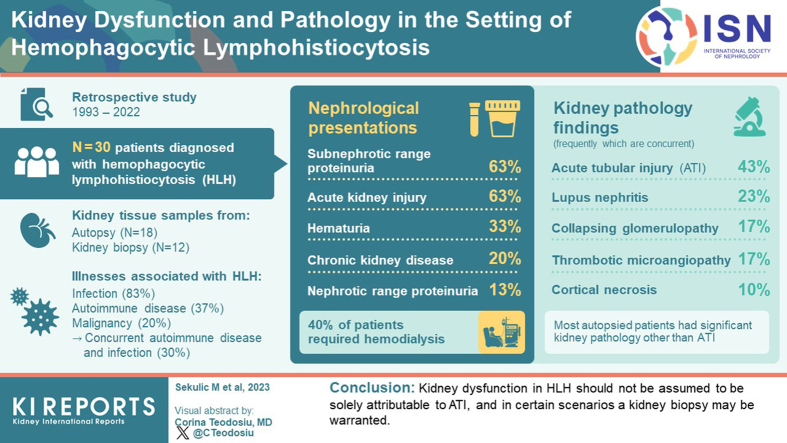
Abstract
Introduction
Hemophagocytic lymphohistiocytosis (HLH) is a clinicopathologic syndrome produced by dysregulated activation of the immune system. Acute kidney injury (AKI) and proteinuria have been infrequently described in the setting of HLH, and investigations of underlying histopathologic changes in the kidney are limited.
Methods
To characterize kidney pathology in HLH, a retrospective review of 30 patients’ clinical and laboratory data, and kidney tissue was performed (18 from autopsy, and 12 biopsied patients).
Results
HLH was associated with infection (83%), autoimmune disease (37%), and malignancy (20%), including 30% with concurrent autoimmune disease and infection. Nephrological presentations included subnephrotic range proteinuria (63%), AKI (63%), hematuria (33%), chronic kidney disease (CKD, 20%), nephrotic range proteinuria (13%), and nephrotic syndrome (7%); and 40% of patients required hemodialysis (HD). Among the 12 patients who underwent kidney biopsy, 6 subsequently showed improved kidney function and the remainder had progressive CKD with most progressing to end-stage kidney disease. Autopsy patients had a median terminal admission of 1 month, and 33% of the biopsied patients died (ranging from 0.3–5 months post-biopsy). Variable pathologies were identified, including acute tubular injury (ATI, 43%), lupus nephritis (LN, 23%), collapsing glomerulopathy (17%), thrombotic microangiopathy (TMA, 17%), and cortical necrosis (10%). Most autopsied patients had significant kidney pathology other than ATI that likely contributed to kidney function decline. A majority of patients with HLH exhibited kidney dysfunction that likely contributed to the poor prognosis.
Conclusion
Kidney dysfunction in HLH should not be assumed to be solely attributable to ATI, and in certain scenarios a kidney biopsy may be warranted.
Keywords: autoimmune disease, hemophagocytic lymphohistiocytosis, histopathology, infection, malignancy
Graphical abstract
HLH is a clinicopathologic entity characterized by cytotoxic T-cell proliferation, increased cytokine production, and immune activation that can result in multiorgan dysfunction and damage.1 Activation of macrophages is a prominent feature of HLH leading to phagocytosis of leukocytes, erythrocytes, platelets, and/or their precursor cells with variable cytopenia, splenomegaly, and hepatomegaly. HLH has been historically designated as either being “primary” when associated with genetic mutations involved in CD8+ T-cell and NK-cell mediated immunity, or, more commonly, “secondary” when developing in the setting of infection, malignancy, immunosuppression, or autoimmune/rheumatologic disease (macrophage activation syndrome is the term specifically used in the setting of autoimmune/rheumatologic disease), which act as triggers to initiate the aberrant immune response of HLH.1,2 Distinction between “primary” and “secondary” forms can be challenging because initial onset of symptoms of some cases of primary HLH can also be initiated by infection.2 Such dichotomous delineation is over-simplistic because it has been suggested that many of these patients have an underlying genotype that predisposes to development of HLH once a sufficient trigger (second hit) is provided. It may instead be more accurate to categorize HLH cases based upon the specific etiologic association: for example, cases with a definitive underlying genetic driver termed as “familial HLH,” cases with a concurrent malignancy be termed as being malignancy-associated HLH, and cases in the setting of autoimmune disease as being autoimmune disease-associated HLH.2
Investigations of patients with HLH and kidney dysfunction and their management have been limited to date, which is surprising considering that kidney dysfunction can be observed in over half of patients.3,4 A French cohort of 95 patients developing AKI with HLH evaluated by Aulagnon et al.4 provided a clinical nephrology perspective of kidney disease associated with HLH. This study was significantly limited due to the lack of histopathologic correlation (only 1 patient underwent kidney biopsy), and follow-up with respect to kidney disease outcome was limited to 6 months. Aside from individual case reports, there is a paucity of investigations examining histopathologic findings in the kidney in the setting of HLH. A small case series by Thaunat et al.5 that included review of kidney biopsy tissue in the setting of nephrotic syndrome and HLH described patients with collapsing glomerulopathy, minimal changes disease (presumed because most cases did not have electron microscopy performed), and TMA. Although informative, patients presenting with a nephrological presentation other than nephrotic syndrome were not included. Herein, we provide the first investigation providing correlation of clinical and laboratory features with underlying pathology of the kidney in patients with HLH.
Methods
Patient Cohort
A retrospective review of the laboratory information system database at Columbia University Medical Center encompassing 29 years (1993–2022) was performed for patients with HLH, including both medical kidney biopsies and autopsies. Patients were diagnosed with HLH based on formal criteria proposed by the Histiocyte Society, known as HLH-2004: exact thresholds/cutoffs as put forth by HLH-2004 were used and noted in Supplementary Table S1.1 The evaluation of autopsy patients with HLH was included to bolster our understanding of patients who did not undergo kidney biopsy ante mortem. The electronic medical record was utilized to obtain clinical, laboratory, and radiologic data for patients. For kidney biopsies received from outside hospitals, clinical, laboratory, and radiologic data were provided by the caring nephrologists. This study was approved by the institutional review board at our institution. AKI, and CKD were defined according to the 2012 Kidney Disease: Improving Global Outcomes guidelines.6,7
Histopathologic Evaluation of Kidney Biopsies and Autopsy-Derived Kidneys
Kidney biopsies had tissue fixed in formalin and embedded in paraffin: sections were evaluated by light microscopy using hematoxylin and eosin, periodic acid-Schiff, Jones methenamine silver, and trichrome stains. All biopsies had fresh tissue triaged and frozen for immunofluorescence microscopy, which was stained for albumin; fibrinogen; C3, C1q, IgG, IgA, and IgM heavy chains; and kappa and lambda light chains. Electron microscopy evaluation was performed on all biopsies.
Kidney tissue collected at the time of autopsy was fixed in formalin. Formalin-fixed paraffin-embedded sections were stained with hematoxylin and eosin and periodic acid-Schiff, and evaluated by light microscopy. For all autopsies, formalin-fixed paraffin-embedded sections were digested with pronase and underwent immunofluorescence staining for IgG, IgA, and IgM heavy chains; kappa and lambda light chains; and C3. For 2 of the autopsies, electron microscopy was performed on formalin-fixed paraffin-embedded tissue.
Histologic parameters of degree of cortical tubular atrophy and interstitial fibrosis, and vascular sclerosis were semiquantitatively evaluated according to the Banff criteria utilized for allograft kidney assessment.8
Primary pathologic diagnoses were determined as the prominent and leading histopathologic findings in the kidneys. Although detailed findings from glomeruli, tubulointerstitium, and vasculature are provided in Tables 1 and 2, the “primary pathologic diagnoses” are highlighted because they are the primary drivers of the histologic findings and clinical presentation. For example, ATI or tubular degenerative changes and interstitial inflammation could be seen in the setting of an active glomerulonephritis (GN), with the active GN being the primary pathologic diagnosis and the ATI and interstitial inflammation interpreted as a consequence of the glomerular disease. ATI was only highlighted as the primary pathologic diagnoses if deemed to represent the predominant process present.
Table 1.
Detailed kidney pathology findings in the setting of HLH
| Patient | Primary pathologic diagnoses | Glomeruli | GS+SS% | Tub | Int | IFTA | Vasc scla | IF | FPE | GBM | TRI | Endo |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1A | Chronic TMA (GVHD, chemotherapy-associated) | Chronic TMA | 0 | Normal | Normal | 5 | Neg | Neg | No EM | No EM | No EM | No EM |
| 2A | ATI, mixed cryo GN (HCV-associated) | Mes proliferative IC mediated GN | 3 | ATI | Normal | 5 | Mild | IC in gloms, VW | No EM | No EM | No EM | No EM |
| 3A | Acute and chronic TMA, focal cortical necrosis, ATI | Acute TMA | 2 | Focal cortical necrosis, ATI | Normal | 25 | Severe, with rare organized fibrin thrombi | Neg | No EM | No EM | No EM | No EM |
| 4A | ATI, 2° FSGS | 2° FSGS | 23 | ATI | Normal | 30 | Mild | Neg | No EM | No EM | No EM | No EM |
| 5A | ATI, LN class III+V | LN class III+V | 1 | ATI | Mild inf | 20 | Neg | IC in gloms | 80 | Spikes | Yes | Normal |
| 6A | Normal | Normal | 0 | ATI | Normal | 0 | Neg | Neg | No EM | No EM | No EM | No EM |
| 7A | Osmotic tubulopathy | 2° FSGS | 4 | ATI, osmotic (radiocontrast) | Normal | 5 | Mild | Neg | No EM | No EM | No EM | No EM |
| 8A | End-stage kidney, nodular DM | Nodular DM, severe | 93 | Normal | Normal | 95 | Severe | Neg | No EM | No EM | No EM | No EM |
| 9A | ATI | Normal | 0 | ATI | Normal | 0 | Neg | Neg | No EM | No EM | No EM | No EM |
| 10A | 2° FSGS, IVLBCL in gloms | 2° FSGS, IVLBCL in gloms | 18 | ATI | Normal | 15 | Mild | Neg | No EM | No EM | No EM | No EM |
| 11A | ATI, nodular DM | Nodular DM, mild | 4 | ATI | Normal | 40 | Mild | Neg | No EM | No EM | No EM | No EM |
| 12A | ATI | 2° FSGS | 3 | ATI | Normal | 0 | Mild | Neg | No EM | No EM | No EM | No EM |
| 13A | ATI, 2° FSGS | 2° FSGS | 3 | ATI | Normal | 0 | Neg | Neg | 25 | Normal | Neg | Swelling |
| 14A | Chronic TMA | Chronic TMA | 23 | Normal | Normal | 30 | Neg | Neg | No EM | No EM | No EM | No EM |
| 15A | Chronic TMA | Chronic TMA | 7 | Normal | Normal | 20 | Moderate | Neg | No EM | No EM | No EM | No EM |
| 16A | ATI | 2° FSGS | 2 | ATI | Normal | 5 | Mild | Neg | No EM | No EM | No EM | No EM |
| 17A | Focal cortical necrosis, ATI | 2° FSGS | 3 | Focal cortical necrosis, ATI | Normal | 10 | Mild | Neg | No EM | No EM | No EM | No EM |
| 18A | Disseminated Aspergillus, focal cortical necrosis, ATI, LN class II | LN class II | 2 | Focal cortical necrosis, ATI | Normal | 0 | Neg | IC in gloms, TBM, VW | No EM | No EM | No EM | No EM |
| 19Bx | Chronic TMA | Chronic TMA | 0 | Normal | Normal | 0 | Neg | Neg | 5 | Normal | Neg | Swelling (diffuse) |
| 20Bx | LN class II | LN class II | 0 | ATI | Normal | 0 | Neg | IC in gloms | 40 | Normal | Neg | Normal |
| 21Bx | Collapsing glomerulopathy | Collapsing glomerulopathy | 6 | ATI | Normal | 20 | Mild | Neg | 70 | Normal | Neg | Normal |
| 22Bx | Glomerular endotheliosis, mesangiolysis, macrophage infiltration, and intraglomerular hemophagocytosisb | Glomerular endotheliosis, mesangiolysis, macrophage infiltration, and intraglomerular hemophagocytosisb | 0 | Normal | Mild inf | 0 | Mild | Neg | 60 | Normal | Neg | Swelling |
| 23Bx | LN class IV+V | LN class IV+V | 7 | ATI | Normal | 5 | Neg | IC in gloms, TBM, VW | 95 | Spikes | Yes | Swelling |
| 24Bx | Collapsing glomerulopathy | Collapsing glomerulopathy | 19 | ATI | Mild inf | 5 | Mild | Neg | 90 | Normal | Neg | Normal |
| 25Bx | Collapsing glomerulopathy and LN II | Collapsing glomerulopathy and LN II | 19 | ATI | Normal | 0 | Neg | IC in gloms | 95 | Normal | Yes | Swelling |
| 26Bx | Collapsing glomerulopathy | Collapsing glomerulopathy | 24 | ATI | Mild inf | 50 | Mild | Neg | 100 | Thickening | Neg | Swelling |
| 27Bx | LN class II, hemophagocytosis in the interstitium | LN class II | 0 | Normal | Hemophagocytosis in the interstitium | 0 | Neg | IC in gloms, TBM, VW | 10 | Normal | Yes | Normal |
| 28Bx | LN class III+V, osmotic tubulopathy | LN class III+V | 0 | ATI, osmotic (radiocontrast) | Normal | 0 | Neg | IC in gloms, TBM, int, VW | 75 | Normal | Yes | Swelling |
| 29Bx | Collapsing glomerulopathy | Collapsing glomerulopathy | 24 | ATI | Mild inf | 20 | Mild | Neg | 100 | Normal | Neg | Normal |
| 30Bx | IgAN | IgAN, mes and endo prolif | 0 | ATI | Normal | 5 | Mild | IC in gloms | 50 | Normal | Neg | Normal |
2° FSGS, secondary/adaptive focal segmental glomerulosclerosis with/without glomerulomegaly; A, autopsy patient; ATI, acute tubular injury; Bx, patient with a kidney biopsy; EM, electron microscopy; Endo, ultrastructural glomerular endothelial cell findings; FPE, percentage of podocyte foot process effacement; GBM, ultrastructural glomerular basement membrane findings; gloms, glomeruli; GS+SS%, percentage of total glomeruli that are globally or segmentally sclerosed; GVHD, graft versus host disease; HCV, viral hepatitis C; IC, immune complex deposition; IF, immunofluorescence microscopy findings; IFTA, percentage of cortex with tubular atrophy and interstitial fibrosis; IgAN, IgA nephropathy; inf, interstitial inflammation; Int, interstitial findings; int, interstitial; IVLBCL, intravascular large B-cell lymphoma; LN, lupus nephritis; mes and endo prolif, mesangial and endocapillary proliferative pattern of glomerular injury; mixed cryo GN, mixed cryoglobulinemic glomerulonephritis; Neg, negative; nodular DM, nodular diabetic glomerulosclerosis; TBM, tubular basement membranes; TMA, thrombotic microangiopathy; TRI, endothelial tubuloreticular inclusions; Tub, tubular findings; Vasc scl, degree of vascular sclerosis; VW, vessel walls.
Vessels in all cases by light microscopic examination were normal or showed varying degrees of sclerosis without other involving pathologies, aside from immune complex deposition when indicated and patient 3A who had rare arteries with organized fibrin thrombi.
The glomerular findings of patient 22Bx have been described by some as “histiocytic glomerulopathy” for which the differential diagnosis includes a glomerulonephritis with very low level (i.e., not detected by IF or EM) glomerular deposition of complement/immune complexes, and TMA.
Table 2.
Summarization of the primary pathologies – frequently concurrent – in the kidney of patients with HLH
| Primary pathology found in the kidney | Number of patients with the given pathology (percentage of all patients) |
|---|---|
| Acute tubular injury | 13 (43%) |
| Osmotic tubulopathy | 3 (10%) |
| Lupus nephritis | 7 (23%) |
| LN class II | 4 (13%) |
| LN class III+V | 2 (7%) |
| LN class IV+V | 1 (3%) |
| Collapsing glomerulopathy | 5 (17%) |
| Thrombotic microangiopathy | 5 (17%) |
| Chronic TMA | 4 (13%) |
| Acute and chronic TMA | 1 (3%) |
| Focal cortical necrosis | 3 (10%) |
| 2° FSGS | 3 (10%) |
| Nodular diabetic glomerulosclerosis | 2 (7%) |
| Mixed cryoglobulinemic glomerulonephritis, IgA nephropathy, hemophagocytosis in the interstitium, “histiocytic glomerulopathy” with intraglomerular hemophagocytosis, intravascular large B-cell lymphoma, disseminated Aspergillus | 1 (3%) of each pathology |
| Normal (i.e., no significant histopathologic findings) | 1 (3%) |
2° FSGS, secondary/adaptive focal segmental glomerulosclerosis; HLH, hemophagocytic lymphohistiocytosis; LN, lupus nephritis; TMA, thrombotic microangiopathy.
Statistical analyses were completed using SPSS (IBM SPSS Statistics, Armonk, NY). For statistical analysis of blood and protein urine dipstick data (scaled at 0/negative, trace, 1+, 2+, 3+, 4+), trace results are arbitrarily equated to a scale value of 0.5. Calculation of estimated glomerular filtration rate was done using the CKD-Epidemiology Collaboration creatinine equation for adult patients, and for pediatric patients (under the age of 18 years old) the “Bedside Schwartz” equation was used.
Results
Demographics and General Clinical Characteristics
We identified 30 cases (18 autopsies and 12 medical kidney biopsies) of patients meeting formal criteria for HLH. One autopsy included a kidney transplant recipient for which the allograft kidney was evaluated (the native kidneys showed advanced chronic parenchymal scarring and their features were not evaluated/described further). Four cases included in this series were individually reported previously in the literature.9, 10, 11, 12
At the time of tissue sampling (whether a biopsy or at the time of autopsy), patients had a median age of 42 years (interquartile range, IQR, 22.5–59.3 years). There were 16 male and 14 female self-identified patients, of which 11 were self-identified as White, 10 as Black, 7 as Hispanic, 1 as Asian, and 1 as Middle Eastern. Other clinical characteristics included a median body mass index of 27.4 kg/m2, diabetes mellitus in 4, and hypertension in 13.
Criteria for Meeting Diagnosis of HLH
One patient had genetic findings diagnostic for HLH (pathogenic mutation of BIRC4), whereas the remaining 29 patients met at least 5 of 8 criteria for the diagnosis of HLH set forth by HLH-2004 (Table 3).1 Only 2 patients underwent genetic testing for gene variants associated with HLH. Twenty-seven patients met the criteria for increased serum ferritin level, 26 had fever, 26 had splenomegaly, 26 had cytopenias affecting ≥2 of 3 lineages in the peripheral blood, and 24 had hypertriglyceridemia and/or hypofibrinogenemia (20 with hypertriglyceridemia, and 7 with hypofibrinogenemia). There was tissue documentation of hemophagocytosis in 26 patients, including involvement of bone marrow in 22, spleen in 5, and lymph nodes in 4; specifically, among biopsied patients, 8 of the 12 had evidence of hemophagocytosis by bone marrow (n = 6) or lymph node (n = 2) biopsy. Low or absent NK-cell activity was document in 1 of 2 patients who underwent testing, whereas increased soluble CD25 (IL-2 receptor) was seen in 9 of 10 patients evaluated. Among the 29 patients that were diagnosed with HLH based on the HLH-2004 criteria (excluding the patient with a detected BIRC4 pathogenic mutation), 15 patients met 5 criteria, 11 met 6 criteria, and 3 exhibited 7 criteria.
Table 3.
Profile of patients’ clinical, laboratory, and pathologic criteria for meeting the diagnosis of HLH
| Criterion (bolded are “major” criteria) | Number of cases with criterion out of total observed/tested (prevalence rate) | Median (interquartile range) |
|---|---|---|
| Fever | 26 of 30 (87%) | |
| Splenomegaly | 26 of 30 (87%) | |
| Cytopenias (affecting ≥2 of 3 lineages in the peripheral blood) | 26 of 30 (87%) | |
| Hemoglobin <90 g/l (in infants <4 weeks: hemoglobin <100 g/l) | 22 of 30 (73%) | 83 g/l (74–94 g/l) |
| Platelets <100 × 109/l | 25 of 30 (83%) | 69.5 × 109/l (26–92 × 109/l) |
| Neutrophils <1.0 × 109/l | 15 of 27 (60%) | 0.8 × 109/l (0.2–9.8 × 109/l) |
| Hypertriglyceridemia and/or hypofibrinogenemia | 24 of 29 (83%) | |
| Fasting triglycerides ≥3.0 mmol/l (i.e., ≥265 mg/dl) | 20 of 27 (74%) | 317 mg/dl (190–484 mg/dl) |
| Fibrinogen ≤1.5 g/l | 7 of 27 (26%) | 2.41 g/l (1.64–4.1 g/l) |
| Hemophagocytosis in bone marrow or spleen or lymph nodes | 26 of 29 (90%) | |
| Hemophagocytosis in bone marrow | 22 of 28 (79%) | |
| Hemophagocytosis in spleen | 5 of 18 (28%) | |
| Hemophagocytosis in lymph node | 4 of 21 (19%) | |
| Low or absent NK-cell activity | 1 of 2 (50%) | |
| Increased ferritin ≥500 μg/l | 27 of 28 (96%) | 3081 μg/l (1222–9000 μg/l) |
| Increased soluble CD25(i.e., soluble IL-2R) ≥2400 U/ml | 9 of 10 (90%) | 3406 U/ml (1719–10903 U/ml) |
HLH, hemophagocytic lymphohistiocytosis; IL-2R, interlukin-2 receptor; NK-cell, natural killer cell.
Associated Conditions With the Development of HLH
HLH can be associated with autoimmune disease, infections, and/or malignancy (Table 4). Eleven patients had a diagnosis of autoimmune disease: 9 with systemic lupus erythematosus (including 1 with concurrent Sjögren syndrome), 1 with Still’s disease, and 1 with Hashimoto thyroiditis. Twenty-five patients had evidence of infection, most commonly cytomegalovirus and Epstein-Barr virus (both n = 6) but also other viral, bacterial, fungal, and/or parasitic infections. Ten patients had evidence of more than 1 infectious organism. Six patients had a diagnosis of malignancy, including 1 patient each with acute lymphoblastic leukemia, acute myeloid leukemia, intravascular large B-cell lymphoma, T-cell/histiocyte rich large B-cell lymphoma, colon cancer, and thyroid cancer. Nine patients had concurrent autoimmune disease and infection, 5 had evidence of concurrent infection and malignancy, and none had evidence of concurrent autoimmune disease and malignancy. Therefore, only 2 of 30 patients had no history of autoimmune disease, infection, or malignancy. None of the patients had evidence of immunocompromise due to primary/genetic immunodeficiency syndrome.
Table 4.
Associated conditions with the development of HLH
| Associated condition | Number of positive cases of total evaluated/tested (prevalence rate) |
|---|---|
| I. Autoimmune disease | |
| Systemic lupus erythematosus | 8 of 30 (32%) |
| Sjögren’s syndrome with systemic lupus erythematosus, Still’s disease, or Hashimoto’s thyroiditis | 1 of 30 (3%) for each disease/syndrome |
| II. Infection | |
| Virus | |
| Cytomegalovirus | 6 of 24 (25%) |
| Epstein-Barr virus | 6 of 20 (30%) |
| Viral hepatitis C | 1 of 26 (4%) |
| Human immunodeficiency virus | 1 of 25 (4%) |
| SARS-CoV-2 | 1 of 7 (14%) |
| BK virus | 1 of 2 (50%) |
| Coxsackievirus | 1 of 1 (100%) |
| Bacteria | |
| Klebsiella pneumoniae, Escherichia coli, Staphylococcus aureus, or Enterococcus faecium | 2 of 25 (8%) for each organism |
| Pseudomonas aeruginosa, Enterobacter cloacae, Klebsiella aeruginosa, Streptococcus pyogenes, or Mycobacterium tuberculosis | 1 of 25 (4%) for each organism |
| Fungus | |
| Aspergillus fumigatus | 2 of 22 (9%) |
| Cryptococcus or Candida dubliniensis | 1 of 22 (5%) for each organism |
| Parasite | |
| Strongyloides | 2 of 11 (18%) |
| Plasmodium falciparum | 1 of 1 (100%) |
| III. Malignancy | |
| Acute lymphoblastic leukemia, acute myeloid leukemia, intravascular large B-cell lymphoma, T-cell/histiocyte rich B-cell lymphoma, colon cancer, or thyroid cancer | 1 of 30 (3%) for each malignancy |
HLH, hemophagocytic lymphohistiocytosis.
Not shown are negative test results for viral hepatitis B (from 21 tested), herpes simplex virus 1 and 2 (from 11 tested), influenza A and B (from 10 tested), and respiratory syncytial virus (from 10 tested).
Additional Clinical, Laboratory, and Pathologic Features Associated With the Development of HLH
Aside from the aforementioned “major” criteria required to meet a diagnosis, patients with HLH can present with other clinical and laboratory features, including neurologic syndromes, lymphadenopathy, edema, skin rash, and hepatobiliary dysfunction. Eight patients exhibited neurologic findings (rate of 27%): 7 with altered mental status and 1 with seizures. Fifteen patients had edema (50%), and 5 had skin rash (17%) on examination. Hepatomegaly was observed in 15 patients (50%), and lymphadenopathy was present in 7 (23%). Coagulation abnormalities (from prothrombin, activated thromboplastin time, and international normalization ratio testing) were observed in 23 patients (77%). Additional laboratory testing for lactate dehydrogenase and liver/biliary functional markers are detailed in Supplementary Table S2. Although many patients revealed elevated markers reflecting hepatobiliary dysfunction, only 2 cases (autopsies) showed hemophagocytosis in the liver, suggesting other mechanisms of injury. Serum levels of IL-18, CXCL9, and sCD163 were not measured, and flow cytometry for cell surface expression of granzyme B, signaling lymphocytic activation molecule associated protein, X-linked inhibitor of apoptosis, and perforin was not performed.
Although hemophagocytosis found in the bone marrow, spleen, or lymph node are contributory to the diagnosis of HLH (vide supra and Table 3), hemophagocytosis was present less frequently in other tissues. Two of the 18 autopsies had hemophagocytosis identified in the liver (11%), 1 of the 18 autopsies showed involvement of the lungs (6%), and only 1 of all 30 patients showed either hemophagocytosis in the glomeruli or in the cortical interstitium of the kidney (3% for each; were previously reported).10,12 Of those patients who exhibited histopathologic evidence of hemophagocytosis, most (n = 21) had apparent evidence of only 1 tissue site of involvement, 3 had concurrent involvement in 2 differing tissue sites, 1 with 3 tissue sites, and 1 with 5 sites showing hemophagocytosis. Not surprisingly, more systemic involvement was identified in autopsy patients, in whom more extensive tissue/organ sampling allowing for its identification.
Nephrological Presentation and Laboratory Markers of Kidney Dysfunction in the Setting of HLH
Kidney dysfunction can manifest as acute and/or chronic depending on the underlying pathogenesis and histologic compartment (glomeruli, tubules, interstitium, and/or vasculature) involved. Nineteen patients exhibited subnephrotic range proteinuria (63%), 19 with AKI (63%), 10 with hematuria (33%), 6 with established CKD (20%), 4 with nephrotic range proteinuria but without the full nephrotic syndrome (13%), and 2 with nephrotic syndrome (7%) (Table 5). Laboratory data used in part to define these nephrological presentations are described in detail in Table 6, and details can also be referenced in Table 7. Patients predominantly presented with elevated serum creatinine (24 of 30 tested, with median of 1.5 mg/dl) and BUN (22 of 30 tested, with median of 36 mg/dl), and decreased serum albumin (29 of 30 tested, with median of 2.3 g/dl). Nearly all of the patients had evidence of proteinuria as demonstrated by urine dipstick (25 of 27 tested), urine protein-to-creatinine ratio (16 of 16 tested, with median of 1.3 g/g), and/or 24-hour urine protein quantification (8 of 8 tested, with median of 2.1 g/24-hours). Six patients had nephrotic range proteinuria with or without the nephrotic syndrome. Whether by urine dipstick (12 of 12 tested) or urine microscopic evaluation (13 of 16 tested, with median 11 red blood cells per high power field), most patients had evidence of hematuria. Just over half of patients exhibited relatively mild leukocyturia (9 of 15 tested, with median of 5 white blood cells per high power field).
Table 5.
Nephrological presentation identified in the setting of HLH
| Nephrological presentation | Number of cases with presentation type out of total (prevalence rate) |
|---|---|
| Subnephrotic range proteinuria | 19 (63%) |
| Acute kidney injury | 19 (63%) |
| Hematuria | 10 (33%) |
| Established chronic kidney disease | 6 (20%) |
| Nephrotic range proteinuria without the full nephrotic syndrome | 4 (13%) |
| Nephrotic syndrome | 2 (7%) |
HLH, hemophagocytic lymphohistiocytosis.
Most patients (n = 23) exhibited more than 1 nephrological presentation.
Table 6.
Kidney functional laboratory testing at the time of kidney tissue sampling in the setting of HLH
| Tested analyte (reference range or scale) | Number of cases of total tested with abnormal values (prevalence rate) | Median (interquartile range) |
|---|---|---|
| Serum creatinine (0.5–0.95 mg/dl) | 24 of 30 elevated (80%) | 1.5 mg/dl (1.1–2.6 mg/dl) |
| Blood urea nitrogen (7–26 mg/dl) | 22 of 30 elevated (73%) | 36 mg/dl (23–63 mg/dl) |
| Serum albumin (3.9–5.2 g/dl) | 29 of 30 decreased (97%) | 2.3 g/dl (1.7–2.7 g/dl) |
| RBC/HPFa (0–2 RBC/HPF) | 13 of 16 increased (81%) | 11 RBC/HPF (5-30 RBC/HPF) |
| WBC/HPFa (0–3 WBC/HPF) | 9 of 15 increased (60%) | 5 WBC/HPF (1–15 WBC/HPF) |
| Hematuria, dipsticka (scale 0 to 3+) | 12 of 12 increased (100%) | 2+ (1–3+) |
| Proteinuria, dipstick (scale 0 to 4+) | 25 of 27 increased (93%) | 2+ (0.5–3+) |
| Urine protein to creatinine ratio (<200 g/g) | 16 of 16 elevated (100%) | 1.3 g/g (1.0–2.6 g/g) |
| 24-hour urine protein quantification (<0.15 g/24-hours) | 8 of 8 elevated (100%) | 2.1 g/24-hours (1.1–2.3 g/24-hours) |
HLH, hemophagocytic lymphohistiocytosis; HPF, high power field; RBC, red blood cells; WBC, white blood cells.
Analysis is restricted to cases in which a sample was collected without a confounding urinary (Foley) catheter in place.
Table 7.
Correlation of clinical, laboratory, and kidney pathology findings in the setting of HLH
| Patient | HLH-associated etiolog(-ies)y | Nephrological presentation | Cre (mg/dl) (eGFR) | Proteinuriaa | Primary pathologic diagnoses | Length of terminal admission or follow-up time (months) | Follow-up eGFR, HD status, death | Follow-up proteinuria |
|---|---|---|---|---|---|---|---|---|
| 1A | Infection, malignancy | CKD | 1.2 (42) | 0 (Dipstick) | Chronic TMA (GVHD, chemotherapy-associated) | 2 | Deceased | NA |
| 2A | Infection | AKI | 1.5 (58) | Trace (Dipstick) | ATI, mixed cryo GN (HCV-associated) | 0.13 | Deceased | NA |
| 3A | Infection | AKI | 2.3 (32) | Trace (Dipstick) | Acute and chronic TMA, focal cortical necrosis, ATI | 0.1 | Deceased | NA |
| 4A | Infection, malignancy | AKI | 1.2 (60) | 0 (Dipstick) | ATI, 2° FSGS | 1 | Deceased | NA |
| 5A | Autoimmune, infection | AKI, nephrotic syndrome, hematuria | 2.3 (29) | 3+ (Dipstick) | ATI, LN class III+V | 2 | Deceased | NA |
| 6A | Familial (BIRC4), infection | Subnephrotic proteinuria | 0.6 (45) | 2+ (Dipstick) | Normal | 4 | Deceased | NA |
| 7A | Autoimmune, infection | AKI, subnephrotic proteinuria | 2.2 (24) | 0.35 g/g, 0.24 g/24-h | Osmotic tubulopathy | 2 | Deceased | NA |
| 8A | Infection | CKD, nephrotic range proteinuria | 3.8 (12) | 3+ (Dipstick) | End-stage kidney, nodular DM | 0.5 | Deceased | NA |
| 9A | Infection | AKI, subnephrotic proteinuria | 1.48 (66) | 1+ (Dipstick) | ATI | 0.75 | Deceased | NA |
| 10A | Malignancy | Subnephrotic proteinuria | 0.94 (80) | 1.3 g/g | 2° FSGS, IVLBCL in gloms | 0.5 | Deceased | NA |
| 11A | Infection | AKI, subnephrotic proteinuria | 8.29 (7) | 2.78 g/g | ATI, nodular DM | 1 | Deceased | NA |
| 12A | Infection | AKI, subnephrotic proteinuria | 1.1 (77) | 0.81 g/g | ATI | 1 | Deceased | NA |
| 13A | Autoimmune | AKI, nephrotic range proteinuria | 4.34 (13) | 5.98 g/g | ATI, 2° FSGS | 0.13 | Deceased | NA |
| 14A | Infection | CKD, Subnephrotic proteinuria | 1.34 (70) | 0.99 g/g | Chronic TMA | 1 | Deceased | NA |
| 15A | Infection, malignancy | CKD, Subnephrotic proteinuria | 1.25 (43) | 1.37 g/g | Chronic TMA | 0.5 | Deceased | NA |
| 16A | Infection | AKI, Subnephrotic proteinuria | 0.97 (89) | Not tested | ATI | 1.6 | Deceased | NA |
| 17A | Infection, malignancy | AKI, subnephrotic proteinuria | 2.05 (36) | 1.77 g/g | Focal cortical necrosis, ATI | 0.35 | Deceased | NA |
| 18A | Autoimmune, infection | AKI | 2.22 (31) | Not tested | Disseminated Aspergillus, focal cortical necrosis, ATI, LN class II | 2 | Deceased | NA |
| 19Bx | Autoimmune, infection | CKD, subnephrotic proteinuria | 1.14 (94) | 2.3 g/24-h | Chronic TMA | 131 | 28, no HD, alive | 0.2 g/g |
| 20Bx | Autoimmune, infection | CKD, subnephrotic proteinuria, hematuria | 2.18 (31) | 2.13 g/24-h | LN class II | 0.3 | 38, no HD, deceased | Not tested |
| 21Bx | Autoimmune | AKI, nephrotic range proteinuria, hematuria | 1.52 (41) | 5.4 g/g | Collapsing glomerulopathy | 5 | 14, HD required, deceased | Not tested |
| 22Bx | No clear associated etiology | AKI, subnephrotic proteinuria, hematuria | 1.9 (38) | 1.76 g/g, 1.7 g/24-h | Glomerular endotheliosis, mesangiolysis, macrophage infiltration, and intraglomerular hemophagocytosisb | 56 | 126, no HD, alive | 0 (Dipstick) |
| 23Bx | No clear associated etiology | Nephrotic syndrome, hematuria | 0.5 (138) | 5.8 g/g | LN class IV+V | 77 | 104, no HD, alive | 0.16 g/g |
| 24Bx | Infection, malignancy | AKI, subnephrotic proteinuria, hematuria | 6.3 (10) | 2.04 g/g, 2.0 g/24-h | Collapsing glomerulopathy | 60 | 107, no HD, alive | 0 (Dipstick) |
| 25Bx | Autoimmune, infection | AKI, subnephrotic proteinuria, hematuria | 0.8 (103) | 1.3 g/g, 0.9 g/24-h | Collapsing glomerulopathy and LN II | 65 | 78, no HD, alive | 0.2 g/g |
| 26Bx | Infection | AKI, subnephrotic proteinuria | 6.6 (10) | 1.0 g/g | Collapsing glomerulopathy | 30 | 96, no HD, alive | 1 (Dipstick) |
| 27Bx | Autoimmune, infection | Subnephrotic proteinuria, hematuria | 0.53 (123) | 0.9 g/g | LN class II, hemophagocytosis in the interstitium | 21 | 117, no HD, alive | 0 (Dipstick) |
| 28Bx | Autoimmune, infection | AKI, nephrotic range proteinuria, hematuria | 3.5 (17) | 4.0 g/24-h | LN class III+V, osmotic tubulopathy | 1 | 122, no HD, deceased | 1.4 g/g |
| 29Bx | Infection | AKI, subnephrotic proteinuria | 10.4 (6) | 2.2 g/24-h | Collapsing glomerulopathy | 5 | 78, no HD, alive | 0.3 g/g |
| 30Bx | Autoimmune, infection | Subnephrotic proteinuria, hematuria | 0.6 (122) | 1.1 g/g | IgAN | 1.1 | 13, HD required, deceased | 2.1 g/g |
2° FSGS, secondary/adaptive focal segmental glomerulosclerosis with/without glomerulomegaly; A, autopsy patient; AKI, acute kidney injury; ATI, acute tubular injury; autoimmune, autoimmune disease; Bx, patient with a kidney biopsy; CKD, established chronic kidney disease; Cre, serum creatinine; eGFR, estimate glomerular filtration rate (ml/min per 1.73 m2); GVHD, graft versus host disease; HCV, viral hepatitis C; HD, hemodialysis; HLH, hemophagocytic lymphohistiocytosis; IgAN, IgA nephropathy; IVLBCL, intravascular large B-cell lymphoma; LN, lupus nephritis; mixed cryo GN, mixed cryoglobulinemic glomerulonephritis; NA, not available; nodular DM, nodular diabetic glomerulosclerosis; TMA, thrombotic microangiopathy; UPCR, urine protein-to-creatinine ratio.
Proteinuria value provided is either from urine dipstick, urine protein-to-creatinine ratio (g/g), and/or 24-hour urine protein collection (g/24-hours); urine dipstick is not shown in the table if either UPCR or 24-hour collection are available.
The glomerular findings of patient 22Bx have been described by some as “histiocytic glomerulopathy” for which the differential diagnosis includes a glomerulonephritis with very low level (i.e., not detected by immunofluorescence or electron microscopy) glomerular deposition of complement/immune complexes, and TMA.
Patient Management in the Setting of HLH
Medical management was directed against underlying etiologies, most commonly infection(s). Twenty-four patients were provided antimicrobial therapy (80%), with 23 receiving antibacterial, 16 antifungal, 10 antiviral, and 3 antiparasitic therapy, including 18 patients who received a combination of antibacterial, antifungal, antiviral, and/or antiparasitic agents.
Twenty-four patients (80%) received immunosuppressive therapy in an effort to reduce the immune activation that characterizes HLH (Table 8 and Supplementary Table S3), of which 17 received more than 1 agent. In some cases, immunosuppressive therapy was also employed in part in the management of an underlying active glomerular pathology (e.g., LN or a collapsing glomerulopathy). Nineteen patients received both immunosuppressive therapy and at least 1 antimicrobial agent (rate of 63%). For management of cytopenia(s), 7 patients received filgrastim (23%), 3 darbepoetin (10%), and 1 romiplostim (3%). Three patients also underwent plasmapheresis (10%). Two autopsy patients received hematopoietic stem cell transplantations 4 months prior to death. One patient received chemotherapy (cyclophosphamide, doxorubicin, vincristine, and prednisolone) directed toward their T-cell or histiocyte-rich large B-cell lymphoma.
Table 8.
Immunosuppressive agents and regimens provided to patients in the setting of HLH
| Immunosuppressive agent(s) | Number of patients receiving (prevalence rate among all patients) |
|---|---|
| Corticosteroid(s) ± calcineurin inhibitor ± MMF ± “other agent(s)” | 12 (40%) |
| Corticosteroid(s) alone | 7 (23%) |
| No immunosuppression provided | 6 (20%) |
| Corticosteroid(s) + MMF | 2 (7%) |
| Corticosteroid(s) + hydroxychloroquine | 2 (7%) |
| Hydroxychloroquine alone | 1 (3%) |
HLH, hemophagocytic lymphohistiocytosis; MMF, mycophenolate mofetil.
Twenty-four patients received immunosuppressive therapy, of which 17 received more than 1 immunosuppressive agent. “Other agent(s)” refer to intravenous immune globulin, anakinra, cyclophosphamide, etoposide, rituximab, daclizumab, antithymocyte globulin, alemtuzumab, infliximab, and/or methotrexate. In Supplementary Table S3, we present further details regarding immunosuppressive agents provided.
HD was required in the setting of HLH for 12 of the 30 patients (40%), including 8 who died and were autopsied, and 4 who underwent kidney biopsy (of which, all 4 recovered kidney function and discontinued HD).
Patient Outcomes in the Setting of HLH
A median follow-up of 25.5 months (IQR of 2.0–64 months) is available for the 12 patients who underwent a kidney biopsy. Median serum creatinine on follow-up of the 12 patients was 1.0 mg/dl (IQR of 0.6–2.7 mg/dL): 6 patients showed improvement of serum creatinine, with 5 of them attaining baseline values. Six patients showed worsening of serum creatinine on follow-up, with 5 progressing to end-stage kidney disease or CKD stage 4 or 5. Two of the patients developed subsequent requirement of HD support, 1.1 and 5.0 months after the kidney biopsies and both died shortly after. By urine dipstick and urine protein-to-creatinine ratio, 7 biopsied patients on follow-up had evidence of relatively mild subnephrotic range proteinuria (n = 6, with median of 0.25 g/g, IQR of 0.25–1.58; n = 1 had 1+ by dipstick) and 3 showed no proteinuria by urine dipstick.
The median length of the terminal admission of the 18 autopsy patients was 1 month (IQR of 0.5–2.0 months). Of the 12 biopsied patients, 4 (33%) have since died at 0.3, 1.0, 1.1, and 5.0 months post-biopsy. Altogether, 4 of the 12 biopsied patients either required HD and/or were deceased on follow-up.
Kidney Pathology in the Setting of HLH
Sampling of the kidneys of patients with HLH for histologic evaluation revealed a variety of acute and/or chronic pathologies involving glomeruli, tubulointerstitium, and/or vasculature (i.e., the histologic compartments of the kidney; Table 1, Table 2 and 7; Figures 1 and 2). It was not uncommon to find more than 1 pathology in a given patient’s kidney. Glomerular pathologies included LN (n = 7, 23%), collapsing glomerulopathy (n = 5, 17%), secondary/adaptive focal segmental glomerulosclerosis (n = 3, 10%), nodular diabetic glomerulosclerosis (n = 2, 7%), and single cases of IgA nephropathy, mixed cryoglobulinemic GN (viral hepatitis C associated), and “histiocytic glomerulopathy” with intraglomerular hemophagocytosis (n = 1 each, 3%). Of the 9 immune complex-mediated GN cases (this includes cases of LN, IgAN, and mixed cryoglobulinemic GN), 4 showed evidence of active endocapillary proliferation/hypercellularity and/or cellular crescents.
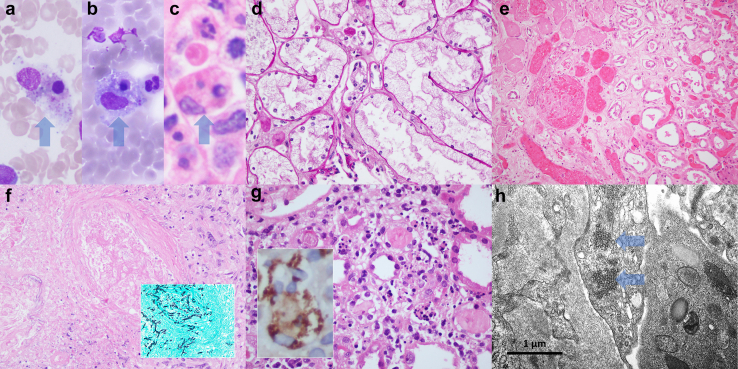
Figure 1.
Representative pathologies observed in patients with HLH. Many patients with HLH revealed evidence of hemophagocytosis in the bone marrow (a–c, with arrows pointing to macrophages containing phagocytosed and degenerate intracellular cellular material). Acute tubular injury was frequently seen in autopsy kidneys; however, the more specific pattern of tubular injury exhibiting features of osmotic tubulopathy (radiocontrast-associated) was also observed (d). Less frequently encountered kidney pathologic findings included cortical coagulative necrosis (e, with necrotic tissue on the left and viable cortex to the right of the figure), disseminated and invasive Aspergillosis (f, with fungal organisms within the wall of an artery; f inset, organisms highlighted with a Grocott’s methenamine silver, GMS, stain), and in a sole case evidence of interstitial hemophagocytosis (g; g inset, immunoperoxidase staining for CD68 highlights interstitial macrophages containing phagocytosed cellular material). By electron microscopy, endothelial tubuloreticular inclusions were identified in patients with systemic lupus erythematosus with or without concomitant viral infection (h, arrows pointing to the endothelial tubuloreticular inclusions). Bone marrow aspirate smears were stained with Wright-Giemsa (a and b). Paraffin sections were stained with hematoxylin and eosin (c, e, f, and g), GMS (f inset), and periodic acid Schiff (d). Original magnifications for e at 200×; for d, f, and f inset at 400×; for a–c, g, and g inset at 600×; and for h at 40,000×. HLH, hemophagocytic lymphohistiocytosis.
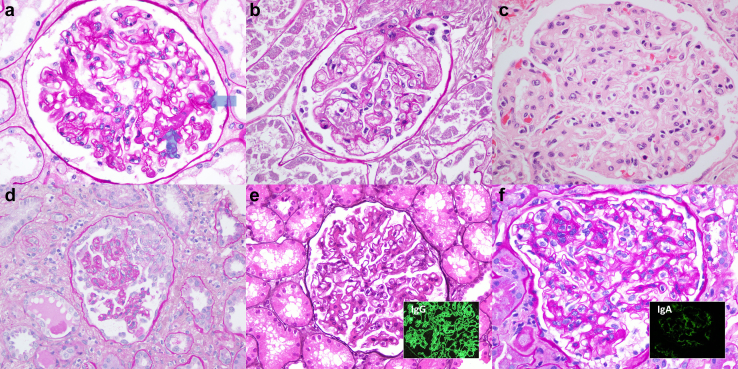
Figure 2.
Select pathologies involving glomeruli of patients in the setting of HLH. Evidence of acute (a) and chronic (b) thrombotic microangiopathy with glomerular involvement was observed, with the former characterized by fresh fibrin thrombi within glomerular capillaries (arrows) and the latter with glomerular basement membrane remodeling and double contour formation. Although 6 patients had a history of malignancy, only a rare patient showed direct kidney involvement by a given malignancy: intravascular large B-cell lymphoma within glomerular capillaries (c). Collapsing glomerulopathy (d) was evident in 5 patients, all of African heritage and 4 with concurrent infections. Lupus nephritis was seen in just under a quarter of the patients, with a proliferative and membranous lupus nephritis illustrated (e, and e inset showing global finely granular glomerular capillary wall and mesangial immune complex deposits strongly reactive for IgG by immunofluorescence microscopy, IF). Although a prevalent glomerulonephritis encountered in practice, only 1 patient in our cohort had IgA nephropathy (f), with the case showing mesangial and endocapillary proliferation (f inset showing global finely granular mesangial deposits strongly reactive for IgA by IF). Paraffin sections were stained with periodic acid Schiff (a, b, d, and f), hematoxylin and eosin (c), and Jones silver (e). Original magnifications for d and e at 400×; for a–c, e inset, f, and f inset at 600×. HLH, hemophagocytic lymphohistiocytosis
All 5 patients who had evidence of collapsing glomerulopathy were of African heritage. Four patients had evidence of ongoing viral or parasitic infection, including 2 with cytomegalovirus, 1 with Strongyloides, and 1 had malaria (Plasmodium falciparum). Two patients with collapsing glomerulopathy also had a history of systemic lupus erythematosus. The patients with collapsing glomerulopathy revealed negative testing for HIV (5 of 5 tested), viral hepatitis B (5 of 5 tested), viral hepatitis C (5 of 5 tested), Epstein-Barr virus (3 of 3 tested), and SARS-CoV-2 (of the 1 tested; the other 4 cases predated the SARS-CoV-2 pandemic).
Tubulointerstitial pathologies included ATI (n = 13, 43%; 2 of which had features of osmotic tubulopathy associated with radiocontrast), focal cortical necrosis (n = 3, 10%), and interstitial hemophagocytosis (n = 1, 3%). Twelve of the 13 cases with ATI as a primary diagnosis were from autopsy patients. Acute and/or chronic interstitial nephritis was not observed.
Endothelial tubuloreticular inclusions were identified in 5 of the 14 cases studied by electron microscopy (36%). In all 5 cases, the patients had LN, with 2 patients testing positive for cytomegalovirus and another positive for HIV.
Additional diagnoses include TMA (acute and/or chronic, and involving glomeruli and/or arterial vessels) in 5 cases (17%) and 1 each with end-stage kidney disease (secondary to diabetes mellitus), renal parenchymal involvement by intravascular large B-cell lymphoma, and disseminated Aspergillus fungal organisms directly infecting the kidney. One patient’s kidney did not show any significant histopathologic findings. The allograft kidney in 1 of the autopsy patients did not show evidence of acute T-cell or active antibody-mediated rejection.
Most of the abovementioned primary diagnoses were acute in nature aside from nodular diabetic glomerulosclerosis, secondary/adaptive focal segmental glomerulosclerosis, etc. Aside from the rare patient with end-stage kidney disease secondary to diabetes mellitus, the cohort’s background chronic kidney parenchymal changes were overall mild: median degree of global and segmental glomerulosclerosis was 3% (IQR of 0%–18%), and median degree of tubular atrophy and interstitial fibrosis was 5% (IQR of 0%–20%). Patients also exhibited predominantly mild vascular sclerosis: 13 with no significant sclerosis, 14 with mild, 1 with moderate, and 2 with severe sclerosis.
Discussion
The low prevalence, complexity of supportive laboratory testing, and nonspecific protean clinical manifestation frequently leads to delays in or misdiagnosis of HLH.13 Even after HLH is identified, potential concomitant autoimmune and infectious disease processes confound clinical management. As in the presented cohort, 30% of the patients had concomitant autoimmune and infectious diseases, creating a conundrum of whether to provide immunosuppression alongside antimicrobial therapy. AKI in the setting of HLH has been previously recognized as a frequent comorbidity, thus highlighting the need for better understanding of the underlying mechanisms of kidney dysfunction: our observed 63% incidence rate of AKI in the setting of HLH was similar to the rate (62%) observed by Aulagnon et al.4 Wang et al.14,15 have described a prediction model based on variables associated with increased risk of developing AKI in HLH (e.g., need for vasopressors, heart failure, and total bilirubin).
Among biopsied patients with HLH in our series, intrinsic kidney etiologies or processes mediate renal injury. Even among autopsy patients, 13 of the 18 had primary pathologic diagnoses other than ATI, indicating that intrinsic kidney disease contributed to acute and/or chronic kidney dysfunction. This suggests that patients are underbiopsied in this setting, potentially reflecting an assumption that ATI would be the sole finding. Although not surprising that ATI was a common finding in autopsied kidneys, this would be expected with agonal multisystem organ dysfunction leading to prerenal ischemia and confounds its true prevalence prior to the terminal course. That said, ATI would not be unexpected in patients with HLH compounded with sepsis, hemodynamic compromise, multisystem organ failure, hypoxemia, and exposure to possible endogenous and/or iatrogenic nephrotoxins. Notably, despite the extensive application of antibiotics and other medications, a primary diagnosis of acute interstitial nephritis was not observed.
Aside from ATI, our findings suggest that many of the observed pathologic diagnoses were associated with HLH and its secondary etiologies, rather than unrelated/incidental findings. First, 9 patients had an immune complex-mediated GN (LN, IgAN, and mixed cryoglobulinemic GN), all of which exhibited relatively mild chronicity with a median degree of global and segmental glomerulosclerosis of only 1% (IQR of 0%–5%), which poses that the glomerular diseases were either of recent onset or significantly exacerbated in the setting of HLH. This phenomenon has been described in other settings of “cytokine storm” and abnormal activation of the immune system (e.g., postvaccination,16 immune checkpoint inhibitor therapy17), raising the possibility that HLH potentiates the development of GNs with endocapillary proliferation or hypercellularity and/or crescents (i.e., an “active” glomerulitis) via immune modulation. That 4 of the 9 patients with an immune complex-GN exhibited “active” glomerulitis supports this point. Particularly in patients with high-risk APOL1 genotypes, collapsing glomerulopathy has also been observed in states of heightened activation of the immune system (e.g., autoimmune disease, infections), and the observation in our cohort and that of Thaunat et al.5 of collapsing glomerulopathy in HLH likely reflects a trigger upon predilecting podocytes.18 Endothelial or vascular injury and a procoagulant state leading to acute features of TMA are also likely mediated by abnormal activation of the immune system in HLH: TMA has been variably reported (13%–70%) to occur in HLH.19,20 Gloude et al.19 describe resolution of TMA and survival after treatment that included eculizumab and emapalumab (an interferon gamma blocker) in a small group of patients developing TMA in HLH. Unsurprisingly, some tested patients were found to harbor complement gene variants, suggesting that in the appropriate genetic setting, HLH may provide the “second-hit.” The finding of endothelial tubuloreticular inclusions in 5 patients with LN, though common in LN, might also reflect immune activation in HLH. The findings of interstitial and intraglomerular hemophagocytosis, each in a single case, are a direct manifestation of HLH in the kidney. Aside from these 2 cases, kidney biopsy alone is insufficient to exclude or support a diagnosis of HLH
Of the 12 patients with HLH who underwent biopsy, 6 improved kidney function on follow-up whereas the others exhibited worsening kidney function. The observation that 4 of the 12 patients expired within 5 months reflects the significant morbidity of HLH. The rapidly progressive disease in some patients with HLH is emphasized by the short duration of the terminal admissions (median one month). These numbers are consistent with the 20% to 50% reported death rate among patients with HLH, many of which rapidly succumb to disease.21,22 Due to the retrospective nature of our series, we could not draw conclusions on the impact of treatments on outcomes.
This retrospective study of patients with HLH is limited by sampling bias toward patients who have died and undergone autopsy, as well as patients undergoing for-indication kidney biopsy, both of which would increase the likelihood of sampling more severe cases of HLH and, in particular, HLH with renal involvement. Bias is also introduced by the protean presentation of HLH, such that milder cases of HLH are likely to be undiagnosed.
We provide the largest investigation characterizing the histopathologic findings in the kidney of patients with HLH and correlate with clinical and laboratory data. Further investigations involving multiple centers and greater numbers of patients are needed, ideally supplemented by widespread molecular/genetic testing for gene variants associated with HLH and possibly genes associated with complement regulation and TMA. Kidney biopsies should be considered in the setting of HLH as a significant proportion of patients have pathologic findings other than ATI that underlie the evident renal dysfunction and, in some cases, may be amenable to directed therapy. Our observations suggest that abnormal activation of the immune system may play a role in HLH-mediated potentiation of the development or worsening of “active” GN’s, collapsing glomerulopathy, and acute TMA: all significant actionable pathologies that a kidney biopsy would illustrate.
Disclosure
All the authors declared no competing interests.
Acknowledgments
The authors thank Hope Dzameshie and Zong Shi Wang (Columbia University Irving Medical Center, Renal Pathology Laboratory) for their invaluable technical assistance.
Footnotes
Table S1. Diagnostic guidelines for HLH as proposed by the Histiocyte Society (HLH-2004, adapted from Henter et al.).
Table S2. Additional laboratory testing for lactate dehydrogenase and liver/biliary functional markers.
Table S3. Detailed list of all immunosuppressive agents provided to patients in the setting of HLH.
Supplementary Material
Table S1. Diagnostic guidelines for HLH as proposed by the Histiocyte Society (HLH-2004, adapted from Henter et al.).
Table S2. Additional laboratory testing for lactate dehydrogenase and liver/biliary functional markers.
Table S3. Detailed list of all immunosuppressive agents provided to patients in the setting of HLH.
References
- 1.Henter J.I., Horne A., Arico M., et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48:124–131. doi: 10.1002/pbc.21039. [DOI] [PubMed] [Google Scholar]
- 2.Jordan M.B., Allen C.E., Greenberg J., et al. Challenges in the diagnosis of hemophagocytic lymphohistiocytosis: recommendations from the North American Consortium for Histiocytosis (NACHO) Pediatr Blood Cancer. 2019;66 doi: 10.1002/pbc.27929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Otrock Z.K., Eby C.S. Clinical characteristics, prognostic factors, and outcomes of adult patients with hemophagocytic lymphohistiocytosis. Am J Hematol. 2015;90:220–224. doi: 10.1002/ajh.23911. [DOI] [PubMed] [Google Scholar]
- 4.Aulagnon F., Lapidus N., Canet E., et al. Acute kidney injury in adults with hemophagocytic lymphohistiocytosis. Am J Kidney Dis. 2015;65:851–859. doi: 10.1053/j.ajkd.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 5.Thaunat O., Delahousse M., Fakhouri F., et al. Nephrotic syndrome associated with hemophagocytic syndrome. Kidney Int. 2006;69:1892–1898. doi: 10.1038/sj.ki.5000352. [DOI] [PubMed] [Google Scholar]
- 6.Group KDIGOKAKIW KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:19–36. doi: 10.1038/kisup.2011.32. [DOI] [Google Scholar]
- 7.Group KDIGOKCW KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2012;3:1–150. doi: 10.1038/ki.2013.243. [DOI] [PubMed] [Google Scholar]
- 8.Roufosse C., Simmonds N., Clahsen-van Groningen M., et al. 2018 reference guide to the Banff classification of renal allograft pathology. Transplantation. 2018;102:1795–1814. doi: 10.1097/TP.0000000000002366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chokshi B., D’Agati V., Bizzocchi L., Johnson B., Mendez B., Jim B. Haemophagocytic lymphohistiocytosis with collapsing lupus podocytopathy as an unusual manifestation of systemic lupus erythematosus with APOL1 double-risk alleles. BMJ Case Rep. 2019;12 doi: 10.1136/bcr-2018-227860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santoriello D., Hogan J., D’Agati V.D. Hemophagocytic syndrome with histiocytic glomerulopathy and intraglomerular hemophagocytosis. Am J Kidney Dis. 2016;67:978–983. doi: 10.1053/j.ajkd.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 11.Shao D., Pena O., Sekulic M., Valdez Imbert R., Vegivinti C.T.R., Jim B. Secondary haemophagocytic lymphohistiocytosis in a patient with new-onset systemic lupus erythematosus: the challenges of timely diagnosis and successful treatment. BMJ Case Rep. 2023;16 doi: 10.1136/bcr-2022-252938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sekulic M., Santoriello D., Masud A., Kudose S. Interstitial hemophagocytosis in hemophagocytic lymphohistiocytosis. Kidney Int. 2023;104:622. doi: 10.1016/j.kint.2023.05.002. [DOI] [PubMed] [Google Scholar]
- 13.Jordan M.B., Allen C.E., Weitzman S., Filipovich A.H., McClain K.L. How I treat hemophagocytic lymphohistiocytosis. Blood. 2011;118:4041–4052. doi: 10.1182/blood-2011-03-278127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang S., Zhou J., Yang J., et al. Clinical features and prognostic factors of acute kidney injury caused by adult secondary hemophagocytic lymphohistiocytosis. J Nephrol. 2022;35:1223–1233. doi: 10.1007/s40620-021-01147-2. [DOI] [PubMed] [Google Scholar]
- 15.Wang S., Yang L., Zhou J., et al. A prediction model for acute kidney injury in adult patients with hemophagocytic lymphohistiocytosis. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.987916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bomback A.S., Kudose S., D’Agati V.D. De novo and relapsing glomerular diseases after COVID-19 vaccination: what do we know so far? Am J Kidney Dis. 2021;78:477–480. doi: 10.1053/j.ajkd.2021.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DiFranza L.T., Chafouleas E., Katipally S., Stokes M.B., Kudose S., Sekulic M. Crescentic fibrillary glomerulonephritis in the setting of immune checkpoint inhibitor therapy: a report of two cases. Glomerular Dis. 2023;3:69–74. doi: 10.1159/000528881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kudose S., Batal I., Santoriello D., et al. Kidney biopsy findings in patients with COVID-19. J Am Soc Nephrol. 2020;31:1959–1968. doi: 10.1681/ASN.2020060802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gloude N.J., Dandoy C.E., Davies S.M., et al. Thinking beyond HLH: clinical features of patients with concurrent presentation of hemophagocytic lymphohistiocytosis and thrombotic microangiopathy. J Clin Immunol. 2020;40:699–707. doi: 10.1007/s10875-020-00789-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Croden J., Bilston L., Taparia M., Grossman J., Sun H.L. Incidence of bleeding and thromboembolism and impact on overall survival in adult patients with hemophagocytic lymphohistiocytosis: a 20-year provincial retrospective cohort study. J Thromb Haemost. 2022;20:671–683. doi: 10.1111/jth.15615. [DOI] [PubMed] [Google Scholar]
- 21.Arca M., Fardet L., Galicier L., et al. Prognostic factors of early death in a cohort of 162 adult haemophagocytic syndrome: impact of triggering disease and early treatment with etoposide. Br J Haematol. 2015;168:63–68. doi: 10.1111/bjh.13102. [DOI] [PubMed] [Google Scholar]
- 22.Riviere S., Galicier L., Coppo P., et al. Reactive hemophagocytic syndrome in adults: a retrospective analysis of 162 patients. Am J Med. 2014;127:1118–1125. doi: 10.1016/j.amjmed.2014.04.034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.