Abstract
Introduction
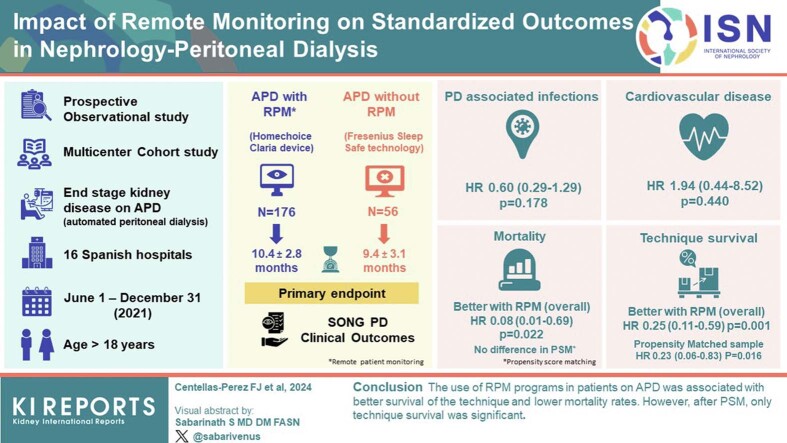
This study aimed to evaluate the association between the use of remote patient monitoring (RPM) in patients on automated peritoneal dialysis (APD) and the Standardized Outcomes in Nephrology in peritoneal dialysis (SONG-PD) clinical outcomes.
Methods
A prospective and multicenter cohort study was conducted on patients with advanced chronic kidney disease on APD, recruited at 16 Spanish Hospitals, between June 1 and December 31, 2021. Patients were divided into 2 cohorts, namely patients on APD with RPM (APD-RPM) and patients on APD without RPM. The primary endpoints were the standardized outcomes of the SONG-PD clinical outcomes: PD-associated infection, cardiovascular disease (CVD), mortality rate, technique survival, and life participation (assessed as health-related quality of life [QoL]). Propensity score matching (PSM) was used to evaluate the association of RPM exposure with the clinical outcomes.
Results
A total of 232 patients were included, 176 (75.9%) in the APD-RPM group and 56 (24.1%) in the APD-without-RPM group. The mean patient follow-up time was significantly longer in the APD-RPM group than in the APD-without-RPM group (10.4 ± 2.8 vs. 9.4 ± 3.1 months, respectively; P = 0.02). In the overall study sample, the APD-RPM group was associated with a lower mortality rate (hazard ratio [HR]: 0.08; 95% confidence interval [CI]: 0.01 to 0.69; P = 0.020) and greater technique survival rate (HR: 0.25; 95% CI: 0.11 to 0.59; P = 0.001). After PSM, APD-RPM continued to be associated with better technique survival (HR: 0.23; 95% CI: 0.06 to 0.83; P = 0.024).
Conclusion
The use of RPM programs in patients on APD was associated with better survival of the technique and lower mortality rates. However, after PSM, only technique survival was significant.
Keywords: chronic kidney disease, peritoneal dialysis, remote patient monitoring, standardized outcomes in nephrology, telemedicine, technique survival
Graphical abstract
PD is a renal replacement therapy that provides continuous, safe, home-based, and cost-effective treatment for patients with end-stage renal disease, with similar or better clinical outcomes than in-center hemodialysis.1 Although PD is home-based therapy, patients still require periodic hospital visits to receive a full assessment of treatment adequacy.2
In addition, the autonomy of the technique, as well as the lack of real-time monitoring, make treatment adherence an issue of great concern. The overall nonadherence rates have been reported as 2.6% to 53% for PD prescriptions and 5% to 20% for APD prescriptions.3 Unfortunately, nonadherence to the technique is usually reported by the patient, without real documentation.3,4
A web-based system for remote monitoring has recently been developed, which allows patient monitoring at home by digital wireless technology.5 This APD system, HomeChoice Claria (Baxter Healthcare, Deerfield, IL), connected to a cloud-based network, was launched in 2015.5,6 It provides real-time monitoring and recording of APD therapy.
This 2-way communication system has an interactive interface that allows both medical and nursing staff to make prescription changes by using a remote connection, thereby reducing the need for frequent in-person visits to the PD center.7,8
This system might be associated with some advantages. Potentially, home treatments could be monitored daily, making it possible to detect problems early and correct potential situations of inadequate dialysis.9,10 Furthermore, this technology allows the clinical team to know many aspects of the therapy at the patient’s home in real time. Moreover, besides detecting patients’ adherence to prescribed therapy, this remote monitoring might offer a safer and better-quality treatment to renal patients.10, 11, 12, 13
Recently, it has been reported that the use of RPM in APD was associated with lower hospitalization rates and length of hospital stay.12 In addition, this system provided other positive outcomes, including blood volume and arterial pressure management,14,15 and longer survival of the technique.16 However, these studies have diverse limitations, such as great data heterogeneity, study design, different interventions, and treatments duration.15,17
As a result, the SONG-PD has developed a consensus with the 5 most important outcomes for patients, caregivers, and healthcare professionals in PD. These would be (in descending order of priority): PD-infection, CVD, mortality, technique survival, and life participation.18, 19, 20 To date, although published papers have dealt with some of these outcomes, none of them have addressed the impact of RPM on all the SONG-PD outcomes in a systematic way.17 Therefore, the current paper aimed to evaluate the association between the use of RPM in patients on APD and the SONG-PD clinical outcomes.
Methods
Design
This prospective, observational, and multicenter cohort study was conducted on patients with end-stage renal disease on APD, recruited at 16 Spanish Hospitals, between June 1 and December 31, 2021.
This study has been approved by the Food Research Ethics Committee of the Albacete General University Hospital with internal code No. 2020/12/145 as of 01/26/2021.
The study was conducted in accordance with the rules of the Declaration of Helsinki and all the study participants provided written informed consent before starting the study.
Study Participants
Women and men from the 16 participating hospitals who were aged ≥18 years, with end-stage renal disease on PD and APD, signed the written consent, and willing to comply with the investigators and protocol indications were included in the study.
Patients with any severe active systemic disease that generates a life expectancy of less than 3 months; those who were participating during the study or have participated in a clinical study during the last 3 months that could interfere with the current study; or pregnant or lactating women were excluded.
Standardized Outcomes in Nephrology-PD Clinical Outcomes
This initiative established a core outcome set for PD studies based on a set of shared priorities for all stakeholders.18,19 It established the 5 most important outcomes for patients, caregivers, and healthcare professionals. They include the following: (i) PD-associated infection, number of peritonitis (defined according to the Peritoneal Dialysis Society criteria)21 per patient during the follow-up; (ii) CVD, incident CVD during follow-up (defined as the occurrence after enrolment of the first one of the following diseases: angina or acute coronary myocardial infarction, ischemic stroke, or peripheral arterial events22); (iii) mortality rate (number of deaths during the follow-up); (iv) technique survival (rewarded from technique failure to be positively framed)19 (defined as composite endpoint of transfer to hemodialysis >30 days or death23; and (v) life participation (it is not uniformly assed in PD studies.17,18 It is related to factors necessary for daily routines and leisure activities.17,18 In the current study, this was assessed by a descriptive questionnaire EuroQol-5D [EQ-5D]).
EQ-5D QoL Questionnaire
EQ-5D is a popular health related QoL instrument used in the clinical evaluation of health care. Each subject self-rates his/her health in terms of 5 dimensions: mobility, self-care, usual activities, pain or discomfort, and anxiety or depression using a 3-level scale.24 The descriptive analysis was reported as a 5-digit number ranging from 11111 (full health) to 33333 (worst health) using the absolute and relative frequency for each profile. Furthermore, the profiles were converted to a single utility index using country-specific value sets and this index was summarized by mean and SD.
Study Groups
Patients were divided into 2 cohorts based on the use of RPM. The patients in the APD-RPM group used the HomeChoice Claria device with Sharesource technology. Patients in the APD-without-RPM used the Fresenius Sleep Safe technology (Fresenius Medical Care, Germany). In each cohort, APD therapy was implemented in accordance with the policies, guidelines, and standard procedures of the hospital clinical practice of each center participating in the study. The inclusion of each patient in the study was carried out during a first visit coinciding with their usual appointment (Patients were informed in detail about the purpose and characteristics of the study and signed the consent).
Patients included in the study had a minimum follow-up of 6 months.
Outcomes
The primary endpoints were the standardized outcomes of the SONG-PD clinical outcomes (i.e., PD-associated infection; CVD; mortality rate; technique survival; and life participation [assessed as health-related QoL, by using a descriptive questionnaire, EQ-5D]). The secondary outcomes included number of unscheduled teleconsultations; number of unscheduled face-to-face visits; number of hospital admissions per patient per year; and number of antihypertensive drugs per patient.
Statistical Analysis
A standard statistical analysis was performed using SAS v9.4 (SAS Institute Inc., Cary, NC) and R v4.2.2 (R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org; 2020).25 Descriptive statistics number (percentage), mean and SD, and mean and 95% CI were used, as appropriate. Data were tested for normal distribution using a Shapiro-Wilks test.
To evaluate the association between RPM exposure and clinical outcomes (PD-associated infection, count of PD-associated infection, CVD, mortality, technique failure, technique failure without death, count of unscheduled teleconsultation, count of unscheduled hospital visit, count of hospitalization, and count of antihypertensive drugs), matching methods were used to compensate for the lack of randomization and to obtain unbiased estimators. PSM was used to evaluate the association of RPM exposure with the clinical outcomes. The propensity score for each subject was calculated from a logistic regression model that included all clinical and demographic variables (including Charlson comorbidity Index) as predictors of the exposure status. A 1:1 matching without replacement utilizing the nearest neighbor within caliper was utilized to match each APD-RPM subject with an APD-without-RPM subject. All APD-without-RPM cases were then randomly ordered and an APD-RPM case with a propensity score closest to the first treatment case was selected. Different calipers were used, and the one that produced a better balance (0.15) between the baseline variables was selected. The balance between groups in the matched sample was evaluated based on standardized differences, with a target value of <0.1. In addition, all categorical variables were compared with Pearson’s χ2 test and continuous variables were analyzed with paired t-test.
The association of RPM exposure with the PD-associated infection, unscheduled teleconsultation, and unscheduled hospital visit was estimated with odds ratio and 95% CI from logistic regression models. The association of RPM exposure with CVD, mortality, and hospitalization were estimated with HR and 95% CI from Cox regression models. The association of RPM exposure with technique survival was estimated with HR and 95% CI from Cox regression models with a transplant as competitive risk. The association of RPM exposure with the count of PD-associated infection, count of unscheduled teleconsultation, count of unscheduled hospital visit, count of hospitalization, and count of antihypertensive drugs per year of patient follow-up was estimated with incidence rate ratio and 95%CI from negative binomial regressions. All regression models included possible confounding factors, as well as the characteristics that presented statistical differences in the descriptive analysis (standardized differences >0.1).
To compare changes in QoL of individuals over time, the changes were classified in an individual’s health state as better (improvement in at least 1 dimension), worse (a deterioration in at least 1 dimension), mixed (improvements and deteriorations in dimensions) or there being no change in the health state (those classified in the no-change group with the 11111 health state can be separated into their own “no problems” group) and were summarized by the absolute and relative frequency. This analysis was also analyzed by each dimension. Finally, change in the index was analyzed by mean and SD. To compare between groups of treatments, the Exact Fisher test (qualitative variable) and t-test student (quantitative variable) were performed.
Results
Descriptive Analysis
A total of 232 patients were included, 176 (75.9%) in the APD-RPM group and 56 (24.1%) in the APD-without-RPM group. The baseline demographic and clinical characteristics of patients are shown in Table 1.
Table 1.
Baseline demographic and clinical characteristics in the overall study sample according to exposure status
| Baseline characteristics | Overall N = 232 |
APD-RPM n = 176 | APD-without-RPM n = 56 | STD | P value |
|---|---|---|---|---|---|
| Demographic data | |||||
| Age, mean (SD) | 55.6 (15.2) | 55.4 (14.7) | 55.9 (16.9) | 0.03 | 0.84 |
| Female sex, n (%) | 80 (34.5) | 61 (34.7) | 19 (33.9) | 0.02 | 1.00 |
| Anthropometric data | |||||
| Height (cm), mean (SD) | 168.3 (9.0) | 168.7 (8.7) | 167.1 (9.7) | 0.16 | 0.28 |
| Weight (kg), mean (SD) | 76.1 (16.9) | 76.8 (16.8) | 74.0 (17.2) | 0.16 | 0.29 |
| BMI (kg/m2), mean (SD) | 26.8 (4.9) | 26.9 (4.6) | 26.4 (5.8) | 0.09 | 0.54 |
| Chronic kidney disease | |||||
| CKD cause, n (%) | 0.32 | 0.85 | |||
| Diabetes | 39 (16.8) | 28 (15.9) | 11 (19.6) | ||
| Cardiovascular disease | 29 (12.5) | 25 (14.2) | 4 (7.1) | ||
| Glomerulonephritis | 52 (22.4) | 36 (20.5) | 16 (28.6) | ||
| Interstitial nephritis | 15 (6.5) | 12 (6.8) | 3 (5.4) | ||
| Polycystic kidney disease | 25 (10.8) | 20 (11.4) | 5 (8.9) | ||
| Systemic diseases | 5 (2.2) | 4 (2.3) | 1 (1.8) | ||
| Family history | 5 (2.2) | 4 (2.3) | 1 (1.8) | ||
| Unknown | 46 (19.8) | 34 (19.3) | 12 (21.4) | ||
| Others | 16 (6.9) | 13 (7.4) | 3 (5.4) | ||
| Previous treatment, n (%) | 73 (31.5) | 52 (29.5) | 21 (37.5) | 0.17 | 0.34 |
| Hemodialysis | 45 (61.6) | 33 (63.5) | 12 (57.1) | ||
| Transplant | 28 (38.4) | 19 (36.5) | 9 (42.9) | ||
| Distance to hospital (km), mean (SD) | 23.1 (27.8) | 22.9 (28.5) | 23.7 (26.0) | 0.03 | 0.85 |
| Cause APD, n (%) | 0.36 | 0.08 | |||
| Patient choice | 143 (61.6) | 104 (59.1) | 39 (69.6) | ||
| Adequation | 72 (31.0) | 61 (34.7) | 11 (19.6) | ||
| Mechanical complications | 17 (7.3) | 11 (6.2) | 6 (10.7) | ||
| Dialysis vintage, n (%) | 0.09 | 0.85 | |||
| <1 yrs | 144 (62.1) | 108 (61.4) | 36 (64.3) | ||
| 1–3 yrs | 69 (29.7) | 54 (30.7) | 15 (26.8) | ||
| >3 yrs | 19 (8.2) | 14 (8.0) | 5 (8.9) | ||
| Residual diuresis (ml), n (%) | 0.11 | 0.80 | |||
| <150 ml/d | 47 (20.3) | 35 (19.9) | 12 (21.4) | ||
| 150–400 ml/d | 17 (7.3) | 14 (8.0) | 3 (5.4) | ||
| >400 ml/d | 168 (72.4) | 127 (72.2) | 41 (73.2) | ||
| Status | 0.07 | 0.79 | |||
| Prevalent patient | 171 (73.7) | 131 (74.4) | 40 (71.4) | ||
| Incidence patient | 61 (26.3) | 45 (25.6) | 16 (28.6) | ||
| Comorbidities | |||||
| Comorbidity index, mean (SD) | 4.9 (2.4) | 4.8 (2.3) | 5.3 (2.8) | 0.22 | 0.14 |
| Hypertension, n (%) | 201 (86.6) | 149 (84.7) | 52 (92.9) | 0.26 | 0.18 |
| Hypertension drugs, mean (SD) | 2.7 (1.8) | 2.7 (1.9) | 2.6 (1.6) | 0.06 | 0.73 |
| SBP (mm Hg), mean (SD) | 136.3 (20.3) | 135.9 (21.0) | 137.9 (18.3) | 0.10 | 0.52 |
| DBP (mm Hg), mean (SD) | 82.6 (11.4) | 82.3 (10.9) | 83.4 (12.8) | 0.10 | 0.51 |
| Diabetes, n (%) | 62 (26.7) | 49 (27.8) | 13 (23.2) | 0.11 | 0.61 |
| Follow-up | |||||
| Follow-up time, mos (mean) | 10.1 (2.9) | 10.4 (2.8) | 9.4 (3.1) | 0.34 | 0.02 |
| Cause of censure, n (%) | 0.83 | 0.03 | |||
| Death | 5 (10.2) | 1 (3.2) | 4 (22.2) | ||
| Technique failure | 20 (40.8) | 11 (35.5) | 9 (50.0) | ||
| Infection | 4 (20.0%) | 2 (18.2%) | 2 (22.2%) | ||
| Inadequate dialysis | 4 (20.0%) | 2 (18.2%) | 2 (22.2%) | ||
| Mechanical | 2 (10.0%) | 2 (18.2%) | 0 (0.0%) | ||
| Social | 1 (5.0%) | 0 (0.0%) | 1 (11.1%) | ||
| Other | 5 (25.0%) | 3 (27.3%) | 2 (22.2%) | ||
| Not reported | 4 (20.0%) | 2 (18.2%) | 2 (22.2%) | ||
| Transplant | 24 (49.0) | 19 (61.3) | 5 (27.8) |
APD, automated peritoneal dialysis; BMI, body mass index; CKD, chronic kidney disease; DBP, diastolic blood pressure; PD, peritoneal dialysis; RPM, remote patient monitoring; SBP, systolic blood pressure; SD, standard deviation; STD, standardized differences.
The mean age was 55.6 ± 15.2 years; 80 (34.5%) patients were women; the mean Charlson index was 4.9 ± 2.4; 62 (26.7%) patients had diabetes; 201 (86.6%) subjects had a history of hypertension, and the proportion of patients with urine output >400 ml was 72.4% (168/232). The mean follow-up time was 10.4 ± 2.8 months in the APD-RPM group and 9.4 ± 3.1 months for APD-RPM and APD-without-RPM, respectively (P = 0.02).
Matching Analysis
Matching analysis was performed to balance the baseline characteristics between cohorts. In Table 2, we present the balance assessment of the matched sample. The analysis shows that baseline characteristics were well-balanced after this process. The final matched sample included 56 patients in the APD-RPM cohort and 56 patients in the APD-without-RPM cohort.
Table 2.
Baseline demographic and clinical characteristics of the final matched sample according to exposure status
| Baseline characteristics | APD-RPM n = 56 | APD-without-RPM n = 56 | STD | P value |
|---|---|---|---|---|
| Demographic data | ||||
| Age, mean (SD) | 56.6 (14.0) | 55.9 (16.8) | 0.04 | 0.81 |
| Female gender, n (%) | 14 (25.0) | 19 (33.9) | 0.19 | 0.41 |
| Anthropometric data | ||||
| BMI (kg/m2), mean (SD) | 27.0 (4.3) | 26.4 (5.8) | 0.09 | 0.56 |
| Chronic kidney disease | ||||
| CKD cause, n (%) | 0.94 | |||
| Diabetes | 13 (23.2) | 11 (19.6) | 0.08 | |
| Cardiovascular disease | 3 (5.4) | 4 (7.1) | 0.07 | |
| Glomerulonephritis | 16 (28.6) | 16 (28.6) | 0.00 | |
| Interstitial nephritis | 3 (5.4) | 3 (5.4) | 0.00 | |
| Polycystic kidney disease | 2 (3.6) | 5 (8.9) | 0.19 | |
| Systemic diseases | 0 (0.0) | 1 (1.8) | 0.13 | |
| Family history | 1 (1.8) | 1 (1.8) | 0.00 | |
| Unknown | 15 (26.8) | 12 (21.4) | 0.13 | |
| Others | 3 (5.4) | 3 (5.4) | 0.00 | |
| Previous treatment, n (%) | 21 (37.5) | 21 (37.5) | 0.00 | 1.00 |
| Hemodialysis | 15 (71.4) | 12 (57.1) | ||
| Transplant | 6 (28.6) | 9 (42.9) | ||
| Distance to hospital (km), mean (SD) | 16.4 (21.6) | 23.7 (26.0) | 0.28 | 0.11 |
| Cause APD, n (%) | 0.97 | |||
| Patient choice | 38 (67.9) | 39 (69.6) | 0.04 | |
| Adequation | 12 (21.4) | 11 (19.6) | 0.05 | |
| Mechanical complications | 6 (10.7) | 6 (10.7) | 0.00 | |
| Dialysis vintage, n (%) | 0.94 | |||
| <1 yrs | 36 (64.3) | 36 (64.3) | 0.00 | |
| 1–3 yrs | 14 (25.0) | 15 (26.8) | 0.04 | |
| >3 yrs | 6 (10.7) | 5 (8.9) | 0.06 | |
| Residual diuresis (ml), n (%) | 0.56 | |||
| <150 ml/d | 14 (25.0) | 12 (21.4) | 0.09 | |
| 150-400 ml/d | 1 (1.8) | 3 (5.4) | 0.16 | |
| >400 ml/d | 41 (73.2) | 41 (73.2) | 0.00 | |
| Status | 0.12 | 0.68 | ||
| Prevalent patient | 37 (66.1) | 40 (71.4) | ||
| Incidence patient | 19 (33.9) | 16 (28.6) | ||
| Comorbidities | ||||
| Comorbidity index, mean (SD) | 5.38 (2.50) | 5.30 (2.78) | 0.02 | 0.89 |
| Hypertension, n (%) | 53 (94.6) | 52 (92.9) | 0.07 | 1.00 |
| Hypertension drugs, mean (SD) | 2.84 (2.10) | 2.62 (1.60) | 0.13 | 0.54 |
| SBP (mm Hg), mean (SD) | 137.3 (18.7) | 137.9 (18.3) | 0.03 | 0.86 |
| DBP (mm Hg), mean (SD) | 84.6 (10.3) | 83.4 (12.8) | 0.09 | 0.60 |
| Diabetes, n (%) | 14 (25.0) | 13 (23.2) | 0.04 | 1.00 |
| Follow-up | ||||
| Follow-up time, months (mean) | 10.4 (2.8) | 9.4 (3.1) | ||
| Cause of censure, n (%) | ||||
| Death | 2 (40.0) | 5 (27.8) | ||
| Technique failure | 2 (40.0) | 9 (50.0) | ||
| Transplant | 1 (20.0) | 4 (22.2) |
APD, automated peritoneal dialysis; BMI, body mass index; CKD, chronic kidney disease; DBP, diastolic blood pressure; PD, peritoneal dialysis; RPM, remote patient monitoring; SBP, systolic blood pressure; SD, standard deviation; STD, standardized differences.
Outcomes
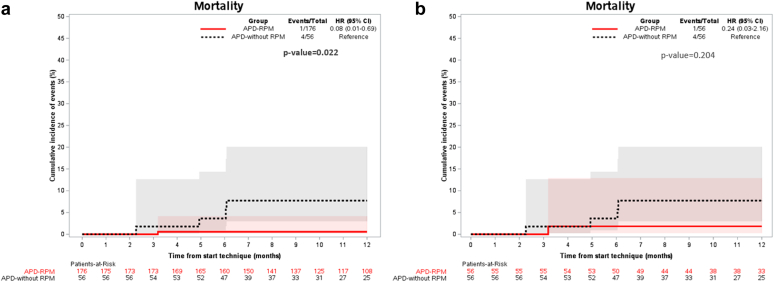
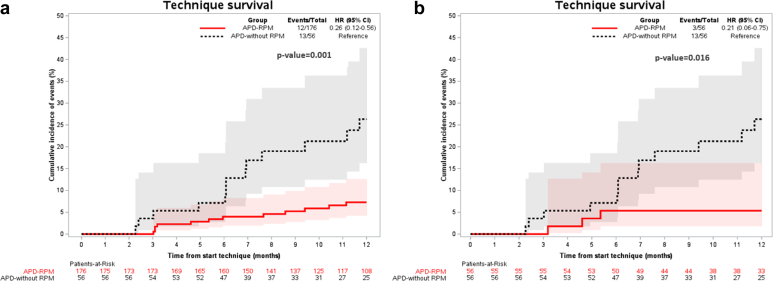
In the overall study sample, as compared to the APD-without-RPM cohort, the APD-RPM group was associated with lower mortality rate (0.6% vs. 7.1%, respectively) (HR: 0.08; 95% CI: 0.01 to 0.69; P = 0.022) (Figure 1a) and greater technique survival rate (6.4% vs. 21.6%, respectively) (HR: 0.25; 95% CI: 0.11 to 0.59; P = 0.001) (Table 3 and Figure 2a).
Figure 1.
Overview of the mortality rates in the overall study population (a) and after propensity score matching (b). (a) In the overall study sample, mortality rates were significantly lower in the APD-RPM group than in the APD-without-RPM group (0.6% vs. 7.1%, respectively) (HR: 0.08; 95% CI: 0.01 to 0.69; P = 0.022). (b) After propensity score matching, no significant differences were observed in mortality rates between the 2 groups. APD, automated peritoneal dialysis; CI, confidence interval; HR, hazard ratio; RPM, remote patient monitoring.
Table 3.
Clinical outcomes associated with rpm in the overall study population
| Outcomes | Overall N = 232 |
APD-RPM n = 176 | APD-without-RPM n = 56 | OR (95% CI)d HR (95% CI) IRR (95% CI) | P value |
|---|---|---|---|---|---|
| Primary outcomes | |||||
| PD-associated infectiona | 40 (17.2) | 27 (15.3) | 13 (23.2) | 0.60 (0.29-1.29) | 0.177 |
| Infections per patient-year | 0.25 (0.62) | 0.23 (0.62) | 0.30 (0.63) | 0.75 (0.36-1.59) | 0.445 |
| Cardiovascular diseaseb | 16 (6.9) | 14 (8.0) | 2 (3.6) | 1.94 (0.44-8.52) | 0.440 |
| Mortality | 5 (2.2) | 1 (0.6) | 4 (7.1) | 0.08 (0.01-0.69) | 0.022 |
| Technique survivalc | 21 (10.1) | 10 (6.4) | 11 (21.6) | 0.25 (0.11-0.59) | 0.001 |
| Secondary outcomes | |||||
| Hospitalization | 72 (31.0) | 51 (29.0) | 21 (37.5) | 0.68 (0.36-1.29) | 0.230 |
| Hospitalizations per patient-year | 0.43 (0.81) | 0.42 (0.86) | 0.45 (0.63) | 0.94 (0.56-1.62) | 0.825 |
| Unscheduled teleconsultation | 164 (74.2) | 125 (75.8) | 39 (69.6) | 1.36 (0.68-2.64) | 0.367 |
| visits per patient-yr | 2.81 (3.43) | 3.03 (3.62) | 2.16 (2.70) | 1.40 (0.97-2.00) | 0.064 |
| Unscheduled hospital visit | 140 (60.3) | 101 (57.4) | 39 (69.6) | 0.59 (0.30-1.10) | 0.105 |
| visits per patient-yr | 1.80 (2.61) | 1.61 (2.38) | 2.38 (3.21) | 0.68 (0.45-1.01) | 0.057 |
| Anti-HBP drugs (drugs per patient-year) | 8.44 (7.18) | 8.82 (7.40) | 7.21 (6.37) | 1.22 (0.92-1.61) | 0.160 |
APD, automated peritoneal dialysis; CI, confidence interval; HBP, high blood pressure; HD, hemodialysis; IRR, incidence rate ratio; OR, odds ratio; HR, hazard ratio; PD, peritoneal dialysis; RPM, remote patient monitoring.
PD-associated infection is defined as the number of peritonitis reports during follow-up.
Cardiovascular disease is defined as the occurrence after enrollment of the first one of the following: angina or acute coronary myocardial infarctions, ischemic stroke, and peripheral arterial events.
Technique survival is defined as composite endpoint of transfer to HD >30 days or death.
OR was calculated in PD-associated infection, unscheduled teleconsultation and unscheduled hospital visit from logistic regression models. HR was calculated in cardiovascular disease, mortality, technique survival and hospitalization from Cox regression models. IRR was calculated in the count of PD-associated infection, count of unscheduled teleconsultation, count of unscheduled hospital visit, count of hospitalization, and count of antihypertensive drugs from negative binomial regressions.
Figure 2.
Overview of the technique survival rates in the overall study population (a) and after propensity score matching (b). (a) In the overall study, population technique survival rate was significantly lower in the APD-without-RPM than in the APD-RPM group (6.4% vs. 21.6%, respectively) (HR: 0.25; 95% CI: 0.11 to 0.59; P = 0.001). (b) After propensity score matching, technique survival continued to be significantly lower in the APD-without-RPM group than in the APD-RPM group (5.6% vs. 21.6%) (HR: 0.23; 95% CI: 0.06 to 0.83; P = 0.016). APD, automated peritoneal dialysis; CI, confidence interval; HR, hazard ratio; RPM, remote patient monitoring.
After PSM (Table 4), there were no differences in mortality rates between both groups (Figure 1b), although APD-RPM compared with APD-without-RPM continued to be associated with more technique survival (5.6% vs. 21.6%) (HR: 0.23; 95% CI: 0.06 to 0.83; P = 0.016). Technique survival cumulative incidence of events (%) using the PSM with competing events (kidney transplant) is shown in Figure 2b. However, there were no significant differences between the 2 groups regarding the rest of both primary and secondary outcomes. In Table 5, we show the results of EQ-5D test in both groups with similar results between them.
Table 4.
Clinical outcomes associated with RPM in the matched sample
| Outcomes | APD-RPM N = 56 |
APD-without RPM N = 56 |
OR (95% CI)d HR (95% CI) IRR (95% CI) | P value |
|---|---|---|---|---|
| Primary outcomes | ||||
| PD-associated infectiona | 12 (21.4) | 13 (23.2) | 0.90 (0.37-2.21) | 0.821 |
| Infections per patient-yr | 0.34 (0.79) | 0.30 (0.63) | 1.11 (0.49-2.56) | 0.792 |
| Cardiovascular diseaseb | 9 (16.1) | 2 (3.6) | 3.92 (0.84-18.2) | 0.081 |
| Mortality | 1 (1.8) | 4 (7.1) | 0.24 (0.03-2.16) | 0.204 |
| Technique survivalc | 3 (5.6) | 11 (21.6) | 0.23 (0.06-0.83) | 0.016 |
| Secondary outcomes | ||||
| Hospitalization | 19 (33.9) | 21 (37.5) | 0.86 (0.39-1.86) | 0.693 |
| Hospitalizations per patient-yr | 0.46 (0.74) | 0.45 (0.63) | 1.04 (0.59-1.81) | 0.890 |
| Unscheduled teleconsultation | 43 (81.1) | 39 (69.6) | 1.87 (0.78-4.71) | 0.168 |
| visits per patient-yr | 3.15 (3.30) | 2.16 (2.70) | 1.46 (0.95-2.23) | 0.081 |
| Unscheduled hospital visit | 31 (55.4) | 39 (69.6) | 0.54 (0.25-1.17) | 0.120 |
| visits per patient-yr | 1.55 (2.56) | 2.38 (3.21) | 0.65 (0.39-1.08) | 0.099 |
| Anti-HBP drugs (drugs per patient-yr) | 9.59 (8.74) | 7.21 (6.37) | 1.33 (0.96-1.84) | 0.087 |
APD, automated peritoneal dialysis; CI, confidence interval; HBP, high blood pressure; HD, hemodialysis; HR, hazard ratio; IRR, incidence rate ratio; OR, odds ratio; PD, peritoneal dialysis; RPM, remote patient monitoring.
PD-associated infection is defined as the number of peritonitis reports during follow-up.
Cardiovascular disease is defined as the occurrence after enrollment of the first one of the following: angina or acute coronary myocardial infarctions, ischemic stroke, and peripheral arterial events.
Technique survival is defined as composite endpoint of transfer to HD >30 days or death.
OR was calculated in PD-associated infection, unscheduled teleconsultation and unscheduled hospital visit from logistic regression models. HR was calculated in cardiovascular disease, mortality, technique survival and hospitalization from Cox regression models. IRR was calculated in the count of PD-associated infection, count of unscheduled teleconsultation, count of unscheduled hospital visit, count of hospitalization, and count of antihypertensive drugs from negative binomial regression.
Table 5.
Quality of life (EQ-5D-3L)
| APD-RPM n = 109 | APD-without RPM n = 37 | P-value | |
|---|---|---|---|
| Baseline, n (%) | |||
| 11111 | 41 (37.6%) | 12 (32.4%) | |
| 11112 | 14 (12.8%) | 5 (13.5%) | |
| 11122 | 9 (8.3%) | 3 (8.1%) | |
| 11121 | 5 (4.6%) | 2 (5.4%) | |
| 11221 | 2 (1.8%) | 2 (5.4%) | |
| 22222 | 6 (5.5%) | 0 (0.0%) | |
| Others | 32 (29.4%) | 13 (35.1%) | |
| EQ Index, mean (SD) | 0.84 (0.24) | 0.74 (0.38) | 0.064 |
| Follow-up | |||
| 11111 | 48(44.0%) | 11 (29.7%) | |
| 11121 | 2 (1.8%) | 4 (10.8%) | |
| 11122 | 6 (5.5%) | 4 (10.8%) | |
| 11112 | 16 (14.7%) | 3 (8.1%) | |
| 11221 | 1 (0.9%) | 2 (5.4%) | |
| 11222 | 3 (2.8%) | 2 (5.4%) | |
| Others | 33 (30.7%) | 11 (29.7%) | |
| EQ Index, mean (SD) | 0.86 (0.25) | 0.80 (0.24) | 0.198 |
| Evolution of QoLa | |||
| Overall | |||
| Change EQ Index, mean (SD) | 0.01 (0.2) | 0.06 (0.3) | 0.262 |
| No problems | 35 (32.1%) | 8 (21.6%) | |
| With problems | 74 (67.9%) | 29 (78.4%) | |
| No change | 32 (43.2%) | 8 (27.6%) | |
| Improve | 24 (32.4%) | 9 (31.0%) | |
| Worsen | 11 (14.9%) | 9 (31.0%) | |
| Mixed change | 7 (9.5%) | 3 (10.3%) | |
| Mobility | 0.594 | ||
| No problems | 78 (71.6%) | 25 (67.6%) | |
| With problems | 31 (28.4%) | 12 (32.4%) | |
| No change | 16 (51.6%) | 5 (41.7%) | |
| Improve | 8 (25.8%) | 5 (41.7%) | |
| Worsen | 7 (22.6%) | 2 (16.7%) | |
| Self-care | 0.853 | ||
| No problems | 95 (87.2%) | 31 (83.8%) | |
| With problems | 14 (12.8%) | 6 (16.2%) | |
| No change | 8 (57.1%) | 4 (66.7%) | |
| Improve | 4 (28.6%) | 1 (16.7%) | |
| Worsen | 2 (14.3%) | 1 (16.7%) | |
| Usual activities | 0.407 | ||
| No problems | 76 (69.7%) | 21 (56.8%) | |
| With problems | 33 (30.3%) | 16 (43.2%) | |
| No change | 17 (51.5%) | 5 (31.2%) | |
| Improve | 9 (27.3%) | 6 (37.5%) | |
| Worsen | 7 (21.2%) | 5 (31.2%) | |
| Pain/discomfort | 0.278 | ||
| No problems | 66 (60.6%) | 17 (45.9%) | |
| With problems | 43 (39.4%) | 20 (54.1%) | |
| No change | 20 (46.5%) | 10 (50.0%) | |
| Improve | 16 (37.2%) | 4 (20.0%) | |
| Worsen | 7 (16.3%) | 6 (30.0%) | |
| Anxiety/depression | 0.607 | ||
| No problems | 58 (53.2%) | 17 (45.9%) | |
| With problems | 51 (46.8%) | 20 (54.1%) | |
| No change | 32 (62.7%) | 10 (50.0%) | |
| Improve | 12 (23.5%) | 6 (30.0%) | |
| Worsen | 7 (13.7%) | 4 (20.0%) |
APD, automated peritoneal dialysis; EQ, EuroQol; PD, peritoneal dialysis; RPM, remote patient monitoring; QoL; quality of life.
Classifies the change in an individual’s health state as better (improvement in at least 1 dimension), worse (a deterioration in at least 1 dimension), mixed (improvements and deteriorations in dimensions), or there being no change in the health state. Those classified in the “no change group” with the 11111 health state can be separated into their own “no problems” group.
Discussion
This study aimed to evaluate whether RPM programs are clinically useful, in terms of SONG-PD, in patients with end-stage renal disease on APD. Other secondary important outcomes were also analyzed.20 To the best of our knowledge, this is the first study assessing the impact of RPM-APD interventions in terms of all SONG-PD clinical outcomes.18, 19, 20
According to the results of the current study, RPM was associated with a better technique survival. Although technique survival is 1 of 5 core patient outcomes identified by the SONG-PD initiative,19 this is a core point that not is always well represented in randomized clinical trials.26 Factors associated with technique survival have been related with patient-related, center-related, and treatment-related.20,27,28 In addition, peritonitis is the most common cause of technique failure.20,21 Moreover, nonadherence to PD treatment could facilitate the development of peritonitis, although we did not observe differences in peritonitis rates in this study. RPM could increase technique survival via increasing PD prescription adherence and contributing to early identification of mechanical problems.12
These findings are consistent with those previously published.16,29,30 Milan Manani et al.29 compared different clinical outcomes and QoL in 2 group of patients undergoing APD, with (35 patients) and without (38 patients) remote monitoring. This study found that RPM reduced the number of emergency visits related to nephrological problems, particularly in those patients with a greater comorbidity score. However, they did not find significant differences in the number of peritonitis or the technique survival between the 2 groups.29 Corzo et al.,16 in a multicenter, retrospective, and observational study, reported less technique failure in 148 patients on APD with RPM than in 148 patients on APD without RPM controls.
Finally, the results of a systematic review and metanalysis, which included 9975 subjects from 5 countries, found that RPM was associated with better clinical outcomes, less technical failure, and better QoL. However, the current evidence is not conclusive.31
The current study agreed that RPM may improve technique survival. This improvement in PD survival may be mainly due to an improvement in adherence, although due to the characteristics of this study, this fact cannot be demonstrated. Therefore, there would be increasing probability of identifying mechanical problems at early stages,12,16 which is a key goal of this type of therapy.
This study did not find differences in terms of number of peritonitis reports or number of hospitalizations, which has been reported to be associated with increased technique survival in other studies.12,16 Other factors, such as center effect or social reasons, that have been associated with peritonitis21 were not evaluated in this study. Technique failure was also associated with other adverse outcomes, including mortality.32 The current study found less mortality in the RPM-APD group in the overall study sample, but not when matching the sample.
According to the results that assessed the impact of an Internet-based instant messaging software on patients on PD, a greater degree of satisfaction and better levels of biochemical biomarkers in the remote monitoring group was observed.33 In addition, they suggested better mortality rates in the remote monitoring group, but the events were no reported.17,33 Conversely, Corzo et al.16 did not report significant differences in mortality rates.
Furthermore, this study evaluated the role of RPM from a patient´s perspective. The current study did not find significant differences in QoL between patients on APD with or without RPM, which was in line with other studies.29,34 This finding might be because QoL questionnaires have not been specifically designed to assess the effect of monitoring home dialysis in life participation.15
Although there are several companies manufacturing different PD systems, the information comparing PD system is very limited.35, 36, 37, 38 This lack of evidence might be due to the thinking that all PD systems are similar, and therefore, will provide similar outcomes. However, there could be potentially clinically relevant differences between PD systems, such as differences in packaging, connectology, solution contents, or other subtle differences that might impact patients’ outcomes.38, 39, 40
This study has some limitations that should be considered when interpreting the results. Lack of randomization and confounding factors might bias the results of the observational studies. Nevertheless, this study used matching methods to compensate for these issues. PSM entails forming matched sets of treated and untreated subjects who share a similar value of the propensity score.41 PSM is a technique used to reduce selection bias in observational studies.42,43 The goal of PSM is to create a sample in which treatment groups are balanced on baseline covariates. Logistic regression (the method used in the current study) is the most used method for estimating the propensity score.41, 42, 43 An additional limitation is comparing 2 devices from 2 different manufacturers. It would be more accurate to use the same device in the 2 groups (APD-RPM vs. APD-without RPM). It has been previously reported that PD systems manufactured by different companies may be associated with important differences in PD technique survival.38 Therefore, we cannot unequivocally conclude that the findings of this study are due exclusively to RPM. An adequately powered randomized study should be conducted to evaluate this issue. Finally, this study included incident patients and might not reflect outcomes in prevalent patients with long dialysis vintage.
Nevertheless, as strength, it should be mentioned its multicenter nature and the fact that it included a large number of patients. The current study was conducted at third-level hospitals, which may reflect the reality of the APD throughout the Spanish territory. We are unaware of previous reports conducted in similar circumstances or populations that have included such many patients. Although there is evidence of studies that have included a larger number of patients,6,16 these were conducted on environments that reflect a very different reality, which therefore limit the applicability of their results to our daily practice.
Conclusions
According to the results of this study, the use of RPM programs in patients on APD was associated with more technique survival. Although in the overall population, the use of RPM programs in patients on APD was associated with lower mortality rate, such findings were not observed after PSM. RPM programs could be a safe and effective strategy for improving PD technique survival. Despite the promising results of the current study, randomized clinical trials using SONG-PD outcomes are needed to confirm these findings.
Disclosure
All the authors declared no competing interests.
Acknowledgments
Medical writing and editorial assistant services were provided by Ciencia y Deporte S.L.
This study was funded by a research grant from Baxter Renal Care CAC program. The funding organization had no role in the design; data collection, analysis, or interpretation of this study; or the decision to submit this report for publication or writing of this manuscript.
Data Availability Statement
The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author Contributions
Conceptualization, methodology, formal analysis, investigation, and supervision were done by FJC-P, AO-C, and JP-M. All authors met the ICMJE authorship criteria. All authors made substantial contributions to conception, design, analysis, and interpretation of data; contributed to writing the article; provided critical revision of the manuscript; and approved the final version.
References
- 1.Li P.K., Chow K.M., Van de Luijtgaarden M.W., et al. Changes in the worldwide epidemiology of peritoneal dialysis. Nat Rev Nephrol. 2017;13:90–103. doi: 10.1038/nrneph.2016.181. [DOI] [PubMed] [Google Scholar]
- 2.Martino F., Adıbelli Z., Mason G., et al. Home visit program improves technique survival in peritoneal dialysis. Blood Purif. 2014;37:286–290. doi: 10.1159/000365168. [DOI] [PubMed] [Google Scholar]
- 3.Griva K., Lai A.Y., Lim H.A., Yu Z., Foo M.W., Newman S.P. Non-adherence in patients on peritoneal dialysis: a systematic review. PLoS One. 2014;9 doi: 10.1371/journal.pone.0089001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Denhaerynck K., Manhaeve D., Dobbels F., Garzoni D., Nolte C., De Geest S. Prevalence and consequences of nonadherence to hemodialysis regimens. Am J Crit Care. 2007;16:222–235. doi: 10.4037/ajcc2007.16.3.222. [DOI] [PubMed] [Google Scholar]
- 5.Whitlow M., Wallace E. Remote patient monitoring: an important tool in advancing home dialysis. Kidney Med. 2019;1:327–328. doi: 10.1016/j.xkme.2019.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanabria M., Rosner M., Vesga J., et al. A remote management program in automated peritoneal dialysis patients in Colombia. Nefrol Latinoam. 2018;15:48–51. doi: 10.24875/NEFRO.18000048. [DOI] [Google Scholar]
- 7.Nayak A., Antony S., Nayak K.S. Remote monitoring of peritoneal dialysis in special locations. Contrib Nephrol. 2012;178:79–82. doi: 10.1159/000337816. [DOI] [PubMed] [Google Scholar]
- 8.Milan Manani S., Rosner M.H., Virzì G.M., et al. Longitudinal experience with remote monitoring for automated peritoneal dialysis patients. Nephron. 2019;142:1–9. doi: 10.1159/000496182. [DOI] [PubMed] [Google Scholar]
- 9.Wood E., McCarthy K., Roper M. Remote monitoring of peritoneal dialysis: evaluating the impact of the Claria Sharesource system. J Kidney Care. 2019;4:16–24. doi: 10.12968/jokc.2019.4.1.16. [DOI] [Google Scholar]
- 10.Bunch A., Vesga J.I., Camargo D.O., et al. Remote automated peritoneal dialysis management in Colombia. Kidney Int Rep. 2019;4:873–876. doi: 10.1016/j.ekir.2019.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bunch A., Ardila F., Castaño R., Quiñonez S., Corzo L. Through the storm: automated peritoneal dialysis with remote patient monitoring during COVID-19 pandemic. Blood Purif. 2021;50:279–282. doi: 10.1159/000511407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanabria M., Buitrago G., Lindholm B., et al. Remote patient monitoring program in automated peritoneal dialysis: impact on hospitalizations. Perit Dial Int. 2019;39:472–478. doi: 10.3747/pdi.2018.00287. [DOI] [PubMed] [Google Scholar]
- 13.Chang M.Y., Chi P.J., Wang H.H., et al. Evaluation of the impact of remote monitoring using the Sharesource connectivity platform on adherence to automated peritoneal dialysis in 51 patients. Med Sci Monit. 2023;29 doi: 10.12659/MSM.939523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yeter H.H., Akcay O.F., Ronco C., Derici U. Automated remote monitoring for peritoneal dialysis and its impact on blood pressure. Cardiorenal Med. 2020;10:198–208. doi: 10.1159/000506699. [DOI] [PubMed] [Google Scholar]
- 15.Yeter H.H., Manani S.M., Ronco C. The utility of remote patient management in peritoneal dialysis. Clin Kidney J. 2021;14:2483–2489. doi: 10.1093/ckj/sfab111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corzo L., Wilkie M., Vesga J.I., et al. Technique failure in remote patient monitoring program in patients undergoing automated peritoneal dialysis: a retrospective cohort study. Perit Dial Int. 2022;42:288–296. doi: 10.1177/0896860820982223. [DOI] [PubMed] [Google Scholar]
- 17.Biebuyck G.K.M., Neradova A., de Fijter C.W.H., Jakulj L. Impact of telehealth interventions added to peritoneal dialysis-care: a systematic review. BMC Nephrol. 2022;23:292. doi: 10.1186/s12882-022-02869-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manera K.E., Tong A., Craig J.C., et al. Standardized outcomes in nephrology-peritoneal dialysis (SONG-PD): study protocol for establishing a core outcome set in PD. Perit Dial Int. 2017;37:639–647. doi: 10.3747/pdi.2017.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manera K.E., Johnson D.W., Craig J.C., et al. Establishing a core outcome set for peritoneal dialysis: report of the SONG-PD (standardized outcomes in nephrology-peritoneal dialysis) consensus workshop. Am J Kidney Dis. 2020;75:404–412. doi: 10.1053/j.ajkd.2019.09.017. [DOI] [PubMed] [Google Scholar]
- 20.Bello A.K., Okpechi I.G., Osman M.A., et al. Epidemiology of peritoneal dialysis outcomes. Nat Rev Nephrol. 2022;18:779–793. doi: 10.1038/s41581-022-00623-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li P.K., Szeto C.C., Piraino B., et al. ISPD peritonitis recommendations: 2016 update on prevention and treatment. Perit Dial Int. 2016;36:481–508. doi: 10.3747/pdi.2016.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma T., Yang Z., Li S., et al. The peritoneal dialysis Telemedicine-assisted Platform Cohort (PDTAP) Study: design and methods. Perit Dial Int. 2022;42:75–82. doi: 10.1177/0896860820962901. [DOI] [PubMed] [Google Scholar]
- 23.Lan P.G., Clayton P.A., Johnson D.W., et al. Duration of hemodialysis following peritoneal dialysis cessation in Australia and New Zealand: proposal for a standardized definition of technique failure. Perit Dial Int. 2016;36:623–630. doi: 10.3747/pdi.2015.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liem Y.S., Bosch J.L., Hunink M.G. Vol. 11. Value Health; 2008. Preference-based quality of life of patients on renal replacement therapy: a systematic review and meta-analysis; pp. 733–741. [DOI] [PubMed] [Google Scholar]
- 25.R Core Team R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. 2020. https://www.R-project.org/ Accessed October 30, 2023.
- 26.Manera K.E., Johnson D.W., Cho Y., et al. Scope and heterogeneity of outcomes reported in randomized trials in patients receiving peritoneal dialysis. Clin Kidney J. 2020;14:1817–1825. doi: 10.1093/ckj/sfaa224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elphick E., Holmes M., Tabinor M., et al. Outcome measures for technique survival reported in peritoneal dialysis: a systematic review. Perit Dial Int. 2022;42:279–287. doi: 10.1177/0896860821989874. [DOI] [PubMed] [Google Scholar]
- 28.Da Luz L.G., Ankawi G., Digvijay K., Rosner M.H., Ronco C. Technique failure in peritoneal dialysis: etiologies and risk assessment. Blood Purif. 2021;50:42–49. doi: 10.1159/000508159. [DOI] [PubMed] [Google Scholar]
- 29.Milan Manani S., Baretta M., Giuliani A., et al. Remote monitoring in peritoneal dialysis: benefits on clinical outcomes and on quality of life. J Nephrol. 2020;33:1301–1308. doi: 10.1007/s40620-020-00812-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chaudhuri S., Han H., Muchiutti C., et al. Remote treatment monitoring on hospitalization and technique failure rates in peritoneal dialysis patients. Kidney360. 2020;1:191–202. doi: 10.34067/KID.0000302019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nygård H.T., Nguyen L., Berg R.C. Effect of remote patient monitoring for patients with chronic kidney disease who perform dialysis at home: a systematic review. BMJ Open. 2022;12 doi: 10.1136/bmjopen-2022-061772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen J.H.C., Johnson D.W., Hawley C., Boudville N., Lim W.H. Association between causes of peritoneal dialysis technique failure and all-cause mortality. Sci Rep. 2018;8:3980. doi: 10.1038/s41598-018-22335-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cao F., Li L., Lin M., Lin Q., Ruan Y., Hong F. Application of instant messaging software in the follow-up of patients using peritoneal dialysis, a randomized controlled trial. J Clin Nurs. 2018;27:3001–3007. doi: 10.1111/jocn.14487. [DOI] [PubMed] [Google Scholar]
- 34.Dey V., Jones A., Spalding E.M. Telehealth: acceptability, clinical interventions and quality of life in peritoneal dialysis. SAGE Open Med. 2016;4 doi: 10.1177/2050312116670188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hall L.J., Kinney R.A., Taber T.E., Hegeman T.F. Comparison of two non-disconnect CAPD delivery systems. Adv Perit Dial. 1989;5:227–228. [PubMed] [Google Scholar]
- 36.Ong L.M., Lim T.O., Hooi L.S., et al. A randomized, multicenter, open-label trial to establish therapeutic equivalence between the Carex and ultra disconnect systems in patients on continuous ambulatory peritoneal dialysis. Perit Dial Int. 2003;23(suppl 2):S139–S143. doi: 10.1177/089686080302302s29. [DOI] [PubMed] [Google Scholar]
- 37.Wong H.S., Ong L.M., Lim T.O., et al. A randomized, multicenter, open-label trial to determine peritonitis rate, product defect, and technique survival between ANDY-Disc and UltraBag in patients on CAPD. Am J Kidney Dis. 2006;48:464–472. doi: 10.1053/j.ajkd.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 38.Boudville N., Ullah S., Clayton P., et al. Differences in peritoneal dialysis technique survival between patients treated with peritoneal dialysis systems from different companies. Nephrol Dial Transplant. 2019;34:1035–1044. doi: 10.1093/ndt/gfy340. [DOI] [PubMed] [Google Scholar]
- 39.Zhou J., Cao X., Lin H., et al. Safety and effectiveness evaluation of a domestic peritoneal dialysis fluid packed in non-PVC bags: study protocol for a randomized controlled trial. Trials. 2015;16:592. doi: 10.1186/s13063-015-1131-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Szeto C.C., Johnson D.W. Low GDP solution and glucose-sparing strategies for peritoneal dialysis. Semin Nephrol. 2017;37:30–42. doi: 10.1016/j.semnephrol.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 41.Rosenbaum P.R., Rubin D.B. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat. 1985;39:33–38. doi: 10.2307/2683903. [DOI] [Google Scholar]
- 42.Morgan C.J. Reducing bias using propensity score matching. J Nucl Cardiol. 2018;25:404–406. doi: 10.1007/s12350-017-1012-y. [DOI] [PubMed] [Google Scholar]
- 43.Austin P.C. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46:399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.