Summary
Background
The incidence of type 2 diabetes in youth is rising, but treatments remain limited. The objective of the study was to assess the efficacy and safety of an empagliflozin dosing regimen versus placebo and linagliptin versus placebo on glycaemic control in youth with type 2 diabetes.
Methods
In this double-blind, placebo-controlled trial, participants with type 2 diabetes (10–17 years; HbA1c 6·5–10·5% [48–91 mmol/mol]) previously treated with metformin and/or insulin were randomised 1:1:1 to oral empagliflozin 10 mg, oral linagliptin 5 mg, or placebo. Participants in the empagliflozin group who failed to achieve HbA1c less than 7·0% (<53 mmol/mol) by week 12 underwent a second double-blinded randomisation at week 14 in a 1:1 ratio, either remaining on 10 mg dose or increasing to 25 mg. Participants on placebo were re-randomised in a double-blinded manner at week 26 to linagliptin or one of the empagliflozin doses (10 or 25 mg) in a 1:1:1 ratio. Investigators remained blinded throughout the trial and received assignments of blinded medication kits through interactive response technology for all participants at the initial randomisation and for the re-randomisations at weeks 14 and 26. The primary outcome was change from baseline in HbA1c at 26 weeks. For empagliflozin, it was based on a pooled analysis for all participants on empagliflozin. Safety was evaluated to week 52. This trial is registered with ClinicalTrials.gov, NCT03429543.
Findings
Between April 2018 and May 2022, of 262 screened participants, 158 were randomly assigned to treatment (53 to placebo, 52 to empagliflozin 10 mg, and 53 to linagliptin). For the primary outcome, the adjusted mean HbA1c change from baseline at week 26 was −0·84% [−9·2 mmol/mol] in the empagliflozin pooled groups versus placebo (95% confidence interval, CI, −1·50 to −0·19 [–16·4 to −2·1]; p=0·012); the corresponding change from baseline for linagliptin versus placebo was −0·34% [−3·8 mmol/mol] (95% CI, −0·99 to 0·30 [−10·8 to 3·3]; p=0·29). Adverse events occurred in 34 (64·2%), 40 (76·9%), and 37 (71·2%) of participants assigned to placebo, empagliflozin pooled doses group, and linagliptin treatment, respectively, up to week 26. Of these, severe adverse events were reported in 3·8%, 1·9%, and 1·9%, respectively. Hypoglycaemia was the most frequently reported adverse event with higher rates for those on active drug treatment. No severe hypoglycaemia cases were reported.
Interpretation
Empagliflozin provided clinically relevant placebo-corrected reductions in HbA1c, whereas linagliptin did not, and may offer a new treatment option for youth with type 2 diabetes.
Introduction
There has been a global upsurge in childhood overweight and obesity,1,2 leading to an increased occurrence of type 2 diabetes in children and adolescents.3–5 Further, youth-onset type 2 diabetes carries extraordinary risk of complications during early adulthood,6,7 placing a significant burden on the individual, their family, and society.1–3
In youth-onset type 2 diabetes, pathologic processes such as early development of insulin resistance and more rapid deterioration in beta-cell function than in individuals diagnosed in adulthood, lead to worse glycaemic control and an elevated risk of premature diabetes-related complications.4,6,8
Over the past few decades many new therapeutic agents, across different drug classes, have been approved for use in adults with type 2 diabetes.9 By contrast, there is a paucity of treatments for type 2 diabetes in youth. Until recently, metformin and insulin were the only approved and established treatments in children and adolescents.10,11 In the United States, agents from one new drug class, glucagon-like peptide-1 receptor agonists (GLP-1RAs), are now gaining approvals for use in youth with type 2 diabetes, following placebo-controlled studies showing notable reductions in glycated haemoglobin in participants treated with either once-daily liraglutide 1.8 mg, once-weekly exenatide 2 mg, or once-weekly dulaglutide 0.75 mg or 1.5 mg.12–14 Metformin, the longest established treatment for type 2 diabetes in youth other than insulin,15 remains the only globally utilised oral agent as the approved GLP-1RAs and insulin both require injection, which may impact treatment adherence. Thus, there remains an unmet need for additional oral therapies for youth with type 2 diabetes, especially for agents targeting diverse mechanisms of action, to effectively address this disorder. Two such agents include dipeptidyl peptidase-4 (DPP-4) inhibitors and sodium–glucose cotransporter 2 (SGLT2) inhibitors.
Efficacy and safety of the DPP-4 inhibitor sitagliptin as initial16 or add-on therapy17 in youth with type 2 diabetes did not yield durable glycaemic control, despite initial reductions in HbA1c.16,17 A recent study evaluated the efficacy and safety of the SGLT2 inhibitor dapagliflozin against placebo in young people, aged 10–24 years, with type 2 diabetes and revealed a significant difference in HbA1c at 24 weeks favouring dapagliflozin, which was evident only in the per protocol but not the intention-to-treat analysis.18
The SGLT2 inhibitor empagliflozin and DPP-4 inhibitor linagliptin are both well-established treatments for adults with type 2 diabetes. Further, empagliflozin has demonstrated improved cardiovascular and renal outcomes in adults with type 2 diabetes and established cardiovascular disease, in patients with heart failure, and in patients with chronic kidney disease.19–23
Empagliflozin and linagliptin have both been investigated in dose-finding paediatric studies,24,25 supporting investigation of their efficacy and safety in placebo-controlled trials in youth with type 2 diabetes.24,25 However, given the recognised challenges with recruitment in such paediatric studies,26 as well as the dearth of evidence regarding the benefits of screening and early treatment for type 2 diabetes in youth,27–29 new paediatric studies, including those with novel approaches to study design, are needed to overcome these deficits.
A major challenge in all studies in paediatric type 2 diabetes is recruitment, with most individual studies to date reporting findings in less than 200 participants.12–16,18 The DIabetes study of liNAgliptin and eMpagliflozin in children and adOlescents (DINAMO) trial was designed to overcome such recruitment difficulties and, as such, included a novel design. The main objective of DINAMO was to assess the efficacy and safety of a daily dosing regimen with empagliflozin, with a potential dose increase from 10 to 25 mg, and a single dose of linagliptin 5 mg, both compared with a shared placebo group, in children and adolescents with type 2 diabetes. Given the proven efficacy of both SGLT2 inhibitors and DDP-4 inhibitors in adults, we hypothesised that these agents compared with placebo would demonstrate improved glycaemic control in children and adolescents with type 2 diabetes receiving background treatment with metformin and/or insulin along with standard lifestyle efforts directed at diet and exercise management.
Methods
Study design
DINAMO was a global, multicentre, randomised, double-blind, placebo-controlled, parallel group trial in 108 centres and 15 countries. A steering committee of academic paediatric endocrinologists/diabetologists designed the study and provided trial oversight. An independent Data Monitoring Committee (DMC) reviewed unblinded safety data during the trial. A list of committee members and investigators appears in the Supplementary Appendix. During the conduct of the study, the DMC met 10 times (at least every 6 months as defined in the charter) to assess the progress of the clinical trial, including an unblinded safety review, and to recommend to the sponsor whether to continue, modify, or stop the trial. No formal analysis of efficacy data was conducted by the DMC.
The trial was conducted in accordance with principles of the Declaration of Helsinki and International Conference on Harmonization Good Clinical Practice guidelines and was approved by local authorities. Independent ethics committees/institutional review boards approved the protocol at participating centres. Participants provided written informed assent (youth) and consent (parents/guardians) before study entry.
Overall, there were 8 versions of the clinical trial protocol, of which 2 versions (Version 1, dated 29 May 2017, and Version 6, dated 14 Jul 2021) were only submitted to the FDA and never implemented due to the requested changes. Six global amendments were issued (dated 3 Oct 2019, 28 Sep 2020, 14 Dec 2020, 14 Jul 2021, 28 Sep 2021, and 23 May 2022), all of which required Independent Ethics Committees and/or Institutional Review Boards’ approval before implementation. Main changes were related to requests from regulators to revise the statistical analysis plan, modifications due to the COVID-19 pandemic, and the addition of an ancillary study (DINAMO MONO, currently ongoing) that is not the subject of this manuscript. Further details are described in the Supplementary Appendix.
Participants
Eligible participants were aged 10–17 years, inclusive, at the time of randomisation, and had type 2 diabetes for at least 8 weeks prior to screening, with HbA1c ≥6·5% to ≤10·5% (48–91 mmol/mol) at screening and a body-mass index ≥85th percentile at entry into run-in. Inclusion criteria included a negative test for both insulinoma antigen 2 and glutamic acid decarboxylase auto-antibodies as measured by the central laboratory at first visit. Exclusion criteria included any antidiabetic medication (except metformin and/or insulin background therapy, which were continued during the study) within 8 weeks prior to the first visit. Other inclusion and exclusion criteria appear in the Supplementary Appendix. Notably, because of the demonstrated dynamic nature of HbA1c in youth with type 2 diabetes,30 potential participants with modifiable exclusion criteria on initial screening (e.g., HbA1c <6·5% [<48 mmol/mol]) could rescreen up to 5 times.
Randomisation and masking
Participants meeting inclusion criteria were randomly assigned in a 1:1:1 ratio to receive oral doses of linagliptin 5 mg, empagliflozin 10 mg, or placebo once daily for 26 weeks as double-blind, double-dummy medication kits, followed by a double-blind active treatment safety extension period to 52 weeks. Randomisation was stratified by age (<15 years; ≥15 to <18 years) with a cap to ensure at least 30% and no more than 70% of the randomised participants were <15 years old. A cap for sex ensured that between 30% and 70% of the randomised participants were female.
Participants in the empagliflozin group who failed to achieve HbA1c less than 7·0% (<53 mmol/mol) by week 12 underwent a second double-blinded randomisation at week 14 in a 1:1 ratio, either remaining on the empagliflozin 10 mg dose or increasing to the 25 mg dose. Participants on placebo were re-randomised in a double-blinded manner at week 26 to linagliptin or one of the empagliflozin doses (10 or 25 mg) in a 1:1:1 ratio. Investigators remained blinded throughout the trial and received assignments of blinded medication kits through interactive response technology (IRT) for all participants at the initial randomisation and for the re-randomisations at weeks 14 and 26.
The randomisation scheme was generated by an independent group of the sponsor who kept the randomisation confidential up to the database lock of DINAMO. Medication kits were provided by the sponsor to the IRT provider, who also managed the re-supply to the investigational sites.
Procedures
The study design is described above and shown in the Supplementary Appendix (Figure S1). Due to the COVID-19 pandemic, screening was suspended between March and July 2020, extending the enrolment timeline from January 2021 to May 2021 (Figure S2, Supplementary Appendix). Participants already on treatment or in follow-up completed primary/secondary endpoint visits onsite if possible. For all other visits, alternative approaches to study performance, such as phone visits, local laboratory testing, and delivery of study medication via courier, were used if onsite visits were not possible. Further details regarding the impact of the COVID-19 pandemic on this trial are described in the Supplementary Appendix.
Of note, a separate ongoing ancillary and exploratory study, DINAMO MONO (operationally under the same protocol NCT03429543) is evaluating the same treatment regimens but in youth who are treatment naïve or not on active treatment after metformin withdrawal. The DINAMO MONO ancillary study was added during the conduct of the DINAMO study based on a request from regulators and is not the subject of this report.
Insulin was the rescue medication, with protocol guidance for initiation. Insulin or increased dose of insulin could be initiated from the first day of treatment until week 52 in case of acute metabolic decompensation and/or repeatedly elevated blood ketone concentrations. If new insulin treatment or insulin treatment at increased dose (i.e., a dose increase of basal insulin of more than 0·1 IU/kg above the baseline prescribed dose) continued for more than 21 consecutive days, including the weaning phase, then the participant was classified as requiring rescue therapy (further details are in the protocol).
Outcome measures
The primary efficacy endpoint was the change in HbA1c from baseline to 26 weeks. For empagliflozin, it was based on a pooled analysis for all participants on empagliflozin. Secondary endpoints included the changes from baseline to 26 weeks in fasting plasma glucose (FPG), body weight, and systolic and diastolic blood pressure. Safety was assessed based upon adverse events that occurred throughout the 52-week treatment period and were coded using Medical Dictionary for Regulatory Activities (MedDRA), version 25.0.
Statistical analysis
The primary family of hypotheses consisted of the two pairwise comparisons of the treatment effect of empagliflozin pooled doses versus placebo and linagliptin versus placebo, followed by the secondary family of hypotheses comparing the effect of each empagliflozin dosing regimen with placebo. In the primary family of hypotheses, the Hochberg procedure accounted for multiple testing of 2 active treatments, empagliflozin and linagliptin, each versus placebo. Statistical significance could be concluded for one treatment if it achieved p<0·025, or for both treatments if both achieved p<0·05. If, and only if, both primary null hypotheses were rejected, the secondary family of hypotheses comparing two empagliflozin dosing regimens versus placebo could be tested in a hierarchical manner (Figure S3, Supplementary Appendix): (1) a regimen with a dose increase to 25 mg for participants not achieving HbA1c less than 7·0% (53 mmol/mol) at week 12, and (2) a regimen of staying on empagliflozin 10 mg throughout the trial.
The modified intention-to-treat (mITT) set was the basis for primary analyses and included all randomised participants treated with at least one dose of study medication and who had a baseline HbA1c measurement. All available on- and off-treatment HbA1c data at week 26 were included in the primary analysis and missing data were replaced by multiple imputation, imputing missing off-treatment values in active treatment groups based on the primary endpoint distribution in the placebo group. This conservative ‘wash-out’ approach in the active treatment groups considered missing off-treatment data being not at random. Missing on-treatment data or data missing in the placebo group were considered missing at random and multiply imputed following the distribution in the respective treatment group. In total, 500 complete trial imputations were generated, evaluated with an analysis of covariance (ANCOVA) model, and the model estimates were combined using Rubin’s rules to determine the p-values for the hypothesis tests.
The primary endpoint was tested by an ANCOVA model with baseline HbA1c as a continuous covariate and with categorical covariates for treatment and age groups (<15 or ≥15–<18 years). A weighted ANCOVA combined the empagliflozin subgroups either achieving HbA1c less than 7·0% (53 mmol/mol) at week 12 (weight=1) or re-randomised to the empagliflozin regimen of interest at week 14 (weight=2) to test the individual hypotheses in the secondary family of hypotheses. Participants not randomised to the empagliflozin regimen of interest at week 14 were assigned a weight=0. This inverse probability weighting31 accounted for the treatment re-assignment of 50% of the re-randomised empagliflozin participants at week 14 (Figures S4 and S5, Supplementary Appendix). For example, in order to compare the dosing regimen of empagliflozin non-responders who were re-randomised to 25 mg dose with the placebo group, those in the empagliflozin non-responder group who were re-randomised to 10 mg were excluded (weight=0), while the contribution of those re-randomised to 25 mg was doubled (weight=2), along with a weighting of 1 for the placebo group and for the empagliflozin responders who did not undergo re-randomisation at 14 weeks.
Mixed models for repeated measurements (MMRM) provided sensitivity analyses of the primary endpoint by either including all available HbA1c values regardless of start of rescue medication and premature treatment discontinuation or by including only on-treatment HbA1c values prior to the start of rescue medication and premature drug discontinuation. A multiple imputation-based per-protocol analysis explored the impact of important protocol deviations on the primary results.
Pre-planned subgroup analyses of the primary endpoint explored the consistency of the treatment effects across subgroups, using the imputation strategy of the primary analysis. Sensitivity and subgroup analyses appear in the Supplementary Appendix. Secondary and further efficacy endpoints and safety endpoints were only analysed descriptively.
All analyses were pre-planned, apart from the analysis of adverse events in the placebo-treated participants following their re-randomisation at week 26, which was post-hoc. Statistical analysis used SAS software version 9.4. Further details of the statistical analyses appear in the Supplementary Appendix. This study is registered with ClinicalTrials.gov, NCT03429543.
Sample size determination
The sample size of 50 randomised participants per group resulted from a balance of clinical, regulatory, feasibility, and statistical considerations. Based upon previous studies of empagliflozin and/or linagliptin in adults with type 2 diabetes receiving background medication of metformin or insulin, the mean differences in the HbA1c change from baseline to week 26 in the treatment groups compared with the placebo group were estimated. Drop-outs from active treatment groups in the adult studies were taken into account and the mean differences were corrected by assigning these drop-outs with the same mean HbA1c change as observed in the placebo group. The drop-out corrected target mean difference to placebo was estimated as −0·55% for the HbA1c change from baseline to week 26, with a standard deviation (SD) of 0·9% and a resulting standardised effect size of −0.61%. Because the target treatment difference was already corrected for drop-outs, no allowance for drop-out was added to the DINAMO sample size. With 50 participants per treatment group, a treatment difference of −0·55% could be detected with 85% power at a two-sided alpha level of 5%.
Alternative scenarios were considered in the protocol (Supplementary Materials, Protocol Section 7.7.1), and the impact on power was explored for higher and lower SDs and for a two-sided alpha level of 2·5% to account for the stricter of the multiplicity-adjusted significance thresholds according to the Hochberg procedure.
Results
The study enrolment, randomisation, and retention appear in Figure 1. The first participant was screened in April 2018 and last treatment was in May 2022. Of 262 children and adolescents screened, 158 (60·3%) were randomly assigned to a treatment group and 157 received treatment. In total, 24 participants were rescreened and 11 of them were randomised (7% of all randomised participants) after the initial modifiable screen failure reason resolved. Among the 11 participants re-screened and randomised, the majority (n=7) initially failed screening due to HbA1c being out of range. Baseline characteristics were balanced among treatment arms (Table 1) and also balanced across empagliflozin responders and non-responders, other than for glycaemic metrics, which were lowest in the empagliflozin 10 mg responder group (Table S1, Supplementary Appendix). Most participants (68·2%) were from North America, with the remainder from South America (17·2%), Europe (11·5%), and Asia (3·2%). By weeks 26 and 52, 17 (10·8%) and 27 (17·2%), respectively, had discontinued study medication prematurely. Upon enrolment, most participants (91·1%, n=143) were treated with metformin or metformin with insulin, 3·2% (n=5) were treated with insulin only, and 5·7% (n=9) received no anti-diabetic therapy other than diet and exercise due to metformin intolerance. The proportion of participants receiving these various background therapies was similar across groups (Table S2, Supplementary Appendix). Treatment adherence by pill-count at week 26 was similar across groups, with adherence at 89·2% in placebo, 89·6% in empagliflozin pooled, and 91·9% in linagliptin groups (Figure S6, Supplementary Appendix). At week 26, there were no missing on-treatment data. HbA1c data at week 26 were missing for 11 of the 17 participants after treatment discontinuation (missing for 7% of treated participants): placebo (n=3), empagliflozin (n=5), and linagliptin (n=3). The main reason for missing data was complete withdrawal of the participant from the trial.
Figure 1: Participant recruitment and disposition.
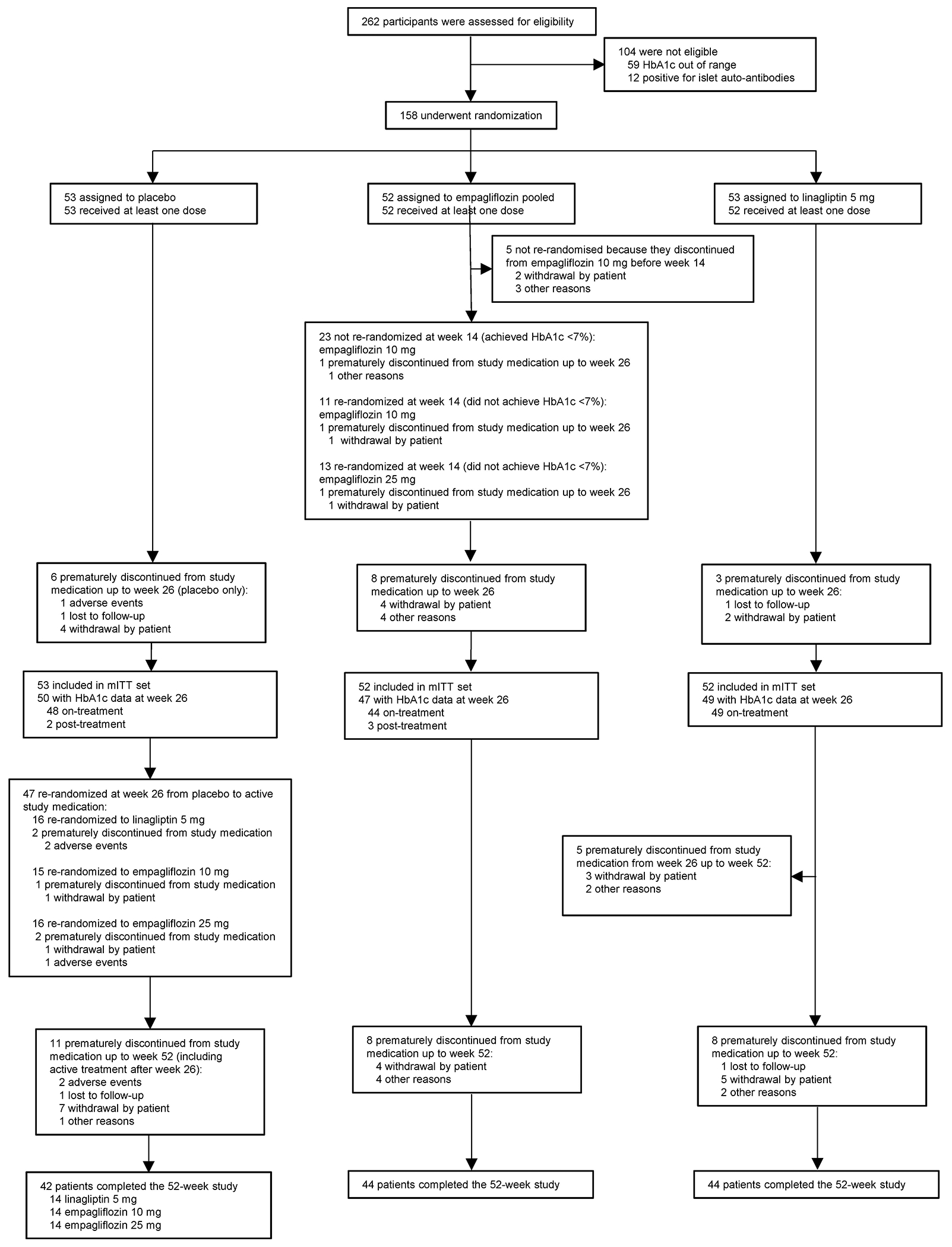
TG1 population.
Among those discontinuing treatment before week 26, on-treatment HbA1c was available for 1 participant.
Reasons for discontinuation of empagliflozin treatment described as ‘other reasons’ included:
1. Exclusionary lab was received more than 2 weeks after randomisation, participant was discontinued per sponsor.
2. Principal investigator decision to discontinue due to non-compliance.
3. Participant has decided to stop taking study medication and, following the study protocol, participant was willing to attend visit 8 only.
4. Despite many attempts to contact participant, person lost to follow-up.
HbA1c, glycated haemoglobin; mITT, modified intention-to-treat; TG, treatment groupings (see Treatment group definitions in this Supplementary Appendix for detailed explanation).
Table 1.
Baseline characteristics of the participants – Treated set
| Characteristic | Placebo (N=53) |
Empagliflozin pooled (N=52) |
Linagliptin 5 mg (N=52) |
Total (N=157) |
|---|---|---|---|---|
| Sex, n (%) | ||||
| Male | 19 (35·8) | 19 (36·5) | 22 (42·3) | 60 (38·2) |
| Female | 34 (64·2) | 33 (63·5) | 30 (57·7) | 97 (61·8) |
| Region, n (%) | ||||
| North America | 34 (64·2) | 36 (69·2) | 37 (71·2) | 107 (68·2) |
| South America | 11 (20·8) | 9 (17·3) | 7 (13·5) | 27 (17·2) |
| Europe | 7 (13·2) | 6 (11·5) | 5 (9·6) | 18 (11·5) |
| Asia | 1 (1·9) | 1 (1·9) | 3 (5·8) | 5 (3·2) |
| Race, n (%) | ||||
| American Indian or Alaska Native | 1 (1·9) | 4 (7·7) | 3 (5·8) | 8 (5·1) |
| Asian | 3 (5·7) | 2 (3·8) | 4 (7·7) | 9 (5·7) |
| Black or African American | 17 (32·1) | 19 (36·5) | 13 (25·0) | 49 (31·2) |
| Native Hawaiian or Other Pacific Islander | 1 (1·9) | 0 (0·0) | 2 (3·8) | 3 (1·9) |
| White | 29 (54·7) | 23 (44·2) | 26 (50·0) | 78 (49·7) |
| All other | 2 (3·8) | 4 (7·7) | 4 (7·7) | 10 (6·4) |
| Ethnicity, n (%) | ||||
| Non-Hispanic or Latino | 32 (60·4) | 35 (67·3) | 30 (57·7) | 97 (61·8) |
| Hispanic or Latino | 21 (39·6) | 17 (32·7) | 22 (42·3) | 60 (38·2) |
| Age, years, mean ± SD | 14·6 ± 1·8 | 14·4 ± 1·9 | 14·6 ± 1·9 | 14·5 ± 1·9 |
| Median (range) | 14·0 (11, 17) | 13·0 (10, 17) | 14·5 (10, 17) | 14·0 (10, 17) |
| Time since diagnosis of diabetes, n (%) | ||||
| <1 year | 18 (34·0) | 17 (32·7) | 16 (30·8) | 51 (32·5) |
| 1 to 3 years | 24 (45·3) | 21 (40·4) | 21 (40·4) | 66 (42·0) |
| >3 years | 11 (20·8) | 14 (26·9) | 15 (28·8) | 40 (25·5) |
| BMI, kg/m2, mean ± SD | 36·07 ± 10·07 | 35·54 ± 7·17 | 36·50 ± 7·55 | 36·04 ± 8·33 |
| Median (range) | 34·62 (65·1, 19·6) | 34·48 (21·1, 56·2) | 34·83 (20·5, 55·2) | 34·65 (19·6, 65·1) |
| BMI z-score, n (%) | ||||
| >2 to ≤3 (Class 1 obesity) | 17 (32·1) | 21 (40·4) | 20 (38·5) | 58 (36·9) |
| >3 (Class 2 to 3 obesity) | 27 (50·9) | 26 (50·0) | 28 (53·8) | 81 (51·6) |
| Weight, kg, mean ± SD | 98·38 ± 29·55 | 98·66 ± 24·35 | 102·76 ± 26·40 | 99·92 ± 26·78 |
| Median (range) | 94·00 (50·7, 168·0) | 93·95 (42·5, 157·0) | 97·55 (43·2, 171·0) | 94·10 (171·0, 42·5) |
| Blood pressure, mean ± SD* | ||||
| SBP | 118·13 ± 11·85 | 120·23 ± 9·97 | 122·31 ± 10·97 | |
| DBP | 72·22 ± 9·27 | 72·03 ± 8·38 | 73·78 ± 8·08 | |
| Fasting C-peptide, nmol/L, mean ± SD | 0·8967 ± 0·4311 | 0·9598 ± 0·5302 | 1·1245 ± 0·6269 | 0·9932 ± 0·5400 |
| Median (range) | 0·8432 (0·036, 1·743) | 0·8449 (0·056, 3·092) | 0·9934 (0·165, 2·980) | 0·8977 (0·036, 3·092) |
| eGFR (Zappitelli), mL/min/1.73 m2, mean ± SD | 124·28 ± 22·96 | 130·09 ± 26·78 | 135·11 ± 36·42 | 129·79 ± 29·39 |
| Median (range) | 123·39 (85·2, 180·0) | 124·62 (90·5, 241·4) | 122·59 (91·3, 282·7) | 123·39 (85·2, 282·7) |
| Tanner scoring score, n (%) | ||||
| 1 | 0 (0·0) | 0 (0·0) | 0 (0·0) | 0 (0·0) |
| 2 to 4 | 21 (39·6) | 24 (46·2) | 19 (36·5) | 64 (40·8) |
| 5 | 32 (60·4) | 28 (53·8) | 33 (63·5) | 93 (59·2) |
| HbA1c, %, mean ± SD | 8·05 ± 1·23 | 8·00 ± 1·29 | 8·05 ± 1·11 | 8·03 ± 1·20 |
| HbA1c, n (%) | ||||
| <8.5% | 37 (69·8) | 36 (69·2) | 31 (59·6) | 104 (66·2) |
| ≥8.5% | 16 (30·2) | 16 (30·8) | 21 (40·4) | 53 (33·8) |
| FPG, mg/dL, mean ± SD | 158·6 ± 53·8 | 154·4 ± 57·8 | 162·8 ± 56·0 | 158·7 ± 55·6 |
| Median (range) | 151·6 (50, 293) | 143·0 (44, 331) | 157·1 (84, 314) | 150·1 (44, 331) |
Data quoted from descriptive analyses over time by treatment, which did not include study totals.
To convert the values for % HbA1c to millimoles per mol, subtract 2·15 and multiply the result by 10·929. To convert the values for plasma glucose to millimoles per liter, multiply by 0·05551. The BMI is the weight in kilograms divided by the square of the height in metres. BMI, body mass index; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose, HbA1c, glycated haemoglobin; mITT, modified intention-to-treat; SBP, systolic blood pressure; SD, standard deviation.
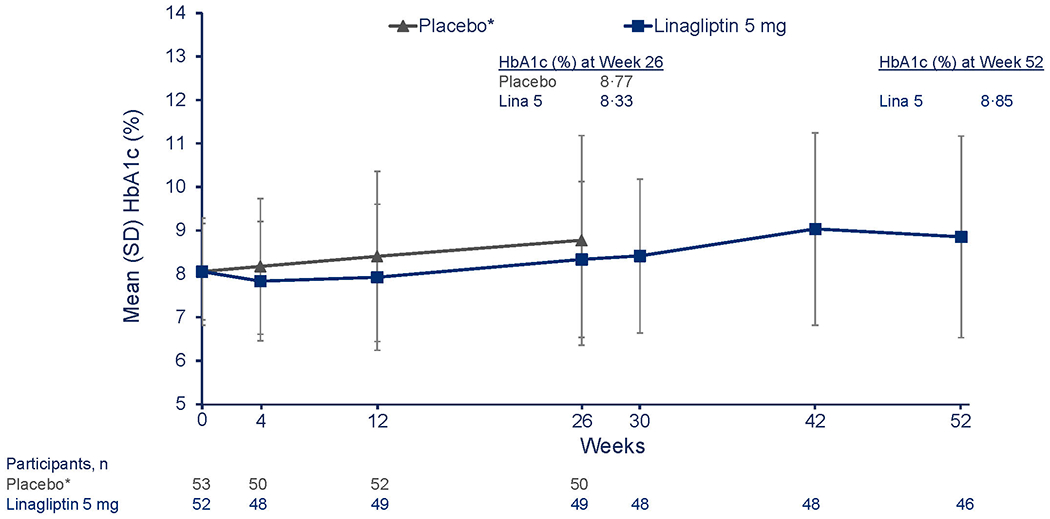
At 26 weeks, the mean change in HbA1c was −0·17% for empagliflozin pooled doses, 0·33% for linagliptin, and 0·68% for the placebo groups (Table 2A). For the primary outcome, the adjusted mean change from baseline in HbA1c at week 26 was −0·84% [−9·2 mmol/mol] in the empagliflozin group versus placebo (95% confidence interval, CI, −1·50 to −0·19 [–16·4 to −2·1]; p=0·012) (Table 2A) and was statistically significant; the corresponding change from baseline for linagliptin versus placebo was −0·34% [–3·8 mmol/mol] (95% CI, −0·99 to 0·30 [−10·8 to 3·3]; p=0·29) (Table 2A). Mean HbA1c through week 52 in the empagliflozin pooled group and linagliptin group appear in Figures 2A and 2B, respectively. In the empagliflozin group, HbA1c decreased by week 4 and remained below the placebo group through week 26, followed by a gradual increase at week 52, albeit below the week 26 value in the placebo group (Figure 2A). In the linagliptin group, there was an initial decrease in HbA1c at week 4, followed by an increase towards baseline values at week 26 (Figure 2B).
Table 2.
Secondary outcomes.
| A. HbA1c [%] change from baseline at Week 26 – mITT set | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Treatment | N analysed | Baseline | Change from baseline | Comparison versus placebo | |||||
| Mean | SD | Adjusted mean | 95% CI | Adjusted mean | 95% CI | ||||
| Primary hypotheses (empagliflozin pooled vs. placebo and linagliptin vs. placebo) | |||||||||
|
| |||||||||
| Placebo | 53 | 8·05 | 1·23 | 0·68 | 0·23 | 1·13 | |||
|
| |||||||||
| Empagliflozin pooled | 52 | 8·00 | 1·29 | −0·17 | −0·64 | 0·31 | −0·84 | −1·50 | −0·19* |
|
| |||||||||
| Linagliptin 5 mg | 52 | 8·05 | 1·11 | 0·33 | −0·13 | 0·79 | −0·34 | −0·99 | 0·30† |
|
| |||||||||
| Secondary hypothesis (empagliflozin responders on 10 mg plus empagliflozin non-responders titrated to 25 mg vs. placebo) | |||||||||
|
| |||||||||
| Placebo | 53 | 8·05 | 1·23 | 0·66 | 0·12 | 1·21 | |||
|
| |||||||||
| Empagliflozin 10 mg +titr 25 mg | 41 | 7·80 | 1·26 | 0·14 | −0·42 | 0·71 | −0·52 | −1·31 | 0·27‡ |
|
| |||||||||
| Secondary hypothesis (empagliflozin responders on 10 mg plus empagliflozin non-responders on 10 mg vs. placebo) | |||||||||
|
| |||||||||
| Placebo | 53 | 8·05 | 1·23 | 0·68 | 0·19 | 1·17 | |||
|
| |||||||||
| Empagliflozin 10 mg +titr 10 mg | 39 | 7·92 | 1·36 | −0·49 | −1·03 | 0·04 | −1·18 | −1·90 | −0·45§ |
| B. Secondary endpoints based on FPG, body weight, SBP and DBP: change from baseline at Week 26 – mITT set | |||||||||
|
| |||||||||
| Treatment | N analysed | Baseline | Change from baseline | Comparison versus placebo | |||||
| Mean | SD | Adjusted mean | 95% CI | Adjusted mean | 95% CI | ||||
|
| |||||||||
| Fasting plasma glucose (mg/dL) | |||||||||
|
| |||||||||
| Placebo | 52 | 158·62 | 53·80 | 15·70 | −0·53 | 31·93 | |||
|
| |||||||||
| Empagliflozin pooled | 48 | 154·43 | 57·78 | −19·48 | −36·39 | −2·57 | −35·18 | −58·61 | −11·74 |
|
| |||||||||
| Linagliptin 5 mg | 51 | 162·81 | 56·01 | 10·29 | −6·12 | 26·69 | −5·41 | −28·49 | 17·67 |
|
| |||||||||
| Body weight (kg) | |||||||||
|
| |||||||||
| Placebo | 52 | 98·87 | 29·62 | −0·04 | −1·40 | 1·32 | |||
|
| |||||||||
| Empagliflozin pooled | 52 | 98·66 | 24·35 | −0·79 | −2·17 | 0·59 | −0·75 | −2·68 | 1·19 |
|
| |||||||||
| Linagliptin 5 mg | 50 | 102·73 | 26·81 | 1·42 | 0·04 | 2·81 | 1·46 | −0·48 | 3·41 |
|
| |||||||||
| Systolic blood pressure (mmHg) | |||||||||
|
| |||||||||
| Placebo | 52 | 118·34 | 11·87 | 1·30 | −1·01 | 3·61 | |||
|
| |||||||||
| Empagliflozin pooled | 52 | 120·23 | 9·97 | −0·12 | −2·47 | 2·24 | −1·42 | −4·72 | 1·88 |
|
| |||||||||
| Linagliptin 5 mg | 50 | 122·39 | 11·13 | 2·21 | −0·14 | 4·56 | 0·91 | −2·40 | 4·22 |
|
| |||||||||
| Diastolic blood pressure (mmHg) | |||||||||
|
| |||||||||
| Placebo | 52 | 72·60 | 8·94 | 0·76 | −1·01 | 2·53 | |||
|
| |||||||||
| Empagliflozin pooled | 52 | 72·03 | 8·38 | 0·78 | −1·04 | 2·60 | 0·02 | −2·52 | 2·56 |
|
| |||||||||
| Linagliptin 5 mg | 50 | 74·01 | 8·13 | 2·26 | 0·46 | 4·05 | 1·50 | −1·03 | 4·02 |
Analyses including all available HbA1c data on treatment, after start of rescue medication and after premature treatment discontinuation
Missing data multiply imputed with ‘wash-out’ approach, using ANCOVA with baseline HbA1c as linear, treatment and age as categorical covariates and applying Rubin’s rules to combine multiple imputations
Mean change from baseline was adjusted for baseline HbA1c and age category for all hypotheses and additionally weighted for secondary hypotheses
Baseline means were not weighted
p-value=0·012,
p-value=0·29,
p-value=0·19,
p-value=0·0015.
To convert the values for % HbA1c to millimoles per mol, subtract 2·15 and multiply the result by 10·929. HbA1c, glycated haemoglobin; mITT, modified intention-to-treat; SD, standard deviation; titr, empagliflozin non-responders titrated to.
Analyses including all available data on treatment, after start of rescue medication and after premature treatment discontinuation
Mean change from baseline was adjusted for baseline value and age category
FPG analysed by ANCOVA with baseline value as linear, treatment and age as categorical covariates, baseline carried forward for missing FPG at week 26
All other endpoints analysed by MMRM including additionally visit as fixed, categorical covariate, interactions of treatment and baseline with visit and patient as random effect with unstructured covariance structure
DBP, diastolic blood pressure; FPG, fasting plasma glucose, mITT, modified intention-to-treat; SBP, systolic blood pressure; SD, standard deviation.
Figure 2: Change in HbA1c from baseline to week 26 and week 52.
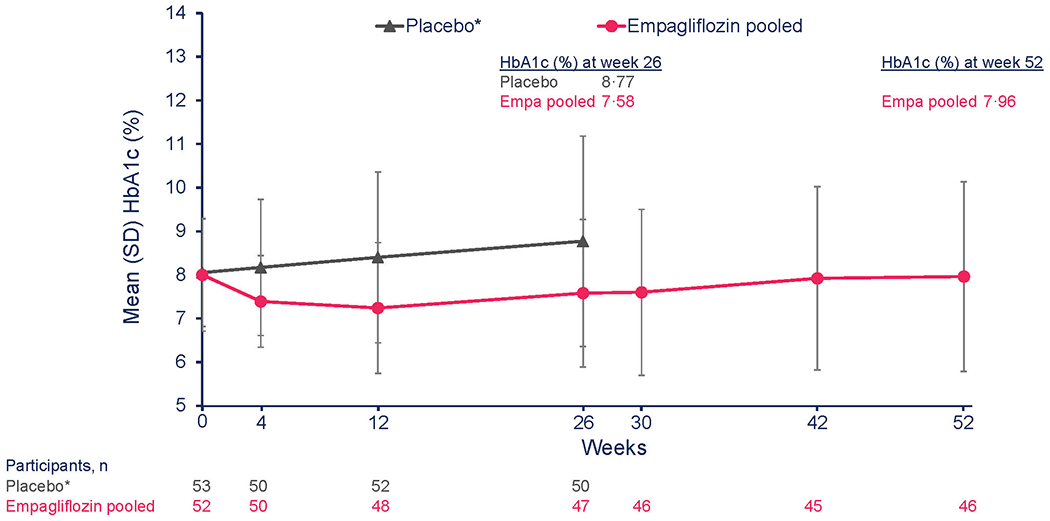
A. Descriptive data reflecting mean HbA1c over time from baseline to week 52 for empagliflozin versus placebo. mITT (TG1, TG5) (OC-AD) population. *Placebo treatment stopped at week 26.
B. Descriptive data reflecting mean HbA1c over time from baseline to week 52 for linagliptin versus placebo. mITT (TG1, TG5) (OC-AD) population. *Placebo treatment stopped at week 26.
To convert the values for % HbA1c to millimoles per mol, subtract 2·15 and multiply the result by 10·929. Empa, empagliflozin; HbA1c; glycated haemoglobin; OC-AD, observed cases (all data, including values after treatment discontinuation and after rescue therapy); Lina, linagliptin; SD, standard deviation; SE, standard error; TG, treatment groupings (see Supplementary Appendix for detailed explanation)
Sensitivity analyses (including MMRM and a per-protocol analysis) were conducted to assess the influence of premature treatment discontinuation, important protocol deviations, and the impact of missing data and its handling on the primary study results. These sensitivity analyses showed treatment differences versus placebo that were generally consistent with the primary analysis for the linagliptin group (Figure S7, Supplementary Appendix). For empagliflozin, the sensitivity analyses showed slightly greater reductions in HbA1c versus placebo. The MMRM (OC) on-treatment analysis, excluding HbA1c values after treatment discontinuation and after rescue medication, resulted in adjusted mean changes of 0.62% (standard error [SE] = 0·22) for placebo and −0·37% (SE = 0·23) for the pooled empagliflozin group with an adjusted mean difference versus placebo of −0·99%.
In addition, subgroup analyses, including sex, age, race, time since diagnosis, and BMI, revealed treatment effects for empagliflozin and linagliptin consistent with the primary endpoint (Figures S8 and S9, Supplementary Appendix). For empagliflozin, there was numerically greater efficacy in participants with high HbA1c and high FPG at baseline (Figure S8, Supplementary Appendix).
By week 26, insulin rescue therapy was initiated in 6 (11·3%) participants in the placebo group, 5 (9·6%) in the empagliflozin pooled group and 4 (7·7%) in the linagliptin group. For the secondary outcomes, the adjusted mean change in FPG was –35·2 mg/dL [–2·0 mmol/L] (95% CI –58·61 to –11·74 [–3·25 to −0·65]) for empagliflozin pooled doses versus placebo and –5·4 mg/dL [−0·3 mmol/L] (95% CI –28·49 to 17·67 [–1·58 to 0·98]) for linagliptin versus placebo (Table 2B). The adjusted mean change in body weight from baseline to week 26 was −0·75 kg in the empagliflozin versus placebo group (95% CI, –2·68 to 1·19); the corresponding change for linagliptin was 1·46 kg (95% CI, −0·48 to 3·41) (Table 2B). The adjusted mean change in systolic blood pressure with empagliflozin versus placebo was –1·42 mmHg (95% CI –4·72 to 1·88), and with linagliptin versus placebo was 0·91 mmHg (95% CI –2·40 to 4·22) (Table 2B). No decreases in diastolic blood pressure from baseline were observed with either agent (Table 2B).
Figure S10 (Supplementary Appendix) demonstrates that, compared with placebo, greater proportions of participants in the empagliflozin pooled group versus placebo group achieved an HbA1c less than 6·5% (48 mmol/mol) (empagliflozin pooled, 11 [21·2%]; placebo, 5 [9·4%]) and less than 7·0% (53 mmol/mol) (empagliflozin pooled, 18 [34·6%]; placebo, 13 [24·5%]) at week 26 (differences versus placebo: 11·7 [95% CI –2·4 to 26·3] and 10·1 [95% CI –7·7 to 28·1], respectively). For empagliflozin, this effect was sustained up to week 52 (Figure S11, Supplementary Appendix).
In a prespecified, descriptive analysis comparing participants receiving empagliflozin 10 and 25 mg doses, the re-randomisation of placebo participants at week 26 (n=47) offered a direct comparison of SGLT2 inhibitor-naïve participants in contrast to those re-randomised at week 14 who failed to achieve HbA1c less than 7·0% (53 mmol/mol) at week 12 on empagliflozin 10 mg. The mean HbA1c values through week 52 in the placebo participants who were re-randomised to empagliflozin 10 (n=15) or 25 mg (n=16) at week 26 appear in Figure S12A (Supplementary Appendix). Mean HbA1c values decreased from weeks 26 to 30 in both the 10 and 25 mg empagliflozin groups of re-randomised placebo participants; from weeks 30 to 52, HbA1c values were lower in participants re-randomised to empagliflozin 25 versus 10 mg. The mean HbA1c from weeks 26 to 52 in those re-randomised to linagliptin at week 26 decreased from weeks 26 to 30, followed by a gradual increase between weeks 30 to 52 (Figure S13, Supplementary Appendix).
The mean HbA1c values between weeks 14 and 52 for the empagliflozin participants who did not achieve target HbA1c less than 7·0% (53 mmol/mol) at week 12 and underwent re-randomisation at week 14 (to empagliflozin 10 mg, n=11; to empagliflozin 25 mg, n=13) appear in Figure S12B (Supplementary Appendix). HbA1c was lower in those re-randomised to empagliflozin 10 versus 25 mg. The weighted adjusted mean change in HbA1c values at week 26 in the dosing regimen up-titrating non-responders to empagliflozin 25 mg was −0·52% [–5·7 mmol/mol] versus the placebo group (95% CI, –1·31 to 0·27 [–14·3 to 2·9]; p=0·19) (Figure S12C, Supplementary Appendix); the corresponding change from baseline in those who remained on 10 mg was –1·18% [–12·9 mmol/mol] (95% CI, –1·90 to −0·45 [–20·8 to –4·9]; p=0·0015) (Figure S12D, Supplementary Appendix).
Safety of active study drug treatment groups was compared with the placebo group up to week 26. Adverse events were reported in 34 (64·2%), 40 (76·9%), and 37 (71·2%) of participants in placebo, empagliflozin pooled, and linagliptin groups at week 26. Of these, severe adverse events were reported in 3·8% of placebo-treated, 1·9% of empagliflozin pooled-treated, and 1·9% of linagliptin-treated participants (Table 3). Hypoglycaemia was the most frequently reported adverse event with higher rates for those on active study drug treatment up to week 26. In the comparison of four groups (placebo, empagliflozin 10 mg responder group, re-randomised 10 mg empagliflozin group, and re-randomised 25 mg empagliflozin group) during weeks 15 to 26, hypoglycaemic events were low and balanced (Table S3, Supplementary Appendix). No severe hypoglycaemia cases requiring assistance were reported. Up to week 26, there was a small number of drug-related adverse events in all groups, with rates across treatment groups generally comparable and driven by the MedDRA preferred term hypoglycaemia. Serious adverse events were reported by 3·8% of participants in each treatment group; none was fatal (Table 3). The occurrence of urinary tract infections was slightly higher in the empagliflozin group than in the placebo and linagliptin treatment groups. No episodes of diabetic ketoacidosis (DKA) nor necrotizing fasciitis were reported with empagliflozin treatment, nor any cases of pancreatitis reported with linagliptin treatment. Overall, from weeks 14 to 26, the rates of adverse events in non-responders re-randomised to empagliflozin 10 and 25 mg were low and comparable with rates in the placebo group (Table S3, Supplementary Appendix ).
Table 3.
Overall summary of adverse events to week 26 – Treated set
| Participants, n (%) | Placebo (N=53) |
Empagliflozin pooled (N=52) |
Linagliptin 5 mg (N=52) |
|---|---|---|---|
| Any adverse event | 34 (64·2) | 40 (76·9) | 37 (71·2) |
| Severe adverse event | 2 (3·8) | 1 (1·9) | 1 (1·9) |
| Drug-related adverse event (investigator-defined) | 6 (11·3) | 9 (17·3) | 9 (17·3) |
| Adverse event leading to discontinuation | 2 (3·8) | 0 (0·0) | 0 (0·0) |
| Serious adverse event | 2 (3·8) | 2 (3·8) | 2 (3·8) |
| Fatal | 0 (0·0) | 0 (0·0) | 0 (0·0) |
| Life-threatening* | 1 (1·9) | 1 (1·9) | 0 (0·0) |
| Persistent or significant disability/incapacity | 0 (0·0) | 0 (0·0) | 0 (0·0) |
| Requiring/prolonging hospitalization* | 2 (3·8) | 2 (3·8) | 0 (0·0) |
| Congenital anomaly/birth defect | 0 (0·0) | 0 (0·0) | 0 (0·0) |
| Other* | 0 (0·0) | 1 (1·9) | 2 (3·8) |
| Other significant adverse events (according to ICH E3) | 1 (1·9) | 0 (0·0) | 0 (0·0) |
| Adverse events of special interest and specific adverse events | |||
| Hypersensitivity reactions | 1 (1·9) | 4 (7·7) | 2 (3·8) |
| Skin lesions | 0 (0·0) | 0 (0·0) | 0 (0·0) |
| Pemphigoid in bullous conditions | 0 (0·0) | 0 (0·0) | 0 (0·0) |
| Pancreatitis | 1 (1·9) | 0 (0·0) | 0 (0·0) |
| Pancreatic cancer | 0 (0·0) | 0 (0·0) | 0 (0·0) |
| Hepatic injury | 1 (1·9) | 2 (3·8) | 2 (3·8) |
| Decreased renal function | 1 (1·9) | 0 (0·0) | 0 (0·0) |
| Diabetic ketoacidosis | 1 (1·9) | 0 (0·0) | 0 (0·0) |
| Increased ketone reported as AE | 2 (3·8) | 2 (3·8) | 4 (7·7) |
| Adverse events leading to lower limb amputation | 0 (0·0) | 0 (0·0) | 0 (0·0) |
| Hypoglycaemia adverse events1) | 5 (9·4) | 12 (23·1) | 10 (19·2) |
| PG <54 mg/dL | 4 (7·5) | 10 (19·2) | 8 (15·4) |
| Severe hypoglycaemia requiring assistance | 0 (0·0) | 0 (0·0) | 0 (0·0) |
| Urinary tract infection | 1 (1·9) | 3 (5·8) | 1 (1·9) |
| Genital infection | 1 (1·9) | 1 (1·9) | 2 (3·8) |
| Acute pyelonephritis or urosepsis | 0 (0·0) | 0 (0·0) | 0 (0·0) |
| Bone fracture | 0 (0·0) | 0 (0·0) | 0 (0·0) |
| Arthralgia | 1 (1·9) | 1 (1·9) | 2 (3·8) |
| Volume depletion | 1 (1·9) | 0 (0·0) | 0 (0·0) |
| Other adverse events in at least >5% of participants | |||
| Infections and infestations | 13 (24·5) | 18 (34·6) | 23 (44·2) |
| Nasopharyngitis | 3 (5·7) | 3 (5·8) | 3 (5·8) |
| Influenza | 0 (0·0) | 0 (0·0) | 3 (5.8) |
| Metabolism and nutrition disorders | 12 (22·6) | 16 (30·8) | 16 (30·8) |
| Vitamin D deficiency | 5 (9·4) | 5 (9·6) | 3 (5·8) |
| Hyperglycaemia | 3 (5·7) | 1 (1·9) | 0 (0·0) |
| Gastrointestinal disorders | 10 (18·9) | 12 (23·1) | 12 (23·1) |
| Abdominal pain | 4 (7·5) | 3 (5·8) | 4 (7·7) |
| Diarrhoea | 5 (9·4) | 2 (3·8) | 3 (5·8) |
| Vomiting | 2 (3·8) | 3 (5·8) | 5 (9·6) |
| Nausea | 3 (5·7) | 3 (5·8) | 3 (5·8) |
| Nervous system disorders | 11 (20·8) | 11 (21·2) | 10 (19·2) |
| Headache | 7 (13·2) | 8 (15·4) | 9 (17·3) |
| Dizziness | 3 (5·7) | 2 (3·8) | 1 (1·9) |
| Respiratory, thoracic and mediastinal disorders | 8 (15·1) | 2 (3·8) | 11 (21·2) |
| Cough | 4 (7·5) | 0 (0·0) | 3 (5·8) |
| Epistaxis | 0 (0·0) | 0 (0·0) | 3 (5·8) |
| Renal and urinary disorders | 3 (5·7) | 1 (1·9) | 6 (11·5) |
| Microalbuminuria | 1 (1·9) | 0 (0·0) | 3 (5·8) |
| Skin and subcutaneous tissue disorders | 1 (1·9) | 5 (9·6) | 4 (7·7) |
| Rash | 0 (0·0) | 3 (5·8) | 1 (1·9) |
| Immune system disorders | 0 (0·0) | 4 (7·7) | 0 (0·0) |
| Seasonal allergy | 0 (0·0) | 4 (7·7) | 0 (0·0) |
TS (TG1) (exposure adjusted) population; MedDRA version used for reporting: 25.0. Definition of serious adverse event includes: death, life-threatening, hospitalisation, prolongation of hospitalization, significant disability, congenital anomaly/birth defect, medical/surgical intervention. Definition of life-threatening adverse event: participant at risk of death (not an event that might cause death if more severe).
For serious adverse events defined as life-threatening, requiring/prolonging hospitalisation, or other, the following were reported. Placebo: 1 patient experienced life-threatening events requiring hospitalisation (splenic vein thrombosis, abdominal pain, pancreatitis acute, systemic inflammatory response syndrome, diabetic ketoacidosis, acute kidney injury, acute respiratory failure, hypovolaemic shock); 1 patient required hospitalisation (hyperglycaemia). Empagliflozin: 1 patient experienced life-threatening events requiring hospitalisation (suicidal ideation) and other events (road traffic accident); 1 patient required hospitalisation (skin candida). Linagliptin: 1 patient experienced other events (breast abscess); 1 patient experienced other events (pneumomediastinum).
MedDRA=Medical Dictionary for Regulatory Activities; TG=treatment groupings (see Supplementary Appendix for detailed explanation); TS=treated set.
Safety was further assessed up to week 52 without a placebo comparison group due to re-randomisation at week 26. Adverse events in the empagliflozin 10 and 25 mg and linagliptin groups following re-randomisation at week 26, as well as adverse events in empagliflozin 10 and 25 mg groups following re-randomisation at week 14 were balanced (Tables S4 and S5, respectively, Supplementary Appendix). Adverse events for empagliflozin pooled doses and linagliptin up to week 52 were also well balanced (Table S6, Supplementary Appendix).
Adverse events of special interest as well as specific adverse events were prespecified in the clinical trial protocol. Adverse events of special interest included hypersensitivity reactions such as angioedema, angioedema-like events, and anaphylaxis (an identified risk with DPP-4 inhibitors), skin lesions (a potential risk with DPP-4 inhibitors), pancreatitis (identified risk with DPP-4 inhibitors), pancreatic cancer (a potential risk with DPP-4 inhibitors), hepatic injury (of potential interest for both investigational drugs), decreased renal function (of potential interest for both investigational drugs), DKA (an identified risk with SGLT2 inhibitors), and events involving lower limb amputation (a potential risk with SGLT2 inhibitors). The analysis of specific adverse events comprised hypoglycaemia, urinary tract infections, genital infections, acute pyelonephritis or urosepsis, bone fracture, arthralgia, pemphigoid in bullous conditions, volume depletion, and ketone measurements reported as an adverse event. Up to weeks 26 and 52, there were few participants with adverse events of special interest, and no observed imbalances in rates among the treatment groups (Table 3 and Table S6, Supplementary Appendix).
Discussion
The DINAMO study is one of the largest studies in youth with type 2 diabetes to date. The study utilised a unique design that included evaluation of two oral treatments – the SGLT2 inhibitor empagliflozin and the DPP-4 inhibitor linagliptin – separately against a single placebo group. The somewhat complex nature of this study design answered the call from regulators and experts for multi-arm efficacy and safety studies in paediatric patients, given the challenges of recruitment in this population,26 and allowed for successful trial completion within four years, despite interruption by the COVID-19 pandemic. All modifications to the study based on the COVID-19 pandemic were assessed based on the CONSERVE 2021 Statement32 and are considered minor changes with no meaningful effect on the study’s objectives or research question, ethical acceptability, internal validity and generalisability, feasibility, or analytical methods and statistical power. Of note, for the primary endpoint analysis, there were no missing HbA1c data at week 26 as a result of the COVID-19 pandemic.
In this study, empagliflozin demonstrated a clinically relevant and statistically significant lowering of HbA1c of about 0·8% versus placebo at week 26. Based on previous paediatric type 2 diabetes studies, a placebo-corrected HbA1c reduction of ≥0·5% is considered as clinically relevant whereas reductions in the range of 0·3% are considered borderline, with uncertainties regarding the clinical relevance.12–18 Similarly, there was a clinically relevant lowering of adjusted mean FPG by about 35 mg/dL at week 26 in the empagliflozin group versus placebo group. During the safety follow-up period, weeks 26 to 52, HbA1c in the empagliflozin pooled group remained lower than the HbA1c in the placebo group at week 26 although the HbA1c had gradually increased, similar to that observed in other paediatric studies,13,18,33 likely reflecting the progressive beta cell failure seen in paediatric patients with T2D.34
In recent studies evaluating the efficacy and safety of the GLP-1RAs dulaglutide (AWARD-PEDS study)12, liraglutide (Ellipse study)13, and exenatide14 in paediatric patients with T2D, the placebo corrected HbA1c reduction were −1·4% for pooled dulaglutide groups; −1·06% with liraglutide, and −0·85% with exenatide respectively. In a recent study with the SGLT2 inhibitor dapagliflozin18, the placebo corrected HbA1c change was −0·75%. Due to differences in trial samples, (e.g., less use of metformin plus insulin in AWARD-PEDS and the Ellipse study, participants aged up to 24 years in the dapagliflozin study), it is difficult to directly compare the efficacy of these different studies. Of note, 43·3% of participants in DINAMO were on insulin therapy, which has been associated with a lack of durable glycaemic control and viewed as a marker of more rapid deterioration of beta cell function.35
Linagliptin did not meet the primary endpoint at week 26. The placebo-corrected HbA1c was lower by about 0·3% with a modest adjusted mean difference in FPG of about −5 mg/dL (−0.3 mmol/L) at week 26 with linagliptin. Furthermore, HbA1c in the linagliptin group gradually increased between weeks 26 and 52, in line with previous paediatric studies of DPP-4 inhibitors, suggesting that these agents do not provide durable improvements in glycaemic control17 in children and adolescents when added to metformin and/or insulin therapy. These agents have demonstrated efficacy in adults with type 2 diabetes, further illustrating that responses to antidiabetic medications in youth with type 2 diabetes differ from those in adults, which may reflect pathophysiologic differences in disease progression.16,34,36 The TODAY33 and RISE34 studies demonstrate the challenges of glycaemic control in youth onset T2D. The challenges appear to arise from a combination of the early development of insulin resistance and more rapid deterioration of beta-cell function in children and adolescents compared with adults with type 2 diabetes. Further, there is the added physiologic insulin resistance of puberty present in adolescents and not adults. Last, BMI in youth onset type 2 diabetes is often higher than that observed in studies of adults with type 2 diabetes.33 These factors may impact the responsiveness to DPP-4 inhibitors in youth with type 2 diabetes.
Other secondary outcomes related to body weight and blood pressure, while in a favourable direction for empagliflozin, did not achieve statistical significance, a finding consistent with other recent paediatric studies of GLP-1RAs12,13 and dapagliflozin18 but in contrast with studies in adults with type 2 diabetes.37 It is unclear why reductions in body weight and blood pressure are not generally seen in paediatric studies.
Re-randomisation of empagliflozin from 10 mg to either 10 or 25 mg at 14 weeks yielded unanticipated results in contrast to the week 52 results among the placebo-treated participants re-randomised to 10 or 25 mg at week 26. The latter group demonstrated the expected dose response, with a larger decrement in HbA1c with 25 versus 10 mg while the former group did not. Although reasons for these contrary observations remain unclear, the small numbers in each subgroup make meaningful interpretation challenging and can yield results susceptible to outliers. Nonetheless, safety profiles of empagliflozin 10 and 25 mg were similar and both empagliflozin and linagliptin treatment demonstrated comparable safety profiles with those established in adults with type 2 diabetes.
There were limitations to the study. Although the novel design of DINAMO helped to overcome the recruitment difficulties seen with previous trials of youth with type 2 diabetes, there was a relatively small number of study participants, limiting detailed subgroup analyses. Thus, future observational studies or clinical trials may help to further assess clinical responsiveness in various subgroups, such as those on insulin only and risk of hypoglycaemia. In addition, much of the clinical trial was conducted during the COVID-19 pandemic, which may have affected the ability of participants to attend in-person clinic visits, limiting personal interactions between study staff and families. Further, the pandemic and its associated travel restrictions may have reduced lifestyle efforts on the part of participants, nonetheless, affecting all treatment groups similarly. Another potential limitation relates to the geographic distribution of the study participants. Although the study was conducted in multiple geographic regions (North and South America, Europe, and Asia), the vast majority of participants was enrolled in the Americas, which may limit generalisability to the broader worldwide population of children and adolescents with type 2 diabetes.
Our findings show that an empagliflozin dosing regimen provides a clinically relevant and statistically significant reduction in HbA1c in youth with type 2 diabetes, whereas linagliptin did not provide statistically significant improvements in glycaemic control. Furthermore, the safety profile of empagliflozin was comparable with that seen in studies in adults, and notable for no episodes of DKA or necrotizing fasciitis reported in these paediatric participants. The results of this trial support the management of type 2 diabetes in youth with orally administered SGLT2 inhibitors that can provide safe and effective lowering of HbA1c.
Supplementary Material
Research in Context.
Evidence before this study
Over the past few decades, there has been a substantial increase in the occurrence of type 2 diabetes in youth due to the epidemic of childhood overweight and obesity. Until recently, there have been limited treatment options for the management of these children and adolescents as only oral metformin and injectable insulin had received regulatory approval for use in youth with type 2 diabetes under 18 years of age. In 2019, the Food and Drug Administration (FDA) and European Medicines Agency (EMA) approved liraglutide as the first daily injectable GLP-1 receptor agonist for use in children aged 10 years and older with type 2 diabetes. The weekly injectable GLP-1 receptor agonists exenatide and dulaglutide have subsequently been studied in placebo-controlled clinical trials in children and adolescents with type 2 diabetes, leading to FDA and EMA regulatory approval of the former and recent FDA approval of the latter with an expectation of EMA approval in early 2023. Nonetheless, despite the availability of multiple oral agents targeting various pathways for the treatment of type 2 diabetes in adults, only one agent, the sodium–glucose cotransporter 2 (SGLT2) inhibitor dapagliflozin, has received regulatory approval from EMA for treatment of type 2 diabetes from age 10 years, based upon a placebo-controlled trial of 72 young people, aged 10 to 24 years old, of whom only 53 were aged 10–17 years.
We searched PubMed from inception to October 25, 2022, with the terms: type 2 diabetes, paediatrics, therapies, and clinical trials to identify studies evaluating pharmacological treatment of type 2 diabetes in children and adolescents. We also assessed screening recommendations for type 2 diabetes in youth and evaluated relevant studies in paediatric type 2 diabetes from the TODAY study group and the Rise consortium. Given the challenges of maintaining durable glycaemic control and the aggressive development of diabetes complications in youth-onset type 2 diabetes, the diabetes community recognised the need to study the efficacy and safety of additional therapeutic agents in children and adolescents with type 2 diabetes.
Added value of the study
In our experience, the DINAMO study is the first successfully completed phase 3 clinical trial utilizing a single placebo group in separate comparisons with two different classes of oral agents for the treatment of type 2 diabetes in youth aged 10 to 17 years old. These two drug classes include the dipeptidyl peptidase-4 (DPP-4) inhibitor linagliptin and the SGLT2 inhibitor empagliflozin, both well studied agents in adults with type 2 diabetes with demonstrated efficacy and safety. The DINAMO study results indicate that oral treatment with empagliflozin provided clinically relevant and statistically significant reductions in HbA1c in youth with type 2 diabetes, whereas linagliptin did not provide statistically significant improvements in glycaemic control. Furthermore, empagliflozin’s safety profile was comparable with that seen in studies of adults with type 2 diabetes.
Implications of all the available evidence
The results of this trial support the management of type 2 diabetes in youth with the SGLT2 inhibitor empagliflozin as it can provide safe and effective lowering of HbA1c. Further, challenges with self-care behaviours related to administration of injectable therapies, such as insulin or GLP-1 receptor agonists, may be avoided with the oral administration of empagliflozin.
Acknowledgements
The authors would like to thank the investigators and participants/parents-guardians of the trial, as well as the Paediatric Type 2 Diabetes Consortium, the Jaeb Center for Health Research Foundation, and Emmes for supporting the conduct of this trial. We also thank Anne Yver, Stefan Hantel, Afshin Salsali, Nima Soleymanlou, and Christy Schroeder from the trial sponsor Boehringer Ingelheim for their invaluable contributions to the planning and management of the trial, and Tess Lam from Syneos Health for her contribution to the analyses. Medical writing assistance, funded by Boehringer Ingelheim, was provided by Charlie Bellinger, BSc of Elevate Scientific Solutions.
Funding:
The Boehringer Ingelheim and Eli Lilly & Company Alliance
Declaration of interests
LML has received consulting fees from Provention, Dompe, Medtronic, Roche, Janssen, Eli Lilly, Dexcom, Novo Nordisk and Vertex. TD has received speaker, advisory panel or research support from AstraZeneca, Bayer, Boehringer Ingelheim, Dexcom, Eli Lilly, Insulet, Lifescan, Medtronic, Novo Nordisk, Roche, and Sanofi, and a shareholder of DreaMed Diabetes, Ltd. GJK has no disclosures to report. WVT has received consulting fees from AstraZeneca, Boehringer Ingelheim, Novo Nordisk, and Medtronic Diabetes. SW has served on a data safety monitoring board for the National Institute of Diabetes and Digestive and Kidney Diseases/NIH, and served on an advisory panel/board for Roche Diagnostics, and Medtronic MiniMed. PZ has consulted for Boehringer Ingelheim, Merck, Eli Lilly, Janssen, I-ACT, and Novo Nordisk. DN and JM are employees of Boehringer Ingelheim.
Data sharing
To ensure independent interpretation of clinical study results and enable authors to fulfil their role and obligations under the ICMJE criteria, Boehringer Ingelheim grants all external authors access to clinical study data pertinent to the development of the publication. In adherence with the Boehringer Ingelheim Policy on Transparency and Publication of Clinical Study Data, scientific and medical researchers can request access to clinical study data when it becomes available on https://vivli.org/, 2 years after publication of the primary manuscript in a peer-reviewed journal. Please visit https://www.mystudywindow.com/msw/datasharing for further information.
References
- 1.Darnton-Hill I, Nishida C, James WP. A life course approach to diet, nutrition and the prevention of chronic diseases. Public Health Nutr 2004; 7(1A): 101–21. [DOI] [PubMed] [Google Scholar]
- 2.Nolan CJ, Damm P, Prentki M. Type 2 diabetes across generations: from pathophysiology to prevention and management. Lancet 2011; 378(9786): 169–81. [DOI] [PubMed] [Google Scholar]
- 3.International Diabetes Federation. IDF Diabetes Atlas. 10th ed. Brussels; 2021. [Google Scholar]
- 4.Al-Saeed AH, Constantino MI, Molyneaux L, et al. An Inverse Relationship Between Age of Type 2 Diabetes Onset and Complication Risk and Mortality: The Impact of Youth-Onset Type 2 Diabetes. Diabetes Care 2016; 39(5): 823–9. [DOI] [PubMed] [Google Scholar]
- 5.Lawrence JM, Divers J, Isom S, et al. Trends in Prevalence of Type 1 and Type 2 Diabetes in Children and Adolescents in the US, 2001-2017. JAMA 2021; 326(8): 717–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.TODAY Study Group. Rapid rise in hypertension and nephropathy in youth with type 2 diabetes: the TODAY clinical trial. Diabetes Care 2013; 36(6): 1735–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.TODAY Study Group, Bjornstad P, Drews KL, et al. Long-Term Complications in Youth-Onset Type 2 Diabetes. N Engl J Med 2021; 385(5): 416–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dabelea D, Stafford JM, Mayer-Davis EJ, et al. Association of Type 1 Diabetes vs Type 2 Diabetes Diagnosed During Childhood and Adolescence With Complications During Teenage Years and Young Adulthood. JAMA 2017; 317(8): 825–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies MJ, Aroda VR, Collins BS, et al. Management of Hyperglycaemia in Type 2 Diabetes, 2022. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arslanian S, Bacha F, Grey M, Marcus MD, White NH, Zeitler P. Evaluation and Management of Youth-Onset Type 2 Diabetes: A Position Statement by the American Diabetes Association. Diabetes Care 2018; 41(12): 2648–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah AS, Zeitler P, Wong J, et al. ISPAD Clinical Practice Consensus Guidelines 2022: Type 2 diabetes mellitus in children and adolescents. Pediatr Diabetes 2022; 23: 872–902. [DOI] [PubMed] [Google Scholar]
- 12.Arslanian SA, Hannon T, Zeitler P, et al. Once-Weekly Dulaglutide for the Treatment of Youths with Type 2 Diabetes. N Engl J Med 2022; 387(5): 433–43. [DOI] [PubMed] [Google Scholar]
- 13.Tamborlane WV, Barrientos-Perez M, Fainberg U, et al. Liraglutide in Children and Adolescents with Type 2 Diabetes. N Engl J Med 2019; 381(7): 637–46. [DOI] [PubMed] [Google Scholar]
- 14.Tamborlane WV, Bishai R, Geller D, et al. Once-Weekly Exenatide in Youth With Type 2 Diabetes. Diabetes Care 2022; 45: 1833–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones KL, Arslanian S, Peterokova VA, Park JS, Tomlinson MJ. Effect of metformin in pediatric patients with type 2 diabetes: a randomized controlled trial. Diabetes Care 2002; 25(1): 89–94. [DOI] [PubMed] [Google Scholar]
- 16.Shankar RR, Zeitler P, Deeb A, et al. A randomized clinical trial of the efficacy and safety of sitagliptin as initial oral therapy in youth with type 2 diabetes. Pediatr Diabetes 2022; 23(2): 173–82. [DOI] [PubMed] [Google Scholar]
- 17.Jalaludin MY, Deeb A, Zeitler P, et al. Efficacy and safety of the addition of sitagliptin to treatment of youth with type 2 diabetes and inadequate glycemic control on metformin without or with insulin. Pediatr Diabetes 2022; 23(2): 183–93. [DOI] [PubMed] [Google Scholar]
- 18.Tamborlane WV, Laffel LM, Shehadeh N, et al. Efficacy and safety of dapagliflozin in children and young adults with type 2 diabetes: a prospective, multicentre, randomised, parallel group, phase 3 study. Lancet Diabetes Endocrinol 2022; 10(5): 341–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anker SD, Butler J, Filippatos G, et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N Engl J Med 2021; 385(16): 1451–61. [DOI] [PubMed] [Google Scholar]
- 20.Packer M, Anker SD, Butler J, et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N Engl J Med 2020; 383(15): 1413–24. [DOI] [PubMed] [Google Scholar]
- 21.Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes. N Engl J Med 2016; 375(4): 323–34. [DOI] [PubMed] [Google Scholar]
- 22.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med 2015; 373(22): 2117–28. [DOI] [PubMed] [Google Scholar]
- 23.Herrington WG, Staplin N, Wanner C, et al. Empagliflozin in Patients with Chronic Kidney Disease. N Engl J Med 2022. [DOI] [PubMed] [Google Scholar]
- 24.Laffel LMB, Tamborlane WV, Yver A, et al. Pharmacokinetic and pharmacodynamic profile of the sodium-glucose co-transporter-2 inhibitor empagliflozin in young people with Type 2 diabetes: a randomized trial. Diabet Med 2018; 35(8): 1096–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tamborlane WV, Laffel LM, Weill J, et al. Randomized, double-blind, placebo-controlled dose-finding study of the dipeptidyl peptidase-4 inhibitor linagliptin in pediatric patients with type 2 diabetes. Pediatr Diabetes 2018; 19(4): 640–8. [DOI] [PubMed] [Google Scholar]
- 26.Karres J, Pratt V, Guettier JM, et al. Joining forces: a call for greater collaboration to study new medicines in children and adolescents with type 2 diabetes. Diabetes Care 2014; 37(10): 2665–7. [DOI] [PubMed] [Google Scholar]
- 27.US Preventative Services Task Force, Mangione CM, Barry MJ, et al. Screening for Prediabetes and Type 2 Diabetes in Children and Adolescents: US Preventive Services Task Force Recommendation Statement. JAMA 2022; 328(10): 963–7. [DOI] [PubMed] [Google Scholar]
- 28.Isganaitis E, Laffel L. Recommendations for Screening Children and Adolescents for Prediabetes and Type 2 Diabetes. JAMA 2022; 328(10): 933–4. [DOI] [PubMed] [Google Scholar]
- 29.Jonas DE, Vander Schaaf EB, Riley S, et al. Screening for Prediabetes and Type 2 Diabetes in Children and Adolescents: Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2022; 328(10): 968–79. [DOI] [PubMed] [Google Scholar]
- 30.Tamborlane WV, Chang P, Kollman C, et al. Eligibility for clinical trials is limited for youth with type 2 diabetes: Insights from the Pediatric Diabetes Consortium T2D Clinic Registry. Pediatr Diabetes 2018; 19(8): 1379–84. [DOI] [PubMed] [Google Scholar]
- 31.Mansournia MA, Altman DG. Inverse probability weighting. BMJ 2016; 352: i189. [DOI] [PubMed] [Google Scholar]
- 32.Orkin AM, Gill PJ, Ghersi D, et al. Guidelines for Reporting Trial Protocols and Completed Trials Modified Due to the COVID-19 Pandemic and Other Extenuating Circumstances: The CONSERVE 2021 Statement. JAMA 2021; 326(3): 257–65. [DOI] [PubMed] [Google Scholar]
- 33.TODAY Study Group, Zeitler P, Hirst K, et al. A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med 2012; 366(24): 2247–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.RISE Consortium. Impact of Insulin and Metformin Versus Metformin Alone on beta-Cell Function in Youth With Impaired Glucose Tolerance or Recently Diagnosed Type 2 Diabetes. Diabetes Care 2018; 41(8): 1717–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bacha F, Cheng P, Gal RL, et al. Initial Presentation of Type 2 Diabetes in Adolescents Predicts Durability of Successful Treatment with Metformin Monotherapy: Insights from the Pediatric Diabetes Consortium T2D Registry. Horm Res Paediatr 2018; 89(1): 47–55. [DOI] [PubMed] [Google Scholar]
- 36.Malik FS, Sauder KA, Isom S, et al. Trends in Glycemic Control Among Youth and Young Adults With Diabetes: The SEARCH for Diabetes in Youth Study. Diabetes Care 2022; 45(2): 285–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsapas A, Karagiannis T, Kakotrichi P, et al. Comparative efficacy of glucose-lowering medications on body weight and blood pressure in patients with type 2 diabetes: A systematic review and network meta-analysis. Diabetes Obes Metab 2021; 23(9): 2116–24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
To ensure independent interpretation of clinical study results and enable authors to fulfil their role and obligations under the ICMJE criteria, Boehringer Ingelheim grants all external authors access to clinical study data pertinent to the development of the publication. In adherence with the Boehringer Ingelheim Policy on Transparency and Publication of Clinical Study Data, scientific and medical researchers can request access to clinical study data when it becomes available on https://vivli.org/, 2 years after publication of the primary manuscript in a peer-reviewed journal. Please visit https://www.mystudywindow.com/msw/datasharing for further information.


