Abstract
Attachment of an array of enteric pathogens to epithelial surfaces is accompanied by recruitment of polymorphonuclear leukocytes (PMN) across the intestinal epithelium. In this report, we examine how Shigella-intestinal epithelium interactions evoke the mucosal inflammatory response. We modeled these interactions in vitro by using polarized monolayers of the human intestinal epithelial cell line, T84, isolated human PMNs, and Shigella flexneri. We show that Shigella attachment to T84-cell basolateral membranes was a necessary component in the signaling cascade for induction of basolateral-to-apical directed transepithelial PMN migration, the direction of PMN transepithelial migration in vivo. In contrast, attachment of Shigella to the T84-cell apical membrane failed to stimulate a directed PMN transepithelial migration response. Importantly, the ability of Shigella to induce PMN migration across epithelial monolayers was dependent on the presence of the 220-kb virulence plasmid. Moreover, examination of Shigella genes necessary to signal subepithelial neutrophils established the requirement of a functional type III secretion system. Our results indicate that the ability of Shigella to elicit transepithelial signaling to neutrophils from the basolateral membrane of epithelial cells represents a mechanism involved in Shigella-elicited enteritis in humans.
The intestinal mucosa is routinely exposed to a wide range of microorganisms and foreign substances and provides both a physical and an immunological barrier to such challenges from the exterior environment. Epithelial cells in the gastrointestinal tract were classically thought to serve the dual purpose of regulating salt, water, and nutrient transport and of providing a barrier to passive diffusion of molecules between the intestinal lumen and tissue space (9, 14). However, it is becoming clear that interactions between intestinal epithelial cells and bacteria may play a role in orchestrating the inflammatory response. For example, attachment of an array of bacterial pathogens, including Salmonella and Shigella, to epithelial cell surfaces is accompanied by recruitment of host defense cells exhibited by transepithelial migration of polymorphonuclear leukocytes (PMN) in the basolateral-to-apical direction. Such transmigration of PMN across intestinal epithelia represents the histological definition of acute intestinal inflammation and is a hallmark of bacterial enterocolitis caused by enteric pathogens such as Salmonella (8, 20, 31, 44, 52) and Shigella (5).
The details of how such host-pathogen interactions evoke the classical histological lesion of PMN transepithelial migration are currently being studied. It is becoming increasing clear that Salmonella-intestinal apical epithelial cell contacts result in the generation of a signaling cascade which directs the trafficking of PMN in the basolateral-to-apical direction (28–30). Salmonella typhimurium induces the intestinal epithelium to secrete a repertoire of chemokines which play an active role in recruiting PMN from the peripheral circulation and directing them across the epithelium to the intestinal lumen (28–30). Such epithelial orchestration of PMN movement is thought to be mediated by polarized secretion of distinct chemokines and neutrophil chemoattractants (29, 30). Interleukin-8, for example, is secreted basolaterally by intestinal epithelial cell lines in vitro (10, 29, 30), as well as in the human colon (19), in response to either proinflammatory cytokines or invasive bacteria. Such secretion leads to gradients of interleukin-8 being formed in the subepithelial extracellular matrix and is largely responsible for the movement of PMN through the extracellular matrices of model epithelia (28). However, to establish gradients which would direct PMN to migrate across the epithelium to the apical surface, these chemokines must be preferentially secreted apically. Recently, the first such chemokine has been described: pathogen-elicited epithelial chemoattractant (30).
The mechanisms which underlie PMN transepithelial migration induced by Shigella-host interactions are not as well characterized, owing, at least in part, to the requirement of Shigella entry into the basolateral domain of the intestinal epithelium. A recent investigation by Perdomo et al. described the ability of S. flexneri to induce PMN transmigration through a confluent epithelial cell monolayer, with the implication being that transmigrating PMN play an active role at early stages of epithelial cell invasion by opening the paracellular pathway for bacterial entry into differentiating colonocytes (42). However, a paradoxical result of their study was the observation that wild-type and avirulent (noninvasive) S. flexneri strains demonstrated equal abilities to induce PMN transepithelial migration across intestinal epithelial cell monolayers.
In the present study, we took a different approach and asked whether positioning Shigella adjacent to the basolateral epithelial membrane domain of their target tissue (i.e., the intestinal epithelial surface through which Shigella invades) promoted the generation of discrete signals necessary to evoke directed migration of PMN across the intestinal monolayer in the basolateral-to-apical direction, analogous to the direction of PMN movement across the intestinal epithelium during active states of inflammation. These studies were performed with Shigella flexneri strains and human peripheral blood PMN in association with polarized monolayers of the human-derived, physiologically confluent, crypt-like cell line T84 to model pathogen-induced intestinal inflammation. We report that model intestinal epithelia respond to basolateral membrane-Shigella interactions by promoting signals essential to drive PMN transepithelial migration in the biologically relevant basolateral-to-apical direction. This signaling response exhibited a strict dependence on Shigella contact with the epithelial basolateral membrane domain, required genes present on the 220-kb large virulence plasmid, and was dependent upon a functional Shigella type III secretion apparatus.
MATERIALS AND METHODS
Cell culture.
T84 intestinal epithelial cells (passages 70 to 95) were grown in a 1:1 mixture of Dulbecco-Vogt-modified Eagle medium and Ham’s F-12 medium supplemented with 15 mM HEPES buffer (pH 7.5), 14 mM NaHCO3, 40 mg of penicillin per ml, 8 mg of ampicillin per ml, 90 mg of streptomycin per ml, and 5% newborn calf serum (22, 36, 39, 40). Monolayers were grown on 0.33-cm2 suspended collagen-coated permeable polycarbonate filters (Costar Corp., Cambridge, Mass.) and used 7 to 14 days after plating, as described previously (22, 39). A steady-state transepithelial cell resistance, approximately 1,500 ohm · cm2, is reached in 5 days, with variability largely related to cell passage number. Monolayers received one weekly feeding following initial plating. Inverted monolayers used to study the transmigration of neutrophils in the physiological basolateral-to-apical direction were constructed as described previously (22, 36, 39, 40).
Bacterial strains and growth conditions.
The bacterial strains used in this study are listed in Table 1.
TABLE 1.
Bacterial strains used in this study and their relevant virulence properties
| Straina | Description | Invasion of HeLa cells | Type III secretion | Refer- ence |
|---|---|---|---|---|
| 2457T | Wild type | + | + | 12 |
| 2457O | 2457T virF::IS2 | − | − | 34 |
| BS103 | 2457T cured of the virulence plasmid | − | − | 27 |
| BS228 | 2457T ipaB::λplac Mu53 | − | +b | 18 |
| BS232 | 2457T mxi::lacZ | − | − | 18 |
| BS545 | 2457T spa33::aphA3 | − | − | 49 |
| BS547 | 2457T mxiM::aphA3 | − | − | 50 |
| M90T | Wild type | + | + | 47 |
| BS176 | M90T cured of the virulence plasmid | − | − | 48 |
All strains are derivatives of S. flexneri 2a except M90T and BS176, which are strains of S. flexneri 5.
Insertion in BS228 results in synthesis of a truncated IpaB product and is polar on the downstream ipa genes; i.e., IpaC, IpaD, and IpaA are not synthesized.
Bacteria were routinely grown at 37°C in tryptic soy broth (TSB; Difco Laboratories, Detroit, Mich.). A 100-μl volume of a stationary-phase culture was used to inoculate 10 ml of TSB, and the bacteria were grown in a shaking incubator for approximately 2 h at 37°C to the mid-exponential phase of growth (optical density at 600 nm of 0.30). Tryptic soy agar is TSB containing 12 g of Bacto Agar (Difco) per liter and 0.025% Congo Red (Sigma Chemical Co., St. Louis, Mo.).
Electrical measurements.
To assess currents, transepithelial potentials, and resistance, a commercial voltage clamp (Bioengineering Department, University of Iowa) was used and interfaced with an equilibrated pair of calomel electrodes submerged in saturated KCl along with a pair of Ag-AgCl electrodes submerged in Hanks balanced salt solution containing Ca2+ and Mg2+ [HBSS(+)]. Agar bridges were used to interface the electrode with the solution on either side of the monolayers (one calomel and one Ag-AgCl electrode in each well), and the short-circuit current and resistance were measured as detailed elsewhere (24).
Shigella invasion into T84 intestinal epithelial cell monolayers.
T84 monolayers were infected by the method of McCormick with slight modifications (29). Inverted monolayers were drained of media and gently washed with HBSS(+) containing 10 mM HEPES (pH 7.4; Sigma). Bacterial samples representing an inoculation ratio of 20 bacteria/epithelial cell were added to the basolateral side of the monolayer, and bacterial invasion was assessed after incubation at 37°C for 90 min. Cell-associated bacteria included populations of bacteria adherent to and/or internalized into the T84 monolayers and were released by incubation with 1% Triton X-100 (Sigma). Internalized bacteria were those obtained from lysis of the epithelial cells with 1% Triton X-100 after the addition of gentamicin (50 μg/ml). Gentamicin, an aminoglycoside antibiotic, does not permeate eukaryotic plasma membranes and is therefore cytolytic only to extracellular populations of bacteria, while intracellular bacteria populations remain viable. For both cell-associated and internalized bacteria, 0.9 ml of Luria-Bertani broth was then added and each sample was vigorously mixed and quantitated by plating for CFU on MacConkey agar medium.
PMN transepithelial migration assay.
The PMN transepithelial migration assay has been detailed previously (16, 39, 40). Human PMN were purified from whole blood (anticoagulated with 13.2 g of citrate and 11.2 g of dextrose in 500 ml of water [pH 6.5]) collected by venipuncture from normal human volunteers of both sexes. The buffy coat was obtained by centrifugation at 400 × g at room temperature. Plasma and mononuclear cells were removed by aspiration, and the majority of erythrocytes were removed by a 2% gelatin sedimentation technique as described previously (40). Residual erythrocytes were then removed by gentle lysis in cold NH4Cl lysis buffer. This technique allowed the rapid isolation (90 min) of functionally active PMN that were 95% pure with 98% viability as determined by trypan blue exclusion. After isolation, PMN were suspended in modified HBSS (without Ca2+ and Mg2+ but with 10 mM HEPES [pH 7.4]) at 4°C at a concentration of 5 × 107/ml and were used for experiments within 1 h after isolation.
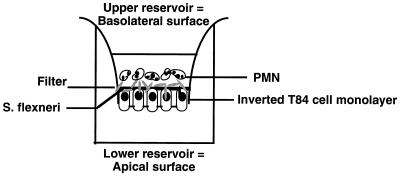
Shigella-epithelial cell-PMN interactions are depicted in Fig. 1. Briefly, before the addition of PMN to this assay system, confluent, inverted T84 polarized monolayers (3.5 × 105 cells/well) (22, 40) were rinsed extensively in HBSS(+) to remove residual serum components. Shigella strains were prepared by washing the bacteria twice in HBSS(+) and resuspending them in 300 μl of buffer per 10 ml of culture (final bacterial concentration, approximately 1.5 × 109 bacteria per ml). For basolateral surface exposure, 25 μl of the bacterial suspension (3.5 × 107 bacteria) was directly added to the upper compartment of inverted T84 monolayers, at a multiplicity of infection of 100 bacteria/epithelial cell, after removal of the basolateral buffer. In studies requiring apical surface exposure, inverted monolayers were removed from each well and placed in a moist chamber such that the epithelial apical membrane (lower compartment) was oriented upward. Again, 25 μl of the bacterial suspension was gently distributed onto the apical surface. For simplicity, the reservoir is referred to by the epithelial membrane domain with which it interfaces (i.e., apical or basolateral). Shigella strains were incubated at either the basolateral or apical epithelial interface for 90 min at 37°C. Nonadherent bacteria were next removed by washing three times in HBSS(+) buffer, and under these conditions it was determined that there were 80 cell-associated bacteria/epithelial cell. The monolayers were then transferred into fresh 24-well tissue culture trays containing 1.0 ml of HBSS buffer in the bottom (apical) compartment and 140 μl in the top (basolateral) compartment. To the basolateral bath, 40 μl of isolated PMN (106 cells) was added to each monolayer and incubated for 150 min at 37°C. Positive control transmigration assays were performed by the addition of chemoattractant (1 μM N-formylmethionylleucyl phenylalanine [fMLP]; Sigma) to the opposing apical reservoir. All experiments were performed in a room whose temperature was 37°C to ensure that the epithelial monolayers, solutions, and plastic ware were maintained at a uniform temperature.
FIG. 1.
Shigella-induced PMN transepithelial migration assay. In this assay, the basolateral surface of inverted T84 monolayers are first colonized by S. flexneri and then washed so that only the cell-associated population remains (cell-adherent plus cell-internalized bacteria). PMN are then placed in the upper (basolateral) reservoir, where they come in contact with the basolateral surface and are subsequently judged for their ability to migrate across these inverted T84 monolayers.
Transmigration was quantified by assaying for the PMN azurophilic granule marker myeloperoxidase, as described previously (22, 39, 40). After each transmigration assay, PMN cell equivalents (CE) were assessed as the number of PMN that had completely traversed the monolayer (i.e., moved into the apical reservoir). Since variation exists in transepithelial cell resistance between groups of monolayers (baseline resistance, 650 to 1,500 ohm · cm2) and in PMN obtained from different donors, individual experiments were performed with large numbers of monolayers and PMN obtained from single blood donors. PMN isolation was restricted to 10 different donors (repetitive donations) over the course of these studies.
Preparation of S. flexneri culture supernatants.
Aerobically grown cultures of S. flexneri at 37°C in TSB (prepared as above) were washed twice in HBSS(+) and resuspended to a final concentration of approximately 5 × 108 cells/ml. After an incubation for 1.5 h at 37°C, the suspensions were centrifuged free of bacteria (6,000 × g for 10 min) and the Shigella conditioned buffer supernatant was collected and passed through a 0.2-μm-pore-size filter. The supernatant was then applied to the basolateral surface of epithelial cell monolayers and assessed for the ability to induce PMN transepithelial migration.
Presentation of data.
PMN transmigration results are represented as PMN CE derived from a daily standard PMN dilution curve. Reservoir-associated PMN (i.e., PMN which had completely traversed the monolayer) are represented as the number of PMN CE per milliliter (total volume, 1 ml). Values are expressed as means and standard deviation (SD) of individual experiments done in triplicate n times. Shigella invasion and myeloperoxidase assay data were compared by Student’s t test.
RESULTS
Basolateral colonization by S. flexneri initiates basolateral-to-apical directed transepithelial migration of PMN.
S. flexneri was initially examined for its ability to adhere to and be internalized by polarized T84 intestinal epithelial cell monolayers. An experiment in which an original inoculum of 20 bacteria/epithelial cell was placed on either the apical or basolateral membrane of polarized T84-cell monolayers for 90 min demonstrated that S. flexneri preferentially entered epithelial cells via the basolateral membrane domain (1.23% ± 0.11% and 0.087% ± 0.004% of the original inoculum was internalized for basolateral and apical membrane association, respectively, of wild-type strain 2457T). These results confirm those of previous investigators (35, 41). The effects of such bacterium-epithelial cell interactions on T84 transepithelial cell resistance were also determined. Transepithelial cell resistance to passive ion flow is an extremely sensitive measure of barrier function in high-resistance epithelia such as T84 monolayers (21). Due to the asymptotic flux-resistance relationship, perturbations so minimal that they induce barely detectable increases in the transepithelial flux of inert solutes routinely elicit sizable decrements in resistance in these high-resistance monolayers (15, 23). To gain insight into the epithelial barrier function integrity during S. flexneri colonization, we investigated the stability of transepithelial cell resistances over time. Transepithelial cell resistance at a final colonization density of 80 cell-associated bacteria/epithelial cell remained high throughout a 4-h colonization period at 37°C. They were observed to fall only after 4 h of bacterial colonization, which also corresponded to a similar fall in resistance from control noncolonized epithelial monolayers. However, physiological confluency was maintained even under these conditions (i.e., resistances in substantial excess of 250 ohm · cm2 [15, 23]). Thus, since the colonized monolayers maintained an appropriate barrier function throughout a 4-h time course, these specific conditions utilizing 7- to 9-day-old monolayers were used for all neutrophil experiments reported below.
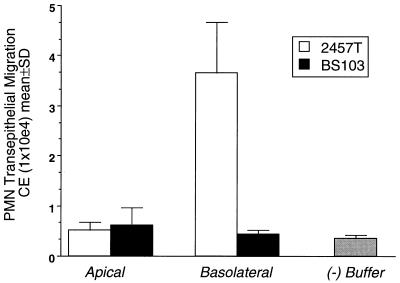
Since recent evidence (35, 41) and our own results (see above) strongly suggest that S. flexneri preferentially enters the intestinal epithelia via the basolateral membrane domain, we investigated whether such basolateral exposure by S. flexneri was a key requirement for induction of the signaling pathway governing PMN transmigration. As shown in Fig. 2, we found that only basolateral colonization of T84 monolayers with S. flexneri elicited a marked neutrophil transepithelial migration (i.e., PMN which had completely traversed the monolayer) response in the basolateral-to-apical direction. In sharp contrast, comparable apical exposure to S. flexneri failed to stimulate detectable PMN transepithelial migration. These data suggest that only S. flexneri exposed to the basolateral surface, rather than to the apical surface, of intestinal epithelia initiate the signals required for PMN transepithelial migration.
FIG. 2.
S. flexneri-T84-cell basolateral membrane association induces PMN transepithelial migration. Polarized monolayers of T84 intestinal epithelial cells were either apically or basolaterally exposed to wild-type S. flexneri 2457T (open bars) or a noninvasive plasmid-cured strain BS103 (solid bars) at a density of 80 cell-associated bacteria/epithelial cell. The ability of S. flexneri to induce PMN transepithelial migration was assessed 90 min later (see Materials and Methods). The negative control (−) (gray bar) represents HBSS(+) buffer in the absence of bacteria or a chemotactic stimulus. A positive control was established by using imposed gradients to the chemotactic peptide fMLP (10−7 M) ([15.12 ± 2.36] × 104 CE). Data are means and SD for four monolayers in a single experiment and are representative of four separate experiments.
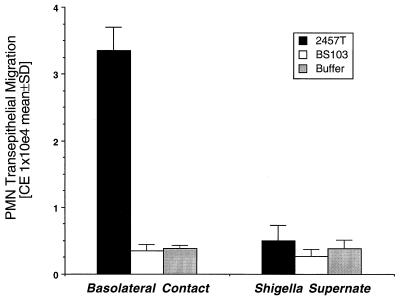
Thus, having established that association of S. flexneri with the epithelial basolateral membrane domain is an important determinant for eliciting PMN transepithelial migration, we investigated whether epithelial cell contact with or simply basolateral exposure to soluble S. flexneri products was essential for induction of PMN transepithelial migration. As shown in Fig. 3, exposure of the basolateral membrane to S. flexneri products under conditions which prevented direct Shigella-epithelial cell contact was ineffective in inducing PMN transepithelial migration. Thus, in the absence of direct bacterium-basolateral membrane contact, PMN transepithelial migration was reduced about 10-fold.
FIG. 3.
Effect of S. flexneri-intestinal epithelial cell contact on the ability to induce PMN transepithelial migration. Shigella-induced PMN transepithelial migration is compared to conditions in which epithelia were exposed to Shigella soluble products at the basolateral membrane interface but in which no direct Shigella-epithelial cell basolateral membrane contact existed. Results are expressed as the mean and SD of triplicate values for each condition and are representative of one of three experiments showing similar results.
S. flexneri-induced PMN transepithelial migration requires the Shigella virulence plasmid.
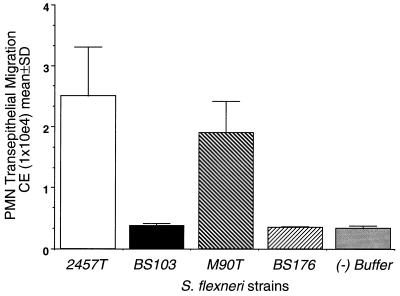
The virulence of Shigella spp. requires expression of genes present on the chromosome, as well as the large virulence plasmid (220 kb), which harbors all of the genes required for invasion (26). We next sought evidence to determine whether the ability to induce the signaling cascade(s) which mediates PMN trafficking across intestinal epithelial cell monolayers is dependent on the presence of the Shigella virulence plasmid. Wild-type S. flexneri (2457T) was compared to its plasmid-cured avirulent derivative (BS103) for the ability to initiate the transepithelial signals required for directing PMN migration in the basolateral-to-apical direction. As shown in Fig. 2, exposure of S. flexneri 2457T to only the basolateral surface specifically induced a directed subepithelial cell-to-lumen PMN transmigration response, while the avirulent strain BS103 failed to elicit PMN transepithelial migration when interfaced with either epithelial membrane domain, despite normal attachment. To address the possibility that such results are strain dependent, we surveyed an additional isogenic pair of S. flexneri strains, wild type (M90T) and plasmid cured (BS176), for their ability to induce transepithelial signaling to PMN. Consistent with the results observed above, we found that only the wild-type strain (M90T) was able to specifically elicit the necessary signal pathway to subepithelial PMN whereas the plasmid-cured avirulent strain (BS176) failed to generate transepithelial signaling to PMN (Fig. 4). These data strongly indicate that the ability to induce PMN signals is dependent on genes on the Shigella virulence plasmid since the loss of the virulence plasmid correlated with the loss of PMN signaling.
FIG. 4.
Comparison of isogenic pairs of plasmid-carrying and plasmid-cured strains of S. flexneri. The negative control (−) (gray bar) represents PMN transmigration to HBSS(+) buffer in the absence of bacteria or a chemotactic stimulus. A positive control was established as described in the legend to Fig. 2 ([30.72 ± 5.32] × 104 CE). Data are means and SD for four monolayers in a single experiment and are representative of four separate experiments, all showing the same result.
Basolateral access is required for S. flexneri to induce PMN transepithelial migration.
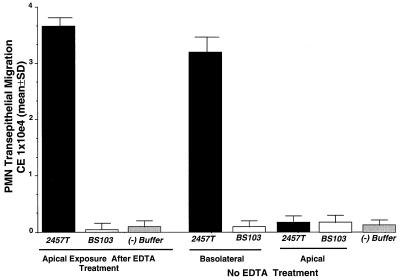
We next sought to investigate whether pathophysiological events which lead to perturbations of epithelial tight junctions could facilitate the generation of signals required for physiologically directed PMN transepithelial migration. Thus, to permit luminal (apical) Shigella access to the basolateral membrane, confluent T84-cell monolayers were transiently perturbed by extracellular Ca2+ depletion (38). Such treatment disrupts intercellular tight junctions, resulting in epithelial cell depolarization while preserving the columnar architecture of the intestinal epithelial cells and permitting access of luminally applied bacteria to basolateral surface ligands (38). As shown in Fig. 5, only under conditions where epithelial tight junctions were perturbed did apical epithelial exposure of wild-type Shigella induce neutrophil transepithelial migration in the basolateral-to-apical direction. In contrast, noninvasive Shigella remained unable to induce PMN transepithelial migration whether or not intercellular tight junctions were perturbed. These data suggest that during events which lead to the disruption of epithelial tight junctions, luminally restricted Shigella is able to gain access to the basolateral epithelial membrane domain required for cell entry and evoke the generation of signals essential for eliciting PMN transepithelial migration.
FIG. 5.
Effects of EDTA treatment on the ability of apically associated S. flexneri to induce PMN transepithelial migration. Diffuse disruption of tight junctions was obtained by means of a brief EDTA exposure. The negative control (−) represents PMN transmigration to HBSS(+) buffer in the absence of bacteria or a chemotactic stimulus. A positive control was established as described in the legend to Fig. 2 ([33.34 ± 4.21] × 104 CE). Data are means and SD for four monolayers in a single experiment and are representative of four separate experiments, all showing the same result.
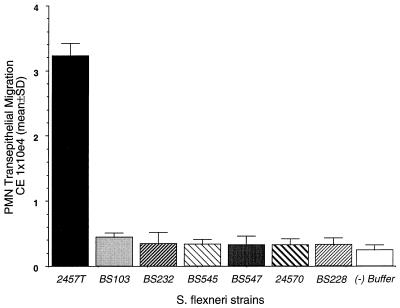
The ability of Shigella to induce signals to PMN requires a functional type III secretion system and/or invasion.
The invasive capacity of Shigella depends upon proteins encoded by three contiguous operons (ipa, mxi, and spa) in a 31-kb region on the virulence plasmid. The ipa locus encodes a set of secreted proteins (Ipa) which are effectors of the entry process (17, 33), while the mxi and spa loci encode the specialized type III secretion apparatus for export of Ipa proteins (1–4, 54). Having established that the Shigella virulence plasmid is necessary to promote signaling to PMN, we investigated whether Shigella invasion and/or the functional type III secretion apparatus is a key component in generating PMN transepithelial signal cascades. Thus, to examine the mechanisms by which S. flexneri induces PMN transepithelial migration, we examined the ability of S. flexneri mutants to induce signals to subepithelial PMN. All five mutants tested were unable to synthesize different proteins required for invasion into epithelial cells. As shown in Fig. 6, basolateral exposure of strains BS232, BS545, and BS547, which harbor independent mutations in the type III secretion apparatus (Table 1), failed to elicit PMN transepithelial migration. BS228, a noninvasive mutant that is secretion competent but synthesizes only a truncated form of IpaB and no IpaD, IpaC, or IpaA, does not elicit PMN transepithelial signaling. Likewise, 2457O, a virF mutant which does not make Ipa proteins or the Mxi-Spa secretion apparatus, failed to generate transepithelial signals required for directed PMN transepithelial migration. It is important to note that although S. flexneri BS228 is not able to invade epithelial cells, it adheres to epithelial cells to a greater extent than 2457T does (data not shown), yet BS228 failed to induce PMN transepithelial migration. These data suggest that failure of BS228 and other mutant strains of Shigella to induce PMN transepithelial migration is not due to the inability of these strains to attach to the epithelial basolateral surface. Therefore, while basolateral exposure of wild-type strain 2457T induced a directed basolateral-to-apical PMN transmigration response, exposure to any one of the invasion-defective strains, irrespective of the mutation, failed to elicit PMN transepithelial migration. These data are consistent with our earlier observation that avirulent, plasmid-cured S. flexneri strains do not initiate the signals important for inducing the PMN transepithelial migration response.
FIG. 6.
The ability of S. flexneri to induce PMN transepithelial migration is dependent upon a functional type III secretion apparatus. The negative control (−) (open bar) represents PMN transmigration to HBSS(+) buffer in the absence of bacteria or a chemotactic stimulus. A positive control was established as described in the legend to Fig. 2 ([15.21 ± 2.72] × 104 CE). Data are means and SD for four monolayers in a single experiment and are representative of four separate experiments, all showing the same result.
DISCUSSION
Shigella species are the causative agents of bacillary dysentery, a disease characterized by bacterial invasion of and multiplication within human colonic epithelial cells (5, 45). Shigella binding to epithelial cells engages a complex signaling cascade which includes bacterium-mediated endocytosis, lysis of the membrane-bound phagocytic vacuole, growth of the bacteria within the cytoplasmic compartment, and intercellular spreading by using the host cytoskeleton as a motor (6, 13, 25, 45). Moreover, recent studies have indicated that in both in vitro (35) and in vivo (41) models of Shigella invasion of intestinal epithelium, invasion occurs preferentially from the basolateral epithelial membrane domain. Hence, the ability of invasive bacteria to reach the basolateral surface of enterocytes seems to be a crucial step in Shigella pathogenesis and is consistent with the observation that invading Shigella bacteria which reach the underlying lamina propria evoke an intense inflammatory response (11). Therefore, we sought to determine whether interfacing Shigella with the basolateral epithelial membrane domain of polarized intestinal T84 epithelial cells (i.e., the same surface mediating Shigella invasion) promoted the generation of discrete signals necessary to elicit PMN transepithelial migration. These findings identify three integral components of Shigella pathogenesis essential for the initiation of signals required for the movement of PMN across cultured intestinal epithelial monolayers. First, this signaling response to subepithelial PMN exhibited a strict dependence on Shigella contact with the epithelial basolateral membrane domain. Second, such signaling required the participation of the 220-kb virulence plasmid. Third, the ability of Shigella to induce signals involved in the transepithelial migration of PMN requires a functional type III secretion apparatus in which Ipa proteins are essential.
Little is known about the nature of Shigella-induced signaling cascades and protein(s) directly involved in evoking the transepithelial signal to neutrophils during active states of enterocolitis. A relevant paradigm is the concept that epithelial orchestration of PMN movement induced by Salmonella typhimurium is mediated by polarized secretion of distinct chemokines (28–30). After apical epithelial cell-S. typhimurium contact, the intestinal epithelium secretes chemokines which play an essential role in recruiting PMN from the peripheral circulation and directing them to migrate across the epithelium to the intestinal lumen (28–30). Unlike S. typhimurium, the capacity of S. flexneri to reach the basolateral surface of enterocytes is an essential step in Shigella pathogenesis. How S. flexneri penetrates the intestinal mucosa to reach the basolateral surface of the epithelium has been an area of intense interest. One possibility is that Shigella bacteria interact with specialized cells, termed M cells, which lie over the Peyer patches (41, 46, 55). These cells function to continually endocytose macromolecules as well as microorganisms from the intestinal lumen. Thus, as a result of M-cell-mediated entry, Shigella bacteria are able to gain access to the basolateral epithelial cell domain. Consistent with these observations, our studies demonstrate that only wild-type S. flexneri strains, when interfaced at the basolateral epithelial membrane domain of intestinal epithelial cell monolayers, generated the appropriate set of signals to drive PMN across the intestinal epithelium. Given that the critical step in Shigella pathogenesis is the ability of the organism to access the basolateral surface of enterocytes, it is not surprising that we were unable to detect measurable amounts of PMN transmigration on exposure of Shigella bacteria to the apical epithelial membrane domain. Our results, however, are in contrast to the findings of Perdomo et al. (42), who reported that apical surface contact by wild-type and plasmid-cured strains of S. flexneri showed an equal ability to induce PMN to transmigrate through a confluent epithelial cell monolayer.
Since only virulent S. flexneri could induce PMN transepithelial migration, we were able to examine Shigella-elicited factors which might drive this response. Notably, we found that Shigella-induced PMN transmigration is dependent upon the presence of the 220-kb virulence plasmid. Strains cured of this plasmid are avirulent and noninvasive and, as we demonstrate in this study, fail to induce signaling to subepithelial PMN. Since Perdomo et al. (42) could not detect this difference in behavior between virulent and avirulent S. flexneri strains, our data reveal a previously unrecognized phenotype associated with Shigella virulence (i.e., the ability to induce PMN transepithelial migration). Such conflicting results cannot be attributed to strain differences. We used the same isogenic pair of plasmid-containing and plasmid-cured strains (M90T and BS176, respectively) as Perdomo et al. (42) and found no PMN induction with the plasmid-cured strain. However, there are two principal differences between our investigation and that by Perdomo et al. First our studies assessed Shigella-epithelial cell interactions from the basolateral membrane domain rather than the apical membrane domain. Given that the entry of S. flexneri into the intestinal barrier occurs via the M cells of follicle-associated epithelium and given that the ability of invasive Shigella to reach the basolateral surface of enterocytes seems to be a crucial step in Shigella pathogenesis, we reasoned, and show in this report, that Shigella-basolateral epithelial cell interactions have a profound effect on the ability of the bacteria to induce an inflammatory response. Second, the interpretation of PMN transepithelial migration differs between our study and that of Perdomo et al. (42). In the gastrointestinal tract, active inflammatory disease characterized by migration of neutrophils across the epithelial lining is a hallmark of both chronic and self-limited diseases (20, 58). To reach the epithelial surface, PMN must travel out of the vascular blood where they encounter matrix components, basement membrane, and finally the epithelium. Still, to cross the intestinal epithelium, PMN must traverse the paracellular space, impale epithelial tight junctions, and move into the luminal compartment (20, 37, 43, 51, 58), where they can interact with the apical epithelial membrane. The sum of these events results in crypt abscesses. In patients with active inflammation, the degree of PMN transepithelial migration correlates with the severity of symptoms (20, 58). Therefore, we define PMN transepithelial migration according to this histopathological definition as those PMN which have completely traversed the monolayer, impaled epithelial tight junctions, and are thus located in the apical epithelial cell compartment. In contrast, Perdomo et al. measured PMN which were associated only with the monolayer (filter) at the basolateral pole, since the number of PMN that traversed the monolayer and appeared in the apical reservoir was too small to be detected and thus largely represents PMN which have migrated only into the monolayer but have not yet crossed the tight junction and hence are trapped in the paracellular and subepithelial spaces (20, 39, 40, 58).
Genes in the Shigella ipa operon (ipaBCD) play crucial roles in the invasion of epithelial cells by Shigella. Mutants unable to synthesize the Ipa proteins not only are incapable of eliciting rearrangement of the actin cytoskeleton around bacterial attachment sites on epithelial cells but also are incapable of disrupting the phagocytic vacuoles surrounding invading bacteria. Secretion of Ipa invasins into the bacterial environment is mediated by the Mxi and Spa proteins (1–4, 54), which form a type III protein secretion system (53). Moreover, secretion of Ipa invasins from Shigella occurs more efficiently upon contact with the basolateral surface of polarized intestinal epithelial cells (32, 57). Although plasmid-cured strains of Shigella failed to elicit transepithelial signaling to PMN, it was not clear whether this signaling event was dependent on the genes in the contiguous ipa, mxi, and spa operons. We show that Shigella strains which either harbor independent mutations in the type III secretion apparatus or fail to make Ipa proteins were unable to initiate the signals required for directed PMN transepithelial migration. Collectively, these observations strongly suggest that transepithelial signaling to PMN is a central virulence mechanism for Shigella-elicited enteritis and depends on plasmid-located genes involved in tissue invasion and secretion of bacterial proteins that mediate invasion. Future experiments should resolve the respective contributions of invasion and type III secretion dependence, imperative for the induction of signals involved in PMN transepithelial migration.
This work identifies a novel Shigella virulence mechanism. We demonstrate that upon S. flexneri-basolateral epithelial cell interactions, basolateral-to-apical directed PMN transepithelial migration ensues, reflecting events relevant to those which occur in the human intestine during acute stages of inflammation. We have previously developed an in vitro model of PMN-intestinal epithelium interactions to investigate the influence of contact of S. typhimurium with intestinal epithelial apical membranes on the subsequent inflammatory response. While we do not completely understand how Salmonella and Shigella induce inflammatory responses, if differences exist they may be because the host cellular events and the signals elicited upon infection by these microorganisms are distinct. For example, Shigella entry into epithelial cells occurs from the basolateral pole and appears to be mediated by the small GTPase rho (56) while Salmonella entry occurs predominantly from the apical pole and is mediated by another GTPase, CDC42 (7). What is clear, however, is that interactions between intestinal epithelial cells and enteric pathogens play a key role in orchestrating the inflammatory response. Significantly, future studies will add to our understanding of the molecular mechanisms important in the regulation of active inflammation characterized by shigellosis and salmonellosis. Perhaps by studying Shigella we will add to our understanding of Salmonella inflammation and vice versa. Furthermore, the most interesting studies may be those that show differences between these important bacterial pathogens.
ACKNOWLEDGMENTS
We thank James L. Madara for critical review of the manuscript.
These studies were supported by NIH grants DK50989 to B.A.M. and AI24656 to A.T.M.
REFERENCES
- 1.Allaoui A, Sansonetti P J, Parsot C. MxiD, an outer membrane protein necessary for the secretion of the Shigella flexneri Ipa invasins. Mol Microbiol. 1993;7:59–68. doi: 10.1111/j.1365-2958.1993.tb01097.x. [DOI] [PubMed] [Google Scholar]
- 2.Allaoui A, Sansonetti P J, Parsot C. MxiJ, a lipoprotein involved in secretion of Shigella Ipa invasins, is homologous to YscJ, a secretion factor of the Yersinia YOP proteins. J Bacteriol. 1992;174:7661–7669. doi: 10.1128/jb.174.23.7661-7669.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrews G P, Hromockyj A E, Coker C, Maurelli A T. Two novel virulence loci, mxiA and mxiB, in Shigella flexneri 2a facilitate secretion of invasion plasmid antigens. Infect Immun. 1991;59:1997–2005. doi: 10.1128/iai.59.6.1997-2005.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andrews G P, Maurelli A T. MxiA of Shigella flexneri 2a, which facilitates export of invasion plasmid antigens, encodes a homolog of the low calcium response protein, LcrD, of Yersinia pestis. Infect Immun. 1992;60:3287–3295. doi: 10.1128/iai.60.8.3287-3295.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bennish, M. L. 1991. Potentially lethal complications of shigellosis. Rev. Infect. Dis. 13(Suppl. 4):319–324. [DOI] [PubMed]
- 6.Bernardini M L, Mounier J, d’Hauteville H, Coquis-Rondon M, Sansonetti P J. Identification of IcsA a plasmid locus of Shigella flexneri that governs bacterial intra- and intercellular spread through interaction of F-actin. Proc Natl Acad Sci USA. 1989;86:3867–3871. doi: 10.1073/pnas.86.10.3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen L, Hobbie S, Galan J. Requirement of CDC42 for Salmonella-induced cytoskeletal and nuclear responses. Science. 1996;274:2115–2118. doi: 10.1126/science.274.5295.2115. [DOI] [PubMed] [Google Scholar]
- 8.Day D W, Mandell B K, Morrson B C. The rectal biopsy appearances in Salmonella colitis. Histopathology. 1978;2:117–131. doi: 10.1111/j.1365-2559.1978.tb01700.x. [DOI] [PubMed] [Google Scholar]
- 9.Diamond J. Twenty-first Bowditch lecture. The epithelial junction: bridge, gate, and fence. Physiologist. 1977;20:10–18. [PubMed] [Google Scholar]
- 10.Eckmann L, Kagnoff M, Fierer J. Epithelial cells secrete the chemokine interleukin-8 in response to bacterial entry. Infect Immun. 1993;61:4569–4574. doi: 10.1128/iai.61.11.4569-4574.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Falkow S, Mekalanos J. Enteric bacilli. In: Davis B D, Dulbecco R, Eisen H N, Ginsberg H S, editors. Microbiology. 4th ed. Philadelphia, Pa: J. B. Lippincott Co.; 1990. pp. 574–576. [Google Scholar]
- 12.Formal S B, Dammin G J, LaBrec E H, Schneider H. Experimental Shigella infections: characteristics of a fatal infection produced in guinea pigs. J Bacteriol. 1958;75:604–610. doi: 10.1128/jb.75.5.604-610.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldberg M B, Sansonetti P J. Shigella subversion of the cellular cytoskeleton: a strategy for epithelial colonization. Infect Immun. 1993;61:4941–4946. doi: 10.1128/iai.61.12.4941-4946.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gumbiner B. The structure, biochemistry, and assembly of epithelial TJs. Am J Physiol. 1987;253:C749–C758. doi: 10.1152/ajpcell.1987.253.6.C749. [DOI] [PubMed] [Google Scholar]
- 15.Hecht G, Pothoulakis C, Lamont J T, Madara J L. Clostridium difficle toxin A perturbs cytoskeletal structure and tight junction permeability of cultured human intestinal epithelial monolayers. J Clin Invest. 1988;82:1516–1524. doi: 10.1172/JCI113760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henson P, Oades Z G. Stimulation of human neutrophils by soluble and insoluble immunoglobulin aggregates. J Clin Invest. 1975;56:1053–1061. doi: 10.1172/JCI108152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.High N, Mounier J, Provost M, Sansonetti P J. IpaB of Shigella flexneri causes entry into epithelial cells and escape from the phagocytic vacuole. EMBO J. 1992;4:1991–1999. doi: 10.1002/j.1460-2075.1992.tb05253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hromockyj A E, Maurelli A T. Identification of Shigella invasion genes by isolation of temperature-regulated inv::lacZ operon fusions. Infect Immun. 1989;57:2963–2970. doi: 10.1128/iai.57.10.2963-2970.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jung H C, Eckmann L, S.-K. Y, Panja A, Fierer J, Morzycka-Wroblewska E, Kagnoff M F. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J Clin Invest. 1995;95:55–65. doi: 10.1172/JCI117676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar N B, Nostrant T T, Appelman H D. The histopathologic spectrum of acute self-limited colitis (acute infectious type colitis) Am J Surg Pathol. 1982;6:523–529. doi: 10.1097/00000478-198209000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Madara J L. Pathobiology of the intestinal epithelial barrier. Am J Pathol. 1990;137:1273–1281. [PMC free article] [PubMed] [Google Scholar]
- 22.Madara J L, Colgan S P, Nusrat A, Delp C, Parkos C A. A simple approach to measurement of electrical parameters of cultured epithelial monolayers: use in assessing neutrophil epithelial interactions. J Tissue Culture Methods. 1992;14:209–213. [Google Scholar]
- 23.Madara J L, Dharmsathaphorn K. Occluding junction structure-function relationships in a cultured monolayer. J Cell Biol. 1985;101:2124–2133. doi: 10.1083/jcb.101.6.2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Madara J L, Parkos C A, Colgan S P, MacLeod R J, Nash S, Matthews J, Delp C, Lencer W S. Cl− secretion in a model intestinal epithelium induced by a neutrophil-derived secretagogue. J Clin Invest. 1992;89:1938–1944. doi: 10.1172/JCI115800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Makino S, Sasakawa C, Kamata K, Kuata T, Yoshikawa M. A genetic determinant required for continuous reinfection of adjacent cells on the large plasmid in Shigella flexneri-2a. Cell. 1986;46:551–555. doi: 10.1016/0092-8674(86)90880-9. [DOI] [PubMed] [Google Scholar]
- 26.Maurelli A, Baudry B, d’Hauteville H, Hale T, Sansonetti P. Cloning of plasmid DNA sequences involved in invasion of HeLa cells by Shigella flexneri. Infect Immun. 1985;49:164–171. doi: 10.1128/iai.49.1.164-171.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maurelli A T, Blackmon B, Curtiss R., III Loss of pigmentation in Shigella flexneri 2a is correlated with loss of virulence and virulence-associated plasmid. Infect Immun. 1984;43:397–401. doi: 10.1128/iai.43.1.397-401.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCormick B, Hofman P, Kim J, Carnes D, Miller S, Madara J. Surface attachment of Salmonella typhimurium to intestinal epithelia imprints the subepithelial matrix with gradients chemotactic for neutrophils. J Cell Biol. 1995;131:1599–1608. doi: 10.1083/jcb.131.6.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCormick B A, Colgan S P, Archer C D, Miller S I, Madara J L. Salmonella typhimurium attachment to human intestinal epithelial monolayers: transcellular signalling to subepithelial neutrophils. J Cell Biol. 1993;123:895–907. doi: 10.1083/jcb.123.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCormick B A, Parkos C A, Colgan S P, Carnes D K, Madara J L. Apical secretion of a pathogen-elicited epithelial chemoattractant (PEEC) activity in response to surface colonization of intestinal epithelia by Salmonella typhimurium. J Immunol. 1998;160:455–466. [PubMed] [Google Scholar]
- 31.McGovern V J, Slavutin L J. Pathology of Salmonella colitis. Am J Surg Pathol. 1979;3:483–490. doi: 10.1097/00000478-197912000-00001. [DOI] [PubMed] [Google Scholar]
- 32.Menard R, Sansonetti P J, Parsot C. The secretion of the Shigella flexneri Ipa invasins is induced by the epithelial cell and controlled by IpaB and IpaD. EMBO J. 1994;13:5293–5302. doi: 10.1002/j.1460-2075.1994.tb06863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Menard R, Sansonetti P J, Parsot C. Non-polar mutagenesis of the ipa genes defines IpaB, IpaC, and IpaD as effectors of Shigella flexneri into epithelial cells. J Bacteriol. 1993;175:5899–5906. doi: 10.1128/jb.175.18.5899-5906.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mills J A, Venkatesan M M, Baron L S, Formal S B. Spontaneous insertion of IS1-like element into the virF gene is responsible for avirulence in opaque colonial variants of Shigella flexneri 2a. Infect Immun. 1992;60:175–182. doi: 10.1128/iai.60.1.175-182.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mounier J, Vasselon T, Hellio R, Lesourd M, Sansonetti P J. Shigella enters human colonic Caco-2 epithelial cells through the basolateral pole. Infect Immun. 1992;60:237–248. doi: 10.1128/iai.60.1.237-248.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nash S, Stafford J, Madara J L. Effects of polymorphonuclear leukocyte transmigration on barrier function of cultured intestinal epithelial monolayers. J Clin Invest. 1987;80:1104–1113. doi: 10.1172/JCI113167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Osborn L. Leukocyte adhesion to endothelium in inflammation. Cell. 1990;62:3–6. doi: 10.1016/0092-8674(90)90230-c. [DOI] [PubMed] [Google Scholar]
- 38.Parkos C A, Colgan S P, Bacarra A E, Nusrat A, Delp-Archer C, Carlson S, Su D H C, Madara J L. Intestinal epithelia (T84) possess basolateral ligands for CD11b/CD18-mediated neutrophil adherence. Am J Physiol. 1995;268:C472–C479. doi: 10.1152/ajpcell.1995.268.2.C472. [DOI] [PubMed] [Google Scholar]
- 39.Parkos C A, Colgan S P, Delp C, Arnaout M A, Madara J L. Neutrophil migration across a cultured epithelial monolayer elicits a biphasic resistance response representing sequential effects on transcellular and paracellular pathways. J Cell Biol. 1992;117:757–764. doi: 10.1083/jcb.117.4.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parkos C A, Delp C, Arnaout M A, Madara J L. Neutrophil migration across a cultured intestinal epithelium: dependence on a CD11b/CD18-mediated event and enhanced efficiency in the physiologic direction. J Clin Invest. 1991;88:1605–1612. doi: 10.1172/JCI115473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perdomo J J, Gounon P, Sansonetti P J. Acute inflammation causes epithelial cell invasion and mucosal destruction in experimental shigellosis. J Exp Med. 1994;180:1307–1319. doi: 10.1084/jem.180.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perdomo J J, Gounon P, Sansonetti P J. Polymorphonuclear leukocyte transmigration promotes invasion of colonic epithelial monolayers by Shigella flexneri. J Clin Invest. 1994;93:633–643. doi: 10.1172/JCI117015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pober J S, Gimbrone M A, LaPierre L A, Mendrick D L, Fiers W, Rothlein R, Springer T A. Overlapping patterns of activation of human endothelial cells by interleukin 1, tumor necrosis factor and immune interferon. J Immunol. 1986;137:1893–1896. [PubMed] [Google Scholar]
- 44.Rout W R, Formal S B, Dammin G J, Giannella R A. Pathophysiology of Salmonella diarrhea in the Rhesus monkey: intestinal transport, morphological and bacteriological studies. Gastroenterology. 1974;67:59–70. [PubMed] [Google Scholar]
- 45.Sansonetti P. Molecular and cellular biology of Shigella flexneri invasiveness: from cell assay systems to shigellosis. Curr Top Microbiol Immunol. 1992;180:1–19. doi: 10.1007/978-3-642-77238-2_1. [DOI] [PubMed] [Google Scholar]
- 46.Sansonetti P J, Arondel J, Cantey J R, Prevost M C, Huerre M. Infection of rabbit Peyers patches by Shigella flexneri: effect of adhesive or invasive bacterial phenotype on follicle associated epithelium. Infect Immun. 1996;64:2752–2764. doi: 10.1128/iai.64.7.2752-2764.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sansonetti P J, Kopecko D J, Formal S B. Involvement of a plasmid in the invasive ability of Shigella flexneri. Infect Immun. 1982;35:852–860. doi: 10.1128/iai.35.3.852-860.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sansonetti P J, Ryter A, Clerc P, Maurelli A T, Mounier J. Multiplication of Shigella flexneri within HeLa cells: lysis of the phagocytic vacuole and plasmid mediated contact hemolysis. Infect Immun. 1986;51:461–469. doi: 10.1128/iai.51.2.461-469.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schuch, R., and A. T. Maurelli. 1997. Unpublished data.
- 50.Schuch, R., and A. T. Maurelli. 1997. Submitted for publication.
- 51.Springer T A. Adhesion receptors of the immune system. Nature. 1990;346:196–197. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- 52.Takeuchi A. Electron microscope studies of experimental Salmonella infection. Am J Pathol. 1967;50:109–119. [PMC free article] [PubMed] [Google Scholar]
- 53.Van Gijsegem F, Genin S, Boucher C. Conservation of secretion pathways for pathogenicity determinants of plant and animal bacteria. Trends Microbiol. 1993;1:175–180. doi: 10.1016/0966-842x(93)90087-8. [DOI] [PubMed] [Google Scholar]
- 54.Venkatesan M M, Buysse J M, Oaks E V. Surface presentation of Shigella flexneri invasion plasmid antigens requires the products of the spa locus. J Bacteriol. 1992;174:1990–2001. doi: 10.1128/jb.174.6.1990-2001.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wassef J, Keren D F, Mailloux J L. Role of M cells in initial bacterial uptake and in ulcer formation in the rabbit intestinal loop model in shigellosis. Infect Immun. 1989;57:858–863. doi: 10.1128/iai.57.3.858-863.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Watarai M, Kamata Y, Kozaki S, Sasakawa C. rho, a small GTP-binding protein, is essential for Shigella invasion of epithelial cells. J Exp Med. 1997;185:281–292. doi: 10.1084/jem.185.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Watarai M, Tobe T, Yoshikawa M, Sasakawa C. Contact of Shigella with host cells triggers release of Ipa invasins and is an essential function of invasiveness. EMBO J. 1995;14:2461–2470. doi: 10.1002/j.1460-2075.1995.tb07243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yardley J H. Pathology of idiopathic inflammatory bowel disease and relevance of specific findings: an overview. In: Yardley J H, editor. Recent developments in the therapy of inflammatory bowel disease. Baltimore, Md: Johns Hopkins University Press; 1996. pp. 3–9. [Google Scholar]