Abstract
IgA nephropathy (IgAN) is the most common primary glomerulonephritis worldwide. Approximately 30% to 45% of patients progress to kidney failure (KF) within 20 to 25 years of diagnosis, and there has long been a lack of effective treatments. The therapeutic landscape in IgAN is rapidly evolving, driven in large part by the acceptance of the surrogate clinical trial end point of proteinuria reduction by regulatory authorities for the accelerated approval of new therapies. Two drugs, targeted release formulation (TRF)-budesonide (nefecon) and sparsentan, have recently been approved under this scheme. Advancing insights into the pathophysiology of IgAN, including the roles of the mucosal immune system, B-cells, the complement system, and the endothelin system have driven development of therapies that target these factors. This review outlines current, recently approved, and emerging therapies for IgAN.
Keywords: APRIL, BAFF, clinical trials, complement, endothelin, IgA nephropathy
First described in 1968, IgAN represents the most common primary glomerulonephritis worldwide.1 Patients present with a wide spectrum of clinical manifestations, including isolated nonvisible hematuria, progressive chronic kidney disease (CKD), nephrotic syndrome, or rapidly progressive glomerulonephritis.2 An estimated 30% to 45% progress to KF within 20 to 25 years of diagnosis.3, 4, 5, 6 However, in a UK registry study of 2299 adults with IgAN, higher rates were reported: over 80% developed KF within 30 years, and a high rate of disease progression was observed even in those with proteinuria <1 g/day.7 Life expectancy with IgAN is reduced by 6 to 10 years, mainly due to complications of KF.5,8,9 IgAN often recurs following kidney transplantation and is a common cause of graft loss.10
In the 2021 Kidney Disease Improving Global Outcomes guidelines for glomerular diseases, it was acknowledged that no specific therapies for IgAN were available.11 The historical lack of drug development in IgAN was largely attributable to the requirement by regulatory authorities for “hard” kidney outcomes (e.g., doubling of serum creatinine or KF) when assessing drug efficacy, and the typical slow progression of IgAN made clinical trials unattractive. In 2019, the Kidney Health Initiative performed an analysis of 13 controlled trials that demonstrated a clear association between an early treatment effect on proteinuria and a composite of time to doubling of serum creatinine, KF, and death.12 Regulatory authorities now accept proteinuria reduction as a reasonably likely surrogate end point for progression to KF and as a basis for the accelerated approval of new treatments in IgAN. This has transformed the IgAN clinical trial landscape, leading to an explosion in clinical trial activity (179 trials registered on clinicaltrials.gov as of August 2023) and the approval of the first 2 drugs specifically for treating IgAN.
Current Guidelines
Diagnosis, Risk Stratification, and Treatment Selection
IgAN can only be diagnosed with a kidney biopsy, which will demonstrate dominant or codominant mesangial IgA deposition.11 Risk stratification should be undertaken using the international IgAN prediction tool, which integrates validated prognostic factors (Table 1) to produce a personalized risk of progression (50% reduction in estimated glomerular filtration rate [eGFR] or development of KF) for up to 7 years from the date of kidney biopsy.13 Despite its value in risk stratification and patient counselling, it is yet to be validated as at tool for guiding treatment. Risk stratification after this period is generally based on proteinuria, blood pressure (BP), and eGFR, each of which are independent prognostic factors.14 Treatment decisions are currently dictated by the extent of proteinuria (after optimization of supportive care) and eGFR (<30 ml/min per 1.73 m2 usually being regarded as the limit below which immunomodulatory treatment would not be used outside a rapidly progressive glomerulonephritis). Traditionally, proteinuria >1 g/day has been used to identify patients at higher risk of KF and suitable for either a clinical trial or immunomodulator. Currently, there are no biomarkers to inform the choice of immunomodulator and the Oxford Classification has not been validated as a tool for treatment selection.
Table 1.
Parameters required by the international IgA nephropathy prediction tool to predict 50% decline in eGFR or kidney failure up to 80 months from biopsy
| Parameters required by the IIgANPT |
|---|
| eGFR at biopsy |
| Systolic blood pressure at biopsy |
| Diastolic blood pressure at biopsy |
| Proteinuria at biopsy |
| Age at biopsy |
| Race |
| ACE inhibitor or ARB at biopsy |
| MEST scorea |
| Immunosuppression at or prior to biopsy |
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; eGFR, estimated glomerular filtration rate; IIgANPT, international IgA nephropathy prediction tool.
MEST score refers to the Oxford Classification scoring for IgA nephropathy, excluding crescents: mesangial hypercellularity (M), endocapillary hypercellularity (E), segmental glomerulosclerosis (S), tubular atrophy/interstitial fibrosis (T).
First Line Management
The 2021 Kidney Disease Improving Global Outcomes guidelines recommend optimized supportive care as initial management, aiming to reduce proteinuria by optimizing BP and maximizing single agent renin-angiotensin system inhibition (RASi), and to address lifestyle and cardiovascular risk factors (e.g., body weight, dietary salt restriction to <2 g/day, smoking cessation, lipid management).11 RASi is recommended when proteinuria is >0.5 g/day, regardless of BP.
The STOP-IgAN trial included a run-in period when comprehensive supportive care was optimized; about one-third of participants responded with a reduction of proteinuria large enough to make them ineligible for the next phase of the trial, highlighting the value of this approach.15
Beyond Supportive Care
Options for those at high risk of progression (defined by Kidney Disease Improving Global Outcomes as having persistent proteinuria >1 g/day despite optimized supportive care) were limited in 2021 and enrolment into clinical trials was encouraged.11 For those with preserved kidney function (i.e., an eGFR ≥30 ml/min per 1.73 m2) and unable to participate in a trial, a course of glucocorticoids was suggested, but only after careful consideration of potential risks and benefits. Previous trials of glucocorticoids in IgAN reported benefit, but their interpretation was often limited by small sample sizes, BP, and RASi not being optimized according to current standards of care, and adverse events not being systematically captured. Two randomized controlled trials (RCTs), STOP-IgAN and TESTING, were designed to address these concerns.
STOP-IgAN (N = 337, from Germany) reported no benefit in terms of full clinical remission with glucocorticoids with or without other immunosuppression compared to supportive care, both within the 3-year study and after approximately 10 years of follow-up. Adverse events were more frequent in the immunosuppression group (Table 2).15
Table 2.
IgAN RCTs evaluating corticosteroids following a run-in period, during which supportive care was optimized
| Trial | Majority ethnicity | Primary outcomes | Key exclusion criteria | Regimen | Outcome |
|---|---|---|---|---|---|
| STOP-IgAN | European | Complete remission: PCR <0.2 g/g and eGFR decrease <5 ml/min per 1.73 m2 from baseline after 3 years | Creatinine clearance <30ml/min, rapidly progressive disease, IgAN variants (e.g., minimal change), secondary IgAN | eGFR ≥60 ml/min per 1.73 m2: 1 g methylprednisolone i.v. for 3 days at month 1, 3, and 5. 0.5mg/kg per 48 h oral prednisolone for 6 months. | 17% experienced remission with treatment vs. 5% with placebo. No difference in rate of eGFR decline. Steroid-related adverse events in treatment group. |
| Progression: eGFR reduction ≥15 ml/min per 1.73 m2 from baseline | eGFR <60 ml/min per 1.73 m2: oral cyclophosphamide 1.5 mg/kg/day for 3 months, then azathioprine 1.5 mg/kg/day + oral prednisolone for 3 years (10 mg/day for 3 months, then 7.6 mg/day) | ||||
| TESTING | Asian | 40% decline in eGFR, ESKD, or death due to kidney disease | IgAN variants (e.g., minimal change), secondary IgAN, >50% crescents on biopsy within 12 months | Original dosing: 0.8 mg/kg/day methylprednisolone | 28% reached end point with treatment vs. 41.3% with placebo (pooled analysis of both doses). More steroid-related adverse events with treatment, including deaths. Benefits lost at 36 months of follow-up. |
| Reduced dosing: 0.4 mg/kg/day methylprednisolone |
eGFR, estimated glomerular filtration rate; ESKD, end-stage kidney disease; IgAN, IgA nephropathy; IIgANPT, international IgAN prediction tool; PCR, protein:creatinine ratio; RCT, randomized controlled trial.
The TESTING study was halted early (total N = 504, predominantly from Asia) due to excess serious adverse events among those receiving methylprednisolone (0.6–0.8 mg/kg/day), mainly due to infection including 2 deaths.16 TESTING was recommenced with a reduced methylprednisolone dose (0.4 mg/kg/day) given for 6 to 8 months with co-trimoxazole prophylaxis. Those treated with either dose of methylprednisolone had a lower rate of meeting the composite primary endpoint (40% reduction in eGFR, KF, or death due to kidney disease) compared to those receiving placebo (28% vs. 41.3%). Although adverse events were not systematically collected (e.g., through validated questionnaires), fewer events were reported with the lower methylprednisolone dose, although 1 death still occurred. Effects on proteinuria reduction were lost by 36 months, implying that potential benefits of systemic glucocorticoids are not sustained.17
Mycophenolate mofetil (MMF) was found to have no benefit beyond RASi in a collection of small RCTs (N = 32–52) performed in Belgium, Canada, and the USA.18, 19, 20 Trials from Asia, however, have reported that MMF treatment is associated with a reduction in proteinuria and preservation of kidney function. An RCT from Hong Kong (N = 40) found that MMF produced a greater reduction in proteinuria compared to placebo, when used at 1.5–2 g/day.21 These benefits were replicated in a recent, larger, open label RCT from China (N = 170); the MAIN trial incorporated a run-in period in which supportive care (RASi, BP control, salt restriction, and smoking cessation) was optimized, after which participants were randomized to receive MMF (1.5 g/day for 12 months, followed by 0.75–1 g/day for at least 6 months) or standard of care therapy only.22 The MAIN trial reported that 7.1% of those receiving MMF met the primary composite outcome of doubling of serum creatinine or KF, versus 21.2% receiving standard-of-care therapy only.22 These results are consistent with a potential benefit of MMF in patients of Chinese origin.
Outside of specific situations (nephrotic syndrome, rapidly progressive glomerulonephritis), evidence to support global use of other immunosuppressive agents is lacking. As with MMF, studies from China reported benefit with hydroxychloroquine; however, these data are yet to be replicated elsewhere.23 Similarly, tonsillectomy is often performed in Japan based on favorable observational studies; however, there are no data outside of Japan supporting benefit.11,24 These differences in treatment response could reflect a heterogeneity in the fundamental pathogenic pathways between races.
Sodium-Glucose Co-Transporter-2 Inhibitors (SGLT2is)
The DAPA-CKD and EMPA-KIDNEY studies of SGLT2is in CKD enrolled large numbers of patients with IgAN (Table 3).25,26 Both were terminated early because of their clear efficacy in preventing kidney function decline, KF, or death. Prespecified analyses confirmed that these effects were equally seen in IgAN participants.27 It should however be noted that DAPA-CKD and EMPA-KIDNEY were CKD-focused trials; baseline eGFR and proteinuria of patients included with IgAN were very different to those enrolled in ongoing or recently reported IgAN-specific trials. Supportive care, including RASi was not optimized during run-in, and the event rate in the placebo arm of the DAPA-CKD IgAN cohort was notably high.28 Whether SGLT2is are as effective in those with preserved kidney function (compared to those with established chronic damage) is not yet clear. Nevertheless, SGLT2is have been shown to be safe and effective treatments for CKD and have emerged as an important addition to supportive care in IgAN.
Table 3.
Summary of SGLT2i RCTs including IgAN participants. eGFR as CKD-EPI
| Trial | Key Inclusion criteria | Primary outcomes | Key exclusion criteria | Regimen | Outcome |
|---|---|---|---|---|---|
| DAPA-CKD | eGFR ≥25 and ≤75 ml/min per 1.73 m2, uPCR ≥200 and ≤5000 mg/g, stable + maximum tolerated single agent RAS blockade for 4 weeks |
Sustained decline of eGFR by 50%, ESKD or death from cardio-renal causes | Immunosuppressive therapy, organ transplantation. | dapagliflozin 10 mg/day | Outcome occurred in 9.2% of treatment group vs. 14.5% in placebo group. |
| EMPA-KIDNEY | eGFR ≥20 and ≤45 ml/min per 1.73 m2 OR eGFR ≥45 and <90 ml/min per 1.73 m2 with uPCR ≥300 mg/g |
Sustained decline of eGFR by 40% or to 10, ESKD, or death from cardio-renal causes | Immunosuppressive therapy, kidney transplant, dual RAS blockade. | empagliflozin 10 mg/day | Outcome occurred in 13.1% of treatment group vs. 16.9% in placebo group. |
CKD-EPI, chronic kidney disease: epidemiology collaboration; eGFR, estimated glomerular filtration rate; ESKD, end-stage kidney disease; IgAN, IgA nephropathy; RAS, renin-angiotensin system; RCT, randomized controlled trial; SGLT2i, sodium-glucose co-transporter-2 inhibitor; uPCR, urine protein-to-creatinine ratio.
Leveraging Novel Insights Into IgAN Pathophysiology
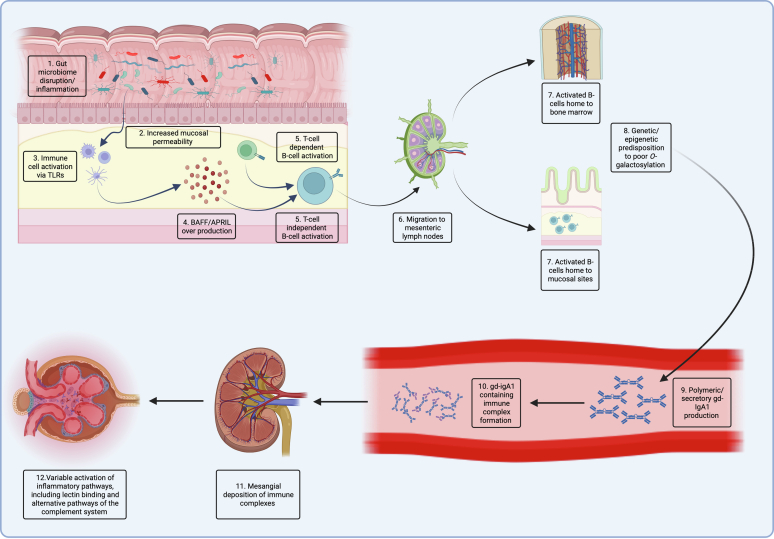
Central to the pathogenesis of IgAN is an increase in circulating IgA1 that lacks galactose at its hinge region (galactose deficient-IgA1 [Gd-IgA1], Hit 1). IgG and IgA autoantibody production occurs in susceptible individuals (Hit 2), with subsequent immune complex formation (Hit 3).29, 30, 31, 32, 33 Deposition of Gd-IgA1-containing immune complexes induces mesangial cell activation and inflammation (Hit 4), driven by several mediators of inflammation, including cytokines, chemokines, and the complement system (Figures 1 and 2), leading to subsequent kidney damage.34 Multiple lines of evidence indicate that circulating Gd-IgA1 originates from the mucosal immune system, particularly the gut-associated and nasal-associated lymphoid tissues.35 Activation of the mucosal immune system can occur in response to environmental triggers such as infectious pathogens, autoinflammatory diseases, and interaction with the prevalent mucosal microbiota. Experimental evidence has shown that activation of mucosal immune cells via toll-like receptors, which may occur via extracellular vesicles produced by pathogens or resident microbiota, can upregulate expression of B-cell activating factor (BAFF) and A PRoliferation Inducing Ligand (APRIL), which are intimately involved in controlling IgA class switch recombination and IgA synthesis by mucosal B/plasma cells.30,36, 37, 38, 39 BAFF and APRIL activate mucosal B-cells via the BAFF receptor, B-cell maturation antigen, and T-cell activator and calcium-modulating ligand interactor.40, 41, 42 Genetic and epigenetic alterations (including micro-RNA dysregulation) within B-cells have been shown to modulate synthesis of Gd-IgA1. Therapies targeting each of these pathways are now being tested in IgAN.
Figure 1.
An overview of the pathophysiology of IgAN. (a) Infections, inflammation, or disruptions to host mucosal microbiota in conjunction with (b) increased mucosal permeability can lead to (c) mucosal immune cells being activated via toll like receptors. (d) This leads to an in increased production of BAFF and APRIL and (e) activation of mucosal B-cells via T-cell independent mechanisms, although these cells may also be activated by T-cells. (f) Activated B-cells traffic to central lymph nodes and (g) then traffic back to mucosal sites or mis-home to systemic sites such as bone marrow. (h) Mucosally-derived plasma cells produce Gd-IgA1 of which the magnitude is in part determined by genetic and epigenetic factors, which take up (i) polymeric and secretory forms. (j) Gd-IgA1 containing circulating immune complexes (k) deposit in the glomerular mesangium, (l) variably activating inflammatory pathways, including the lectin and alternative pathways of the complement system.
BAFF, B-cell activating factor; Gd-IgA1, galactose deficient-IgA1; IgAN, IgA nephropathy.
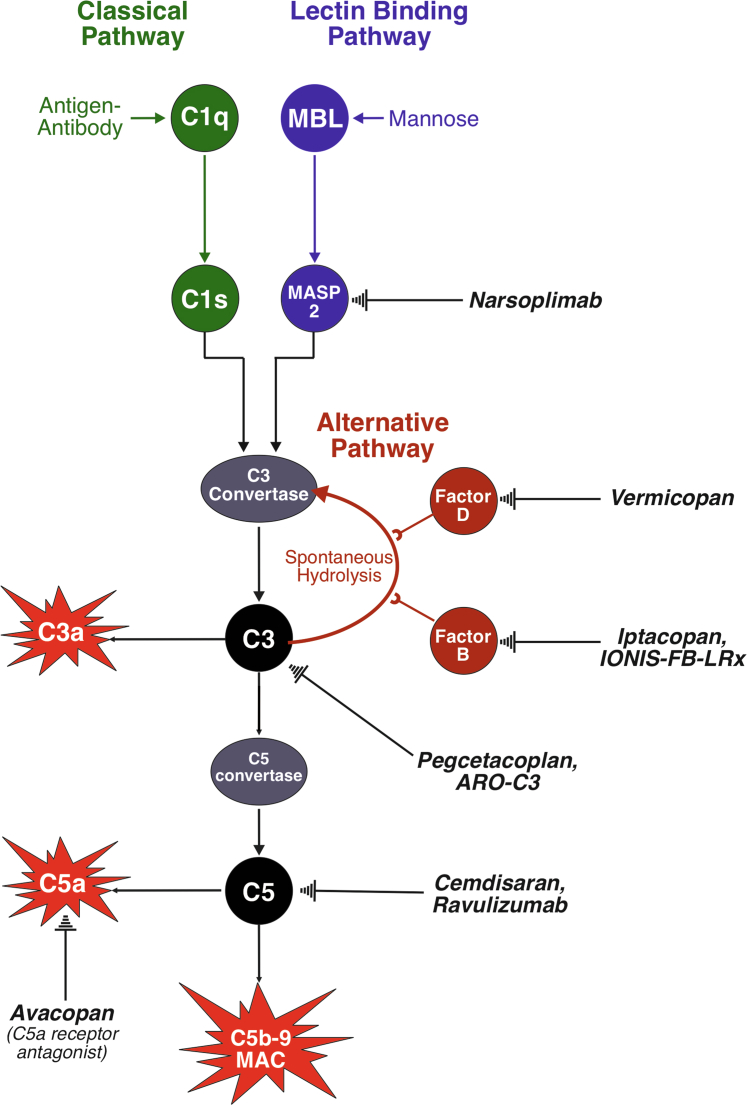
Figure 2.
The complement system. The complement system can be activated by the classical, lectin, or alternative pathways. The classical pathway (green) is activated by antigen-antibody complexes, which bind C1q allowing C1r to cleave C1s which eventually leads to the formation of a C3 convertase downstream. There is little evidence of complement system activation via the classical pathway in IgAN. The lectin pathway (blue) is activated by mannose moieties commonly found on microbial surfaces, but also on Gd-IgA1, which bind mannose binding lectin to activate MASP1 and MASP2. MASP2 activation mirrors that of C1s, eventually leading to the formation of a C3 convertase. MASP2 is inhibited by narsoplimab. The alternative pathway (red) is constantly activated by the hydrolysis of C3; the pathway is promoted by factor B and factor D. Factor B is inhibited by iptacopan and IONIS-FB-LRx, whereas factor D is inhibited by vermicopan. The common pathway (black) is activated by any C3 convertase, cleaving C3 (inhibited by pegcetacoplan and ARO-C3) to C3a, an inflammatory mediator, and C3b. C3b is further acted upon by the C3 convertases to produce a C5 convertase, which cleaves C5 (can be inhibited by cemdisaran and ravuluzimab) to produce C5a, an inflammatory mediator (the C5a receptor can be blocked by avacopan), and C5b. This eventually leads to the formation of the membrane attack complex, which is capable of cell lysis. Gd-IgA1, galactose deficient-IgA1; IgAN, IgA nephropathy; MASP1, mannose-binding lectin serine protease 1; MASP2, mannose-binding lectin serine protease 2.
Recently Approved Therapies
Nefecon
Nefecon (Tarpeyo [US]/Kinpeygo [EU]) comprises budesonide packaged within a pH-sensitive capsule, engineered to release active drug at peak concentration within the terminal ileum to act on the immune cells responsible for mucosal IgA production within Peyer’s patches.43 The drug undergoes extensive first pass metabolism, minimizing systemic absorption and side effects. In the phase 2 NEFIGAN trial, nefecon treatment resulted in a 27.3% reduction in proteinuria at 9 months, with preservation of kidney function up to 12 months.44, 45, 46 In parallel, reductions in serum levels of BAFF, APRIL, Gd-IgA1, secretory IgA, and IgA-IgG-immune complexes occurred.45 The phase 3 NefIgArd trial evaluated TRF-budesonide 16 mg/day for 9 months versus placebo. Results from part A of the study (N = 199) demonstrated a 27% reduction in mean urine protein-to-creatinine ratio and preservation of kidney function (3.87 ml/min per 1.73 m2 benefit) at 9 months. Based on these data, nefecon became the first disease-specific treatment to receive accelerated approval for IgAN.47 Sustained and significant improvements in kidney function and proteinuria were recently reported at 24 months (including 15 months off treatment), although proteinuria rose and eGFR started to decline in both groups after 12 months.48 The drug was generally well-tolerated; however, hypertension (15.% vs. 2%), edema (15.5% vs. 4%), muscle spasms (13.4% vs. 4%), and acne (11.3% vs. 2%) were reported more frequently with nefecon compared to placebo.
Sparsentan
Sparsentan (Filspari [US]) is a dual endothelin type A receptor (ETA-R) and angiotensin receptor antagonist. Endothelin receptors are widely expressed G-protein coupled receptors,49 existing as either ETA-R or endothelin type B receptor subtypes, and are activated by endothelin 1, 2, or 3). ETA-Rs are mainly expressed on vascular smooth muscle and cause potent vasoconstriction. In vitro, ETA-R activation leads to mesangial cell activation and inflammation.50 In the ddY mouse model of IgAN, ETA-R blockade suppressed the development of histological lesions and reduced proteinuria.51 In humans, endothelin 1-related polymorphisms associate with IgAN, and glomerular ET1 expression correlates with a worse prognosis.52,53 Sparsentan is being evaluated in the phase 3 PROTECT trial (NCT03762850). An interim analysis demonstrated a 49.8% mean proteinuria reduction at 9 months in those treated with sparsentan, approximately 3 times greater than that achieved with irbesartan (15.1%).54 This effect was independent of changes in BP. Sparsentan was well-tolerated and there were no edema-related drug discontinuations, in distinction to the experience of endothelin receptor antagonism in SONAR, a diabetic kidney disease trial.55 Nevertheless, the incidence of edema (14% vs. 9%), hypotension (14% vs. 6%) and dizziness (13% vs. 5%) were more frequent with sparsentan compared to irbesartan alone. Sparsentan was approved by the US Food and Drug Administration in 2023 for patients with IgAN at high risk of KF. Two-year eGFR data were recently reported.56 Compared to irbesartan, treatment with sparsentan led to slower eGFR decline, with a reduction in the total 2-year eGFR slope (day 1 to week 110 of treatment) by 1.0 ml/min per 1.73 m2/year (P = 0.058; −2.9 ml/min per 1.73 m2/year with sparsentan vs. −3.9 ml/min per 1.73 m2/year with irbesartan) and in the chronic 2-year eGFR slope (week 6–week 110 of treatment) by 1.1 ml/min per 1.73 m2/year (P = 0.037, eGFR change = −2.7 ml/min per 1.73 m2/year with sparsentan vs. −3.8 ml/min per 1.73 m2/year with irbesartan). The difference in the 2 slope measurements (total vs. chronic) may be explained by the initial acute eGFR decrease after commencing either sparsentan and irbesartan due to their glomerular hemodynamic effects, and therefore chronic slope may be a better measure of nephroprotection with these classes of medications. The reduction in proteinuria with sparsentan observed in the interim analysis was maintained throughout the study period. In addition, the number of events in the composite kidney outcome of 40% eGFR reduction, end-stage kidney disease or all-cause mortality trended in favor of sparsentan treatment, although the study was not powered for this outcome.56 An open-label experimental medicine study (SPARTAN; NCT04663204) is ongoing and is exploring the mechanistic actions of sparsentan in IgAN with repeat kidney biopsies, cardiac and kidney MRI, and extensive serum and urine biomarker studies.
Therapies in Phase 2 and Phase 3 Studies in 2023
Endothelin Receptor Antagonists
In addition to sparsentan, atrasentan is an ETA-R antagonist being evaluated in a phase 3 trial (ALIGN), a phase 2 open-label basket study (AFFINITY; NCT04573478), and the ASSIST cross-over study examining combination of atrasentan with SGLT2is (NCT05834738). Interim analysis of AFFINITY demonstrated a 58.5% mean proteinuria reduction in patients with IgAN treated with atrasentan at 24 weeks, and a favorable safety profile.57 ALIGN also met its primary endpoint on interim analysis, with treatment with atrasentan resulting in a statistically significant reduction in proteinuria compared to placebo at 36 weeks.58
B-Cell Directed Treatments
As the source of Gd-IgA1, B-cells are central to the pathogenesis of IgAN (Figure 1). Rituximab, an anti-CD20 chimeric monoclonal antibody that depletes peripheral B-cells, demonstrated no benefit in terms of proteinuria reduction, kidney function, or in reducing levels of Gd-IgA1 in an open-label study in IgAN. This may reflect the inability of rituximab to target tissue-resident (e.g., mucosal) B-cells or CD20-/CD38+ plasma cells.59,60
BAFF and APRIL play critical roles in IgA class switch recombination and B-cell proliferation and survival. Serum levels of BAFF and APRIL correlate with IgAN disease severity, and genome wide association studies identified the gene encoding APRIL (TNFSF13) as an IgAN risk locus.61, 62, 63, 64 BAFF-overexpressing mice develop an IgAN-like phenotype, and blocking APRIL inhibits spontaneous IgAN in ddY mice.65,66
Sibeprenlimab (VIS649) and zigakibart (BION-1301) are APRIL-neutralizing monoclonal antibodies currently being evaluated in phase 3 studies (VISIONARY; NCT05248646 and BEYOND; NCT05852938 respectively). In the phase 2 ENVISION study, sibeprenlimab demonstrated rapid suppression of serum Gd-IgA1, IgA, and APRIL, and a 44% mean placebo-adjusted proteinuria reduction at 9 months, in a prespecified interim analysis of 72 participants.67 Full results of the ENVISION study for 155 participants have recently been reported.68 At 12 months, sibeprenlimab produced a dose dependent reduction in proteinuria of 47.2% to 62% compared to 20% by placebo, whereas eGFR remained stable in the sibeprenlimab-treated groups (change from baseline was -2.7 to 0.2 ml/min per 1.73 m2) compared to a 7.4 ml/min per 1.73 m2 reduction in the placebo group. Zigakibart is being evaluated in a single-arm open-label phase 1/2 study of up to 30 patients with IgAN. Interim results from 17 participants report that the drug is well-tolerated and suppresses Gd-IgA1, APRIL, and proteinuria, with a 67% mean proteinuria reduction at 52 weeks.69
Atacicept, telitacicept and povetacicept are fusion proteins containing extracellular portions of T-cell activator and calcium-modulating ligand interactor, which bind and inhibit both BAFF and APRIL. Atacicept is being evaluated in the phase 2b ORIGIN trial; interim results have demonstrated reductions in serum IgA and Gd-IgA1, a 43% reduction in mean proteinuria, and eGFR stabilization at 36 weeks (vs. 8.5% decline with placebo).70 A phase 3 trial (ORIGIN 3, NCT04716231) is being initiated. Telitacicept was studied in a phase 2 IgAN RCT in China (N = 44) (NCT04905212); treatment produced a 49% mean proteinuria reduction at the 240 mg dose, with eGFR remaining stable.71 Povetacicept is being studied in an open label basket trial (RUBY-3) including patients with IgAN (NCT05732402).
An alternative B cell targeting approach, utilizing monoclonal antibodies that bind CD38+ plasma cells and being developed to treat multiple myeloma, is also being tested in IgAN. Felzartamab is being assessed in a phase 2 RCT for proteinuria reduction (IGNAZ; NCT05065970) and mezagitamab is being studied in a phase 1 trial (NCT05174221).
At present, there are insufficient data to determine if targeting APRIL alone, APRIL and BAFF, or CD38 is likely to be of greatest benefit in IgAN.
Modulating the Complement System
The complement system comprises over 20 proteins, capable of mediating inflammation as part of the innate immune response.34 It is activated via the classical, lectin, or alternative pathways (Figure 2). Glomerular C3 codeposition occurs in >90% of IgAN cases and the absence of C1q in most cases implies that classical pathway activation does not play a role in IgAN.34 Multiple therapies are being developed to block individual proteins of the complement cascade and many of these are being studied in IgAN (Figure 2; see Cheung et al.72 for a comprehensive review). Complement inhibitors are likely to soon offer a realistic alternative to systemic glucocorticoids as a means to limit intrarenal inflammation and glomerular injury in IgAN.
Alternative Pathway Inhibition
There is evidence for alternative pathway activation in most cases of IgAN, and increased mesangial deposition of alternative pathway components is associated with a worse clinical outcome.34 Drugs in development in IgAN target factor B (FB) and factor D (Figure 2).
Iptacopan, an oral small molecule FB inhibitor, was assessed in a phase 2 dose-finding study. Iptacopan inhibited alternative pathway activation and reduced proteinuria by 23% compared to placebo by month 3.73,74 Iptacopan is being evaluated in a phase 3 RCT (APPLAUSE-IgAN, NCT04578834).75 IONIS-FB-LRx is an antisense oligonucleotide which blocks hepatic FB production through RNA interference. Interim analysis of an ongoing single arm phase 2 IgAN study (N = 10) demonstrated reductions in plasma FB, serum alternative pathway activity, urine Ba, and 44% proteinuria reduction by week 29 (NCT04014335),76 and a phase 3 trial is being initiated (IMAGINATION; NCT05797610). Vermicopan (ALXN2050), a small molecule factor D inhibitor, is currently being evaluated in a phase 2 study (NCT05097989).
Lectin Pathway Inhibition
Glomerular deposition of lectin pathway components, including mannose-binding lectin serine protease 1 and 2 (MASP1/2) and C4d, are detected in 30% to 40% of IgAN cases, and their presence correlates with a worse outcome.34 Narsoplimab, a monoclonal antibody against mannose-binding lectin serine protease 2 (Figure 2), was tested in a small phase 2 trial. A 72% mean proteinuria reduction occurred by week 18 in those who entered the study on corticosteroids. Twelve patients continued with open-label extended narsoplimab treatment, which resulted in a 38% mean proteinuria reduction after 22 months, and preserved eGFR compared to a matched retrospective cohort.77 Narsoplimab was subsequently assessed in the phase 3 ARTEMIS-IgAN trial (NCT03608033). A recent interim analysis found no statistically significant evidence of proteinuria reduction with narsoplimab treatment compared to placebo, and the trial has now been discontinued.78 Further analysis of the ARTEMIS-IgAN trial is awaited.
Common Pathway Inhibition
Drugs targeting complement proteins in the common pathway (C3, C5, and C5a receptor) are also being evaluated in IgAN (Figure 2). C3 is being targeted in phase 2 studies with the peptide pegcetacoplan (NCT03453619), and with ARO-C3, an RNA interference treatment designed to suppress hepatic C3 production (NCT05083364). C5 inhibition is being studied in phase 2 studies with the monoclonal antibody ravulizumab (SANCTUARY; NCT04564339) and the RNA interference treatment cemdisiran, designed to suppress hepatic C5 production (NCT03841448). Interim results from the cemdisiran phase 2 study demonstrated a 37.4% reduction in mean proteinuria at 32 weeks, and stabilization of kidney function (N = 22).79 An interim analysis of SANCTUARY at 6 months (N = 43 on ravulizumab vs. 23 on placebo) found a 40.3% reduction in proteinuria in those treated with ravulizumab compared to 10.9% with placebo, and no safety concerns.80 Avacopan, a small molecule C5a receptor antagonist, was studied in a small open-label study. Three of 7 patients had a 50% reduction in proteinuria at week 12, and urinary monocyte chemoattractant protein-1-to-creatinine ratio was reduced by 30% by week 8, indicating suppression of renal inflammation.81
Future Directions
The increased understanding of IgAN pathogenesis and the development of new treatment options is transforming the therapeutic landscape for IgAN. The Kidney Disease Improving Global Outcomes 2021 guidelines are already in need of updating, and new treatment strategies have been proposed for the management of IgAN.82 As more therapies become available, clinicians will soon be faced with having to select the most appropriate treatment strategy for their patients. Effective strategies are almost certainly going to involve combinations of drugs; however, there is likely to be limited trial data available on these approaches for the foreseeable future.
At present, initiation and escalation of treatment is guided by levels of proteinuria and eGFR, which are nonspecific and often late indicators of kidney damage. Wider use of the international IgAN prediction tool to define those patients most at risk of progression where intervention is needed, alongside identification of additional biomarkers to improve prognostic precision of the international IgAN prediction tool is required. Biomarkers are also needed to predict response to the new therapeutic approaches, and also to monitor response, so that those not responding can be switched to alternative treatments quickly. Each of the phase 3 trials in IgAN has generated a large biorepository of samples and the hope is that through collaboration between academia and industry, these samples can be studied to find the biomarkers we need to deliver truly personalized medicine to those living with IgAN.
Disclosure
HS reports no competing interests. JB reports receiving consulting and speaker fees from Alnyam, Argenx, Astellas, BioCryst, Calliditas, Chinook, Dimerix, Galapagos, Novartis, Omeros, Otsuka, Travere Therapeutics, Vera Therapeutics, and Visterra; reports receiving grant support from Argenx, Calliditas, Chinook, Galapagos, GSK, Novartis, Omeros, Travere Therapeutics, and Visterra; reports serving as scientific/medical advisor to Alnylam, Astellas, Calliditas, Chinook, Galapagos, GlaxoSmithKline, Novartis, Omeros, Roche, Travere Therapeutics, UCB, and Visterra, Inc.; is a member of Kidney Health Initiative; and has lectured, chaired, or participated in symposia/panel discussions for Calliditas, Omeros, and Travere Therapeutics. CKC reports receiving consulting and speaker fees from Alpine Immune Sciences, Calliditas, Chinook, CSL Vifor, George Clinical, Novartis, Otsuka, Stada, Travere Therapeutics, Vera Therapeutics; receiving grant support from Travere Therapeutics; and being on a data monitoring committee for Roche.
Acknowledgments
The figures were created with BioRender.com.
References
- 1.Berger J., Hinglais N. [Intercapillary deposits of IgA-IgG] J Urol Nephrol (Paris) 1968;74:694–695. [PubMed] [Google Scholar]
- 2.Galla J.H. IgA nephropathy. Kidney Int. 1995;47:377–387. doi: 10.1038/ki.1995.50. [DOI] [PubMed] [Google Scholar]
- 3.Reich H.N., Troyanov S., Scholey J.W., Cattran D.C., Toronto Glomerulonephritis Registry Remission of proteinuria improves prognosis in IgA nephropathy. J Am Soc Nephrol. 2007;18:3177–3183. doi: 10.1681/ASN.2007050526. [DOI] [PubMed] [Google Scholar]
- 4.D’Amico G., Colasanti G., Barbiano di Belgioioso G., et al. Long-term follow-up of IgA mesangial nephropathy: clinico-histological study in 374 patients. Semin Nephrol. 1987;7:355–358. [PubMed] [Google Scholar]
- 5.Hastings M.C., Bursac Z., Julian B.A., et al. Life expectancy for patients from the Southeastern United States with IgA nephropathy. Kidney Int Rep. 2018;3:99–104. doi: 10.1016/j.ekir.2017.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moriyama T., Tanaka K., Iwasaki C., et al. Prognosis in IgA nephropathy: 30-year analysis of 1,012 patients at a single center in Japan. PLoS One. 2014;9 doi: 10.1371/journal.pone.0091756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pitcher D., Braddon F., Hendry B., et al. Long-term outcomes in IgA nephropathy. Clin J Am Soc Nephrol. 2023;18:727–738. doi: 10.2215/CJN.0000000000000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jarrick S., Lundberg S., Welander A., et al. Mortality in IgA nephropathy: a nationwide population-based cohort study. J Am Soc Nephrol. 2019;30:866–876. doi: 10.1681/ASN.2018101017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knoop T., Vikse B.E., Svarstad E., Leh S., Reisæter A.V., Bjørneklett R. Mortality in patients with IgA nephropathy. Am J Kidney Dis. 2013;62:883–890. doi: 10.1053/j.ajkd.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 10.Uffing A., Pérez-Saéz M.J., Jouve T., et al. Recurrence of igA nephropathy after kidney transplantation in adults. Clin J Am Soc Nephrol. 2021;16:1247–1255. doi: 10.2215/CJN.00910121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.KDIGO K. Clinical practice guideline for glomerulonephritis. Kidney Int Suppl (2011) 2012;2:209–217. doi: 10.1038/kisup.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thompson A., Carroll K., Inker A., et al. Proteinuria reduction as a surrogate end point in trials of IgA nephropathy. Clin J Am Soc Nephrol. 2019;14:469–481. doi: 10.2215/CJN.08600718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barbour S.J., Coppo R., Zhang H., et al. Evaluating a new international risk-prediction tool in IgA nephropathy. JAMA Intern Med. 2019;179:942–952. doi: 10.1001/jamainternmed.2019.0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Selvaskandan H., Shi S., Twaij S., Cheung C.K., Barratt J. Monitoring immune responses in IgA nephropathy: biomarkers to guide management. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.572754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rauen T., Eitner F., Fitzner C., et al. Intensive supportive care plus immunosuppression in IgA nephropathy. N Engl J Med. 2015;373:2225–2236. doi: 10.1056/NEJMoa1415463. [DOI] [PubMed] [Google Scholar]
- 16.Lv J., Zhang H., Wong M.G., et al. Effect of oral methylprednisolone on clinical outcomes in patients with IgA nephropathy: the TESTING randomized clinical trial. JAMA. 2017;318:432–442. doi: 10.1001/jama.2017.9362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lv J., Wong M.G., Hladunewich M.A., et al. Effect of oral methylprednisolone on decline in kidney function or kidney failure in patients with IgA nephropathy: the TESTING randomized clinical trial. JAMA. 2022;327:1888–1898. doi: 10.1001/jama.2022.5368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maes B.D., Oyen R., Claes K., et al. Mycophenolate mofetil in IgA nephropathy: results of a 3-year prospective placebo-controlled randomized study. Kidney Int. 2004;65:1842–1849. doi: 10.1111/j.1523-1755.2004.00588.x. [DOI] [PubMed] [Google Scholar]
- 19.Hogg R.J., Bay R.C., Jennette J.C., et al. Randomized controlled trial of mycophenolate mofetil in children, adolescents, and adults with IgA nephropathy. Am J Kidney Dis. 2015;66:783–791. doi: 10.1053/j.ajkd.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 20.Frisch G., Lin J., Rosenstock J., et al. Mycophenolate mofetil (MMF) vs placebo in patients with moderately advanced IgA nephropathy: a double-blind randomized controlled trial. Nephrol Dial Transplant. 2005;20:2139–2145. doi: 10.1093/ndt/gfh974. [DOI] [PubMed] [Google Scholar]
- 21.Tang S., Leung J.C.K., Chan L.Y.Y., et al. Mycophenolate mofetil alleviates persistent proteinuria in IgA nephropathy. Kidney Int. 2005;68:802–812. doi: 10.1111/j.1523-1755.2005.00460.x. [DOI] [PubMed] [Google Scholar]
- 22.Hou F.F., Xie D., Wang J., et al. Effectiveness of mycophenolate mofetil among patients with progressive IgA nephropathy: a randomized clinical trial. JAMA Netw Open. 2023;6 doi: 10.1001/jamanetworkopen.2022.54054. [DOI] [PubMed] [Google Scholar]
- 23.Liu L., Yang Y., Shi S., et al. Effects of hydroxychloroquine on proteinuria in IgA nephropathy: a randomized controlled trial. Am J Kidney Dis. 2019;74:15–22. doi: 10.1053/j.ajkd.2019.01.026. [DOI] [PubMed] [Google Scholar]
- 24.Selvaskandan H., Cheung C.K., Muto M., Barratt J. New strategies and perspectives on managing IgA nephropathy. Clin Exp Nephrol. 2019;23:577–588. doi: 10.1007/s10157-019-01700-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heerspink H.J.L., Stefánsson B.V., Correa-Rotter R., et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383:1436–1446. doi: 10.1056/NEJMoa2024816. [DOI] [PubMed] [Google Scholar]
- 26.The EMPA-KIDNEY Collaborative Group. Herrington W.G., Staplin N., Wanner C., et al. Empagliflozin in patients with chronic kidney disease. N Engl J Med. 2023;388:117–127. doi: 10.1056/NEJMoa2204233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wheeler D.C., Toto R.D., Stefánsson B.V., et al. A pre-specified analysis of the DAPA-CKD trial demonstrates the effects of dapagliflozin on major adverse kidney events in patients with IgA nephropathy. Kidney Int. 2021;100:215–224. doi: 10.1016/j.kint.2021.03.033. [DOI] [PubMed] [Google Scholar]
- 28.Barratt J., Floege J. SGLT-2 inhibition in IgA nephropathy: the new standard of care? Kidney Int. 2021;100:24–26. doi: 10.1016/j.kint.2021.04.002. [DOI] [PubMed] [Google Scholar]
- 29.Hu S., Bao H., Xu X., et al. Increased miR-374b promotes cell proliferation and the production of aberrant glycosylated IgA1 in B cells of IgA nephropathy. FEBS Lett. 2015;589(24):4019–4025. doi: 10.1016/j.febslet.2015.10.033. [DOI] [PubMed] [Google Scholar]
- 30.Qin W., Zhong X., Fan J.M., Zhang Y.J., Liu X.R., Ma X.Y. External suppression causes the low expression of the Cosmc gene in IgA nephropathy. Nephrol Dial Transplant. 2008;23:1608–1614. doi: 10.1093/ndt/gfm781. [DOI] [PubMed] [Google Scholar]
- 31.Xing Y., Li L., Zhang Y., et al. C1GALT1 expression is associated with galactosylation of IgA1 in peripheral B lymphocyte in immunoglobulin A nephropathy. BMC Nephrol. 2020;21:18. doi: 10.1186/s12882-019-1675-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Serino G., Sallustio F., Cox S.N., Pesce F., Schena F.P. Abnormal miR-148b expression promotes aberrant glycosylation of IgA1 in IgA nephropathy. J Am Soc Nephrol. 2012;23:814–824. doi: 10.1681/ASN.2011060567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gale D.P., Molyneux K., Wimbury D., et al. Galactosylation of IgA1 is associated with common variation in C1GALT1. JASN: ASN. 2017;28:2158–2166. doi: 10.1681/ASN.2016091043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tortajada A., Gutierrez E., Pickering M.C., Praga Terente M., Medjeral-Thomas N. The role of complement in IgA nephropathy. Mol Immunol. 2019;114:123–132. doi: 10.1016/j.molimm.2019.07.017. [DOI] [PubMed] [Google Scholar]
- 35.Selvaskandan H., Cheung C.K., Barratt J. Immunological drivers of IgA nephropathy: exploring the mucosa-kidney link. J Immunogenet. 2022;49:8–21. doi: 10.1111/iji.12561. [DOI] [PubMed] [Google Scholar]
- 36.Kano T., Suzuki H., Makita Y., Fukao Y., Suzuki Y. Nasal-associated lymphoid tissue is the major induction site for nephritogenic IgA in murine IgA nephropathy. Kidney Int. 2021;100:364–376. doi: 10.1016/j.kint.2021.04.026. [DOI] [PubMed] [Google Scholar]
- 37.Blaas S.H., Stieber-Gunckel M., Falk W., Obermeier F., Rogler G. CpG-oligodeoxynucleotides stimulate immunoglobulin A secretion in intestinal mucosal B cells. Clin Exp Immunol. 2009;155:534–540. doi: 10.1111/j.1365-2249.2008.03855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuznik A., Bencina M., Svajger U., Jeras M., Rozman B., Jerala R. Mechanism of endosomal TLR inhibition by antimalarial drugs and imidazoquinolines. J Immunol. 2011;186:4794–4804. doi: 10.4049/jimmunol.1000702. [DOI] [PubMed] [Google Scholar]
- 39.Tan J., Ni D., Taitz J., et al. Dietary protein increases T-cell-independent sIgA production through changes in gut microbiota-derived extracellular vesicles. Nat Commun. 2022;13:4336. doi: 10.1038/s41467-022-31761-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Litinskiy M.B., Nardelli B., Hilbert D.M., et al. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat Immunol. 2002;3:822–829. doi: 10.1038/ni829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.MacLennan I., Vinuesa C. Dendritic cells, BAFF, and APRIL: innate players in adaptive antibody responses. Immunity. 2002;17:235–238. doi: 10.1016/s1074-7613(02)00398-9. [DOI] [PubMed] [Google Scholar]
- 42.Stavnezer J., Guikema J.E.J., Schrader C.E. Mechanism and regulation of class switch recombination. Annu Rev Immunol. 2008;26:261–292. doi: 10.1146/annurev.immunol.26.021607.090248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smerud H.K., Bárány P., Lindström K., et al. New treatment for IgA nephropathy: enteric budesonide targeted to the ileocecal region ameliorates proteinuria. Nephrol Dial Transplant. 2011;26:3237–3242. doi: 10.1093/ndt/gfr052. [DOI] [PubMed] [Google Scholar]
- 44.Molyneux K., Scionti K., Wolski W., et al. FC050: Nefecon® selectively modifies the composition of circulating immune complexes in IGA nephropathy. Nephrol Dial Transplant. 2022;37(suppl 3) doi: 10.1093/ndt/gfac107.002. Supplement_3. [DOI] [Google Scholar]
- 45.Molyneux K., Wimbury D., Barratt J. P0344NEFECON® (budesonide) selectively reduces circulating levels of BAFF (BLYS) and soluble BCMA and TACI in IGA nephropathy. Nephrol Dial Transplant. 2020;35(suppl 3) doi: 10.1093/ndt/gfaa142.P0344. Supplement_3. [DOI] [Google Scholar]
- 46.Fellström B.C., Barratt J., Cook H., et al. Targeted-release budesonide versus placebo in patients with IgA nephropathy (NEFIGAN): a double-blind, randomised, placebo-controlled phase 2b trial. Lancet. 2017;389:2117–2127. doi: 10.1016/S0140-6736(17)30550-0. [DOI] [PubMed] [Google Scholar]
- 47.BARRATT J., Stone A., Kristensen J. POS-830 NEFECON FOR THE TREATMENT OF IgA NEPHROPATHY IN PATIENTS AT RISK OF PROGRESSING TO END-STAGE RENAL DISEASE: the NEFIGARD PHASE 3 TRIAL RESULTS. Kidney Int Rep. 2021;6:S361. doi: 10.1016/j.ekir.2021.03.868. [DOI] [Google Scholar]
- 48.Lafayette R., Kristensen J., Stone A., et al. Efficacy and safety of a targeted-release formulation of budesonide in patients with primary IgA nephropathy (NefIgArd): 2-year results from a randomised phase 3 trial. Lancet. 2023;402:859–870. doi: 10.1016/S0140-6736(23)01554-4. [DOI] [PubMed] [Google Scholar]
- 49.Kedzierski R.M., Yanagisawa M. Endothelin system: the double-edged sword in health and disease. Annu Rev Pharmacol Toxicol. 2001;41:851–876. doi: 10.1146/annurev.pharmtox.41.1.851. [DOI] [PubMed] [Google Scholar]
- 50.Chinook Therapeutics. Human renal mesangial cell activation induced by endothelin-1 or IgA nephropathy patient-derived immune complexes is blocked by selective ETA antagonist atrasentan. https://www.chinooktx.com/file.cfm/52/docs/Atrasentan_Human_Renal_Mesangial_Cell_Activation_WCN_FINAL.pdf
- 51.Nakamura T., Ebihara I., Fukui M., Tomino Y., Koide H. Effect of a specific endothelin receptor A antagonist on glomerulonephritis of ddY mice with IgA nephropathy. Nephron. 1996;72:454–460. doi: 10.1159/000188912. [DOI] [PubMed] [Google Scholar]
- 52.Maixnerová D., Merta M., Reiterová J., et al. The influence of three endothelin-1 polymorphisms on the progression of IgA nephropathy. Folia Biol. 2007;53:27–32. https://www.ncbi.nlm.nih.gov/pubmed/17328840 [PubMed] [Google Scholar]
- 53.LEHRKE I., WALDHERR R., RITZ E., WAGNER J. Renal endothelin-1 and endothelin receptor type B expression in glomerular diseases with proteinuria. J Am Soc Nephrol. 2001;12:2321–2329. doi: 10.1681/ASN.V12112321. [DOI] [PubMed] [Google Scholar]
- 54.Heerspink H., Radhakrishnan J., Alpers C., et al. Sparsentan in patients with IgA nephropathy: a prespecified interim analysis from a randomised, double-blind, active-controlled clinical trial. Lancet. 2023;401:1584–1594. doi: 10.1016/S0140-6736(23)00569-X. [DOI] [PubMed] [Google Scholar]
- 55.Perkovic V., Santos J., Fraenkel M., et al. Atrasentan and renal events in patients with type 2 diabetes and chronic kidney disease (SONAR): a double-blind, randomised, placebo-controlled trial. Lancet. 2019;393:1937–1947. doi: 10.1016/S0140-6736(19)30772-X. [DOI] [PubMed] [Google Scholar]
- 56.Brad R., Barratt J., Heerspink H., et al. Efficacy and safety of Sparsentan Versus Irbesartan in Patients With IgA Nephropathy (PROTECT): 2-Year results from a randomised, active-controlled, phase 3 trial. Lancet. 2023;402:2077–2090. doi: 10.1016/S0140-6736(23)02302-4. [DOI] [PubMed] [Google Scholar]
- 57.Kim S., Vo N., Lee S., et al. FC052: atrasentan for the treatment of IGA nephropathy: interim results from the affinity study. Nephrol Dial Transplant. 2022;37(suppl 3) doi: 10.1093/ndt/gfac107.004. Supplement_3. [DOI] [Google Scholar]
- 58.Novartis. Novartis investigational atrasentan Phase III study demonstrates clinically meaningful and highly statistically significant proteinuria reduction in patients with IgA nephropathy (IgAN). Novartis. 2023. https://www.novartis.com/news/media-releases/novartis-investigational-atrasentan-phase-iii-study-demonstrates-clinically-meaningful-and-highly-statistically-significant-proteinuria-reduction-patients-iga-nephropathy-igan Accessed 12 November.
- 59.Lafayette R.A., Canetta P.A., Rovin B.H., et al. A randomized, controlled trial of rituximab in IgA nephropathy with proteinuria and renal dysfunction. J Am Soc Nephrol. 2017;28:1306–1313. doi: 10.1681/ASN.2016060640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mei H.E., Frölich D., Giesecke C., et al. Steady-state generation of mucosal IgA+ plasmablasts is not abrogated by B-cell depletion therapy with rituximab. Blood. 2010;116:5181–5190. doi: 10.1182/blood-2010-01-266536. [DOI] [PubMed] [Google Scholar]
- 61.Xin G., Shi W., Xu L., Su Y., Yan L., Li K. Serum BAFF is elevated in patients with IgA nephropathy and associated with clinical and histopathological features. J Nephrol. 2013;26:683–690. doi: 10.5301/jn.5000218. [DOI] [PubMed] [Google Scholar]
- 62.Sallustio F., Curci C., Chaoul N., et al. High levels of gut-homing immunoglobulin A+ B lymphocytes support the pathogenic role of intestinal mucosal hyperresponsiveness in immunoglobulin A nephropathy patients. Nephrol Dial Transplant. 2021;36:452–464. doi: 10.1093/ndt/gfaa264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhai Y., Zhu L., Shi S., Liu L., Lv J., Zhang H. Increased APRIL expression induces IgA1 aberrant glycosylation in IgA nephropathy. Med (Baltim) 2016;95:e3099. doi: 10.1097/MD.0000000000003099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kiryluk K., Li Y., Scolari F., et al. Discovery of new risk loci for IgA nephropathy implicates genes involved in immunity against intestinal pathogens. Nat Genet. 2014;46:1187–1196. doi: 10.1038/ng.3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McCarthy D.D., Chiu S., Gao Y., Summers-deLuca L.E., Gommerman J.L. BAFF induces a hyper-IgA syndrome in the intestinal lamina propria concomitant with IgA deposition in the kidney independent of LIGHT. Cell Immunol. 2006;241:85–94. doi: 10.1016/j.cellimm.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 66.McCarthy D.D., Kujawa J., Wilson C., et al. Mice overexpressing BAFF develop a commensal flora-dependent, IgA-associated nephropathy. J Clin Invest. 2011;121:3991–4002. doi: 10.1172/JCI45563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chan D.T.M., Kanjanabuch T., Liew A., et al. WCN23-0684 interim biomarker analysis from a randomized, double-blind, placebo-controlled, PHASE 2 trial of sibeprenlimab (VIS649) in participants with immunoglobulin A nephropathy. Kidney Int Rep. 2023;8:S76–S77. doi: 10.1016/j.ekir.2023.02.169. [DOI] [Google Scholar]
- 68.Mathur M., Barratt J., Chacko B., et al. A Phase 2 trial of sibeprenlimab in patients with IgA nephropathy. N Engl J Med. 2024;390:20–31. doi: 10.1056/NEJMoa2305635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barratt J., Hour B., Sibley C., et al. Interim results of PHASE 1 AND 2 trials to investigate the safety, tolerability, pharmacokinetics, pharmacodynamics, and clinical activity of BION-1301 in patients with IGA nephropathy. Nephrol Dial Transplant. 2021;36(Supplement_1):FC040. [Google Scholar]
- 70.Lafayette R., Maes B., Lin C., et al. 36-week Efficacy & Safety of atacicept 150 mg in the ORIGIN Randomized, Double-blind, Placebo-controlled Phase 2b study in IgAN and Persistent Proteinuria. 60th ERA Congress. 2023 [Google Scholar]
- 71.Lv J., Liu L., Hao C., et al. Randomized Phase 2 trial of telitacicept in patients with IgA nephropathy with persistent proteinuria. Kidney Int Rep. 2023;8:499–506. doi: 10.1016/j.ekir.2022.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cheung C.K., Dormer J.P., Barratt J. The role of complement in glomerulonephritis—are novel therapies ready for prime time? Nephrol Dial Transplant. 2022;38:1789–1797. doi: 10.1093/ndt/gfac296. [DOI] [PubMed] [Google Scholar]
- 73.Barratt J., Rovin B., Zhang H., et al. POS-546 EFFICACY AND SAFETY OF IPTACOPAN IN IgA NEPHROPATHY: RESULTS OF A RANDOMIZED DOUBLE-BLIND PLACEBO-CONTROLLED PHASE 2 STUDY AT 6 months. Kidney Int Rep. 2022;7:S236. doi: 10.1016/j.ekir.2022.01.577. [DOI] [PubMed] [Google Scholar]
- 74.Zhang H., Rizk D.V., Perkovic V., et al. Results of a randomized double-blind placebo-controlled Phase 2 study propose iptacopan as an alternative complement pathway inhibitor for IgA nephropathy. Kidney Int. 2024;105:189–199. doi: 10.1016/j.kint.2023.09.027. [DOI] [PubMed] [Google Scholar]
- 75.Rizk D.V., Rovin B.H., Zhang H., et al. Targeting the alternative complement pathway with iptacopan to treat IgA nephropathy: design and rationale of the APPLAUSE-IgAN study. Kidney Int Rep. 2023;8:968–979. doi: 10.1016/j.ekir.2023.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Barbour S., Hladunewich M., Irvine J., et al. IONIS-FB-LRx, for Treatment of IgA Nephropathy; 2022. An Exploratory Trial of an Investigational RNA Therapeutic. [Google Scholar]
- 77.Lafayette R.A., Rovin B.H., Reich H.N., Tumlin J.A., Floege J., Barratt J. Safety, tolerability and efficacy of narsoplimab, a novel MASP-2 inhibitor for the treatment of IgA nephropathy. Kidney Int Rep. 2020;5:2032–2041. doi: 10.1016/j.ekir.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Omeros Omeros corporation provides update on interim analysis of Artemis-IGAN PHASE 3 trial of narsoplimab in IGA nephropathy. Omeros Website. https://investor.omeros.com/news-releases/news-release-details/omeros-corporation-provides-update-interim-analysis-artemis-igan
- 79.Barratt J., Yeo S.C., Fernstrom A., et al. Exploratory Results From the Phase 2 Study of Cemdisiran in Patients With IgA Nephropathy. ASN Kidney Week. November 4, 2022 https://www.asn-online.org/education/kidneyweek/2022/program-abstract.aspx?controlId=3797891 Accessed December 20, 2023. [Google Scholar]
- 80.Barratt J., Rocha Castilla J.L., Garlo K., Rice K., Lafayette R. Efficacy and safety of ravulizumab in a Phase 2 randomized controlled trial in IgA nephropathy. ASN Kidney Week. November 2, 2023 https://www.asn-online.org/education/kidneyweek/2023/program-abstract.aspx?controlId=3979029 Accessed December 19, 2023. [Google Scholar]
- 81.Bruchfeld A., Nachman P., Parikh S., et al. TO012C5A receptor inhibitor avacopan in IGA nephropathy study. Nephrol Dial Transplant. 2017;32(suppl 3) doi: 10.1093/ndt/gfx129.TO012. iii82:iii82-iii82. [DOI] [Google Scholar]
- 82.Gleeson P.J., O’Shaughnessy M.M., Barratt J. IgA nephropathy in adults - Treatment Standard. Nephrol Dial Transplant. 2023;38:2464–2473. doi: 10.1093/ndt/gfad146. [DOI] [PMC free article] [PubMed] [Google Scholar]