Introduction
Focal segmental glomerulosclerosis (FSGS) combines a heterogeneous group of different etiologies with defined glomerular histopathology and accounts for approximately 10% of nephrotic syndrome cases in adults and 20% of cases in children in Europe. Currently, there is no specific therapy available for FSGS, with the majority of patients being treated with systemic corticosteroids, calcineurin inhibitors and renin-angiotensin-system inhibitors.1 Although some patients respond to therapy, a significant proportion remains unresponsive, ultimately requiring kidney replacement therapy, resulting in a significant burden to the individual patient as well as to the health care system.
Drug repurposing represents an attractive possibility to study approved drugs for new clinical indications, especially in rare diseases. Utilizing a network-based drug repurposing approach, a molecular model of FSGS pathophysiology has previously been generated and applied in a systems biology workflow to conduct a computational screen for novel compounds to treat FSGS.2 Recently, this approach has identified the antiplatelet drug clopidogrel as a novel repositioning candidate.3 Clopidogrel is known to inhibit the P2Y12 subtype of ADP receptor, which is important in activation of platelets, but also demonstrates anticoagulant, anti-inflammatory, antioxidant, immunosuppressive, and proautophagic effects that could counterbalance dysregulated FSGS pathways (Supplementary Figure S1, Supplementary Methods). Significant reduction of proteinuria and improved renal histomorphology in the adriamycin FSGS mouse model provided experimental validation of the in silico prediction and suggests that clopidogrel is a promising candidate for clinical testing in patients with FSGS.3
Discussion
Here, we present ClopiD4FSGS as a single-arm multicenter proof-of-concept phase 2 trial over 24 weeks, with a planned interim analysis after the first 10 patients (EudraCT Nr: 2022-003313-11) (Figure 1, Supplementary Methods). Patients aged 18 to 75 years with primary or genetic forms of FSGS will be enrolled (Supplementary Tables S1 and S2). All patients will receive clopidogrel 75 mg daily. The primary end point of the study is defined as the change of urinary protein-to-creatinine ratio assessed through a 24-hour urine collection at baseline compared to the measurement at 24 weeks. As secondary end point, the proportion of patients who achieved the FSGS partial remission end point, defined as urinary protein-to-creatinine ratio <1.5 g/g and a >40% reduction in urinary protein-to-creatinine ratio from baseline will be assessed (Figure 2).
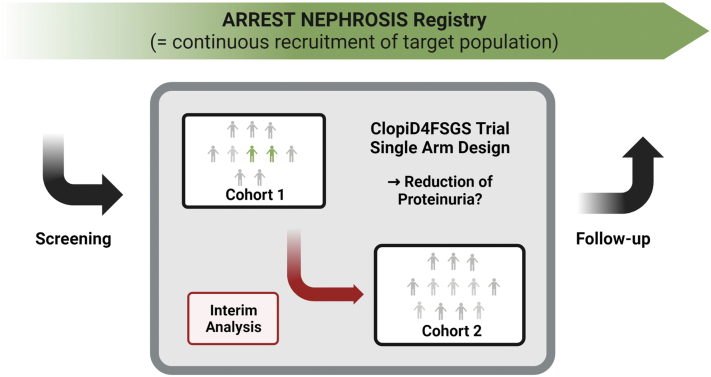
Figure 1.
ClopiD4FSGS trial overview. Patients enrolled in the ARREST NEPHROSIS registry are screened for eligibility. A total of up to 22 patients will be recruited. After the recruitment of 10 patients (= cohort 1), an interim analysis for early futility will be performed after visit 2 (= 84 days after baseline visit). The futility assessment will be based on a secondary parameter only. If no more than 1 patient reaches the focal segmental glomerulosclerosis partial remission end point, the study will be stopped for early futility. Otherwise, recruitment of the remaining patients will be completed, and the primary end point be tested in the final analysis of 22 patients. The primary outcome is the percentage change of the urinary protein-to-creatinine ratio from baseline to week 24. Focal segmental glomerulosclerosis partial remission end point is defined as a urinary protein-to-creatinine ratio <1.5 and a >40% reduction in urinary protein-to-creatinine ratio compared to baseline, which will be reported for each visit. Registry data will be continuously obtained during and following the ClopiD4FSGS trial.
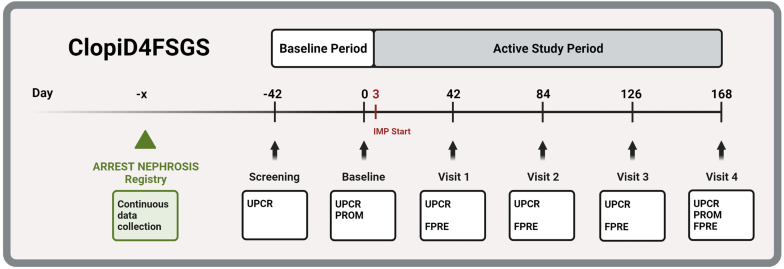
Figure 2.
ClopiD4FSGS study design. The baseline visit will be conducted 7 weeks after the screening assessment (= baseline period), followed by 24-week treatment period with 75 mg clopidogrel daily (= active study period). UPCR as assessed by 24-hour-urine collections will be assessed at baseline and at each study visit. The primary end point is defined as the percent proteinuria change from baseline to visit 4 (week 24). Individual patient trajectories will be reported. The secondary end point is the proportion of patients who achieve the FPRE after visit 4 (week 24). As tertiary end points, core outcomes as defined by the “Standardized Outcomes in Nephrology” (SONG) initiative will be assessed, including PROM for life participation. Patients are recruited from the ARREST-NEPHROSIS registry (green triangle, green box), registry data will be continuously obtained during and following the ClopiD4FSGS trial. IMP, investigational medicinal product; PROM, patient reported outcome measures; UPCR, urinary protein-to-creatinine ratio.
FPRE is defined as UPCR <1.5 and a >40% reduction in UPCR.
The ClopiD4FSGS trial primarily aims to generate sufficient clinical evidence regarding the expected benefit-to-risk ratio to justify allocation of appropriate resources of pharmaceutical companies for further clinical development. Drug repurposing allows for trial designs that focus on proof of efficacy, because prior evidence provides data regarding the side effect profile in other indications: the safety profile of clopidogrel in its primary indications has been assessed in multiple clinical trials, also in patients with chronic kidney disease.4
Including patients with genetic and primary FSGS is consistent with the original network-based analyses that identified clopidogrel to interfere with FSGS development and progression via several signaling cascades, mediated by its anticoagulant, anti-inflammatory, antioxidant, immunosuppressive, and proautophagic functions.3 Interestingly, recent experimental and clinical data also suggest that the P2Y12 subtype of ADP receptor is a driver of renal fibrosis and the use of clopidogrel might slow the decline of estimated glomerular filtration rate in patients with chronic kidney disease.5 The similarity of inclusion (Supplementary Table S1) and exclusion criteria (Supplementary Table S2) to the recent DUET trial will also allow comparison of the results of our study with this study, which includes a similar unselected population with primary FSGS.6
The overall goal of any drug for treatment of FSGS is the preservation of kidney function, being highly relevant both for the patients affected and for the healthcare system to reduce the enormous costs for treating patients with end-stage kidney disease, necessitating adequately powered long-term randomized controlled trials. In the ClopiD4FSGS trial, as standard in early FSGS trials, proteinuria will be the short-term measure of treatment efficacy.6 Assessment of urinary protein-to-creatinine ratio in timed urine specimen is also performed as a routine parameter in the ARREST NEPHROSIS registry, because changes in this continuous clinical index are classically considered as informative for kidney function prognosis.
Because of the single-arm design, clopidogrel will be added to each patient’s standard of care. Allowing other forms of treatment, such as immunosuppression and antiproteinuric drugs (including renin-angiotensin-system inhibitors as well as sodium-glucose cotransporter-2 inhibitors) ensures that effective therapies will not be withheld from patients included in ClopiD4FSGS. Single-arm studies of “add on” therapies facilitate recruitment and are therefore considered as a preferable design in early trials of rare diseases with a small number of eligible patients in whom no effective standard of care exists.7 The low likelihood of spontaneous improvement in patients with FSGS under standard treatment formed another important rationale for our single-arm trial design with a predefined futility threshold.6,8 The interim analysis will lead to early detection of futility; therefore, the number of patients exposed to an ineffective drug should be minimized. This analysis allows us to quickly assess the potential of clopidogrel to achieve comparable effects as observed with sparsentan (= FSGS partial remission end point in about 30% of treated patients).6 If this predefined threshold is not likely to be reached (= no more than one of the first 10 patients achieves FSGS partial remission end point), the study will be terminated early. The interim futility assessment will be based on the secondary (surrogate) parameter and no formal hypothesis testing for the primary end point will be performed. Taken together, these measures should increase acceptability of the participation in this trial. This approach is consistent with other studies previously conducted in rare diseases, such as early oncology trials.
Patient registries are another critical tool to facilitate timely recruitment of a sufficient sample size for clinical research in rare diseases. As an international example, the Nephrotic Syndrome Study Network project assesses genotype-phenotype correlations, clinical management and long-term outcomes, searching for novel genetic entities, diagnostic and prognostic biomarkers for glomerular diseases in patients who have been enrolled in 33 study sites in North America and is supposed to facilitate the initiation of clinical trials (www.neptune-study.org). The ARREST-NEPHROSIS registry set out to achieve similar goals in Austria, and should also facilitate the recruitment of patients for ClopiD4FSGS (NCT06162546). Importantly, continuous collection of patient data in this academic registry will allow independent traceability of the course of disease in these patients after the discontinuation of the study medication. Thus, effects during the active phase of the study can be tested for reversibility after discontinuation of the study medication over an extended period.
Conclusion
In conclusion, the ClopiD4FSGS trial will apply innovative elements in the design of a proof-of-concept study in order to effectively collect evidence about whether a repositioned drug should undergo further clinical development in a rare kidney disease. First, the repositioning approach per se will allow a focus on efficacy, due to the known side effect profile of the drug in its original indication, and thus allow use of a single arm design. Second, population and outcome selection are adapted to recent studies resulting in a low likelihood of spontaneous remission that should allow assessment of threshold crossing as a cost-effective way to estimate therapeutic benefit.8 Third, single-arm study design (with the omission of a placebo arm), timely futility decision through interim analysis with the intention of avoiding unnecessary exposure and networking with a nationwide registry study are measures to increase acceptability of both patients and investigators, thereby allowing improved recruitment rates. Findings from this proof-of-concept study may further support the development and evaluation of clopidogrel for the treatment of FSGS; and may also serve as a template for feasible early clinical development to evaluate the potential of repositioned drugs in rare kidney diseases.
Disclosure
LD-F, MA, DC, KE, PG, BO, MR, MDS, MWie, MWin, EZ, AK, CA are investigators for the ARREST NEPHROSIS registry. CA, MA, DC, KE, PG, BO, AK, MR, MDS, GS-P, MWie, MWin, and EZ have acted as consultants (ClopiD4FSGS Advisory Board) to Delta4 and have received consulting fees. CA holds Delta4 stocks. LD-F received research grants from Delta 4. BO reports receiving research grants from CSL Vifor and Otsuka and speaking fees and travel support from Otsuka. AK reports receiving research grants from CSL Vifor and Otsuka, and has received consulting fees from CSL Vifor, Otsuka, Walden Biosciences, and Catalyst Biosciences. PG received speaking fees from Otsuka and consulting fees from CSL Vifor.
Acknowledgments
This work and the ARREST NEPHROSIS registry are sponsored by the Medical University of Vienna and endorsed by the Austrian Society of Nephrology. The ClopiD4FSGS trial is sponsored by Delta 4 (Delta 4 GmbH, Alser Straße 23 / Top 301080 Vienna, office@delta4.ai, represented by Kurt Herpel) with support of the Vienna Business Agency (project 4502705). The study sponsors have been actively involved in study design and have taken an active role in the decision to submit this report for publication. The authors were involved in the decision to submit this manuscript and will take public responsibility for all aspects of the publication. All figures have been created with BioRender.com.
Footnotes
Supplementary Methods.
Supplementary References.
Figure S1. Modeled impact of clopidogrel on FSGS pathophysiology.
Table S1. ClopiD4FSGS inclusion criteria.
Table S2. ClopiD4FSGS exclusion criteria.
Table S3. ClopiD4FSGS study assessment schedule.
Supplementary Patient Information and Informed Consent.
SPIRIT Checklist.
Supplementary Material
Supplementary Methods.
Supplementary References.
Figure S1. Modeled impact of clopidogrel on FSGS pathophysiology.
Table S1. ClopiD4FSGS inclusion criteria.
Table S2. ClopiD4FSGS exclusion criteria.
Table S3. ClopiD4FSGS study assessment schedule.
Supplementary Patient Information and Informed Consent.
SPIRIT Checklist.
References
- 1.Hodson E.M., Sinha A., Cooper T.E. Interventions for focal segmental glomerulosclerosis in adults. Cochrane Database Syst Rev. 2022;2:CD003233. doi: 10.1002/14651858.CD003233.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boehm M., Bukosza E.N., Huttary N., et al. A systems pharmacology workflow with experimental validation to assess the potential of anakinra for treatment of focal and segmental glomerulosclerosis. PLoS One. 2019;14 doi: 10.1371/journal.pone.0214332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gebeshuber C.A., Daniel-Fischer L., Regele H., et al. Computational drug repositioning of clopidogrel as a novel therapeutic option for focal segmental glomerulosclerosis. Transl Res. 2023;259:28–34. doi: 10.1016/j.trsl.2023.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Natale P., Palmer S.C., Saglimbene V.M., et al. Antiplatelet agents for chronic kidney disease. Cochrane Database Syst Rev. 2022;2:CD008834. doi: 10.1002/14651858.CD008834.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen J., Tang Y., Zhong Y., et al. P2Y12 inhibitor clopidogrel inhibits renal fibrosis by blocking macrophage-to-myofibroblast transition. Mol Ther. 2022;30:3017–3033. doi: 10.1016/j.ymthe.2022.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trachtman H., Nelson P., Adler S., et al. DUET: a phase 2 study evaluating the efficacy and safety of sparsentan in patients with FSGS. J Am Soc Nephrol. 2018;29:2745–2754. doi: 10.1681/ASN.2018010091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davi R., Mahendraratnam N., Chatterjee A., Dawson C.J., Sherman R. Informing single-arm clinical trials with external controls. Nat Rev Drug Discov. 2020;19:821–822. doi: 10.1038/d41573-020-00146-5. [DOI] [PubMed] [Google Scholar]
- 8.Eichler H.G., Bloechl-Daum B., Bauer P., et al. “Threshold-crossing”: a useful way to establish the counterfactual in clinical trials? Clin Pharmacol Ther. 2016;100:699–712. doi: 10.1002/cpt.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.