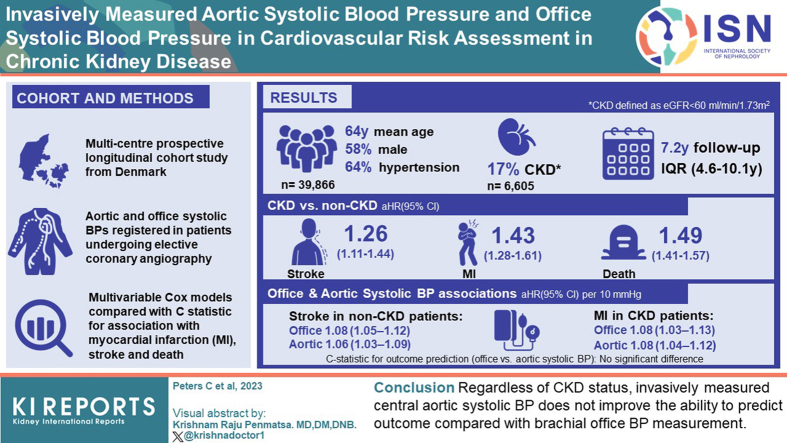
Abstract
Introduction
Central aortic blood pressure (BP) could be a better risk predictor than brachial BP. This study examined whether invasively measured aortic systolic BP improved outcome prediction beyond risk prediction by conventional cuff-based office systolic BP in patients with and without chronic kidney disease (CKD).
Methods
In a prospective, longitudinal cohort study, aortic and office systolic BPs were registered in patients undergoing elective coronary angiography (CAG). CKD was defined as estimated glomerular filtration rate (eGFR) <60 ml/min per 1.73 m2. Multivariable Cox models were used to determine the association with incident myocardial infarction (MI), stroke, and death.
Results
Aortic and office systolic BPs were available in 39,866 patients (mean age: 64 years; 58% males; 64% with hypertension) out of which 6605 (17%) had CKD. During a median follow-up of 7.2 years (interquartile range: 4.6–10.1 years), 1367 strokes (CKD: 353), 1858 MIs (CKD: 446), and 7551 deaths (CKD: 2515) occurred. CKD increased the risk of stroke, MI, and death significantly. Office and aortic systolic BP were both associated with stroke in non-CKD patients (adjusted hazard ratios with 95% confidence interval per 10 mm Hg: 1.08 [1.05–1.12] and 1.06 [1.03–1.09], respectively) and with MI in patients with CKD (adjusted hazard ratios: 1.08 [1.03–1.13] and 1.08 [1.04–1.12], respectively). There was no significant difference between prediction of outcome with office or aortic systolic BP when adjusted models were compared with C-statistics.
Conclusion
Regardless of CKD status, invasively measured central aortic systolic BP does not improve the ability to predict outcome compared with brachial office BP measurement.
Keywords: cardiovascular disease, chronic kidney disease, cuff-measured brachial blood pressure, invasive aortic blood pressure, mortality, systolic blood pressure
Graphical abstract
High BP is frequent in patients with CKD and often, several antihypertensive drugs are required to lower BP and control hypertension.1,2 Currently, cuff-based brachial artery BP is predominantly used when measuring BP. However, vital organs such as the brain, heart and kidneys are exposed to the central rather than the brachial BP. Central aortic systolic BP is normally lower than the corresponding brachial value due to pulse pressure amplification although this difference is highly variable between individuals.3,4 Perhaps more importantly, the BP response to antihypertensive treatment can differ substantially between the aorta and the brachial artery.5, 6, 7 Central BP has therefore been suggested as a better risk predictor than brachial BP in terms of end-organ damage including CKD progression and future cardiovascular events.8, 9, 10 Commercially available equipment (SphymoCor, Mobil-O-Graph, Arteriograph, etc.) can be used for noninvasive estimation of the central BP.11, 12, 13 Estimated central BP can predict cardiovascular events8,14; however, whether it is a better predictor than brachial BP is debatable.6,8,14, 15, 16, 17 In a recent study with 2875 patients with CKD, estimated central BP was not superior to brachial BP in terms of outcome prediction.18 This could perhaps be explained by the inherent inaccuracies concerning estimated central BP.19, 20, 21, 22, 23, 24, 25 In patients with CKD, this is even more pronounced with progressive underestimation of the central systolic BP with decreasing renal function and increasing arterial stiffness.13 Consequently, invasively measured central BP in the ascending aorta is considered gold standard. Only a few studies have previously assessed the association between invasively measured central aortic BP and cardiovascular risk yielding conflicting results.26, 27, 28, 29 Although patients with CKD are at a high risk of cardiovascular events,30,31 no previous studies have focused on this group. The aim of the present cohort study was to examine whether invasively measured aortic systolic BP improves prediction of stroke, MI, and all-cause mortality beyond risk prediction by office systolic BP in patients with and without CKD who underwent elective CAG.
Methods
We included all patients undergoing CAG in Western Denmark from January 1, 2003 to December 31, 2016 who were registered in the Western Denmark Heart Registry (WDHR).32 This registry collects patient and procedure data from hospitals in Western Denmark. In the case of multiple examination of the same patient during the inclusion period, the first CAG was used as the index CAG. The data were linked to outcome data in the Danish National Patient Registry (DNPR),33 the Danish National Prescription Registry,34 and the Danish Civil Registration System,35 using the unique central personal registration number.35,36
Brachial office BP and invasively obtained aortic BP from the WDHR were used. We focused on systolic BP, because systolic BP increases when the pulse wave progresses from the aorta toward the peripheral arteries due to pulse wave amplification, while diastolic BP remains constant within a few mm Hg.4 The brachial office BP was measured using a cuff by the referring general practitioner or during the CAG admission. Information regarding measurement method (auscultatory or oscillometric) or number of BP measurements was not available. The invasively obtained aortic BP was measured in the ascending aorta during the CAG procedure using a fluid-filled catheter. Typically, a 6F Boston Scientific Expo Angiographic catheter (Boston Scientific, Natick, MA) with a length of 100 cm and an internal diameter of 1.4 mm attached to a NAMIC transducer (Navilyst Medical, Marlborough, MA) or similar were used although not routinely registered. Transducers were placed at the midaxillary line and calibrated to zero before each examination according to local standard-operation procedures. Catheters were inserted through a femoral sheath into the ascending aorta and invasive BP was measured prior to the angiography when the BP was stable. Patients undergoing CAG via brachial or radial artery access (n = 9418) were excluded to ensure use of invasive aortic BP measurements.
CKD was defined as eGFR below 60 ml/min per 1.73 m2. Plasma creatinine at the time of CAG as recorded in the WDHR was used for calculation of eGFR using the CKD-Epidemiology Collaboration equation.37 The eGFR estimate was also used for classification of CKD stages 1–5 according to Kidney Disease: Improving Global Outcomes guidelines.38 There was no data on proteinuria. Dialysis treatment was classified as either a dialysis diagnosis or a dialysis access diagnosis (arteriovenous fistula, dialysis catheter, peritoneal dialysis catheter) and absence of a renal transplantation diagnosis in the DNPR before CAG. Diabetes was defined as follows: either (i) treatment with insulin ± oral glucose-lowering drugs, oral glucose-lowering drugs alone, or nonpharmacological dietary treatment for diabetes, as recorded in the WDHR; (ii) a diabetes diagnosis recorded in the DNPR before or 1 month after CAG; or (iii) redemption of ≥1 prescription(s) for diabetes medication within 6 months before or 1 month after CAG, as recorded in Danish National Prescription Registry.34
Hypertension was defined as receipt of treatment for hypertension at the time of CAG as recorded in the WDHR or a diagnosis of hypertension registered in the DNPR. Prescription records for statins, antiplatelets, and antihypertensive drugs were obtained from Danish National Prescription Registry.34
Extent of coronary artery disease, that is, the number of coronary arteries with obstructive coronary artery disease (defined as ≥50% angiographic stenosis) was recorded as: 0-vessel, 1-vessel, 2-vessel or 3-vessel disease, or diffuse nonobstructive vessel disease. Comorbidities were evaluated using the Charlson Comorbidity Index score based on discharge diagnoses registered in the DNPR.39 We used a full look-back period of patient history before the study inclusion date.
Outcome Definition
The DNPR was used to identify admissions for MI (International Classification of Diseases, Tenth Revision codes DI21–21.9) and stroke (International Classification of Diseases, Tenth Revision DI60–61, DI629, DI63–64). Analyses were made separately for the first stroke and the first MI event occurring after the index CAG date. Information on all-cause death was obtained from the Civil Registration System.35
Ethical Considerations
The study was approved by the Danish Data Protection Agency (record no. 1-16-02-193-18). According to Danish law, approval from an ethics committee and informed consent from the patients were not required for registry-based studies.
Statistical Analysis
Continuous variables are reported as mean with SD for normally distributed data and as median (range) for skewed data. Normality was assessed by histograms and QQ-plots. The associations between BP with stroke, MI, and all-cause death were assessed in Cox regression models and are reported as hazard ratio per 10 mm Hg. The proportional hazards assumption was assessed by log-log plots and found to be fulfilled. Information on smoking status was missing in 4.6% of patients, height in 1.9% of patients, and weight in 1.7% of patients. These missing data were imputed by 20 imputations using chained equations as recommended for Cox-models.40 To avoid double registration of procedure-related events, follow-up as well as event registration was initiated 30 days after CAG. The following predefined variables were used in multivariable regression models: CKD (eGFR ≥60 ml/min per 1.73 m2 vs. eGFR <60 ml/min per 1.73 m2), age, sex, systolic BP, smoking (never/former/active), a categorized Charlson Comorbidity Index-score (0, 1, 2, or >2 points), hypertension (yes/no), antihypertensive treatment (0, 1, 2, or >2 drugs), statin treatment (yes/no), antiplatelet therapy (aspirin or adenosine diphosphate receptor inhibitors), extent of coronary artery disease (none, diffuse nonobstructive, 1, 2, or 3 vessel disease), atrial fibrillation (yes/no), diabetes (yes/no), and body mass index category in kg/m2 (<18.5 [underweight], 18.5–24.9 [normal], 25–29.9 [overweight], 30–34.9 [class 1 obesity], 35–39.9 [class 2 obesity], ≥40 [class 3 obesity]). These variables were assessed for inclusion in multivariable Cox models with and without stratification for CKD. We tested for interaction between the effects of CKD and the BP variables on outcomes. Significant interaction terms were observed in the stroke and all-cause mortality models (crude analysis) and for office systolic BP and MI (adjusted analysis). Second-order polynomials of the BP variables were tested for inclusion in all models and was significant in the all-cause mortality model and was therefore evaluated as a categorical variable. The added prediction, discrimination, and reclassification by invasive aorta BP compared to office BP were assessed using different strategies as follows: (i) aortic and office BP were included in the same Cox models, (ii) discrimination was assessed with Harrell’s C calculated for Cox models fitted with either aortic systolic BP or office systolic BP using bootstrapping with 50 replications,41 (iii) reclassification was assessed by the continuous net reclassification index after adding aortic systolic BP to models with office systolic BP,42 and (iv) the incremental value of adding aortic systolic BP to a model with brachial systolic BP was tested with a likelihood ratio test for goodness-of-fit of the model. A 2-tailed P-value <0.05 was considered to indicate statistical significance. Data was analyzed using Stata version 18 (StataCorp LP, TX).
Results
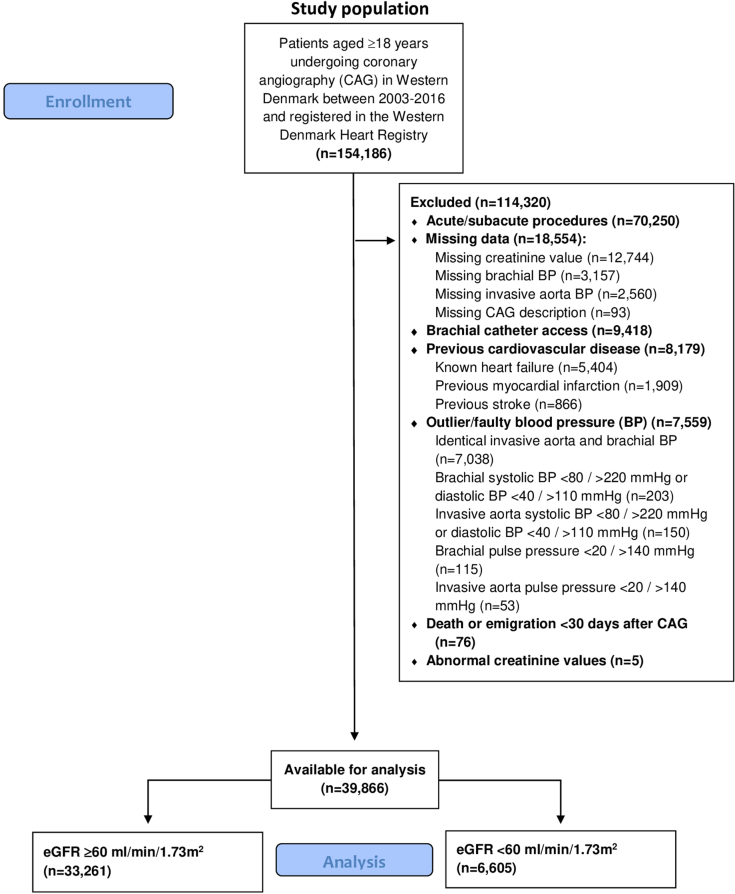
A total of 39,866 patients were included of which 6605 had CKD (eGFR <60 ml/min per 1.73 m2). Patients undergoing acute or subacute CAG, patients with known heart failure, patients with previous stroke or MI, and patients with faulty BP-values were excluded as shown in Figure 1. During a median follow-up of 7.2 years (interquartile range 4.6–10.1 years), 1367 strokes (353 in patients with CKD), 1858 MIs (446 in patients with CKD), and 7551 deaths (2515 in patients with CKD) were recorded. Baseline characteristics for the total cohort and CKD stratified are shown in Table 1. Most patients with renal disease had stage 3 CKD (88%) and only 5% were on dialysis treatment.
Figure 1.
Study flow chart describing patient selection process. BP, blood pressure; eGFR, estimated glomerular filtration rate.
Table 1.
Patient demographics
| Baseline characteristics | All |
eGFR ≥60 ml/min per 1.73 m2 |
eGFR <60 ml/min per 1.73 m2 |
P-value |
|---|---|---|---|---|
| N = 39,866 | n = 33,261 | n = 6605 | ||
| Age | 64 (11) | 63 (10) | 72 (10) | <0.001 |
| Sex (males) | 58% (23,259) | 60% (19,988) | 50% (3271) | <0.001 |
| Weight (kg)a | 81.3 (16.7) | 81.8 (16.8) | 78.7 (15.4) | <0.001 |
| Height (cm)a | 172 (9) | 172 (9) | 169 (9) | <0.001 |
| BMI (kg/m2)a | 27.5 (4.8) | 27.5 (4.8) | 27.4 (4.7) | 0.01 |
| Smokinga | <0.001 | |||
| Active | 21% (8564) | 23% (7617) | 14% (947) | |
| Never | 34% (13,432) | 33% (11,098) | 35% (2334) | |
| Former | 40% (16,043) | 40% (13,172) | 43% (2871) | |
| Unknown or missing status | 5% (1827) | 4% (1374) | 7% (453) | |
| Charlson comorbidity index | <0.001 | |||
| 0 | 70.3% (28,023) | 73.0% (24,287) | 56.6% (3736) | |
| 1 | 15.4% (6159) | 15.4% (5121) | 15.7% (1038) | |
| 2 | 8.1% (3210) | 7.0% (2314) | 13.6% (896) | |
| >2 | 6.2% (2474) | 4.6% (1539) | 14.2% (935) | |
| Diabetes | 16% (6521) | 15% (5126) | 21% (1395) | <0.001 |
| Hypertension | 64% (25,515) | 61% (20,283) | 79% (5232) | <0.001 |
| Atrial fibrillation before CAG | 11% (4405) | 10% (3292) | 17% (1113) | <0.001 |
| Antihypertensive treatment (drugs) | <0.001 | |||
| 0 | 16.0% (6368) | 18.1% (6006) | 5.5% (362) | |
| 1 | 29.7% (11,830) | 31.8% (10,581) | 18.9% (1249) | |
| 2 | 27.6% (10,998) | 27.3% (9072) | 29.2% (1926) | |
| >2 | 26.8% (10,670) | 22.9% (7602) | 46.4% (3068) | |
| ACE inhibitor treatment | 29% (11,432) | 27% (9036) | 36% (2396) | <0.001 |
| ARB treatment | 19% (7581) | 17% (5724) | 28% (1857) | <0.001 |
| CCB treatment | 35% (13,972) | 33% (11,011) | 45% (2961) | <0.001 |
| Beta-blocker treatment | 57% (22,701) | 55% (18,381) | 65% (4320) | <0.001 |
| Thiazide treatment | 18% (7304) | 17% (5670) | 25% (1634) | <0.001 |
| Loop diuretic treatment | 14% (5699) | 11% (3509) | 33% (2190) | <0.001 |
| MRA treatment | 3% (1385) | 3% (881) | 8% (504) | <0.001 |
| Statin treatment | 72% (28,803) | 72% (24,043) | 72% (4760) | 0.72 |
| Antiplatelet therapy | 76% (30,163) | 76% (25,200) | 75% (4963) | 0.28 |
| Aspirin treatment | 72% (28,802) | 72% (24,071) | 72% (4731) | 0.22 |
| ADP-inhibitor treatment | 18% (7367) | 18% (6132) | 19% (1235) | 0.62 |
| VKA/NOAC treatment | 10% (3965) | 9% (2932) | 16% (1033) | <0.001 |
| VKA treatment | 8% (3385) | 7% (2464) | 14% (921) | <0.001 |
| NOAC treatment | 2% (684) | 2% (540) | 2% (144) | 0.001 |
| CAG indication | <0.001 | |||
| SAP | 70% (27,827) | 71% (23,759) | 62% (4068) | |
| Valve disease | 10% (4061) | 9% (2933) | 17% (1128) | |
| Unspecified chest pain | 7% (2887) | 8% (2615) | 4% (272) | |
| Other | 5% (1998) | 4% (1,443) | 8% (555) | |
| Cardiomyopathy | 2% (869) | 2% (665) | 3% (204) | |
| Missing | 2% (798) | 2% (621) | 3% (177) | |
| Arythmia | 2% (781) | 2% (673) | 2% (108) | |
| UAP | 1% (242) | 1% (220) | 0% (22) | |
| Control/Complication/STEMI | <1% (57) | <1% (42) | <1% (15) | |
| Coronary artery disease | <0.001 | |||
| 0 VD | 44% (17,711) | 46% (15,259) | 37% (2452) | |
| Diffuse VD | 13% (5270) | 13% (4404) | 13% (866) | |
| 1 VD | 20% (7824) | 20% (6524) | 20% (1300) | |
| 2 VD | 12% (4596) | 11% (3719) | 13% (877) | |
| 3 VD | 11% (4465) | 10% (3355) | 17% (1110) | |
| eGFR (ml/min per 1.73 m2) | 80 (66–92) | 84 (73–94) | 51 (42–56) | <0.001 |
| CKD-stage | <0.001 | |||
| 1 | 28% (11,300) | 34% (11,300) | ||
| 2 | 55% (21,961) | 66% (21,961) | ||
| 3 | 15% (5828) | 88% (5828) | ||
| 4 | 1% (332) | 5% (332) | ||
| 5 | 0% (129) | 2% (129) | ||
| 5d | 1% (316) | 5% (316) | ||
| Brachial/Office systolic BP (mm Hg) | 143 (20) | 143 (19) | 145 (21) | <0.001 |
| Brachial/Office diastolic BP (mm Hg) | 81 (11) | 81 (11) | 79 (11) | <0.001 |
| Invasive aortic systolic BP (mm Hg) | 145 (23) | 144 (23) | 147 (24) | <0.001 |
| Invasive aortic diastolic BP (mm Hg) | 72 (12) | 72 (12) | 69 (13) | <0.001 |
| Brachial/Office pulse pressure (mm Hg) | 62 (17) | 61 (17) | 66 (17) | <0.001 |
| Invasive aortic pulse pressure (mm Hg) | 72 (21) | 72 (21) | 78 (22) | <0.001 |
ACE, angiotensin-converting enzyme; ADP, adenosine diphosphate receptor inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; BP, blood pressure; CAG, coronary angiography; CCB, calcium channel blocker; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; MRA, mineralocorticoid receptor antagonists; NOAC, non-vitamin K antagonist oral anticoagulants; SAP, stable angina pectoris; STEMI, ST-elevation myocardial infarction; UAP, unstable angina pectoris; VD, vessel disease; VKA, vitamin K antagonist treatment.
Weight: n = 675 (all), n = 541 (eGFR ≥60), n = 134 (eGFR <60).
Height: n = 749 (all), n = 595 (eGFR ≥60), n = 154 (eGFR <60).
BMI: n = 855 (all), n = 677 (eGFR ≥60), n = 178 (eGFR <60).
Smoking: n = 1827 (all), n = 1374 (eGFR ≥60), n = 453 (eGFR <60).
Missing data on weight, height, and smoking status were imputed as described in the methods section.
Denotes baseline variables with missing data:
Cuff-Based Office Systolic BP Versus Invasive Aortic Systolic BP
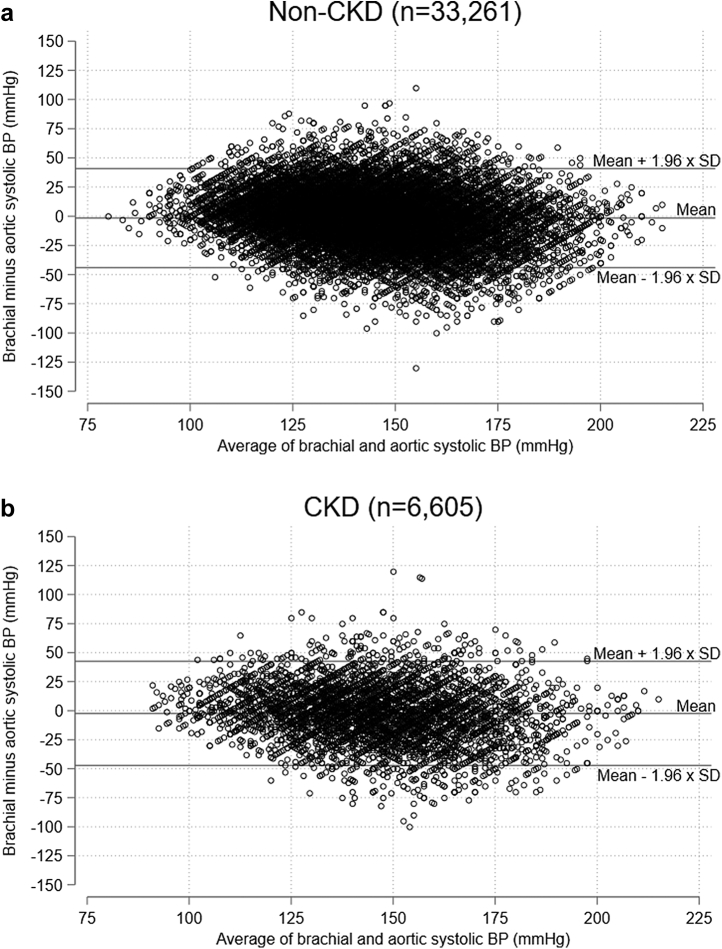
The difference between brachial cuff-based systolic BP and invasive aortic systolic BP was compared with Bland-Altman plots for non-CKD patients (Figure 2a) and patients with CKD (Figure 2b). As illustrated, the limits of agreement were wide regardless of CKD status, most likely reflecting the fact that brachial BP and invasive aortic BP were not obtained simultaneously. The mean difference with 95% confidence interval between cuff-based brachial systolic BP and invasive aortic BP was −1.6 (−1.8 to −1.3) mm Hg for non-CKD patients and −2.4 (−2.9 to −1.8) mm Hg for patients with CKD. The difference was 0.8 (0.2–1.4) mm Hg higher in non-CKD patients (P = 0.001). Apart from renal function, the difference between cuff-based brachial systolic BP and invasive aortic BP was also influenced by age, sex, pulse pressure, beta-blocker treatment, and heart rate (see Supplementary Figures S1–S7 and Supplementary Tables S1–S3).
Figure 2.
Bland-Altman plots: (a) Non-CKD patients; (b) CKD patients, showing difference between cuff-based brachial office systolic BP and invasively obtained aortic systolic BP. BP, blood pressure; CKD, chronic kidney disease; SD, standard deviation.
Associations Between CKD and Stroke, MI, and All-Cause Mortality
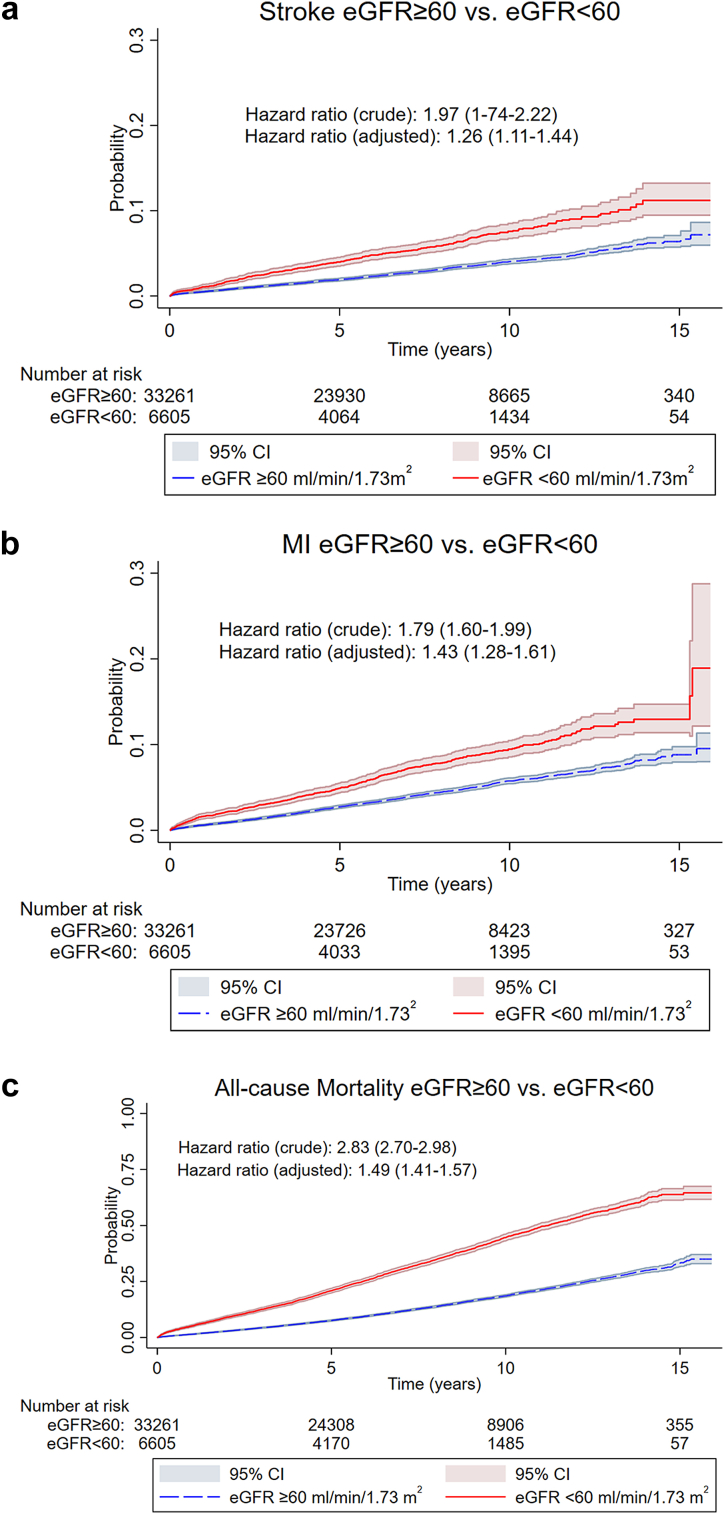
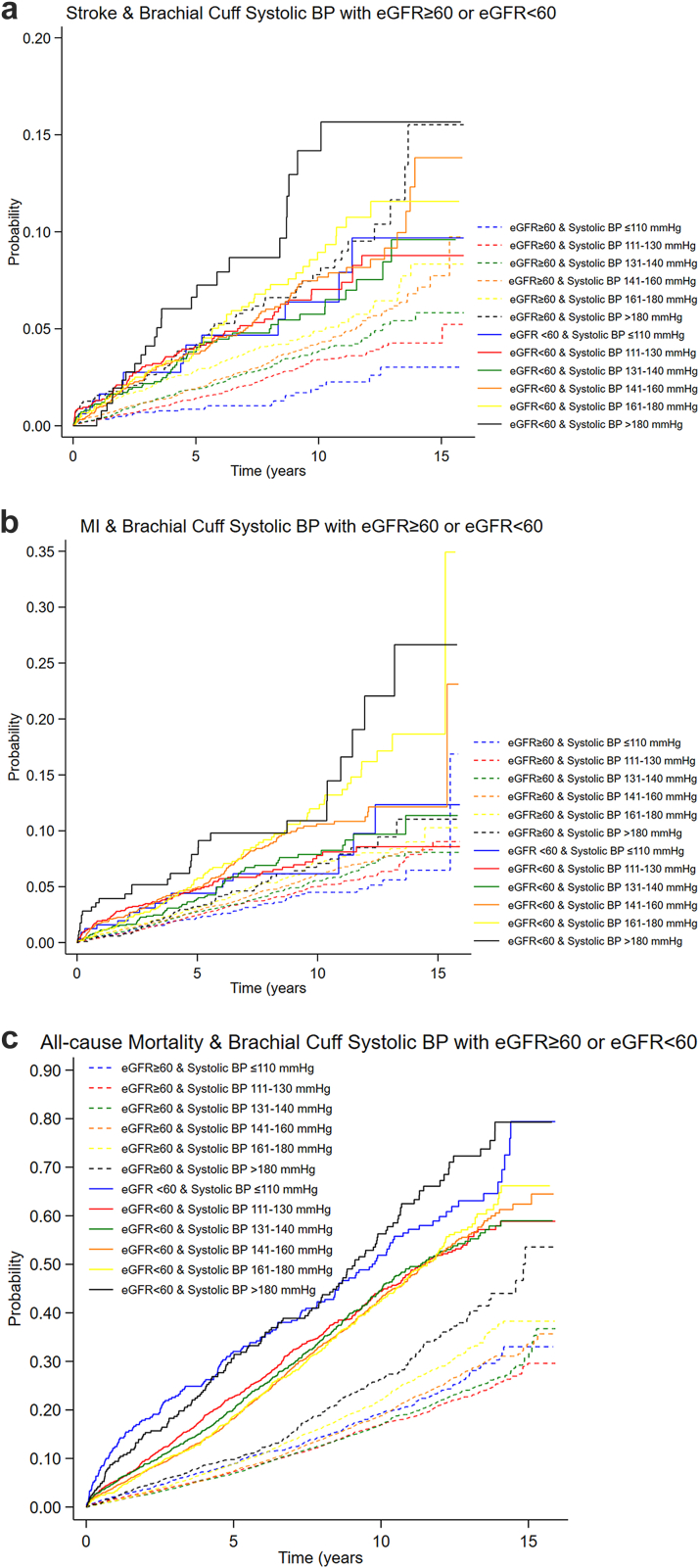
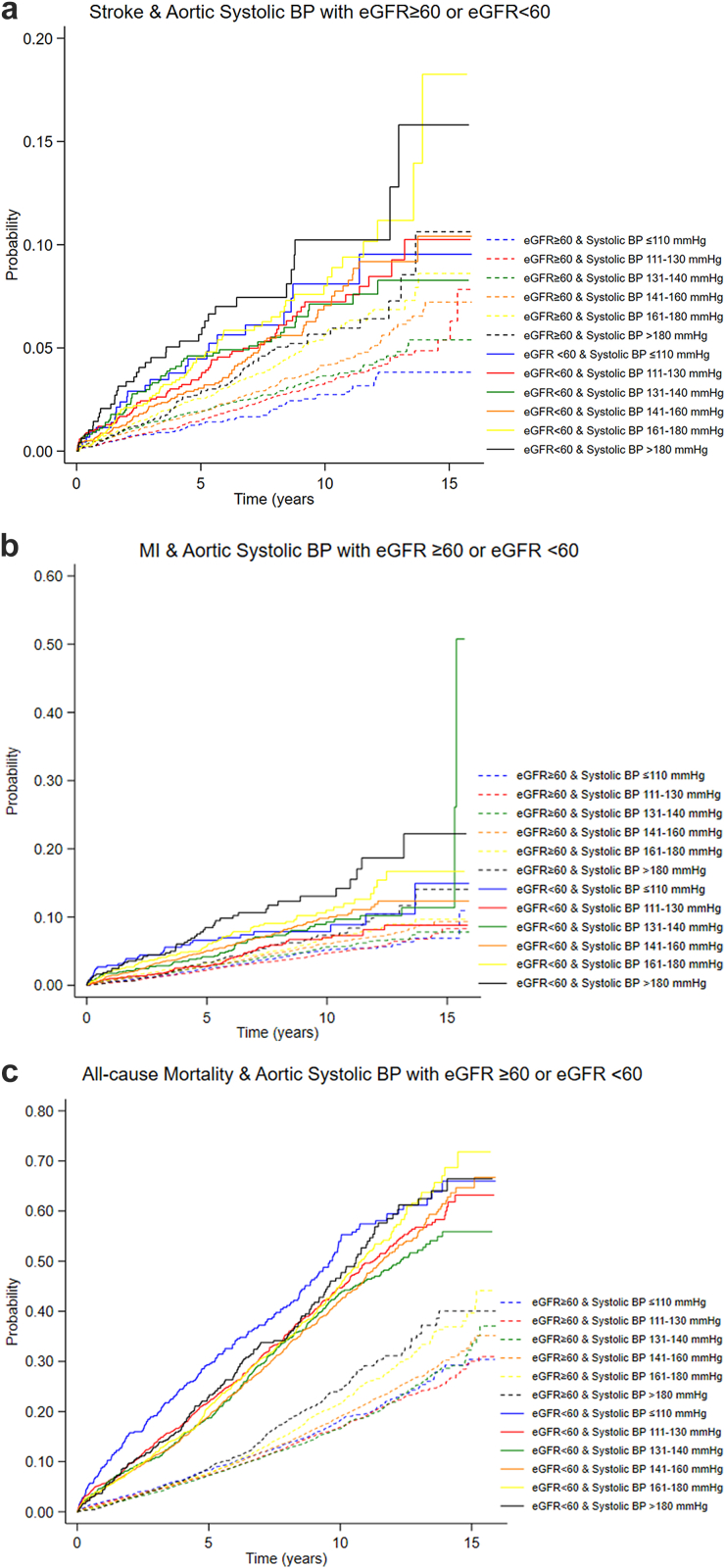
CKD was significantly associated with increased risk of stroke, MI, and all-cause mortality as illustrated in Figure 3a–c with crude and adjusted hazard ratios and Kaplan-Meier failure curves. In Figures 4 and 5, we show Kaplan-Meier failure curves stratified for CKD and BP-level based on either cuff-based office systolic BP or invasive aortic systolic BP.
Figure 3.
(a) Kaplan Meier failure curves with stroke as outcome according to CKD status. (b) Kaplan Meier failure curves with MI as outcome. (c) Kaplan Meier failure curves with all-cause mortality as outcome.
Parameters included in the CKD stratified adjusted models:
Stroke: age, sex, smoking (never, former, and active), number of diseased vessels (none, diffuse coronary atherosclerosis without significant [>50%] stenosis/1, 2, or 3 vessel disease), atrial fibrillation (yes/no), diabetes (yes/no), statin treatment (yes/no), antiplatelet treatment (yes/no), antihypertensive drugs prescribed (0, 1, 2, or >2), and BMI category (kg/m2): (<18.5 [underweight], 18.5–24.9 [normal], 25–29.9 [overweight], 30–34.9 [class 1 obesity], 35–39.9 [class 2 obesity], ≥40 [class 3 obesity]).
MI: age, sex, smoking (never, former, and active), number of diseased vessels (none, diffuse coronary atherosclerosis without significant [>50%] stenosis/1, 2, or 3 vessel disease), diabetes (yes/no), hypertension (yes/no), statin treatment (yes/no), antiplatelet treatment (yes/no), and BMI category (kg/m2): (<18.5 [underweight], 18.5–24.9 [normal], 25–29.9 [overweight], 30–34.9 [class 1 obesity], 35–39.9 [class 2 obesity], ≥40 [class 3 obesity]).
All-cause mortality: age, sex, smoking (never, former, active), modified Charlson comorbidity index (0/1/2/>2), number of diseased vessels (none, diffuse coronary atherosclerosis without significant [>50%] stenosis/1, 2, or 3 vessel disease), and BMI category (kg/m2): (<18.5 [underweight], 18.5–24.9 [normal], 25–29.9 [overweight], 30–34.9 [class 1 obesity], 35–39.9 [class 2 obesity], ≥40 [class 3 obesity]). BMI, body mass index; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; MI, myocardial infarction; 95% CI, 95% confidence interval.
Figure 4.
Outcomes: (a) Stroke; (b) MI; (c) All-cause mortality, based on cuff-based brachial office systolic BP and stratified for CKD (eGFR <60 ml/min per 1.73 m2). BP, blood pressure; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; MI, myocardial infarction.
Figure 5.
Outcomes: (a) Stroke; (b) MI; (c) All-cause mortality, based on invasive aortic systolic BP and stratified for CKD (eGFR<60 ml/min per 1.73 m2). BP, blood pressure; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; MI, myocardial infarction.
Associations Between Brachial Office BP and Aortic Systolic BP and Stroke
CKD significantly modified the association with stroke for both office and aortic systolic BP in crude analyses but not in adjusted analyses. Accordingly, results are presented stratified by CKD (Table 2). In the adjusted analyses including all patients, both office and aortic systolic BP were significantly associated with stroke. In non-CKD patients, both office and aortic systolic BP were significantly associated with stroke in both crude and adjusted analyses. In patients with CKD, only office BP was significantly associated with stroke in crude analysis. In multivariable analyses in patients with CKD, both office and aortic systolic BP were borderline significant in terms of stroke prediction, and in adjusted analyses based on complete cases (omitting patients with missing body mass index and smoking status values) both office and aortic systolic BP were significant (Supplementary Table S4).
Table 2.
Hazard ratios with 95% CI for the association between office or aortic systolic BP and the incidence of stroke, MI, and all-cause mortality
| Hazard ratios and outcome prediction |
All (N = 39,866) |
Patients with eGFR ≥60 ml/min per 1.73 m2 (n = 33,261) |
Patients with eGFR <60 ml/min per 1.73 m2 (n = 6605) |
||||
|---|---|---|---|---|---|---|---|
| Outcome | Office Systolic BP |
Aortic Systolic BP |
Office Systolic BP |
Aortic Systolic BP |
Office Systolic BP |
Aortic Systolic BP |
|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | ||
| Stroke | |||||||
| Crude | d(P = 0.006) | d(P = 0.04) | 1.15 (1.11–1.18)c | 1.10 (1.07–1.13)c | 1.055 (1.004–1.109)a | 1.040 (0.995–1.087) | |
| Adjusted | 1.08 (1.05–1.11)c | 1.06 (1.03–1.08)c | 1.08 (1.05–1.12)c | 1.06 (1.03–1.09)c | 1.05 (1.00–1.10) | 1.043 (0.999–1.090) | |
| MI | Crude | 1.08 (1.05–1.10)c | 1.08 (1.06–1.10)c | 1.06 (1.03–1.09)c | 1.07 (1.04–1.09)c | 1.10 (1.05–1.15)c | 1.09 (1.05–1.13)c |
| Adjusted | d(P = 0.01) | 1.05 (1.03–1.07)c | 1.01 (0.99–1.04) | 1.04 (1.02–1.07)b | 1.08 (1.03–1.13)b | 1.08 (1.04–1.12)c | |
| All-cause mortality | Crude | ||||||
| ≤110 | 1.27 (1.14–1.41)c | 1.12 (1.02–1.23)a | 1.18 (1.03–1.34)a | 1.06 (0.94–1.18) | 1.35 (1.13–1.60)b | 1.26 (1.08–1.48)b | |
| 111–130 | 1 | 1 | 1 | 1 | 1 | 1 | |
| 131–140 | 1.02 (0.95–1.10) | d(P = 0.006) | 1.03 (0.94–1.12) | 1.01 (0.92–1.10) | 0.97 (0.86–1.09) | 0.90 (0.79–1.03) | |
| 141–160 | d(P = 0.002) | d(P < 0.001) | 1.15 (1.06–1.24)c | 1.11 (1.03–1.20)b | 0.93 (0.84–1.04) | 0.93 (0.83–1.04) | |
| 161–180 | d(P < 0.001) | d(P < 0.001) | 1.37 (1.25–1.50)c | 1.28 (1.17–1.40)c | 0.95 (0.83–1.08) | 1.02 (0.90–1.16) | |
| >180 | 1.77 (1.58–1.97)c | d(P < 0.001) | 1.69 (1.47–1.95)c | 1.43 (1.26–1.61)c | 1.46 (1.22–1.75)c | 1.08 (0.92–1.28) | |
| Adjusted | |||||||
| ≤110 | 1.32 (1.19–1.47)c | 1.14 (1.04–1.25)b | 1.30 (1.14–1.48)c | 1.16 (1.04–1.30)b | 1.36 (1.15–1.62)c | 1.12 (0.96–1.32) | |
| 111–130 | 1 | 1 | 1 | 1 | 1 | 1 | |
| 131–140 | 0.94 (0.88–1.01) | 0.90 (0.84–0.97)b | 0.94 (0.86–1.02) | 0.90 (0.83–0.99)a | 0.95 (0.84–1.08) | 0.89 (0.79–1.02) | |
| 141–160 | 0.95 (0.89–1.01) | 0.93 (0.87–0.99)a | 0.97 (0.90–1.04) | 0.93 (0.86–1.00) | 0.90 (0.81–1.01) | 0.92 (0.82–1.03) | |
| 161–180 | 0.97 (0.90–1.05) | 0.94 (0.88–1.01) | 0.99 (0.91–1.09) | 0.93 (0.85–1.01) | 0.91 (0.80–1.03) | 0.94 (0.83–1.07) | |
| >180 | 1.21 (1.08–1.35)b | 0.99 (0.89–1.09) | 1.08 (0.94–1.25) | 0.93 (0.82–1.06) | 1.41 (1.17–1.69)c | 1.04 (0.88–1.23) | |
BMI, body mass index; BP, blood pressure; eGFR, estimated glomerular filtration rate; HR, hazard ratio; MI, myocardial infarction; 95% CI, 95% confidence interval.
Results are presented for all participants and stratified by CKD status (eGFR<60 or eGFR<60 ml/min per 1.73m2). Results for stroke and MI are presented per 10 mm Hg difference. Results for all-cause mortality are presented per BP category in comparison to the reference category (111–130 mm Hg).
Adjusted models:.
Stroke: age, sex, smoking (never, former, and active), number of diseased vessels (none, diffuse coronary atherosclerosis without significant [>50%] stenosis/1, 2, or 3 vessel disease), atrial fibrillation (yes/no), diabetes (yes/no), statin treatment (yes/no), antiplatelet treatment (yes/no), antihypertensive drugs prescribed (0, 1, 2, or >2), and BMI category (kg/m2): (<18.5 [underweight], 18.5–24.9 [normal], 25–29.9 [overweight], 30–34.9 [class 1 obesity], 35–39.9 [class 2 obesity], ≥40 [class 3 obesity]).
MI: age, sex, smoking (never, former, and active), number of diseased vessels (none, diffuse coronary atherosclerosis without significant [>50%] stenosis/1, 2, or 3 vessel disease), diabetes (yes/no), hypertension (yes/no), statin treatment (yes/no), antiplatelet treatment (yes/no), and BMI category (kg/m2): (<18.5 [underweight], 18.5–24.9 [normal], 25–29.9 [overweight], 30–34.9 [class 1 obesity], 35–39.9 [class 2 obesity], ≥40 [class 3 obesity]).
All-cause mortality: age, sex, smoking (never, former, active), modified Charlson comorbidity index (0/1/2/>2), number of diseased vessels (none, diffuse coronary atherosclerosis without significant [>50%] stenosis/1, 2, or 3 vessel disease), and BMI category (kg/m2): (<18.5 [underweight], 18.5–24.9 [normal], 25–29.9 [overweight], 30–34.9 [class 1 obesity], 35–39.9 [class 2 obesity], ≥40 [class 3 obesity]).
P < 0.05
P < 0.01
P < 0.001
Indicates that the interaction term eGFR <60 ml/min x office systolic BP/aortic systolic BP was significant.
Associations Between Brachial Office BP and Aortic Systolic BP and MI
In the total study population, both office and aortic BP were associated with MI in crude analyses. This association was not significantly modified by the presence of CKD. However, in adjusted analyses, the association between office BP and MI was significantly modified by the presence of CKD and adjusted analyses were done stratified for CKD. The association between aortic systolic BP and MI was not significantly modified by the presence of CKD in adjusted analysis. In non-CKD patients, office BP lost significance in terms of MI prediction; however, aortic systolic BP remained significant. In patients with CKD, both office and aortic BP were significantly associated with MI in adjusted analyses (Table 2).
Associations Between Office and Aortic Systolic BP and All-Cause Mortality
CKD significantly modified the association with all-cause mortality for both office and aortic systolic BP in crude analyses. Accordingly, results are presented stratified by CKD (Table 2). In non-CKD patients, both office and aortic systolic BP were significantly associated with death in crude analyses. In patients with CKD, only low (≤110 mm Hg) or high (>180 mm Hg) office BP were significantly associated with death in crude analysis. Only aortic systolic BP ≤110 mm Hg was significantly associated with death in crude analysis in CKD. In multivariable analysis, CKD did not significantly modify the association with all-cause mortality. In the total study population, only low (≤110 mm Hg) or high (>180 mm Hg) office systolic BP were significant in adjusted analysis. Low (≤110 mm Hg) aortic systolic BP was also associated with increased risk of death in the total study population in adjusted analysis. Aortic systolic BP categories 131–140 mm Hg and 141–160 mm Hg were associated with a lower risk of death in the total study population in adjusted analysis. When stratified for CKD both office and aortic systolic BP ≤110 mm Hg were significantly associated with increased risk of death in non-CKD patients in adjusted analyses. In patients with CKD, office systolic BP ≤110 mm Hg or >180 mm Hg were significantly associated with death. Aortic systolic BP lost significance in patients with CKD in adjusted analysis.
Relative Prognostic Contribution of Office Versus Aortic Systolic BP
The relative prognostic contribution of office versus aortic systolic BP was assessed for the associations where both BP indices were significantly associated with the outcome using patients with complete data shown in Supplementary Table S1. There was no significant difference between office and aortic systolic BP in the crude models, but Harrel’s C-values were quite low regardless of using aortic or office systolic BP (Table 3). The adjusted models performed better with Harrel’s C-values around 0.7 indicating good models in terms of predicting outcome. Yet, still there was no significant difference between prediction of outcome with office or aortic systolic BP in adjusted models. Continuous net reclassification index was barely significant with stroke as outcome in non-CKD patients but otherwise nonsignificant when aortic BP was added to adjusted Cox models with office BP. In adjusted models, the likelihood ratio test was only significant with stroke as outcome (all patients) and MI as outcome (patients with CKD), suggesting that the incremental model fit from adding aortic systolic BP to a multivariable model with office BP is limited most likely due to a high degree of collinearity between office and aortic systolic BP.
Table 3.
Evaluation of the prognostic value of aortic systolic BP as compared with office systolic BP
| Stroke,all patients (N = 37,316) | |||
| Cox regression with both BPvariables in the same model | HR (95% CI) | P-value | |
| Adjusted | office BP | 1.06 (1.03–1.10) | <0.001 |
| aortic BP | 1.03 (1.01–1.06) | 0.02 | |
| C-statistics | Harrel’s C (95% CI) | ||
| Cox regression with office BP versus Cox regression with aortic BP | office BP | aortic BP | |
| Adjusted | 0.70 | 0.70 | |
| Difference | 0.003 (-0.005 to 0.006) | 0.10 | |
| Reclassification (aortic BP added to model with office BP) | continuous net reclassification index (95% CI) | ||
| Adjusted | 4.78 (-8.4 to 10.41) % | 0.10 | |
| Likelihood ratio test (model with office BP vs. model with aortic BP and office BP) for goodness-of-fit | |||
| Adjusted | 0.02 | ||
| Stroke, nonrenal patients (eGFR ≥60 ml/min per 1.73 m2) (n = 31,306) | |||
| Cox regression with both BP variables in the same model | HR (95% CI) | P-value | |
| Crude | office BP | 1.11 (1.07–1.15) | <0.001 |
| aortic BP | 1.05 (1.02–1.08) | 0.001 | |
| Adjusted | office BP | 1.07 (1.03–1.11) | <0.001 |
| aortic BP | 1.031 (0.999–1.065) | 0.06 | |
| C-statistics | Harrel's C (95% CI) | ||
| Cox model with office BP versus Cox model with aortic BP | office BP | aortic BP | |
| Crude | 0.58 | 0.56 | |
| Difference | 0.013 (−0.007 to 0.032) | 0.21 | |
| Adjusted | 0.70 | 0.70 | |
| Difference | 0.0030 (−0.0003 to 0.0064) | 0.07 | |
| Reclassification (aortic BP added to model with office BP) | continuous net reclassification index (95% CI) | ||
| Crude | 8.64 (2.38–14.89) % | 0.007 | |
| Adjusted | 6.90 (0.44–13.36) % | 0.04 | |
| Likelihood ratio test (model with office BP vs. model with aortic BP and office BP) for goodness-of-fit | |||
| Crude | 0.001 | ||
| Adjusted | 0.06 | ||
| Stroke, patients with CKD (eGFR <60 ml/min per 1.73 m2) (n = 6010) | |||
| Cox regression with both BP variables in the same model | HR (95% CI) | P-value | |
| Adjusted | office BP | 1.04 (0.98–1.11) | 0.20 |
| aortic BP | 1.03 (0.98–1.09) | 0.21 | |
| C-statistics | Harrel’s C (95% CI) | ||
| Cox model with office BP vs. Cox model with aortic BP | office BP | aortic BP | |
| Adjusted | 0.64 | 0.64 | |
| Difference | 0.002 (−0.005 to 0.010) | 0.55 | |
| Reclassification (aortic BP added to model with office BP) | continuous net reclassification index (95% CI) | ||
| Adjusted | −2.07 (−13.56 to 9.41) % | 1.28 | |
| Likelihood ratio test (model with office BP vs. model with aortic BP and office BP) for goodness-of-fit | |||
| Adjusted | 0.21 | ||
| MI, patients with CKD (eGFR <60 ml/min per 1.73 m2) (n = 6010) | |||
| Cox regression with both BP variables in the same model | HR (95% CI) | P-value | |
| Crude | office BP | 1.07 (1.02–1.12) | 0.01 |
| aortic BP | 1.06 (1.01–1.11) | 0.01 | |
| Adjusted | office BP | 1.052 (0.999–1.109) | 0.06 |
| aortic BP | 1.051 (1.004–1.100) | 0.03 | |
| C-statistics | Harrel's C (95% CI) | ||
| Cox model with office BP vs. Cox model with aortic BP | office BP | aortic BP | |
| Crude | 0.54 | 0.55 | |
| Difference | −0.0017 (−0.044 to 0.009) | 0.20 | |
| Adjusted | 0.72 | 0.72 | |
| Difference | −0.0006 (−0.0045 to 0.0033) | 0.77 | |
| Reclassification (aortic BP added to model with office BP) | continuous net reclassification index (95% CI) | ||
| Crude | 10.45 (0.84–20.06) % | 0.03 | |
| Adjusted | 5.43 (−4.72 to 15.59) | 0.29 | |
| Likelihood ratio test (model with office BP vs. model with aortic BP and office BP) for goodness-of-fit | |||
| Crude | 0.01 | ||
| Adjusted | 0.03 | ||
BMI, body mass index; BP, blood pressure; CKD, chronic kidney disease; HR, hazard ratio; MI, myocardial infarction; 95% CI, 95% confidence interval.
HR results for stroke and MI are presented per 10 mm Hg difference. Evaluation of aortic versus office systolic BP was only performed in the subgroups in which both BP indices were associated with the outcome (stroke and MI) when tested in separate Cox models, using patients with complete data (n = 37,316 hereof n = 6010 with eGFR <60 ml/min per 1.73 m2 due to missing covariate values for BMI and smoking) (Supplementary Table S4).
The prognostic contribution of aortic and office systolic BP was assessed using complementary statistical strategies as follows: (i) by simultaneously including both BP indices in the same Cox models (crude and adjusted), (ii) discrimination was assessed based on Harrell’s C calculated for Cox models (crude and adjusted) fitted with either aortic systolic BP or office systolic BP, (iii) improvement in reclassification by adding aortic systolic BP to models with office systolic BP (crude and adjusted) was assessed by the continuous net reclassification index, and (iv) the incremental value of adding aortic systolic BP to a model with brachial systolic BP was tested with a likelihood ratio test for goodness-of-fit of the model with or without aortic systolic BP in the model.
Adjusted models for stroke includes: age, sex, smoking (never, former, and active), number of diseased vessels (none, diffuse coronary atherosclerosis without significant [>50%] stenosis/1, 2, or 3 vessel disease), atrial fibrillation (yes/no), diabetes (yes/no), statin treatment (yes/no), antiplatelet treatment (yes/no), antihypertensive drugs prescribed (0, 1, 2, or >2), and BMI category (kg/m2): (<18.5 [underweight], 18.5–24.9 [normal], 25–29.9 [overweight], 30–34.9 [class 1 obesity], 35–39.9 [class 2 obesity], ≥40 [class 3 obesity]).
Adjusted models for MI includes: age, sex, smoking (never, former, and active), number of diseased vessels (none, diffuse coronary atherosclerosis without significant [>50%] stenosis/1, 2, or 3 vessel disease), diabetes (yes/no), hypertension (yes/no), statin treatment (yes/no), antiplatelet treatment (yes/no), BMI category (kg/m2): (<18.5 [underweight], 18.5–24.9 [normal], 25–29.9 [overweight], 30–34.9 [class 1 obesity], 35–39.9 [class 2 obesity], ≥40 [class 3 obesity]).
Discussion
The main finding of this study is that event prediction in terms of stroke, MI, and all-cause mortality was not improved by invasively obtained central aortic systolic BP compared to conventional cuff-based systolic BP regardless of CKD status. Our study elaborates previous findings but included more patients with longer follow-up. More importantly, it provided additional insight regarding the presence of renal disease and cardiovascular outcome. Therefore, as a novelty, in a large cohort of patients with CKD, our study compared invasively measured aortic systolic BP and cuff-based brachial systolic BP in terms of outcome prediction focusing on stroke, MI, and all-cause mortality.
For many years, both in patients with CKD 43,44 and those without CKD,8,14,16 central aortic BP has been proposed as a potentially better risk marker than conventional cuff-based brachial BP. The rationale behind this idea originates mainly from physiology and because of the difference in BP-response to antihypertensive medication when aortic and peripheral BP were compared as demonstrated in the CAFE study.6 Systolic BP is amplified because the pulse wave generated by the cardiac systole progresses from the aorta to the peripheral arteries.4 This amplification varies between individuals, and the systolic BP in the brachial artery may differ significantly from the aortic BP, to which the heart, brain, and kidneys are exposed.3,4 Despite the plausible physiology and the fact that antihypertensive medications can exert differential effects on brachial and central pressure,5, 6, 7 the added prognostic value of aortic BP remains debated45,46 and the latest study in patients with CKD did not find central BP superior to conventional cuff BP.18 The latter study used noninvasive assessment of central aortic BP and could be affected by inherent inaccuracies and calibration problems as previously demonstrated by our group.13,47 Therefore, noninvasive estimates of central BP progressively underestimate invasive central systolic BP with decreasing renal function and increasing arterial stiffness in patients with CKD.13 Use of invasive aortic measurements obviously bypasses this problem. The mean difference between cuff-based brachial systolic BP and invasive aortic systolic BP in the present study was similar to previous findings by our group (non-CKD: −1.4 [−3.9 to 1.0] mm Hg and CKD: 3.0 [−0.3 to 6.3] mm Hg)13 although the difference between non-CKD and CKD was smaller in the present study and with much wider limits of agreement, most likely caused by nonsimultaneous and nonstandardized cuff-based office BP and invasive aortic BP measurements in the present study.
Until now, the prognostic impact of invasively measured aortic BP has been assessed in 5 previous studies.26, 27, 28, 29,48 Three of these focused on the strength of the association between aortic BP with major cardiovascular events and mortality, and did not report associations with brachial office BP.26,27,29 The present study and 2 previous studies from our group28,48 expanded on the available studies by presenting the discrimination of aortic BP with C-index and its confidence limits for models, including aortic BP and established risk markers. Ideally, a new risk marker should demonstrate added predictive value beyond current best practice, in this case cuff-based office BP, by the improvement of discrimination and risk classification.49 The present study found no indication of superiority of invasively measured systolic BP compared with office systolic BP. When discrimination and reclassification were assessed, our data did not show improvement in discrimination and reclassification in models with aortic systolic BP as compared with office systolic BP. However, it should be acknowledged that both office systolic BP and aortic systolic BP performed rather poorly in crude analyses regardless of CKD status with Harrel’s C-values close to 0.5, which equals random chance in terms of prognostic value indicating the importance of additional factors (age, smoking status, diabetes, and number of diseased coronary vessels, etc.). Moreover, it highlights the fact that our population overall is a high-risk population in which BP, not necessarily, is the best predictor in terms of outcome. Therefore, in younger patients with lower cardiovascular risk where differences between aortic and brachial BP are found to be higher observations may be different. Unfortunately, it is highly unlikely that invasive aortic BP measurements will become available for comparison with office BP measurements from a young low-risk population because such patients do not undergo aortic catheterization.
In line with previous studies, patients with CKD in our cohort had elevated risk of stroke, MI, and all-cause mortality compared to non-CKD patients demonstrating a clear association between progression of cardiovascular risk and decline in renal function.30,31 Previous studies in patients with CKD have demonstrated a U-shaped association between systolic BP and cardiovascular outcome. This U-shaped association has been shown to disappear after adjustment for known cardiovascular disease.50 We omitted patients with known previous cardiovascular disease (heart failure, MI, and stroke) from our cohort and found a linear relationship with both office and aortic systolic BP for stroke and MI as outcome. Regarding all-cause mortality, our data suggest a U-shaped association with a higher risk of death with both office and aortic systolic BP <110 mm Hg. This indicates reverse causation due to undiagnosed premorbid conditions (heart failure and frailty) which inevitably weakens associations with systolic BP, but fits well with previous observations of a decline in systolic BP prior to death.51
Strengths and Limitations
This study is the largest to-date based on data from invasive aortic BP measurements and at the same time including patients with CKD. Our study was conducted in Denmark, which has a national tax-based universal healthcare system, thereby reducing selection bias caused by selective inclusion of specific hospitals, health insurances, or age groups. Follow-up and outcome ascertainment were standardized through high-quality national registers,36 and patients were followed-up with until death, emigration, or end of follow-up. Information bias or differential outcome misclassification are therefore unlikely to have affected our results.36 Office BP was measured by the referring physician or during the admission for the CAG and therefore not under the same standardized conditions as invasively measured aortic BP. However, this source of additional random error in office BP measures would a priori confer a prognostic advantage for the invasive BP. Pulse wave data were not available for analysis and arterial stiffness was not measured. The study population consisted of patients referred for elective CAG on suspicion of coronary artery disease. Therefore, our results may neither be directly applicable to patients in the general population with hypertension nor to all patients with CKD. The uneven representation of CKD stages with the majority belonging to CKD stage 3 mirrors the real world but could arguably be perceived as a potential limitation especially for comparison with advanced stages of CKD including dialysis patients. As in all observational studies, residual confounding may still affect the association between BP and outcomes; however, it is unlikely to substantially affect the comparisons of office versus aortic systolic BP.
Conclusion
In conclusion, we did not observe a clinically meaningful improvement in discrimination in the present high-risk population, including patients with CKD when assessing systolic aortic BP in our prediction model. Therefore, the effects of systolic BP when obtained via the classical cuff applied to the brachial artery seem to capture most of the prognostic information carried by systolic BP and our data do not indicate that central BP would improve risk classification during long term follow-up.
Perspectives
Cardiovascular risk assessment based on aortic BP has been suggested as potentially superior to office BP both in patients with CKD and those without CKD. So far, no previous studies in patients with CKD have assessed whether invasively obtained aortic BP provides added discrimination or reclassification beyond office BP. In our large study, we found strong evidence that the predictive ability of invasively measured aortic systolic BP, the gold-standard method, does not exceed office systolic BP and that invasively measured aortic systolic BP does not improve discrimination or reclassification beyond office BP regardless of the presence of CKD. Moreover, our findings suggest that there is little to be gained by introducing noninvasive estimates of aortic systolic BP, such as the ones usually obtained by commercial devices. Pulse wave analysis may potentially provide additional information beyond office BP; however, whether this will improve discrimination and reclassification in terms of cardiovascular outcome remains to be elucidated.
Disclosure
All the authors declared no competing interests.
Acknowledgments
This work was supported by research grants from Karen Elise Jensens Fond, the Central Denmark Region, Denmark, The Novo Nordisk Foundation, and the A.P. Møller Foundation for the Advancement of Medical Science. The funding entities were not involved in any aspects of the design or conduct of the study or collection, analysis, or interpretation of the data nor in the review or approval of the manuscript.
Data Availability
Data for this article was based on Danish health data collected, stored, and managed in national health registers at The Danish Health Data Authority. The dataset is only available via The Secure Research Platform (Forskermaskinen) in a closed environment without access to the internet which prevent us from sharing our dataset.
Footnotes
Figure S1. The difference (cuff-invasive systolic BP) for all patients according to CKD stage.
Figure S2. The difference (cuff-invasive systolic BP) versus age stratified for CKD.
Figure S3. The difference (cuff-invasive systolic BP) versus age quintiles in non-CKD patients.
Figure S4. The difference (cuff-invasive systolic BP) versus sex (males vs. females).
Figure S5. The difference (cuff-invasive systolic BP) versus pulse pressure stratified for CKD.
Figure S6. The difference (cuff-invasive systolic BP) versus beta-blocker treatment stratified for CKD.
Figure S7. The difference (cuff-invasive systolic BP) versus heart rate stratified for beta-blocker status.
Table S1. Systolic BP comparisons according to beta-blocker treatment status (all patients).
Table S2. Systolic BP comparisons according to beta-blocker treatment status (non-CKD patients).
Table S3. Systolic BP comparisons according to beta-blocker treatment status (CKD patients).
Table S4. Hazard ratios for stroke, myocardial infarction and all-cause mortality based on patients without missing covariate data (nonimputed data).
STROBE Statement (PDF).
Supplementary Material
Figure S1. The difference (cuff-invasive systolic BP) for all patients according to CKD stage.
Figure S2. The difference (cuff-invasive systolic BP) versus age stratified for CKD.
Figure S3. The difference (cuff-invasive systolic BP) versus age quintiles in non-CKD patients.
Figure S4. The difference (cuff-invasive systolic BP) versus gender (males vs. females).
Figure S5. The difference (cuff-invasive systolic BP) versus pulse pressure stratified for CKD.
Figure S6. The difference (cuff-invasive systolic BP) versus beta-blocker treatment stratified for CKD.
Figure S7. The difference (cuff-invasive systolic BP) versus heart rate stratified for beta-blocker status.
Table S1. Systolic BP comparisons according to beta-blocker treatment status (all patients).
Table S2. Systolic BP comparisons according to beta-blocker treatment status (non-CKD patients).
Table S3. Systolic BP comparisons according to beta-blocker treatment status (CKD patients).
Table S4. Hazard ratios for stroke, myocardial infarction and all-cause mortality based on patients without missing covariate data (nonimputed data).
STROBE Statement (PDF).
References
- 1.Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group KDIGO 2021 Clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int. 2021;99:S1–S87. doi: 10.1016/j.kint.2020.11.003. [DOI] [PubMed] [Google Scholar]
- 2.De Nicola L., Minutolo R., Zamboli P., et al. Italian audit on therapy of hypertension in chronic kidney disease: the TABLE-CKD study. Semin Nephrol. 2005;25:425–430. doi: 10.1016/j.semnephrol.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 3.Picone D.S., Schultz M.G., Otahal P., et al. Accuracy of cuff-measured blood pressure: systematic reviews and meta-analyses. J Am Coll Cardiol. 2017;70:572–586. doi: 10.1016/j.jacc.2017.05.064. [DOI] [PubMed] [Google Scholar]
- 4.Nichols W.W., O’Rourke M.F., Vlachopoulos C. 6th ed. Hodder Arnold; 2011. McDonald’s Blood Flow in Arteries: Theoretical, Experimental and Clinical Principles. [Google Scholar]
- 5.Khatir D.S., Carlsen R.K., Ivarsen P., et al. Effects of enhanced versus reduced vasodilating treatment on brachial and central blood pressure in patients with chronic kidney disease: a randomized controlled trial. J Hypertens. 2021;39:2232–2240. doi: 10.1097/HJH.0000000000002942. [DOI] [PubMed] [Google Scholar]
- 6.Williams B., Lacy P.S., Thom S.M., et al. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation. 2006;113:1213–1225. doi: 10.1161/CIRCULATIONAHA.105.595496. [DOI] [PubMed] [Google Scholar]
- 7.Boutouyrie P., Achouba A., Trunet P., Laurent S., Group E.T. Amlodipine-valsartan combination decreases central systolic blood pressure more effectively than the amlodipine-atenolol combination: the EXPLOR study. Hypertension. 2010;55:1314–1322. doi: 10.1161/HYPERTENSIONAHA.109.148999. [DOI] [PubMed] [Google Scholar]
- 8.Roman M.J., Devereux R.B., Kizer J.R., et al. Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: the Strong Heart Study. Hypertension. 2007;50:197–203. doi: 10.1161/HYPERTENSIONAHA.107.089078. [DOI] [PubMed] [Google Scholar]
- 9.Cohen D.L., Townsend R.R. Central blood pressure and chronic kidney disease progression. Int J Nephrol. 2011;2011 doi: 10.4061/2011/407801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuan M., Moody W.E., Townend J.N. Central blood pressure in chronic kidney disease: latest evidence and clinical relevance. Curr Hypertens Rev. 2014;10:99–106. doi: 10.2174/1573402111666141231145931. [DOI] [PubMed] [Google Scholar]
- 11.Laugesen E., Rossen N.B., Peters C.D., et al. Assessment of central blood pressure in patients with type 2 diabetes: a comparison between SphygmoCor and invasively measured values. Am J Hypertens. 2014;27:169–176. doi: 10.1093/ajh/hpt195. [DOI] [PubMed] [Google Scholar]
- 12.Rossen N.B., Laugesen E., Peters C.D., et al. Invasive validation of arteriograph estimates of central blood pressure in patients with type 2 diabetes. Am J Hypertens. 2014;27:674–679. doi: 10.1093/ajh/hpt162. [DOI] [PubMed] [Google Scholar]
- 13.Carlsen R.K., Peters C.D., Khatir D.S., et al. Estimated aortic blood pressure based on radial artery tonometry underestimates directly measured aortic blood pressure in patients with advancing chronic kidney disease staging and increasing arterial stiffness. Kidney Int. 2016;90:869–877. doi: 10.1016/j.kint.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 14.Pini R., Cavallini M.C., Palmieri V., et al. Central but not brachial blood pressure predicts cardiovascular events in an unselected geriatric population: the Icare Dicomano Study. J Am Coll Cardiol. 2008;51:2432–2439. doi: 10.1016/j.jacc.2008.03.031. [DOI] [PubMed] [Google Scholar]
- 15.Huang C.M., Wang K.L., Cheng H.M., et al. Central versus ambulatory blood pressure in the prediction of all-cause and cardiovascular mortalities. J Hypertens. 2011;29:454–459. doi: 10.1097/HJH.0b013e3283424b4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vlachopoulos C., Aznaouridis K., O’Rourke M.F., Safar M.E., Baou K., Stefanadis C. Prediction of cardiovascular events and all-cause mortality with central haemodynamics: a systematic review and meta-analysis. Eur Heart J. 2010;31:1865–1871. doi: 10.1093/eurheartj/ehq024. [DOI] [PubMed] [Google Scholar]
- 17.Dart A.M., Gatzka C.D., Kingwell B.A., et al. Brachial blood pressure but not carotid arterial waveforms predict cardiovascular events in elderly female hypertensives. Hypertension. 2006;47:785–790. doi: 10.1161/01.HYP.0000209340.33592.50. [DOI] [PubMed] [Google Scholar]
- 18.Rahman M., Hsu J.Y., Desai N., et al. Central blood pressure and cardiovascular outcomes in chronic kidney disease. Clin J Am Soc Nephrol. 2018;13:585–595. doi: 10.2215/CJN.08620817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zuo J.L., Li Y., Yan Z.J., et al. Validation of the central blood pressure estimation by the SphygmoCor system in Chinese. Blood Press Monit. 2010;15:268–274. doi: 10.1097/MBP.0b013e3283386866. [DOI] [PubMed] [Google Scholar]
- 20.Davies J.I., Band M.M., Pringle S., Ogston S., Struthers A.D. Peripheral blood pressure measurement is as good as applanation tonometry at predicting ascending aortic blood pressure. J Hypertens. 2003;21:571–576. doi: 10.1097/01.hjh.0000052476.40108.5b. [DOI] [PubMed] [Google Scholar]
- 21.Hope S.A., Meredith I.T., Cameron J.D. Effect of non-invasive calibration of radial waveforms on error in transfer-function-derived central aortic waveform characteristics. Clin Sci (Lond) 2004;107:205–211. doi: 10.1042/CS20030294. [DOI] [PubMed] [Google Scholar]
- 22.Smulyan H., Siddiqui D.S., Carlson R.J., London G.M., Safar M.E. Clinical utility of aortic pulses and pressures calculated from applanated radial-artery pulses. Hypertension. 2003;42:150–155. doi: 10.1161/01.HYP.0000084051.34269.A9. [DOI] [PubMed] [Google Scholar]
- 23.Cloud G.C., Rajkumar C., Kooner J., Cooke J., Bulpitt C.J. Estimation of central aortic pressure by SphygmoCor requires intra-arterial peripheral pressures. Clin Sci (Lond) 2003;105:219–225. doi: 10.1042/CS20030012. [DOI] [PubMed] [Google Scholar]
- 24.Horváth I.G., Németh A., Lenkey Z., et al. Invasive validation of a new oscillometric device (arteriograph) for measuring augmentation index, central blood pressure and aortic pulse wave velocity. J Hypertens. 2010;28:2068–2075. doi: 10.1097/HJH.0b013e32833c8a1a. [DOI] [PubMed] [Google Scholar]
- 25.Weber T., Wassertheurer S., Rammer M., et al. Validation of a brachial cuff-based method for estimating central systolic blood pressure. Hypertension. 2011;58:825–832. doi: 10.1161/HYPERTENSIONAHA.111.176313. [DOI] [PubMed] [Google Scholar]
- 26.Chirinos J.A., Zambrano J.P., Chakko S., et al. Relation between ascending aortic pressures and outcomes in patients with angiographically demonstrated coronary artery disease. Am J Cardiol. 2005;96:645–648. doi: 10.1016/j.amjcard.2005.04.036. [DOI] [PubMed] [Google Scholar]
- 27.Jankowski P., Kawecka-Jaszcz K., Czarnecka D., et al. Pulsatile but not steady component of blood pressure predicts cardiovascular events in coronary patients. Hypertension. 2008;51:848–855. doi: 10.1161/HYPERTENSIONAHA.107.101725. [DOI] [PubMed] [Google Scholar]
- 28.Laugesen E., Knudsen S.T., Hansen K.W., et al. Invasively measured aortic systolic blood pressure and office systolic blood pressure in cardiovascular risk assessment: a prospective cohort study. Hypertension. 2016;68:768–774. doi: 10.1161/HYPERTENSIONAHA.116.07495. [DOI] [PubMed] [Google Scholar]
- 29.Wu H.P., Lin M.J. Central aortic pressure and long-term outcome in hypertensive patients undergoing percutaneous coronary intervention. Sci Rep. 2020;10 doi: 10.1038/s41598-020-74619-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Go A.S., Chertow G.M., Fan D., McCulloch C.E., Hsu C.Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 31.Anavekar N.S., McMurray J.J., Velazquez E.J., et al. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med. 2004;351:1285–1295. doi: 10.1056/NEJMoa041365. [DOI] [PubMed] [Google Scholar]
- 32.Schmidt M., Maeng M., Jakobsen C.J., et al. Existing data sources for clinical epidemiology: the Western Denmark Heart Registry. Clin Epidemiol. 2010;2:137–144. doi: 10.2147/clep.s10190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andersen T.F., Madsen M., Jørgensen J., Mellemkjoer L., Olsen J.H. The Danish National Hospital. A valuable source of data for modern health sciences. Dan Med Bull. 1999;46:263–268. [PubMed] [Google Scholar]
- 34.Pottegård A., Schmidt S.A.J., Wallach-Kildemoes H., Sørensen H.T., Hallas J., Schmidt M. Data resource profile: the Danish national prescription registry. Int J Epidemiol. 2017;46:798-798f. doi: 10.1093/ije/dyw213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pedersen C.B., Gøtzsche H., Møller J.O., Mortensen P.B. The Danish civil registration system. A cohort of eight million persons. Dan Med Bull. 2006;53:441–449. [PubMed] [Google Scholar]
- 36.Gregersen R., Wiingreen R., Rosenberg J. Health-related register-based research in Denmark. Ugeskr Laeger. Article in Danish. Ugeskr Laeger. 2018:180. [PubMed] [Google Scholar]
- 37.Levey A.S., Stevens L.A., Schmid C.H., et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levin A., Stevens P.E., Bilous R.W., et al. Kidney disease: improving global outcomes (KDIGO) CKD work group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. doi: 10.1038/kisup.2012.73. [DOI] [Google Scholar]
- 39.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 40.White I.R., Royston P. Imputing missing covariate values for the Cox model. Stat Med. 2009;28:1982–1998. doi: 10.1002/sim.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Newson R.B. Comparing the predictive powers of survival models using Harrell’s C or Somers’ D. STATA J. 2010;10:339–358. doi: 10.1177/1536867X1001000303. [DOI] [Google Scholar]
- 42.Pencina M.J., D’Agostino R.B., Sr., Steyerberg E.W. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30:11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Safar M.E., Blacher J., Pannier B., et al. Central pulse pressure and mortality in end-stage renal disease. Hypertension. 2002;39:735–738. doi: 10.1161/hy0202.098325. [DOI] [PubMed] [Google Scholar]
- 44.London G.M., Blacher J., Pannier B., Guérin A.P., Marchais S.J., Safar M.E. Arterial wave reflections and survival in end-stage renal failure. Hypertension. 2001;38:434–438. doi: 10.1161/01.hyp.38.3.434. [DOI] [PubMed] [Google Scholar]
- 45.McEniery C.M., Cockcroft J.R., Roman M.J., Franklin S.S., Wilkinson I.B. Central blood pressure: current evidence and clinical importance. Eur Heart J. 2014;35:1719–1725. doi: 10.1093/eurheartj/eht565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mitchell G.F. Central pressure should not be used in clinical practice. Artery Res. 2015;9:8–13. doi: 10.1016/j.artres.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carlsen R.K., Peters C.D., Khatir D.S., et al. The authors reply. Kidney Int. 2017;91:254. doi: 10.1016/j.kint.2016.09.027. [DOI] [PubMed] [Google Scholar]
- 48.Laugesen E., Knudsen S.T., Hansen K.W., et al. Invasive aortic pulse pressure is not superior to cuff pulse pressure in cardiovascular risk prediction. J Hypertens. 2021;39:607–613. doi: 10.1097/HJH.0000000000002694. [DOI] [PubMed] [Google Scholar]
- 49.Hlatky M.A., Greenland P., Arnett D.K., et al. Criteria for evaluation of novel markers of cardiovascular risk: a scientific statement from the American Heart Association. Circulation. 2009;119:2408–2416. doi: 10.1161/CIRCULATIONAHA.109.192278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Herrington W., Staplin N., Judge P.K., et al. Evidence for reverse causality in the association between blood pressure and cardiovascular risk in patients with chronic kidney disease. Hypertension. 2017;69:314–322. doi: 10.1161/HYPERTENSIONAHA.116.08386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ravindrarajah R., Hazra N.C., Hamada S., et al. Systolic blood pressure trajectory, frailty, and all-cause mortality >80 years of age: cohort study using electronic health records. Circulation. 2017;135:2357–2368. doi: 10.1161/CIRCULATIONAHA.116.026687. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data for this article was based on Danish health data collected, stored, and managed in national health registers at The Danish Health Data Authority. The dataset is only available via The Secure Research Platform (Forskermaskinen) in a closed environment without access to the internet which prevent us from sharing our dataset.