDs or their combination in postoperative pain management: a qualitative review. Br J Anaesth. 88(2):199–214. [DOI] [PubMed] [Google Scholar]
- Kean J, Monahan PO, Kroenke K, Wu J, Yu Z, Stump TE, Krebs EE. 2016. Comparative Responsiveness of the PROMIS Pain Interference Short Forms, Brief Pain Inventory, PEG, and SF-36 Bodily Pain Subscale. Med Care. 54(4):414–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manchikanti L, Helm S, II, Fellows B, Janata JW, Pampati V, Grider JS, Boswell MV. 2012. Opioid epidemic in the United States. Pain Physician. 15(3 Suppl):ES9–E38. [PubMed] [Google Scholar]
- McCabe SE, West BT, Boyd CJ. 2013. Leftover prescription opioids and nonmedical use among high school seniors: a multi-cohort national study. J Adolesc Health. 52(4):480–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe SE, Boyd CJ, Evans-Polce RJ, McCabe VV, Schulenberg JE, Veliz PT. 2020. Pills to powder: a 17-year transition from prescription opioids to heroin among US adolescents followed into adulthood.J Addict Med. 15(3):241–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlisch DR. 2002. The efficacy of combination analgesic therapy in relieving dental pain.J Am Dent Assoc. 133(7):861–871. [DOI] [PubMed] [Google Scholar]
- Mehlisch DR, Aspley S, Daniels SE, Bandy DP. 2010. Comparison of the analgesic efficacy of concurrent ibuprofen and paracetamol with ibuprofen or paracetamol alone in the management of moderate to severe acute postoperative dental pain in adolescents and adults: a randomized, double-blind, placebo-controlled, parallel-group, single-dose, two-center, modified factorial study. Clin Ther. 32(5):882–895. [DOI] [PubMed] [Google Scholar]
- Mehlisch DR, Aspley S, Daniels SE, Southerden KA, Christensen KS. 2010. A single-tablet fixed-dose combination of racemic ibuprofen/paracetamol in the management of moderate to severe postoperative dental pain in adult and adolescent patients: a multicenter, two-stage, randomized, double-blind, parallel-group, placebo-controlled, factorial study. Clin Ther. 32(6):1033–1049. [DOI] [PubMed] [Google Scholar]
- Mehlisch DR, Frakes LA. 1984. A controlled comparative evaluation of acetaminophen and aspirin in the treatment of postoperative pain. Clin Ther. 7(1):89–97. [PubMed] [Google Scholar]
- Mehlisch DR, Sollecito WA, Heffrick JF, Leibold DG, Markowitz R, Schow CE, Shultz R, Waite DE. 1990. Multicenter clinical trial of ibuprofen and acetaminophen in the treatment of postoperative dental pain. J Am Dent Assoc. 121(2):257–263. [DOI] [PubMed] [Google Scholar]
- Menhinick KA, Gutmann JL, Regan JD, Taylor SE, Buschang PH. 2004. The efficacy of pain control following nonsurgical root canal treatment using ibuprofen or a combination of ibuprofen and acetaminophen in a randomized, double-blind, placebo-controlled study. Int Endodontic J. 37(8):531–541. [DOI] [PubMed] [Google Scholar]
- Moore PA, Ziegler KM, Lipman RD, Aminoshariae A, Carrasco-Labra A, Mariotti A. 2018. Benefits and harms associated with analgesic medications used in the management of acute dental pain: an overview of systematic reviews. J Am Dent Assoc. 149(4):256–265.e3. [DOI] [PubMed] [Google Scholar]
- Moore RA, Derry S, Aldington D, Wiffen PJ. 2015. Single dose oral analgesics for acute postoperative pain in adults—an overview of Cochrane reviews. Cochrane Database of Systematic Reviews. 2015(9):CD008659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RA, Wiffen PJ, Derry S, Maguire T, Roy YM, Tyrrell L. 2015. Non-prescription (OTC) oral analgesics for acute pain—an overview of Cochrane reviews. Cochrane Database Syst Rev. 11:CD010794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okunev I, Frantsve-Hawley J, Tranby E. 2021. Trends in national opioid prescribing for dental procedures among patients enrolled in Medicaid. J Am Dent Assoc. 152(8):622–630.e3. [DOI] [PubMed] [Google Scholar]
- Ong CKS, Seymour RA, Lirk P, Merry AF. 2010. Combining paracetamol (acetaminophen) with nonsteroidal anti-inflammatory drugs: a qualitative systematic review of analgesic efficacy for acute postoperative pain. Anesth Analg. 110(4):1170–1179. [DOI] [PubMed] [Google Scholar]
- Somerman MJ, Volkow ND. 2018. The role of the oral health community in addressing the opioid overdose epidemic. J Am Dent Assoc. 149(8):663–665. [DOI] [PubMed] [Google Scholar]
- Suda KJ, Zhou J, Rowan SA, McGregor JC, Perez RI, Evans CT, Gellad WF, Calip GS. 2020. Overprescribing of opioids to adults by dentists in the U.S., 2011–2015. Am J Prevent Med. 58(4):473–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zisapel N, Nir T. 2003. Determination of the minimal clinically significant difference on a patient visual analog sleep quality scale.J Sleep Res. 12(4):291–298. [DOI] [PubMed] [Google Scholar]