Abstract
Background
Smoking is a significant health hazard and contributes to cardiovascular and pulmonary diseases. It can increase postoperative complications during oral and maxillofacial surgery due to its topical effect on the oral mucosa. New alternatives to traditional tobacco products are gaining popularity, in particular, electronic cigarettes.
Objectives
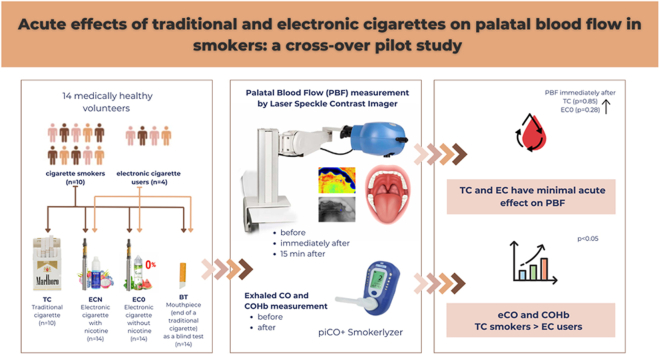
This pilot study investigated the acute effects of nicotine-containing and nicotine-free electronic cigarettes on palatal blood flow (PBF), and compared their effects to traditional cigarettes.
Materials and methods
14 medically healthy volunteers (8 males, 6 females, age: 34.7 ± 7.0) were recruited for the study. All patients (N = 14) were requested to smoke nicotine-containing (ECN) and nicotine-free electronic cigarettes (EC0) and a mouthpiece (end of a traditional cigarette) as a control sham smoking blind test (BT). EC users did not smoke a traditional cigarette (TC), resulting in 10 people in the TC group. Palatal blood flow was measured by Laser Speckle Contrast Imager before, immediately after, and 15 min after the exposures. Exhaled carbon monoxide (eCO) and carboxyhemoglobin (COHb) were measured before and immediately after smoking with a piCO+ Smokerlyzer machine.
Results
In all groups, no significant differences were observed in the changes of palatal blood flow between time points. Exhaled carbon monoxide and carboxyhemoglobin were significantly higher in the traditional cigarette (TC) group compared to the nicotine-containing electronic cigarette (ECN) and nicotine-free electronic cigarette (EC0) groups, both before and after the exposure (p < 0.05).
Conclusion
Acute use of either traditional or electronic cigarettes may have minimal impact on palatal blood flow, but additional studies are required to clarify their impact on the mucosa.
Keywords: E-cigarette, Electronic nicotine delivery systems, Cigarette, Smoking, Oral microcirculation, Palatal blood flow
Graphical abstract
1. Introduction
Smoking is a public health concern, with over 1.1 billion smokers globally.1 By causing endothelial dysfunction and atherosclerosis, smoking raises the risk of coronary artery disease, myocardial infarction, stroke, and peripheral artery disease.2 Furthermore, smoking is linked to postoperative complications. Smoking affects postoperative wound healing after surgical and nonsurgical tooth extractions, routine maxillofacial surgeries, implant surgeries, and periodontal therapies.3
When tobacco smoke is inhaled, oral tissues are directly exposed to various chemical pollutants, including nicotine, carbon monoxide, and hydrogen cyanide.4 By the action of nicotine on noradrenaline secretion, smoking has a vasoconstrictive effect on the microcirculation of the gingiva, leading to a decrease in blood flow within the gingival tissues.5 While no differences in capillary densities have been measured between smokers and nonsmokers,4 chronic smoking habits induce significant morphologic changes in the microcirculation of the human labial mucosa.6
Vaping and electronic cigarette (EC) use has increased dramatically in recent years, increasing from an estimated 68 million users (0.9 % of the global population) in 2020, to a projected 86 million in 2023.7 Electronic cigarettes are often touted as a purportedly safer alternative to traditional cigarettes, but there is ongoing controversy regarding their safety and potential risks.8
Only one previous article9 investigated the effect of electronic cigarettes on capillary blood flow of the oral mucosa, finding a small but significant rise due to nicotine vaping. However, the effect was not compared to traditional cigarettes. Therefore, the aim of this study was to investigate the acute effects of nicotine-containing and nicotine-free electronic cigarettes on palatal blood flow (PBF) and to compare their effect to that of traditional cigarettes.
2. Materials and methods
2.1. Participants
Fourteen medically healthy volunteers (8 males, 6 females) participated in the trial. Their ages ranged from 21 to 49 (mean age: 34.7 ± 7.0). All patients were evaluated as having good oral hygiene and did not have any general diseases. Otherwise, the inclusion criterion was that each participant should be a smoker or electronic cigarette user for at least half a year. Exclusion criteria were breastfeeding, pregnancy, or planning on being pregnant in the next six months; BMI over 30; suffering an acute infection in the previous two weeks before the visit; chronic disease (e.g., diabetes, cardiovascular diseases, COPD); use of any medications; radiotherapy in the previous three years before the study; keeping a diet which changes the pH of the mouth; and severe periodontal disease.
This study was conducted according to the Helsinki Declaration, approved by the Semmelweis University Regional and Institutional Committee of Science and Research Ethics, and has a ClinicalTrials.gov protocol registration with the ID of NCT03011710. All patients received written consent information about the details and possible risks of the measurement. Signed consent was obtained from each subject.
2.2. Treatment assignments
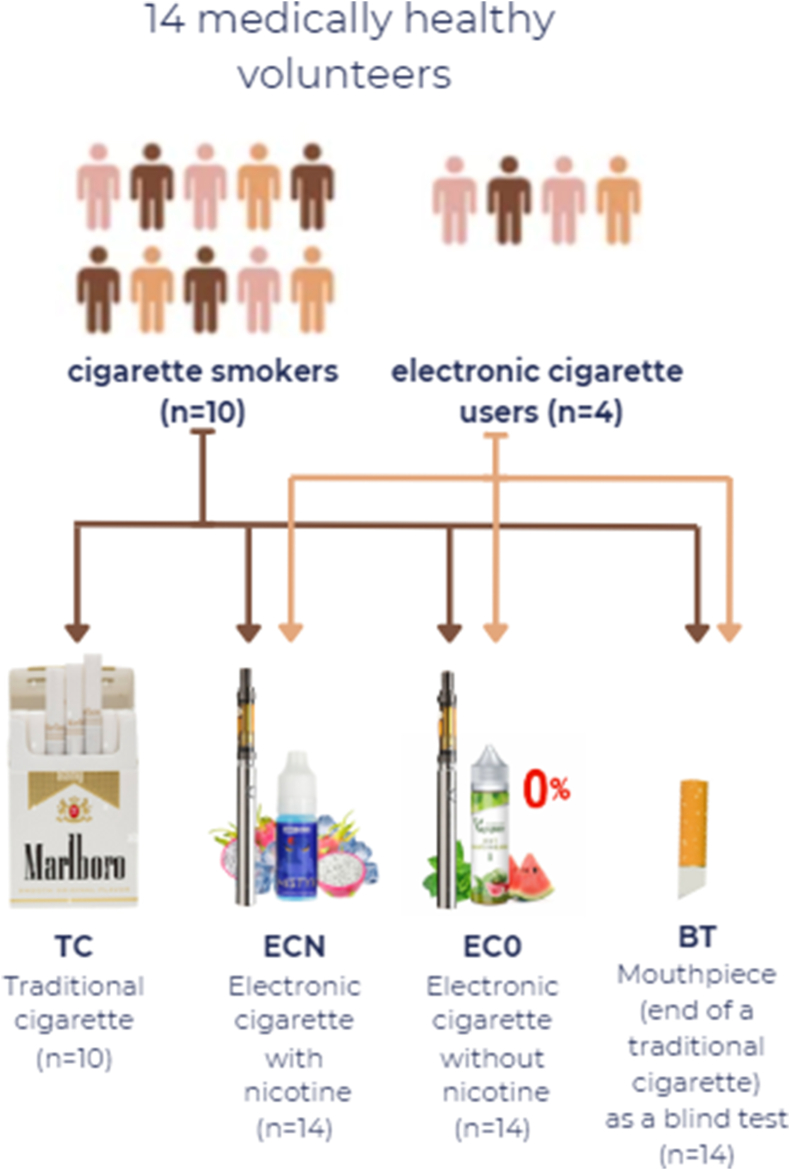
Each patient was randomly assigned to an exposure sequence (cross-over design). All patients (n = 14) were included in the nicotine-free e-cigarette (EC0), nicotine-containing e-cigarette (ECN), and blind test (BT) treatment. Ten patients were included in the traditional cigarette (TC) group, as the other four patients used only e-cigarettes before the experiment; as a result, they were not compelled to consume traditional cigarettes (Fig. 1).
Fig. 1.
Assignment to treatment groups. All patients were included in the EC0, ECN, and BT treatment group, but only cigarette smokers were included in the TC group.
Marlboro Gold was used in the TC group, with each cigarette containing 0,6 mg/cigarette nicotine. A ProVari v2.5 was used in the electronic cigarette, with two types of liquid, either Mystic Juice containing 6 mg/ml (ECN) or 0 mg/ml nicotine (EC0). In the blind test (BT) group, the end of a traditional cigarette was used as a mouthpiece (Fig. 1). The products were provided for the participants by the research team. The acute treatment lasted 5 min.
2.2.1. PBF measurement
PBF was measured by Laser Speckle Contrast Imager (785 nm PeriCam PSI HR System, Perimed AB, Stockholm, Sweden) (LSCI) with a 10 cm focal distance. The resolution was set at 60 μm/pixel. LSCI has demonstrated excellent reliability in oral mucosa blood flow measurement.10,11 It has a high temporal and spatial resolution. The measured area covered about 2 × 3 cm, and ten images/second, were recorded.
The participants were asked not to eat, smoke, chew a piece of gum, or drink anything except water for 8 h before the study. They were also asked not to brush their teeth in the 1 h prior to the measurement, in order to avoid any stimulus affecting the blood flow.
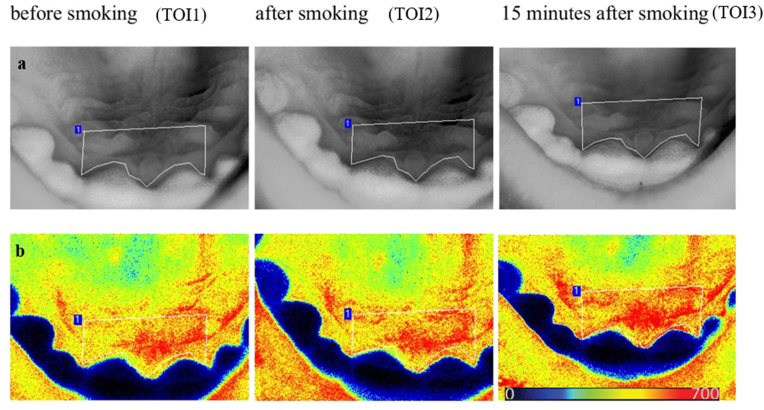
Perfusion images were evaluated by the Pimsoft software (Perimed AB, Stockholm, Sweden). A fixed-size region of interest (ROI) was applied behind the incisors to analyze the blood flow measurement (Fig. 2). Data were collected in 90-s periods as a time of interest (TOI), including the pre-smoking phase (TOI1), immediately after smoking (TOI2), and 15 min after smoking (TOI3).
Fig. 2.
Native (a) and perfusion images (b) from Pimsoft software at different times of interest (TOI). The palatal area, which is statistically analyzed (the region of interest), is outlined on the native image by a white line. TOI1, before exposure; TOI2, immediately after exposure; TOI3, 15 min after exposure. Warmer colors (red) represent higher perfusion, and colder colors (blue) represent lower perfusion on a scale of 0–700 laser speckle perfusion unit (LSPU).
2.3. Carbon dioxide and carboxyhemoglobin measurements
Following the PBF measurement, exhaled carbon monoxide (eCO) and carboxyhemoglobin (COHb) were measured before and immediately after smoking. The participants were asked to hold their breath for 15 s and blow into the mouthpiece of the piCO+ Smokerlyzer (Bedfont® Scientific Ltd., Harrietsham, United Kingdom). The results were displayed on the machine's screen in terms of ppm and percentage.
2.4. Protocol
Each participant rested for at least 15 min in a dental chair in a supine position at a constant temperature before the measurements. After resting, a blood flow measurement lasting 90 s began on the palate, and the eCO and COHb were measured. Then, patients were asked to use one of the devices for 5 min, and eCO and COHb were measured again. Another 90-sec PBF measurement was followed by a 15 min resting period. Then, a final 90-sec blood flow measurement was carried out.
2.5. Statistical analysis
Results are expressed as the mean ± standard deviation of the mean (SD) and sample size (N) for each treatment group. The normality of data was checked by applying the Shapiro-Wilk's test, and the homogeneity of variances was assessed through Levene's test. Since the criteria for normality were not met, non-parametric statistical procedures were used in the analysis.
Comparisons between different smoking groups were performed with the non-parametric Kruskal-Wallis test to determine significant differences between groups. Within smoking groups, the values in different time points were compared with non-parametric Friedman ANOVA (repeated measurements). In pairwise comparison, the significance level was adjusted with Bonferroni correction. The analysis was two-sided with a level of significance of α = 0.05. All statistical analyses were performed using the SPSS 28 (IBM®) software package.
3. Results
3.1. Changes in PBF
3.1.1. Average and relative PBF change
No statistically significant differences were found when examining mean difference (MD) and standard deviation (SD) for PBF before (TOI1), immediately after (TOI2), and 15 min after (TOI3) exposure (Table 1). This may be owing to the fact that the pre-exposure measurements of different individuals varied substantially (range 145–370). However, measuring relative differences in PBF between different time points revealed that EC0 caused a significantly higher increase between TOI1 and TOI2 (MD: 8.3; SD:16.9) (p < 0.05) and between TOI1 and TOI3 (MD: 7.1; SD:18.9) (p < 0.05) compared to BT. (Table 2). These findings should be taken with caution, since the high SD values obscured any possible differences in interaction.
Table 1.
Mean and standard deviation of PBF measured in laser speckle perfusion unit (LSPU) at different Times of Interest (TOI). TOI1, before exposure; TOI2, immediately after exposure; TOI3, 15 min after exposure; TC, traditional cigarette; EC0, nicotine-free electronic cigarette; ECN, nicotine-containing electronic cigarette; BT, blind test.
| Treatment groups | TOI1 |
TOI2 |
TOI3 |
|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | |
| TC | 240.4 ± 79.1 | 238.6 ± 56.2 | 232.2 ± 49.9 |
| EC0 | 211.5 ± 64.4 | 219.8 ± 69.4 | 218.6 ± 64.3 |
| ECN | 231.7 ± 77.6 | 223.9 ± 69.1 | 228.2 ± 81.1 |
| BT | 226.9 ± 75.4 | 210.4 ± 59.8 | 208.4 ± 49.4 |
| All Groups | 226.3 ± 71.9 | 222.6 ± 63.0 | 221.4 ± 61.5 |
Table 2.
Relative PBF changes (in laser speckle perfusion units LSPU) from the baseline (TOI1) immediately (TOI2) and 15 after the exposure (TOI3). Significant differences in changes compared to the blind test (BT) are indicated by * (p < 0.05). TC, traditional cigarette; EC0, nicotine-free electronic cigarette; ECN, nicotine-containing electronic cigarette.
| Treatment groups | TOI2 vs. TOI1 |
TOI3 vs. TOI1 |
|---|---|---|
| Mean ± SD | Mean ± SD | |
| TC | −1.8 ± 30.3 | −8.2 ± 30.4 |
| EC0 | 8.3 ± 16.9* | 7.1 ± 18.9* |
| ECN | −7.7 ± 27.9 | −3.5 ± 25.2 |
| BT | −16.5 ± 24.2 | −18.5 ± 30.3 |
| All Groups | −3.6 ± 25.7 | −4.9 ± 26.9 |
3.1.2. Percent PBF change from baseline
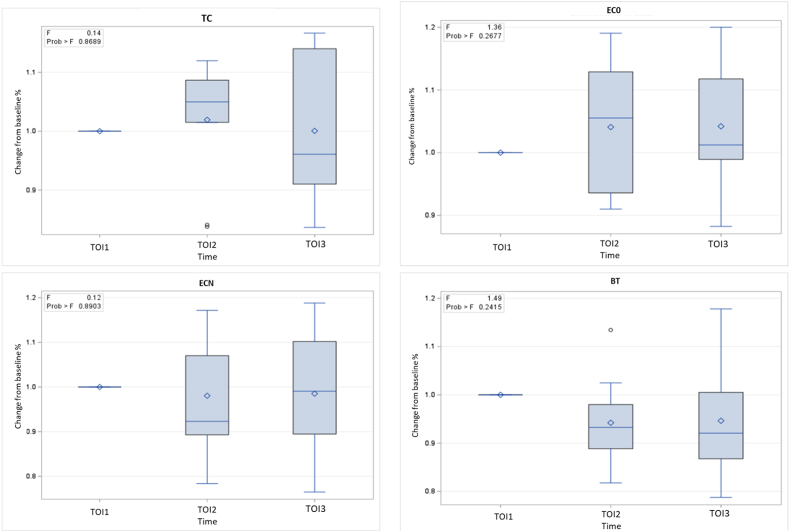
Values were also expressed as percentages of baseline for each group (Fig. 3). In all groups, no significant changes were observed in percent PBF between time points. However, a slight increase was observed in the PBF immediately after TC (+1.9 %) (p = 0.85) and EC0 (+4.1 %) (p = 0.28) exposure. In group TC, the PBF returned to baseline in TOI3, while in group EC0, it remained elevated (+4.2 %). In groups ECN and BT, a slight decrease was observed in TOI2 (−2.0 %, p = 0.85; −3.8 %, p = 0.22 respectively) and TOI3 (−1.5 %, p = 91; −3.4 %, p = 0.27 respectively).
Fig. 3.
The box plot indicates the percent PBF change from baseline after traditional cigarette smoking (a) nicotine-free electronic cigarette (b) nicotine-containing electronic cigarette (c) and blind test (d). TOI1, before exposure; TOI2, immediately after exposure; TOI3, 15 min after exposure; TC, traditional cigarette; EC0, nicotine-free electronic cigarette; ECN, nicotine-containing electronic cigarette; BT, blind test.
3.2. eCO and COHb
In group TC, an increased eCO and COHb was observed (Table 3). The eCO was significantly higher in group TC (MD:18.0; SD: 9.9) compared to group ECN (MD:8.2; SD: 6.4) and EC0 (MD:9.1; SD:7.5) before the exposure (p < 0.05). The eCO was significantly higher in group TC (MD:24.2; SD: 13.2) compared to groups ECN (MD:8.9; SD: 6.9) and EC0 (MD:9.3; SD:7.2) after the exposure (p < 0.05). The COHb was significantly higher in group TC (MD:3.6; SD:1.8) compared to groups ECN (MD:2.0; SD:1.2) and EC0 (MD:2.2; SD:1.2) before the exposure (p < 0.05). The COHb was significantly higher in group TC (MD:4.5; SD: 2.2) compared to groups ECN (MD:2.8; SD:2.1) and EC0 (MD:2.2; SD:1.1) after the exposure (p < 0.05).
Table 3.
Mean difference (MD) and standard deviation (SD) of exhaled carbon-monoxide (eCO) and carboxyhemoglobin (COHb) in ppm before and after smoking. # indicates statistically significant (p < 0.05) differences compared with traditional cigarettes. * indicates statistically significant changes (p < 0.05) from the baseline (before exposure). TC, traditional cigarette; EC0, nicotine-free electronic cigarette; ECN, nicotine-containing electronic cigarette; BT, blind test.
| Variables | eCO Before | eCO After | COHb Before | COHb After |
|---|---|---|---|---|
| TC | 18.0 ± 9.9 | 24.2 ± 13.2* | 3.6 ± 1.8 | 4.5 ± 2.2* |
| EC0 | 9.1 ± 7.5# | 9.3 ± 7.2# | 2.2 ± 1.2# | 2.2 ± 1.1# |
| ECN | 8.2 ± 6.4# | 8.9 ± 6.9# | 2.0 ± 1.2# | 2.8 ± 2.1 |
| BT | 11.3 ± 8.5 | 12.1 ± 8.6# | 2.4 ± 1.4# | 2.6 ± 1.4# |
4. Discussion
The objective of the present study was to evaluate the acute alteration of traditional and electronic cigarette smoking on palatal blood flow. In all groups, no significant differences were observed in changes of PBF between time points. Although not statistically significant, a slight increase was observed in the PBF immediately after TC and EC0 exposure, probably due to the local heat emitted by the tobacco products. Molnár et al.12 found that local application of heat causes a rapid and transient increase in gingival blood flow. The observed increase may be attributed only to the rise in the average speed of blood cells, while the concentration of moving blood cells is unchanged.
Besides the heat, nicotine is the primer responsible for most of the acute effects of cigarettes on oral blood perfusion. It is rapidly absorbed to a peak concentration at the end of smoking, followed by a rapid decline during the first 15–20 min.13 Nicotine is known to act as a local irritant in a number of tissues, including the oral mucosa; it has been hypothesized that it stimulates sensory neurons to release vasodilator substances, thereby constituting the axon reflex.14 Nicotine stimulates the release of calcitonin gene-related peptide (CGRP) from afferent nerve terminals, inducing neurogenic inflammation that temporarily increases perfusion.15 On the other hand, nicotine directly affects the smooth muscle of gingival blood vessels by causing vasoconstriction through noradrenaline secretion.16
The effect of smoking on oral microvascular perfusion depends on the form and duration of use.17 In line with this, ECN tended to decrease the blood flow in the current investigation, probably due to the nicotine content. E-cigarettes imitate traditional smoking through e-liquid evaporation which contains individually controlled amounts of nicotine in its solvent.18 In contrast, the EC0 tended to increase the microcirculation due to the heating effect and the lack of nicotine content. E-liquids can contain numerous substances, propylene glycol, vegetable glycerin, and flavorings.19 Carbonyl compounds such as formaldehyde, acetaldehyde, and acrolein are produced by the thermal degradation of these compounds20 and can act as local irritants to the gingiva, thus causing hyperaemia. The increase caused by TC may be attributed to local heat and the complex responses to the vasoactive hormones. However, the complex stimuli in the TC and ECN groups might cancel each other out, making it difficult to detect any statistically significant changes. The palatal blood flow also seems to be decreased during the mouthpiece simulation, probably due to the negative pressure.
Reuther et al.9 reported a slight increase in blood flow after both nicotine-containing and nicotine-free electronic cigarettes, but this was most pronounced in those containing nicotine. Meekin et al.21 discovered that cigarette smoking caused a slight decrease in gingival blood flow in heavy smokers and increased gingival blood flow in light smokers. Neither change, however, was statistically significant. Mavropoulos16 found that cigarette smoking induced a modest hyperaemic response in the gingiva. Baab et al.13 experienced acute increased perfusion in the gingival sulcus but not in the gingival crest of young habitual smokers. Differences in opinion regarding whether cigarette smoking induces an increase or decrease in blood flow in periodontal tissues may be attributable to the absence of confounding factors. Study design and study populations may also have an impact on the findings.
While in previous studies9 13 16 21 laser Doppler flowmeter was used to record the relative blood flow of the mucosa, in this study, laser speckle contrast imaging (LSCI) was applied. Laser Doppler flowmetry is only capable of one-point measurement, while LSCI is capable of complex surface monitoring.22 By averaging out local variation in microcirculation, the LSCI has a lower coefficient of variation than LDF tool.11 It is a noncontact, fast, simple technique that can be used to visualize perfusion in various tissues, as it can create 2-D perfusion maps of large surfaces. It is based on the principle that backscattered light from a coherent laser light illuminated tissue forms a random interference pattern at the detector called the speckle pattern. The movement of red blood cells in tissues causes fluctuations in this speckle pattern resulting in the blurring of speckle images. LSCI is a widespread method used in rheumatology, dermatology, burn injuries, ophthalmology, neurology, and gastrointestinal tract surgery.23 Dental applications have also been reported to assess the blood flow of the gingiva24 and the dental pulp.25
CO is a toxic component of cigarette smoke and a biological marker of cigarette smoking exposure. Therefore, it was used to estimate smoking status in this study. CO typically interacts with hemoglobin, producing CO–Hb, which reduces the blood's capacity to transport oxygen.26 The eCO and blood COHb% levels can be used to quantify the severity of smoking, as these parameters correlate with the number of cigarettes smoked per day and the duration of smoking.27 According to the manufacturer's protocol, the standard values for non-smokers, moderate, and heavy smokers are 1–7. 8–19. And 20 ppm or more, respectively.28 Accordingly, smoking a traditional cigarette increased the CO and CO-Hb. However, e-cigarettes, regardless of nicotine content, failed to increase these parameters. Unlike conventional cigarettes, electronic cigaretes do not require combustion to deliver nicotine; consequently, fewer harmful and potentially harmful constituents, including CO, are emitted.29
4.1. Strengths and limitations
This pilot study was the first to compare the effect of electronic cigarettes on PBF to traditional cigarettes. In addition, the innovative LSCI approach was employed instead of the more traditional laser Doppler flowmetry. However, the present study had some limitations. First, we only used one type of e-cigarette, and it would be useful to conduct a wider study of the various devices on the market, as different devices may have different nicotine delivery efficiency.30 Achieving visibility of the palate for optical blood flow measurement is challenging due to the extraoral positioning of the LSCI, which imposes certain limitations.31 The study was conducted with a small number of only 14 medically healthy volunteers. While it is referred to as a pilot study, the limited sample size may affect the generalizability and statistical power of the findings. The study compares traditional and electronic cigarettes' effects on PBF but does not include a control group of non-smokers. A non-smoking control group could provide a baseline for comparison and help to differentiate the acute and chronic effects of smoking. However, asking non-smokers to smoke is not ethically sound.
4.2. Implication for practice and research
More effort should be made to educate the public about the risks associated with using electronic cigarettes. Further research is needed with a larger sample size, and chronic long-term effects should be examined to clarify the role of electronic cigarette smoking on mucosal microcirculation.
5. Conclusion
Within the limitations of this study we can say that in contrast to cigarette smoking, the use of electronic cigarettes did not elevate eCO and COHb levels. Acute exposure to cigarette smoking and electronic cigarette use has minimal effect on palatal blood flow. ECN tended to decrease, whereas TC and EC0 tended to increase the palatal blood flow, though the effects were not significant. Additional studies are required to clarify the impact of electronic cigarettes on oral circulation, periodontal and surgical treatment results.
References
- 1.Who . third ed. Dec. 18. 2019. WHO Global Report on Trends in Prevalence of Tobacco Use 2000-2025.https://www.who.int/publications/i/item/who-global-report-on-trends-in-prevalence-of-tobacco-use-2000-2025-third-edition 2019 [Google Scholar]
- 2.Klein J., Diaba-Nuhoho P., Giebe S., et al. Regulation of endothelial function by cigarette smoke and next-generation tobacco and nicotine products. Pflügers Archiv. 2023;475(7):835–844. doi: 10.1007/s00424-023-02824-w. [published Online First: 20230607] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balaji S.M. Tobacco smoking and surgical healing of oral tissues: a review. Indian J Dent Res. 2008;19(4):344–348. doi: 10.4103/0970-9290.44540. [DOI] [PubMed] [Google Scholar]
- 4.Lindeboom J.A., Mathura K.R., Harkisoen S., et al. Effect of smoking on the gingival capillary density: assessment of gingival capillary density with orthogonal polarization spectral imaging. J Clin Periodontol. 2005;32(12):1208–1212. doi: 10.1111/j.1600-051X.2005.00854.x. [DOI] [PubMed] [Google Scholar]
- 5.Shuler R.L. Effect of cigarette smoking on the circulation of the oral mucosa. J Dent Res. 1968;47(6):910–915. doi: 10.1177/00220345680470065201. [DOI] [PubMed] [Google Scholar]
- 6.Lova R.M., Miniati B., Macchi C., et al. Morphologic changes in the microcirculation induced by chronic smoking habit: a videocapillaroscopic study on the human labial mucosa. Am Heart J. 2002;143(4):658. doi: 10.1067/mhj.2002.121461. [DOI] [PubMed] [Google Scholar]
- 7.Jerzynski T., Stimson G.V., Shapiro H., Krol G. Estimation of the global number of e-cigarette users in 2020. Harm Reduct J. 2021;18(1):109. doi: 10.1186/s12954-021-00556-7. [published Online First: 20211023] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Almeida-da-Silva C.L.C., Matshik Dakafay H., O'Brien K., et al. Effects of electronic cigarette aerosol exposure on oral and systemic health. Biomed J. 2021;44(3):252–259. doi: 10.1016/j.bj.2020.07.003. [published Online First: 20200724] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reuther W.J., Hale B., Matharu J., et al. Do you mind if I vape? Immediate effects of electronic cigarettes on perfusion in buccal mucosal tissue--a pilot study. Br J Oral Maxillofac Surg. 2016;54(3):338–341. doi: 10.1016/j.bjoms.2015.12.001. [published Online First: 20160122] [DOI] [PubMed] [Google Scholar]
- 10.Molnar B., Molnar E., Fazekas R., et al. Assessment of palatal mucosal wound healing following connective-tissue harvesting by laser speckle contrast imaging: an observational case series study. Int J Periodontics Restor Dent. 2019;39(2):e64–e70. doi: 10.11607/prd.3878. [published Online First: 2019/02/23] [DOI] [PubMed] [Google Scholar]
- 11.Molnar E., Fazekas R., Lohinai Z., et al. Assessment of the test-retest reliability of human gingival blood flow measurements by Laser Speckle Contrast Imaging in a healthy cohort. Microcirculation. 2018;25(2) doi: 10.1111/micc.12420. [DOI] [PubMed] [Google Scholar]
- 12.Molnar E., Lohinai Z., Demeter A., et al. Assessment of heat provocation tests on the human gingiva: the effect of periodontal disease and smoking. Acta Physiol Hung. 2015;102(2):176–188. doi: 10.1556/036.102.2015.2.8. [DOI] [PubMed] [Google Scholar]
- 13.Baab D.A., Oberg P.A. The effect of cigarette smoking on gingival blood flow in humans. J Clin Periodontol. 1987;14(7):418–424. doi: 10.1111/j.1600-051x.1987.tb01547.x. [DOI] [PubMed] [Google Scholar]
- 14.Warner D.O., Joyner M.J., Charkoudian N. Nicotine increases initial blood flow responses to local heating of human non-glabrous skin. J Physiol. 2004;559(Pt 3):975–984. doi: 10.1113/jphysiol.2004.062943. [published Online First: 20040722] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Su C. Actions of nicotine and smoking on circulation. Pharmacol Ther. 1982;17(1):129–141. doi: 10.1016/0163-7258(82)90050-x. [DOI] [PubMed] [Google Scholar]
- 16.Mavropoulos A., Aars H., Brodin P. Hyperaemic response to cigarette smoking in healthy gingiva. J Clin Periodontol. 2003;30(3):214–221. doi: 10.1034/j.1600-051x.2003.10284.x. [DOI] [PubMed] [Google Scholar]
- 17.Mavropoulos A., Aars H., Brodin P. The acute effects of smokeless tobacco (snuff) on gingival blood flow in man. J Periodontal Res. 2001;36(4):221–226. doi: 10.1034/j.1600-0765.2001.036004221.x. [DOI] [PubMed] [Google Scholar]
- 18.Grana R., Benowitz N., Glantz S.A. E-cigarettes: a scientific review. Circulation. 2014;129(19):1972–1986. doi: 10.1161/CIRCULATIONAHA.114.007667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ooi B.G., Dutta D., Kazipeta K., Chong N.S. Influence of the E-cigarette emission profile by the ratio of glycerol to propylene glycol in E-liquid composition. ACS Omega. 2019;4(8):13338–13348. doi: 10.1021/acsomega.9b01504. [published Online First: 20190805] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nicholson T., Scott A., Newton Ede M., Jones S.W. The impact of E-cigarette vaping and vapour constituents on bone health. J Inflamm. 2021;18(1):16. doi: 10.1186/s12950-021-00283-7. [published Online First: 20210505] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meekin T.N., Wilson R.F., Scott D.A., et al. Laser Doppler flowmeter measurement of relative gingival and forehead skin blood flow in light and heavy smokers during and after smoking. J Clin Periodontol. 2000;27(4):236–242. doi: 10.1034/j.1600-051x.2000.027004236.x. [DOI] [PubMed] [Google Scholar]
- 22.Tew G.A., Klonizakis M., Crank H., et al. Comparison of laser speckle contrast imaging with laser Doppler for assessing microvascular function. Microvasc Res. 2011;82(3):326–332. doi: 10.1016/j.mvr.2011.07.007. [published Online First: 20110722] [DOI] [PubMed] [Google Scholar]
- 23.Heeman W., Steenbergen W., van Dam G., Boerma E.C. Clinical applications of laser speckle contrast imaging: a review. J Biomed Opt. 2019;24(8):1–11. doi: 10.1117/1.JBO.24.8.080901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Regan C., White S.M., Yang B.Y., et al. Design and evaluation of a miniature laser speckle imaging device to assess gingival health. J Biomed Opt. 2016;21(10) doi: 10.1117/1.JBO.21.10.104002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dick S.K., Chistyakova G.G., Terekh A.S., et al. Characterization of blood flow rate in dental pulp by speckle patterns of backscattered light from an in vivo tooth. J Biomed Opt. 2014;19(10) doi: 10.1117/1.JBO.19.10.106012. [DOI] [PubMed] [Google Scholar]
- 26.Low E.C., Ong M.C., Tan M. Breath carbon monoxide as an indication of smoking habit in the military setting. Singap Med J. 2004;45(12):578–582. [PubMed] [Google Scholar]
- 27.Herath P., Wimalasekera S., Amarasekara T., et al. Adverse effects of cigarette smoking on exhaled breath carbon monoxide, blood carboxyhemoglobin, and hematological parameters amongst Sri Lankan adult tobacco smokers: a descriptive study. Population Medicine. 2021;3(October):1–10. doi: 10.18332/popmed/143076. [DOI] [Google Scholar]
- 28.Morozumi T., Kubota T., Sato T., et al. Smoking cessation increases gingival blood flow and gingival crevicular fluid. J Clin Periodontol. 2004;31(4):267–272. doi: 10.1111/j.1600-051X.2004.00476.x. [DOI] [PubMed] [Google Scholar]
- 29.Nga J.D.L., Hakim S.L., Bilal S. Comparison of end tidal carbon monoxide levels between conventional cigarette, electronic cigarette and heated tobacco product among asiatic smokers. Subst Use Misuse. 2020;55(12):1943–1948. doi: 10.1080/10826084.2020.1781180. [published Online First: 20200619] [DOI] [PubMed] [Google Scholar]
- 30.Farsalinos K.E., Spyrou A., Tsimopoulou K., et al. Nicotine absorption from electronic cigarette use: comparison between first and new-generation devices. Sci Rep. 2014;4:4133. doi: 10.1038/srep04133. [published Online First: 20140226] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fazekas R., Molnar E., Lohinai Z., et al. Functional characterization of collaterals in the human gingiva by laser speckle contrast imaging. Microcirculation. 2018;25(3) doi: 10.1111/micc.12446. [DOI] [PubMed] [Google Scholar]