Abstract
Background:
Since treatment options for youth with T2D are limited, we assessed the efficacy and safety of dapagliflozin as add-on therapy in adolescents and young adults with T2D receiving metformin, insulin or both.
Methods:
Participants with T2D (aged 10–<25 years; HbA1c 6·5–11%) were randomized 1:1 to dapagliflozin 10mg or placebo during a 24-week double-blind period, followed by a 28-week open-label safety extension where all participants received dapagliflozin (NCT02725593). Primary outcome was between-group differences in change in HbA1c from baseline to 24-weeks (intent-to-treat analysis). A pre-specified sensitivity analysis of the primary outcome was also assessed in the per-protocol population.
Findings:
Seventy-two participants (19 [26·4%] of whom aged 18–<25 years) were randomized (39 dapagliflozin, 33 placebo). Mean age was 16 years. After 24-weeks, mean change (95%CI) in HbA1c was −0·25% (−0·85, 0·34) for dapagliflozin and +0·50% (−0·18, 1·17) for placebo; between group difference of −0·75% (95%CI: −1·65, 0·15; p=0·101) favoring dapagliflozin. In the per-protocol population (34 dapagliflozin, 26 placebo) after 24-weeks, mean change (95%CI) was −0·51% (−1·07, 0·05) for dapagliflozin and +0·62% (−0·04, 1·27) for placebo; between group difference of −1·13% (95%CI: −1·99, −0·26; p=0·012). Adverse events (AEs) occurred in 27 (69·2%) dapagliflozin- and 19 (57·6%) placebo-treated participants over 24-weeks, and in 29 (74·4%) participants receiving dapagliflozin over 52-weeks. Hypoglycemia occurred in 11 (28·2%) dapagliflozin- and 6 (18·2%) placebo-treated participants over 24-weeks and in 13 participants (33·3%) receiving dapagliflozin over 52-weeks; none were considered as serious AEs. No AEs of diabetic ketoacidosis occurred.
Interpretation:
Addition of dapagliflozin to standard-of-care in youth with T2D improved glycemic control with a clinically relevant decrease in HbA1c and a low risk of severe hypoglycemia, providing evidence for the efficacy and safety of dapagliflozin as an additional treatment option in this unique population of children, adolescents and young adults living with T2D.
Funding:
AstraZeneca
Introduction
Management of type 2 diabetes (T2D) in youth is complex, and with the majority of clinical studies in adult populations only, treatment options for young people are limited due to the lack of approval of new drugs.1–4 Despite requirements from regulatory agencies to test the efficacy and safety of new T2D drugs in pediatric populations,5,6 such studies have been extremely difficult to carry out for a multitude of reasons, especially with difficulties in recruiting younger people in sufficiently high numbers.3,7
Historically, metformin (approved for use in 1999) is the first-line therapeutic treatment for the majority of people aged ≥10 years with diabetes1,4. Indeed, until 2019, metformin was the only drug approved for youth with T2D based on the results of a randomized clinical trial.8 Metformin has been comprehensively studied in the Treatment Options for type 2 Diabetes in Adolescents and Youth (TODAY) study series. One analysis from TODAY demonstrated that failure of metformin monotherapy rapidly developed in ~50% of participants by 12 months.9 This is believed to be due, at least in part, to gastrointestinal side-effects which can affect treatment adherence and quality-of-life.10,11 Rescue treatment with insulin therapy can be initiated, but unfortunately may not be sufficiently effective and/or tolerated in some young people. Indeed, one analysis demonstrated that only one-third of youth with T2D have a consistent HbA1c decrease of ≥0·5% after one year of add-on insulin treatment.12
In 2019, liraglutide, a glucagon-like peptide 1 receptor agonist which requires daily subcutaneous self-injections13 was approved in the United States and Europe for use in youth with T2D. In July 2021, the once-weekly extended-release preparation of the glucagon-like peptide-1 receptor agonist, exenatide,14 was approved for subcutaneous self-injection in people ≥10 years of age with T2D in the United States. Due to weight gain and increased risk of hypoglycemia associated with insulin therapy in particular2 and adherence issues with injectable therapies in general,1,15 additional oral therapy options which do not result in weight gain and do not increase the risk of hypoglycemia in youth with T2D would be a welcomed addition to the treatment armamentarium.
Dapagliflozin is an orally active sodium−glucose co-transporter-2 (SGLT-2) inhibitor approved for use in adults with T2D. In October 2021, the Committee for Medicinal Products for Human Use at the European Medicines Agency adopted a positive opinion recommending a change to the terms of the marketing authorization of dapagliflozin to include children ≥10 years of age with uncontrolled T2D. A final decision by the European Commission is expected by January 2022. Dapagliflozin reduces reabsorption of filtered glucose in the kidney thereby increasing urinary glucose excretion and facilitating weight loss. It has been shown in adult populations of T2D to lower HbA1c both as monotherapy or add-on therapy with other glucose-lowering agents, including metformin and/or insulin.16 More recently dapagliflozin has been shown to reduce the risk of heart failure, kidney disease, and death in two large Phase 3 studies involving adults with and without T2D.17,18 The pharmacokinetics (PK) and pharmacodynamics (PD) of dapagliflozin in children and adolescents with T2D have already been assessed and showed similar characteristics to that observed in adult populations.19,20
The aim of this study was to evaluate the efficacy and safety of dapagliflozin 10 mg as add-on therapy in children and young adults with T2D receiving standard of care (metformin alone, insulin alone or metformin+insulin).
Methods
Study design
This was a multicenter, 24-week, placebo-controlled, double-blind, randomized Phase 3 study with a 4-week lead-in period and a 28-week open-label safety extension (NCT02725593). Participants were randomized from 30 centers in five countries (Hungary [two centers], Israel [four], Mexico [six], Russia [six] and US [12]). The study was performed in accordance with the Declaration of Helsinki and applicable regulatory requirements. Institutional review boards and independent ethics committees associated with each center approved the study before any procedures were implemented.
As shown in Figure S1, after a 4-week placebo lead-in period, participants were randomized 1:1 to receive dapagliflozin or placebo during the 24-week double-blind period. Subsequently, participants were able to enter a 28-week open-label safety extension period where all participants received dapagliflozin, followed by a 4-week post-treatment safety follow-up period. Participants maintained background therapies throughout the study. Those who prematurely discontinued the study drug entered a non-treatment, follow-up phase, with modified assessments at the scheduled visits until study completion.
Participants
Participants aged 10–<25 years with T2D were eligible for inclusion. Inclusion criteria were HbA1c 6·5–11%, FPG ≤14·2 mmol/L (≤256 mg/dL) and a stable dose of either metformin (≥1000 mg daily), insulin, or a combination of metformin (≥1000 mg daily) plus insulin for a minimum of 8 weeks. These were the only drugs approved for use in youth with T2D at the time of study. Those with a previous diagnosis of T1D, monogenic diabetes, genetic disorders with strong associations with insulin resistance/diabetes and/or obesity, or secondary diabetes were excluded from the study. A list of the inclusion and exclusion criteria are provided in Table S1. Participants (or parents/guardians for those aged 10–<18 years) provided written informed consent; minors <18 years of age also had to provide written assent.
Randomization and masking
Participants were stratified by sex, age (10–15, >15–<18, ≥18–<25 years) and background medication (metformin alone, insulin alone, or insulin+metformin). A priori recruitment of participants aged 18–<25 years was limited to <40% of the total population, while recruitment of participants aged 10–15 years was to comprise ≥20% of the total population. An interactive web/voice response system randomly assigned treatment (placebo or study drug) to each participant. During the 24-week efficacy period, participants and study personnel were blinded to treatment. Treatment during the subsequent 28-week extension period was open-label, although blinding with respect to treatment received in the initial 24-week period was maintained. The sponsor was responsible for randomization and blinding.
Procedures
The study treatments were oral, once-daily, dapagliflozin 10 mg or placebo added to standard of care (metformin alone, insulin alone or metformin+insulin). Participants were assessed weekly during the 4-week lead-in period, at baseline, during the double-blind period (Week 1, 2, 4, 8, 10, 12, 16, 20, 24), during the open-label safety extension (Week 28, 32, 36, 40, 46, 52) and 4 weeks post treatment. Basal insulin was initiated or up-titrated as open-label rescue for participants meeting prespecified criteria for lack of glycemic control: FPG >13·3 mmol/L (>240 mg/dL) during the double-blind period; FPG >10 mmol/L (>180 mg/dL) or HbA1c >8.0% during the open-label period (Table S2). Rescued participants continued treatment with the study drug and continued in the study. Those receiving insulin (as background or as glycemic rescue) underwent dose adjustments as per investigators’ discretion.
Outcome measures
Efficacy
The primary efficacy outcome was mean change from baseline to 24 weeks in HbA1c with dapagliflozin 10 mg versus placebo. Values after glycemic rescue or permanent discontinuation from study drug were excluded. A prespecified sensitivity analysis was to be performed if >10% of participants in either treatment group had protocol deviations predefined as affecting the primary efficacy results. These predefined protocol deviations are described in Table S3. The primary efficacy endpoint was also described according to subgroups: sex, race (white, non-white), baseline HbA1c (<8%, ≥8%) and background medication (insulin±metformin, metformin only).
Secondary endpoints, in order of hierarchical testing, were mean change from baseline to 24 weeks in FPG, percentage of participants who received glycemic rescue or discontinued study due to lack of glycemic control up to 24 weeks, and percentage of participants with baseline HbA1c ≥7% who achieved HbA1c <7% at 24 weeks.
Safety
Safety and tolerability were assessed throughout and included reporting of adverse events (AEs), serious AEs (SAEs), discontinuation due to AEs, hypoglycemia, diabetes ketoacidosis (adjudicated by committee), hepatic laboratory parameters, and vital signs (height, weight, BMI z-score and blood pressure).
Hypoglycemia was defined according to American Diabetes Association (ADA) criteria: severe (required assistance to administer carbohydrate, glucagon or other actions to promote neurological recovery), documented symptoms (typical symptoms and/or plasma glucose [PG] ≤3·9 mmol/L [≤70 mg/dL]), asymptomatic (no symptoms, PG ≤3·9 mmol/L [≤70 mg/dL]), probable symptomatic (typical symptoms without a glucose measurement) and relative (any typical symptom but with PG >3·9 mmol/L [>70 mg/dL]).21 Hypoglycemia definitions according to International Society for Pediatric Diabetes (ISPAD) 2014 criteria was also applied to participants aged <18 years old at study entry;22 severe hypoglycemia defined as an event associated with severe neuroglycopenia usually resulting in coma or seizure and requiring parenteral therapy (glucagon or intravenous glucose) and mild/moderate hypoglycemia defined as all other events that did not meet the definition of severe.
Statistical analysis
Based on an estimated treatment difference of 0·78% and assuming a SD of 0·9% for change from baseline in HbA1c at 24 weeks, a sample size of 25 participants per treatment group would provide 85% power to demonstrate the superiority of dapagliflozin to placebo at a 2-sided alpha level of 5%. To ensure that ≥50 participants would have Week 24 assessments while on treatment, ≥66 participants were planned to be randomised. Expected improvement over placebo from simulation suggested that this relatively small sample size provided adequate statistical power, with expected variability obtained from adult experience. The primary outcome was assessed in both intent-to-treat (ITT) and per-protocol analyzes. All secondary endpoints were assessed in ITT analyses only.
The primary endpoint was based on a Mixed Model Repeated Measures (MMRM) approach, excluding values after glycemic rescue or permanent discontinuation from study drug, with fixed factors for treatment, week, treatment-by-week interaction, randomization strata and covariates for baseline HbA1c and baseline HbA1c measurement-by-week interaction. To account for within-participant variability, an unstructured covariance matrix was used. Degrees of freedom were estimated using the Kenward-Roger approach. Point estimates and 95% Confidence Intervals (CI) for the least square mean change in HbA1c for each treatment group and their contrast as dapagliflozin treatment group minus placebo (with associated p-value at 24 weeks) were calculated. For the analysis of the primary endpoint by subgroups, interaction factors for subgroup, subgroup-by-week, and subgroup-by-week-by-treatment were added and interaction p-values (representing the treatment by subgroup interaction at Week 24) calculated.
Analysis of the secondary endpoint of change from baseline in FPG at Week 24 used the MMRM approach as described above, replacing covariate terms for HbA1c with FPG. For the secondary endpoints of percentage of participants who received glycemic rescue or who discontinued the study due to lack of glycemic control up to 24 weeks, and the percentage of participants with baseline HbA1c ≥7% who achieved HbA1c <7% at 24 weeks, unadjusted proportions and difference in proportion (95% CI) were based on the Chan-Zhang method with p-values from a Fisher’s exact test.
The family-wise Type I error rate related to the primary and secondary efficacy endpoints was controlled at the 2-sided 0·05 level by using a hierarchical closed testing procedure, starting with the primary endpoint and then the secondary endpoints ordered as listed in the previous section. If a comparison between groups did not achieve statistical significance, nominal p-values were presented for the remaining endpoints (without statistical inference).
Safety analyses included data after glycemic rescue and are descriptive only; no statistical tests were performed. An independent data monitoring committee (pediatric and endocrine specialists and statisticians) regularly reviewed trial data.
This study is registered with ClinicalTrials.gov (NCT02725593).
Role of funding source
The study funder (AstraZeneca) was involved in study design, data collection, data analysis, data interpretation and writing of the report.
Role of the Pediatric Diabetes Consortium
Representatives from the study sites located in the United States who were members of the Pediatric Diabetes Consortium had monthly meetings with AstraZeneca to evaluate and facilitate recruitment of the patients into the study.
Results
The first patient was enrolled into the study on 22 June 2016, and the last enrolled patient completed the last study visit on 06 April 2020. Of the 168 participants screened, 80 entered the 4-week, placebo lead-in period. Failure to meet randomization criteria was the most common reason for not entering the lead-in period (n=85) (Table S4). Seventy-two participants were randomized to treatment: 39 to dapagliflozin and 33 to placebo (Figure 1). The per-protocol group consisted of 34 participants in the dapagliflozin group and 26 participants in the placebo group. In total 34/39 (87.2%) dapagliflozin and 27/33 (81.8%) placebo participants completed the 24-week double-blind period, with almost all still receiving treatment (32/39 [82.1%] and 25/33 [75.8%] participants, respectively). Withdrawal by participant/guardian was the most common reason for study discontinuation of randomized treatment (n=4 for dapagliflozin and n=5 for placebo).
Figure 1: Participant disposition.
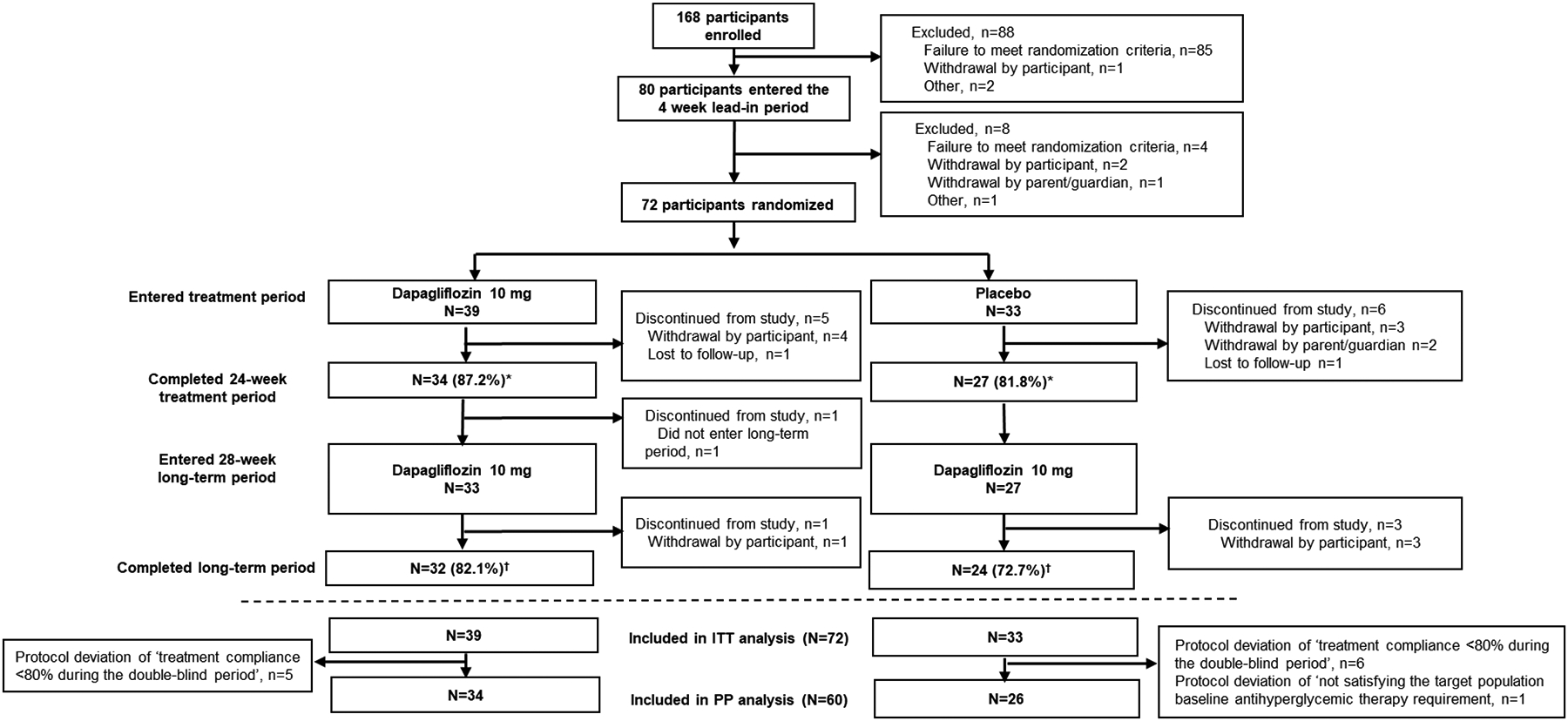
*32/39 (82.1%) and 25/33 (75.8%) participants in the dapagliflozin and placebo groups, respectively, were still receiving study drug at the end of the double-blind short-term period; †30/39 (76.9%) and 24/33 (72.7%) participants, respectively, were still receiving open-label dapagliflozin treatment at the end of the long-term period; ITT=intent-to-treat; PP=per-protocol
The 28-week open-label safety extension was included in the study design to encourage enrollment in the study and to limit the duration of placebo treatment. All of the 27 placebo-treated participants who completed the double-blind period switched to treatment with dapagliflozin in the 28-week open-label extension. One participant in the dapagliflozin group who completed the double-blind period did not enter the open-label extension period. A high proportion of participants completed the 28-week, open-label period; namely, 32/39 (82.1%) initially randomized to dapagliflozin and 24/33 (72.7%) initially randomized to placebo who switched to dapagliflozin, with almost all still receiving treatment at the end of the study (30/39 [76.9%] and 22/33 [72.7%] participants, respectively) (Figure 1).
Demographics and baseline characteristics of the 72 randomized participants in the ITT population are described in Table 1. Overall mean age was 16 years, with 42% aged 10–15 years and 32% aged >15–<18 years. There were more white and European participants randomized to dapagliflozin than to placebo. The dapagliflozin group also had lower FPG and BMI (including standardized z-score) and greater use of insulin (± metformin). Other clinical characteristics are shown in Table 1.
Table 1:
Demographics and baseline characteristics
| Dapagliflozin 10 mg (N=39) |
Placebo (N=33) |
Total (N=72) |
|
|---|---|---|---|
| Age, years | 16·1 (3·3) | 16·2 (3·6) | 16·1 (3·4) |
| Age group (years), n (%) | |||
| ≥10 and ≤15 | 16 (41·0) | 14 (42·4) | 30 (41·7) |
| >15 and <18 | 13 (33·3) | 10 (30·3) | 23 (31·9) |
| ≥18 and <25 | 10 (25·6) | 9 (27·3) | 19 (26·4) |
| Female, n (%) | 24 (61·5) | 19 (57·6) | 43 (59·7) |
| Race, n (%) | |||
| White | 28 (71·8) | 16 (48·5) | 44 (61·1) |
| Black or African-American | 8 (20·5) | 10 (30·3) | 18 (25·0) |
| American Indian or Alaska Native | 2 (5·1) | 3 (9·1) | 5 (6·9) |
| Other* | 1 (2·6) | 4 (12·1) | 5 (6·9) |
| Geographic region, n (%) | |||
| North America | 16 (41·0) | 16 (48·5) | 32 (44·4) |
| Latin America | 7 (17·9) | 9 (27·3) | 16 (22·2) |
| Europe | 16 (41·0) | 8 (24·2) | 24 (33·3) |
| Duration of T2D, years | 3.10 (2·67) | 3.15 (3·05) | 3.12 (2·83) |
| Duration of T2D (years), n (%) | |||
| <3 | 22 (56·4) | 21 (63·6) | 43 (59·7) |
| ≥3 and ≤10 | 15 (38·5) | 10 (30·3) | 25 (34·7) |
| >10 | 2 (5·1) | 2 (6·1) | 4 (5·6) |
| HbA1c, % | 7·95 (1·59) | 7·85 (1·19) | 7·90 (1·41) |
| HbA1c † , n (%) | |||
| <6·5% | 5 (12·8) | 2 (6·1) | 7 (9·7) |
| ≥6·5 and <9% | 25 (64·1) | 24 (72·7) | 49 (68·1) |
| ≥9 and ≤11% | 7 (17·9) | 7 (21·2) | 14 (19·4) |
| >11% | 2 (5·1) | 0 | 2 (2·8) |
| Fasting plasma glucose, mmol/L [mg/dL] | 8·66 (3·09) [156·0 (55·7)] | 9·27 (3·51) [167·0 (63·2)] |
8.94 (3·28) [161·1 (59·1)] |
| BMI, kg/m 2 | 31·38 (7·51) | 33·55 (8·81) | 32·38 (8·14) |
| Standardized BMI (z-score) ‡ | 1·69 (0·91) | 1·84 (1·08) | 1·76 (0·98) |
| eGFR, mL/min/1·73 m 2 | 121·5 (22·4) | 122·2 (26·0) | 121·8 (23·9) |
| Systolic blood pressure, mmHg | 119·4 (12·9) | 118·2 (15·2) | 118·8 (13·9) |
| Diabetes treatments, n (%) | |||
| Metformin | 17 (43·6) | 20 (60·6) | 37 (51·4) |
| Insulin | 7 (17·9) | 5 (15·2) | 12 (16·7) |
| Metformin + insulin | 15 (38·5) | 8 (24·2) | 23 (31·9) |
| Metformin, mg | n=32 | n=28 | n=60 |
| Mean (SD) | 1666 (431) | 1625 (565) | 1647 (494) |
| Median (min–max) | 1700 (1000–2550) | 1600(1000–2550) | 1700 (1000–2550) |
| Insulin, IU | n=22 | n=13 | n=35 |
| Mean (SD) | 58·0 (42·7) | 57·2 (48·1) | 57·7 (44·1) |
| Median (min–max) | 44·5 (5–170) | 38·0 (3–170) | 40·0 (3–170) |
Asian, Native Hawaiian or other Pacific Islander, Arab, white/native American or mixed;
All participants met the study criteria of HbA1c ≥6·5–11% at screening, but after the 4-week placebo lead-in period a small number of participants had a HbA1c <6·5% (7 [9·7%] participants) or >11% (2 [2·8%] participants);
Adjusted for age and sex based on 2000 Center for Disease Control and Prevention z-score (derived using age expressed in months with participants aged ≥20 years set as 239·5 months)
All data are mean (SD) unless otherwise stated; T2D=type 2 diabetes; eGFR=estimated glomerular filtration rate.
For the primary endpoint, the adjusted mean change from baseline in HbA1c at 24 weeks was −0·25% for dapagliflozin and +0·50% for placebo, resulting in a between group difference of −0·75% (95% CI: −1·65, 0·15; p=0·101) favoring dapagliflozin in the ITT population (Figure 2A). In the sensitivity analysis in the per-protocol population, mean change from baseline was −0·51% for dapagliflozin and +0·62% for placebo, resulting in a difference of −1·13% (95% CI: −1·99, −0·26; p=0·012) favoring dapagliflozin (Figure 2B). When assessed by subgroup (Figure S2), the benefit of dapagliflozin versus placebo was maintained across sex, race, baseline HbA1c and background medication.
Figure 2: Efficacy outcomes.
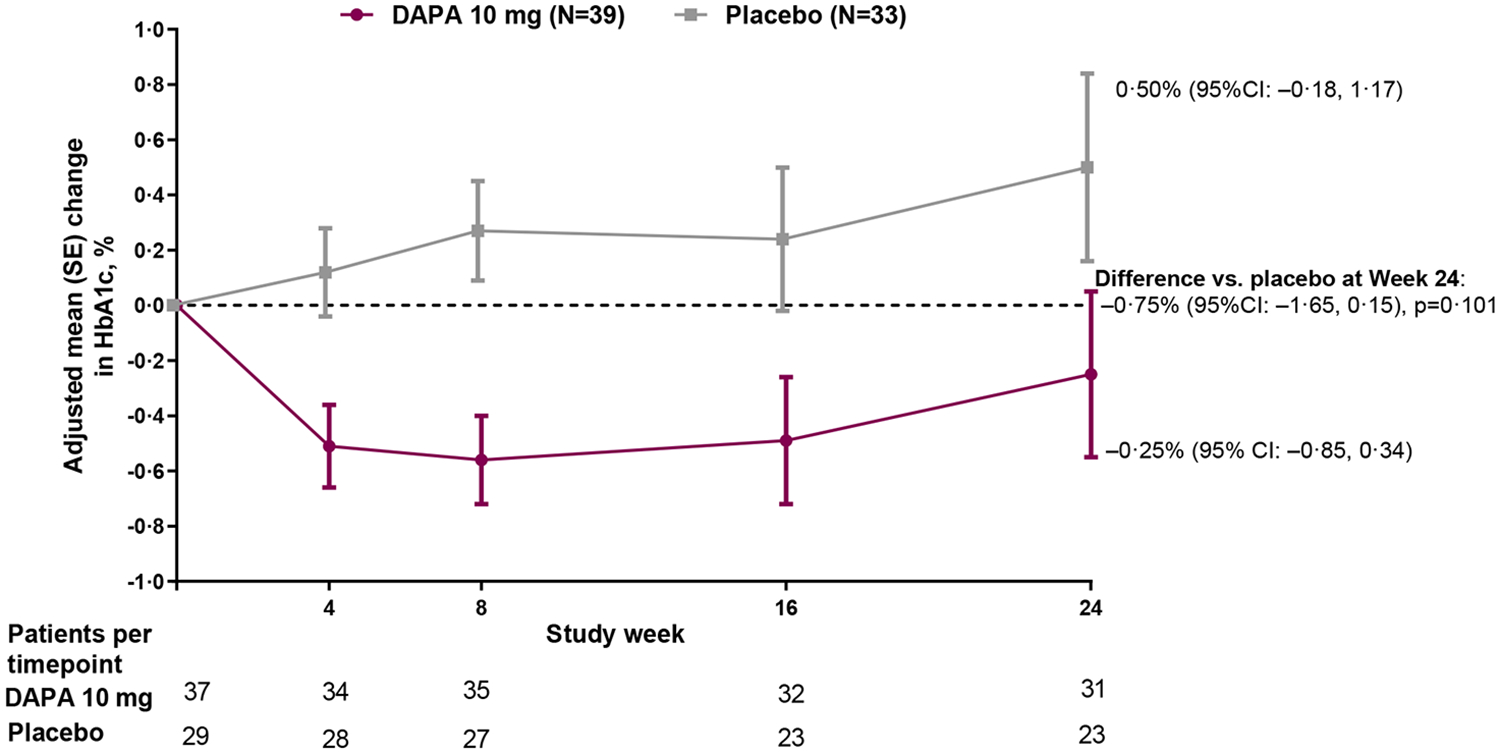
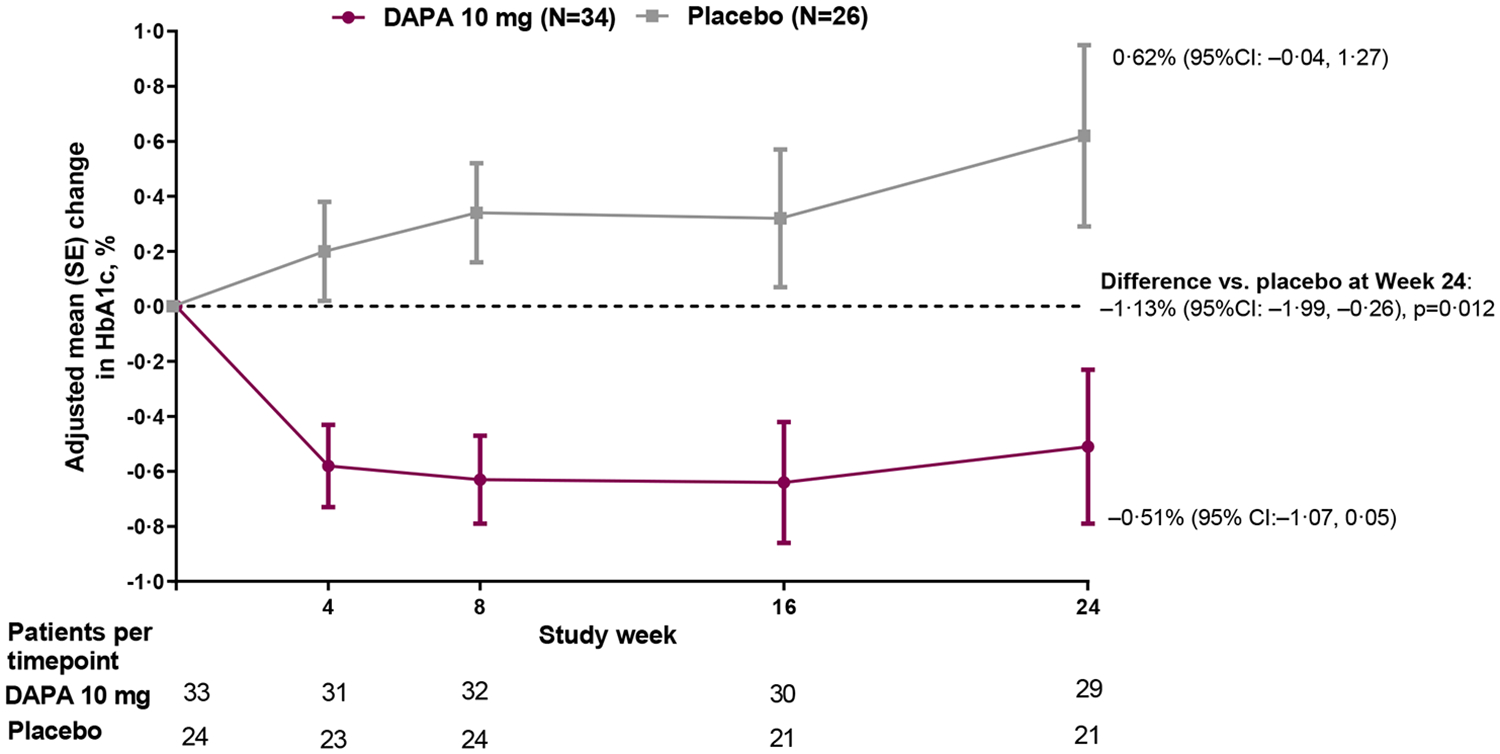
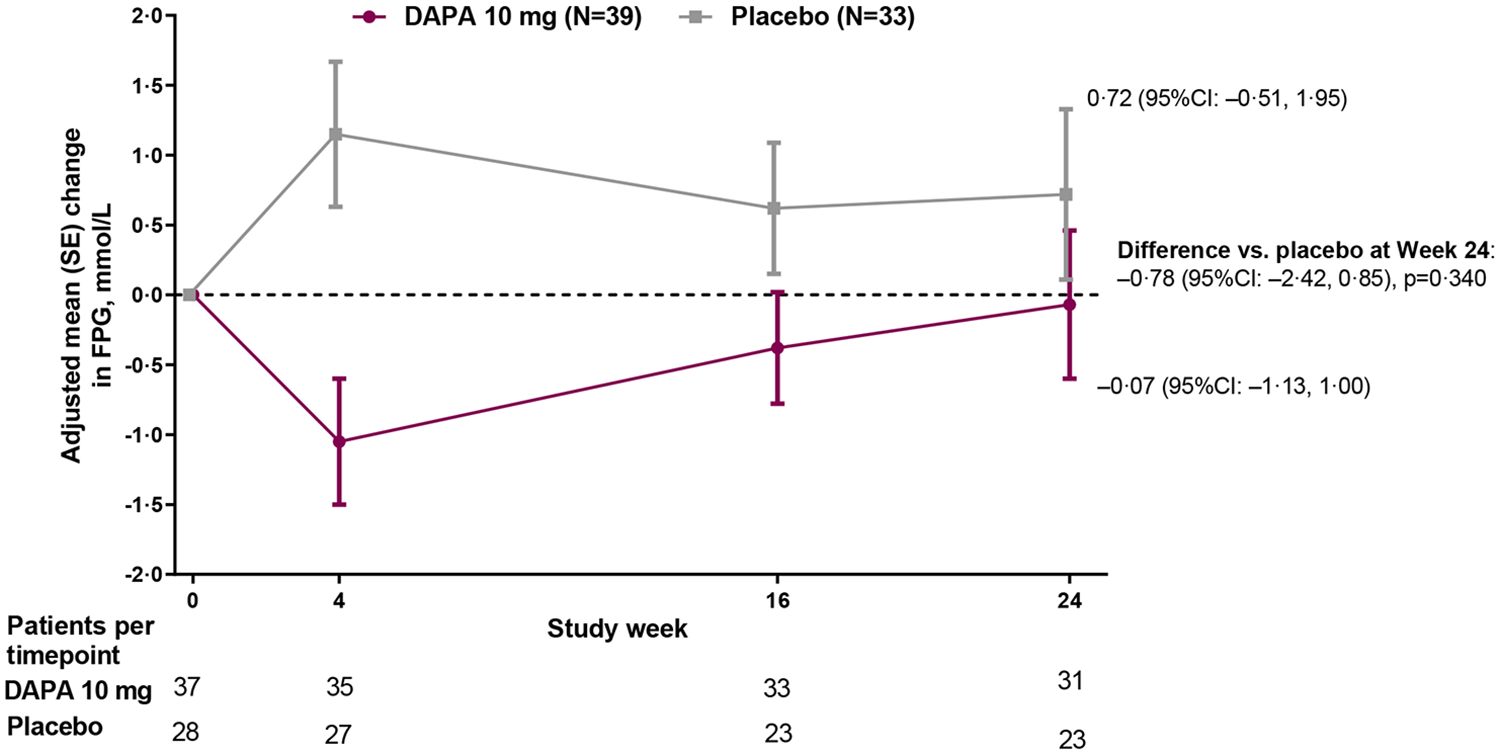
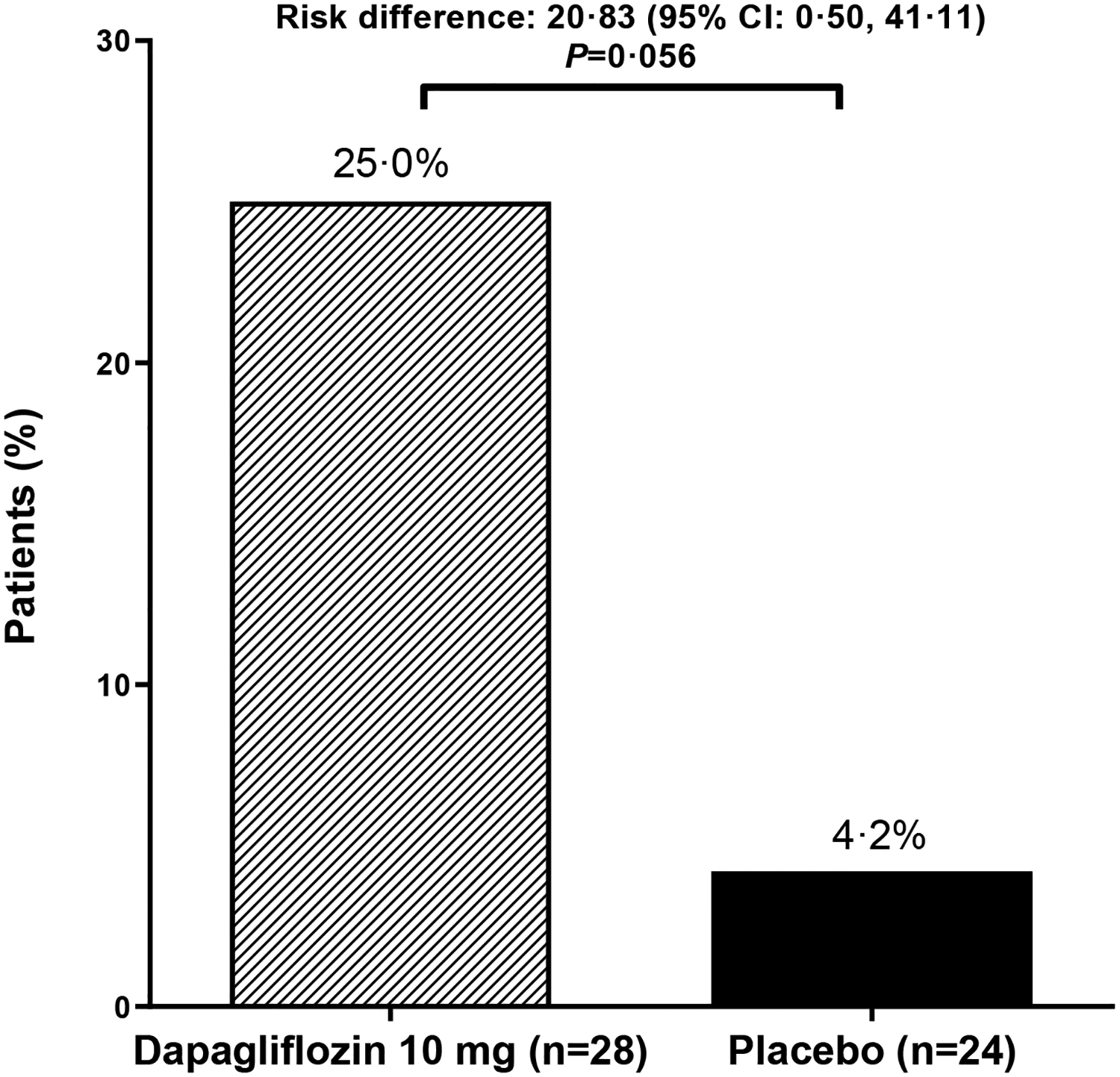
(A) Primary outcome of adjusted mean change from baseline to Week 24 in HbA1c; (B) Sensitivity analysis of the primary outcome in the per-protocol population (excludes participants with relevant protocol deviations); (C) Secondary outcome of adjusted mean change from baseline to Week 24 in FPG; (D) Secondary outcome of proportion of participants with baseline HbA1c ≥7% who achieved HbA1c <7% at Week 24; Analyses exclude values after glycemic rescue or permanent discontinuation from study drug; The primary outcome did not achieve statistical significance in the ITT population and all other p-values should be considered as nominal p-values; DAPA=dapagliflozin; FPG=fasting plasma glucose; ITT=intent-to-treat
There was an initial decrease in FPG with dapagliflozin and an increase with placebo, but by 24 weeks FPG had returned to baseline values in the dapagliflozin group (Figure 2C). However, as FPG increased in the placebo group, by 24 weeks there was a between group difference of −0·78 (95% CI: −2·42, 0·85) mmol/L (−14·1 [−43·6, 15·3] mg/dL). By 24 weeks, only 2 (5·1%) participants in the dapagliflozin group and 3 (9·1%) participants in the placebo group received glycemic rescue or discontinued the study due to lack of glycemic control. Approximately three quarters of participants in each group had HbA1c ≥7% at baseline (28/39 [71·8%] in the dapagliflozin group and 24/33 [72·7%] in the placebo group). Of these, a larger proportion of participants in the dapagliflozin group achieved HbA1c <7% at 24 weeks; 7 (25·0%]) in the dapagliflozin group versus 1 (4·2%) in the placebo group (Figure 2D).
During the 24-week double-blind period, AEs occurred in 27 (69·2%) and 19 (57·6%) participants in the dapagliflozin and placebo groups, respectively (Table 2). Over the entire 52 weeks of the study, AEs occurred in 29 (74·4%) participants randomized to dapagliflozin, the most common of which were headache, nasopharyngitis and vitamin D deficiency. One AE led to treatment discontinuation; a genital infection in a female participant randomized to dapagliflozin during the 24-week double-blind period (subsequently resolved). SAEs occurred in five participants; two randomized to dapagliflozin during the entire 52-week study (depression and lower abdominal pain), two randomized to placebo (spontaneous abortion and hyperglycemia) and one placebo-treated participant who switched to dapagliflozin during the 28-week extension period (hyperglycemia). No SAEs led to discontinuation of dapagliflozin treatment and no deaths or episodes of diabetic ketoacidosis occurred in any participants in the study. Changes in clinical laboratory hepatic parameters (Table S5) were generally small and transient. There was no notable effect of treatment on vital signs, including BMI z-score or blood pressure (Table S6).
Table 2:
Safety outcomes
| Double-blind short-term period only (24 weeks) |
Open-label long-term period only (28 weeks) |
Double-blind period plus open-label period (52 weeks) |
||
|---|---|---|---|---|
| n (%) | Dapagliflozin 10 mg (N=39) |
Placebo (N=33) |
Placebo switched to dapagliflozin 10 mg (N=27) |
Dapagliflozin 10 mg (N=39) |
| Adverse events | ||||
| ≥1 AE | 27 (69·2) | 19 (57·6) | 4 (14·8) | 29 (74·4) |
| AE leading to discontinuation of study drug | 1 (2·6) | 0 | 0 | 1 (2·6) |
| ≥1 SAE | 1 (2·6) | 2 (6·1) | 1 (3·7) | 2 (5·1) |
| Most common AEs * | ||||
| Headache | 4 (10·3) | 3 (9·1) | 1 (3·7) | 5 (12·8) |
| Nasopharyngitis | 4 (10·3) | 0 | 2 (7·4) | 5 (12·8) |
| Vitamin D deficiency | 4 (10·3) | 1 (3·0) | 1 (3·7) | 5 (12·8) |
| Oropharyngeal pain | 3 (7·7) | 1 (3·0) | 0 | 4 (10·3) |
| Nausea | 3 (7·7) | 0 | 0 | 3 (7·7) |
| Urinary tract infection | 2 (5·1) | 1 (3·0) | 0 | 3 (7·7) |
| Cough | 2 (5·1) | 1 (3·0) | 1 (3·7) | 2 (5·1) |
| Diarrhea | 2 (5·1) | 2 (6·1) | 0 | 2 (5·1) |
| Gastroenteritis viral | 2 (5·1) | 0 | 0 | 2 (5·1) |
| Hypertension | 2 (5·1) | 1 (3·0) | 0 | 2 (5·1) |
| Pharyngitis streptococcal | 2 (5·1) | 0 | 1 (3·7) | 2 (5·1) |
| Sinus congestion | 2 (5·1) | 0 | 0 | 2 (5·1) |
| Vomiting | 2 (5·1) | 0 | 0 | 2 (5·1) |
| Increased weight | 2 (5·1) | 0 | 0 | 2 (5·1) |
| Hypertriglyceridaemia | 1 (2·6) | 2 (6·1) | 1 (3·7) | 1 (2·6) |
| Toothache | 1 (2·6) | 2 (6·1) | 0 | 1 (2·6) |
| Hyperglycemia | 0 | 3 (9·1) | 1 (3·7) | 0 |
≥5% of participants in either treatment group during the double-blind period; All safety analyses include data after glycemic rescue; AEs are recorded up to and including 4 days after the last dose or the end of treatment period, and SAEs up to and including 30 days after last dose or end of treatment period; AE=adverse event; SAE=serious adverse event
Hypoglycemia (classified per ADA criteria) occurred in the double-blind period in 11 (28·2%) participants randomized to dapagliflozin and 6 (18·2%) randomized to placebo (Table 3). Of these participants, 8 and 5 (respectively) were also receiving insulin. In participants treated with dapagliflozin throughout the 52-week study, hypoglycemia occurred in 13 participants (33·3%), with most events being asymptomatic (26 of 37 events); 10 of these participants were also receiving insulin. Severe hypoglycemia occurred in three participants randomized to dapagliflozin (two in the 24-week double-blind period and one in the 28-week extension period), and in one placebo group participant who switched to dapagliflozin during the 28-week extension period. No hypoglycemia events were considered SAEs and none led to discontinuation of treatment. Observations by ISPAD classification (participants aged <18 years only) were similar, with almost all events classified as mild/moderate (Table 3).
Table 3:
Hypoglycemia
| Hypoglycemia category* | Double-blind short-term period only (24 weeks) |
Open-label long-term period only (28 weeks) |
Double-blind period plus open-label period (52 weeks) |
|
|---|---|---|---|---|
| Dapagliflozin 10 mg (N=39) |
Placebo (N=33) |
Placebo switched to dapagliflozin 10 mg (N=27) |
Dapagliflozin 10 mg (N=39) |
|
| ADA classification | ||||
| ≥1 Hypoglycemia (any category), n (%) | 11 (28·2) | 6 (18·2) | 3 (11·1) | 13 (33·3) |
| Number of events | 21 | 27 | 44 | 37 |
| ≥1 Severe † , n (%) | 2 (5·1) | 0 | 1 (3·7) | 3 (7·7) |
| Number of events | 2 | 0 | 1 | 3 |
| ≥1 Documented symptomatic ‡ , n (%) | 3 (7·7) | 1 (3·0) | 2 (7.4·0) | 4 (10·3) |
| Number of events | 4 | 7 | 6 | 6 |
| ≥1 Asymptomatic § , n (%) | 8 (20·5) | 4 (12·1) | 3 (11·1) | 10 (25·6) |
| Number of events | 14 | 19 | 37 | 26 |
| ≥1 Probable symptomatic ‖ , n (%) | 0 | 0 | 0 | 0 |
| Number of events | 0 | 0 | 0 | 0 |
| ≥1 Relative ¥ , n (%) | 1 (2·6) | 1 (3·0) | 0 | 2 (5·1) |
| Number of events | 1 | 1 | 0 | 2 |
| ISPAD classification | ||||
| N# | 29 | 24 | 24 | 29 |
| ≥1 Hypoglycemia (any category), n (%) | 8 (27·6) | 2 (8·3) | 3 (12·5) | 10 (34·5) |
| Number of events | 18 | 16 | 36 | 34 |
| ≥1 Severe ** , n (%) | 2 (6·9) | 0 | 0 | 3 (10·3) |
| Number of events | 2 | 0 | 0 | 3 |
| ≥1 Mild/moderate †† , n (%) | 8 (27·6) | 1 (4·2) | 3 (12·5) | 10 (34·5) |
| Number of events | 16 | 15 | 36 | 30 |
| ≥1 Unclassified ‡‡ , n (%) | 0 | 1 (4·2) | 0 | 1 (3·4) |
| Number of events | 0 | 1 | 0 | 1 |
All analyses include data after glycemic rescue; Data are number of participants and percentage (n (%)) and number of events;
Participants with multiple events in the same category are counted only once in that category. Participants with events in more than one category are counted once in each of those categories;
Requires assistance from another person to actively administer carbohydrate, glucagon, or take other corrective actions to promote neurological recovery;
Typical symptoms and plasma glucose ≤3·9 mmol/L (70 mg/dL);
No symptoms and plasma glucose ≤3·9 mmol/L (≤70 mg/dL);
Typical symptoms without a glucose measurement but presumably caused by plasma glucose ≤3·9 mmol/L (≤70 mg/dL);
Any typical symptom but with plasma glucose >3·9 mmol/L (>70 mg/dL);
Hypoglycemic event with severe cognitive impairment (including coma and convulsions) requiring external assistance by another person to actively administer carbohydrates, glucagon, or take other corrective actions;
Hypoglycemic event that didn’t meet the definition of severe hypoglycemia or unclassified hypoglycemia;
Hypoglycemic event that meets criteria of ‘probable symptomatic’ or ‘relative’ according to ADA classifications; N# is the number of participants aged <18 years at the beginning of the study (applicable to ISPAD classifications only);
ADA=American Diabetes Association; AE=adverse event; DAPA=dapagliflozin; ISPAD=International Society for Pediatric Diabetes; PBO=placebo
Discussion
In this Phase 3 study of youth with T2D receiving metformin, insulin or metformin+insulin, addition of dapagliflozin resulted in a decrease in HbA1c versus placebo (between group difference of −0·75% [95 CI: −1·65, 0·05]; p=0·101) after 24 weeks. Since it is well documented that children and young adults with T2D may have challenges with self-care and adherence,3,7 a sensitivity analysis of only protocol-compliant participants was pre-specified in the study design. This analysis – which excluded 12 (17%) participants for protocol deviations directly related to the primary efficacy results – demonstrated a statistically significant decrease in HbA1c with dapagliflozin versus placebo (between group difference of −1·13% [−1·99, −0·26]; p=0·012). The increase in HbA1c over time observed in the placebo group (which included some participants considered well-controlled at baseline) in our study is in line with previous studies in youth with T2D.13,23 The decrease in HbA1c observed with dapagliflozin is also consistent with a previous single dose PK/PD study in a pediatric population19 which showed an increase in urinary glucose excretion broadly similar to studies in adult populations.20 Finally, subgroup analyses of the primary endpoint showed the benefit of dapagliflozin regardless of baseline characteristics such as race, HbA1c and type of background therapy.
The sample size of this study was based on modelling and simulation using data from both the dapagliflozin pediatric PK/PD study and from previous studies of dapagliflozin in adults, in agreement with the European Medicines Agency, and focused on change in HbA1c. No statistically significant between-group differences were observed in the secondary endpoints. For FPG, between group changes from baseline to 24 weeks of treatment indicated a −0·78 (95% CI: −2·42, 0·85) mmol/L (−14·1 [−43·6, 15.3] mg/dL) decrease, favoring dapagliflozin. The other secondary endpoints at Week 24 (percentage of participants who received glycemic rescue or discontinued study due to lack of glycemic control, and percentage of participants with baseline HbA1c ≥7% who achieved HbA1c <7%) were numerically in favor of dapagliflozin, although the small number of participants with these outcomes limits interpretation. It is also noteworthy in this study that all of the participants in the placebo group who completed the 24-week, double-blind period continued to the 28-week open-label period. Indeed, providing an opportunity to switch treatment from placebo to dapagliflozin during the 28-week open-label period proved to be an important factor in our ability to enroll participants in the study.
Clinical trials of new drugs for youth with T2D provide an opportunity to examine the safety and tolerability, as well as the efficacy, of these agents. No episodes of diabetic ketoacidosis were observed and the only AE that led to the discontinuation of dapagliflozin treatment was a genital infection (a known AE of dapagliflozin from adult studies). Dapagliflozin has a low propensity to cause hypoglycemia when used as monotherapy or in combination with most other glucose-lowering therapies. However, when used with insulin, the risk of hypoglycemia increases due to the lower glucose levels achieved with the combination of treatment.16 In our study, hypoglycemia occurred in one-third of participants who received dapagliflozin over 52 weeks, with almost all (10 out of 13 patients) also receiving insulin. Most events were asymptomatic or mild/moderate. The higher use of insulin in the dapagliflozin versus placebo group at baseline (56 vs 39%) may have influenced the frequency of occurrence of hypoglycemia between the two treatment groups. It should be noted that both the European and US prescribing information for the newly approved glucose-lowering agent, liraglutide, also describe the higher rate of hypoglycemia versus placebo in the pediatric population.24,25
It could be considered somewhat surprising that there were no notable effects of treatment with dapagliflozin on body weight, BMI, BMI z-score or blood pressure in our study, as previous studies of dapagliflozin in adult populations have reported significant changes in weight and blood pressure.16 This may reflect, however, that the young participants were experiencing (or had recently emerged from) puberty, when considerable increases and fluctuations in growth, maturation and development occur.
A unique aspect of our study was the inclusion of a subset of young adult participants (18–<25 years), an age range consistently under-represented in clinical trials in adults with T2D. For example, in a large National Institutes of Health sponsored study of >5000 adults with T2D, people <30 years of age were excluded.26 It is also unfortunate, however, that some regulatory agencies exclude participants >17 years of age from participation in pediatric T2D trials. As disease characteristics of T2D in young adults are similar to pediatric T2D, with many people diagnosed in childhood, we believe that inclusion of this group in pediatric studies is warranted. Our study has some limitations. Despite including both children and young adults with T2D, there is still a relatively small number of participants for a Phase 3 study, owing to well-known issues with recruitment of youth with T2D.3,7 Furthermore, although the study did enroll patients of different ethnicities across three major geographic regions (North American, Latin America and Europe), findings may have somewhat limited generalizability to a broader population of youth with T2D due to the relatively small number of participants included. In clinical trials of adults with T2D, dapagliflozin has been studied in diverse populations across the globe with no suggestion of significant ethnic or regional differences in treatment outcomes.16,27
With an increasing population of youth with T2D,28 there is an unmet need for additional treatment options which are effective, well-tolerated and easy to administer. Dapagliflozin is the first oral glucose-lowering therapy since metformin to demonstrate a clinically relevant decrease in HbA1c and a low risk of hypoglycemia in youth with T2D receiving standard of care. These observations are in line with the efficacy predicted in the pediatric PK/PD study19,20 and with findings from previous studies of dapagliflozin in adult populations.16 Given the adverse cardiovascular profile of many young people with T2D, in addition to recent evidence suggesting that risk of long-term complications increases steadily over time,29 the non-glycemic benefits of dapagliflozin reported in adults with/without T2D17,18 is also an important consideration. In summary, this study provides evidence for the efficacy and safety of dapagliflozin as an additional treatment option in this unique population of children, adolescents and young adults living with T2D.
Supplementary Material
Research in context.
Evidence before this study
We searched PubMed (not limited by date) using the following search terms: type 2 diabetes, youth, youth-onset, young, children, adolescents, pediatric, paediatric. We then reviewed the titles and abstracts of the search results to identify clinical trials assessing pharmacological treatment of type 2 diabetes (T2D) in young people. We also considered recommendations and treatment guidelines from any relevant associations and societies. The reference lists of selected articles were also used to further inform our search.
There are numerous analyses from the TODAY (Treatment Options for type 2 Diabetes in Adolescents and Youth) and RISE Peds (Restoring Insulin Secretion Pediatric Medication) clinical trials evaluating oral metformin, or oral metformin plus injectable insulin, in youth with T2D. Both of these agents are approved for use in people ≥10 years of age with T2D and recommended in 2016 consensus guidance from the American Diabetes Association, the American Academy of Pediatrics, the International Society for Pediatric and Adolescent Diabetes, and the Pediatric Endocrine Society.
There is a single Phase 3 study of an oral sulfonylurea (glimepiride) demonstrating comparable efficacy and safety versus metformin but with greater weight gain, and a single Phase 3 study of an injectable glucagon-like peptide-1 receptor agonist (liraglutide) added to metformin with or without insulin, which demonstrated superior efficacy versus the control group but with an increased frequency of gastrointestinal adverse events. Liraglutide, injected subcutaneously once daily, was approved for use in 2019 in people ≥10 years of age with T2D, and is included in the 2020 update of the Standards of Medical Care in Diabetes by the American Diabetes Association for use in children and adolescents with T2D. A second glucagon-like peptide-1 receptor agonist, exenatide extended-release (injected subcutaneously once-weekly), was approved for use in the United States in July 2021 in people ≥10 years of age with T2D.
We also identified a number of Phase 2 studies of other oral glucose-lowering agents in this patient population, including a thiazolidinedione (pioglitazone), dipeptidyl peptidase-4 inhibitors (alogliptin, linagliptin and sitagliptin) and sodium−glucose co-transporter-2 inhibitors (canagliflozin, dapagliflozin, empagliflozin). In October 2021, the European Medicines Agency adopted a positive opinion recommending a change to the terms of the marketing authorization of dapagliflozin to include children ≥10 years of age with T2D, with a final decision by the European Commission expected by January 2022.
Added value of the study
Here we present results from the first Phase 3 study of a sodium−glucose co-transporter-2 inhibitor, dapagliflozin, added to standard of care (metformin, insulin or both) in young people aged 10–<25 years with T2D. We show that dapagliflozin treatment improved glycemic control, with a meaningful decrease in HbA1c over 24 weeks, a low risk of hypoglycemia and no episodes of diabetic ketoacidosis. Dapagliflozin is the first oral glucose-lowering therapy since metformin to demonstrate clinical efficacy and safety in this patient population and provides a significant contribution to the evidence base. A unique aspect of this study was the inclusion of a subset of young adults aged 18–<25 years, an age range consistently under-represented in clinical trials of adults with T2D.
Implications of all the available evidence
Despite multiple agents across several drug classes available to adults with T2D, treatment of youth with T2D is complex and approved treatment options are considerably more limited. Currently only one oral treatment (metformin) and three injectable therapies (insulin, liraglutide and extended-release exenatide) are available, with the potential of an additional oral therapy (dapagliflozin) soon becoming available in Europe. With an increasing population of youth with T2D, and due to weight gain and increased risk of hypoglycemia associated with insulin therapy in particular and adherence issues with injectable therapies in general, oral therapies that are effective, well-tolerated and easy to administer are increasingly desirable treatment options. Phase 2 and 3 studies in youth with T2D (using agents with established efficacy and safety in adult populations), are beginning to emerge. The results of this Phase 3 study show that oral treatment with dapagliflozin represents an additional treatment option for youth with T2D. Large outcome studies have demonstrated the cardiovascular and renal benefits of sodium−glucose co-transporter-2 inhibitors in adult populations. Given that the risk of long-term complications has been shown to increase steadily over time in youth with T2D, treatments that could potentially mitigate this risk are increasingly valuable in this patient population.
Acknowledgements
This study was funded by AstraZeneca. The authors wish to acknowledge Róisín O’Connor, inScience Communications, Springer Healthcare, for medical writing assistance, funded by AstraZeneca.
Declaration of interests
WVT has received consulting fees from AstraZeneca, Boehringer Ingelheim, Novo Nordisk and Medtronic Diabetes. LML has received consulting fees from Provention, Dompe, Insulet, Medtronic, Roche, Janssen, Eli Lilly, Convatec, Dexcom and Novo Nordisk. NS has received grants for medical research from Novo Nordisk, has participated in a Data Safety Monitoring Board or Advisory Board and has received consulting fees and payment/honoraria from Novo Nordisk, Eli Lilly, Boehringer Ingelheim, Sanofi, AstraZeneca and Abbott, and has received support for attending meetings and/or travel from Novo Nordisk, Eli Lilly, Boehringer Ingelheim, Sanofi, AstraZeneca. EI and MVN have nothing to declare. JR is an employee of AstraZeneca. CK and EN are employees and stockholders of AstraZeneca.
Data sharing
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure. The study protocol is available from clinicaltrials.gov (https://clinicaltrials.gov/ct2/show/study/NCT02725593)
References
- 1.Arslanian S, Bacha F, Grey M, Marcus MD, White NH, Zeitler P. Evaluation and Management of Youth-Onset Type 2 Diabetes: A Position Statement by the American Diabetes Association. Diabetes Care 2018; 41(12): 2648–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davies MJ, D’Alessio DA, Fradkin J, et al. Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018; 41(12): 2669–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nadeau KJ, Anderson BJ, Berg EG, et al. Youth-Onset Type 2 Diabetes Consensus Report: Current Status, Challenges, and Priorities. Diabetes Care 2016; 39(9): 1635–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeitler P, Arslanian S, Fu J, et al. ISPAD Clinical Practice Consensus Guidelines 2018: Type 2 diabetes mellitus in youth. Pediatr Diabetes 2018; 19 Suppl 27: 28–46. [DOI] [PubMed] [Google Scholar]
- 5.European Medicine Agency. Paediatric Regulation. 2007. https://www.ema.europa.eu/en/human-regulatory/overview/paediatric-medicines/paediatric-regulation (accessed June 2021.
- 6.US Food & Drug Administration. Pediatric Research Equite Act (PREA). 2003. https://www.fda.gov/drugs/development-resources/pediatric-research-equity-act-prea (accessed June 2021.
- 7.Zeitler P, Chou HS, Copeland KC, Geffner M. Clinical trials in youth-onset type 2 diabetes: needs, barriers, and options. Curr Diab Rep 2015; 15(5): 28. [DOI] [PubMed] [Google Scholar]
- 8.Jones KL, Arslanian S, Peterokova VA, Park JS, Tomlinson MJ. Effect of metformin in pediatric patients with type 2 diabetes: a randomized controlled trial. Diabetes Care 2002; 25(1): 89–94. [DOI] [PubMed] [Google Scholar]
- 9.Today Study Group, Zeitler P, Hirst K, et al. A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med 2012; 366(24): 2247–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Group TS. Safety and tolerability of the treatment of youth-onset type 2 diabetes: the TODAY experience. Diabetes Care 2013; 36(6): 1765–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meyers AG, Hudson J, Cravalho CKL, et al. Metformin treatment and gastrointestinal symptoms in youth: Findings from a large tertiary care referral center. Pediatr Diabetes 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bacha F, El Ghormli L, Arslanian S, et al. Predictors of response to insulin therapy in youth with poorly-controlled type 2 diabetes in the TODAY trial. Pediatr Diabetes 2019; 20(7): 871–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tamborlane WV, Barrientos-Perez M, Fainberg U, et al. Liraglutide in Children and Adolescents with Type 2 Diabetes. N Engl J Med 2019; 381(7): 637–46. [DOI] [PubMed] [Google Scholar]
- 14.Tamborlane WV, Bishai R, Geller D, et al. Once-Weekly Exenatide in Youth with Type 2 Diabetes: A Pivotal Phase III Randomized Study. Diabetes 2021; 70(Supplement 1): 91-LB. [Google Scholar]
- 15.Polonsky WH, Henry RR. Poor medication adherence in type 2 diabetes: recognizing the scope of the problem and its key contributors. Patient Prefer Adherence 2016; 10: 1299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dhillon S Dapagliflozin: A Review in Type 2 Diabetes. Drugs 2019; 79(10): 1135–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med 2019; 381(21): 1995–2008. [DOI] [PubMed] [Google Scholar]
- 18.Heerspink HJL, Stefansson BV, Correa-Rotter R, et al. Dapagliflozin in Patients with Chronic Kidney Disease. N Engl J Med 2020; 383(15): 1436–46. [DOI] [PubMed] [Google Scholar]
- 19.Tirucherai GS, LaCreta F, Ismat FA, Tang W, Boulton DW. Pharmacokinetics and pharmacodynamics of dapagliflozin in children and adolescents with type 2 diabetes mellitus. Diabetes Obes Metab 2016; 18(7): 678–84. [DOI] [PubMed] [Google Scholar]
- 20.Parkinson J, Tang W, Johansson CC, Boulton DW, Hamren B. Comparison of the exposure-response relationship of dapagliflozin in adult and paediatric patients with type 2 diabetes mellitus. Diabetes Obes Metab 2016; 18(7): 685–92. [DOI] [PubMed] [Google Scholar]
- 21.Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care 2013; 36(5): 1384–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ly TT, Maahs DM, Rewers A, et al. ISPAD Clinical Practice Consensus Guidelines 2014. Assessment and management of hypoglycemia in children and adolescents with diabetes. Pediatr Diabetes 2014; 15 Suppl 20: 180–92. [DOI] [PubMed] [Google Scholar]
- 23.Tamborlane WV, Chang P, Kollman C, et al. Eligibility for clinical trials is limited for youth with type 2 diabetes: Insights from the Pediatric Diabetes Consortium T2D Clinic Registry. Pediatr Diabetes 2018; 19(8): 1379–84. [DOI] [PubMed] [Google Scholar]
- 24.European Medicines Agency. Victoza Summary of Product Characteristics. https://www.ema.europa.eu/en/documents/product-information/victoza-epar-product-information_en.pdf (accessed April 2021).
- 25.US Food and Drug Administration. Victoza Prescribing Information https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/022341s031lbl.pdf (accessed April 2021).
- 26.Wexler DJ, Krause-Steinrauf H, Crandall JP, et al. Baseline Characteristics of Randomized Participants in the Glycemia Reduction Approaches in Diabetes: A Comparative Effectiveness Study (GRADE). Diabetes Care 2019; 42(11): 2098–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khunti K, Kosiborod M, Kim DJ, et al. Cardiovascular outcomes with sodium-glucose cotransporter-2 inhibitors vs other glucose-lowering drugs in 13 countries across three continents: analysis of CVD-REAL data. Cardiovasc Diabetol 2021; 20(1): 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Imperatore G, Boyle JP, Thompson TJ, et al. Projections of type 1 and type 2 diabetes burden in the U.S. population aged <20 years through 2050: dynamic modeling of incidence, mortality, and population growth. Diabetes Care 2012; 35(12): 2515–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Group TS, Bjornstad P, Drews KL, et al. Long-Term Complications in Youth-Onset Type 2 Diabetes. N Engl J Med 2021; 385(5): 416–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure. The study protocol is available from clinicaltrials.gov (https://clinicaltrials.gov/ct2/show/study/NCT02725593)


