Abstract
Introduction
Data on longitudinal trajectory of kidney function decline and fluctuation in albuminuria leading to end-stage kidney disease (ESKD) is sparse in patients with type 2 diabetes.
Methods
Using data from an observational study of patients with type 2 diabetes and biopsy-confirmed diabetic kidney disease (DKD), generalized additive mixed models (GAMMs) were performed to quantify patterns of longitudinal trajectory of estimated glomerular filtration rate (eGFR) decline to ESKD associated with repeated measures of urine albumin-to-creatinine ratio (ACR).
Results
Over a median follow-up period of 3.3 years, 155 of 319 patients progressed to ESKD. Among these patients, 91.6% exhibited a curvilinear pattern in their eGFR trajectory. The median coefficient of variation for ACR, representing the variability in ACR measurements, was 48.9 (interquartile range: 36.9, 68.2). The median compound annual growth rate (CAGR) for ACR, reflecting the variation in ACR progression over time, was 43.6% (interquartile range: 0.0, 102.5); and 84.5% of patients developed nephrotic-range albuminuria, with a majority remaining nephrotic and subsequently progressing to ESKD. There was a positive association between the instantaneous speed of eGFR decline and ACR.
Conclusion
The observed curvilinear pattern in eGFR trajectory, high variability in ACR progression over time, and positive correlation between the speed of eGFR decline and ACR highlight the complex dynamics of disease progression and emphasize close monitoring of ACR fluctuation over time in patients with DKD.
Keywords: diabetic kidney disease, glomerular filtration rate, albuminuria, end-stage kidney disease, trajectory of eGFR, fluctuation in albuminuria
Graphical abstract
See Commentary on Page 194
DKD is a major microvascular complication of diabetes that affects up to 50% of patients with type 2 diabetes worldwide.1, 2, 3 The clinical course of DKD is characterized by an increase in albuminuria and/or a decline in glomerular filtration rate (GFR), which ultimately leads to ESKD.4, 5, 6 However, how patients with DKD progress to ESKD, with the dynamics of GFR decline and albuminuria levels over time, has not been fully investigated.
The natural history of DKD was conceptualized through observational studies conducted before the current era of standard care of renoprotective drugs or through cross-sectional studies.7 It has been postulated to show a uniform clinical presentation: GFR is assumed to decline linearly and increase in albuminuria is a unidirectional process.8 Nowadays, however, accumulating evidence suggest that trajectories of GFR decline are not always linear and levels of albuminuria are often fluctuating, and these are widely variable between and within patients in various chronic kidney disease (CKD) conditions over long periods of observation.9, 10, 11, 12 For example, Li et al.9 reported that 41.6% of patients with CKD with at least 3 years of follow-up and 8 measurements of GFR showed a greater than 90% probability of having nonlinear eGFR trajectory. These findings suggest that the substantial number of GFR measurements and relatively long period of follow-up time may be prerequisites for determining the longitudinal trajectories of GFR decline and changes in albuminuria because small fluctuations in GFR and levels of albuminuria are common and should not be interpreted as an overall GFR trajectory and changes in albuminuria.
Although several longitudinal studies in patients with DKD have been published,13, 14, 15 the major challenges of these studies relate to the sparsity of cohorts of patients with DKD with sufficient longitudinal follow-up to ESKD to allow for determining the trajectories of GFR decline to ESKD, the lack of accounting for interindividual and intraindividual variability of eGFR, and the assumption of linear trajectory of GFR decline. GAMM allows not only for nonlinear relationship between the dependent variable and the independent variables by fitting smooth functions to each independent variable but also for the inclusion of random effects, which capture the variability of observations within groups or individuals.16 Therefore, we applied this method to investigate the dynamics of GFR decline and albuminuria levels over time, delineating serial measurements of GFR and albuminuria, thereby leading to ESKD from a cohort of biopsy-confirmed DKD.
Methods
Data Sources
The Toranomon Natural History Study of Diabetic Kidney Disease is an ongoing longitudinal cohort study that follows the course of DKD in patients with type 2 diabetes and biopsy-confirmed DKD to better understand how DKD develops and how it may be treated. Briefly, the source population included 319 patients with type 2 diabetes aged 30 to 82 years who underwent clinical kidney biopsy and were diagnosed with DKD as the only kidney disease diagnosis at Toranomon Hospital (Tokyo, Japan) and Toranomon Hospital Kajigaya (Kanagawa, Japan) between 1990 and 2020. The majority of the study population, mainly inhabiting in the Tokyo metropolitan area, was under the care of each hospital or its satellite clinics every 3 months and was followed-up with from the date of kidney biopsy until the earliest date of: (i) developing ESKD (defined as initiation of hemodialysis, peritoneal dialysis, kidney transplantation, or death from uremia), (ii) all-cause death, or (iii) censoring (censoring for loss to follow-up or administrative censoring occurring on the end of December 2021).
Considering that our primary interest was to evaluate how patients with DKD progress to ESKD, with the time course of GFR decline and albuminuria levels, we focused our research on patients who developed ESKD. The Toranomon Natural History Study of Diabetic Kidney Disease was approved by the institutional review boards of Toranomon Hospital and Toranomon Hospital Kajigaya and was conducted in accordance with the declaration of Helsinki.
Kidney Function, Albuminuria Status, and Other Measurements
Serum creatinine, ACR, and other laboratory data were gathered from the medical records of each patient on each subsequent visit from the time of the kidney biopsy to the time of developing ESKD. eGFR was calculated using the CKD Epidemiology Collaboration equation modified by a Japanese coefficient.17 Albuminuria status was classified as follows: normal to mildly increased albuminuria (formerly known as normoalbuminuria) (ACR <30 mg/g Crea), moderately increased albuminuria (formerly known as microalbuminuria) (30 to <300 mg/g Crea), severely increased albuminuria (formerly known as macroalbuminuria [not including nephrotic-range albuminuria]) (ACR 300 to <3000 mg/g Crea), and nephrotic-range albuminuria (ACR ≥3000 mg/g Crea). The other obtained data included age, sex, body mass index (BMI), known duration of diabetes, glycated hemoglobin (HbA1c), systolic blood pressure (SBP), diastolic blood pressure, total cholesterol, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein cholesterol, triglycerides, medication usage including renin-angiotensin system (RAS) blocker, glucose-lowering agent (oral hypoglycemics and/or insulin) and statin, medical history of cardiovascular disease and smoking status. Pathological findings were evaluated according to the Renal Pathology Society Classification of Diabetic Nephropathy.18 In brief, pathological findings were classified as follows: (i) class I was glomerular basement thickening and only mild, nonspecific changes on light microscopy; (ii) class II was mild (IIa) or severe (IIb) mesangial expansion without either nodular lesions or global sclerosis in >50% of the glomeruli; (iii) class III was nodular lesions without global sclerosis in >50% of the glomeruli; and (iv) class IV was global sclerosis in >50% of the glomeruli. Considering that the typical pathological lesion of DKD is nodular lesions (in glomerular lesions), we separated the categories according to whether nodular lesions were included or not (class I, IIa & IIb vs. class III & IV). These procedures were conducted by three pathologists.
Statistical Analyses
First, we described the baseline characteristics of the study population according to the category of baseline eGFR (as >60, 30–60, and <30 ml/min per 1.73 m2). Baseline characteristics were quantified using median and interquartile range for continuous variables and percentages for categorical variables. Then, these characteristics were compared across groups, with P values calculated by analysis of variance for continuous variables and χ2 tests for categorical variables and P values for trend calculated by a nonparametric test for trend across ordered groups developed by Cuzick.19
Second, we quantified the pattern of trajectory of eGFR decline. Because most eGFR trajectories appeared curvilinear on inspection, we applied GAMM to eGFR data in each patient to find the eGFR trajectory of best fit for each patient. The heterogeneity among individuals was accounted for by assuming random effects between individuals. To avoid overfitting, we compared these models with the linear model based on the following fit statistics: Coefficient of determination (R2), Akaike information criterion, and F test calculated by analysis of variance test. The final model was selected based on F test calculated by analysis of variance test.
Third, we assessed the fluctuation of albuminuria level in each patient. Because our data showed a high interindividual and intraindividual variability in ACR, we used coefficient of variation and CAGR to compare fluctuation in ACR between patients. The formula for CAGR for ACR is given by:
Where, N is the number of ACR measurements, tn is the time of ACR measurement at time n, and tm is the time of ACR measurement at time m (m>n). We also quantified the pattern of albuminuria. To avoid taking a transient fluctuation as a perpetual fluctuation, the determination of albuminuria status was based on being in the same status at least 3 consecutive occasions or 2 serial occasions separated by more than 6 months. Progression of albuminuria was defined as a permanent transition from a lower status to a higher status, and a decrease in albuminuria was defined as the opposite. Remaining in the same status as the baseline status was defined as no change. We also calculated average ACR and number of patients who developed nephrotic-range albuminuria.
Finally, we evaluated the instantaneous speed of eGFR decline (the slope of eGFR) by albuminuria level over time. Instantaneous speed of eGFR decline is given by:
Where is the derivative of the function , and is the function of eGFR at time . Then we plotted the instantaneous speed of eGFR by albuminuria level over time. To further estimate the association between pattern of albuminuria (progression, regression, and stable) and ESKD, Cox proportional hazards regression models were used to test associations of patterns of albuminuria with the onset of ESKD adjusting for clinical variables.
In addition, we performed panel data analysis to evaluate the effects of repeated measurements of risk factors on albuminuria status. Based on the guideline risk definition,20 we used the following cutoffs to define presence of the risk factors: age ≥65 years, BMI ≥23 kg/m2 for Asian,21 SBP ≥130 mm Hg, HbA1c ≥7.0 %, LDL cholesterol ≥100 mg/dl, and current smoking. We used mixed-effects logistic model for repeated measures.
All statistical analyses were performed using R V.4.2.0 (R Foundation for Statistical Computing, Vienna, Austria). The command “GAMM” from the mgcv package was used for the GAMMs and trajectory was plotted using the command “plot”. All statistical tests were 2 sided, and we considered P value <0.05 to be statistically significant.
Results
Baseline Characteristics
Among 319 patients in the entire cohort, 163 developed ESKD, 27 died before ESKD, and 129 were censored. Eight (4.9%) of 163 who developed ESKD were excluded due to having simultaneous measurements of eGFR and ACR less than 5 times or developing acute kidney injury, leaving 155 patients for analysis. The study flow and selection of study population are summarized in Figure 1. In Table 1, we show the baseline characteristics of the patients, overall and stratified by baseline eGFR. The characteristics of overall cohort were as follows: median (interquartile range) age of 60 (50–67) years old, men were 78.1%, BMI 24.2 (22.0–26.8), known duration of diabetes of 14 (9–20) years, SBP 144 (130–160) mm Hg, diastolic blood pressure 80 (71–90) mm Hg, HbA1c 7.1% (6.3–8.4), LDL cholesterol 122 (96–160) mg/dl, eGFR 31.6 (20.4–46.1) ml/min per 1.73 m2, and ACR 2205 (963–4793) mg/g Crea. Between subgroups, there appeared to be a trend for the baseline eGFR to be lower with lower HbA1c, higher ACR, higher prevalence of ever-smoker, and higher severity of pathological lesions at baseline.
Figure 1.
Study flow and selection of study population. ACR, urine albumin-to-creatinine ratio; DKD, diabetic kidney disease; eGFR, estimated glomerular filtration rate; ESKD, end-stage kidney disease.
Table 1.
Baseline characteristics of study population in total and by category of estimated glomerular filtration rate
| Characteristic | Total |
Baseline eGFR (ml/min per 1.73 m2) |
P valuea | P value for trendb | ||
|---|---|---|---|---|---|---|
| >60 |
30–60 |
<30 |
||||
| (n = 155) | (n = 17) | (n = 65) | (n = 73) | |||
| Age (years) | 60 (50, 67) | 56 (49, 60) | 58 (50, 66) | 63 (52, 67) | 0.235 | 0.056 |
| Male sex | 121 (78.1%) | 12 (70.6%) | 50 (76.9%) | 59 (80.8%) | 0.629 | 0.344 |
| BMI (kg/m2) | 24.2 (22.0, 26.8) | 24.0 (23.1, 25.1) | 24.4 (21.4, 27.4) | 24.2 (22.0, 26.6) | 0.978 | 0.803 |
| Duration of diabetes (years) | 14 (9, 20) | 13 (8, 20) | 13 (10, 18) | 15 (9, 22) | 0.398 | 0.225 |
| Systolic BP (mm Hg) | 144 (130, 160) | 140 (130, 154) | 144 (133, 160) | 145 (129, 160) | 0.666 | 0.735 |
| Diastolic BP (mm Hg) | 80 (71, 90) | 80 (72, 90) | 80 (70, 90) | 80 (72, 90) | 0.992 | 0.971 |
| HbA1c (%) | 7.1 (6.3, 8.4) | 8.5 (6.6, 10.2) | 7.2 (6.3, 8.4) | 6.7 (6.2, 7.8) | 0.015 | 0.005 |
| LDL cholesterol (mg/dl) | 122 (96, 160) | 139 (95, 173) | 122 (101, 177) | 117 (89, 157) | 0.608 | 0.154 |
| Estimated GFR (ml/min per 1.73 m2) | 31.6 (20.4, 46.1) | 76.2 (67.6, 83.4) | 40.3 (35.6, 48.9) | 19.8 (15.5, 24.6) | <0.001 | <0.001 |
| ACR (mg/g Crea) | 2205 (963, 4793) | 1296 (465, 1897) | 2271 (886, 4090) | 2902 (1259, 5975) | 0.035 | 0.011 |
| ACR status | ||||||
| <30 | 2 (1.3%) | 0 (0.0%) | 1 (1.5%) | 1 (1.4%) | 0.242 | 0.073 |
| 30 to <300 | 9 (5.8%) | 3 (17.6%) | 2 (3.1%) | 4 (5.5%) | ||
| 300 to <3000 | 78 (50.3%) | 10 (58.8%) | 35 (53.8%) | 33 (45.2%) | ||
| ≥3000 | 66 (42.6%) | 4 (22.2%) | 27 (41.5%) | 35 (47.9%) | ||
| History of cardiovascular disease | 35 (22.6%) | 2 (11.8%) | 12 (18.5%) | 21 (28.8%) | 0.186 | 0.070 |
| Retinopathy | 108 (69.7%) | 12 (70.6%) | 44 (67.7%) | 52 (71.2%) | 0.900 | 0.799 |
| Ever-smoker | 87 (56.1%) | 7 (41.2%) | 33 (50.8%) | 47 (64.4%) | 0.115 | 0.039 |
| Antihypertensive drug use | 141 (91.0%) | 14 (82.4%) | 59 (90.8%) | 68 (93.2%) | 0.375 | 0.203 |
| ACE inhibitor or ARB use | 106 (68.4%) | 13 (76.5%) | 45 (69.2%) | 48 (65.8%) | 0.681 | 0.398 |
| Oral antihyperglycemic drug use | 68 (43.9%) | 11 (64.7%) | 28 (43.1%) | 29 (39.7%) | 0.172 | 0.114 |
| Insulin use | 86 (55.5%) | 8 (47.1%) | 31 (47.7%) | 47 (64.4%) | 0.109 | 0.057 |
| Statin use | 56 (36.1%) | 6 (35.3%) | 22 (33.8%) | 28 (38.4%) | 0.857 | 0.661 |
| Pathologic classification | ||||||
| Class I, IIa, & IIb | 70 (45.2%) | 13 (76.5%) | 38 (58.5%) | 19 (26.0%) | <0.001 | <0.001 |
| Class III & VI | 85 (54.8%) | 4 (23.5%) | 27 (41.5%) | 54 (74.0%) | ||
ACE, angiotensin-converting enzyme; ACR, urine albumin-to-creatinine ratio; ARB, angiotensin receptor blocker; BMI, body mass index; BP, blood pressure; eGFR, estimated glomerular filtration rate; GFR, glomerular filtration rate; HbA1c, glycated hemoglobin; LDL, low-density lipoprotein.
Data are presented as median (interquartile range) for continuous measures, and n (%) for categorical measures.
P value calculated by analysis of variance for continuous variables and χ2 tests for categorical variables and represents differences across categories of eGFR.
P value calculated by a nonparametric test for trend across ordered groups.
Patterns of Trajectory of eGFR Decline
In Table 2 and Figure 2a, we show the pattern of eGFR trajectory. Among all patients, 91.6% had a curvilinear shape of eGFR trajectory. Between subgroups, there appeared to be no trends for patterns of eGFR trajectory. Trajectories of eGFR decline on selected patients are shown in Figure 2c. Fit statistics and eGFR trajectories of each patient can be found in Supplementary Table S1 and Supplementary Figure S1. Partial residual plots for each predictor can be found in Supplementary Figure S2.
Table 2.
Follow-up data in total and by baseline eGFR
| Total |
Baseline eGFR (ml/min per 1.73 m2) |
aP value | bP value for trend | |||
|---|---|---|---|---|---|---|
| >60 |
30–60 |
<30 |
||||
| (n = 155) | (n = 17) | (n = 65) | (n = 73) | |||
| No. of simultaneous measurements of eGFR and ACR | 21 (11, 46) | 41 (25, 64) | 25 (14, 51) | 15 (10, 27) | 0.024 | <0.001 |
| Time to ESKD (yr) | 3.3 (1.6, 6.0) | 8.8 (5.6, 11.5) | 4.2 (2.5, 8.2) | 2.2 (1.0, 3.9) | <0.001 | <0.001 |
| eGFR | ||||||
| cPattern of eGFR | 0.269 | 0.107 | ||||
| Linear | 13 (8.4%) | 1 (5.9%) | 3 (4.6%) | 9 (12.3%) | ||
| Curvilinear | 142 (91.6%) | 16 (94.1%) | 62 (95.4%) | 64 (87.7%) | ||
| Coefficient of variation for eGFR | 43.1 (34.4, 56.1) | 58.7 (56.1, 66.9) | 46.3 (38.4, 58.8) | 36.8 (29.0, 44.2) | <0.001 | <0.001 |
| ACR | ||||||
| dPattern of albuminuria | 0.373 | 0.142 | ||||
| Progression | 51 (32.9%) | 9 (52.9%) | 21 (32.3%) | 21 (28.8%) | ||
| Regression | 17 (11.0%) | 2 (11.8%) | 6 (9.2%) | 9 (12.3%) | ||
| No change | 87 (56.1%) | 6 (35.3%) | 38 (58.5%) | 43 (58.9%) | ||
| Coefficient of variation for albuminuria | 48.9 (36.9, 68.2) | 57.8 (48.7, 70.6) | 50.8 (37.4, 69.8) | 42.2 (30.2, 59.1) | 0.077 | 0.003 |
| Compound annual growth rate for albuminuria (%) | 43.6 (0.0, 102.5) | 50.4 (32.9, 93.8) | 39.1 (3.9, 100.1) | 49.9 (-8.0, 118.9) | 0.925 | 0.633 |
| No. of patients who developed nephrotic-range albuminuria | 131 (84.5%) | 15 (88.2) | 55 (84.6) | 61 (83.6) | 0.891 | 0.661 |
ACR, urine albumin-to-creatinine ratio; eGFR, estimated glomerular filtration rate; ESKD, end-stage kidney disease.
Data are presented as median (interquartile range) for continuous measures, and n (%) for categorical measures.
P value calculated by analysis of variance for continuous variables and χ2 tests for categorical variables and represents differences across categories of eGFR.
P value calculated by a nonparametric test for trend across ordered groups.
The pattern of eGFR in each patient was determined based on the analysis of variance F-test by applying a generalized additive mixed model and a linear model to each patient’s eGFR data (See Statistical Analyses in the Methods section and Supplementary Table S1).
The pattern of albuminuria was quantified as follows: progression of albuminuria was defined as a permanent transition from a lower albuminuria category to a higher albuminuria category, and a decrease in albuminuria was defined as the opposite. Remaining in the same category as the baseline status was defined as no change (See Statistical Analyses in the Methods section).
Figure 2.
Trajectories of eGFR decline and ACR to end-stage kidney disease. (a) Trajectories of eGFR decline to end-stage kidney disease in the entire cohort. (b) Trajectories of ACR to end-stage kidney disease in the entire cohort. (c) Trajectories of eGFR decline to end-stage kidney disease with observed measurements of eGFR and ACR in selected patients. ACR, urine albumin-to-creatinine ratio; eGFR, estimated glomerular filtration rate.
The median coefficient of variation for eGFR was 43.1 (34.4, 56.1). Between subgroups, there appeared to be a trend for the coefficient of variation for eGFR to be smaller with lower eGFR categories at baseline.
Patterns of Albuminuria and Fluctuations of Albuminuria
In Table 2 and Figure 2b, we show the patterns of albuminuria. Among all patients, 32.9% of patients progressed to a higher albuminuria status and 11.0% regressed to a lower albuminuria status, whereas 56.1% of patients remained in the same albuminuria status as the same status at baseline. Between subgroups, there appeared to be no trend for patterns of albuminuria.
The median coefficient of variation for albuminuria was 48.9 (36.9, 68.2). Between subgroups, there appeared to be a trend for the coefficient of variation for albuminuria to be smaller with lower eGFR categories at baseline. The median CAGR for albuminuria was 43.6% (0.0, 102.5) and 84.5% of patients developed nephrotic-range albuminuria, many of whom remained nephrotic and developed ESKD. There appeared to be no trend for CAGR for albuminuria.
Association Between eGFR Decline and Albuminuria Level
Instantaneous Speed of eGFR Decline by Albuminuria Level Over Time
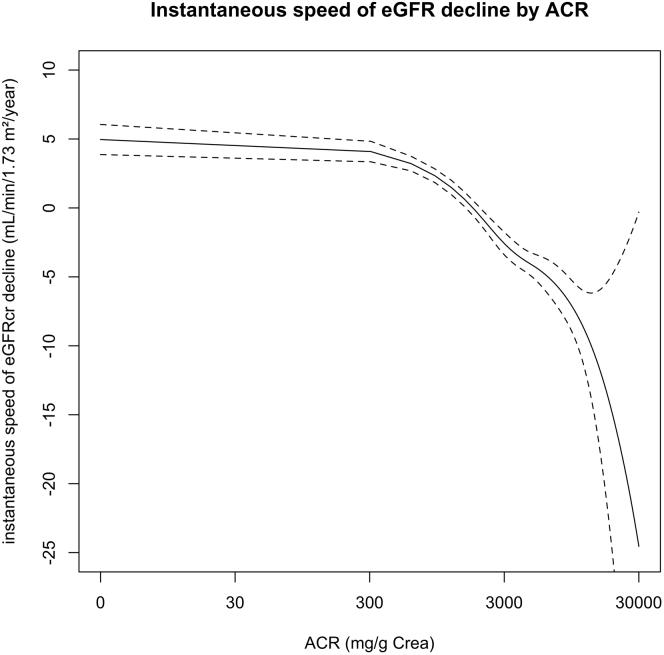
In Figure 3, we show the instantaneous speed of eGFR decline (eGFR slope) by albuminuria levels. The instantaneous speed of eGFR decline increased with increasing albuminuria level.
Figure 3.
Instantaneous speed of eGFR decline by ACR. Instantaneous speed of eGFR (slope of eGFR) was calculated by the derivative of nonlinear function of eGFR trajectory and was plotted by albuminuria level over time. ACR, urine albumin-to-creatinine ratio; eGFR, estimated glomerular filtration rate.
Longitudinal Association of Metabolic Risk Factors With Albuminuria Status
In Table 3, we show the longitudinal association of metabolic risk factors with albuminuria levels. In adjusted analysis, increase above the threshold in BMI, SBP, HbA1c, and LDL levels throughout the course of DKD were associated with albuminuria status.
Table 3.
Longitudinal effect of metabolic risk factors on albuminuria
| Albuminuria category |
95% CI | P value | ≥3000 |
95% CI | P value | |
|---|---|---|---|---|---|---|
| 300 to <3000 | ||||||
| RRR (SE) | RRR (SE) | |||||
| Age ≥65 yr | 0.77 (0.25) | 0.41, 1.45 | 0.424 | 0.69 (0.36) | 0.24, 1.94 | 0.479 |
| Male sex | 0.94 (0.66) | 0.24, 3.70 | 0.926 | 0.63 (0.69) | 0.07, 5.37 | 0.673 |
| BMI ≥23 | 1.11 (0.39) | 0.55, 2.21 | 0.774 | 1.10 (0.50) | 0.45, 2.70 | 0.837 |
| Systolic BP ≥130 mm Hg | 2.04 (0.35) | 1.46, 2.86 | <0.001 | 3.37 (0.86) | 2.05, 5.54 | <0.001 |
| HbA1c ≥7.0 % | 1.52 (0.33) | 0.99, 2.33 | 0.054 | 0.81 (0.25) | 0.44, 1.47 | 0.489 |
| LDL cholesterol ≥100 mg/dl | 1.63 (0.01) | 1.13, 2.36 | 0.009 | 3.79 (1.16) | 2.08, 6.91 | <0.001 |
| eGFR category | ||||||
| >60 | 1.12 (0.35) | 0.60, 2.06 | 0.727 | 4.58 (2.75) | 1.41, 14.9 | 0.730 |
| 30–60 | 1.48 (0.57) | 0.69, 3.16 | 0.309 | 9.25 (3.40) | 2.53, 22.6 | 0.163 |
| <30 | 0.76 (0.30) | 0.35, 1.65 | 0.486 | 7.90 (5.28) | 2.14, 29.2 | 0.156 |
| Ever smoked | 2.33 (1.39) | 0.72, 7.48 | 0.156 | 3.66 (3.40) | 0.59, 22.6 | 0.163 |
| ACE inhibitor or ARB use | 1.18 (0.28) | 0.75, 1.88 | 0.475 | 1.15 (0.45) | 0.53, 2.47 | 0.730 |
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; BMI, body mass index; BP, blood pressure; CI, confidence interval; HbA1c, glycated hemoglobin; LDL, low-density lipoprotein; RRR, relative risk ratio; SE, standard error.
RRR (95% CI) and P values were determined for demographic and laboratory characteristics by multinomial logistic model for repeated measurements (with albuminuria category <300 as reference category).
The Association Between Albuminuria and SBP
In Figure 4, we show the association between albuminuria and SBP. SBP increased with increasing albuminuria level.
Figure 4.
Association between albuminuria and systolic blood pressure. Each dot represents ACR and systolic blood pressure at all measurement time points, and the smooth line (blue line) with 95% confidence interval (gray stripe) were obtained by fitting a GAMM spline. ACR, urine albumin-to-creatinine ratio; GAMM, generalized additive mixed model.
Discussion
In this analysis of a cohort study of biopsy-confirmed DKD, we found that majority of patients with DKD who ended up with ESKD showed a curvilinear eGFR trajectory with a large fluctuation of ACR. Although there was a wide diversity of trajectory of eGFR decline and a high interindividual and intraindividual variability in ACR, the speed of eGFR decline changed over time depending on the albuminuria level. We also found that albuminuria status was longitudinally associated with increase above the threshold in BMI, SBP, HbA1c, and LDL cholesterol levels.
Given the lack of generally accepted classification of eGFR trajectories and fluctuation of albuminuria that show diversity, we thoroughly investigated repeated measurements of eGFR and ACR in each patient and determined each pattern of trajectory of eGFR decline and fluctuation in albuminuria leading to ESKD. We observed that majority of patients showed a nonlinear pattern of eGFR decline regardless of baseline eGFR; and 84.5% of patients developed nephrotic-range albuminuria, many of whom remained nephrotic and developed ESKD, although there were a high interindividual and intraindividual variability in ACR. We also observed that albuminuria levels were associated with the speed of eGFR decline. Most of previous studies only reported that baseline eGFR and ACR are independently associated with progression to ESKD in patients with DKD.4, 5, 6 However, the speed of eGFR decline can be constantly modified by various conditions throughout the course of DKD, which may result in nonlinear trajectories of eGFR decline. Albuminuria can also vary substantially between and within individuals. To address these issues, we adopted GAMMs, which allow not only for nonlinear relationship between longitudinal eGFR and ACR by fitting smooth functions to each variable but also for the inclusion of random effects, to capture the variability of observations within groups or individuals. Using this approach, we observed that most of patients showed a nonlinear trajectory of eGFR decline with a fluctuation of ACR and that the instantaneous speed was positively correlated with ACR. Furthermore, we also observed that there was a longitudinal association between albuminuria status and metabolic factors such as BMI, SBP, HbA1c, and LDL cholesterol levels, over the course of DKD. We especially focused on the association of blood pressure and albuminuria, because the clinical course of DKD is characterized by an increase in albuminuria and/or a decline in GFR, often accompanied by an elevation in blood pressure. We assessed the association between albuminuria and SBP, using a smooth technique (a generalized additive model), and we observed the positive correlation between them.
Defining the natural history of DKD has been challenging for various reasons. First, cohorts of patients with DKD with sufficient longitudinal follow-up is sparse. The substantial number of GFR measurements and relatively long period of follow-up time are prerequisites for determining the longitudinal trajectories of GFR and changes in albuminuria because small fluctuations in GFR and levels of albuminuria are common and should not be interpreted as an overall GFR trajectory and changes in albuminuria.7 To address this issue, one way to observe the natural history of DKD is to reconstruct the average eGFR trajectory to ESKD for the average patient. If we can collect eGFR data from multiple patients at different time points, we should theoretically be able to reconstruct the average eGFR trajectory to ESKD for the average patient. However, the major drawback of this method is that it includes eGFR data from patients who did not develop ESKD and does not accurately represent the trajectory to ESKD. Second, progression of DKD is heterogeneous; trajectories of GFR decline and levels of albuminuria are widely variable between and within patients in DKD.7 Again, reconstructing the average eGFR trajectory from pooled eGFR data from multiple patients at different times does not address this issue because it combines trajectories of eGFR with large interindividual variability into a single trajectory. Third, there is a limitation in assuming a linear trajectory in the natural history of DKD. In 1976, a linear model of renal decline was conceptualized on the assumption that the rate of renal decline is constant.22 Since then, the reciprocal of serum creatinine had been used as an approximation of GFR and the plot of them had been used to predict ESKD for a period of times.23, 24, 25 Although some patients fit this linear model, evidence suggests that the reciprocal serum creatinine plot is not accurate in predicting ESKD in a substantial portion of patients with CKD because of a wide variation in the renal function.24,25 Subsequently, eGFR prediction equations were developed that more closely approximates measured GFR;26,27 however, there is still a large body of evidence that trajectories of eGFR are not always linear and these are widely variable between and within patients in various kidney conditions over long periods of observation.9, 10, 11, 12
Cross-sectional studies reveal that a proportion of patients with diabetes have an eGFR decline but without albuminuria.28 Recent studies have shown that these patients without albuminuria have a slower eGFR decline and lower risk of ESKD than patients with albuminuria.29,30 However, these findings were based on a single measurement of baseline albuminuria; therefore no conclusion has yet been reached on whether patients without albuminuria at baseline developed ESKD without ever developing albuminuria throughout the history of DKD. One study from the Steno Diabetes Center reported that nearly 20% of patients with DKD developed ESKD without ever developing macroalbuminuria.12 However, contrary to this study, none of our patients developed ESKD without ever developing macroalbuminuria. A very small number of patients in our cohort had no albuminuria for a relatively long period of time and indeed their kidney function declined very slowly but they developed macroalbuminuria eventually or at least once during course of DKD. This difference may come from the difference in the study population. The study population in our study comprised biopsy-confirmed DKD, whereas that of the Steno Diabetes Center included patients with clinically diagnosed DKD, which possibly included other interstitial dominant disease or ischemic renal disease, such as interstitial nephritis and extrarenal arteriosclerotic vascular disease. We think that patients with DKD who develop ESKD without ever developing macroalbuminuria are extremely exceptional, and it is unlikely that such a course is attributable to diabetes.
A key strength of our study is the use of a study population from a real-world cohort of biopsy-confirmed DKD, who had not only clinically but also pathologically diagnosed with DKD, and who had serial measurements of eGFR and ACR and determined onset of ESKD, which enabled robust analysis of the trajectories of eGFR and changes of ACR leading to ESKD. Other strengths include the use of GAMM to estimate the effects of repeated measurements of ACR on eGFR decline and to handle heterogeneity, nonlinearity of eGFR, and ACR. However, our findings should be interpreted in the context of several limitations. First, even though the study population is from a real-world cohort, there may be a selection bias. There is a possibility that the study population was biopsied because they were suspected to have any kind of kidney disease rather than DKD. In contrast, we believe that the use of biopsy-confirmed DKD rather than inaccurate clinical diagnosis of DKD provides a clear picture of the clinical course of DKD. Further, we believe that our findings are clinically versatile because they demonstrate that the relationship between eGFR and albuminuria over time affects renal prognosis, even when pathological findings are comparable at baseline. Second, half of the study population was censored (did not developed ESKD or died by the end of the data collection [the end of December 2021]), which may not capture the whole picture of clinical course of DKD. Again, this is the nature of the real-world ongoing cohort study. Third, the study population comprised Asian, mostly Japanese patients, and our findings may not apply to populations from different geographical origins. Fourth, the eGFR calculated by the CKD Epidemiology Collaboration equation modified by a Japanese coefficient based on serum creatinine was used in our study, although measured GFR using plasma or urinary clearance of exogeneous filtration marker is considered the gold standard for assessment of kidney function. However, it is unrealistic in clinical practice to perform the measured GFR at each visit. Fifth, the measurement of urinary albumin excretion in our study was determined by measurement of ACR, although the 24-hour urine collection is the gold standard for measurement of albuminuria. However, previous studies have shown that the ACR value demonstrated the noninferiority of predictive capability for renal disease progression.31 Moreover, as with the measured GFR, it is unrealistic in clinical practice to measure 24-hour urine collection at each visit. Sixth, the study population was not given contemporary standard care. The percentages of patients who were prescribed the maximal dose of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers at baseline were 0.0% in ACR <30, 16.7% in ACR of 30 to <300, 14.0% in ACR of 300 to <3000, and 22.7% in ACR ≥3000. There would be much room for improving the prognosis of our patients by increasing to maximal dose of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers. Now that sodium-glucose cotransporter-2 inhibitors have proved to be effective in reducing the rate of progression to ESKD.32,33 However, none of the patients in our study received sodium-glucose cotransporter-2 inhibitors, because most patients had been enrolled in our study before they had access to the most recent standard care for patients with DKD. Whether use of sodium-glucose cotransporter-2 inhibitors would change trajectories of eGFR and levels of albuminuria and reduce ESKD events resulting in changing the course of DKD must wait for future analyses of this ongoing cohort.
In conclusion, this analysis demonstrated that the majority of patients with biopsy-confirmed DKD who developed ESKD showed a curvilinear eGFR trajectory with fluctuation in ACR. Despite high interindividual and intraindividual variability in changes in eGFR and ACR, longitudinal ACR was inversely associated with eGFR decline to ESKD. We also found that albuminuria status was longitudinally associated with BMI, SBP, HbA1c, and LDL cholesterol levels. These results highlight the complex dynamics of disease progression and emphasize close monitoring of ACR fluctuation over time to detect eGFR decline to ESKD in patients with DKD.
Disclosure
All the authors declared no conflicts of interests.
Acknowledgments
The authors thank various people for their contribution to this project; Dr. Yoko Yoshida, Ms. Yuki Inoue, The Institute for Adult Disease, Asahi Life Foundation, Ms. Yurina Takaishi, Ms. Keiko Sahara and Ms. Tokiko Hoshikawa, Toranomon Hospital, for their help in collecting the data. This study was supported in part by a Ministry of Health, Labour and Welfare Grant-in-Aid for Diabetic Nephropathy and Nephrosclerosis Research (JP17ek0310003) and a grant for medical research from the Okinaka Memorial Institute for Medical Research, Tokyo, Japan. The funding source had no role in study design or execution, data analysis, manuscript writing, or manuscript submission.
Author Contributions
MY, KF, and TW designed the study protocol, researched data, contributed to the discussion, wrote the manuscript, and reviewed and edited the manuscript. All authors contributed to the discussion and reviewed the manuscript. SN is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Figure S1. eGFR trajectories of each patient.
Figure S2. Partial residual plots for each predictor.
Table S1. Fit statistics for linear and GAMM model.
STROBE Statement (PDF).
Contributor Information
Masayuki Yamanouchi, Email: m.yamanouchi@toranomon.gr.jp.
Takashi Wada, Email: twada@staff.kanazawa-u.ac.jp.
Supplementary Material
Figure S1. eGFR trajectories of each patient.
Figure S2. Partial residual plots for each predictor.
Table S1. Fit statistics for linear and GAMM model.
STROBE Statement (PDF).
References
- 1.Tuttle K.R., Bakris G.L., Bilous R.W., et al. Diabetic kidney disease: a report from an ADA Consensus Conference. Diabetes Care. 2014;37:2864–2883. doi: 10.2337/dc14-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Afkarian M., Sachs M.C., Kestenbaum B., et al. Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol. 2013;24:302–308. doi: 10.1681/ASN.2012070718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group KDIGO 2020 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int. 2020;98:S1–S115. doi: 10.1016/j.kint.2020.06.019. [DOI] [PubMed] [Google Scholar]
- 4.Ninomiya T., Perkovic V., de Galan B.E., et al. Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J Am Soc Nephrol. 2009;20:1813–1821. doi: 10.1681/ASN.2008121270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Astor B.C., Matsushita K., Gansevoort R.T., et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int. 2011;79:1331–1340. doi: 10.1038/ki.2010.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wada T., Haneda M., Furuichi K., et al. Clinical impact of albuminuria and glomerular filtration rate on renal and cardiovascular events, and all-cause mortality in Japanese patients with type 2 diabetes. Clin Exp Nephrol. 2014;18:613–620. doi: 10.1007/s10157-013-0879-4. [DOI] [PubMed] [Google Scholar]
- 7.Oshima M., Shimizu M., Yamanouchi M., et al. Trajectories of kidney function in diabetes: a clinicopathological update. Nat Rev Nephrol. 2021;17:740–750. doi: 10.1038/s41581-021-00462-y. [DOI] [PubMed] [Google Scholar]
- 8.Mogensen C.E., Christensen C.K., Vittinghus E. The stages in diabetic renal disease. With emphasis on the stage of incipient diabetic nephropathy. Diabetes. 1983;32(suppl 2):64–78. doi: 10.2337/diab.32.2.s64. [DOI] [PubMed] [Google Scholar]
- 9.Li L., Astor B.C., Lewis J., et al. Longitudinal progression trajectory of GFR among patients with CKD. Am J Kidney Dis. 2012;59:504–512. doi: 10.1053/j.ajkd.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weldegiorgis M., de Zeeuw D., Li L., et al. Longitudinal estimated GFR trajectories in patients with and without Type 2 diabetes and nephropathy. Am J Kidney Dis. 2018;71:91–101. doi: 10.1053/j.ajkd.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 11.Jiang G., Luk A.O.Y., Tam C.H.T., et al. Progression of diabetic kidney disease and trajectory of kidney function decline in Chinese patients with Type 2 diabetes. Kidney Int. 2019;95:178–187. doi: 10.1016/j.kint.2018.08.026. [DOI] [PubMed] [Google Scholar]
- 12.Vistisen D., Andersen G.S., Hulman A., Persson F., Rossing P., Jørgensen M.E. Progressive decline in estimated glomerular filtration rate in patients with diabetes after moderate loss in kidney function-even without albuminuria. Diabetes Care. 2019;42:1886–1894. doi: 10.2337/dc19-0349. [DOI] [PubMed] [Google Scholar]
- 13.Mogensen C.E. Progression of nephropathy in long-term diabetics with proteinuria and effect of initial anti-hypertensive treatment. Scand J Clin Lab Investig. 1976;36:383–388. doi: 10.1080/00365517609055274. [DOI] [PubMed] [Google Scholar]
- 14.Parving H.H., Smidt U.M., Friisberg B., Bonnevie-Nielsen V., Andersen A.R. A prospective study of glomerular filtration rate and arterial blood pressure in insulin-dependent diabetics with diabetic nephropathy. Diabetologia. 1981;20:457–461. doi: 10.1007/BF00253407. [DOI] [PubMed] [Google Scholar]
- 15.Viberti G.C., Bilous R.W., Mackintosh D., Keen H. Monitoring glomerular function in diabetic nephropathy. A prospective study. Am J Med. 1983;74:256–264. doi: 10.1016/0002-9343(83)90624-1. [DOI] [PubMed] [Google Scholar]
- 16.Baayen R.H., Linke M. In: A Practical Handbook of Corpus Linguistics. Paquot M., Gries S.T., editors. Springer; 2020. Generalized additive mixed models. [DOI] [Google Scholar]
- 17.Horio M., Imai E., Yasuda Y., Watanabe T., Matsuo S. Modification of the CKD epidemiology collaboration (CKD-EPI) equation for Japanese: accuracy and use for population estimates. Am J Kidney Dis. 2010;56:32–38. doi: 10.1053/j.ajkd.2010.02.344. [DOI] [PubMed] [Google Scholar]
- 18.Tervaert T.W., Mooyaart A.L., Amann K., et al. Pathologic classification of diabetic nephropathy. J Am Soc Nephrol. 2010;21:556–563. doi: 10.1681/ASN.2010010010. [DOI] [PubMed] [Google Scholar]
- 19.Cuzick J. A Wilcoxon-type test for trend. Stat Med. 1985;4:87–90. doi: 10.1002/sim.4780040112. [DOI] [PubMed] [Google Scholar]
- 20.de Boer I.H., Khunti K., Sadusky T., et al. Diabetes management in chronic kidney disease: a consensus report by the American Diabetes Association (ADA) and kidney disease: improving global outcomes (KDIGO) Diabetes Care. 2022;45:3075–3090. doi: 10.2337/dci22-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsu W.C., Araneta M.R., Kanaya A.M., Chiang J.L., Fujimoto W. BMI cut points to identify at-risk Asian Americans for type 2 diabetes screening. Diabetes Care. 2015;38:150–158. doi: 10.2337/dc14-2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones R.H., Hayakawa H., Mackay J.D., Parsons V., Watkins P.J. Progression of diabetic nephropathy. Lancet. 1979;1:1105–1106. doi: 10.1016/s0140-6736(79)91788-4. [DOI] [PubMed] [Google Scholar]
- 23.Bleyer A.J. A reciprocal graph to plot the reciprocal serum creatinine over time. Am J Kidney Dis. 1999;34:576–578. doi: 10.1016/s0272-6386(99)70089-2. [DOI] [PubMed] [Google Scholar]
- 24.Shah B.V., Levey A.S. Spontaneous changes in the rate of decline in reciprocal serum creatinine: errors in predicting the progression of renal disease from extrapolation of the slope. J Am Soc Nephrol. 1992;2:1186–1191. doi: 10.1681/ASN.V271186. [DOI] [PubMed] [Google Scholar]
- 25.Szeto C.C., Leung C.B., Wong T.Y., et al. Extrapolation of reciprocal creatinine plot is not reliable in predicting the onset of dialysis in patients with progressive renal insufficiency. J Intern Med. 2003;253:335–342. doi: 10.1046/j.1365-2796.2003.01121.x. [DOI] [PubMed] [Google Scholar]
- 26.Levey A.S., Coresh J., Greene T., et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 27.Levey A.S., Stevens L.A. Estimating GFR using the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation: more accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis. 2010;55:622–627. doi: 10.1053/j.ajkd.2010.02.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Porrini E., Ruggenenti P., Mogensen C.E., et al. ERA-EDTA diabesity working group. Non-proteinuric pathways in loss of renal function in patients with type 2 diabetes. Lancet Diabetes Endocrinol. 2015;3:382–391. doi: 10.1016/S2213-8587(15)00094-7. [DOI] [PubMed] [Google Scholar]
- 29.Shimizu M., Furuichi K., Toyama T., et al. Long-term outcomes of Japanese type 2 diabetic patients with biopsy-proven diabetic nephropathy. Diabetes Care. 2013;36:3655–3662. doi: 10.2337/dc13-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamanouchi M., Furuichi K., Hoshino J., et al. Nonproteinuric versus proteinuric phenotypes in diabetic kidney disease: a propensity score-matched analysis of a nationwide, biopsy-based cohort study. Diabetes Care. 2019;42:891–902. doi: 10.2337/dc18-1320. [DOI] [PubMed] [Google Scholar]
- 31.Ying T., Clayton P., Naresh C., Chadban S. Predictive value of spot versus 24-hour measures of proteinuria for death, end-stage kidney disease or chronic kidney disease progression. BMC Nephrol. 2018;19:55. doi: 10.1186/s12882-018-0853-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perkovic V., Jardine M.J., Neal B., et al. Canagliflozin and renal outcomes in Type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295–2306. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 33.Heerspink H.J.L., Stefánsson B.V., Correa-Rotter R., et al. Committees and investigators. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383:1436–1446. doi: 10.1056/NEJMoa2024816. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.