Abstract
Human β-defensin-1 (hBD-1) is a member of the family of small cationic antimicrobial peptides that have been identified in several mucosal epithelia. Because human gingival epithelium is a site that is constantly challenged by oral microorganisms, we examined the expression of hBD-1 in human gingival epithelial and fibroblast cell cultures and tissue samples. Cell cultures were challenged with cell wall extracts of Porphyromonas gingivalis or Fusobacterium nucleatum, Escherichia coli lipopolysaccharide, tumor necrosis factor alpha, or phorbol myristate acetate. hBD-1 mRNA was detected in unstimulated and stimulated cultures by reverse transcription (RT)-PCR using several primer sets specific for hBD-1. Gingival epithelial cells, but not gingival fibroblasts, expressed a product of the predicted size for hBD-1 mRNA. The sequence of the PCR product was identical to that of hBD-1. hBD-1 mRNA expression was not significantly modulated by any of the stimulants tested. Human gingival tissues from noninflamed and inflamed sites were also analyzed by RT-PCR. hBD-1 mRNA was expressed in all tissue samples. The relative expression of hBD-1 mRNA was similar in noninflamed and inflamed tissues obtained from each of four patients undergoing treatment for periodontitis. However, the relative expression of hBD-1 mRNA varied in gingival biopsies obtained from 15 different normal individuals, and the relative hBD-1 expression was unrelated to interleukin-8 expression. Our findings show the constitutive expression of hBD-1 mRNA in cultured epithelial cells and gingival tissues but not gingival fibroblasts. These findings suggest that expression of hBD-1 may play a role as part of the innate host defenses in maintaining normal gingival health.
Human oral gingival epithelium is a dense, cornified epithelium that has a protective surface, while the sulcular and junctional regions of the gingival epithelium are noncornified and, hence, more readily susceptible to infection (reviewed in reference 40). These epithelia are constantly exposed to a variety of microbial challenges, notably from dental plaque, that can lead to gingivitis and bacterially induced periodontal disease which involves disruption of the epithelial barrier as an early event. Gingival epithelium functions as a mechanically protective barrier, but in addition, the gingival epithelial cells produce various cytokines in response to periodontal microorganisms, for example, interleukin-8 (IL-8), a powerful inducer of neutrophil and T-lymphocyte chemotaxis (29, 47). Neutrophils release granules that contain several types of microbicidal agents, including members of the defensin family of cationic antimicrobial peptides (reviewed in references 9, 23, and 28). Examples include members of the α-defensin subfamily, such as human neutrophil peptides 1 to 4 in azurophilic granules of polymorphonuclear neutrophils, and numerous members of both the α- and β-defensin subfamilies in neutrophils of other vertebrates (17, 41–43). It has recently been shown that mucosal epithelia also express related defensin family members, suggesting that the neutrophils and epithelial cells use similar antimicrobial peptides in innate host defense mechanisms in resisting infection. In human intestinal epithelium, the α-defensins, defensins 5 and 6, are located in granules of Paneth cells (21, 32). Antimicrobial peptides of the β-defensin subfamily that are expressed in epithelia include bovine tracheal and lingual antimicrobial β-defensins (TAP and LAP, respectively) (8, 39) and human β-defensin-1 (hBD-1) and hBD-2. hBD-1 was first identified in plasma filtrate (1) and subsequently found in mucosal epithelia from urogenital and respiratory tracts (33, 49); hBD-2 was identified in psoriatic skin (16). These peptides may provide a first line of defense for mucosal tissues (2, 20). Our hypothesis is that the gingival epithelium, which is constantly exposed to microorganisms of supra- and subgingival plaque, may express these natural antibiotics as part of its protective function. We have initiated a new line of investigations to test the possibility that these defensin peptides are generated by human gingival epithelial (HGE) cells and tissue and have a role in maintaining oral health.
In this study, we showed that cultured HGE cells and gingival tissue express hBD-1 mRNA. The expression of hBD-1 mRNA in cultured cells was constitutive and was not significantly modulated by cytokine- or bacterium-mediated stimulation. Moreover, while there was little or no difference in hBD-1 mRNA between noninflamed and inflamed tissues from the same patient, hBD-1 mRNA levels differed between normal individuals. Our findings suggest that HGE cells constitutively express this antimicrobial peptide as part of the innate host defense mechanisms.
MATERIALS AND METHODS
Culture of HGE cells.
Healthy gingival samples were obtained from the tissue overlying impacted third molar teeth of adult humans. Tissue (dimensions were about 5 by 7 mm) was rinsed twice in HEPES-buffered saline containing 1% penicillin, streptomycin, and 1-μl/ml amphotericin B (Fungizone; GIBCO-BRL, Life Technologies, Grand Island, N.Y.) and cut into small pieces (2 by 2 mm). The explants were treated with 6-mg/ml dispase (Sigma Chemical Co., St. Louis, Mo.) in HEPES-buffered saline overnight at 4°C to separate the epithelium from the underlying fibrous connective tissue. After enzymatic separation, the epithelium was readily lifted off and then incubated at 37°C in 5 ml of trypsin-EDTA (0.05% trypsin, 0.53 mM EDTA; GIBCO-BRL) for 10 min. The epithelial sheets were repeatedly pipetted to prepare a single-cell suspension, and the trypsinization was stopped by addition of an equal amount of Dulbecco’s modified Eagle medium (DMEM) (GIBCO-BRL) supplemented with 10% fetal calf serum (Gemini, Calabasas, Calif.). The cell pellets were collected and resuspended in a serum-free keratinocyte growth medium (Clonetics Corporation, San Diego, Calif.) supplemented with human recombinant epidermal growth factor, hydrocortisone, bovine insulin, bovine pituitary extract, gentamicin sulfate, amphotericin B, and 0.15 mM CaCl2 (35). Resuspended cells were plated in T-25 flasks (Corning Glass Works, Corning, N.Y.) and grown to near confluence in a humidified incubator at 37°C and 5% CO2. Cell lines were frozen at 5 × 105 cells/vial at the first passage by standard procedures. Frozen cell lines used in these studies were thawed and cultured for one additional passage (passage 2) to expand their numbers prior to bacterial and cytokine stimulation.
Culture of HGFs.
Human gingival fibroblast (HGF) cell lines at passage 22 were provided by Martine Michel, Department of Oral Biology, University of Washington. These cells were thawed, plated at 2 × 105/ml in 100-mm-diameter dishes, and cultured in DMEM (GIBCO-BRL) supplemented with l-glutamine, 10% fetal calf serum, and 1% penicillin-streptomycin. Primary HGFs were derived from normal gingival connective tissue taken from gingival biopsies overlying impacted third molars after the epithelium was removed. Connective tissue was incubated in DMEM supplemented with 10% fetal calf serum and 1% penicillin-streptomycin in a 60-mm-diameter petri dish for 2 to 3 weeks or until a sufficient number of fibroblasts spread from the tissue. A 3-ml volume of trypsin-EDTA was used to release and collect fibroblasts surrounding the tissue. An equal volume of DMEM plus 10% fetal calf serum was added to stop the action of trypsin. Primary HGFs were plated into two 100-mm-diameter petri dishes and then passaged for one additional passage after confluency to expand their number prior to bacterial and cytokine (tumor necrosis factor alpha [TNF-α]) stimulation.
Bacterial crude cell wall preparation.
Anaerobic cultures of Fusobacterium nucleatum ATCC 25586 and Porphyromonas gingivalis ATCC 33277 were grown, and crude cell walls were prepared by differential centrifugation as previously described (24). Briefly, cells were scraped from plates and suspended in phosphate-buffered saline without serum. The cells were broken by passage through a French pressure cell at 15,000 lb/in2 in the presence of a cocktail of protease inhibitors which included 2 mM (final concentration) each Pefabloc SC (Boehringer GmbH, Mannheim, Germany), Nα-p-tosyl-l-lysine chloromethyl ketone (TLCK)-HCl, and benzamidine (Sigma Chemical Co.). Unbroken cells were removed by low-speed centrifugation at 2,200 × g for 10 min at 4°C. The crude cell wall fraction was collected from the supernatant by high-speed centrifugation at 30,000 × g for 20 min at 4°C. The extract was resuspended in 0.5 ml of phosphate-buffered saline for total protein determinations. Protein concentration was determined by bicinchoninic acid assay (Pierce Chemical Co., Rockford, Ill.) as described in the manufacturer’s instructions.
Cell stimulation.
Cultures of HGFs or HGE cells were grown to approximately 80% cell confluence and then stimulated for 24 h with 10- or 100-μg/ml F. nucleatum or P. gingivalis cell wall extract, with 1-, 10-, or 100-ng/ml E. coli 026:B6 lipopolysaccharide (LPS; Sigma Chemical Co.), or with 1-, 10-, or 100-ng/ml recombinant human TNF-α (R&D Systems, Minneapolis, Minn.) in the presence or absence of 1% human serum. After stimulation, cells were lysed directly with RNA extraction buffer.
RNA preparation and analysis.
Total RNA was harvested and purified by using an RNA-Stat 30 kit (Tel-Test “B,” Inc., Friendswood, Tex.) in accordance with the manufacturer’s protocol. The total RNA concentration in each sample was calculated from the A260. cDNA was synthesized from 3 μg of total RNA by using the SUPERSCRIPT Preamplification System (GIBCO-BRL) in accordance with the manufacturer’s instructions. Digestion of genomic DNA possibly contaminating RNA samples was performed by using DNase I (GIBCO-BRL) prior to reverse transcription (RT) for some samples. Ten microliters of a 1:5 dilution of cDNA in a total volume of 50 μl was used for PCR analysis. PCR amplification was performed by using 0.25 μl of Amplitaq DNA polymerase (Perkin Elmer, Branchburg, N.J.), 1 μl of each 10 mM deoxynucleoside triphosphate, 6.25 μl of GeneAmp 10× PCR buffer II, 5 μl of 25 mM MgCl2, 1 μl of each specific upstream and downstream primer at 25 μM, and water with the hot-start method to enhance the sensitivity and specificity of amplification. Upper and lower mixture reagents were prepared and then separated by melted Ampliwax PCR Gem 100 (Roche Molecular Systems, Inc., Foster City, Calif.). The sequences of the oligonucleotide primers and the specific annealing temperatures used in the PCR are summarized in Table 1. The denaturing and polymerizing temperatures were 94 and 72°C, respectively. The oligonucleotide primers were synthesized by GIBCO-BRL. For the locations of the hBD-1 primers in the cDNA, see Fig. 2. These are intron-spanning primers. In most experiments, the DNA targets were amplified for 28 and 35 cycles or for 22, 25, and 28 cycles as a means to more accurately interpret the differences between the relative amounts of amplified products obtained under the different conditions. Results were evaluated in the region of increasing amplification for each primer. The gene for ribosomal phosphoprotein (RPO), a housekeeping gene, was amplified as a control for equal loading in all samples. In addition, keratin 5, an epithelial cell product, was amplified in tissue samples for comparing the contribution of cDNA in the epithelial compartment, excluding the connective tissue and other cell types that may have been present. The PCR products were separated by electrophoresis on a 1.5% agarose gel, and their sizes were compared with a standard DNA marker, φX174RF HaeIII fragments (GIBCO-BRL). The largest DNA band (from the 5.1 and 3.1 hBD-1 primer pair) was cut from a low-melting-point agarose gel (GIBCO-BRL) and further purified by S&S Elutip minicolumns (Schleicher & Schuell, Inc., Keene, N.H.). The identity of each purified PCR product was confirmed by sequencing analysis using internal primers (5.1 and 3.1) at the DNA Sequencing Facility, Department of Biochemistry, University of Washington, and compared with the full cDNA sequence of hBD-1 in the GenBank database (accession no. X92744). An hBD-1 plasmid was used as a positive control in initial studies. This plasmid was a generous gift of Mark G. Anderson, Magainin Pharmaceuticals Inc. All experiments were replicated two or three times, and similar results were observed.
TABLE 1.
Primer sequences and annealing temperatures
| Primer | Sequence | Annealing temp (°C) |
|---|---|---|
| 5.1 | ATG AGA ACT TCC TAC CTT CTG CT | 52 |
| 5.2 | CTC TGC TTA CTT TTG TCT G | 52 |
| 3.1 | TCA CTT GCA GCA CTT | 52 |
| 3.2 | CTG TGT AAC AGG TGC C | 52 |
| IL-8 (5′) | TTT CTG ATG GAA GAG AGC TCT GTC TGG | 60 |
| IL-8 (3′) | AGT GGA ACA AGG ACT TGT GGA TCC TGG | 60 |
| RPO (5′) | AGC AGG TCT TCG ACA ATG GCA | 47 |
| RPO (3′) | ACT CTT CCT TGG CTT CAA CC | 47 |
| Keratin 5 (5′) | GTC CTC TCC ATG GAC AAC AAC | 49 |
| Keratin 5 (3′) | TGT CAA TCT CGG CTC TCA GCC | 49 |
FIG. 2.
Comparison of the translated region of the hBD-1 DNA sequence (upper line) and the nucleotide sequence of a PCR product generated from primers 5.1 and 3.1 (lower line). Bold and double-underlined letters represent the location of primer 5.1, italic and double-underlined letters represent that of primer 5.2, italic and underlined letters represent that of primer 3.2, and bold and underlined letters represent that of primer 3.1. The sequence is identical to the hBD-1 sequence (GenBank accession no. X92744).
Densitometric analysis of PCR results.
Ethidium bromide-stained gels were analyzed by densitometry and compared in a semiquantitative manner within a single experiment by using Kodak 1D gel analysis software. The relative ratio of the net intensities of the hBD-1 and keratin 5 bands from the same subject (less the background intensity) was determined to show the relative amount of hBD- 1 mRNA expression between subjects and to examine the possible correlation between these ratios and the presence of IL-8 mRNA expression, an indicator for tissue activation. The values were calculated for 22 and 25 cycles of PCR amplification to avoid overamplification of PCR products at 28 cycles.
Human gingival tissue samples.
Normal gingival tissue samples were obtained from tissue overlying impacted third molars (age range, 17 to 30 years) from 15 different patients. Additional gingival tissues were collected from 12 adult patients undergoing periodontal surgery at the Graduate Periodontal Clinic and Hospital Dentistry, School of Dentistry, University of Washington, in accordance with an approved human subjects protocol. Consent was obtained from all subjects. The weight and dimensions of each tissue sample were determined. Total RNA was immediately harvested by homogenizing fresh tissue samples with 1 ml of TRIZOL reagent (GIBCO-BRL) per 50 to 100 mg of tissue in accordance with the manufacturer’s protocol, followed by phenol-chloroform separation and alcohol precipitation. The resulting RNA pellets were resuspended in 100 μl of diethyl pyrocarbonate-treated water, and the RNA yields were determined by UV absorbance. cDNA was synthesized from 3 μg of total RNA by using the SUPERSCRIPT Preamplification System (GIBCO-BRL) in accordance with the manufacturer’s instructions. Genomic DNA digestion by DNase I (GIBCO-BRL) was always done before RT. RT-PCR and PCR product analyses were then performed as described above.
RESULTS
Expression of hBD-1 mRNA in cultured HGE cells.
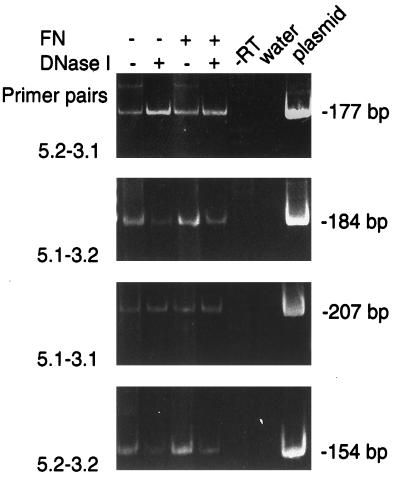
hBD-1 mRNA was amplified from both unstimulated HGE cells and cells stimulated with the F. nucleatum cell wall extract (Fig. 1). An hBD-1 plasmid served as a positive control, while water was a negative control for the PCR. The sizes of DNA bands were as predicted when each of the four primer pairs was used. DNase I digestion prior to RT clearly eliminated the nonspecific binding of the primers to DNA (for example, Fig. 1, primers 5.2 and 3.1) that was a minor contaminant in RNA samples. The PCR product from the largest DNA product (primers 5.1 and 3.1) was purified and sequenced by using internal primers. The sequence was confirmed to be identical to the cDNA sequence of hBD-1 in the translated region (Fig. 2).
FIG. 1.
Expression of hBD-1 mRNA in cultured HGE cells stimulated with F. nucleatum cell wall extract (FN) as analyzed by 35 cycles of RT-PCR. HGE cells were cultured in serum-free keratinocyte growth medium and incubated overnight with 100-μg/ml F. nucleatum cell wall extract (+) or were unstimulated (−). DNase I was used in some samples to digest the genomic DNA possibly contaminating the RNA samples. PCR products, as described in Materials and Methods, were separated by electrophoresis on a 1.5% agarose gel and stained with ethidium bromide. For the locations of the four primers, see Fig. 2. −RT denotes a control in which the reverse transcriptase enzyme was omitted. The water lane was the negative control. The molecular sizes of PCR products from RNA samples and the hBD-1 plasmid (plasmid lane) were predicted from the sequences and were in accordance with the molecular size markers (φX174RF HaeIII fragments [not shown]).
Assessment of regulation of hBD-1 mRNA expression.
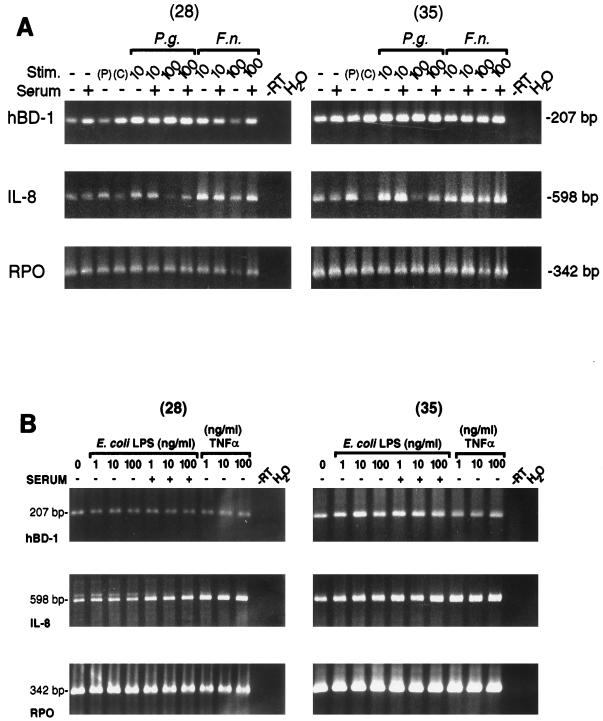
To examine regulation of hBD-1 mRNA expression, HGE cells were stimulated with P. gingivalis or F. nucleatum cell wall extracts, phorbol myristate acetate (PMA), E. coli LPS, or TNF-α. IL-8, which is known to be responsive to cell activation (47), was used as a control. hBD-1 mRNA was not significantly different in control cells and cells activated by any of the stimuli used (Fig. 3A and B). Similar results were seen in three separate analyses. In contrast, IL-8 expression appeared to be up-regulated in cells challenged by the F. nucleatum cell wall extract at 10 and 100 μg/ml (Fig. 3A) and TNF-α (Fig. 3B). This result was readily confirmed in additional experiments using shorter stimulation times. It was interesting that the IL-8 mRNA seemed to be down-regulated in a dose-dependent manner in HGE cells challenged by the P. gingivalis cell wall extract (Fig. 3A), consistent with results of others (4, 31), although there was little or no effect on hBD-1 expression.
FIG. 3.
(A) Constitutive expression of hBD-1 mRNA in cultured HGE cells stimulated (Stim.) either with 10- or 100-μg/ml P. gingivalis (P.g.) or F. nucleatum (F.n.) cell wall extract in the presence or absence of 1% human serum or with 10-ng/ml PMA (P) overnight. C represents the control lane for PMA (only dimethyl sulfoxide, used as the vehicle for PMA, was added). PCR amplification was performed for 28 or 35 cycles by using primer pairs for hBD-1 (5.1-3.1 primer pair), IL-8 (a control marker indicating cellular response), and RPO, a control housekeeping gene for equivalent loading. It was evident that the apparent decrease in hBD-1 mRNA from HGE cells challenged by 100-μg/ml F. nucleatum cell wall extract in the absence of serum resulted from loading less of the RNA sample in the RT reaction mixture, since this lane gave a consistently lower signal. The sizes of PCR products were as predicted. Note the reduced signal for IL-8 with the higher dose of P. gingivalis. (B) Constitutive expression of hBD-1 mRNA in cultured HGE cells stimulated overnight either with various doses (1, 10, or 100 ng/ml) of E. coli LPS in the presence or absence of 1% human serum or with TNF-α (1, 10, or 100 ng/ml) in the absence of human serum. Three different primer pairs were included in the PCR amplification for 28 or 35 cycles, as for panel A. Note that hBD-1 expression is consistent in all lanes.
Lack of hBD-1 mRNA expression by cultured HGFs.
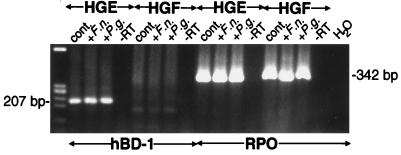
A comparison of hBD-1 expression in HGFs and HGE cells is shown in Fig. 4. Unlike cultured HGE cells, unstimulated or stimulated HGFs did not express hBD-1 mRNA. RPO served as a PCR control and a control for equivalent loading of RNA. hBD-1 was not detected in either primary HGFs (data not shown) or in HGFs (Fig. 4) that had been maintained in culture for multiple passages.
FIG. 4.
hBD-1 is expressed in cultured HGE cells and not HGFs. No hBD-1 expression was detected in control (cont.) HGF cells and cells stimulated with 100-μg/ml F. nucleatum (F.n.) or P. gingivalis (P.g.) cell wall extract overnight (HGFs from passage 22). Unlike HGFs, both control and stimulated cultured HGE cells constitutively expressed hBD-1 mRNA. PCR amplification was done for 35 cycles to enhance the detection of a small signal in the HGFs. A housekeeping gene (RPO) served as a positive control for RT-PCR and RNA quality in samples. Similar results were obtained when primary HGFs were either left unstimulated or stimulated with E. coli LPS and TNF-α and then tested for hBD-1 expression.
Expression of hBD-1 mRNA in gingival tissue samples.
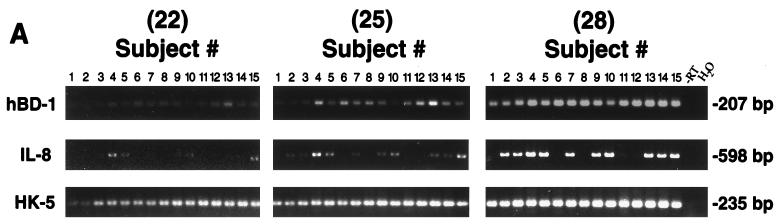
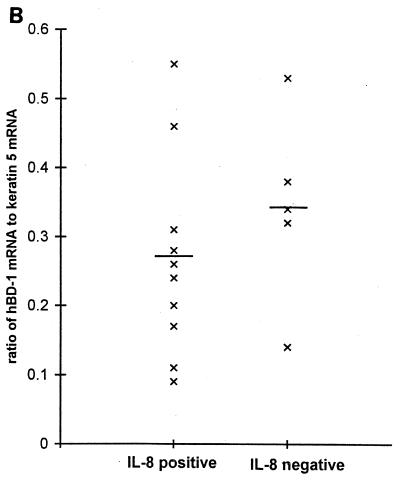
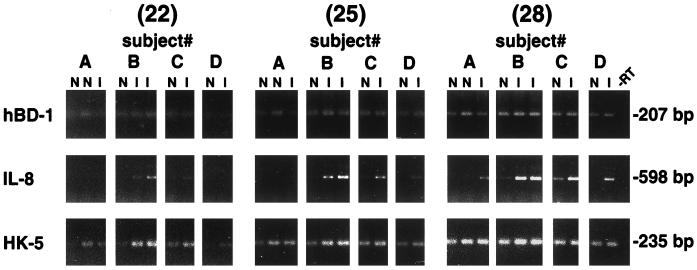
To determine expression of hBD-1 in gingival tissue in vivo, RNA was extracted from tissue freshly obtained from 15 normal individuals undergoing third molar extraction and from 12 patients undergoing periodontal surgery. Multiple normal samples, as well as inflamed and noninflamed tissue samples, were analyzed. hBD-1 mRNA was expressed in every sample tested. Results in Fig. 5A show RT-PCR results from 15 normal subjects, and densitometric analysis results are shown in Fig. 5B. For comparisons of tissue samples, an epithelial protein, keratin 5, was used to evaluate the relative contribution of the epithelial compartment. The relative ratio of net intensities of hBD-1 and keratin 5 mRNA expression varied between normal individuals (Fig. 5A and also in a graphical format in Fig. 5B), suggesting differential hBD-1 expression. Although relative PCR is only semiquantitative, this variation is most evident at a low PCR cycle number, when overamplification of cDNA targets is avoided. IL-8 mRNA expression was readily detected in a subset of the normal gingival samples (n = 10) (Fig. 5A), suggesting tissue activation even though inflammation was not clinically evident. IL-8-positive samples were also positive for TNF-α expression (data not shown), another indicator of tissue inflammation (38). However, the relative level of hBD-1 mRNA expression was not correlated with IL-8 expression (Student’s t test; P = 0.40).
FIG. 5.
(A) RT-PCR analysis of hBD-1 mRNA expression in normal human gingival tissue samples. hBD-1 expression was detected as a 207-bp fragment, IL-8 expression was detected as a 598-bp fragment, and keratin 5 was detected as a 235-bp fragment. All products were amplified for 22, 25, and 28 cycles. Note the variation in hBD-1 expression which is most evident at 25 cycles. (B) Relative expression of hBD-1 is not related to IL-8 expression. hBD-1 expression was analyzed relative to keratin 5 expression by densitometry of the results in panel A and shown in a graphical format. The y axis represents relative ratios of hBD-1 PCR signal at 25 cycles to the keratin 5 signal at 25 cycles. The density values of hBD-1 expression at 25 cycles were chosen for comparisons between individuals because of the obvious differences in expression seen in panel A. Each point represents the relative expression of hBD-1 from a single individual; the population is divided into IL-8-positive (n = 10; mean ± standard deviation; 0.27 ± 0.15) and IL-8-negative (n = 5; 0.34 ± 0.14) groups. The mean value of each group is indicated by a horizontal line. The relative level of hBD-1 mRNA expression was not correlated with IL-8 expression (Student’s t test; P = 0.40).
For a limited number of subjects, noninflamed and inflamed tissue samples were available from the same subject (n = 4). The results of the RT-PCR assay for hBD-1 in these samples are shown in Fig. 6. Expression of IL-8 is enhanced in inflamed regions in these cases (especially in subjects A and C), consistent with the results of others (47). In contrast, hBD-1 expression is seen in every sample and the levels of hBD-1 detected are similar in noninflamed and inflamed samples taken from the same subject, especially when evaluated relative to the keratin 5 signal.
FIG. 6.
Comparison of the expression of hBD-1 mRNA in clinically noninflamed tissue (N) and clinically inflamed tissue (I) from the same subjects (A to D). Due to restricted tissue sample availability and size, tissues from only four different subjects could be compared. The predicted sizes of the DNA fragments generated from the hBD-1, IL-8, and keratin 5 primers were the same as in Fig. 5A. All products were amplified for 22, 25, and 28 cycles. Note that although the IL-8 signal varies between the normal and inflamed sites, the hBD-1 signal is quite consistent, especially relative to the keratin 5 signal.
DISCUSSION
This report is the first to show expression of β-defensin-specific mRNA in human oral mucosal epithelial cells or tissue. Our findings demonstrate that the β-defensin hBD-1, previously found in the human kidney, lung, and urogenital tract (33, 48, 49), is also expressed in the human gingiva surrounding the teeth, a site of constant microbial challenge and frequent inflammation. Interestingly, while other mammalian models, such as the bovine tracheal and lingual epithelium, demonstrate β-defensin stimulation at sites of infection or inflammation (5, 39), our findings indicate that hBD-1 mRNA is constitutively expressed in both clinically normal and inflamed tissues and in cultured HGE cells. Moreover, while E. coli LPS and TNF-α enhance β-defensin mRNA expression in bovine tracheal epithelial cells (7), these agents fail to stimulate hBD-1 mRNA expression in cultured HGE cells. Our results coincide with those of others who have reported constitutive expression of hBD-1 in human airway and endocervical epithelium (33, 48, 49). These results imply that different signal transduction pathways mediate hBD-1 induction in human gingival epithelium and that of the bovine β-defensins (TAP and LAP) in their respective cell types. Interestingly, the binding site for nuclear factor κB, the mammalian transcription factor which has been shown to mediate the induction of a variety of genes, including those involved in immune and inflammatory responses (25), is present in the upstream regulatory region of the bovine TAP gene (6) and presumably also in the LAP gene (39). It is absent in the hBD-1 gene (30, 33) (GenBank accession no. U50930 and U50931), and this could explain why E. coli LPS and TNF-α do not up-regulate hBD-1 in primary HGE cell cultures. The gene for hBD-1 does, however, contain nuclear factor-IL-6 and gamma interferon consensus sites in its upstream flanking region, suggesting that specific inflammatory mediator involvement may have a role in regulating hBD-1 mRNA. Thus, further studies of the induction of hBD-1 in primary epithelial cell lines by inflammatory mediators could lead to understanding of how this β-defensin is regulated in human mucosa in the oral cavity and at other body sites. On the other hand, if hBD-1 expression is not enhanced by inflammation, other factors, such as growth factors, steroids, and cell differentiation and development, may play a role in its regulation (18, 19).
Recent findings implicate hBD-1 in the normal defense of the human airway epithelium. Tracheal epithelial cells from cystic fibrosis (CF) patients are highly susceptible to microbial colonization, in contrast to normal airway cells (45). The apparent cause for susceptibility is that the defect in the CF gene product, the CF transmembrane conductance regulator, leads to elevated NaCl in airway surface fluid (11, 22), which dramatically decreases the antimicrobial activity of airway surface fluid (45). One factor which is produced by airway epithelial cells, and is inactivated in the high-salt milieu of CF, is hBD-1 (12). The potential role of hBD-1 was shown by using hBD-1 antisense, which abolished bactericidal activity from xenographs (12). Lack of function of hBD-1 is believed, therefore, to contribute to bacterial adherence and colonization and subsequent inflammation and tissue destruction in CF. By analogy with CF, the inflammation of periodontal disease might be viewed as a situation in which epithelial antimicrobial peptides could offer the first line of host defense, but their action could be inhibited by specific bacterial products, i.e., bacterial proteases (3, 27, 37); by host-regulated factors; and/or by physiological surface conditions.
Periodontitis is an inflammatory disorder resulting from a complex biofilm of “friendly” commensals and periopathogenic bacteria and their products, especially the gram-negative organisms Bacteroides forsythus, P. gingivalis, and Treponema denticola (13, 14, 34, 46), while F. nucleatum is considered to be an oral commensal microorganism found at both healthy and diseased sites. The recognized shift in composition from gram-positive, aerobic, fermentative microorganisms to predominantly gram-negative, anaerobic, chemoorganotrophic, and proteolytic organisms has long been correlated with periodontal tissue breakdown (44). In addition, the severity of tissue breakdown has been associated with the degree of host “predisposition” (10), undoubtedly involving a complex interplay between the host and the resident oral microorganisms. Such predisposing factors may range from behavioral ones (i.e., stress, smoking) to genetic susceptibility relating to host responses. In this light, the first demonstration of a variant in a specific genetic marker leading to overproduction of a cytokine was recently reported showing the IL-1β genotype as a severity factor in adult periodontal disease (26). In this report, we show that the level of hBD-1 mRNA expression relative to that of keratin 5 varies between individuals (Fig. 5A and B), although we recognize that relative PCR is only semiquantitative and suggest the need for further verification. The pattern of variation in hBD-1 mRNA expression was not found to be correlated with age or cytokine (IL-8 and TNF-α) expression in these normal subjects. Further evaluation of a possible correlation of hBD-1 expression or that of other gingival epithelial antimicrobial peptides with health and disease could lead to the identification of additional genetic markers predisposing to periodontitis or to specific forms of periodontal disease.
Epithelial defensin peptides contain both hydrophilic and hydrophobic portions which facilitate their insertion into phospholipid membranes of microorganisms, leading to selective toxicity of these peptides for a wide range of bacterial species. Due to the lack of a specific lipid and receptor requirement on target cells, these peptides have a broad range of antimicrobial activities (reviewed in references 15 and 23). In light of an increasing problem with microbial resistance to conventional antibiotics, identification of naturally synthesized antimicrobial peptides, such as defensins, has potential significance for therapeutic applications. While some antimicrobial peptides may be cytotoxic, i.e., α-defensins of human neutrophils (36), there is no evidence that β-defensins are cytotoxic to mammalian cells. hBD-1, for example, is presumed to be secreted and to function in the extracellular environment (12, 48). The β-defensin peptides may be especially important at mucosal sites and have a natural role in diseases whose etiologies involve multiple bacterial species and multiple host inflammatory mediators, such as periodontal disease.
ACKNOWLEDGMENTS
We are grateful to Mark G. Anderson, Magainin Pharmaceuticals Inc., for providing hBD-1 plasmid DNA. We thank Philip Fleckman and Martine Michel for assistance with the cell culture and use of facilities and Robert O’Neal, the Graduate Periodontics Clinic, and Rutger Persson for providing human gingival tissue.
This study was supported by a Hack Research Endowment grant, the UW Royalty Research Fund (to B.A.D.), and NIH T35 DE07150 and NIH DE10329 (to A.W.).
REFERENCES
- 1.Bensch K W, Raida M, Magert H J, Schulz K-P, Forssmann W G. hBD 1: a novel beta defensin from human plasma. FEBS Lett. 1995;368:331–335. doi: 10.1016/0014-5793(95)00687-5. [DOI] [PubMed] [Google Scholar]
- 2.Bevins C L. Antimicrobial peptides as agents of mucosal immunity. Ciba Found Symp. 1994;186:250–260. doi: 10.1002/9780470514658.ch15. [DOI] [PubMed] [Google Scholar]
- 3.Curtis M A, Ramakrishnan M, Slaney J M. Characterization of the trypsin like enzymes of Porphyromonas gingivalis W83 using a radiolabelled active site directed inhibitor. J Gen Microbiol. 1993;139:949–955. doi: 10.1099/00221287-139-5-949. [DOI] [PubMed] [Google Scholar]
- 4.Darveau R P, Belton C M, Reife R A, Lamont R J. Local chemokine paralysis: a novel mechanism of bacterial persistence. Infect Immun. 1998;66:1660–1665. doi: 10.1128/iai.66.4.1660-1665.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diamond G, Bevins C L. Endotoxin upregulates expression of an antimicrobial peptide gene in mammalian airway epithelial cells. Chest. 1994;105(3 Suppl.):51S–52S. doi: 10.1378/chest.105.3_supplement.51s. [DOI] [PubMed] [Google Scholar]
- 6.Diamond G, Jones D E, Bevins C L. Airway epithelial cells are the site of expression of a mammalian antimicrobial peptide gene. Proc Natl Acad Sci USA. 1993;90:4596–4600. doi: 10.1073/pnas.90.10.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diamond G, Russell J P, Bevins C L. Inducible expression of an antibiotic peptide gene in lipopolysaccharide-challenged tracheal epithelial cells. Proc Natl Acad Sci USA. 1996;93:5156–5160. doi: 10.1073/pnas.93.10.5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diamond G, Zasloff M, Eck H, Brasseur M, Maloy W L, Bevins C L. Tracheal antimicrobial peptide, a cysteine rich peptide from mammalian tracheal mucosa: peptide isolation and cloning of a cDNA. Proc Natl Acad Sci USA. 1991;88:3952–3956. doi: 10.1073/pnas.88.9.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ganz T, Lehrer R I. Antimicrobial peptides of leukocytes. Curr Opin Hematol. 1997;4:53–58. doi: 10.1097/00062752-199704010-00009. [DOI] [PubMed] [Google Scholar]
- 10.Genco R J. Host responses in periodontal diseases: current concepts. J Periodontol. 1992;63:338–355. doi: 10.1902/jop.1992.63.4s.338. [DOI] [PubMed] [Google Scholar]
- 11.Gilljam H, Ellin A, Strandvik B. Increased bronchial chloride concentration in cystic fibrosis. Scand J Clin Lab Invest. 1989;49:121–124. doi: 10.3109/00365518909105409. [DOI] [PubMed] [Google Scholar]
- 12.Goldman M J, Anderson G M, Stolzenberg E D, Kari U P, Zasloff M, Wilson J M. Human beta defensin 1 is a salt sensitive antibiotic in lung that is inactivated in cystic fibrosis. Cell. 1997;88:553–560. doi: 10.1016/s0092-8674(00)81895-4. [DOI] [PubMed] [Google Scholar]
- 13.Haffajee A D, Cugini M A, Tanner A, Pollack R P, Smith C, Kent R L., Jr Subgingival microbiota in healthy, well-maintained elder and periodontitis subjects. J Clin Periodontol. 1998;25:346–353. doi: 10.1111/j.1600-051x.1998.tb02454.x. [DOI] [PubMed] [Google Scholar]
- 14.Haffajee A D, Socransky S. Microbial etiological agents of destructive periodontal diseases. Periodontol 2000. 1994;5:78–111. doi: 10.1111/j.1600-0757.1994.tb00020.x. [DOI] [PubMed] [Google Scholar]
- 15.Hancock R E W. Peptide antibiotics. Lancet. 1997;349:418–422. doi: 10.1016/S0140-6736(97)80051-7. [DOI] [PubMed] [Google Scholar]
- 16.Harder J, Bartels J, Christophers E, Schroder J M. A peptide antibiotic from human skin. Nature. 1997;387:861. doi: 10.1038/43088. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 17.Harwig S S, Swiderek K M, Kokryakow V N, Tan L, Lee T D, Panyutich E A, Aleshina G M, Shamova O V, Lehrer R I. Gallinacins: cysteine-rich antimicrobial peptides of chicken leukocytes. FEBS Lett. 1994;342:281–285. doi: 10.1016/0014-5793(94)80517-2. [DOI] [PubMed] [Google Scholar]
- 18.Herwig S, Su Q, Zhang W, Ma Y S, Tempst P. Distinct temporal patterns of defensin mRNA regulation during drug-induced differentiation of human myeloid leukemia cells. Blood. 1996;87:350–364. [PubMed] [Google Scholar]
- 19.Huttner K M, Brezinski-Caliguri D J, Mahoney M M, Diamond G. Antimicrobial peptide expression is developmentally regulated in the ovine gastrointestinal tract. J Nutr. 1998;128:297S–299S. doi: 10.1093/jn/128.2.297S. [DOI] [PubMed] [Google Scholar]
- 20.Jones D E, Bevins C L. Defensin 6 mRNA in human Paneth cells: implications for antimicrobial peptides in host defense of the human bowel. FEBS Lett. 1993;315:187–192. doi: 10.1016/0014-5793(93)81160-2. [DOI] [PubMed] [Google Scholar]
- 21.Jones D E, Bevins C L. Paneth cells of the human small intestine express an antimicrobial peptide gene. J Biol Chem. 1992;267:23216–23225. [PubMed] [Google Scholar]
- 22.Joris L, Dab I, Quinton P M. Elemental composition of human airway surface fluid in healthy and diseased airways. Am Rev Respir Dis. 1993;148:1633–1637. doi: 10.1164/ajrccm/148.6_Pt_1.1633. [DOI] [PubMed] [Google Scholar]
- 23.Kagan B L, Ganz T, Lehrer R I. Defensins: a family of antimicrobial and cytotoxic peptides. Toxicology. 1994;87:131–149. doi: 10.1016/0300-483x(94)90158-9. [DOI] [PubMed] [Google Scholar]
- 24.Kennell W, Holt S C. Comparative studies of the outer membranes of Bacteroides gingivalis, strains ATCC 33277, W50, W83, 381. Oral Microbiol Immunol. 1990;5:121–130. doi: 10.1111/j.1399-302x.1990.tb00409.x. [DOI] [PubMed] [Google Scholar]
- 25.Kopp E B, Ghosh S. NF-κB and Rel proteins in innate immunity. Adv Immunol. 1995;58:1–27. doi: 10.1016/s0065-2776(08)60618-5. [DOI] [PubMed] [Google Scholar]
- 26.Kornman K S, Crane A, Wang H Y, deGiovine F S, Newman M G, Pirk R W, Wilson T G, Higginbottom F L, Duff G W. The interleukin-1 genotype as a severity factor in adult periodontal disease. J Clin Periodontol. 1997;24:72–77. doi: 10.1111/j.1600-051x.1997.tb01187.x. [DOI] [PubMed] [Google Scholar]
- 27.Lantz M S, Allen R D, Ciborowski P, Holt S C. Purification and immunolocalization of a cysteine protease from Porphyromonas gingivalis. J Periodontal Res. 1993;28:467–469. doi: 10.1111/j.1600-0765.1993.tb02104.x. [DOI] [PubMed] [Google Scholar]
- 28.Lehrer R I, Ganz T. Endogenous vertebrate antibiotics. Defensins, protegrins, and other cysteine rich antimicrobial peptides. Ann N Y Acad Sci. 1996;797:228–239. doi: 10.1111/j.1749-6632.1996.tb52963.x. [DOI] [PubMed] [Google Scholar]
- 29.Li J, Ireland G W, Farthing R M, Thornhill M H. Epidermal and oral keratinocytes are induced to produce RANTES and IL-8 by cytokine stimulation. J Invest Dermatol. 1996;106:661–666. doi: 10.1111/1523-1747.ep12345482. [DOI] [PubMed] [Google Scholar]
- 30.Liu L, Zhao C, Heng H H Q, Ganz T. The human beta defensin-1 and alpha-defensins are encoded by adjacent genes: two families with differing disulfide topology share a common ancestry. Genomics. 1997;43:316–320. doi: 10.1006/geno.1997.4801. [DOI] [PubMed] [Google Scholar]
- 31.Madianos P N, Papapanou P N, Sandros J. Porphyromonas gingivalis infection of oral epithelium inhibits neutrophil transepithelial migration. Infect Immun. 1997;65:3983–3990. doi: 10.1128/iai.65.10.3983-3990.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mallow E B, Harris A, Salzman N, Russell J P, DeBerardinis R J, Ruchelli E, Bevins C L. Human enteric defensins. J Biol Chem. 1996;271:4038–4045. doi: 10.1074/jbc.271.8.4038. [DOI] [PubMed] [Google Scholar]
- 33.McCray P B, Bentley L. Human airway epithelia express a β-defensin. Am J Respir Cell Mol Biol. 1997;16:343–349. doi: 10.1165/ajrcmb.16.3.9070620. [DOI] [PubMed] [Google Scholar]
- 34.Moore W E C, Moore L V H. The bacteria of periodontal diseases. Periodontology 2000. 1994;5:66–77. doi: 10.1111/j.1600-0757.1994.tb00019.x. [DOI] [PubMed] [Google Scholar]
- 35.Oda D, Watson E. Human oral epithelial cell culture. I. Improved conditions for reproducible culture in serum free medium. In Vitro Cell Dev Biol. 1990;26:589–595. doi: 10.1007/BF02624208. [DOI] [PubMed] [Google Scholar]
- 36.Okrent D G, Lichtenstein A K, Ganz T. Direct cytotoxicity of polymorphonuclear leukocyte granule proteins to human lung-derived cells and endothelial cells. Am Rev Respir Dis. 1990;141:179–185. doi: 10.1164/ajrccm/141.1.179. [DOI] [PubMed] [Google Scholar]
- 37.Pike R, McGraw W, Potempa J, Travis J. Lysine and arginine specific proteinases from Porphyromonas gingivalis. Isolation, characterization, and evidence for the existence of complexes with hemagglutinins. J Biol Chem. 1994;269:406–411. [PubMed] [Google Scholar]
- 38.Roberts F A, McCaffery K A, Michalek S M. Profile of cytokine mRNA expression in chronic adult periodontitis. J Dent Res. 1997;76:1833–1839. doi: 10.1177/00220345970760120501. [DOI] [PubMed] [Google Scholar]
- 39.Schonwetter B S, Stolzenberg E D, Zasloff M A. Epithelial antibiotics induced at sites of inflammation. Science. 1995;267:1645–1648. doi: 10.1126/science.7886453. [DOI] [PubMed] [Google Scholar]
- 40.Schroeder H E, Listgarten M A. The gingival tissues: the architecture of periodontal protection. Periodontol 2000. 1997;13:91–120. doi: 10.1111/j.1600-0757.1997.tb00097.x. [DOI] [PubMed] [Google Scholar]
- 41.Selsted M E, Brown D M, DeLange R J, Harwig S S, Lehrer R I. Primary structures of six antimicrobial peptides of rabbit peritoneal neutrophils. J Biol Chem. 1985;260:4579–4584. [PubMed] [Google Scholar]
- 42.Selsted M E, Harwig S S, Ganz T, Schilling J W, Lehrer R I. Primary structures of three human neutrophil defensins. J Clin Invest. 1985;76:1436–1439. doi: 10.1172/JCI112121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Selsted M E, Tang Y Q, Morris W L, McGuire P A, Novotny M J, Smith W, Henschen A H, Cullor J S. Purification, primary structures, and antibacterial activities of beta defensins, a new family of antimicrobial peptides from bovine neutrophils. J Biol Chem. 1993;268:6641–6648. [PubMed] [Google Scholar]
- 44.Slots J, Rams T E. Microbiology of periodontal disease. In: Slots J, Taubman M A, editors. Contemporary oral microbiology and immunology. St. Louis, Mo: Mosby Year Book; 1992. pp. 425–443. [Google Scholar]
- 45.Smith J J, Travis S M, Greenberg E P, Welsh M J. Cystic fibrosis airway epithelia fail to kill bacteria because of abnormal airway surface fluid. Cell. 1996;85:229–236. doi: 10.1016/s0092-8674(00)81099-5. [DOI] [PubMed] [Google Scholar]
- 46.Socransky S S, Haffajee A D, Cugini M A, Smith C, Kent R L J. Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25:134–144. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 47.Tonetti M S, Imboden M A, Gerber L, Lang N P, Laissue J, Mueller C. Localized expression of mRNA for phagocyte-specific chemotactic cytokines in human periodontal infections. Infect Immun. 1994;62:4005–4014. doi: 10.1128/iai.62.9.4005-4014.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Valore E V, Park C H, Quayle A, Wiles K R, McCray P B J, Ganz T. Human β-defensin-1, an antimicrobial peptide of urogenital tissues. J Clin Invest. 1998;101:1633–1642. doi: 10.1172/JCI1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao C, Wang I, Lehrer R I. Widespread expression of beta-defensin hBD-1 in human secretory glands and epithelial cells. FEBS Lett. 1996;396:319–322. doi: 10.1016/0014-5793(96)01123-4. [DOI] [PubMed] [Google Scholar]