Abstract
Introduction
Peritoneal dialysis (PD)-associated peritonitis due to tuberculosis (TB) is associated with poor outcomes and optimal treatment strategies for this condition remain unknown. Our study aimed to: (i) systematically review the published literature on peritonitis caused by Mycobacterium tuberculosis in patients on PD and (ii) review cases of peritonitis due to M tuberculosis in patients on PD reported in Australia and New Zealand to determine the epidemiology, management strategies, and outcomes of this condition.
Methods
A literature search of Medline, Scopus, Embase, ClinicalTrials.gov, Cochrane CENTRAL Register of Controlled Trials and Google Scholar for articles published from inception date to June 2022 was conducted. To be eligible, articles had to describe patient characteristics, initial anti-TB therapy, and treatment outcomes in all patients on PD with peritonitis caused by M tuberculosis. Data from the Australia and New Zealand Dialysis and Transplant (ANZDATA) Registry of patients on PD who developed peritonitis due to M tuberculosis between September 2001 and December 2020 were included and analyzed.
Results
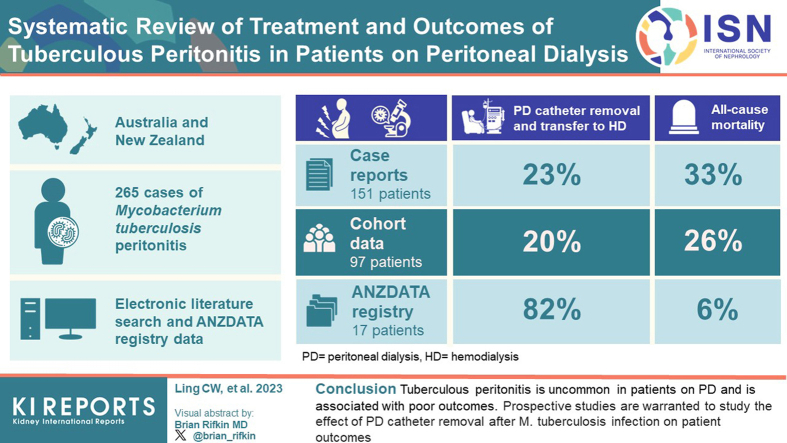
The systematic literature review identified 70 case studies (151 patients) and 8 cohort studies (97 patients), whereas the ANZDATA Registry identified 17 cases of peritonitis due to M tuberculosis. Overall, in patients diagnosed with peritonitis due to M tuberculosis, the rates of PD catheter removal and permanent transfer to hemodialysis (HD) were numerically higher in the ANZDATA Registry cases (82%) than in the case studies (23%) and cohort studies (20%). Observed all-cause mortality rates were also higher as observed in the case studies (33%) and cohort studies (26%) than in the ANZDATA Registry cases (6%).
Conclusion
Tuberculous peritonitis is uncommon in patients on PD and is associated with poor outcomes. Prospective studies are warranted to study the effect of retaining PD catheters after M tuberculosis infection on patient outcomes.
Keywords: antituberculosis regimen, Mycobacterium tuberculosis, outcomes, peritoneal dialysis, peritonitis, treatment
Graphical abstract
Peritonitis is a serious complication in patients receiving PD. M tuberculosis is an uncommon cause of peritonitis,1 which has been associated with the permanent transition to HD and high mortality rates.2 Compared to the general population, the risk of TB in patients with kidney failure was reported to be 100-fold higher.3 This is likely due to the impaired cellular immunity in kidney failure4 and a compromised immune system that can trigger the reactivation of latent TB in the peritoneum caused by the hematogenous spread from previous pulmonary, active pulmonary, or miliary TB.5
To date, there have not been robust data to guide optimal management strategies for patients with PD-associated tuberculous peritonitis. Whereas the recent International Society for Peritoneal Dialysis (ISPD) peritonitis guidelines make a level 2C recommendation suggesting that anti-TB therapy without PD catheter removal,6 several reports have shown conflicting results.7, 8, 9, 10, 11 Furthermore, the current recommendations for treating tuberculous peritonitis are extrapolated from the guidelines for treating pulmonary and other extrapulmonary TB,12 which is further limited by expert opinion and case reports.6,13 Therefore, given the variation in anti-TB regimens and outcomes in patients on PD, it is imperative to identify optimal management strategies to improve the survival rates and prolong the length of time on PD. Therefore, the objectives of this study were to: (i) systematically review the published literature on peritonitis caused by M tuberculosis in patients on PD and (ii) review cases of peritonitis due to M tuberculosis in patients on PD reported in Australia and New Zealand to determine the epidemiology, management strategies, and outcomes of this condition.
Methods
We conducted our study in 2 parts: (i) literature review and (ii) ANZDATA Registry analysis.
Literature Review
Search Methodology
We conducted a systematic review of the literature following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses.14 All publications and conference papers published from date of inception to June 2022 were identified from Medline, Scopus, Embase, ClinicalTrials.gov, Cochrane CENTRAL Register of Controlled Trials and Google Scholar (Supplementary Tables S1-S3). The following search terms were used: “Mycobacterium tubercul∗ OR tubercul∗ OR tuberculous, “peritonitis”, and “peritoneal dialysis” (Supplementary Figures S1 and S2). This protocol was registered in PROSPERO (registration number CRD42022348318).
Inclusion and Exclusion Criteria and Study Selection
To be eligible, articles had to describe patient characteristics, initial anti-TB therapy, and treatment outcomes in all patients on PD with peritonitis caused by M tuberculosis. In Table 1, we summarize the variables extracted from the included articles. Articles describing M tuberculosis not causing PD-associated peritonitis, tuberculous peritonitis in patients not on PD, conference abstracts on tuberculous peritonitis in which numbers did not tally correctly, and those that only described adverse effects from anti-TB therapy were excluded.
Table 1.
Variables extracted from the articles on tuberculous peritonitis in Supplementary Tables S1 and S2
| The following data were extracted for the included articles: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
HD, hemodialysis; PD, peritoneal dialysis.
Racial origin from endemic countries or high TB incidence (upper-moderate) was defined as >100-299 or > 50–99 new and relapse cases per 100,000 population.15
Concomitant or within 4 weeks of previous peritonitis was defined as the presence of other organisms in the PD effluent identified on the same day that M tuberculosis was identified or peritonitis episode 30 days before the current tuberculous peritonitis.
The outcomes were PD-catheter removal, death related to tuberculous peritonitis, and the dialysis modality during the course of anti-TB treatment. PD-catheter removal was defined as the removal of the PD-catheter during the peritonitis episode within 30 days of diagnosis of tuberculous peritonitis. Permanent transfer to HD was defined as transfer to HD for more than 30 days. Death related to tuberculous peritonitis was defined as death reported within 30 days of onset and/or diagnosis of tuberculous peritonitis, whichever was earlier, or death due to tuberculous peritonitis as reported by the authors on the case studies and retrospective cohort studies.
Data Extraction
Two independent reviewers (CWL and RLC) screened the abstracts of all articles and then reviewed the short-listed full-text articles of the published articles for eligibility and relevance. Any disagreement was resolved by discussion with the team.
ANZDATA Registry Analysis
A retrospective analysis of data obtained from the ANZDATA Registry was conducted in all patients on PD who developed peritonitis between September 2001 and December 2020. Peritonitis episodes due to M tuberculosis were reviewed. The data collection and management details are available on the ANZDATA website.16 This study was approved by the Western Sydney Local Health District (2019/ETH10518).
Data Collection
The data collected included patient demographics, comorbidities (chronic lung disease, coronary artery disease, peripheral vascular disease, cerebrovascular disease, diabetes mellitus), body mass index subcategorized according to the World Health Organization classification, primary cause of kidney disease, PD start date, date of peritonitis, concomitant or within 4 weeks of previous peritonitis (defined as the presence of other organisms in the PD effluent identified on the same day that M tuberculosis was identified or peritonitis episode 30 days before the current tuberculous peritonitis), PD modality at the time of infection, number of peritonitis episodes (and causative organisms) during the study period, country and state of the PD unit, empirical antibiotic regimen, anti-TB regimen, PD catheter removal, duration from diagnosis of tuberculous peritonitis to catheter removal, and outcomes following tuberculous peritonitis (Supplementary Table S4).
The outcomes measured were PD-catheter removal, dialysis modality while receiving anti-TB drugs, and death related to tuberculous peritonitis. PD catheter removal was defined as the removal of the catheter within 30 days of diagnosis of tuberculous peritonitis. Death related to tuberculous peritonitis was defined as death as reported in the ANZDATA to be due to tuberculous peritonitis or within 30 days of onset of symptoms and/or diagnosis of tuberculous peritonitis. The results were presented using descriptive analysis.
Results
Literature Review
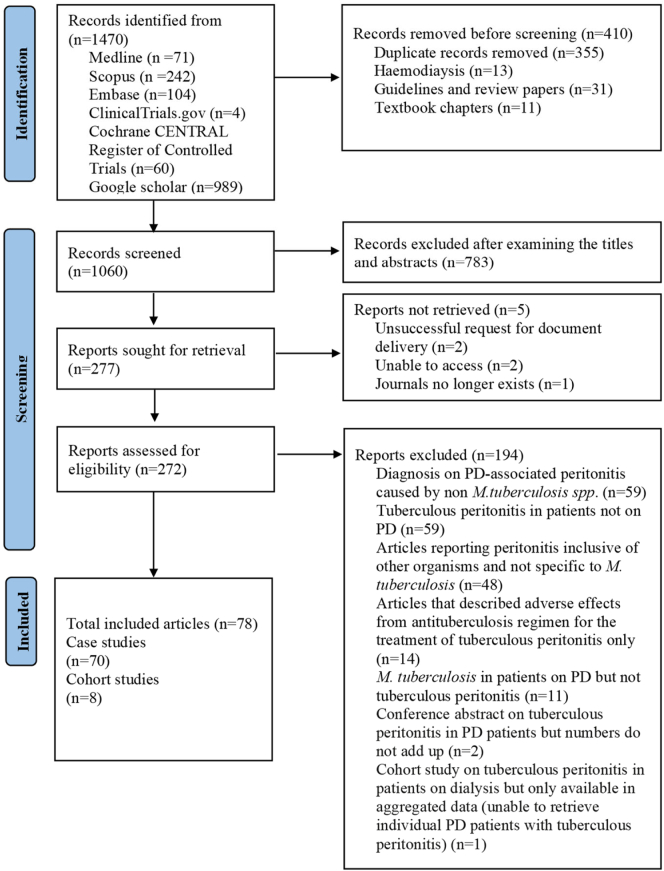
A total of 1470 articles were identified, and 1060 abstracts were screened for relevance after duplicates were removed. From these 272 full-text articles were assessed for eligibility. A further 194 studies were excluded, and 78 articles were included in the final study. Of the included studies, 70 articles were case studies and 8 were cohort studies (Figure 1). A total of 151 patients were identified in the case studies and 97 patients in the cohort studies of the literature review (Supplementary Tables S1, S2, and S3). The median (interquartile range [IQR]) time from PD commencement to tuberculous peritonitis in the case studies and aggregated mean duration reported in the cohort studies were 12 (6–27) and 17 months, respectively. In Table 2, we summarize the patient characteristics identified in the case studies of the literature review. In Table 3, we summarize the additional risk factors for tuberculous peritonitis in patients on PD identified in the case studies and cohort studies in the literature review. The most common additional risk factors identified in the case studies and cohort studies in the literature search were racial origin from the endemic region and a high TB incidence country15 (24.5%, n = 37/151 and 57.7%, n = 56/97), respectively (Table 3). Of 151 patients identified in the case studies, 29 (19.2%) had concomitant nontuberculous peritonitis or within 4 weeks of previous nontuberculous peritonitis. On the other hand, only 2.1% (n = 2/97) in the cohort studies had concomitant or within 4 weeks of previous nontuberculous peritonitis. Of these, 1.0% (n = 1/97) had peritonitis with Klebsiella pneumoniae 3 weeks before the diagnosis of tuberculous peritonitis; the patient died 3 weeks after tuberculous peritonitis was diagnosed, and 1.0% (n = 1/97) had concomitant fungal peritonitis at the time of tuberculous peritonitis, and the PD catheter was removed.
Figure 1.
Search strategy and selection of studies. PD, peritoneal dialysis.
Table 2.
Characteristics of patients with tuberculous peritonitis in the literature review
| Characteristics | All patients (N = 151) |
|---|---|
| Age (years), mean ± SD | 49.0 ± 15.1 |
| Male gender, n (%) | 81 (53.6%) |
| PD modality, n (%) | |
| CAPD | 126 (83.4%) |
| APD | 11 (7.3%) |
| Not reported | 14 (9.3%) |
| Primary kidney disease, n (%) | |
| Diabetic nephropathy | 37 (24.5%) |
| Not reported | 31 (20.5%) |
| Glomerulonephritis | 27 (17.9%) |
| Hypertensive nephrosclerosis | 24 (15.9%) |
| Interstitial nephritis | 21 (13.9%) |
| Other | 11 (7.3%) |
| Country of the study, n (%)b | |
| UK | 20 (13.2%) |
| Othersa | 18 (11.9%) |
| Turkey | 17 (11.3%) |
| Hong Kong | 16 (10.6%) |
| Taiwan | 16 (10.6%) |
| USA | 14 (9.3%) |
| India | 13 (8.6%) |
| South Africa | 12 (7.9%) |
| Spain | 10 (6.6%) |
| Saudi Arabia | 8 (5.3%) |
| New Zealand | 4 (2.6%) |
| Morocco | 3 (2.0%) |
APD, automated peritoneal dialysis; CAPD, continuous ambulatory peritoneal dialysis; SD, standard deviation; UK, United Kingdom; USA, United States of America.
Others: Australia, Canada, France, Indonesia, Japan, Korea, Malaysia, Portugal, Singapore, Tunisia.
May not round up to 100% due to rounding.
Table 3.
Risk factors for tuberculous peritonitis in patients on peritoneal dialysis in the literature review
| Risk factors identified from the case studies | All patients (N = 151) |
|---|---|
| Unknown/not reported | 26 (17.2%) |
| Single risk factorsa | 79 (52.3%) |
| Endemic region/originate from high TB incidence country | 37 (24.5%) |
| Diabetes mellitus | 12 (7.9%) |
| Previous history of pulmonary TB or history of positive PPD (Mantoux test) | 11 (7.3%) |
| Active pulmonary TB | 6 (4.0%) |
| Hypoalbuminemia/malnutrition | 5 (3.3%) |
| Taking steroids/immunosuppressants | 3 (2.0%) |
| Other extra-pulmonary TB | 3 (2.0%) |
| Close family contact with active pulmonary TB/occupational exposure to bovine TB | 2 (1.3%) |
| Combination risk factorsa | 46 (30.5%) |
| Endemic region/originate from high TB incidence country + hypoalbuminemia/malnutrition | 12 (7.9%) |
| Endemic region/originate from high TB incidence country+ diabetes mellitus | 9 (6.0%) |
| Diabetes mellitus + hypoalbuminemia/malnutrition | 5 (3.3%) |
| Taking steroids/immunosuppressants + previous history of TB | 2 (1.3%) |
| Endemic region/originate from high TB incidence country + hypoalbuminemia/malnutrition + diabetes mellitus | 2 (1.3%) |
| Endemic region/originate from high TB incidence country + other extra-pulmonary TB | 2 (1.3%) |
| Endemic region/originate from high TB incidence country + taking steroids/immunosuppressants | 2 (1.3%) |
| Diabetes mellitus + hypoalbuminemia/malnutrition + active pulmonary TB/other extra-pulmonary TB | 2 (1.3%) |
| Diabetes mellitus + past history of pulmonary TB | 2 (1.3%) |
| Active pulmonary TB + other extra-pulmonary TB + hypoalbuminemia + taking steroids/immunosuppressants | 1 (0.7%) |
| Endemic region/originate from high TB incidence country + active pulmonary TB + taking steroids/immunosuppressants | 1 (0.7%) |
| Taking steroids/immunosuppressants + hypoalbuminemia/malnutrition + other extra-pulmonary TB | 1 (0.7%) |
| Diabetes mellitus + other extra-pulmonary TB | 1 (0.7%) |
| Endemic region/originate from high TB incidence country + previous history of TB | 1 (0.7%) |
| Taking steroids/immunosuppressants + active pulmonary TB | 1 (0.7%) |
| Endemic region/originate from high TB incidence country + diabetes mellitus + other extra-pulmonary TB | 1 (0.7%) |
| Endemic region/originate from high TB incidence country + active pulmonary TB | 1 (0.7%) |
| Risk factors identified from the cohort studiesa | All patients (n = 97) |
|---|---|
| Unknown | 11 (11.3%) |
| Single risk factors | 68 (70.1%) |
| Racial origin of endemic region /originate from high TB incidence country | 56 (57.7%) |
| Latent TB from occupational exposure (i.e., coal miner) | 5 (5.2%) |
| Diabetes mellitus | 4 (4.1%) |
| Malignant disease (i.e., cervix carcinoma) | 1 (1.0%) |
| Positive PPD (Mantoux test) | 2 (2.1%) |
| Combination risk factorsa | 18 (18.6%) |
| Endemic region/originate from high TB incidence country + diabetes mellitus | 16 (16.5%) |
| Endemic region/originate from high TB incidence country + malignant disease | 1 (1.0%) |
| Endemic region/originate from high TB incidence country + past close family history of pulmonary TB | 1 (1.0%) |
TB, tuberculosis; PPD, purified protein derivative.
May not total up to 100% due to rounding.
ANZDATA Registry Cases
Patient Characteristics
A total of 15,849 episodes of peritonitis in 21,741 patients on PD were identified during the study period (September 2001 to December 2020). Of these, only 17 episodes (0.1%) were caused by M tuberculosis. The median (IQR) time from PD start date to developing tuberculous peritonitis was 27 (5–39) months. The baseline patient characteristics and clinical information of the patients at the time of peritonitis due to M tuberculosis are summarized in Supplementary Table S4.
Treatment of Tuberculous Peritonitis in the Case Studies and Cohort Studies in the Literature Review and ANZDATA Registry Cases
In Supplementary Tables S5, S6, and S7, we summarize the choice of anti-TB regimen in the case studies, cohort studies, and ANZDATA Registry cases, respectively. The most common anti-TB regimen used included rifampicin + isoniazid + pyrazinamide in the case studies and cohort studies of the literature review (27.2%, n = 41/151), (12.4%, n = 12/97) and ANZDATA Registry cases (23.5%, n = 4/17), respectively.
The median (IQR) duration of anti-TB treatment was 9 (9–12) months in the case studies of the literature review, whereas the aggregated mean duration of the anti-TB treatment in the cohort study ranged from 9 to 18 months. The median (IQR) time anti-TB therapy started from the onset of symptoms of tuberculous peritonitis in the case studies of the literature review was 6 (3–11) weeks.
Clinical Outcomes in the Case Studies and Cohort Studies of the Literature Review and ANZDATA Registry Cases
All-Cause Mortality
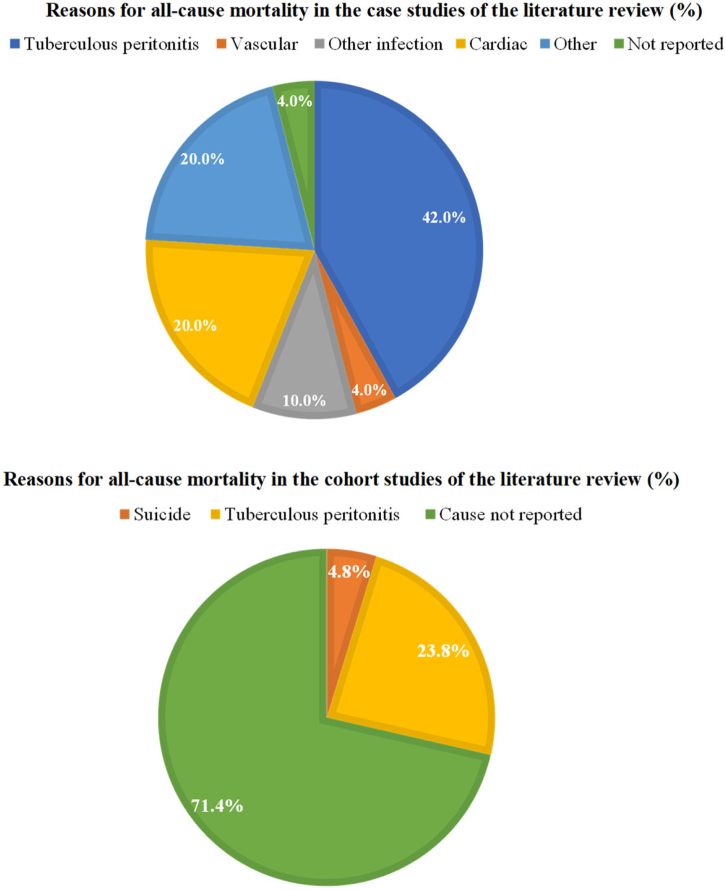
Overall, the all-cause mortality rates of patients with tuberculous peritonitis in the case studies and cohort studies of the literature review and ANZDATA Registry cases were 33.1% (n = 50/151), 25.8% (n = 25/97) and 5.9% (n = 1/17), respectively (Table 4 and Figure 2). In Figure 2, we illustrate the reasons for all-cause mortality in the case studies and cohort studies in the literature review group.
Table 4.
Clinical outcomes of tuberculous peritonitis from the case studies in the literature review
| Outcomes–case studies | All patients (N = 151) |
|---|---|
| PD-catheter removal,n(%) | |
| Yesa | 87 (57.6%) |
| No | 60 (39.7%) |
| Not reported | 4 (2.6%) |
| Status of PD while receiving antituberculosis regimen for tuberculous peritonitis, n (%) | |
|---|---|
| Died related to tuberculous peritonitisb | 28 (18.5%) |
| Died while receiving antituberculosis therapy for tuberculous peritonitisc | 22 (14.6%) |
| Transferred to permanent HDd | 34 (22.5%) |
| Remained on PD | 30 (19.9%) |
| Not reported | 29 (19.2%) |
| Returned to PD | 6 (4.0%) |
| Simultaneous PD catheter removal and replacemente | 1 (0.7%) |
| Kidney transplant 2 months after diagnosis of tuberculous peritonitis | 1 (0.7%) |
| All-cause mortality f, n (%) | |
|---|---|
| No | 98 (64.9%) |
| Yes | 50 (33.1%) |
| Not reported | 3 (2.0%) |
| Outcomes-cohort studies | All patients (n = 97) |
|---|---|
| PD-catheter removal,n(%) | |
| Yes | 49 (50.5%) |
| No | 47 (48.5%) |
| Not reported | 1 (1.8%) |
| Status of dialysis while receiving antituberculosis regimen for tuberculous peritonitis, n (%) | All patients (n = 97) |
|---|---|
| Not reported | 34 (35.1%) |
| Died | 25 (25.8%) |
| Transferred to HD | 19 (19.6%) |
| Returned to PD | 10 (10.3%) |
| Remained on PD | 9 (9.3%) |
| All-cause mortality,fn (%) | |
|---|---|
| No | 72 (74.2%) |
| Yes | 25 (25.8%) |
HD, hemodialysis, PD, peritoneal dialysis; TB, tuberculosis.
Inclusive of simultaneous removal and replacement of PD catheter
Death (i) before tuberculous peritonitis was diagnosed or (ii) while receiving antituberculosis treatment for tuberculous peritonitis and the status of the dialysis modality remained unknown.
Within 30 days of diagnosis of TB peritonitis or during the treatment of tuberculous peritonitis.
Remained alive after permanent transfer to HD.
Remained alive.
All-cause mortality (death from tuberculous peritonitis or while receiving antituberculosis treatment for tuberculous peritonitis).
Figure 2.
Reasons for all-cause mortalitya in the literature review.b
aAll-cause mortality was defined as death due to tuberculous peritonitis and all deaths while receiving antituberculosis treatment for tuberculous peritonitis.
bPercentage is computed based on the total number of deaths in the case studies and cohort studies, respectively.
Need for PD Catheter Removal
The majority of the patients in the case studies and cohort studies of the literature review had PD catheter removed (58% [n = 87/151] and 51% [49/97], respectively), whereas 82% (n = 14/17) of patients in the ANZDATA Registry cases group had PD catheters removed. Of these, 23% (n = 34/151) in the case studies and 20% (n = 19/97) cohort studies of the literature review group and 82% (n = 14/17) in the ANZDATA Registry cases group were reported to have permanently transferred to HD. In the case studies group, 6% (n = 9/151) had PD catheters removed due to inadequate ultrafiltration while receiving an anti-TB regimen for tuberculous peritonitis. The median (IQR) time from diagnosis of tuberculous peritonitis to the removal of the PD catheter reported in the case studies and ANZDATA Registry cases was 13 (7–20) and 7 (6–10) days, respectively.
Of the 40% (n = 60/151) who did not have PD catheter removal in the case studies group, 6.0% (n = 9/151) died from causes related to tuberculous peritonitis after a median (IQR) time of 20 (13–30) days after diagnosis of tuberculous peritonitis. Twenty-three patients (15%) in the case studies group who did not have PD catheter removal remained on PD after completing the anti-TB regimen for tuberculous peritonitis. Of those, 2% (n = 3/151) were subsequently transferred to HD after a mean duration of 12 months following tuberculous peritonitis due to inadequate ultrafiltration. In Table 5, we illustrate the relationship between mortality and PD catheter removal in the case studies and ANZDATA groups (these data could not be obtained in the cohort studies group).
Table 5.
Relationship between mortality and peritoneal dialysis catheter removal
|
Status of the PD catheter |
Case studies (N = 151) |
|||
|---|---|---|---|---|
| Death related to tuberculous peritonitis,an (%) | Died while receiving antituberculosis therapy for tuberculous peritonitis,bn (%) | Alive, n (%) | Living status not reported by the authors, n (%) | |
| PD catheter removed | 17 (11.3%) | 12 (7.9%) | 55 (36.4%) | 3 (2.0%) |
| PD catheter not removed | 9 (6.0%) | 10 (6.6%) | 41 (27.2%) | 0 (0.0%) |
| Status of PD catheter removal not reported | 2 (1.3%) | 0 (0.0%) | 2 (1.3%) | 0 (0.0%) |
| ANZDATA Registry (n = 17) |
||
|---|---|---|
| Death related to tuberculous peritonitis,cn (%)d | Alive, n (%)d | |
| PD catheter removed | 0 (0%) | 14 (82%) |
| PD catheter not removed | 1 (6%) | 2 (12%) |
PD, peritoneal dialysis.
Death related to tuberculous peritonitis as reported by the authors or within 30 days of diagnosis of tuberculous peritonitis.
Death that occurred >30 days of diagnosis of tuberculous peritonitis and unrelated to tuberculous peritonitis while receiving antituberculosis therapy for tuberculous peritonitis.
Death within 30 days of diagnosis of tuberculous peritonitis.
Did not sum up to % due to rounding.
Among 82% (n = 14/17) of patients in the ANZDATA Registry cases group who were permanently transferred to HD, 29% (n = 4/14) of patients died while on HD (due to cachexia or withdrawal of HD) after a median (IQR) time of 44 (25–87) months after permanent transfer to HD.
Recommencement of PD
In the case studies, 1% (n = 2/151) of patients were reported to have had PD catheter reinserted within 4 weeks of PD catheter removal. Of these, 1 patient survived, whereas the other patient died from tuberculous peritonitis. Of the patients who returned to PD, 4% (n = 6/151) were in the case studies group and 10% (n = 10/97) were from the cohort studies (Table 4), whereas none of the patients in the ANZDATA Registry cases group was reported to have returned to PD after completing the anti-TB treatment.
Remaining on PD
The proportion of patients who remained on PD during anti-TB treatment were 9% (n = 9/97) in the cohort studies, 20% (n = 30/151) in the case studies, and 18% (n = 3/17) in the ANZDATA Registry cases. Among the 18% (n = 3/17) patients who remained on PD in the ANZDATA Registry cases group, 12% (n = 2/17) had a permanent transition to HD 22.1 and 17.0 months after the diagnosis of tuberculous peritonitis due to inadequate solute clearance, and 6% (n = 1/17) patients died on the day tuberculous peritonitis was diagnosed.
Mortality Attributed to Tuberculous Peritonitis
Overall, 19% (n = 28/151) of patients in the case studies and 26% (n = 25/97) of patients in the cohort studies died related to tuberculous peritonitis.
Discussion
Our study is the first to examine the epidemiology, management strategies, and outcomes of tuberculous peritonitis through a systematic literature review and multicenter binational registry analysis. Although M tuberculosis is an uncommon cause of peritonitis, it is associated with poor outcomes, such as high transfer rates to HD and mortality. M tuberculosis has also been reported to form a biofilm17,18 which can limit the penetration and efficacy of anti-TB drugs, increasing the risk of treatment failure.
Although our study observed PD catheter removal in the majority of the patients in the ANZDATA Registry cases, cohort studies, and case studies of the literature review groups, a higher proportion of patients in the ANZDATA Registry cases group had their PD catheters removed. A direct comparison of the outcomes of tuberculous peritonitis between the case studies, cohort studies, and ANZDATA Registry groups cannot be performed. This is due to the potential of practice variation for the management of peritonitis across different eras (i.e., case studies that were diagnosed and managed before the year 2000 versus our ANZDATA Registry analysis that included patients between the year 2000 and 2020) and in different countries. Moreover, the ISPD recommendations for the management of PD-related peritonitis have also evolved since the first set of recommendations was published in 2005. Nevertheless, we observed that no patients in the ANZDATA Registry cases group were reported to have had either concomitant nontuberculous peritonitis or nontuberculous peritonitis within 4 weeks of diagnosis of peritonitis due to M tuberculosis as compared to the literature review group, where 19% patients were reported to have had nontuberculous peritonitis either concomitantly or within the previous 4 weeks. This is consistent with previous studies19,20 where recurrent peritonitis was associated with higher odds of mortality.
There remains conflicting evidence regarding the need for PD catheter removal in patients with tuberculous peritonitis. Although several studies have reported successful treatment of tuberculous peritonitis without PD catheter removal,2,10,21, 22, 23, 24, 25, 26 others reported that PD catheter removal was necessary.7, 8, 9, 10, 11 Of note, the ISPD peritonitis guidelines have provided 2C recommendations to treat tuberculous peritonitis with anti-TB drugs instead of PD catheter removal as the first-line treatment. In addition, a scoping review by Thomson et al.2 based on the case studies demonstrated that PD catheter removal in patients with tuberculous peritonitis was not associated with higher survival rates. Compared to other bacterial peritonitis infections, where identification of the causative organism on culture usually takes a few days, M tuberculosis can take a few weeks because of its insidious growth in culture media. This does not only result in a delay in diagnosis of M tuberculosis infection; however, patients with this infection are also often diagnosed initially as culture-negative peritonitis and are treated with empiric antibiotics. Given that these “culture-negative” episodes do not respond to the initial empirical antibiotic regimens, most clinicians will consider PD catheter removal based on the current ISPD peritonitis guidelines suggesting 1D recommendations to remove PD catheters in patients with PD effluent that fails to clear after 5 days of appropriate antibiotics,6 an approach that has been associated with lower mortality and preservation of peritoneal membrane function.6
In our study, compared to the case studies and cohort studies, we have demonstrated earlier PD catheters removal in patients in the ANZDATA cases group (7 [6–10] vs. 13 [7–20]) days and had better survival (94% vs. 65% and 74%, respectively). However, direct comparison between the groups is impossible because of the difference in patient populations and unmeasured confounders (i.e., variation in the treatment protocols and experience across different PD units and treating physicians for “refractory” peritonitis). In centers where peritonitis due to M tuberculosis can be diagnosed early, by either rapid diagnostic assays such as Xpert MTB/RIF (Gene Xpert) and Xpert MTB/RIF Ultra (Xpert Ultra) or peritoneal biopsies, the ISPD peritonitis guidelines’ 2C suggestion of anti-TB treatment while retaining the PD catheter could be implemented.
The long-term outcomes in patients with tuberculous peritonitis who remained on PD and restarted PD following interim HD remain scarce. In our study, 2 patients from the ANZDATA Registry cases group who remained on PD were permanently transferred to HD after a mean duration of 20 months after the diagnosis of tuberculous peritonitis due to inadequate small solute transport. Although preserving the length of PD therapy is one of the determinants of outcomes, previous studies have reported alterations in peritoneal transport characteristics, such as the decline in ultrafiltration and an increase in the dialysate/peritoneal creatinine ratio after severe bacterial peritonitis27, 28, 29 and several studies are required to evaluate the peritoneal membrane function and outcomes in patients who restarted PD. On the other hand, in the ANZDATA Registry cases group, 29% of the patients who were permanently transferred to HD after tuberculous peritonitis while on HD died after a median duration of 44 months due to cachexia and withdrawal of HD. Therefore, our findings highlight the need for more studies to determine the optimal time to restart PD and evaluate whether returning to PD after an interim HD will lead to better patient outcomes, including improved survival.
Notably, 2 patients in the case studies had PD catheter removal and replacement within 4 weeks of removal. Of these, 1 patient died from tuberculous peritonitis, whereas the remaining survived, although the long-term outcomes remain unknown. Nonetheless, given the high mortality rates with tuberculous peritonitis in patients on PD and limited data on outcomes if a PD catheter is reinserted within 4 weeks of removal, more studies are required on the optimal time to restart PD after PD catheter removal.
Another important aspect is the variation in the anti-TB regimen observed in our study. Of note, the recommended doses of the anti-TB regimen on the ISPD peritonitis guidelines were extrapolated from the recommendations used to treat pulmonary and other extrapulmonary TB and were limited by expert opinion and case series.6 Although our study demonstrated that rifampicin, isoniazid, and pyrazinamide are the most common anti-TB regimens observed in the literature review and ANZDATA Registry case groups, the choice of anti-TB therapy should be center-specific and demographic-specific. Successful treatment of tuberculous peritonitis is contingent on adequate drug concentrations in the peritoneal cavity and the organism's susceptibility to anti-TB drugs. However, this may not always be possible due to various factors such as the low bioavailability of the rifampicin in the dialysate when administered orally,30 treatment discontinuation owing to its dose-dependent adverse effects,31 and the increasing prevalence of the multidrug-resistant M tuberculosis strain, especially in high multidrug-resistant burden countries. More pharmacokinetic studies are warranted to evaluate if intraperitoneal rifampicin with other commonly used anti-TB drugs will enable dialysate drug concentrations to remain above the minimum inhibitory concentrations for M tuberculosis until the subsequent scheduled dosing.
Finally, our study demonstrated that the median time to develop tuberculous peritonitis from PD commencement in the literature review group and ANZDATA Registry cases were 12 and 27 months, respectively. Furthermore, the majority (82.3%) of the patients in the literature review group had additional risk factors for tuberculous peritonitis. These findings highlight the need to identify and manage risk factors associated with tuberculous peritonitis. These include diabetes, long-term steroids and immunosuppressants (i.e., antiretrovirals for human immunodeficiency disease (HIV) and antineoplastic drugs), history of pulmonary TB, and malnutrition.32,33
There were several limitations to this study. First, given the limitations of data collection in ANZDATA Registry, we could not identify several important risk factors for tuberculous peritonitis, such as information on the serum albumin level and the use of steroids and other immunosuppressants at the time of development of tuberculous peritonitis, and determine the reason for PD catheter removal (i.e., whether the PD catheter was removed after initiating anti-TB treatment due to loss of ultrafiltration or refractory peritonitis after failure to respond to 5 days of empirical antibiotics because this information is not collected in the ANZDATA Registry). Second, there was an absence of a control group to compare the characteristics and outcomes of peritonitis due to M tuberculosis to other organisms due to the small number of peritonitis episodes due to M tuberculosis identified in this study. In addition, we could not perform a multivariable analysis to examine the predictors of the outcomes of tuberculous peritonitis between the ANZDATA Registry cases and the literature review group due to insufficient case numbers. Third, the incomplete data sets in the ANZDATA Registry cases, particularly on the anti-TB regimen and its treatment end date, limited the ability to compare the treatment strategy with the literature review. We were also unable to determine the association of the anti-TB regimen with the treatment outcomes due to the missing data. Thus, we could not determine the optimal anti-TB regimen in this study. Fourth, loss to follow-up in the long-term outcomes in the literature review, particularly alterations to the peritoneal membrane function, return to PD after the interim HD and effect on the mortality rates after tuberculous peritonitis, cannot be excluded. Fifth, given that the nature of the case series and retrospective study design are susceptible to missing data bias, we could not determine the time to death in patients on PD who died due to tuberculous peritonitis without PD catheter removal. Finally, because of the retrospective nature of this study, the date of diagnosis of peritonitis due to M tuberculosis infection can vary because there may be heterogeneity in how some studies could report this date depending on signs or symptoms and definite laboratory identification of M tuberculosis. This can make the interpretation of the effect of PD catheter removal on outcomes somewhat speculative. Nonetheless, the strength of this study lies in its ability to compare the treatment strategy and outcomes of tuberculous peritonitis between Australia and New Zealand and worldwide through a systematic review and large, bi-national registry analysis.
In summary, peritonitis due to M tuberculosis is relatively uncommon but is associated with poor outcomes. Although early laboratory diagnosis of this infection will likely improve patient outcomes, prospective studies are warranted to study the effect on retaining PD catheters after M tuberculosis infection is diagnosed while patients receive anti-TB treatment. We acknowledge that the 2022 ISPD guidelines6 and Thomson et al.2 have discouraged routine PD catheter removal in patients with tuberculous peritonitis; however, our findings from the ANZDATA Registry suggest that prompt PD catheter removal may have contributed to better survival in patients on PD with tuberculous peritonitis, challenging the ISPD 2022 peritonitis guidelines’ 2C recommendation that suggests anti-TB therapy without PD catheter removal in these patients. Our findings also highlight the need for robust prospective studies to identify the optimal choice, combination, and duration of the anti-TB regimen for treating tuberculous peritonitis in optimizing outcomes while maintaining the feasibility of maintaining long-term PD.
Disclosure
KS has received speaker’s honoraria from Baxter Healthcare and is on the medical advisory board of Fresenius Medical Care for Australia and New Zealand. DWJ has received consultancy fees, research grants, speaker’s honoraria, and travel sponsorships from Baxter Healthcare and Fresenius Medical Care; consultancy fees from AstraZeneca, Bayer, and AWAK; speaker’s honoraria from ONO and Boehringer Ingelheim & Lilly; and travel sponsorships from Ono and Amgen. He is a current recipient of an Australian National Health and Medical Research Council Leadership Investigator Grant. All the other authors declared no conflicting interests.
Acknowledgments
The authors would like to acknowledge the Australia and New Zealand Dialysis and Transplant Registry (ANZDATA) for their assistance and provision of the information from the ANZDATA database. The data reported here have been supplied by ANZDATA. The interpretation of data reported in this paper is that of the authors and does not reflect the official policy or opinion of the ANZDATA Registry. The authors would also like to acknowledge Ms. Isabelle Raisin for providing library support in the systematic review component of this manuscript. The author received no financial support for the research, authorship, and publication of this article.
Footnotes
Figure S1. PRISMA checklist.
Figure S2. Search strategy.
Table S1. Patient characteristics with tuberculous peritonitis in patients on peritoneal dialysis - case studies.
Table S2. Other patient characteristics and outcomes of patients on peritoneal dialysis with tuberculous peritonitis - case studies.
Table S3. Summary of the cohort studies of patients on peritoneal dialysis with tuberculous peritonitis.
Table S4. Patient characteristics in patients with tuberculous peritonitis in the ANZDATA Registry analysis.
Table S5. Antituberculosis regimen in the literature review - case studies.
Table S6. Antituberculosis regimen in the literature review - cohort studies.
Table S7. Antituberculosis regimen in the ANZDATA Registry analysis.
Supplementary References.
Supplementary Material
Figure S1. PRISMA checklist.
Figure S2. Search strategy.
Table S1. Patient characteristics with tuberculous peritonitis in patients on peritoneal dialysis - case studies.
Table S2. Other patient characteristics and outcomes of patients on peritoneal dialysis with tuberculous peritonitis - case studies.
Table S3. Summary of the cohort studies of patients on peritoneal dialysis with tuberculous peritonitis.
Table S4. Patient characteristics in patients with tuberculous peritonitis in the ANZDATA Registry analysis.
Table S5. Antituberculosis regimen in the literature review - case studies.
Table S6. Antituberculosis regimen in the literature review - cohort studies.
Table S7. Antituberculosis regimen in the ANZDATA Registry analysis.
Supplementary References
References
- 1.Lye W.C. Rapid diagnosis of Mycobacterium tuberculous peritonitis in two continuous ambulatory peritoneal dialysis patients, using DNA amplification by polymerase chain reaction. Adv Perit Dial. 2002;18:154–157. [PubMed] [Google Scholar]
- 2.Thomson B.K.A., Vaughan S., Momciu B. Mycobacterium tuberculosis peritonitis in peritoneal dialysis patients: a scoping review. Nephrol (Carlton) 2022;27:133–144. doi: 10.1111/nep.13997. [DOI] [PubMed] [Google Scholar]
- 3.Moore D.A., Lightstone L., Javid B., Friedland J.S. High rates of tuberculosis in end-stage renal failure: the impact of international migration. Emerg Infect Dis. 2002;8:77–78. doi: 10.3201/eid0801.010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Romanowski K., Clark E.G., Levin A., Cook V.J., Johnston J.C. Tuberculosis and chronic kidney disease: an emerging global syndemic. Kidney Int. 2016;90:34–40. doi: 10.1016/j.kint.2016.01.034. [DOI] [PubMed] [Google Scholar]
- 5.Vadivel N., Tucker J.K., Trikudanathan S., Heher E., Singh A.K. Tuberculous peritonitis: a race against time. Kidney Int. 2006;70:969–972. doi: 10.1038/sj.ki.5001610. [DOI] [PubMed] [Google Scholar]
- 6.Li P.K., Chow K.M., Cho Y., et al. ISPD peritonitis guideline recommendations: 2022 update on prevention and treatment. Perit Dial Int. 2022;42:110–153. doi: 10.1177/08968608221080586. [DOI] [PubMed] [Google Scholar]
- 7.Waness A., Al Shohaib S. Tuberculous peritonitis associated with peritoneal dialysis. Saudi J Kidney Dis Transpl. 2012;23:44–47. [PubMed] [Google Scholar]
- 8.MacCormick J.O.C.M. Tuberculous peritonitis in patients on CAPD-the importance of Lympholytosis in the peritoneal fluid. Perit Dial Int. 1980;1:106. [Google Scholar]
- 9.Khanna R., Fenton S., Cattran D., Thompson D., Deitel M., Oreopoulos D. Tuberculous peritonitis in patients undergoing continuous ambulatory peritoneal dialysis (CAPD) Perit Dial Int. 1980;1:10–12. doi: 10.1177/089686088000100302. [DOI] [Google Scholar]
- 10.Cheng I.K., Chan P.C., Chan M.K. Tuberculous peritonitis complicating long-term peritoneal dialysis. Report of 5 cases and review of the literature. Am J Nephrol. 1989;9:155–161. doi: 10.1159/000167956. [DOI] [PubMed] [Google Scholar]
- 11.Yorioka N., Oda H., Joarder Z.H., Kobayashi M., Harada S., Yamakido M. Tuberculous peritonitis in a patient undergoing continuous ambulatory peritoneal dialysis. Hiroshima J Med Sci. 1988;37:93–95. [PubMed] [Google Scholar]
- 12.Unsal A., Ahbap E., Basturk T., et al. Tuberculosis in dialysis patients: a nine-year retrospective analysis. J Infect Dev Ctries. 2013;7:208–213. doi: 10.3855/jidc.2664. [DOI] [PubMed] [Google Scholar]
- 13.Akpolat T. Tuberculous peritonitis. Perit Dial Int. 2009;29(suppl 2):166–169. doi: 10.1177/089686080902902S32. [DOI] [PubMed] [Google Scholar]
- 14.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO global lists of high burden countries for tuberculosis (TB), TB/HIV and multidrug/rifampicin-resistant TB (MDR/RR-TB), 2021-2025. World Health Organization. https://cdn.who.int/media/docs/default-source/hq-tuberculosis/who_globalhbcliststb_2021-2025_backgrounddocument.pdf?sfvrsn=f6b854c2_9
- 16.Australia & New Zealand dialysis & transplant registry (ANZDATA). Data forms. https://www.anzdata.org.au/anzdata/services/data-management/data-forms/
- 17.Esteban J., García-Coca M. Mycobacterium biofilms. Front Microbiol. 2017;8:2651. doi: 10.3389/fmicb.2017.02651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brennan M.J. Biofilms and Mycobacterium tuberculosis. Infect Immun. 2017;85:e00411–e00417. doi: 10.1128/IAI.00411-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burke M., Hawley C.M., Badve S.V., et al. Relapsing and recurrent peritoneal dialysis-associated peritonitis: a multicenter registry study. Am J Kidney Dis. 2011;58:429–436. doi: 10.1053/j.ajkd.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 20.Szeto C.C., Kwan B.C., Chow K.M., et al. Recurrent and relapsing peritonitis: causative organisms and response to treatment. Am J Kidney Dis. 2009;54:702–710. doi: 10.1053/j.ajkd.2009.04.032. [DOI] [PubMed] [Google Scholar]
- 21.Mallat S.G., Brensilver J.M. Tuberculous peritonitis in a CAPD patient cured without catheter removal: case report, review of the literature, and guidelines for treatment and diagnosis. Am J Kidney Dis. 1989;13:154–157. doi: 10.1016/s0272-6386(89)80135-0. [DOI] [PubMed] [Google Scholar]
- 22.Vathsala A., Thomas A., Ng B.L., Lim C.H. An unusual case of peritonitis. Ann Acad Med Singap. 1987;16:666–670. [PubMed] [Google Scholar]
- 23.Tan D., Fein P.A., Jorden A., Avram M.M. Successful treatment of tuberculous peritonitis while maintaining patient on CAPD. Adv Perit Dial. 1991;7:102–104. [PubMed] [Google Scholar]
- 24.Mousson C., Bonnin A., Dumas M., Chevet D., Rifle G. Peritoneal tuberculosis and continuous ambulatory peritoneal dialysis. Nephrologie. 1993;14:139–142. [PubMed] [Google Scholar]
- 25.Tsai T.C., Hsu J.C., Chou L.H., Lee M.L. Tuberculous peritonitis in a child undergoing continuous ambulatory peritoneal dialysis. Zhonghua Min Guo Xiao Er Ke Yi Xue Hui Za Zhi. 1994;35:455–459. [PubMed] [Google Scholar]
- 26.Herrera C.M., Montes Delgado R., Guerrero Riscos A., et al. Mycobacterium tuberculosis as a cause of peritonitis in a patient undergoing continuous ambulatory peritoneal dialysis. Nephron. 1996;73:318–319. doi: 10.1159/000189062. [DOI] [PubMed] [Google Scholar]
- 27.Szeto C.-C., Chow K.-M., Wong T.Y.-H., et al. Feasibility of resuming peritoneal dialysis after severe peritonitis and Tenckhoff catheter removal. J Am Soc Nephrol. 2002;13:1040–1045. doi: 10.1681/ASN.V1341040. [DOI] [PubMed] [Google Scholar]
- 28.Wong T.Y.H., Szeto C.-C., Lai K.-B., Lam C.W.K., Lai K.-N., Li P.K.T. Longitudinal study of peritoneal membrane function in continuous ambulatory peritoneal dialysis: relationship with peritonitis and fibrosing factors. Perit Dial Int. 2000;20:679–685. doi: 10.1177/089686080002000617. [DOI] [PubMed] [Google Scholar]
- 29.Ram R., Swarnalatha G., Dakshinamurty K.V. Reinitiation of peritoneal dialysis after catheter removal for refractory peritonitis. J Nephrol. 2014;27:445–449. doi: 10.1007/s40620-014-0048-1. [DOI] [PubMed] [Google Scholar]
- 30.Ahn C., Oh K.-H., Kim K., et al. Effect of peritoneal dialysis on plasma and peritoneal fluid concentrations of isoniazid, pyrazinamide, and rifampin. Perit Dial Int. 2003;23:362–367. doi: 10.1177/089686080302300409. [DOI] [PubMed] [Google Scholar]
- 31.Saito N., Yoshii Y., Kaneko Y., et al. Impact of renal function-based anti-tuberculosis drug dosage adjustment on efficacy and safety outcomes in pulmonary tuberculosis complicated with chronic kidney disease. BMC Infect Dis. 2019;19:374. doi: 10.1186/s12879-019-4010-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rohit A., Abraham G. Peritoneal dialysis related peritonitis due to Mycobacterium spp.: a case report and review of literature. J Epidemiol Glob Health. 2016;6:243–248. doi: 10.1016/j.jegh.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feleke B.E., Feleke T.E., Biadglegne F. Nutritional status of tuberculosis patients, a comparative cross-sectional study. BMC Pulm Med. 2019;19:182. doi: 10.1186/s12890-019-0953-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.