Summary
Background
The Americas region has the lowest (North America) and the second highest (Latin America and Caribbean) cervical cancer (CC) mortality worldwide. The lack of reliable data on screening coverage in the region hinders proper monitoring of the World Health Organization (WHO) CC elimination initiative.
Methods
For this synthetic analysis, we searched data on CC screening coverage from official sources and national health surveys, supplemented with a formal WHO country consultation. Context data were obtained from official sources (income, health expenditure, inequality-adjusted human development index -IHDI-, universal health coverage, CC incidence/mortality). Country age-specific coverages for 2019 by screening interval were computed. Missing data were imputed through a multi-step algorithm. Beta-regression and Poisson-regression models were used to analyse associations between context variables, screening coverage, and CC mortality.
Findings
We included data from 37 countries in the Americas. Data on coverage of HPV testing was scarce, and for many countries only Pap-smear coverage data was available. Overall, 78%, 34%, 60%, and 67% of women aged 25–65 years have been screened ever in their lifetime, and in the previous year, 3 years, and 5 years, respectively. By sub-region, 3-year coverage ranges from 48% (South America) to 72% (North America). Twenty-four countries showed screening coverage below 70%. Income and health system type were associated with screening coverage, but coverage was not associated with CC mortality.
Interpretation
In the Americas region 35.1% and 56.8% of countries report 3-year and 5-year coverage over 70%, respectively. Inequalities remain a major challenge for screening programs in the region. The elimination campaign should reinforce the transition to HPV testing and strengthen surveillance systems.
Funding
Instituto de Salud Carlos III, European Regional Development Fund, Secretariat for Universities and Research of the Department of Business and Knowledge of the Government of Catalonia, and Horizon 2020.
Keywords: Uterine cervical neoplasms, Early detection of cancer, Mass screening, Americas
Research in context.
Evidence before this study
The WHO launched a global initiative to eliminate cervical cancer (CC) as a public health problem. Screening coverage over 70% is a key elimination target; thus, reliable data are essential for monitoring the progress towards CC elimination. Most countries in the Americas region lack organized screening, and standardized and comparable coverage estimates are not yet available. We recently reported global CC screening coverage using an innovative methodology that allows for comparability; however, detailed data at the country level is not yet published. In March 2020, we carried out a search from 2009 to 2019 considering reports about national coverage of CC screening in the Americas region. The search was done in LILACS (DeCS terms “cáncer cervical” AND “tamizaje” AND “cobertura”) and PubMed (terms “North America” [Mesh] OR “Caribbean Region” [Mesh] OR “West Indies” [Mesh] OR “Latin America” [Mesh] OR “South America” [Mesh] OR “Central America” [Mesh] AND “Mass Screening” [Mesh] OR “Early Detection of Cancer” [Mesh] OR “Diagnostic Screening Programs” [Mesh] OR “Early Diagnosis” [Mesh] AND ∗Coverage OR Participation∗ OR “Screening coverage” OR “Invitation coverage” OR “Up-to-date screening” OR “adherence” OR “uptake” OR “Effective screening” OR “Examination rate” AND “Uterine Cervical Neoplasms” [Mesh] OR “Uterine Cervical Dysplasia” [Mesh] OR “Cervical Intraepithelial Neoplasia” [Mesh] OR “cervical cancer” OR “cervical precancer” OR “cervical precancerous lesions”). We found 498 reports but only 27 articles used national data on CC screening coverage, mostly from the USA and Brazil. Only one study reported binational data (USA and Canada) and a narrative review reported coverage for 14 Latin American countries; however, no standard definition of coverage was used regarding target populations, screening intervals, or screening tests; in consequence, coverage data are highly heterogenous without any standardization or data processing.
Added value of this study
We used an innovative methodology to present baseline estimates of CC screening coverage for the 37 WHO member states in the Americas region. In addition, we collected and reported data on contextual factors with a potential influence on screening coverage and CC mortality. For every country, we report coverage data for the most common target populations in national programmes (women 25–65 years old), and for the target population defined in the WHO elimination strategy (women 35–45 years old). All data are reported for 1-, 3-, and 5-year screening intervals and ever in lifetime. Coverage estimates are analysed against contextual variables including income level, health expenditure, human development index, universal health coverage, programmatic approach, and predominant type of health system (segmented/not-segmented). The methodology enables the comparison of estimates between countries in the region with similar contextual conditions.
Implications of all the available evidence
CC control remains an unmet goal for most countries in the Americas region. Although low screening coverage has been suggested as a major cause for the lack of impact on CC mortality, no significant progress on programme monitoring is observed for the majority of countries. Previous reports on screening coverage are disconnected from screening guidelines and lack uniformity in terms of target populations and screening intervals. Using innovative methods to overcome these limitations, we estimate that 60% of women aged 25–65 years in the Americas region have been screened during the previous three years, as recommended by most national guidelines on cytology-based screening; however, coverage estimates increase to 67% with an extended screening interval (five years), suggesting that an improved transition to human papillomavirus (HPV) testing will bring the region closer to the WHO elimination target. Socioeconomic disparities remain a major challenge to increasing screening coverage, but we provide insights regarding the characteristics of health systems and screening programmes to help countries understand the determinants of successful screening. Providing standardized information is not only a call to improve monitoring systems by using common indicators but also an opportunity to learn from neighbouring countries with similar cultural and socioeconomic backgrounds.
Introduction
The World Health Organization (WHO) launched in 2020 an initiative for the elimination of cervical cancer aimed at reducing incidence below 4 per 100,000.1 Target goals to achieve this objective include having, 90% of girls fully vaccinated against human papillomavirus (HPV) at 15 years of age, 70% of women screened twice between 35 and 45 years old using a high-performance test, and 90% or more identified precancerous lesions and invasive cancers treated by 2030.1 Accordingly, the Pan American Health Organization (PAHO) considers strengthening information systems a critical component of cervical cancer prevention and control programmes to allow proper monitoring of the progress towards elimination targets.2
Significant disparities in the socioeconomic determinants of cervical cancer incidence and mortality have been described for the Americas region.3 Although the burden of disease has decreased in some countries, there are still significant differences between and within countries, without substantial changes for the most disadvantaged settings, where cervical cancer mortality has increased.3,4 Accordingly, Latin America and the Caribbean is the region with the second highest cervical cancer mortality worldwide after Africa, whereas North America has the lowest incidence and mortality globally.5
Deficient organization of screening programmes challenges both the achievement of coverage targets and monitoring of programme performance.6 Previous reports on screening coverage for the Americas region have failed to provide reliable data given not only the varied quality of information sources but also the lack of standardized reports regarding coverage definition, target populations, and screening intervals.7 Indeed, several countries' reports do not provide information aligned with screening policies, making it difficult to properly assess the programme's performance.8
Recently, an accurate method for providing standardized information on screening coverage was reported by Bruni and colleagues.9 This methodology enables comparability of the estimates despite the heterogeneity of screening policies and variability of available coverage data. However, the initial report does not provide detailed data for different screening intervals and age groups for individual countries.9 Using this approach, we present detailed standardized estimates of cervical cancer screening coverage in the region, supplemented by a specific analysis to explore the association between contextual factors, screening coverage, and cervical cancer mortality.
Methods
The methods for data extraction and the statistical analysis to provide standardized coverage estimates have been described elsewhere.9
A detailed description of the systematic search and inclusion and exclusion criteria is presented in the Supplementary Material.
Data sources
Briefly, from July 2019 to October 2020, we searched official websites and data from national surveys and governmental reports of different screening coverage intervals (previous one year, previous two years, previous three years, previous five years, and ever in lifetime). Complementary information from international data sources was also retrieved (WHO and USAID databases). After the first review, if no coverage information was available, we conducted a systematic search in the PubMed database (via Medline) without language or date restrictions (Supplementary Material, Appendix 1). If more than one source was found we consulted country experts about data reliability. Finally, to review the data collected and preliminary estimates on cervical cancer screening coverage, a formal consultation round with WHO member states was done from November 2020 to February 2021, resulting in the update of screening coverage data for the Bahamas, Brazil, Canada, Colombia, Jamaica, Peru, the United States, and Uruguay.
We examined changes in the screening programme (including screen-and-treat approaches),10 income level, and health system reforms, in order to qualitatively assess the representativeness of coverage data for each country's situation by 2019 before the COVID-19 pandemic; thus, only national, population-based screening data representative of the country's situation in 2019 entered the final database and criteria for such representativeness have been previously described.9
We collected data on contextual variables from the United Nations (UN population prospects and UN inequality-adjusted human development index -IHDI-,11,12 the World Bank (income level),13 and the WHO Global Health Observatory (Universal Health Coverage and per capita health expenditure).14 For the classification of health systems, we found an extensive number of typologies and the coexistence of multiple models; however, no classification including all countries in the region was found.15,16 Consequently, we classified health systems into four categories as proposed by Chung M17,18: national health systems with a unique payer and provider (NHS: Beveridge model), national health insurance systems (NHI: individual basis) based on public funding but with public or private providers, fragmented health systems with social security provision for workers and public health provision for the remaining population (SS: Bismarck model), and countries with predominantly out-of-pocket funding, including private insurance and health services provision (OP). Two senior investigators independently categorized health systems considering the main funding source and care provision model based on multiple sources of information; disagreements were solved by consensus between the two. There is no health system with a unique funding source or provision model; thus, we considered the models covering the majority of the population to be the predominant type.19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38
Statistical analysis
Estimated data on cervical cancer incidence and mortality (2020) were retrieved from the WHO-IARC databases.5,39 The Average Annual Percentage Change (AAPC) of mortality rates was estimated for the last 15 years of available data, with the corresponding 95% confidence intervals (95% CI).40
We extracted screening coverage data by age group for any available screening interval. Coverage rates were transformed into single-age data points by assigning the same coverage to all ages in the corresponding age group. Missing data were assumed at random and imputed through a multi-step algorithm using different statistical techniques,9 and a sensitivity analysis was done to assess and validate the methodology. Briefly, we used linear interpolation between screening intervals, imputations per missing datapoint using predictive mean matching methods, last observation carried forward or next observation carried backwards, and ponderation rates based on coverage from countries with similar income and screening algorithms.9
Country-specific estimates were computed from the estimation of the number of screened women for each age group, screening interval, and the country as the numerator and the UN populations as the denominator. Bootstrap 95% confidence intervals were calculated using the percentile method with 3000 bootstrap replications. Country-specific estimates were aggregated by age group (25–65 years and 35–45 years) and contextual variables.
Additionally, in this paper, we used a Beta regression model to analyse the association of contextual variables with screening coverage as a dependent variable in the form of percentages, and we used a Poisson regression model to evaluate the association between screening coverage and contextual variables with cervical cancer mortality (Age Standardized Rate -ASR-per 100,000 women). For both models, we ran bivariate and multivariate analyses. For the multivariate analysis, only variables with p-values <0.05 were included. The contextual variables considered in the models include income level (four categories: high-, upper-middle-, lower-middle-, and low-) IHDI (four categories: very high, high, medium, and low), predominant health system model (two categories: universal financial coverage and integrated health care provision –NHS/NHI- and fragmented financial coverage and healthcare provision –SS/OP-), predominant health system financing (three categories: public revenues, mixed, and private), predominant health services provision (two categories: public and public/private), health system steering role (two categories: national and territorial), public health expenditure (as continuous and in two categories: <70% and ≥70%), cervical cancer screening scheme (four categories: one approach, different approaches—same population, different approaches—different population, and no programme), screen-and-treat approach (two categories: yes and no/no programme),10 rural population (as continuous in percentage), and Universal Health Coverage (UHC) service capacity index (as continuous).
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
We collected data from 37 countries in North America (3), Central America (8), South America (12), and the Caribbean (14). In total, 12 countries were categorized as high-income, 20 as upper-middle-income, 4 as lower-middle-income, and only Haiti as low-income; however, socioeconomic determinants largely varied within these income categories (for example, in high-income countries, the percentage of the rural population ranged from 0 to 69 and health expenditure per capita from USD$875 to USD$10,624). Accordingly, the countries’ IHDI categorizations did not match their income levels (Table 1). Despite the differences in socioeconomic determinants, most countries showed a significant decrease in cervical cancer mortality rates in the last 15 years, with only Ecuador and Paraguay showing a significant increase for the period analysed (AAPC for Ecuador: 1.1 [95% CI, 0.2–2.0] and AAPC for Paraguay: 2.2 [0.5–4.0]; Table 1).
Table 1.
Country's contextual characteristics.
| Country | Socioeconomic characteristics |
Health system characteristics |
Cervical cancer |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Population 2019(a) | Rural pop 2019 (%)(a) | Income level(b) | Inequality-adjusted HDI 2019(c) | Predominant health system Type(d) | UHC index services capacity & access 2017(e) | Health expenditure per capita (US $) 2018(e) | Incidence 2020 (ASR per 100,000)(f) | Mortality 2020 (ASR per 100,000)(f) | Average annual percentage change (AAPC) mortality (ASR)—15 years(g) |
||
| Last year | AAPC (95% CI) | ||||||||||
| North America | |||||||||||
| Bermuda | 63,000 | 0.0 | High | N/A | SS | N/A | N/A | N/A | N/A | N/A | N/A |
| Canada | 37,411,000 | 18.5 | High | Very high | NHI | 100 | 4995 | 5.5 | 1.9 | 2015 | −1.4 (−3.8, 1.1) |
| United States of America | 329,065,000 | 17.5 | High | Very high | OP | 100 | 10,624 | 6.2 | 2.1 | 2016 | −0.7b (−0.9, −0.5) |
| Central America | |||||||||||
| Belize | 390,000 | 54.1 | Upper-middle | Medium | NHI | 56 | 286 | 19.1 | 14.8 | 2016 | −0.7 (−3.7, 2.5) |
| Costa Rica | 5,048,000 | 19.7 | Upper-middle | Medium | NHI | 77 | 910 | 11.7 | 5.4 | 2014 | −3.4b (−5.2, −1.6) |
| El Salvador | 6,454,000 | 27.2 | Lower-middle | Low | SS | 79 | 289 | 13.1 | 7.4 | 2014 | −2.9b (−4.1, −1.7) |
| Guatemala | 17,581,000 | 48.6 | Upper-middle | Low | SS | 32 | 260 | 20.3 | 11.9 | 2016 | 2.0 (−0.2, 4.2) |
| Honduras | 9,746,000 | 41.5 | Lower-middle | Low | SS | 45 | 176 | 19.5 | 12.5 | N/A | N/A |
| Mexico | 127,576,000 | 20.3 | Upper-middle | Medium | SS | 80 | 520 | 12.6 | 5.7 | 2016 | −3.9b (−4.4, −3.3) |
| Nicaragua | 6,546,000 | 40.0 | Lower-middle | Low | SS | 73 | 174 | 21.3 | 12.6 | 2017 | −1.7b (−2.7, −0.7) |
| Panama | 4,246,000 | 31.8 | High | Medium | SS | 89 | 1132 | 14.0 | 7.5 | 2016 | −1.9b (−3.4, −0.4) |
| South America | |||||||||||
| Argentina | 44,781,000 | 8.1 | Upper-middle | High | SS | 89 | 1128 | 16.7 | 8.7 | 2016 | −0.2 (−1.1, 0.6) |
| Bolivia | 11,513,000 | 29.9 | Lower-middle | Low | SS | 82 | 224 | 36.6 | 18.0 | N/A | N/A |
| Brazil | 211,050,000 | 13.3 | Upper-middle | Medium | NHS | 99 | 848 | 12.7 | 6.3 | 2016 | −0.7b (−0.9, −0.6) |
| Chile | 18,952,000 | 12.0 | High | High | SS | 94 | 1456 | 11.1 | 5.2 | 2016 | −3.1b (−4.6, −1.6) |
| Colombia | 50,339,000 | 18.7 | Upper-middle | Medium | NHI | 85 | 513 | 14.9 | 7.4 | 2015 | −3.0b (−4.2, −1.8) |
| Ecuador | 17,374,000 | 35.4 | Upper-middle | Medium | SS | 86 | 516 | 16.0 | 8.2 | 2016 | 1.1b (0.2, 2.0) |
| Guyana | 783,000 | 73.7 | Upper-middle | Medium | NHI | 70 | 296 | 29.5 | 15.1 | N/A | N/A |
| Paraguay | 7,045,000 | 37.8 | Upper-middle | Medium | SS | 63 | 400 | 34.1 | 19.0 | 2016 | 2.2b (0.5, 4.0) |
| Peru | 32,510,000 | 22.2 | Upper-middle | Medium | SS | 81 | 369 | 22.2 | 11.5 | 2015 | 0.6 (−1.0, 2.3) |
| Suriname | 581,000 | 33.4 | Upper-middle | Low | NHI | 78 | 474 | 23.7 | 14.1 | 2014 | −1.1 (−3.8, 1.7) |
| Uruguay | 3,462,000 | 4.6 | High | High | NHI | 94 | 1590 | 11.7 | 5.6 | 2016 | −2.4b (−4.0, −0.9) |
| Venezuela | 28,516,000 | 13.5 | Upper-middle | Medium | SS | 75 | 257 | 22.2 | 12.5 | 2013 | −1.2b (−1.9, −0.5) |
| Caribbean | |||||||||||
| Antigua & Barbuda | 97,000 | 81.4 | High | N/A | NHI | 70 | 875 | NA | NA | N/A | N/A |
| Bahamas | 389,000 | 17.5 | High | N/A | NHI | 81 | 2013 | 14.9 | 10.6 | N/A | N/A |
| Barbados | 287,000 | 69.0 | High | Medium | NHS | 78 | 1165 | 15.2 | 9.0 | 2013a | −2.5b (−0.8, −3.3) |
| Cuba | 11,333,000 | 23.2 | Upper-middle | N/A | NHS | 100 | 987 | 13.9 | 6.9 | 2016 | −1.5b (−2.1, −1.0) |
| Dominica | 72,000 | 30.6 | Upper-middle | N/A | NHS | NA | 491 | NA | NA | N/A | N/A |
| Dominican Republic | 10,739,000 | 18.6 | Upper-middle | Medium | SS | 75 | 462 | 17.9 | 11.7 | 2013 | 0.4 (−2.1, 2.9) |
| Grenada | 112,000 | 61.6 | Upper-middle | N/A | NHS | 64 | 475 | NA | NA | N/A | N/A |
| Haiti | 11,263,000 | 43.7 | Low | Low | NHS | 30 | 64 | 11.6 | 9.0 | N/A | N/A |
| Jamaica | 2,948,000 | 43.4 | Upper-middle | Medium | NHI | 74 | 321 | 21.6 | 13.6 | N/A | N/A |
| Puerto Rico | 2,933,000 | 8.0 | High | N/A | SS | N/A | N/A | 8.0 | 2.8 | N/A | N/A |
| Saint Kitts & Nevis | 53,000 | 73.6 | High | N/A | NHS | N/A | 993 | N/A | N/A | N/A | N/A |
| Saint Lucia | 183,000 | 80.3 | Upper-middle | Medium | NHS | 59 | 465 | 16.6 | 11.0 | N/A | N/A |
| Saint Vincent & the Grenadines | 111,000 | 46.8 | Upper-middle | N/A | NHS | 59 | 329 | NA | NA | N/A | N/A |
| Trinidad & Tobago | 1,395,000 | 46.2 | High | N/A | NHS | 78 | 1123 | 19.8 | 11.9 | 2012 | −1.8 (−3.7, 0.1) |
IHDI: Inequality-Adjusted Human Development Index, UHC: Universal Health Coverage, ASR: Age Standardized Rate, CI: Confidence Interval, NHS: health systems with unique payer, SS: health systems based on social security for workers and public health provision for the remaining population, NHI: national health insurance (individual) based on public funding but public and private providers, OP: predominantly out of pocket health services provision including private insurance. N/A: Non-Available.
Includes only the last 14 years.
Statistically significant.
Sources: (a) United Nations Populational10 Prospects 2019, (b) World Bank,12(c) United Nations, IHDI classification11: Very high (≥80), High (0.70–0.79), Medium (0.55–0.69), Low: <0.55, (d) Health system type,18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37(e) WHO 2019,13(f) IARC 2020,5(g) WHO cancer mortality database.38
At the time of data collection, most countries’ reports on coverage data corresponded to cytology-based screening and no specific data on coverage of screen-and-treat approaches was identified. Only governments from Brazil, Colombia, and Uruguay reported data from administrative sources (Brazil: cancer information system, Colombia: registry of specific protection and early detection activities, and Uruguay: cervical cancer screening programme); data from the remaining countries were from population-based household surveys. For Antigua and Barbuda, Panama, Suriname, and Venezuela, we did not find representative data for 2019.
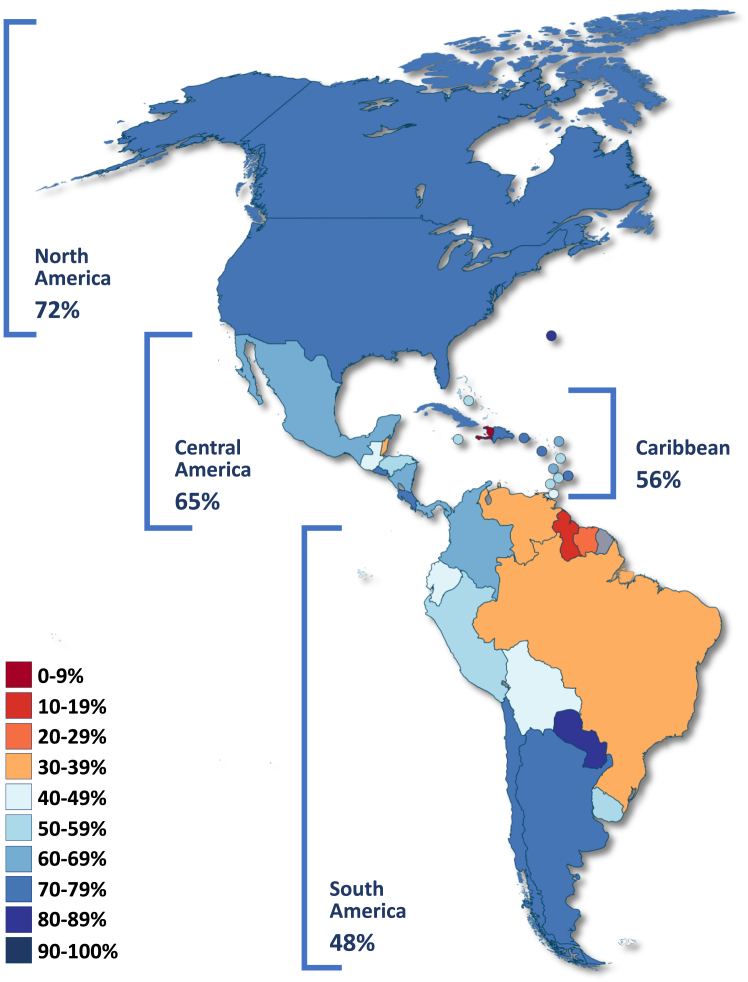
Overall, in the Americas region, 78% of women aged 25–65 years have been screened ever in their lifetime; 34% have been screened in the previous year, 60% in the previous three years, and 67% in the previous five years (Table 2). Among subregions, in a three-year screening interval, South America and the Caribbean showed the lowest coverage (48% and 56%, respectively) and North America and Central America the highest (72% and 65%, respectively) (Fig. 1), with high-income countries registering higher coverage estimates than low- and middle-income countries (LMIC) (Table 2). Moreover, countries with higher IHDI and higher public health expenditures had higher coverage. Similar trends were observed for coverage estimates at previous one-, five-year screening intervals and ever in their lifetime.
Table 2.
Estimated number of screened women and cervical cancer screening coverage by subgroups of analysis, 2019 (women aged 25–65 years).
| Area | Previous year |
Previous 3 years |
Previous 5 years |
Ever in lifetime |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of screened women in millions N (95% CI) | Coverage % (95% CI) | Number of screened women in millions N (95% CI) | Coverage % (95% CI) | Number of screened women in millions N (95% CI) | Coverage % (95% CI) | Number of screened women in millions N (95% CI) | Coverage % (95% CI) | |||||||||
| Total | 90.4 | (78.6–103.4) | 34% | (29–39%) | 160.0 | (140.1–182.0) | 60% | (52–68%) | 178.0 | (156.1–202.0) | 67% | (58–76%) | 208.8 | (183.1–236.6) | 78% | (69–89%) |
| By subregion | ||||||||||||||||
| North America | 42.0 | (32.9–51.3) | 43% | (34–52%) | 70.8 | (56.1–85.9) | 72% | (57–88%) | 76.6 | (60.8–92.9) | 78% | (62–95%) | 85.6 | (68.0–103.6) | 87% | (69–100%) |
| Central America | 16.8 | (13.4–20.4) | 37% | (30–46%) | 29.1 | (22.9–35.7) | 65% | (51–80%) | 33.2 | (26.1–40.8) | 74% | (58–91%) | 37.9 | (29.9–46.6) | 85% | (67–100%) |
| South America | 28.0 | (25.1–30.9) | 25% | (22–27%) | 54.2 | (48.1–60.5) | 48% | (42–53%) | 61.4 | (54.3–69.0) | 54% | (48–61%) | 77.8 | (67.2–89.6) | 68% | (59–79%) |
| Caribbean | 3.6 | (3.1–4.2) | 34% | (29–40%) | 6.0 | (5.1–7.0) | 56% | (48–65%) | 6.8 | (5.8–7.8) | 63% | (54–73%) | 7.4 | (6.4–8.5) | 69% | (59–79%) |
| By income level | ||||||||||||||||
| High income | 45.5 | (34.7–57.3) | 43% | (33–54%) | 76.8 | (59.0–96.1) | 72% | (55–90%) | 83.2 | (64.0–104.1) | 78% | (60–98%) | 93.3 | (71.8–116.6) | 87% | (67–100%) |
| Low and middle income | 44.8 | (40.1–49.6) | 28% | (25–31%) | 83.3 | (74.2–92.6) | 52% | (46–58%) | 94.8 | (84.3–105.7) | 59% | (52–66%) | 115.5 | (101.6–130.3) | 72% | (63–81%) |
| Upper middle income | 42.2 | (37.7–47.0) | 28% | (25–31%) | 78,6 | (69.8–88.0) | 52% | (46–59%) | 89.5 | (79.3–100.5) | 60% | (53–67%) | 109.5 | (95.9–124.2) | 73% | (64–83%) |
| Lower middle income | 2.6 | (2.4–2.8) | 33% | (30–36%) | 4.6 | (4.3–4.9) | 58% | (54–62%) | 5.1 | (4.8–5.5) | 65% | (60–69%) | 5.8 | (5.4–6.2) | 73% | (68–78%) |
| Low income | 52 k | (42 k–63 k) | 2% | (2–3%) | 122 k | (110 k–137 k) | 5% | (4–5%) | 185 k | (166 k–204 k) | 7% | (7–8%) | 245 k | (219 k–272 k) | 10% | (9–11%) |
| By inequality-adjusted human development indexa | ||||||||||||||||
| Very high (≥ 0.80) | 42.0 | (34.2–49.4) | 43% | (35–51%) | 70.7 | (58.4–82.7) | 72% | (60–84%) | 76.6 | (63.3–89.4) | 78% | (65–91%) | 85.6 | (70.8–99.7) | 87% | (72–100%) |
| High (0.70–0.79) | 8.1 | (6.9–9.4) | 46% | (39–54%) | 13.0 | (11.2–15.0) | 75% | (64–86%) | 13.8 | (11.9–15.9) | 79% | (68–91%) | 15.7 | (13.6–17.9) | 90% | (78–100%) |
| Medium (0.55–0.69) | 34.4 | (30.1–38.9) | 26% | (23–29%) | 66.3 | (57.8–75.1) | 50% | (44–57%) | 76.3 | (66.4–86.7) | 57% | (50–65%) | 94.8 | (81.7–109.0) | 71% | (62–82%) |
| Low (0.55) | 3.8 | (3.3–4.2) | 26% | (23–30%) | 6.5 | (5.8–7.1) | 45% | (41–50%) | 7.4 | (6.7–8.1) | 51% | (47–56%) | 8.5 | (7.7–9.3) | 60% | (54–65%) |
| By universal health coverage indexb | ||||||||||||||||
| >82 | 68.0 | (57.6–79.1) | 34% | (29–40%) | 119.2 | (102.0–137.6) | 60% | (51–69%) | 131.1 | (112.4–151.1) | 66% | (57–76%) | 154.9 | (132.9–178.2) | 78% | (67–90%) |
| 74–81 | 18.7 | (15.2–22.4) | 34% | (27–40%) | 34.6 | (28.2–41.3) | 62% | (51–74%) | 39.8 | (32.6–47.6) | 71% | (58–85%) | 46.0 | (37.7–54.9) | 83% | (68–98%) |
| <74 | 3.2 | (2.8–3.8) | 27% | (23–31%) | 5.5 | (4.8–6.3) | 46% | (40–53%) | 6.3 | (5.5–7.1) | 52% | (45–59%) | 7.1 | (6.2–8.0) | 59% | (52–67%) |
| By predominant health system type | ||||||||||||||||
| National health system | 8.8 | (6.8–10.9) | 14% | (10–17%) | 23.0 | (17.4–29.0) | 35% | (27–44%) | 27.2 | (20.4–34.5) | 42% | (31–53%) | 38.3 | (27.9–49.9) | 59% | (43–77%) |
| National health insurance (individual) | 5.2 | (4.1–6.4) | 37% | (29–45%) | 10.1 | (7.8–12.4) | 72% | (56–88%) | 10.9 | (8.5–13.4) | 77% | (60–95%) | 12.0 | (9.4–14.7) | 85% | (67–100%) |
| Social security | 38.0 | (33.9–42.3) | 38% | (34–42%) | 63.6 | (56.6–70.8) | 64% | (57–71%) | 71.2 | (63.2–79.3) | 71% | (63–80%) | 81.3 | (72.3–90.6) | 82% | (73–91%) |
| Out of pocket (Private) | 38.3 | (29.8–46.5) | 43% | (34–53%) | 63.4 | (49.5–76.8) | 72% | (56–87%) | 68.8 | (53.8–83.1) | 78% | (61–94%) | 77.1 | (60.5–93.0) | 87% | (69–100%) |
| By public health expenditure (% Total Health Expenditure)c | ||||||||||||||||
| >65 | 14.5 | (12.2–16.9) | 44% | (37–51%) | 22.8 | (19.5–26.3) | 69% | (59–79%) | 24.7 | (21.1–28.4) | 75% | (64–86%) | 27.1 | (23.2–31.1) | 82% | (70–94%) |
| 50–64 | 62.7 | (51.9–74.2) | 41% | (34–48%) | 107.2 | (89.4–126.2) | 70% | (58–82%) | 118.2 | (98.8–138.8) | 77% | (64–90%) | 134.1 | (112.4–157.1) | 87% | (73–100%) |
| <49 | 12.8 | (10.7–14.9) | 16% | (13–19%) | 29.3 | (23.6–35.3) | 37% | (30–45%) | 34.4 | (27.5–41.7) | 43% | (35–53%) | 46.8 | (36.3–58.4) | 59% | (46–74%) |
CI: Confidence Interval.
Countries of Antigua and Barbuda, Bahamas, Bermuda, Cuba, Dominica, Grenada, Puerto Rico, Saint Kitts and Nevis, Saint Vincent and the Grenadines, Trinidad and Tobago are excluded because of missing data.
Countries of Bermuda, Dominica, Puerto Rico and Saint Kitts and Nevis are excluded because of missing data.
Countries of Bermuda and Puerto Rico are excluded because of missing data.
Fig. 1.
Three-year interval cervical cancer screening coverage for women aged 25–65 years in the Americas region, estimates until 2019.
On the basis of individual countries, Haiti (Caribbean) and Guyana (South America) revealed the lowest coverage for a three-year screening interval (4% and 13%, respectively) in women aged 25–65 years, and among the remaining sub-regions, Belize (Central America) and the USA (North America) showed the lowest coverage (39% and 71%, respectively) (Table 3). For women 35–45 years old and a five-year screening interval, representing a proxy to the target population and the screening interval proposed for the WHO elimination strategy, the same countries revealed the lowest coverage in their corresponding subregions (8%, 18%, 50%, and 82%, respectively) and 12 countries (32.4%) remained under the target of 70% coverage (only 3 between 60% and 69%) (Supplementary Material, Appendix 2).
Table 3.
Estimated cervical cancer screening coverage in 2019 for women aged 25–65 years.
| Country | Target population in thousands | Previous year % (95% CI) | Previous 3 years % (95% CI) | Previous 5 years % (95% CI) | Ever in lifetime % (95% CI) |
|---|---|---|---|---|---|
| North America | |||||
| Bermuda | 19.4 | 63 (59–67) | 86 (80–92) | 89 (83–96) | 93 (86–100) |
| Canada | 10,457.9 | 38 (37–40) | 76 (73–78) | 81 (78–84) | 88 (84–91) |
| United States of America | 87,449.4 | 43 (41–45) | 71 (68–74) | 77 (75–80) | 87 (86–88) |
| Central America | |||||
| Belize | 91.1 | 23 (18–31) | 39 (32–46) | 45 (38–52) | 60 (54–67) |
| Costa Rica | 1378.1 | 49 (45–52) | 74 (69–78) | 75 (71–80) | 77 (73–81) |
| El Salvador | 1661.1 | 44 (38–49) | 73 (65–81) | 80 (71–89) | 88 (79–97) |
| Guatemala | 3743.6 | 28 (21–36) | 46 (38–53) | 54 (46–61) | 64 (57–72) |
| Honduras | 2151.2 | 34 (28–39) | 55 (46–64) | 62 (53–72) | 70 (60–80) |
| Mexico | 33,090.4 | 37 (34–40) | 66 (59–73) | 76 (68–84) | 88 (79–96) |
| Nicaragua | 1605.2 | 40 (34–46) | 67 (58–76) | 76 (65–86) | 84 (73–95) |
| Panama | 1043.6 | 44 (37–51) | 68 (60–76) | 77 (69–84) | 87 (79–95) |
| South America | |||||
| Argentina | 11,288.7 | 49 (45–54) | 77 (72–82) | 81 (76–87) | 90 (83–96) |
| Bolivia | 2513.2 | 19 (15–24) | 42 (36–48) | 47 (40–55) | 57 (47–67) |
| Brazil | 58,778.6 | 12 (11–12) | 34 (31–36) | 40 (37–44) | 58 (50–67) |
| Chile | 5278.8 | 43 (40–45) | 72 (68–75) | 76 (72–80) | 89 (84–94) |
| Colombia | 13,525.0 | 53 (50–57) | 68 (62–73) | 73 (67–79) | 80 (72–88) |
| Ecuador | 4165.3 | 26 (20–32) | 45 (39–51) | 52 (45–58) | 66 (61–71) |
| Guyana | 181.1 | 5 (2–8) | 13 (11–15) | 17 (15–29) | 21 (19–23) |
| Paraguay | 1579.4 | 41 (36–46) | 80 (70–90) | 82 (73–92) | 85 (76–94) |
| Peru | 8255.2 | 17 (16–19) | 58 (51–64) | 70 (62–79) | 84 (74–95) |
| Suriname | 144.0 | 15 (10–20) | 26 (20–33) | 31 (25–39) | 40 (32–49) |
| Uruguay | 897.5 | 23 (21–25) | 53 (49–57) | 63 (58–67) | 89 (85–92) |
| Venezuela | 7237.7 | 24 (18–32) | 39 (31–48) | 45 (37–54) | 54 (45–65) |
| Caribbean | |||||
| Antigua & Barbuda | 28.1 | 31 (25–37) | 60 (53–67) | 73 (66–79) | 85 (78–91) |
| Bahamas | 109.8 | 35 (29–41) | 54 (49–59) | 64 (59–68) | 73 (70–77) |
| Barbados | 81.3 | 50 (47–53) | 77 (73–79) | 85 (83–87) | 94 (91–96) |
| Cuba | 3261.5 | 47 (42–51) | 77 (72–82) | 85 (79–91) | 89 (83–96) |
| Dominica | 19.5 | 28 (26–31) | 57 (52–61) | 67 (62–73) | 78 (71–84) |
| Dominican Republic | 2594.4 | 48 (43–52) | 73 (66–79) | 79 (73–86) | 86 (79–93) |
| Grenada | 28.9 | 26 (24–29) | 54 (50–59) | 69 (64–74) | 84 (79–89) |
| Haiti | 2498.5 | 2 (1–2) | 4 (4–5) | 7 (6–8) | 9 (8–10) |
| Jamaica | 768.6 | 22 (18–26) | 52 (46–58) | 67 (62–73) | 83 (77–88) |
| Puerto Rico | 831.0 | 48 (43–53) | 79 (74–84) | 86 (82–91) | 91 (87–96) |
| Saint Kitts & Nevis | 15.1 | 46 (42–50) | 71 (66–75) | 78 (74–82) | 85 (81–89) |
| Saint Lucia | 52.6 | 31 (27–34) | 63 (58–68) | 76 (71–82) | 90 (84–96) |
| Saint Vincent & the Grenadines | 28.3 | 23 (21–25) | 54 (50–58) | 68 (64–73) | 83 (77–88) |
| Trinidad & Tobago | 401.3 | 22 (20–24) | 44 (41–47) | 54 (51–58) | 65 (62–69) |
CI: Confidence Interval. Countries in bold letters correspond to countries providing data based on administrative sources. Data from the remaining countries correspond to population-based household surveys except for Antigua & Barbuda, Panama, Suriname, and Venezuela were no available data were found.
Income level, predominant health system type, public health expenditure, and UHC service capacity index were associated with screening coverage in the bivariate analysis (Supplementary Material, Appendix 3); however, only income level and health system type were significantly associated in the multivariate analyses (Table 4). In particular, the estimated odds of screening coverage decreased to 0.05 (95% CI: 0.01–0.29) for low-income compared to high-income countries, and countries with an SS/OP health system had an estimated odds ratio (OR) of 0.59 (95% CI: 0.39–0.90) compared to countries with no-fragmented health system (NHS/NHI). The factors associated with cervical cancer mortality in the bivariate analysis were income level, IHDI, predominant health system, public health expenditure, percentage of rural population, the presence of screen-and-treat approaches, UHC service capacity index, and screening coverage (Supplementary Material, Appendix 3); however, only the IHDI and the presence of screen-and-treat approaches showed a significant effect on mortality in the multivariate analysis (Table 4). Specifically, countries with medium and low IHDI had an increase in mortality rate of 92% (rate ratio (RR): 1.92, 95% CI: 1.24–3.10) and 91% (RR: 1.91, 95% CI: 1.15–3.27), respectively, compared to countries with a high or very high index, and countries implementing a screen-and-treat approach had an increase in mortality rate of 38% (RR: 1.38, 95% CI: 1.03–1.84) compared to countries that did not implement this strategy.
Table 4.
Context variables associated with screening coverage and cervical cancer mortality in multivariate analyses.
| Independent variable | N countries | OR | 95% CI | p-value |
|---|---|---|---|---|
| A. Screening coveragea | ||||
| Income level | <0.001 | |||
| High | 12 | Ref | Ref | |
| Upper-middle | 20 | 0.58 | 0.38–0.89 | |
| Lower-middle | 4 | 0.52 | 0.25–1.07 | |
| Low | 1 | 0.05 | 0.01–0.29 | |
| Predominant health system type | 0.02 | |||
| No-fragmented (NHS/NHI) | 19 | Ref | Ref | |
| Fragmented (SS/OP) | 18 | 0.59 | 0.39–0.90 | |
| Independent variable | N countries | RR | 95%CI | p-value |
| B. Cervical cancer mortalityb | ||||
| Screen and treat approach | 0.03 | |||
| No/no program | 18 | Ref | Ref | |
| Yes | 12 | 1.38 | 1.03–1.84 | |
| Missing | 1 | – | – | |
| Inequality-adjusted HDI | 0.01 | |||
| Very high/High | 5 | Ref | Ref | |
| Medium | 7 | 1.92 | 1.24–3.10 | |
| Low | 4 | 1.91 | 1.15–3.27 | |
| Missing | 5 | – | – | |
OR: Odds Ratio, RR: Rate Ratio, CI: Confidence Interval, NHS: National Health system, NHI: National Health Insurance, SS: Social Security, OP: Out of pocket, HDI: Human Development Index. Data in bold correspond to statistically significant associations.
Beta-regression model.
Poisson-regression model. Coverage for women aged 25–65 years, 3-years interval. For cervical cancer mortality screening coverage is included as an independent variable and missing categories are not included in the regression models.
Discussion
To our knowledge, this is the first report to provide detailed and standardized estimates on cervical cancer screening coverage for every country in the Americas region, based on an innovative methodological approach.
Based on coverage estimates for the previous three years among women 25–65 years old, we found that all North American countries already report a screening coverage of over 70%; however, only 10 out of 34 countries in Latin America and the Caribbean meet this level of coverage for a three-year interval. Yet, according to the total number of women screened, we estimate that 60% of women 25–65 years old in the Americas have been screened in the previous three years, and 67% have been screened in the previous five years.
This shows that the Americas region, as a whole, is close to the WHO target to screen 70% women for CC; however, inequalities remain a major challenge for screening programmes, which are expressed not only as differences in screening determinants but also in screening rates and cervical cancer incidence and mortality. The highest mortality rate is nine times higher than the lowest (Paraguay vs Canada), and the difference in screening coverage between the lowest and the highest income levels is 67% (previous three years among women 25–65 years old). In addition, although cervical cancer mortality has decreased in several countries, inequality is evident within countries such as Colombia, Argentina, and Brazil, where mortality can be up to five times higher in the most disadvantaged regions compared to the regions with the lowest mortality.3,41, 42, 43
Indeed, we found an inverse association between income level and screening coverage and lower coverage for countries classified as having fragmented health systems (SS/OP) (Table 4). As indicated in the methods section, the classification of health systems was done by the research team, which might be a major source of bias for this estimate; however, fragmented health systems usually have different screening guidelines and levels of programme organization making it difficult to achieve large population impact at the country level. Despite the significant association observed between screening coverage and the type of health system, careful interpretation is warranted since countries classified as having no-fragmented health systems could have differential services for population groups and this category is composed mainly of small countries from the Caribbean. In contrast, Brazil, a country with a large population which is in this category, reports one of the lowest coverages in the region. The Brazilian data are based on the official cancer information system (SISCAN)9; however, governmental institutions such as the Brazilian National Cancer Institute describe the lack of consolidation of the system as a weakness and use the National Health Survey as an alternative source of information, reporting 81.3% coverage for the previous three years.44 In fact, the low coverage reported by Brazil does not correspond to the mortality rate as compared with the remaining upper-middle-income countries in the region.
A lack of agreement between screening coverage and cervical cancer mortality has been previously reported in Latin America.45,46 Although it could be the result of lag time, it also highlights the relevance of other programme components, such as proper follow-up for positive-screened women (diagnostic work-up and CIN2+ treatment rates), particularly in areas with low access to health care.47, 48, 49, 50 In agreement with previous data, we found no association between screening coverage and cervical cancer mortality but did find a direct association of mortality with high inequality levels and higher mortality with the presence of screen-and-treat programmes. The latter may be a result of the higher percentage of women residing in distant areas with low access to health care even in countries with no-fragmented health systems such as Colombia10; however, due to the relatively low number of observations (37 countries) these results should be cautiously interpreted.
Previous data on cervical cancer screening coverage have been reported in different studies, but it reflects the difficulties for standardized measurements between countries: different target populations (age ranges), different screening intervals, different periods, and different reports for the same country. This makes it difficult to compare countries or benchmarks among them to identify progress or a lack of programme organization. A recent report by the International Agency for Research on Cancer shows data on cervical cancer screening coverage from selected countries globally, highlighting the use of program data exclusively; despite the relevant effort and complex methodology, the report makes evident the challenges of deficient program organization (no standard denominators), the potential limitations of program data (most data correspond to a number of tests per year rather than coverage by screening interval and data outside the program are considered overestimation rather than low program coverage), and consequently the scarcity of robust data (only five countries from the Americas region and three of them with fragmented health systems reporting only the public sector).51 An additional source of data on screening coverage has been the WHO survey on cancer country profiles.52 This report was traditionally restricted to a single coverage value, with origins in non-standardized sources and including data only from the public sector excluding the social security in countries with fragmented health systems. Recently, cervical cancer profiles have been updated to include data based on the same methodology used here, as previously published.9
Our report has several strengths including the systematic search, the supplement with governmental data, and the standardised estimates; however, it also bears several limitations. Only three countries provided administrative data, and only Uruguay's data came from the cervical cancer screening programme, as data from Brazil and Colombia were generated from other sources of administrative information, such as reimbursement claims. As a result, the sources of information are mainly the same as those used in the previous reports we have criticized (e.g. household surveys). However, for a more accurate estimate and prediction we filtered by quality of data, gathered data directly from the source whenever possible, and developed strong statistical algorithms for data validation and analysis if required due to missing data. To assess the reliability of the methodology, we did an exhaustive sensitivity analysis of the missing data treatment assuming over and underestimation of coverage, obtaining almost identical coverage estimates.9 This approach reduced, but did not eliminate, the recall bias from household surveys; therefore, the 1-year screening interval might provide more accurate and reliable data than longer intervals. Moreover, we cannot control consent bias or other respondent biases in household surveys.53 In consequence, coverage overestimation could be present, with variable impact among the different countries.
We reflect that improving the quality of program data is imperative as a critical component of program organization; however, due to the limited capacity and resources at present, this objective may take a long time to achieve in LMIC. Nevertheless, the improvement of program organization will not solve challenges regarding health systems fragmentation. Therefore, strengthening health surveys might be a complementary measure by reviewing methodologies to reduce recall and social desirability biases.54
Despite the limitations described, we used the most accurate source of information currently available, which may be the case for a medium to long period of time given the lack of population-based programmes in most countries of the region. We consider our report to be a baseline measure, and we expect periodic updates to help countries develop stronger monitoring systems and build stronger cervical cancer screening programmes as an essential component of the WHO elimination strategy.
Contributors
LB and RM conceptualised the project. LB, BS, ER and RM designed the study and planned the analysis. BS designed the data-extraction form, did the literature search. BS, GFD, MCM and RM contributed to the collection of data. ER and JSC did the formal statistical analysis. LB and BS supervised the statistical analyses. LB, ER and BS did WHO country consultation. BS, ER, RM, JSC cross-checked data. GFD, JSC, RM and ER prepared the tables. GFD and RM prepared the first draft of the manuscript. All authors contributed to data interpretation, critically revised subsequent drafts, and read and approved the submitted version. All authors had full access to all data in the study and had final responsibility for the decision to submit for publication.
Data sharing statement
The study's findings are supported by data available in public online repositories and data available upon request from the data provider. Produced estimates are published on WHO Global Health Observatory Data Repository (https://www.who.int/data/gho). Upon request, computer code is available in the IDIBELL repository (https://repository.idibell.cat).
Editor note
The Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Declaration of interests
The Cancer Epidemiology Research Programme (with which LB, BS, and ER are affiliated) has received sponsorship for grants from Merck Sharp & Dohme and HPV test kits at no cost from Roche for research purposes. RM reports consulting fee from International Atomic Energy Agency. All other authors report no competing interests. JC currently works for Pharma Industry related with Cancer but not related with the topic of the articleThe findings and conclusions in this report are those of the authors and do not represent the official position of PAHO or WHO.
Acknowledgements
This study was funded by Instituto de Salud Carlos III through the projects PI18/01137, PI21/00982 and CIBERESP CB06/02/0073 (Co-funded by European Regional Development Fund. ERDF, a way to build Europe) also this project has received funding from the European Union's Horizon 2020 research and innovation programme under grant agreement No 847845. With the support of the Secretariat for Universities and Research of the Department of Business and Knowledge of the Government of Catalonia. Grants to support the activities of research groups (2021SGR01029). We thank CERCA Programme/Generalitat de Catalunya for institutional suport.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lana.2024.100689.
Contributor Information
Ginna Fernández-Deaza, Email: fernandez.ginna@javeriana.edu.co.
Beatriz Serrano, Email: bscarro@iconcologia.net.
Esther Roura, Email: eroura@iconcologia.net.
Juan Sebastián Castillo, Email: juan_castillo@javeriana.edu.co.
María Caicedo-Martínez, Email: caicedom.maria@gmail.com.
Laia Bruni, Email: lbruni@iconcologia.net.
Raúl Murillo, Email: rmurillo@husi.org.co.
Appendix A. Supplementary data
References
- 1.World Health Organization . 2020. Global strategy to accelerate the elimination of cervical cancer as a public health problem.https://www.who.int/publications/i/item/9789240014107 Accessed September 06, 2022. [Google Scholar]
- 2.Pan American Health Organization . 2018. Plan of action for cervical cancer prevention and control 2018-2030.https://www.paho.org/hq/index.php?option=com_docman&view=download&category_slug=mandates-strategies-3448&alias=47584-plan-cervical-cancer-2018-2030&Itemid=270&lang=en Accessed September 06, 2022. [Google Scholar]
- 3.Murillo R. Reducing social inequalities in cancer: evidence and priorities for research. IARC Scientific Publication; 2019. Social inequalities in cancer in Latin America; pp. 223–226. [Google Scholar]
- 4.Serrano B., Brotons M., Bosch F.X., Bruni L. Epidemiology and burden of HPV-related disease. Best Pract Res Clin Obstet Gynaecol. 2018;47:14–26. doi: 10.1016/j.bpobgyn.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 5.International Agency for Research on Cancer . Cancer Today; 2020. Global cancer observatory.https://gco.iarc.fr/today/home Accessed September 06, 2022. [Google Scholar]
- 6.Screening Performance Indicators Working Group. Cervical Cancer Prevention and Control Network Executive summary--performance monitoring for cervical cancer screening programs in Canada. Chronic Dis Can. 2010;31(1):45–48. [PubMed] [Google Scholar]
- 7.International Agency for Research on Cancer . IARC handbooks of cancer prevention; 2022. Cervical cancer screening.https://publications.iarc.fr/604 [Google Scholar]
- 8.Murillo R., Herrero R., Sierra M.S., Forman D. Cervical cancer in Central and South America: burden of disease and status of disease control. Cancer Epidemiol. 2016;44:S121–S130. doi: 10.1016/j.canep.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 9.Bruni L., Serrano B., Roura E., et al. Cervical cancer screening programmes and age-specific coverage estimates for 202 countries and territories worldwide: a review and synthetic analysis. Lancet Glob Health. 2022;10(8):e1115–e1127. doi: 10.1016/S2214-109X(22)00241-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernández-Deaza G., Caicedo-Martínez M., Serrano B., et al. Cervical cancer screening programs in Latin America: current recommendations for facing elimination challenges. Salud Publica Mex. 2022;64:415–423. doi: 10.21149/13204. [DOI] [PubMed] [Google Scholar]
- 11.United Nations . 2019. World population prospects.https://population.un.org/wpp/Download/Standard/Population/ Accessed June 11, 2021. [Google Scholar]
- 12.United Nations . 2019. Human development reports. Inequality adjusted human development index.http://hdr.undp.org/en/content/inequality-adjusted-human-development-index-ihdi Accessed August 08 2021. [Google Scholar]
- 13.The World Bank . 2020. World Bank country and leading groups.https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups Accessed August 08 2021. [Google Scholar]
- 14.World Health Organization . 2019. The global health observatory.https://apps.who.int/gho/portal/uhc-cabinet-wrapper-v2.jsp?id=1010501 Accessed August 08 2021. [Google Scholar]
- 15.Toth F. Classification of healthcare systems: can we go further? Health Policy. 2016;120(5):535–543. doi: 10.1016/j.healthpol.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 16.de Carvalho G., Schmid A., Fischer J. Classifications of health care systems: do existing typologies reflect the particularities of the Global South? Global Soc Pol. 2021;21(2):278–300. [Google Scholar]
- 17.Chung M. Princeton Publich Health Review; 2017. Health care reform: learning from other mayor health care systems.https://pphr.princeton.edu/2017/12/02/unhealthy-health-care-a-cursory-overview-of-major-health-care-systems/ Accessed August 08 2021. [Google Scholar]
- 18.Kulesher R.R., Forrestal E. International models of health systems financing. J Hosp Adm. 2014;3(4):127. [Google Scholar]
- 19.Lorenzoni L., Pinto D., Guanais F., Plaza-Reneses T., Frederic D., Auraaen A. OECD Health Working Papers; 2019. Health systems characteristics: a survey of 21 Latin America and Caribbean countries.https://www.oecd-ilibrary.org/social-issues-migration-health/health-systems-characteristics_0e8da4bd-en Accessed August 08 2021. [Google Scholar]
- 20.Frenk J., Gómez-Dantés O. Health systems in Latin America: the search for universal health coverage. Arch Med Res. 2018;49(2):79–83. doi: 10.1016/j.arcmed.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 21.Acosta M., Sáenz M.R., Gutiérrez B., Bermúdez J.L. Sistema de salud de El Salvador. Salud Publica Mex. 2011;53(2):S188–S196. [PubMed] [Google Scholar]
- 22.Alcalde-Rabanal E.J., Lazo-González O., Nigenda G. Sistema de salud de Perú. Salud Publica Mex. 2011;53(2):S243–S254. [PubMed] [Google Scholar]
- 23.Aran D., Laca H. Sistema de salud de Uruguay. Salud Publica Mex. 2011;53(2):S265–S274. [PubMed] [Google Scholar]
- 24.Becerril-Montekio V., López-Dávila L. Sistema de salud de Guatemala. Salud Publica Mex. 2011;53(2):S197–S208. [PubMed] [Google Scholar]
- 25.Becerril-Montekio V., Reyes J.D., Manuel A. Sistema de salud de Chile. Salud Publica Mex. 2011;53(2):S132–S143. [PubMed] [Google Scholar]
- 26.Belló M., Becerril-Montekio V.M. Sistema de salud de Argentina. Salud Publica Mex. 2011;53(2):S96–S108. [PubMed] [Google Scholar]
- 27.Bermúdez-Madriz J.L., Sáenz M.R., Muiser J., Acosta M. Sistema de salud de Honduras. Salud Publica Mex. 2011;53(2):S209–S219. [PubMed] [Google Scholar]
- 28.Bonvecchio A., Becerril-Montekio V., Carriedo-Lutzenkirchen Á., Landaeta-Jiménez M. Sistema de salud de Venezuela. Salud Publica Mex. 2011;53(2):S275–S286. [PubMed] [Google Scholar]
- 29.Dantés O.G., Sesma S., Becerril V.M., Arreola H. Sistema de salud de México. Salud Publica Mex. 2011;53(2):S220–S232. [PubMed] [Google Scholar]
- 30.Domínguez-Alonso E., Zacca E. Sistema de salud de Cuba. Salud Publica Mex. 2011;53(2):S168–S176. [PubMed] [Google Scholar]
- 31.Guerrero R., Gallego A.I., Becerril-Montekio V., Vásquez J. Sistema de Salud de Colombia. Salud Publica Mex. 2011;53(2):S144–S155. [PubMed] [Google Scholar]
- 32.Ledo C., Soria R. Sistema de salud de Bolivia. Salud Publica Mex. 2011;53(2):S109–S119. [PubMed] [Google Scholar]
- 33.Lucio R., Villacrés N., Henríquez R. Sistema de salud de Ecuador. Salud Publica Mex. 2011;53(2):S177–S187. [PubMed] [Google Scholar]
- 34.Montekio V.B., Medina G., Aquino R. Sistema de salud de Brasil. Salud Publica Mex. 2011;53(2):S120–S131. [PubMed] [Google Scholar]
- 35.Muiser J., del Rocio Sáenz M., Bermúdez J.L. Sistema de salud de Nicaragua. Salud Publica Mex. 2011;53(2):S233–S242. [PubMed] [Google Scholar]
- 36.del Rocio Sáenz M., Acosta M., Muiser J., Bermúdez J.L. Sistema de salud de costa rica. Salud Publica Mex. 2011;53(2):S156–S167. [PubMed] [Google Scholar]
- 37.Rathe M., Moliné A. Sistema de salud de república dominicana. Salud Publica Mex. 2011;53(2):S255–S264. [PubMed] [Google Scholar]
- 38.Pan American Health Organization . 2017. Health in the Americas: summary: regional outlook and country profiles.https://iris.paho.org/handle/10665.2/34469 Accessed August 08, 2021. [Google Scholar]
- 39.World Health Organization, Department of Information Evidence and research, mortality database. https://www-dep.iarc.fr/WHOdb/WHOdb.htm Accessed June 11, 2021.
- 40.National Cancer Institute Joinpoint regression program. https://surveillance.cancer.gov/joinpoint/ Accessed June 11, 2021.
- 41.Pardo Ramos C., de Vries E., Buitrago Reyes L.A., Gamboa Garay O. 4th ed. Instituto Nacional de Cancerología - ESE; 2017. Atlas de mortalidad por cáncer en Colombia; pp. 1–124. [Google Scholar]
- 42.Instituto Nacional del Cáncer Argentina. Estadísticas – mortalidad. https://www.argentina.gob.ar/salud/instituto-nacional-del-cancer/estadisticas/mortalidad Available from: Accessed November 11, 2022.
- 43.Instituto Nacional de Câncer, Ministério da Saúde Brasil . 2021. Controle do câncer do colo do útero.https://www.inca.gov.br/controle-do-cancer-do-colo-do-utero/conceito-e-magnitude Accessed November 11, 2022. [Google Scholar]
- 44.Instituto Nacional de Câncer . Dados e Números sobre Câncer de Colo do Útero. Relatório Anual. 2022. Coordenação de Prevenção e Vigilância Divisão de Detecção Precoce e Apoio à Organização de Rede.https://www.inca.gov.br/sites/ufu.sti.inca.local/files/media/document/dados_e_numeros_colo_22setembro2022.pdf Available from: Accessed September 12, 2023. [Google Scholar]
- 45.Chocontá-Piraquive L.A., Alvis-Guzman N., De la Hoz-Restrepo F. How protective is cervical cancer screening against cervical cancer mortality in developing countries? The Colombian case. BMC Health Serv Res. 2010;10:270. doi: 10.1186/1472-6963-10-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murillo R., Almonte M., Pereira A., et al. Cervical cancer screening programs in Latin America and the caribbean. Vaccine. 2008;26:L37–L48. doi: 10.1016/j.vaccine.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 47.Murillo R., Wiesner C., Cendales R., Piñeros M., Tovar S. Comprehensive evaluation of cervical cancer screening programs: the case of Colombia. Salud Publica Mex. 2011;53(6):469–477. [PubMed] [Google Scholar]
- 48.Garcés-Palacio I.C., Altarac M., Kirby R., McClure L.A., Mulvihill B., Scarinci I.C. Contribution of health care coverage in cervical cancer screening follow-up: findings from a cross-sectional study in Colombia. Int J Gynecol Cancer. 2010;20(7):1232–1239. doi: 10.1111/igc.0b013e3181e8dfb8. [DOI] [PubMed] [Google Scholar]
- 49.Cremer M.L., Maza M., Alfaro K.M., et al. Introducing a high-risk HPV DNA test into a public sector screening program in El Salvador. J Low Genit Tract Dis. 2016;20(2):145–150. doi: 10.1097/LGT.0000000000000188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paolino M., Gago J., Le Pera A., Cinto O., Thouyaret L., Arrossi S. Adherence to triage among women with HPV-positive self-collection: a study in a middle-low income population in Argentina. Ecancer. 2020;14:1138. doi: 10.3332/ecancer.2020.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang L., Mosquera I., Lucas E., et al. CanScreen5, a global repository for breast, cervical and colorectal cancer screening programs. Nat Med. 2023;29(5):1135–1145. doi: 10.1038/s41591-023-02315-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pan American Health Organization . Cancer country profiles 2020. 2020. Cancer country profiles 2020.https://www.paho.org/hq/index.php?option=com_docman&view=list&slug=4-cancer-country-profiles-2020&Itemid=270&lang=es Available from: Accessed August 08 2021. [Google Scholar]
- 53.Dillon A., Mensah E.R. Respondent biases in household surveys. SSRN J. 2021:21–103. https://www.ssrn.com/abstract=3801258 Available from: Accessed August 08 2021. [Google Scholar]
- 54.Althubaiti A. Information bias in health research: definition, pitfalls, and adjustment methods. J Multidiscip Healthc. 2016;9:211–217. doi: 10.2147/JMDH.S104807. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.