Abstract
The combination of hyaluronic acid and iodine (Hyodine) has sparked interest in wound care and could have valuable applications in treating burn injuries. We aimed to provide valuable insight into the potential advantages, limitations, and implications of using Hyodine in burn wound management. We studied 25 male rats to assess the clinical outcomes and wound‐healing effects of Hyodine. Each rat received a deep second‐degree burn wound on their back using metal stamps. Subsequently, the rats were then randomly split into two groups. The first group was treated with a layer of Hyodine gel, while the second group received Vaseline. The burn sites were photographed on days 1, 7, 14, and 21 using a digital camera. After excision of the burn wounds, histopathology slides were stained and evaluated in terms of the degree of epithelialization, angiogenesis, inflammatory cells' infiltration, and collagen amount and arrangement. Despite a non‐significant difference regarding the extent of burn wound area between intervention and control groups in the first day of experiment, the rats that were treated with Vaseline showed a significant decrease compared to those who received Hyodine in the second and third weeks (p = 0.02). On the other hand, epithelialization, pathology score, and collagen synthesis were significantly different between days 7, 14, and 21 of each group. However, collagen arrangement and neovascularization were only significantly different between days 7, 14, and 21 in Hyodine group (p = 0.02 and p = 0.03, respectively). The Hyodine gel may offer beneficial outcomes in patients with a burn wound. Based on our findings, despite a non‐significant difference in the extent of burn wound area, using Hyodine revealed a significant improvement in different histopathological variables including neovascularization, and collagen arrangement.
Keywords: burn wound, wound healing, hyaluronic acid, histopathological outcomes, Hyodine, Vaseline
1. INTRODUCTION
Burn injuries, especially deep second‐degree burns, remain a significant medical challenge due to their potential complications, extended recovery periods, and the risk of enduring physical and psychological effects. 1 The pursuit of an ideal therapeutic agent for these injuries is ongoing, with various treatment modalities having been explored over the years.
Hyaluronic acid (HA), a naturally occurring glycosaminoglycan found in the extracellular matrix of various tissues has been identified in numerous locations in the human body, such as the eye, skin, and soft tissue. 2 Its role in cell differentiation, embryological development, inflammation, wound healing, and other biological processes has been well established. 3 , 4 Furthermore, HA's contribution to each stage of normal wound healing has been examined, emphasising its importance in the wound‐healing process. 5 On the other hand, iodine, a long‐standing antiseptic, is renowned for its broad‐spectrum antimicrobial activity, effective against a wide range of pathogens, including antibiotic‐resistant strains. 6 , 7 The innovative combination of HA and iodine, particularly in gel form, offers a promising approach in burn wound management by integrating the wound‐healing properties of HA with the antimicrobial benefits of iodine. 2
Several studies have already delved into the potential benefits of HA and iodine in wound healing. A study highlighted the effectiveness of HA in skin abrasions, showing accelerated re‐epithelialization compared to other treatments. 8 Another study on Hyodine, a mixture of high molecular weight HA with the iodine complex KI, 3 demonstrated its ability to stimulate wound contraction and epidermal proliferation, while also maintaining wound moisture. 6 Additionally, the application of esterified HA has shown promise in the treatment of non‐progressive wounds, including venous leg ulcers and diabetic foot ulcers. 1 Furthermore, a unique system for wound treatment based on a combination of high molecular weight sodium hyaluronate with an iodine complex has shown potential in the treatment of non‐healing diabetic foot wounds. 9 Such findings underscore the potential of combining HA and iodine in wound care, suggesting a synergistic effect that could be potentially beneficial for burn injuries. 10
However, while these studies provide valuable insights, the combined efficacy of HA and iodine, especially in the context of deep second‐degree burns, remains underexplored. This gap in knowledge underscores the significance of our research. Using a rat model, we aim to compare the therapeutic potential of a commercial brand of HA and iodine complex, named Hyodine gel, with that of Vaseline in treating deep second‐degree burn wounds. Through this investigation, we hope to offer a fresh perspective and valuable insights into the potential advantages, limitations, and implications of using Hyodine gel in burn wound management, thereby contributing to the broader understanding of burn care.
2. METHODS
We recruited 25 adult male Sprague–Dawley rats based on the standard of compliance with animal rights and the instructions of our Medical Ethics Committee (IR.IUMS.AEC.1401.012) on the use and care of laboratory animals. This study was conducted in Fatima Plastic and Reconstructive Hospital, Tehran, Iran in 2023.
After inducing general anesthesia with Ketamin 10% (Alfasan Inc., Woerden, The Netherlands) (70 mg/kg) and Xylazin 2% (Alfasan Inc., Woerden, The Netherlands) (9 mg/kg), the hairs on the back of the rats were shaved with an electric lathe. A deep second‐degree burn wound with the dimensions of 2 × 2 cm2 was created on the back of the rats using metal stamps. Immediately after creating the burn wound, 10 cc of normal saline was injected intraperitoneally to all of the rats in order to compensate for fluids. A total of 50 mg/kg of dipyrone, diluted in normal saline was injected to rats for pain control and it was used twice a day for 24–48 h. Rats were kept in separate shelves. Subsequently, the rats were randomly divided into two groups. In the first group, 12 rats were treated with a layer of Hyodine gel and in the second group, 13 rats were treated with Vaseline. Hyodine is a commercial brand of HA and iodine in a form of the gel. Both groups had daily, non‐occlusive dressings until the 21st day. On days 1, 7, 14, and 21, the burn sites were photographed using a digital camera, Nikon D300 (Nikon Inc., Tokyo, Japan) with Macro Lens with focal length of 60 mm, 1:10 magnification, from the distance of 80 cm with a ruler. The extent of the wound in each photo was calculated using the ImageJ software (ver. 1.45, NIH, Maryland, USA).
On each of days 7, 14, 21, the burn wounds of 4 rats were excised after anesthesia with a surgical blade in order to undergo morphological and pathological measurements and evaluations. The rats with excised burn wounds were euthanazed in a standard way using a high dose of Thiopental. Histopathology slides were stained using H&E staining, in terms of the degree of epithelialization, angiogenesis, infiltration of acute and chronic inflammatory cells, and the collagen amount and arrangement. Pathology score was calculated as the average score of epithelialization, acute and chronic inflammatory cells, neovascularization, collagen synthesis and arrangement, and fibroblasts.
2.1. Statistical analysis
The Kolmogorov–Smirnov test was used to ensure that normality of the quantitative variables. Mann–Whitney U test and One‐way ANOVA were used for evaluating the differences between variables. Mauchly's Test of Sphericity and ANOVA repeated measure were used for differences of mean area and pathology scores within and between subjects of both groups. p‐value less than 0.05 was assumed as a significance level.
3. RESULTS
On the first day, 25 male rats were randomly assigned to control and Hyodine group, 13 rats in control group, and 12 rats in Hyodine group. On the fifth day, one of the rats from the Hyodine group was deceased.
On Day 1, the mean ± SD area of the burn wound in the control group was 4.50 ± 0.26, and in the Hyodine group it was 4.51 ± 0.31 which was not significantly different. On Days 14 and 21, the differences between the mean area of the two groups were statistically significant with the Vaseline group having less mean area of burn wound (p‐values of 0.02) (Table 1). On Day 21, 8 rats (5 rats in the control group and 3 rats in the Hyodine group) were remaining, which the progress of their area of burn wounds individually are presented in Figure 1.
TABLE 1.
Evaluation of mean area of burn wound in the Hyodine and control groups.
| Control group | Hyodine group | p‐value | |||||
|---|---|---|---|---|---|---|---|
| Mean | Std. Deviation | Std. Error Mean | Mean | Std. Deviation | Std. Error Mean | ||
| Day 1, Vaseline group n = 13, Hyodine group n = 12 | 4.50 | 0.26 | 0.07 | 4.51 | 0.31 | 0.09 | 0.85 |
| Day 7, Vaseline group n = 13, Hyodine group n = 11 | 4.58 | 0.99 | 0.28 | 4.88 | 1.83 | 0.55 | 0.69 |
| Day 14, Vaseline group n = 9, Hyodine group n = 7 | 2.07 | 1.31 | 0.43 | 3.19 | 1.01 | 0.38 | 0.02 |
| Day 21, Vaseline group n = 5, Hyodine group n = 3 | 0.22 | 0.23 | 0.10 | 1.57 | 0.99 | 0.57 | 0.03 |
Note: Mann–Whitney U test was used in this table.
FIGURE 1.
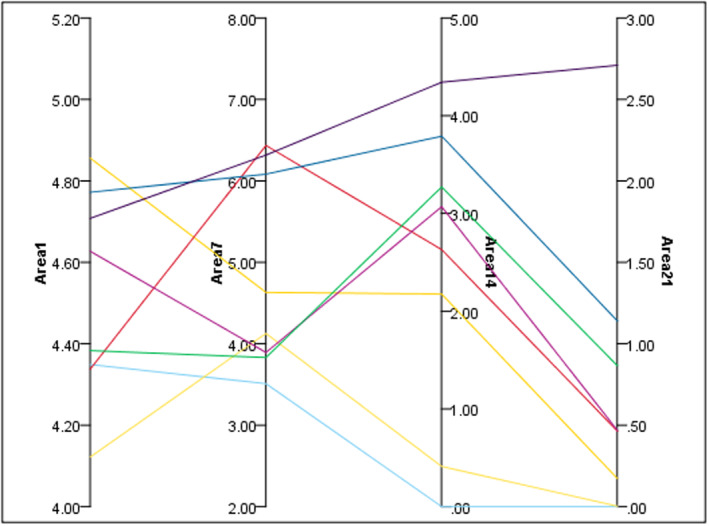
Parallel visualisation of the area of the eight rats that made it to Day 21.
ANOVA repeated measure tests of the mean of areas of burn wound, showed no significant difference between the two groups or within the subjects in each group (Table 2, Figure 2).
TABLE 2.
ANOVA repeated measure of area.
| ANOVA repeated measure | Type III sum of squares | Df | Mean square | F | Sig. | Partial Eta squared | |
|---|---|---|---|---|---|---|---|
| Within subjects | Sphericity assumed | 3.883 | 3 | 1.294 | 2.521 | 0.090 | 0.296 |
| Between subjects | Sphericity assumed | 9.698 | 1 | 9.698 | 5.557 | 0.056 | 0.481 |
FIGURE 2.
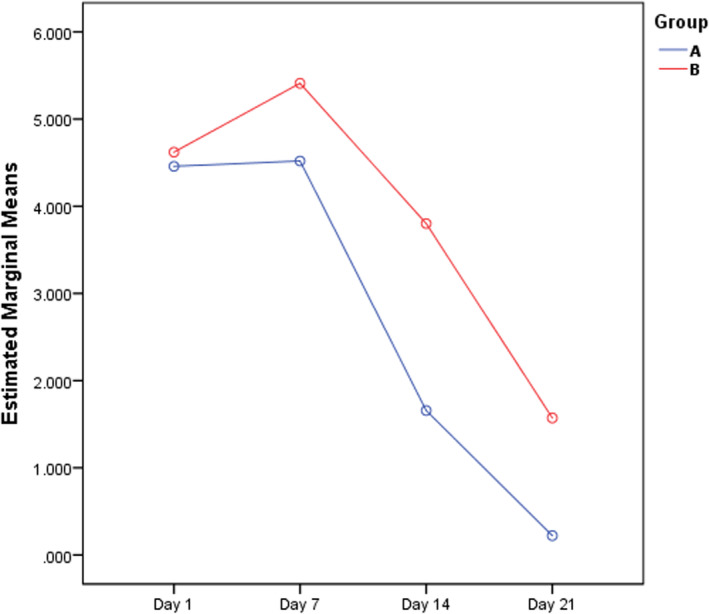
Estimated marginal means of the area of burn wound by repeated measure during Days 7, 14, and 21 (Group A: Hyodine group; Group B: Vaseline group).
Epithelialization, acute and chronic inflammatory cells, neovascularization, collagen synthesis and arrangement, fibroblasts, and pathology score were assessed in the excised burn wounds on Days 7, 14, 21. None of them showed statistically significant differences (Table 3).
TABLE 3.
Evaluation of pathology variables in 1st, 2nd, and 3rd weeks.
| Day 7 | Day 14 | Day 21 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Vaseline (n = 4) | Hyodine (n = 3) | p‐value | Vaseline (n = 4) | Hyodine (n = 4) | p‐value | Vaseline (n = 5) | Hyodine (n = 3) | p‐value | |
| Epithelialization | 0.00 ± 0.00 | 0.00 ± 0.00 | 1.00 | 2.75 ± 1.70 | 1.75 ± 0.95 | 0.48 | 5.00 ± 0.00 | 2.66 ± 2.08 | 0.14 |
| Acute inflammatory cells | 0.75 ± 0.50 | 1.00 ± 0.00 | 0.62 | 1.25 ± 1.89 | 1.25 ± 0.50 | 0.48 | 3.60 ± 0.54 | 2.00 ± 1.73 | 0.25 |
| Chronic inflammatory cells | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 | 1.60 ± 0.54 | 1.33 ± 0.57 | 0.57 |
| Neovascularization | 3.50 ± 1.00 | 4.00 ± 0.00 | 0.62 | 2.25 ± 0.50 | 2.25 ± 0.50 | 1.00 | 2.40 ± 0.54 | 1.66 ± 0.57 | 0.25 |
| Collagen synthesis | 0.25 ± 0.50 | 0.00 ± 0.00 | 0.62 | 1.50 ± 0.57 | 1.50 ± 0.57 | 1.00 | 2.60 ± 0.54 | 2.33 ± 0.57 | 0.57 |
| Collagen arrangement | 0.50 ± 1.00 | 0.00 ± 0.00 | 0.62 | 2.00 ± 0.00 | 2.00 ± 0.00 | 1.00 | 1.40 ± 0.54 | 1.66 ± 0.57 | 0.57 |
| Fibroblasts | 3.00 ± 0.00 | 3.00 ± 0.00 | 1.00 | 1.75 ± 0.95 | 1.75 ± 0.50 | 0.88 | 2.00 ± 0.00 | 1.33 ± 0.57 | 0.14 |
| Pathology score | 1.28 ± 0.11 | 1.28 ± 0.00 | 1.00 | 1.78 ± 0.66 | 1.64 ± 0.18 | 1.00 | 2.65 ± 0.32 | 1.85 ± 0.74 | 0.25 |
Note: All variables were evaluated by Mann–Whitney U test.
ANOVA repeated measure tests of the pathology score, showed no significant difference between the two groups or within the subjects of each group (Table 4, Figure 3). The results of Kruskar Wallis test within the rats of each group showed that epithelialization, pathology score and collagen synthesis were significantly different between Days 7, 14, and 21. However, collagen arrangement and neovascularization were significantly different between Days 7, 14, and 21 only in the Hyodine group (p‐value of 0.020 and 0.025, respectively) (Table 5, Figures 4 and 5).
TABLE 4.
ANOVA repeated measure of pathology score.
| ANOVA repeated measure | Type III sum of squares | Df | Mean square | F | Sig. | |
|---|---|---|---|---|---|---|
| Within subjects | Sphericity assumed | 0.27 | 2 | 0.13 | 0.54 | 0.60 |
| Between subjects | Sphericity assumed | 0.38 | 1 | 0.38 | 2.85 | 0.16 |
FIGURE 3.
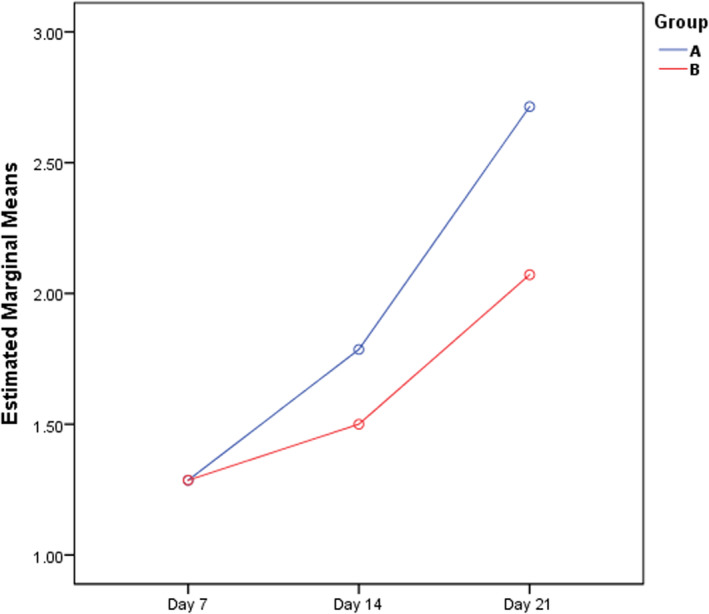
Estimated marginal means of the pathology score of burn wounds by repeated measure during Days 7, 14, and 21 (Group A: Hyodine group; Group B: Vaseline group).
TABLE 5.
Evaluation of important pathology variables in 1st, 2nd, and 3rd weeks between subjects of each group.
| Vaseline group | Hyodine group | |||||||
|---|---|---|---|---|---|---|---|---|
| Day 7 | Day 14 | Day 21 | p‐value | Day 7 | Day 14 | Day 21 | p‐value | |
| Epithelialization | 0.00 ± 0.00 | 2.75 ± 1.70 | 5.00 ± 0.00 | 0.005 | 0.00 ± 0.00 | 1.75 ± 0.95 | 2.66 ± 2.08 | 0.045 |
| Pathology score | 1.28 ± 0.11 | 1.78 ± 0.66 | 2.65 ± 0.32 | 0.024 | 1.28 ± 0.00 | 1.64 ± 0.18 | 1.85 ± 0.74 | 0.048 |
| Collagen synthesis | 0.25 ± 0.50 | 1.50 ± 0.57 | 2.60 ± 0.54 | 0.008 | 0.00 ± 0.00 | 1.50 ± 0.57 | 2.33 ± 0.57 | 0.024 |
| Collagen arrangement | 0.50 ± 1.00 | 2.00 ± 0.00 | 1.40 ± 0.54 | 0.056 | 0.00 ± 0.00 | 2.00 ± 0.00 | 1.66 ± 0.57 | 0.020 |
| Neovascularization | 3.50 ± 1.00 | 2.25 ± 0.50 | 2.40 ± 0.54 | 0.119 | 4.00 ± 0.00 | 2.25 ± 0.50 | 1.66 ± 0.57 | 0.025 |
Note: One Way ANOVA was used for evaluation of all variables in each group.
FIGURE 4.

Representative macroscopic images of the wound in both groups in Days 1, 7, 14, and 21.
FIGURE 5.

Representative microscopic images of the wound in both groups in Days 1, 7, 14, and 21.
4. DISCUSSION
Burn wounds, particularly deep second‐degree burns, present a significant challenge in medical treatment due to their complexity and the potential for complications. The primary objective of this study was to compare the efficacy of Hyodine gel, a commercial brand of Hyaluronic acid and iodine complex, with Vaseline in the treatment of deep second‐degree burn wounds in rats. The results of this study provide valuable insights into the potential benefits and limitations of using Hyodine gel for burn wound treatment.
Our preliminary findings suggest that while there was no significant difference in the mean area of burn wounds between the control (Vaseline) and Hyodine groups on Day 1, by Days 14 and 21, the control group exhibited a significantly smaller mean area of burn wounds. This suggests that Vaseline may have a more pronounced effect in reducing the size of burn wounds over time compared to Hyodine gel. However, the pathology scores, which evaluated various parameters such as epithelialization, inflammation, neovascularization, and collagen synthesis, did not show any significant differences between the two groups. This indicates that while Vaseline may be more effective in reducing wound size, both treatments might have comparable effects on the quality of wound healing at the cellular and tissue levels. Additionally, our findings indicated progressive improvements in epithelialization and collagen synthesis over time for both groups. While both exhibited changes in collagen arrangement and neovascularization, the differences were statistically significant only in the Hyodine group, suggesting its potential in promoting a more organised and vascularized wound healing process.
Our study's findings on the efficacy of Hyodine gel in accelerating burn wound healing align with previous research on the benefits of HA in post‐surgical infections, as discussed in the study on onychocryptosis by Fernández et al. 11 The combination of HA and iodine, as highlighted in a study by Mikonsinski et al., 12 offers a synergistic effect that promotes faster wound healing while preventing infections. This dual action is crucial in burn wound management, where the risk of infection is high. The use of hydrogel dressings, as mentioned in a review on hydrogels by Surowiecka et al., 13 presents an innovative approach to burn care. These dressings not only provide a moist environment conducive to healing but also offer cooling effects, reducing pain and discomfort. Our study's findings on the soothing effects of Hyodine gel resonate with this, suggesting that the gel's formulation might share similarities with hydrogel dressings. Furthermore, the role of hyaluronic acid in wound healing cannot be understated; as research elucidates, hyaluronic acid plays a pivotal role in various stages of wound healing, from inflammation to tissue remodelling. 11 While our study observed accelerated wound healing in the control group, the significant changes in collagen arrangement and neovascularization in the Hyodine group provide insights into the specific effects of HA. Proper collagen arrangement ensures the structural integrity of the newly formed tissue, and neovascularization ensures adequate blood supply to the healing tissue. The pronounced effects of Hyodine gel on these critical components of wound healing underscore its potential benefits in promoting a more organised and vascularized wound healing process, likely attributed to the properties of HA. Lastly, the inflammatory response in wound healing, as seen in our study, can be related to the findings of the study by Said et al. which highlights the role of resident dendritic cells in inflammation, which is a crucial stage in wound healing. 14 , 15 Understanding this inflammatory response could certainly offer insights into improving wound care treatments.
The combination of HA and iodine in a gel form for use in animal studies has raised some concerns. A study by De Bouelle et al. on the efficacy and safety of topical iodine products in wound care highlighted potential cytotoxic effects of iodine on fibroblasts and keratinocytes, which can delay wound healing, especially at high concentrations. 15 On the other hand, the study by Zheng et al. on the biocompatibility and efficacy of a linearly cross‐linked sodium hyaluronic acid hydrogel found it to be well tolerated with no adverse effects on retinal function and morphology, suggesting its potential as a promising retinal patch for sealing retinal breaks. 16 Iodine, particularly in the form of povidone‐iodine, has been a staple in wound care for its antimicrobial properties for many years. Its efficacy against a broad spectrum of pathogens, including antibiotic‐resistant strains, is well documented. 17 As a long‐standing antiseptic, iodine offers broad‐spectrum antimicrobial activity, making it effective against a wide range of pathogens, including antibiotic‐resistant strains. 18 While traditionally used for infectious wounds, its role in non‐infectious wounds is debated. Some clinicians argue that iodine may delay healing due to its potential cytotoxic effects on fibroblasts and keratinocytes, essential for wound repair. 19 , 20 However, a systematic review found that iodine did not necessarily prolong wound healing. 20 Another study indicated that povidone iodine's impact on wound healing can vary, 19 and certain iodine concentrations might even promote wound healing by influencing macrophage behaviour. 21 The combination of iodine with hyaluronic acid in Hyodine introduces a dynamic that might not be fully understood yet, especially considering the potential interactions between the antimicrobial properties of iodine and the wound‐healing properties of hyaluronic acid.
5. STRENGTHS AND LIMITATIONS
In terms of strengths, our study employed a rigorous and comprehensive methodology, ensuring that the data collected was both accurate and representative of the population under study. This robust approach enhances the reliability of our findings. By comparing our results with previous studies, we were able to identify patterns and trends that are consistent across different research settings. This not only validates our findings but also provides a broader context for understanding burn wound healing. The utilisation of the latest technologies in wound assessment and healing measurement allowed for more precise and detailed data collection, leading to more nuanced insights. Furthermore, the use of control groups in our study allowed for a clearer understanding of the effects of the interventions being tested, ruling out potential confounding factors.
However, there are certain limitations to consider. First, numerous external factors, such as nutrition, stress, and other health conditions, can affect wound healing, and our study may not have accounted for all these variables, potentially influencing the results. Second, while animal models, such as mice, offer specific advantages in research, they may not completely mimic the wound‐healing process of humans. This can lead to differences in how certain treatments or interventions may work in humans compared to animals. On top of that, our focus on immediate and short‐term outcomes means that long‐term effects and outcomes, especially in chronic wounds, might provide different insights. Third, as with any study, there is a potential for biases in participant selection, data interpretation, or even in the reporting of results. While we took measures to minimise these biases, they cannot be entirely ruled out. Last, the limited number of rats used in our study poses a limitation in terms of sample size and lack of sample diversity, potentially affecting the robustness and generalizability of our findings.
6. CONCLUSION
In conclusion, our study offers valuable insights into the effects of the gel‐formulated combination of hyaluronic acid and iodine versus Vaseline on deep second‐degree burn wounds in rats, laying a foundation for future research. While our findings contribute to the understanding of burn wound healing, it is essential to recognise the study's limitations and the broader context. The Hyodine gel could offer promising results in burn wound healing. However, the dynamic nature of scientific research necessitates continuous exploration and validation. As we present our conclusions, we also emphasise the importance of ongoing inquiry in the realm of burn wound healing to further deepen our understanding and improve patient outcomes.
FUNDING INFORMATION
This work was supported by Nikan Teb Kimia, Tehran, Iran.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Farokh Forghani S, Bagheri T, Naderi Gharahgheshlagh S, et al. The effect of hyaluronic acid and iodine complex gel compared to Vaseline on deep second‐degree burn wound in rats. Int Wound J. 2024;21(2):e14738. doi: 10.1111/iwj.14738
Contributor Information
Sara Ghorbanian Kelachayeh, Email: s.ghorbanian@nitkaco.com.
Rana Irilouzadian, Email: ranairilouzadian@gmail.com.
DATA AVAILABILITY STATEMENT
The data that are not presented in the manuscript are available upon request from the co‐corresponding author.
REFERENCES
- 1. Schneider HP, Landsman A. Preclinical and clinical studies of hyaluronic acid in wound care: a case series and literature review. Wounds. 2019;31(2):41‐48. [PubMed] [Google Scholar]
- 2. Cutting KF. Wound healing through synergy of hyaluronan and an iodine complex. J Wound Care. 2011;20:424‐428. [DOI] [PubMed] [Google Scholar]
- 3. Juncan AM, Moisă DG, Santini A, et al. Advantages of hyaluronic acid and its combination with other bioactive ingredients in cosmeceuticals. Molecules. 2021;26(15):4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Papakonstantinou E, Roth M, Karakiulakis G. Hyaluronic acid: a key molecule in skin aging. Dermatoendocrinology. 2012;4(3):253‐258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Frenkel JS. The role of hyaluronan in wound healing. Int Wound J. 2014;11(2):159‐163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Slavkovsky R, Kohlerova R, Jiroutova A, et al. Effects of hyaluronan and iodine on wound contraction and granulation tissue formation in rat skin wounds. Clin Exp Dermatol. 2010;35(4):373‐379. [DOI] [PubMed] [Google Scholar]
- 7. Gershenfeld L, Witlin B. Iodine as an antiseptic. Ann N Y Acad Sci. 1950;53(1):172‐182. [DOI] [PubMed] [Google Scholar]
- 8. Leite MN, Frade MAC. Efficacy of 0.2% hyaluronic acid in the healing of skin abrasions in rats. Heliyon. 2021;7(7):e07572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sobotka L, Smahelova A, Pastorova J, Kusalova M. A case report of the treatment of diabetic foot ulcers using a sodium hyaluronate and iodine complex. Int J Low Extrem Wounds. 2007;6(3):143‐147. [DOI] [PubMed] [Google Scholar]
- 10. Chen RF, Wang CT, Chen YH, et al. Hyaluronic acid‐povidone‐iodine compound facilitates diabetic wound healing in a streptozotocin‐induced diabetes rodent model. Plast Reconstr Surg. 2019;143(5):1371‐1382. [DOI] [PubMed] [Google Scholar]
- 11. Fernández AN, Gómez‐Carrión A, Zaragoza‐García I, et al. Management of post‐surgical infection of onychocryptosis with topical application of hyaluronic acid versus antibacterial ointments. Heliyon. 2022;8(8):e10099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mikosinski J, Di Landro A, Łuczak‐Szymerska K, et al. Efficacy and safety of a hyaluronic acid‐containing gauze pad in the treatment of chronic venous or mixed‐origin leg ulcers: a prospective, multicenter, randomized controlled trial. Wounds. 2021;33(6):147‐157. [PubMed] [Google Scholar]
- 13. Surowiecka A, Strużyna J, Winiarska A, Korzeniowski T. Hydrogels in burn wound management‐a review. Gels. 2022;8(2):122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Said A, Weindl G. Regulation of dendritic cell function in inflammation. J Immunol Res. 2015;2015:743169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. De Boulle K, Heydenrych I. Patient factors influencing dermal filler complications: prevention, assessment, and treatment. Clin Cosmet Investig Dermatol. 2015;8:205‐214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zheng C, Xi H, Wen D, et al. Biocompatibility and efficacy of a linearly cross‐linked sodium hyaluronic acid hydrogel as a retinal patch in Rhegmatogenous retinal detachment repairment. Front Bioeng Biotechnol. 2022;10:914675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Smith R, Russo J, Fiegel J, Brogden N. Antibiotic delivery strategies to treat skin infections when innate antimicrobial defense fails. Antibiotics. 2020;9(2):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Drosou A, Falabella A, Kirsner R. Antiseptics on wounds: an area of controversy. Wounds. 2003;15:149‐166. [Google Scholar]
- 19. Bigliardi PL, Alsagoff SAL, El‐Kafrawi HY, Pyon J‐K, Wa CTC, Villa MA. Povidone iodine in wound healing: a review of current concepts and practices. Int J Surg. 2017;44:260‐268. [DOI] [PubMed] [Google Scholar]
- 20. Vermeulen H, Westerbos SJ, Ubbink DT. Benefit and harm of iodine in wound care: a systematic review. J Hosp Infect. 2010;76(3):191‐199. [DOI] [PubMed] [Google Scholar]
- 21. Cooper RA. Iodine revisited. Int Wound J. 2007;4(2):124‐137. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that are not presented in the manuscript are available upon request from the co‐corresponding author.


