Abstract
Introduction
Kidney disease is a well-known extraintestinal manifestation (EIM) associated with inflammatory bowel disease (IBD), with a variety of underlying etiologies. However, little is known about the overall outcomes and predictors.
Methods
This is a retrospective, observational cohort study. Patients with IBD in whom a native kidney biopsy was performed at Mayo Clinic (Rochester, MN) between 1994 and 2022, were included. Demographic, clinical, and histologic characteristics of prognostic interest were collected. The main outcomes were kidney failure, disease remission, kidney function changes at last follow-up, and death.
Results
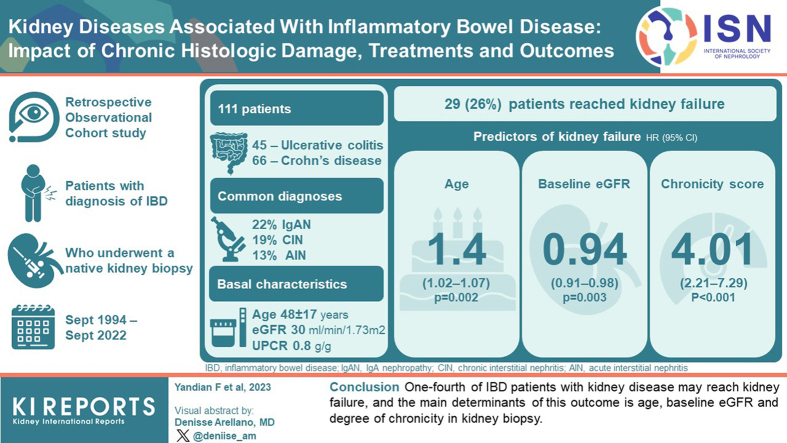
From a total cohort of 318 patients, we selected a study group of 111 patients followed-up with at our institution (45 ulcerative colitis [UC] and 66 Crohn’s disease [CD]), with a mean age of 48 ± 17 years (40% females). IgA nephropathy (IgAN), chronic interstitial nephritis (CIN), and acute interstitial nephritis (AIN) were the most common diagnoses (22%, 19%, 13%, respectively). Median estimated glomerular filtration rate (eGFR) at presentation was 30 ml/min per 1.73 m2 (interquartile range [IQR]: 17–54) and urinary protein-to-creatinine ratio [UPCR] 0.8 g/g (0.3–3.4), without differences between IBD types. During a median follow-up of 59 months (12–109), 29 patients (26%) reached kidney failure. By multivariable analysis, the main predictors of kidney failure were age (hazard ratio [HR]: 1.04; P = 0.002), baseline eGFR (HR: 0.94; P = 0.003) and histologic chronicity score (HR: 4.01; P < 0.001). Therapeutic management varied according to underlying etiology. Global survival (kidney failure + death) was significantly better in patients who achieved complete or partial remission, or stabilization or improvement of kidney function.
Conclusion
One-fourth of patients with IBD with kidney disease may reach kidney failure, and the main determinants of this outcome is age, baseline eGFR, and degree of chronicity in kidney biopsy.
Keywords: IgA nephropathy, inflammatory bowel disease, interstitial nephritis, kidney failure, total chronicity score
Graphical abstract
IBD is a chronic inflammatory disorder of the gastrointestinal tract, and the main types are CD and UC.1, 2, 3 Although the exact underlying pathogenic mechanisms remain largely unknown, evidence suggests that the disease may be primarily due to dysregulation of intestinal T-cells in genetically susceptible individuals, although idiosyncratic reactions to drugs, other environmental factors, and gut microbiota dysbiosis may also contribute to the disease.1,4, 5, 6
EIMs can occur in up to 5% to 50% of individuals with IBD, potentially affecting any organ system, including the musculoskeletal system, skin, kidneys, liver, lungs, and eyes.7, 8, 9 EIMs can be classified into those reactive manifestations in the setting of inflammatory bowel activity (e.g., arthritis, erythema nodosum, or uveitis), and those autoimmune diseases independent of bowel activity (e.g., ankylosing spondylitis, primary biliary cirrhosis, or autoimmune thyroid disease), which largely reflect an increased susceptibility to immune system dysregulation.7, 8, 9, 10
Kidney involvement has been described in 4% to 23% of patients with IBD and may be caused by several factors, including the primary systemic disease itself, secondary complications due to persistent chronic inflammation, malnutrition, dehydration episodes associated with intestinal losses, or drug-related side effects.5,9,11,12 The most common causes of kidney injury in patients with IBD include urolithiasis, fistulas, and ureteral obstruction.12, 13, 14 However, the spectrum of kidney diseases associated with IBD may be broader, including glomerular or interstitial diseases.11,12 The most common diagnosis described in a previous case series of 83 consecutive patients with IBD15 included, IgAN, tubulointerstitial nephritis, arterio-nephrosclerosis, acute tubular necrosis, or podocytopathies with minimal change or focal segmental glomerulosclerosis lesions, among others. Kidney disease associated with IBD has a significant impact on patients’ quality of life and is associated with increased patient morbidity and even mortality.5,9,11,15 Despite this, kidney outcomes of these patients and their main predictors have scarcely been evaluated.
Therefore, the aim of this study was to comprehensively analyze the major etiologies of kidney disease, treatment strategies, and outcomes of a large cohort of patients with IBD who underwent native kidney biopsy during the past 28 years at Mayo Clinic, as a referral center for IBD.
Methods
Study Patients
Adult patients previously diagnosed with IBD who underwent a native kidney biopsy at Mayo Clinic (Rochester, Minnesota) between September 1994 and September 2022, were enrolled. Diagnosis of IBD, either UC or CD, was confirmed by the treating gastroenterologists based on clinical, endoscopic, and histopathologic findings of colorectal specimens, before kidney disease diagnosis. Kidney transplant patients, those with insufficient kidney tissue to establish an accurate diagnosis, or those in which the kidney biopsy report was unavailable, were excluded. For the evaluation of outcomes, only patients with available follow-up were included in the analysis.
Given the retrospective nature of the study and the complete anonymization of all patient data, informed consent was waived for individual patients. The study was conducted in accordance with the tenets of the Declaration of Helsinki and approved by the Institutional Review Board at Mayo Clinic.
Clinical, Laboratory, and Histopathologic Data
Baseline and follow-up data were compiled from medical records, and the main demographic, clinical, and biochemical parameters of prognostic interest were collected at baseline and after 6, 12, 18, 24, 36, 48, 60 months and/or last follow-up. All biochemical parameters were analyzed using routine laboratory methods.
All kidney biopsy specimens were evaluated according to a standardized protocol that included light microscopy (hematoxylin and eosin, Jones' silver methenamine, Masson's trichrome, and periodic acid-Schiff), immunofluorescence staining (IgG, IgA, IgM, C3, C1q, kappa, lambda, fibrinogen, and albumin), and electron microscopy. The degree of disease chronicity was assessed using a semiquantitative grading scale16 for glomerulosclerosis, tubular atrophy, interstitial fibrosis (as <10%, 10%–25%, 26%–50%, or >50%), and presence/absence of arteriosclerosis, extracted from the original biopsy report. Total chronicity score in kidney biopsy was then calculated for each patient as the sum of individual chronicity scores.16 Grades of chronic changes were as follows: minimal (score 0–1), mild (score 2–4), moderate (score 5–7), or severe (≥8).
Outcomes and Definitions
The main outcomes were the following: (i) kidney failure, defined as eGFR <15 ml/min per 1.73 m2 by the Chronic Kidney Disease – Epidemiology Collaboration equation,17 maintenance dialysis or preemptive kidney transplantation; (ii) disease remission in proteinuric kidney diseases, and stabilization or improvement of kidney function in those not characterized by the presence of proteinuria; and (iii) composite outcome of kidney failure or death.
For patients with an associated proteinuric glomerular disease, complete remission was defined as eGFR ≥60 ml/min per 1.73 m2 (or return to ±15% of baseline value if eGFR was <60), and UPCR <0.5 g/g. Partial remission was defined as a reduction of proteinuria >50% (or UPCR <3.5 g/g in those with nephrotic range proteinuria), plus stabilization (±25% of baseline eGFR) or improvement in kidney function. For kidney diseases not associated with a glomerular disease or proteinuria (e.g., acute tubular necrosis or CIN), only the changes in kidney function over time were evaluated (stabilization, improvement or decline of eGFR over follow-up time).
Baseline was defined as the time at which the kidney biopsy was performed, and follow-up period was defined as the interval of time elapsed between kidney biopsy and last outpatient visit or kidney failure. Hematuria was defined as ≥5 red blood cell per high-power field. Leukocyturia was defined as leukocyte esterase more than trace positive or higher on urinalysis. IgA vasculitis was defined as a vasculitis with IgA1-dominant immune deposits, affecting small vessels (predominantly capillaries, venules, or arterioles), with associated cutaneous, gastrointestinal, and/or articular involvement.
Statistical Analyses
This is a retrospective, observational cohort study. Descriptive statistics are presented as mean and SD, or median and IQR for continuous variables; and frequencies or percentages for categorical variables.
Comparisons of continuous variables between 2 groups were assessed by using the unpaired t test or the Mann-Whitney U test, where appropriate. χ2-square test, or Fisher’s exact test were used for categorical variables.
Cox proportional hazards regression models were used to analyze the main determinants of kidney failure, using a backward progressive conditional elimination process. Distributions of time to kidney failure were depicted by survival curves using the Kaplan-Meier method.
A P-value <0.05 was considered to be significant. All P-values are reported 2-sided. Analyses were performed using IBM SPSS Statistics 24.0 (IBM Corp. Armonk, NY) and GraphPad Prism version 7.00 (GraphPad Software, La Jolla, CA).
Results
Cohort Description
During the study period, data from 348 patients were retrieved, of whom 27 cases were excluded due to kidney transplantation, and 3 patients due to missing kidney biopsy report, leaving 318 patients (median age 54 years, IQR: 38–68), 156 (49%) with UC and 162 (51%) with CD. Of these, complete clinical follow-up data at Mayo were obtained in 111 patients (Supplementary Figure S1).
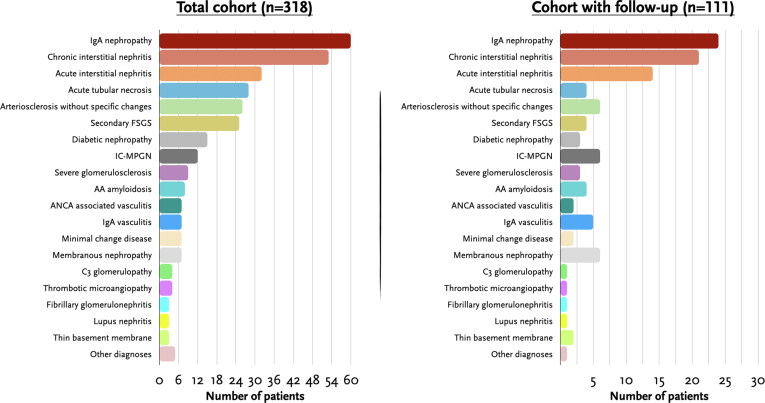
In Figure 1a and Supplementary Table S1, we summarize the major underlying etiologies of kidney disease. In the overall cohort (N = 318), 60 patients (19%) had IgAN, 53 (17%) had CIN, 32 (10%) had AIN, 26 patients (8%) with arteriosclerosis without significant glomerular or interstitial damage, 25 (8%) had secondary focal segmental glomerulosclerosis, and 15 (8%) had diabetic nephropathy. Of the 53 patients with CIN, 4 (8%) had granulomas; whereas of the 32 cases with AIN, granulomas were present in 13 (40%). Other less common kidney diagnoses included immune complex-mediated membranoproliferative glomerulonephritis (IC-MPGN) (12 patients, 4%), AA amyloidosis (8 cases, 3%), antineutrophil cytoplasmic antibody vasculitis (7 cases, 2%), IgA vasculitis (7 patients, 2%), minimal change disease (7 patients, 2%), and membranous nephropathy (7 patients, 2%), among others. The proportions were similar in the 111 patients with available clinical follow-up data at our institution (Figure 1b).
Figure 1.
Summary of main etiologies of kidney disease in the total cohort (N = 138), and the cohort of patients with follow-up at our institution (n = 111).
In Table 1, we display the baseline clinical characteristics of patients with available follow-up (n = 111), and according to type of IBD. Mean age was 48±17 years, and 44 (40%) were female. Eleven had concomitant type 2 diabetes mellitus (10%). Seventy patients (63%) had been treated with corticosteroids for the IBD, whereas 68 patients (61%) had received 5-aminosalicylilate agents (5-ASA). Fifteen of the 21 patients (71%) with CIN had been treated with 5-ASA agents, whereas 8 of the 14 with AIN (57%) had received 5-ASA. On the other hand, 6 of the 17 (35%) patients with granulomas had been treated with 5-ASA. Other less frequent treatments included azathioprine in 36 patients (32%), infliximab in 33 (30%), and adalimumab in 20 cases (18%). Three patients (21%) with AIN and 7 (33%) with CIN had been treated with azathioprine. Median eGFR at baseline was 30 ml/min per 1.73 m2 (IQR: 17–54), and UPCR 0.8 g/g (IQR: 0.3–3.4). Hematuria was a frequent finding, being present in 55 patients (59%). No significant differences in baseline comorbidities or clinical characteristics were observed between patients with UC or CD.
Table 1.
Clinical characteristics of study population, and by type of inflammatory bowel disease
| Characteristic | Total (N = 111) | Ulcerative colitis (n = 45) | Crohn’s disease (n = 66) | P value |
|---|---|---|---|---|
| Baseline | ||||
| Age, yr | 48 ± 17 | 47 ± 16 | 48 ± 18 | 0.84 |
| Sex, female (%) | 44 (40) | 18 (40) | 26 (39) | 0.94 |
| Hypertension, n (%) | 46 (41) | 18 (40) | 28 (42) | 0.79 |
| Diabetes mellitus, n (%) | 11 (10) | 2 (4) | 9 (14) | 0.11 |
| Previous treatments for IBD, n (%) | 0.43 | |||
| None | 12 (11) | 5 (11) | 7 (11) | |
| Corticosteroids | 70 (63) | 24 (53) | 46 (70) | |
| 5–ASA | 68 (61) | 30 (67) | 38 (58) | |
| Azathioprine | 36 (32) | 9 (20) | 27 (41) | |
| Vedolizumab | 5 (5) | 1 (2) | 4 (6) | |
| Infliximab | 33 (30) | 6 (13) | 27 (41) | |
| Certolizumab | 2 (2) | 0 (0) | 2 (3) | |
| Adalimumab | 20 (18) | 3 (7) | 17 (26) | |
| Othera | 2 (2) | 1 (2) | 1 (2) | |
| Diagnosis of kidney disease | ||||
| Systolic blood pressure, mm Hg | 127 ± 20 | 124 ± 18 | 128 ± 22 | 0.34 |
| Diastolic blood pressure, mm Hg | 76 ± 11 | 76 ± 9 | 77 ± 13 | 0.59 |
| Body mass index, kg/m2 | 27 ± 6 | 26 ± 6 | 26 ± 6 | 0.21 |
| Serum creatinine, mg/dl | 2.3 (1.6–3.5) | 2.2 (1.2–3.3) | 2.4 (1.6–3.7) | 0.54 |
| eGFR, ml/min per 1.73 m2 | 30 (17–54) | 32 (17–66) | 29 (17–47) | 0.48 |
| Serum albumin, g/dl | 3.9 (3.2–4.2) | 3.8 (2.7–4.2) | 4 (3.5–4.3) | 0.09 |
| Hemoglobin, g/dl | 11.7 (10–13) | 11.7 (10–13) | 11.8 (10–13) | 0.81 |
| UPCR, g/g | 0.8 (0.3–3.4) | 1.1 (0.3–5.6) | 0.7 (0.3–2.5) | 0.37 |
| Nephrotic syndrome, n (%) | 25 (23) | 13 (29) | 12 (18) | 0.19 |
| Hematuriab, n (%) | 55 (59) | 28 (62) | 27 (41) | 0.16 |
| Leukocyturiab, n (%) | 27 (28) | 9 (20) | 18 (27) | 0.22 |
5–ASA, 5-aminosalicylic acid; eGFR, estimated glomerular filtration rate; IBD, inflammatory bowel disease; UPCR: urinary protein-to-creatinine ratio.
Including anakinra (n = 1) and ustekinumab (n = 1).
Available in 95 (86%) patients.
Treatments After Kidney Disease Diagnosis
In Table 2, we display immunosuppressive management and kidney outcomes, by type of kidney disease. Of note, 6 patients (25%) with IgAN were treated with corticosteroids, 2 (8%) with combination of corticosteroids plus mycophenolate mofetil, whereas 16 (67%) received nonimmunosuppressive therapy (one of which was under treatment with budesonide for the IBD). The majority of patients with CIN (n = 11, 52%) and AIN (n = 6, 43%) were treated with corticosteroids alone or combined with mycophenolate mofetil. As expected, none of the patients with arteriosclerosis without specific changes, severe glomerulosclerosis, secondary focal segmental glomerulosclerosis, or diabetic nephropathy received immunosuppression. Conversely, of the 6 patients with membranous nephropathy, 2 (33%) were treated with calcineurin inhibitors, 3 (50%) with rituximab, and 1 (17%) was not treated with immunosuppression. Of the 7 patients with IC-MPGN or C3 glomerulopathy, 2 (29%) were treated with corticosteroids, 1 (14%) with corticosteroids plus mycophenolate mofetil, 1 (14%) with rituximab, 1 (14%) with azathioprine, whereas the rest received conservative non-immunosuppressive treatments.
Table 2.
Major kidney disease etiologies, and detailed information on baseline characteristics, treatments, and outcomes
| Kidney disease | N | eGFR | UPCR | Chronicity Score | IS treatment | IBD treatment modification after kidney disease diagnosis | Kidney failure | Remission or changes in KF |
|---|---|---|---|---|---|---|---|---|
| IgAN | 24 (22) | 37 (24–81) | 0.7 (0.4–1.4) | 3 (2–3) | None: 16 (67) CS: 6 (25) CS + MMF: 2 (8) |
None: 18 (75) Ustekinumab: 3 (13) Infliximab: 2 (8) Adalimumab: 2 (8) |
5 (21) | No remission: 14 (58) Partial: 3 (13) Complete: 7 (29) |
| IgA vasculitis | 5 (5) | 47 (34-104) | 1.3 (0.9-2.3) | 1 (1–2) | None: 1 (20) CS: 2 (40) CS + MMF: 2 (40) |
None: 4 (80) Vedolizumab: 1 (20) |
0 (0) | No remission: 2 (40) Partial: 1 (20) Complete: 2 (40) |
| CIN | 21 (19) | 26 (15–33) | 0.7 (0.2–0.7) | 3 (3–4) | None: 9 (43) CS: 11 (52) CS+MMF: 1 (5) |
None: 16 (75) Azathioprine: 2 (10) Adalimumab: 1 (5) Vedolizumab: 1 (5) Budesonide: 1 (5) |
8 (38) | Decline of KF: 15 (71) Stabilization of KF: 4 (19) Improvement of KF: 2 (10) |
| AIN | 14 (13) | 21 (13–32) | 0.4 (0.1–1.5) | 2 (1–2) | None: 5 (36) CS: 6 (43) CS+MMF: 3 (21) |
None: 6 (44) Ustekinumab: 3 (21) Vedolizumab: 1 (7) Budesonide: 1 (7) Azathioprine: 1 (7) Infliximab: 2 (14) |
2 (14) | Decline of KF: 7 (50) Stabilization of KF: 3 (21) Improvement of KF: 4 (29) |
| ATN | 4 (4) | 10 (7–13) | 0.7 (0.2–1.9) | 2 (1–2) | None: 3 (75) CS: 1 (25) |
Adalimumab: 3 (75) Azathioprine: 1 (25) |
0 (0) | Stabilization of KF: 2 (50) Improvement of KF: 2 (50) |
| Arteriosclerosis without specific changes and severe glomerulosclerosis | 9 (8) | 22 (17–42) | 0.3 (0.2–1.6) | 3 (3–4) | None: 9 (100) | None: 7 (78) Adalimumab: 2 (22) |
5 (56) | Decline of: 8 (89) Stabilization of KF: 1 (11) |
| Secondary FSGS | 4 | 54 (27–86) | 1 (0.6–2.9) | 3 (2–3) | None: 4 (100) | Vedolizumab: 1 (25) | 1 (25) | No remission: 4 (100) |
| Diabetic nephropathy | 3 | 30 (29–48) | 9 (7–11) | 3 (2–3) | None: 3 (100) | None: 3 (100) | 2 (67) | Decline of KF: 3 (100) |
| IC-MPGN and C3G | 7 | 30 (14–55) | 1.3 (0.7–8) | 2 (2–4) | None: 2 (29) CS: 2 (29) CS+MMF: 1 (14) RTX: 1 (14) Aza: 1 (14) |
None: 6 (86) Infliximab: 1 (14) |
2 (29) | No remission: 4 (57) Partial remission: 3 (43) |
| AA amyloidosis | 4 | 29 (13–50) | 1.2 (0.9–4.4) | 3 (2–4) | None: 4 (100) | Budesonide: 1 (25) Adalimumab: 2 (50) Infliximab: 1 (25) |
1 (25) | No remission: 3 (75) Complete remission: 1 (25) |
| ANCA vasculitis | 2 | 27 | 0.7 | 2 | CS+CyC+Aza: 2 (100) | None: 2 (100) | 0 (0) | Complete remission: 2 (100) |
| MCD | 2 | 77 | 8 | 1 | CS+CNI: 2 (100) | None: 1 (50) Vedolizumab: 1 (50) |
0 (0) | Partial remission: 1 (50) Complete remission: 1 (50) |
| MN | 6 | 45 (30–84) | 6 (6–16) | 2 (1–4) | None: 1 (17) CS+CNI: 2 (33) RTX: 3 (50) |
None: 5 (6) | 3 (50) | No remission: 4 (67) Partial remission: 1 (17) Complete remission: 1 (17) |
| TMA | 1 | 14 | 3 | 3 | PEX: 1 (100) | None: 1 (100) | 0 (0) | Partial remission: 1 (100) |
| Fibrillary GN | 1 | 35 | 8 | 3 | None (100) | None: 1 (100) | 0 (0) | No remission: 1 (100) |
| Lupus nephritis | 1 | 107 | 5.3 | 1 | CS+MMF+CNI | None: 1 (100) | 0 (0) | Complete remission: 1 (100) |
| Thin basement membrane | 2 | 43 | 0.04 | 1 | None: 1 (100) | None: 2 (100) | 0 (0) | Improvement of KF: 2 (100) |
| LCPT | 1 | 81 | 5 | 1 | Clone-targeted therapy | None: 1 (100) | 0 (0) | Complete remission: 1 (100) |
AIN, acute interstitial nephritis; ANCA, antineutrophil cytoplasmic antibody; ATN, acute tubular necrosis; Aza, azathioprine; C3G, C3 glomerulopathy; CIN, chronic interstitial nephritis; CNI, calcineurin inhibitor; CS, corticosteroids; CyC, cyclophosphamide; eGFR, estimated glomerular filtration rate; GN, glomerulonephritis; IC-MPGN, immune complex-mediated membranoproliferative glomerulonephritis; IgAN, Immunoglobulin A nephropathy; IS, immunosuppressive; KF, kidney function; LCPT, light chain proximal tubulopathy; MCD, minimal change disease; MMF, mycophenolate mofetil; MN, membranous nephropathy; RTX, rituximab; TMA, thrombotic microangiopathy; UPCR, urinary protein-to-creatinine ratio.
Outcome: Kidney Failure
During a median follow-up time of 59 months (IQR: 12–109), 29 patients (26%) progressed to kidney failure. The clinical characteristics of patients and treatments received according to this outcome are displayed in Table 3 and Supplementary Table S2. Patients who progressed to kidney failure were significantly older, had lower baseline eGFR, nephrotic syndrome, and lower hemoglobin levels. In addition, there was a nonsignificant trend toward lower baseline serum albumin, higher UPCR, and lower frequency of hematuria.
Table 3.
Histologic diagnosis, degree of disease chronicity, and treatments received, according to development of kidney failure at last follow-up
| Characteristic | Total (N = 111) | Kidney failure (n = 29) | No kidney failure (n = 82) | P value |
|---|---|---|---|---|
| Kidney disease diagnosis | 0.47 | |||
| IgA nephropathy | 24 (22) | 5 (17) | 19 (23) | |
| Chronic interstitial nephritis | 21 (19) | 8 (28) | 13 (16) | |
| Acute interstitial nephritis | 14 (13) | 2 (7) | 12 (15) | |
| Acute tubular necrosis | 4 (4) | 0 (0) | 4 (5) | |
| Arteriosclerosis without specific changes | 6 (5) | 3 (10) | 3 (4) | |
| Secondary FSGS | 4 (4) | 1 (3) | 3 (4) | |
| Diabetic nephropathy | 3 (3) | 2 (7) | 1 (1) | |
| IC-MPGN | 6 (5) | 2 (7) | 4 (5) | |
| Severe glomerulosclerosis | 3 (3) | 2 (7) | 1 (1) | |
| AA amyloidosis | 4 (4) | 1 (3) | 3 (4) | |
| ANCA associated vasculitis | 2 (2) | 0 (0) | 2 (2) | |
| IgA vasculitis | 5 (5) | 0 (0) | 5 (6) | |
| Minimal change disease | 2 (2) | 0 (0) | 2 (2) | |
| Membranous nephropathy | 6 (5) | 3 (10) | 3 (4) | |
| C3 glomerulopathy | 1 (1) | 0 (0) | 1 (1) | |
| Thrombotic microangiopathy | 1 (1) | 0 (0) | 1 (1) | |
| Fibrillary glomerulonephritis | 1 (1) | 0 (0) | 1 (1) | |
| Lupus nephritis | 1 (1) | 0 (0) | 1 (1) | |
| Thin basement membrane | 2 (2) | 0 (0) | 2 (2) | |
| Light-chain proximal tubulopathy | 1 (1) | 0 (0) | 1 (1) | |
| Disease chronicity in kidney biopsy | ||||
| Glomerulosclerosis | 1 (0–2) | 2 (2–3) | 1 (0–2) | <0.001 |
| Interstitial fibrosis | 1 (0–2) | 2 (2–3) | 1 (0–2) | <0.001 |
| Tubular atrophy | 1 (0–2) | 2 (2–3) | 1 (0–2) | <0.001 |
| Arteriosclerosis | 1 (0–1) | 1 | 1 (0–1) | 0.001 |
| Total chronicity score | 3 (2–3) | 4 (3–5) | 2 (1–3) | <0.001 |
| Total chronicity score, N (%) | <0.001 | |||
| Minimal (0–1) | 27 (24) | 0 (0) | 27 (33) | |
| Mild (2–4) | 24 (22) | 0 (0) | 24 (29) | |
| Moderate (5–7) | 39 (35) | 13 (45) | 26 (32) | |
| Severe (≥8) | 21 (19) | 16 (55) | 5 (6) | |
| Immunosuppressive treatments received after kidney disease diagnosis | 0.65 | |||
| None | 59 (53) | 18 (62) | 41 (50) | |
| Corticosteroids | 30 (27) | 7 (24) | 23 (28) | |
| Corticosteroids + MMF | 10 (9) | 2 (7) | 8 (10) | |
| Corticosteroids + CNI | 4 (4) | 1 (3) | 3 (4) | |
| Rituximab | 4 (4) | 1 (3) | 3 (4) | |
| Other | 4 (4) | 0 (0) | 4 (5) | |
| Follow-up, months | 59 (12–109) | 13 (4–38) | 87 (23–127) | <0.001 |
AA, amyloid A; ANCA, antineutrophil cytoplasmic antibodies; CNI, calcineurin inhibitor; FSGS, focal segmental glomerulosclerosis; IC-MPGN, immune-complex membranoproliferative glomerulonephritis; IgA, immunoglobulin A; MMF, mycophenolate mofetil.
No significant differences were observed in the etiologies of kidney disease according to this outcome, although a greater percentage of patients with CIN, arteriosclerosis without specific changes, diabetic nephropathy, severe glomerulosclerosis, and membranous nephropathy, reached kidney failure (Table 3). Regarding histologic findings, patients who progressed to kidney failure had significantly greater degree of glomerulosclerosis, interstitial fibrosis, tubular atrophy, and arteriosclerosis (Table 3). Median total chronicity score of patients who reached kidney failure was 4 (IQR: 3–5), compared to 2 (IQR: 1–3) in those who did not (P < 0.001). No significant differences were observed in the outcomes of patients with AIN or CIN treated or not with 5-ASA (Supplementary Table S3).
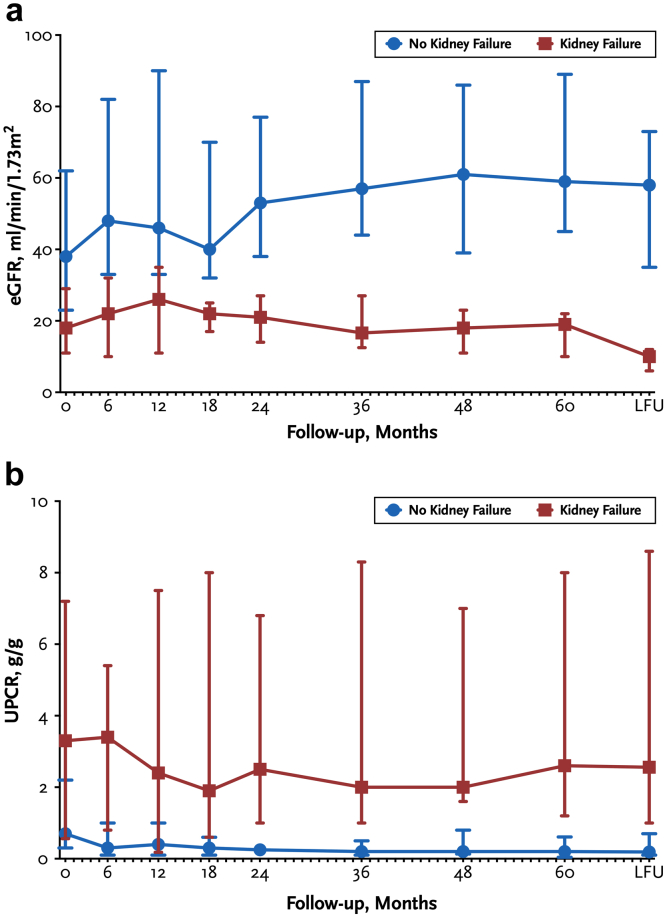
In Figure 2, we show the longitudinal evolution of eGFR and UPCR over time, according to the development of kidney failure. A progressive decline in eGFR and trend toward an increase in UPCR was observed in patients who progressed to kidney failure.
Figure 2.
(a) Evolution of eGFR according to the development of kidney failure at last follow-up; (b) Evolution of UPCR according to the development of kidney failure. Data are presented as median (interquartile range). eGFR, estimated glomerular filtration rate; UPCR, urinary protein-to-creatinine ratio.
By multivariable Cox regression analysis (Table 4), the main predictors of kidney failure were age (HR: 1.04; 95% confidence interval: 1.02–1.07; P = 0.002), baseline eGFR (HR: 0.94; 95% confidence interval: 0.91–0.98; P = 0.003) and histologic total chronicity score (HR: 4.01; 95% confidence interval: 2.21–7.29; P < 0.001).
Table 4.
Cox proportional hazard regression analysis for association between covariables and kidney failure
| Variable | Univariable Analysis |
P | Multivariable Analysis |
P value |
|---|---|---|---|---|
| Hazard ratio (95% CI) | Hazard ratio (95% CI) | |||
| Age (per year increment) | 1.03 (1.01–1.07) | 0.009 | 1.04 (1.02–1.07) | 0.002 |
| Sex | 0.93 | |||
| Female | 1.00 (reference) | |||
| Male | 0.96 (0.46–2.03) | |||
| Type of IBD | 0.56 | |||
| Ulcerative colitis | 1.00 (reference) | |||
| Crohn’s disease | 1.25 (0.59–2.64) | |||
| Baseline eGFR (per ml/min increment) | 0.94 (0.92–0.97) | <0.001 | 0.94 (0.91–0.98) | 0.003 |
| Baseline UPCR (per 1 g/g increment) | 1.13 (1.06–1.21) | <0.001 | ||
| Total chronicity score (per grade increment) | 5.93 (3.24–10.85) | <0.001 | 4.01 (2.21–7.29) | <0.001 |
CI, confidence interval; eGFR, estimated glomerular filtration rate; IBD, inflammatory bowel disease; UPCR, urinary protein-to-creatinine ratio.
Number of events: 29.
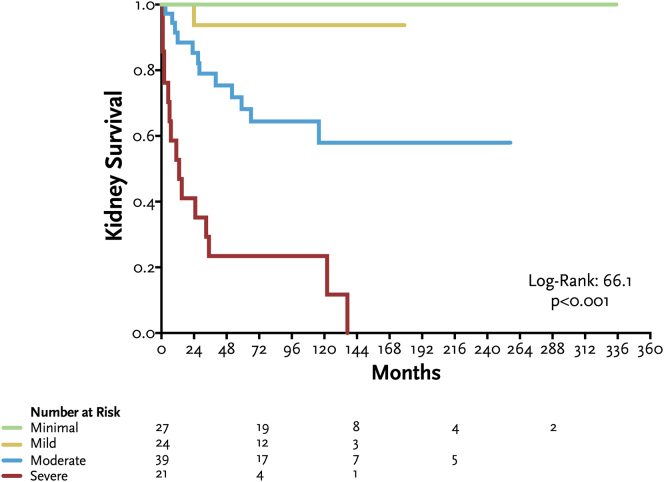
In Figure 3, we show Kaplan-Meier curves for kidney survival according to chronicity grades in kidney biopsy. Patients with severe disease chronicity score had a significantly worse kidney survival.
Figure 3.
Kaplan-Meier curves for kidney survival according to grades of histologic total disease chronicity score in kidney biopsy.
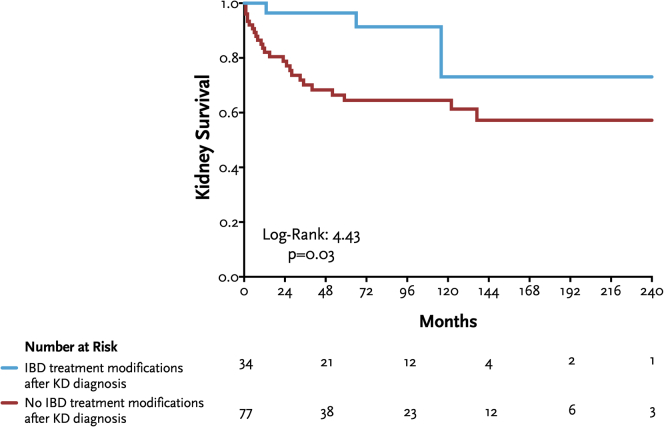
Thirty-four patients (31%) underwent modifications in the treatment of IBD after the diagnosis of kidney diseases (Table 2) and showed a significantly better kidney survival compared to those without treatment adjustments (Figure 4).
Figure 4.
Kaplan-Meier curves for kidney survival according to IBD treatment modifications after kidney disease (KD) diagnosis. IBD, inflammatory bowel disease.
Outcome: Disease Remission or Improvement in Kidney Function
Overall, 61 patients (55%) had kidney diseases characterized by the presence of proteinuria, of which 16 (26%) achieved complete remission, 9 (15%) achieved partial remission, and 36 (59%) had no remission (Table 2). Notably, of the 24 patients with IgAN, 7 (29%) achieved complete remission whereas 3 (13%) achieved partial remission after immunosuppression. Likewise, of the 5 patients with IgA vasculitis, 2 (40%) achieved complete remission, 1 (20%) achieved partial remission, and 2 (40%) had no remission. Of the 7 patients with IC-MPGN or C3 glomerulopathy, 3 (43%) achieved partial remission, whereas 4 (57%) did not achieve remission. Of the 6 patients with membranous nephropathy, 4 had resistant disease despite immunosuppression, 1 (17%) achieved partial remission, and 1 (17%) achieved complete remission at last follow-up. Of the 4 patients with AA amyloidosis, only 1 (25%) achieved complete remission, whereas the rest did not achieve remission.
On the other hand, 50 patients (45%) had nonproteinuric kidney diseases, of which 20 (40%) showed stabilization or improvement of kidney function, whereas 30 (60%) showed progressive kidney function decline (Table 2). Interestingly, of the 21 patients with CIN (19%), only 2 (10%) showed improvement of kidney function, whereas in 4 (19%), kidney function stabilized. Conversely, of the 14 with AIN, 4 (29%) showed improvement of kidney function, 3 (21%) showed stabilization, and 7 (50%) showed progressive decline of kidney function despite treatment.
Composite Outcome: Kidney Failure or Death
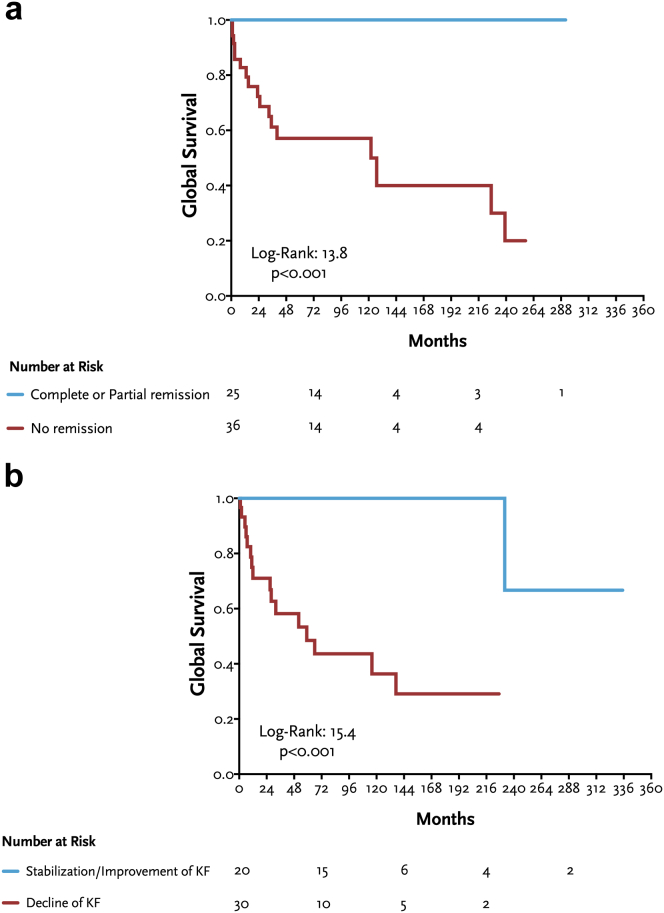
At last follow-up, 13 patients (12%) died: 2 patients (15%) with AA amyloidosis, 2 (15%) with AIN, 2 (15%) with CIN, 2 (15%) with diabetic nephropathy, 1 (8%) with fibrillary glomerulonephritis, 1 (8%) with IC-MPGN, 1 (8%) with IgAN, 1 (8%) with secondary focal segmental glomerulosclerosis, and 1 (8%) with arteriosclerosis without specific changes. In Figure 5, we show Kaplan-Meier curves for global survival (composite outcome) according to disease remission (a) or stabilization or improvement of kidney function (b). Patients who achieved partial or complete remission of kidney disease or stabilization or improvement of kidney function had a significantly better survival.
Figure 5.
(a) Kaplan-Meier curves for global survival (kidney failure + death) according to remission status (complete or partial versus no remission); (b) Kaplan-Meier curves for global survival (kidney failure + death) according to changes in kidney function (stabilization or improvement versus declining kidney function).
Discussion
Herein, we comprehensively evaluated the major etiologies of kidney disease and outcomes of a large cohort of patients with IBD. There are several major findings in this study. First, our results confirm previous findings that IgAN, CIN, and AIN are the most commonly associated kidney diagnoses in patients with IBD, although other glomerular or systemic autoimmune diseases may also be associated. Second, despite immunosuppressive therapy, up to 26% of patients with IBD with kidney disease may develop kidney failure. Third, higher age at kidney disease diagnosis and higher degree of disease chronicity in kidney biopsy are the most important predictors of kidney failure, whereas higher eGFR predicts a reduced risk of kidney failure. Fourth, up to 41% of patients with proteinuric kidney diseases associated with IBD may achieve some degree of remission (partial or complete) after treatments, and up to 40% of patients with nonproteinuric kidney diseases may show stabilization or improvement of kidney function. Finally, those patients who achieve partial or complete remission of kidney disease or stabilization or improvement of kidney function had a significantly better overall survival.
To our knowledge, this is the largest study to evaluate the spectrum of kidney disease associated with IBD and the predictors of kidney outcomes in this patient population.
IgAN was the most frequent glomerular disease in our cohort, and this is consistent with previous studies.15,18, 19, 20 Patients with IgAN had a significant kidney impairment at the time of diagnosis, along with a high disease chronicity in kidney biopsy. Although the association between IgAN and IBD may be coincidental, emerging evidence suggests a potential common underlying mechanism in disease pathogenesis.21,22 Recent studies have identified risk loci in genes involved in intestinal mucosal integrity, and common risk alleles for both IgAN and IBD.23,24 The gut-kidney axis hypothesis supports a key role for imbalances between immunity and microbiota in the development or exacerbation of IgAN.25,26 Gut microbiota can lead to B cell activation via B cell activation factor, and this overstimulation promotes a significant shift from IgA2 to IgA1 production.27,28 In addition, an aberrant O-linked glycosylation of IgA is observed in patients with CD.29 A Swedish population-based cohort study found that patients with IgAN had an increased risk of IBD both before and after the diagnosis of kidney disease.30 The cumulative incidence of kidney failure in this population has been reported to be up to 50%, although other studies have found no significant difference between IBD-related IgAN and primary IgAN (17%).30,31 In our study, only 5 patients (21%) reached kidney failure at last follow-up, although the median follow-up was shorter compared to the former study, which could partially explain this difference. Interestingly, a previous study described a remarkably higher percentage of glomerulosclerosis and a greater extent of interstitial fibrosis and tubular atrophy in IgAN associated with CD, together with a higher macrophage infiltration, suggesting that these patients may have a more aggressive clinical phenotype of IgAN.32 Despite that, the optimal therapeutic approach of these patients remains to be elucidated. Moreover, although the novel targeted-release formulation of budesonide appears to be a promising therapeutic strategy for the management of both IBD and IgAN,33,34 other generic forms of budesonide currently available for the treatment of IBD should also be compared in these patients.
Another major group of etiologies of kidney disease associated with IBD were CIN and AIN, representing 19% and 13% of study patients, respectively, with frequencies similar to those reported in other cohorts.15,35 Although the underlying pathogenic mechanisms of the association between interstitial kidney disease and IBD have not been elucidated, it has been suggested that interstitial damage may be the result of prolonged exposure to certain drugs, repeated episodes of acute kidney injury in the setting of IBD activity, or a specific form of EIM in patients with IBD.35, 36, 37 Although some studies have suggested that the incidence of 5-ASA-associated nephrotoxicity is exceptional,38, 39, 40 5-ASA represents the most commonly associated culprit drug in adult patients with IBD and interstitial kidney disease.39,41,42 The underlying pathogenesis is not fully understood, although it may represent an idiosyncratic delayed-type hypersensitivity reaction.39,43 In fact, the molecular structure of 5-ASA is very similar to that of acetylsalicylic acid or phenacetin, which have also been implicated in the occurrence of nonsteroidal antiinflammatory drug-induced nephropathy.39,44, 45, 46 In our study, the majority of patients with CIN had been treated with 5-ASA, strongly suggesting the involvement of this drug in the development of kidney disease. However, the retrospective design of our study prevents us from establishing definitive causal conclusions. In addition, it should be noted that patients with IBD may experience AIN unrelated to 5-ASA, which poses a significant diagnostic challenge.20 Interestingly, in our study up to two-thirds of patients had granulomatous interstitial kidney disease unrelated to 5-ASA exposure, supporting the idea that this histologic feature may represent a genuine EIM.47 Another immunosuppressive agent that has been associated with the development of AIN and CIN is azathioprine,48,49 although in our study, only a small subset of cases had received this treatment for IBD.
Early identification and withdrawal of the potential offending drug represents a mainstay in the management of drug-induced AIN.50 However, this can be particularly problematic in patients taking multiple medications,50 such as those with IBD. Although the evidence for the use of immunosuppression in AIN is primarily based on observational studies, treatment with corticosteroids has been associated with better recovery of kidney function.51, 52, 53 In our study, the majority of patients with CIN and AIN were treated with corticosteroids, or the combination of corticosteroids plus mycophenolate mofetil (as a corticosteroid-sparing agent), at the discretion of the treating physician. As expected, AIN demonstrated a significantly higher rate of kidney function recovery compared to CIN, suggesting that only a subset of patients was diagnosed early enough to prevent the progression of irreversible interstitial fibrosis. However, it is important to note that because our study used kidney biopsy as the starting point for the evaluation of kidney outcomes, the variable delay in diagnosis may have contributed to the prognosis in some cases.
In addition to the aforementioned etiologies, our study provides insight into several other potential underlying diseases associated with IBD, such as IC-MPGN, C3 glomerulopathy, membranous nephropathy, podocytopathy with minimal change disease, antineutrophil cytoplasmic antibody vasculitis, or AA amyloidosis, previously described on the basis of single case reports. Nonetheless, although the exact etiologic link between IBD and these etiologies remains uncertain, it is conceivable to speculate that patients with IBD might possess genetic and immunologic characteristics that render them more susceptible to these processes.
The main determinants of kidney failure in the entire cohort were advanced age at diagnosis, and the extent of disease chronicity observed in kidney biopsy, whereas higher eGFR predicted a lower risk of kidney failure. These findings are frequently encountered in other kidney diseases and may suggest either a delayed diagnosis of kidney disease, or a more aggressive presentation with limited response to conventional therapies. These insights can help in guiding the adjustment of immunosuppressive treatments, tailoring their intensity accordingly. Conversely, patients who underwent IBD treatment modifications after kidney disease diagnosis exhibited better kidney survival compared to those in whom no modifications were made, indicating a potential kidney benefit linked to the management and control of IBD.
Several limitations should be acknowledged. First, because of the observational and retrospective nature of the study, no causal relationships can be established. Second, the diverse range of kidney diseases observed in our study hindered a more detailed analysis of outcomes specific to each individual disease. Third, only variables known to be determinants of prognosis were included in the basic clinical model, and therefore, we cannot rule out that other unmeasured confounders could have a prognostic influence. Fourth, follow-up data was unavailable in 207 patients in the overall cohort which could limit the generalizability of some of the findings. Notwithstanding these limitations, our study collected a large series of well-characterized patients with IBD who underwent kidney biopsy at out institution. This comprehensive approach enabled us to thoroughly analyze the histologic features and outcomes of this particular population.
In conclusion, our study findings underscore the critical importance of early detection and effective management of kidney disease in patients with IBD. Regular assessment and monitoring of kidney function, together with a multidisciplinary approach and potential interventions aimed at slowing disease progression, offer potential benefits for this patient population. Given that kidney disease contributes to heightened morbidity and mortality, and significantly impacts patients' quality of life, these findings emphasize the need for proactive measures in this area. Further research with larger, multicenter cohorts and prospective study designs would be needed to confirm and extend these findings.
Disclosure
All the authors declared no conflicting interests.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.
Author Contributions
The conception and analysis and interpretation of data was by FY, FC-F, and FF. Drafting of the article was by FY, FC-F, MJS, and FF. Final approval of the version to be published was provided by all authors.
Footnotes
Figure S1. Flowchart of study patients.
Table S1. Histologic diagnosis in kidney biopsies in overall cohort, and by type of inflammatory bowel disease.
Table S2. Clinical characteristics of study population, according to the development of kidney failure.
Table S3. Clinical characteristics of patients with acute and chronic interstitial nephritis, according to 5-ASA treatment exposure.
Contributor Information
Federico Yandian, Email: fedeuru141@hotmail.com.
Fernando Caravaca-Fontán, Email: fcaravacaf@gmail.com.
Fernando C. Fervenza, Email: fervenza.fernando@mayo.edu.
Supplementary Material
Figure S1. Flowchart of study patients.
Table S1. Histologic diagnosis in kidney biopsies in overall cohort, and by type of inflammatory bowel disease.
Table S2. Clinical characteristics of study population, according to the development of kidney failure.
Table S3. Clinical characteristics of patients with acute and chronic interstitial nephritis, according to 5-ASA treatment exposure.
References
- 1.Loddo I., Romano C. Inflammatory bowel disease: genetics, epigenetics, and pathogenesis. Front Immunol. 2015;6:551. doi: 10.3389/fimmu.2015.00551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flynn S., Eisenstein S. Inflammatory bowel disease presentation and diagnosis. Surg Clin North Am. 2019;99:1051–1062. doi: 10.1016/j.suc.2019.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Ng S.C., Shi H.Y., Hamidi N., et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390:2769–2778. doi: 10.1016/S0140-6736(17)32448-0. [DOI] [PubMed] [Google Scholar]
- 4.Babickova J., Gardlik R. Pathological and therapeutic interactions between bacteriophages, microbes and the host in inflammatory bowel disease. World J Gastroenterol. 2015;21:11321–11330. doi: 10.3748/wjg.v21.i40.11321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mutalib M. Renal involvement in paediatric inflammatory bowel disease. Pediatr Nephrol. 2021;36:279–285. doi: 10.1007/s00467-019-04413-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xavier R.J., Podolsky D.K. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 7.Annese V. A review of extraintestinal manifestations and complications of inflammatory bowel disease. Saudi J Med Sci. 2019;7:66–73. doi: 10.4103/sjmms.sjmms_81_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Danese S., Semeraro S., Papa A., et al. Extraintestinal manifestations in inflammatory bowel disease. World J Gastroenterol. 2005;11:7227–7236. doi: 10.3748/wjg.v11.i46.7227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oikonomou K., Kapsoritakis A., Eleftheriadis T., Stefanidis I., Potamianos S. Renal manifestations and complications of inflammatory bowel disease. Inflamm Bowel Dis. 2011;17:1034–1045. doi: 10.1002/ibd.21468. [DOI] [PubMed] [Google Scholar]
- 10.Larsen S., Bendtzen K., Nielsen O.H. Extraintestinal manifestations of inflammatory bowel disease: epidemiology, diagnosis, and management. Ann Med. 2010;42:97–114. doi: 10.3109/07853890903559724. [DOI] [PubMed] [Google Scholar]
- 11.Dincer M.T., Dincer Z.T., Bakkaloglu O.K., et al. Renal manifestations in inflammatory bowel disease: a cohort study during the biologic era. Med Sci Monit. 2022;28 doi: 10.12659/MSM.936497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ambruzs J.M., Larsen C.P. Renal manifestations of inflammatory bowel disease. Rheum Dis Clin North Am. 2018;44:699–714. doi: 10.1016/j.rdc.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 13.Shield D.E., Lytton B., Weiss R.M., Schiff M. Urologic complications of inflammatory bowel disease. J Urol. 1976;115:701–706. doi: 10.1016/s0022-5347(17)59341-6. [DOI] [PubMed] [Google Scholar]
- 14.Pardi D., Tremaine W.J., Sandborn W.J., McCarthy J.T. Renal and urologic complications of inflammatory bowel disease. Am J Gastroenterol. 1998;93:504–514. doi: 10.1111/j.1572-0241.1998.156_b.x. [DOI] [PubMed] [Google Scholar]
- 15.Ambruzs J.M., Walker P.D., Larsen C.P. The histopathologic spectrum of kidney biopsies in patients with inflammatory bowel disease. Clin J Am Soc Nephrol. 2014;9:265–270. doi: 10.2215/CJN.04660513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sethi S., D’Agati V.D., Nast C.C., et al. A proposal for standardized grading of chronic changes in native kidney biopsy specimens. Kidney Int. 2017;91:787–789. doi: 10.1016/j.kint.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 17.Levey A.S., Stevens L.A., Schmid C.H., et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayashi R., Ueno Y., Tanaka S., et al. Clinical characteristics of inflammatory bowel disease patients with immunoglobulin A nephropathy. Intest Res. 2021;19:430–437. doi: 10.5217/ir.2020.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Filiopoulos V., Trompouki S., Hadjiyannakos D., Paraskevakou H., Kamperoglou D., Vlassopoulos D. IgA nephropathy in association with Crohn’s disease: a case report and brief review of the literature. Ren Fail. 2010;32:523–527. doi: 10.3109/08860221003710554. [DOI] [PubMed] [Google Scholar]
- 20.Yang Z., Xu X., Dong Y., Zhang Y. The pathological and outcome characteristics of renal lesions in Crohn’s disease. BMC Nephrol. 2022;23:256. doi: 10.1186/s12882-022-02883-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monteiro R.C. Recent advances in the physiopathology of IgA nephropathy. Néphrologie Ther. 2018;14(suppl 1):S1–S8. doi: 10.1016/j.nephro.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 22.Xiao M., Ran Y., Shao J., Lei Z., Chen Y., Li Y. Causal association between inflammatory bowel disease and IgA nephropathy: a bidirectional two-sample Mendelian randomization study. Front Genet. 2022;13 doi: 10.3389/fgene.2022.1002928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kiryluk K., Li Y., Scolari F., et al. Discovery of new risk loci for IgA nephropathy implicates genes involved in immunity against intestinal pathogens. Nat Genet. 2014;46:1187–1196. doi: 10.1038/ng.3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi D., Zhong Z., Wang M., et al. Identification of susceptibility locus shared by IgA nephropathy and inflammatory bowel disease in a Chinese Han population. J Hum Genet. 2020;65:241–249. doi: 10.1038/s10038-019-0699-9. [DOI] [PubMed] [Google Scholar]
- 25.Han S., Shang L., Lu Y., Wang Y. Gut microbiome characteristics in IgA nephropathy: qualitative and quantitative analysis from observational studies. Front Cell Infect Microbiol. 2022;12 doi: 10.3389/fcimb.2022.904401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shah N.B., Nigwekar S.U., Kalim S., et al. The gut and blood microbiome in IgA nephropathy and healthy controls. Kidney360. 2021;2:1261–1274. doi: 10.34067/KID.0000132021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brandtzaeg P., Carlsen H.S., Halstensen T.S. The B-cell system in inflammatory bowel disease. Adv Exp Med Biol. 2006;579:149–167. doi: 10.1007/0-387-33778-4_10. [DOI] [PubMed] [Google Scholar]
- 28.Xin G., Shi W., Xu L.-X., Su Y., Yan L.J., Li K.S. Serum BAFF is elevated in patients with IgA nephropathy and associated with clinical and histopathological features. J Nephrol. 2013;26:683–690. doi: 10.5301/jn.5000218. [DOI] [PubMed] [Google Scholar]
- 29.Inoue T., Iijima H., Tajiri M., et al. Deficiency of N-acetylgalactosamine in O-linked oligosaccharides of IgA is a novel biologic marker for Crohnʼs disease. Inflamm Bowel Dis. 2012;18:1723–1734. doi: 10.1002/ibd.22876. [DOI] [PubMed] [Google Scholar]
- 30.Rehnberg J., Symreng A., Ludvigsson J.F., Emilsson L. Inflammatory bowel disease is more common in patients with IgA nephropathy and predicts progression of ESKD: a Swedish population-based cohort study. J Am Soc Nephrol. 2021;32:411–423. doi: 10.1681/ASN.2020060848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joher N., Gosset C., Guerrot D., et al. Immunoglobulin A nephropathy in association with inflammatory bowel diseases: results from a national study and systematic literature review. Nephrol Dial Transplant. 2022;37:531–539. doi: 10.1093/ndt/gfaa378. [DOI] [PubMed] [Google Scholar]
- 32.Akiyama M., Shimomura K., Yoshimoto H., et al. Crohn’s disease may promote inflammation in IgA nephropathy: a case-control study of patients undergoing kidney biopsy. Virchows Arch. 2022;481:553–563. doi: 10.1007/s00428-022-03373-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fellström B.C., Barratt J., Cook H., et al. Targeted-release budesonide versus placebo in patients with IgA nephropathy (NEFIGAN): a double-blind, randomised, placebo-controlled phase 2b trial. Lancet. 2017;389:2117–2127. doi: 10.1016/S0140-6736(17)30550-0. [DOI] [PubMed] [Google Scholar]
- 34.Barratt J., Lafayette R., Kristensen J., et al. Results from part A of the multi-center, double-blind, randomized, placebo-controlled NefIgArd trial, which evaluated targeted-release formulation of budesonide for the treatment of primary immunoglobulin A nephropathy. Kidney Int. 2023;103:391–402. doi: 10.1016/j.kint.2022.09.017. [DOI] [PubMed] [Google Scholar]
- 35.Pohjonen J., Nurmi R., Metso M., et al. Inflammatory bowel disease in patients undergoing renal biopsies. Clin Kidney J. 2019;12:645–651. doi: 10.1093/ckj/sfz004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Praga M., Sevillano A., Aunon P., González E. Changes in the aetiology, clinical presentation and management of acute interstitial nephritis, an increasingly common cause of acute kidney injury. Nephrol Dial Transplant. 2015;30:1472–1479. doi: 10.1093/ndt/gfu326. [DOI] [PubMed] [Google Scholar]
- 37.Caravaca-Fontán F., Fernández-Juárez G., Praga M. Acute kidney injury in interstitial nephritis. Curr Opin Crit Care. 2019;25:558–564. doi: 10.1097/MCC.0000000000000654. [DOI] [PubMed] [Google Scholar]
- 38.Gisbert J.P., Luna M., González-Lama Y., et al. Effect of 5-aminosalicylates on renal function in patients with inflammatory bowel disease: 4-year follow-up study. Gastroenterol Hepatol (N Y) 2008;31:477–484. doi: 10.1157/13127088. [DOI] [PubMed] [Google Scholar]
- 39.Gisbert J.P., González-Lama Y., Maté J. 5-aminosalicylates and renal function in inflammatory bowel disease: a systematic review. Inflamm Bowel Dis. 2007;13:629–638. doi: 10.1002/ibd.20099. [DOI] [PubMed] [Google Scholar]
- 40.Herrlinger K.R., Noftz M.K., Fellermann K., Schmidt K., Steinhoff J., Stange E.F. Minimal renal dysfunction in inflammatory bowel disease is related to disease activity but not to 5-ASA use. Aliment Pharmacol Ther. 2001;15:363–369. doi: 10.1046/j.1365-2036.2001.00940.x. [DOI] [PubMed] [Google Scholar]
- 41.Zand L., Kattah A., Fervenza F.C., et al. C3 glomerulonephritis associated with monoclonal gammopathy: a case series. Am J Kidney Dis. 2013;62:506–514. doi: 10.1053/j.ajkd.2013.02.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adiga A., Goldfarb D.S. The association of mesalamine with kidney disease. Adv Chronic Kidney Dis. 2020;27:72–76. doi: 10.1053/j.ackd.2019.09.002. [DOI] [PubMed] [Google Scholar]
- 43.Arend L.J., Springate J.E. Interstitial nephritis from mesalazine: case report and literature review. Pediatr Nephrol. 2004;19:550–553. doi: 10.1007/s00467-004-1411-6. [DOI] [PubMed] [Google Scholar]
- 44.Margetts P.J., Churchill D.N., Alexopoulou I. Interstitial nephritis in patients with inflammatory bowel disease treated with mesalamine. J Clin Gastroenterol. 2001;32:176–178. doi: 10.1097/00004836-200102000-00019. [DOI] [PubMed] [Google Scholar]
- 45.DeBore M., Stolear J., Nouwen E., Elseviers M.M. 5-Aminosalycylic acid (5-ASA) and chronic tubulointerstitial nephritis in patients with chronic inflammatory bowel disease: is there a link? Nephrol Dial Transplant. 1997;12:1839–1841. doi: 10.1093/ndt/12.9.1839. [DOI] [PubMed] [Google Scholar]
- 46.Praga M., González E. Acute interstitial nephritis. Kidney Int. 2010;77:956–961. doi: 10.1038/ki.2010.89. [DOI] [PubMed] [Google Scholar]
- 47.Timmermans S., Christiaans M.H.L., Abdul-Hamid M.A., Stifft F., Damoiseaux J.G., van Paassen P. Granulomatous interstitial nephritis and Crohn’s disease. Clin Kidney J. 2016;9:556–559. doi: 10.1093/ckj/sfw041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reinhold-Keller E., Schmitt W.H., Gross W.L. Azathioprine toxicity mimicking a relapse of Wegener’s granulomatosis. Rheumatology (Oxford) 2001;40:831–832. doi: 10.1093/rheumatology/40.7.831. [DOI] [PubMed] [Google Scholar]
- 49.Bir K., Herzenberg A.M., Carette S. Azathioprine induced acute interstitial nephritis as the cause of rapidly progressive renal failure in a patient with Wegener’s granulomatosis. J Rheumatol. 2006;33:185–187. [PubMed] [Google Scholar]
- 50.Perazella M.A., Markowitz G.S. Drug-induced acute interstitial nephritis. Nat Rev Nephrol. 2010;6:461–470. doi: 10.1038/nrneph.2010.71. [DOI] [PubMed] [Google Scholar]
- 51.Gonzalez E., Gutierrez E., Morales E., et al. Factors influencing the progression of renal damage in patients with unilateral renal agenesis and remnant kidney. Kidney Int. 2005;68:263–270. doi: 10.1111/j.1523-1755.2005.00401.x. [DOI] [PubMed] [Google Scholar]
- 52.Fernandez-Juarez G., Perez J.V., Caravaca-Fontán F., et al. Duration of treatment with corticosteroids and recovery of kidney function in acute interstitial nephritis. Clin J Am Soc Nephrol. 2018;13:1851–1858. doi: 10.2215/CJN.01390118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Preddie D.C., Markowitz G.S., Radhakrishnan J., et al. Mycophenolate mofetil for the treatment of interstitial nephritis. Clin J Am Soc Nephrol. 2006;1:718–722. doi: 10.2215/CJN.01711105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.