Key Points
Question
In patients with acute ischemic stroke undergoing thrombectomy, does a potential benefit associated with intravenous thrombolysis vary according to treatment times?
Findings
In this individual participant data meta-analysis (n = 2313) of 6 randomized clinical trials, intravenous thrombolysis plus thrombectomy was significantly associated with a favorable shift in functional outcome at 90 days vs thrombectomy alone if the time from symptom onset to expected administration of intravenous thrombolysis was within 2 hours 20 minutes. Thereafter, there was no statistically significant association.
Meaning
The findings indicate that the benefit associated with intravenous thrombolysis prior to thrombectomy was time dependent and lessened with longer times between symptom onset and expected administration of intravenous thrombolysis.
Abstract
Importance
The benefit of intravenous thrombolysis (IVT) for acute ischemic stroke declines with longer time from symptom onset, but it is not known whether a similar time dependency exists for IVT followed by thrombectomy.
Objective
To determine whether the benefit associated with IVT plus thrombectomy vs thrombectomy alone decreases with treatment time from symptom onset.
Design, Setting, and Participants
Individual participant data meta-analysis from 6 randomized clinical trials comparing IVT plus thrombectomy vs thrombectomy alone. Enrollment was between January 2017 and July 2021 at 190 sites in 15 countries. All participants were eligible for IVT and thrombectomy and presented directly at thrombectomy-capable stroke centers (n = 2334). For this meta-analysis, only patients with an anterior circulation large-vessel occlusion were included (n = 2313).
Exposure
Interval from stroke symptom onset to expected administration of IVT and treatment with IVT plus thrombectomy vs thrombectomy alone.
Main Outcomes and Measures
The primary outcome analysis tested whether the association between the allocated treatment (IVT plus thrombectomy vs thrombectomy alone) and disability at 90 days (7-level modified Rankin Scale [mRS] score range, 0 [no symptoms] to 6 [death]; minimal clinically important difference for the rates of mRS scores of 0-2: 1.3%) varied with times from symptom onset to expected administration of IVT.
Results
In 2313 participants (1160 in IVT plus thrombectomy group vs 1153 in thrombectomy alone group; median age, 71 [IQR, 62 to 78] years; 44.3% were female), the median time from symptom onset to expected administration of IVT was 2 hours 28 minutes (IQR, 1 hour 46 minutes to 3 hours 17 minutes). There was a statistically significant interaction between the time from symptom onset to expected administration of IVT and the association of allocated treatment with functional outcomes (ratio of adjusted common odds ratio [OR] per 1-hour delay, 0.84 [95% CI, 0.72 to 0.97], P = .02 for interaction). The benefit of IVT plus thrombectomy decreased with longer times from symptom onset to expected administration of IVT (adjusted common OR for a 1-step mRS score shift toward improvement, 1.49 [95% CI, 1.13 to 1.96] at 1 hour, 1.25 [95% CI, 1.04 to 1.49] at 2 hours, and 1.04 [95% CI, 0.88 to 1.23] at 3 hours). For a mRS score of 0, 1, or 2, the predicted absolute risk difference was 9% (95% CI, 3% to 16%) at 1 hour, 5% (95% CI, 1% to 9%) at 2 hours, and 1% (95% CI, −3% to 5%) at 3 hours. After 2 hours 20 minutes, the benefit associated with IVT plus thrombectomy was not statistically significant and the point estimate crossed the null association at 3 hours 14 minutes.
Conclusions and Relevance
In patients presenting at thrombectomy-capable stroke centers, the benefit associated with IVT plus thrombectomy vs thrombectomy alone was time dependent and statistically significant only if the time from symptom onset to expected administration of IVT was short.
This individual participant data meta-analysis assesses treatment time from stroke symptom onset and disability level after treatment with intravenous thrombolysis plus thrombectomy vs thrombectomy alone among patients who presented directly at thrombectomy-capable stroke centers.
Introduction
Current guidelines recommend administration of intravenous thrombolysis (IVT) before thrombectomy in all eligible patients with large-vessel anterior circulation stroke.1 The Improving Reperfusion Strategies in Ischemic Stroke (IRIS) Collaborators2 pooled individual participant data from 6 randomized clinical trials comparing IVT plus thrombectomy vs thrombectomy alone in participants presenting directly to a thrombectomy-capable center. The results were statistically inconclusive.3 The noninferiority of thrombectomy alone could not be demonstrated using a noninferiority margin of 5% for the absolute difference in the rates of functional independence at 90 days; however, the superiority of IVT plus thrombectomy was also not shown.2
No randomized clinical trial has ever proven a benefit of IVT in patients directly admitted to thrombectomy-capable stroke centers and undergoing thrombectomy. Certain subgroups of patients may benefit from a treatment strategy that combines IVT with thrombectomy, while other subgroups may not benefit or may experience harm from prior IVT.4,5 The time from stroke symptom onset to treatment is one potential factor that could influence the relative effectiveness and safety of administering IVT before thrombectomy.6,7
This study aimed to determine whether the association of treatment with IVT plus thrombectomy vs thrombectomy alone and better outcomes was modified by the time from stroke symptom onset to treatment. Based on previous results and pathophysiological considerations, we hypothesized that there is a benefit associated with IVT plus thrombectomy if IVT is administered early after stroke symptom onset.
Methods
Study Design and Inclusion Criteria
The IRIS Collaborators conducted an individual participant data meta-analyses of randomized clinical trials comparing IVT plus thrombectomy vs thrombectomy alone in participants presenting directly to a thrombectomy-capable stroke center. Only randomized clinical trials using second-generation thrombectomy devices were eligible if published before March 9, 2023.
A systematic search strategy was used (eMethods 1 in Supplement 1) to identify the following 6 randomized clinical trials including 2334 participants: the Direct Endovascular Thrombectomy vs Combined IVT and Endovascular Thrombectomy for Patients With Acute Large Vessel Occlusion in the Anterior Circulation Trial (DEVT),8 the Direct Intraarterial Thrombectomy in Order to Revascularize Acute Ischemic Stroke Patients With Large Vessel Occlusion Efficiently in Chinese Tertiary Hospitals: a Multicenter Randomized Clinical Trial (DIRECT-MT),9 A Randomized Controlled Trial of DIRECT Endovascular Clot Retrieval vs Standard Bridging Thrombolysis With Endovascular Clot Retrieval Within 4.5 Hours of Stroke Onset (DIRECT-SAFE),10 the Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands Investigating the Added Benefit of Intravenous Alteplase (MR CLEAN-NO IV),11 the Direct Mechanical Thrombectomy in Acute LVO Stroke Trial (SKIP),12 and the Solitaire With the Intention for Thrombectomy Plus Intravenous t-PA vs DIRECT Solitaire Stent-Retriever Thrombectomy in Acute Anterior Circulation Stroke Trial (SWIFT DIRECT).13
The IRIS Collaborators agreed to participate in the current collaboration and the anonymized individual patient data were pooled. An overview of the characteristics for each trial appear in eTable 1 in Supplement 1. This preplanned secondary study excluded the participants with basilar artery occlusions.
For the individual trials that were included, written informed consent was obtained from patients before randomization or deferred consent was used in accordance with national legislation in the participating countries. For deferred consent, patients or their legal representatives were asked to provide written informed consent as soon as possible after randomization. All participants or proxies provided consent for data collection in the original trials and all data were anonymized before pooling.
This meta-analysis was prospectively designed and approved by the IRIS executive committee. The methods of data pooling and organizational aspects have been described2,14 and a new dedicated statistical analysis plan for this subanalysis was published online15 and was finalized before conducting the current analysis.
The results and study methods are presented according to the Preferred Reporting Items for Systematic Review and Meta-Analyses of Individual Participant Data.16 The interaction being investigated was evaluated for its credibility using the Instrument to Assess the Credibility of Effect Modification Analyses.17
Definition of Time Parameters and Rationale
In all trials, the time of symptom onset was recorded as either the time point at which the onset of stroke symptoms was observed, or the time at which the participant was last seen well. The time point of randomization was automatically captured in the electronic database. The time point of IVT administration and arterial access for thrombectomy was registered by the local investigators of each trial. The time between the onset of stroke symptoms and the expected administration of IVT was chosen as the main time metric to evaluate the modification of the association of IVT plus thrombectomy with better outcomes.
We used the time between symptom onset and the expected administration of IVT rather than the time between symptom onset and randomization so that reported time metrics are in accordance with international guidelines for IVT.18 These guidelines refer to the times between symptom onset and the administration of IVT derived from randomized clinical trials, which evaluated the heterogeneity of the treatment effect for IVT vs placebo with time.18,19,20 Moreover, the power calculation for this substudy was based on time between symptom onset and administration of IVT. Lastly, there were time delays between randomization and administration of IVT in the included trials,8,9,10,12,13 indicating that the time from symptom onset to randomization would not be the best approximation for treatment times.
For all participants, the time between symptom onset and expected administration of IVT was derived by adding the respective trial’s mean time from randomization to administration of IVT (derived from patients allocated to IVT and thrombectomy and receiving IVT) to the time interval between symptom onset and randomization of each individual patient.20,21 Further explanations and the underlying rationale for this approach appear in eMethods 2 in Supplement 1.
Primary and Secondary Outcomes
The primary outcome was disability at 90 days after stroke according to the ordinal modified Rankin Scale score (7-level variable with scores ranging from 0 [no symptoms] to 6 [death]; minimal clinically important difference for the rates of a modified Rankin Scale score of 0, 1, or 2: 1.3%).22 The secondary outcomes included dichotomized functional outcomes (modified Rankin Scale score of 0, 1, or 2 [referred to as functional independence] vs a score of 3, 4, 5, or 6), early recanalization (defined as absence of treatable occlusion on the first angiography run or successful reperfusion [an expanded Thrombolysis in Cerebral Infarction scale score of 2b, 2c, or 313,23 on the first angiography runs compared with the occlusion on baseline cross-sectional imaging]), and final successful reperfusion (expanded Thrombolysis in Cerebral Infarction scale score of 2b, 2c, or 3 vs 0, 1, or 2a) at the end of the endovascular procedure. The safety outcomes were intracranial hemorrhage (any subtype) and symptomatic intracranial hemorrhage; the ratings and definitions from each individual trial were used in the current analysis.
Statistical Analysis
A detailed description of the analytic approach appears in the statistical analysis plan,15 which was registered online on May 7, 2023 (updated on May 15, 2023, and the updated version was registered on May 21, 2023; eMethods 3 in Supplement 1). The primary research hypothesis was that the benefit associated with IVT plus thrombectomy vs thrombectomy alone is modified by the time between symptom onset to expected administration of IVT.
The research hypothesis was that participants with shorter times between symptom onset and expected administration of IVT would show a greater benefit associated with IVT plus thrombectomy vs thrombectomy alone than participants in whom the times were longer. We assumed a linear association between the log of the adjusted common odds ratio (OR), reflecting the benefit associated with IVT plus thrombectomy, and better outcomes. Other nonlinear model assumptions were also tested (eMethods 3 in Supplement 1).
The analyses were carried out using a superiority framework. This decision was based on the investigators concerns whether it is reasonable to assume that the burden of proof should lie with the simpler rather than the more complex treatment, even when there is no established evidence to support the latter in the studied population.2 Among patients undergoing thrombectomy who are eligible for IVT, there are no data showing a statistically significant benefit associated with IVT, and the burden of proof should therefore lie with IVT plus thrombectomy rather than with thrombectomy alone.2 The power considerations of the proposed analysis appear in eMethods 3 in Supplement 1.
For the primary hypothesis, patients were analyzed according to their randomization group and no exclusions or adjustments were made on the basis of crossover. The analysis was performed using mixed-effects ordinal regression (cumulative link mixed model) to test for an interaction between the treatment allocation and the time from symptom onset to expected administration of IVT and to calculate the odds of a 1-point shift on the modified Rankin Scale score in the direction of better outcomes, which was expressed with the adjusted common OR.
Treatment allocation, time from symptom onset to expected administration of IVT (linear), and their interaction term were used as covariates. Trial and trial × treatment were used as random intercepts and random slopes. The analyses were adjusted for the following variables with previously shown influence on functional outcomes: age, baseline Alberta Stroke Program Early CT Score, atrial fibrillation, occlusion location on baseline computed tomographic angiogram or magnetic resonance angiogram, baseline National Institutes of Health Stroke Scale score, and prestroke modified Rankin Scale score. The model was interpreted by plotting the conditional association of IVT plus thrombectomy with better outcomes against time from symptom onset to expected administration of IVT and by reporting the following cutoff values: the time point at which the lower boundary of the 95% CI of the adjusted common OR (association of IVT plus thrombectomy with better outcomes vs thrombectomy alone) crossed 1 (the association is statistically significant until this time point) and when it crossed 1.053 (until this time point, the association is 97.5% certain to be larger than an absolute difference of 1.3% for a modified Rankin Scale score of 0, 1, or 2 [corresponding to the minimal clinically important difference]) (eMethods 4 in Supplement 1).
Stacked bar charts were used to display the distribution of the modified Rankin Scale scores per allocation group stratified by the time from symptom onset to expected administration of IVT before and after the lower boundary of the 95% CI of the adjusted common OR crossed 1. This stratification was chosen post hoc. In addition, the following cutoffs were defined post hoc: (1) the time point at which the point estimate of the adjusted common OR crossed 1 (the time point at which the model suggests there are no differences in functional outcomes among patients allocated to IVT plus thrombectomy vs thrombectomy alone); (2) the time point at which the upper boundary of the 95% CI for the adjusted common OR crossed 1.222 (the time point at which the model suggests certainty by 97.5% that the difference in functional outcomes is smaller than an absolute difference of 5% in modified Rankin Scale scores of 0-2); and (3) the time point at which the upper boundary of the 95% CI for the adjusted common OR crossed 1.128 (the time point at which the model suggests certainty by 97.5% that the difference in functional outcomes is smaller than an absolute difference of 3% in the modified Rankin Scale scores of 0-2).24 The rationale for cutoff selection, details on the sensitivity analysis performed, and assessment of the model assumptions appear in eMethods 3-4 in Supplement 1.
For exploratory purposes, we also evaluated potential interactions of IVT plus thrombectomy vs thrombectomy alone according to the time between randomization to thrombectomy start (arterial puncture) using the same methods as outlined above. This exploratory analysis was limited to the prespecified outcomes of ordinal modified Rankin Scale score (shift analysis), early recanalization, and symptomatic intracranial hemorrhage (further details appear in eMethods 3 in Supplement 1).
All results are presented so that an adjusted common OR or an OR greater than 1 indicates better outcomes or higher risk (for safety outcomes) with IVT plus thrombectomy vs thrombectomy alone. The absolute risk differences (ARDs) and the corresponding numbers needed to treat were calculated from the primary model based on marginal effects at the mean, bootstrapped simulation procedures, or generalized ORs (eMethods 4 in Supplement 1). The ARDs are provided as rate differences for a modified Rankin Scale score of 0, 1, or 2 at 90 days and for patients with a modified Rankin Scale score at least 1 point lower (less disability).
Missing data were handled with multiple imputation (eMethods 3 in Supplement 1). A total of 5 imputation sets were produced with 10 iterations. All analyses were based on pooled results from the 5 imputed data sets in accordance with Rubin rules (unless stated otherwise). For descriptive results (including tables and stacked bar charts), only the observed values are reported. Two investigators (J.K. and F.C.) and 3 statisticians (L.B., H.L., and D.N.) performed the statistical analyses.
All analyses were conducted using R version 4.2.1 (R Foundation for Statistical Computing; additional details appear in eMethods 4 in Supplement 1). All estimates of association include 95% CIs. The P values are 2-sided with values less than .05 considered statistically significant for the primary outcome analysis. All secondary outcome analyses are exploratory and were not adjusted for type I error inflation. An independent nonauthor senior statistician repeated the primary outcome analysis based on the published statistical analysis plan using another statistical software package (Stata version 17.0; StataCorp).
Results
Six randomized clinical trials comparing IVT plus thrombectomy vs thrombectomy alone were identified (eFigure 1 in Supplement 1). Between January 2017 and July 2021, 2334 participants were enrolled at 190 sites in 15 countries, spanning 4 continents. Participants enrolled in the DIRECT-SAFE trial10 presenting with a basilar artery occlusion were excluded (n = 21), leaving 2313 participants with an anterior-circulation large-vessel occlusion stroke in the final analyses. The pooled data availability was 97.4% for the time metrics (time from symptom onset to randomization and time from randomization to administration of IVT) and all prespecified primary and secondary outcomes; there were 14 650 observations in 15 038 data points (eTable 2 in Supplement 1). The risk of bias was considered low for the included trials (eFigure 2 in Supplement 1).
Of the 2313 participants included (median age, 71 years [IQR, 62-78 years]; 44.3% were female and 55.7% were male; Table 1), 1160 were allocated to IVT (0.9 mg/kg of alteplase, maximum dose of 90 mg [n = 1032]; 0.6 mg/kg of alteplase, maximum dose of 90 mg [n = 103]; or 0.25 mg/kg of tenecteplase [n = 25]) plus thrombectomy and 1153 were allocated to thrombectomy alone. The baseline characteristics by treatment group and by quartiles of time from symptom onset to expected administration of IVT appear in Table 1.
Table 1. Baseline Characteristics Stratified by Treatment Group and Quartiles of Time From Symptom Onset to Expected Administration of Intravenous Thrombolysis (IVT).
| Time from symptom onset to expected administration of IVT, mina | All patients (N = 2313) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 21-106 | 107-148 | 149-197 | 198-742 | |||||||
| IVT + thrombectomy (n = 279) | Thrombectomy alone (n = 294) | IVT + thrombectomy (n = 263) | Thrombectomy alone (n = 322) | IVT + thrombectomy (n = 303) | Thrombectomy alone (n = 273) | IVT + thrombectomy (n = 315) | Thrombectomy alone (n = 264) | IVT + thrombectomy (n = 1160) | Thrombectomy alone (n = 1153) | |
| Age, median (IQR), y | 69 (61-76) | 71 (62-78) | 71 (61-80) | 71 (62-80) | 72 (64-79) | 71 (61-78) | 70 (60-78) | 72 (63-78) | 71 (62-78) | 71 (62-78) |
| Sex, No. (%) | ||||||||||
| Female | 107 (38) | 123 (42) | 129 (49) | 153 (48) | 143 (47) | 112 (41) | 132 (42) | 126 (48) | 511 (44) | 514 (45) |
| Male | 172 (62) | 171 (58) | 134 (51) | 169 (52) | 160 (53) | 161 (59) | 183 (58) | 138 (52) | 649 (56) | 639 (55) |
| NIH Stroke Scale score, median (IQR) [No.]b | 17 (13-20) [279] | 17 (12-21) [292] | 16 (12-20) [262] | 16.5 (12-21) [322] | 16 (12-21) [303] | 16 (11-20) [273] | 17 (12-21) [315] | 16 (12-20) [264] | 16 (12-21) [1160] | 16 (12-20) [1153] |
| Modified Rankin Scale score, No./total (%)c | ||||||||||
| 0 | 225/278 (81) | 237/293 (81) | 229/263 (87) | 261/322 (81) | 257/303 (85) | 237/273 (87) | 278/315 (88) | 231/264 (88) | 989/1159 (85) | 966/1152 (84) |
| 1 | 38/278 (14) | 39/293 (13) | 23/263 (9) | 43/322 (13) | 32/303 (11) | 29/273 (11) | 22/315 (7) | 22/264 (8) | 115/1159 (10) | 133/1152 (12) |
| 2 | 12/278 (4) | 10/293 (3) | 9/263 (3) | 16/322 (5) | 11/303 (4) | 7/273 (3) | 10/315 (3) | 9/264 (3) | 42/1159 (4) | 42/1152 (4) |
| >2 | 3/278 (1) | 7/293 (2) | 2/263 (1) | 2/322 (1) | 3/303 (1) | 0/273 | 5/315 (2) | 2/264 (1) | 13/1159 (1) | 11/1152 (1) |
| History, No./total (%) | ||||||||||
| Atrial fibrillation | 83/279 (30) | 96/292 (33) | 99/263 (38) | 125/322 (39) | 135/303 (45) | 121/273 (44) | 125/315 (40) | 117/263 (44) | 442/1160 (38) | 459/1150 (40) |
| Ischemic stroke | 34/278 (12) | 44/291 (15) | 42/259 (16) | 48/320 (15) | 37/303 (12) | 36/271 (13) | 48/314 (15) | 33/263 (13) | 161/1154 (14) | 161/1145 (14) |
| Diabetes | 42/256 (16) | 42/275 (15) | 36/219 (16) | 43/262 (16) | 60/270 (22) | 37/241 (15) | 47/271 (17) | 40/229 (17) | 185/1016 (18) | 162/1007 (16) |
| Arterial hypertension | 144/279 (52) | 148/292 (51) | 157/259 (61) | 190/320 (59) | 199/301 (66) | 160/272 (59) | 175/315 (56) | 145/264 (55) | 675/1154 (58) | 643/1148 (56) |
| Occlusion location, No./total (%)d | ||||||||||
| Internal carotid artery | 72/279 (26) | 80/287 (28) | 67/262 (26) | 97/320 (30) | 77/301 (26) | 73/269 (27) | 86/312 (28) | 71/261 (27) | 302/1154 (26.0) | 321/1137 (28) |
| First/second segment of the middle cerebral artery | 207/279 (74) | 207/287 (72) | 195/262 (74) | 223/320 (70) | 224/301 (74) | 196/269 (73) | 226/312 (72) | 190/261 (73) | 852/1154 (74) | 816/1137 (72) |
| Tandem carotid lesion, No./total (%)e | 51/273 (19) | 53/286 (19) | 37/257 (14) | 57/313 (18) | 29/299 (9.7) | 29/270 (11) | 44/307 (14) | 40/262 (15) | 161/1136 (14) | 179/1131 (16) |
| Alberta Stroke Program Early CT Score, median (IQR) [No.]f | 9 (8-10) [276] | 9 (8-10) [291] | 9 (7-10) [258] | 8 (7-10) [313] | 9 (7-10) [298] | 9 (7-10) [267] | 8 (6-10) [309] | 8 (7-10) [258] | 9 (7-10) [1141] | 9 (7-10) [1129] |
| Symptom onset to randomization, median (IQR), min | 76.4 (65-86) | 76.0 (63-87) | 118 (107-128) | 119 (107-129) | 162 (152-176) | 163 (150-175) | 221 (203-244) | 223 (204-243) | 144 (98-193) | 134 (96-182) |
| Randomization to administration of IVT, ming | ||||||||||
| Median (IQR) [No.] | 5 (3-11) [248] | 8 (4-13) [241] | 8 (4-13) [268] | 6 (4-12) [278] | 7 (4-12) [1035] | |||||
| Mean (SD) [No.] | 8.4 (9.3) [268] | 10.0 (9.7) [268] | 10.0 (9.7) [268] | 9.2 (9.4) [278] | 9.5 (9.4) [1035] | |||||
| Symptom onset to arterial puncture, median (IQR), min [No.] | 110 (95-125) [270] | 105 (90-121) [283] | 154 (140-168) [260] | 150 (132-163) [317] | 196 (180-215) [301] | 192 (175-210) [269] | 260 (235-282) [304] | 253 (235-275) [260] | 180 (135-230) [1135] | 166 (127-220) [1129] |
| BP, median (IQR), mm Hg [No.] | ||||||||||
| Systolic | 146 (130-166) [256] | 147 (130-167) [278] | 148 (132-168) [219] | 148 (131-163) [266] | 150 (133-166) [273] | 145 (132-160) [242] | 149 (131-165) [270] | 153 (133-168) [230] | 148 (131-167) [1018] | 148 (131-164) [1016] |
| Diastolic | 82 (74-94) [256] | 83 (75-95) [276] | 82 (72-92) [219] | 84 (73-94) [266] | 82 (74-92) [273] | 83 (75-94) [242] | 84 (74-95) [270] | 83 (75-95) [230] | 82 (73-93) [1017] | 83 (74-95) [1014] |
| Glucose level, median (IQR), mg/dL [No.] | 118 (105-138) [254] | 117 (103-139) [278] | 125 (105-150) [219] | 123 (108-141) [259] | 126 (108-157) [267] | 120 (105-144) [234] | 122 (106-151) [261] | 126 (106-153) [227] | 123 (106-148) [1001] | 121 (105-144) [998] |
| Participants by study, No. (%) | ||||||||||
| DEVT8 | 6 (2) | 12 (4) | 22 (8) | 26 (8) | 51 (17) | 40 (15) | 39 (12) | 38 (14) | 118 (10) | 116 (10) |
| DIRECT-MT9 | 38 (14) | 39 (13) | 60 (22) | 73 (23) | 93 (31) | 104 (38) | 138 (44) | 111 (42) | 329 (28) | 327 (28) |
| DIRECT-SAFE10 | 21 (8) | 15 (5) | 42 (16) | 55 (17) | 30 (10) | 31 (11) | 44 (14) | 34 (13) | 137 (12) | 135 (12) |
| MR CLEAN-NO IV11 | 146 (52) | 150 (51) | 44 (17) | 58 (18) | 37 (12) | 34 (13) | 39 (12) | 31 (12) | 266 (23) | 273 (24) |
| SKIP12 | 26 (9) | 31 (11) | 24 (9) | 29 (9) | 34 (11) | 21 (8) | 19 (6) | 20 (8) | 103 (9) | 101 (9) |
| SWIFT DIRECT13 | 42 (15) | 47 (16) | 71 (27) | 81 (25) | 58 (19) | 43 (16) | 36 (11) | 30 (11) | 207 (18) | 201 (17) |
Abbreviations: BP, blood pressure; DEVT, Direct Endovascular Thrombectomy vs Combined IVT and Endovascular Thrombectomy for Patients With Acute Large Vessel Occlusion in the Anterior Circulation Trial; DIRECT-MT, Direct Intraarterial Thrombectomy in Order to Revascularize Acute Ischemic Stroke Patients With Large Vessel Occlusion Efficiently in Chinese Tertiary Hospitals: a Multicenter Randomized Clinical Trial; DIRECT-SAFE, A Randomized Controlled Trial of DIRECT Endovascular Clot Retrieval vs Standard Bridging Thrombolysis With Endovascular Clot Retrieval Within 4.5 Hours of Stroke Onset; MR CLEAN-NO IV, Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands Investigating the Added Benefit of Intravenous Alteplase; NIH, National Institutes of Health; SKIP, Direct Mechanical Thrombectomy in Acute LVO Stroke Trial; SWIFT DIRECT, Solitaire With the Intention for Thrombectomy Plus Intravenous t-PA vs DIRECT Solitaire Stent-Retriever Thrombectomy in Acute Anterior Circulation Stroke Trial.
SI conversion factor: To convert glucose to mmol/L, multiply by 0.0555.
The times were calculated as outlined in the Methods section.
Scores range from 0 to 42; higher scores indicate more severe neurological deficits.
Scores range from 0 (no symptoms) to 6 (death); higher scores indicate more severe disability.
Detected by computed tomographic angiography or magnetic resonance angiography.
Defined as high-grade carotid stenosis or occlusion based on admission imaging (computed tomographic angiography or magnetic resonance angiography) or first catheter angiography series during endovascular treatment, depending on the information available within each trial.
Scores range from 0 to 10; lower scores indicate a large extend of imaging signs indicating likely irreversible ischemia.
Calculated for patients allocated to IVT plus thrombectomy.
The median time from symptom onset to randomization was 2 hours 18 minutes (IQR, 1 hour and 37 minutes to 3 hours 7 minutes). Among the participants who were allocated to and received IVT before thrombectomy, the median time was 7 minutes (IQR, 4-12 minutes) from randomization to administration of IVT and the mean time was 9.5 minutes (SD, 9.4 minutes) (eFigure 3 in Supplement 1). The median time from symptom onset to expected administration of IVT was 2 hours 28 minutes (IQR, 1 hour and 46 minutes to 3 hours 17 minutes) (eFigure 4 in Supplement 1; an overview of treatment times appears in eFigure 5 in Supplement 1). In the overall cohort, there was no statistically significant between-group difference in the degree of disability at 90 days (adjusted common OR, 1.12 [95% CI, 0.96-1.32]).2
Primary Outcome Analysis
The descriptive outcome data appear in Table 2. In the overall population, the grade of disability at 90 days significantly increased with longer times from symptom onset to expected administration of IVT (adjusted common OR, 0.81 [95% CI, 0.75-0.87] for better outcomes per hour delay in all patients derived from the primary model). In the early time window, there was a significant benefit associated with IVT plus thrombectomy vs thrombectomy alone (Figure 1). This beneficial association lessened with longer times from symptom onset to expected administration of IVT. For every hour of delay, there was a significant reduction in the association of IVT plus thrombectomy with better outcomes (ratio of adjusted common OR, 0.84 [95% CI, 0.72-0.97] per hour delay, P = .02 for interaction; Table 3).
Table 2. Primary and Secondary Outcomes Stratified by Treatment Group and Time From Symptom Onset to Expected Administration of Intravenous Thrombolysis (IVT).
| Time from symptom onset to expected administration of IVT, mina | ||||||||
|---|---|---|---|---|---|---|---|---|
| 21-106 | 107-148 | 149-197 | 198-742 | |||||
| IVT + thrombectomy (n = 279) | Thrombectomy alone (n = 294) | IVT + thrombectomy (n = 263) | Thrombectomy alone (n = 322) | IVT + thrombectomy (n = 303) | Thrombectomy alone (n = 273) | IVT + thrombectomy (n = 315) | Thrombectomy alone (n = 264) | |
| Primary outcome b | ||||||||
| Modified Rankin Scale score, median (IQR) [No.] | 2 (1-4) [279] | 2 (1-4) [293] | 2 (1-4) [263] | 2 (1-4) [322] | 2 (1-4) [303] | 3 (1-5) [273] | 2 (1-4) [314] | 3 (1-5) [263] |
| Modified Rankin Scale score, No./total (%)c | ||||||||
| 0 | 49/279 (18) | 29/293 (10) | 38/263 (14) | 49/322 (15) | 45/303 (15) | 42/273 (15) | 33/314 (11) | 23/263 (9) |
| 1 | 39/279 (14) | 51/293 (17) | 60/263 (23) | 65/322 (20) | 53/303 (17) | 46/273 (17) | 37/314 (12) | 39/263 (15) |
| 2 | 85/279 (30) | 77/293 (26) | 48/263 (18) | 52/322 (16) | 56/303 (18) | 42/273 (15) | 45/314 (14) | 49/263 (19) |
| 3 | 34/279 (12) | 41/293 (14) | 36/263 (14) | 39/322 (12) | 56/303 (18) | 40/273 (15) | 45/314 (14) | 55/263 (21) |
| 4 | 20/279 (7) | 29/293 (10) | 25/263 (10) | 37/322 (11) | 32/303 (11) | 26/273 (10) | 48/314 (15) | 26/263 (10) |
| 5 | 20/279 (7) | 26/293 (9) | 23/263 (9) | 39/322 (12) | 28/303 (9) | 28/273 (10) | 44/314 (14) | 19/263 (7) |
| 6 | 32/279 (11) | 40/293 (14) | 33/263 (13) | 41/322 (13) | 45/303 (15) | 49/273 (18) | 62/314 (20) | 52/263 (20) |
| Secondary outcomes, No./total (%) | ||||||||
| Modified Rankin Scale score of 0, 1, or 2d | 173/279 (62) | 157/293 (54) | 146/263 (56) | 166/322 (52) | 154/303 (51) | 130/273 (48) | 115/314 (37) | 111/263 (42) |
| Early recanalization | 7/269 (3) | 5/277 (2) | 7/259 (3) | 8/313 (3) | 18/296 (6) | 5/263 (2) | 13/301 (4) | 1/255 (<1) |
| Expanded Thrombolysis in Cerebral Infarction scale score of 2b, 2c, or 3e | 238/264 (90) | 220/268 (82) | 228/251 (91) | 270/310 (87) | 258/290 (89) | 219/263 (83) | 249/296 (84) | 212/252 (84) |
| Intracranial hemorrhage | ||||||||
| Any | 76/263 (29) | 73/278 (26) | 84/262 (32) | 93/318 (29) | 108/296 (36) | 96/271 (35) | 139/309 (45) | 93/260 (36) |
| Symptomatic | 11/279 (4) | 10/294 (3) | 16/262 (6) | 12/322 (4) | 13/301 (4) | 10/272 (4) | 23/314 (7) | 18/264 (7) |
The times were calculated as outlined in the Methods section.
The primary outcome analysis tested whether the association between the allocated treatment (IVT + thrombectomy vs thrombectomy alone) and disability at 3 months (assessed using the modified Rankin Scale scores) varied with times from symptom onset to expected administration of IVT.
Scores were used to assess 90-day disability. A score of 0 indicates no symptoms and a score of 6 indicates death.
Scores of 0, 1, and 2 indicate functional independence.
A score of 2b indicates good reperfusion; 2c, near-perfect reperfusion; and 3, complete or 100% reperfusion.
Figure 1. Influence of the Time From Symptom Onset to Expected Administration of Intravenous Thrombolysis on the Benefit Associated With Intravenous Thrombolysis Plus Thrombectomy.
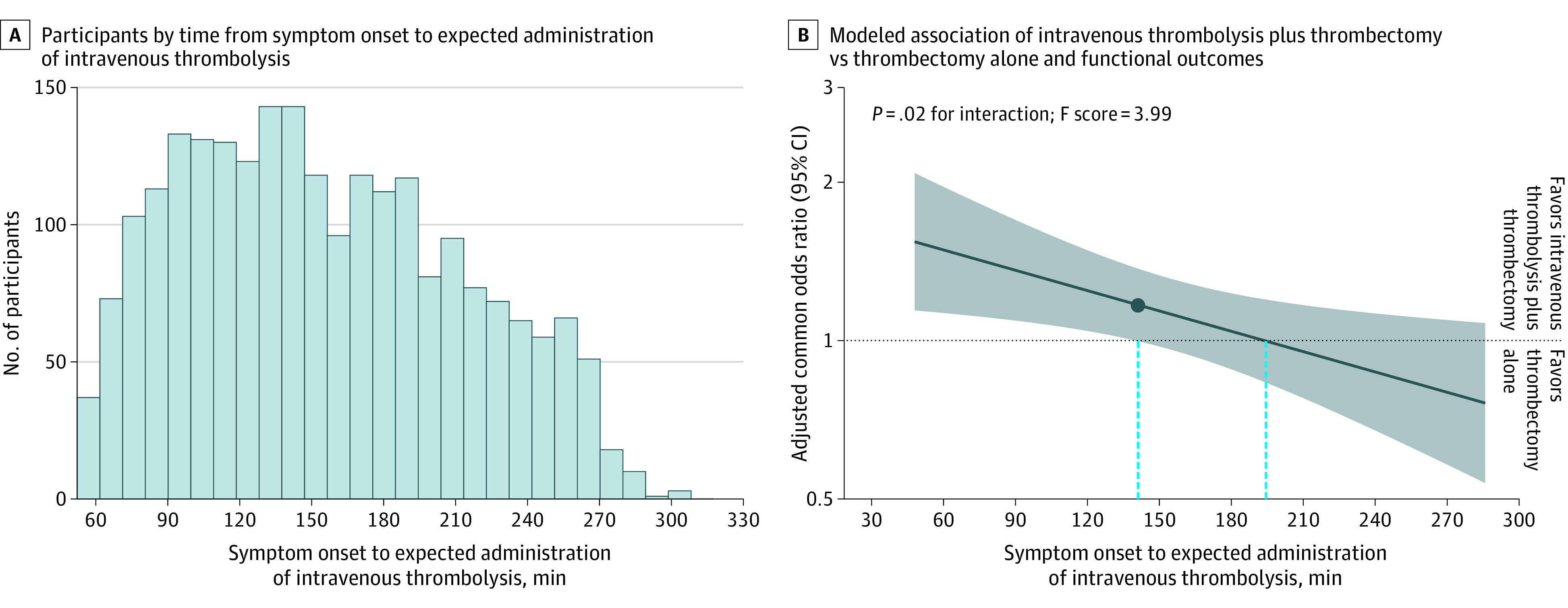
In A, there were 14 patients omitted from the graph (had symptom onset to expected administration of intravenous thrombolysis times of <45 minutes or >320 minutes). In B, the solid dark blue line indicates the best model fit of the log odds ratio for a favorable shift in the modified Rankin Scale scores at 90 days associated with intravenous thrombolysis plus thrombectomy vs thrombectomy alone and treatment delay between symptom onset to expected administration of intravenous thrombolysis. The shaded area reflects the 95% CI for the adjusted common odds ratio. An adjusted common odds ratio greater than 1 indicates better outcomes (favorable shifts in the modified Rankin Scale scores) associated with treatment with intravenous thrombolysis plus thrombectomy. The dashed blue lines indicate the time points at which the lower bound of the 95% CI and the point estimate crossed 1.0.
Table 3. Interaction of Symptom Onset to Expected Administration of Intravenous Thrombolysis (IVT) With the Association of Treatment Allocation and Outcomes.
| Estimated association for time from symptom onset to expected treatment with IVT + thrombectomy, adjusted OR (95% CI)a | Ratio of adjusted OR (95% CI) per 1-h delay | P value for interaction | Direction of the association with increasing time from symptom onset to expected administration of IVT | ||||
|---|---|---|---|---|---|---|---|
| At 1 h | At 2 h | At 3 h | At 4 h | ||||
| Primary outcome | |||||||
| Shift in modified Rankin Scale score, adjusted common OR (95% CI) | 1.49 (1.13 to 1.96) | 1.25 (1.04 to 1.49) | 1.04 (0.88 to 1.23) | 0.87 (0.68 to 1.13) | 0.84 (0.72 to 0.97) | .02 | The benefit associated with IVT + thrombectomy lessened |
| Predictions based on the primary outcome | |||||||
| Predicted ARD for participants with better outcomes (≥1 point lower on modified Rankin Scale), % (95% CI)b,c | 12 (3 to 21) | 7 (1 to 12) | 1 (−4 to 6) | −4 (−13 to 4) | |||
| Predicted NNT (95% CI) so that 1 additional patient has a better outcome (≥1 point lower on modified Rankin Scale)c,d | 9 (5 to 30) | 16 (9 to 99) | 91 (16 to −23) | −22 (27 to −7) | |||
| Predicted ARD for participants with a modified Rankin Scale score of 0, 1, or 2, % (95% CI)e | 9 (3 to 16) | 5 (1 to 9) | 1 (−3 to 5) | −3 (−8 to 3) | |||
| Predicted NNT (95% CI) so that 1 additional patient has a modified Rankin Scale score of 0, 1, or 2 | 11 (7 to 35) | 20 (11 to 106) | 107 (22 to −35) | −34 (40 to −11) | |||
| Secondary outcomes | |||||||
| Modified Rankin Scale score of 0, 1, or 2f | 1.54 (1.10 to 2.17) | 1.26 (1.02 to 1.56) | 1.03 (0.84 to 1.27) | 0.84 (0.61 to 1.17) | 0.82 (0.68 to 0.98) | .03 | The benefit associated with IVT + thrombectomy lessened |
| Early recanalization | 0.95 (0.36 to 2.54) | 1.82 (0.89 to 3.73) | 3.48 (1.44 to 8.42) | 6.66 (1.76 to 25.15) | 1.91 (1.05 to 3.48) | .03 | The benefit associated with IVT + thrombectomy increased |
| Expanded Thrombolysis in Cerebral Infarction score of 2b, 2c, or 3 at the end of the interventiong | 2.06 (1.29 to 3.29) | 1.77 (1.26 to 2.47) | 1.52 (1.10 to 2.08) | 1.30 (0.85 to 1.99) | 0.86 (0.66 to 1.11) | .26 | No statistically significant interaction |
| Intracranial hemorrhage | |||||||
| Any | 1.10 (0.76 to 1.58) | 1.17 (0.93 to 1.47) | 1.25 (1.02 to 1.52) | 1.33 (0.98 to 1.80) | 1.07 (0.89 to 1.28) | .49 | No statistically significant interaction |
| Symptomatic | 1.46 (0.68 to 3.11) | 1.42 (0.83 to 2.40) | 1.37 (0.88 to 2.15) | 1.33 (0.74 to 2.39) | 0.97 (0.70 to 1.35) | .86 | No statistically significant interaction |
Abbreviations: ARD, absolute risk difference; NNT, number needed to treat; OR, odds ratio.
The associations were derived from the primary analysis model. Instead of an adjusted OR, the first row contains adjusted common ORs (as indicated in column 1). Under predictions based on the primary outcome, 2 of the rows contain NNTs and 2 of the rows contain ARDs (as indicated in column 1). The bottom half of the Table (under secondary outcomes) contains adjusted ORs.
Positive values represent patients having a better outcome (≥1 point lower on the modified Rankin Scale indicates less disability) if treated with IVT plus thrombectomy. Negative values represent patients having a poorer outcome (≥1 point higher on the modified Rankin Scale indicates more disability).
The ARDs and NNTs were estimated using simulation processes, marginal probabilities at the mean, and generalized ORs (eMethods 4 in Supplement 1), therefore, the results and cutoff points may differ slightly compared with the model presented in Figure 1.
The NNTs are expressed so that 1 patient has at least 1 point lower on the modified Rankin Scale (less disability) if treated with IVT plus thrombectomy. Negative values represent the number needed to harm so that 1 patient has at least 1 point higher on the modified Rankin Scale (more disability) if treated with IVT plus thrombectomy.
The ARDs were derived from predicted probabilities of a modified Rankin Scale score of 0, 1, or 2 derived directly from the ordinal model (primary analysis) using marginal probabilities at the mean (eMethods 4 in Supplement 1).
Scores of 0, 1, and 2 indicate functional independence.
A score of 2b indicates a good recanalization result; 2c, near-perfect reperfusion; and 3, complete or 100% reperfusion.
At 1 hour after symptom onset, the adjusted common OR representing the estimated association of IVT before thrombectomy and improvement in the modified Rankin Scale score by 1 point was 1.49 (95% CI, 1.13-1.96) with a predicted ARD of 9% (95% CI, 3%-16%) to achieve a modified Rankin Scale score of 0, 1, or 2. At 1 hour and 30 minutes after symptom onset, the adjusted common OR was 1.36 (95% CI, 1.09-1.70) with a predicted ARD of 7% (95% CI, 2%-13%) to achieve a modified Rankin Scale score of 0, 1, or 2. At 2 hours after symptom onset, the adjusted common OR was 1.25 (95% CI, 1.04-1.49) with a predicted ARD of 5% (95% CI, 1%-9%) to achieve a modified Rankin Scale score of 0, 1, or 2 (Table 3 and eFigure 6 in Supplement 1). The numbers needed to treat so that 1 more participant has a lower score by at least 1 point (less disability) on the modified Rankin Scale at 90 days were 9 (95% CI, 5-30) at 1 hour, 11 (95% CI, 7-42) at 1 hour and 30 minutes, and 16 (95% CI, 9-99) at 2 hours (Table 3 and eFigure 6 in Supplement 1).
The estimated symptom onset to expected time of administration of IVT was 2 hours 20 minutes at which the lower boundary of the 95% CI for the estimated benefit associated with IVT before thrombectomy first crossed the line of no association (1.0). For the times from symptom onset to expected administration of IVT that were longer than 2 hours 20 minutes, there was no statistically significant association. The point estimate crossed the line of no association at 3 hours 14 minutes (Figure 1).
The benefit associated with IVT plus thrombectomy was estimated to be statistically significantly greater than the minimal clinically important difference if the expected administration of IVT occurred within 1 hour and 55 minutes after symptom onset (eFigure 7 in Supplement 1). According to the model, the association of IVT plus thrombectomy and better outcomes with ARDs greater than 5% or 3% in the rates for modified Rankin Scale scores of 0, 1, or 2 can be excluded with 97.5% certainty if the time from onset to expected administration of IVT was longer than 3 hours 3 minutes or longer than 3 hours 59 minutes, respectively (eFigure 7 in Supplement 1). The credibility of the interaction analysis was rated as moderate according to the Instrument to Assess the Credibility of Effect Modification Analyses17 (eResults in Supplement 1).
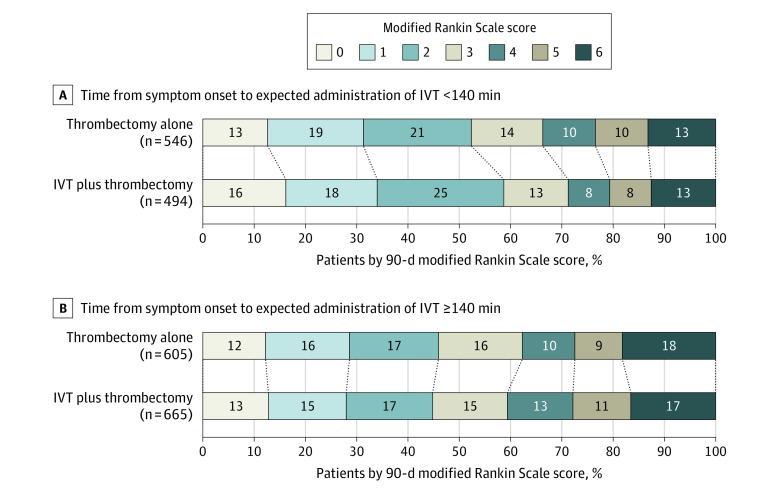
The decline in the association of IVT before thrombectomy with better outcomes was qualitatively comparable with the data reported in the trials comparing IVT with placebo; however, the overall magnitude of the observed associations was smaller in the data included in the current study (eFigure 8 in Supplement 1). Stratifying participants by expected treatment before vs after 2 hours 20 minutes showed favorable shifts in functional outcomes associated with IVT plus thrombectomy in the early time window (adjusted common OR, 1.31 [95% CI, 1.04-1.66] for <2 hours 20 minutes and a median modified Rankin Scale score of 2 [IQR, 1-4] for IVT plus thrombectomy vs 2 [IQR, 1-4] for thrombectomy alone), but not in the later time window (adjusted common OR, 0.97 [95% CI, 0.79-1.19] for ≥2 hours 20 minutes and a median modified Rankin Scale score of 3 [IQR, 1-5] for IVT plus thrombectomy vs 3 [IQR, 1-5] for thrombectomy alone) (Figure 2).
Figure 2. Distribution of the 90-Day Modified Rankin Scale Scores by Treatment Group and Dichotomized Intervals From Symptom Onset to Expected Administration of Intravenous Thrombolysis (IVT).
If IVT was administered within 2 hours 20 minutes after stroke onset, there was a favorable shift across the modified Rankin Scale scores at 90 days associated with IVT plus thrombectomy vs thrombectomy alone (median modified Rankin Scale score of 2 [IQR, 1-4] for the IVT plus thrombectomy group vs 2 [IQR, 1-4] for the thrombectomy alone group; adjusted common odds ratio, 1.31 [95% CI, 1.04-1.66]). No between-group differences were observed in participants treated after 2 hours 20 minutes (median modified Rankin Scale score of 3 [IQR, 1-5] for the IVT plus thrombectomy group vs 3 [IQR, 1-5] for the thrombectomy alone group; adjusted common odds ratio, 0.97 [95% CI, 0.79-1.19]). The modified Rankin Scale scores at 90 days were available for 2310 of 2313 participants (99.9%). The modified Rankin Scale scores were missing for 1 patient treated with thrombectomy alone before 2 hours 20 minutes and 1 patient in each group treated later than 2 hours 20 minutes.
Sensitivity Analyses for the Primary Outcome
The prespecified sensitivity analyses yielded similar results. For all models, the estimated time at which the lower boundary of the 95% CI for the adjusted common OR first crossed 1.0 for the estimated association of IVT before thrombectomy with better outcomes varied between 2 hours 11 minutes to 2 hours 30 minutes (eTable 3 in Supplement 1). The nonlinear associations of time from symptom onset to expected administration of IVT, the influence of relaxing the proportional odds assumption, and the analyses regarding individual trial effects confirmed the robustness of the primary outcome result (eTables 4-5 and eFigures 9-14 in Supplement 1).
In a post hoc sensitivity analysis using time from symptom onset to randomization instead of time from symptom onset to expected administration of IVT, the estimated time at which the lower boundary of the 95% CI for the estimated association of IVT plus thrombectomy with better outcomes first crossed 1.0 was at 2 hours 11 minutes, whereas the point estimate crossed the null association at 3 hours 4 minutes.
Secondary Outcomes
For functional independence (modified Rankin Scale score of 0, 1, or 2), there was a significant interaction of the benefit associated with IVT plus thrombectomy vs thrombectomy alone and time from symptom onset to expected administration of IVT (ratio of adjusted OR per 1-hour delay, 0.82 [95% CI, 0.68-0.98], P = .03 for interaction; Table 3 and eFigure 15 in Supplement 1). If treated early, the rates of functional independence were significantly higher in participants treated with IVT plus thrombectomy at 1 hour (adjusted OR, 1.54 [95% CI, 1.10-2.17]) and at 2 hours (adjusted OR, 1.26 [95% CI, 1.02-1.56]).
Early recanalization occurred more often in participants allocated to IVT plus thrombectomy (4.0% vs 1.7% for thrombectomy alone), and the association was stronger in participants treated later from symptom onset (adjusted OR, 0.95 [95% CI, 0.36-2.54] at 1 hour; adjusted OR, 3.48 [95% CI, 1.44-8.42] at 3 hours); and the ratio of adjusted OR per 1-hour delay was 1.91 (95% CI, 1.05-3.48), P = .03 for interaction; Table 3 and eFigure 16 in Supplement 1). This interaction was not confirmed in analyses that left out 1 of each included trial of this individual participant meta-analysis (eTable 6 in Supplement 1). The estimated odds for an association between IVT plus thrombectomy vs thrombectomy alone and higher rates of successful reperfusion declined with extended time from symptom onset to expected administration of IVT (adjusted OR, 2.06 [95% CI, 1.29-3.29] at 1 hour; adjusted OR, 1.77 [95% CI, 1.26-2.47] at 2 hours; and adjusted OR, 1.52 [95% CI, 1.10-2.08] at 3 hours), but this interaction was not statistically significant (ratio of adjusted OR per 1-hour delay, 0.86 [95% CI, 0.66-1.11], P = .26 for interaction; eFigure 17 in Supplement 1).
No statistically significant interaction with time from symptom onset to expected administration of IVT was observed for the outcomes of symptomatic or any intracranial hemorrhage (Table 3 and eFigures 18-19 in Supplement 1). In addition, there was no statistically significant interaction between treatment allocation and the time interval between randomization and thrombectomy start (arterial puncture) (eTable 7 in Supplement 1).
Discussion
In participants with an anterior-circulation large-vessel occlusion stroke who presented directly to endovascular treatment centers, the benefit associated with IVT in the setting of endovascular thrombectomy was time dependent. The observed interaction was in the direction of a beneficial association of IVT with improved functional outcomes when administered earlier. Using the only available pooled individual participant randomized clinical trial data of patients undergoing thrombectomy with vs without IVT, there was a significant benefit associated with IVT plus thrombectomy compared with thrombectomy alone if the time between symptom onset and expected administration of IVT was within 2 hours 20 minutes.
The magnitude of the association between delay to expected administration of IVT and the change in the magnitude of the association between IVT plus thrombectomy and better outcomes was clinically meaningful. Our results suggest a reasonably large benefit associated with IVT plus thrombectomy if IVT was administered early. However, similar to trials comparing IVT vs placebo, we observed a rapid increase in the estimated number of patients needed to treat to achieve benefit when there were longer treatment delays.19,25
Notably, the change in the adjusted common OR appeared to follow a similar relative decline to the change in OR over time observed in trials comparing IVT vs placebo (end point of modified Rankin Scale score of 0 or 1).19 Although the overall association of IVT with better outcomes was smaller in patients who underwent thrombectomy compared with patients who did not, this may suggest a partially shared pathophysiological mechanism.19
Our findings were consistent with the data for observational comparisons. The Highly Effective Reperfusion Evaluated in Multiple Endovascular Stroke (HERMES) Collaborators21 found that the rates of good outcomes declined more rapidly in patients undergoing thrombectomy who received IVT than in patients ineligible for IVT, suggesting a potential differential effect of time. In addition, recent observational registry data from the US showed that shorter times from door to administration of IVT were associated with improved outcomes in patients undergoing thrombectomy.7
After the publication of the HERMES results,6,21 theoretical considerations for and against the use of IVT before thrombectomy were presented.4,26 This equipoise was not only theoretical, but shared among treating physicians. In a multicenter analysis using the German Stroke Registry,27 37% of patients directly admitted to a comprehensive stroke center did not receive IVT before thrombectomy despite the absence of contraindications.
After the main results of the trials included in this meta-analysis were presented,2 an expedited update of the European Stroke Organisation-European Society for Minimally Invasive Neurological Therapy thrombectomy guidelines1 unequivocally recommended the use of IVT before thrombectomy in all eligible patients. However, the lack of superiority of IVT before thrombectomy led to ongoing discussions regarding this recommendation and the applicability and interpretation of the meta-analytic findings.2 In particular, the cost-effectiveness, the chosen designs, the potential influence of ethnic origin, and the margin deemed acceptable to establish noninferiority were questioned.1,24,28,29
Based on recent international recommendations, the credibility of this subgroup analysis was moderate, suggesting that an interaction is likely present and separate associations within the respective subgroups should be assumed.17 Therefore, we suggest that the time between symptom onset and expected administration of IVT should be considered in the decision-making process for patients who are directly admitted at stroke centers to undergo thrombectomy, although there is uncertainty with regard to the association of IVT plus thrombectomy and improved outcomes beyond the time point indicating a loss of a significant association. For the decision-making process, it should be taken into consideration that the analysis provided high certainty that beyond 3 hours IVT was not associated with a clinical benefit larger than an ARD of 5% for a modified Rankin Scale score of 0, 1, or 2.
The abovementioned intervals appear meaningful for clinical routine and future trials. Median treatment delay of IVT before thrombectomy (from symptom onset to thrombolysis administration) in patients directly admitted to thrombectomy-capable centers varied in recent reports, with 1 hour and 22 minutes to 1 hour and 30 minutes being reported in large Western registries,30,31,32 and 1 hour and 57 minutes reported in a metropolitan Japanese registry.33 The median time from symptom onset to arrival in the emergency department for patients directly admitted to endovascular treatment–capable centers was 1 hour and 5 minutes in HERMES6 and 2 hours 35 minutes in the Transfer to the Closest Local Stroke Center vs Direct Transfer to Endovascular Stroke Center of Acute Stroke Patients With Suspected Large Vessel Occlusion in the Catalan Territory (RACECAT) trial.34
Hence, depending on the geographic setting, up to approximately 50% of patients eligible for IVT may receive a treatment that showed no statistically significant association with better outcomes according to the data in the current study. If the current study’s findings are validated, they can help to stratify cohorts by patients undergoing thrombectomy for which IVT is likely associated with a significant benefit, patients in whom there is uncertainty regarding a beneficial association of IVT plus thrombectomy with better outcomes, and patients in whom it appears very unlikely that a potential beneficial association of IVT plus thrombectomy is present.
The mechanism by which IVT was associated with increased odds for better functional outcomes in the early but not in the late time window could not be determined in the current study. Even though IVT clearly favors early recanalization before thrombectomy, the absolute differences were very small and, surprisingly, this association was found to be stronger in patients treated later. Hence, the classic infarct progression concept usually put forward to explain a considerable proportion of the time dependency observed in IVT vs placebo trials does not seem to explain the time dependency of associations observed in the current study. Therefore, further research is needed to elucidate the role of different contributors to the interaction observed.
Limitations
First, our results are applicable only to patients presenting directly at centers with endovascular facilities. Second, central adjudication of images was at the study level and images were not reevaluated centrally.
Third, the use of admission imaging to exclude potential trial participants with high volumes of likely irreversibly infarcted brain tissue based on the protocols of the trials included may have influenced the direction and magnitude of the observed associations with treatment times. Fourth, the results of the interaction analyses using times from randomization to thrombectomy start should be interpreted cautiously because inclusion of the covariates observed after randomization can lead to biased estimates.35,36,37,38
Fifth, the included trials were designed so that eligibility for thrombectomy had to be assessed before randomization, potentially delaying the administration of IVT depending on institutional standards of the participating centers. Sixth, because eligibility for thrombectomy (including anticipated difficulty of arterial access among others) was evaluated for inclusion of participants into the trials, the results may not be generalizable to cases when eligibility for thrombectomy is questionable (eg, arterial access is deemed more technically challenging).
Seventh, almost all participants allocated to IVT plus thrombectomy were treated with intravenous alteplase, and only 25 participants were treated with intravenous tenecteplase. This number was too small to conduct meaningful subgroup analyses. Therefore, the results may not be generalizable to IVT with tenecteplase. The updated thrombectomy guidelines from the European Stroke Organisation-European Society for Minimally Invasive Neurological Therapy39 currently recommend administration of 0.25 mg/kg of tenecteplase instead of 0.9 mg/kg of alteplase in patients presenting with large-vessel occlusion stroke who are eligible for IVT. The expert consensus favored IVT with tenecteplase followed by thrombectomy instead of thrombectomy alone despite the lack of randomized clinical trial evidence to support this recommendation.39 It should be noted, however, that superiority of intravenous tenecteplase vs intravenous alteplase in directly admitted patients undergoing thrombectomy is not well established.
In patients undergoing thrombectomy included in the Intravenous Tenecteplase Compared With Alteplase for Acute Ischemic Stroke in Canada (AcT) trial,40 no significant difference in outcomes between patients allocated to IVT with alteplase vs tenecteplase was observed. Although the influence of time on clinical outcomes appears similar in patients treated with alteplase and in patients treated with tenecteplase,41 the potentially higher efficacy of tenecteplase in patients with large-vessel occlusion stroke undergoing thrombectomy observed in the Tenecteplase versus Alteplase Before Endovascular Therapy for Ischemic Stroke (EXTEND-IA TNK) trial42 may lead to different magnitudes and cutoffs in the observed associations. Ongoing trials,43 including the Randomization to Endovascular Treatment Alone or Preceded by Systemic Thrombolysis With Tenecteplase in Ischemic Stroke (DIRECT-TNK) trial (NCT05199194), aim to answer this specific question.
Conclusions
In patients presenting at thrombectomy-capable stroke centers, the benefit associated with IVT plus thrombectomy vs thrombectomy alone was time dependent and statistically significant only if the time from symptom onset to expected administration of IVT was short.
eMethods 1. Systematic Search Strategy
eMethods 2. Details and Rational regarding the estimated time metrics
eMethods 3. Statistical Analysis Plan
eMethods 4. Further statistical methods
eResults. Credibility assessment
eTable 1. Characteristics of the trials included
eTable 2. Availability of Time Metrics and Outcomes
eTable 3. Sensitivity analyses of the primary analysis
eTable 4. Assessment of non-linear relationships
eTable 5. Formal testing for evidence of nominal and scaling effects
eTable 6. Leave-One-Study-Out analysis regarding the interaction of onset-to-expected- IVT times and the association of IVT plus thrombectomy vs thrombectomy with early recanalization
eTable 7. Interaction of time from randomization to thrombectomy start and associations of intravenous thrombolysis plus thrombectomy with outcomes
eFigure 1. PRISMA Flow Chart
eFigure 2. Risk of Bias Across the Included Randomized-Controlled Trials
eFigure 3. Distribution of randomization-to-observed-IVT times
eFigure 4. Distribution of onset-to-expected-IVT times
eFigure 5. Histogram of other treatment metrics
eFigure 6. Number needed to treat and absolute risk difference based on predicted probabilities
eFigure 7. Influence of onset-to-expected-IVT times on the benefit associated with IVT plus thrombectomy (additional cut-offs)
eFigure 8. Comparison to trials comparing intravenous thrombolysis versus placebo
eFigure 9. Change in the benefit associated with IVT plus thrombectomy over onset-to- expected –IVT time quantiles
eFigure 10. Effect of relaxing the proportional odds assumptions
eFigure 11. Contribution of participants of each trial before and after 2 hours 20 minutes
eFigure 12. Study-level associations
eFigure 13. Leave-one-study-out associations
eFigure 14. Correlation matrix of covariates in the primary model
eFigure 15. Change in the association between intravenous thrombolysis plus thrombectomy versus thrombectomy alone and rates of mRS 0-2
eFigure 16. Change in the association of intravenous thrombolysis plus thrombectomy versus thrombectomy alone and early recanalization on first angiographic images
eFigure 17. Change in the association of intravenous thrombolysis plus thrombectomy versus thrombectomy alone and successful reperfusion (TICI2b-3) at the end of the endovascular intervention
eFigure 18. Change in the risk of symptomatic intracranial hemorrhage after intravenous thrombolysis plus thrombectomy versus thrombectomy alone
eFigure 19. Change in the risk of any intracranial hemorrhage after intravenous thrombolysis plus thrombectomy versus thrombectomy alone
eReferences
Nonauthor collaborators
Data sharing statement
References
- 1.Turc G, Tsivgoulis G, Audebert HJ, et al. European Stroke Organisation (ESO)-European Society for Minimally Invasive Neurological Therapy (ESMINT) expedited recommendation on indication for intravenous thrombolysis before mechanical thrombectomy in patients with acute ischemic stroke and anterior circulation large vessel occlusion. J Neurointerv Surg. 2022;14(3):209-227. doi: 10.1136/neurintsurg-2021-018589 [DOI] [PubMed] [Google Scholar]
- 2.Majoie CB, Cavalcante F, Gralla J, et al. ; IRIS Collaborators . Value of intravenous thrombolysis in endovascular treatment for large-vessel anterior circulation stroke: individual participant data meta-analysis of six randomised trials. Lancet. 2023;402(10406):965-974. doi: 10.1016/S0140-6736(23)01142-X [DOI] [PubMed] [Google Scholar]
- 3.Piaggio G, Elbourne DR, Altman DG, Pocock SJ, Evans SJW; CONSORT Group . Reporting of noninferiority and equivalence randomized trials: an extension of the CONSORT statement. JAMA. 2006;295(10):1152-1160. doi: 10.1001/jama.295.10.1152 [DOI] [PubMed] [Google Scholar]
- 4.Fischer U, Kaesmacher J, Mendes Pereira V, et al. Direct mechanical thrombectomy versus combined intravenous and mechanical thrombectomy in large-artery anterior circulation stroke: a topical review. Stroke. 2017;48(10):2912-2918. doi: 10.1161/STROKEAHA.117.017208 [DOI] [PubMed] [Google Scholar]
- 5.Nogueira RG, Tsivgoulis G. Large vessel occlusion strokes after the DIRECT-MT and SKIP trials: is the alteplase syringe half empty or half full? Stroke. 2020;51(10):3182-3186. doi: 10.1161/STROKEAHA.120.030796 [DOI] [PubMed] [Google Scholar]
- 6.Goyal M, Menon BK, van Zwam WH, et al. ; HERMES Collaborators . Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723-1731. doi: 10.1016/S0140-6736(16)00163-X [DOI] [PubMed] [Google Scholar]
- 7.Man S, Solomon N, Mac Grory B, et al. Shorter door-to-needle times are associated with better outcomes after intravenous thrombolytic therapy and endovascular thrombectomy for acute ischemic stroke. Circulation. 2023;148(1):20-34. doi: 10.1161/CIRCULATIONAHA.123.064053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zi W, Qiu Z, Li F, et al. ; DEVT Trial Investigators . Effect of endovascular treatment alone vs intravenous alteplase plus endovascular treatment on functional independence in patients with acute ischemic stroke: the DEVT randomized clinical trial. JAMA. 2021;325(3):234-243. doi: 10.1001/jama.2020.23523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang P, Zhang Y, Zhang L, et al. ; DIRECT-MT Investigators . Endovascular thrombectomy with or without intravenous alteplase in acute stroke. N Engl J Med. 2020;382(21):1981-1993. doi: 10.1056/NEJMoa2001123 [DOI] [PubMed] [Google Scholar]
- 10.Mitchell PJ, Yan B, Churilov L, et al. ; DIRECT-SAFE Investigators . Endovascular thrombectomy versus standard bridging thrombolytic with endovascular thrombectomy within 4·5 h of stroke onset: an open-label, blinded-endpoint, randomised non-inferiority trial. Lancet. 2022;400(10346):116-125. doi: 10.1016/S0140-6736(22)00564-5 [DOI] [PubMed] [Google Scholar]
- 11.LeCouffe NE, Kappelhof M, Treurniet KM, et al. ; MR CLEAN–NO IV Investigators . A randomised trial of intravenous alteplase before endovascular treatment for stroke. N Engl J Med. 2021;385(20):1833-1844. doi: 10.1056/NEJMoa2107727 [DOI] [PubMed] [Google Scholar]
- 12.Suzuki K, Matsumaru Y, Takeuchi M, et al. ; SKIP Study Investigators . Effect of mechanical thrombectomy without vs with intravenous thrombolysis on functional outcome among patients with acute ischemic stroke: the SKIP randomized clinical trial. JAMA. 2021;325(3):244-253. doi: 10.1001/jama.2020.23522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fischer U, Kaesmacher J, Strbian D, et al. ; SWIFT DIRECT Collaborators . Thrombectomy alone versus intravenous alteplase plus thrombectomy in patients with stroke: an open-label, blinded-outcome, randomised non-inferiority trial. Lancet. 2022;400(10346):104-115. doi: 10.1016/S0140-6736(22)00537-2 [DOI] [PubMed] [Google Scholar]
- 14.IRIS Collaborators . IRIS Collaboration pooled analysis: statistical analysis plan. https://mrclean-noiv.nl/DOC/IRIS_SAP_220928.pdf
- 15.IRIS Collaborators . IRIS—time to treatment individual participant meta-analysis—update SAP. Published 2023. Accessed January 26, 2024. doi: 10.17605/OSF.IO/Y9BNW [DOI]
- 16.Stewart LA, Clarke M, Rovers M, et al. ; PRISMA-IPD Development Group . Preferred Reporting Items for Systematic Review and Meta-Analyses of Individual Participant Data: the PRISMA-IPD statement. JAMA. 2015;313(16):1657-1665. doi: 10.1001/jama.2015.3656 [DOI] [PubMed] [Google Scholar]
- 17.Schandelmaier S, Briel M, Varadhan R, et al. Development of the Instrument to Assess the Credibility of Effect Modification Analyses (ICEMAN) in randomized controlled trials and meta-analyses. CMAJ. 2020;192(32):E901-E906. doi: 10.1503/cmaj.200077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berge E, Whiteley W, Audebert H, et al. European Stroke Organisation (ESO) guidelines on intravenous thrombolysis for acute ischaemic stroke. Eur Stroke J. 2021;6(1):I-LXII. doi: 10.1177/2396987321989865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Emberson J, Lees KR, Lyden P, et al. ; Stroke Thrombolysis Trialists’ Collaborative Group . Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. 2014;384(9958):1929-1935. doi: 10.1016/S0140-6736(14)60584-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stroke Thrombolysis Trialists’ Collaborative Group . Details of a prospective protocol for a collaborative meta-analysis of individual participant data from all randomized trials of intravenous rt-PA vs control: statistical analysis plan for the Stroke Thrombolysis Trialists’ Collaborative meta-analysis. Int J Stroke. 2013;8(4):278-283. doi: 10.1111/ijs.12040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saver JL, Goyal M, van der Lugt A, et al. ; HERMES Collaborators . Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: a meta-analysis. JAMA. 2016;316(12):1279-1288. doi: 10.1001/jama.2016.13647 [DOI] [PubMed] [Google Scholar]
- 22.Cranston JS, Kaplan BD, Saver JL. Minimal clinically important difference for safe and simple novel acute ischemic stroke therapies. Stroke. 2017;48(11):2946-2951. doi: 10.1161/STROKEAHA.117.017496 [DOI] [PubMed] [Google Scholar]
- 23.Liebeskind DS, Bracard S, Guillemin F, et al. ; HERMES Collaborators . eTICI reperfusion: defining success in endovascular stroke therapy. J Neurointerv Surg. 2019;11(5):433-438. doi: 10.1136/neurintsurg-2018-014127 [DOI] [PubMed] [Google Scholar]
- 24.Kaesmacher J, Mujanovic A, Treurniet K, et al. Perceived acceptable uncertainty regarding comparability of endovascular treatment alone versus intravenous thrombolysis plus endovascular treatment. J Neurointerv Surg. 2023;15:227-232. doi: 10.1136/neurintsurg-2022-018665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lees KR, Bluhmki E, von Kummer R, et al. ; ECASS, ATLANTIS, NINDS and EPITHET rt-PA Study Group . Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet. 2010;375(9727):1695-1703. doi: 10.1016/S0140-6736(10)60491-6 [DOI] [PubMed] [Google Scholar]
- 26.Chandra RV, Leslie-Mazwi TM, Mehta BP, et al. Does the use of IV tPA in the current era of rapid and predictable recanalization by mechanical embolectomy represent good value? J Neurointerv Surg. 2016;8(5):443-446. doi: 10.1136/neurintsurg-2015-012231 [DOI] [PubMed] [Google Scholar]
- 27.Schlemm L, Siebert E, Kleine JF, et al. ; GSR-ET Investigators . Decline of thrombolysis rates before endovascular therapy in patients with acute anterior circulation large vessel occlusion ischemic stroke: a multicenter analysis from the German Stroke Registry. Eur Stroke J. 2023;8(3):610-617. doi: 10.1177/23969873231177774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Campbell BCV, Kappelhof M, Fischer U. Role of intravenous thrombolytics prior to endovascular thrombectomy. Stroke. 2022;53(6):2085-2092. doi: 10.1161/STROKEAHA.122.036929 [DOI] [PubMed] [Google Scholar]
- 29.Fiehler J, Zeleňák K, Zapf A. The price of certainty: when is a new therapy good enough? J Neurointerv Surg. 2021;13(12):1065-1066. doi: 10.1136/neurintsurg-2021-018392 [DOI] [PubMed] [Google Scholar]
- 30.Schaefer JH, Kurka N, Keil F, et al. Endovascular treatment for ischemic stroke with the drip-and-ship model—insights from the German Stroke Registry. Front Neurol. 2022;13:973095. doi: 10.3389/fneur.2022.973095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Froehler MT, Saver JL, Zaidat OO, et al. ; STRATIS Investigators . Interhospital transfer before thrombectomy is associated with delayed treatment and worse outcome in the STRATIS Registry (Systematic Evaluation of Patients Treated With Neurothrombectomy Devices for Acute Ischemic Stroke). Circulation. 2017;136(24):2311-2321. doi: 10.1161/CIRCULATIONAHA.117.028920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Venema E, Groot AE, Lingsma HF, et al. Effect of interhospital transfer on endovascular treatment for acute ischemic stroke. Stroke. 2019;50(4):923-930. doi: 10.1161/STROKEAHA.118.024091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ueda T, Hasegawa Y, Takeuchi M, et al. ; K-NET Registry Investigators . Primary results of mechanical thrombectomy for acute ischemic stroke: the K-NET registry in the Japanese metropolitan area. Int J Stroke. 2023;18(5):607-614. doi: 10.1177/17474930221138014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pérez de la Ossa N, Abilleira S, Jovin TG, et al. ; RACECAT Trial Investigators . Effect of direct transportation to thrombectomy-capable center vs local stroke center on neurological outcomes in patients with suspected large-vessel occlusion stroke in nonurban areas: the RACECAT randomized clinical trial. JAMA. 2022;327(18):1782-1794. doi: 10.1001/jama.2022.4404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen X, Liu M, Zhang J. A note on postrandomization adjustment of covariates. Drug Inf J. 2005;39(4):373-383. doi: 10.1177/009286150503900405 [DOI] [Google Scholar]
- 36.US Food and Drug Administration . Adjusting for covariates in randomized clinical trials for drugs and biological products; guidance for industry; availability. Published May 26, 2023. Accessed January 26, 2024. https://www.federalregister.gov/documents/2023/05/26/2023-11263/adjusting-for-covariates-in-randomized-clinical-trials-for-drugs-and-biological-products-guidance
- 37.European Medicines Agency . Guideline on adjustment for baseline covariates in clinical trials. Published February 26, 2015. Accessed January 26, 2024. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-adjustment-baseline-covariates-clinical-trials_en.pdf
- 38.Rochon J. Issues in adjusting for covariates arising postrandomization in clinical trials. Drug Inf J. 1999;33(4):1219-1228. doi: 10.1177/009286159903300425 [DOI] [Google Scholar]
- 39.Alamowitch S, Turc G, Palaiodimou L, et al. European Stroke Organisation (ESO) expedited recommendation on tenecteplase for acute ischaemic stroke. Eur Stroke J. 2023;8(1):8-54. doi: 10.1177/23969873221150022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Menon BK, Buck BH, Singh N, et al. ; AcT Trial Investigators . Intravenous tenecteplase compared with alteplase for acute ischaemic stroke in Canada (AcT): a pragmatic, multicentre, open-label, registry-linked, randomised, controlled, non-inferiority trial. Lancet. 2022;400(10347):161-169. doi: 10.1016/S0140-6736(22)01054-6 [DOI] [PubMed] [Google Scholar]
- 41.Singh N, Almekhlafi MA, Bala F, et al. Effect of time to thrombolysis on clinical outcomes in patients with acute ischemic stroke treated with tenecteplase compared to alteplase: analysis from the AcT randomized controlled trial. Stroke. 2023;54(11):2766-2775. doi: 10.1161/STROKEAHA.123.044267 [DOI] [PubMed] [Google Scholar]
- 42.Campbell BCV, Mitchell PJ, Churilov L, et al. ; EXTEND-IA TNK Investigators . Tenecteplase versus alteplase before thrombectomy for ischemic stroke. N Engl J Med. 2018;378(17):1573-1582. doi: 10.1056/NEJMoa1716405 [DOI] [PubMed] [Google Scholar]
- 43.Singh N, Menon BK, Dmytriw AA, Regenhardt RW, Hirsch JA, Ganesh A. Replacing alteplase with tenecteplase: is the time ripe? J Stroke. 2023;25(1):72-80. doi: 10.5853/jos.2022.02880 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods 1. Systematic Search Strategy
eMethods 2. Details and Rational regarding the estimated time metrics
eMethods 3. Statistical Analysis Plan
eMethods 4. Further statistical methods
eResults. Credibility assessment
eTable 1. Characteristics of the trials included
eTable 2. Availability of Time Metrics and Outcomes
eTable 3. Sensitivity analyses of the primary analysis
eTable 4. Assessment of non-linear relationships
eTable 5. Formal testing for evidence of nominal and scaling effects
eTable 6. Leave-One-Study-Out analysis regarding the interaction of onset-to-expected- IVT times and the association of IVT plus thrombectomy vs thrombectomy with early recanalization
eTable 7. Interaction of time from randomization to thrombectomy start and associations of intravenous thrombolysis plus thrombectomy with outcomes
eFigure 1. PRISMA Flow Chart
eFigure 2. Risk of Bias Across the Included Randomized-Controlled Trials
eFigure 3. Distribution of randomization-to-observed-IVT times
eFigure 4. Distribution of onset-to-expected-IVT times
eFigure 5. Histogram of other treatment metrics
eFigure 6. Number needed to treat and absolute risk difference based on predicted probabilities
eFigure 7. Influence of onset-to-expected-IVT times on the benefit associated with IVT plus thrombectomy (additional cut-offs)
eFigure 8. Comparison to trials comparing intravenous thrombolysis versus placebo
eFigure 9. Change in the benefit associated with IVT plus thrombectomy over onset-to- expected –IVT time quantiles
eFigure 10. Effect of relaxing the proportional odds assumptions
eFigure 11. Contribution of participants of each trial before and after 2 hours 20 minutes
eFigure 12. Study-level associations
eFigure 13. Leave-one-study-out associations
eFigure 14. Correlation matrix of covariates in the primary model
eFigure 15. Change in the association between intravenous thrombolysis plus thrombectomy versus thrombectomy alone and rates of mRS 0-2
eFigure 16. Change in the association of intravenous thrombolysis plus thrombectomy versus thrombectomy alone and early recanalization on first angiographic images
eFigure 17. Change in the association of intravenous thrombolysis plus thrombectomy versus thrombectomy alone and successful reperfusion (TICI2b-3) at the end of the endovascular intervention
eFigure 18. Change in the risk of symptomatic intracranial hemorrhage after intravenous thrombolysis plus thrombectomy versus thrombectomy alone
eFigure 19. Change in the risk of any intracranial hemorrhage after intravenous thrombolysis plus thrombectomy versus thrombectomy alone
eReferences
Nonauthor collaborators
Data sharing statement