Abstract
To determine the effects of interleukin-4 (IL-4) on bacterial clearance from the mouse lung, transgenic mice expressing IL-4 in respiratory epithelial cells under the control of the Clara cell secretory protein promoter (CCSP-IL-4 mice) were infected intratracheally with Pseudomonas aeruginosa. Survival of CCSP-IL-4 mice following bacterial administration was markedly improved compared with that of control mice. While bacteria proliferated in lungs of wild-type mice, a rapid reduction in the number of bacteria was observed in the IL-4 mice as early as 6 h postinfection. Similarly, intranasal administration of IL-4 enhanced bacterial clearance from the lungs of wild-type mice. While acute and chronic IL-4 increased the numbers of neutrophils in bronchoalveolar lavage fluid, bacterial infection was associated with acute neutrophilic pulmonary infiltration, and this response was similar in the presence or absence of IL-4. Local administration or expression of IL-4 in the mouse lung enhanced pulmonary clearance of P. aeruginosa in vivo and decreased mortality following infection.
Interleukin-4 (IL-4) is a pleiotropic cytokine produced primarily by the Th2 cell subset of T lymphocytes and by mast cells. IL-4 induces the differentiation of uncommitted precursor CD4+ T cells toward the Th2 subset and inhibits the differentiation of Th1 cells but also enhances differentiation, proliferation, and activation of various inflammatory cells. Antigen presentation in the presence of IL-4 leads to the formation of immunoglobulin E antibodies and enhances the migration of eosinophils and mast cells into the sites of infection or inflammation (3). While IL-4 has been implicated in the response to parasitic infection (17, 32, 58), allergy (19, 30, 49), and chronic inflammation (48, 59), its potential role in bacterial host defense remains unclear.
Pseudomonas aeruginosa is a common cause of acute and chronic pneumonia in humans. Pulmonary infection caused by P. aeruginosa is an important factor contributing to the morbidity and mortality associated with cystic fibrosis (CF). Chronic lung infection with P. aeruginosa in CF is associated with acute and chronic inflammation that is dominated by neutrophilic infiltration. Lung injury associated with P. aeruginosa infection is related to the destructive effects of the organism on the lung parenchyma and may be further exacerbated by the activation of neutrophils and other inflammatory mediators that lead to the progressive obstruction and fibrosis in small airways, causing the progressive deterioration of lung function seen in CF (5). Furthermore, pulmonary infection caused by P. aeruginosa is associated with increased production of various cytokines, including IL-1, IL-6, IL-8, and tumor necrosis factor alpha (TNF-α), which may be involved in neutrophil recruitment and activation, critical to the resolution of the infection, or play a role in the chronic inflammatory disease seen in CF (8, 35).
Acute clearance of P. aeruginosa from the respiratory tract is mediated by a variety of factors that may play a role in Pseudomonas infection in CF. Increased bacterial growth was observed in lungs from mice administered TNF-α neutralizing antibody prior to intratracheal (i.t.) infection with P. aeruginosa (22). Local phagocytic infiltrates at the site of infection were also thought to be important in the pathogenesis of respiratory infection (1, 10, 43). In vivo depletion of alveolar macrophages decreased the initial neutrophil influx and reduced the concentrations of TNF-α and macrophage inhibitory protein 2 in mouse lungs infected with P. aeruginosa. However, in mice depleted of alveolar macrophages, increased neutrophil recruitment was associated with decreased bacterial clearance and decreased animal survival after i.t. infection with Klebsiella pneumoniae (9). C5a receptor-deficient mice were susceptible to i.t. infection with P. aeruginosa despite increased pulmonary neutrophil infiltration (25).
Surfactant protein A (SP-A) and SP-D are members of the collectin group of mammalian lectins with an amino-terminal collagen-like domain and a carboxy-terminal carbohydrate recognition domain (45). SP-A enhances binding and phagocytosis of bacterial pathogens by macrophages. SP-A increases macrophage clearance by binding directly to the surface of bacterial pathogens such as Haemophilus influenzae, Streptococcus pneumoniae, group A streptococci, K. pneumoniae, and Mycobacterium bovis BCG (28, 41, 57, 60). Binding of SP-A to these pathogens is Ca2+ dependent, directly implicating the carbohydrate recognition domain in SP-A binding. In addition, SP-A enhances the serum-dependent phagocytosis of Staphylococcus aureus (42) and stimulates macrophages for enhanced clearance of P. aeruginosa, Escherichia coli, K. pneumoniae, and Mycobacterium tuberculosis (20, 28, 40, 42, 60). The importance of SP-A in innate immunity of the lung was recently demonstrated in vivo, using mice with a targeted deletion of the SP-A gene (34, 37). These mice had impaired clearance of group B streptococci and P. aeruginosa (37, 38). The role of SP-D in host defense is less well studied in vivo; however, SP-D binds to many bacterial pathogens such as E. coli (36). Recent studies with the CCSP-IL-4 mouse (see below) demonstrated marked accumulation of SP-A or SP-D in alveolar airspaces (26), raising the possibility that host defense mediated by SP-A may be altered in this mouse model.
To ascertain the role of chronic airway inflammation in pulmonary infection by P. aeruginosa, we have used transgenic mice in which the murine IL-4 cDNA was selectively expressed in the conducting airway epithelium under the control of the Clara cell secretory protein (CCSP) promoter (CCSP-IL-4 transgenic mice) (47). Local, chronic overexpression of IL-4 in the mouse lung caused an age-dependent increase in airway inflammation associated with increased pulmonary macrophages, neutrophils, lymphocytes, and eosinophils. Epithelial cell hypertrophy, mucus-like cell metaplasia, and increased SP-A were noted in the lungs from CCSP-IL-4 mice (26, 47, 56). Because of the similarity of the pulmonary findings in the CCSP-IL-4 mice in clinical conditions with chronic pulmonary inflammation such as asthma and CF, we assessed whether CCSP-IL-4 mice were susceptible to bacterial infection. To study the effect of IL-4 on the clearance of P. aeruginosa, CCSP-IL-4 mice were infected i.t. with the bacteria. Clearance of P. aeruginosa from the mouse lungs was markedly enhanced by chronic or acute exposure to IL-4.
MATERIALS AND METHODS
Animals.
Mice were housed and studied under Institutional Animal Care and Use Committee-approved protocols and virus-free conditions in the animal facility at The Children’s Hospital Research Foundation, Cincinnati, Ohio. The generation of CCSP-IL-4 mice was described previously (26, 57). Transgenic mice contain a construct in which the rat CCSP promoter directs expression of the murine IL-4 cDNA. Two founder CCSP-IL-4 transgenic mice (founder line 29) were crossed into FVBN mice (Charles River Laboratories, Wilmington, Mass.) and bred to produce homozygous transgenic mice, which were identified by PCR analysis of tail DNA as described previously (26). Five-week-old, sixth- or seventh-generation, homozygous CCSP-IL-4 mice in the FVBN background and age-matched FVBN mice (Harlan Sprague Dawley Inc., Indianapolis, Ind.) were used in all experiments. Wild-type mice were treated intranasally (i.n.) with recombinant murine IL-4 (R&D Systems, Minneapolis, Minn.) at 1- and 0.5-μg doses, given 16 and 1 h prior to P. aeruginosa administration.
Intratracheal administration of P. aeruginosa.
A mucoid P. aeruginosa strain was obtained from a clinical isolate, kindly provided by J. R. Wright, Duke University, Durham, N.C. Bacteria were suspended in sterile phosphate-buffered saline (PBS) with 20% glycerol (Sigma Chemical Co., St. Louis, Mo.), and aliquots were frozen at −80°C. To minimize variability related to bacterial culture, aliquots were taken from the same initial stock for the experiments. Prior to each experiment, bacteria were plated overnight on 2× yeast-tryptone (YT) agar; single colonies were then inoculated in 4 ml of 2× YT broth and grown in a shaker incubator overnight at 37°C. The broth was centrifuged at 1,200 × g for 10 min at 4°C; the bacteria were then washed once with sterile PBS and resuspended in 4 to 8 ml of sterile PBS. The concentration of bacteria was determined by spectrophotometry. P. aeruginosa (5 × 107 CFU) was administered i.t. to CCSP-IL-4 transgenic mice and wild-type mice after administration of IL-4 i.n.
Mice were anesthetized with isofluorane, and an anterior midline incision was used to expose the trachea. A tuberculin syringe with a 30-gauge needle was used to administer 100 μl of the bacteria into the trachea. The incision was closed with a drop of Nexaband. Control mice were injected with nonpyrogenic PBS.
Bacterial clearance.
Animals were sacrificed 6 and 24 h after infection with a lethal intraperitoneal injection of sodium pentobarbital. The abdomen was opened by a midline incision, and the animals were exsanguinated by transection of the inferior vena cava to reduce pulmonary hemorrhage. The lung and spleen were removed, weighed, and separately homogenized in 2 ml of sterile PBS. Serial dilutions of the homogenates were plated on 2× YT agar plates to quantitate bacteria.
BAL.
Animals were sacrificed as described above, and the lungs were lavaged three times with 1-ml aliquots of sterile PBS. The recovered bronchoalveolar lavage (BAL) fluid was pooled, and the volume was measured. Numbers of viable cells were assessed by trypan blue (Gibco BRL) exclusion, using a hemocytometer. Samples were centrifuged, and differential cell counts were performed on the cytospin preparations after staining with Diff-Quik (American Scientific Products, McGaw Park, Ill.).
MPO assay.
Neutrophil accumulation in the lung was quantitated by measuring myeloperoxidase (MPO) activity in lung homogenates 6 h after bacterial infection (54). Lungs were harvested, weighed, and homogenized in 3 ml of homogenate buffer (100 mM sodium acetate [pH 6.0], 20 mM EDTA [pH 7.0], 1% hexadecyltrimethylammonium bromide). Lung homogenates were sonicated for 15 s and then centrifuged at 10,000 × g for 15 min at 4°C. The supernatants were diluted 1:10 in the homogenate buffer, samples were pipetted as duplicates into 96-well microtiter plates (Falcon, Franklin Lakes, N.J.) and then mixed with an equal volume of assay buffer (1 mM hydrogen peroxide, 1% hexadecyltrimethylammonium bromide, 3.2 mM 3,3′5,5′-tetramethylbenzidine), and the plate was read at 650 nm over a period of 4 min.
Analysis of IL-4, TNF-α, and IL-1β.
Lung homogenates were stored at −20°C prior to use. On the day of the assay, homogenates were thawed and centrifuged at 1,200 × g to remove cell debris. Levels of IL-4, TNF-α, and IL-1β were quantitated in diluted (1:2 to 1:10) samples by using quantitative murine sandwich enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems) as described by the manufacturer. Levels of IL-4 were measured by using an ELISA kit from Endogen (Woburn, Mass.) as prescribed by the manufacturer.
Analysis of surfactant proteins.
Western blot analysis for SP-A and SP-D was performed on lung homogenates as described previously (37). Briefly, lungs were homogenized in 5 ml of sucrose buffer containing protease inhibitors. The homogenate was centrifuged at 250 × g for 10 min at 2°C, and the supernatant was centrifuged at 120,000 × g for 18 h at 4°C. Resulting pellets were resuspended in 300 μl of buffer, and 15 μl was loaded on sodium dodecyl sulfate–10 to 27% polyacrylamide gradient gels. After separation, proteins were transblotted to polyvinylidene difluoride membranes (Bio-Rad, Hercules, Calif.) and blocked with 5% bovine serum albumin in Tris-buffered saline (25 mM Tris [pH 7.6], 0.15 M NaCl, 0.1% Tween 20). The membranes were incubated with guinea pig anti-rat SP-A (29) or rabbit anti-rat SP-D antibodies (kindly provided by E. Crouch, Washington University, St. Louis, Mo.). Proteins were visualized by enhanced chemiluminescence detection (Amersham, Arlington Heights, Ill.) after incubation with appropriate horseradish peroxidase-conjugated secondary antibodies (Calbiochem, San Diego, Calif.). Immunoreactive SP-A and SP-D protein bands were identified by exposing the membranes to XAR film (Eastman Kodak Co., Rochester, N.Y.).
Statistics.
Statistical analyses were performed by natural log transformation of the data, as the distribution of variables, bacterial counts, number of neutrophils, MPO activity, and SP-A, TNF-α, and IL-1β concentrations were not normally distributed. Analysis of variance (ANOVA) and Student’s t test were performed to assess differences between groups. P values of <0.05 were considered significant. Values reported are means ± standard errors of the means (SEM).
RESULTS
Rapid clearance of P. aeruginosa from lungs of CCSP-IL-4 transgenic mice.
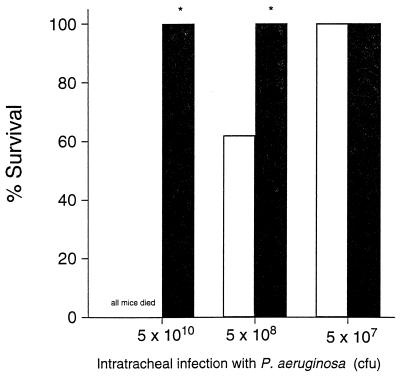
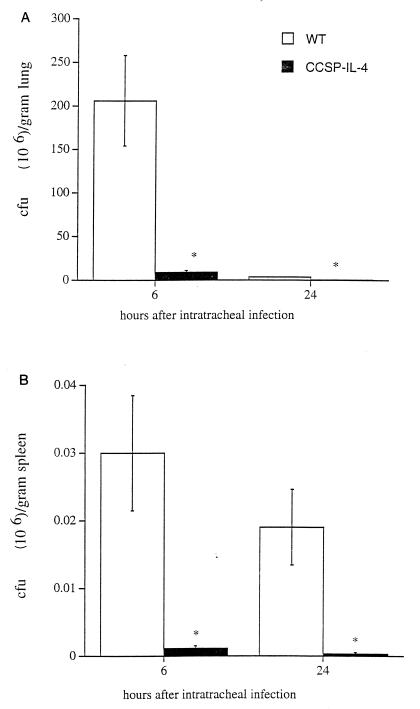
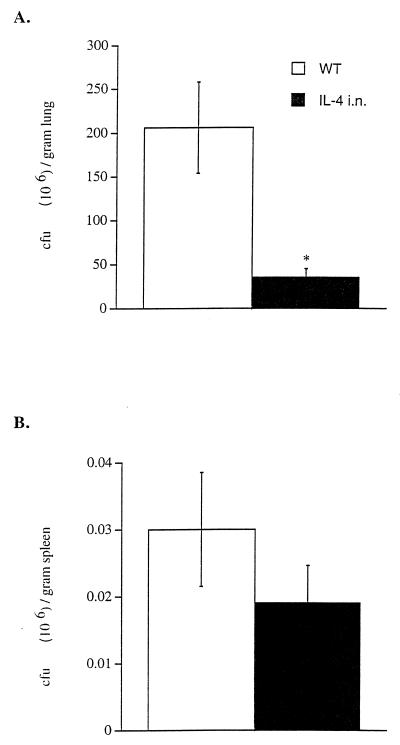
All wild-type mice died 48 h after i.t. infection with 5 × 1010 CFU of P. aeruginosa, while all CCSP-IL-4 mice survived (Fig. 1). Survival of CCSP-IL-4 mice infected i.t. with 5 × 108 CFU of P. aeruginosa was significantly greater than in the control group (Fig. 1). All CCSP-IL-4 mice had completely cleared the inoculated bacteria 48 h later (data not shown). For subsequent experiments, mice were infected i.t. with a sublethal dose of 5 × 107 CFU of P. aeruginosa. At this dose, all wild-type and CCSP-IL-4 mice survived the bacterial infection. Six hours after infection with 5 × 107 CFU, bacteria proliferated in lungs of wild-type mice, whereas bacteria were rapidly cleared from CCSP-IL-4 mouse lungs (Fig. 2A). Twenty-four hours after the administration of P. aeruginosa, both wild-type and CCSP-IL-4 mice had cleared most bacteria; however, more efficient bacterial clearance was noted in CCSP-IL-4 mouse lungs. Inconsistent systemic spread of P. aeruginosa was seen, as four of eight wild-type and two of eight CCSP-IL-4 mouse spleen homogenates contained bacteria at 6 and 24 h postinfection, and bacterial counts were significantly lower in CCSP-IL-4 mice than in wild-type mice (Fig. 2B).
FIG. 1.
Increased survival of CCSP-IL-4 mice after infection with P. aeruginosa. Survival was determined 48 h after wild-type (open bars) and CCSP-IL-4 (closed bars) mice were infected i.t. with P. aeruginosa. Data represent eight mice per group. ∗, P < 0.05 compared to wild-type infected mice, as assessed by ANOVA.
FIG. 2.
Enhanced clearance of P. aeruginosa in CCSP-IL-4 mouse lungs. P. aeruginosa counts (CFU) were determined by quantitative cultures of lung and spleen homogenates harvested 6 and 24 h after administration of 5 × 107 CFU of P. aeruginosa in wild-type (WT) and CCSP-IL-4 mice. (A) Bacterial CFU numbers were significantly higher in wild-type mouse lung homogenates than in those of CCSP-IL-4 transgenic mice at 6 and 24 h postinfection. (B) Bacteria were detected in wild-type and CCSP-IL-4 mouse spleens. Data represent means ± SEM for eight mice per group. ∗, P < 0.05 compared to wild-type infected mice, as assessed by ANOVA.
Neutrophilic infiltration after P. aeruginosa infection.
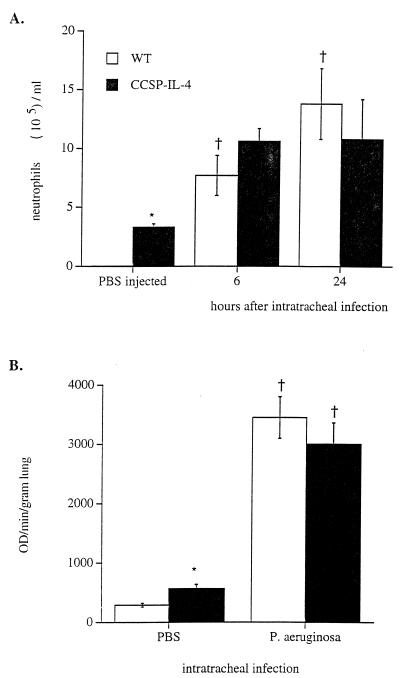
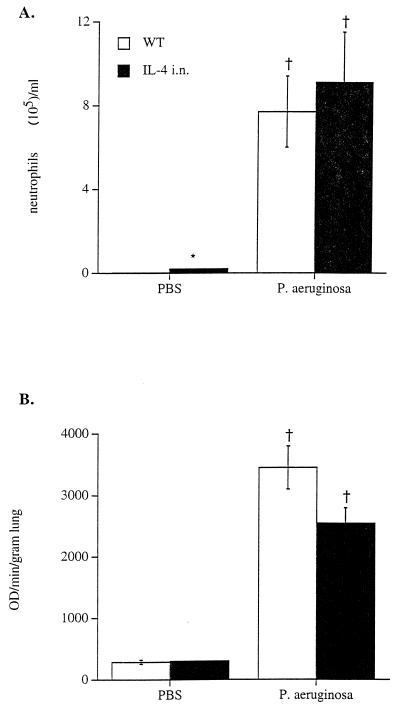
As described previously, increased numbers of macrophages, neutrophils, and lymphocytes were observed in lungs from CCSP-IL-4 transgenic mice (26). The numbers of neutrophils in BAL fluid from CCSP-IL-4 and wild-type mice increased after i.t. administration of P. aeruginosa (Fig. 3A). Six and 24 h after administration of the bacteria, the numbers of neutrophils in BAL fluid were similar in CCSP-IL-4 and wild-type mice. In addition to neutrophils, macrophage numbers were also increased in both wild-type or CCSP-IL-4 mice after infection (data not shown).
FIG. 3.
Increased leukocytic infiltration and MPO activity after bacterial infection. Wild-type (WT) and CCSP-IL-4 mice were infected i.t. with 5 × 107 P. aeruginosa CFU. (A) Increased numbers of neutrophils were observed in BAL fluid of wild-type mice at 6 and 24 h after bacterial infection compared to PBS-treated control mice. The numbers of neutrophils were similar in BAL fluids from P. aeruginosa-infected CCSP-IL-4 transgenic mice, CCSP-IL-4 control mice, and wild-type infected mice. (B) MPO activity was significantly increased in both wild-type and CCSP-IL-4 mouse lung homogenates at 6 and 24 h after P. aeruginosa infection compared to PBS-treated control mice. Values are means ± SEM for approximately 8 mice per group. †, P < 0.05 compared to PBS-treated control mice; ∗, P < 0.05 compared to wild-type mice, as assessed by ANOVA.
Lung MPO activity was measured to estimate total neutrophil influx into the lung. Prior to bacterial administration, MPO activity was significantly greater in CCSP-IL-4 mice than in control mice (Fig. 3B). MPO activity was increased to similar levels in both wild-type and CCSP-IL-4 mice 6 h after administration of P. aeruginosa.
TNF-α and IL-1β concentrations in lung homogenates.
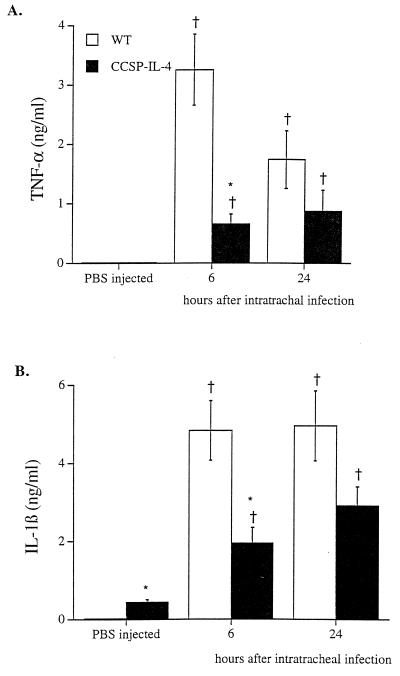
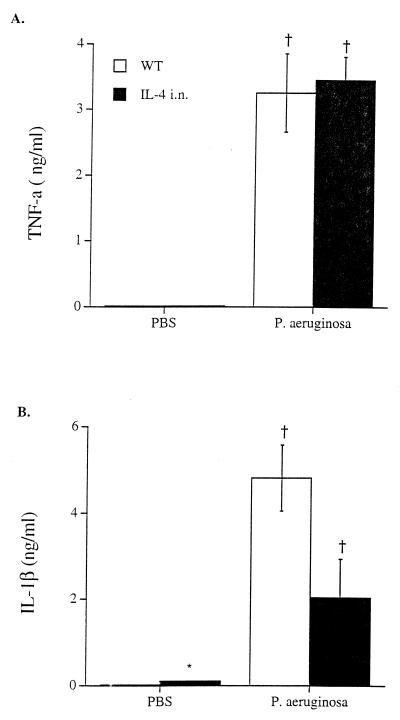
Infection with P. aeruginosa significantly increased concentrations of TNF-α and IL-1β in lung homogenates from wild-type and CCSP-IL-4 transgenic mice (Fig. 4). Following the administration of bacteria, concentrations of TNF-α and IL-1β were lower in lung homogenates from CCSP-IL-4 mice than in those from wild-type mice.
FIG. 4.
Effects of P. aeruginosa infection on lung TNF-α and IL-1β concentrations. TNF-α and IL-1β concentrations were assessed in lung homogenates from wild-type (WT) and CCSP-IL-4 mice. (A) Increased concentrations of TNF-α were observed in wild-type and CCSP-IL-4 mice after i.t. infection with P. aeruginosa compared to PBS-treated control mice. (B) Bacterial infection increased IL-1β concentrations in wild-type and CCSP-IL-4 mouse lung homogenates in comparison to PBS-injected control mice. Values are means ± SEM for six mice per group. †, P < 0.05 compared to PBS-treated control mice; ∗, P < 0.05 compared to wild-type mice, as assessed by ANOVA.
SP-A and SP-D.
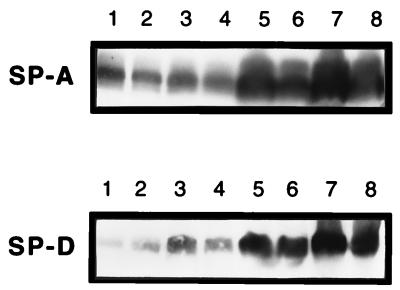
Concentrations of SP-A and SP-D were higher in lung homogenates from CCSP-IL-4 mice than in those from controls (Fig. 5). ELISA confirmed the increased concentration of SP-A in the lung homogenates from CCSP-IL-4 mice (Table 1). Twenty-four hours postinfection, SP-D concentrations were increased in lung homogenates of both wild-type and CCSP-IL-4 mice (Fig. 5). In contrast, there was a small but significant decrease in the concentration of SP-A in lung homogenates from both wild-type and CCSP-IL-4 mice after infection with P. aeruginosa.
FIG. 5.
Surfactant analysis. Lungs from wild-type and CCSP-IL-4 mice were homogenized 24 h after administration of PBS or 5 × 107 CFU of P. aeruginosa. SP-A and SP-D proteins were assessed by Western blot analysis. Lanes: 1 and 2, PBS-injected wild-type mice; 3 and 4, wild-type mice administered P. aeruginosa; 5 and 6, PBS-treated control CCSP-IL-4 mice; 7 and 8, P. aeruginosa-injected CCSP-IL-4 mice. Increased SP-A and SP-D concentrations were noted in PBS-treated or infected CCSP-IL-4 mice. Increased SP-D was observed in lung homogenates from wild-type mice infected with P. aeruginosa.
TABLE 1.
Bacterial infection decreased SP-A concentrations in lung homogenates
| Mice | SP-A concn (ng/ml) in lung homogenate from mice injected with P. aeruginosaa:
|
||
|---|---|---|---|
| PBS |
P. aeruginosa
|
||
| 6 h postinfection | 24 h postinfection | ||
| Wild type | 357 ± 19 | 267 ± 85 | 197 ± 9† |
| CCSP-IL-4 | 953 ± 73* | 682 ± 72* | 357 ± 11*† |
| Treated i.n. with IL-4 | 378 ± 148 | 263 ± 79 | ND |
Mean ± SEM for three mice per group. ND, not done; *, P < 0.05 compared to wild-type mice, as assessed by ANOVA; †, P < 0.05 compared to PBS-injected control mice, as assessed by ANOVA.
Acute effects of i.n. IL-4 administration on bacterial clearance.
To assess the role of transient increase in IL-4 levels in the mouse lung, recombinant murine IL-4 was administered in to the lungs 16 and 1 h prior to infection with P. aeruginosa. As with CCSP-IL-4 mice, increased numbers of neutrophils and macrophages were measured in BAL fluid after IL-4 treatment. Bacteria did not proliferate in lungs of mice treated with IL-4 compared to wild-type mice 6 h postinfection (Fig. 6A). Bacteria, albeit in low numbers, were detected in spleen homogenates from both wild-type mice and IL-4-treated mice, indicating systemic spread of P. aeruginosa (Fig. 6B).
FIG. 6.
Effect of acute IL-4 on bacterial clearance. IL-4 was administered i.n. to the lungs of wild-type mice 16 and 1 h prior to bacterial infection. Lungs and spleens from wild-type (WT) and wild-type mice administered IL-4 i.n. were harvested 6 h after infection with 5 × 107 CFU of P. aeruginosa. (A) Bacterial counts were significantly higher in wild-type mouse lung homogenates at 6 h postinfection than in lung homogenates from wild-type mice treated with IL-4. (B) Bacteria were detected in spleen homogenates from wild-type and IL-4-treated mice. Data are means ± SEM for eight mice per group. *, P < 0.05 compared to wild-type mice, as assessed by ANOVA.
Following infection with P. aeruginosa, the increases in neutrophils in BAL fluid from wild-type mice and IL-4-treated mice were similar (Fig. 7A). The increase in lung MPO activity was similar in both IL-4-treated and control mice (Fig. 7B).
FIG. 7.
Increased pulmonary leukocytic infiltration and MPO activity in mice treated with IL-4. Wild-type (WT) mice and wild-type mice administered IL-4 i.n. were sacrificed 6 h after i.t. administration of PBS or 5 × 107 CFU of P. aeruginosa. (A) Increased neutrophils were observed in BAL fluid from both control and IL-4-treated mice after P. aeruginosa infection. (B) Increased MPO activity was observed in lung homogenates from IL-4-treated and control mice after infection with bacteria. Data represent means ± SEM for eight mice per group. †, P < 0.05 compared to PBS control mice; ∗, P < 0.05 compared to wild-type mice, as assessed by ANOVA.
Concentrations of TNF-α and IL-1β in lung homogenates were increased following infection in both control and IL-4-treated mice (Fig. 8). IL-1β was significantly decreased in lung homogenates from mice treated with IL-4 compared to those from wild-type mice after infection with P. aeruginosa. Acute i.n. administration of IL-4 did not alter the SP-A and SP-D concentrations in lung homogenates before or after bacterial infection (Table 1 and data not shown).
FIG. 8.
TNF-α and IL-1β concentrations after infection. Lungs from wild-type (WT) mice and mice administered IL-4 i.n. were homogenized 6 h after i.t. injection with PBS or 5 × 107 CFU of P. aeruginosa. Concentrations of TNF-α (A) and IL-1β (B) were increased after P. aeruginosa infection. Values represent means ± SEM for eight per group. †, P < 0.05 compared to PBS control mice; ∗, P < 0.05 compared to wild-type mice, as assessed by ANOVA.
Endogenous IL-4 levels following infection.
The levels of IL-4 were measured in lung homogenates from wild-type mice, wild-type mice treated with IL-4 intranasally, and CCSP-IL-4 mice before or after infection with P. aeruginosa. IL-4 was undetectable in lungs from uninfected wild-type mice. After i.n. administration of IL-4, the levels of IL-4 recovered in lung homogenates varied considerably, from 750 to 12,965 pg/ml (n = 6), and were significantly higher than the levels of IL-4 in CCSP-IL-4 mice, which ranged from 271 to 348 pg/ml (n = 3). Six hours after infection, IL-4 was detected in only two of five wild-type mice (11.5 and 198.5 pg/ml of homogenate) and was detectable in only one of six wild-type mice (14 pg/ml of homogenate) 24 h after infection. The concentrations of IL-4 in lung homogenates of the IL-4-treated mice 6 h postinfection were reduced, and IL-4 was detected in five of six mice tested at concentrations ranging from 0 to 4,005 pg/ml. The concentration of IL-4 in lung homogenates of CCSP-IL-4 mice was 161 to 223 pg/ml 6 h after infection (n = 3) and decreased to 3 to 348 pg/ml (n = 7) after 24 h. Exogenous IL-4 added to lung homogenates from wild-type mice, both before and after infection with P. aeruginosa, was completely recovered.
DISCUSSION
This work demonstrates increased clearance of P. aeruginosa from the lungs of transgenic mice expressing IL-4 under the control of CCSP promoter and from mice acutely treated with IL-4 prior to bacterial infection. Increased bacterial clearance was associated with improved survival of the mice following a large i.t. inoculation of the bacteria. Infection was associated with intense leukocyte infiltration, increased TNF-α and IL-1β, and decreased SP-A concentrations in both wild-type and CCSP-IL-4 mice. IL-4-dependent protection from P. aeruginosa infection was not directly related to enhanced production of the cytokines TNF-α and IL-1β, leukocyte infiltration, or changes in SP-A content. IL-4 conferred surprising protection from Pseudomonas pneumonia, supporting the concept that the activation of host defenses by chronic or acute IL-4 treatment may be useful in prevention or therapy of pulmonary infection.
While both acute and chronic exposure of the lung to IL-4 enhances bacterial clearance of P. aeruginosa, the mechanisms by which IL-4 protects the lung from infection is unclear. In vitro studies indicate that IL-4 is a modulator of leukocyte function. IL-4 enhances the expression of complement receptors CR1, CR3, and CR4 on the surface of neutrophils, monocytes, and macrophages and increases complement-dependent phagocytosis by these cells (11, 12, 50). In addition, IL-4 is a potent stimulator of mannose receptor expression on macrophages (55). Both complement- and mannose-dependent clearance mechanisms have been reported for P. aeruginosa (53). IL-4 promotes the maturation, differentiation, proliferation, and survival of neutrophils and macrophages (6, 7, 11, 12, 21, 46). IL-4 reduces the production of superoxide radicals in macrophages by suppressing the expression of gp91-phox, the heavy subunit of NADPH oxidase, and reduces the production of nitric oxide by macrophages (2, 27, 44, 62). Although IL-4 exerts an antagonistic effect on nitric oxide production by lipopolysaccharide or TNF-α-stimulated macrophages, IL-4 synergizes with TNF-α to maintain prolonged expression of inducible nitric oxide synthase in airway epithelial cells (23, 27, 44). Consistent with our findings, IL-4 suppresses the secretion of the inflammatory cytokines IL-1β and TNF-α by lipopolysaccharide-stimulated macrophages (16). In CCSP-IL-4 mice in the present study, the levels of IL-1β and TNF-α were increased but were lower than in wild-type mice (Fig. 5). Intranasally administered IL-4 did not influence the levels of TNF-α after infection compared to controls but was associated with decreased IL-1β. The difference in effect between acute and chronic exposure on TNF-α production may be related to the levels or duration of IL-4 exposure prior to infection. IL-4 and IL-10 share some anti-inflammatory properties on macrophages (11, 44, 46), and IL-4 has been reported to modulate the synthesis of IL-10 (29). More recently, it was shown that pretreatment of mice with IL-10 led to decreased lung injury and increased survival of mice after i.t. infection with PA103, a cytotoxic strain of P. aeruginosa. Infection with PA103 led to significantly increased expression of IL-4, IL-10, IL-6, and TNF-α, suggesting that the balance of inflammatory and proinflammatory cytokines is a critical factor in determining the outcome of lung host defense against bacterial pneumonia (51).
Surfactant proteins play an important role in host defense against bacterial pathogens. SP-A and SP-D stimulate macrophage chemotaxis and enhance binding of bacteria to macrophages (57, 61). SP-A gene-deficient mice are susceptible to bacterial infection with group B streptococci and fail to efficiently clear P. aeruginosa after i.t. administration (37, 38). Thus, the increased concentration of SP-A and SP-D in BAL fluid from CCSP-IL-4 mice may have contributed to the observed increased bacterial clearance. In both wild-type and CCSP-IL-4 mice, SP-A concentrations decreased after infection with P. aeruginosa, consistent with previous studies in adult humans with bacterial pneumonia (4, 39). In contrast, SP-D concentrations increased in lungs of both wild-type and CCSP-IL-4 mice 24 h after infection with P. aeruginosa. Whether this increased SP-D contributes to bacterial clearance is unknown. However, the finding that SP-A and SP-D concentrations were unchanged from those in mice treated acutely with IL-4 suggests that protection was also conferred independently of SP-A and SP-D.
P. aeruginosa is the principal cause of morbidity and mortality in patients with CF (5). P. aeruginosa presents the host with numerous immunoevasive activities that hamper its clearance and exacerbate the inflammatory response, with deleterious effects on the host’s tissue (5, 31). The biochemical events that lead to bacterial invasion and colonization in the CF lung are not known. However, recent studies indicate that the onset of the inflammatory response in children with CF occurs prior to bacterial colonization (31). The importance of T-cell-derived cytokines was deduced from studies in a rat model of P. aeruginosa infection, where it was reported that systemic or mucosal immunization with killed bacteria led to successful clearance of a lethal i.t. dose of P. aeruginosa in association with a 25-fold increase in alveolar neutrophil numbers (14, 15).
In summary, this study demonstrates a role of IL-4 in pulmonary clearance of P. aeruginosa in vivo. Inflammation, necessary for the effective clearance of bacteria, was greater with chronic and acute exposure of the lung to IL-4. Exogenous administration of IL-4 enhanced clearance of P. aeruginosa from wild-type mice and therefore may represent a strategy to prevent or treat pulmonary infections.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grant HL 51832 and the Cystic Fibrosis Foundation.
We thank Joseph Kitzmiller for help with the MPO assay and P. Gartside for assistance with statistical analysis.
REFERENCES
- 1.Amura C R, Fontan P A, Sanjuan N, Sordelli D O. The effect of treatment with interleukin-1 and tumor necrosis factor on Pseudomonas aeruginosa lung infection in a granulocytopenic mouse model. Clin Immunol Immunopathol. 1994;73:261–266. doi: 10.1006/clin.1994.1196. [DOI] [PubMed] [Google Scholar]
- 2.Andersson A, Grunewald S M, Duschl A, DiSanto J P. Mouse macrophage development in the absence of the common γ chain: defining receptor complexes responsible for IL-4 and IL-13 signalling. Eur J Immunol. 1997;27:1762–1768. doi: 10.1002/eji.1830270725. [DOI] [PubMed] [Google Scholar]
- 3.Banchereau J, Rybak M E. Interleukin-4. In: Thomson A, editor. The cytokine handbook. San Diego, Calif: Academic Press; 1995. pp. 99–126. [Google Scholar]
- 4.Baughman R P, Sternberg R I, Hull W, Buchsbaum J A, Whitsett J A. Decreased surfactant protein A in patients with bacterial pneumonia. Am Rev Respir Dis. 1993;147:653–657. doi: 10.1164/ajrccm/147.3.653. [DOI] [PubMed] [Google Scholar]
- 5.Berger M. Inflammation in the lung in cystic fibrosis. Clin Rev Allergy. 1991;9:119–142. doi: 10.1007/978-1-4612-0475-6_8. [DOI] [PubMed] [Google Scholar]
- 6.Bober L A, Waters T A, Pugliese-Sivo C C, Sullivan L M, Narula S K. IL-4 induces neutrophilic maturation of HL-60 cells and activation of human peripheral blood neutrophils. Clin Exp Immunol. 1995;99:129–136. doi: 10.1111/j.1365-2249.1995.tb03483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boey H, Rosenbaum R, Castracane J, Borish L. Interleukin-4 is a neutrophil activator. J Allergy Clin Immunol. 1989;83:978–984. doi: 10.1016/0091-6749(89)90115-2. [DOI] [PubMed] [Google Scholar]
- 8.Bonfield T L, Panuska J R, Konstan M W, Hilliard K A, Hilliard J B, Ghnaim H, Berger M. Inflammatory cytokines in cystic fibrosis lungs. Am J Respir Crit Care Med. 1995;152:2111–2118. doi: 10.1164/ajrccm.152.6.8520783. [DOI] [PubMed] [Google Scholar]
- 9.Broug-Holub E, Toews G B, van Iwaarden J F, Strieter R M, Kunkel S L, Paine R, Standiford T J. Alveolar macrophages are required for protective pulmonary defenses in murine Klebsiella pneumonia: elimination of alveolar macrophages increases neutrophil recruitment but decreases bacterial clearance and survival. Infect Immun. 1997;65:1139–1146. doi: 10.1128/iai.65.4.1139-1146.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buret A, Dunkley M L, Pang G, Clancy R L, Cripps A W. Pulmonary immunity to Pseudomonas aeruginosa in intestinally immunized rats: role of alveolar macrophages, tumor necrosis factor alpha, and interleukin-1α. Infect Immun. 1994;62:5335–5343. doi: 10.1128/iai.62.12.5335-5343.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Capsoni F, Minonzio F, Ongari A A, Carbonelli V, Galli A, Zanussi C. IL-10 up-regulates human monocyte phagocytosis in the presence of IL-4 and IFNγ. J Leukocyte Biol. 1995;58:351–358. doi: 10.1002/jlb.58.3.351. [DOI] [PubMed] [Google Scholar]
- 12.Chen B D M, Sensenbrenner L, Fan K, Run Q. Murine recombinant IL-4 is a bifunctional regulator of macrophage growth induced by colony-stimulating factors. J Immunol. 1992;148:753–759. [PubMed] [Google Scholar]
- 13.Coyle A J, Le Gros G, Bertnand C, Tsuyuki S, Heusser C H, Kopf M, Anderson G P. IL-4 is required for the induction of lung Th2 mucosal immunity. Am J Respir Cell Mol Biol. 1995;13:54–59. doi: 10.1165/ajrcmb.13.1.7598937. [DOI] [PubMed] [Google Scholar]
- 14.Cripps A W, Dunkley M L, Clancy R L, Kyd J. Pulmonary immunity to Pseudomonas aeruginosa. Immunol Cell Biol. 1995;73:418–424. doi: 10.1038/icb.1995.65. [DOI] [PubMed] [Google Scholar]
- 15.Dunkley M L, Pabst R, Cripps A W. An important role for intestinally derived T cells in respiratory defense. Immunol Today. 1995;16:231–236. doi: 10.1016/0167-5699(95)80165-0. [DOI] [PubMed] [Google Scholar]
- 16.Essner R, Rhoades K, McBride W H, Morton D L, Economou J S. IL-4 downregulates IL-1 and TNF gene expression in human monocytes. J Immunol. 1989;142:3857–3861. [PubMed] [Google Scholar]
- 17.Finkelman F D, Holmes J, Katona I M, Urban J F, Beckmann M P, Schooley K A, Coffman R L, Mosmann T R, Paul W E. Lymphokine control of in vivo immunoglobulin isotype selection. Annu Rev Immunol. 1990;8:303–333. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- 18.Garlepp M J, Rose A H, Dench J E, Robinson B W. Clonal analysis of lung and blood T cells in patients with sarcoidosis. Thorax. 1994;49:577–585. doi: 10.1136/thx.49.6.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garlisi C G, Falcone A, Kung T T, Stelts D, Pennline K J, Beavis A J, Smith S R, Egan R W, Umland S P. T cells are necessary for Th2 cytokine production and eosinophil accumulation in airways of antigen-challenged allergic mice. Clin Immunol Immunopathol. 1995;75:75–83. doi: 10.1006/clin.1995.1055. [DOI] [PubMed] [Google Scholar]
- 20.Gaynor C D, McCormack F X, Voelker D R, McGowan S E, Schlesinger L S. Pulmonary surfactant protein A mediates enhanced phagocytosis of Mycobacterium tuberculosis by a direct interaction with human macrophages. J Immunol. 1995;155:5343–5351. [PubMed] [Google Scholar]
- 21.Girard D, Paquin D, Beaulieu A D. Responsiveness of human neutrophils to interleukin-4: induction of cytoskeletal rearrangements, de novo protein synthesis and delay of apoptosis. Biochem J. 1997;325:147–153. doi: 10.1042/bj3250147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gosselin D, DeSanctis J, Boule M, Skamene E, Matouk C, Radzioch D. Role of tumor necrosis factor alpha in innate resistance to mouse pulmonary infection with Pseudomonas aeruginosa. Infect Immun. 1995;63:3272–3278. doi: 10.1128/iai.63.9.3272-3278.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo F H, Uetani K, Haque S J, Williams B R G, Dweik R A, Thunnissen F B J M. Interferon γ and interleukin 4 stimulate expression of inducible nitric oxide synthase in human airway epithelium through synthesis of soluble mediators. J Clin Invest. 1997;100:829–838. doi: 10.1172/JCI119598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hashimoto S, Pittet J, Hong K, Folkesson H, Bagby G, Kobzik L, Frevert C, Watanbe K, Tsurufuji S, Weiner-Kronish J. Depletion of alveolar macrophages decreases neutrophil chemotaxis to Pseudomonas airspace infections. Am J Physiol. 1996;270:L819–L828. doi: 10.1152/ajplung.1996.270.5.L819. [DOI] [PubMed] [Google Scholar]
- 25.Hopken U E, Lu B, Gerard N P, Gerard C. The C5a chemoattractant receptor mediates mucosal defense to infection. Nature. 1996;383:86–89. doi: 10.1038/383086a0. [DOI] [PubMed] [Google Scholar]
- 26.Jain-Vora S, Wert S E, Temann U-A, Rankin J A, Whitsett J A. Interleukin-4 alters epithelial cell differentiation and surfactant homeostasis in the postnatal mouse lung. Am J Respir Cell Mol Biol. 1997;17:541–551. doi: 10.1165/ajrcmb.17.5.2883. [DOI] [PubMed] [Google Scholar]
- 27.Jungi T W, Brcic M, Sager H, Dobbelaere D A E, Furger A, Roditi I. Antagonistic effects of IL-4 and interferon γ on inducible nitric oxide synthase expression in bovine macrophages exposed to gram-positive bacteria. Clin Exp Immunol. 1997;109:431–438. doi: 10.1046/j.1365-2249.1997.4891384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kabha K, Schmegner J, Keisari Y, Parolis H, Schlepper-Schaefer J, Ofek I. SP-A enhances phagocytosis of Klebsiella by interaction with capsular polysaccharides and alveolar macrophages. Am J Physiol. 1997;272:L344–L352. doi: 10.1152/ajplung.1997.272.2.L344. [DOI] [PubMed] [Google Scholar]
- 29.Kambayashi T, Jacob C O, Stassmann G. IL-4 and IL-13 modulate IL-10 release in endotoxin-stimulated murine peritoneal mononuclear phagocytes. Cell Immunol. 1996;171:153–158. doi: 10.1006/cimm.1996.0186. [DOI] [PubMed] [Google Scholar]
- 30.Kay A B, Ying S, Varney V, Gaga M, Durham S R, Moqbel R, Wardlaw A J, Hamid Q. Messenger RNA expression of the cytokine gene cluster, interleukin-3 (IL-3), IL-4, IL-5 and granulocyte/macrophage colony-stimulating factor, in allergen-induced late-phase cutaneous reactions in atopic subjects. J Exp Med. 1991;173:775–778. doi: 10.1084/jem.173.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khan T Z, Wagener J S, Bost T, Martinez J, Accurso F J, Riches D W. Early pulmonary inflammation in infants with cystic fibrosis. Am J Respir Crit Care Med. 1995;151:1075–1082. doi: 10.1164/ajrccm/151.4.1075. [DOI] [PubMed] [Google Scholar]
- 32.King C L, Ottesen E A, Nutman T B. Cytokine regulation of antigen-driven immunoglobulin production in filarial parasite infections in humans. J Clin Invest. 1990;85:1810–1815. doi: 10.1172/JCI114639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ko Y H, Delannoy M, Pedersen P L. Cystic fibrosis, lung infections, and human tracheal antimicrobial peptide (hTAP) FEBS Lett. 1997;405:200–208. doi: 10.1016/s0014-5793(97)00189-0. [DOI] [PubMed] [Google Scholar]
- 34.Korfhagen T R, Bruno M D, Ross G F, Huelsman K M, Ikegami M, Jobe A H, Wert S E, Stripp B R, Morris R E, Glasser S W, Bachurski C J, Iwamoto H S, Whitsett J A. Altered surfactant function and structure in SP-A gene targeted mice. Proc Natl Acad Sci USA. 1996;93:9594–9599. doi: 10.1073/pnas.93.18.9594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kronberg G, Hansen M B, Svenson M, Fomsgaard A, Hoiby N, Bendtzen K. Cytokine in sputum and serum from patients with cystic fibrosis and chronic Pseudomonas aeruginosa infection as markers of destructive inflammation in the lungs. Pediatr Pulmonol. 1993;15:292–297. doi: 10.1002/ppul.1950150506. [DOI] [PubMed] [Google Scholar]
- 36.Kuan S-F, Rust K, Crouch E. Interactions of surfactant protein D with bacterial lipopolysaccharide. J Clin Invest. 1992;90:97–106. doi: 10.1172/JCI115861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.LeVine A M, Bruno M D, Huelsman K M, Ross G F, Whitsett J A, Korfhagen T R. Surfactant protein A-deficient mice are susceptible to group B streptococcal infection. J Immunol. 1997;158:4336–4340. [PubMed] [Google Scholar]
- 38.LeVine, A. M., K. E. Kurak, M. D. Bruno, J. M. Stark, J. A. Whitsett, and T. R. Korfhagen. Surfactant protein-A deficient mice are susceptible to Pseudomonas aeruginosa infection. Am. J. Respir. Cell Mol. Biol., in press. [DOI] [PubMed]
- 39.LeVine A M, Lotze A, Stanley S, Stroud C, O’Donnell R, Whitsett J, Pollack M M. Surfactant content in children with inflammatory lung disease. Crit Care Med. 1996;24:1062–1067. doi: 10.1097/00003246-199606000-00029. [DOI] [PubMed] [Google Scholar]
- 40.Manz-Keinke H, Plattner H, Schlepper-Schafer J. Lung surfactant protein A (SP-A) enhances serum-independent phagocytosis of bacteria by alveolar macrophages. Eur J Cell Biol. 1992;57:95–100. [PubMed] [Google Scholar]
- 41.McNeely T B, Coonrod J D. Aggregation and opsonization of type A but not type B Haemophilus influenzae by surfactant protein A. Am J Respir Cell Mol Biol. 1994;11:114–122. doi: 10.1165/ajrcmb.11.1.8018334. [DOI] [PubMed] [Google Scholar]
- 42.McNeely T B, Coonrod J D. Comparison of the opsonic activity of human surfactant protein A for Staphylococcus aureus and Streptococcus pneumoniae with rabbit and human macrophages. J Infect Dis. 1993;167:91–97. doi: 10.1093/infdis/167.1.91. [DOI] [PubMed] [Google Scholar]
- 43.Morissette C, Skamene E, Gervais F. Endobronchial inflammation following Pseudomonas aeruginosa infection in resistant and susceptible strains of mice. Infect Immun. 1995;63:1718–1724. doi: 10.1128/iai.63.5.1718-1724.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perreti M, Szabo C, Thiemmertmann C. Effect of interleukin 4 and interleukin 10 on leukocyte migration and nitric oxide production in the mouse. Br J Pharmacol. 1995;116:2251–2257. doi: 10.1111/j.1476-5381.1995.tb15061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pison U, Max M, Neuendank A, Weibbach S, Pietschmann S. Host defense capacities of pulmonary surfactant: evidence for ‘non-surfactant’ functions of the surfactant system. Eur J Clin Invest. 1994;24:586–599. doi: 10.1111/j.1365-2362.1994.tb01110.x. [DOI] [PubMed] [Google Scholar]
- 46.Poe J C, Wagner D H, Miller R W, Stout R D, Suttles J. IL-4 and IL-10 modulation of CD40-mediated signalling of monocyte IL-1b synthesis and rescue from apoptosis. J Immunol. 1997;159:846–852. [PubMed] [Google Scholar]
- 47.Rankin J A, Picarella D E, Geba G, Temann U-A, Prasad B, DiCosmo B, Tarallo A, Stripp B, Whitsett J A, Flavell R A. Phenotypic and physiologic characterization of transgenic mice expressing IL-4 in the lung. Proc Natl Acad Sci USA. 1996;93:7821–7825. doi: 10.1073/pnas.93.15.7821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rivas D, Mozo L, Zamorano J, Gayo A, Torre-Alonso J C, Rodriguez A, Guiterrez C. Upregulated expression of IL-4 receptors and increased levels of IL-4 in rheumatoid arthritis patients. J Autoimmun. 1995;8:587–600. doi: 10.1016/0896-8411(95)90010-1. [DOI] [PubMed] [Google Scholar]
- 49.Robinson D S, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bantley A M, Corrigan C, Durham S R, Kay A B. Predominant Th2-like bronchoalveolar T lymphocyte population in atopic asthma. N Engl J Med. 1992;326:298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- 50.Sampson L L, Heuser J, Brown E J. Cytokine regulation of complement receptor-mediated ingestion by mouse peritoneal macrophages. GM-CSF and IL-4 activate phagocytosis by a common mechanism requiring autostimulation by IFN-β. J Immunol. 1991;146:1005–1013. [PubMed] [Google Scholar]
- 51.Sawa T, Corry D B, Gropper M A, Ohara M, Kurahashi K, Wiener-Kronish J P. IL-10 improves lung injury and survival in Pseudomonas aeruginosa pneumonia. J Immunol. 1997;159:2858–2866. [PubMed] [Google Scholar]
- 52.Smith J J, Travis S M, Greenberg E P, Welsh M J. Cystic fibrosis airway epithelia fail to kill bacteria because of abnormal airway surface fluid. Cell. 1996;85:229–236. doi: 10.1016/s0092-8674(00)81099-5. [DOI] [PubMed] [Google Scholar]
- 53.Speert D P, Wright S D, Silverstein S C, Mah B. Functional characterization of macrophage receptors for in vitro phagocytosis of unopsonized Pseudomonas aeruginosa. J Clin Invest. 1988;82:872–879. doi: 10.1172/JCI113692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stark J, van Egmond A, Zimmerman J, Carabell S, Tosi M. Detection of enhanced neutrophil adhesion to parainfluenza-infected airway epithelial cells using a modified myeloperoxidase assay in a microtiter format. J Virol Methods. 1992;40:225–242. doi: 10.1016/0166-0934(92)90071-k. [DOI] [PubMed] [Google Scholar]
- 55.Stein M, Keshav S, Harris N, Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunology macrophage activation. J Exp Med. 1992;176:287–292. doi: 10.1084/jem.176.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Temann U-A, Prasad B, Gallap M W, Basbaum C, Ho S B, Flavell R A, Rankin J A. A novel role for murine IL-4 in vivo: induction of MUC5AC gene expression and mucin hypersecretion. Am J Respir Cell Mol Biol. 1997;16:471–478. doi: 10.1165/ajrcmb.16.4.9115759. [DOI] [PubMed] [Google Scholar]
- 57.Tino M J, Wright J R. Surfactant protein A stimulates phagocytosis of specific pulmonary pathogens by alveolar macrophages. Am J Physiol. 1996;270:L677–L688. doi: 10.1152/ajplung.1996.270.4.L677. [DOI] [PubMed] [Google Scholar]
- 58.Urban J F, Madden K B, Svetic A, Cheever A, Trotta P P, Gause W C, Katona I M, Finkelman F D. The importance of Th2 cytokines in protective immunity to nematodes. Immunol Rev. 1992;127:205–220. doi: 10.1111/j.1600-065x.1992.tb01415.x. [DOI] [PubMed] [Google Scholar]
- 59.Wallace W A, Ramage E A, Lamb D, Howie S E. A type 2 (Th2-like) pattern of immune response predominates in the pulmonary interstitium of patients with cryptogenic fibrosing alveolitis (CFA) Clin Exp Immunol. 1995;101:436–441. doi: 10.1111/j.1365-2249.1995.tb03131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weikert L F, Edwards E, Chroneos Z C, Hager C, Hoffman L, Shepherd V L. SP-A enhances uptake of bacillus Calmette-Guerin by macrophages through a specific SP-A receptor. Am J Physiol. 1997;272:L989–L995. doi: 10.1152/ajplung.1997.272.5.L989. [DOI] [PubMed] [Google Scholar]
- 61.Wright J R, Youmans D C. Pulmonary surfactant protein A stimulates chemotaxis of alveolar macrophages. Am J Physiol. 1993;264:L338–L344. doi: 10.1152/ajplung.1993.264.4.L338. [DOI] [PubMed] [Google Scholar]
- 62.Zhou Y, Lin G, Murtaugh M P. Interleukin-4 suppresses the expression of macrophage NADPH oxidase heavy chain subunit (gp91-phox) Biochim Biophys Acta. 1994;1265:40–48. doi: 10.1016/0167-4889(94)00207-u. [DOI] [PubMed] [Google Scholar]