Abstract
Chronic wounds have emerged as a significant healthcare burden, affecting millions of patients worldwide and presenting a substantial challenge to healthcare systems. The diagnosis and management of chronic wounds are notably intricate, with inappropriate management contributing significantly to the amputation of limbs. In this work, we propose a compact, wireless, battery-free, and multimodal wound monitoring system to facilitate timely and effective wound treatment. The design of this monitoring system draws on the principles of higher-order parity-time symmetry, which incorporates spatially balanced gain, neutral, and loss, embodied by an active −RLC reader, an LC intermediator, and a passive RLC sensor, respectively. Our experimental results demonstrate that this wireless wound sensor can detect temperature (T), relative humidity (RH), pressure (P), and pH with exceptional sensitivity and robustness, which are critical biomarkers for assessing wound healing status. Our in vitro experiments further validate the reliable sensing performance of the wound sensor on human skin and fish. This multifunctional monitoring system may provide a promising solution for the development of futuristic wearable sensors and integrated biomedical microsystems.
Keywords: biomedical sensors, wearable sensors, wireless sensors, wound healing, parity-time symmetry
Graphical Abstract

I. Introduction
Wearable sensors which monitor the physical and chemical conditions of the human body have gained increasing importance in the past decade [1]-[5]. To date, various wearable sensors, such as glucose sensors [1], blood pressure sensors [2], and electrocardiogram sensors [3], [4] have been proposed to assist clinical decision making, treatment, and health management. Despite these recent advances, relatively less attention has been paid to chronic wound monitoring. Chronic wounds that fail to heal in an orderly manner within expected timeframes affect more than 6.5 million people in the United States alone and cost the economy over $25 billion annually [6], [7]. This burden is still surging due to the rising prevalence of obesity, diabetes, and aging population [6], [7]. In current clinical practice, wound assessment relies primarily on the visual inspection of the wound size and depth, as well as signs of inflammation [8]. The reliability of visual assessment depends largely on the experience of clinicians and is inevitably influenced by a degree of subjectivity. Especially for chronic wounds whose early symptoms are subtle and difficult to distinguish from those of normally healing wounds, the traditional assessment method may fail to provide sufficient information of the wound, leading to improper or delayed treatment. Recently, researchers have disclosed the underlying relationship between wound status and various pivotal biomarkers (e.g., temperature, moisture, pH, strain, and oxygen levels) [9]-[14]. Monitoring these physiological parameters may provide a more quantitative evaluation of the wound healing process and allow for more rapid and accurate diagnosis of chronic wounds.
In this context, a plethora of wearable biosensors have been developed to effectively monitor temperature [9], [10], humidity [11], [12], and other crucial parameters in the vicinity of the wound site [7], [13], [14]. The objective, sensor-based assessment is definitely an immense improvement compared with conventional methods. Yet, most reported wound sensors still suffer from one or more critical limitations, such as high manufacturing cost, insufficient sensitivity, unavoidable battery usage, and redundant wire connections linking sensors to external reading equipment (which restrict the activities of patients) [15]-[18]. Moreover, some of the sensing systems can only detect a single parameter of wound healing [14], [19]-[21], which is obviously inadequate since wound healing is a complex and multi-stage process accompanied by dynamic changes in multiple physiological parameters. To address the existing limitations, we present a battery-free, multiplex sensing platform for monitoring the wound healing process. The platform leverages the concept of generalized higher-order parity-time (PT) symmetry [22]-[24]. As depicted in Fig. 1(a), the wound site parameters, including temperature (T), relative humidity (RH), pressure (P), and pH level, can be captured by detecting the resonant dip shifts in the reflection spectrum. These measured indicators are important for assessing the wound status. Typically, normal skin or a healing wound has a temperature of 30.2–36.9 °C and a slightly acidic pH of 5.0–6.5, while an infected wound experiences a suddenly increased temperature and a slightly basic pH (above 7.4) due to the presence of certain types of enzymes and bacteria [19], [20], [25], [26]. Moisture is a parameter that must be carefully managed in the wound environment, since dry wounds usually show a slower epithelialization, while moist wounds may have an increased risk of infection [27]. Pressure is predominantly linked to cell proliferation and neovascularization [11].
Fig. 1.
(a) Schematic of the wireless wound monitoring system, where the parameters to be detected (e.g., temperature, relative humidity, pressure, and pH) are obtained from the shifts of narrow resonant dips. (b) Equivalent circuit diagram of the third-order PTX-symmetric sensing system, which consists of an active −RLC reader, an LC intermediator and passive RLC sensors.
In this paper, we present a systematic theoretical study and experimental demonstration of the generalized higher-order PT-symmetric wound sensing system, which can monitor multiple wound healing indicators with excellent sensitivity. Although PT-symmetric telemetry has been applied to several wireless sensing applications, experimental demonstrations to date have been detecting single resistive or reactive change on the sensor [28]-[30]. To the best of our knowledge, this is the first time that multiple physiological parameters are sensed at the time using the PT-symmetric telemetry. In this work, we experimentally verified that a PT-symmetric wound sensor can be used to simultaneously monitor temperature (20–50 °C), relative humidity (30%–90%), pH (4–10), and pressure (0–200 mmHg). The sensing ranges are carefully designed to cover all possible environmental conditions surrounding a wound or surgical site. The proposed PT-symmetric multimodal sensing platform enables a myriad of healthcare applications, such as wound management, wearable IoT devices, biotelemetry, and telemedicine.
II. Theoretical Analysis
PT symmetry is an essential milestone in non-Hermitian physics, unveiling that non-conservative Hamiltonians that satisfy parity (P) and time-reversal (T) operators can possess completely real eigenspectra [28], [31], [32]. This revolutionary discovery has made an enormous difference in the fields of optics [33], [34], electronics [35]-[37], electromagnetics [38]-[41], and acoustics [42]. Standard PT-symmetric electronic systems have shown significant advancements in sensing applications, exhibiting superior sensitivity compared to traditional RLC sensors [21], [23], [28], [37]. The enhanced sensitivity of the standard second-order PT-symmetric system can be attributed to eigenfrequency bifurcation near the exceptional point (EP), which allows significant resonance frequency shift in response to small impedance perturbations. Despite their effectiveness in improving the sensing performance, the second-order PT-symmetric system can only detect one type of resistive or reactive change. The third (or higher)-order PT-symmetric telemetry with more than three eigenfrequencies offers a greater degree of freedom in data interpretation. Furthermore, PT-reciprocal scaling (PTX) symmetry has been introduced as a generalized form of PT symmetry [23], [39], [43]. In principle, PT-symmetric systems necessitate a precise balance of gain and loss, whereas such a weak constraint can be removed in the PTX-symmetric systems, even though PT and PTX systems share the same eigenspectrum but different eigenmodes [23]. Hence, PTX-symmetric systems may provide more design degrees of freedom.
Figure 1(b) shows the schematic of the generalized higher-order PT-symmetric wound sensing system, which comprises an active −RLC reader (gain), an intermediator (neutral), and two passive RLC sensors (loss). The two RLC tank sensors, which share the same reader, respectively make the system fulfill the PT- and PTX-symmetric conditions. As shown in Fig. 1(b), the PTX-symmetric system is similar to its PT-symmetric counterpart, but with all elements in the tank sensor scaled in a specific manner (i.e., , , where is the reciprocal scaling factor). Here, the PT sensor includes a thermistor and a capacitive humidity sensor, and the PTX sensor consists of a resistive pressure sensor and a pH sensor. This telemetry system can simultaneously monitor the temperature, humidity, pressure, and pH at the wound site. The PT- and PTX-symmetric systems can be described by Kirchhoff’s laws, which provide us with the effective non-Hermitian Hamiltonian of the system [23], [28], [36]. For the PT/PTX-symmetric system shown in Fig. 1(b), there exist eigenfrequencies which can be by letting . Under the PT/PTX-symmetric condition (i.e., , ), the three eigenfrequencies can be written as:
| (1a) |
| (1b) |
| (1c) |
where denotes the gain-loss parameter or the effective -factor of the resonant tank (), is the coupling coefficient between the neighboring resonators (, where is the mutual inductance), and is the resonant angular frequency of the neutral tank (). We note that the PT- and PTX-symmetric systems share the same eigenfrequencies. The real parts of eigenfrequencies are plotted in Fig. 2(a), where the theoretical and experimental results are presented by colored contours and dots, respectively. In Fig. 2(a), an EP can be clearly observed at:
| (2) |
where an eigenfrequency bifurcation occurs along the EP loci. When , the system is in the exact PT-symmetric phase whereby the eigenfrequencies are purely real and correspond to the resonant (angular) frequencies.
Fig. 2.
(a) Real parts of eigenfrequencies of the PT/PTX-symmetric system in Fig. 1(b), where the calculated and measured results are represented by clolor countours and scattered points, respectively. (b) Contours of reflection coefficients as a function of the gain-loss parameter and the normalized angular frequency ; here .
Figure 2(b) shows the contours of reflection as a function of frequency and gain-loss parameter for the third-order PT/PTX-symmetric system, where the reflection minima occur at the eigenfrequencies (or resonant frequencies). Unlike the standard second-order PT- and PTX-symmetric systems that display dissimilar resonance line shapes [23], [43], the third-order PT- and PTX-symmetric systems possess exactly identical reflection spectra. Noticeably, the PTX system may offer wider design flexibility as it allows for adjusting/scaling the lumped element values on the sensor side. As evident from Figs. 2(a) and 2(b), when the system operates at the exact PT-symmetric phase, the two resonant frequencies, and , are sensitive to the gain-loss parameter and coupling coefficient . Especially when is around , even a tiny resistive or reactive perturbation that alters can lead to noticeable shifts of , and . On the other hand, the eigenfrequency is fixed to .
When monitoring wound parameters, the system experiences changes in both resistance and capacitance, and should be able to distinguish between changes in each. These variations could slightly break the PT/PTX symmetry of the system. Considering a small resistive perturbation applied on the sensor, i.e., and (), the eigenfrequencies of the third-order PT/PTX system can be approximately expressed as:
| (3a) |
| (3b) |
| (3c) |
We note that in case , which conveys that the system satisfies the PT/PTX symmetry, Eq. (3) is equivalent to Eq. (1). Equation (3) states that with slightly unbalanced resistance in the sensor and reader, and are influenced by the resistive perturbation while remains unchanged. Therefore, we conclude that whether the system is symmetric or slightly asymmetric in resistance, is only related to and values, and is independent of and .
Since the coil inductance and coupling coefficient are fixed, the equivalent capacitance C can be determined from the offset of . After the equivalent capacitance of the sensor is obtained, the effective resistance of the sensor can be obtained by tracking or , which varies with . One can first adjust the varactors on both the reader and intermediator to attain a distinct resonance at . Subsequently, by fine-tuning the effective resistance of the reader, three resonant dips can be observed in the reflection spectrum (i.e., the exact PT symmetry). This step enables retrieving the sensor’s resistance R. As a consequence, the third-order PT-symmetric sensing system allows for simultaneously detecting resistive and capacitive changes on the sensor, which may not be possible with standard second-order PT-symmetric system [23], [28], [37] or traditional passive wireless sensing methods [21], [44], [45]. In the pursuit of optimal wound management, we utilize the third-order PT/PTX-symmetric system with two tank sensors, as shown in Fig. 1(b), for simultaneous monitoring of multiple biomarkers crucial to the healing process. This approach allows us to precisely track two resistive parameters, namely temperature and pressure, along with two capacitive parameters, humidity and pH. It is worth highlighting that through judicious adaptations of the sensors and transducers, the PT/PTX telemetry platform may also facilitate concurrent detection of other wound site parameters and biological indicators, including but not limited to oxygen, thermal conductivity, and uric acid.
III. Experimental Demonstration
Figure 3(a) shows the photograph of the PT/PTX sensing platform (left) and the flexible, wearable wound sensor embedded in a smart bandage (right). When monitoring wound status, the smart bandage can be easily affixed onto the wound site, while the associated reader is directly connected to a vector network analyzer (VNA) to acquire the reflection spectrum. As shown in Fig. 3(a), the PT/PTX-symmetric wound sensor consists of (1) a negative temperature coefficient (NTC) thermistor from Murata Electronics (NCP15XC680E03RC), (2) a capacitive humidity sensor from Innovative Sensor Technology (P14-W), (3) a force sensing resistor from Ohmite (FSR06BE), and (4) a custom-made pH sensor, fabricated by polyaniline modification of laser-induced graphene electrodes. The integrated smart bandage is based on a single flexible polyimide substrate with a thickness of only 0.16 mm, rendering it eminently suited for deployment in wearable technology. The wound parameters to be monitored can be derived from the resonance dips in the measured reflection spectrum, provided that the coil inductance and coupling strength are fixed at appropriate values. As shown in Fig. 3(b), a reflection minimum was first found at by tuning the capacitance of the intermediator and reader, the resonance frequency is used as a sign of balance in capacitance (i.e., ); here, the three resonant frequencies are denoted by , . Three reflection dips were then acquired by adjusting the resistance of the reader until the PT symmetry is achieved (i.e., ) [Fig. 3(c)]. By this simple, two-step tracking of resonance frequencies of the third-order PT/PTX-symmetric system, and values can be obtained with high accuracy. It is evidently seen from Fig. 3(c) that resonance at is dominated by the capacitance of the system and is unaffected if the resistance values of the reader and the sensor are slightly different. What observed from Figs. 3(b) and 3(c) is consistent with the theoretical result in Eq. (3), indicating that the proposed sensing mechanism can be effective for multiplexed sensing of the wound site. During the experiment, the coupling coefficient , associated with the distance between the neighboring coils, was kept at a fixed value of 0.45. The value of is chosen to make the system operate close to the EP.
Fig. 3.
(a) Photograph of experimental setup for the wound monitoring system (left) and the compact, flexible, and wearable wound sensor (right). Measured reflection spectra for two scenarios: (b) when both and are unknown, and one first tries to determine , and (c) after is known, one tries to find the exact value of .
A. Temperature and Humidity Monitoring
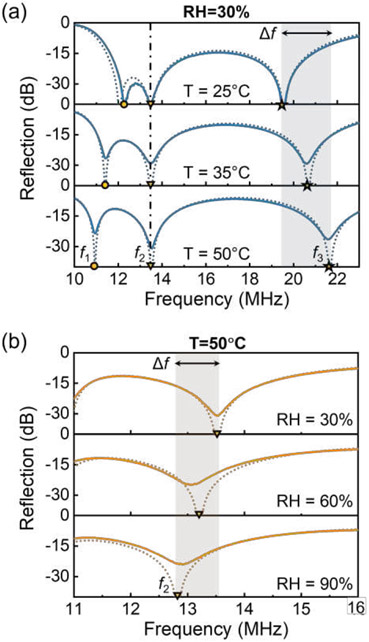
During the measurement of temperature and humidity, the coil inductance is fixed at 1120 nH, and the equivalent resistance and capacitance of the sensor are influenced by temperature and relative humidity, respectively. The NTC thermistor, with a parallel connected resistor of 182 Ω, exhibits an equivalent resistance of 48.7 Ω at room temperature (25 °C). The capacitance of the capacitive humidity sensor is measured to be 126.6 pF at 30% RH, such that at 25 °C and 30% RH, and the system operates around the EP. Figure 4(a) shows the reflection spectra of the wound sensing system under different temperature conditions; here, RH = 30%. The experimental results (solid lines) show great consistency with theoretical ones (dashed lines). It is observed from Fig. 4(a) that and respond sensitively to temperature variations, while remains constant. This agrees with the previous theoretical investigation that is only affected by the sensor’s effective capacitance related to relative humidity. We note that while both and are responsive to variations in temperature, the latter one shows a more remarkable shift due to the eigenfrequency bifurcation effect discussed in the previous section [Fig. 2]. Therefore, the detection of wound temperature is undertaken by tracing the shifts of the third resonant frequency or the frequency ratio . Figure 4(b) plots the resonance at with different relative humidity, which further demonstrates the feasibility of monitoring relative humidity levels through the detection of shifts in the resonant frequency .
Fig. 4.
Reflection spectra of the PT/PTX-symmetric wound monitoring system with (a) 30% RH and different temperature, and (b) 50 °C and different RH. Here, the solid and dashed lines respectively represent the measured and calculated results.
The influence of relative humidity on the capacitance of the humidity sensor is presented in Fig. 5(a), where a notable increase from 126.6 pF to 138.1 pF is observed upon an increase in relative humidity from 30% to 90%. The increase in capacitance results in a downshifting . The frequency shift as a function of relative humidity is shown in Fig. 5(b); here . As relative humidity ascends from 30% to 90%, the increase in capacitance leads to a drop of by approximately 600 kHz, indicating a sensitivity of 9.3 kHz/%RH. Figure 5(c) depicts the variation in the equivalent resistance of the NTC thermistor, which is proportional to temperature changes. We find that the equivalent resistance decreases from 48.7 Ω to 26.6 Ω, as temperature increases from 25 °C to 50 °C. The reduction in resistance increases the value of , and thus the frequency ratio increases with temperature, as presented in Fig. 5(d). When the relative humidity is 30% and 90%, the sensitivity of monitoring temperature is 81.1 kHz/°C and 84.7 kHz/°C, respectively. Sensitivity increases slightly with the moisture level, as higher RH corresponds to a higher capacitance and thus a lower gain-loss parameter (closer to ). The sensing ranges (i.e., 25–50 °C and 30%–90% RH) may cover all possible variations in temperature and humidity around the wound site. It is evident from Figs. 5(b) and 5(d) that the relationship between and RH remains constant irrespective of temperature. Furthermore, once RH is ascertained, the relationship between and temperature becomes apparent. [Eq. (1c)]. Therefore, the third-order PT/PTX-symmetric sensor possesses the capability to simultaneously acquire wound temperature and humidity. Moreover, the sensitivity levels for both temperature and humidity sensing are highly commendable. In particular, the sensitivity of resistive temperature sensing experiences a considerable enhancement due to the eigenfrequency bifurcation occurring around the EP, and can be further improved when the system is operated near the divergent EP (DEP) [22], [24]. In addition, there are several deficiencies in experimental measurements, including asymmetric coupling, fabrication errors, and parasitic effects of the circuit boards, which slightly destroy the PT-symmetric condition and could affect the accuracy of the measurement results. The performance of the PT-symmetric wound sensor may be further improved through several strategies. For instance, a feedback control system could be utilized to precisely pitch the positions of the reader and intermediator, ensuring consistency of the coupling strength between the reader and intermediator and that between the intermediator and sensor. A properly designed dielectric spacer made of low-permittivity materials can also provide better coil alignment. In addition, the errors due to parasitic effects and manufacturing flaws can be significantly suppressed by using the advanced complementary metal-oxide semiconductor (CMOS) integrated circuit and packaging technology (e.g., on-chip RF reader).
Fig. 5.
(a) Measured capacitance of the capacitive humidity sensor versus relative humidity. (b) Frequency shift as a function of relative humidity. (c) Measured equivalent resistance of the NTC thermistor versus temperature. (d) Frequency ratio as a function of temperature. The error bars are generated from three measurements.
B. Pressure and pH Monitoring
To obtain pressure and pH around the wound, the reader and intermediator used are the same as those used in temperature and humidity monitoring, namely that their coil inductance is still 1120 nH. The passive RLC tank sensor follows the reciprocal scaling rule where the scaling factor x = 3.8. Here, the variations in resistance and capacitance respectively correspond to the changes in pressure and pH. The force sensing resistor (in parallel with a 187-Ω resistor to fulfill PTX-symmetric condition) has an equivalent resistance of ~187 Ω at 0 mmHg. The pH sensor is connected in parallel with a voltage-controlled varactor diode (MAVR-000405-0287FT, MACOM Technology Solutions). Thus, changes in the potential difference between pH electrodes (i.e., a reference electrode, RE, and a sensing electrode, SE) can be translated to capacitance variations. At pH = 10, the equivalent capacitance of the sensor is measured to be 31.8 pF. The gain-loss parameter is thus 1.96 at 0 mmHg and 10 pH. Figures 6(a) and 6(b) plot the reflection coefficients with different pressure and pH, respectively, where the solid (dashed) lines denote the measured (calculated) results. As with our prior analysis, the pressure that corresponds to the resistance value can be monitored by tracking the alteration in the third resonant frequency or the frequency ratio , and the pH level that corresponds to the capacitance value can be obtained directly from the second resonant frequency .
Fig. 6.
Reflection spectra of the PT/PTX-symmetric wound monitoring system with (a) pH=10 and different pressures, and (b) P = 200 mmHg and different pH values. Here, the solid and dashed lines respectively represent the measured and calculated results.
Figure 7(a) presents the measured electrode potential and capacitance at different pH levels. The electrode potential, which serves as the bias voltage for the varactor diode, decreases with increasing pH. As pH increases from 4 to 10, an increase in the capacitance from 22.5 pF to 31.8 pF can be observed, giving rise to a left shift of . Figure 7(b) illustrates the frequency drift with different pH levels, showing a ~2500 kHz frequency change when pH varies from 4 to 10. It is seen that the pH can be read from regardless of the pressure conditions. Sensitivity of monitoring pH remains at 434.0 kHz per unit change in pH. After the pH level is acquired, a direct correspondence can be found between the pressure and the frequency ratio . Figure 7(c) shows the equivalent resistance of the force-sensing resistor as a function of pressure. We see that its equivalent resistance is ~187 Ω at 0 mmHg, and as pressure increases to 200 mmHg, the resistance drops to ~120 Ω. With pressure increases, the resistance drop results in an increase in the gain-loss parameter , and also an increase in , as described in Fig. 7(d). Sensitivity of pressure monitoring, calculated as the shift of , is 6.4 kHz/mmHg when pH = 4 and is 7.8 kHz//mmHg when pH = 10. Again, the sensitivity slightly increases with pH, as higher pH pushes the system closer to the EP. By virtue of the proposed PT/PTX-symmetric sensing platform, the pressure and pH are simultaneously detected with excellent linearity and sensitivity. The sensing performance of the PT/PTX-symmetric wound sensor could be further improved by using a set of and closer to the EP or DEP and reducing the parasitic effects and losses in the circuit components. The wound parameters monitored in this work (i.e., temperature, humidity, pressure, and pH) play an important role in assessing the wound healing process. Besides these, the PT/PTX sensing system may also be applied to detect other clinically significant biomarkers like strain, uric acid, and oxygen concentration. We envision that the proposed PT/PTX-symmetric sensing system would serve as a steppingstone toward chronic wound management, wearable biotelemetry, as well as various non-invasive techniques.
Fig. 7.
(a) Potential difference between the pH electrodes (left axis) and equivalent capacitance of the sensor (right axis) versus pH level. (b) Frequency shift as a function of pH level. Inset: schematic of the tank sensor when performing pressure and pH measurement. (c) Equivalent resistance of the force sensing resistor versus pressure. (d) Frequency ratio as a function of pressure. The error bars are generated from three measurements.
C. In Vitro Demonstration
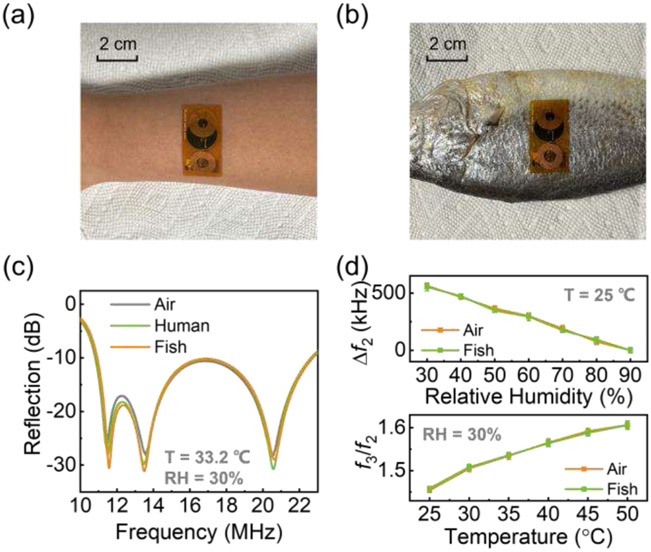
To further validate the wound healing sensing system, a series of in vitro experiments are conducted. Specifically, the performance of the wound sensor is compared when the sensor tag is placed in the air, affixed to human skin, and affixed to a fish. As clearly illustrated in Figs. 8(a) and 8(b), the ultrathin and flexible characteristics of the wound sensor afford it the ability to conform seamlessly to the curved surfaces of human skin and piscine organisms. Figure 8(c) displays the reflection spectrum of the system under identical temperature and relative humidity conditions, with the sensor tag positioned in three different settings, namely, in the air, on human skin, and on a fish. The temperature employed in this investigation is 33.2 °C, corresponding to the typical skin temperature. To ensure uniformity in temperature conditions, a hotplate is employed to sustain a temperature of 33.2 °C for the sensor tag located in the air and affixed to the fish, which closely approximates the temperature of human skin. Furthermore, the ambient RH is consistently maintained at a level of 30%. As seen in Fig. 8(c), despite the tag being placed on different substrates (air, human, fish), the measured reflection spectra are nearly the same as long as the same temperature and RH are ensured. The resonant frequencies, or resonant dips, which encode the information of sensing parameters, are barely affected by the presence of the human body or animal tissue. This is due to the fact that the communication between the reader and sensor tag is through inductive links. The coupling strength between coil inductors is unrelated to the permittivity, but only related to the permeability of the dielectric media. For most dielectric media, including biological tissues and liquids, the permeability has a constant value of 1. Consequently, the presence of human, fish, or other biological tissues has little impact on the inductive coupling strength and resonant frequency of the wound sensing system.
Fig. 8.
Photographs of the wound sensor (a) affixed to human skin and (b) affixed to a fish. The reader setup is exactly the same as that shown in Fig. 3(a). (c) Measured reflection spectrum of the wound sensing system with T = 33.2 °C and RH = 30%. (d) Frequency shift as a function of relative humidity with T = 25 °C (top). Frequency ratio as a function of temperature with RH = 30% (bottom). (c) and (d) indicate that the resonant frequency and sensing efficacy of the wound sensor exhibit remarkable consistency when deployed in air, adhered to human skin, and adhered to a fish.
Fig. 8(d) compares the performance of the wound sensor when used to monitor RH (top; temperature is fixed at 25 °C) and temperature (bottom; RH is fixed at 30%). It can be seen that the frequency shift as a function of RH and the ratio as a function of temperature are almost unaffected whether the tag is placed in the air or affixed to a fish. Our in vitro experiments demonstrate that the proposed wound monitoring system performs with good consistency and reliability, even in the presence of biological tissues. These findings underscore the sensor’s potential as an appealing solution for wound monitoring and diverse wearable medical and health monitoring applications.
IV. Conclusion
We have proposed the multifunctional PT/PTX-symmetric sensing platform with great potential for applications in realtime wireless wound monitoring and management. We have theoretically studied and practically implemented the proposed sensing platform, which enables simultaneous monitoring of multiple physiological parameters associated with capacitive and resistive perturbations on the sensor. Specifically, we have experimentally demonstrated the simultaneous in situ detection of body temperature, relative humidity, pressure, and pH, which are all relevant indicators of wound status. In addition, we have performed in vitro tests to verify and validate that the effectiveness, uniformity and reliability of the proposed wireless wound monitoring technique when the compact wearable sensor is in contact with the human skin and animal tissue. Such a fully passive and wireless multiplex sensing platform may be beneficial for developing the next-generation wearable biomedical devices and systems.
Acknowledgments
This material is based upon work supported by the National Science Foundation under Grant No. ECCS-CCSS 1914420 and National Institute of Health under Grant No. R01EB033371.
Biographies

Zhilu Ye received the B.S. degree in microelectronics from Huazhong University of Science and Technology, China, in 2018 and the master’s degree in electrical engineering from Arizona State University, USA, in 2019. She received her Ph.D. degree from University of Illinois at Chicago in 2023. Since then, she has been serving as an assistant professor in the School of Life Science and Technology at Xi’an Jiaotong University, China.
Her research area includes applied electromagnetics, antennas, radio-frequency and microwave circuits, and their applications in wireless biomedical sensors, wearable and implantable devices, telemedicine, and healthcare internet-of-things. She was granted the 2022 Dean’s Scholar Fellowship from University of Illinois at Chicago, the USNC-URSI 2022 Travel Fellowship from the National Academy of Sciences, the nomination for the IEEE Sensors 2022 Best Paper Award, and the second place for the IEEE AP-S/URSI 2022 Student Design Contest.

Minye Yang received his bachelor’s degree in optical and electronic information from Huazhong University of Science and Technology, Wuhan, China, in 2018 and his M. Sc. degree in electrical engineering from Wayne State University, Detroit, MI, USA, in 2019. He received his Ph.D. degree from the University of Illinois Chicago, Chicago, IL, USA, in 2023. He is now serving as the assistant professor in the School of Electronics Science and Technology at Xi’an Jiaotong University, China.
His research mainly focuses on applied electromagnetics, including non-Hermitian physics, ultrasensitive sensors, radio-frequency/microwave circuits, noninvasive biomedical sensing, soft/wearable electronics, and electromagnetically-enabled hardware securities. He was a recipient of the 2022 URSI-NRSM travel grant and the nomination for the best paper award of IEEE Sensors 2022. He was awarded the Young Talented Scientist by Xi’an Jiaotong University.

Mohamed Farhat received his Ph.D. in Optics and Electromagnetism from Aix-Marseille University in 2010 where he obtained as well his Master degree in Theoretical Physics in 2006. His PhD dissertation was titled by “Metamaterials for Harmonic and Biharmonic Cloaking and Superlensing.” He has authored over 100 publications, including 1 edited book, 94 journal papers, 7 book chapters, and 5 international patents, as well as over 90 conference papers, with over 4700 citations, as of January 2023. He has organized several special sessions at the Meta conferences, and is active reviewer for many international journals in Physics including Physical Review Letters and Nature Physics. He has co-edited the book “Transformation Wave Physics: Electromagnetics, Elastodynamics and Thermodynamics” at Pan Stanford Publishing. His research is in the fields of plasmonics and metamaterials with applications spanning optical and acoustical waves.

Mark M.-C. Cheng received the B.S. and Ph.D. degrees from National Tsing-Hua University, Hsinchu, Taiwan, in 1995 and 2003, respectively. He was a Research Assistant Professor with the Department of Nanomedicine and Biomedical Engineering, the University of Texas Health Science Center at Houston, Houston, TX, USA, from 2006 to 2007. In 2008, he joined Wayne State University (WSU) and became a Full Professor in 2019. At WSU, he initialized curriculum in Nanoengineering and Cyber-Physical Systems (CPS). In 2019, he joined the Department of electrical and Computer Engineering, the university of Alabama, Tuscaloosa, AL, USA, where he is currently a professor. He has authored approximately 120 articles in peer-reviewed journal and conference proceedings. He has been involved in multidisciplinary research in microsystem design, biomedical devices, biosensor, new materials, wearable sensors, and environmental Interne-of-Things (IoT).
Dr. Cheng was a recipient of the National Science (NSF) CAREER Award, the ONR Summer Faculty Fellowship, and the President Research Enhancement Award.

Pai-Yen Chen (Senior Member, IEEE) is an Associate Professor in the Department of Electrical and Computer Engineering at the University of Illinois, Chicago (UIC). He received the Ph.D. degree from the University of Texas at Austin in 2013. He received M.S. and B.S. degrees from National Chiao Tung University in Taiwan in 2006 and 2004. He previously served as an Assistant Professor at the Wayne State University (WSU) during 2014-2018, a Research Scientist at Intellectual Ventures' Metamaterial Commercialization Center during 2013-2014, and a Research Staff in the Taiwan Semiconductor Research Institute (TSRI) during 2006-2009. He has been involved in multidisciplinary research on applied electromagnetics, RF and microwave antennas and circuits, metamaterials, metasurfaces, wireless sensors and systems, nanoelectromagnetism, plasmonics, nanophotonics, and nanoelectronics. He has received quite a few prestigious awards, including National Science Foundation (NSF) CAREER Award, IEEE Sensors Council Young Professional Award, IEEE Raj Mittra Travel Grant (RMTG) Award, SPIE Rising Researcher Award, ACES Early Career Award, PIERS Young Scientist Award, Young Scientist Awards from URSI General Assembly and URSI Commission B: Electromagnetics, IOP Measurement Science and Technology Emerging Leader, Air Force Research Laboratory Faculty Fellowship, UIC College of Engineering Faculty Research Award, WSU College of Engineering Faculty Research Excellence Award, Donald Harrington Fellowship, Taiwan Ministry of Education Study Abroad Award, United Microelectronics Corporation Scholarship, and quite a few best paper awards and travel grants from major IEEE conferences, including IEEE Antennas and Propagation Symposium (2011, 2013, 2016 and 2021), IEEE International Microwave Symposium (2015), IEEE Sensors Conference (2016), IEEE Wireless Power Transfer Conference (2021), and USNC-URSI Ernest K. Smith Student Paper Award (2012). He currently serves as Associate Editor of IEEE Sensors Journal, IEEE Transactions on Antennas and Propagation, and IEEE Antennas and Wireless Propagation Letters, and Lead Guest Editor of IEEE Journal of Selected Areas in Sensors. He was a former Associate Editor of Applied Electromagnetics, IEEE Journal of Radio Frequency Identification, and IEEE Journal of Electromagnetics, RF and Microwaves in Medicine and Biology (IEEE-JERM), and former Guest Editor of IEEE Transactions on Antennas and Propagation.
Contributor Information
Zhilu Ye, Department of Electrical and Computer Engineering, University of Illinois at Chicago, Chicago, IL 60607, USA.
Minye Yang, Department of Electrical and Computer Engineering, University of Illinois at Chicago, Chicago, IL 60607, USA.
Mohamed Farhat, Division of Computer Electrical and Mathematical Sciences and Engineering, King Abdullah University of Science and Technology (KAUST), Thuwal 23955-6900, Saudi Arabia.
Mark M.-C. Cheng, Department of Electrical and Computer Engineering, University of Alabama, Tuscaloosa, AL, USA.
Pai-Yen Chen, Department of Electrical and Computer Engineering, University of Illinois at Chicago, Chicago, IL 60607, USA.
References
- [1].Oliver NS, Toumazou C, Cass AEG, and Johnston DG, “Glucose sensors: a review of current and emerging technology,” Diabetic Medicine, vol. 26, no. 3, Art. no. 3, 2009. doi: 10.1111/j.1464-5491.2008.02642.x. [DOI] [PubMed] [Google Scholar]
- [2].Fassbender H et al. , “Fully implantable blood pressure sensor for hypertonic patients,” in 2008 IEEE SENSORS, Oct. 2008, pp. 1226–1229. doi: 10.1109/ICSENS.2008.4716664. [DOI] [Google Scholar]
- [3].Yu B, Xu L, and Li Y, “Bluetooth Low Energy (BLE) based mobile electrocardiogram monitoring system,” in 2012 IEEE International Conference on Information and Automation, Jun. 2012, pp. 763–767. doi: 10.1109/ICInfA.2012.6246921. [DOI] [Google Scholar]
- [4].Zheng K, Chen S, Zhu L, Zhao J, and Guo X, “Large Area Solution Processed Poly (Dimethylsiloxane)-Based Thin Film Sensor Patch for Wearable Electrocardiogram Detection,” IEEE Electron Device Letters, vol. 39, no. 3, Art. no. 3, Mar. 2018, doi: 10.1109/LED.2018.2792022. [DOI] [Google Scholar]
- [5].Ye Z et al. , “A Breathable, Reusable, and Zero-Power Smart Face Mask for Wireless Cough and Mask-Wearing Monitoring,” ACS Nano, vol. 16, no. 4, Art. no. 4, Apr. 2022, doi: 10.1021/acsnano.1c11041. [DOI] [PubMed] [Google Scholar]
- [6].Olsson M et al. , “The humanistic and economic burden of chronic wounds: A systematic review,” Wound Repair and Regeneration, vol. 27, no. 1, pp. 114–125, 2019, doi: 10.1111/wrr.12683. [DOI] [PubMed] [Google Scholar]
- [7].Derakhshandeh H, Kashaf SS, Aghabaglou F, Ghanavati IO, and Tamayol A, “Smart Bandages: The Future of Wound Care,” Trends in Biotechnology, vol. 36, no. 12, Art. no. 12, Dec. 2018, doi: 10.1016/j.tibtech.2018.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Powers JG, Higham C, Broussard K, and Phillips TJ, “Wound healing and treating wounds: Chronic wound care and management,” Journal of the American Academy of Dermatology, vol. 74, no. 4, Art. no. 4, Apr. 2016, doi: 10.1016/j.jaad.2015.08.070. [DOI] [PubMed] [Google Scholar]
- [9].Lim R, Wai Choong DS, and Cheng M-Y, “Development of Integrated Pressure and Temperature Sensing Strips for Monitoring Venous Leg Ulcer Application,” in 2020 IEEE 70th Electronic Components and Technology Conference (ECTC), Jun. 2020, pp. 1586–1591. doi: 10.1109/ECTC32862.2020.00249. [DOI] [Google Scholar]
- [10].Tang N et al. , “Wearable Sensors and Systems for Wound Healing-Related pH and Temperature Detection,” Micromachines, vol. 12, no. 4, Art. no. 4, Apr. 2021, doi: 10.3390/mi12040430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lu S-H et al. , “Multimodal sensing and therapeutic systems for wound healing and management: A review,” Sensors and Actuators Reports, vol. 4, p. 100075, Nov. 2022, doi: 10.1016/j.snr.2022.100075. [DOI] [Google Scholar]
- [12].Gomez D, Morgan SP, Gill BRH, and Korposh S, “Polymeric fibre optic sensor based on a SiO2 nanoparticle film for humidity sensing on wounds,” in Sixth European Workshop on Optical Fibre Sensors, SPIE, May 2016, pp. 294–297. doi: 10.1117/12.2236845. [DOI] [Google Scholar]
- [13].Kassal P et al. , “Smart bandage with wireless connectivity for uric acid biosensing as an indicator of wound status,” Electrochemistry Communications, vol. 56, pp. 6–10, Jul. 2015, doi: 10.1016/j.elecom.2015.03.018. [DOI] [Google Scholar]
- [14].Rahimi R et al. , “A low-cost flexible pH sensor array for wound assessment,” Sensors and Actuators B: Chemical, vol. 229, pp. 609–617, Jun. 2016, doi: 10.1016/j.snb.2015.12.082. [DOI] [Google Scholar]
- [15].Ochoa M et al. , “Integrated sensing and delivery of oxygen for next-generation smart wound dressings,” Microsyst Nanoeng, vol. 6, no. 1, Art. no. 1, May 2020, doi: 10.1038/s41378-020-0141-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Farooqui MF and Shamim A, “Low Cost Inkjet Printed Smart Bandage for Wireless Monitoring of Chronic Wounds,” Sci Rep, vol. 6, no. 1, Art. no. 1, Jun. 2016, doi: 10.1038/srep28949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jose M et al. , “Stretchable printed device for the simultaneous sensing of temperature and strain validated in a mouse wound healing model,” Sci Rep, vol. 12, no. 1, Art. no. 1, Jun. 2022, doi: 10.1038/s41598-022-13834-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hattori Y et al. , “Multifunctional Skin-Like Electronics for Quantitative, Clinical Monitoring of Cutaneous Wound Healing,” Advanced Healthcare Materials, vol. 3, no. 10, Art. no. 10, 2014, doi: 10.1002/adhm.201400073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Tamayol A et al. , “Flexible pH-Sensing Hydrogel Fibers for Epidermal Applications,” Advanced Healthcare Materials, vol. 5, no. 6, Art. no. 6, 2016, doi: 10.1002/adhm.201500553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Guinovart T, Valdés-Ramírez G, Windmiller JR, Andrade FJ, and Wang J, “Bandage-Based Wearable Potentiometric Sensor for Monitoring Wound pH,” Electroanalysis, vol. 26, no. 6, Art. no. 6, 2014, doi: 10.1002/elan.201300558. [DOI] [Google Scholar]
- [21].Deng W-J, Wang L-F, Dong L, and Huang Q-A, “LC Wireless Sensitive Pressure Sensors With Microstructured PDMS Dielectric Layers for Wound Monitoring,” IEEE Sensors Journal, vol. 18, no. 12, Art. no. 12, Jun. 2018, doi: 10.1109/JSEN.2018.2831229. [DOI] [Google Scholar]
- [22].Sakhdari M, Ye Z, Farhat M, and Chen P-Y, “Generalized Theory of PT-Symmetric Radio-Frequency Systems With Divergent Exceptional Points,” IEEE Transactions on Antennas and Propagation, vol. 70, no. 10, pp. 9396–9405. Oct. 2022, doi: 10.1109/TAP.2022.3179528. [DOI] [Google Scholar]
- [23].Chen P-Y et al. , “Generalized parity-time symmetry condition for enhanced sensor telemetry,” Nat Electron, vol. 1, no. 5, Art. no. 5, May 2018, doi: 10.1038/s41928-018-0072-6. [DOI] [Google Scholar]
- [24].Sakhdari M, Hajizadegan M, Zhong Q, Christodoulides DN, El-Ganainy R, and Chen P-Y, “Experimental Observation of PT Symmetry Breaking near Divergent Exceptional Points,” Phys. Rev. Lett, vol. 123, no. 19, Art. no. 19, Nov. 2019, doi: 10.1103/PhysRevLett.123.193901; [DOI] [PubMed] [Google Scholar]; Sakhdari M, Hajizadegan M, and Chen PY, “Robust Extended-Range Wireless Power Transfer Using a Higher-Order PT-Symmetric Platform,” Physical Review Research, Vol. 2, 013152 (2020). [Google Scholar]
- [25].Lin Y-H, Chen Y-C, Cheng K-S, Yu P-J, Wang J-L, and Ko N-Y, “Higher Periwound Temperature Associated with Wound Healing of Pressure Ulcers Detected by Infrared Thermography,” Journal of Clinical Medicine, vol. 10, no. 13, Art. no. 13, Jan. 2021, doi: 10.3390/jcm10132883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Fierheller M and Sibbald RG, “A Clinical Investigation into the Relationship between Increased Periwound Skin Temperature and Local Wound Infection in Patients with Chronic Leg Ulcers,” Advances in Skin & Wound Care, vol. 23, no. 8, Art. no. 8, Aug. 2010, doi: 10.1097/01.ASW.0000383197.28192.98. [DOI] [PubMed] [Google Scholar]
- [27].Okan D, Woo K, Ayello EA, and Sibbald G, “The Role of Moisture Balance in Wound Healing,” Advances in Skin & Wound Care, vol. 20, no. 1, Art. no. 1, Jan. 2007. [DOI] [PubMed] [Google Scholar]
- [28].Sakhdari M, Hajizadegan M, Li Y, Cheng MM-C, Hung JCH, and Chen P-Y, “Ultrasensitive, Parity-Time-Symmetric Wireless Reactive and Resistive Sensors,” IEEE Sensors Journal, vol. 18, no. 23, Art. no. 23, Dec. 2018, doi: 10.1109/JSEN.2018.2870322. [DOI] [Google Scholar]
- [29].Yang M, Ye Z, Zhu L, Farhat M, and Chen P-Y, “Recent advances in coherent perfect absorber-lasers and their future applications,” J. Cent. South Univ, vol. 29, no. 10, pp. 3203–3216, Oct. 2022, doi: 10.1007/s11771-022-5160-0. [DOI] [Google Scholar]
- [30].Yang M, Ye Z, Farhat M, and Chen P-Y, “Enhanced Radio-Frequency Sensors Based on a Self-Dual Emitter-Absorber,” Phys. Rev. Applied, vol. 15, no. 1, Art. no. 1. Jan. 2021, doi: 10.1103/PhysRevApplied.15.014026. [DOI] [Google Scholar]
- [31].Yang M, Ye Z, and Chen P-Y, “A Quantum-Inspired Biotelemetry System for Robust and Ultrasensitive Wireless Intracranial Pressure Monitoring,” in 2021 IEEE Sensors, Sydney, Australia: IEEE, Oct. 2021, pp. 1–4. doi: 10.1109/SENSORS47087.2021.9639684. [DOI] [Google Scholar]
- [32].Ye Z, Yang M, and Chen P-Y, “Multi-Band Parity-Time-Symmetric Wireless Power Transfer Systems for ISM-Band Bio-Implantable Applications,” IEEE J. Electromagn. RF Microw. Med. Biol, vol. 6, no. 2, Art. no. 2, Jun. 2022, doi: 10.1109/JERM.2021.3120621. [DOI] [Google Scholar]
- [33].El-Ganainy R, Makris KG, Christodoulides DN, and Musslimani ZH, “Theory of coupled optical PT-symmetric structures,” Opt. Lett, vol. 32, no. 17, Art. no. 17, Sep. 2007, doi: 10.1364/OL.32.002632. [DOI] [PubMed] [Google Scholar]
- [34].Miri M-A and Alù A, “Exceptional points in optics and photonics,” Science, vol. 363, no. 6422, Art. no. 6422, Jan. 2019, doi: 10.1126/science.aar7709. [DOI] [PubMed] [Google Scholar]
- [35].Ye Z, Yang M, and Chen P-Y, “Multi-Band Parity-Time-Symmetric Wireless Power Transfer Systems for ISM-Band Bio-Implantable Applications,” IEEE Journal of Electromagnetics, RF and Microwaves in Medicine and Biology, pp. 1–8, 2021, doi: 10.1109/JERM.2021.3120621. [DOI] [Google Scholar]
- [36].Yang M, Ye Z, Farhat M, and Chen P-Y, “Ultrarobust Wireless Interrogation for Sensors and Transducers: A Non-Hermitian Telemetry Technique,” IEEE Trans. Instrum. Meas, vol. 70, pp. 1–9, 2021, doi: 10.1109/TIM.2021.3107057.33776080 [DOI] [Google Scholar]
- [37].Yang M, Ye Z, Alsaab N, Farhat M, and Chen P-Y, “In-Vitro Demonstration of Ultra-Reliable, Wireless and Batteryless Implanted Intracranial Sensors Operated on Loci of Exceptional Points,” IEEE Transactions on Biomedical Circuits and Systems, vol. 16, no. 2, Art. no. 2, Apr. 2022, doi: 10.1109/TBCAS.2022.3164697. [DOI] [PubMed] [Google Scholar]
- [38].Farhat M, Yang M, Ye Z, and Chen P-Y, “PT-Symmetric Absorber-Laser Enables Electromagnetic Sensors with Unprecedented Sensitivity,” ACS Photonics, vol. 7, no. 8, Art. no. 8, Aug. 2020, doi: 10.1021/acsphotonics.0c00514. [DOI] [Google Scholar]
- [39].Ye Z, Yang M, Zhu L, and Chen P-Y, “PTX-symmetric metasurfaces for sensing applications,” Front. Optoelectron, vol. 14, no. 2, Art. no. 2, Jun. 2021, doi: 10.1007/s12200-021-1204-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Yang M, Ye Z, Farhat M, and Chen P-Y, “Cascaded PT-symmetric artificial sheets: multimodal manipulation of self-dual emitter-absorber singularities, and unidirectional and bidirectional reflectionless transparencies,” J. Phys. D: Appl. Phys, vol. 55, no. 8, Art. no. 8, Nov. 2021, doi: 10.1088/1361-6463/ac3300. [DOI] [Google Scholar]
- [41].“Metasurface Absorber-Emitter for Humidity Sensing,” RSL, vol. 3, 2021, doi: 10.46620/21-0029. [DOI] [Google Scholar]
- [42].Fleury R, Sounas D, and Alù A, “An invisible acoustic sensor based on parity-time symmetry,” Nat Commun, vol. 6, no. 1, Art. no. 1, May 2015, doi: 10.1038/ncomms6905. [DOI] [PubMed] [Google Scholar]
- [43].Ye Z, Farhat M, and Chen P-Y, “Tunability and switching of Fano and Lorentz resonances in PTX-symmetric electronic systems,” Appl. Phys. Lett, vol. 117, no. 3. Art. no. 3, Jul. 2020, doi: 10.1063/5.0014919. [DOI] [Google Scholar]
- [44].Chen P-J, Saati S, Varma R, Humayun MS, and Tai Y-C, “Wireless Intraocular Pressure Sensing Using Microfabricated Minimally Invasive Flexible-Coiled LC Sensor Implant,” Journal of Microelectromechanical Systems, vol. 19, no. 4, pp. 721–734, Aug. 2010, doi: 10.1109/JMEMS.2010.2049825. [DOI] [Google Scholar]
- [45].Huang Q-A, Dong L, and Wang L-F, “LC Passive Wireless Sensors Toward a Wireless Sensing Platform: Status, Prospects, and Challenges,” Journal of Microelectromechanical Systems, vol. 25, no. 5, Art. no. 5, Oct. 2016, doi: 10.1109/JMEMS.2016.2602298. [DOI] [Google Scholar]